Abstract
It is widely accepted that the distinctive aroma and flavour traits of Brassicaceae crops are produced by glucosinolate (GSL) hydrolysis products (GHPs) with other non-GSL derived compounds also reported to contribute significantly to their aromas. This study investigated the flavour profile and glucosinolate content of four Brassicaceae species (salad rocket, horseradish, wasabi, and watercress). Solid-phase microextraction followed by gas chromatography-mass spectrometry and gas chromatography-olfactometry were used to determine the volatile compounds and odorants present in the four species. Liquid chromatography-mass spectrometry was used to determine the glucosinolate composition, respectively. A total of 113 compounds and 107 odour-active components were identified in the headspace of the four species. Of the compounds identified, 19 are newly reported for ‘salad’ rocket, 26 for watercress, 30 for wasabi, and 38 for horseradish, marking a significant step forward in understanding and characterising aroma generation in these species. There were several non-glucosinolate derived compounds contributing to the ‘pungent’ aroma profile of the species, indicating that the glucosinolate-derived compounds are not the only source of these sensations in Brassicaceae species. Several discrepancies between observed glucosinolates and hydrolysis products were observed, and we discuss the implications of this for future studies.
Keywords: volatile compounds, odorants, glucosinolate, Brassicaceae, ‘salad’ rocket, wasabi, horseradish, watercress
1. Introduction
Crops of the Brassicaceae family are grown all over the world, and they form an important part of many different cuisines and cultures [1]. Some species are noted for their distinctive, and often very strong, tastes and flavours. Armoracia rusticana (horseradish), Eruca sativa (‘salad’ rocket), Eutrema japonicum (wasabi), and Nasturtium officinale (watercress) are four such examples, which are noted for their pungent, peppery, and aromatic organoleptic properties [2,3,4].
Horseradish and wasabi produce large roots that are grated and used as a condiment in many cultures across the world, most notably in Eastern Europe and the United Kingdom (horseradish) and Japan (wasabi; [5]). Horseradish is a vegetative perennial that grows widely in temperate regions [6], whereas wasabi can only be grown in a very few locations, owing to its sensitivity to temperature change and root oxygen availability [7]. Wasabi is traditionally cultivated in damp river valleys of Japan, although commercial operations have been established elsewhere, such as in the UK.
Salad rocket originates from the Middle East and has spread throughout the Mediterranean basin [8]. It has become naturalised on every inhabited continent and is considered an invasive weed in some regions. Watercress has similarly become naturalised (for example in North America) and grows in shallow rivers and streams. It can be cultivated commercially on large scales using artificial growing ‘pools’ flooded with stream water. Its leaves and shoots are a popular salad and sandwich garnish, and they can also be made into soups [9].
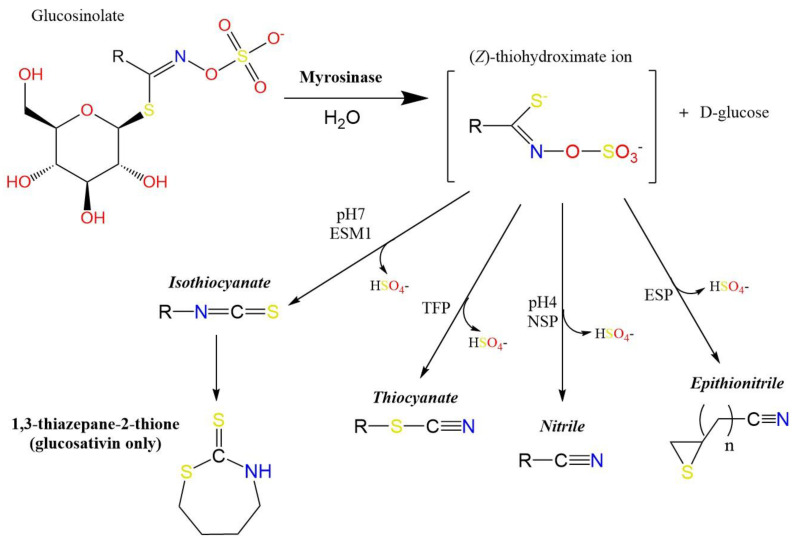
It is widely accepted that the distinctive aroma and flavour traits are produced by glucosinolate (GSL) hydrolysis products (GHPs [10]). GSLs are sulphur-containing secondary metabolites produced by Brassicales plants in response to biotic and abiotic stress [11]. Myrosinase enzymes are responsible for the hydrolysis of GSLs in water to form a plethora of GHPs, the conformation of which can be determined by the presence of enzyme cofactors, pH level, metallic ion concentration, and the precursor GSLs side-chain structure [8]. These products include: isothiocyanates (ITCs), nitriles, epithionitriles, indoles, oxazolidine-2-thiones, and other diverse products that result from tautomeric rearrangements (Figure 1).
Figure 1.
The glucosinolate–myrosinase reaction and hydrolysis products. Abbreviations: ESM1, epithiospecifier modifier protein 1; TFP, thiocyanate forming protein; NSP, nitrile specifier forming protein; ESP, epithiospecifier protein.
Previous work has reported olfactometry data for each of these crops; however, data are generally very scarce. Only five studies of E. sativa volatile compounds have been published in the last twenty years [3,12,13,14,15]. Very little information regarding wasabi root volatile composition and aroma is available outside of Japanese language journals [7,16]. Similarly, very little information is available for watercress, with only four papers published in the last 40 years [17,18,19,20]. Horseradish is the most well characterised of these four species, but still, only six studies of note have been published in the last 50 years [6,21,22,23,24,25].
There is also an ‘elephant in the room’ regarding previous reports of GHPs present in aroma profiles of these Brassicaceae vegetables. Many of the reported GHP compounds are derived from GSL precursors that are not regularly reported as part of the profile for each respective species. In E. sativa, for example, GHPs such as methyl ITC, 3-butenyl ITC, 1-isothiocyanato-4-methylpentane, and 5-(methylsulfanyl)pentanenitrile have all been previously reported [13]. The GSL precursors to these compounds (glucocapparin, GCP; gluconapin, GNP; 4-methylpentyl GSL, 4MP; and glucoberteroin, GBT; respectively) have never been reliably or consistently reported as being part of the GSL profile in this species [26,27]. This begs the question whether these identifications are correct or if the reports of GSL components in these species are incomplete. This may be due to a lack of sensitivity in reported mass spectrometry methods or because there is a lack of analytical standards to confirm compound identities. Another possibility is that there is a post-hydrolysis modification of GHPs, either by enzymatic means or through reactions with other phytochemical components, or as part of thermolytic reactions during gas chromatography. Very few examples of such modifications have been reported within the literature [28], but this may explain the presence of some GHPs within profiles of species where the GSL precursor is absent.
Other non-GSL derived compounds have also been reported to contribute significantly to the aromas of Brassicaceae crops. 2-Isopropyl-3-methoxypyrazine has been found to produce a strong pea, or green, pepper-like aroma in horseradish, for example ([6] Figure 2). In rocket, ‘green-leaf’ volatiles such as 3-hexenal and 1-penten-3-one have also been highlighted as having high odour potency [12]. The role of these compounds in aroma generation in GSL-containing crops is often not fully appreciated, and there are many diverse compounds with equally high odour intensities to GHPs present within the volatile bouquet.
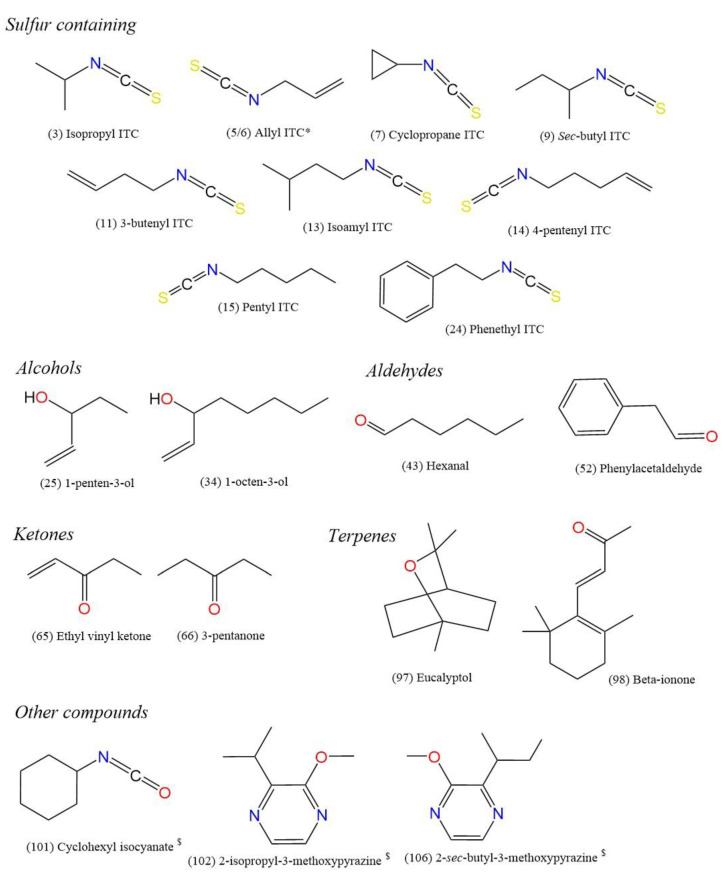
Figure 2.
Chemical structures of volatile compounds found in horseradish, rocket, wasabi, and watercress samples (numbers in parentheses refer to compound codes in Table 2); * two separate peaks present for this compound; $ tentatively identified.
The aims of this study were to (i) identify and describe in detail the key odorants of four Brassicaceae species (salad rocket, horseradish, wasabi, and watercress) by gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS), and (ii) associate the observed GHPs with their respective GSL profiles by liquid chromatography-mass spectrometry (UPLC-MS/MS). Our goal was to improve upon existing compound characterisation and odour descriptors for compounds in salad rocket, horseradish, wasabi, and watercress. The contribution of pungency by GHPs to aroma profiles is well studied; however, other aroma traits generated by non-GHPs are not well described for these species, and they likely create distinctive and subtle sensory characteristics. Additionally, detailed GSL compositions and MS/MS spectra for rocket, wasabi, and watercress is presented, highlighting discrepancies with observed hydrolysis products.
2. Materials and Methods
2.1. Samples
Individual horseradish and wasabi roots were purchased from Morrison’s supermarket (Reading, UK) and The Wasabi Company (Dorchester, UK) respectively. Watercress was purchased as whole bags of leaves from ASDA supermarket (Reading, UK). Salad rocket was grown in controlled environment conditions at the University of Reading using seeds donated by Elsoms Seeds Ltd. (Spalding, UK) and designated RS4 and RS8. Seeds of each cultivar were sown into module trays containing peat-based seedling compost and germinated at 30 °C (daytime; 25 °C night). Lighting conditions were set to a long-day cycle (16 h light, 8 h dark). Light intensity was set to 380 µmol m−2 s−1. Humidity was ambient. Plants were considered mature upon the development of 10 to 15 leaves. Harvested leaves were taken intact and placed inside a Zip-loc bag.
Roots and leaves were placed in a fridge upon either purchase or harvest (4 °C) until further analysis was performed.
2.2. Chemicals
For headspace solid-phase-microextraction (HS-SPME), calcium chloride and the alkane standards C6-C25 (100 μg/mL) in diethyl ether were obtained from Sigma-Aldrich (now Merck; Poole, UK). For ultra-high performance liquid chromatography mass spectrometry (UPLC-MS), authentic compounds of glucoiberin (GIB; 99.61%, HPLC), progoitrin (PRO; 99.07%, HPLC), sinigrin (SIN; 99%, HPLC), glucoraphanin (GRA; 99.86%, HPLC), glucoalyssin (GAL, 98.8%, HPLC), gluconapin (GNP, 98.66%, HPLC), 4-hydroxyglucobrassicin (4HGB; 96.19%, HPLC), glucobrassicanapin (GBN; 99.22%, HPLC), glucotropaeolin (GTP; 99.61%, HPLC), glucoerucin (GER; 99.68%, HPLC), glucobrassicin (GBR; 99.38%, HPLC), and gluconasturtiin (GNT; 98.38%, HPLC) were purchased from PhytoPlan (Heidelberg, Germany). Methanol (HPLC grade), formic acid (LC-MS grade), and acetonitrile (LC-MS grade) were purchased from VWR (Leicestershire, UK).
2.3. Volatile Compounds
2.3.1. Headspace Solid Phase Microextraction (SPME)
Samples of respective leaf and root tissues were homogenised by means of a commercial blender for 30 s, and 2 g of each was weighed into a SPME vial of 15 mL fitted with a screw cap. Samples were left aside for 10 min for the enzymatic hydrolysis of GSLs to take place. After exactly 10 min, 2 mL of saturated CaCl2 was added in order to cease the enzymatic reactions. After equilibration at 40 °C for 10 min, a 50/30 μm DVB/CAR/PDMS fibre was exposed to the headspace above the sample for 20 min. Three biological replicates were prepared for GC-MS analysis, and two replicates for each of the three assessors were prepared for the GC-O analysis.
2.3.2. GC-MS Analysis of SPME Extracts
After extraction, the SPME device was inserted into the injection port of an Agilent 7890A gas chromatography system coupled to an Agilent 5975C detection system equipped with an automated injection system (CTC-CombiPAL). A capillary column HP-5MS (30 m × 0.25 mm × 0.25 μm film thickness) (Agilent, Santa Clara, CA, USA) coated with (5% Phenyl Methyl Silox) was used for the chromatographic separation of volatile compounds. The oven temperature program used was 2 min at 40 °C isothermal and an increase of 4 °C/min to 250 °C. Helium was used at 3 mL/min as carrier gas. The sample injection mode was splitless. Mass spectra were measured in electron ionisation mode with an ionisation energy of 70 eV, the scan range from 20 to 280 m/z and the scan rate of 5.3 scans/s. The data were controlled and stored by the HP G1034 Chemstation system. Identities were confirmed by running the samples on a Stabilwax-DA (30 m × 0.25 mm × 0.25 μm film thickness) polar column from Restek (Bellefonte, PA, USA). Volatile compounds were identified or tentatively identified by comparison of each mass spectrum with spectra from authentic compounds analysed in our laboratory, or from the NIST mass spectral database (NIST/EPA/NIH Mass Spectral database, 2014), or spectra published elsewhere (see Supplementary Data S1 for GC-MS chromatograms and compound fragmentation spectra). A spectral quality value >80 was used alongside linear retention index to support the identification of compounds where no authentic standards were available. LRI was calculated for each volatile compound using the retention times of a homologous series of C6-C25 n-alkanes and by comparing the LRI with those of authentic compounds analysed under similar conditions. The compound peak areas were normalised and converted to the relevant abundance of each component as a percentage of the total peak area.
