Abstract
Objectives:
To embed pharmacy residents in an interprofessional nephrology clinic to conduct medication reconciliation in targeted high-risk patients with nondialysis kidney disease.
Setting:
This pilot was a prospective quality improvement initiative conducted in an interprofessional outpatient nephrology clinic.
Practice description:
The nephrology clinic team includes nephrology providers, a social worker, and a geriatrician. The team is responsible for the management of conditions such as nondialysis kidney disease, resistant hypertension, acute kidney injury, proteinuria, and nephropathy.
Evaluation:
Primary outcomes included the number and type of medication discrepancies and drug therapy problems identified. Secondary outcomes included the changes in care process directly resulting from the pharmacy residents’ recommendations. The perceived value of the pharmacy residents to the interprofessional team was assessed through postintervention anonymous surveys and semistructured interviews.
Results:
The pharmacy residents conducted 118 visits for 87 unique patients (mean age 73 years, 97% male) with nondialysis kidney disease (89% stages III–V), polypharmacy (87% of patients taking > 10 medications), and a heavy comorbidity burden (85% hypertension, 80% dyslipidemia, 59% diabetes mellitus type II) from January to October 2017. Pharmacists identified 344 medication discrepancies and 301 drug therapy problems, resulting in 398 changes in care process. The most frequently identified discrepancies and drug therapy problems were the omission of an active medication from the medication list (86 of 344 discrepancies, 25%) and potentially inappropriate medications (106 of 301 drug therapy problems, 35%). Pharmacists recommended 228 medication changes, provided 76 adherence devices, facilitated 24 consults or referrals, and communicated with the primary care team on 70 occasions. The interprofessional team members all strongly agreed that patients and the team benefited from the pharmacists’ involvement.
Conclusion:
Pharmacy resident–led medication reconciliation resulted in the identification and resolution of medication discrepancies and drug therapy problems, leading to changes in the care process.
In a recent population-based retrospective cohort study of 2 million adults, patients seen by a nephrologist had the highest number of comorbidities, mean number of prescribed medications, highest rate of death, and highest rate of placement in a long-term care facility.1 Indeed, the majority of patients seen by outpatient nephrology providers in the United States are adults with chronic kidney disease (CKD) and complex medical conditions,1–3 who are at increased risk of cognitive decline,4 frailty,5 and difficulty completing activities of daily living as they age.6,7 Owing to a high comorbidity burden and diminishing kidney function, older adults with CKD also have an elevated risk for polypharmacy, with potentially inappropriate medications or inappropriately dosed medications based on the level of kidney impairment, all of which may lead to adverse outcomes.1,8,9 Adults with kidney disease are subject to many medication-related problems, including adverse drug reactions, drug–drug interactions, and inappropriate renal dosing. These types of medication-related problems contribute to 1 in 6 hospitalizations and an estimated annual United States health care cost of $175 billion.10
As kidney disease progresses toward end-stage renal disease (ESRD), medication burden grows: in a cohort study of dialysis-dependent adults in the United States, one-fourth were taking 25 or more daily medications, which was significantly associated with reduced medication adherence, decreased physical function, and lower health-related quality of life.11 The associated annual cost of medication nonadherence in the United States has been estimated to be $300 billion, with an estimated annual cost of prescription drug–related morbidity and mortality of more than $528 billion.12,13
Medication reconciliation may provide a solution for the detection and resolution of medication-related problems, polypharmacy, and medication nonadherence in patients with kidney disease. Using a structured process to compare patients’ medication lists, prescription and nonprescription medication bottles, medication management behaviors, and adherence is critical for patients with kidney disease, who take a high number of medications with frequent dose adjustments.10,13–16 Medication reconciliation is paramount after any transition of care, including a hospitalization, and should be conducted at routine intervals to account for frequent medication changes or adverse effects in patients with kidney disease.13
Pharmacists are proficient in medication reconciliation and management, and intervention supports improved clinical outcomes and medication adherence, as well as reduced hospitalization and health care costs for older high-risk patients.13,17,18 Because medication management is critical to preventing the progression of kidney disease to ESRD, pharmacists’ use of a proactive approach to medication optimization and deprescribing is key.14 The 2012 Kidney Disease Improving Global Outcomes “Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease” support involving pharmacists in the medication management and review of patients with kidney disease.19
Although much has been published regarding the role of pharmacists in reducing medication burden and associated costs in dialysis16,20–27 and transplant28–33 patients, evidence for pharmacist intervention for patients with nondialysis kidney disease in the outpatient setting is sparse and hetero-geneous.34 Several models have used pharmacist expertise effectively in the management of anemia in patients with nondialysis kidney disease; however, they did not use medication reconciliation to target the issues of medication-related problems, polypharmacy, and nonadherence.35–37 In 2 French outpatient clinics, inclusion of a pharmacist consultation service for patients with CKD led to the detection of significantly more drug-related problems compared with usual renal care, with more drug-related problems associated with older age and higher number of daily medications.38,39 Similarly, pharmacists within a quality improvement initiative in a community health center detected an average of 3.2 drug-related problems per CKD patient, but fewer than half of the pharmacist recommendations were accepted and implemented by physicians.40 That study showed a significant correlation between a greater number of drug-related problems and more advanced kidney disease.14,34,40 Although those studies highlighted the importance of pharmacist medication review and reconciliation in identifying drug therapy problems, there was minimal description of the pharmacist’s role in resolving medication discrepancies, communicating with non-nephrology providers, assessing adherence, and triggering consultations or referrals to needed services. In addition, the clinical models had the pharmacist and nephrology providers working separately rather than collaborating in person.
Four published interprofessional care models report on patient outcomes for those with a pharmacist on their care team compared with usual care.41–44 Collectively, these models demonstrated a slower rate of estimated glomerular filtration rate decline,41 improved parathyroid hormone monitoring and adherence to guideline-recommended antihypertensive medications,42 and improved proteinuria screening; however, there was no significant impact on mortality or acute care utilization in those with a pharmacist on their team versus usual care.44 These studies did not report on the specific pharmacist workflow or changes in care process resulting from pharmacist assessment. Therefore, it was difficult to determine how or if the pharmacist contributed to improved clinical care in the outpatient CKD population.
