Abstract
Statins are among the most widely used drug classes in the world. Apart from their basic mechanism of action, which is lowering cholesterol levels, many pleiotropic effects have been described so far, such as anti-inflammatory and antiatherosclerotic effects. A growing number of scientific reports have proven that these drugs have a beneficial effect on the functioning of the nervous system. The first reports proving that lipid-lowering therapy can influence the development of neurological and psychiatric diseases appeared in the 1990s. Despite numerous studies about the mechanisms by which statins may affect the functioning of the central nervous system (CNS), there are still no clear data explaining this effect. Most studies have focused on the metabolic effects of this group of drugs, however authors have also described the pleiotropic effects of statins, pointing to their probable impact on the neurotransmitter system and neuroprotective effects. The aim of this paper was to review the literature describing the impacts of statins on dopamine, serotonin, acetylcholine, and glutamate neurotransmission, as well as their neuroprotective role. This paper focuses on the mechanisms by which statins affect neurotransmission, as well as on their impacts on neurological and psychiatric diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), vascular dementia (VD), stroke, and depression. The pleiotropic effects of statin usage could potentially open floodgates for research in these treatment domains, catching the attention of researchers and clinicians across the globe.
Keywords: dopamine, acetylcholine, glutamate, BDNF, serotonin, neurotransmitters, statins, neurodegenerative diseases, stroke, depression
1. Introduction
Statins are the most widespread group of lipid-lowering drugs in the world [1]. For this reason, they are recommended for the primary and secondary prevention of cardiovascular events [2]. For many years, other effects of this group of drugs have been well known, which are primarily focused on anti-inflammatory activity [3,4]. The first scientific reports on the impacts of antilipid therapy on psychiatric and neurological diseases appeared in the 1990s. In 1990, Muldoon et al. proved that cholesterol-lowering therapy increases the risk of death in men as a result of accidents and suicide [5]. Subsequent reports also showed a relationship between cholesterol levels and the occurrence of anxiety, depression, and related suicide [6,7]. Moreover, despite very ambiguous results concerning these effects, meta-analyses have shown that statins reduce depressive symptoms and the frequency of hospitalization caused by intensification of these symptoms [8,9]. At the same time, reports began to appear in which researchers described the relationship between cholesterol level and the symptom severity in neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [10,11]. These observations prompted researchers to look for a potential mechanism of action by which statins act on neurotransmitter systems to influence neurological and psychiatric disorders.
This study aims to systematize the current knowledge about the potential mechanisms by which statins affect cholinergic, dopaminergic, glutaminergic, and serotonergic transmission, as well as the impact of these interactions on the development and progression of neurodegenerative diseases and psychiatric disorders. Obviously, the effects of statins on neurological diseases through their lowering of the amount of total cholesterol and antiatherosclerotic effects seem not to be overlooked. However, in our manuscript, we only focus on their effects on neurotransmission and their neuroprotective role, because this topic is still a subject of discussion among scientists and requires further clinical research [12,13]. In this publication, we try to systematically review the current scientific data from international reports. For this purpose, the PubMed databases were reviewed in order to isolate reports according to the following key phrases: “statins and neurotransmission”, “lipid signaling and neurotransmission”, “statins and neurodegenerative diseases”, and “statins and psychiatric disorders”.
2. Statins–Structure and Permeability
Statins are drugs whose primary mechanism of action is to inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR). This is related to the ability of the pharmacophore, which for all statins is a dihydroheptanoic acid, to lower HMGCR activity. However, it is not a pharmacophore but rather the covalently related hydrophobic ring system that determines the chemical properties of individual statins, such as their solubility or pharmacokinetic properties.
Statins are divided into two categories: type 1, natural or semi-synthetic (these include lovastatin, simvastatin, and pravastatin); and type 2, otherwise known as fully synthetic [14]. One of the differences between the types of statins is their ability to bind to HMGCR. Type 2 statins, such as atorvastatin and rosuvastatin, are able to interact more strongly with HMGCR due to their greater hydrogen binding capacity [15]. The second difference is their different hydrophilicity. Lovastatin, simvastatin, fluvastatin, pitavastatin, and cerivastatin are more lipophilic, while rosuvastatin and pravastatin are more hydrophilic. This feature is very important in the context of the pleiotropic effects of this group of drugs. Lipophilic statins have a greater ability to passively pass from blood to tissues, including the ability to cross the blood–brain barrier (BBB). This results, among other things, in a greater severity of side effects. Hydrophilic statins, due to the necessity to penetrate inside the cells by active transport, show a more hepatoselective effect, which means that other effects, apart from lipid-lowering activity, are less intense. Recent studies confirm this difference in the ability to produce pleiotropic effects after taking type 1 and type 2 statins [16].
In summary, the basic differences between the two types of statins consist of their differences in chemical structure, which result in different pharmacokinetics for both types and the ability to penetrate into different types of tissues. Thereby, this results in the differentially expressed capacity to induce pleiotropic effects by different types of statins, including actions on the central nervous system (CNS).
3. Statins and Dopaminergic Neurotransmission
3.1. Structure and Synthesis of Dopamine
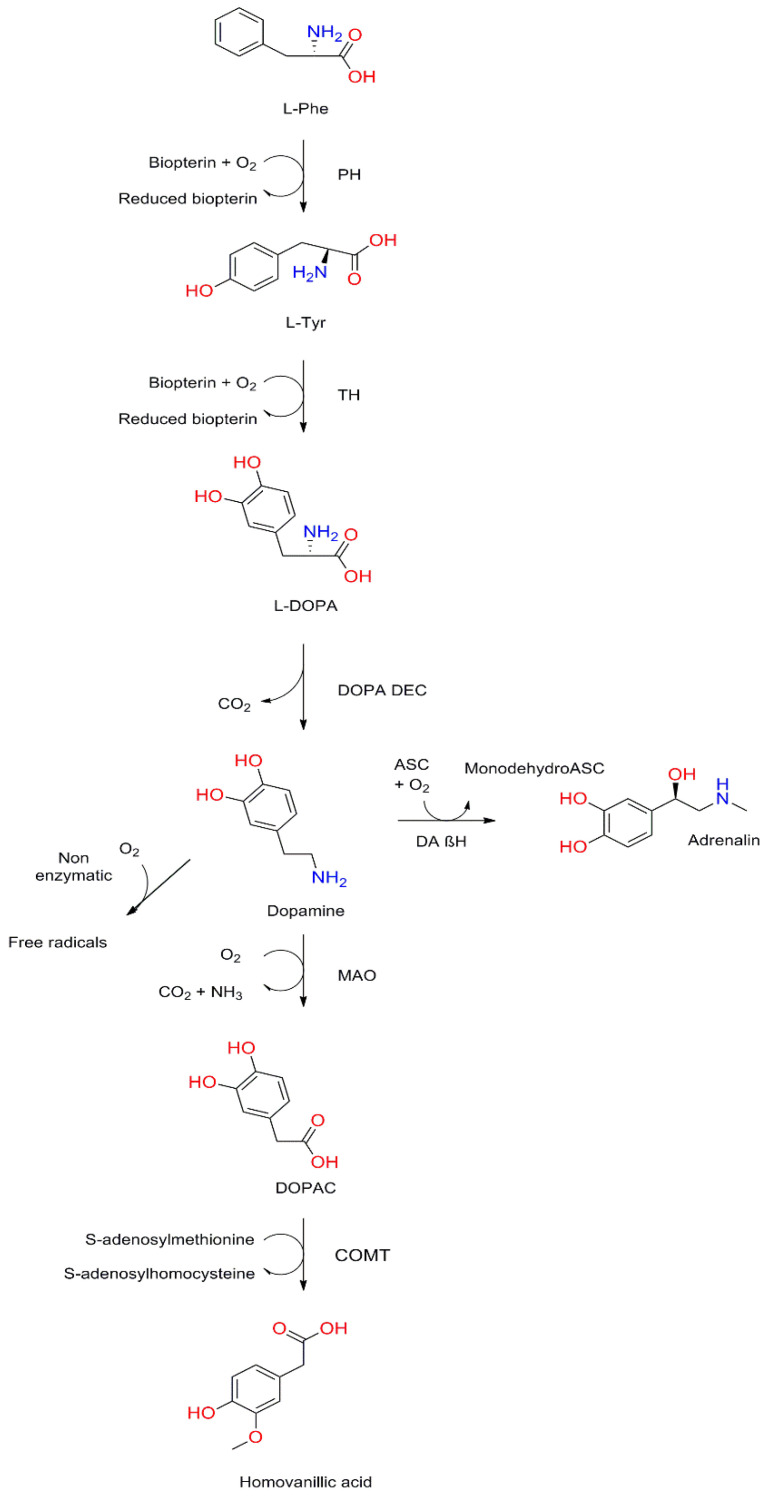
The chemical 4-(2-aminoethyl)-1,2-benzenediol, known as dopamine (DA), is one of the most important neurotransmitters in the human nervous system. It is synthesized from phenylalanine (Phe), which is converted by phenylalanine hydroxylase (PH) to tyrosine (Tyr), which is a precursor of several important bioactive molecules. Two enzymes are involved in the conversion of Tyr to DA: L-tyrosine hydroxylase (TH), used as a marker for dopamine-producing cells, and levo-dopa decarboxylase (DOPA DEC). DA synthesized in cells can be used and is then degraded, but in some cells with dopamine beta-hydroxylase (DAβH), such as adrenal gland cells, it takes part in the synthesis of norepinephrine (NA). The detailed synthesis and degradation process is shown in Figure 1.
Figure 1.
Dopamine synthesis and degradation pathways. L-Phe, L-phenylalanine; PH, phenylalanine hydroxylase; L-Tyr, L-tyrosine; TH, tyrosine hydroxylase; L-DOPA, levo-dopa; DOPA DEC, L-DOPA decarboxylase; ASC, ascorbic acid; DA βH, dopamine β-hydroxylase; MAO, monoamine oxidase; DOPAC, 3,4-dihydroxyphenylacetic acid; COMT, catechol-o-methyltransferase.