2.3.3. GC-O Analysis of SPME Extracts
After extraction, the SPME device was inserted into the injection port of an Agilent 7890B Series ODO 2 (SGE) GC-O system equipped with a non-polar HP-5MS column (30 m × 0.25 mm × 0.25 μm film thickness). The outlet was split between a flame ionisation detector and a sniffing port. The contents of the SPME fibre were desorbed for 3 min in a split/splitless injection port, in splitless mode, onto five small loops of the column in a coil, which were cooled in solid carbon dioxide and contained within a 250 mL beaker. The injector and detector temperatures were maintained at 280 °C and 250 °C, respectively. During desorption, the oven was held at 40 °C. After desorption, the solid carbon dioxide was removed from the oven. The oven was maintained at 40 °C for a further 2 min and then, the temperature was raised at 4 °C/min to 200 °C and at 8 °C/min to 300 °C. Helium was the carrier gas, and the flow rate was 2.0 mL/min. Three assessors were used for the detection and verbal description of the odour active components of the SPME extracts. Each assessor participated in three training sessions for each sample species prior to scoring sessions. Each assessor evaluated by sniffing each sample in duplicate and documented the odour description, retention time, and odour intensity (OI) on a seven-point scale (2–8), where <3 = weak, 5 = medium, and 7 = strong. Only those odours that were detected by all three assessors were recorded in the results. n-Alkanes C6-C25 were analysed under the same conditions to obtain LRI values for comparison with the GC-MS data.
2.4. Non-Volatile Compounds
2.4.1. Glucosinolate (GSL) Extraction
GSL extraction was performed as per the protocol presented by [29] with modifications. Briefly, 40 mg of dried leaf powder was placed into Eppendorf tubes and put into a heat block (80 °C for ten minutes). Afterwards, 1 mL of preheated methanol water (70% v/v) was added to dried powder, vortexed vigorously, and placed in a water bath (75 °C) for 20 min. Samples were cooled and centrifuged at full speed for five minutes at room temperature (≈22 °C); the supernatant was collected and filtered (0.22 µm PVDF Acrodisc syringe filters; VWR, Lutterworth, UK). Crude extracts were dried using a centrifugal evaporator and re-suspended in 1 mL of LC-MS-grade H2O and stored at −80 °C until analysis. Immediately before analysis by UPLC-MS, samples were diluted five-fold with LC-MS-grade H2O.
2.4.2. UPLC-MS Analysis of GSL Extracts
UPLC-MS was performed on a Shimadzu Nexera X2 series UHPLC, coupled with an 8050 triple quadrupole mass spectrometer system (Shimadzu UK Ltd., Milton Keynes, UK). Separation of standards and samples was achieved using a Waters BEH C18 Acquity column (100 × 2.1 mm, 1.7 µm; Waters Corp., Wilmslow, UK) with an Acquity in-line filter. Mobile phases consisted of 0.1% formic acid in LC-MS grade H2O (A), and 0.1% formic acid in LC-MS grade acetonitrile (B) and GSLs were separated during a five minute run with the following gradient timetable: (i) 0–50 s (A-B, 98:2, v/v), (ii) 50 s–3 min (A-B, 70:30, v/v), (iii) 3–3 min 10 s (A-B, 5:95, v/v), (iv) 3 min 10 s–4 min (A-B, 5:95, v/v), (v) 4–4 min 10 s (A-B, 98:2, v/v), (vi) 4 min 10 s–5 min (A-B, 98:2, v/v). The flow rate was 0.4 mL per min and the column oven temperature was 35 °C.
Two MS methods were used for the identification and quantification of GSLs. First, a Product Ion Scan (PIS) method was established to identify GSLs based on known primary ion masses ([M-H]-) characteristic fragment ions (357, 258, and 97 m/z; Table 1). Then, MS/MS spectra were compared to authentic standards and available literature sources [30,31,32,33,34,35,36,37,38]. Pentyl GSL (PEN), isobutyl GSL (ISO), glucoputranjivin (GPJ), and butyl GSL (BUT) were tentatively identified due to the possible presence of isomers [38], and/or no reliable reference MS spectra could be found in the literature. Total ion chromatograms of glucosinolates identified were included in the Supplementary Data (S2).
Table 1.
Glucosinolate compounds identified in salad rocket, wasabi, and horseradish by UPLC-MS/MS.
| Glucosinolate | Common Name | Abbreviation | Rt | LOD (nmol g−1) | LOQ (nmol g−1) | R2 | Precursor Ion ([M-H]-) | Quantification Transition | Confirmation Transition | MS/MS of [M-H]- Ion m/z (Relative Intensity) |
References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-(methylsulfinyl)propyl | glucoiberin * | GIB | 1.061 | 0.053 | 0.161 | 0.969 | 422 | 422 > 357 | 422 > 97 | 357(57), 97(100), 95(61) | [30,31] |
| pentyl a,$ | - | PEN | 1.08 | - | - | - | 388 | 388 > 75 | 388 > 97 | 273(15), 258(13), 194(14), 192(13), 97(100), 89(24), 79(18),75(71), 74(11) | - |
| (R)-2-hydroxy-3-butenyl | progoitrin * | PRO | 1.336 | 0.066 | 0.199 | 0.933 | 388 | 388 > 74 | 388 > 97 | 97(100), 74(75) | [32] |
| allyl | sinigrin * | SIN | 1.523 | 0.105 | 0.319 | 0.982 | 358 | 358 > 258 | 358 > 97 | 258(10), 97(100), 74(25) | [30,32,33] |
| isobutyl a,$ | - | ISO | 1.56 | - | - | - | 374 | 374 > 240 | 374 > 115 | 277(11), 274(20), 258(86), 257(62), 240(95), 115(100), 97(26), 95(38) | - |
| 4-(methylsulfinyl)butyl | glucoraphanin * | GRA | 1.598 | 0.059 | 0.179 | 0.989 | 436 | 436 > 371 | 436 > 97 | 371(80), 97(100) | [30,32] |
| 4-(cystein-S-yl)butyl | glucorucolamine a,$ | GRM | 1.654 | - | - | - | 494 | 494 > 406 | 494 > 217 | 413(32), 406(66), 404(38), 295(32), 291(17), 275(38), 250(66), 217(100), 209(33), 195(20), 171(38), 145(38), 129(17), 114(17), 112(40), 97(47), 96(32), 75(16) | - |
| 5-(methylsulfinyl)pentyl | glucoalyssin * | GAL | 2.421 | 0.064 | 0.194 | 0.971 | 450 | 450 > 206 | 450 > 97 | 386(42), 275(49), 263(21), 208(21), 206(100), 190(71), 97(51), 75(49) | [31,32,34] |
| 1-methylethyl | glucoputranjivin a,$ | GPJ | 2.447 | - | - | - | 360 | 360 > 75 | 360 > 97 | 359(24), 119(13), 97(100), 94(34), 75(54) | - |
| 3-butenyl | gluconapin * | GNP | 2.489 | 0.151 | 0.459 | 0.953 | 372 | 372 > 258 | 372 > 97 | 258(6), 97(100) | [30,31] |
| 4-(β-D-glucopyrano syldisulfanyl)butyl |
diglucothiobeinin a,$ | DGTB | 2.501 | - | - | - | 600 | 600 > 290 | 600 > 97 | 290(7), 97(100) | [32] |
| 5-(methylthio)pentyl | glucoberteroin a,$ | GBT | 2.593 | - | - | - | 434 | 434 > 95 | 434 > 97 | 146(60), 97(73), 95(100) | [31,35] |
| 4-hydroxy-3-indolylmethyl | 4-hydroxygluco brassicin * |
4HGB | 2.619 | 0.15 | 0.454 | 0.943 | 463 | 463 > 285 | 463 > 97 | 285(17), 97(100) | [30,32] |
| 1-methylpropyl | glucocochlearin a,$ | GCL | 2.682 | - | - | - | 374 | 374 > 75 | 374 > 97 | 293(10), 275(19), 98(58), 97(100), 95(31), 84(10), 75(81) | [33] |
| 4-mercaptobutyl | glucosativin a,$ | GSV | 2.684 | - | - | - | 406 | 406 > 74 | 406 > 97 | 259(12), 97(100), 76(19), 74(23) | [32] |
| 7-(methylsulfinyl)heptyl a,$ | - | 7MSH | 2.737 | - | - | - | 478 | 478 > 413 | 478 > 97 | 413(46), 259(12), 219(11), 98(68), 97(100), 75(12) | [36] |
| 4-pentenyl | glucobrassicanapin a | GBN | 2.819 | 0.081 | 0.245 | 0.945 | 386 | 386 > 75 | 386 > 96 | 97(100), 96(44), 75(50) | [33] |
| dimeric 4-mercaptobutyl a,$ | - | DMB | 2.839 | - | - | - | 811 | 405 > 80 | 405 > 97 | 208(11), 97(100), 81(15), 80(16), 75(15) | [32] |
| 2(S)-hydroxy-2-phenylethyl | glucobarbarin a,$ | GBB | 2.847 | - | - | - | 438 | 438 > 98 | 438 > 96 | 437(16), 332(20), 274(17), 195(11), 137(29), 135(18), 98(48), 96(100), 74(30) | [37] |
| benzyl | glucotropaeolin * | GTP | 2.879 | 0.129 | 0.39 | 0.962 | 408 | 408 > 259 | 408 > 97 | 259(10), 97(100) | [30,31] |
| 4-(methylthio)butyl | glucoerucin * | GER | 2.919 | 0.04 | 0.121 | 0.948 | 420 | 420 > 74 | 420 > 96 | 258(16), 241(17), 178(15), 96(100), 75(13), 74(27) | [30,35] |
| indolyl-3-methyl | glucobrassicin * | GBC | 3.102 | 0.096 | 0.29 | 0.955 | 447 | 447 > 259 | 447 > 97 | 259(10), 97(100) | [31,32] |
| 4-methoxyindolyl-3-methyl | 4-methoxygluco brassicin b,$ |
4MGB | 3.173 | - | - | - | 477 | 477 > 75 | 477 > 97 | 259(12), 258(13), 127(10), 119(13), 98(53), 97(100), 84(15), 75(32), 74(15) | [30,31,33,34] |
| 2-phenethyl | gluconasturtiin * | GNT | 3.419 | 0.062 | 0.189 | 0.968 | 422 | 422 > 259 | 422 > 97 | 259(10), 97(100) | [32,33] |
| 1-methoxyindolyl-3-methyl | neoglucobrassicin b,$ | NGB | 3.526 | - | - | - | 477 | 477 > 75 | 477 > 97 | 144(13), 97(100), 84(16), 82(11), 75(22) | [32] |
| 4-methylpentyl a,$ | - | 4MP | 3.695 | - | - | - | 402 | 402 > 259 | 402 > 97 | 275(11), 259(24), 195(16), 179(10), 159(20), 97(100), 85(12) | - |
| hexyl a,$ | - | HEX | 3.726 | - | - | - | 402 | 402 > 119 | 402 > 97 | 401(22), 274(15), 241(59), 226(32), 204(14), 198(15), 197(14), 168(14), 161(56), 160(32), 138(32), 121(32), 119(64), 116(15), 114(14), 98(46), 97(100), 96(65), 85(15), 79(15) | [31,32] |
| 7-(methylthio)heptyl a,$ | - | 7MTH | 4.109 | - | - | - | 462 | 462 > 75 | 462 > 97 | 283(11), 275(11), 274(11), 220(17), 97(100), 75(32) | [36] |
| butyl a,$ | - | BUT | 4.176 | - | - | - | 375 | 375 > 256 | 375 > 180 | 328(48), 316(41), 307(41), 260(41), 256(58), 235(41), 207(41), 195(50), 185(20), 180(100), 143(20), 120(63), 97(41) | - |
Ions in bold agree with previous studies; ions underlined indicate characteristic ions associated with glucosinolates. * Authentic standard; a quantified using sinigrin; b quantified using glucobrassicin; $ tentative identification.
MS/MS settings for the PIS method were as follows: samples were analysed in the negative ion mode with a scan range of 70–820 m/z. A collision energy of 25 eV and a scan speed of 30,000 u per s−1. For the quantification of GSLs, a Multiple Reaction Monitoring (MRM) method was established. Based on the fragmentation observed in the PIS method, confirmation and quantification transitions were established (Table 1). Dwell times for each precursor and product ion were set to 5 s.
Authentic GSL compounds were run as external standards. Limits of detection (LOD) and limits of quantification (LOQ) were established for each and are presented in Table 1. As standard compounds are not available for all GSLs, SIN was used to semi-quantify glucorucolamine (GRM), glucoputranjivin (GPJ), diglucothiobeinin (DGTB), glucoberteroin (GBT), glucocochlearin (GCL), glucosativin (GSV), dimeric 4-mercaptobutyl GSL (DMB), glucobarbarin (GBB), and tentatively identified GSL compounds. Similarly, GBR was used to semi-quantify the indolic GSLs 4-methoxyglucobrassicin (4MGB) and neoglucobrassicin (NGB).
3. Results and Discussion
3.1. Volatile Compounds
The volatile compounds identified in the headspace of the four Brassicales species are listed in Table 2, detailing their PubChem compound identification (PubChem CID) as well as their linear retention indices (LRI) in a polar and non-polar column. Semiquantitative characterisation results are also shown in Table 2 as relative area. ITCs and alcohols were the chemical classes of compounds dominating the volatile profile of the samples with other compounds such as aldehydes, esters, and terpenes also present.
Table 2.