Objectives
To our knowledge, our model is the first to describe embedding pharmacy residents in an outpatient clinic to conduct medication reconciliation for patients with nondialysis kidney disease, working with nephrology providers in person and in real time. We applaud efforts to use medication reconciliation in the dialysis and transplant settings, and we build on previously published models within the nondialysis population to target the issues of medication-related problems, polypharmacy, and nonadherence. In this quality improvement initiative, we assess the impact of pharmacy residents within an interprofessional nephrology clinic conducting medication reconciliation on targeted high-risk patients with kidney disease. We describe the technical aspects of the pharmacist workflow to allow for replication in other nephrology and potentially other subspecialty clinic settings. We also detail the pharmacist’s role in the detection and resolution of medication discrepancies and drug therapy problems, with a focus on specific changes in care process that resulted from pharmacist assessment.
Setting
This pilot was a prospective quality improvement initiative conducted in a Veterans Affairs (VA) interprofessional outpatient nephrology clinic from January to October 2017. The nephrology clinic receives requests for formal consultation from other providers for the management of conditions such as nondialysis CKD, resistant hypertension, acute kidney injury, proteinuria, and nephropathy. Consultation requests may be placed by primary care providers or medical specialists within the VA Boston Healthcare System. Patients are followed in a different clinic if they proceed dialysis; thus the present study population includes only those with nondialysis kidney disease.
Practice description
The nephrology clinic team includes nephrology providers, a social worker, and a geriatrician. Figure 1 details the roles and responsibilities of each team member in the pilot. Within the outpatient nephrology clinic, Veterans are seen for an initial in-clinic visit after the placement of a consultation. Follow-up visits may take place as needed: as frequently as every 2 weeks for acute issues, and as infrequently as yearly for those with stable kidney disease. The nephrology providers are responsible for the evaluation of renal disease. Nephrology providers are the sole parties responsible for associated billing for the visits. Nephrology providers frequently order laboratory monitoring and counsel on diet and self-care. Patients see the social worker and geriatrician on an as-needed basis in clinic. Although these providers document their encounters with the patients, they do not bill for the visits. Their workload is documented through the encounter and the billing for all encounters with the interprofessional team is completed by the nephrology provider at the end of that day’s visit. At times, providers may follow-up with patients via telephone; providers document and bill for these encounters accordingly. The purpose of the pilot was to embed 2 pharmacy residents into the clinic model to conduct in-person medication reconciliation to improve medication management for high-risk patients with nondialysis kidney disease. The pilot was approved by the facility’s internal Research and Development Committee as a quality improvement initiative and was exempt from further Institutional Review Board oversight.
Figure 1.
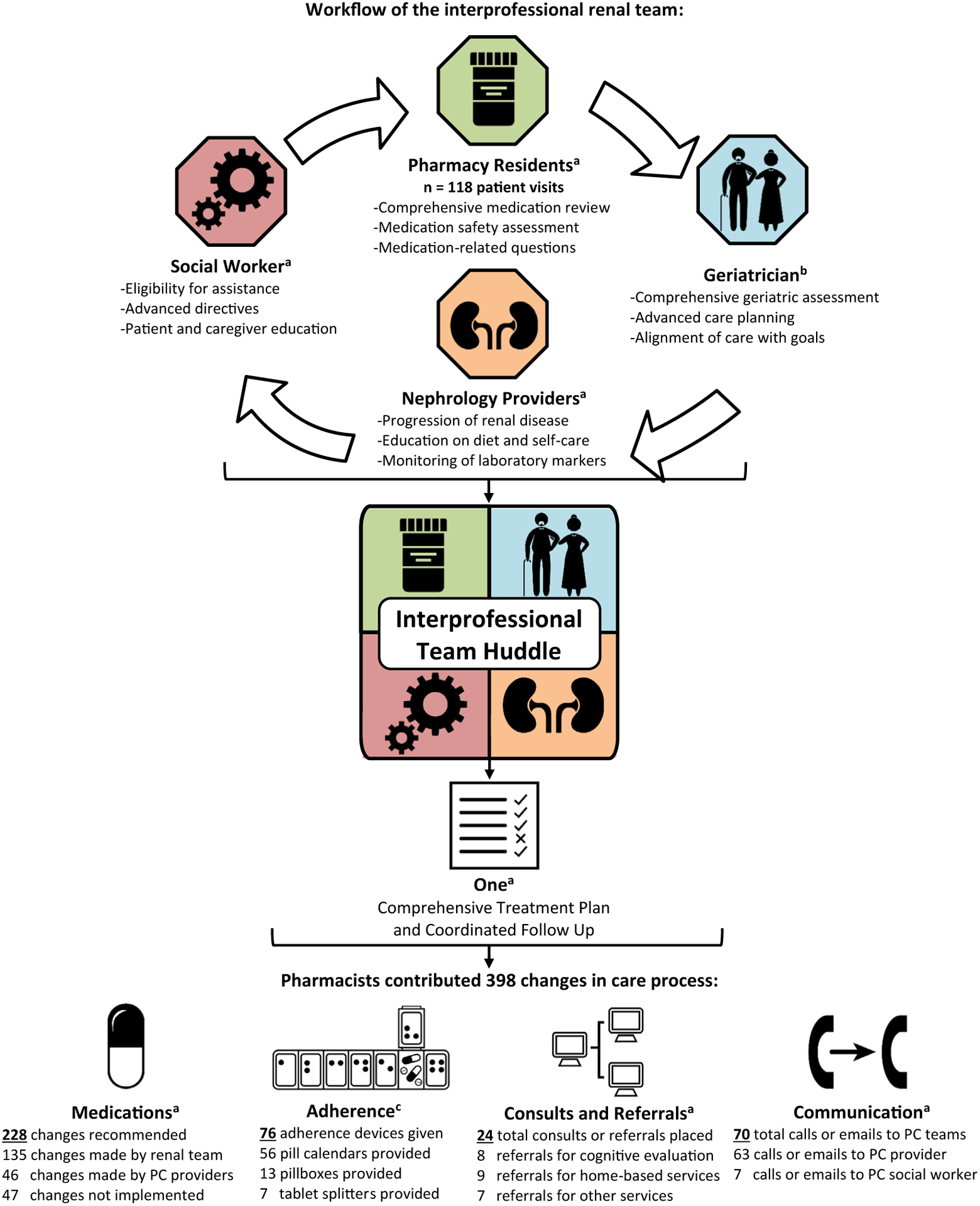
A schematic illustration of the interprofessional clinic process, team member roles, and resulting changes in the care process due to the pharmacy residents’ interventions. The interprofessional team consisted of a geriatrician, 2 pharmacy residents, a social worker, and nephrology providers. The pharmacy residents completed 118 medication reconciliation visits for targeted patients. Patients had the option to meet with the geriatrician and social worker as needed. After all individual visits were completed, the interprofessional team huddled to create a comprehensive treatment plan and coordinate follow-up. After retrospective review, the pharmacy residents contributed 398 changes in care process: 228 medication-related recommendations, 76 adherence devices, 24 consultations or referrals, and 70 telephone calls or e-mails to primary care (PC) teams. a Images by Bakunetsu Kaito from the Noun Project. b Image by Marie van den Broeck from the Noun Project. c Image by Michael Thompson from the Noun Project.
