Abstract
Introduction:
Focal impulse and rotor modulation (FIRM) ablation can be used to target nonpulmonary vein (PV) sources of atrial fibrillation (AF). No published studies have compared freedom from atrial fibrillation (FFAF) after pulmonary vein reisolation (PVRI) plus FIRM to PVRI alone in patients with reconnected PVs undergoing repeat ablation.
Methods:
A 3:1 matched retrospective cohort study was performed on 21 patients with recurrent AF and PV reconnection who underwent PVRI plus FIRM-guided ablation and 63 patients with recurrent AF treated with PVRI alone at a single institution. All patients in the PVRI-alone cohort had cryoballoon PVRI at the time of repeat ablation without additional lesion sets for AF. Cases were matched based on the type of AF (paroxysmal vs nonparoxysmal), left atrial diameter (±4 mm), left ventricular ejection fraction (±10%), duration of AF (±18 months), and age (±5 years). The primary endpoint was FFAF after a 3-month blanking period.
Results:
Out of 53 total FIRM cases performed at Northwestern Memorial Hospital between 2015 and 2017, 21 patients had PVRI plus FIRM for recurrent AF with PV reconnection. These patients had an average of 3.3 ± 2.1 rotors (60% left atrial) ablated. Over a median follow-up time of 24.7 months (interquartile range, 13–36 months), patients in the PVRI-alone cohort demonstrated a higher rate of FFAF (n = 35; 55.6%) than patients in the PVRI plus FIRM-guided ablation cohort (n = 7; 33.3%) (logrank P = .049).
Conclusion:
In patients undergoing repeat ablation for AF with PV reconnection, PVRI plus FIRM did not increase FFAF compared to PVRI alone.
Keywords: atrial fibrillation ablation, cryoballoon ablation, focal impulse and rotor modulation
1 |. INTRODUCTION
Pulmonary vein isolation (PVI) is the cornerstone of ablative treatment for atrial fibrillation (AF). Despite advances in technology, 1-year freedom from atrial fibrillation (FFAF) after a single PVI ranges from approximately 50% to 80%.1–3 If patients have recurrent AF after PVI, a second ablation is often pursued. FFAF after pulmonary vein reisolation (PVRI) remains suboptimal at 40% to 75%, with lower rates of FFAF if patients have nonparoxysmal atrial fibrillation (NPAF) before PVRI.4–7 There are limited data to guide ablation technique in patients who have recurrent AF after PVI.8,9
Potential mechanisms for recurrent AF after PVI are pulmonary vein (PV) reconnection and activity of non-PV sources of AF initiation and maintenance including right and left atrial rotors. Prior studies have shown that rotors may drive activation and sustain AF in both human and animal models.10 Data are conflicting on the role of targeting these focal rotors through focal impulse and rotor modulation (FIRM) in addition to PVI during catheter ablation.10–14 The REAFFIRM trial concluded that rotor ablation has equivalent effectiveness to PVI alone in patients with NPAF undergoing the first ablation. The ongoing REDO-FIRM trial will assess the utility of FIRM during redo ablations, but there are presently no data on the efficacy of rotor ablation with PVRI for recurrent AF when PVs are reconnected.15–17
The goal of the present study is to compare procedural and clinical success rates of PVRI plus FIRM-guided ablation to PVRI alone in patients undergoing repeat ablation for AF with PV reconnection.
2 |. METHODS
2.1 |. Patients
A retrospective analysis of a prospectively maintained single-center database of all AF ablation cases performed at Northwestern Memorial Hospital between 2012 and 2017 was conducted. Out of 53 total FIRM cases, 21 consecutive patients who had PVRI plus FIRM ablation for recurrent AF with PV reconnection were included in this study. A 3:1 matched cohort of 63 cases was selected from a prospectively maintained database of patients with recurrent AF and PV reconnection who underwent PVRI alone.18–20 The index procedures in both cohorts were either cryoballoon ablation (CBA), radiofrequency ablation (RFA), or a surgical Maze procedure. In the PVRI plus FIRM cohort, the energy source for PVRI was either RFA or CBA. In the PVRI-alone cohort, the energy source for PVRI was CBA in all cases. Isolated PVRI cases were retrospectively matched to PVRI plus FIRM cases based on the type of AF (paroxysmal vs NPAF), left atrial diameter (±4 mm), left ventricular ejection fraction (±10%), duration of AF diagnosis (±18 months), and age (±5 years). The primary endpoint was FFAF, defined as no return of AF greater than 30 seconds after a 3-month blanking period.21 The study was approved by the Northwestern University Institutional Review Board.
2.2 |. Procedural details: FIRM
Patients provided informed consent before the procedure. FIRM mapping was typically completed in both atria unless precluded by the presence of intracardiac leads that would interfere with mapping. After a comprehensive evaluation, a FIRM-mapping basket was advanced into the left or right atrium under fluoroscopic and intracardiac ultrasound guidance (Figure 1). The size of the basket catheter used was determined by measuring the length of the interatrial septum to the posterior wall of the left atrium using intracardiac echocardiography during the procedure. If the patient presented to the electrophysiology lab in normal sinus rhythm (NSR), AF was induced and sustained for more than 5 minutes before FIRM mapping. The decision of which atrium to map first was operator dependent. Rotors were visualized as a rotational activity within the atrium using the FIRM software while the patient was in AF (Abbott, Menlo Park, CA) (Figure 2). Three-dimensional maps of the left and right atria were created with NAVX (St. Jude Medical, St. Paul, MN) or CARTO3 (Biosense Webster, St. Baldwin Park, CA) (Figure 3). Rotors were ablated as appropriate with a 64-electrode basket catheter (Abbott) in the right and left atria using 25 to 35 W for 20 to 60 seconds, or until electrograms were reduced. Remapping to assess for residual rotors was operator dependent. All patients in this group had PVRI for reconnected PVs with RFA or CBA along with FIRM-guided ablation. The decision to reisolate PVs before or after rotor ablation was operator dependent.