Volatile compounds identified in the headspace of four Brassicales species analysed by HS-SPME GC-MS.
| Compound Number | Compound | PubChem ID | LRI a | ID b | Peak Areas (% of total) | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-5MS | Stabilwax | Salad Rocket | Wasabi | Horseradish | Watercress | ||||||
| Sulphur-containing compounds | |||||||||||
| 1 | carbon disulphide | 6348 | <600 | 738 | B | 0.43 * | 0.04 * | 0.02 | 0.11 * | [21] | |
| 2 | methyl thiocyanate | 11168 | 711 | 1266 | B | 0.13 | nd | nd | 0.48 | [12] | |
| 3 | isopropyl ITC | 75263 | 835 | 1176 | B | nd | 4.07 | 0.03 | nd | [7,23] | |
| 4 | allyl thiocyanate | 69816 | 870 | 1358 | B | nd | 0.48 * | 0.73 | nd | [22] | |
| 5 | allyl ITC ^ | 5971 | 881 | 1371 | B | nd | 8.61 | 7.35 | nd | [7,14,18,24] | |
| 6 | allyl ITC ^ | 890 | 1392 | B | nd | 52.11 | 39.34 | nd | |||
| 7 | cyclopropane ITC | 92463 | 899 | 1223 | B | nd | 0.07 * | 0.05 * | nd | - | |
| 8 | cyclopentyl-1-thiaethane | 138938 | 922 | 1068 | B | nd | nd | nd | 0.51 * | - | |
| 9 | sec-butyl ITC | 78151 | 933 | 1265 | B | 0.46 * | 4.95 | 1.84 | nd | [7,23] | |
| 10 | isobutyl ITC | 68960 | 955 | 1316 | B | nd | 1.80 | 0.45 | nd | [7,21] | |
| 11 | 3-butenyl ITC | 76922 | 982 | 1455 | B | 0.19 | 4.24 | 1.52 | nd | [3,7,22] | |
| 12 | butyl ITC | 11613 | 998 | 1597 | B | 0.67 | 0.12 | 0.32 | nd | [7,15] | |
| 13 | isoamyl ITC | 79086 | 1059 | 1431 | B | 0.10 | 0.41 * | 0.55 | 0.07 | [3,18,22] | |
| 14 | 4-pentenyl ITC | 87436 | 1086 | 1543 | B | nd | 12.42 | 2.26 | nd | [7,22] | |
| 15 | pentyl ITC | 69415 | 1098 | 1488 | B | 0.56 * | 0.12 * | 0.20 | nd | [21] | |
| 16 | 1-isothiocyanato-4-methylpentane | 519452 | 1162 | 1522 | B | 4.96 | 0.01 * | 0.04 | 0.18 | [3,18,21] | |
| 17 | cyclohexyl ITC | 14289 | 1177 | 1650 | B | nd | 0.01 * | nd | nd | - | |
| 18 | <unidentified ITC> | - | 1193 | nd | 8.57 | nd | nd | - | |||
| 19 | ibervirin | 62351 | 1315 | 1991 | B | 0.58 | 0.29 | 1.02 | nd | [3,7,22] | |
| 20 | sativin | 85704368 | 1353 | >2000 | B | 0.55 | nd | nd | nd | [12] | |
| 21 | octyl ITC | 78161 | 1372 | 1760 | B | nd | nd | nd | 0.16 * | - | |
| 22 | benzyl ITC | 2346 | 1372 | >2000 | B | nd | 0.03 * | 1.14 | nd | [12,22] | |
| 23 | erucin | 78160 | 1440 | >2000 | B | 7.16 | 0.01 | 0.02 | nd | [7,12,22] | |
| 24 | phenethyl ITC | 16741 | 1477 | >2000 | B | nd | 0.68 | 32.47 | 30.72 | [7,12,18,24] | |
| Total sulphur-containing compounds | 15.79 | 99.07 | 89.78 | 32.92 | |||||||
| Alcohols | |||||||||||
| 25 | 1-penten-3-ol | 12020 | 678 | 1159 | A | 0.78 | nd | 0.10 * | 8.06 | [3,7,18] | |
| 26 | pentan-1-ol | 6276 | 763 | 1251 | A | 0.13 * | 0.02 * | 0.02 * | 0.25 * | - | |
| 27 | (E)-2-penten-1-ol | 5364919 | 763 | 1312 | A | 0.17 * | nd | nd | 0.31 * | - | |
| 28 | (Z)-2-penten-1-ol | 15306 | 767 | 1322 | A | 0.76 | nd | 0.06 | 4.78 | [3,22] | |
| 29 | 1-propoxy-2-propanol | 15286 | 840 | B | nd | nd | nd | 0.24 * | - | ||
| 30 | (E)-3-hexen-1-ol | 5284503 | 850 | 1365 | A | nd | nd | nd | 0.20 | [3,19,22] | |
| 31 | (Z)-3-hexen-1-ol | 5281167 | 856 | 1387 | A | 40.88 | nd | 1.88 | 35.99 | [3,18,22] | |
| 32 | 2-hexen-1-ol | 5318042 | 863 | 1405 | A | 1.25 | nd | nd | 0.35 | [7,13,18,22] | |
| 33 | hexan-1-ol | 8103 | 866 | 1355 | A | 4.82 * | nd | 0.22 | 2.61 | [7,18] | |
| 34 | 1-octen-3-ol | 18827 | 977 | A | 0.18 | 0.01 * | 0.02 * | 0.06 * | [14] | ||
| 35 | 2-ethylhexanol | 7720 | 1025 | 1501 | B | 0.12 * | 0.01 * | nd | 0.12 * | - | |
| 36 | benzyl alcohol | 244 | 1035 | A | nd | nd | 0.05 * | nd | - | ||
| 37 | 2-phenylethanol | 6054 | 1116 | 1930 | A | 0.16 * | nd | 0.03 * | 2.64 * | - | |
| 38 | 1-nonanol | 8914 | 1167 | A | nd | 0.03 * | nd | nd | - | ||
| 39 | terpinen-4-ol | 11230 | 1182 | A | nd | 0.01 * | nd | nd | - | ||
| Total alcohols | 49.25 | 0.09 | 2.38 | 55.62 | |||||||
| Aldehydes | |||||||||||
| 40 | 2-pentanal | 7895 | 699 | A | nd | 0.02 * | nd | nd | - | ||
| 41 | 2-pentenal | 5364752 | 753 | 1136 | A | 0.31 | nd | 0.02 * | 0.23 * | [3] | |
| 42 | 3-hexenal | 643139 | 796 | 1178 | A | 1.72 | nd | 0.04 | 0.23 | [3,18,22] | |
| 43 | hexanal | 6184 | 798 | 1094 | A | 8.64 | <0.01 * | 0.37 | 1.16 * | [12,21] | |
| 44 | (E)-2-hexenal | 5281168 | 852 | 1224 | A | 14.30 | nd | 0.31 | 2.10 | [3,7,18,21] | |
| 45 | 4-heptenal | 5283318 | 901 | A | 0.08 * | nd | nd | 0.51 | - | ||
| 46 | heptanal | 8130 | 902 | A | 0.13 | nd | 0.02 | 0.10 * | [13,21] | ||
| 47 | 2,4-hexadienal | 637564 | 908 | B | 0.09 | nd | 0.01 * | nd | [3,18] | ||
| 48 | benzaldehyde | 240 | 964 | 1654 | A | 0.17 | 0.02 * | nd | nd | [12,22] | |
| 49 | 2,4-heptadienal (isomer 1) | 5283321 | 994 | B | 0.09 | nd | nd | nd | [12] | ||
| 50 | octanal | 454 | 1001 | A | nd | 0.02 * | nd | nd | - | ||
| 51 | 2,4-heptadienal (isomer 2) | 5283324 | 1009 | 1472 | B | 0.15 | nd | 0.25 * | 0.10 * | [12] | |
| 52 | phenylacetaldehyde | 998 | 1047 | A | nd | nd | 0.02 | 0.24 * | [22] | ||
| 53 | 2-octenal | 5283324 | 1058 | A | nd | nd | 0.06 * | nd | - | ||
| 54 | nonanal | 31289 | 1101 | A | 0.41 | 0.10 * | 0.04 | 0.31 * | [13,21] | ||
| 55 | decanal | 8175 | 1202 | A | nd | 0.12 * | 0.04 | 0.19 * | [21] | ||
| 56 | vanillin | 1183 | 1403 | A | 0.02 | nd | 0.01 * | nd | [13] | ||
| Total aldehydes | 26.11 | 0.28 | 1.25 | 5.17 | |||||||
| Esters | |||||||||||
| 57 | (Z)-pent-2-en-1-yl acetate | 5363400 | 910 | B | nd | nd | nd | 0.07 * | - | ||
| 58 | 3-hexenyl acetate | 5352557 | 1003 | 1284 | A | 2.54 | nd | nd | 0.50 | [14,18] | |
| 59 | (Z)-3-hexenyl butanoate | 5352438 | 1181 | 1440 | A | 0.99 | nd | nd | nd | [14] | |
| 60 | methyl salicylate | 4133 | 1201 | A | nd | nd | 0.04 * | nd | - | ||
| 61 | ethyl decanoate | 8048 | 1389 | A | 0.06 * | nd | 0.03 * | nd | - | ||
| 62 | methyl dodecanoate | 8139 | 1521 | B | 0.04 | 0.01 * | 0.02 * | 0.08 * | [14] | ||
| 63 | diethyl phthalate | 6781 | 1588 | B | 0.08 * | nd | nd | nd | - | ||
| 64 | ethyl laurate | 7800 | 1591 | B | nd | nd | 0.06 * | nd | - | ||
| Total esters | 3.71 | 0.01 | 0.15 | 0.65 | |||||||
| Ketones | |||||||||||
| 65 | ethyl vinyl ketone | 15394 | 682 | 1035 | A | 0.43 | nd | 0.05 * | nd | [3] | |
| 66 | 3-pentanone | 7288 | 697 | 993 | A | 0.66 | nd | 0.05 * | 1.86 | [3,19] | |
| 67 | 2,3-octanedione | 11449 | 980 | A | nd | nd | 0.01 | nd | [22] | ||
| 68 | 6-methyl-5-hepten-2-one | 9862 | 983 | A | 0.55 | nd | nd | 0.23 * | [12] | ||
| 69 | 2,2,6-trimethylcyclohexanone | 17000 | 1038 | B | nd | nd | nd | 0.11 * | - | ||
| 70 | (E,E)-3,5-octadien-2-one | 5352876 | 1070 | B | nd | nd | 0.08 * | nd | - | ||
| 71 | 3,5-octadien-2-one | 5352876 | 1092 | B | nd | nd | 0.09 * | nd | - | ||
| 72 | dihydro-2H-thiopyran-3(4H)-one | 140474 | 1160 | 1856 | B | 0.58 | nd | nd | nd | - | |
| 73 | geranylacetone | 1549778 | 1451 | A | 0.10 * | 0.02 * | nd | nd | - | ||
| Total ketones | 2.32 | 0.02 | 0.27 | 2.21 | |||||||
| Nitriles | |||||||||||
| 74 | 3-butenenitrile | 8009 | 654 | B | nd | 0.15 | 0.37 | nd | [7,21] | ||
| 75 | 5-methylhexanenitrile | 29593 | 943 | B | 0.27 | nd | nd | nd | [13] | ||
| 76 | 6-heptenenitrile | 4140856 | 971 | B | nd | 0.08 * | nd | nd | - | ||
| 77 | thiiraneacetonitrile | 148821 | 1004 | B | nd | nd | 3.58 * | nd | [39] | ||
| 78 | phenylacetonitrile | 8794 | 1142 | B | nd | nd | 0.01 * | nd | - | ||
| 79 | 5-(methylsulfanyl)pentanenitrile | 93320 | 1200 | B | 1.13 | nd | nd | nd | [12] | ||
| 80 | 4-(methylthio)-butanenitrile | 100962 | 1213 | B | nd | nd | 0.11 * | nd | - | ||
| 81 | benzenepropanenitrile | 12581 | 1243 | >2000 | B | nd | nd | 0.75 | 1.47 | [17,22] | |
| 82 | <unidentified nitrile> | - | 1559 | B | nd | 0.22 | nd | nd | - | ||
| Total nitriles | 1.39 | 0.45 | 4.83 | 1.47 | |||||||
| Hydrocarbons | |||||||||||
| 83 | 2,2,4,6,6-pentamethylheptane | 26058 | 991 | 947 | A | nd | nd | nd | 0.69 * | - | |
| 84 | undecane | 14257 | 1096 | A | 0.23 | nd | 0.01 * | nd | [15] | ||
| 85 | 1-dodecene | 8183 | 1187 | A | nd | 0.06 * | 0.12 * | 0.64 * | - | ||
| 86 | dodecane | 8182 | 1196 | A | nd | nd | 0.01 | nd | [22] | ||
| 87 | tridecane | 12388 | 1296 | A | nd | nd | 0.03 | nd | |||
| 88 | tetradecane | 12389 | 1396 | A | 0.10 | 0.01 * | 0.06 * | nd | [15] | ||
| 89 | pentadecane | 12391 | 1497 | A | nd | nd | 0.09 * | nd | - | ||
| 90 | hexadecane | 11006 | 1599 | A | nd | nd | 0.10 * | nd | - | ||
| 91 | heptadecane | 12398 | 1699 | A | nd | nd | 0.06 * | nd | - | ||
| 92 | octadecane | 11635 | 1799 | A | nd | nd | 0.03 * | nd | - | ||
| 93 | nonadecane | 12401 | 1899 | A | nd | nd | 0.01 * | nd | - | ||
| Total hydrocarbons | 0.39 | 0.06 | 0.62 | 1.33 | |||||||
| Terpenes | |||||||||||
| 94 | p-cymene | 7463 | 1023 | 1250 | A | nd | <0.01 * | 0.01 * | 0.06 | [18] | |
| 95 | o-cymene | 10703 | 1027 | A | 0.12 * | 0.01 * | nd | nd | - | ||
| 96 | d-limonene | 440917 | 1033 | 1169 | A | nd | <0.01 * | 0.07 * | nd | - | |
| 97 | eucalyptol | 2758 | 1037 | A | nd | <0.01 * | nd | nd | - | ||
| 98 | β-ionone | 638014 | 1494 | 1952 | A | 0.16 | nd | nd | 0.43 | [12,20] | |
| Total terpenes | 0.28 | 0.01 | 0.08 | 0.49 | |||||||
| Other compounds | |||||||||||
| 99 | allyl isocyanate | 15123 | 645 | B | nd | nd | 0.02 * | nd | - | ||
| 100 | 2-ethylfuran | 18554 | 701 | A | 0.16 | nd | 0.02 | 0.14 * | [3,21] | ||
| 101 | cyclohexyl isocyanate | 18502 | 961 | B | nd | 0.01 * | nd | nd | - | ||
| 102 | 1-isopropyl-3-methoxypyrazine | 33166 | 1093 | B | 0.25 | nd | 0.09 | nd | [15,25] | ||
| 103 | 1,2,3,5-tetramethylbenzene | 10695 | 1124 | B | 0.06 * | nd | nd | nd | - | ||
| 104 | veratrole | 7043 | 1144 | B | nd | nd | 0.09 * | nd | - | ||
| 105 | octanoic acid | 379 | 1157 | B | 0.10 | nd | 0.06 | nd | [14,22] | ||
| 106 | 2-sec-butyl-3-methoxypyrazine | 520098 | 1172 | B | 0.15 * | nd | 0.43 | nd | [25] | ||
| 107 | phenethyl isocyanate | 160602 | 1226 | 1807 | B | nd | nd | 0.41 * | 0.70 * | - | |
| 108 | quinoline | 7047 | 1247 | B | nd | nd | 0.02 * | nd | - | ||
| 109 | caprolactam | 7768 | 1251 | B | 0.05 * | nd | nd | nd | - | ||
| 110 | 4-bromophenol | 7808 | 1283 | B | 0.02 * | nd | nd | nd | - | ||
| 111 | 6-methylquinoline | 7059 | 1331 | B | nd | nd | 0.01 * | nd | - | ||
| 112 | methyleugenol | 7127 | 1400 | B | 0.02 * | nd | nd | nd | - | ||
| 113 | benzyl tiglate | 250096 | 1504 | B | nd | nd | 0.01 * | nd | - | ||
| Total other compounds | 0.77 | 0.01 | 0.63 | 0.14 | |||||||
a Linear retention index on a HP-5MS and Stabilwax columns. b A, mass spectrum and LRI agree with those of authentic compound; B, mass spectrum agrees with reference spectrum in the NIST/EPA/NIH mass spectra database and LRI agree with those in the literature, tentatively identified. ^ Based on library identification and spectra but two separate peaks were present; nd, not detected; * newly reported for species.