Evaluation
Primary outcomes included the number and type of medication discrepancies and drug therapy problems identified. Secondary outcomes included the changes in care process directly resulting from the pharmacy residents’ assessment. The perceived value of the pharmacy residents to the interprofessional team was assessed. Data collection on patient baseline characteristics, medication reconciliation data, and changes in care process45 occurred via retrospective chart review of encounter notes, medication lists, medication refills, and consultations or referrals up to 1 year after the end of the pilot period. Descriptive statistics are summarized. This pilot did not include a control or comparator group, so no statistical analysis was completed.
The value of pharmacy residents’ inclusion in the interprofessional team model was evaluated through anonymous surveys and semistructured interviews derived from previously published assessments of interprofessional teams46–48 and were completed by all team members (n = 6) after pilot completion. Team members were given a paper survey with questions addressing the perceived benefit of pharmacists (e.g., I am satisfied by the care provided by the pharmacists in clinic; My patients benefit from pharmacist-led medication reconciliation; Findings or recommendations by the pharmacist influence my care plan). Team members indicated their level of agreement for each question on a 5-point Likert scale. After the anonymous survey, a clinical pharmacist who was previously involved in the pilot as a pharmacy resident used the above questions to facilitate a semistructured interview with the team member. The pharmacist asked team members to provide any additional comment for each of the 9 questions as the team members saw fit. Surveys were scored, and interview data were compiled and analyzed for common themes.
Practice innovation
During the pilot period, 2 pharmacy residents were embedded in the clinic to conduct medication reconciliation for targeted high-risk patients: 1 postgraduate year (PGY) 2 resident specializing in geriatric pharmacy and 1 PGY-1 resident. Both residents had experience in geriatrics and medication reconciliation through outpatient residency rotations but had not received prior formal training in nephrology. Pharmacy residents were precepted by the attending nephrologist in the clinic and conducted patient visits separately. Although clinical pharmacy specialists may work under a scope of practice with a collaborating physician, allowing the pharmacist to prescribe medications and order necessary laboratory tests within that scope of practice, in the present model the nephrology providers were responsible for the prescription of medications and ordering of necessary laboratory tests.
Each week, the pharmacy residents reviewed the charts of scheduled nephrology clinic patients in the electronic medical record (EMR) to identify targeted patients for medication reconciliation who had any of the criteria in Figure 2. Before or after their visits with the nephrology provider, these targeted patients were offered a visit with a pharmacy resident to review their medications. Patients who may not have been targeted in the screening process may have been referred to the pharmacy resident in real time if a nephrology provider identified the need for pharmacist assessment during their clinic visit that day. If a patient was seen by a pharmacy resident at his or her previous renal visit, he or she was not excluded from an additional medication reconciliation at subsequent visits. Patients seen by a pharmacy resident were not required to have a diagnosis of CKD and may have been seen in the nephrology clinic for other reasons.
Figure 2.
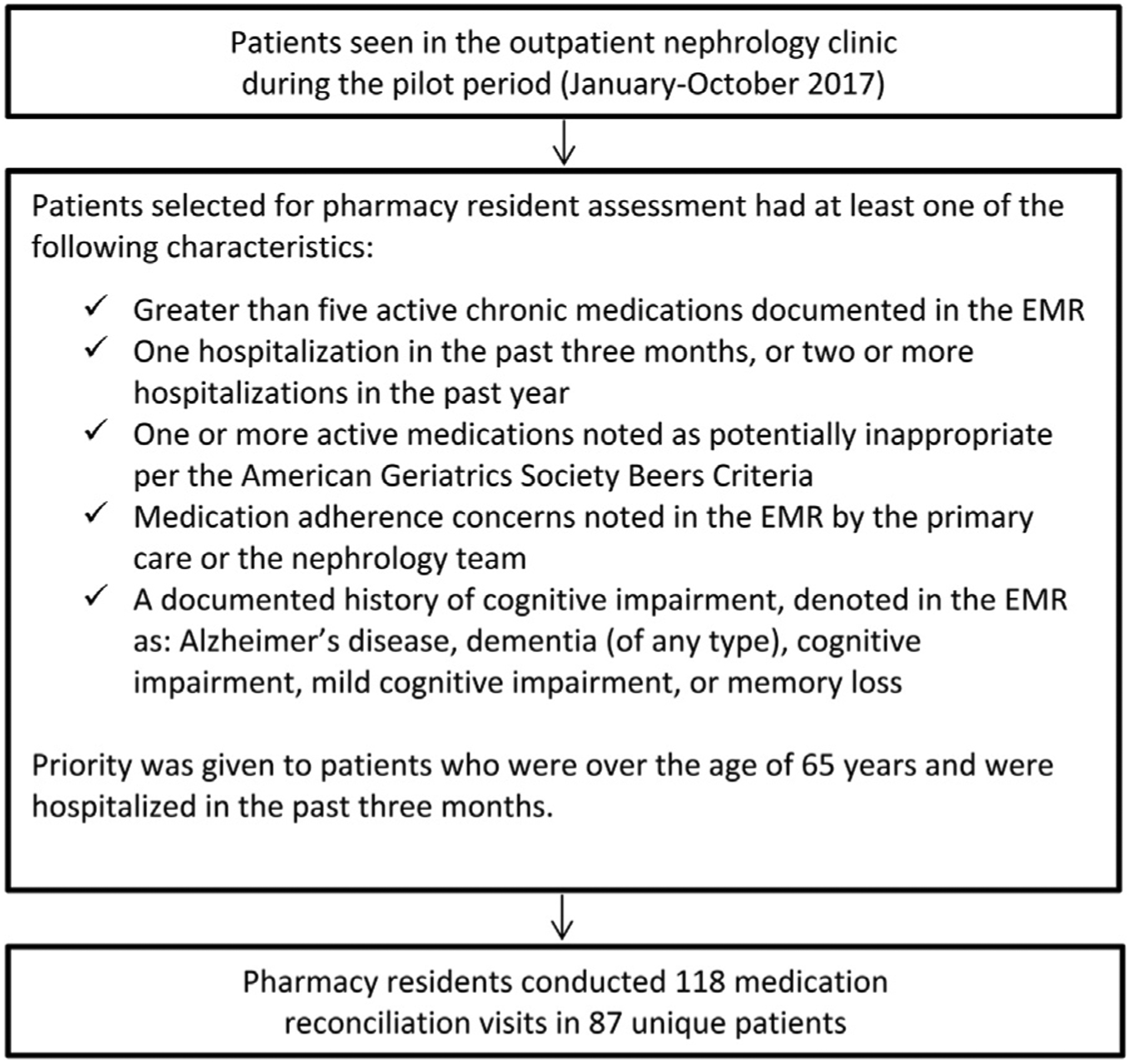
A diagram of the pharmacy resident’s method for identifying clinic patients for medication reconciliation. Each week, the pharmacy residents reviewed the charts of scheduled patients with the use of the electronic medical record (EMR), targeting patients with the characteristics described within the figure. Patients were also referred to the pharmacy residents by the interprofessional team as needed. Before or after their visit with the nephrology provider, targeted patients were offered a visit with a pharmacy resident to review their medications.
