Abstract
In tomato (Solanum lycopersicum), there are at least three SlMLO (Mildew resistance Locus O) genes acting as susceptibility genes for the powdery mildew disease caused by Oidium neolycopersici, namely SlMLO1, SlMLO5 and SlMLO8. Of the three homologs, the SlMLO1 gene plays a major role since a natural mutant allele called ol-2 can almost completely prevent fungal penetration by formation of papillae. The ol-2 allele contains a 19-bp deletion in the coding sequence of the SlMLO1 gene, resulting in a premature stop codon within the second cytoplasmic loop of the predicted protein. In this study, we have developed a new genetic resource (M200) in the tomato cv. Micro-Tom genetic background by means of ethyl methane sulfonate (EMS) mutagenesis. The mutant M200 containing a novel allele (the m200 allele) of the tomato SlMLO1 gene showed profound resistance against powdery mildew with no fungal sporulation. Compared to the coding sequence of the SlMLO1 gene, the m200 allele carries a point mutation at T65A. The SNP results in a premature stop codon L22* located in the first transmembrane domain of the complete SlMLO1 protein. The length of the predicted protein is 21 amino acids, while the SlMLO1 full-length protein is 513 amino acids. A high-resolution melting (HRM) marker was developed to distinguish the mutated m200 allele from the SlMLO1 allele in backcross populations. The mutant allele conferred recessive resistance that was associated with papillae formation at fungal penetration sites of plant epidermal cells. A comprehensive list of known mlo mutations found in natural and artificial mutants is presented, which serves as a particularly valuable resource for powdery mildew resistance breeding.
Keywords: Solanum lycopersicum, Micro-Tom, EMS mutagenesis, powdery mildew, Oidium neolycopersici, SlMLO1
1. Introduction
Tomato (Solanum lycopersicum L.) is a model crop species of high economic value with interesting developmental features such as compound leaves, fleshy fruits, and sympodial shoot branching. The amount of information currently available for the domesticated tomato is abundant. Its genome [1], transcriptome (Tomato Functional Genomics Database, http://ted.bti.cornell.edu/) and metabolome [2] are available, as well as functional genomic tools, like RNA interference (RNAi, [3,4,5]), transcription activator-like effector nucleases (TALENs, [6]), and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9-based directed mutagenesis [7,8].
An important aspect of the domesticated tomato is its lack of genetic diversity because of years of selection for a limited set of traits, such as fruit shape and size [9]. However, given the upcoming challenges for agriculture regarding climate change and food safety, it has become a prominent issue to improve tomato also for resistance or tolerance to biotic and abiotic stresses [10]. One way to achieve this goal is to use the diversity present in wild relatives. It has been a general practice in tomato breeding to use wild relatives as a donor for introgression of valuable traits present in tomato varieties. Another way to increase genetic diversity is to introduce new mutations artificially. Chemical and physical mutagenesis are frequently used for this purpose in most of the economically important crop species [11]. Of the chemical mutagens, ethyl methane sulfonate (EMS) is most commonly used. EMS selectively alkylates guanine bases, which, during DNA replication, are preferably coupled with a thymine over a cytosine residue, resulting in a random point mutation. Most of these mutations (70–99%) consist of substitutions from C to T or from G to A (abbreviated as C/G to T/A) [12,13,14]. Five EMS tomato populations were developed during the last years, two of which used the tomato cultivar Micro-Tom (MT) [15,16,17,18,19]. In contrast to most of the cultivated tomatoes, MT is a miniature determinate tomato cultivar (8–10 cm when grown in 14 cm diameter pots) and has a short life cycle (70–90 d from sowing to fruit ripening) [15,20]. MT has been compared to Arabidopsis as a model system to carry out molecular research in tomato. The Japanese mutant database, TOMATOMA has become available, together with MT’s genome and a whole-genome resequencing analysis of EMS-MT mutants [21,22]. Collectively, these features make MT a suitable cultivar for large-scale mutagenesis studies.
Breeders aim at finding and introducing durable resistance in cultivated crops. One way to achieve this consists of using impaired plant susceptibility genes (S-genes) [23,24]. The Mildew resistance locus o (MLO) gene is the best characterized example of S-genes in several crops. Natural and EMS-induced loss-of-function mutants of MLO were first detected in powdery mildew (PM)-resistant barley. This mlo-based resistance has been successfully employed in agriculture for nearly five decades [25,26,27].
MLO is a member of a medium-sized gene family [28]. The MLO genes encode plant transmembrane proteins which typically span across the plasma membrane seven times and end in the cytoplasm with a C-terminal domain. MLO proteins seem to be involved in many biological processes, although their core biochemical function is still unknown. These proteins likely act in signal transduction in a calcium and calmodulin dependent manner [29,30]. In Arabidopsis and barley, pleiotropic effects associated with the disruption of MLO function consist of aberrant root architecture (AtMLO4 and AtMLO11) [31,32], reduced fertility (AtMLO7) [33], induced lesions (HvMLO1 and HvMLO3) [34,35], early leaf senescence (HvMLO5) [36], reduced root colonization by mycorrhizal fungi [37], and susceptibility to several hemibiotrophic and necrotrophic pathogens [30,38,39,40,41].
The MLO gene conferring PM resistance is highly conserved in plant species and can be tracked back to green algae [42]. Each plant species contains a certain number of MLO paralogs. In a given species, identification of the respective MLO paralogs that confer PM susceptibility is a prerequisite for the subsequent utilization of mlo alleles. Members of clade IV in monocots and V in dicots are described as susceptibility factors towards pathogens causing the PM disease [42,43,44,45]. Functional proteins of these genes are required by adapted PM pathogens to be able to penetrate the cell wall and cause disease [30,46,47]. In tomato, the SlMLO gene family comprises 16 homologs, of which four belong to clade V, namely SlMLO1, SlMLO3, SlMLO5 and SlMLO8 [48].
In addition to barley, natural mlo mutants of different types (i.e., transposon insertions, single nucleotide polymorphisms, and small indels) have been found in many plant species, including cucumber (CsaMLO8 [49]), melon (CmMLO2 [50]), pea (er-1, -2, -3, and -4 [51], er-6 [52], and er-7 [53]), rose (RhMLO4 [54]), apple (MdMLO19 [55]), and tobacco (NtMLO2 [56]). These examples demonstrate that naturally occurring allelic variants represent a rich source for mlo-mutants. In addition, loss-of-function mutations have been obtained through targeted genome editing technologies. These include TALEN-induced Tamlo triple-mutant lines and CRISPR/Cas9-mediated mutagenesis of TaMLO-A1 allele in hexaploid wheat [57], CRISPR/Cas9-induced SlMLO1 mutant in tomato [58], and CRISPR/Cas9-induced VvMLO3 mutant in grapevine [59]. When looking at all the mlo mutant alleles obtained with mutagens, the highest number is found in barley (33 [60]), followed by wheat (16 [61]), pea (3 [62,63]), and petunia (2 [64]). In tomato and several other plant species (apple, melon, pea, tobacco) no pleiotropic effects are described for mutants of MLO genes conferring resistance to adapted PM species [30].
A naturally mutated allele of the SlMLO1 gene Solyc04g049090.3, called ol-2, was described in the past years [65,66,67,68]. The ol-2 variant contains a 19-bp deletion in the coding sequence resulting in a premature stop codon within the second cytoplasmic loop of the predicted protein. This mutation, first identified in S. lycopersicum var. cerasiforme, when in homozygous state, mediates broad-spectrum resistance to Oidium neolycopersici, recently also referred to as Pseudoidium neolycopersici (Mycobank database; https://www.mycobank.org/page/Basic%20names%20search). The ol-2 conferred resistance is characterized by the formation of papillae beneath the fungal appressoria, which can significantly reduce the fungal penetration [69]. In the following years, transgenic RNAi lines were developed to silence simultaneously multiple clade V-SlMLO homologs [48,69]. One construct, in particular, was described to silence SlMLO1, SlMLO5 and SlMLO8. When ol-2 plants were compared to plants of the RNAi lines, a higher level of resistance was observed associated with the latter. Because of these results, it was concluded that the three SlMLO genes contribute to the tomato susceptibility towards PM, with SlMLO1 having the major role [48].
In the present study, we describe the in-house development of an EMS mutant population of the tomato cultivar MT. The purpose of this EMS population is to select mutants resistant to different tomato pathogens, and to identify the causal mutant S-genes. In this EMS population, a mutant called M200 was uncovered that showed profound resistance to tomato PM. It was shown to be defective in the SlMLO1 gene. Then, we performed a comparison of the novel allele with the ol-2 mutation in different genetic backgrounds as well as RNAi lines in which three clade V SlMLO homologues are silenced. Results and implications are further presented and discussed in the context of mlo mutations occurring in other plant species.
2. Materials and Methods
2.1. Development of the Micro-Tom EMS Population
Seeds of the tomato cultivar Micro-Tom (MT) were obtained from the Beekenkamp Plants B.V. company (Maasdijk, The Netherlands). First, to determine which concentration of EMS (ethyl methane sulfonate) solution should be used for efficient mutagenesis, a pilot experiment was performed. A batch of approximately 1000 MT seeds (M0) was presoaked in distilled water for 8 h and treated overnight with three concentrations of an EMS solution, 0.5% (v/v), 0.75% (v/v) and 1% (v/v), respectively. The obtained M1 seeds were then thoroughly washed with distilled water, sown in the greenhouse of Unifarm of Wageningen University and Research, The Netherlands, and grown at a day/night temperature of 21/19 °C and relative humidity of 60% during a 16 h day/8 h night regime. Three-week-old seedlings were transplanted individually to 14 cm pots and grown until 5 to 10 fruits per plant could be harvested. M2 seeds were collected from these fruits, surface sterilized in 2% (v/v) of HCl (Hydrogen chloride) and disinfected in phosphate solution for a minimum of one hour, followed by air drying.