GC-olfactometry analysis of the samples yielded a total of 107 odorants across the four species, which are presented in Table 3. Qualitative differences were observed between the samples with horseradish and watercress yielding a total of 52 and 51 odorants, respectively. Green/grassy, radish, sulphury, and horseradish were some of the terms that were mostly used by the assessors to describe the odours. Additionally, a total of 46 odorants of unknown identity were detected within the headspace of the four Brassicales analysed that may contribute to the odour profiles of these crops. These compounds matched no corresponding peaks and LRI values within the GC-MS data. This suggests that the compounds responsible for generating the perceived aromas were present at levels below the detection threshold of the instrumentation used. The number and diversity of unidentified compounds and aromas is indicative of the fact that characterisation of the species’ volatile profiles is far from complete, and it is likely that many more will be discovered in future studies.
Table 3.
Odorants identified by HS-SPME GC-O in the headspace of four Brassicales species.
| Compound Number | Compound | LRI a | Odour Description | Odour Intensity c | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| This Study | Previous Description(s) b | Salad Rocket | Wasabi | Horseradish | Watercress | ||||
| 114 | <unknown> | <600 | sulphury | - | nd | 3 | 3 | 4 | - |
| 115 | <unknown> | <600 | cooked onions | - | nd | nd | nd | 4 | - |
| 116 | <unknown> | <600 | buttery | - | 3 | 5 | 5 | 5 | - |
| 117 | <unknown> | <600 | sulphury, horseradish, rancid | - | 3 | 6 | 4 | nd | - |
| 118 | <unknown> | 602 | horseradish | - | nd | nd | 5 | nd | - |
| 119 | <unknown> | 609 | mustard, horseradish | - | nd | 6 | nd | nd | - |
| 120 | <unknown> | 615 | rotten cabbage | - | nd | nd | 5 | 4 | - |
| 121 | <unknown> | 622 | onions | - | nd | 6 | nd | nd | - |
| 99 | allyl isocyanate $ | 648 | musty, burnt plastic † | - | nd | nd | 2 * | nd | - |
| 122 | <unknown> | 655 | sulphury, cabbage-like | - | 6 | nd | nd | nd | - |
| 74 | 3-butenenitrile $ | 657 | sulphury, green, pungent † | - | nd | 3 | 4 | nd | [21] |
| 25 | 1-penten-3-ol | 676 | cabbage, sulphury | pungent, horseradish-like | 3 | nd | nd | 7 | [40] |
| 65 | ethyl vinyl ketone | 680 | pungent, rotten, green | pungent | 5 | nd | nd | nd | [41] |
| 66 | 3-pentanone | 687 | green, grassy, floral | acetone-like | nd | nd | nd | 6 | [42] |
| 123 | <unknown> | 713 | sulphury, garlic | - | nd | 2 | 3 | nd | - |
| 2 | methyl thiocyanate $ | 719 | sulphury, oniony | sulphur | 4 | nd | nd | 4 * | - |
| 124 | <unknown> | 737 | sulphury, rotten onion | - | 3 | nd | nd | 3 | - |
| 40 | 2-pentenal | 750 | apple, green | green, apple-like | 3 | nd | 3 * | 4 * | [43] |
| 125 | <unknown> | 758 | sulphury, cooked onion, pungent | - | nd | nd | nd | 6 | - |
| 126 | <unknown> | 775 | sulphury, oniony | - | nd | nd | nd | 4 | - |
| 42 | 3-hexenal | 796 | grassy, green, floral | leafy, green | 4 | nd | 3 | 4 | [25] |
| 43 | hexanal | 799 | green, grassy, pungent | green, grass-like, leafy | 5 * | nd | 4 | 4 * | [44] |
| 127 | <unknown> | 811 | green, parsley | - | nd | nd | nd | 4 | - |
| 128 | <unknown> | 812 | mustard, pungent, oniony | - | 5 | 6 | 3 | 6 | - |
| 3 | isopropyl ITC $ | 834 | pungent, grassy notes | pungent | nd | 5 | nd | nd | [12] |
| 44 | (E)-2-hexenal | 852 | green, fresh, apples (weak) | green | 3 | nd | 2 | 4 | [45] |
| 30 | (Z)-3-hexen-1-ol | 856 | green, radishy | green | 2 | nd | nd | 4 | [46] |
| 32 | 2-hexen-1-ol | 864 | green, leafy | green, leafy | 3 | nd | nd | nd | [47] |
| 129 | <unknown> | 866 | cooked, roasted chicken, chicken soup | - | nd | 3 | 3 | 5 | - |
| 130 | <unknown> | 870 | nutty, spicy | - | nd | nd | 3 | nd | - |
| 4 | allyl thiocyanate $ | 879 | peppery, horseradish, pungent | strong, pungent, mustard-like | nd | nd | 4 | nd | [48] |
| 131 | <unknown> | 892 | green, sour apples | - | 5 | nd | nd | 6 | - |
| 5 | allyl ITC $ | 895 | pungent, horseradish | strong, pungent, mustard-like | nd | nd | 4 | nd | [48] |
| 6 | allyl ITC $ | 898 | garlic, mustard, horseradish, very pungent | nd | 7 | 7 | nd | ||
| 7 | cyclopropane ITC $ | 900 | horseradish, garlic, onion, sulphur † | - | nd | 5 * | 7 * | nd | [49] |
| 45 | 4-heptenal | 901 | grassy, green † | mushroom-like, fatty, fishy, cooked, potato | 4 * | nd | nd | 3 * | [50] |
| 46 | heptanal | 902 | fatty, green | fatty, green | 4 | nd | nd | 5 * | [13] |
| 132 | <unknown> | 907 | apples, grass | - | nd | nd | nd | 4 | - |
| 47 | 2,4-hexadienal $ | 907 | green, rotten | green | 3 | nd | 3 * | nd | [51] |
| 57 | (Z)-2-pent-2-en-1-yl acetate $ | 914 | sulphury, rotten † | - | nd | nd | nd | 3 * | - |
| 8 | cyclopentyl-1-thiaethane | 918 | sulphur, sweaty † | - | nd | nd | nd | 3 * | - |
| 133 | <unknown> | 921 | potato | - | nd | 5 | nd | 4 | - |
| 9 | sec-butyl ITC $ | 934 | radish, vegetative | green | nd | 5 | 3 | nd | [52] |
| 10 | isobutyl ITC $ | 956 | cooked, pungent, sulphury † | - | nd | 4 | 5 | nd | [21] |
| 101 | cyclohexyl isocyanate $ | 965 | cooked, peppery, potato † | - | nd | 5 * | nd | nd | [53] |
| 34 | 1-octen-3-ol | 978 | mushroom | mushroom | 5 | 5 * | 3 * | 5 * | - |
| 11 | 3-butenyl ITC $ | 983 | green, pungent, aromatic | aromatic, pungent | 5 | 6 | 4 | nd | [54] |
| 68 | 6-methyl-5-hepten-2-one | 984 | perfume, floral, citrus | citrus, lemongrass | 4 | nd | nd | 5 * | [55] |
| 12 | butyl ITC $ | 997 | peppery, sulphurous, oniony | sulphury | nd | 4 | 2 | nd | [21] |
| 77 | thiiraneacetonitrile $ | 1005 | sweaty, gas-like † | - | nd | nd | 4 | nd | [56] |
| 134 | <unknown> | 1010 | grassy | - | nd | nd | nd | 3 | - |
| 135 | <unknown> | 1020 | earthy, musty, petrol, aromatic | - | nd | nd | nd | 5 | - |
| 136 | <unknown> | 1025 | bread-like | - | nd | nd | nd | 5 | - |
| 137 | <unknown> | 1032 | green | - | nd | nd | nd | 3 | - |
| 96 | d-limonene | 1034 | lemon, vegetable | citrus, herbal | nd | nd | 3 * | nd | [57] |
| 36 | benzyl alcohol | 1037 | fruity, medicinal, wine | phenolic | nd | nd | 4 * | nd | [58] |
| 97 | eucalyptol | 1037 | eucalyptus, mint | eucalyptus | nd | 5 * | nd | nd | [42] |
| 69 | 2,2,6-trimethylcyclohexanone $ | 1038 | floral, green with citrus notes | thujonic | nd | nd | nd | 4 * | [59] |
| 52 | phenylacetaldehyde | 1044 | honey-sweet | honey, sweet | nd | nd | 4 | 7 * | [21] |
| 138 | <unknown> | 1051 | oniony | - | 5 | nd | nd | nd | - |
| 139 | <unknown> | 1053 | soily, earthy | - | 6 | nd | nd | nd | - |
| 13 | isoamyl ITC $ | 1057 | pungent, grassy | green | nd | 6 * | 3 | nd | [3] |
| 140 | <unknown> | 1066 | medicinal, floral | - | nd | nd | nd | 5 | - |
| 141 | <unknown> | 1076 | gas, sulphur, burnt, roasted | - | 3 | nd | 5 | 4 | - |
| 142 | <unknown> | 1080 | roasted, smoky | - | 3 | nd | nd | 3 | - |
| 14 | 4-pentenyl ITC $ | 1084 | pungent, peppery, sulphurous, musty | mustard, horseradish-like | nd | 5 | 3 | nd | [48] |
| 71 | 3,5-octadien-2-one $ | 1090 | green, pungent | fruity | nd | nd | 4 * | nd | - |
| 102 | 2-isopropyl-3-methoxypyrazine $ | 1091 | rotten, potato, vegetative | pea-like, earthy, bean-like | 6 | nd | 6 | nd | [60] |
| 15 | pentyl ITC $ | 1095 | cabbage, green, rotten | green | 5 * | 4 * | 6 * | nd | - |
| 143 | <unknown> | 1095 | cucumber, floral, flowers | - | nd | nd | nd | 3 | - |
| 54 | nonanal | 1101 | fatty, green | green, fatty | nd | nd | 4 | 5 * | [40] |
| 37 | 2-phenylethanol | 1114 | floral | floral | 3 * | nd | nd | 4 * | [58] |
| 144 | <unknown> | 1118 | spicy, chemical | - | 3 | nd | nd | nd | - |
| 145 | <unknown> | 1122 | sulphury, gas-like | - | 4 | nd | 2 | nd | - |
| 146 | <unknown> | 1133 | sulphury, garlic | - | nd | 3 | 2 | nd | - |
| 147 | <unknown> | 1147 | petrol, aromatic | - | nd | nd | nd | 6 | - |
| 148 | <unknown> | 1153 | cucumber | - | nd | nd | nd | 6 | - |
| 149 | <unknown> | 1153 | spicy, cinnamon-like, nutty | - | nd | 6 | nd | nd | - |
| 150 | <unknown> | 1155 | fresh cucumber, rotten, vegetable | - | 2 | 5 | nd | nd | - |
| 16 | 1-isothiocyanato-4-methylpentane $ | 1162 | musty † | - | 2 | 4 * | 2 | nd | - |
| 106 | 2-sec-butyl-3-methoxypyrazine $ | 1173 | earthy, rotten potatoes, vegetable-like | musty, green, pea-like, bell pepper-like | 3 * | nd | 7 | nd | - |
| 59 | (Z)-3-hexenyl butanoate | 1181 | green, wine-like | green, fruity | 4 | nd | nd | nd | [61] |
| 18 | <unidentified ITC> | 1192 | radish, green | - | nd | 4 | nd | nd | - |
| 151 | <unknown> | 1198 | grassy, fruity, chemical, dried fruit | - | nd | nd | nd | 6 | - |
| 79 | 5-(methylsulfanyl)pentanenitrile $ | 1200 | radish | broccoli-like, cabbage-like | 2 | nd | nd | nd | [62] |
| 152 | <unknown> | 1200 | sweet, floral, violets, perfume | - | - | 3 | 4 | - | - |
| 60 | methyl salicylate | 1201 | medicinal, camphorous | wintergreen, mint | nd | nd | 4 * | nd | [40] |
| 153 | <unknown> | 1203 | peppery, green, earthy | - | nd | 5 | nd | 5 | - |
| 107 | phenethyl isocyanate | 1224 | ground pepper, pungent, horseradish † | - | nd | nd | 4 * | nd | - |
| 81 | benzenepropanenitrile $ | 1242 | herbal, green, floral † | - | nd | 3 | 3 | 4 | [21] |
| 154 | <unknown> | 1249 | liquorice, medicinal | - | nd | 4 | nd | nd | - |
| 155 | <unknown> | 1266 | cooked, cabbage, sulphur | - | nd | nd | 3 | nd | - |
| 156 | <unknown> | 1275 | green, radish, potato | - | nd | nd | nd | 4 | - |
| 157 | <unknown> | 1279 | soapy, pungent | - | nd | nd | nd | 5 | - |
| 158 | <unknown> | 1285 | soapy, grassy, floral | - | nd | 2 | nd | 6 | - |
| 19 | ibervirin $ | 1314 | horseradish, radish, vegetative | vegetative, horseradish-like, gooseberry-like | nd | 3 | 5 | nd | [63] |
| 159 | <unknown> | 1318 | medicinal, soapy | - | nd | nd | nd | 5 | - |
| 111 | 6-methylquinoline $ | 1334 | hydrogen sulphide, egg | tobacco, fecal | nd | nd | 3 * | nd | - |
| 20 | sativin $ | 1349 | burnt, rubbery, soily † | rocket-like | 3 | nd | nd | nd | [12] |
| 21 | octyl ITC $ | 1370 | green, vegetative † | - | nd | nd | nd | 5 * | [64] |
| 22 | benzyl ITC $ | 1378 | rotten grass, cooked | watercress-like | nd | 3 * | 4 | - | [48] |
| 61 | ethyl decanoate | 1391 | green, waxy | waxy, apple | nd | nd | 4 * | nd | [65] |
| 23 | erucin $ | 1441 | radishy | radish-like, cabbage-like | 2 | nd | nd | nd | [12] |
| 160 | <unknown> | 1478 | minty, cooling, fresh | - | nd | nd | 4 | nd | - |
| 24 | phenethyl ITC $ | 1480 | radish, gooseberry, sweet | horseradish-like, gooseberry-like | nd | nd | 6 | 7 | [48] |
| 98 | β-ionone | 1495 | soapy, fusty | floral, woody, fruity | 2 | nd | nd | 6 | [41] |
| 113 | benzyl tiglate $ | 1500 | musty | earthy, mushroom-like | nd | nd | 4 * | nd | - |
a Linear retention index on a HP-5MS column; for identification please check Table 2. b Odour description of compound present in the Good Scents online database: http://www.thegoodscentscompany.com/ (accessed on 1 April 2021) and literature sources; † tentative new odour description. c Average of intensities recorded by three assessors evaluating each sample in duplicate (scoring scale: weak = 3, medium = 5, strong = 7); nd, not detected; * newly reported for species; $ compound tentatively identified.