A pharmacy resident completed an in-person comprehensive medication review in a designated examination room within the clinic space, using best practices for medication reconciliation.49–51 The medication review was conducted before the nephrology clinic visit. During this time, patients would normally have been seated in the reception area waiting to be roomed. Instead, the pharmacy resident escorted patients to the examination room, completed the medication review, and communicated with the nephrology provider before the nephrology provider met with the patient. This did not extend the clinic visit and allowed the nephrology provider to obtain vital information before their nephrology assessment.
The pharmacy resident reconciled the clinical indication, dose, route, frequency, and timing of medication administration with the prescription orders in the system-wide medical record. Alternate sources for medication reconciliation were used when applicable or available (e.g., information from home nurse or caregiver, contacting non-VA pharmacies via telephone, patients’ own medication bottles or pillboxes). Discrepancies and drug therapy problems were identified using open-ended motivational interviewing, refill records, and pill count when available.49–51
Medication nonadherence was defined as a patient report of missing > 80% of medication doses in an average week. This was extrapolated from the Pharmacy Quality Alliance which recommends a proportion of days covered threshold of 80% to classify medication nonadherence.52 Because the pharmacy resident targeted many older adults with kidney dysfunction for assessment, the pharmacy resident assessed medication appropriateness according to patient age and renal function and completed an in-person side-effects screening related to the patient’s current medications.9,53–55 All data from the pharmacy residents’ assessment were recorded in a templated encounter note in the EMR. In addition to medication reconciliation, the pharmacy resident provided in-person patient education about medication instructions, indication, adverse effects, interactions between medications, and appropriate dosing with the use of IBM Micromedex during the visits as needed. Patients had the opportunity to ask questions during the visit.
After all members of the interprofessional team completed their patient encounter, the team gathered for an in-person huddle. During this discussion, findings and recommendations from all members of the team, including the pharmacy resident, were integrated to yield awritten comprehensive treatment plan which was discussed with the patient. The pharmacy residents provided the patient with adherence devices such as medication calendars (a tabular medication list organized by administration time of day, designed to make filling a pillbox easier), pillboxes, tablet splitters, and tablet crushers as needed. The pharmacy resident completed any necessary medication refills or renewals through the facility’s outpatient pharmacy. After the care plan was completed, the pharmacy resident ensured that the medication list in the EMR was reconciled to reflect the patient’s current medication regimen, including any changes made that day, and documented all pharmacist actions in their encounter note in the EMR (Figure 1).
In response to the pharmacy resident’s medication reconciliation, changes to any medications managed by the nephrology providers were made in real time. The pharmacy resident contacted primary care providers and other specialists through secure e-mail regarding any recommendations or notable findings from the medication reconciliation performed during the encounter. The pharmacy resident assisted providers in facilitating medication changes or referrals. At times, the pharmacy resident subsequently performed follow-up telephone calls to patients to assess changes, such as tapering of proton pump inhibitors, liberalizing diabetes or hypertensive regimens, titration of new medications, and monitoring of medication adverse effects, and documented these follow-up encounters in the EMR.
Results
During the pilot period, the pharmacy residents completed a total of 118 medication reconciliation visits for 87 unique patients. Seventeen patients were seen 2 to 4 times by the pharmacy residents, and 1 patient was seen 6 times (discussed below).
Patients seen by the pharmacy residents were most often white non-Latino (78%) men (97%) with a mean age of 73 years. The majority of these patients had advanced CKD, with 29% having CKD stage III, 48% stage IV, and 12% stage V. Patients seen by the pharmacy residents had a heavy comorbidity burden, including hypertension (85%), dyslipidemia (80%), diabetes mellitus type II (59%), and a history of clinical atherosclerotic cardiovascular disease56 (51%). Fourteen patients (16%) had a diagnosis of cognitive impairment, and 6 of those were newly diagnosed as a direct result of the interprofessional nephrology clinic visit. All but 1 of the 87 patients met the definition for polypharmacy (more than 5 active medications),57 and 87% of the patients had more than 10 medications at the time of the visit. On average, patients received outpatient prescriptions from 11 ± 6 unique prescribers over the past year, including postdischarge prescriptions from inpatient prescribers (Table 1).
Table 1.
Characteristics of patients seen by pharmacists (n = 87)
| Characteristic | n (%) |
|---|---|
| Age, y, mean ± SD | 73 ± 10 |
| Male | 84 (97) |
| Race | |
| White, non-Latino | 68 (78) |
| Black, non-Latino | 15 (17) |
| Other or not specified | 4 (5) |
| CKD stage by eGFR (mL/min/1.73m3) | |
| I, eGFR > 90 | 1 (1) |
| III, eGFR ≥30–59 | 25 (29) |
| IV, eGFR ≥15–29 | 42 (48) |
| Nondialysis V, eGFR < 15 | 10 (12) |
| Seen for non-CKD-related renal conditiona | 9 (10) |
| Comorbid conditionsb | |
| Hypertension | 74 (85) |
| Dyslipidemia | 70 (80) |
| Diabetes mellitus type II | 51 (59) |
| Clinical ASCVDc | 44 (51) |
| Obesity | 32 (37) |
| Depression | 21 (24) |
| Heart failure | 19 (22) |
| Atrial fibrillation | 16 (18) |
| Posttraumatic stress disorder | 15 (17) |
| Asthma or COPD | 15 (17) |
| Cognitive impairmentd | 14 (16) |
| Existing diagnosis, n | 8 |
| New diagnosis resulting from nephrology visit, n | 6 |
| Number of medications | |
| 4–10 | 12 (13) |
| 11–15 | 41 (47) |
| 16–20 | 17 (20) |
| > 20 | 17 (20) |
| Patients with medication adherence issues | 33 (38) |
| Number of prescribers in 1 year, mean ± SD | 11 ± 6 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimate glomerular filtration rate.
