Abstract
Older adults with chronic kidney disease (CKD) often experience polypharmacy, a recognized predictor of prescribing problems including inappropriately dosed medications, drug–drug and drug–disease interactions, morbidity and mortality. Polypharmacy is also associated with nonadherence, which leads to recurrent hospitalizations and poorer hemodialysis outcomes in CKD patients. Further complicating medication management in this vulnerable population are the physiologic changes that occur with both age and CKD. This guide for pharmacists and prescribers offers considerations in medication evaluation and management among older adults with CKD. Careful prescribing with the aid of tools such as the American Geriatrics Society Beers Criteria can support safe medication use and appropriate prescribing. Polypharmacy may be systematically addressed through ‘deprescribing,’ an evidence-based process that enables identification and elimination of unnecessary or inappropriate medications. Detailed guidance for deprescribing in older adults with CKD has not been published previously. We highlight three specific targets for medication optimization and deprescribing in older adults with CKD: (1) proton pump inhibitors, (2) oral hypoglycemic agents, including newer classes of agents, and (3) statins. These medication classes have been chosen as they represent three of the most commonly prescribed classes of medications in the United States. For each area, we review considerations for medication use in older adults with CKD and provide strategies to avoid, modify, or discontinue these medications when clinically indicated. By utilizing deprescribing techniques, pharmacists are well positioned to help decrease the medication burden in older adults with CKD, thereby potentially reducing the risk of morbidity and mortality associated with polypharmacy.
1. Introduction
Older adults with chronic kidney disease (CKD) are highly vulnerable to polypharmacy. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines define CKD as an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 for greater than 3 months [1]. In older adults, a common criticism is that CKD is ‘overdiagnosed’ given the use of a single absolute threshold [2]. Older adults with CKD often have complex comorbidities, have multiple prescribers, and are underrepresented in the literature, resulting in medication management challenges in this population [3]. By the time CKD patients progress to end-stage renal disease and hemodialysis, they frequently experience polypharmacy, commonly defined as the use of five or more medications, the prevalence of which is increasing in older adults in the United States (US) (from 31% in 2005 to 36% in 2011) [4, 5]. In a single cross-sectional analysis performed in the US in 2009, a quarter of hemodialysis-dependent patients were on 25 or more daily medications [6]. The number of medications that a patient takes is a recognized predictor of prescribing problems including inappropriately dosed medications, drug–drug interactions and drug–disease interactions [7]. Medication-related factors including pill burden, medication regimen complexity, and number of phosphate binders prescribed have been associated with poorer adherence in dialysis patients [8].
The purpose of this review is to discuss the challenges to medication management in older adults with CKD. We aim to propose a framework for safe medication management in this vulnerable population. First, we review physiologic changes in older adults with CKD that make some medications potentially dangerous in the context of the aging kidney. Second, we propose deprescribing, the process of eliminating or reducing unnecessary and/or inappropriate medications [9, 10], as a systematic solution to the polypharmacy problem. And finally, given the lack of deprescribing guidance for older adults with CKD, we highlight three specific targets for medication optimization and deprescribing in this particular population: (1) proton pump inhibitors (PPIs), (2) oral hypoglycemic agents, including newer classes of hypoglycemic agents, and (3) statins. For each area, we review considerations for medication use in older adults with CKD and provide strategies to avoid, modify, or discontinue these medications when clinically indicated, thus adding specific and concrete CKD examples to more generalized deprescribing approaches [9, 10]. These are three of the most commonly prescribed classes of medications for older adults in the US, but may be potentially unsafe or unnecessary in older adults with CKD [5, 11–13].
2. The Effect of the Aging Kidney on Medication Management
Both aging and CKD result in physiologic changes that affect medication pharmacokinetics (PK) and pharmacodynamics (PD) [13–15]. The kidney plays a significant role in drug PK, resulting in alterations in absorption, distribution, metabolism and elimination in older patients with CKD. With increased age and reduced renal function, there is an increase in gastric pH. As a result, medications that require an acidic environment for absorption are less readily absorbed. Albumin levels affect drug distribution, and both aging and impaired kidney function are associated with lower albumin levels. Medications that are highly protein-bound will therefore yield more drug freely available to have an effect. Additionally, reduced activity of the cytochrome P450 enzymes with age and impaired kidney function causes decreased drug metabolism. Most notably, with older age and impaired kidney function, medication clearance is reduced overall, which may lead to drug accumulation and increased risk for adverse effects [13–15]. Moreover, calculations to evaluate kidney function in frail older adults are less reliable and may overestimate clearance due to reduced muscle mass and thus a falsely low serum creatinine [16]. Strategies have been employed including rounding serum creatinine to 1.0 mg/dL; however, it has been demonstrated that this is not appropriate and should be avoided [16]. These challenges have led to dose calculation errors for many drugs, particularly for individuals with severe renal impairment [16].
PD properties also change with age, affecting the number, affinity and sensitivity of receptors in the body [14]. As a result, older adults may be more susceptible to age-induced orthostatic hypotension with antihypertensive medications and have a diminished response to beta-blockers related to changes in the receptor sites [14]. Older adults also may experience increased central nervous system adverse effects (i.e., dizziness, confusion) from benzodiazepines related to greater permeability of the blood–brain barrier and a more lipophilic body composition [14]. All of these factors contribute to the complexity of medication use in this vulnerable population. It is important to consider PK and PD changes in older adults with CKD to assess the appropriateness and safety of medications.
3. Potentially Inappropriate Medications for the Aging Kidney
As the number of medications a patient takes increases, the number of potential adverse outcomes increases concurrently [17]. In a national study of older veterans conducted in the US, 45% of patients with kidney impairment received one or more drugs that were contraindicated or prescribed at an excessive dose [18]; a study examining their non-veteran counterparts in the community revealed that one-third of medications prescribed for older adults with renal disease were inappropriate [9]. The strongest predictor of renally inappropriate prescribing was the number of medications used, with more than five times the risk among individuals taking ten or more medications compared with those taking one to three medications [18].