3.1.1. ‘Salad’ Rocket
A total of 57 volatile compounds were identified or tentatively identified in the headspace of E. sativa leaf samples. Nineteen compounds are newly reported for this species, some of which make up relatively large portions of the total volatile compounds’ bouquet (Table 2). Compounds with the greatest relative abundances were (Z)-3-hexen-1-ol (31, 40.9%), (E)-2-hexenal (44, 14.3%), hexanal (43, 8.6%), erucin (23, 7.2%), and 1-isothiocyanato-4-methylpentane (16, 5%; Figure 2). These observations are broadly in agreement with previous studies of ‘salad’ rocket [3,12].
Despite its high relative abundance, (Z)-3-hexen-1-ol produced only a weak, green, radishy aroma (Table 3) in rocket leaves. (E)-2-Hexenal by comparison was 2.9-fold less abundant in relative terms but produced a slightly stronger aroma, described by assessors as green, and apple-like. Hexanal (43) produced a pungent, green, grassy aroma of relatively high intensity, which has not been previously described in rocket to our knowledge.
2-Isopropyl-3-methoxypyrazine (102) by comparison was of low relative abundance in the rocket headspace (0.3%; Table 2) but was found to have one of the strongest aromas in rocket (rotten, potato-like, vegetative; Table 3). 1-Isothiocyanato-4-methylpentane had a weak aroma and was given a tentative new description of ‘musty’, as no previous studies have reported an odour for this compound.
The ITC erucin (23) is known for its anticarcinogenic properties, but its aroma was only recently described [12]. In agreement with a previous report, this compound produced a radishy aroma of weak intensity. Interestingly, the compound previously associated with characteristic “rocket-like” aroma (sativin, 20) [12] was relatively weak-smelling. In this study, the compound was found to have a burnt, rubbery, and soil-like aroma. This suggests that sativin is not the main driver of pungency or aroma in ‘salad’ rocket. Other compounds such as ethyl vinyl ketone (65; [41]), hexanal, 3-butenyl ITC (11) [48,54], and several unknown compounds (see final paragraph in this section) all had descriptions of pungency at higher intensities than sativin (Table 3). It may also be likely that no single compound is responsible for this attribute of rocket aroma but rather several.
Pentyl ITC (15, 0.6%) not previously identified in ‘salad’ rocket produced a strong odour, which was characterised as cabbage-like, green, and rotten (Table 3, Figure 2). As will be discussed in Section 3.2, we have tentatively identified pentyl GSL (Table 1) as a significant and previously unreported component of the GSL profile of ‘salad’ rocket, which gives rise to this ITC compound.
Other compounds not previously identified in ‘salad’ rocket included 2-phenylethanol, 4-heptenal and 2-sec-butyl-3-methoxypyrazine. 2-Phenylethanol (37, 0.2%) was noted to impart a floral aroma at a medium-weak intensity (Table 3). This compound is derived from phenylalanine and has been found to contribute to aroma and flavour in many foods, such as tomatoes [66]. 4-Heptenal (45) occurred in rocket leaves with a relative abundance of 0.1% (Table 2). Despite this low amount in terms of the overall volatile profile, the compound was perceived at a medium intensity by the assessors (Table 3) and described as grassy and green. This compound has been variously described as mushroom-like [50], fatty and fishy [67], and potatoey [68]. The variation in these descriptions may be associated with the isomerisation of the compound, which could not be resolved in this study. 2-sec-Butyl-3-methoxypyrazine (106, 0.2%) has been reported in several Brassicales species, such as white mustard, rapeseed [60], and horseradish [25], but not in E. sativa (Figure 2). It has been variously described as having a pea-like, musty, green, and bell pepper-like aroma. Assessors described the compound as earthy, similar to rotten potatoes, and vegetable-like. Despite its relatively low abundance, it was perceived as a medium-weak smelling compound in the sample headspace.
A total of 13 unidentified odorants were also detected in the headspace of rocket by GC-O (Table 3). These varied in intensity but all were distinguished and reported by assessors. Odour descriptions for these compounds were buttery (116), sulphury (117, 122, 124, 141, 145), horseradish-like, rancid (117), cabbage-like (122), rotten onion (124), mustard, pungent (128), oniony (128, 138), green, sour apples (131), soily, earthy (139), gas (141, 145), burnt (141), roasted (141, 142), smoky (142), spicy, chemical (144), fresh cucumber, rotten, and vegetable-like (150).
3.1.2. Wasabi
A total of 43 compounds were identified or tentatively identified in the headspace of wasabi roots (Table 2) with 30 compounds newly described for the species, making this a significant step forward in the understanding of aroma composition in wasabi roots. Two peaks of near-identical spectra were observed and identified as allyl ITC (5/6, 8.6%/52.1%) and were the most abundant compounds, which agrees with previous observations [7]. It is unknown why two distinct peaks were formed in this manner, and further investigation may be required to determine the isomeric differences responsible for the separation. 4-Pentenyl ITC (14, 12.4%) was also found to be high in terms of overall relative abundance.
Despite having near identical spectra, compounds 5 and 6 presented distinct differences in aroma and intensity. Peak 6 was characterised as being very pungent (Table 3) and having garlic, mustard, and horseradish-like qualities. Allyl ITC is one of the most well characterised ITCs and is well known for these properties [1,48,63] (Figure 2). Peak 5 by contrast had no discernible aroma in wasabi but was apparent in horseradish (see Section 3.1.3). 4-Pentenyl ITC likewise exhibited a pungent aroma and strong odour intensity but also had peppery, sulphurous, and musty notes. This compound is commonly reported in Brassicaceae crops [69]. 3-Butenyl ITC (11, 4.2%) was scored as a high odour intensity compound, despite its much lower relative abundance and was described as having a pungent, green, and aromatic odour.
Several other GHP odorants are newly reported in wasabi including cyclopropane ITC, isoamyl ITC, pentyl ITC, 1-isothiocyanato-4-methylpentane, and benzyl ITC. Cyclopropane ITC (7, 0.1%) is likely to be a cyclic reaction product of allyl ITC and has been previously reported in brown mustard [49] (Figure 2). To our knowledge, no odour description of this compound has been previously made, but assessors described it as sulphurous, horseradish, garlic, and onion-like (Table 3). The high odour intensity score indicates that it is a significant component of wasabi aroma. Isoamyl ITC (13, 0.4%) was described as having a pungent grassy aroma and being of high odour intensity. This compound is not commonly reported in Brassicales species, but it is used as a food additive [70]. Most ITC compounds are noted for their sulphurous and mustard-like potency in Brassicales; however, the contribution of grassy aroma ITCs to volatile compound bouquets has not been previously appreciated or fully understood. Pentyl ITC (15, 0.1%) and 1-isothiocyanato-4-methylpentane (16, <0.1%) were observed and shared the same odour characteristics as in ‘salad’ rocket (see Section 3.1.1.). Benzyl ITC (22, <0.1%) was reported to have a rotten grass and cooked aroma of medium-weak intensity. Similar to isoamyl ITC, this compound is not regularly reported as a constituent of Brassicales headspace, but these data indicate that even in very low relative abundance, it is odour active.
Another interesting compound was also found in wasabi headspace: cyclohexyl isocyanate (101, <0.1%; Figure 2). This has been previously reported in black mustard [53], though it is unclear if it is related to or derived from GHPs. Assessors perceived this odour having a medium-strong intensity and described it as peppery, cooked, and potato-like (Table 3). This is a tentative new odour description for this compound, and our data suggest it to be an important constituent of wasabi aroma.
Two additional compounds not previously identified in wasabi were 1-octen-3-ol and eucalyptol. 1-Octen-3-ol (34, <0.1%), despite its very low relative intensity in root tissue headspace (Table 2, Figure 2), exhibited a high odour intensity imparting a mushroom-like odour in agreement with previous descriptions [50]. Eucalyptol (97, <0.1%), previously observed in Brassicales crops [18] but not in wasabi (Figure 2), was found to have a medium-strong, characteristic eucalyptus, and mint aroma, and it is likely an important component of the overall volatile bouquet.
Similar to rocket, 17 unidentified odorants were detected in wasabi root samples (Table 3). Reported aromas were sulphury (114, 117, 123, 146), buttery (116), horseradish-like (117, 119), rancid (117), mustard (119, 128), onion (121, 128), garlic (123, 146), pungent (128), cooked, roasted chicken, chicken soup (129), potato (133), spicy, cinnamon-like, nutty (149), fresh cucumber, rotten, vegetable-like (150), radish (79), green (79, 153), sweet (152), floral (152, 158), violets, perfume (152), peppery, earthy (153), liquorice, medicinal (154), soapy, and grassy (158), confirming our statement that wasabi’s volatile profile is far from complete.
3.1.3. Horseradish
A total of 75 compounds were identified or tentatively identified in the headspace of horseradish roots, 38 of which are newly reported (Table 2). As with wasabi, the peaks with the highest relative abundances were dominated by GHPs. Compounds with the highest relative abundances were allyl ITC (5/6, 7.4%/39.3%), phenethyl ITC (24, 32.5%), thiiraneacetonitrile (77, 3.6%), 4-pentenyl ITC (14, 2.3%), (Z)-3-hexen-1-ol (31, 1.9%), and sec-butyl ITC (9, 1.8%; Figure 2).
As stated in Section 3.1.2, allyl ITC was identified as two distinct peaks (5 and 6). As in wasabi, 6 was of the greatest abundance and odour intensity, producing a very strong, pungent, garlic, mustard, and horseradish-like aroma (Table 3), whereas 5 produced a medium intensity, pungent horseradish smell. Thus far, the presence of two peaks has not been addressed or explained satisfactorily within the literature, with only one previous paper reporting the same phenomenon of separate and distinct allyl ITC peaks [71]. Sec-butyl ITC (9) produced a medium-weak intensity aroma that was vegetative and radish-like (Table 3). The compound was also present in wasabi at a medium intensity. The compound has been previously reported in horseradish as having green, chemical, and mustard like aromas [21], and it is known to activate the human Transient Receptor Potential Ankyrin 1 (TRPA1). This receptor is known to act in response to environmental irritants, and several ITCs identified in this study are known to activate it to varying degrees (isopropyl ITC, 3; isobutyl ITC, 10; allyl ITC, 5/6; 3-butenyl ITC, 11; 4-pentenyl ITC, 14; benzyl ITC, 22; phenylethyl ITC, 24; [72]). Phenylethyl ITC (24) is known to be a key constituent of horseradish aroma, and our data are in agreement with previous reports [73]. Assessors described the compound as radish and gooseberry-like, with a sweet note. It had a high odour intensity and contributed significantly to the odour profile of roots. Likewise, 4-pentenyl ITC (14) was observed to have the same odour attributes as previous reports [1] and those found for wasabi in this study, but at a lower intensity. By contrast, pentyl ITC (15) was present at much lower relative intensities to other GHPs (0.2%, Table 2) but produced a strong, green, rotten, and cabbage-like aroma.
Thiiraneacetonitrile (77) has been previously reported in horseradish [22] and is an epithionitrile hydrolysis product of sinigrin. To our knowledge, no previous studies have described the odour of this compound. We found it to have a sweaty, gas-like aroma of medium intensity (Table 3).
2-Isopropyl-3-methoxypyrazine (102, 0.1%) and 2-sec-butyl-3-methoxypyrazine (106, 0.4%) have been previously described and characterised in horseradish roots [25] as having green and pepper-like aromas. Our data agree with previous reports but found the compounds to be of very high aroma intensity, despite relatively low abundances within the headspace (Table 3). Assessors described the compounds as rotten, earthy, potato-like, and vegetative.
We report several compounds previously unidentified in horseradish including GHPs, isocyanates, alcohols, aldehydes, and a ketone and ester. As in wasabi root, cyclopropane ITC (7, 0.1%) produced an intense aroma containing horseradish, garlic, onion, and sulphur notes (Table 3). Therefore, it is likely to be a significant contributor to root odour and the volatile profile, despite its very low abundance, which may be a reason why it has not been previously detected and/or reported.
As discussed in Section 3.1.2, it is unknown if the presence of isocyanates is linked with GSLs and their hydrolysis products. Allyl isocyanate (99, <0.1%) and phenethyl isocyanate (107, 0.4%) were both observed for the first time in horseradish roots. Given that high abundances of allyl ITCs (5/6) and phenethyl ITC (24) were observed, it seems likely that isocyanates may be derived from them and/or directly from parent GSLs. Isocyanates are not commonly reported in the literature, and their formation may be because of as-yet-unstudied enzymatic or post-hydrolysis modification processes. Allyl isocyanate was described as having a weak musty and burnt plastic aroma; and phenethyl isocyanate was described as being pungent, with ground pepper and horseradish-like quality at a medium intensity. We are not aware of any previous odour descriptions for these compounds, so these are tentative new characterisations.
1-Octen-3-ol (34, <0.1%) was identified, and as in wasabi root, it produced a mushroom-like aroma of medium-weak intensity (Table 3). Benzyl alcohol (36, 0.1%) has been previously reported in Brassica oleracea [74] and rapeseed [58], but not in horseradish. It was characterised as having a medium intensity aroma, described as fruity, medicinal, and wine-like. Its low abundance but relatively high odour intensity may make it a subtle but key constituent of the root aroma profile. Two aldehyde compounds were also found to contribute to odour within the headspace. 2-Pentenal (41, <0.1%) produced a green, apple-like aroma [43], and 2,4-hexadienal (47, <0.1%) produced a green, rotten smell, both of medium-weak intensity (Table 3; [51]). A ketone, 3,5-octadien-2-one (71, 0.1%) was also tentatively identified (Table 2) and described as having a pungent green aroma of medium intensity (Table 3).
In the esters group, methyl salicylate (60, <0.1%), a common compound throughout the plant kingdom, has previously been identified in Brassicales as part of systemic acquired resistance response to herbivory [75], and it was identified for the first time as a volatile constituent of horseradish headspace (Table 2). Its aroma is characteristic of, and present in, plants such as wintergreen. Assessors described its odour as medicinal and camphorous at a medium intensity (Table 3). Ethyl decanoate (61, <0.1%), known to be present in B. oleracea [59], was described by the assessors as green and waxy (Table 3; [65]). Benzyl tiglate (113, <0.1%) was reported at low relative abundance, but a perceptible musty aroma was apparent for this compound.
The presence of D-limonene (96, 0.1%) is reported for the first time in horseradish root. It is known to be a constituent of B. oleracea headspace [76], but its sensory contribution to Brassicales is not well defined. Assessors found this compound to have a medium-weak intensity aroma of lemon and being vegetable-like (Table 3 [57]). This agrees with previous descriptions of the odour properties of the compound.