In the CNS, the process described above is carried out by groups of neurons called dopaminergic neurons, which can be found in many different parts of the CNS but are mostly concentrated in the substantia nigra pars compacta (SNpc). These neurons are responsible for receiving signals traveling from the striatum, then processing them and further transmitting them to other parts of the CNS, such as the globus pallidus (GP), thalamus, or substantia nigra pars reticulata (SNpr). DA, through the signaling pathways described above, participates in many processes regulated by the CNS, from the control of motor functions to cognition. Its action is based on two well-known mechanisms. The first one, called wiring transmission, involves the release of DA by neurons into the synaptic cleft, which then the released neurotransmitter acts on receptors in the postsynaptic membrane. The second mechanism, which is much more interesting, is called volume transmission, in which DA released from the presynaptic membrane reaches the extracellular space and binds to the dopaminergic receptors of neurons, which are not in direct contact with the cell from which it is released [17,18,19].
3.2. Dopamine Receptors
So far, five dopamine receptors (D1, D2, D3, D4, and D5) have been described. They belong to the G-protein-coupled receptor (GPCR) family. It is considered that the binding of DA to these receptors leads to changes in the concentration of cyclic adenosine monophosphate (cAMP), which changes the activity of kinase DA- and cAMP-regulated phosphoprotein of 32 kDa molecular weight (DARPP32), which is a key protein in dopaminergic neurotransmission. This is mediated by G proteins associated with the individual dopaminergic receptors. The Gs protein, associated with D1 and D5 receptors, causes the activation of adenylate cyclase (AC), which causes an increase in cAMP concentration, while the Gi protein, associated with D2, D3, and D4 receptors, causes inactivation of AC and a decrease in cAMP concentration [20]. This process is shown in Figure 2. Importantly, dopamine receptors can be found not only in the brain, but also in other types of tissues, which leads to the conclusion that DA is more than just a neurotransmitter [21,22,23].
Figure 2.
Mechanism of action of dopaminergic receptors. The binding of dopamine to the D1 and D5 receptors causes the activation of adenylate cyclase (AC) via the Gs protein. Activation of AC causes an increase in the concentration of cyclic adenosine monophosphate (cAMP), which results in an increase in the concentration of DA- and cAMP-regulated phosphoprotein of 32 kDa molecular weight (DARPP32), which penetrates into the cell nucleus, inducing a physiological response of the cell to dopamine. The reverse reaction is caused by the binding of dopamine to the D2, D3, and D4 receptors, which causes the inhibition of AC through the Gi protein.
Researchers have repeatedly described the presence of different variants of dopamine receptors and many polymorphisms of the genes encoding these receptors. Importantly, some of these polymorphisms may be associated with some types of addiction, such as alcohol or drug addiction [24,25,26,27]. The variety of DA’s effects and the variety of drugs affecting dopaminergic transmission come from the ability of dopamine receptors to form complexes in which they combine with each other or with other types of membrane receptors. Importantly, each of the heteromers formed in this way transmits a different signal inside the cell after activation by DA, so each has a different physiological role and pharmacological properties [28,29]. Examples of such heteromers are homeotropic heteromers D1–D3 [30], D2–D3 [31], D2–D5 [32], and D2–D4 [33] and heterotropic heteromers A1–D1 [34], A2A–D2 [35], D1–H3 [36], D2–H3 [37] and D4-adrenergic [38]. The presence of these heteromers is important not only in physiological mechanisms, such as the regulation of melatonin production by the pineal gland [39], but also in the pathogenesis of diseases such as PD. One of the main causes of this disease is the antagonism between dopaminergic transmission and purinergic regulation of neurotransmitter release caused by the presence of A1–D1 and A2A–D2 heteromers [40,41].
3.3. Cholesterol and Dopaminergic Transmission
Because disorders of dopaminergic transmission were found to be among the main causes of PD development, researchers have also described other mechanisms that are responsible for such disorders. One of the described mechanisms is a disorder of DA release and reuptake regulated by the dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) proteins. These proteins are key regulators of DA release into the synaptic cleft. Because the structure of DAT consists of two conserved cholesterol-like molecules, it is suggested that the protein may interact directly with cholesterol. In the absence of cholesterol, changes occur in the conformation of this protein that enhance DA reuptake, and in the presence of bound cholesterol these conformational changes are inhibited [42]. Moreover, cholesterol strengthens H-bonds, which bind DA and levo-dopa (L-DOPA) to the cell membrane, influencing their metabolism [43]. It is worth noting that the relationship between cholesterol and DA is not one-sided. Excess DA is responsible for the increase in cholesterol synthesis by activating the c-Jun N-terminal kinase (JNK3)/sterol regulatory element-binding protein 2 (SREBP2) signaling pathway in astrocyte colonies [44].
Another described mechanism by which cholesterol levels may influence the development of PD is an increased concentration of oxysterols produced from cholesterol. Evidence from studies shows that an elevated concentration of 24-hydroxycholesterol (24-OHC) in the cerebrospinal fluid of patients suffering from PD correlates with the worst prognosis [45]. Accordingly, it has been proposed that 24-OHC becomes a biomarker in PD. Other studies also indicate the effect of 27-hydroxycholesterol (27-OHC), another oxysterol. In dopaminergic neurons, this causes an increase in α-synuclein concentration by inhibiting proteasomes and activating the liver X receptors (LXRs) [46,47]. Moreover, 27-OHC induces inhibition of the estrogen receptor, which leads to inhibition of the expression of TH, and thus slows down the synthesis of DA [48].
The last mechanism by which cholesterol metabolism may affect neurodegenerative processes within dopaminergic neurons is related to the relationship between cholesterol and accumulated α-synuclein deposits [49]; α-synuclein is a protein whose overexpression may inhibit the transport and release of neurotransmitters from synaptic vesicles [50]. The α-synuclein molecule is made up of 140 amino acids and can be broken down into three domains: the N-terminal lipid-binding α-helix, the amyloid-binding central domain (known as NAC), and the C-terminal acidic tail. Importantly, its structure is characterized by a tandem repeat in the α-helix similar to those found in apolipoproteins. It follows that this protein has a structure similar to apolipoproteins [51,52]. The two cholesterol binding domains thus give the α-synuclein molecule a strong tendency to bind to lipid membranes, especially in cholesterol-rich regions. Moreover, studies conducted in vitro and in animal models show that α-synuclein could play a role in cholesterol transport [53,54,55]. Studies have reported that cholesterol may affect the interaction between α-synucelin oligomers and the cell membrane, which leads to membrane destruction, and thus cell death [56]. Moreover, with a low concentration of apolipoprotein E (APOE), α-synuclein is more prone to aggregation, which suggests that these two proteins may be competitively bound to cholesterol [57]. The mechanisms described above are illustrated in Figure 3.
Figure 3.
Cholesterol metabolism in Parkinson’s disease. After endocytosis of apolipoprotein E (APOE)-cholesterol particles, cholesterol is metabolized to 27-hydroxylcholesterol (27-OHC) and other oxysterols. Furthermore, 27-OHC can increase α-synuclein synthesis, downregulate tyrosine hydroxylase (TH) activity, and cause oxidative stress and apoptosis. In addition, excessive cholesterol and oxysterol can promote α-synuclein aggregation, and aggregated α-synuclein will eventually form Lewy bodies (LBs).
It is important to emphasize that the last two described mechanisms concerning oxysterols and the deposition of α-synuclein are responsible for neurodegeneration not only within dopaminergic neurons, but also within other types of neurons, which may result in the occurrence of diseases such as AD [58,59] or Lewy body dementia (LBD) [60].
3.4. Influence of Statins on Dopaminergic Transmission
Due to the above-described mechanisms involving the influence of cholesterol on neurodegenerative processes and dopaminergic transmission, researchers’ attention has been drawn to the influence of lipid-lowering therapy with statins on the course of neurodegenerative diseases such as PD and AD. Studies show that chronic statin treatment exerts an anti-inflammatory effect, inhibits oxidative stress, and has a preventive effect on apoptosis of neurons, including dopaminergic neurons [61,62]. This effect is mainly focused on inhibiting the release of pro-inflammatory cytokines and the activation of nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) cells [61]. It has also been proven in cell models that simvastatin, by inhibiting N-methyl-D-aspartate receptor 1 (NMDAR1), inhibits the inflammatory process within nerve cells [63]. Another mechanism by which statins inhibit neurodegenerative processes is in vitro reduction of beta-amyloid (Aβ) concentration in nerve cells [64], as well as activation of a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and increased activity of phospholipid transporter (PLTP), which reduces the concentration of plasma-phosphorylated tau181 (p-tau181) [65]. So far, however, there are no reports describing the influence of statins on the process of dopaminergic transmission by modifying cholesterol levels. All preclinical effects of statins on the process of neurotransmission and neuroprotection discussed in this article are summarized in the Table S1.
In connection with the above-described mechanisms, many clinical trials have been conducted to determine the effects of lipid-lowering therapy on the course of PD and AD. In the case of AD, previous studies have shown that statin therapy reduces the risk of AD by up to 70% [66,67]. However, later studies showed no correlation between this therapy and the risk of dementia [68]. These differences may be caused not only by differences in disease severity between patients, but also by the different chemical properties of the statins. For example, lipophilic statins, due to the ease of crossing BBB, show a stronger effect than hydrophilic ones in inhibiting the progression of AD [69]. In the case of PD research, the divergence is even greater. According to a meta-analysis prepared by Sheng et al., most observational studies show that statins can reduce the risk of PD by up to 26% [70], while several clinical studies have shown that statins are harmful to patients suffering from PD. Studies on the efficacy of statins for the prevention of PD and AD are summarized in Table 1.