The American Geriatrics Society (AGS) Beers Criteria consist of potentially inappropriate medications in older adults and serve as a guideline for healthcare professionals to improve the safety of medication prescribing [19]. While the AGS Beers Criteria include various high-risk medications (i.e., opioids, antipsychotics, and benzodiazepines), they also provide a section on drugs that should be avoided or dose-adjusted in individuals with kidney impairment [19, 20]. Commonly prescribed medications on this list include anticoagulants, central nervous system agents, gastrointestinal agents and hyperuricemia agents (see Table 1). Regular review of the medication lists of older adults with CKD is of utmost importance to allow for identification of inappropriate medications based on age and level of kidney impairment.
Table 1.
Dosing recommendations in kidney impairment adapted from the 2015 AGS Beers Criteria [19]
| Medications | CrCl (mL/min) | Recommendationa, rationale |
|---|---|---|
| Anticoagulants | ||
| Apixaban | < 25 | Avoid, increased bleeding risk |
| Edoxaban | < 30 or > 95b | |
| Rivaroxaban | < 30 | |
| Dabigatran | ||
| Fondaparinux | ||
| Enoxaparinc | ||
| Diuretics | ||
| Amiloride | < 30 | Avoid, risk for increased potassium and/or decreased sodium |
| Spironolactone | ||
| Triamterene | ||
| Central nervous system | ||
| Duloxetine | < 30 | Avoid, increased gastrointestinal adverse effects |
| Levetiracetam | < 80 | Reduce dose, central nervous system adverse effects |
| Gabapentin | < 60 | |
| Pregabalin | ||
| Tramadol | < 30 | Reduce dose (maximum 200 mg/day), central nervous system adverse effects (avoid ER formulation) |
| Gastrointestinal | ||
| Cimetidine | < 50 | Reduce dose, risk of delirium |
| Famotidine | ||
| Nizatidine | ||
| Ranitidine | ||
| Hyperuricemia | ||
| Colchicine | < 30 | Reduce dose, increased gastrointestinal, neuromuscular or bone marrow toxicity |
| Probenecid | Avoid, loss of effectiveness |
CrCl creatinine clearance, ER extended release, FDA Food and Drug Administration
Beers Criteria dosing recommendations may differ from FDA labeled dosing
Increased risk of clotting with edoxaban use in patients with CrCl > 95 mL/min
50% dose reduction required with use of enoxaparin in patients with CrCl < 30 mL/min; use of unfractionated heparin is preferred in patients with kidney impairment
4. Deprescribing: A Systematic Solution
The concept of ‘deprescribing’ involves eliminating unnecessary and/or inappropriate medications [10, 17, 21] and is associated with improved patient satisfaction, decreased cost, and decreased healthcare utilization, without the risk of adverse events [17, 21]. Deprescribing may be performed via a systematic process [10], which enables the identification and mitigation of inappropriate prescribing. A sample deprescribing protocol suggests the following five steps: (1) reconcile all medications according to indication; (2) assess the appropriateness of each medication considering the risks and benefits of use; (3) assess each medication for eligibility to be discontinued; (4) prioritize medications for discontinuation; and (5) implement and monitor medication discontinuation [10]. Criteria for discontinuation include lack of a valid indication, result of the prescribing cascade (a medication prescribed to treat an adverse drug effect), potential cause of harm, failure to control disease/symptom or disease/symptom has resolved, preventative medication unlikely to confer benefit over patient’s remaining lifespan or unacceptable treatment burden [10]. All patients should be evaluated on an individual basis with careful consideration of their complete medication regimen, comorbid disease states, disease-state goals and patient-specific goals. Emphasis should be placed on patient involvement within this process as successful deprescribing requires patient buy-in and clear communication between healthcare professionals and patients/caregivers [10].
There is robust literature to support deprescribing in older adults. Efforts have demonstrated that the use of specific deprescribing tools and/or algorithms allows for a reduction in polypharmacy by decreasing medication burden [22–24]. Additionally, the use of deprescribing has been associated with decreased mortality, fewer referrals to nursing homes, reduced costs, and improvements in patients’ perception of their global health [10, 22–24]. No increased risks have been associated with deprescribing [10, 22–24].
5. Targets for Deprescribing in Older Adults with CKD
Published approaches to deprescribing in the pre-dialysis CKD population are lacking [12, 21, 25]. While one generalized approach has been published, this example lacks specificity and concrete examples of how to deprescribe targeted medications in older adults with CKD [12]. In light of the paucity of literature on the topic, here we review specific deprescribing considerations in the evaluation and management of polypharmacy among older adults with CKD. We focus on PPIs, oral hypoglycemic agents, and statins. For each medication category, we review considerations for medication use in older adults with CKD and provide strategies to avoid, modify, or discontinue these medications when clinically indicated, thus adding specific and concrete CKD examples to more generalized deprescribing approaches [9, 10]. PPIs, oral hypoglycemic agents, and statins are three of the most commonly prescribed classes of medications for older adults in the US [5, 11] and have been cited by several recent publications as potentially unsafe agents or targets for deprescribing in older adults with CKD [11–13].
5.1. Proton Pump Inhibitors
PPIs are among the most commonly prescribed medications in the US, with a prevalence of 18.5% among community-dwelling older adults in 2010–2011 [5]. However, their indication for use is not always clear. In hemodialysis patients taking PPIs, more than a quarter of the time the indication was unclear or unknown [21]. While the most commonly reported side effects associated with PPI use (i.e., diarrhea, abdominal pain, constipation and headache) are generally minor, significant negative consequences can arise from long-term use. The AGS Beers Criteria recognize PPIs as potentially inappropriate medications and suggest avoidance of use beyond 8 weeks without justification, because of their potential associations with progression of kidney disease, bone fracture, small intestinal bacterial overgrowth, Clostridium difficile infection, pneumonia, micronutrient deficiencies, and gastrointestinal malignancies [19, 26]. Of particular concern to patients with CKD is the potentially negative effect of PPIs on kidney function. Among 2.6 million subjects, PPI users experienced a significantly higher risk of acute kidney injury [risk ratio (RR) 1.44; 95% confidence interval (CI) 1.09–1.91] and CKD (RR 1.36; 95% CI 1.07–1.71) compared with non-PPI users [27].