Finally, 6-methylquinoline (111, <0.1%), an aromatic compound, produced an unpleasant hydrogen sulphide and egg-like aroma of medium-weak intensity, and it has not been previously reported.
Similar to the other species tested, 15 unidentified odorants were detected in horseradish root samples (Table 3) and were of weak to medium intensity. Reported aromas were sulphury (114, 117, 123, 141, 145, 146, 155), buttery (116), horseradish-like (117, 118), rancid (117), rotten (120), cabbage-like (120, 155), garlic, (123, 146), mustard, pungent, oniony (128), cooked (129, 155), roasted chicken, chicken soup (129), nutty, spicy (130), gas (141, 145), burnt, roasted (141), sweet, floral, violets, perfume (152), minty, cooling, and fresh (160).
3.1.4. Watercress
A total of 42 compounds were identified or tentatively identified in the headspace of watercress, 26 of which are newly reported (Table 2). The headspace profile was dominated by alcohol and ITC compounds: (Z)-3-hexen-1-ol (31, 36%), phenethyl ITC (24, 30.7%), 1-penten-3-ol (25, 8.1%), and (Z)-2-penten-1-ol (28, 4.8%; Figure 2).
1-Penten-3-ol is a compound present widely in Brassicales species [77]. It exhibited a high intensity in watercress leaves, producing a sulphurous and cabbage-like aroma. These attributes are often attributed to ITCs and other sulphur-containing compounds; however, our data suggest that some of these characteristics in watercress could be attributed to this alcohol. (Z)-3-Hexen-1-ol by comparison was much higher in relative abundance but produced a medium intensity aroma that was green and radishy (Table 3).
Phenethyl ITC (aroma attributes described in Section 3.1.2.) had one of the highest intensity aromas in watercress, along with phenylacetaldehyde (52, 0.2%). The latter, similar to phenethyl ITC, is derived from phenylalanine, but occurs in much lower abundance (Table 2, Figure 2). Its aroma was described as honey-sweet (Table 3) and is likely a significant contributor to watercress odour that has previously gone unrecognised.
Other compounds contributing high odour intensities despite low relative abundances were 3-pentanone (66, 1.9%) and β-ionone (98, 0.4%; Figure 2). 3-Pentanone is regularly reported in Brassicales [3] and was described as high intensity, green, grassy, and floral smelling (Table 3, Figure 2). β-Ionone is common to many plant species as a degradation product of carotenoids [78], and it was described as soapy and fusty by assessors, with a high intensity.
Several sulphur-containing compounds, aldehydes, alcohols, ketones, not previously identified in watercress were identified. Methyl thiocyanate (2, 0.5%) imparted a sulphury and oniony note, with a medium intensity. This compound is known to be a GSL hydrolysis product of GCP (methyl GSL), but as will be discussed in Section 3.2, this compound was not detected in the UPLC-MS/MS analysis. Therefore, we suggest that it is not directly derived from this GSL and may be a degradation product of other GSL hydrolysis products within the tissues and headspace of the tested Brassicaceae.
Cyclopentyl-1-thiaethane (8, 0.5%) was detected, and uniquely present in watercress compared with the other three species tested (Table 2). Little is known about this compound in a biological context. It produced a sweaty, sulphury, medium-weak intensity aroma that is tentatively described in this species for the first time (Table 3).
Five aldehydes, two alcohol and two ketones, are newly reported for watercress, which produced perceptible odours within the headspace bouquet: 2-pentenal (41, 0.5%), hexanal (43, 1.2%), 4-heptenal (45, 0.5%), heptenal (46, 0.1%), and nonanal (54, 0.3%). Both heptenal and nonanal exhibited fatty, green aromas of medium intensity (Table 3) and are common to other Brassicaceae species [77]. 1-Octen-3-ol (34, 0.1%) produced a medium strength mushroom-like aroma (as described in Section 3.1.3.), and 2-phenylethanol (37, 2.6%) produced a floral scent. 6-Methyl-5-hepten-2-one (68, 0.2%) has been previously observed in ‘wild’ rocket and described as having a citrus aroma [55,79]. In this study, it was also identified with this characteristic, but also as floral and perfume-like, in both watercress and ‘salad’ rocket. It produced a medium-strong aroma in watercress. 2,2,6-Trimethylcyclohexanone (69, 0.1%) has been variously described as thujonic, menthol-like, and camphorous [80]. Here, it was described by assessors as imparting floral and green odours, with citrus notes.
One ester, (Z)-pent-2-en-1-yl acetate (57, 0.1%), was only detected in watercress, and it produced a medium-weak aroma. It was described as sulphury and rotten, and we are not aware of any previous odour attributes associated with this compound. As such, this is a tentative first description.
Octyl ITC (21, 0.2%) has been previously identified in horseradish [64] but not watercress to our knowledge. Its exact derivation and parent GSL are unclear in the literature, though watercress has been reported to contain glucohirsutin (GHS, (RS)-8methylsulfinyl)octyl GSL; [38]). This will be discussed further in Section 3.2 and Section 3.3. Assessors found the compound to be of medium aroma strength having a green and vegetative character. Again, we are unaware of previous odour descriptions for this compound.
Finally, there were 29 unidentified odorants detected by GC-O, making this the highest number of the four species analysed (Table 3). Aromas described by assessors were sulphury (114, 124, 125, 126, 141), cooked onions (115, 125), buttery (116), rotten, cabbage-like (120), rotten onion (124), pungent (125, 128, 157), oniony (126, 128), green (127, 131, 137, 153, 156), parsley (127), mustard (128), cooked, roasted chicken, chicken soup (129), sour apples (131), apples (132), grass (132, 134, 151, 158), potato (133, 156), earthy (135, 153), musty (135), petrol, aromatic (135, 147), bread-like (136), medicinal (140, 159), floral (140, 143, 158), gas, burnt, roasted (141, 142), smoky (142), cucumber (143, 148), flowers (143), fruity, chemical, dried fruit (151), peppery (153), radish (156), and soapy (157, 158, 159). This indicates that the volatile profile of watercress is far from complete, and further research is required to elucidate these compounds.
3.2. Non-Volatile Compounds (Glucosinolates)
GSL composition and concentrations for ‘salad’ rocket, wasabi, and watercress are presented in Table 4. Due to an unforeseen termination of supply, it was not possible to include horseradish roots in this analysis.
Table 4.
Glucosinolate concentrations of ‘salad’ rocket, wasabi, and watercress determined by UPLC-MS/MS.
| Glucosinolate a | Abbreviation | Concentration b | ||
|---|---|---|---|---|
| Salad Rocket | Wasabi | Watercress | ||
| glucoiberin | GIB | 0.007 ± 0.001 | 0.003 ± <0.001 | nd |
| pentyl GSL $ | PEN | 4.915 ± 1.633 | nd | nd |
| progoitrin | PRO | 0.074 ± 0.067 | 0.001 ± <0.001 | nd |
| sinigrin | SIN | 0.002 ± 0.001 | 11.121 ± 0.247 | 0.001 ± <0.001 |
| isobutyl GSL $ | ISO | nd | 3.382 ± 0.762 | nd |
| glucoraphanin | GRA | 1.761 ± 0.508 | 0.001 ± <0.001 | 0.006 ± <0.001 |
| glucorucolamine | GRM | 7.571 ± 1.208 | nd | nd |
| glucoalyssin | GAL | 0.215 ± 0.044 | 2.123 ± 0.477 | 0.470 ± 0.026 |
| glucoputranjivin $ | GPJ | 0.058 ± 0.024 | 2.289 ± 0.515 | nd |
| gluconapin | GNP | 0.001 ± 0.001 | 0.010 ± 0.001 | nd |
| diglucothiobeinin | DGTB | 4.622 ± 1.144 | nd | nd |
| glucoberteroin | GBT | 0.412 ± 0.086 | nd | nd |
| 4-hydroxyglucobrassicin | 4HGB | 0.012 ± 0.002 | 0.062 ± 0.002 | 0.006 ± <0.001 |
| glucocochlearin | GCL | nd | 2.184 ± 0.166 | nd |
| glucosativin | GSV | 6.639 ± 2.402 | nd | nd |
| 7-(methylsulfinyl)heptyl GSL | 7MSH | nd | 4.55 ± 0.393 | 21.472 ± 1.219 |
| glucobrassicanapin | GBN | nd | 0.146 ± 0.005 | nd |
| dimeric 4-mercaptobutyl GSL | DMB | 78.861 ± 8.384 | nd | nd |
| glucobarbarin | GBB | nd | nd | 0.730 ± 0.037 |
| glucotropaeolin | GTP | 0.027 ± 0.002 | 0.001 ± <0.001 | 0.001 ± <0.001 |
| glucoerucin | GER | 0.733 ± 0.088 | nd | nd |
| glucobrassicin | GBC | 0.027 ± 0.008 | 0.001 ± <0.001 | 0.118 ± 0.006 |
| 4-methoxyglucobrassicin | 4MGB | 0.366 ± 0.113 | nd | 2.001 ± 0.104 |
| gluconasturtiin | GNT | 0.001 ± <0.001 | nd | 1.514 ± 0.040 |
| neoglucobrassicin | NGB | 2.682 ± 0.433 | 1.525 ± 0.229 | 1.596 ± 0.037 |
| 4-methylpentyl GSL | 4MP | 1.293 ± 0.836 | nd | nd |
| hexy GSL | HEX | 0.225 ± 0.071 | nd | 0.434 ± 0.031 |
| 7-(methylthio)heptyl GSL | 7MTH | nd | 0.132 ± 0.035 | 2.663 ± 0.104 |
| butyl GSL $ | BUT | 0.301 ± 0.081 | nd | 0.606 ± 0.359 |
| Total | 111.098 ± 14.633 | 27.532 ± 2.831 | 31.645 ± 1.353 | |
a GSL: glucosinolate; $ = tentative identification. b Concentration in μmol g−1 dry weight; means are from six replicates for salad rocket, five replicates for wasabi and eight replicates for watercress; nd, not detected.
3.2.1. ‘Salad’ Rocket
‘Salad’ rocket contained the highest dry weight concentrations of GSLs (111.1 ± 14.6 µmol g−1 dw). This was predominantly due to high amounts of DMB (78.9 ± 8.4 µmol g−1 dw). Other GSLs of note included GSV (6.6 ± 2.4 µmol g−1 dw), GRM (7.6 ± 1.2 µmol g−1 dw), and DGTB (4.6 ± 1.1 µmol g−1 dw), which are unique to the genera Eruca and Diplotaxis. Other routinely reported GSLs for this species were GRA (1.8 ± 0.5 µmol g−1 dw) and NGB (2.7 ± 0.4 µmol g−1 dw).
Interestingly, several other GSLs that have not been, or are rarely reported for the species, were also detected; some in relatively high concentrations: GIB (<0.1 ± <0.1 µmol g−1 dw), PEN (4.9 ± 1.6 µmol g−1 dw), GPJ (<0.1 ± <0.1 µmol g−1 dw), GBT (0.4 ± <0.1 µmol g−1 dw), GTP (<0.1 ± <0.1 µmol g−1 dw), 4MP (1.3 ± 0.8 µmol g−1 dw), HEX (0.2 ± <0.1 µmol g−1 dw), and BUT (0.3 ± <0.1 µmol g−1 dw).
Of note is the high abundance of PEN (m/z 388). It seems unlikely that a GSL of such relatively high concentration has gone undetected in previous analyses. Therefore, we postulate that previous studies may have attributed the negative ion mass incorrectly to that of PRO, which is also m/z 388 (Table 1). The authentic standard of PRO did not match the retention time or MS/MS spectra of PEN, and it was found in only very low concentrations by comparison. While PEN is only a tentative identification (due to the possibility of other isomeric GSLs such as glucojiaputin and 3-methylbutyl GSL), the presence of pentyl ITC (17) within the headspace of rocket makes this the most likely identification. See Section 3.3 for further discussion.
3.2.2. Wasabi
Sixteen GSL compounds were identified in wasabi roots, totaling 27.5 ± 2.8 µmol g−1 dw (Table 4). The most abundant compound was SIN (11.1 ± 0.2 µmol g−1 dw), which agrees with previous studies [81]. Wasabi is known to have a diverse GSL profile, and we observed relatively high abundances for ISO (3.4 ± 0.8 µmol g−1 dw), GAL (2.1 ± 0.5 µmol g−1 dw), GPJ (2.3 ± 0.5 µmol g−1 dw), GCL (2.2 ± 0.2 µmol g−1 dw), 7MSH (4.6 ± 0.4 µmol g−1 dw), and NGB (1.5 ± 0.2 µmol g−1 dw). Other compounds occurring in low abundance that are not frequently reported were GIB and GRA.
3.2.3. Watercress
Fourteen GSLs were found in watercress leaves, amounting to 31.6 ± 1.4 µmol g−1 dw (Table 4). In most previous studies of this species, GNT (1.5 ± <0.1 µmol g−1 dw) has been found to have the greatest abundance [4]; however, our analysis revealed that 7MSH had the highest total concentration (21.5 ± 1.2 µmol g−1 dw), dominating the overall profile in these samples. There were also relatively high concentrations of 7MTH (2.7 ± 0.1 µmol g−1 dw), and the indolic GSLs 4MGB (2 ± 0.1 µmol g−1 dw), and NGB (1.6 ± <0.1 µmol g−1 dw). Minor amounts of SIN, GRA, and GTP were also observed, and they are not frequently reported in this species.
3.3. Discrepancies between Identified Glucosinolate Hydrolysis Products and Glucosinolate Profile Precursors
There is often an ‘elephant in the room’ regarding volatile GSL hydrolysis products and reported GSL profiles in Brassicales crops: there are often GSLs found with no corresponding hydrolysis products, or more troublingly, hydrolysis products observed but no GSL precursor. Table 5 presents a list of the GSL-derived compounds identified within the headspace of ‘salad’ rocket, wasabi, and watercress, alongside their expected GSL precursors. It is apparent from our data that the present study is no exception when it comes to discrepancies of this nature, and there is a need to find a robust solution to prevent the inaccurate reporting of both GSLs and their volatile hydrolysis products.
Table 5.