FIGURE 1.
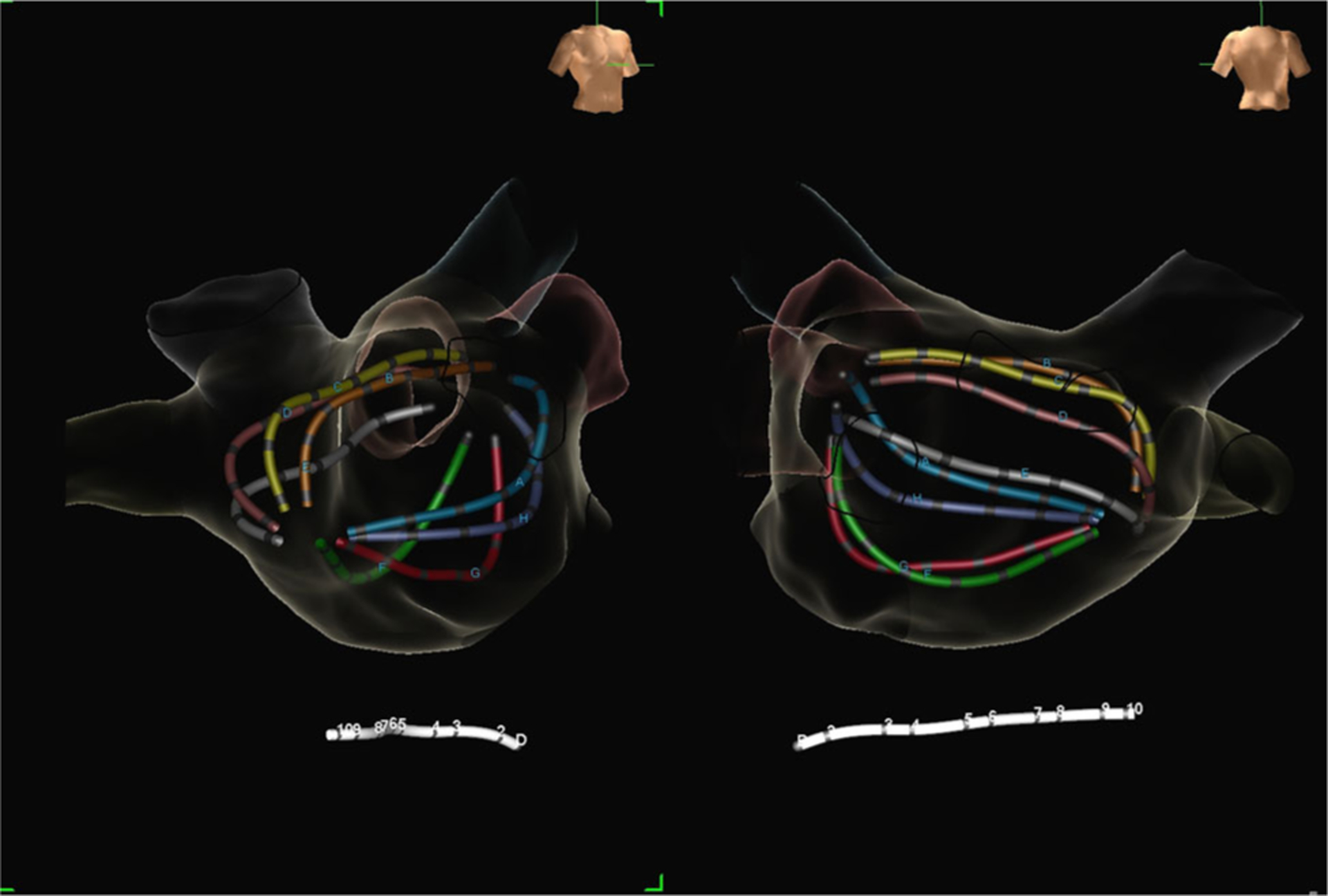
Right anterior oblique (left) and posteroanterior (right) views of the 64-electrode FIRM-mapping basket catheter (Topera, Abbott, Menlo Park, CA) in the left atrium. FIRM, focal impulse and rotor modulation
FIGURE 2.
