Abstract
Simple Summary
Cyathostomins (small strongyles) are a multispecies group of intestinal parasites in horses and the main target of deworming efforts by horse owners. It is not known whether species of cyathostomins have individual responses to dewormers. The objective of this study was to identify differences between cyathostomin species in reemergence rates following commercial dewormer treatment. This study used gene sequencing to profile the presence/absence of cyathostomin species in fecal samples at 2-week intervals following deworming to determine how quickly each species reinfected horses. Moxidectin was found to be the most effective at slowing the overall reemergence of these parasites, followed by Ivermectin, then Pyrantel. Seven species were resistant to all three deworming products. This study demonstrates that dewormer sensitivity differs between cyathostomin species, which could lead to more targeted control measures.
Abstract
Cyathostomins are a multispecies parasite ubiquitous in Equids. Cyathostomins have developed resistance to all but one class of anthelmintics, but species-level sensitivity to anthelmintics has not been shown. This study measured reinfection rates of cyathostomin species following the administration of three commercial dewormers. Nine treated horses were compared with 90 untreated controls during June-September 2017–2019. Ivermectin (IVM) (n = 6), Moxidectin (MOX) (n = 8) or Pyrantel (PYR) (n = 8) were orally administered. Fecal samples were collected every 14 d for 98 d. Fecal egg count reductions (FECR) were calculated using a modified McMaster technique. Nineteen cyathostomin species were identified by 5.8S-ITS-2 profiling using amplicon sequencing. Data were analyzed in QIIME1 and R statistical software using presence/absence methods. MOX had the lowest numbers of species present over the time course, followed by PYR then IVM (7.14, 10.17, 11.09, respectively); however, FECR was fastest for PYR. The presence of seven species: Coronocyclus labiatus, Cyathostomum catinatum, Cyathostomum tetracanthum, Cylicocylus elongatus, Cylicodontophorus bicoronatus, Cylicostephanus minutus, and Cylicostephanus goldi were unaffected by treatment (p > 0.05) points to species-specific differences in dewormer sensitivity and environmental persistence. Identifying resistance patterns at the species level will enable mechanistic understandings of cyathostomin anthelmintic resistance and targeted approaches to control them.
Keywords: equine, strongyle, anthelmintic, cyathostomin, fecal egg count, resistance, amplicon sequencing
1. Introduction
Cyathostomins are the most prevalent equine intestinal parasite group comprising 89–100% of the worm burden in horses [1,2]. Over 50 cyathostomin species from 14 genera have been described, and a single horse may harbor from 1 to 26 species at a time [3,4]. Cyathostomins can be found in horses of all ages and as early as 4 months, and while cyathostomin burdens can vary, the presence of the parasitic worms remains constant in the gut for the animal’s entire life [5]. Young horses have higher infective rates due to naïve immune systems when compared to older horses [5] and horses who live on pasture will have higher infective rates from grazing on or near fecal material when compared to stalled horses [6]. Luminal and encysted parasites in the equine gut can cause a myriad of health concerns, including weight loss, poor feed efficiency, dull coat, diarrhea, intermittent colic, and decreased performance [7]. The spontaneous eruption of larvae encysted in cecal or colonic tissue (larval cyathostominosis) carries a 50% fatality rate due to tissue damage [6,8,9,10,11]. In Canada, larval cyathostominosis has been suggested as an emerging equine disease that may presage trends for horses in the United States [8].
Benzimidazoles, tetrahydropyrimidines (pyrantel pamoate (PYR)), and macrocyclic lactones (Ivermectin (IVM) and Moxidectin (MOX)) are the main anthelmintic drug classes used to control cyathostomins in horses [12]. Due to high frequency and prolonged use, the development of resistance to benzimidazole followed by tetrahydropyrimidines has left one effective option, macrocyclic lactones, against all stages of cyathostomins [13,14]; however, resistance to this class appears to be emerging [15,16]. Some cyathostomins even exhibit multidrug class resistance [15,17,18,19].
When MOX, IVM, and PYR were first introduced to the market, the MOX egg reappearance period (ERP) was 16–22 weeks [20,21,22], 9–13 weeks [22,23,24], and 5–6 weeks [24,25], respectively. The ERP of these three anthelmintics is currently reported at 10–12 weeks, 6–8 weeks, and 4–5 weeks, respectively [26].
Four mechanisms have been suggested for the rise of anthelmintic resistance in horses; (1) pre-existing alleles for resistance, (2) spontaneous mutations before or at the time of anthelmintic exposure, (3) frequent mutations for the reappearance of resistant alleles, or (4) host migration of resistant alleles is spread through new populations [16,27,28,29].
Thus far, efforts to understand the mechanisms of resistance of cyathostomins have largely considered them to be a monolithic group [19,30]. It is unlikely that drivers of resistance act uniformly across the 50 cyathostomin species, but little is known about species-specific sensitivity to anthelmintic drugs or the environmental factors favoring the success of individual cyathostomin species [31]. Species and genera contributions to shortened egg reappearance rates have been studied via morphological identification of adult worms [32], PCR-ELISA [33,34], and Reverse Line Blot [35,36,37] techniques. All three of these study techniques are laborious and difficult to conduct on a large number of horses, particularly morphological identification, because horses must be euthanized and necropsied [38]. The AAEP guidelines for the fecal egg count reduction test remain the gold-standard and most widely adopted method to determine anthelmintic resistance [26,39]. However, the interpretation from the FECR to the ERP still varies between researchers and makes comparisons with the literature difficult. While DNA sequencing has been used to identify cyathostomins at the species level since the 1990s [40,41,42], the use of marker genes to survey cyathostomin populations via NGS of fecal material (as is commonly performed for bacteria) is a novel approach.
The objective of this study was to use next-generation sequencing (NGS) to track the presence of cyathostomin species in equine fecal samples following treatment with three commercial anthelmintics: Moxidectin, Ivermectin, and Pyrantel compared with untreated controls. We hypothesize that species-specific differences in response to anthelmintic drugs underly the ability of cyathostomins to develop resistance. This research describes species-level differences in the response and reemergence of cyathostomins to each anthelmintic and demonstrates the efficacy of a noninvasive sequence-based methodology for identifying the presence of cyathostomin species from fecal samples.
2. Materials and Methods
2.1. Study Design
This experiment was approved by the University of Delaware Animal Care and Use Committee (#AUP90R).
Horses housed at two locations were enrolled in the study (Table 1).
Table 1.
Description of horse subjects.
| Horse ID | Sex 1 | Age (Years) | Breed | Weight (kg) | Farm ID 2 |
|---|---|---|---|---|---|
| 1 | G | 10 | Arabian | 456 | 1 |
| 2 | M | 10 | Arabian | 449 | 1 |
| 3 | G | 8 | Quarter Horse | 600 | 1 |
| 4 | M | 10 | Quarter Horse | 534 | 1 |
| 5 | G | 7 | Thoroughbred | 490 | 1 |
| 6 | G | 16 | Standardbred | 470 | 1 |
| 7 * | G | 19 | Saddlebred | 493 | 2 † |
| 8 | M | 18 | Standardbred | 498 | 2 |
| 9 * | G | 33 | Morgan | 392 | 2 |
1 Sex is defined as gelding (G) or mare (M). 2 Farm 1 is located in Newark, DE, Farm 2 is located in Elkton, MD. * Horse 7 was not included in the sequencing data for MOX and Horse 9 for PYR due to unsuccessful amplification for >50% of the time points. † Farm 2 was eliminated from the IVM trial due to previous deworming within 180 d prior to trial enrollment.
All horses were considered to be idle with occasional pleasure riding, lived in mixed-sex pastures, and had been residents of their respective herds for a minimum of 2 years prior to the study. The horses had not received anthelmintic treatment or antibiotics for 180 days prior to the beginning of the study. All horses were housed in grass pastures with year-round ad libitum access to forage, pasture, water, and mineral salt blocks and received grain supplementation only as needed to maintain body condition. The study tested three different anthelmintics during the summer months (May–September) over 3 years (2017–2019) with Moxidectin (MOX) (n = 8) conducted in 2017, Pyrantel (PYR) (n = 8) conducted in 2018 (n = 8), and Ivermectin (IVM) (n = 6) conducted in 2019. The summer season across all three treatments/years (average 24.15 °C, 11.92 inches precipitation) was fairly equivalent to normal DE summer season conditions of warm and wet conditions (average 24 °C, 12” inches precipitation) [43]. To evaluate the natural fluctuations of cyathostomin species for the duration of the study period, untreated control (CON) fecal samples (n = 90) were collected from pasture-managed horses in the mid-Atlantic region who had not received anthelmintic treatment within the last 180 days parallel to the sampling points of the horses enrolled to the study (Supplementary, Table S1).
