Abstract
In this study, magnetite nanoparticles were prepared and coated with poly(ethylene glycol) terminated by alendronate to ensure firm binding to the iron oxide surface. Magnetic nanoparticles, designated as magnetite coated with poly(ethylene glycol)-alendronate (Fe3O4@PEG-Ale), were characterized in terms of number-average (Dn) and hydrodynamic (Dh) size, ζ-potential, saturation magnetization, and composition. The effect of particles on blood pressure, vascular functions, nitric oxide (NO), and superoxide production in the tissues of spontaneously hypertensive rats, as well as the effect on red blood cell (RBC) parameters, was investigated after intravenous administration (1 mg Fe3O4/kg of body weight). Results showed that Fe3O4@PEG-Ale particles did negatively affect blood pressure, heart rate and RBC deformability, osmotic resistance and NO production. In addition, Fe3O4@PEG-Ale did not alter functions of the femoral arteries. Fe3O4@PEG-Ale induced increase in superoxide production in the kidney and spleen, but not in the left heart ventricle, aorta and liver. NO production was reduced only in the kidney. In conclusion, the results suggest that acute intravenous administration of Fe3O4@PEG-Ale did not produce negative effects on blood pressure regulation, vascular function, and RBCs in hypertensive rats.
Keywords: magnetic, alendronate, nanoparticles, cardiovascular, red blood cells
1. Introduction
Iron oxide-based magnetic nanoparticles (NPs) exhibiting superparamagnetic properties due to their nanoscale size are promising in a variety of bioapplications [1,2]. Such particles were already approved by the Food and Drug Administration (FDA) for magnetic resonance imaging (MRI) of sentinel lymph nodes, liver, spleen, and bowel [3] or treatment of iron deficiency [4]. Examples of commercial polysaccharide-coated magnetic nanoparticles involve Lumirem®, Feridex®, EndoremTM, Feraheme®, and GastroMARK® [5]. However, some of them were later withdrawn from the market due to the lack of interest in the medical community, rentability of production, and potential health risks. A major concern relates to their toxic effects on the cells, as well as on the living organism, mainly due to possible interference with iron metabolism. Behavior of iron oxide nanoparticles administered intravenously in the organism depends on their physicochemical properties, such as composition, size, ζ-potential, coating, colloidal stability, concentration, etc. They are excluded mostly by kidneys, if size is <6–15 nm [6]; larger particles are excreted via hepatobiliary clearance and some are internalized in the spleen. Elevated amounts of iron were observed also in the lungs, brain, heart, aorta, and other tissues [7]. A recent study showed superparamagnetic poly(ethylene glycol) (PEG)-coated magnetite NPs did not alter blood pressure and plasma corticosterone levels, but produced tissue-dependent changes in nitric oxide (NO) production in normotensive rats [8]. Importantly, iron oxide NPs altered vascular function in terms of enhanced NO-dependent components of acetylcholine-induced endothelium-dependent relaxation. Circulating nanoparticles may potentially influence red blood cells (RBCs) and damage their membranes. As NPs can pass into RBC cytoplasm, NPs can affect the intracellular environment of RBCs.
The two main forms of iron oxide are magnetite (Fe3O4) and its oxidized analogue maghemite (γ-Fe2O3). Their selection depends on the purpose of the application. While some authors prefer higher magnetization of magnetite [9,10], others emphasize oxidation stability of maghemite [11]. NPs were frequently modified to achieve stealth behavior against adaptive immune systems and prolonged circulation in the blood stream, and vice versa, to increase attractivity for some specific cells, e.g., the cancer ones [12,13]. Suitable coating also reduces toxicity of NPs associated mainly with oxidative stress, DNA damage or hemolysis [14,15]. Coatings include low-molecular weight, as well as polymer molecules, and various cell lysates to mask nanoparticles in the blood stream. To anchor polymer coatings on particle surface, various ligands were used, including phosphate [16], (bis)phosphonate [17], sulfo [18], and carboxyl groups [19], or polymer was covalently crosslinked on magnetic nanoparticles [20]. Examples of polymers suitable as particle coatings include dextran, carboxydextran [21], PEG [22], and polyvinylpyrrolidone [23], which are FDA-approved and biocompatible.