Given the potential risks associated with long-term PPI use, we recommend: (1) reviewing the indication for PPI use, (2) weighing the risks versus benefits of continued use in those with a valid indication, and (3) considering deprescribing if clinically indicated. Clinical judgment must be used to appropriately determine who is eligible for deprescribing. For example, in a 72-year-old patient with a history of a non-steroidal anti-inflammatory drug (NSAID)-induced ulcer who is no longer taking an NSAID and has no other risk factors, deprescribing the PPI would be considered appropriate. Alternatively, in a 72-year-old patient with Barrett’s esophagus on long-term anticoagulant therapy and chronic steroids, the gastrointestinal protective benefits of PPI use likely outweigh the risks.
Once deprescribing eligibility is established, a deprescribing strategy that tapers PPI use is recommended [28] since abrupt PPI discontinuation could potentially result in rebound symptoms of acid hypersecretion until the normal balance between acid and gastrin production is restored [29]. The majority of the tapering strategies support a reduction of the PPI maintenance dose by 50% in 1- to 2-week intervals. For example, for a patient initially on omeprazole 40 mg twice daily, reduce to 20 mg twice daily for 1–2 weeks, then reduce to 20 mg once daily for 1–2 weeks followed by 20 mg every other day for 1–2 weeks, then stop altogether. Close monitoring for acid-related symptoms is required while tapering. Non-pharmacologic symptom management methods, including dietary and lifestyle modifications, are also highly encouraged (i.e., eating smaller meals, elevating the head of the bed, avoiding lying down after eating, avoiding alcoholic/caffeinated beverages and fried/fatty foods). In addition, calcium-based antacids may be used on an as-needed basis for intermittent acid-related symptoms. If acid-related symptoms persist, the provider and patient must decide on a threshold for restarting PPI therapy based on the risks and benefits of use.
5.2. Oral Hypoglycemic Agents
An estimated 40% of patients with diabetes will develop CKD; however, adequate management of hyperglycemia can delay CKD progression, especially when managed prior to the onset of diabetic kidney damage [12, 30]. The KDIGO and the American Diabetes Association (ADA) guidelines support relaxed hemoglobin A1c (HbA1c) goals (less than 8–8.5%) for most patients with CKD, due to their risk of hypoglycemia and the likelihood of significant microvascular disease burden [1, 31]. The AGS recommends higher HbA1c targets (8–9%) for older adults with comorbid conditions, poor health, and a limited life expectancy, due to evidence that tighter control yields minimal cardiovascular benefit and a greater risk of hypoglycemia [32–34].
Many oral hypoglycemic agents pose a risk for hypoglycemia and related consequences (i.e., falls). Additionally, there are renal dosing restrictions or contraindications to consider (see Fig. 1). Older adults are disproportionately vulnerable to hypoglycemia and its associated complications [35–37]. Likewise, intensive glucose lowering is associated with significantly higher rates of hypoglycemia in patients with CKD compared with the general population [38, 39]. Metformin, a mainstay in diabetes management, has a low risk of hypoglycemia, but its use is at times precluded because of renal dosing restrictions. It is not recommended to be initiated if the eGFR drops below 45 mL/min/1.73 m2, is contraindicated when the eGFR is < 30 mL/min/1.73 m2, and may require dose reduction for eGFR levels in between [9, 40]. Newer hypoglycemic agents may present a safe and effective alternative for diabetes management, especially when renal function, hypoglycemic risk, or comorbidities may not allow for conventional agents (see Table 2) [30, 38, 41].
Fig. 1.
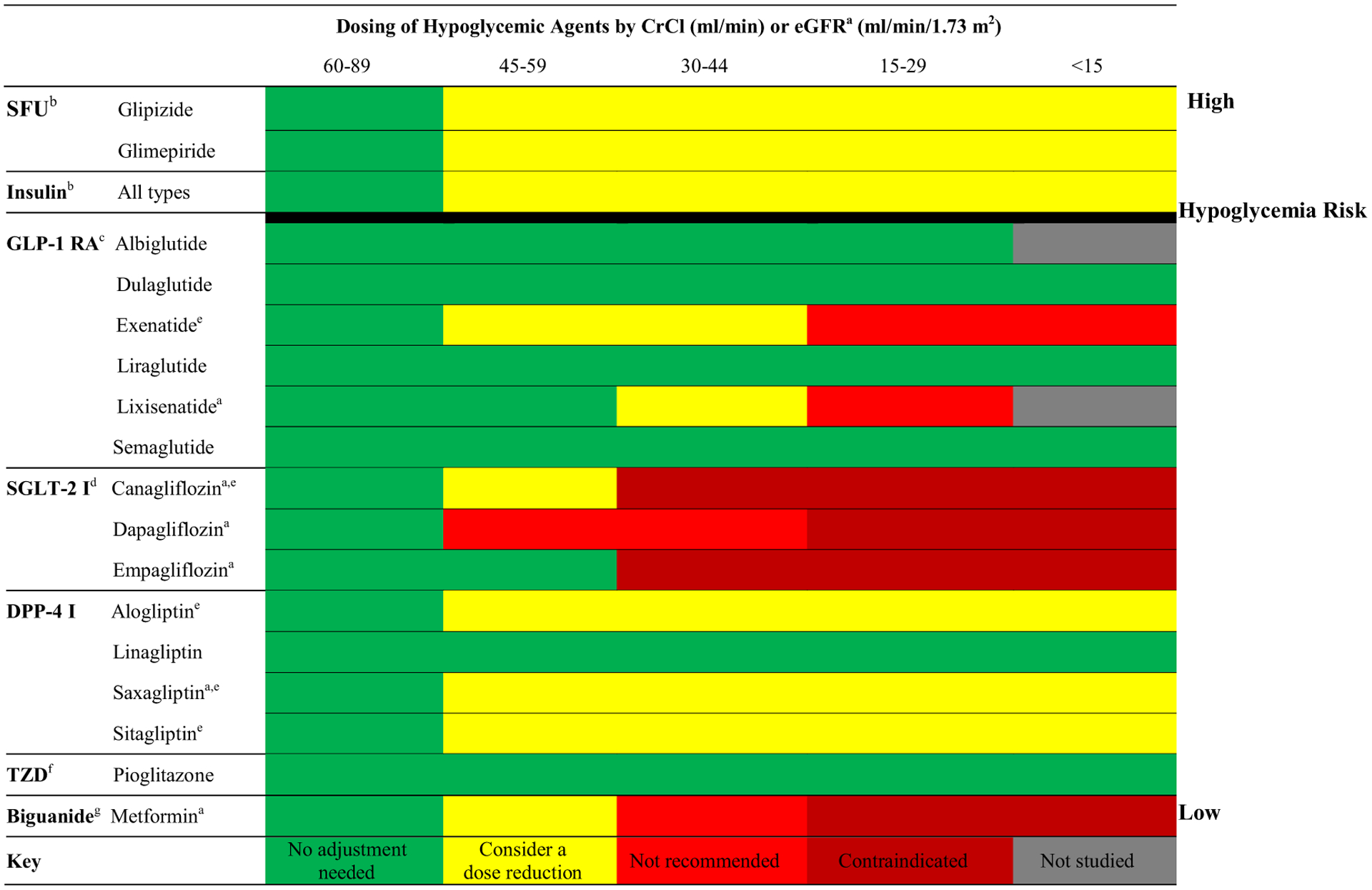
Dose adjustments of hypoglycemic agents by CrCl or eGFR in CKD [30, 38, 41, 45–47]. CKD chronic kidney disease, CrCl creatinine clearance, DPP-4 I dipeptidyl peptidase-4 inhibitor, eGFR estimated glomerular filtration rate, FDA Food and Drug Administration, GLP-1 RA glucagon-like peptide-1 receptor agonist, SFU sulfonylurea, SGLT-2 I sodium-glucose co-transporter-2 inhibitor, TZD thiazolidinedione. aDosing calculated by eGFR rather than CrCl for lixisenatide, canagliflozin, dapagliflozin, empagliflozin, saxagliptin, and metformin. bRisk in kidney failure due to increased risk of hypoglycemia. cRisk in kidney failure due to possible association with acute kidney injury, under investigation. dContraindicated in kidney failure due to inefficacy and increased side effects. eDose reductions required for these agents, starting in the 45–59 mL/min (CrCl) or 45–59 mL/min/1.73 m2 (eGFR) range per FDA; see package inserts of individual agents for details. fRisk in kidney failure due to risk of edema with medication use. gRisk in kidney failure due to risk of lactic acidosis