2.2. Fecal Sample Collection
Pre-treatment control samples were obtained on Day 0, and anthelmintics were orally administered according to the manufacturer’s instructions. Equine weights were estimated using a horse and pony weigh tape (Coburn, Whitewater, WI), and weights were rounded up to prevent underdosing horses. Post-treatment fecal samples were collected every 14 d for 98 d total. Fecal samples were obtained by picking up feces within 5 min of defecation with an inverted Ziploc bag, and the air was expelled. Immediately, approximately 4 mL of fecal material was aliquoted from the inside of a fecal ball using a sterile spoon into a 5 mL tube containing 1 mL of DNA/RNA Shield preservative (Zymo, Tustin, CA, USA) and shaken vigorously. This sample was placed at −20 °C until nucleic acid extraction could be performed (within 1 month). Fecal samples were stored in individual Ziploc bags at 4 °C until the FEC could be performed (within 48 h).
2.3. Fecal Egg Count Reduction and Egg Reappearance Period Tests
Fecal egg counts were conducted using the Paracount-EPG Kit (Chalex LLC, Park City, UT, USA) according to the manufacturer’s instructions. Fecal egg count reductions (FECR) [26] were calculated for each individual horse and were determined as:
| (1) |
Day 0 FEC (FEC0d) represents the pre-treatment survey, and FEC conducted on each subsequent sampling day (Day 14, 28, 42, 56, 70, 98) for each treatment was used for FECpost treatment. FECR values were averaged for each treatment at each time point, and an FECR cut-off of ≤90% was used for MOX and IVM, and an FECR cut-off of ≤80% was used for PYR as outlined by Nielsen et al. [26] to determine a shortened fecal egg reappearance period (ERP) as a measure of anthelmintic resistance [14,44].
2.4. DNA Extraction and Sequencing
DNA was extracted after thawing using a commercial kit (QIAGEN QIAmp Powerfecal DNA Isolation Kit, Germantown, MD, USA). DNA triplicates were tested for quantity and quality using Qubit (Thermo Fisher, Waltham, MA, USA) and Nanodrop (Thermo Fisher, Waltham, MA, USA) according to manufacturer instructions. Amplification of the 5.8S-ITS-2 rRNA and attachment was performed using custom region-specific primers: forward primer 5′-GACTAGCTTCAGCGATGGA-3′ and reverse primer 5′-AACGYTGTCATACAGGCACT-3′. Primers were designed using Primer1 [45] to produce amplicons that could be used on the Illumina MiSeq platform (450–480 basepairs), targeting the highly conserved 5.8S and ITS-2 rRNA gene regions [46,47] of the 19 equine cyathostomins included in this study (Table 2). Primer specificity for equine cyathostomins was validated through morphological and molecular identification of adult cyathostomins from equine feces (unpublished) (Supplementary, Table S2).
Table 2.
Species accession numbers of aligned sequences of cyathostomins for taxonomy assignments. The naming conventions of Lichtenfels et al. [34] were used in this paper.
| Taxa | Accession Number |
|---|---|
| Cylicocyclus (CY) ashworthi | Y08586 |
| Cylicocyclus (CY) leptostomus | KP693432 |
| Cylicocyclus (CY) nassatus | Y08585 |
| Cylicocyclus (CY) radiatus | JQ906423 |
| Cyathostomum (CS) tetracanthum | KF850629 |
| Cylicocyclus (CY) insigne | Y08588 |
| Cylicocyclus (CY) auriculatus | JQ906414 |
| Coronocyclus (CO) labiatus | JN786947 |
| Coronocyclus (CO) labratum | AJ004838 |
| Cylicostephanus (CT) calicatus | KM085356 |
| Cylicocylus (CY) elongatus | JQ906417 |
| Cyathostomum (CS) pateratum | KF850627 |
| Cyathostomum (CS) catinatum | KF850626 |
| Cylicostephanus (CT) goldi | KM085357 |
| Cylicostephanus (CT) longibursatus | KM085358 |
| Cylicodontophorus (CD) bicoronatus | KP693441 |
| Cylicostephanus (CT) minutus | KM085361 |
| Coronocyclus (CO) coronatus | JN786951 |
| Poteriostomum (POT) imparidentatum | KP693433 |
PCR products were pooled and sequenced via Illumina MiSeq platform by RTL Genomics (Lubbock, TX). Paired ends were joined using FLASh (v.1.2.11) [48]. Quality filtering was performed in QIIME1 [49] using the split_seqs.py command, and taxonomic assignments were conducted using the map_reads_to_reference.py command with the aligned sequences of 19 cyathostomins (Table 2) using a QIIME 1 [49] pipeline. The identification and naming conventions by Lichtenfiels et al. [50] were used in this study. Sequence data have been submitted to the NCBI Sequence Read Archive within PRJNA716069.
2.5. Phylogenetic Analysis
Phylogenetic trees were constructed of the 19 cyathostomin species genomic sequences listed in Table 2. Strongylus equinus (KM605251), Strongylus vulgaris (AP017698), and Syngamus trachea (GQ888718) were included as outgroups. Bootstrap analysis with 500 replicates was used to assess the confidence limits of the branches of the maximum likelihood trees. Trees were drawn using MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets: (https://www.megasoftware.net/ accessed on 17 September 2020) [51].
2.6. Statistical Analysis and Species Frequency Reductions
Due to variable cell numbers and DNA content for different cyathostomin development stages [52], species abundance estimates could not be made, and data were analyzed using presence/absence methods [38]. All data were evaluated in R statistical software [53]. Cyathostomin species frequency of presence was determined by the percentage of horses harboring the species at each given time point and treatment and analyzed with ANOVA and Tukey all-pair comparison method with significance determined at (p ≤ 0.05) and a tendency toward significance at (0.05 ≤ p ≥ 0.10). Cyathostomin species frequency of presence reductions (SFPR) was determined as:
| (2) |
Cyathostomin species frequency of presence (SFP0d) represents the pre-treatment survey, and SFP of each subsequent sampling day (Day 14, 28, 42, 56, 70, 98) for each treatment were used for SFPpost treatment.
To predict the probability of a species’ presence at each given time point post-treatment (MOX, IVM, and PYR), binomial logistic regression models were employed compared to the CON samples. Spearman’s coefficient, r, was used to determine correlations between cyathostomin species and anthelmintic treatment with significance determined at (−0.3 ≤ r ≥ 0.3). The Spearman correlation, r, is considered to be fairly significant at (≥+/−0.3–<+/−0.5), moderate with (≥+/−0.5–<+/−0.7), strong with (≥+/−0.7–<+/−0.9), and substantial with (≥+/−0.9–+/−1.0).
3. Results
3.1. Sequencing Results
Amplicon sequencing yielded an average of 14,773 joined reads per sample (standard deviation = 5699). One horse was removed from the MOX and PYR trials due to inadequate amplification for more than 50% of the time points. POT. imparidentatum was not observed in this study and is among the less common cyathostomin species and was removed from the study [5]. CS. tetracanthum was observed at a very low rate and is also a lesser found species but was not removed from the analysis. CS. teteracanthum was observed at Day 0 and 14 of the CON samples but was then no longer detected in any samples except for Day 70 during MOX treatment in 38% of the samples (Supplementary, Table S3).
3.2. Fecal Egg and Species Count Reductions
FECR tests revealed an ERP of 100% 14 d post-administration for both MOX and IVM and 98.37% for PYR. PYR reached a shortened egg reappearance rate (ERP < 80%) by 28 d, followed by IVM at 42 d and MOX at 84 d (ERP < 90%) (Table 3). The overall total number of species observed in the fecal material was reduced the most by MOX, followed by IVM and PYR (Table 3). On average, horses enrolled in the study harbored 9.39 (±3.16 s.d.) species at 0 d (Supplementary, Table S4).
Table 3.