Bisphosphonate-containing molecules belong to drugs for treatment of bone illnesses. Alendronate (Ale) is a bisphosphonate medication used for treatment of osteoporosis in women who have undergone menopause, as well as in men, in whom hypertension is frequent comorbidity, as they form complexes with calcium [24]. In addition, endothelial dysfunction is a hallmark of arterial hypertension and any further damage of the endothelium (inner monolayer of the arteries) would worsen the already existing disease state. That is why any NPs used in medical applications, especially when they are administered intravenously, should not damage the endothelium and/or decrease the release of the endothelium-derived relaxing factors (mainly release of NO) or increase the endothelium-derived contracting factors [25].
In this study, Ale was used as a ligand for anchoring PEG on the surface of the iron oxide nanoparticles. The advantage of Ale consists in that it is used in human medicine, and it was used previously as an additive to coating of metals due to its generally good chelating properties [26]. We investigated the influence of magnetite NPs coated with PEG-alendronate (Fe3O4@PEG-Ale) on certain biological parameters, such as blood pressure, heart rate, vascular function and nitric oxide and superoxide production in the organs and tissues of spontaneously hypertensive rats. In addition, we determined red blood cell fundamental physiological parameters—deformability, osmotic resistance and NO production.
2. Materials and Methods
2.1. Materials
Sodium salt of (4-amino-1-hydroxy-1-phosphonobutyl)phosphonic acid trihydrate (alendronate; Ale) was purchased from TCI (Tokyo, Japan). α-Methoxy poly(ethylene glycol) succinimidyl ester (PEG-NHS; molar mass = 5000 g/mol) was purchased from Rapp Polymere (Tuebingen, Germany). Ferric chloride hexahydrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ammonium hydroxide and Na2HPO4·12∙H2O and KH2PO4 used for the preparation of 0.5 M phosphate buffer (PB) were obtained from Lach-Ner (Neratovice, Czech Republic). Ultrapure Q-water that was ultrafiltered using a Milli-Q Gradient A10 system (Millipore, Molsheim, France) was used in all experiments.
2.2. Synthesis of PEG-Alendronate
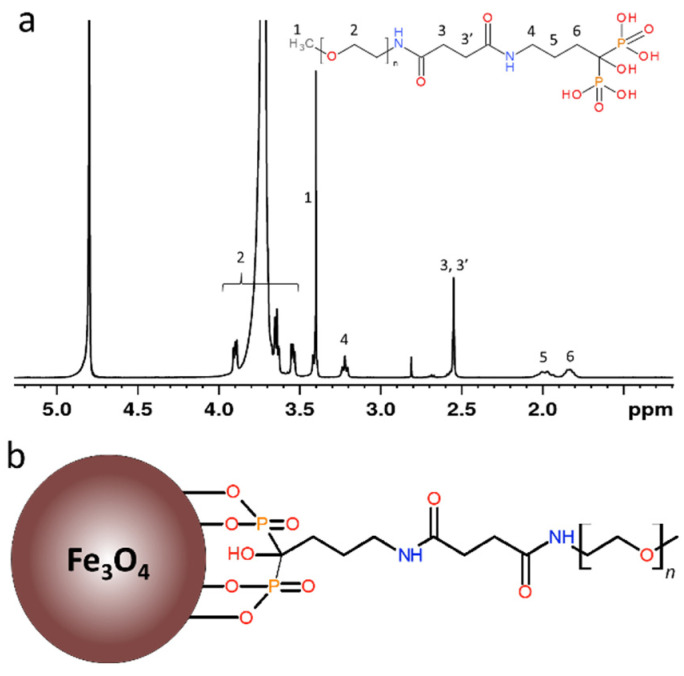
Ale (0.14 g) was dissolved in 0.5 M PB (2 mL; pH 7.4) at 0 °C and pH of the solution was adjusted to 7.4 by addition of 4 M aqueous NaOH. PEG-NHS (0.5 g) was then added and the reaction mixture was stirred at 0 °C for 6 h and at room temperature for 16 h. The mixture was acidified with 4 M HCl to pH 2, PEG-alendronate (PEG-Ale) was extracted with CH2Cl2 (3 × 8 mL) and then combined organic layers were filtered through a 0.45 µm polytetrafluoroethylene filter (Millet, Milwaukee, WI, USA). CH2Cl2 was removed on a rotary evaporator at 30 °C and the resulting product was vacuum-dried at 60 °C over phosphorus pentoxide. Chemical structure of PEG-Ale in D2O was analyzed at 23 °C by 1H NMR spectrum (Figure 1a).