EMS treatment of approximately 1000 MT seeds (M0) was repeated four more times (five batches in total). Since several studies showed that the 1% EMS concentration yielded almost two fold more mutations per genome than other concentrations, like 0.5% or 0.75%, without affecting too much the rate of viability [19,21], only the 1% EMS dilution was used for the latter four seed batches.
2.2. Powdery Mildew Disease Assays and Quantification of Relative Fungal Biomass
Multiple disease assays were performed to test for PM resistance. These assays involved the screening of approximately 2000 M1 plants originating from the first two batches of EMS treated M0 seeds, testing segregating families (BC1S1 families derived from M200 × MT and F2 from M200 × MM) for linkage analysis of PM resistance and the m200 allele, as well as further generations and control genotypes for PM phenotypic evaluation and histological analysis. Four-week-old plants were inoculated with a fresh suspension of Oidium neolycopersici (On-Wageningen isolate) conidiospores. The On isolate was maintained on tomato cv. Moneymaker (MM) as previously described [69]. The suspension was made by rinsing heavily sporulating leaves of the cultivar MM with tap water and adjusting this suspension to a concentration of 2 × 104 spores per milliliter. Ten to fifteen days after inoculation, the plants were visually inspected. To each plant, a score was given based on a disease index (DI) varying from 0 to 3, where 0 indicates that no fungal sporulation is visible and three that fungal colonies cover most of the surface of the inoculated leaves, as in the cv. MM.
For the quantification of relative PM fungal biomass in infected mutant, RNAi-silenced and control genotypes (see Section 2.3 Plant Materials), the third and fourth true leaf of each infected plant were harvested and snap-frozen in liquid nitrogen. The samples were ground in liquid nitrogen with mortar and pestle. Plant and fungal genomic DNAs (gDNA) were isolated using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Isolated DNA was used for qPCR with the primer pairs On_ITS, designed on O. neolycopersici internal transcribed spacer (ITS) sequence (GenBank accession number EU047564), and SlEF1α, designed on the tomato Elongation Factor 1α (Ef1α) as reference gene for normalization (Table S1). qPCR was performed using the CFX96 Real-Time PCR machine (Bio-Rad, Hercules, CA, USA). Each 10 μL reaction contained 300 nM of each primer, 1 μL (10ng) gDNA template and 1 × iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). Cycling conditions initiated with a denaturation step at 95 °C for 3 min., followed by 40 cycles of 10 sec. denaturation at 95 °C and 30 sec. annealing and extension at 60 °C, finished by a melt cycle of 0.5 °C increment per 10 sec. from 65 °C to 95 °C. Relative fold-change of the ratio between fungal and tomato gDNAs was calculated by the 2−∆∆Ct method [70]. Four biological replicates and two technical replicates were used in this experiment. Tukey’s multiple comparison test was performed in order to assess significant differences between the genotypes.
2.3. Plant Materials
The identified mutant M200 showing resistance to powdery mildew was either crossed with Moneymaker (MM) or backcrossed to MT to obtain F1 or BC1 seeds which were harvested from each fruit and kept separately. BC1 plants derived from three individual crosses (fruits) of M200 × MT were tested with powdery mildew and four plants per family were kept for self-pollination and seed production. Two of the three corresponding progenies (BC1S1) were further tested with powdery mildew and selected for seed production if showing a resistant phenotype. Three F1 plants from the cross M200 × MM were allowed to self-pollinate. Their progenies (F2) were tested with powdery mildew. The disease test and the visual inspection of further generations were performed as for the M1 plants. Individual F2 plants were selected that were MM-like in their morphology (lacking the dwarf and determinate growth characteristics of cultivar MT), and were homozygous for the m200 allele. The selected F2 plants were kept for the production of F3 seeds and subsequently F4 progeny was obtained.
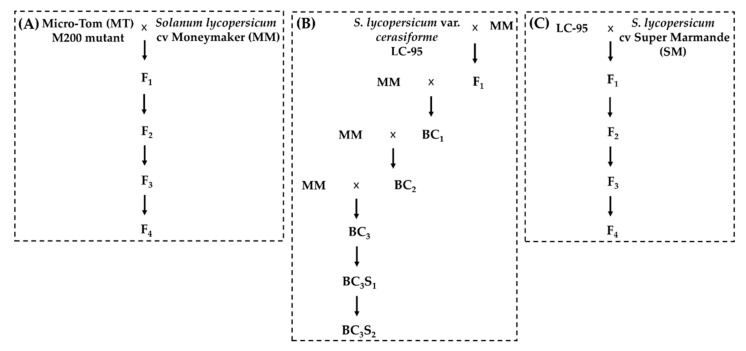
For histological analysis, eight F4 plants carrying the m200 allele derived from two original crosses M200 × MM were chosen (Figure 1). In addition, three plants of two BC3S2 lines derived from a cross between a resistant plant homozygous for the ol-2 allele and MM were included (Figure 1) [48,65]. Moreover, we added three resistant F4 plants also carrying the ol-2 allele derived from the self-pollination of the F1 from a cross between the original line LC-95 of S. lycopersicum var. cerasiforme and the cv. Super Marmande (SM) (Figure 1). For simplicity during the description of Figures and Tables, the first ol-2 genotype is referred to as ol-2_MM and the second as ol-2_SM. Furthermore, three transgenic plants of a T2 family carrying the RNAi construct able to silence SlMLO1, SlMLO5, and SlMLO8 as described in Zheng et al. [48] were selected. As susceptible control, three MM plants were included in this experiment. The transgenic plants carrying the RNAi construct were selected by standard PCR performed on DNA isolated with the 2% CTAB method [71] from all the germinated seedlings, using two primer pairs, one targeting the NPTII gene and the other the 35S promoter. Primer sequences are shown in Table S1.
Figure 1.
Pedigree scheme of (A) F4 plants homozygous for the m200 allele, (B) BC3S2 lines homozygous for the ol-2 allele in Solanum lycopersicum cv. Moneymaker background (ol-2_MM) and (C) F4 plants carrying the ol-2 allele in cv. Super Marmande background (ol-2_SM).
2.4. Cloning of the SlMLO1 Coding Sequence from the Mutagenized Resistant Micro-Tom Plant M200
The third and fourth true leaves of the M200 plant and two MT plants (not subjected to the EMS treatment) were collected after the powdery mildew test and immediately frozen in liquid nitrogen. The samples were ground in liquid nitrogen with mortar and pestle. Total RNA was isolated with the RNeasy® plant mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The concentration of the total RNA was measured using the Nanodrop. Approximately 1 μg of RNA was treated with DNase (Invitrogen) to remove any DNA contamination. This treated RNA was used in a one-step PCR with the SuperScript® III (Invitrogen, Waltham, MA, USA) and the specific primers for the SlMLO1 gene used in Zheng et al. [48] (sequences in Table S1). The amplified PCR products were run on a 1% agarose gel. The bands with the desired product size (1743-bp) were excised from the gel and the products recovered using the QIAquick gel extraction kit (Qiagen). The eluted PCR products were sequenced (Table S1) with primers used for amplification of the full-length cDNA as well as two primers (SlMLO1_seqA and SlMLO1_seqB) located in between and the obtained sequences aligned with the known SlMLO1 coding sequence (cds) of Heinz (Solyc04g049090.3) using the package MegAlign of the software DNASTAR® Lasergene8. The predicted protein derived from the SlMLO1 sequence cloned from the M200 plant was analyzed using the TMHMM software (http://www.cbs.dtu.dk/services/TMHMM/, accessed on 9 November 2020) and the PROTTER web tool to predict sequence features and visualize the protein [72].
2.5. Development of a HRM Marker for Detection of the Mutation in the SlMLO1 Gene
In order to follow the segregation of the SNP associated with the m200 allele in BC1 generation (19 plants) of the backcrosses between the M200 plant × MT, the selfing BC1S1 progenies (144 plants) and F2 families (115 plants) of the crosses between M200 plant × MM, the DNA of each plant was isolated using 2% CTAB in a protocol adapted for a 96-well plate [71]. The quantity and integrity of genomic DNA were determined using the Nanodrop and running 1 μL of the isolated DNA on an agarose gel (1%), respectively.
Primers amplifying a gDNA fragment of 406-bp containing the mutation site were designed for a high-resolution melting assay (HRM). The sequences of these primers are reported in the Table S1. PCR amplifications were carried out in a 10 μL reaction mixture containing 10 ng of genomic DNA, 2 μL of 5× PCR buffer, 0.4 μL of 5 mM dNTPs, 0.5 U Phire™ Hot Start II DNA Polymerase (ThermoFisher, Waltham, MA, USA), 0.25 μM of forward and reverse primer (10 mM each) and 1 µL of LC GreenPlus (Idaho Technology Inc., Salt Lake City, UT, USA). The amplification included an initial denaturation at 98 °C for 30 s, followed by 41 cycles of 98 °C for 5 s, 60 °C for 5 s and 72 °C for 15 s, and finishing with a final elongation at 72 °C for 30 s. The HRM genotyping was performed on a Light Scanner instrument (HR96 model, Idaho technology Inc., Salt Lake City, UT, USA) with continuous melting curve acquisition (10 acquisitions per °C) during a 0.1 °C/s ramp from 40 to 95 °C. Data were retrieved and analyzed using the Light Scanner software followed by manual curation of the obtained genotype calls. DNA samples from MT or MM plants (homozygous for the wild-type SlMLO1 allele), M200 plants (homozygous for the mutated m200 allele) and BC1 (M200 × MT) or F1 (M200 × MM) plants (heterozygous) were used as controls to establish the reference HRM curves.