Reappearance of cyathostomins over 98 days post deworming with three different anthelmintics.
| FECR 1/ERP 2 | Total Number of Species 3 | ||||||
|---|---|---|---|---|---|---|---|
| Day | IVM (%) | MOX (%) | PYR (%) | CON | IVM | MOX | PYR |
| 0 | -- | -- | -- | 13.16 ± 3.7 | 10.5 ± 5.4 | 10.9 ± 4.6 | 7.88 ± 4.6 |
| 14 | 100.00 | 100.00 | 98.37 | 13.16 ± 3.7 | 6.50 ± 7.2 | 7.13 ± 3.9 | 7.88 ± 4.1 |
| 28 | 96.15 | 100.00 | 72.29 * | 9.00 ± 5.0 | 10.7 ± 5.4 | 5.13 ± 3.3 | 5.50 ± 4.8 |
| 42 | 81.73 * | 98.58 | 49.46 | 9.00 ± 5.0 | 12.2 ± 2.6 | 5.63 ± 3.4 | 9.13 ± 4.9 |
| 56 | 53.85 | 96.45 | 7.63 | 14.33 ± 2.3 | 10.2 ± 5.7 | 4.25 ± 5.2 | 10.8 ± 4.1 |
| 70 | 67.31 | 93.85 | −91.29 | 14.33 ± 2.3 | 12.0 ± 3.3 | 6.00 ± 3.7 | 11.8 ± 3.3 |
| 84 | 34.62 | 83.91 * | −152.69 | 10.3 ± 3.7 | 10.7 ± 5.1 | 4.75 ± 3.1 | 11.5 ± 4.4 |
| 98 | 0.00 | 60.25 | −167.36 | 10.3 ± 5.7 | 9.33 ± 4.6 | 6.88 ± 6.2 | 9.75 ± 3.9 |
1 Fecal egg count reduction and 2 egg reappearance period. 3 Average number of the total species present at each timepoint ± the standard deviation. * indicates a shortened ERP using an FECR cut-off of ≤90% for IVM and MOX and ≤80% for PYR as outlined by Nielsen et al. [26]. CON–control (untreated), IVM–Ivermectin, MOX–Moxidectin, PYR–Pyrantel. The total number of species present per timepoint allows tracking of the total number of present cyathostomins to see if horses are reinfected by many species or specific species that increase numerically to make up the majority of the parasite burden.
Natural fluctuations of cyathostomin species presence was demonstrated by the CON group, which showed that 12 species were reduced at Day 28–42, then rose back to 0 d infection rates at 56–70 d but then reduced again at 84–98 d (Figure 1). Only five species (CY. radiatus, CY. nassatus, CY. ashworthi, CT. longibursatus, CS. catinatum) in the CON samples appeared to be consistently present and not demonstrating this natural environmental response.
Figure 1.
Heatmap of cyathostomin SPFR to anthelmintic treatments. SFPR demonstrates the natural variation of species populations in the CON group and how the species, in turn, respond to anthelmintic treatment. CON-control, IVM-Ivermectin, MOX-Moxidectin, PYR-Pyrantel.
Six species demonstrated IVM resistance (CY. elongatus, CY. auriculatus, CT. minutus, CS. tetracanthum, CO. labratum, CO. labiatus), showing no reduction at 14 d post-treatment (Figure 1). IVM reduced species infection rates for 11 species at 14 d post-treatment, but a 100% reduction in any species was not observed, as was achieved by MOX and PYR (Figure 1). Cyathostomin species treated with IVM returned to 0 d infection rates or greater by 28 d in all species except for CY. leptostomus (42 d), CO.coronatus (42 d), and CD. bicoronatus (56 d). The extended period of CD. bicoronatus reduction may be a factor of environmental and natural species patterns of the region as reflected in the CON group. MOX was able to achieve 100% reduced SFP rates in four species 14 d post-treatment (CT. minutus, CO. labiatus, CO. coronatus, CD. bicoronatus) and continue to reduce rates for at least four weeks. Five species (CY. leptostomus, CY. insigne, CY. elongatus, CY. auriculatus, and CS. catinatum) were reduced by 100% at later time points but were able to reinfect horses more quickly than the four species showing more sensitivity toward MOX (Figure 1).
3.3. Anthelmintic Resistance
Over the entire course of the study, seven species, CO. labiatus (p = 0.403), CS. catinatum (p = 0.066), CS. tetracanthum (p = 0.281), CY. elongatus (p = 0.106), CD. bicoronatus (p = 0.108), CT. minutus (p = 0.074) and CT. goldi (p = 0.189), exhibited multidrug resistance to all three anthelmintics (Treatment, p > 0.05) (Table 4).
Table 4.
ANOVA and Spearman correlations based on frequency of presence.
| p-Value | Spearman Correlation (r) | |||||
|---|---|---|---|---|---|---|
| Worm Species | CON | IVM | MOX | PYR | Treatment | MOX |
| CO. coronatus | 1.00 a | 0.77 ac | 0.20 b | 0.64 c | 3.36 × 10−6 *** | −0.44 |
| CO. labiatus | 0.17 a | 0.08 a | 0.06 a | 0.04 a | 0.403 | |
| CO. labratum | 0.19 a | 0.04 a | 0.24 a | 2.7 × 10−17 a | 0.038 * | 0.36 |
| CS. catinatum | 1.00 a | 0.83 a | 0.56 a | 0.83 a | 0.066 | |
| CS. tetracanthum | 0.02 a | 0.0 a | 0.06 a | 0.00 a | 0.281 | |
| CY. ashworthi | 1.00 a | 0.83 a | 0.47 b | 0.75 a | 0.0004 *** | −0.38 |
| CY. auriculatus | 0.42 a | 0.31 a | 0.21 a,b | 0.24 a | 0.045 * | |
| CY. insigne | 0.57 a | 0.52 a,c | 0.21 b | 0.46 b,c | 9.2 × 10−5 *** | −0.31 |
| CY. leptostomus | 0.76 a | 0.67 a | 0.27 b | 0.44 b | 1.13 × 10−5 *** | −0.32 |
| CY. nassatus | 0.85 a | 0.83 a | 0.47 b | 0.79 a | 3.04 × 10−5 *** | −0.34 |
| CY. radiatus | 0.97 a | 0.71 a | 0.33 b | 0.69 a | 0.0004 *** | −0.41 |
| CS. pateratum | 0.91 a | 0.83 a | 0.67 a | 0.81 a | 0.00159 *** | |
| CY. elongatus | 0.56 a | 0.31 a | 0.22 a | 0.38 a | 0.106 | |
| CD. bicoronatus | 0.48 a | 0.42 a | 0.16 b | 0.36 a | 0.108 | |
| CT. calicatus | 0.97 a | 0.77 a,c | 0.36 b | 0.61 b,c | 0.0005 *** | −0.31 |
| CT. goldi | 0.92 a | 0.79 a | 0.74 a | 0.78 a | 0.189 | |
| CT. longibursatus | 1.00 a | 0.83 a,b | 0.71 b | 0.89 a,b | 0.025 * | |
| CT. minutus | 0.57 a | 0.54 a | 0.13 b | 0.46 a | 0.074 | −0.36 |
CON–control; MOX–Moxidectin; IVM–Ivermectin; PYR–Pyrantel. Superscripts with different letters within row demonstrate p < 0.05 with Tukey’s all-pair comparison testing. Spearman correlations for IVM and PYR are not shown because no significant correlations were found. ANOVA significance was determined at (p ≤ 0.05) and a tendency toward significance at (0.05 ≤ p ≥ 0.10). Spearman’s coefficient, r, significance was determined at (0.3 ≤ r ≥ −0.3). The Spearman correlation, r, is considered to be fairly significant at (≥+/−0.3–<+/−0.5), moderate with (≥+/−0.5–<+/−0.7), strong with (≥+/−0.7–<+/−0.9), and substantial with (≥ +/−0.9–+/−1.0).