Figure 1.
(a) 1H NMR spectrum of PEG-Ale and (b) scheme of Fe3O4@PEG-Ale nanoparticles. PEG-Ale, poly(ethylene glycol)-alendronate.
2.3. Preparation of Fe3O4 Nanoparticles and Their Modification with PEG-Ale
Aqueous FeCl3·6H2O (10 mmol) solution (50 mL) was added with vigorous stirring to an iron(II) hydroxide dispersion prepared from aqueous solution (25 mL) of FeCl2·4H2O (5 mmol) and ammonium hydroxide (40 mmol; 3.7 mL). Resulting black precipitate was magnetically separated and washed with water ten times (100 mL each). Ammonium hydroxide (100 µL) was then added, the product was washed with water three times (50 mL each), and sonicated with a UP400S ultrasonic processor (Hielscher Ultrasonics, Teltow, Germany) for 5 min. To determine magnetic properties and iron oxide concentration, a small part of the colloid was lyophilized. PEG-Ale (22 mg) was then added to an aqueous dispersion of Fe3O4 nanoparticles (10 mL; 4.4 mg of Fe3O4/mL) that was then sonicated for 2 min (10% power; Bandeline Sonoplus, Berlin, Germany) and filtered through a sterile 0.45 µm filter to reach a concentration of 4.4 mg of Fe3O4@PEG-Ale per mL.
2.4. Characterization of Nanoparticles
The particles were visualized on a Tecnai Spirit G2 transmission electron microscope (TEM; FEI, Brno, Czech Republic). The number-average (Dn), weight-average diameter (Dw), and dispersity (Ð) were calculated from TEM micrographs, counting at least 500 individual particles: Dn = ∑Di/N, Dw = ∑Di4/∑Di3, Ð = Dw/Dn, where Di is the diameter of particle i and N is the total number of counted particles. Dynamic light scattering (DLS) was measured on a ZEN 3600 Zetasizer Nano Instrument (Malvern Instruments, Malvern, UK) providing hydrodynamic diameter Dh, polydispersity (PD), and ζ-potential. Superconducting quantum interference device magnetometry was performed on a Quantum Design MPMS XL device (San Diego, CA, USA). The magnetization curves were measured up to the fields of 3183 kA/m at 5 and 300 K. The zero-field-cooled and field-cooled (ZFC-FC) measurements were carried out in a magnetic field of H = 1.59 kA/m. Weight (Mw)- and number-average molar mass (Mn), and polydispersities of the polymers were determined on a Shimadzu high-performance size-exclusion liquid chromatograph (SEC, Tokyo, Japan) equipped with a UV-Vis diode array, OptilabrEX refractive index and DAWN EOS multiangle light scattering detectors (Wyatt, Santa Barbara, CA, USA), and a TSK SuperAW3000 column with methanol/sodium acetate buffer (80/20 v/v) eluent (Ph 6.5) at a flow rate of 0.6 mL/min. The 1H NMR spectrum was measured by a Bruker AscendTM 400 spectrometer operating at 400 MHz. Fourier-transform infrared (FTIR) spectrometer (PerkinElmer, Waltham, MA, USA) was equipped with a Specac MKII Golden Gate single attenuated total reflection. Amount of coating was evaluated by a PerkinElmer thermogravimetric analyzer (TGA). The grafting density (σ) of PEG on the particle surface was calculated according to Equation (1):
| (1) |
where mPEG and mFe3O4 are weight percentages of PEG and magnetite in the particles according to TGA, respectively, ρ is magnetite density (5.18 kg/m3), V is volume of a particle (approximated by the volume of sphere), NA is Avogadro’s number, Mn is number-average molar mass of PEG-Ale (5249 g/mol), and S is surface area of a particle (approximated by the surface area of sphere).