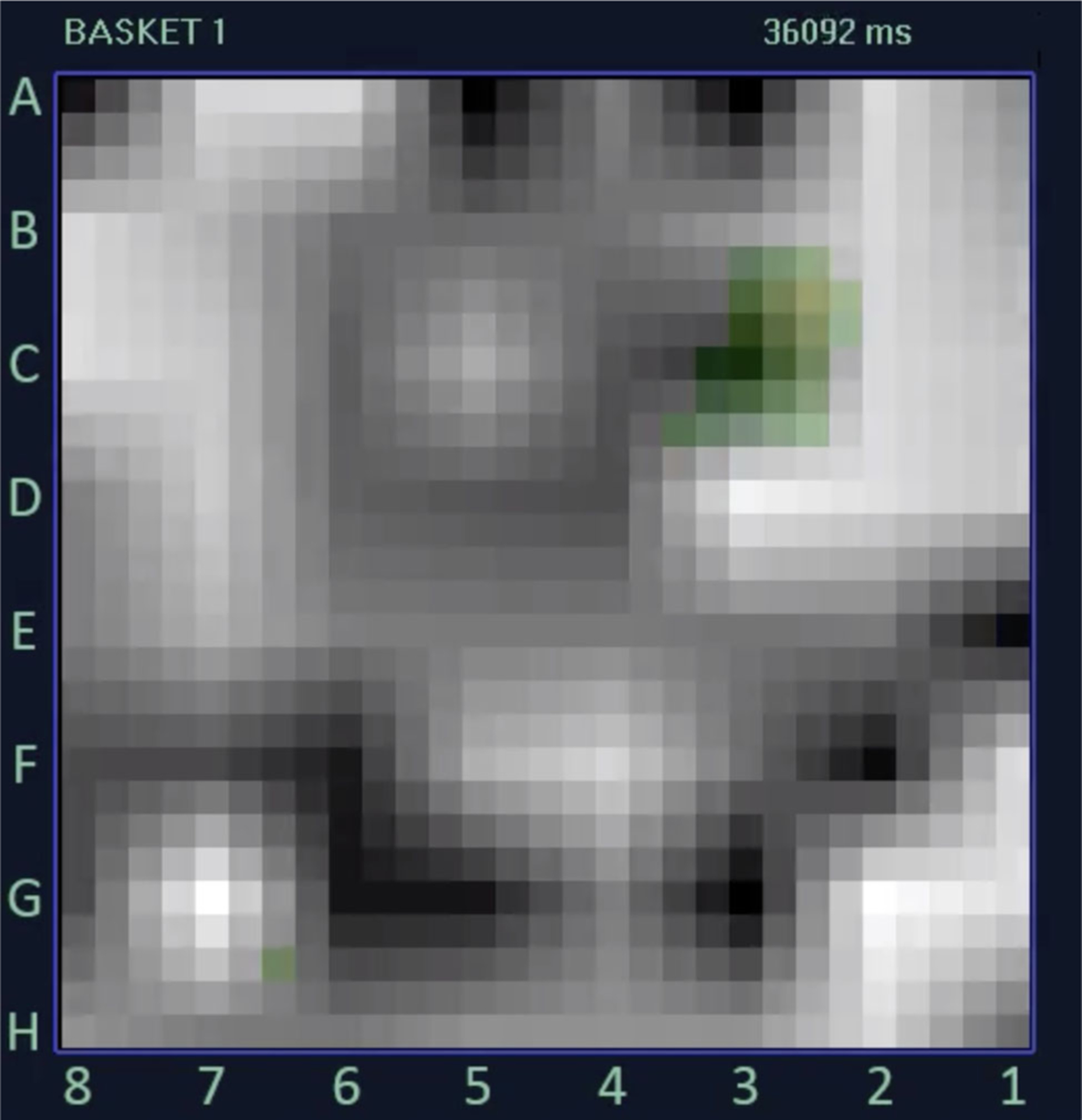
Image of a rotor map from a 72-year-old woman undergoing FIRM plus PVRI using data obtained from the 64-electrode FIRM-mapping basket catheter. This FIRM map demonstrates a clockwise rotational activity profile centered around electrode C3 by automated annotation (green squares). FIRM, focal impulse and rotor modulation; PVRI, pulmonary vein reisolation
FIGURE 3.
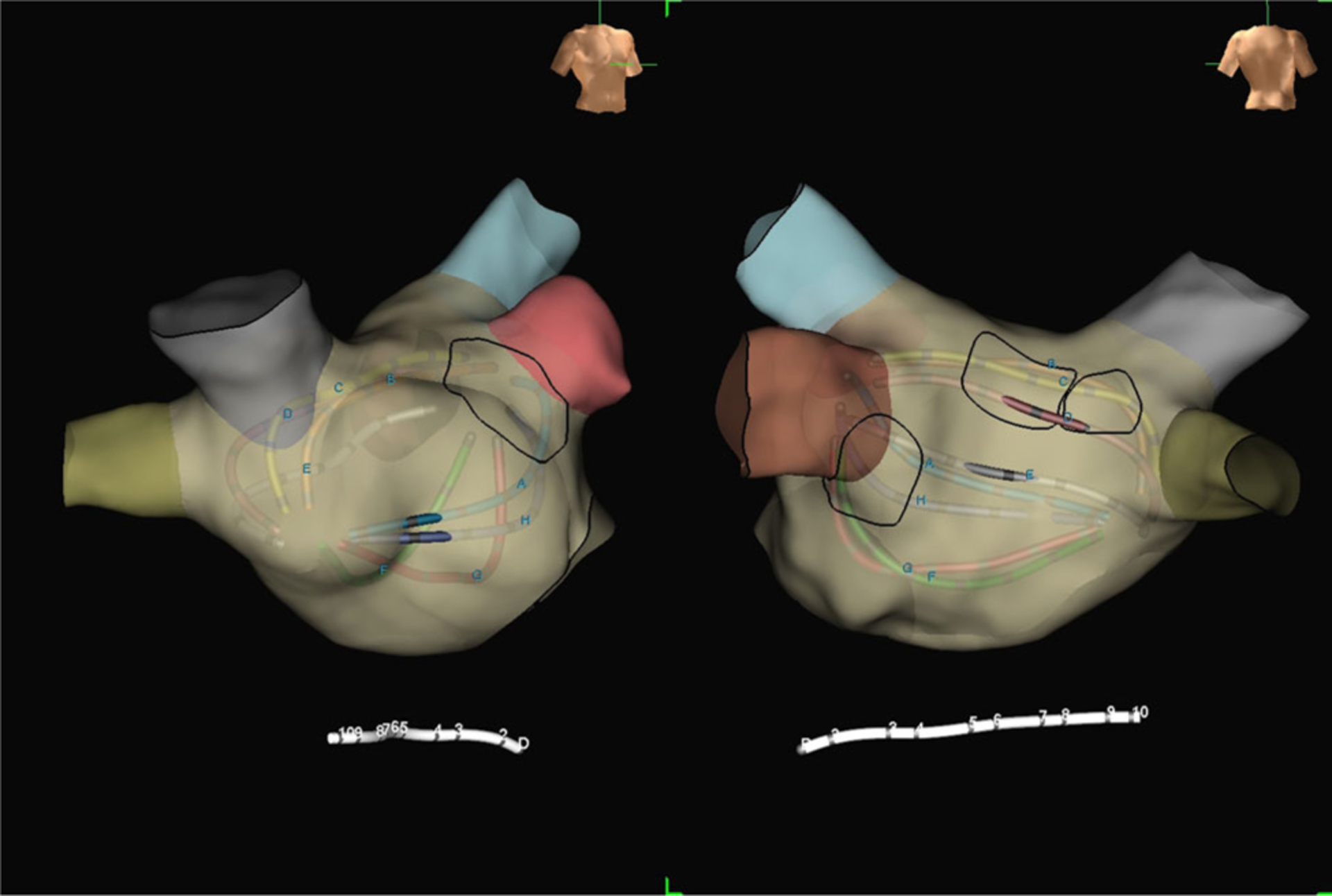
Right anterior oblique (left) and posteroanterior (right) electroanatomic maps of the left atrium from the same 72-year-old patient undergoing FIRM plus PVRI for recurrent AF. The black circles outline three rotors (two on the midposterior left atrial wall, one adjacent to the left atrial appendage) based on mapping with FIRM software. These three rotors were subsequently targeted for ablation. AF, atrial fibrillation; FIRM, focal impulse and rotor modulation; PVRI, pulmonary vein reisolation
2.3 |. Procedural details: Radiofrequency PVRI
After transseptal catheterization, a mapping catheter (Lasso; Biosense Webster or PentaRay; Biosense Webster) and irrigated force-sensing RFA catheter (ThermoCool SmartTouch; Biosense Webster or TactiCath; Abbott) were advanced into the left atrium. All PVs were interrogated for reconnection using the mapping catheter. PV isolation of all reconnected PVs was performed using the irrigated RFA catheter with a target temperature of 42°C and power of 20 to 35 W for 20 to 40 seconds or a force-time integral of 400 gs when available. Entry and exit block were confirmed after PVI. Following ablation, intracardiac echocardiography was used to confirm the absence of pericardial effusion. Provocative maneuvers after RFA to induce atrial arrhythmias were performed at the discretion of the operator. Cardioversion to sinus rhythm was performed if a patient remained in AF after RFA.22
2.4 |. Procedural details: Cryoballoon PVRI
Following transseptal catheterization, an Arctic Front Advance (Medtronic Inc, Minneapolis, MN) cryoballoon catheter and lasso catheter (Biosense Webster) were introduced into the left atrium using the CryoSheath (Medtronic Inc). All PVs were interrogated for reconnection using the lasso catheter. Three-dimensional mapping was used at the discretion of the operator. A 28-mm cryoballoon was used in nearly all (>95%) cases. CBA was delivered at the ostium of each PV, with pulmonary venography performed before each ablation to confirm the appropriate location and balloon occlusion, and using entrance and exit block as endpoints. Lesion duration evolved over time from two 4-minute freezes per vein to two 3-minute freezes per vein, with some operators limiting veins to a single 3-minute application if time to effect was less than 60 seconds. Target