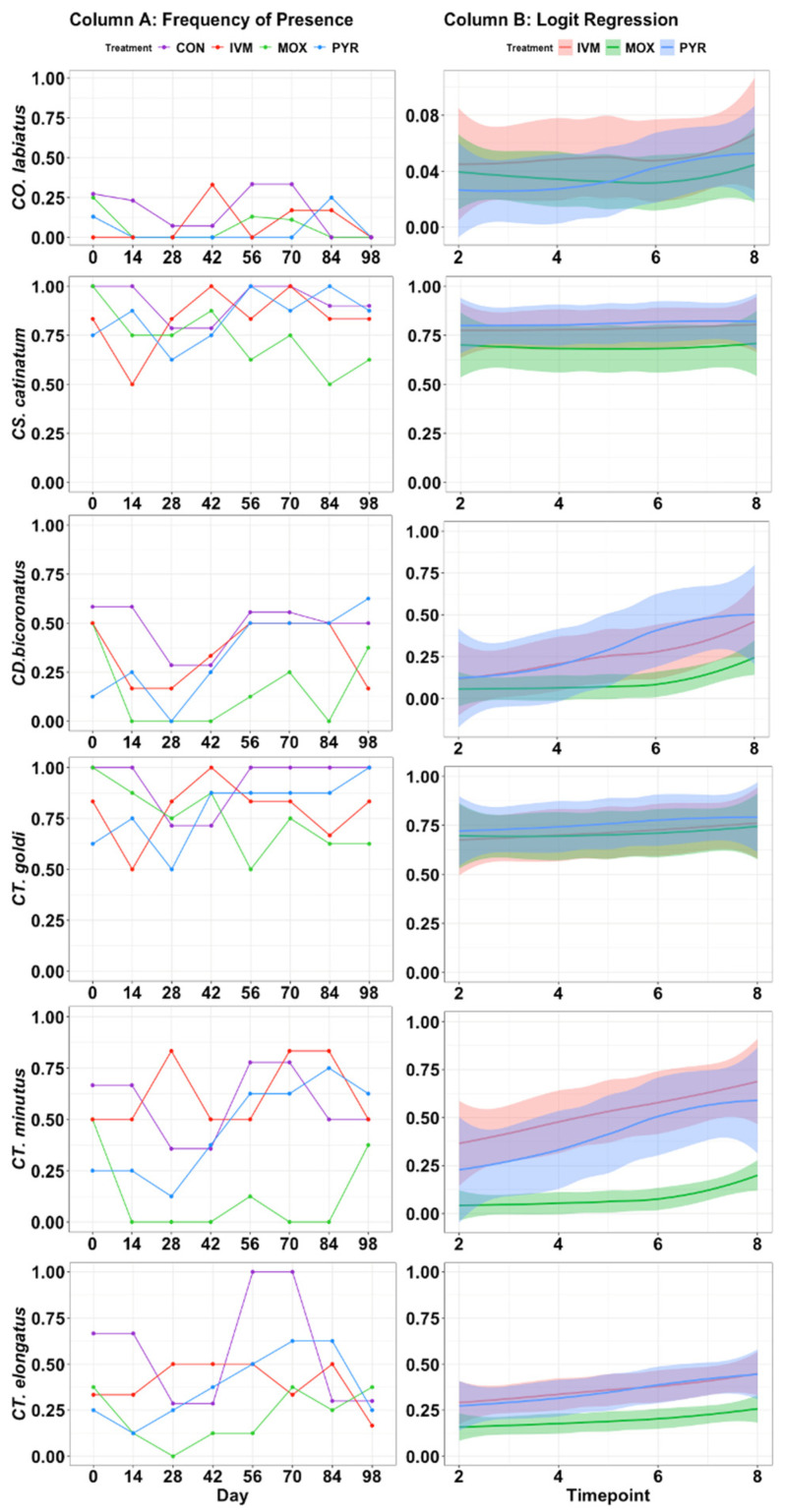
These seven species appear to be acutely responsive to treatment, but the quick reinfection is a demonstration of multidrug resistance (Figure 2, Column A). Frequency of presence infection rates were used to predict the species’ ability to reinfect the herd (Figure 2, Column B).
Figure 2.
Frequency of presence and logit regression models of the multidrug-resistant species presence in the study herd. Column A contains frequency of presence at each time point, and column B contains the binomial logistic regression predicted probability of presence plots based on CON samples. Timepoint 2 = Day 14, Timepoint 3 = Day 28, Timepoint 4 = Day 42, Timepoint 5 = Day 56, Timepoint 6 = Day 70, Timepoint 7 = Day 84, Timepoint 8 = Day 98. Shaded areas indicate 95% confidence intervals.
CS. catinatum and CT. goldi demonstrated the highest level of anthelmintic resistance with a ≥50% chance of observing these species in the herd 14 d post-treatment and no change in the probability of infection regardless of treatment or time (Figure 2, Column B). Three species, CO. labratum, CY. auriculatus, and CT. longibursatus showed a tendency to develop multidrug resistance (p (0.5 ≥ p ≤ 0.10)), and multidrug resistance could not be detected in the last seven species (p ≥ 0.10) (Table 4).
Tukey’s all-pair comparison testing determined that MOX reduced 10 species populations (CO. coronatus, CY. ashworthi, CY. insigne, CY. leptostomus, CY. nassatus, CY. radiatus, CY. elongatus, CD. bicoronatus, CT. calicatus, CT. longibursatus, and CT. minutus) and PYR reduced four species (CO. coronatus, CY. insigne, CY. leptostomus, and CT. calicatus) when compared to the CON group (p < 0.05) but none could be determined to be reduced following IVM treatment (Table 4).
Spearman correlation testing revealed that eight species (CO. coronatus, CY. ashworthi, CY. insigne, CY. leptostomus, CY. nassatus, CY. radiatus, CT. calicatus, and CT. minutus) were negatively correlated with MOX treatment (Table 4), while CO. labratum was found to be positively correlated.
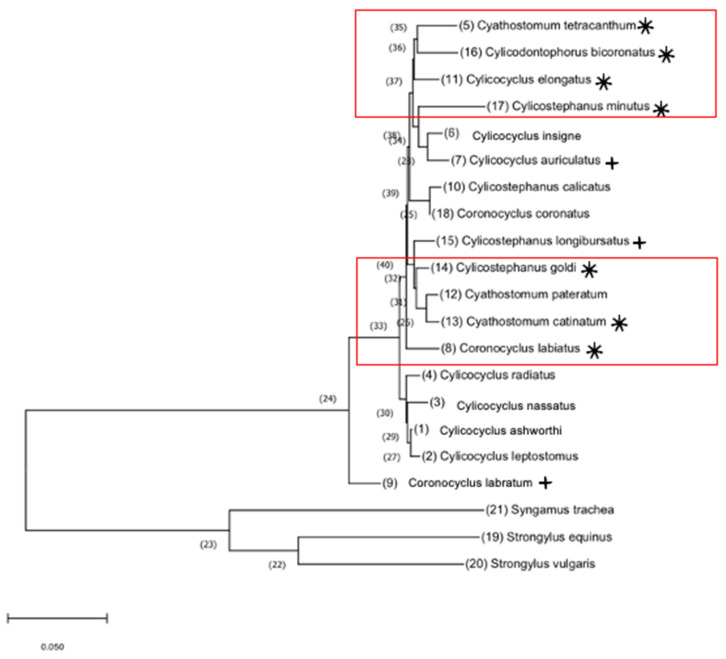
3.4. Phylogeny
The maximum likelihood tree showed that the seven species demonstrating multidrug class resistance (CO. labiatus, CS. catinatum, CS. tetracanthum, CY. elongatus, CD. bicoronatus, CT. minutus, and CT. goldi) form two closely related clades (Figure 3). The three species that showed a tendency toward multidrug resistance did not group closely together, although CY. auriculatus and CT. longibursatus emerged between the two highly resistant clades.
Figure 3.
Phylogenetic relationships of equine cyathostomins based on full-length gene sequences. Maximum likelihood tree using bootstrap inference of equine cyathostomins in this study (accession numbers listed in Table 2). Syngamus trachea (KM605251), Strongylus equinus (AP017698), and Strongylus vulgaris (GQ888718) were included as outgroups. * indicates resistance to IVM, MOX, and PYR (p ≥ 0.05). + indicates trending resistance (0.01 ≥ p < 0.05). to IVM, MOX, and PYR. Red boxes indicate the clustering of species presenting resistance.
4. Discussion
Using noninvasive sequence-based methodology for identifying the presence of cyathostomin species from fecal samples, this research demonstrates species-specific differences in the response and reemergence of cyathostomins to three commonly used anthelmintics. This work shows the efficacy of NGS strategies for profiling cyathostomins from fecal samples and challenges the prevailing strategy of treating these parasites as a monolithic group.