2.5. Animal Experiments
Male, spontaneously hypertensive rats (SHR) were obtained from the certified animal facility of the Department of Toxicology and Laboratory Animal Breeding, Centre of Experimental Medicine, Dobrá Voda, Slovakia. Rats, 13–16 weeks old, were housed under standard conditions at 22–24 °C and 12-h light/dark cycle and fed with pelleted diet Altromin formula 1320, variant P (Altromin Spezialfutter, Lage, Germany) and tap water ad libitum. All the procedures used in this study were approved by the State Veterinary and Food Administration of the Slovak Republic in accordance with the European Union Directive 2010/63/EU.
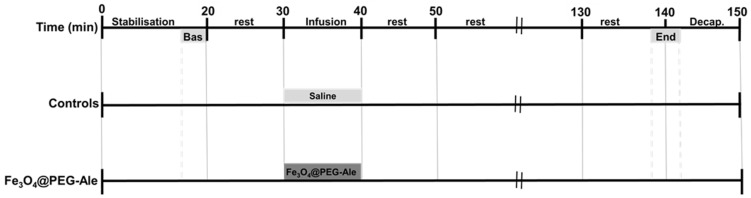
Animals were organized into two groups. The control (Cont) group (n = 6–7) obtained saline infusion, while the nanoparticle group received Fe3O4@PEG-Ale nanoparticles (n = 5–6). The nanoparticles (1 mg Fe3O4/kg body weight) dispersed in saline were administered intravenously (IV) for 10 min into the jugular vein. Experimental protocol is shown in the Figure 2. After the experiment, rats were exposed to brief CO2 anesthesia and decapitated within 5 min of the final mean arterial pressure (MAP) recording.
Figure 2.
Time course of the experimental protocol. Basal recordings of blood pressure and heart rate were determined at the beginning (Bas) and at the end of the experiment as the average values of ~120-s time periods between 16–20 min and 138–142 min of the experiment. Then, the rats were decapitated (Decap.). Bas, baseline; Fe3O4@PEG-Ale, Fe3O4@PEG-alendronate nanoparticles.
2.6. Measurement of Blood Pressure and Heart Rate
One day before the experiment, two fine bore polyethylene catheters (Smiths Medical International, Kent, UK) were implanted under 2.5–3% isoflurane anesthesia. One catheter was inserted into the left carotid artery for determination of arterial blood pressure (BP) and heart rate (HR) and the second one was inserted into the jugular vein for IV infusion of NPs or saline as described previously [8]. The experiments were performed in the quiet room to avoid any non-specific stimuli affecting BP and HR, with sampling rate 1 kHz. During the experiments, the conscious rats were placed into a dark plastic box, which allowed their free movement. Arterial catheter was attached to BP recording PowerLab data acquisition system (ADInstruments, Bella Vista, Australia). MAP and HR were recorded during the entire experiment and both parameters were evaluated during 120-s time periods between 10 and 14 min before nanoparticle administration (Bas), during the entire 10 min of NPs infusion, as well as during 120-s time periods ~100 min after the NPs administration (end of the experiment). Results were calculated using LabChart Pro version 8 (ADInstruments, Bella Vista, Australia).
2.7. Determination of Activity of Nitric Oxide Synthase
Activity of nitric oxide synthase (NOS) (expressed as pkat/g of protein) was assessed in 20% tissue homogenates by determining [3H]-L-citrulline formation from [3H]-L-arginine (ARC, St. Louis, MO, USA) as described in detail earlier [8]. Protein concentration was determined using the Lowry method [27].
2.8. Production of Superoxide
Tissues (10–25 mg) were placed into ice-cold modified Krebs–Henseleit solution (physiological saline solution, PSS; in mmol/L: 119 NaCl, 4.7 KCl, 1.17 MgSO4·7H2O, 25 NaHCO3, 1.18 KH2PO4, 0.03 Na2EDTA, 2.5 CaCl2·2H2O, 5.5 glucose). Lucigenin (50 µmol/L), as well as tissue samples alone, were added to PSS bubbled with pneumoxide (5% CO2 and 95% O2) at 37 °C and pH 7.4 and preincubated in the dark for 20 min. After the preincubation, either background lucigenin-enhanced chemiluminescence or lucigenin-enhanced chemiluminescence produced by tissue samples were measured for 6 min using a TriCarb 2910TR liquid scintillation analyzer (TriCarb, Perkin Elmer, Waltham, MA, USA) [28]. Background counts were subtracted from those of tissue samples and expressed as counts per min per mg of tissue (cpm/mg).