temperatures were −30°C to −55°C for all patients and esophageal temperature monitoring was used for those patients receiving general anesthesia. Entry and exit block were confirmed after PVI. During isolation of the right-sided PVs, a catheter was positioned in the superior vena cava to perform high-output pacing to monitor for phrenic nerve injury. Provocative maneuvers after CBA to induce atrial arrhythmias were performed at the discretion of the operator. Cardioversion to sinus rhythm was performed if a patient remained in AF after CBA.22,23
2.5 |. Clinical follow up
Antiarrhythmic drugs (AAD) were routinely stopped after a 3-month postprocedure blanking period. All patients had scheduled clinical follow up with a cardiologist every 6 months beginning 2 to 3 months postablation. Routine electrocardiogram (ECGs) at the time of office visits, external monitoring for 7 to 21 days, downloads from implanted devices, and readings from Kardia smartphone monitors (AliveCor, Mountain View, CA) were used for rhythm assessment. Additional rhythm assessments were performed during symptoms suggestive of arrhythmia recurrence.
2.6 |. Endpoints and data analysis
The primary endpoint for this study was FFAF from the end of a 90-day blanking period through the final rhythm evaluation at the time of data collection irrespective of the duration of AADs.21 Patient demographics, arrhythmia evaluation, and procedural complications were analyzed from the electronic medical record, and data were entered into an Institutional Review Board approved database. Statistical analyses were completed using SPSS Version 25 (IBM, Armonk, NY) to complete χ2 or Fisher exact tests for categorical variables, and Student t tests or Mann-Whitney U tests for continuous variables as appropriate. Kaplan-Meier curves were created to compare procedural success over time using Prism Software Version 8 (GraphPad, La Jolla, CA). P < .05 were considered to be significant in this study. Numerical results are reported as mean ± standard deviation, median (interquartile [IQR] range), or number (%).
3 |. RESULTS
3.1 |. Patient characteristics
There were 21 patients who underwent PVRI plus FIRM ablation and 63 control cases of PVRI alone included in this study. Among the patients who underwent PVRI plus FIRM ablation, 11 (52.4%) previously had RFA, 8 (38.1%) previously had CBA, and 2 (9.5%) previously had a surgical Maze procedure. Of the patients who underwent PVRI alone, 44 (69.8%) had prior RFA, and 19 (30.2%) had prior CBA. Patients in the FIRM cohort had a higher rate of diabetes mellitus, fewer prior AADs, and fewer PVs reconnected than patients in the PVRI-alone cohort (Table 1). No other major differences in baseline demographics between the two groups were observed (Table 1).