4.1. Fecal Egg Count Reductions and Species Frequency of Presence Reductions
While cyathostomin species variation is observed between study populations due to geography and climate, a globally recognized ‘core’ group of 10–12 species (CS. catinatum, CS. pateratum, CO. coronatus, CO. labiatus, CO. labratum, CY. nassatus, CY. leptostomus, CY. insigne, CT. longibursatus, CT. goldi, CT. calicatus, CT. minutus) has been recognized that comprises up to 99% of the cyathostomin burden in horses [4,13,54,55,56,57,58,59]. While the methods used in this study cannot measure the species composition of the parasite burden of individual horses, we did observe that five species of the ‘core’ group (CT. longibursatus, CS. catinatum, CS. pateratum, CY. ashworthi, CT. goldi) plus an additional two species (CY. radiatus, and CY. nassatus) were found in ≥80% of all CON samples (Supplementary, Table S5). The high prevalence rates of CY. radiatus, and CY. nassatus may be a result of the small sample size used in this study.
Cyathostomin transmission is known to be seasonally regulated [55,60] based on both larvae and adult worm preferences for temperature and moisture. The optimum temperature for the development of strongyle eggs and larvae ranges from 25–33 °C [1,3,55] with an upper limit of 38 °C [61] and the optimal fecal moisture level of the closely related ruminant trichostrongylids is 57–63% with larval development not occurring below 20% [62,63]. The cyathostomin lifecycle poorly tolerates desiccation [55] and freeze/thaw cycles [64,65]. This study demonstrated through the CON samples that a natural species-specific temporal response specific to the mid-Atlantic region could be observed. The fluctuation of infectivity rates between cyathostomin species could indicate increased or decreased abilities to adapt to environmental conditions to ensure survival in addition to their heightened anthelmintic resistance responses. This study did not experience any temperature or wet conditions outside of the optimal range reported for cyathostomins.
This study observed similar ERP for MOX and IVM to the ERP reported elsewhere [26]; however, our results observed a shorter ERP for PYR than previous reports [21]. The shortened ERP for PYR could be a reflection of a heightened herd specific response; however, the horses enrolled in the study did not have an extensive history of PYR treatment, and the FECR for PYR at 14 d is higher than reported in other studies that reported values of 87.1% [66] and 73.4% [15], which makes this explanation unlikely. These results may indicate that the ERP for PYR has been further reduced since reported by the AAEP Parasite Control Guidelines [26].
Anthelmintic resistance has been perpetuated by selective pressure caused by overuse and overexposure to anthelmintics. Therefore, it is expected that the species that are known to be the first infectors and members of the ‘core’ harbor the highest levels of resistance traits. In Kentucky, USA [35], foals and yearlings were found to already be infected with IVM-resistant cyathostomins (CY. nassatus, CT. longibursatus, CT. calicatus, and CT. minutus), and another foal study [67] found that the highly resistant species, CS. catinatum and CT. goldi, are among the first to infect foals suggesting similar patterns of species-specific resistance observed in this study.
This study also demonstrates differences between drug efficacy and parasite resistance as defined by Barnes et al. [55] and Dargatz et al. [68,69]. In general, anthelmintics with poor absorption rates (such as tetrahydropyrimidines (PYR)) present cyathostomins with less selective pressure to develop resistance than drugs with higher absorption rates (macrocyclic lactones (IVM and MOX)) [70]. Using targeted deworming strategies such as using drugs with lower absorption rates and treating horses with the highest FEC serves to preserve a refugia of less resistant parasites for the population as a whole [26,70]. The present study observed that PYR had a low efficacy overall (indicated by ERP and total species prevalence rates) and low resistance since 100% reduced SFP rates in some species were observed. On the other hand, IVM had higher efficacy and a higher level of resistance observed by low SFP rates. IVM demonstrated a 100% FECR as expected; however, it was unexpected to observe no differences between CON and IVM species prevalence rates. This suggests the presence of infective larvae from the environment or recently emerged adults that would be detected by NGS methods and not by the McMaster FEC. The contrasting FECR and SFPR results observed in this study may be a reflection of quick reinfection by a contaminated pasture or the emergence of the encysted larvae from the mucosal tissues. The physiology underlying excystment rates and triggers are still undiscovered because of the difficulty of observing and noninvasively detecting encysted larvae [71,72].
The FEC method has been criticized for underestimating parasite burdens due to its inability to detect the larval population [71], whereas NGS can detect the genetic material of all lifecycle stages. However, a limitation of the molecular tools is that they cannot estimate parasite abundances because an individual can contribute multiple copies of the genomic material based on the lifecycle stage [52].
The preservation of the refugia population has been praised as a positive deworming management practice because the refugia have undergone less selective pressure for anthelmintic resistance and thus preserves the anthelmintic-sensitive genetics [1,30,70]. When the refugia population is lost, the genetic pool is dominated by highly resistant genetics.
4.2. Anthelmintic Resistance
Similar to our results, Lyons et al. [73] observed multidrug class resistance in CT. goldi, CT. minutus, and CS. catinatum in a herd of benzimidazole-resistant Shetland ponies following pyrantel and oxibendazole anthelmintics. The same study [73] also observed resistance in CY. nassatus, CO. coronatus, CT. longibursatus, and CT. calicatus that was not observed in the present study, although this study found that CT. longibursatus may be showing a sign of the beginning signs of resistance to PYR.
It is possible that correlations between species and treatments were only found in MOX because of this drug’s capacity to target encysted larvae reducing a higher proportion of the parasite burden when compared to IVM and PYR. Three of the five species that persisted following MOX treatment (CY. leptostomus, CY. insigne, and CS. catinatum) appear to be part of the ‘core’ group and most likely have higher anthelmintic resistance levels. Over time these species may have decreased due to prolonged exposure to MOX treatment and its increased lipophilicity when compared to PYR and IVM [70,74]. Interestingly, CO. labratum was found to be positively correlated with MOX treatment (r = 0.36). This could either indicate less efficacy of MOX to this species, differential host response, or a seasonal effect.
4.3. Phylogeny
It has been hypothesized that closely related species may be the next to acquire multidrug resistance [27,28]. Based on the phylogenetic relationships of the cyathostomin gene sequences, the results of this study suggest emerging resistance in CY. auriculatus and CT. longibursatus.
The inheritance of the anthelmintic resistance traits is not well understood in cyathostomins due to the lack of complete resistance and inadequate models. The genetics of resistance traits in rumen gut nematodes have been reported to be expressed as incomplete dominant, complete dominant, incomplete recessive, sex-linked recessive, and autosomal recessive and will vary between parasites and drugs [75]. The H. contortus (Trichostrongylidae) rumen parasite with complete anthelmintic resistance is a close relative to equine cyathostomins (Strongylidae) as members of the Strongylida order [76,77]. In H. contortus, mutations in multiple isotype-I and -II genes and GluCl channels may be responsible for IVM and bendazole resistance [13]. In equine cyathostomins, mutations of the isotype-I and -II genes have been found in bendazole and IVM-resistant CY. nassatus and CS. catinatum. CS. catinatum, CS. tetracanthum, CY. nassatus, and CS. goldi possess a GluCl α4 subunit but demonstrate a 12% inter-specific variation within the species with an additional 4% intra-specific variation within CY. nassatus [78,79]. This demonstrates that although there is low rDNA diversity between Strongylida taxa [42,80], there is large mDNA diversity at the species level [81]. The use of molecular methods enables noninvasive monitoring and tracking of resistance traits such as these.
5. Conclusions
Anthelmintic resistance has been a persistent problem for controlling cyathostomins in horses. It is imperative to slow the rate of resistance before complete resistance occurs. This study uses NGS profiling to demonstrate that there are species-specific differences between cyathostomins in response to anthelmintic treatment. Seven species were identified to demonstrate multidrug resistance and nine species to be acutely sensitive to MOX. MOX remains the most effective and PYR the least effective according to FECR and ERP measures but the most resistance is observed with IVM. Early detection of model organisms that demonstrate complete anthelmintic resistance will enable the discovery of genetic and ecophysiological differences between anthelmintic-sensitive and -resistant species to develop more targeted deworming strategies to control the resistant populations.