Identified volatile glucosinolate hydrolysis products in the headspace of ‘salad’ rocket, wasabi, and watercress, and the presence/absence of their respective glucosinolate precursor.
| Precursor Glucosinolate | Glucosinolate Hydrolysis Product (Compound No.) | Glucosinolate Observed? | Hydrolysis Product Observed? | ||||
|---|---|---|---|---|---|---|---|
| Salad Rocket | Wasabi | Watercress | Salad Rocket | Wasabi | Watercress | ||
| sinigrin | 3-butenenitrile (74) $ | √ | √ | √ | x | √ | x |
| allyl thiocyanate (4) $ | √ | √ | √ | x | √ | x | |
| allyl ITC (5/6) $ | √ | √ | √ | x | √ | x | |
| cyclopropane ITC (7) $ | √ | √ | √ | x | √ | x | |
| thiiraneacetonitrile (77) $ | √ | √ | √ | x | x | x | |
| glucocapparin | methyl thiocyanate (2) $ | x | x | x | √ * | x | √ * |
| glucoputranjivin $ | isopropyl ITC (3) $ | √ | √ | x | √ | x | x |
| glucocochlearin $ | sec-butyl ITC (9) $ | x | √ | x | √ * | √ | x |
| 4-methylpentyl GSL $ | 5-methylhexanenitrile (75) $ | √ | x | x | √ | x | x |
| 1-isothiocyanato-4-methylpentane (16) $ | √ | x | x | √ | √ * | √ * | |
| isobutyl GSL | isobutyl ITC (10) $ | x | √ | x | x | √ | x |
| <unknown> | 6-heptenenitrile (76) $ | - | - | - | x | √ * | x |
| gluconapin | 3-butenyl ITC (11) $ | √ | √ | x | √ | √ | x |
| butyl GSL | butyl ITC (12) $ | √ | x | √ | √ | √ * | x |
| 3-methylbutyl GSL | isoamyl ITC (13) $ | x | x | x | √ * | √ * | √ * |
| glucobrassicanapin | 4-pentenyl ITC (14) $ | x | √ | x | x | √ | x |
| pentyl GSL | pentyl ITC (15) $ | √ | x | x | √ | √ * | x |
| glucotropaeolin | phenylacetonitrile (78) $ | √ | √ | √ | x | x | x |
| benzyl ITC (22) $ | √ | √ | √ | x | √ | x | |
| hexyl GSL | cyclohexyl ITC (17) $ | √ | x | √ | x | √ * | x |
| glucoberteroin | 5-(methylsulfanyl)pentanenitrile (79) $ | √ | x | x | √ | x | x |
| glucoerucin | 4-(methylthio)butanenitrile (80) $ | √ | x | x | x | x | x |
| erucin (23) $ | √ | x | x | √ | √ * | x | |
| gluconasturtiin | benzenepropanenitrile (81) $ | √ | x | √ | x | x | √ |
| glucoiberverin | iberverin (19) $ | x | x | x | √ * | √ * | x |
| glucosativin | sativin (20) $ | √ | x | x | √ | x | x |
| <unknown> | octyl ITC (21) $ | x | x | x | x | x | √ * |
| gluconasturtiin | phenylethyl ITC (24) $ | √ | x | √ | x | √ * | √ |
* Hydrolysis product observed but not glucosinolate precursor; $ tentatively identified.
As discussed in previous sections, compounds such as methyl thiocyanate (2) may be produced from degradation of other hydrolysis products. Others such as the presence of sec-butyl ITC (9) in rocket, butyl ITC (12) in wasabi, and octyl ITC (21) in watercress cannot be so easily explained. There are several explanations with varying levels of likelihood: firstly, the most likely is that the ITCs and other GSL hydrolysis products have been identified incorrectly, and that they belong to other parent GSLs present within the analysed tissues. Despite researchers’ best efforts to obtain authentic standards to test and match MS profiles, this is not always possible or affordable, and so there is a heavy reliance upon reference libraries. These are often incomplete and not always accurate. It is also possible that the high temperatures utilised in GC-MS cause thermolytic reactions to occur in GHPs, thus yielding compounds not ‘naturally’ produced by tissues. The next possibility is that the GSLs responsible for producing hydrolysis products are below the LOD of the MS/MS method. In this case, this is unlikely, as the LOD for each standard GSL was low, and it is likely much more sensitive and accurate than volatile compound measurements by GC-MS. Thirdly and perhaps least likely is that there is some as-yet-unknown mechanism(s) by which GSL hydrolysis products are modified post-hydrolysis. This is speculation, but there have been recent reports of previously unknown tautomeric rearrangements [82] and enzymatic actions [28] upon hydrolysis products that do not preclude this as an impossibility. Indeed, the dynamics of reactions occurring within the headspace of Brassicales is virtually unstudied, and so some may be produced through the degradation or rearrangement of structurally similar compounds. This is an area that requires much more detailed scrutiny.
4. Conclusions
This study has highlighted numerous newly identified and tentatively identified volatile compounds present in the headspace of ‘salad’ rocket, wasabi, horseradish, and watercress. Many of these appear to contribute strongly to the aroma profiles of each respective crop. We have also found 46 aroma traits present in the headspace of the samples that have no association with the identified compounds. This suggests that there are many as-yet undiscovered odour-active compounds present within Brassicales headspace.
Our data also highlight the need for more detailed studies on the volatilome of Brassicales species, and that the sole focus should not be upon GSL hydrolysis products. While accounting for a large proportion of the respective volatile profiles and odour active components of species, we have identified numerous instances where non-GSL derived compounds have odour intensities greater than those that are GSL-derived, such as 1-penten-3-ol (25), phenylacetaldehyde (52), 2-sec-butyl-3-methoxypyrazine (106), and β-ionone (98). Several non-GSL derived compounds also share similar pungent odour characteristics with GHPs, indicating that the latter may not be the only source of these sensations in Brassicales crops. There is also a clear need for the improvement of mass spectral libraries and the availability of GSL and GHP standards in order to overcome discrepancies between GSL profiles and the reported volatiles derived therefrom.
Acknowledgments
The authors would like to thank Richard Tudor of Elsoms Seeds Ltd. (Spalding, UK) for providing Eruca sativa seeds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10051055/s1, Supplementary Data S1. Compound fragmentation spectra with corresponding library matches (where available) and GC-MS chromatograms.
Author Contributions
Conceptualization, L.B. and S.L.; methodology, L.B. and S.L.; software, L.B., E.K., O.O.O. and S.L.; validation, L.B., O.O.O. and S.L; formal analysis, L.B., E.K. and S.L.; investigation, L.B., E.K. and S.L.; resources, L.B., E.K., O.O.O. and S.L.; data curation, L.B., E.K., O.O.O. and S.L.; writing—original draft preparation, L.B.; writing—review and editing, L.B., E.K., O.O.O. and S.L.; visualization, L.B., O.O.O. and S.L.; supervision, L.B. and S.L.; project administration, L.B. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bell L., Oloyede O.O., Lignou S., Wagstaff C., Methven L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018;62:1700990. doi: 10.1002/mnfr.201700990. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran P.N., Pillai G.S., Divakaran M. Other herbs and spices: Mango ginger to wasabi. In: Peter K.V., editor. Handbook of Herbs and Spices. 2nd ed. Woodhead Publishing; Cambridge, UK: 2012. pp. 557–582. [DOI] [Google Scholar]
- 3.Bell L., Spadafora N.D., Müller C.T., Wagstaff C., Rogers H.J. Use of TD-GC–TOF-MS to assess volatile composition during post-harvest storage in seven accessions of rocket salad (Eruca sativa) Food Chem. 2016;194:626–636. doi: 10.1016/j.foodchem.2015.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giallourou N., Oruna-Concha M.J., Harbourne N. Effects of domestic processing methods on the phytochemical content of watercress (Nasturtium officinale) Food Chem. 2016;212:411–419. doi: 10.1016/j.foodchem.2016.05.190. [DOI] [PubMed] [Google Scholar]
- 5.Sultana T., Savage G.P., Mcneil D.L., Porter G.P., Clark B. Comparison of flavour compounds in wasabi and horseradish. J. Food Agric. Environ. 2003;1:117–121. [Google Scholar]
- 6.Kroener E.-M., Buettner A. Unravelling important odorants in horseradish (Armoracia rusticana) Food Chem. 2017;232:455–465. doi: 10.1016/j.foodchem.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Depree J.A., Howard T.M., Savage G.P. Flavour and pharmaceutical properties of the volatile sulphur compounds of Wasabi (Wasabia japonica) Food Res. Int. 1999;31:329–337. doi: 10.1016/S0963-9969(98)00105-7. [DOI] [Google Scholar]
- 8.Bell L., Wagstaff C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia) J. Agric. Food Chem. 2014;62:4481–4492. doi: 10.1021/jf501096x. [DOI] [PubMed] [Google Scholar]
- 9.Palaniswamy U.R. Watercress: A salad crop with chemopreventive potential. Horttechnology. 2001;11:622–626. doi: 10.21273/HORTTECH.11.4.622. [DOI] [Google Scholar]
- 10.Siegmund B. Biogenesis of aroma compounds: Flavour formation in fruits and vegetables. In: Parker J.K., Elmore J.S., Methven L., editors. Flavour Development, Analysis and Perception in Food and Beverages. Woodhead Publishing; Cambridge, UK: 2015. pp. 127–149. [DOI] [Google Scholar]
- 11.Kim J.I., Dolan W.L., Anderson N.A., Chapple C. Indole glucosinolate biosynthesis limits phenylpropanoid accumulation in Arabidopsis thaliana. Plant Cell. 2015;27:1529–1546. doi: 10.1105/tpc.15.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffo A., Masci M., Moneta E., Nicoli S., Sánchez del Pulgar J., Paoletti F., Pulgar D. Characterization of volatiles and identification of odor-active compounds of rocket leaves. Food Chem. 2018;240:1161–1170. doi: 10.1016/j.foodchem.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Blazevic I., Mastelic J. Free and bound volatiles of rocket (Eruca sativa Mill.) Flavour Fragrance J. 2008;23:278–285. doi: 10.1002/ffj.1883. [DOI] [Google Scholar]
- 14.Jirovetz L., Smith D., Buchbauer G. Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC−MS, and olfactometry. J. Agric. Food Chem. 2002;50:4643–4646. doi: 10.1021/jf020129n. [DOI] [PubMed] [Google Scholar]
- 15.Miyazawa M., Maehara T., Kurose K. Composition of the essential oil from the leaves of Eruca sativa. Flavour Fragrance J. 2002;17:187–190. doi: 10.1002/ffj.1079. [DOI] [Google Scholar]
- 16.Nakanishi A., Miyazawa N., Haraguchi K., Watanabe H., Kurobayashi Y., Nammoku T. Determination of the absolute configuration of a novel odour-active lactone, cis-3-methyl-4-decanolide, in wasabi (Wasabia japonica Matsum.) Flavour Fragrance J. 2014;29:220–227. doi: 10.1002/ffj.3196. [DOI] [Google Scholar]
- 17.Kameoka H., Hashimoto S. Volatile flavor components from wild Wasabia japonica Matsum. (Wasabi) and Nasturtium officinale R. Br. (Orandagarashi) J. Agric. Chem. Soc. Japan. 1982;56:441–443. [Google Scholar]
- 18.Liu Y., Zhang H., Umashankar S., Liang X., Lee H., Swarup S., Ong C. Characterization of plant volatiles reveals distinct metabolic profiles and pathways among 12 Brassicaceae vegetables. Metabolites. 2018;8:94. doi: 10.3390/metabo8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva M.F., Campos V.P., Barros A.F., Terra W.C., Pedroso M.P., Gomes V.A., Ribeiro C.R., Silva F.J. Volatile emissions of watercress (Nasturtium officinale) leaves and passion fruit (Passiflora edulis) seeds against Meloidogyne incognita. Pest Manag. Sci. 2020;76:1413–1421. doi: 10.1002/ps.5654. [DOI] [PubMed] [Google Scholar]
- 20.Spence R.-M.M., Tucknott O.G. An apparatus for the comparative collection of headspace volatiles of watercress. J. Sci. Food Agric. 1983;34:412–416. doi: 10.1002/jsfa.2740340415. [DOI] [Google Scholar]
- 21.Agneta R., Möllers C., Rivelli A.R. Horseradish (Armoracia rusticana), a neglected medical and condiment species with a relevant glucosinolate profile: A review. Genet. Resour. Crop Evol. 2013;60:1923–1943. doi: 10.1007/s10722-013-0010-4. [DOI] [Google Scholar]
- 22.Dekić M.S., Radulović N.S., Stojanović N.M., Randjelović P.J., Stojanović-Radić Z.Z., Najman S., Stojanović S. Spasmolytic, antimicrobial and cytotoxic activities of 5-phenylpentyl isothiocyanate, a new glucosinolate autolysis product from horseradish (Armoracia rusticana P. Gaertn., B. Mey. & Scherb., Brassicaceae) Food Chem. 2017;232:329–339. doi: 10.1016/j.foodchem.2017.03.150. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert J., Nursten H.E. Volatile constituents of horseradish roots. J. Sci. Food Agric. 1972;23:527–539. doi: 10.1002/jsfa.2740230414. [DOI] [Google Scholar]
- 24.Pilipczuk T., Kusznierewicz B., Chmiel T., Przychodzeń W., Bartoszek A. Simultaneous determination of individual isothiocyanates in plant samples by HPLC-DAD-MS following SPE and derivatization with N -acetyl- l -cysteine. Food Chem. 2017;214:587–596. doi: 10.1016/j.foodchem.2016.07.125. [DOI] [PubMed] [Google Scholar]
- 25.Kroener E.-M., Buettner A. Sensory-analytical comparison of the aroma of different horseradish varieties (Armoracia rusticana) Front. Chem. 2018;6 doi: 10.3389/fchem.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasini F., Verardo V., Caboni M.F., D’Antuono L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD–MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012;133:1025–1033. doi: 10.1016/j.foodchem.2012.01.021. [DOI] [Google Scholar]
- 27.Bell L., Wagstaff C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. X. 2019;1:100002. doi: 10.1016/j.fochx.2018.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agerbirk N., Matthes A., Erthmann P.Ø., Ugolini L., Cinti S., Lazaridi E., Nuzillard J.-M., Müller C., Bak S., Rollin P., et al. Glucosinolate turnover in Brassicales species to an oxazolidin-2-one, formed via the 2-thione and without formation of thioamide. Phytochemistry. 2018;153:79–93. doi: 10.1016/j.phytochem.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Bell L., Oruna-Concha M.J., Wagstaff C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC–MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015;172:852–861. doi: 10.1016/j.foodchem.2014.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochfort S.J., Trenerry V.C., Imsic M., Panozzo J., Jones R. Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry. 2008;69:1671–1679. doi: 10.1016/j.phytochem.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Andini S., Dekker P., Gruppen H., Araya-Cloutier C., Vincken J.-P. Modulation of glucosinolate composition in Brassicaceae seeds by germination and fungal elicitation. J. Agric. Food Chem. 2019;67:12770–12779. doi: 10.1021/acs.jafc.9b05771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cataldi T.R.I., Rubino A., Lelario F., Bufo S.A. Naturally occurring glucosinolates in plant extracts of rocket salad (Eruca sativa L.) identified by liquid chromatography coupled with negative ion electrospray ionization and quadrupole ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:2374–2388. doi: 10.1002/rcm.3101. [DOI] [PubMed] [Google Scholar]