TABLE 1.
Baseline demographics
| FIRM + PVRI (n = 21) | PVRI alone (n = 63) | P value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, y | 59.8 ± 14.6 | 62.5 ± 9.9 | .43 |
| Male sex | 16 (76.2%) | 46 (67%) | .77 |
| AF type | |||
| Paroxysmal AF | 2 (9.5%) | 6 (9.5%) | 1.00 |
| Nonparoxysmal AF | 19 (90.5%) | 57 (90.5%) | 1.00 |
| Months of AF | 84 (32–101) | 72 (36–108) | .70 |
| Left atrial diameter, mm | 44.4 ±7.9 | 40.4 ±6.2 | .05 |
| Left ventricular ejection fraction (%) | 49.8 ±11.7 | 54.9 ± 9.4 | .05 |
| Implantable cardioverter-defibrillator/pacemaker present before ablation | 1 (4.8%) | 8 (12.7%) | .31 |
| PVs reconnected | 2.3 ± 1.2 | 3.2 ±0.98 | <.01 |
| 0 PVs reconnected | 1 (4.8%) | 0 (0%) | .25 |
| 1 PV reconnected | 5 (23.8%) | 3 (4.8%) | .02 |
| 2 PVs reconnected | 5 (23.8%) | 14 (22.2%) | 1.00 |
| 3 PVs reconnected | 5 (23.8%) | 9 (14.3%) | .32 |
| 4 PVs reconnected | 4 (19.0%) | 34 (54.0%) | <.01 |
| CHA2DS2VASc score | 2.3 ± 1.5 | 1.8 ±1.1 | .12 |
| # Prior AADs | 0.95 ±0.59 | 1.3 ±0.74 | .03 |
| Comorbid conditions | |||
| Hypertension | 9 (42.9%) | 27 (42.9%) | 1.00 |
| Diabetes | 5 (23.8%) | 2 (3.2%) | .01 |
| Cerebrovascular accident | 2 (9.5%) | 4 (6.3%) | .64 |
| Coronary artery disease | 4 (19.0%) | 10 (15.9%) | .74 |
| Hyperlipidemia | 10 (47.6%) | 20 (31.7%) | .43 |
Abbreviations: AAD, antiarrhythmic drugs; AF, atrial fibrillation; FIRM, focal impulse and rotor modulation; PV, pulmonary veins; PVRI, pulmonary vein reisolation.
3.2 |. Procedural results
Among patients who underwent FIRM ablation, rotors were found and ablated in 20 (95.2%) cases, with an average 3.2 ± 2.0 rotors per procedure. Rotors were exclusively located in the left atrium in four (19.0%) cases, while six patients (28.6%) had exclusively right atrial rotors. Rotors were ablated in both atria in 10 (47.6%) cases (Figure 4). An average of 12.6 ± 4.6 lesions was delivered per rotor site per FIRM case. PVs were reisolated in all FIRM cases with RFA used in 18 (85.7%) cases and CBA used in 3 (14.3%) cases. All patients in the PVRI-alone cohort underwent PVI using CBA without additional lesion sets for AF.
FIGURE 4.
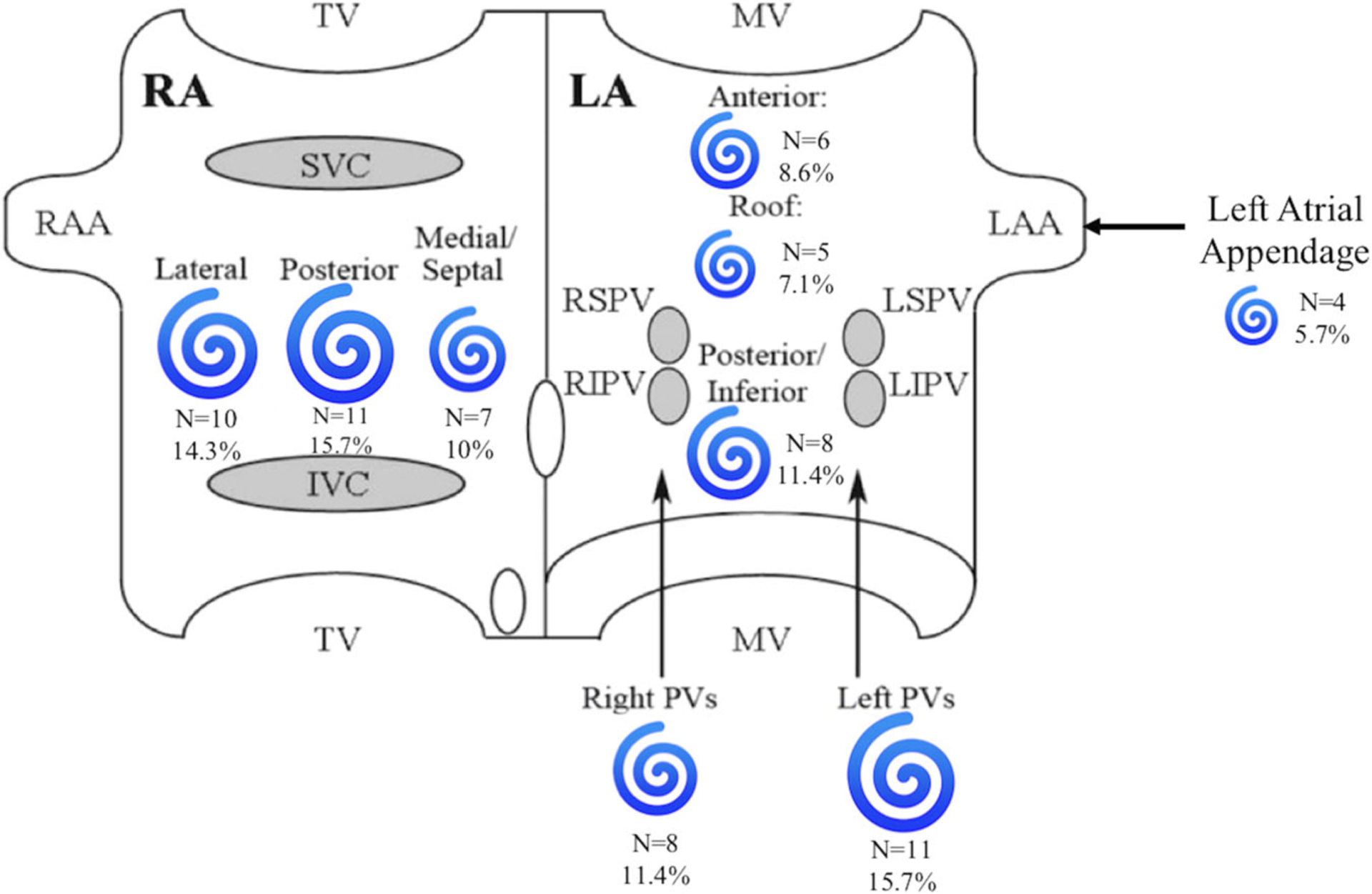
Location and number of rotors in the right and left atria among patients who underwent PVRI plus FIRM ablation. Image adapted from Miller et al12. FIRM, focal impulse and rotor modulation; IVC, inferior vena cava; LA, left atrium; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; PV, pulmonary vein; PVRI, pulmonary vein reisolation; RA, right atrium; RAA, right atrial appendage; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SVC, superior vena cava; TV, tricuspid valve