2.6. Histological Analysis
The powdery mildew disease assay was performed on four-week-old plants as described in Section 2.2, but using a higher concentration of On spores equal to 3 × 105 conidia/mL.
Samples from four plants of each genotype were collected 72 h postinoculation, bleached in a 1:3 (v/v) acetic acid/ethanol solution, stained 48 h later by boiling in 0.005% trypan blue in lactophenol: ethanol (1:2 v/v) solution for 3-5 min and finally cleared in a nearly saturated aqueous solution of chloral hydrate (5:2 w/v). Analysis was conducted using a Zeiss Axiophot bright field microscope. For quantification of fungal structures approximately 100 infection units were analyzed per genotype, from at least two different plants per genotype. An infection unit (IU) was defined as a spore with a germination tube. For each IU, the presence of haustorium or papilla was recorded. For some IU, photos were taken using the 100x magnification coupled with the differential interface contrast (DIC) technique at different focus to be able to observe all the fungal structures eventually developed.
3. Results
3.1. A Novel EMS mlo Mutant (M200) Shows Resistance to Powdery Mildew
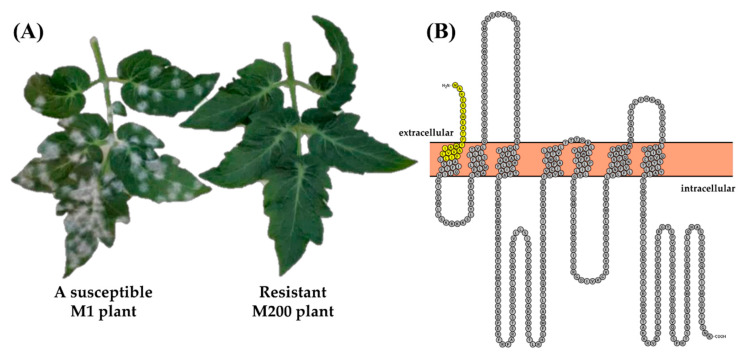
An EMS-mutagenized population of tomato cv. MT was developed and phenotypically screened for resistance to the powdery mildew pathogen O. neolycopersici (On). During the EMS treatment, the 1% v/v EMS concentration was mostly used to maximize the genomic variation with a minimum decrease in viability. The M1 plants derived from the first two batches of EMS treatment (about 1000 seeds per batch) were inoculated with spores of the pathogen On by spray inoculation. In the first group of approximately 1000 M1 plants, one plant (M200) showed no fungal sporulation, while all other plants were severely infected (Figure 2A).
Figure 2.
A novel EMS mlo mutant (M200) shows resistance to powdery mildew. (A) Contrasting phenotypes of susceptible leaves of an M1 plant and resistant leaves of the M200 plant after Oidium neolycopersici inoculation. (B) Schematic representation of the SlMLO1 protein of the cv. Heinz. The predicted truncated m200 protein is indicated in yellow, while the region that is absent in m200 is indicated in grey.
The M1 plants were allowed to self-pollinate and M2 seeds were collected. All the tested M200 M2 plants were free of PM symptoms, and thus resistant. Except for the resistant phenotype, no other morphological differences were observed in M200 M1 and M2 plants compared to wild-type MT (not subjected to the EMS treatment).
To find the causal mutation for the highly resistant phenotype of the M200 plant and its M2 progeny, SlMLO1 was chosen as the first candidate gene. The coding sequence (cds) of the SlMLO1 gene in M200 was obtained. A SNP (T65A; SL4.0ch04:38,795,717 coding strand position) was detected in the SlMLO1 cds of the M200 plant compared to the sequence in MT and tomato cultivar Heinz (Figure S1). This point mutation results in a premature stop codon (L22*). This stop codon at position 22 in the full-length SlMLO1 protein sequence of Heinz is located in the first transmembrane domain (Figure 2B). The resulting truncated protein contains 21 amino acids (aa) instead of 513 aa (Figure 2B). Although the N-terminal domain of the wild-type SlMLO1 protein is predicted to be extracellular, the hypothetical truncated 21 aa protein of the M200 mutant does not contain a functional transmembrane region and is predicted to be located in the intracellular space. The premature stop codon location from M200 differs from the stop codon identified in the ol-2 allele, located in the second intracellular loop [68]. This loss-of-function allele of the SlMLO1 gene is therefore novel and was named m200.
3.2. The m200 Allele Is a Unique SlMlo1 Loss-of-Function Allele
The T-to-A tranversion in the m200 mutant is not a typical EMS-induced mutation. In order to verify whether any natural impaired SlMLO1 allele is already present in MT, the full-length nucleotide sequence of the Heinz SlMLO1 mRNA (1878-bp) was compared with the full length transcript AK322443 (from clone LEFL1037DE09) of MT SlMLO1 (1847-bp), obtained from NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 3 June 2016). Additionally, successful cloning of full-length cDNA sequence was accomplished by PCR amplification of mRNA derived from our wild-type MT plants. A multialignment of these SlMLO1 sequences did not reveal any mutation (Figure S1). Thus, these findings indicated that the SlMLO1 gene in MT does not differ from the one in other cultivated tomatoes, like Heinz and MM. In addition, we searched for any predicted mutations of the SlMLO1 gene among the sequenced 360 tomato accessions in the Tomato 360 variants SL2.50 genome browser at SGN (https://solgenomics.net/jbrowse_solgenomics/, accessed on 1 April 2021) for position SL2.50ch04:39557939 [73]. The output of this analysis also revealed that there are no predicted natural mutations at the T65 position where the m200 SNP occurs. These results suggest that the point mutation in the SlMLO1 gene in the M200 mutant is a new and unique mutation.
3.3. The Resistant Phenotype Fully Cosegregates with the Novel m200 Allele
To analyze the association of PM resistant phenotype with the presence of the m200 allele, the M200 mutant was backcrossed to MT and additionally crossed to MM (Figure 1). Initially, three BC1 families derived from different fruits of the cross between M200 and MT were tested with On. All 19 BC1 plants (12 plants of BC1 family 1, three of family 2, and four of family 3) showed clear fungal sporulation, and were as susceptible as the controls, MM and MT. A high-resolution melting (HRM) marker was developed (Table S1) which could clearly distinguish the SlMLO1 allele carried by the wild-type MT/MM from the mutated m200 allele. All 19 BC1 plants were heterozygous for the m200 allele.
Two BC1S1 families derived from M200 × MT and three F2 from M200 × MM were produced and their phenotypic responses to On were assessed (Tables S2 and S3). All BC1S1 and F2 resistant plants were homozygous for the m200 allele, and all susceptible plants were either homozygous or heterozygous for the MT/MM allele. Overall, these results confirm that PM resistance cosegregates with the m200 allele.
3.4. Full Resistance Provided by the m200 Allele
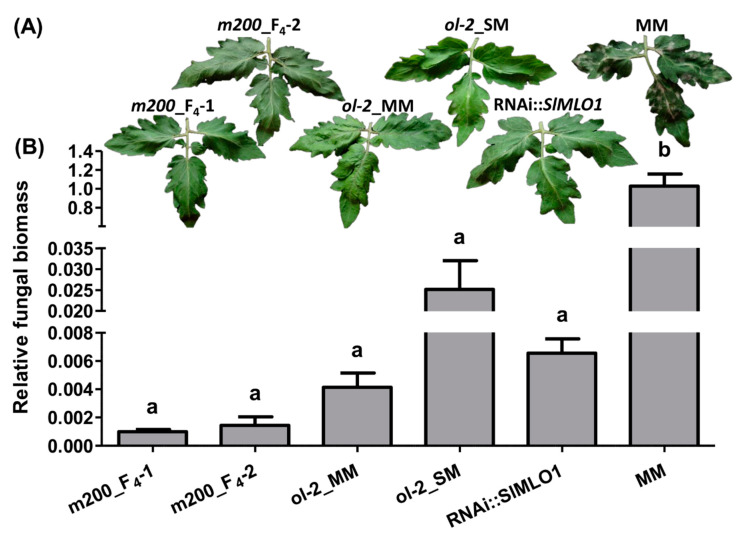
To compare the level of resistance conferred by the newly identified m200 allele with other mutants of the SlMLO1 gene, we performed a disease test where we included the m200 mutant in MM background (F4 generation), the ol-2 mutant in two different genetic backgrounds (MM and Super Marmande [SM]; Figure 1), as well as the RNAi::SlMLO1 line in which the SlMLO1, SlMLO5 and SlMLO8 genes are silenced [48].
The control MM plants were heavily infected at 18 days post inoculation (dpi) and showed significantly higher fungal biomass when compared with all the other genotypes (Figure 3). For the ol-2 mutant in SM background (hereafter, ol-2_SM), no fungal sporulation was observed on the third and fourth leaves (Figure 3A). Occasionally, weak mycelium growth could be seen on the first and second true leaves, while no fungal sporulation was observed on all plants of the m200 mutant (hereafter m200_F4), the ol-2 mutant in MM background (hereafter, ol-2_MM) and the RNAi::SlMLO1 line, throughout the entire disease assay (Figure 3A). Compared to the other mutants, although not significant, fungal biomass was reduced in plants carrying the m200 allele (Figure 3B).