Acknowledgments
We thank Brian Arisman, Ariel Strouse, Anthony Pompetti, and Rebecca Davis for their help during the trial and the Equine Microbiome Project at the University of Delaware.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11051345/s1, Table S1: Description of CON horse subjects, Table S2: Comparison of morphological and molecular ID of cyathostomin species, Table S3: Frequency of presence of cyathostomins following treatment, Table S4: Total species variation per horse at Day 0, Table S5: Species prevalence in CON samples.
Author Contributions
Conceptualization, A.S.B. and A.C.B.J.; Methodology, A.S.B. and A.C.B.J.; Formal Analysis, A.C.B.J.; Investigation, A.C.B.J.; Writing–Original Draft Preparation, A.C.B.J.; Writing–Review and Editing, A.S.B.; Supervision, A.S.B.; Project Administration, A.S.B.; Funding Acquisition, A.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Morris Animal Foundation, grant # D18EQ-802.
Institutional Review Board Statement
This experiment was approved by the University of Delaware Animal Care and Use Committee (#AUP90R).
Data Availability Statement
Sequence data has been submitted to the NCBI Sequence Read Archive within PRJNA716069.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nielsen M.K., Kaplan R.M., Thamsborg S.M., Monrad J., Olsen S.N. Climatic Influences on Development and Survival of Free-Living Stages of Equine Strongyles: Implications for Worm Control Strategies and Managing Anthelmintic Resistance. Vet. J. 2007;174:23–32. doi: 10.1016/j.tvjl.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Morariu S., Mederle N., Badea C., Dărăbuş G., Ferrari N., Genchi C. The Prevalence, Abundance and Distribution of Cyathostomins (Small Stongyles) in Horses from Western Romania. Vet. Parasitol. 2016;223:205–209. doi: 10.1016/j.vetpar.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Mfitilodze M.W., Hutchinson G.W. Prevalence and Abundance of Equine Strongyles (Nematoda: Strongyloidea) in Tropical Australia. J. Parasitol. 1990;76:487–494. doi: 10.2307/3282826. [DOI] [PubMed] [Google Scholar]
- 4.Bucknell D.G., Gasser R.B., Beveridge I. The Prevalence and Epidemiology of Gastrointestinal Parasites of Horses in Victoria, Australia. Int. J. Parasitol. 1995;25:711–724. doi: 10.1016/0020-7519(94)00214-9. [DOI] [PubMed] [Google Scholar]
- 5.Chapman M.R., French D.D., Klei T.R. Gastrointestinal helminths of ponies in louisiana: A comparison of species currently prevalent with those present 20 years ago. J. Parasitol. 2002;88:1130–1134. doi: 10.1645/0022-3395(2002)088[1130:GHOPIL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Love S., Murphy D., Mellor D. Pathogenicity of Cyathostome Infection. Vet. Parasitol. 1999;85:113–122. doi: 10.1016/S0304-4017(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 7.Garcia A., Brady H.A., Nichols W.T., Prien S. Equine Cyathostomin Resistance to Fenbendazole in Texas Horse Facilities. J. Equine Vet. Sci. 2013;33:223–228. doi: 10.1016/j.jevs.2012.06.005. [DOI] [Google Scholar]
- 8.Peregrine A.S., McEwen B., Bienzle D., Koch T.G., Weese J.S. Larval Cyathostominosis in Horses in Ontario: An Emerging Disease? Can. Vet. J. 2006;47:80–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlinger C. Equine Small Strongyles: Epidemiology, Pathology, and Control. [(accessed on 10 February 2021)];Compend. Contin. Educ. Pract. Vet. 1991 :863. Available online: https://agris.fao.org/agris-search/search.do?recordID=US9143595. [Google Scholar]
- 10.Murphy D., Love S. The Pathogenic Effects of Experimental Cyathostome Infections in Ponies. Vet. Parasitol. 1997;70:99–110. doi: 10.1016/S0304-4017(96)01153-3. [DOI] [PubMed] [Google Scholar]
- 11.Mair T.S., Cripps P.J., Ricketts S.W. Diagnostic and Prognostic Value of Serum Protein Electrophoresis in Horses with Chronic Diarrhoea. Equine Vet. J. 1993;25:324–326. doi: 10.1111/j.2042-3306.1993.tb02973.x. [DOI] [PubMed] [Google Scholar]
- 12.Gokbulut C., McKellar Q.A. Anthelmintic Drugs Used in Equine Species. Vet. Parasitol. 2018;261:27–52. doi: 10.1016/j.vetpar.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan R.M. Anthelmintic Resistance in Nematodes of Horses. Vet. Res. 2002;33:491–507. doi: 10.1051/vetres:2002035. [DOI] [PubMed] [Google Scholar]
- 14.Von Samson-Himmelstjerna G., Fritzen B., Demeler J., Schürmann S., Rohn K., Schnieder T., Epe C. Cases of Reduced Cyathostomin Egg-Reappearance Period and Failure of Parascaris Equorum Egg Count Reduction Following Ivermectin Treatment as Well as Survey on Pyrantel Efficacy on German Horse Farms. Vet. Parasitol. 2007;144:74–80. doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan R.M., Klei T.R., Lyons E.T., Lester G., Courtney C.H., French D.D., Tolliver S.C., Vidyashankar A.N., Zhao Y. Prevalence of Anthelmintic Resistant Cyathostomes on Horse Farms. J. Am. Vet. Med. Assoc. 2004;225:903–910. doi: 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen M.K., Banahan M., Kaplan R.M. Importation of Macrocyclic Lactone Resistant Cyathostomins on a US Thoroughbred Farm. Int. J. Parasitol. Drugs Drug Resist. 2020;14:99–104. doi: 10.1016/j.ijpddr.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traversa D., von Samson-Himmelstjerna G., Demeler J., Milillo P., Schürmann S., Barnes H., Otranto D., Perrucci S., di Regalbono A.F., Beraldo P., et al. Anthelmintic Resistance in Cyathostomin Populations from Horse Yards in Italy, United Kingdom and Germany. Parasit. Vectors. 2009;2:S2. doi: 10.1186/1756-3305-2-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic Resistance in Important Parasites of Horses: Does It Really Matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Canever R.J., Braga P.R.C., Boeckh A., Grycajuck M., Bier D., Molento M.B. Lack of Cyathostomin Sp. Reduction after Anthelmintic Treatment in Horses in Brazil. Vet. Parasitol. 2013;194:35–39. doi: 10.1016/j.vetpar.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs D.E., Hutchinson M.J., Parker L., Gibbons L.M. Equine Cyathostome Infection: Suppression of Faecal Egg Output with Moxidectin. Vet. Rec. 1995;137:545. doi: 10.1136/vr.137.21.545. [DOI] [PubMed] [Google Scholar]
- 21.DiPietro J.A., Hutchens D.E., Lock T.F., Walker K., Paul A.J., Shipley C., Rulli D. Clinical Trial of Moxidectin Oral Gel in Horses. Vet. Parasitol. 1997;72:167–177. doi: 10.1016/S0304-4017(97)01108-4. [DOI] [PubMed] [Google Scholar]
- 22.Demeulenaere D., Vercruysse J., Dorny P., Claerebout E. Comparative Studies of Ivermectin and Moxidectin in the Control of Naturally Acquired Cyathostome Infections in Horses. Vet. Rec. 1997;141:383–386. doi: 10.1136/vr.141.15.383. [DOI] [PubMed] [Google Scholar]
- 23.Borgsteede F.H.M., Boersma J.H., Gaasenbeek C.P.H., van der Burg W.P.J. The Reappearance of Eggs in Faeces of Horses after Treatment with Ivermectin. Vet. Q. 1993;15:24–26. doi: 10.1080/01652176.1993.9694363. [DOI] [PubMed] [Google Scholar]
- 24.Boersema J.H., Eysker M., Maas J., van der Aar W.M. Comparison of the Reappearance of Strongyle Eggs in Foals, Yearlings, and Adult Horses after Treatment with Ivermectin or Pyrantel. Vet. Q. 1996;18:7–9. doi: 10.1080/01652176.1996.9694602. [DOI] [PubMed] [Google Scholar]
- 25.Boersema J.H., Borgsteede F.H.M., Eysker M., Saedt I. The Reappearance of Strongyle Eggs in Faeces of Horses Treated with Pyrantel Embonate. Vet. Q. 1995;17:18–20. doi: 10.1080/01652176.1995.9694524. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen M.K., Mittel L., Grice A., Erksine M., Graves E., Vaala W., Tully R.C., French D.D., Bowman R., Kaplan R.M. AAEP Parasite Control Guidelines 2019. [(accessed on 10 February 2021)]; Available online: http://www.aaep.org/custdocs/ParasiteControlGuidelinesFinal.pdf.