Table 2.
| Class and agents | HbA1c reduction | Administration | Mechanism of action | Renal benefit | CV benefit |
|---|---|---|---|---|---|
| DPP-4 inhibitors | |||||
| Alogliptin, linagliptin, saxagliptin, sitagliptin | 0.6–0.8% via prandial glucose reduction | Oral, once daily | Inhibits DPP-4 enzyme to reduce the breakdown of incretins which increases insulin secretion and suppresses glucagon release in a glucose-dependent manner | Saxagliptin (SAVOR-TIMI 53 [48–50]): reduction in albuminuria | Saxagliptin (SAVOR-TIMI 53 [50]), alogliptin (EXAMINE [51, 52]), sitagliptin (TECOS [53]): non-inferiority vs. placebo Linagliptin (CARMELINA [54]): study ongoing |
| GLP-1 agonists | |||||
| Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide | 0.5–1.6% via primarily prandial glucose reduction, some fasting reduction | Injectable, once daily to once weekly depending on agent | Synthetic form of GLP-1 that increases insulin secretion, decreases glucagon secretion, and delays gastric emptying in a glucose-dependent manner | Liraglutide (LEADER [56, 60]): preserved eGFR, reduced microalbuminuria, less nephropathy, lower new onset macroalbuminuria vs. placebo | Dulaglutide (REWIND [55]): study ongoing Lixisenatide (ELIXA [57]), exenatide (EXSCEL [58]), semaglutide (SUSTAIN-6 [59]): non-inferiority vs. placebo Liraglutide (LEADER [60]): significantly reduced CV mortality, nonfatal MI, nonfatal stroke, all-cause mortality |
| SGLT-2 inhibitors | |||||
| Canagliflozin, dapagliflozin, empagliflozin | 0.5–1.5% via prandial and fasting glucose reduction | Oral, once daily | Inhibits SGLT-2, a glucose transporter, lowering the threshold for renal glucose excretion to increase urinary glucose excretion by blocking glucose reabsorption in the proximal tubules | Empagliflozin (EMPA-REG OUTCOME [ESRD] [62–64] and EMPA-REG OUTCOME [65]): reduced Scr doubling, albuminuria, renal replacement therapy and eGFR decline; EMPA KIDNEY [73]: study is ongoing Canagliflozin (CANVAS R [69, 70]): decreased albuminuria and composite outcome of eGFR reduction, renal replacement therapy and death; CREDENCE [66, 67]: study stopped early due to benefit, data to be published Dapagliflozin (Dapa-CKD [71]): study ongoing |
Empagliflozin (EMPA-REG OUTCOME [65]), canagliflozin (CANVAS [68]): significantly reduced risk of CV death, nonfatal MI, nonfatal stroke, all-cause mortality Dapagliflozin (DECLARETIMI58 [72]): study ongoing |
CV cardiovascular, DPP-4 dipeptidyl peptidase-4, eGFR estimated glomerular filtration rate, GLP-1 glucagon-like peptide-1, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c hemoglobin A1c, MI myocardial infarction, SGLT-2 sodium-glucose co-transporter-2, SCr serum creatinine
In addition to hypoglycemic risk and renal dose adjustments, it is required to consider the precautions, contraindications, and benefits of certain medications based on comorbid conditions. Published trials have reported on cardiovascular benefits and eGFR preserving capabilities of newer hypoglycemic agents, while several ongoing trials are investigating hypothesized ‘renoprotective’ qualities of these agents (see Table 2) [38, 42–44]. While novel treatment options such as sodium-glucose co-transporter-2 (SGLT-2) inhibitors and some glucagon-like peptide-1 (GLP-1) receptor agonists are not Food and Drug Administration (FDA)-approved for patients with advanced kidney disease, dipeptidyl peptidase-4 (DPP-4) inhibitors may be dose-adjusted and considered for these patients (see Fig. 1 for specific dosing) [30, 45]. DPP-4 inhibitors are oral, once-daily medications (compared to the injectable GLP-1 receptor agonists) that have shown effects on HbA1c reduction equipotent to sulfonylureas in patients with diabetes and CKD [41, 46], with significantly less risk of hypoglycemia [41, 46, 47]. Currently, all DPP-4 inhibitors available in the US can be used in all stages of CKD and dialysis with respective dose adjustments [38]. Randomized trials have demonstrated acceptable short-term safety of novel agents in CKD patients as well as the potential for cardiovascular and renal benefits (see Table 2 [48–73]), though data on longer-term use remain limited [38].