Figure 3.
Phenotypic evaluation of the powdery mildew symptoms and relative fungal biomass quantification. Panel (A) shows leaves collected 18 days after the pathogen inoculation. Panel (B) refers to fungal biomass measured by relative quantification of the ratio between Oidium neolycopersici and plant gDNAs on different genotypes (F4 plants carrying the m200 allele, plants carrying the ol-2 allele in Moneymaker (MM) and Super Marmande background, a plant carrying the RNAi::SlMLO1 construct, and MM). Bars show standard errors based on four plants. Columns labeled with different letters are significantly different at p < 0.05 according to Tukey’s multiple comparison test.
3.5. Papilla Formation Is Associated with Resistance in the m200 Mutant
A histological experiment was conducted to (1) study the resistance mechanism of the M200 resistant mutant and (2) compare the level of resistance conferred by the m200 mutant allele in MM background with other genotypes including the ol-2_MM, ol-2_SM and RNAi::SlMLO1 lines.
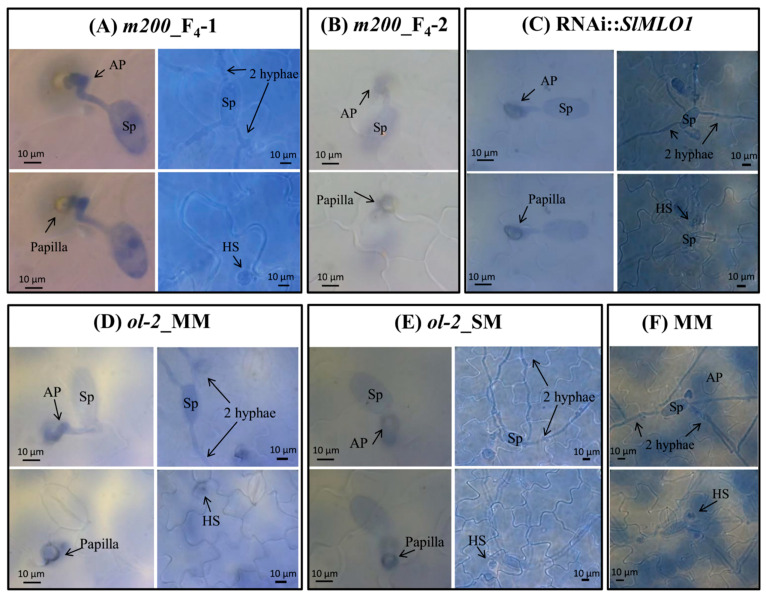
Compared to MM, fungal growth on all individuals of the M200 mutant was considerably reduced due to the formation of a papilla beneath the appressorium (Figure 4 and Table 1). In MM, hardly any papillae were formed. In contrast, the percentage of papilla formation per infection unit (IU) was higher than 32% in the mutant genotypes, varying from 32.7% in the m200_F4-2 to 71.6% in RNAi::SlMLO1 (Figure 4 and Table 1). Simultaneously, the percentage of haustorium formation per infection unit was drastically decreased in all the tested mutant genotypes compared to MM. No haustoria were observed in m200_F4-2, while in the other mutants haustoria were observed at a rate of 10.3% in m200_F4-1, 4.4% in ol-2_MM, 11% in ol-2_SM, and 5.5% in RNAi::SlMLO1 (Figure 4 and Table 1).
Figure 4.
Microscopic observations on powdery mildew infection and development of the infection units (IU) of Oidium neolycopersici on six different genotypes. In each panel photos are taken from (A) and (B) F4 plants carrying the m200 allele, (C) a plant carrying the RNAi::SlMLO1 construct, (D) and (E) plants carrying the ol-2 allele in Moneymaker (MM) and Super Marmande (SM), (F) MM, respectively. Photos of two IU/genotype are shown, except for the MM and F4-2 carrying the m200 allele where only one IU is shown. Each photo is taken with different focus to observe all the fungal structures and papillae, from the most superficial to the deepest ones. Sp = spore, AP = appressorium; HS = haustorium; 2 hyphae = secondary hyphae.
Table 1.
Oidium neolycopersici development 72 h after the artificial inoculation. Approximately 100 infection units (IU = fungal spore producing a germination tube) per genotype were observed and the number of papillae and haustoria were counted. Subsequently, the percentage of IU showing a papilla or haustorium was calculated.
| Genotype | Number of Fungal/Plant Structures Observed | %Papilla/IU | %Haustorium/IU | ||
|---|---|---|---|---|---|
| IU | Papilla | Haustorium | |||
| m200_F4-1 | 97 | 34 | 10 | 35.1 | 10.3 |
| m200_F4-2 | 101 | 33 | 0 | 32.7 | 0 |
| ol-2_MM | 90 | 55 | 4 | 61.1 | 4.4 |
| ol-2_SM | 100 | 51 | 11 | 51 | 11 |
| RNAi::SlMLO1 | 109 | 78 | 6 | 71.6 | 5.5 |
| MM | 102 | 1 | 92 | 0.98 | 90.2 |
4. Discussion
Powdery mildew disease can be a problem in greenhouses and field tomato cultivations. The humidity that forms at the leaf surface when cold nights change to warm days or when plants are grown in crowded locations without sufficient air circulation is enough to ignite an infection [74]. The availability of resistant cultivars is, therefore, essential to control this disease in a sustainable way. The resistance can be achieved in several ways. Although for crop improvement mainly conventional breeding methods are used, major limitations such as lack of genetic diversity are frequently observed in the domesticated tomato [9]. Thus, genetic modification technologies including genome editing approaches are considered as an extension of traditional breeding methods. However, the deployment of transgenic plants in plant breeding and agriculture is still socially and politically debated in many parts of the world. In Europe, the plants obtained with genome editing tools are subjected to the same stringent regulations as transgenic organisms [75]. Therefore, currently, nontransgenic strategies are favored to uncover novel alleles. One of the ways consists of inducing mutations in PM susceptibility genes artificially with chemical mutagens, such as EMS.
In this work, we describe the set-up of an EMS mutant population of the tomato cv. MT with which we aimed at finding new sources of resistance to various diseases. Here we focused in particular on finding sources of resistance to the PM disease caused by O. neolycoperisici. By screening the EMS plants, a new loss-of-function allele of the SlMLO1 gene, designated m200 was identified, which confers full resistance against PM. Histological study showed that the resistance of the M200 mutant is associated with papilla formation.
4.1. Is the m200 Mutation a Real Product of the EMS Mutagenesis?
A PM disease test was performed on the M1 plants initially obtained with the intention of finding dominant mutations. The M200 mutant was found, and the sequence analysis showed that the resistance was due to a nonsense mutation (T65A) leading to a stop codon in the coding region of the SlMLO1 gene (Figure 2B). It is unexpected that a recessive mutation occurred in homozygous state in an M1 plant since the probability of having a mutation on both alleles has been shown to be extremely low [76]. The m200 allele seems to be a real product of the EMS treatment given that the SlMLO1 gene in wild-type MT is identical with the one in cultivated tomatoes, Heinz and MM. This is to be expected since MT originated from two cultivated tomatoes [20].
However, it is important to notice that the mutation detected in the m200 allele is not typically produced by the EMS mutagen. EMS treatment mainly triggers transitions, e.g., purine replaced by purine A ↔ G, and pyrimidine replaced by pyrimidine C ↔ T (indicated as G/C → A/T, [77,78]). In Arabidopsis, almost all the EMS mutations described correspond to G/C to A/T transitions [12]. So far, EMS mlo null alleles were reported in wheat (16 mlo alleles), barley (11), and petunia (2) [60,61,64]. In all cases, except three, the mutagenized treatment produced the expected base substitutions (G/C → A/T). In contrast, the barley mutants mlo-13 (T → A) [79], mlo-26 (T → A) [79] and mlo-30 (A → T) [36] are characterized by transversions (purine replaced by a pyrimidine, and vice-versa), as observed in m200 (T → A). In mlo-13 and mlo-26, the transversion caused two missense mutations, V30E and L27H respectively, which in both cases lead to the loss-of-function of the protein. In mlo-30, the mutation occurred in intron sequences which affected transcript splicing [36] and resulted in one transcript containing an 18-nucleotide deletion of exon 12 and another containing the entire unspliced intron 11. Therefore, although not common, the type of mutation observed in the M200 mutant is not an exception.
The fact that the mutation occurred homozygously in an M1 plant, can also lead to the hypothesis that it spontaneously occurred. Spontaneous mutations in Arabidopsis are known to take place at a rate of 10−7 to 10−8 bp/generation [80,81]. However, the large majority of spontaneous mutations are transition mutations [82]. The occurrence of the m200 mutation might also be explained by a gene conversion event involving a paralog SlMlo gene or a point mutation created at the break point of a gene conversion event [83].