Table 1.
Studies on the efficacy of statins for prevention of Alzheimer’s disease (AD) and Parkinson’sdisease (PD).
| Statins | Model | Group Size | Effects | References |
|---|---|---|---|---|
| All types | Rotterdam study | 6992 | Reduced risk of late-onset AD | Haag et al. [71] |
| Prospective study | 15,291 | Incerased risk of PD | Huang et al. [72] | |
| Retrospective case–control analysis | 2322 | Lipophilic statins increased risk of PD and hydrophilic statins did not affect incidence of PD | Liu et al. [73] | |
| Population-based cohort study | 232,877 | Statins did not affect incidence of PD | Rozani et al. [74] | |
| Meta-analysis | 3,845,303 | Statins, especially atorvastatin, reduced risk of PD | Yan et al. [75] | |
| Meta-analysis | 3,513,209 | Decreased risk of PD | Bai et al. [76] | |
| Meta-analysis | 2,787,249 | Statins reduced risk of PD | Sheng et al. [70] | |
| Atorvastatin | Randomized controlled trial | 640 | No therapeutic effect in AD | Feldman et al. [77] |
| Randomized controlled trial | 63 | AD progressed slowly | Sparks et al. [78] | |
| Lovastatin | Randomized controlled trial | 160 | Decreased serum Aβ | Friedhoff et al. [79] |
Because of these uncertainties regarding the research on groups of patients with PD and AD, well-designed controlled trials are needed to clearly demonstrate the effects of these groups of drugs on neurodegenerative diseases.
4. Statins and Cholinergic Neurotransmission
4.1. Cholinergic Transmission in Pathogenesis of Vascular Dementia
Vascular dementia (VD) is the second most frequent subtype of cognitive disorders after AD [80]. Chronic cerebral hypoperfusion (CCH), the crucial factor, which is caused by negative modification of cerebral blood vessels and associated with the initiation and progression of VD, results in numerous molecular changes inside the brain cells and neuronal junctions, including neurotransmitter and lipid metabolism disturbance, mitochondrial dysfunction, alteration of growth factors, neuroinflammation, and overproduction of reactive oxygen species (ROS) [81].
Acetylcholine (ACh) plays an important role in the physiological functioning of the CNS. The neuronal synthesis of Ach from choline and acetyl-CoA is catalyzed by acetylcholine transferase enzyme (ChAT). Subsequently, Ach, transported in vesicles with the involvement of vesicular acetylcholine transporter (VAChT), is released into the synaptic cleft, where it can bind to receptors. Within the synapse, ACh is degraded by acetylcholinesterase (AChE), resulting in the formation of acetic acid and choline, a precursor for the synthesis of new ACh [82,83].
There are two types of ACh receptors: metabotropic muscarinic receptors (mAChRs) and ionotropic nicotinic receptors (nAChRs). The family of mACHRs contains five subtypes of GPCR, M1–M5. The larger group, with pentameric nAChRs made up of α and β subunits, contains nonselective cation channels. The effects of binding ACh to cholinergic receptors can result in stimulation or inhibition of neuronal signaling, depending on the receptor subtype and its location on a pre- or postsynaptic membrane [84,85,86].
The basal forebrain cholinergic system, comprising the medial septal nucleus, the nucleus of the diagonal band of Broca, and the nucleus basalis of Meynert, is widely accepted as a crucial structure of cognitive functions. It is involved in the regulation of memory, attention, and emotions [87]. There is some evidence that cholinergic mechanisms are also responsible for the control of cerebral blood flow [88,89]. This may partially explain the pathogenesis of VD and deterioration in the course of disease. The ongoing neuroinflammation in patients with VD may also be attenuated by activation of the cholinergic system (α7 nAChRs) [90].
Ischemic lesions observed in various areas of the brain in patients with VD can cause decreased amounts of ACh, gamma-aminobutyric acid (GABA), or DA [81]. The most profound deficits of common cholinergic markers, such as ChAT, AChE, and VAChT, appear in the temporal cortex and hippocampus [91]. However, the latest research suggests that more evident loss of cholinergic function occurs in the brains of patients with mixed dementia [92]. A decreased Ach level is also observed in cerebrospinal fluid [93,94].Findings concerning changes in cholinergic receptor numbers are contradictory for mAChRs [95,96]. The amount of nAChRs seems to be preserved in VD [97]. The cholinergic reductions observed in the course of VD may be responsible for the cognitive impairment [98].
4.2. Influence of Statins on Cholinergic Transmission
Statins, due to their pluripotential pleiotropic effects on brain cells and vessels beyond lipid-lowering actions, have been widely tested as drugs for the treatment of VD [99]. In L-methionine-induced VD, the use of simvastatin ameliorated behavioral status and increased the amount of ACh in the brain tissue of rats [100]. These encouraging observations have not been seen in human patients with VD. Moreover, some studies indicated potential harmful effects of statin therapy on neuropsychological tests of attention and psychomotor speed [101]. Recent assessments of randomized, placebo-controlled trials did not confirm the clinical significance of these observations [102]. Although statin therapy is useful in primary and secondary prevention of vascular incidents, including strokes, to date there is no conclusive proof that statins have a major influence on the prevention, incidence, or progression of VD [80,103].
5. Statins and Glutamatergic Neurotransmission
5.1. Structure and Synthesis of Glutamate
Glutamate (Glu), the anion of glutamic acid, acts as a neurotransmitter. It is the major excitatory transmitter within the human nervous system, accounting for over 85% of the synaptic connections in the CNS. Glu can be produced de novo from α-ketoglutaric acid as part of the citric acid cycle. In CNS, Glu is synthesized in the glutamate–glutamine cycling mechanism. These reactions occur in presynaptic neurons or glial cells. Glu is transported within presynaptic neurons by vesicular glutamate transporters and then released into the synaptic cleft. Inside the synaptic cleft, anions of glutamic acid can bind several different postsynaptic receptor types, named according to their agonists: kainite receptor (KAR), α-amino-3-hydroxy-5-methyl4-isoxazole propionic acid receptor (AMPAR), and N-methyl-D-aspartate receptor (NMDAR). Glu binds to these receptors with different affinity and induces differential effects on target postsynaptic neurons [104,105]. For this part of the review, we would like to focus on NMDARs.
5.2. N-Methyl-D-Aspartate Receptor
Belonging to the neurotransmitter receptors, NMDARs constitute the largest subclass of glutamate-gated ion channels in human excitatory synapses, which have a main part in neuroplasticity, neuronal development, and learning and memory processes [106]. NMDARs are heteromeric molecules formed of one obligatory GluN1 (also referred to asNR1) incorporated with various constellations of GluN2 (also named NR2) and GluN3 subunits, which take several variants: the single GluN1 subunit with eight isoforms, four GluN2 subunits (GluN2A–GluN2D), and two GluN3 subunits. Both the GluN1 and GluN2 subunits participate in the development of the NMDAR ion channel. Each NMDAR has a similar membrane subunit topology, which is dominated by a large extracellular N-terminus, a membrane region containing three transmembrane segments, a re-entrant loop, and an extracellular loop between the transmembrane segments. Intracellularly, it is situated in a carboxyl (C) domain of various sizes, and miscellaneous proteins interact in this site [107,108,109,110,111].
NMDAR is extraordinary in that the opening of the channel requires the merging of two different agonists, Glu and glycine (Gly). Glu binds to the GluN2 subunit, while the binding site for Gly, the co-agonist, is located on the GluN1 subunits. The NMDAR ion channel is permeable to monovalent cations, such as Na+ and K+, and divalent cations, especially Ca2+. It is regulated by voltage-dependent Mg2+ blockade. Accordingly, both depolarization of the postsynaptic neurons and presynaptic release of Glu is needed for maximal current flow through the NMDAR channel. The concentration of Gly in most synapses is usually enough to allow for efficient NMDAR activation [108,110,111,112,113]. NMDAR is mainly located at dendritic spines, where through specific interactions it connects to intracellular molecules of the postsynaptic multiprotein network known as the postsynaptic density (PSD); for the subunit GluN1thisis neurofilament light protein (NF-L), while for GluN2 these are PSD-95, PSD-93, and synapse-associated protein 102(SAP102). In addition to their function as PSD cytoskeleton proteins, PSD-95 and SAP102 are involved in transporting newly synthesized NMDA receptors to the PSD. Build or behavior irregularities for these molecules could disturb receptor signaling, interfere with NMDAR trafficking, and finally affect neurotransmission [107]. The number of NMDARs can be modified, which contributes to the mechanism regulating synaptic efficacy and their remodeling [114]. With disorder in the NMDA signal pathway, glutamatergic transmission could exacerbate brain diseases, including psychiatric, neurodegenerative, and excitotoxic disorders [112].
5.3. Role of Glutamatergic Transmission in the Pathogenesis of Stroke
Excitotoxicity is a pathological process that causes cell death as the result of the toxic actions of excitatory amino acids. Considering that Glu is the main excitatory neurotransmitter in the human CNS, excitotoxicity typically refers to the trauma and death of neurons that occur from prolonged exposition to Glu. It comes from overloading the cell with ions, mainly calcium, which is notably neurotoxic and leads to the activation of enzymes that degrade proteins, nucleic acids, and other components of the cell. It is considered that Ca2+ inflow through NMDA channels is a common pathway of neuronal cell death. Excess levels of Glu in the CNS are associated with increased intracellular calcium ions levels, which cause a rise in their concentration in sensitive organelles such as mitochondria and the endoplasmic reticulum (ER) [115]. The mitochondrial uptake of calcium results in the production of ROS [116].