Within the PVRI plus FIRM-guided ablation cohort, six (28.6%) patients presented in NSR. After induction of AF for the procedure, zero of these six patients required chemical or electrical cardioversion to return to NSR at the end of the case. Of the remaining 15 (71.4%) patients in the PVRI plus FIRM cohort who presented in AF, 13 (86.7%) required electrical cardioversion to return to NSR at the conclusion of the case and 2 (13.3%) patients converted to NSR during the case. Among the patients in the PVRI-alone cohort, 46 (73%) presented in NSR, of whom 36 (78.3%) remained in NSR throughout the procedure. The remaining 10 patients in the PVRI-alone cohort who presented in NSR developed AF during the procedure, with electrical cardioversion required in 8 (17.4%) patients, and spontaneous cardioversion observed in 2 (4.4%) patients. Among the 17 (27%) patients in the PVRI-alone cohort who presented in AF, 12 (70.6%) patients were electrically cardioverted, 1 patient (5.9%) was chemically cardioverted, and 4 patients (23.5%) spontaneously converted to NSR. All patients in both cohorts were in NSR at the end of the cases. There were no major procedural complications in either cohort.
3.3 |. Long-term results
Over a median follow-up time of 24.7 months (IQR, 13–36 months), patients in the PVRI-alone cohort (n = 35; 55.6%) demonstrated a higher rate of FFAF than patients in the PVRI plus FIRM-guided ablation cohort (n = 7; 33.3%) (logrank P = .049) (Figure 5). Among patients with recurrence of AF, the median time to recurrence in the FIRM cohort was 9.0 months (IQR, 4.8–18.7 months) compared to 8.8 months (IQR, 5.5–19.7 months) in the PVRI-alone cohort (Figure 2). There were no significant differences in the ECG recording methods for determining recurrent AF between the two groups.
FIGURE 5.
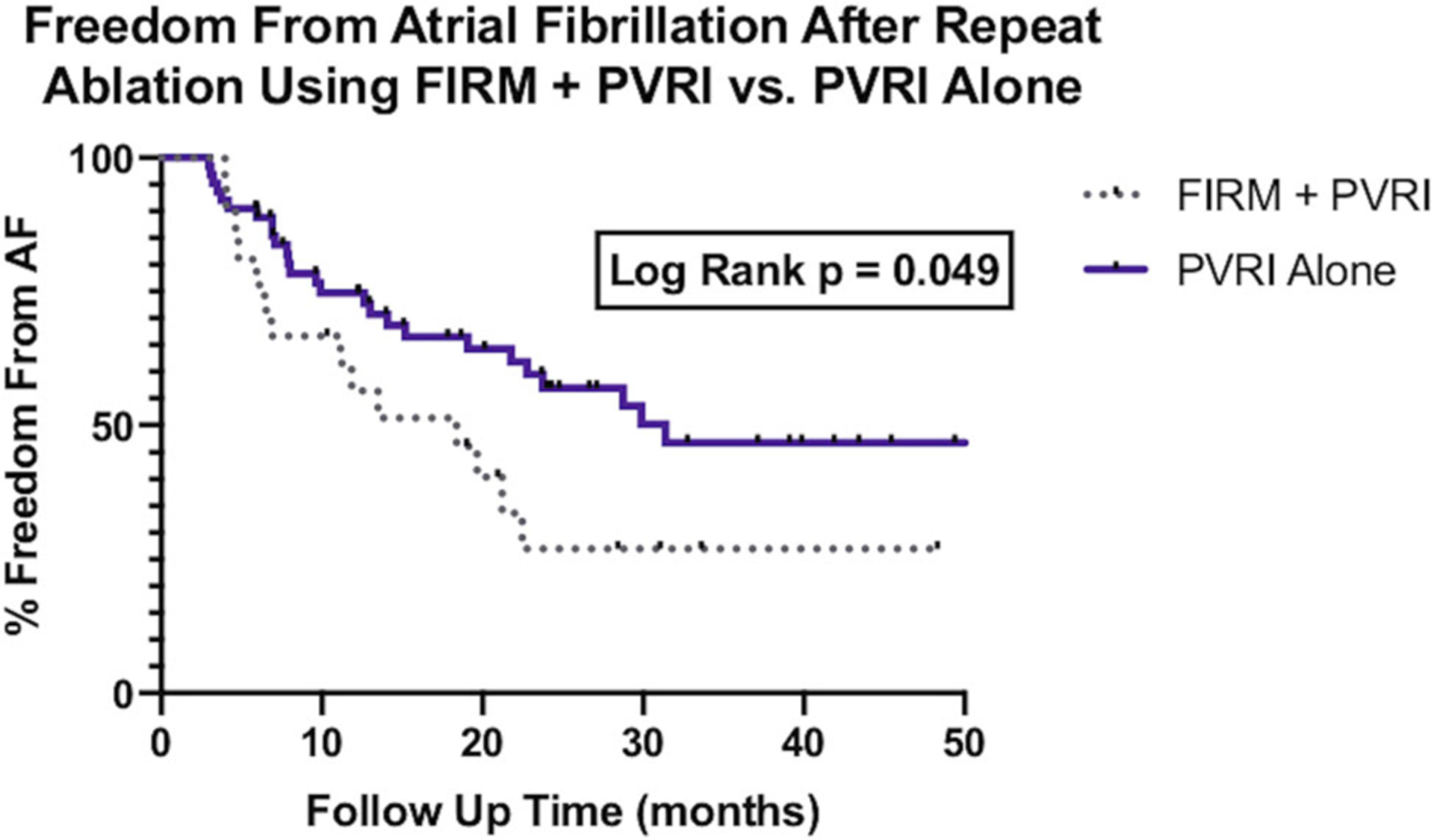
Survival analysis of freedom from atrial fibrillation after PVRI plus FIRM ablation vs PVRI alone. FIRM, focal impulse and rotor modulation; PVRI, pulmonary vein reisolation
4 |. DISCUSSION
In patients undergoing redo ablations for AF with reconnected PVs, long-term FFAF remains suboptimal. While repeat ablation has been shown to be superior to medical therapy for patients with recurrent AF after ablation, there are no clear guidelines to recommend the best ablation technique for this population.4,9,24 Studies on various techniques for substrate modification including left atrial roof lines, left atrial posterior wall lines, posterior wall isolation, superior vena cava isolation, and ablation of complex fractionated electrograms have yielded variable results.5,25
There are multiple mechanisms that allow AF to be initiated and sustained. The objective of mapping of rotational activity and focal impulses with FIRM software is to find and ablate sources of arrhythmogenic tissue that may trigger AF beyond commonly perceived sources.10 In contrast to surgical approaches, or other anatomically-based ablation techniques, foci of arrhythmia are selectively targeted for ablation in FIRM. The FIRM procedure is dependent on the assumption that AF is driven by localized sources, rather than global disorganization.10,15 FIRM success relies on interrupting the activation of rotors, which produce focal areas of disorganized electrical activity within the right and left atria.10,24,26