4.2. Is the Resistance Level of Slmlo Mutants Dependent on Papilla Formation?
The Slmlo1 mutants and silenced plants showed a large increase in the percentage of IU to which the plant had responded with the formation of a papilla, compared to the susceptible control MM plants (Table 1). This is in line with observations in other mlo mutants [26,69,84]. Consonni et al. [46] reported that the mlo-based resistance is characterized at the cellular level by the timely cell-wall deposition of papillae at the attempted fungal penetration sites which lead to early termination of fungal infection. However, more recent reports on mlo mutants or silenced plants show that papillae can be observed in both resistant and susceptible plants, and a distinction should be made between effective and ineffective papillae [85]. In apple, papillae are larger in resistant mlo lines than in susceptible wild-type lines [55]. In barley, effective papillae show higher concentrations of callose, arabinoxylan and cellulose than ineffective papillae [85]. Nevertheless, callose deposition in papillae is not required for mlo-mediated penetration resistance [86]. In Arabidopsis, PMR4/GSL5 callose synthase (POWDERY MILDEW RESISTANCE4/GLUCAN SYNTHASE-LIKE5) is responsible for the spontaneous callose deposition in mlo2 mutant, however, no differences in the level of PM resistance were observed between mlo2 single and mlo2 pmr4 double mutants [86]. In addition, it was shown that resistance conferred by mlo is not dependent on salicylic acid (SA) accumulation [86]. Further research is required to assess the importance of papillae formation for mlo-based resistance.
4.3. Is the Level of mlo-Based Resistance Influenced by the Position of the Mutation?
The full resistance of the m200 plant is hypothesized to be caused by the severe truncation of this mutant Slmlo1 allele. After reviewing the available literature on barley mlo mutants, three interesting cases, namely mlo-13, mlo-17 and mlo-32, were found [79,87,88]. All three mutants carry mutations leading to a stop codon in the first transmembrane of the HvMlo protein, which corresponds to the same region where the m200 mutation is found. They were all indicated as completely resistant mutants. Moreover, another barley mutant, the mlo-43, was found to carry a stop codon in the second intracellular domain, the same as the nonsense mutation identified in tomato ol-2 mutant [60]. The mlo-43 is a mutant of the cv. Bonus and it was also described as completely resistant [89]. A mutant of the same cultivar, mlo-36, was described to contain a nonsense mutation at W357, in the sixth transmembrane domain [60,89]. Both mutants were only phenotypically scored, and considered highly resistant, with mlo-36 even annotated as immune [89].
Other more recent evaluations of barley impaired alleles have not been found due to premature protein truncation. The reason is that it was shown that defective protein variants would probably not pass the quality test of the ERAD machinery (endoplasmic reticulum-associated protein degradation, [90]). The ER-localized quality control system monitors and validates proper folding and modification of proteins, among which the membrane proteins. If this holds true, the extremely truncated m200 protein, as well as the ol-2 variant, should be subjected to a dramatic reduction in accumulation. Thus, both variants should lead to a similar level of resistance, if compared in the same background. Though it is currently largely unknown which signatures classify malformed membrane proteins, previous studies indicated that the second cytoplasmic loop and the transmembrane regions are the major quality determinant of the HvMlo protein variants [60,90]. Premature truncations heavily affect protein folding. Therefore, mutants containing amino acid substitutions were preferred to truncated mlo alleles in studies addressed at evaluating the biological activity of the Mlo variant.
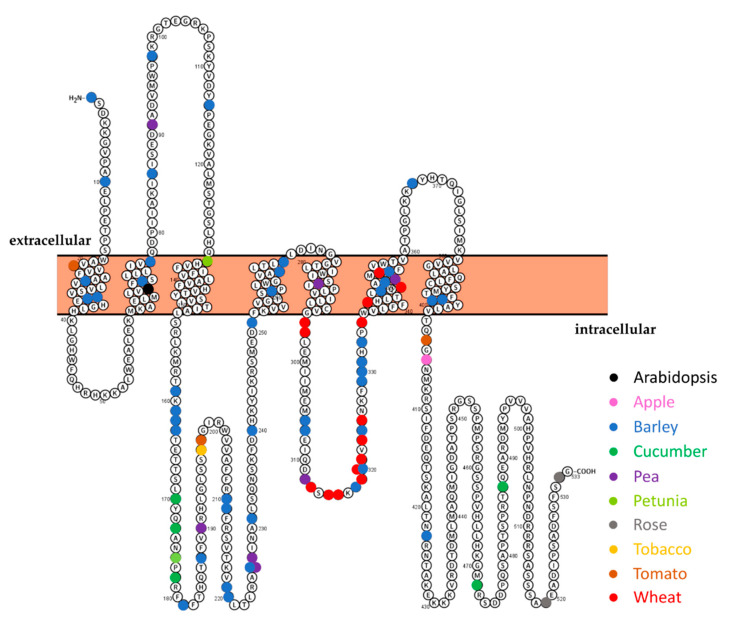
Alternatively, it is possible to exploit amino acid residues that are crucial for the powdery mildew susceptibility-conferring function of the MLO protein (Figure 5, Figure S2). With the increasing number of MLO sequences being functionally characterized in various plant species, multiple protein alignments point out the occurrence of highly conserved residues/regions (Figure S2). These amino acids have been previously shown to be invariable in MLO orthologs involved in the interaction with PM fungi and therefore predicted to have an important functional/structural role for the PM susceptibility-conferring function [42,91]. In addition, a codon-based evolutionary analysis was conducted that resulted in the identification of 130 codons under negative selection, thus predicted to be conserved during evolution (Figure S2) [92]. Amino acids specific for monocot and dicot MLO proteins which do not seem to influence the interaction with PM pathogens were also highlighted (Figure S2). Mining the available literature revealed 21 naturally occurring mlo alleles as well as 74 chemically and radiation-induced mlo alleles, comprising 77 single amino acid substitutions that result in loss-of-MLO function (Table S4). We combined the information of amino acids with the actual mutations found in natural and artificial mutants to map functionally important sites of the MLO protein as being sensitive to functional impairment by mutational perturbation (Figure 5, Table S4). The large majority of the mutations are found in the second (21) and third (23) cytoplasmic domains, which have already been identified as relevant regions for the MLO proteins acting as PM-susceptibility factors (Figure 5). Transmembrane (TM) regions are additional sites of loss-of-function mutations in 24 cases, with the predominant occurrence in the sixth transmembrane (7) (Figure 5), indicating that TM domains harbor important sites for protein conformational changes. These sites/regions are critical for the susceptibility-conferring activity of the MLO protein.
Figure 5.
Schematic representation of the complete barley HvMLO protein. The orange bar represents the plant membrane. Colored dots indicate the amino acids of the corresponding mlo-mutants in different plant species. Overview of the depicted mlo-mutants are shown in Table S4.
Any novel MLO protein characterized in a certain crop species can be added to this alignment provided by Figure S2 to select predicted amino acid positions that, being under negative selection, can represent targets of protein loss of function. If artificial or natural mutants are not available, the information of Figure 5 can be usefully coupled with the genome editing technologies to obtain loss-of-function mutations, especially within the protein domains/sites that act as determinants of PM susceptibility.
5. Conclusions
The use of impaired MLO genes in plant breeding against PM is a promising strategy due to its broad-spectrum and durable characteristics. In this study, we developed a new genetic resource in MT background by means of EMS mutagenesis, and transferred it to a genetic background resembling MM by means of crossing and selfing. The mutant M200 containing a novel allele (the m200 allele) of the tomato SlMLO1 gene showed profound PM resistance with no fungal sporulation and hardly detectable fungal biomass. Thus, it represents a valuable new mutant allele that can be used in breeding PM-resistant tomato cultivars.
Acknowledgments
D.G. was funded by a fellowship from the China Scholarship Council.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12050719/s1, Figure S1: Nucleotide alignment of the SlMLO1 sequence experimentally obtained from the tomato cv. Micro-Tom (MT), the full-length transcript AK322443 of MT SlMLO1 obtained from NCBI, the SlMLO1 gene in M200, MM leaf cDNA sequence of SlMLO1 obtained from Zheng et al. [22] and the one from the cv. Heinz as in the SGN database (Solyc04g49090.3). Arrow indicates the base change T → A responsible for the premature stop codon in M200 plant, Figure S2: Protein alignment of functionally characterized MLO sequences of Arabidopsis thaliana AtMLO2, -6, and -12 (GenBank accession numbers NP172598, NP176350, and NP565902) [17], Pisum sativum (pea) PsMLO1 (GenBank accession number FJ463618) [1], Medicago truncatula (barrel clover) MtMLO1 (GenBank accession number HQ446457) [1], Lotus japonicus LjMLO1 (GenBank accession number AY967410) [1], Capsicum annuum (pepper) CaMLO2 (GenBank accession number AFH68055) [23], Cucumis sativus (cucumber) CsaMLO1 and -8 (GenBank accession numbers Csa1M085890.1 and Csa5M623470.1) [12,14], Solanum lycopersicum (tomato) SlMLO1 (GenBank accession number NP001234814) [7], Nicotiana tabacum (tobacco) NtMLO1 (GenBank accession number KM244716) [15], S. melongena (eggplant) SmMLO1 (GenBank accession number KM244717) [24], Malus domestica (apple) MdMLO19 (GenBank accession number MDP0000168714) [10], Triticum aestivum (wheat) TaMLO-A1b, TaMLO-B1a, and TaMLO-D1 (GenBank accession numbers AX063298, AF361932, and AX063296) [25,26], Hordeum vulgare (barley) HvMLO (GenBank accession number Z83834) [27] and Oryza sativa (rice) OsMLO2 (GenBank accession number AF384030) [25]. Highlighted in green and in light blue are the conserved amino acids among the whole MLO family indicated by Kusch et al. [28] and by Elliott et al. [29], respectively. Amino acids highlighted in gray refer to the ones reported to be under negative selection by Appiano et al. [30]. Letters displayed in green, light blue or gray indicate synonymous amino acid exchanges in each of three categories above described. Letters in red bold indicate amino acids identified in mlo-mutants for each of the plant species described above. Black lines indicate the position of the transmembrane domains which have been numbered with romans numbers, Table S1: Primer pairs used in this study, Table S2: Genotyping and phenotyping of eight progenies (BC1S1) derived from two (i.e., BC1_1 and BC1_3) of the three BC1 crosses M200 × MT, Table S3: Genotyping and phenotyping of the progenies (F2) of three crosses between the resistant M200 plant and the tomato cv. Moneymaker (M200 × MM), Table S4: Overview of the mlo-mutants described in the literature.