An evidence-based antihyperglycemic deprescribing guideline and algorithm has been described and supports efforts to reduce or stop agents that are most likely to contribute to hypoglycemia based on individualized HbA1c targets [74]. Although this algorithm is not specific to older adults with CKD, in combination with information described in Fig. 1 and Table 2, high-risk agents may be identified and deprescribed in this population as well as replaced with safer alternatives. We emphasize the use of agents with a glucose-dependent mechanism of action and therefore low risk of hypoglycemia (i.e., metformin, DPP-4 inhibitors, SGLT-2 inhibitors and GLP-1 receptor agonists) as well as ensuring all oral hypoglycemic medications are dosed appropriately based on the level of kidney impairment. An example of safe deprescribing might include tapering a sulfonylurea (i.e., reduce glipizide by 5 mg/day each week until discontinued); if needed, the sulfonylurea may be replaced by a renally dosed DPP-4 inhibitor. HbA1c goals and antihyperglycemic regimens should be routinely reviewed for safety and efficacy in older adults with CKD to reduce polypharmacy and prevent hypoglycemia in these vulnerable patients [35–37].
5.3. Statins
Statins have been identified as the most commonly prescribed medication class in community-dwelling older adults [11], yet evidence of statin benefit in patients aged 75 years and older and frail individuals with multiple comorbidities such as CKD is marginal and conflicting [75–77]. Although patients with CKD are at increased risk of developing cardiovascular disease [78], they are also likely to be older, frail, with multiple comorbidities and polypharmacy and are possibly at higher risk for statin-induced myopathy [79, 80]. The KDIGO guidelines recommend initiation of statin therapy in all patients aged 50 years and older with CKD stages 3–5a who are not on hemodialysis and in patients aged 18–49 years with CKD and known cardiovascular risk factors or coronary disease [81]. Despite these recommendations, evidence for the continued benefit of statins in these patients remains limited [75, 82, 83]. Within hemodialysis and non-dialysis CKD patients, statins may be a target for deprescribing.
In regards to statin use in patients undergoing hemodialysis, the data for discontinuation of statins for primary and secondary prevention in patients on hemodialysis are fairly robust but nuanced (see Table 3) [76, 80, 81, 83]. Emerging evidence also suggests that statins may accelerate vascular calcifications through calcification of plaques and calcium accumulation in the arterial system, which can be harmful in the dialysis population [77]. Consideration must also be given to the prognosis of older adults undergoing hemodialysis and the time to benefit of preventative medication interventions. For many medications like statins, we must compare an individual’s life expectancy and prognosis to the time to benefit of the medication intervention; for statins, this time to benefit has been recently proposed as 2–5 years for primary prevention [84, 85]. Over 250,000 adults aged 75 years and older started dialysis in the US in the last 10 years despite evidence that many older adults experience a substantial decline in functional status and quality of life after dialysis initiation [86, 87]. Most of these adults only survive an additional 16 months after starting dialysis [86, 88], less than the proposed statin time to benefit [84, 85]. Given that the evidence for continued statin use in hemodialysis patients has shown little to no benefit and the limited life expectancy of older adults undergoing hemodialysis, statins should be a target for deprescribing in the dialysis population. A 2015 randomized controlled trial showed that stopping statins in patients with a limited life expectancy (estimated 12 months or less), including those with renal disease, was safe, improved quality of life, and did not affect survival [89, 90]. Statins do not need to be tapered and may be stopped abruptly [90].
Table 3.