Stroke is a major cause of death, causing approximately 9% of deaths worldwide. Up to 80% of the global burden of stroke is attributed to ischemic stroke. This is a type of stroke characterized by a temporary or permanent reduction in blood perfusion due to embolic or thrombotic occlusion in cerebral arteries. Most cases of focal ischemia result from occlusion of the middle cerebral artery [117]. There is evidence that stroke leads to the release of large amounts of Glu, which activates NMDARs, and that glutamate-induced excitotoxicity participates in the neuronal death observed after stroke [118]. The first step of excitotoxicity during acute ischemia is a sudden increase of Glu levels in the ischemic region of the brain. Activation of NMDARs does not always lead to excitotoxicity. There is evidence that this receptor has dual effects, depending on the subunit subpopulation. GluN2A tends to promote neuronal survival and protects the brain against excitotoxic injury, whereas the GluN2B subunit promotes neuronal death. Cerebral ischemia triggering excessive activation of NMDARs induces rapid and specific upregulation of GluN2B [119].
Previous studies found that the excitotoxic process connected with acute ischemia is responsible for redistributed microtubule-associated proteins (MAP2) and loss of microtubule stability as a consequence. Normally these proteins are engaged in the regulation of vesicle transport during the creation or recovery of neuronal pathways [120]. Complexes of cadherin or catenins and actin are involved in maintaining the structure of the scaffolding proteins. Cerebral ischemia leads to structural damage of the cytoskeleton mediated by RhoGTPasas imbalance, Ras homolog family member A (RhoA) activation, and inactivation of Ras-related C3 botulinum toxin substrate (Rac), related to the rupture of adhesion. A study by Cespedes-Rubio showed that RhoA activity is increased in cell death processes due to excitotoxicity [121]. The inflammatory response induced by ischemia triggers the activation of signaling pathways, finally leading to neuronal cell death. There is evidence confirming that the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) signaling pathway is one of the serious signaling paths taking part in neuronal apoptosis. Glycogen synthase kinase-3β (GSK-3β) is an important protein downstream of Akt. Sustained activation of GSK-3β is pro-apoptotic in cerebral ischemia because it leads to hyperphosphorylation of tau, with consequent microtubule destabilization [122].
5.4. Influence of Statins on Glutamatergic Transmission and Their Neuroprotective Effect
Researchers continue to look for new effects of statin treatment in stroke, in primary and secondary prevention and in the acute phase of ischemia. Statins exert protective effects in vivo and in experimental models of stroke. Recent meta-analyses showed that statin therapy significantly reduces the overall risk and mortality rate of stroke, in both primary and secondary prevention, which confirms that accurate control of the lipid profile is needed [123,124]. Beyond their effects on the lipid profile, statins are also credited with pleiotropic effects. Among the pleiotropic effects reported in cerebral ischemia is improved endothelial function, stabilized atherosclerotic plaque, impaired inflammation with a concomitant decrease in ROS, and inhibition of the thrombogenic response [125]. Increasingly, studies are examining the effects of statin treatment on NMDARs and the process of excitotoxicity after acute ischemia. The precise mechanisms involved in these actions are not completely known. Studies indicate that NMDA channels are involved in the neuroprotective mechanism induced by statins to promote neuronal recovery after cerebral focal ischemia.
Gutierrez-Vargas et al. examined the influence of a high dose of atorvastatin on NMDA receptors after cerebral ischemia in laboratory rats. This work suggests that atorvastatin protects neurons after ischemia, restoring the balance of subunits by decreasing GluN2B upregulation [106]. Additionally, the same study described that treatment with atorvastatin improves the adhesion protein complex of NMDARs associated with PSD-95, influences Akt activation in promoting cell survival, and in turn promotes synaptic plasticity. Statins inhibit the synthesis of valid isoprenoids, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which are important intermediates for the post-translational modification of Rho GTPases, leading to the modulation of various cellular functions, e.g., decreased structural damage of the cytoskeleton [125]. Additionally, Gutierrez-Vargas et al. proved that atorvastatin used after ischemic stroke influences the recovery of the actin cytoskeleton and stabilizes microtubules by increased activity of Rac and RhoA reduction [126]. Another mechanism of neuroprotection by statins involves their influence on inflammation through a number of proinflammatory cytokines. Tuttolomondo et al., in the first human randomized trial, proved that early administration of high-dose atorvastatin caused a significantly lower serum level of inflammatory markers and may be related to a better prognosis after stroke [127]. Additionally, Campos-Martorell et al. showed that simvastatin used after acute ischemia had an influence on decreased oxidative stress [128]. Brain-derived neurotrophic factor (BDNF) induces neuronal proliferation and synaptogenesis and is also involved in the regulation of neurogenesis. After injury, it takes part in the recovery of neuronal tissue. Cerebral ischemia decreased levels of BDNF [129]. Atorvastatin used in the treatment of cerebral ischemia in animals led to recovered BDNF levels [106].
Considering that cerebral ischemia is one of the major global health problems with great costs for rehabilitation and recovery, more effective and accessible methods are needed to immediately reduce postischemic injury. Statins meet these criteria: they are cheap and easily available. Experimental models, experiments on rats, and preclinical studies have shown that they influence neuronal cells differently and could be used to reduce neurodegeneration after stroke. The above studies prove that large multi-center clinical studies are needed.
6. Statins and Serotoninergic Neurotransmission
6.1. Structure and Synthesis of Serotonin
Serotonin (5-HT) is one of the oldest neurotransmitters; it is estimated that its receptors appeared 700–800 million years ago in unicellular eukaryotes, such as Paramecium caudatum [130]. It is a monoamine produced within both the CNS and the peripheral nervous system (PNS). In the CNS, serotonergic neurons can be found in the dorsalraphe nucleus (DRN) and median raphe nucleus (MRN) [131]. In the PNS, it is synthesized in the gastrointestinal (GI) system by gut neurons and enterochromaffin cells. The substrate for its production is tryptophan and the synthesis process follows the scheme shown in Figure 4 [132].
Figure 4.
Serotonin synthesis and degradation pathways.
In the CNS, serotonergic neurons from DRN and MRN communicate with various areas within the cerebral cortex, limbic system, midbrain, and cerebellum [133]. Serotonin communication occurs mainly through volume transmission (VT) in the extracellular space and the cerebrospinal fluid (CSF). Serotonin travels from the source to target cells (neurons and astroglia) through energy gradients, leading to its diffusion and convection [134]. By interacting with its receptors, 5-HT is responsible for the regulation of many processes important for life, which include perception, mood, anxiety, aggression, cognitive functions, attention, sexual functions, and the circadian rhythm [131,135,136].
6.2. Serotonin Receptors and Transporters
Thirteen G-protein-coupled heptahelial serotonin receptors (5-HTRs) and one ligand-gated ion channel have been identified and are divided into seven distinct classes (5-HT1–7) [132,134]. All 5-HTRs are heteroreceptors associated with the postsynaptic membrane on nonserotonergic neurons. Presynaptically located autoreceptors (5-HT1A,1B,1D) respond to the regulation of 5-HT release through negative feedback and influence the neuronal firing rate. The 5-HTRs are located within the CNS, PNS, and other tissues, and the exact mechanisms of their action and the effects of stimulation are presented in Table 2 [132,137].
Table 2.
Serotonin (5-HT) receptor subtypes. CNS, central nervous system; cAMP, cyclic adenosine monophosphate; AC, adenylate cyclase; GIT, gastrointestinal tract; IP3, inositol-1,4,5-triphosphate; PKC, protein kinase C.
| Receptor | Location | Mechanism of Action | Functions |
|---|---|---|---|
| 5-HT1A | CNS | Decreased cAMP concentration by inhibition of AC | Learning and memory, depression, anxiety-like behaviors |
| 5-HT1B | CNS, vascular smooth muscle | Decreased cAMP concentration by inhibition of AC | Aggression, antimigraine effects and vasoconstriction, depression and anxiety-like behaviors |
| 5-HT1C | CNS, limfocytes | Not completely understood | Not completely understood |
| 5-HT1D | CNS, vascular smooth muscle | Decreased cAMP concentration by inhibition of AC | Pain perception, antimigraine effects, and vasoconstriction |
| 5-HT1E | CNS | Decreased cAMP concentration by inhibition of AC | Not completely understood |
| 5-HT1F | CNS, uterus, heart, GIT | Decreased cAMP concentration by inhibition of AC | Pain perception, antimigraine effects, andanxiety-like behaviors |
| 5-HT2A | CNS, PNS, thrombocytes, smooth muscles | Enhanced AC activity and IP3 | Pain perception, sensorimotor, motivation, emotionalregulation, vasoconstriction, smooth muscles cell constriction, thrombocyte aggregation |
| 5-HT2B | CNS, stomach | Enhanced PKC activity and IP3 | Anxiety-like behaviors, smooth muscle cell constriction |
| 5-HT2C | CNS, limfocytes | Enhanced PKC activity and IP3 | Anxiogenesis, sexual behavior, pain perception, regulation of serotonergic neuron activity |
| 5-HT3 | CNS, PNS | Opening of Na+, Ca2+, and K+ channels, depolarization of plasma membrane | Vomiting reflex, anxiety-like behaviors |
| 5-HT4 | CNS, PNS | Increased cAMP concentration by activation of AC | Anxiety-like behaviors, learning and memory |
| 5-HT5A | CNS | Decreased cAMP concentration by inhibition of AC | Learning and memory, emotional behaviors, acquisition of adaptive behavior, circadian rhythm |
| 5-HT6 | CNS, leukocytes | Increased cAMP concentration by activation of AC | Anxiety-like behaviors, learning and memory, cognition |
| 5-HT7 | CNS, GIT, vascular smooth muscles | Increased cAMP concentration by activation of AC | Regulation of sleep and circadian rhythm, thermoregulation, learning and memory, regulation of 5-HT release |
One of the new concepts of depression is that disturbances in integrated allosteric receptor–receptor interactions in highly sensitive 5-HT1A heteroreceptor complexes may contribute to the induction of major depression (MD). For example, disruption or dysfunction in 5-HT1A-FGFR1 heteroreceptor complexes in the suture–hippocampal serotonin neuron systems may contribute to the development of MD [134].