Included idiopathic membranous nephropathy (2), partial nephrogenic diabetes insipidus (1), poorly controlled hypertension (2), acute kidney injury (2), and proteinuria (2).
Identified with the use of patient “problem list” in the electronic medical record.
As defined in the 2018 AHA/ACC guideline.55
Consisted of mild cognitive impairment (n = 6), vascular dementia (3), dementia (2), memory loss (2), and cognitive impairment (1).
Pharmacy residents identified 344 medication discrepancies and 301 drug therapy problems. The most common discrepancies were classified as an omitted drug from the medication list (n = 86; 25%; e.g., over-the-counter medication omitted from medication list), continuation of a previously discontinued medication (n = 65; 19%; e.g., diuretic discontinued after previous hospitalization but patient continued taking it), and taking a medication differently from prescribed (n = 55; 16%; e.g., self-adjusting basal insulin doses based on blood glucose readings). The majority of the identified drug therapy problems stemmed from potentially inappropriate prescribing53–55 (n = 106; 35%; most often American Geriatrics Society Beers Criteria medications) leading to a potential or actual adverse drug reaction (n = 81; 27%). On average, the pharmacists identified 2.9 medication discrepancies and 2.6 drug therapy problems per visit (Table 2).
Table 2.
Pharmacist findings on medication reconciliation
| Characteristic | n (%) |
|---|---|
| Medication discrepancies | 344 |
| Omitted drug | 86 (25%) |
| Previously stopped medication | 65 (19%) |
| Patient not taking as prescribed | 55 (16%) |
| Incorrect or missing dose | 46 (13%) |
| Incorrect frequency | 38 (11%) |
| Additional drug | 34 (10%) |
| Duplicate therapy | 17 (5%) |
| Incorrect drug | 3 (1%) |
| Mean: 2.9 discrepancies per visit | |
| Drug therapy problems | 301 |
| Potentially inappropriate medication | 106 (35%) |
| Potential of actual adverse drug reaction | 81 (27%) |
| Adherence issue | 42 (14%) |
| Unnecessary drug therapy | 27 (9%) |
| Dose too high | 15 (5%) |
| Needs drug therapy | 12 (4%) |
| Dose too low | 9 (3%) |
| Needs different drug product | 9 (3%) |
| Mean: 2.6 drug therapy problems per visit | |
| Anticholinergic medicationsa identified | 46 |
| Inappropriately dosed medicationsb | 4 |
| Gabapentin | 2 |
| Allopurinol | 1 |
| Metformin | 1 |
| AGS Beers criteriac medications identified | 99 |
| Long-term use of PPIs in low-risk patientsd | 33 (34%) |
| Benzodiazepines | 12 (12%) |
| Opioids for chronic noncancer pain | 11 (11%) |
| Nonbenzodiazepine sedative hypnotics | 9 (9%) |
| Alpha-blockers for BP management | 8 (8%) |
| Diphenhydramine | 5 (5%) |
| Use of scheduled oral NSAIDs | 5 (5%) |
| Oxybutynin | 4 (4%) |
| Tricyclic antidepressants | 4 (4%) |
| Skeletal muscle relaxants | 3 (3%) |
| Non-DHP CCBs for BP management in HFrEF | 2 (2%) |
| Doxepin > 6 mg/d | 1 (1%) |
| Hydroxyzine | 1 (1%) |
| Paroxetine | 1 (1%) |
Abbreviations: AGS, American Geriatrics Society; BP, blood pressure; HFrEF, heart failure with a reduced ejection fraction; CCBs, calcium channel blockers; DHP, dihydropyridine; NSAIDs, nonsteroidal antiinflammatory drugs; PPIs, proton pump inhibitors.
Based on the Anticholinergic Risk Scale.53
Medications were inappropriately dosed based on package insert and patient’s renal function.
American Geriatrics Society 2015 Beers Criteria Update Expert Panel.54
Per the AGS Beers criteria, long-term use of PPIs is considered to be appropriate in certain high-risk patients. Patients noted here did not meet the criteria for high-risk patients and therefore, per the Beers criteria, their use of long-term PPIs was inappropriate.54
The pharmacy residents retrospectively identified 398 changes in the care process that resulted directly from their own interventions. The pharmacy residents collectively made 228 recommendations to optimize medication management, including evidence-based strategies for deprescribing.9,58 The nephrology providers implemented 135 medication changes (59% of recommendations) and primary care providers implemented 46 changes (20% of recommendations); 47 recommendations (21%) were not implemented, 25 of which were refused by the patient and 22 not implemented for unknown reasons. Pharmacy residents identified medication nonadherence in 42 (36%) of the visits and provided 76 medication adherence devices (56 pill calendars, 13 pillboxes, 6 tablet splitters, and 1 tablet crusher). The pharmacy residents facilitated 24 consultations or referrals, most frequently consultations for further geriatric or cognitive evaluation (n = 8) or home-based primary care (n = 9). Pharmacy residents communicated directly with the patient’s primary care team on 70 occasions (Figure 1).
For the particular patient who was seen 6 times by the pharmacy resident, multiple visits with all members of the interprofessional team led directly to a comprehensive geriatric assessment and cognitive evaluation by the embedded geriatrician, which yielded a dementia diagnosis, triggering the nephrology and primary care teams to provide the patient and caregiver with increased support through home-based primary care services, social work services, and pharmacy resident assistance at all nephrology visits.
In postintervention surveys and interviews, all members of the interprofessional team strongly agreed that patients and staff benefited from the pharmacy resident-provided care and medication reconciliation within the nephrology clinic. Quotes from the semistructured interviews highlighted the added value of the pharmacists:
“The patients love it. They come out of other doctors’ visits and complain that they are in and out in 15 minutes. I have had families and patients tell me that they really feel taken care of … they value the service and the extra time.”
— Nephrology provider
“The pharmacists are collaborative and make for an improved interdisciplinary team. Our patients are complex. Medication management is one of the main things that keeps our patients from heading toward dialysis, so having a pharmacist is really important.”
— Social worker
“[Patient name] was especially someone that I needed help with— he had so many medication changes, wasn’t able to do his meds on his own, and it was just impossible to keep track of everything. For these complicated patients, which unfortunately is a lot of our patients, having more heads looking [at them] is much better than just me, the doctor.”