Relevant studies of outcomes associated with statin use in CKD
| Study design | N | Comparison groups | Outcomes | Results (HR, 95% CI) | Conclusion |
|---|---|---|---|---|---|
| Statin use among dialysis patients | |||||
| Wanner et al. 2005 [81] | |||||
| Randomized controlled trial | 1255 | Statin vs. placebo | Composite cardiac death, non-fatal MI, and stroke | 0.92 (0.77–1.10), p = 0.37 | No significant decrease in composite endpoints |
| Fellstrom et al. 2009 [80] | |||||
| Randomized controlled trial | 2776 | Composite cardiac death, non-fatal MI, and nonfatal stroke | 0.96 (0.84–1.11), p = 0.59 | No significant decrease in composite endpoints | |
| Palmer et al. 2012 [76] | |||||
| Meta-analysis | 8289 | Statin vs. no statin or placebo | CV eventa ACM |
0.95 (0.88–1.03) 0.96 (0.90–1.02) |
Little to no benefit on decreasing CV events, mortality |
| Herrington et al. 2016 [83] | |||||
| Meta-analysis | 183,419 | Statin vs. no statin | Major vascular eventb Major coronary eventc ACM |
0.94 (0·79–1.11), p for trend = 0.008 0.89 (0.70–1.14), p for trend = 0.01 0.97 (0.88–1.08), p for trend = 0.03 |
Effect on primary outcomes decreased as eGFR declined No apparent benefit |
| Study design | N | Stages of CKD (n, %) | Outcomes | Results (HR, 95% CI) | Conclusion |
| Statin use among non-dialysis patients with CKD | |||||
| Messow and Isles 2017 [91] | |||||
| Meta-analysis | 31,104 | CKD 3 (19,386, 62.3%) | MACEd CMd ACM |
MACE: 0.72 (0.67–0.78), p < 0.001 CM: 0.75 (0.52–1.09), p = 0.130 ACM: 0.82 (0.73–0.91), p < 0.001 |
Statins reduced MACE, ACM |
| CKD 4 (2565, 8.2%) | MACE: 0.78 (0.62–0.99), p = 0.041 | Statins reduced MACEe | |||
| CKD 5 (7051, 22.7%) | MACE: 0.92 (0.84–1.02), p = 0.099 CM: 0.92 (0.75–1.13), p = 0.409 ACM: 0.95 (0.87–1.04), p = 0.870 |
No benefit for all outcomes | |||
| Baigent et al. 2011 [79]f | |||||
| Randomized controlled trial | 9270 | CKD 2 (88, 1%) CKD 3 (2155, 37%) CKD 4 (2562, 28%) CKD 5 (1221, 20%) |
MAEg | MAE: 0.83 (0.74–0.94), p = 0.002 | Statin plus ezetimibe significantly reduced MAE |
| Chung et al. 2017 [82] | |||||
| Retrospective cohort study | 16,428 | CKD 5 onlyh | ACM | ACM: 0.73 (0.68–0.80), p < 0.001 | Statins reduced ACM |
ACM all-cause mortality, CI confidence interval, CHD coronary heart disease, CKD chronic kidney disease, CM cardiac mortality, CV cardiovascular, eGFR estimated glomerular filtration rate, HR hazard ratio, MACE major cardiovascular events, MAE major atherosclerotic events MI myocardial infarction
Non-fatal MI, coronary death, non-hemorrhagic stroke, arterial revascularization, fatal or non-fatal stroke
Non-fatal MI or death from coronary heart disease from a vascular cause
Non-fatal MI or death from coronary heart disease from a non-vascular cause
Variable definitions for MACE and CM; see Appendix 1 of Messow and Isles [91]
No data for CKD 4 group for CM and ACM
Included subjects for primary prevention only; all others included patients receiving statins for primary and secondary prevention
Included non-fatal MI, CHD death, stroke, and revascularization procedures; study groups were statin plus ezetimibe vs. placebo with all stages of CKD
Included CKD stage 5 patients prescribed an erythropoietin-stimulating agent with dyslipidemia
Whether statins should be continued in older adults with CKD who are not yet on dialysis is unclear. A recent meta-analysis concluded that statins reduced major cardiovascular events in patients with CKD stages 3–4 and decreased all-cause mortality in patients with CKD stage 3, but found no apparent benefit for all outcomes in patients with CKD stage 5 and on dialysis (see Table 3) [91]. This included the notable Study of Heart and Renal Protection (SHARP) randomized controlled trial, which examined the benefit of statins for primary prevention only in non-dialysis patients and found a significant reduction in the composite endpoint of major atherosclerotic events for the population, but did not elaborate on the benefit of statins by CKD stage [79]. A few studies have proposed a renoprotective effect of statins beyond lipid-lowering, related to a reduction in inflammation and proteinuria, leading to improvements in eGFR [92, 93]. The evidence for statin benefit in CKD stages 2–4 is mixed; statins may be a target for deprescribing in older non-dialysis CKD patients with risk factors for statin-induced myopathy, limited life expectancy, or polypharmacy [11].
6. Conclusion
Older adults with CKD often have a high medication burden and are at risk for adverse outcomes associated with polypharmacy. This review discussed the challenges to medication management in older adults with CKD and proposed a framework for safe medication use via consideration of physiologic changes in older adults with CKD, appropriate prescribing and deprescribing. We described deprescribing as a methodical, evidence-based intervention for this vulnerable population. In our review, we highlighted three prevalently used medication classes and deprescribing opportunities in older adults with CKD: (1) PPIs, (2) oral hypoglycemic agents, including newer classes of hypoglycemic agents, and (3) statins. For each of these medication categories, we recommended that providers review appropriateness based on indication and evidence for benefits in older adults with CKD; we encouraged deprescribing when clinically indicated, using tapering strategies when necessary (i.e., for PPIs and oral hypoglycemic agents) [28, 74]. Collaboration with an interdisciplinary team is of utmost importance in caring for this complex population. Pharmacists are well positioned within the team to aid in the evaluation of patients’ medication lists, review appropriateness and provide recommendations for successful deprescribing [12]. Further research is needed to determine the association between deprescribing and specific outcomes among older adults with CKD.
Key Points.
Older adults with chronic kidney disease (CKD) often have a high medication burden and are at risk for adverse outcomes associated with polypharmacy; however, specific guidance for deprescribing in this population does not exist.
Identification of potentially inappropriate medications and consideration of the risks and benefits for continued medication use is fundamental to deprescribing in older adults with CKD.
Further research is needed to determine the association between deprescribing and specific outcomes among older adults with CKD.
Acknowledgements
The authors would like to acknowledge John Roe-faro, PharmD, BCGP, FASHP and Bryan Wood, PharmD, pharmacy residency program directors to Laura K. Triantafylidis and Chelsea E. Hawley at VA Boston Healthcare System. We thank them for their continued support.
Funding
No funding was received for the preparation of this article.