Another important membrane protein involved in serotonergic transmission is the serotonin reuptake transporter (SERT). It is responsible for the removal of free 5-HT from the synaptic cleft, which directly affects the duration of 5-HTR activation. Some transporter-regulatory proteins, such as syntaxin 1A (Syn1A) and secretory carrier membrane protein 2 (SCAMP2), are involved in regulating the activity of SERT [138]. It is also known that some polymorphisms in the SERT gene are associated with the occurrence of depression, anxiety disorders, autism, and suicidality [139]; therefore, the process of 5-HT reuptake has become one of the most important points in therapy for depression disorders.
6.3. Influence of Statins on Serotoninergic Transmission
Due to the influence of statins on neurodegenerative diseases and cognitive disorders known from many studies, consideration was also given to their potential influence on psychiatric disorders. A possible mechanism of their action is to increase serotonin reuptake through the SERT receptor in a manner independent of the cholesterol synthesis pathway, as described in animal models [140]. The range of concentrations in which statins increase SERT uptake is wide and includes concentrations achieved in acute systemic treatment [140,141]. Such a mechanism would suggest a potential effect of intensifying or inducing depressive symptoms. However, a cohort study of the Swedish population published in 2020 suggested that the incidence of depressive disorders in the group of people taking statins was lower than in the general population [142].
Possible mechanisms underlying the antidepressant effects of statins may include anti-inflammatory, antioxidant, and lipid-lowering properties [143]. The potential anti-inflammatory effects of statins include lowering C-reactive protein (CRP) levels [144] andantioxidant activity [145], inhibiting the production of pro-inflammatory cytokines by monocytes [146], inhibiting lymphocytes by blocking the function of antigen-1 leukocytes (LFA-1) [147], and blocking T-cell activation [148]. The antidepressant mechanism of statins may also be related to their antiatherosclerotic effect and their influence on damage to small white matter vessels, which underlies the hypothesis of vascular depression [149]. Such injuries may predispose people to depression, accelerate its course, and reduce the effectiveness of antidepressants [143].
Despite the mechanisms described above and the retrospective studies conducted so far, the influence of statins on the incidence of depressive disorders is still unclear and requires further research.
7. Conclusions
To date, researchers have described a number of mechanisms by which cholesterol influences neuronal transmission. These mechanisms can also be influenced by statins, which has been confirmed in animal and cellular models. Additionally, many retrospective studies have described the beneficial effects of this group of drugs on neurological diseases and psychiatric disorders. So far, however, there have been no clinical trials that have unequivocally proven their beneficial effects on the diseases described in our paper. This opens up a wide field for researchers, especially as statins still remain one of the most widely used drug groups in the general population.
Supplementary Materials
The following are available online. Table S1: Preclinical effects of statins on neurotransmission and neuroprotection.
Author Contributions
Conceptualization, M.K. and J.S.-K.; methodology, M.K. and J.S.-K.; resources, M.K., J.S.-K., M.H., M.B., M.M., and G.M.; writing—original draft preparation, M.K., J.S.-K., M.H., and M.M.; writing—review and editing, M.K., M.B., R.P., and B.O.; supervision, R.P. and B.O.; project administration, M.K., J.S.-K., and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical University of Silesia, grant number PCN-1-185/N/9/O.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koushki K., Shahbaz S.K., Mashayekhi K., Sadeghi M., Zayeri Z.D., Taba M.Y., Banach M., Al-Rasadi K., Johnston T.P., Sahebkar A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2021;60:175–199. doi: 10.1007/s12016-020-08791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabar S., Harker M., O’Flynn N., Wierzbicki A.S., On behalf of the Guideline Development Group Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: Summary of updated NICE guidance. BMJ. 2014;349:g4356. doi: 10.1136/bmj.g4356. [DOI] [PubMed] [Google Scholar]
- 3.Altaf A., Qu P., Zhao Y., Wang H., Lou D., Niu N. NLRP3 inflammasome in peripheral blood monocytes of acute coronary syndrome patients and its relationship with statins. Coron. Artery Dis. 2015;26:409–421. doi: 10.1097/MCA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 4.de Bont N., Netea M.G., Rovers C., Smilde T., Demacker P.N., van der Meer J.W., Stalenhoef A.F. LPS-induced cytokine pro-duction and expression of LPS-receptors by peripheral blood mononuclear cells of patients with familial hypercholesterolemia and the effect of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;139:147–152. doi: 10.1016/S0021-9150(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 5.Muldoon M.F., Manuck S.B., Matthews K.A. Lowering cholesterol concentrations and mortality: A quantitative review of primary prevention trials. BMJ. 1990;301:309–314. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang T.-L., Wu S.-C., Chiang Y.-S., Chen J.-F. Correlation between serum lipid, lipoprotein concentrations and anxious state, depressive state or major depressive disorder. Psychiatry Res. 2003;118:147–153. doi: 10.1016/S0165-1781(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 7.Vevera J., Zukov I., Morcinek T., Papezová H. Cholesterol concentrations in violent and non-violent women suicide attempters. Eur. Psychiatry. 2003;18:23–27. doi: 10.1016/S0924-9338(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 8.Parsaik A.K., Singh B., Hassan M.M., Singh K., Mascarenhas S.S., Williams M.D., Lapid M.I., Richardson J.W., West C.P., Rummans T.A. Statins use and risk of depression: A systematic review and meta-analysis. J. Affect. Disord. 2014;160:62–67. doi: 10.1016/j.jad.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Yatham M.S., Yatham K.S., Ravindran A.V., Sullivan F. Do statins have an effect on depressive symptoms? A systematic review and meta-analysis. J. Affect. Disord. 2019;257:55–63. doi: 10.1016/j.jad.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan F., Van Rhijn A., Ijomah G., McIntyre F., Skinner E., Horrobin D., Ward N. Tin and fatty acids in dementia. Prostaglandins, Leukot. Essent. Fat. Acids. 1991;43:229–238. doi: 10.1016/0952-3278(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 11.Dexter D.T., Holley A.E., Flitter W.D., Slater T.F., Wells F.R., Daniel S.E., Lees A.J., Jenner P., Marsden C.D. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: An HPLC and ESR study. Mov. Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 12.Kivipelto M., Solomon A. Cholesterol as a risk factor for Alzheimer’s disease—Epidemiological evidence. Acta Neurol. Scand. Suppl. 2006;185:50–57. doi: 10.1111/j.1600-0404.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 13.Wei Q., Wang H., Tian Y., Xu F., Chen X., Wang K. Reduced Serum Levels of Triglyceride, Very Low Density Lipoprotein Cholesterol and Apolipoprotein B in Parkinson’s Disease Patients. PLoS ONE. 2013;8:e75743. doi: 10.1371/journal.pone.0075743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2004;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 15.Davidson M.H. Rosuvastatin: A highly efficacious statin for the treatment of dyslipidaemia. Expert Opin. Investig. Drugs. 2002;11:125–141. doi: 10.1517/13543784.11.1.125. [DOI] [PubMed] [Google Scholar]
- 16.Irwin J.C., Fenning A.S., Vella R.K. Statins with different lipophilic indices exert distinct effects on skeletal, cardiac and vascular smooth muscle. Life Sci. 2020;242:117225. doi: 10.1016/j.lfs.2019.117225. [DOI] [PubMed] [Google Scholar]
- 17.Fuxe K., Borroto-Escuela D.O. Volume transmission and receptor-receptor interactions in heteroreceptor complexes: Understanding the role of new concepts for brain communication. Neural Regen. Res. 2016;11:1220–1223. doi: 10.4103/1673-5374.189168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuxe K., Agnati L.F., Marcoli M., Borroto-Escuela D.O. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem. Res. 2015;40:2600–2614. doi: 10.1007/s11064-015-1574-5. [DOI] [PubMed] [Google Scholar]
- 19.Zoli M., Torri C., Ferrari R., Jansson A., Zini I., Fuxe K., Agnati L.F. The emergence of the volume transmission concept. Brain Res. Rev. 1998;26:136–147. doi: 10.1016/S0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 20.Alexander S.P., Christopoulos A., Davenport A.P., Kelly E., Mathie A., Peters J.A., Veale E.L., Armstrong J.F., Faccenda E., Harding S.D., et al. The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019;176:21–141. [Google Scholar]
- 21.Ricci A., Mignini F., Tomassoni D., Amenta F. Dopamine receptor subtypes in the human pulmonary arterial tree. Auton. Autacoid Pharmacol. 2006;26:361–369. doi: 10.1111/j.1474-8673.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 22.Hussain T., Lokhandwala M.F. Renal Dopamine Receptors and Hypertension. Exp. Biol. Med. 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 23.Aslanoglou D., Bertera S., Sánchez-Soto M., Benjamin Free R., Lee J., Zong W., Xue X., Shrestha S., Brissova M., Logan R.W., et al. Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Transl. Psychiatry. 2021;11:59. doi: 10.1038/s41398-020-01171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kranzler H.R., Edenberg H.J. Pharmacogenetics of Alcohol and Alcohol Dependence Treatment. Curr. Pharm. Des. 2010;16:2141–2148. doi: 10.2174/138161210791516387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Foll B., Gallo A., Le Strat Y., Lu L., Gorwood P. Genetics of dopamine receptors and drug addiction: A comprehensive review. Behav. Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- 26.Smith L., Watson M., Gates S., Ball D., Foxcroft D. Meta-Analysis of the Association of the Taq1A Polymorphism with the Risk of Alcohol Dependency: A HuGE Gene-Disease Association Review. Am. J. Epidemiol. 2007;167:125–138. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- 27.Tyndale R.F. Genetics of alcohol and tobacco use in humans. Ann. Med. 2003;35:94–121. doi: 10.1080/07853890310010014. [DOI] [PubMed] [Google Scholar]