Author Contributions
A.-M.A.W., Y.B. and R.G.F.V. conceived the study. M.A., A.v.T., F.M.-D., D.S., D.G. and R.H. performed the experiments and analyzed the data. Z.Y. and M.A. designed the Figures. Z.Y. and M.A. drafted the manuscript; Z.Y., R.G.F.V., Y.B. und A.-M.A.W. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Tomato Genome Consortium The tomato genome sequence provides insights into fleshy fruit evolution. Nat. Cell Biol. 2012;485:635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moco S., Bino R.J., Vorst O., Verhoeven H.A., De Groot J., Van Beek T.A., Vervoort J., De Vos C.H.R. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant. Physiol. 2006;141:1205–1218. doi: 10.1104/pp.106.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong A.-S., Yao Q.-H., Peng R.-H., Li X., Han P.-L., Fan H.-Q. Different effects on ACC oxidase gene silencing triggered by RNA interference in transgenic tomato. Plant. Cell Rep. 2004;23:639–646. doi: 10.1007/s00299-004-0887-7. [DOI] [PubMed] [Google Scholar]
- 4.Schijlen E.G., de Vos C.R., Martens S., Jonker H.H., Rosin F.M., Molthoff J.W., Tikunov Y.M., Angenent G.C., van Tunen A.J., Bovy A.G. RNA Interference Silencing of Chalcone Synthase, the First Step in the Flavonoid Biosynthesis Pathway, Leads to Parthenocarpic Tomato Fruits. Plant. Physiol. 2007;144:1520–1530. doi: 10.1104/pp.107.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jong M., Wolters-Arts M., Feron R., Mariani C., Vriezen W.H. The Solanum lycopersicumauxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant. J. 2009;57:160–170. doi: 10.1111/j.1365-313X.2008.03671.x. [DOI] [PubMed] [Google Scholar]
- 6.Lor V.S., Starker C.G., Voytas D.F., Weiss D., Olszewski N.E. Targeted Mutagenesis of the Tomato PROCERA Gene Using Transcription Activator-Like Effector Nucleases. Plant. Physiol. 2014;166:1288–1291. doi: 10.1104/pp.114.247593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks C., Nekrasov V., Lippman Z.B., Van Eck J. Efficient Gene Editing in Tomato in the First Generation Using the Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated9 System. Plant. Physiol. 2014;166:1292–1297. doi: 10.1104/pp.114.247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T., Zhang H., Zhu H. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic. Res. 2019;6:1–13. doi: 10.1038/s41438-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez G.R., Muños S., Anderson C., Sim S.-C., Michel A., Causse M., Gardener B.B.M., Francis D., van der Knaap E. Distribution of SUN, OVATE, LC, and FAS in the Tomato Germplasm and the Relationship to Fruit Shape Diversity. Plant. Physiol. 2011;156:275–285. doi: 10.1104/pp.110.167577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kissoudis C., Van De Wiel C., Visser R.G., Van Der Linden G. Future-proof crops: Challenges and strategies for climate resilience improvement. Curr. Opin. Plant. Biol. 2016;30:47–56. doi: 10.1016/j.pbi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Shu Q.-Y., Forster B.P., Nakagawa H. Principles and applications of plant mutation breeding. In: Shu Q.-Y., Forster B.P., Nakagawa H., editors. Plant Mutation Breeding and Biotechnology. CABI; Wallingford, UK: 2012. pp. 301–325. [DOI] [Google Scholar]
- 12.Greene E.A., Codomo C.A., Taylor N.E., Henikoff J.G., Till B.J., Reynolds S.H., Enns L.C., Burtner C., Johnson J.E., Odden A.R., et al. Spectrum of Chemically Induced Mutations from a Large-Scale Reverse-Genetic Screen in Arabidopsis. Genetics. 2003;164:731–740. doi: 10.1093/genetics/164.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Till B.J., Reynolds S.H., Weil C., Springer N., Burtner C., Young K., Bowers E., Codomo C.A., Enns L.C., Odden A.R., et al. Discovery of induced point mutations in maize genes by TILLING. BMC Plant. Biol. 2004;4:12. doi: 10.1186/1471-2229-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Till B.J., Cooper J., Tai T.H., Colowit P., Greene E.A., Henikoff S., Comai L. Discovery of chemically induced mutations in rice by TILLING. BMC Plant. Biol. 2007;7:19. doi: 10.1186/1471-2229-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner R., Jacobson Y., Melamed S., Levyatuv S., Shalev G., Ashri A., Elkind Y., Levy A. A new model system for tomato genetics. Plant. J. 1997;12:1465–1472. doi: 10.1046/j.1365-313x.1997.12061465.x. [DOI] [Google Scholar]
- 16.Menda N., Semel Y., Peled D., Eshed Y., Zamir D. In silico screening of a saturated mutation library of tomato. Plant J. 2004;38:861–872. doi: 10.1111/j.1365-313X.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 17.Gady A.L., Hermans F.W., Van De Wal M.H., Van Loo E.N., Visser R.G., Bachem C.W. Implementation of two high through-put techniques in a novel application: Detecting point mutations in large EMS mutated plant populations. Plant. Methods. 2009;5:13. doi: 10.1186/1746-4811-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito T., Asamizu E., Mizoguchi T., Fukuda N., Matsukura C., Ezura H. Mutant Resources for the Miniature Tomato (Solanum lycopersicum L.) ‘Micro-Tom’. J. Jpn. Soc. Hortic. Sci. 2009;78:6–13. doi: 10.2503/jjshs1.78.6. [DOI] [Google Scholar]
- 19.Minoia S., Petrozza A., D’Onofrio O., Piron F., Mosca G., Sozio G., Cellini F., Bendahmane A., Carriero F. A new mutant genetic resource for tomato crop improvement by TILLING technology. BMC Res. Notes. 2010;3:69. doi: 10.1186/1756-0500-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott J.W., Harbaugh B.K. Micro-Tom: A Miniature Dwarf Tomato. Agricultural Experiment Station, Institute of Food and Agricultural Sciences, University of Florida; Gainesville, FL, USA: 1989. p. 370. [Google Scholar]
- 21.Saito T., Ariizumi T., Okabe Y., Asamizu E., Hiwasa-Tanase K., Fukuda N., Mizoguchi T., Yamazaki Y., Aoki K., Ezura H. TOMATOMA: A Novel Tomato Mutant Database Distributing Micro-Tom Mutant Collections. Plant. Cell Physiol. 2011;52:283–296. doi: 10.1093/pcp/pcr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirasawa K., Hirakawa H., Nunome T., Tabata S., Isobe S. Genome-wide survey of artificial mutations induced by ethyl methanesulfonate and gamma rays in tomato. Plant. Biotechnol. J. 2015;14:51–60. doi: 10.1111/pbi.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavan S., Jacobsen E., Visser R.G.F., Bai Y. Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Mol. Breed. 2009;25:1–12. doi: 10.1007/s11032-009-9323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Schie C.C., Takken F.L. Susceptibility Genes 101: How to Be a Good Host. Annu. Rev. Phytopathol. 2014;52:551–581. doi: 10.1146/annurev-phyto-102313-045854. [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen J.H. Comparison of Induced Mutant Genes to Spontaneous Genes in Barley Conditioning Resistance to Powdery Mildew. International Atomic Energy Agency; Vienna, Austria: 1971. pp. 117–124. [Google Scholar]
- 26.Jørgensen I.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica. 1992;63:141–152. doi: 10.1007/BF00023919. [DOI] [Google Scholar]
- 27.Brown J.K. Durable Resistance of Crops to Disease: A Darwinian Perspective. Annu. Rev. Phytopathol. 2015;53:513–539. doi: 10.1146/annurev-phyto-102313-045914. [DOI] [PubMed] [Google Scholar]
- 28.Devoto A., Piffanelli P., Nilsson I., Wallin E., Panstruga R., von Heijne G., Schulze-Lefert P. Topology, Subcellular Localization, and Sequence Diversity of the Mlo Family in Plants. J. Biol. Chem. 1999;274:34993–35004. doi: 10.1074/jbc.274.49.34993. [DOI] [PubMed] [Google Scholar]
- 29.Kim M.C., Panstruga R., Elliott C., Müller J., Devoto A., Yoon H.W., Park H.C., Cho M.J., Schulze-Lefert P. Calmodulin interacts with MLO protein to regulate defence against mildew in barley. Nat. Cell Biol. 2002;416:447–451. doi: 10.1038/416447a. [DOI] [PubMed] [Google Scholar]
- 30.Kusch S., Panstruga R. mlo-Based Resistance: An Apparently Universal “Weapon” to Defeat Powdery Mildew Disease. Mol. Plant. Microbe Interact. 2017;30:179–189. doi: 10.1094/MPMI-12-16-0255-CR. [DOI] [PubMed] [Google Scholar]