Footnotes
Conflict of interest Laura K. Triantafylidis, Chelsea E. Hawley, Laura P. Perry and Julie M. Paik have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. 10.7326/0003-4819-158-11-20136040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25–9. 10.1159/000445450. [DOI] [PubMed] [Google Scholar]
- 3.Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. Challenges and enablers of deprescribing: a general practitioner perspective. PLoS One. 2016;11(4):e0151066. 10.1371/journal.pone.0151066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koronkowski MJ, Semla TP, Schmader KE, Hanlon JT. Recent literature update on medication risk in older adults, 2015–2016. J Am Geriatr Soc. 2017;65(7):1401–5. 10.1111/jgs.14887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473–82. 10.1001/jamainternmed.2015.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–96. 10.2215/cjn.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman MA, Miao Y, Boscardin WJ, Komaiko KD, Schwartz JB. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014;29(10):1379–86. 10.1007/s11606-014-2924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghimire S, Castelino RL, Lioufas NM, Peterson GM, Zaidi ST. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One. 2015;10(12):e0144119. 10.1371/journal.pone.0144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SA, Bhandari S. The prevalence of potentially inappropriate medication prescribing in elderly patients with chronic kidney disease. Postgrad Med J 1051;2013(89):247–50. 10.1136/postgradmedj-2012-130889. [DOI] [PubMed] [Google Scholar]
- 10.Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–34. 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- 11.Merel SE, Paauw DS. Common drug side effects and drug–drug interactions in elderly adults in primary care. J Am Geriatr Soc. 2017;65(7):1578–85. 10.1111/jgs.14870. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker CF, Miklich MA, Patel RS, Fink JC. Medication safety principles and practice in CKD. Clin J Am Soc Nephrol. 2018. 10.2215/cjn.00580118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponticelli C, Sala G, Glassock RJ. Drug management in the elderly adult with chronic kidney disease: a review for the primary care physician. Mayo Clin Proc. 2015;90(5):633–45. 10.1016/j.mayocp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Wooten JM. Pharmacotherapy considerations in elderly adults. Southern Med J. 2012;105(8):437–45. 10.1097/SMJ.0b013e31825fed90. [DOI] [PubMed] [Google Scholar]
- 15.Liles AM. Medication considerations for patients with chronic kidney disease who are not yet on dialysis. Nephrol Nurs J. 2011;38(3):263–70. [PubMed] [Google Scholar]
- 16.Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy. 2013;33(9):912–21. 10.1002/phar.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–95. 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Chang F, O’Hare AM, Miao Y, Steinman MA. Use of renally inappropriate medications in older veterans: a national study. J Am Geriatr Soc. 2015;63(11):2290–7. 10.1111/jgs.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Geriatrics Society. Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 20.Hanlon JT, Aspinall SL, Semla TP, Weisbord SD, Fried LF, Good CB, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57(2):335–40. 10.1111/j.1532-5415.2008.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kid Dis. 2017;70(5):611–8. 10.1053/j.ajkd.2017.02.374. [DOI] [PubMed] [Google Scholar]
- 22.Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014;10:Cd008165. 10.1002/14651858.cd008165.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–54. 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 24.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54. 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 25.Pai AB, Boyd A, Depczynski J, Chavez IM, Khan N, Manley H. Reduced drug use and hospitalization rates in patients undergoing hemodialysis who received pharmaceutical care: a 2-year, randomized, controlled study. Pharmacotherapy. 2009;29(12):1433–40. 10.1592/phco.29.12.1433. [DOI] [PubMed] [Google Scholar]
- 26.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706–15. 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331–42. 10.1093/ndt/gfw470. [DOI] [PubMed] [Google Scholar]
- 28.Haastrup P, Paulsen MS, Begtrup LM, Hansen JM, Jarbol DE. Strategies for discontinuation of proton pump inhibitors: a systematic review. Fam Pract. 2014;31(6):625–30. 10.1093/fampra/cmu050. [DOI] [PubMed] [Google Scholar]
- 29.Reeve E, Andrews JM, Wiese MD, Hendrix I, Roberts MS, Shakib S. Feasibility of a patient-centered deprescribing process to reduce inappropriate use of proton pump inhibitors. Ann Pharmacother. 2015;49(1):29–38. 10.1177/1060028014558290. [DOI] [PubMed] [Google Scholar]
- 30.Di Lullo L, Mangano M, Ronco C, Barbera V, De Pascalis A, Bellasi A, et al. The treatment of type 2 diabetes mellitus in patients with chronic kidney disease: what to expect from new oral hypoglycemic agents. Diabetes Metab Syndr. 2017;11(Suppl 1):S295–305. 10.1016/j.dsx.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Improving care and promoting health in populations. standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S7–12. 10.2337/dc18-S001. [DOI] [PubMed] [Google Scholar]
- 32.Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020–6. 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno G, Mangione CM. Management of cardiovascular disease risk factors in older adults with type 2 diabetes mellitus: 2002–2012 literature review. J Am Geriatr Soc. 2013;61(11):2027–37. 10.1111/jgs.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342–56. 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Simplification of insulin regimen in older adults and risk of hypoglycemia. JAMA Intern Med. 2016;176(7):1023–5. 10.1001/jamainternmed.2016.2288. [DOI] [PubMed] [Google Scholar]
- 36.Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Liberating A1C goals in older adults may not protect against the risk of hypoglycemia. J Diabetes Complications. 2017;31(7):1197–9. 10.1016/j.jdiacomp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Weinstock RS, DuBose SN, Bergenstal RM, Chaytor NS, Peterson C, Olson BA, et al. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2016;39(4):603–10. 10.2337/dc15-1426. [DOI] [PubMed] [Google Scholar]
- 38.Neumiller JJ, Alicic RZ, Tuttle KR. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol. 2017;28(8):2263–74. 10.1681/asn.2016121372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121–7. 10.2215/cjn.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2016 [Google Scholar]
- 41.Singh-Franco D, Harrington C, Tellez-Corrales E. An updated systematic review and meta-analysis on the efficacy and tolerability of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes with moderate to severe chronic kidney disease. SAGE Open Med. 2016;4:2050312116659090. 10.1177/2050312116659090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng D, Fei Y, Liu Y, Li J, Chen Y, Wang X, et al. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysis. PLoS ONE. 2014;9(10):e111543. 10.1371/journal.pone.0111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zavattaro M, Caputo M, Sama MT, Mele C, Chasseur L, Marzullo P, et al. One-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine. 2015;50(3):620–6. 10.1007/s12020-014-0519-0. [DOI] [PubMed] [Google Scholar]