- 28.Franco N., Franco R. Understanding the Added Value of G-Protein-Coupled Receptor Heteromers. Scientifica. 2014;2014:362937. doi: 10.1155/2014/362937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco R., Casadó V., Cortés A., Ferrada C., Mallol J., Woods A., Lluís C., Canela E.I., Ferre S. Basic Concepts in G-Protein-Coupled Receptor Homo- and Heterodimerization. Sci. World J. 2007;7:48–57. doi: 10.1100/tsw.2007.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcellino D., Ferré S., Casadó V., Cortés A., Le Foll B., Mazzola C., Drago F., Saur O., Stark H., Soriano A., et al. Identification of dopamine D1-D3 receptor heteromers: Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarselli M., Novi F., Schallmach E., Lin R., Baragli A., Colzi A., Griffon N., Corsini G.U., Sokoloff P., Levenson R., et al. D2/D3 Dopamine Receptor Heterodimers Exhibit Unique Functional Properties. J. Biol. Chem. 2001;276:30308–30314. doi: 10.1074/jbc.M102297200. [DOI] [PubMed] [Google Scholar]
- 32.Hasbi A., Fan T., Alijaniaram M., Nguyen T., Perreault M.L., O’Dowd B.F., George S.R. Calcium signaling cascade links do-pamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. USA. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borroto-Escuela D.O., Van Craenenbroeck K., Romero-Fernandez W., Guidolin D., Woods A.S., Rivera A., Haegeman G., Agnati L.F., Tarakanov A.O., Fuxe K. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor–receptor inter-actions. Biochem. Biophys. Res. Commun. 2011;404:928–934. doi: 10.1016/j.bbrc.2010.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginés S., Hillion J., Torvinen M., Le Crom S., Casadó V., Canela E.I., Rondin S., Lew J.Y., Watson S., Zoli M., et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA. 2000;97:8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillion J., Canals M., Torvinen M., Casadó V., Scott R., Terasmaa A., Hansson A., Watson S., Olah M.E., Mallol J., et al. Coaggregation, Cointernalization, and Codesensitization of Adenosine A2A Receptors and Dopamine D2Receptors. J. Biol. Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 36.Ferrada C., Moreno E., Casadó V., Bongers G., Cortés A., Mallol J., Canela E.I., Leurs R., Ferré S., Lluís C., et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br. J. Pharmacol. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrada C., Ferré S., Casadó V., Cortés A., Justinova Z., Barnes C., Canela E.I., Goldberg S.R., Leurs R., Lluis C., et al. Inter-actions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borroto-Escuela D.O., Brito I., Romero-Fernandez W., Di Palma M., Oflijan J., Skieterska K., Duchou J., Van Craenenbroeck K., Suárez-Boomgaard D., Rivera A., et al. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub com-ponents. Int. J. Mol. Sci. 2014;15:8570–8590. doi: 10.3390/ijms15058570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez S., Moreno-Delgado D., Moreno E., Pérez-Capote K., Franco R., Mallol J., Cortés A., Casadó V., Lluis C., Ortiz J., et al. Circadian-Related Heteromerization of Adrenergic and Dopamine D4 Receptors Modulates Melatonin Synthesis and Release in the Pineal Gland. PLoS Biol. 2012;10:e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro G., Borroto-Escuela D.O.D.O., Fuxe K., Franco R. Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology. 2015;104:161–168. doi: 10.1016/j.neuropharm.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Fuxe K., Agnati L., Jacobsen K., Hillion J., Canals M., Torvinen M., Tinner-Staines B., Staines W., Rosin D., Terasmaa A., et al. Receptor heteromerization in adenosine A2A receptor signaling: Relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–S23. doi: 10.1212/01.WNL.0000095206.44418.5C. [DOI] [PubMed] [Google Scholar]
- 42.Zeppelin T., Ladefoged L.K., Sinning S., Periole X., Schiøtt B. A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput. Biol. 2018;14:e1005907. doi: 10.1371/journal.pcbi.1005907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orłowski A., Grzybek M., Bunker A., Pasenkiewicz-Gierula M., Vattulainen I., Männistö P.T., Róg T. Strong preferences of dopamine and l-dopa towards lipid head group: Importance of lipid composition and implication for neurotransmitter metabolism. J. Neurochem. 2012;122:681–690. doi: 10.1111/j.1471-4159.2012.07813.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhuge W., Wen F., Ni Z., Zheng Z., Zhu X., Lin J., Wang J., Zhuge Q., Ding S. Dopamine Burden Triggers Cholesterol Overload Following Disruption of Synaptogenesis in Minimal Hepatic Encephalopathy. Neuroscience. 2019;410:1–15. doi: 10.1016/j.neuroscience.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 45.Björkhem I., Lövgren-Sandblom A., Leoni V., Meaney S., Brodin L., Salveson L., Winge K., Pålhagen S., Svenningsson P. Oxysterols and Parkinson’s disease: Evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci. Lett. 2013;555:102–105. doi: 10.1016/j.neulet.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Cheng D., Kim W.S., Garner B. Regulation of α-synuclein expression by liver X receptor ligands in vitro. NeuroReport. 2008;19:1685–1689. doi: 10.1097/WNR.0b013e32831578b2. [DOI] [PubMed] [Google Scholar]
- 47.Schommer J., Marwarha G., Schommer T., Flick T., Lund J., Ghribi O. 27-Hydroxycholesterol increases α-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci. 2018;19:17. doi: 10.1186/s12868-018-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Marwarha G., Rhen T., Schommer T., Ghribi O. The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hy-droxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors--relevance to Parkinson’s disease. J. Neurochem. 2011;119:1119–1136. doi: 10.1111/j.1471-4159.2011.07497.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Nakamura K., Mori F., Tanji K., Miki Y., Yamada M., Kakita A., Takahashi H., Utsumi J., Sasaki H., Wakabayashi K. Iso-pentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathology. 2015;35:432–440. doi: 10.1111/neup.12204. [DOI] [PubMed] [Google Scholar]
- 50.Scott D., Roy S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 2012;32:10129–10135. doi: 10.1523/JNEUROSCI.0535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krüger R., Vieira-Saecker A.M., Kuhn W., Berg D., Müller T., Kühnl N., Fuchs G.A., Storch A., Hungs M., Woitallam D., et al. Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann. Neurol. 1999;45:611–617. doi: 10.1002/1531-8249(199905)45:5<611::AID-ANA9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 52.Fantini J., Carlus D., Yahi N. The fusogenic tilted peptide (67–78) of α-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta (BBA) Biomembr. 2011;1808:2343–2351. doi: 10.1016/j.bbamem.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Hsiao J.-H.T., Halliday G.M., Kim W.S. α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules. 2017;22:1769. doi: 10.3390/molecules22101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sui Y.-T., Bullock K.M., Erickson M.A., Zhang J., Banks W. Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barceló-Coblijn G., Golovko M.Y., Weinhofer I., Berger J., Murphy E.J. Brain neutral lipids mass is increased in α-synuclein gene-ablated mice. J. Neurochem. 2006;101:132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- 56.Van Maarschalkerweerd A., Vetri V., Vestergaard B. Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett. 2015;589:2661–2667. doi: 10.1016/j.febslet.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Emamzadeh F.N., Aojula H., McHugh P.C., Allsop D. Effects of different isoforms of apoE on aggregation of the α-synuclein protein implicated in Parkinson’s disease. Neurosci Lett. 2016;618:146–151. doi: 10.1016/j.neulet.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 58.Heverin M., Bogdanovic N., Lütjohann D., Bayer T., Pikuleva I., Bretillon L., Diczfalusy U., Winblad B., Björkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 2004;45:186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Lütjohann D., Von Bergmann K. 24S-Hydroxycholesterol: A Marker of Brain Cholesterol Metabolism. Pharmacopsychiatry. 2003;36:102–106. doi: 10.1055/s-2003-43053. [DOI] [PubMed] [Google Scholar]
- 60.Sokratian A., Ziaee J., Kelly K., Chang A., Bryant N., Wang S., Xu E., Li J.Y., Wang S.-H., Ervin J., et al. Heterogeneity in α-synuclein fibril activity correlates to disease phenotypes in Lewy body dementia. Acta Neuropathol. 2021;141:547–564. doi: 10.1007/s00401-021-02288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sierra S., Ramos M.C., Molina P., Esteo C., Vázquez J.A., Burgos J.S. Statins as Neuroprotectants: A Comparative In Vitro Study of Lipophilicity, Blood-Brain-Barrier Penetration, Lowering of Brain Cholesterol, and Decrease of Neuron Cell Death. J. Alzheimer’s Dis. 2011;23:307–318. doi: 10.3233/JAD-2010-101179. [DOI] [PubMed] [Google Scholar]
- 62.Yan J., Xu Y., Zhu C., Zhang L., Wu A., Yang Y., Xiong Z., Deng C., Huang X.-F., Yenari M.A., et al. Simvastatin Prevents Dopaminergic Neurodegeneration in Experimental Parkinsonian Models: The Association with Anti-Inflammatory Responses. PLoS ONE. 2011;6:e20945. doi: 10.1371/journal.pone.0020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan J., Sun J., Huang L., Fu Q., Du G. Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J. Neurosci. Res. 2014;92:634–640. doi: 10.1002/jnr.23329. [DOI] [PubMed] [Google Scholar]