- 31.Bidzinski P., Noir S., Shahi S., Reinstädler A., Gratkowska D.M., Panstruga R. Physiological characterization and genetic modifiers of aberrant root thigmomorphogenesis in mutants of Arabidopsis thaliana MILDEW LOCUS O genes. Plant. Cell Environ. 2014;37:2738–2753. doi: 10.1111/pce.12353. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z., Noir S., Kwaaitaal M., Hartmann H.A., Wu M.-J., Mudgil Y., Sukumar P., Muday G., Panstruga R., Jones A.M. Two Seven-Transmembrane Domain MILDEW RESISTANCE LOCUS O Proteins Cofunction in Arabidopsis Root Thigmomorphogenesis. Plant. Cell. 2009;21:1972–1991. doi: 10.1105/tpc.108.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler S.A., Shimosato-Asano H., Keinath N.F., Wuest S.E., Ingram G., Panstruga R., Grossniklaus U. Conserved Molecular Components for Pollen Tube Reception and Fungal Invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 34.Lorrain S., Vailleau F., Balagué C., Roby D. Lesion mimic mutants: Keys for deciphering cell death and defense pathways in plants? Trends Plant. Sci. 2003;8:263–271. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 35.Wolter M., Hollricher K., Salamini F., Schulze-Lefert P. The mlo resistance alleles to powdery mildew infection in barley trigger a developmentally controlled defence mimic phenotype. Mol. Genet. Genom. 1993;239:122–128. doi: 10.1007/BF00281610. [DOI] [PubMed] [Google Scholar]
- 36.Piffanelli P., Zhou F., Casais C., Orme J., Jarosch B., Schaffrath U., Collins N.C., Panstruga R., Schulze-Lefert P. The Barley MLO Modulator of Defense and Cell Death Is Responsive to Biotic and Abiotic Stress Stimuli. Plant. Physiol. 2002;129:1076–1085. doi: 10.1104/pp.010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Lozano J.M., Gianinazzi S., Gianinazzi-Pearson V. Genes involved in resistance to powdery mildew in barley differentially modulate root colonization by the mycorrhizal fungus Glomus mosseae. Mycorrhiza. 1999;9:237–240. doi: 10.1007/s005720050273. [DOI] [Google Scholar]
- 38.Jansen C., von Wettstein D., Schäfer W., Kogel K.-H., Felk A., Maier F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA. 2005;102:16892–16897. doi: 10.1073/pnas.0508467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarosch B., Kogel K.-H., Schaffrath U. The Ambivalence of the Barley Mlo Locus: Mutations Conferring Resistance Against Powdery Mildew (Blumeria graminis f. sp. hordei) Enhance Susceptibility to the Rice Blast Fungus Magnaporthe grisea. Mol. Plant. Microbe Interact. 1999;12:508–514. doi: 10.1094/mpmi.1999.12.6.508. [DOI] [Google Scholar]
- 40.Kumar J., Hückelhoven R., Beckhove U., Nagarajan S., Kogel K.-H. A Compromised Mlo Pathway Affects the Response of Barley to the Necrotrophic Fungus Bipolaris sorokiniana (Teleomorph: Cochliobolus sativus) and Its Toxins. Phytopathology. 2001;91:127–133. doi: 10.1094/PHYTO.2001.91.2.127. [DOI] [PubMed] [Google Scholar]
- 41.McGrann G.R.D., Stavrinides A., Russell J., Corbitt M.M., Booth A., Chartrain L., Thomas W.T.B., Brown J.K.M. A trade off between mlo resistance to powdery mildew and increased susceptibility of barley to a newly important disease, Ramularia leaf spot. J. Exp. Bot. 2014;65:1025–1037. doi: 10.1093/jxb/ert452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusch S., Pesch L., Panstruga R. Comprehensive Phylogenetic Analysis Sheds Light on the Diversity and Origin of the MLO Family of Integral Membrane Proteins. Genome Biol. Evol. 2016;8:878–895. doi: 10.1093/gbe/evw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feechan A., Jermakow A.M., Torregrosa L., Panstruga R., Dry I.B. Identification of grapevine MLO gene candidates involved in susceptibility to powdery mildew. Funct. Plant. Biol. 2008;35:1255–1266. doi: 10.1071/FP08173. [DOI] [PubMed] [Google Scholar]
- 44.Acevedo-Garcia J., Kusch S., Panstruga R. Magical mystery tour: MLO proteins in plant immunity and beyond. New Phytol. 2014;204:273–281. doi: 10.1111/nph.12889. [DOI] [PubMed] [Google Scholar]
- 45.Panstruga R. Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochem. Soc. Trans. 2005;33:389–392. doi: 10.1042/BST0330389. [DOI] [PubMed] [Google Scholar]
- 46.Consonni C., Humphry M.E., Hartmann H.A., Livaja M., Durner J., Westphal L., Vogel J., Lipka V., Kemmerling B., Schulze-Lefert P., et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006;38:716–720. doi: 10.1038/ng1806. [DOI] [PubMed] [Google Scholar]
- 47.Hückelhoven R. Powdery mildew susceptibility and biotrophic infection strategies. FEMS Microbiol. Lett. 2005;245:9–17. doi: 10.1016/j.femsle.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Zheng Z., Appiano M., Pavan S., Bracuto V., Ricciardi L., Visser R.G.F., Wolters A.-M.A., Bai Y. Genome-Wide Study of the Tomato SlMLO Gene Family and Its Functional Characterization in Response to the Powdery Mildew Fungus Oidium neolycopersici. Front. Plant. Sci. 2016;7:380. doi: 10.3389/fpls.2016.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berg J.A., Appiano M., Martínez M.S., Hermans F.W.K., Vriezen W.H., Visser R.G.F., Bai Y., Schouten H.J. A transposable element insertion in the susceptibility gene CsaMLO8 results in hypocotyl resistance to powdery mildew in cucumber. BMC Plant. Biol. 2015;15:243. doi: 10.1186/s12870-015-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng H., Kong W., Lv J., Li J. Analysis of powdery mildew resistance in wild melon MLO mutants. Hortic. Plant. J. 2015;1:165–171. doi: 10.16420/j.issn.2095-9885.2015-0036. [DOI] [Google Scholar]
- 51.Humphry M., Reinstädler A., Ivanov S., Bisseling T., Panstruga R. Durable broad-spectrum powdery mildew resistance in pea er1 plants is conferred by natural loss-of-function mutations in PsMLO1. Mol. Plant. Pathol. 2011;12:866–878. doi: 10.1111/j.1364-3703.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun S., Fu H., Wang Z., Duan C., Zong X., Zhu Z. Discovery of a Novel er1 Allele Conferring Powdery Mildew Resistance in Chinese Pea (Pisum sativum L.) Landraces. PLoS ONE. 2016;11:e0147624. doi: 10.1371/journal.pone.0147624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun S., Deng D., Wang Z., Duan C., Wu X., Wang X., Zong X., Zhu Z. A novel er1 allele and the development and validation of its functional marker for breeding pea (Pisum sativum L.) resistance to powdery mildew. Theor. Appl. Genet. 2016;129:909–919. doi: 10.1007/s00122-016-2671-9. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann H., Qiu X., Wehmeyer J., Debener T. Isolation, Molecular Characterization, and Mapping of Four Rose MLO Orthologs. Front. Plant. Sci. 2012;3:244. doi: 10.3389/fpls.2012.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pessina S., Angeli D., Martens S., Visser R.G., Bai Y., Salamini F., Velasco R., Schouten H.J., Malnoy M. The knock-down of the expression of MdMLO19 reduces susceptibility to powdery mildew (Podosphaera leucotricha) in apple (Malus domestica) Plant. Biotechnol. J. 2016;14:2033–2044. doi: 10.1111/pbi.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimura T., Sato S., Tajima T., Arai M. Powdery mildew resistance in the Japanese domestic tobacco cultivar Kokubu is associated with aberrant splicing of MLO orthologues. Plant. Pathol. 2016;65:1358–1365. doi: 10.1111/ppa.12498. [DOI] [Google Scholar]
- 57.Wang Y., Cheng X., Shan Q., Zhang Y., Liu J., Gao C., Qiu J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014;32:947–951. doi: 10.1038/nbt.2969. [DOI] [PubMed] [Google Scholar]
- 58.Nekrasov V., Wang C., Win J., Lanz C., Weigel D., Kamoun S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017;7:1–6. doi: 10.1038/s41598-017-00578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan D.-Y., Guo Y., Cheng Y., Hu Y., Xiao S., Wang Y., Wen Y.-Q. CRISPR/Cas9-mediated mutagenesis of VvMLO3 results in enhanced resistance to powdery mildew in grapevine (Vitis vinifera) Hortic. Res. 2020;7:1–14. doi: 10.1038/s41438-020-0339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinstädler A., Müller J., Czembor J.H., Piffanelli P., Panstruga R. Novel induced mlo mutant alleles in combination with site-directed mutagenesis reveal functionally important domains in the heptahelical barley Mlo protein. BMC Plant. Biol. 2010;10:31. doi: 10.1186/1471-2229-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acevedo-Garcia J., Spencer D., Thieron H., Reinstädler A., Hammond-Kosack K., Phillips A.L., Panstruga R. mlo-based powdery mildew resistance in hexaploid bread wheat generated by a non-transgenic TILLING approach. Plant. Biotechnol. J. 2016;15:367–378. doi: 10.1111/pbi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavan S., Schiavulli A., Appiano M., Marcotrigiano A.R., Cillo F., Visser R.G.F., Bai Y., Lotti C., Ricciardi L. Pea powdery mildew er1 resistance is associated to loss-of-function mutations at a MLO homologous locus. Theor. Appl. Genet. 2011;123:1425–1431. doi: 10.1007/s00122-011-1677-6. [DOI] [PubMed] [Google Scholar]