- 27.Scare J.A., Lyons E.T., Wielgus K.M., Nielsen M.K. Combination Deworming for the Control of Double-Resistant Cyathostomin Parasites–Short and Long Term Consequences. Vet. Parasitol. 2018;251:112–118. doi: 10.1016/j.vetpar.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Gilleard J.S., Beech R.N. Population Genetics of Anthelmintic Resistance in Parasitic Nematodes. Parasitology. 2007;134.8:1133. doi: 10.1017/S0031182007000066. [DOI] [PubMed] [Google Scholar]
- 29.Redman E., Whitelaw F., Tait A., Burgess C., Bartley Y., Skuce P.J., Jackson F., Gilleard J.S. The Emergence of Resistance to the Benzimidazole Anthlemintics in Parasitic Nematodes of Livestock Is Characterised by Multiple Independent Hard and Soft Selective Sweeps. PLoS Negl. Trop. Dis. 2015;9:e0003494. doi: 10.1371/journal.pntd.0003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leathwick D.M., Sauermann C.W., Nielsen M.K. Managing Anthelmintic Resistance in Cyathostomin Parasites: Investigating the Benefits of Refugia-Based Strategies. Int. J. Parasitol. Drugs Drug Resist. 2019;10:118–124. doi: 10.1016/j.ijpddr.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corning S. Equine Cyathostomins: A Review of Biology, Clinical Significance and Therapy. Parasit. Vectors. 2009;2:S1. doi: 10.1186/1756-3305-2-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellaw J.L., Krebs K., Reinemeyer C.R., Norris J.K., Scare J.A., Pagano S., Nielsen M.K. Anthelmintic Therapy of Equine Cyathostomin Nematodes–Larvicidal Efficacy, Egg Reappearance Period, and Drug Resistance. Int. J. Parasitol. 2018;48:97–105. doi: 10.1016/j.ijpara.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkinson J.E., Freeman K.L., Lichtenfels J.R., Palfreman S., Love S., Matthews J.B. Identification of Strongyle Eggs from Anthelmintic-Treated Horses Using a PCR-ELISA Based on Intergenic DNA Sequences. Parasitol. Res. 2005;95:287–292. doi: 10.1007/s00436-004-1289-z. [DOI] [PubMed] [Google Scholar]
- 34.Traversa D., Iorio R., Otranto D., Giangaspero A., Milillo P., Klei T.R. Species-Specific Identification of Equine Cyathostomes Resistant to Fenbendazole and Susceptible to Oxibendazole and Moxidectin by Macroarray Probing. Exp. Parasitol. 2009;121:92–95. doi: 10.1016/j.exppara.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Ionita M., Howe D.K., Lyons E.T., Tolliver S.C., Kaplan R.M., Mitrea I.L., Yeargan M. Use of a Reverse Line Blot Assay to Survey Small Strongyle (Strongylida: Cyathostominae) Populations in Horses before and after Treatment with Ivermectin. Vet. Parasitol. 2010;168:332–337. doi: 10.1016/j.vetpar.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Čerňanská D., Paoletti B., Kráľová-Hromadová I., Iorio R., Čudeková P., Milillo P., Traversa D. Application of a Reverse Line Blot Hybridisation Assay for the Species-Specific Identification of Cyathostomins (Nematoda, Strongylida) from Benzimidazole-Treated Horses in the Slovak Republic. Vet. Parasitol. 2009;160:171–174. doi: 10.1016/j.vetpar.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 37.Kooyman F.N.J., van Doorn D.C.K., Geurden T., Wagenaar J.A. Semi-Quantitative Differentiation of Cyathostomin Larval Cultures by Reverse Line Blot. Vet. Parasitol. 2016;216:59–65. doi: 10.1016/j.vetpar.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Bredtmann C.M., Krücken J., Murugaiyan J., Kuzmina T., von Samson-Himmelstjerna G. Nematode Species Identification—Current Status, Challenges and Future Perspectives for Cyathostomins. Front. Cell. Infect. Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Doorn D.C.K., Ploeger H.W., Eysker M., Geurden T., Wagenaar J.A., Kooyman F.N.J. Cylicocyclus Species Predominate during Shortened Egg Reappearance Period in Horses after Treatment with Ivermectin and Moxidectin. Vet. Parasitol. 2014;206:246–252. doi: 10.1016/j.vetpar.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Hodgkinson J.E., Lichtenfels J.R., Mair T.S., Cripps P., Freeman K.L., Ramsey Y.H., Love S., Matthews J.B. A PCR–ELISA for the Identification of Cyathostomin Fourth-Stage Larvae from Clinical Cases of Larval Cyathostominosis. Int. J. Parasitol. 2003;33:1427–1435. doi: 10.1016/S0020-7519(03)00140-1. [DOI] [PubMed] [Google Scholar]
- 41.Hodgkinson J.E., Love S., Lichtenfels J.R., Palfreman S., Ramsey Y.H., Matthews J.B. Evaluation of the Specificity of Five Oligoprobes for Identification of Cyathostomin Species from Horses. Int. J. Parasitol. 2001;31:197–204. doi: 10.1016/S0020-7519(00)00161-2. [DOI] [PubMed] [Google Scholar]
- 42.Chilton N.B., Hoste H., Hung G.-C., Beveridge I., Gasser R.B. The 5.8S RDNA Sequences of 18 Species of Bursate Nematodes (Order Strongylida): Comparison with Rhabditid and Tylenchid Nematodes. Int. J. Parasitol. 1997;27:119–124. doi: 10.1016/S0020-7519(96)00158-0. [DOI] [PubMed] [Google Scholar]
- 43.Leathers D., Office of The Delaware State Climatologist Off. [(accessed on 10 February 2021)];Del. State Climatol. Available online: http://climate.udel.edu/search-results/
- 44.Larsen M.L., Ritz C., Petersen S.L., Nielsen M.K. Determination of Ivermectin Efficacy against Cyathostomins and Parascaris Equorum on Horse Farms Using Selective Therapy. Vet. J. 2011;188:44–47. doi: 10.1016/j.tvjl.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Collins A., Ke X. Primer1: Primer Design Web Service for Tetra-Primer ARMS-PCR. Open Bioinforma. J. 2012;6:55–58. doi: 10.2174/1875036201206010055. [DOI] [Google Scholar]
- 46.Elela S.A., Nazar R.N. Role of the 5.8S RRNA in Ribosome Translocation. Nucleic Acids Res. 1997;25:1788–1794. doi: 10.1093/nar/25.9.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimitrova A.D., Gecheff K.I., Ananiev E.D. Methylation pattern of ribosomal rna genes in nor-deleted and nor- reconstructed barley lines (hordeum vulgare L.). organization of igs in rdna repeat unit. Genet. Plant. Phys. 2012;2:3–14. [Google Scholar]
- 48.Magoc T., Salzberg S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichtenfels J.R., Kharchenko V.A., Dvojnos G.M. Illustrated Identification Keys to Strongylid Parasites (Strongylidae: Nematoda) of Horses, Zebras and Asses (Equidae) Vet. Parasitol. 2008;156:4–161. doi: 10.1016/j.vetpar.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pecson B.M., Barrios J.A., Johnson D.R., Nelson K.L. A Real-Time PCR Method for Quantifying Viable Ascaris Eggs Using the First Internally Transcribed Spacer Region of Ribosomal DNA. APPL Env. Microbiol. 2006;72:9. doi: 10.1128/AEM.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.R Core Team . R: A Language and Environment for Statistical Computing. R Foundatioon for Statistical Computing; Vienna, Austria: 2018. [Google Scholar]
- 54.Lyons E.T., Tolliver S.C., Drudge J.H. Historical Perspective of Cyathostomes: Prevalence, Treatment and Control Programs. Vet. Parasitol. 1999;85:97–112. doi: 10.1016/S0304-4017(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 55.Ogbourne C.P. Observations on the Free-Living Stages of Strongylid Nematodes of the Horse. Parasitology. 1972;64:461–477. doi: 10.1017/S0031182000045534. [DOI] [PubMed] [Google Scholar]