- 64.Fassbender K., Simons M., Bergmann C., Stroick M., Lutjohann D., Keller P., Runz H., Kuhl S., Bertsch T., von Bergmann K., et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kojro E., Gimpl G., Lammich S., Marz W., Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the -secretase ADAM 10. Proc. Natl. Acad. Sci. USA. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolozin B., Kellman W., Ruosseau P., Celesia G.G., Siegel G. Decreased Prevalence of Alzheimer Disease Associated With 3-Hydroxy-3-Methyglutaryl Coenzyme A Reductase Inhibitors. Arch. Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 67.Rockwood K., Kirkland S., Hogan D.B., Macknight C., Merry H., Verreault R., Wolfson C., McDowell I. Use of Lipid-Lowering Agents, Indication Bias, and the Risk of Dementia in Community-Dwelling Elderly People. Arch. Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 68.Rea T.D., Breitner J.C., Psaty B.M., Fitzpatrick A.L., Lopez O.L., Newman A.B., Hazzard W.R., Zandi P.P., Burke G.L., Lyketsos C.G., et al. Statin use and the risk of incident dementia: The Cardiovascular Health Study. Arch. Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 69.Lin F.C., Chuang Y.S., Hsieh H.M., Lee T.C., Chiu K.F., Liu C.K., Wu M.T. Early Statin Use and the Progression of Alzheimer Disease: A Total Population-Based Case-Control Study. Medicine. 2015;94:e2143. doi: 10.1097/MD.0000000000002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheng Z., Jia X., Kang M. Statin use and risk of Parkinson’s disease: A meta-analysis. Behav. Brain Res. 2016;309:29–34. doi: 10.1016/j.bbr.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 71.Haag M.D.M., Hofman A., Koudstaal P.J., Stricker B.H.C., Breteler M.M.B. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J. Neurol. Neurosurg. Psychiatry. 2008;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 72.Huang X., Alonso A., Guo X., Umbach D.M., Lichtenstein M.L., Ballantyne C.M., Mailman R.B., Mosley T.H., Chen H. Statins, plasma cholesterol, and risk of Parkinson’s disease: A prospective study. Mov. Disord. 2015;30:552–559. doi: 10.1002/mds.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu G., Sterling N.W., Kong L., Lewis M.M., Mailman R.B., Chen H., Leslie D., Huang X. Statins may facilitate Parkinson’s disease: Insight gained from a large, national claims database. Mov. Disord. 2017;32:913–917. doi: 10.1002/mds.27006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rozani V., Giladi N., El-Ad B., Gurevich T., Tsamir J., Hemo B., Peretz C. Statin adherence and the risk of Parkinson’s disease: A population-based cohort study. PLoS ONE. 2017;12:e0175054. doi: 10.1371/journal.pone.0175054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan J., Qiao L., Tian J., Liu A., Wu J., Huang J., Shen M., Lai X. Effect of statins on Parkinson’s disease: A systematic review and meta-analysis. Medicine. 2019;98:e14852. doi: 10.1097/MD.0000000000014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai S., Song Y., Huang X., Peng L., Jia J., Liu Y., Lu H. Statin Use and the Risk of Parkinson’s Disease: An Updated Meta-Analysis. PLoS ONE. 2016;11:e0152564. doi: 10.1371/journal.pone.0152564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feldman H., Doody R.S., Kivipelto M., Sparks D.L., Waters D.D., Jones R.W., Schwam E., Schindler R., Hey-Hadavi J., Demicco D.A., et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 78.Sparks D.L., Sabbagh M.N., Connor D.J., Lopez J., Launer L.J., Browne P., Wasser D., Johnson-Traver S., Lochhead J., Ziol-wolski C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: Preliminary results. Arch. Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 79.Friedhoff L.T., Cullen E.I., Geoghagen N.S., Buxbaum J.D. Treatment with controlled-release lovastatin decreases serum con-centrations of human beta-amyloid (A beta) peptide. Int. J. Neuropsychopharmacol. 2001;4:127–130. doi: 10.1017/S1461145701002310. [DOI] [PubMed] [Google Scholar]
- 80.Appleton J.P., Scutt P., Sprigg N., Bath P.M. Hypercholesterolaemia and vascular dementia. Clin. Sci. 2017;131:1561–1578. doi: 10.1042/CS20160382. [DOI] [PubMed] [Google Scholar]
- 81.Du S.-Q., Wang X.-R., Xiao L.-Y., Tu J.-F., Zhu W., He T., Liu C.-Z. Molecular Mechanisms of Vascular Dementia: What Can Be Learned from Animal Models of Chronic Cerebral Hypoperfusion? Mol. Neurobiol. 2016;54:3670–3682. doi: 10.1007/s12035-016-9915-1. [DOI] [PubMed] [Google Scholar]
- 82.Maurer S.V., Williams C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017;8:1489. doi: 10.3389/fimmu.2017.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun Y., Zhao Z., Li Q., Wang C., Ge X., Wang X., Wang G., Qin Y. Dl-3-n-butylphthalide regulates cholinergic dysfunction in chronic cerebral hypoperfusion rats. J. Int. Med. Res. 2020;48:300060520936177. doi: 10.1177/0300060520936177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Picciotto M.R., Higley M.J., Mineur Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol. Sci. 2003;24:414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 86.Picciotto M.R., Caldarone B.J., King S.L., Zachariou V. Nicotinic Receptors in the Brain Links between Molecular Biology and Behavior. Neuropsychopharmacolohy. 2000;22:451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- 87.Everitt B.J., Robbins T.W. Central Cholinergic Systems and Cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 88.Sato A., Sato Y., Uchida S. Activation of the intracerebral cholinergic nerve fibers originating in the basal forebrain increases regional cerebral blood flow in the rat’s cortex and hippocampus. Neurosci. Lett. 2004;361:90–93. doi: 10.1016/j.neulet.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 89.Sato A., Sato Y., Uchida S. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Int. J. Dev. Neurosci. 2001;19:327–337. doi: 10.1016/S0736-5748(01)00017-X. [DOI] [PubMed] [Google Scholar]
- 90.Pavlov V.A., Tracey K.J. Controlling inflammation: The cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 2006;34:1037–1040. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 91.Perry E.K., Gibson P.H., Blessed G., Perry R.H., Tomlinson B.E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977;34:247–265. doi: 10.1016/0022-510X(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 92.Sharp S.I., Francis P.T., Elliott M.S., Kalaria R.N., Bajic N., Hortobágyi T., Ballard C.G. Choline Acetyltransferase Activity in Vascular Dementia and Stroke. Dement. Geriatr. Cogn. Disord. 2009;28:233–238. doi: 10.1159/000239235. [DOI] [PubMed] [Google Scholar]
- 93.Tohgi H., Abe T., Kimura M., Saheki M., Takahashi S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J. Neural. Transm. 1996;103:1211–1220. doi: 10.1007/BF01271206. [DOI] [PubMed] [Google Scholar]
- 94.Jia J.P., Jia J.M., Zhou W.D., Xu M., Chu C.B., Yan X., Sun Y.X. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Chin. Med. J. 2004;117:1161–1164. [PubMed] [Google Scholar]
- 95.Sakurada T., Alufuzoff I., Winblad B., Nordberg A. Substance P-like immunoreactivity, choline acetyltransferase activity and cholinergic muscarinic receptors in Alzheimer’s disease and multi-infarct dementia. Brain Res. 1990;521:329–332. doi: 10.1016/0006-8993(90)91561-T. [DOI] [PubMed] [Google Scholar]
- 96.Waller S.B., Ball M.J., Reynolds M.A., London E.D. Muscarinic Binding and Choline Acetyltransferase in Postmortem Brains of Demented Patients. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1986;13:528–532. doi: 10.1017/S0317167100037252. [DOI] [PubMed] [Google Scholar]
- 97.Martin-Ruiz C., Court J., Lee M., Piggott M., Johnson M., Ballard C., Kalaria R., Perry R., Perry E. Nicotinic receptors in de-mentia of Alzheimer, Lewy body and vascular types. Acta Neurol. Scand. Suppl. 2000;176:34–41. doi: 10.1034/j.1600-0404.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 98.Damodaran T., Müller C.P., Hassan Z. Chronic cerebral hypoperfusion-induced memory impairment and hippocampal long-term potentiation deficits are improved by cholinergic stimulation in rats. Pharmacol. Rep. 2019;71:443–448. doi: 10.1016/j.pharep.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 99.Sodero A.O., Barrantes F.J. Pleiotropic effects of statins on brain cells. Biochim. Biophys. Acta (BBA) Biomembr. 2020;1862:183340. doi: 10.1016/j.bbamem.2020.183340. [DOI] [PubMed] [Google Scholar]
- 100.El-Dessouki A.M., Galal M.A., Awad A.S., Zaki H.F. Neuroprotective Effects of Simvastatin and Cilostazol in l-Methionine-Induced Vascular Dementia in Rats. Mol. Neurobiol. 2016;54:5074–5084. doi: 10.1007/s12035-016-0051-8. [DOI] [PubMed] [Google Scholar]
- 101.Muldoon M.F., Barger S.D., Ryan C.M., Flory J.D., Lehoczky J.P., Matthews K.A., Manuck S.B. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 2000;108:538–546. doi: 10.1016/S0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 102.Collins R., Reith C., Emberson J., Armitage J., Baigent C., Blackwell L., Blumenthal R., Danesh J., Smith G.D., DeMets D., et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 103.Sinha K., Sun C., Kamari R., Bettermann K. Current status and future prospects of pathophysiology-based neuroprotective drugs for the treatment of vascular dementia. Drug Discov. Today. 2020;25:793–799. doi: 10.1016/j.drudis.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Davoudian P.A., Wilkinson S.T. Clinical Overview of NMDA-R Antagonists and Clinical Practice. Volume 89. Elsevier BV; Amsterdam, The Netherlands: 2020. pp. 103–129. [DOI] [PubMed] [Google Scholar]
- 105.Meldrum B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 106.Gutierrez-Vargas J.A., Muñoz-Manco J.I., Garcia-Segura L.M., Cardona-Gómez G.P. GluN2B N-methyl-D-aspartic acid receptor subunit mediates atorvastatin-Induced neuroprotection after focal cerebral ischemia. J. Neurosci. Res. 2014;92:1529–1548. doi: 10.1002/jnr.23426. [DOI] [PubMed] [Google Scholar]