Previous nonrandomized studies that have evaluated FIRM in patients undergoing repeat ablations have yielded variable results. In a cohort of 52 patients, all of whom had a prior ablation, FIRM with PVRI produced 69.2% FFAF 12 months following the procedure.27 However, other studies of FIRM in populations mainly consisting of patients with NPAF undergoing PVRI plus FIRM ablation have reported FFAF rates as low as 20%.14 The location of rotors in patients undergoing a redo ablation for paroxysmal AF (pAF) has been found to be more similar to the location of rotors in patients with NPAF than those undergoing a first ablation for pAF,24 but the incremental benefit of FIRM in patients with recurrent AF and PV reconnection undergoing a repeat ablation has not previously been delineated.16 The present study suggests that PVRI alone is associated with a higher rate of FFAF than PVRI plus FIRM in patients undergoing repeat ablation for recurrent AF with PV reconnection. Rotor ablation may have been associated with higher rates of recurrent AF due to the creation of areas of re-entry within the left and right atria. Previous work investigating the addition of FIRM to PVI has demonstrated that use of FIRM in these cases may be proarrhythmic via the creation macroreenterant circuits.28
There are several limitations of the present study including small sample size, absence of randomization, and the use of a comparison group that was not contemporaneous. Furthermore, though the control group was matched on several important variables, there are clinical variables that were not specifically matched including the number of reconnected veins before the repeat ablation and the proportion of patients presenting in sinus rhythm. The more advanced substrate in the PVRI+FIRM group may have contributed to higher rates of recurrent AF. Lastly, while operator discretion resulted in differences in the method of PVRI between the two groups, prior studies have shown similar rates of FFAF with RFA and CBA.22,29 Though our findings do support the conclusion that PVRI plus FIRM does not result in a higher rate of FFAF than PVRI alone, future studies, such as the upcoming RE DO-FIRM trial, will compare PVRI plus FIRM ablation to PVRI alone in a randomized prospective manner.17
5 |. CONCLUSION
There are limited data to guide ablation techniques for patients with recurrent AF and PV reconnection after a prior ablation. In patients undergoing repeat ablations for AF with PV reconnection, the addition of FIRM-guided ablation to reisolation of the PVs did not increase FFAF. Upcoming randomized-controlled studies will further investigate this approach.
Footnotes
Disclosure: Nishant Verma receives honoraria for speaking from Medtronic, Inc. Bradley P. Knight receives honoraria for consulting and speaking for Medtronic Inc. Rod S. Passman receives research support, consulting fees, and speaker fees from Medtronic, and royalties from UpToDate; Northwestern University receives fellowship support from Medtronic, Inc. Other authors: No disclosures.