- 56.Silva A.V.M., Costa H.M.A., Santos H.A., Carvalho R.O. Cyathostominae (Nematoda) Parasites of Equus Caballus in Some Brazilian States. Vet. Parasitol. 1999;86:15–21. doi: 10.1016/S0304-4017(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 57.Kuzmina T.A., Kharchenko V.A., Starovir A.I., Dvojnos G.M. Analysis of the Strongylid Nematodes (Nematoda: Strongylidae) Community after Deworming of Brood Horses in Ukraine. Vet. Parasitol. 2005;131:283–290. doi: 10.1016/j.vetpar.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Reinemeyer C.R., Smith S.A., Gabel A.A., Herd R.P. The Prevalence and Intensity of Internal Parasites of Horses in the U.S.A. Vet. Parasitol. 1984;15:75–83. doi: 10.1016/0304-4017(84)90112-2. [DOI] [PubMed] [Google Scholar]
- 59.Zanet S., Battisti E., Labate F., Oberto F., Ferroglio E. Reduced Efficacy of Fenbendazole and Pyrantel Pamoate Treatments against Intestinal Nematodes of Stud and Performance Horses. Vet. Sci. 2021;8:42. doi: 10.3390/vetsci8030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauermann C.W., Leathwick D.M., Lieffering M., Nielsen M.K. Climate Change Is Likely to Increase the Development Rate of Anthelmintic Resistance in Equine Cyathostomins in New Zealand. Int. J. Parasitol. Drugs Drug Resist. 2020;14:73–79. doi: 10.1016/j.ijpddr.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rupasinghe D., Ogbourne C.P. Laboratory Studies on the Effect of Temperature on the Development of the Free-Living Stages of Some Strongylid Nematodes of the Horse. Z. Für Parasitenkd. 1978;55:249–253. doi: 10.1007/BF00390377. [DOI] [Google Scholar]
- 62.Mfitilodze M.W., Hutchinson G.W. Development and Survival of Free-Living Stages of Equine Strongyles under Laboratory Conditions. Vet. Parasitol. 1987;23:121–133. doi: 10.1016/0304-4017(87)90030-6. [DOI] [PubMed] [Google Scholar]
- 63.Rossanigo C.E., Gruner L. Moisture and Temperature Requirements in Faeces for the Development of Free-Living Stages of Gastrointestinal Nematodes of Sheep, Cattle and Deer. J. Helminthol. 1995;69:357–362. doi: 10.1017/S0022149X00014954. [DOI] [PubMed] [Google Scholar]
- 64.Von Ober-Blöbaum W. Untersuchungen Über Die Einwirkungen Physikalischer Einflü Sse Auf Die Larven von Pferdestrongyliden. Tierartzl Rundsch. 1932;47:812–815. [Google Scholar]
- 65.Lucker J.T. Survival and development at low tempera tures of eggs and preinfective larvae horse strongyles. J. Agric. Res. 1941;63:193. [Google Scholar]
- 66.Kaplan R.M., West E.M., Norat-Collazo L.M., Vargas J. A Combination Treatment Strategy Using Pyrantel Pamoate and Oxibendazole Demonstrates Additive Effects for Controlling Equine Cyathostomins. Equine Vet. Educ. 2014;26:485–491. doi: 10.1111/eve.12201. [DOI] [Google Scholar]
- 67.Lyons E.T., Kuzmina T.A., Tolliver S.C., Collins S.S. Observations on Development of Natural Infection and Species Composition of Small Strongyles in Young Equids in Kentucky. Parasitol. Res. 2011;109:1529–1535. doi: 10.1007/s00436-011-2460-y. [DOI] [PubMed] [Google Scholar]
- 68.Barnes E.H., Dobson R.J. Population Dynamics of Trichostrongylus Colubriformis in Sheep: Computer Model to Simulate Grazing Systems and the Evolution of Anthelmintie ResistancE. Int. J. Parasitol. 1990;20:823–831. doi: 10.1016/0020-7519(90)90019-J. [DOI] [PubMed] [Google Scholar]
- 69.Dargatz D.A., Traub-Dargatz J.L., Sangster N.C. Antimicrobic and Anthelmintic Resistance. Vet. Clin. North. Am. Equine Pract. 2000;16:515–536. doi: 10.1016/S0749-0739(17)30093-7. [DOI] [PubMed] [Google Scholar]
- 70.Sangster N.C. Pharmacology of Anthelmintic Resistance in Cyathostomes: Will It Occur with the Avermectin/Milbemycins? Vet. Parasitol. 1999;85:189–204. doi: 10.1016/S0304-4017(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 71.Dowdall S.M.J., Matthews J.B., Mair T., Murphy D., Love S., Proudman C.J. Antigen-Specific IgG(T) Responses in Natural and Experimental Cyathostominae Infection in Horses. Vet. Parasitol. 2002;106:225–242. doi: 10.1016/S0304-4017(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 72.Stratford C.H., McGorum B.C., Pickles K.J., Matthews J.B. An Update on Cyathostomins: Anthelmintic Resistance and Diagnostic Tools. Equine Vet. J. 2011;43:133–139. doi: 10.1111/j.2042-3306.2011.00397.x. [DOI] [PubMed] [Google Scholar]
- 73.Lyons E.T., Tolliver S.C., Drudge J.H., Collins S.S., Swerczek T.W. Continuance of Studies on Population S Benzimidazole-Resistant Small Strongyles in a Shetland Pony Herd in Kentucky: Effect of Pyrantel Pamoate (1992–1999) Vet. Parasitol. 2001;94:247–256. doi: 10.1016/S0304-4017(00)00382-4. [DOI] [PubMed] [Google Scholar]
- 74.Gokbulut C., Nolan A.M., Mckellar Q.A. Plasma Pharmacokinetics and Faecal Excretion of Ivermectin, Doramectin and Moxidectin Following Oral Administration in Horses. Equine Vet. J. 2001;33:494–498. doi: 10.2746/042516401776254835. [DOI] [PubMed] [Google Scholar]
- 75.Dobson R.J., Lejambre L., Gill J.H. Management of Anthelmintic Resistance: Inheritance of Resistance and Selection with Persistent Drugs. Int. J. Parasitol. 1996;26:993–1000. doi: 10.1016/S0020-7519(96)80078-6. [DOI] [PubMed] [Google Scholar]
- 76.Terrill T.H., Kaplan R.M., Larsen M., Samples O.M., Miller J.E., Gelaye S. Anthelmintic Resistance on Goat Farms in Georgia: Efficacy of Anthelmintics against Gastrointestinal Nematodes in Two Selected Goat Herds. Vet. Parasitol. 2001;97:261–268. doi: 10.1016/S0304-4017(01)00417-4. [DOI] [PubMed] [Google Scholar]
- 77.Durette-Desset M.-C., Beveridge I., Spratt D.M. The Origins and Evolutionary Expansion of the Strongylida (Nematoda) Int. J. Parasitol. 1994;24:1139–1165. doi: 10.1016/0020-7519(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 78.Cwiklinski K., Merga J.Y., Lake S.L., Hartley C., Matthews J.B., Paterson S., Hodgkinson J.E. Transcriptome Analysis of a Parasitic Clade V Nematode: Comparative Analysis of Potential Molecular Anthelmintic Targets in Cylicostephanus Goldi. Int. J. Parasitol. 2013;43:917–927. doi: 10.1016/j.ijpara.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Tandon R., LePage K.T., Kaplan R.M. Cloning and Characterization of Genes Encoding α and β Subunits of Glutamate-Gated Chloride Channel Protein in Cylicocyclus Nassatus. Mol. Biochem. Parasitol. 2006;150:46–55. doi: 10.1016/j.molbiopara.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Dorris M., De Ley P., Blaxter M.L. Molecular Analysis of Nematode Diversity and the Evolution of Parasitism. Parasitol. Today. 1999;15:188–193. doi: 10.1016/S0169-4758(99)01439-8. [DOI] [PubMed] [Google Scholar]
- 81.Blouin M.S., Yowell C.A., Courtney C.H., Dame J.B. Host Movement and the Genetic Structure of Populations of Parasitic Nematodes. Genetics. 1995;141:1007–1014. doi: 10.1093/genetics/141.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data has been submitted to the NCBI Sequence Read Archive within PRJNA716069.