- 33.Agneta R., Rivelli A.R., Ventrella E., Lelario F., Sarli G., Bufo S.A. Investigation of glucosinolate profile and qualitative aspects in sprouts and roots of horseradish (Armoracia rusticana) using LC-ESI–hybrid linear ion trap with fourier transform ion cyclotron resonance mass spectrometry and infrared multiphoton dissociation. J. Agric. Food Chem. 2012;60:7474–7482. doi: 10.1021/jf301294h. [DOI] [PubMed] [Google Scholar]
- 34.Maldini M., Foddai M., Natella F., Petretto G.L., Rourke J.P., Chessa M., Pintore G. Identification and quantification of glucosinolates in different tissues of Raphanus raphanistrum by liquid chromatography tandem-mass spectrometry. J. Food Compos. Anal. 2017;61:20–27. doi: 10.1016/j.jfca.2016.06.002. [DOI] [Google Scholar]
- 35.Shi H., Zhao Y., Sun J., Yu L., Chen P. Chemical profiling of glucosinolates in cruciferous vegetables-based dietary supplements using ultra-high performance liquid chromatography coupled to tandem high resolution mass spectrometry. J. Food Compos. Anal. 2017;61:67–72. doi: 10.1016/j.jfca.2017.01.018. [DOI] [Google Scholar]
- 36.Mellon F.A., Bennett R.N., Holst B., Williamson G. Intact glucosinolate analysis in plant extracts by programmed cone voltage electrospray LC/MS: Performance and comparison with LC/MS/MS methods. Anal. Biochem. 2002;306:83–91. doi: 10.1006/abio.2002.5677. [DOI] [PubMed] [Google Scholar]
- 37.Bianco G., Agerbirk N., Losito I., Cataldi T.R.I. Acylated glucosinolates with diverse acyl groups investigated by high resolution mass spectrometry and infrared multiphoton dissociation. Phytochemistry. 2014;100:92–102. doi: 10.1016/j.phytochem.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Blažević I., Montaut S., Burčul F., Olsen C., Burow M., Rollin P., Agerbirk N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry. 2019;169:112100. doi: 10.1016/j.phytochem.2019.112100. [DOI] [PubMed] [Google Scholar]
- 39.Al-Gendy A.A., Lockwood G.B. GC-MS analysis of volatile hydrolysis products from glucosinolates in Farsetia aegyptia var. ovalis. Flavour Fragrance J. 2003;18:148–152. doi: 10.1002/ffj.1163. [DOI] [Google Scholar]
- 40.Wang C., Xing J., Chin C.-K., Ho C.-T., Martin C.E. Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry. 2001;58:227–232. doi: 10.1016/S0031-9422(01)00233-3. [DOI] [PubMed] [Google Scholar]
- 41.Buttery R.G., Teranishi R., Ling L.C., Turnbaugh J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agric. Food Chem. 1990;38:336–340. doi: 10.1021/jf00091a074. [DOI] [Google Scholar]
- 42.Deasy W., Shepherd T., Alexander C.J., Birch A.N.E., Evans K.A. Development and validation of a SPME-GC-MS method for in situ passive sampling of root volatiles from glasshouse-grown broccoli plants undergoing blow-ground herbivory by larvae of cabbage root fly, Delia radicum L. Phytochem. Anal. 2016;27:375–393. doi: 10.1002/pca.2637. [DOI] [PubMed] [Google Scholar]
- 43.Moshonas M.G., Shaw P.E. Some newly found orange essence components including trans-2-pentenal. J. Food Sci. 1973;38:360–361. doi: 10.1111/j.1365-2621.1973.tb01429.x. [DOI] [Google Scholar]
- 44.Berger R.G., Drawert F., Kollmannsberger H. The flavour of cape gooseberry (Physalis peruviana L.) Z. Für Lebensm. -Unters. und Forsch. 1989;188:122–126. doi: 10.1007/BF01042735. [DOI] [Google Scholar]
- 45.Hatanaka A., Harada T. Formation of cis-3-hexenal, trans-2-hexenal and cis-3-hexenol in macerated Thea sinensis leaves. Phytochemistry. 1973;12:2341–2346. doi: 10.1016/0031-9422(73)80435-2. [DOI] [Google Scholar]
- 46.D’Auria J.C., Pichersky E., Schaub A., Hansel A., Gershenzon J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007;49:194–207. doi: 10.1111/j.1365-313X.2006.02946.x. [DOI] [PubMed] [Google Scholar]
- 47.Angerosa F. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000;68:283–287. doi: 10.1016/S0308-8146(99)00189-2. [DOI] [Google Scholar]
- 48.Kato M., Imayoshi Y., Iwabuchi H., Shimomura K. Kinetic changes in glucosinolate-derived volatiles by heat-treatment and myrosinase activity in nakajimana (Brassica rapa L. cv. nakajimana) J. Agric. Food Chem. 2011;59:11034–11039. doi: 10.1021/jf201626z. [DOI] [PubMed] [Google Scholar]
- 49.Sharma A., Rai P.K., Prasad S. GC–MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchem. J. 2018;138:488–493. doi: 10.1016/j.microc.2018.01.015. [DOI] [Google Scholar]
- 50.Song H., Liu J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018;114:187–198. doi: 10.1016/j.foodres.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 51.Aparicio R., Morales M.T., Alonso V. Authentication of European virgin olive oils by their chemical compounds, sensory attributes, and consumers’ attitudes. J. Agric. Food Chem. 1997;45:1076–1083. doi: 10.1021/jf960659h. [DOI] [Google Scholar]
- 52.Hossain S., Bergkvist G., Berglund K., Glinwood R., Kabouw P., Mårtensson A., Persson P. Concentration- and time-dependent effects of isothiocyanates produced from Brassicaceae shoot tissues on the pea root rot pathogen Aphanomyces euteiches. J. Agric. Food Chem. 2014;62:4584–4591. doi: 10.1021/jf501776c. [DOI] [PubMed] [Google Scholar]
- 53.Kask K., Kännaste A., Talts E., Copolovici L., Niinemets Ü. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant Cell Environ. 2016;39:2027–2042. doi: 10.1111/pce.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruce T.J.A. Glucosinolates in oilseed rape: Secondary metabolites that influence interactions with herbivores and their natural enemies. Ann. Appl. Biol. 2014;164:348–353. doi: 10.1111/aab.12128. [DOI] [Google Scholar]
- 55.Hansen M., Laustsen A.M., Olsen C.E., Poll L., SØRensen H. Chemical and sensory quality of Broccoli (Brassica oleracea L. Var italica) J. Food Qual. 1997;20:441–459. doi: 10.1111/j.1745-4557.1997.tb00486.x. [DOI] [Google Scholar]
- 56.Hanschen F.S., Kaufmann M., Kupke F., Hackl T., Kroh L.W., Rohn S., Schreiner M. Brassica vegetables as sources of epithionitriles: Novel secondary products formed during cooking. Food Chem. 2018;245:564–569. doi: 10.1016/j.foodchem.2017.10.124. [DOI] [PubMed] [Google Scholar]
- 57.Fernandes F., Pereira D.M., Guedes de Pinho P., Valentão P., Pereira J.A., Bento A., Andrade P.B. Headspace solid-phase microextraction and gas chromatography/ion trap-mass spectrometry applied to a living system: Pieris brassicae fed with kale. Food Chem. 2010;119:1681–1693. doi: 10.1016/j.foodchem.2009.09.046. [DOI] [Google Scholar]
- 58.Bartlet E., Blight M.M., Lane P., Williams I.H. The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol. Exp. Appl. 1997;85:257–262. doi: 10.1046/j.1570-7458.1997.00256.x. [DOI] [Google Scholar]
- 59.Fernandes F., Guedes de Pinho P., Valentão P., Pereira J.A., Andrade P.B. Volatile constituents throughout Brassica oleracea L. Var. acephala germination. J. Agric. Food Chem. 2009;57:6795–6802. doi: 10.1021/jf901532m. [DOI] [PubMed] [Google Scholar]
- 60.Ortner E., Granvogl M., Schieberle P. Elucidation of thermally induced changes in key odorants of white mustard seeds (Sinapis alba L.) and rapeseeds (Brassica napus L.) using molecular sensory science. J. Agric. Food Chem. 2016;64:8179–8190. doi: 10.1021/acs.jafc.6b03625. [DOI] [PubMed] [Google Scholar]
- 61.Smid H.M., Van Loon J.J.A., Posthumus M.A., Vet L.E.M. GC-EAG-analysis of volatiles from brussels sprouts plants damaged by two species of Pieris caterpillars: Olfactory receptive range of a specialist and a generalist parasitoid wasp species. Chemoecology. 2002;12:169–176. doi: 10.1007/PL00012665. [DOI] [Google Scholar]
- 62.Klopsch R., Witzel K., Börner A., Schreiner M., Hanschen F.S. Metabolic profiling of glucosinolates and their hydrolysis products in a germplasm collection of Brassica rapa turnips. Food Res. Int. 2017;100:392–403. doi: 10.1016/j.foodres.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Angelino D., Jeffery E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods. 2014;7:67–76. doi: 10.1016/j.jff.2013.09.029. [DOI] [Google Scholar]
- 64.Petrović S., Drobac M., Ušjak L., Filipović V., Milenković M., Niketić M. Volatiles of roots of wild-growing and cultivated Armoracia macrocarpa and their antimicrobial activity, in comparison to horseradish, A. rusticana. Ind. Crops Prod. 2017;109:398–403. doi: 10.1016/j.indcrop.2017.08.056. [DOI] [Google Scholar]
- 65.Tohar N., Mohd M.A., Jantan I., Awang K. A comparative study of the essential oils of the genus Plumeria Linn. from Malaysia. Flavour Fragr. J. 2006;21:859–863. doi: 10.1002/ffj.1617. [DOI] [Google Scholar]
- 66.Tieman D., Taylor M., Schauer N., Fernie A.R., Hanson A.D., Klee H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA. 2006;103:8287–8292. doi: 10.1073/pnas.0602469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Triqui R., Reineccius G. Changes in flavor profiles with ripening of anchovy (Engraulis encrasicholus) J. Agric. Food Chem. 1995;43:1883–1889. doi: 10.1021/jf00055a024. [DOI] [Google Scholar]
- 68.Parker J.K. Introduction to aroma compounds in foods. In: Parker J.K., Elmore J.S., Methven L., editors. Flavour Development, Analysis and Perception in Food and Beverages. Woodhead Publishing; Cambridge, UK: 2015. pp. 3–30. [DOI] [Google Scholar]
- 69.Schreiner M., Krumbein A., Ruppel S. Interaction between plants and bacteria: Glucosinolates and phyllospheric colonization of cruciferous vegetables by Enterobacter radicincitans DSM 16656. J. Mol. Microbiol. Biotechnol. 2009;17:124–135. doi: 10.1159/000226589. [DOI] [PubMed] [Google Scholar]
- 70.Deng Q., Zinoviadou K.G., Galanakis C.M., Orlien V., Grimi N., Vorobiev E., Lebovka N., Barba F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Eng. Rev. 2015;7:357–381. doi: 10.1007/s12393-014-9104-9. [DOI] [Google Scholar]
- 71.Park H.-W., Choi K.-D., Shin I.-S. Antimicrobial activity of isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root against oral microorganisms. Biocontrol Sci. 2013;18:163–168. doi: 10.4265/bio.18.163. [DOI] [PubMed] [Google Scholar]
- 72.Terada Y., Masuda H., Watanabe T. Structure–activity relationship study on isothiocyanates: Comparison of TRPA1-activating ability between allyl isothiocyanate and specific flavor components of wasabi, horseradish, and white mustard. J. Nat. Prod. 2015;78:1937–1941. doi: 10.1021/acs.jnatprod.5b00272. [DOI] [PubMed] [Google Scholar]
- 73.Masuda H., Harada Y., Tanaka K., Nakajima M., Tabeta H. Biotechnology for Improved Foods and Flavors. Volume 637. American Chemical Society; Washington, DC, USA: 1996. Characteristic odorants of wasabi (Wasabia japonica matum), Japanese horseradish, in comparison with those of horseradish (Armoracia rusticana) pp. 67–78. [Google Scholar]
- 74.Lv J., Wu J., Zuo J., Fan L., Shi J., Gao L., Li M., Wang Q. Effect of Se treatment on the volatile compounds in broccoli. Food Chem. 2017;216:225–233. doi: 10.1016/j.foodchem.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Blight M.M., Pickett J.A., Wadhams L.J., Woodcock C.M. Antennal perception of oilseed rape, Brassica napus (Brassicaceae), volatiles by the cabbage seed weevil Ceutorhynchus assimilis (Coleoptera, Curculionidae) J. Chem. Ecol. 1995;21:1649–1664. doi: 10.1007/BF02033667. [DOI] [PubMed] [Google Scholar]
- 76.Chen H.-Z., Zhang M., Guo Z. Discrimination of fresh-cut broccoli freshness by volatiles using electronic nose and gas chromatography-mass spectrometry. Postharvest Biol. Technol. 2019;148:168–175. doi: 10.1016/j.postharvbio.2018.10.019. [DOI] [Google Scholar]
- 77.Müller-Maatsch J., Gurtner K., Carle R., Björn Steingass C. Investigation into the removal of glucosinolates and volatiles from anthocyanin-rich extracts of red cabbage. Food Chem. 2019;278:406–414. doi: 10.1016/j.foodchem.2018.10.126. [DOI] [PubMed] [Google Scholar]
- 78.Davies K. Annual Plant Reviews, Plant Pigments and Their Manipulation. Volume 14 Blackwell Publishing; Oxford, UK: 2004. [Google Scholar]
- 79.Mastrandrea L., Amodio M.L., Pati S., Colelli G. Effect of modified atmosphere packaging and temperature abuse on flavor related volatile compounds of rocket leaves (Diplotaxis tenuifolia L.) J. Food Sci. Technol. 2017;54:2433–2442. doi: 10.1007/s13197-017-2685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva Souza M.A., Peres L.E., Freschi J.R., Purgatto E., Lajolo F.M., Hassimotto N.M. Changes in flavonoid and carotenoid profiles alter volatile organic compounds in purple and orange cherry tomatoes obtained by allele introgression. J. Sci. Food Agric. 2020;100:1662–1670. doi: 10.1002/jsfa.10180. [DOI] [PubMed] [Google Scholar]
- 81.Li L., Lee W., Lee W.J., Auh J.H., Kim S.S., Yoon J. Extraction of allyl isothiocyanate from wasabi (Wasabia japonica Matsum) using supercritical carbon dioxide. Food Sci. Biotechnol. 2010;19:405–410. doi: 10.1007/s10068-010-0057-3. [DOI] [Google Scholar]
- 82.Fechner J., Kaufmann M., Herz C., Eisenschmidt D., Lamy E., Kroh L.W., Hanschen F.S. The major glucosinolate hydrolysis product in rocket (Eruca sativa L.), sativin, is 1,3-thiazepane-2-thione: Elucidation of structure, bioactivity, and stability compared to other rocket isothiocyanates. Food Chem. 2018;261:57–65. doi: 10.1016/j.foodchem.2018.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study is available on request from the corresponding author.