— Nephrology trainee
Discussion
We have described the first model of embedding pharmacy residents in an outpatient clinic to conduct medication reconciliation for patients with nondialysis kidney disease. Targeted patients for pharmacy resident intervention in our pilot were older with advanced kidney disease and a high medication and comorbidity burden, highlighting the importance of vigilant medication review in this vulnerable population. Our findings also demonstrate the value of adding the pharmacist to an interprofessional nephrology team, contributing 398 changes in care process over a 9-month pilot period. Anonymous survey evaluation and semistructured interviews noted the value of the pharmacy resident and the interprofessional team to patients and providers.
In line with previous literature,1,8–11,13–16 all but 1 of the 87 patients exhibited polypharmacy and multiple prescribers.57,59,60 Our results were similar to a population-based cohort study that noted that the patients seen by a nephrologist had a high mean number of comorbidities (4.2, 95% CI 4.2–4.3) and a high mean number of prescribed medications (14.2, 95% CI 14.2–14.3).1 This further underscores the need for proactive pharmacist-led medication reconciliation in patients with nondialysis kidney disease at regular intervals to prevent adverse outcomes.10,13–16,19 We gave priority to older patients with a recent hospitalization, because patients seen by a nephrologist have significantly higher rates of death (6.6%, 95% CI 6.3%–6.9%) and placement in a long-term care facility (2.0%, 95% CI 1.8%–2.2%) compared with adults not seen by a nephrologist.1 We found that 38% of patients were non-adherent to their complex medication regimens, possibly related to difficulties managing complex medical conditions,2,3 cognitive decline,4 frailty,5 and functional decline.6,7 We did not assess if pharmacy resident intervention resulted in improved medication adherence, mortality, or affected nursing home placement. This is a target for future study.38–43
Pharmacist interventions led to similar to discrepancy detection rates in other studies; however, those studies lacked description of pharmacist interventions related to managing polypharmacy and deprescribing in the population of the present model.38–40 When inappropriate medications were identified, pharmacy residents used CKD-specific deprescribing techniques,8,9,58 although further research is needed to help reduce the medication burden in older patients with nondialysis kidney disease. Pharmacy resident intervention resulted in 328 changes in care process; although published literature describes the high detection rate of drug-related problems in this population, they fail to describe the pharmacist’s role in the resolution of these problems, especially related to communication and coordination of care.38–40 Future directions for this model may include targeted pharmacist medication reconciliation after discharge for enrollees in the outpatient nephrology clinic.61,62
There are potential limitations to our model, which was conducted in a VA facility, an integrated health care system with a unique funding structure and shared medical record. The use of pharmacy residents eliminated the need for assessment of the number of full-time-equivalent pharmacists needed per panel of kidney disease patients, which has not yet been defined.10 Nonresident clinical pharmacists did not provide direct patient care in this model. This was intentional, because the pilot model described here could lead to the justification for funds to hire a full-time nonresident clinical pharmacist to conduct medication reconciliation, deprescribing, and associated follow-up. A cost-benefit analysis is underway to translate pharmacy resident interventions to cost-avoidance and cost savings; this analysis will provide the basis for advocating for funds to compensate 1 clinical pharmacist for the 4 hours/week clinic time slot. Future directions will include scalability of that clinical pharmacist to all 4 weekly clinic sessions depending on cost-benefit analysis. An alternate approach may be the use of specially trained pharmacy technicians for medication review under the supervision of 1 pharmacist. In the private sector, pharmacists collect reimbursement for medication reconciliation and medication therapy management services in the ambulatory care setting for patients with high drug costs and chronic conditions such as CKD.10,63–66 However, changes to the Medicare Part D system may preclude dialysis patients from qualifying for medication therapy management services in the future based on cost of medications alone.10
The pharmacy residents also targeted older high-risk patients with kidney disease, which may have led to more discrepant medication reviews and medication management challenges. This pilot study lacked a control group; although members of the interprofessional team valued the pharmacist-led medication reconciliation in semistructured interviews, it is possible that members of the nephrology team may have identified these medication errors during their own respective visits. We did not report on patient-centered outcomes, such as health-related quality of life, that have been negatively associated with medication burden.11,14 Retrospective review for changes in care process was completed through chart review, which may not have shown unsuccessful attempts to taper or change medications. Although pharmacy residents communicated with providers frequently, they did not contact providers to understand why pharmacy resident recommendations were not implemented.
Conclusion
The addition of pharmacy residents to an interprofessional nephrology clinic model led to the detection and resolution of hundreds of medication-related problems. The collaboration of the interprofessional team yielded changes in the care process and is a valuable model for caring for older adults with nondialysis kidney disease. Pending cost-benefit analysis, resources may be allocated to allow for clinical pharmacists to be embedded within interprofessional care teams to further improve how we care for older adults in subspecialty settings and to allow for further study of outcomes related to the financial and clinical value of pharmacists in these settings.
Key Points.
Background:
Pharmacy residents were embedded in an interprofessional nephrology clinic to conduct medication reconciliation for targeted older high-risk patients with nondialysis kidney disease and complex medical conditions.
Findings:
Through 118 medication reconciliation visits with 87 unique patients, pharmacy residents identified 344 medication discrepancies and 301 drug therapy problems (~4 per patient), resulting in 398 changes in care process.
All members of the interprofessional team strongly agreed that patients and staff benefited from the pharmacy resident involvement within the nephrology clinic.
Acknowledgments
The authors acknowledge the VA Boston interprofessional nephrology team, including the geriatrician Laura P. Perry, for their clinical support of this intervention and their commitment to improving care for older adults with kidney disease. The authors thank Michael Takach and Riza Usta for their clinical support as PGY-1 pharmacy residents within this intervention and Emily Hillel and Catherine Smith for their assistance in data collection.
Funding:
This was an unfunded quality improvement initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the U.S. government.
Biography
Chelsea E. Hawley, PharmD, Advanced Fellow in Geriatrics, New England Geriatric Research, Education, and Clinical Center, and Clinical Pharmacist, Department of Pharmacy, VA Boston Healthcare System, Boston, MA
Laura K. Triantafylidis, PharmD, Geriatric Pharmacist and Clinical Pharmacy Specialist, Department of Pharmacy, VA Boston Healthcare System, Boston, MA
Julie M. Paik, MD, ScD, MPH, Investigator, New England Geriatric Research, Education, and Clinical Center, and Attending Nephrologist, Renal Section, VA Boston Healthcare System; Associate Epidemiologist, Department of Pharmacoepidemiology and Pharmacoeconomics, and Attending Nephrologist, Renal Division, Brigham and Women’s Hospital; and Attending Nephrologist, Department of Medicine, Harvard Medical School, Boston, MA
Footnotes
Disclosure: The authors declare no relevant conflicts of interest or financial relationships.