REFERENCES
- 1.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boveda S, Metzner A, Nguyen DQ, et al. Single-procedure outcomes and quality-of-life improvement 12 months post-cryoballoon ablation in persistent atrial fibrillation: results from the multicenter CRYO4-PERSISTENT AF trial. JACC Clin Electrophysiol. 2018;4(11):1440–1447. [DOI] [PubMed] [Google Scholar]
- 3.Aryana A, Kowalski M, O’Neill PG, et al. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: short- and long-term results of a multicenter study. Heart Rhythm. 2016;13(12):2306–2313. [DOI] [PubMed] [Google Scholar]
- 4.Barakat AF, Wazni OM, Saliba WI, et al. Repeat ablation or medical management alone for recurrent arrhythmias after ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29(4):551–558. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg GR, Ono M, Aryana A, et al. The incremental benefit of non-pulmonary vein left atrial ablation in patients undergoing a repeat persistent atrial fibrillation ablation procedure. J Interv Card Electrophysiol. 2017;48(2):185–191. [DOI] [PubMed] [Google Scholar]
- 6.De Regibus V, Iacopino S, Abugattas JP, et al. Repeat procedures using the second-generation cryoballoon for recurrence of atrial fibrillation after initial ablation with conventional radiofrequency. J Interv Card Electrophysiol. 2017;49(2):119–125. [DOI] [PubMed] [Google Scholar]
- 7.Coutiño HE, de Asmundis C, Mugnai G, et al. Repeat procedures after second-generation cryoballoon ablation as an index procedure for persistent atrial fibrillation: one-year follow-up. J Interv Card Electrophysiol. 2016;47(3):365–371. [DOI] [PubMed] [Google Scholar]
- 8.Lin D, Santangeli P, Zado ES, et al. Electrophysiologic findings and long-term outcomes in patients undergoing third or more catheter ablation procedures for atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26(4):371–377. [DOI] [PubMed] [Google Scholar]
- 9.Darby AE. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation. 2016;9(1):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baykaner T, Lalani GG, Schricker A, Krummen DE, Narayan SM. Mapping and ablating stable sources for atrial fibrillation: summary of the literature on focal impulse and rotor modulation (FIRM). J Interv Card Electrophysiol. 2014;40(3):237–244. [DOI] [PubMed] [Google Scholar]
- 11.Tilz RR, Lin T, Rillig A, et al. Focal impulse and rotor modulation for the treatment of atrial fibrillation: locations and 1 year outcomes of human rotors identified using a 64-electrode basket catheter. J Cardiovasc Electrophysiol. 2017;28(4):367–374. [DOI] [PubMed] [Google Scholar]
- 12.Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25(9):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JM, Kalra V, Das MK, et al. Clinical benefit of ablating localized sources for human atrial fibrillation: the Indiana University FIRM registry. J Am Coll Cardiol. 2017;69(10):1247–1256. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg JS, Shah Y, Bhatt A, et al. Focal impulse and rotor modulation: acute procedural observations and extended clinical follow-up. Heart Rhythm. 2017;14(2):192–197. [DOI] [PubMed] [Google Scholar]
- 15.Stiles MK, Sanders P, Lau DH. Targeting the substrate in ablation of persistent atrial fibrillation: recent lessons and future directions. Front Physiol. 2018;9:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brachmann J. Prospective randomized comparison of rotor ablation vs. conventional ablationfor treatment of persistent atrial fibrillation—The REAFFIRM trial. Paper presented at: Heart Rhythm Society Scientific Sessions; 2019; San Fransisco, CA. [Google Scholar]
- 17.Randomized Evaluation of Redo Ablation Procedures of Atrial Fibrillation With FIRM Guided Procedures (REDO-FIRM). Clinical-Trials.gov. Accessed 22 July 2019.
- 18.Setia MS. Methodology series module 2: case-control studies. Indian J Dermatol. 2016;61(2):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewallen S, Courtright P. Epidemiology in practice: case-control studies. Community Eye Health. 1998;11(28):57–58. [PMC free article] [PubMed] [Google Scholar]
- 20.Hennessy S, Bilker WB, Berlin JA, Strom BL. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol. 1999;149(2):195–197. [DOI] [PubMed] [Google Scholar]
- 21.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. [DOI] [PubMed] [Google Scholar]
- 22.Wasserlauf J, Pelchovitz DJ, Rhyner J, et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015;38(4):483–489. [DOI] [PubMed] [Google Scholar]
- 23.Peigh G, Kaplan RM, Bavishi A, et al. A novel risk model for very late return of atrial fibrillation beyond 1 year after cryoballoon ablation: the SCALE-CryoAF score. J Interv Card Electrophysiol. 2019:1–9. 10.1007/s10840-019-00588-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaman JAB, Baykaner T, Clopton P, et al. Recurrent post-ablation paroxysmal atrial fibrillation shares substrates with persistent atrial fibrillation: an 11-center study. JACC Clin Electrophysiol. 2017;3(4): 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S, Barakat AF, Saliba WI, et al. Recurrent atrial fibrillation after initial long-term ablation success: electrophysiological findings and outcomes of repeat ablation procedures. Circ Arrhythm Electrophysiol. 2018;11(4):e005785. [DOI] [PubMed] [Google Scholar]
- 26.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112(5):849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer SG, Károlyi L, Rämmler C, et al. Treatment of recurrent nonparoxysmal atrial fibrillation using focal impulse and rotor mapping (FIRM)-guided rotor ablation: early recurrence and long-term outcomes. J Cardiovasc Electrophysiol. 2017;28(1):31–38. [DOI] [PubMed] [Google Scholar]
- 28.Buch E, Share M, Tung R, et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm. 2016;13(3):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–2245. [DOI] [PubMed] [Google Scholar]