- 44.Cheng JWM, Badreldin HA, Patel DK, Bhatt SH. Antidiabetic agents and cardiovascular outcomes in patients with heart diseases. Curr Med Res Opin. 2017;33(6):985–92. 10.1080/03007995.2017.1284052. [DOI] [PubMed] [Google Scholar]
- 45.Williams ME, Garg R. Glycemic management in ESRD and earlier stages of CKD. Am J Kid Dis. 2014;63(2 Suppl 2):S22–38. 10.1053/j.ajkd.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 46.Howse PM, Chibrikova LN, Twells LK, Barrett BJ, Gamble JM. Safety and efficacy of incretin-based therapies in patients with type 2 diabetes mellitus and CKD: a systematic review and meta-analysis. Am J Kid Dis. 2016;68(5):733–42. 10.1053/j.ajkd.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Garg R, Williams ME. Diabetes management in the kidney patient. Med Clin North Am. 2013;97(1):135–56. 10.1016/j.mcna.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, et al. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care. 2017;40(1):69–76. 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 49.Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 Trial. Diabetes Care. 2015;38(4):696–705. 10.2337/dc14-1850. [DOI] [PubMed] [Google Scholar]
- 50.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 51.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 52.White WB, Kupfer S, Zannad F, Mehta CR, Wilson CA, Lei L, et al. Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care. 2016;39(7):1267–73. 10.2337/dc16-0303. [DOI] [PubMed] [Google Scholar]
- 53.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 54.Rosenstock J, Perkovic V, Alexander JH, Cooper ME, Marx N, Pencina MJ, et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA((R))): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetology. 2018;17(1):39. 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ClinicalTrials.gov. Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND)—NCT01394952. 2011. https://clinicaltrials.gov/ct2/show/NCT01394952. Accessed 1 Aug 2018 [DOI] [PubMed]
- 56.Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48. 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 57.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 58.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 60.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kid Dis. 2018;72(2):267–77. 10.1053/j.ajkd.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 63.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137(2):119–29. 10.1161/circulationaha.117.028268. [DOI] [PubMed] [Google Scholar]
- 64.Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610–21. 10.1016/s2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 65.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 66.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study: rationale, design, and baseline characteristics. Am J Nephrol. 2017;46(6):462–72. 10.1159/000484633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Janssen Pharmaceutical Companies of Johnson & Johnson: phase 3 CREDENCE renal outcomes trial of INVOKANA® (canagliflozin) is being stopped early for positive efficacy findings. PR Newswire, 2018. [Google Scholar]
- 68.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137(4):323–34. 10.1161/circulationaha.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 70.Perkovic V, Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018. 10.1016/s2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 71.ClinicalTrials.gov. A study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease (Dapa-CKD)—NCT03036150. 2017. https://clinicaltrials.gov/ct2/show/NCT03036150. Accessed 1 Aug 2018.
- 72.ClinicalTrials.gov. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI58)—NCT01730534. 2012. https://clinicaltrials.gov/ct2/show/results/NCT01730534. Accessed 1 Aug 2018.
- 73.Boehringer Ingelheim and Lilly announce an academic collaboration with University of Oxford to investigate the effects of empagliflozin in people with chronic kidney disease. Ingelheim, Germany and Indianapolis, IN: Boehringer Ingelheim; 2018. [Google Scholar]
- 74.Farrell B, Black C, Thompson W, McCarthy L, Rojas-Fernandez C, Lochnan H, et al. Deprescribing antihyperglycemic agents in older persons: evidence-based clinical practice guideline. Can Fam Phys. 2017;63(11):832–43. [PMC free article] [PubMed] [Google Scholar]
- 75.Gurwitz JH, Go AS, Fortmann SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA. 2016;316(19):1971–2. 10.1001/jama.2016.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;157(4):263–75. 10.7326/0003-4819-157-4-201208210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Vriese AS. Should statins be banned from dialysis? J Am Soc Nephrol. 2017;28(6):1675–6. 10.1681/asn.2017020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burmeister JE, Mosmann CB, Costa VB, Saraiva RT, Grandi RR, Bastos JP, et al. Prevalence of cardiovascular risk factors in hemodialysis patients - the CORDIAL study. Arq Bras Cardiol. 2014;102(5):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tom-son C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. 10.1016/s0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–407. 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 81.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–48. 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 82.Chung CM, Lin MS, Hsu JT, Hsiao JF, Chang ST, Pan KL, et al. Effects of statin therapy on cerebrovascular and renal outcomes in patients with predialysis advanced chronic kidney disease and dyslipidemia. J Clin Lipidol. 2017;11(2):422–31.e2. 10.1016/j.jacl.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 83.Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4(10):829–39. 10.1016/s2213-8587(16)30156-5. [DOI] [PubMed] [Google Scholar]
- 84.Lee SJ, Kim CM. Individualizing prevention for older adults. J Am Geriatr Soc. 2018;66(2):229–34. 10.1111/jgs.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. 2013;310(24):2609–10. 10.1001/jama.2013.282612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–47. 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Unruh ML, Newman AB, Larive B, Dew MA, Miskulin DC, Greene T, et al. The influence of age on changes in health-related quality of life over three years in a cohort undergoing hemodialysis. J Am Geriatr Soc. 2008;56(9):1608–17. 10.1111/j.1532-5415.2008.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schlanger LE, Bailey JL, Sands JM. Geriatric nephrology: old or new subspecialty. Clin Geriatr Med. 2009;25(3):311–24. 10.1016/j.cger.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175(5):701–2. 10.1001/jamainternmed.2015.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kutner JS, Blatchford PJ, Taylor DH Jr, Ritchie CS, Bull JH, Fair-clough DL, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med. 2015;175(5):691–700. 10.1001/jamainternmed.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Messow CM, Isles C. Meta-analysis of statins in chronic kidney disease: who benefits? QJM. 2017;110(8):493–500. 10.1093/qjmed/hcx040. [DOI] [PubMed] [Google Scholar]
- 92.Mikolasevic I, Zutelija M, Mavrinac V, Orlic L. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovas Dis. 2017;10:35–45. 10.2147/ijnrd.S101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mesquita J, Varela A, Medina JL. Dyslipidemia in renal disease: causes, consequences and treatment. Endocrinol Nutr. 2010;57(9):440–8. 10.1016/j.endonu.2010.06.003. [DOI] [PubMed] [Google Scholar]