- 107.Kristiansen L.V., Huerta I., Beneyto M., Meador-Woodruff J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 108.Salussolia C.L., Prodromou M.L., Borker P., Wollmuth L.P. Arrangement of Subunits in Functional NMDA Receptors. J. Neurosci. 2011;31:11295–11304. doi: 10.1523/JNEUROSCI.5612-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Groc L., Bard L., Choquet D. Surface trafficking of N-methyl-d-aspartate receptors: Physiological and pathological perspectives. Neuroscience. 2009;158:4–18. doi: 10.1016/j.neuroscience.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 110.Loftis J.M., Janowsky A. The N-methyl-d-aspartate receptor subunit NR2B: Localization, functional properties, regulation, and clinical implications. Pharmacol. Ther. 2003;97:55–85. doi: 10.1016/S0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 111.Furukawa H., Singh S.K., Mancusso R., Gouaux E. Subunit arrangement and function in NMDA receptors. Nat. Cell Biol. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 112.Kalia L.V., Kalia S.K., Salter M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dobrek Ł., Thor P. Glutamate NMDA Receptors in Pathophysiology and Pharmacotherapy of Selected Nervous System Diseases. PHMD. 2011;65:338–346. doi: 10.5604/17322693.946637. [DOI] [PubMed] [Google Scholar]
- 114.Lau C.G., Zukin R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 115.Dong X.-X., Wang Y., Qin Z.-H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009;30:379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynolds I.J., Hastings T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Donnan G.A., Fisher M., Malcolm Macleod S.M.D. Emergency and Comprehensive Care for Stroke Needed. Lancet. 2008;373:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [Google Scholar]
- 118.Lo E.H., Moskowitz M.A., Jacobs T.P. Exciting, Radical, Suicidal: How Brain Cells Die after Stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 119.Lai T.W., Zhang S., Wang Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 120.Hoskison M., Yanagawa Y., Obata K., Shuttleworth C. Calcium-dependent NMDA-induced dendritic injury and MAP2 loss in acute hippocampal slices. Neuroscience. 2007;145:66–79. doi: 10.1016/j.neuroscience.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jurado F.W., Cardona-go G.P. P120 Catenin/a N-Catenin Are Molecular Targets in the Neuroprotection and Neuronal Plasticity Mediated by Atorvastatin after Focal Cerebral Ischemia. J. Neurosci. Res. 2010;88:3621–3634. doi: 10.1002/jnr.22511. [DOI] [PubMed] [Google Scholar]
- 122.Valerio A., Bertolotti P., Delbarba A., Perego C., Dossena M., Ragni M., Spano P., Carruba M.O., De Simoni M.G., Nisoli E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J. Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 123.Tramacere I., Boncoraglio G.B., Banzi R., Del Giovane C., Kwag K.H., Squizzato A., Moja L. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: A systematic review and network meta-analysis. BMC Med. 2019;17:1–12. doi: 10.1186/s12916-019-1298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yebyo H.G., Aschmann H.E., Kaufmann M., Puhan M.A. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am. Hear. J. 2019;210:18–28. doi: 10.1016/j.ahj.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 125.Wang C.-Y., Liu P.-Y., Liao J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gutiérrez-Vargas J.A., Cespedes-Rubio A., Cardona-Gómez G.P. Perspective of synaptic protection after post-infarction treatment with statins. J. Transl. Med. 2015;13:1–9. doi: 10.1186/s12967-015-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tuttolomondo A., Di Raimondo D., Pecoraro R., Maida C., Arnao V., Corte V.D., Simonetta I., Corpora F., Di Bona D., Maugeri R., et al. Early High-Dosage Atorvastatin Treatment Improved Serum Immune-Inflammatory Markers and Functional Outcome in Acute Ischemic Strokes Classified as Large Artery Atherosclerotic Stroke: A Randomized Trial. Medicine. 2016;95:e3186. doi: 10.1097/MD.0000000000003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campos-Martorell M., Salvador N., Monge M., Canals F., García-Bonilla L., Hernández-Guillamon M., Ayuso M.I., Chacon P., Rosell A., Alcázar A., et al. Brain proteomics identifies potential simvastatin targets in acute phase of stroke in a rat embolic model. J. Neurochem. 2014;130:301–312. doi: 10.1111/jnc.12719. [DOI] [PubMed] [Google Scholar]
- 129.Ploughman M., Windle V., MacLellan C.L., White N., Doré J.J., Corbett D. Brain-Derived Neurotrophic Factor Contributes to Recovery of Skilled Reaching after Focal Ischemia in Rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 130.Hannon J., Hoyer D. Molecularbiology of 5-HT receptors. Behav. Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 131.Abela A.R., Browne C.J., Sargin D., Prevot T.D., Ji X.D., Li Z., Lambe E.K., Fletcher P.J. Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology. 2020;168:107985. doi: 10.1016/j.neuropharm.2020.107985. [DOI] [PubMed] [Google Scholar]
- 132.Rang H.P., Dale M.M., Ritter J.M., Flower R.J., Henderson G. Rang & Dale’s pharmacology. 7th ed. Elsevier Churchill Livingstone; Edinburgh, UK: 2012. pp. 199–200. [Google Scholar]
- 133.Huang K.W., Ochandarena N.E., Philson A.C., Hyun M., Birnbaum J.E., Cicconet M., Sabatini B.L. Molecular and anatomicalorganization of the dorsalraphenucleus. Elife. 2019;8:e46464. doi: 10.7554/eLife.46464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borroto-Escuela D.O., Ambrogini P., Chruścicka B., Lindskog M., Crespo-Ramirez M., Hernández-Mondragón J.C., Perez de la Mora M., Schellekens H., Fuxe K. The Role of Central Serotonin Neurons and 5-HT Heteroreceptor Complexes in the Pathophysiology of Depression: A HistoricalPerspective and Future Prospects. Int. J. Mol. Sci. 2021;22:1927. doi: 10.3390/ijms22041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He J., Hommen F., Lauer N., Balmert S., Scholz H. Serotonin transporter dependent modulation of food-seeking behavior. PLoS ONE. 2020;15:e0227554. doi: 10.1371/journal.pone.0227554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paulus E.V., Mintz E.M. Circadianrhythms of clockgeneexpression in the cerebellum of serotonin-deficient Pet-1 knockout mice. Brain Res. 2016;1630:10–17. doi: 10.1016/j.brainres.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 137.Pourhamzeh M., Moravej F.G., Arabi M., Shahriari E., Mehrabi S., Ward R., Ahadi R., Joghataei M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2021:1–22. doi: 10.1007/s10571-021-01064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Müller H.K., Wiborg O., Haase J. Subcellular Redistribution of the Serotonin Transporter by Secretory Carrier Membrane Protein 2. J. Biol. Chem. 2006;281:28901–28909. doi: 10.1074/jbc.M602848200. [DOI] [PubMed] [Google Scholar]
- 139.White K.J., Walline C.C., Barker E.L. Serotonin transporters: Implications for antidepressantdrug development. AAPS J. 2005;7:e421–433. doi: 10.1208/aapsj070242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deveau C.M., Rodriguez E., Schroering A., Yamamoto B.K. Serotonin transporter regulation by cholesterol-independent lipid signaling. Biochem. Pharmacol. 2021;183:114349. doi: 10.1016/j.bcp.2020.114349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson-Anuna L.N., Eckert G.P., Keller J.H., Igbavboa U., Franke C., Fechner T., Schubert-Zsilavecz M., Karas M., Müller W.E., Wood W.G. Chronic administration of statins altersmultiplegene expression patterns in mouse cerebral cortex. J. Pharmacol. Exp. Ther. 2005;312:786–793. doi: 10.1124/jpet.104.075028. [DOI] [PubMed] [Google Scholar]
- 142.Molero Y., Cipriani A., Larsson H., Lichtenstein P., D’Onofrio B.M., Fazel S. Associations between statin use and suicidality, depression, anxiety, and seizures: A Swedish total-population cohort study. Lancet Psychiatry. 2020;7:982–990. doi: 10.1016/S2215-0366(20)30311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Köhler-Forsberg O., Otte C., Gold S.M., Østergaard S.D. Statins in the treatment of depression: Hype or hope? Pharmacol. Ther. 2020;215:107625. doi: 10.1016/j.pharmthera.2020.107625. [DOI] [PubMed] [Google Scholar]
- 144.Jialal I., Stein D., Balis D., Grundy S.M., Adams-Huet B., Devaraj S. Effect of hydroxymethylglutarylcoenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.CIR.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 145.Shishehbor M.H., Aviles R.J., Brennan M.L., Fu X., Goormastic M., Pearce G.L., Gokce N., Keaney J.F., Penn M.S., Sprecher D.L., et al. Association of nitrotyrosinelevels with cardiovasculardisease and modulation by statintherapy. JAMA. 2003;289:1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 146.Ferro D., Parrotto S., Basili S., Alessandri C., Violi F. Simvastatininhibits the monocyteexpression of proinflammatorycytokines in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2000;36:427–431. doi: 10.1016/S0735-1097(00)00771-3. [DOI] [PubMed] [Google Scholar]
- 147.Weitz-Schmidt G., Welzenbach K., Brinkmann V., Kamata T., Kallen J., Bruns C., Cottens S., Takada Y., Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrinsite. Nat. Med. 2001;6:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 148.Bu D.X., Tarrio M., Grabie N., Zhang Y., Yamazaki H., Stavrakis G., Maganto-Garcia E., Pepper-Cunningham Z., Jarolim P., Aikawa M., et al. Statin-induced Krüppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J. Clin. Investig. 2010;120:1961–1970. doi: 10.1172/JCI41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van Agtmaal M.J.M., Houben A.J.H.M., Pouwer F., Stehouwer C.D.A., Schram M.T. Association of Microvascular Dysfunction With Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:729–739. doi: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.