Previous presentations: Preliminary results of this model were presented as an abstract at the 2017 American Society for Health-System Pharmacists Midyear Clinical Meeting and the 2018 American Geriatrics Society Annual Scientific Meeting in Orlando, Florida.
Contributor Information
Chelsea E. Hawley, New England Geriatric Research, Education, and Clinical Center,; Department of Pharmacy, VA Boston Healthcare System, Boston, MA
Laura K. Triantafylidis, Department of Pharmacy, VA Boston Healthcare System, Boston, MA
Julie M. Paik, New England Geriatric Research, Education, and Clinical Center, Renal Section, VA Boston Healthcare System; Brigham and Women’s Hospital; Department of Medicine, Harvard Medical School, Boston, MA
References
- 1.Tonelli M, Wiebe N, Manns BJ, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1:e184852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisberg LS. The patient-centered medical home and the nephrologist. Adv Chronic Kidney Dis. 2011;18:450–455. [DOI] [PubMed] [Google Scholar]
- 3.Rosner M, Abdel-Rahman E, Williams ME. American Society of Nephrology Advisory Group on Geriatric Nephrology. Geriatric nephrology: responding to a growing challenge. Clin J Am Soc Nephrol. 2010;5:936–942. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee G, Karia S, Varley J, Brown EA. Cognitive impairment in elderly renal inpatients: an under-identified phenomenon. Nephron Clin Pract. 2014;126:19–23. [DOI] [PubMed] [Google Scholar]
- 5.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triantafylidis LK, Hawley CE, Perry LP, Paik JM. The role of deprescribing in older adults with chronic kidney disease. Drugs Aging. 2018;35(11): 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker CF, Miklich MA, Patel RS, Fink JC. Medication safety principles and practice in CKD. Clin J Am Soc Nephrol. 2018;13(11):1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Peter WL, Wazny LD, Patel UD. New models of chronic kidney disease care including pharmacists: improving medication reconciliation and medication management. Curr Opin Nephrol Hypertens. 2013;22:656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe JH, McInnis T, Hirsch JD. Cost of prescription drug-related morbidity and mortality. Ann Pharmacother. 2018;52:829–837. [DOI] [PubMed] [Google Scholar]
- 13.Mason NA. Polypharmacy and medication-related complications in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20: 492–497. [DOI] [PubMed] [Google Scholar]
- 14.Stemer G, Lemmens-Gruber R. The clinical pharmacist’s contributions within the multidisciplinary patient care team of an intern nephrology ward. Int J Clin Pharm. 2011;33:759–762. [DOI] [PubMed] [Google Scholar]
- 15.Manley HJ, Cannella CA, Bailie GR, St Peter WL. Medication-related problems in ambulatory hemodialysis patients: a pooled analysis. Am J Kidney Dis. 2005;46:669–680. [DOI] [PubMed] [Google Scholar]
- 16.Mirkov S Implementation of a pharmacist medication review clinic for haemodialysis patients. N Z Med J. 2009;122:25–37. [PubMed] [Google Scholar]
- 17.Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc (2003). 2008;48:203–214. [DOI] [PubMed] [Google Scholar]
- 18.Moore JM, Shartle D, Faudskar L, Matlin OS, Brennan TA. Impact of a patient-centered pharmacy program and intervention in a high-risk group. J Manag Care Pharm. 2013;19:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 20.Hug BL, Witkowski DJ, Sox CM, et al. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009;76:1192–1198. [DOI] [PubMed] [Google Scholar]
- 21.Pai AB, Boyd A, Chavez A, Manley HJ. Health-related quality of life is maintained in hemodialysis patients receiving pharmaceutical care: a 2-year randomized, controlled study. Hemodial Int. 2009;13:72–79. [DOI] [PubMed] [Google Scholar]
- 22.Ledger S, Choma G. Medication reconciliation in hemodialysis patients. CANNT J. 2008;18:41–43. [PubMed] [Google Scholar]
- 23.Pai AB, Boyd A, Depczynski J, Chavez IM, Khan N, Manley H. Reduced drug use and hospitalization rates in patients undergoing hemodialysis who received pharmaceutical care: a 2-year, randomized, controlled study. Pharmacotherapy. 2009;29:1433–1440. [DOI] [PubMed] [Google Scholar]
- 24.McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70:611–618. [DOI] [PubMed] [Google Scholar]
- 25.Patricia NJ, Foote EF. A pharmacy-based medication reconciliation and review program in hemodialysis patients: a prospective study. Pharm Pract (Granada). 2016;14:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Peter WL. Improving medication safety in chronic kidney disease patients on dialysis through medication reconciliation. Adv Chronic Kidney Dis. 2010;17:413–419. [DOI] [PubMed] [Google Scholar]
- 27.Qudah B, Albsoul-Younes A, Alawa E, Mehyar N. Role of clinical pharmacist in the management of blood pressure in dialysis patients. Int J Clin Pharm. 2016;38:931–940. [DOI] [PubMed] [Google Scholar]
- 28.Migliozzi DR, Zullo AR, Collins C, Elsaid KA. Achieving blood pressure control among renal transplant recipients by integrating electronic health technology and clinical pharmacy services. Am J Health Syst Pharm. 2015;72:1987–1992. [DOI] [PubMed] [Google Scholar]
- 29.Chisholm MA, Mulloy LL, Jagadeesan M, DiPiro JT. Impact of clinical pharmacy services on renal transplant patients’ compliance with immunosuppressive medications. Clin Transplant. 2001;15:330–336. [DOI] [PubMed] [Google Scholar]
- 30.Chisholm MA, Mulloy LL, Jagadeesan M, Martin BC, DiPiro JT. Effect of clinical pharmacy services on the blood pressure of African-American renal transplant patients. Ethn Dis. 2002;12:392–397. [PubMed] [Google Scholar]
- 31.Wang HY, Chan AL, Chen MT, Liao CH, Tian YF. Effects of pharmaceutical care intervention by clinical pharmacists in renal transplant clinics. Transplant Proc. 2008;40:2319–2323. [DOI] [PubMed] [Google Scholar]
- 32.Aberger EW, Migliozzi D, Follick MJ, Malick T, Ahern DK. Enhancing patient engagement and blood pressure management for renal transplant recipients via home electronic monitoring and web-enabled collaborative care. Telemed J E Health. 2014;20:850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldonado AQ, Weeks DL, Bitterman AN, et al. Changing transplant recipient education and inpatient transplant pharmacy practices: a single-center perspective. Am J Health Syst Pharm. 2013;70:900–904. [DOI] [PubMed] [Google Scholar]