- 63.Santo T., Rashkova M., Alabaça C., Leitão J. The ENU-induced powdery mildew resistant mutant pea (Pisum sativum L.) lines S(er1mut1) and F(er1mut2) harbour early stop codons in the PsMLO1 gene. Mol. Breed. 2013;32:723–727. doi: 10.1007/s11032-013-9889-x. [DOI] [Google Scholar]
- 64.Jiang P., Chen Y., Wilde H.D. Identification and mutagenesis of disease susceptibility genes of Petunia hybrida. Plant. Cell Tissue Organ. Cult. 2016;126:117–125. doi: 10.1007/s11240-016-0982-9. [DOI] [Google Scholar]
- 65.Ciccarese F., Amenduni M., Schiavone D., Cirulli M. Occurrence and inheritance of resistance to powdery mildew (Oidium lycopersici) in Lycopersicon species. Plant. Pathol. 1998;47:417–419. doi: 10.1046/j.1365-3059.1998.00254.x. [DOI] [Google Scholar]
- 66.De Giovanni C., Dell’Orco P., Bruno A., Ciccarese F., Lotti C., Ricciardi L. Identification of PCR-based markers (RAPD, AFLP) linked to a novel powdery mildew resistance gene (ol-2) in tomato. Plant. Sci. 2004;166:41–48. doi: 10.1016/j.plantsci.2003.07.005. [DOI] [Google Scholar]
- 67.Pavan S., Zheng Z., Borisova M., Van Den Berg P., Lotti C., De Giovanni C., Lindhout P., De Jong H., Ricciardi L., Visser R.G.F., et al. Map- vs. homology-based cloning for the recessive gene ol-2 conferring resistance to tomato powdery mildew. Euphytica. 2007;162:91–98. doi: 10.1007/s10681-007-9570-8. [DOI] [Google Scholar]
- 68.Bai Y., Pavan S., Zheng Z., Zappel N.F., Reinstädler A., Lotti C., De Giovanni C., Ricciardi L., Lindhout P., Visser R., et al. Naturally Occurring Broad-Spectrum Powdery Mildew Resistance in a Central American Tomato Accession Is Caused by Loss of Mlo Function. Mol. Plant. Microbe Interac. 2008;21:30–39. doi: 10.1094/MPMI-21-1-0030. [DOI] [PubMed] [Google Scholar]
- 69.Bai Y., Van Der Hulst R., Bonnema G., Marcel T.C., Meijer-Dekens F., Niks R.E., Lindhout P. Tomato Defense to Oldium neolycopersici: Dominant Ol Genes Confer Isolate-Dependent Resistance Via a Different Mechanism Than Recessive ol-2. Mol. Plant. Microbe Interact. 2005;18:354–362. doi: 10.1094/MPMI-18-0354. [DOI] [PubMed] [Google Scholar]
- 70.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 71.Doyle J.J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- 72.Omasits U., Ahrens C.H., Müller S., Wollscheid B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 73.The 100 Tomato Genome Sequencing Consortium. Aflitos S.A., Schijlen E., De Jong H., De Ridder D., Smit S., Finkers R., Wang J., Zhang G., Li N., et al. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant. J. 2014;80:136–148. doi: 10.1111/tpj.12616. [DOI] [PubMed] [Google Scholar]
- 74.Horst R.K. Powdery mildews. In: Horst K.R., editor. Westcott’s Plant Disease Handbook. Springer; Dordrecht, The Netherlands: 2013. pp. 285–293. [DOI] [Google Scholar]
- 75.Callaway E. CRISPR plants now subject to tough GM laws in European Union. Nature. 2018;560:16. doi: 10.1038/d41586-018-05814-6. [DOI] [PubMed] [Google Scholar]
- 76.Oladosu Y., Rafii M.Y., Abdullah N., Hussin G., Ramli A., Rahim H.A., Miah G., Usman M. Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 2016;30:1–16. doi: 10.1080/13102818.2015.1087333. [DOI] [Google Scholar]
- 77.Sega G.A. A review of the genetic effects of ethyl methanesulfonate. Mutat. Res. Genet. Toxicol. 1984;134:113–142. doi: 10.1016/0165-1110(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 78.Griffiths J.F., Griffiths A.J., Miller J.H., Suzuki D.T. An Introduction to Genetic Analysis. 7th ed. WH Freeman and Company; New York, NY, USA: 2000. [Google Scholar]
- 79.Büschges R., Hollricher K., Panstruga R., Simons G., Wolter M., Frijters A., van Daelen R., van der Lee T., Diergaarde P., Groenendijk J., et al. The Barley Mlo Gene: A Novel Control Element of Plant Pathogen Resistance. Cell. 1997;88:695–705. doi: 10.1016/S0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- 80.Kovalchuk I., Kovalchuk O., Hohn B. Genome-wide variation of the somatic mutation frequency in transgenic plants. EMBO J. 2000;19:4431–4438. doi: 10.1093/emboj/19.17.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bashir T., Sailer C., Gerber F., Loganathan N., Bhoopalan H., Eichenberger C., Grossniklaus U., Baskar R., Vanholme B., Vanholme R., et al. Hybridization Alters Spontaneous Mutation Rates in a Parent-of-Origin-Dependent Fashion in Arabidopsis. Plant. Physiol. 2014;165:424–437. doi: 10.1104/pp.114.238451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ossowski S., Schneeberger K., Lucas-Lledó J.I., Warthmann N., Clark R.M., Shaw R.G., Weigel D., Lynch M. The Rate and Molecular Spectrum of Spontaneous Mutations in Arabidopsis thaliana. Science. 2010;327:92–94. doi: 10.1126/science.1180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mondragon-Palomino M., Gaut B.S. Gene Conversion and the Evolution of Three Leucine-Rich Repeat Gene Families in Arabidopsis thaliana. Mol. Biol. Evol. 2005;22:2444–2456. doi: 10.1093/molbev/msi241. [DOI] [PubMed] [Google Scholar]
- 84.Feechan A., Jermakow A.M., Ivancevic A., Godfrey D., Pak H., Panstruga R., Dry I.B. Host Cell Entry of Powdery Mildew Is Correlated with Endosomal Transport of Antagonistically Acting VvPEN1 and VvMLO to the Papilla. Mol. Plant. Microbe Interact. 2013;26:1138–1150. doi: 10.1094/MPMI-04-13-0091-R. [DOI] [PubMed] [Google Scholar]
- 85.Chowdhury J., Henderson M., Schweizer P., Burton R.A., Fincher G.B., Little A. Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei. New Phytol. 2014;204:650–660. doi: 10.1111/nph.12974. [DOI] [PubMed] [Google Scholar]
- 86.Consonni C., Bednarek P., Humphry M., Francocci F., Ferrari S., Harzen A., van Themaat E.V.L., Panstruga R. Tryptophan-Derived Metabolites Are Required for Antifungal Defense in the Arabidopsis mlo2 Mutant. Plant. Physiol. 2010;152:1544–1561. doi: 10.1104/pp.109.147660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molina-Cano J.L., Simiand J.P., Sopena A., Pérez-Vendrell A.M., Dorsch S., Rubiales D., Swanston J.S., Jahoor A. Mildew-resistant mutants induced in North American two- and six-rowed malting barley cultivars. Theor. Appl. Genet. 2003;107:1278–1287. doi: 10.1007/s00122-003-1362-5. [DOI] [PubMed] [Google Scholar]
- 88.Panstruga R., Reinstädler A., Müller J., Molina-Cano J.L. Molecular characterization of mlo mutants in North American two- and six-rowed malting barley cultivars. Mol. Plant. Pathol. 2005;6:315–320. doi: 10.1111/j.1364-3703.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 89.Lundqvist U. Plant Mutation Breeding for Crop Improvement. International Atomic Energy Agency (IAEA); Vienna, Austria: 1991. Swedish mutation research in barley with plant breeding aspects. A historical review; pp. 135–147. [Google Scholar]
- 90.Müller J., Piffanelli P., Devoto A., Miklis M., Elliott C., Ortmann B., Schulze-Lefert P., Panstruga R. Conserved ERAD-Like Quality Control of a Plant Polytopic Membrane Protein. Plant. Cell. 2005;17:149–163. doi: 10.1105/tpc.104.026625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elliott C., Müller J., Miklis M., Bhat R.A., Schulze-Lefert P., Panstruga R. Conserved extracellular cysteine residues and cytoplasmic loop–loop interplay are required for functionality of the heptahelical MLO protein. Biochem. J. 2005;385:243–254. doi: 10.1042/BJ20040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Appiano M., Catalano D., Martínez M.S., Lotti C., Zheng Z., Visser R.G.F., Ricciardi L., Bai Y., Pavan S. Monocot and dicot MLO powdery mildew susceptibility factors are functionally conserved in spite of the evolution of class-specific molecular features. BMC Plant. Biol. 2015;15:1–10. doi: 10.1186/s12870-015-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and supplementary material.