- 34.Salgado TM, Moles R, Benrimoj SI, Fernandez-Llimos F. Pharmacists’ interventions in the management of patients with chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2012;27:276–292. [DOI] [PubMed] [Google Scholar]
- 35.Allenet B, Chen C, Romanet T, Vialtel P, Calop J. Assessing a pharmacist-run anaemia educational programme for patients with chronic renal insufficiency. Pharm World Sci. 2007;29:7–11. [DOI] [PubMed] [Google Scholar]
- 36.Bucaloiu ID, Akers G, Bermudez MC, et al. Outpatient erythropoietin administered through a protocol-driven, pharmacist-managed program may produce significant patient and economic benefits. Manag Care Interface. 2007;20:26–30. [PubMed] [Google Scholar]
- 37.Joy MS, Candiani C, Vaillancourt BA, Chin H, Hogan SL, Falk RJ. Reengineering clinical operations in a medical practice to optimize the management of anemia of chronic kidney disease. Pharmacotherapy. 2007;27:734–744. [DOI] [PubMed] [Google Scholar]
- 38.Belaiche S, Romanet T, Allenet B, Calop J, Zaoui P. Identification of drug-related problems in ambulatory chronic kidney disease patients: a 6-month prospective study. J Nephrol. 2012;25:782–788. [DOI] [PubMed] [Google Scholar]
- 39.Belaiche S, Romanet T, Bell R, Calop J, Allenet B, Zaoui P. Pharmaceutical care in chronic kidney disease: experience at Grenoble University Hospital from 2006 to 2010. J Nephrol. 2012;25:558–565. [DOI] [PubMed] [Google Scholar]
- 40.Patel HR, Pruchnicki MC, Hall LE. Assessment for chronic kidney disease service in high-risk patients at community health clinics. Ann Pharmacother. 2005;39:22–27. [DOI] [PubMed] [Google Scholar]
- 41.Bayliss EA, Bhardwaja B, Ross C, Beck A, Lanese DM. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol. 2011;6:704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooney D, Moon H, Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrol. 2015;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang AR, Evans M, Yule C, et al. Using pharmacists to improve risk stratification and management of stage 3A chronic kidney disease: a feasibility study. BMC Nephrol. 2016;17:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishani A, Christopher J, Palmer D, et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68:41–49. [DOI] [PubMed] [Google Scholar]
- 45.Hall RK, Haines C, Gorbatkin SM, et al. Incorporating geriatric assessment into a nephrology clinic: preliminary data from two models of care. J Am Geriatr Soc. 2016;64:2154–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson CG, Park I, Sutherland SE, Ray L. Assessing pharmacist-led annual wellness visits: interventions made and patient and physician satisfaction. J Am Pharm Assoc (2003). 2015;55:449–454. [DOI] [PubMed] [Google Scholar]
- 47.Collins C, Kramer A, O’Day ME, Low MB. Evaluation of patient and provider satisfaction with a pharmacist-managed lipid clinic in a Veterans Affairs medical center. Am J Health Syst Pharm. 2006;63: 1723–1727. [DOI] [PubMed] [Google Scholar]
- 48.Drummond KL, Painter JT, Curran GM, et al. HIV patient and provider feedback on a telehealth collaborative care for depression intervention. AIDS Care. 2017;29:290–298. [DOI] [PubMed] [Google Scholar]
- 49.Mueller SK, Kripalani S, Stein J, et al. A toolkit to disseminate best practices in inpatient medication reconciliation: multi-center medication reconciliation quality improvement study (MARQUIS). Jt Comm J Qual Patient Saf. 2013;39:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salanitro AH, Kripalani S, Resnic J, et al. Rationale and design of the Multicenter Medication Reconciliation Quality Improvement Study (MARQUIS). BMC Health Serv Res. 2013;13:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cipolle RJ, Strand LM, Morley PC. Drug therapy problems. Chapter 5. In: Cipolle RJ, Strand LM, Morley PC, eds. Pharmaceutical Care Practice: The Patient-Centered Approach to Medication Management Services. 3rd ed. New York: McGraw-Hill; 2012. [Google Scholar]
- 52.Pharmacy Quality Alliance. PQA adherence measures. 2018. Available at: https://www.pqaalliance.org/adherence-measures. Accessed April 19, 2019.
- 53.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045–1051. [DOI] [PubMed] [Google Scholar]
- 54.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508–513. [DOI] [PubMed] [Google Scholar]
- 55.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 56.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. [e-pub ahead of print]. 10.1016/j.jacc.2018.11.003. [DOI] [Google Scholar]
- 57.Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65: 989–995. [DOI] [PubMed] [Google Scholar]
- 58.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175: 827–834. [DOI] [PubMed] [Google Scholar]
- 59.Koronkowski MJ, Semla TP, Schmader KE, Hanlon JT. Recent literature update on medication risk in older adults, 2015–2016. J Am Geriatr Soc. 2017;65:1401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang F, O’Hare AM, Miao Y, Steinman MA. Use of renally inappropriate medications in older veterans: a national study. J Am Geriatr Soc. 2015;63:2290–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musgrave CR, Pilch NA, Taber DJ, et al. Improving transplant patient safety through pharmacist discharge medication reconciliation. Am J Transplant. 2013;13:796–801. [DOI] [PubMed] [Google Scholar]
- 62.Sebaaly J, Parsons LB, Pilch NA, Bullington W, Hayes GL, Easterling H. Clinical and financial impact of pharmacist involvement in discharge medication reconciliation at an academic medical center: a prospective pilot study. Hosp Pharm. 2015;50:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatah E, Braund R, Tordoff J, Duffull SB. A systematic review and meta-analysis of pharmacist-led fee-for-services medication review. Br J Clin Pharm. 2014;77:102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenz TL, Monaghan MS. Pay-for-performance model of medication therapy management in pharmacy practice. J Am Pharm Assoc (2003). 2011;51:425–431. [DOI] [PubMed] [Google Scholar]
- 65.Lloyd KB, Evans RL. Reimbursement model for pharmacist-directed medication therapy management. J Am Pharm Assoc (2003). 2012;52: 161–169. [DOI] [PubMed] [Google Scholar]
- 66.Reinke T Medication therapy management program in N.C. saves $13 million. Manag Care. 2011;20:17–18. [PubMed] [Google Scholar]