2.9. Determination of Vascular Functions
Isolated and cleaned femoral arteries with intact endothelium were cut into segments (two segments of each rat) and placed in Mulvany-Halpern isometric myograph (Dual Wire Myograph system 410A; Danish Myo Technology, Aarhus, Denmark) to investigate vascular function as described in detail previously [8]. Contractions induced by 125 mmol/L K+ and serotonin (5-HT; 10−6 mol/L) were investigated in the absence and presence of NO synthase inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME, 3 × 10−4 mol/L, 30 min pre-incubation). After 20 min of stabilization of 5-HT-induced contraction, endothelium-dependent relaxations were induced by administration of cumulative concentrations of acetylcholine (ACh, 10−9 to 10−5 mol/L) into the organ chamber. After washing and stabilization of the arteries, NOS inhibitor L-NAME (3 × 10−4 mol/L, 30 min incubation time) was added into the organ chamber and ACh-induced relaxations were repeatedly evaluated. Endothelium-independent relaxations produced by vascular smooth muscle cells were investigated using exogenous NO donor sodium nitroprusside (SNP, 10−9 to 10−5 mol/L).
2.10. Determination of Red Blood Cell Parameters
Red blood cell parameters were determined as described in detail previously [29]. Briefly, RBC deformability assessed by filtration method was expressed as a percentage of RBCs that were able to pass through the filters with pores 5 μm in diameter (Ultrafree-MC SV Centrifugal Filter, Merck Millipore, Ireland). For osmotic resistance, hemolytic assay was applied. RBCs were suspended in solutions with varying concentrations of NaCl (0.1–0.9%), incubated for 30 min and centrifuged. Intensity of hemolysis was determined spectrophotometrically and NaCl concentration in which 50% hemolysis occurred (IC50) was calculated from obtained data. NO production by RBCs was determined using 4,5-diaminofluorescein diacetate (Abcam, Cambridge, UK). NO dependent fluorescence was observed using a Nikon Eclipse Ti fluorescence microscope (Tokyo, Japan) and quantified using ImageJ software.
2.11. Statistical Analyses
Statistical analysis was performed by unpaired or paired Student’s t-test, where appropriate. MAP, HR, and vascular functions were analyzed by analysis of variance (ANOVA) for repeated measures. ANOVA analyses were followed by the Bonferroni post hoc test. To assess the difference in RBC parameters before and after the NP administration paired Student’s t-test or Wilcoxon test (depending on data normality) were used. The values were found to significantly differ when p < 0.05. The data were presented as mean ± standard error of mean (SEM). GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) and Statistica 13.5 (StatSoft, Hamburg, Germany) were used for the statistical analyses.
3. Results
3.1. Fe3O4 Nanoparticles Preparation
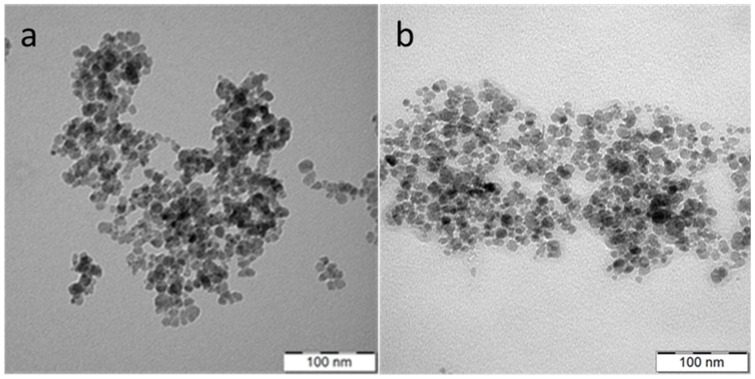
Magnetic Fe3O4 nanoparticles were synthetized by a coprecipitation method with a base. The technique is advantageous due to its simplicity, possibility of large-scale production, and high reaction yield. Resulting Fe3O4 nanoparticles had number-average diameter Dn = 11 nm and moderately high dispersity Ð = 1.24 according to TEM micrograph (Figure 3a), while hydrodynamic size (Dh = 100 nm) and polydispersity (PD = 0.11) were determined by DLS.
Figure 3.
Transmission electron microscope micrographs of (a) Fe3O4 and (b) Fe3O4@PEG-Ale nanoparticles.
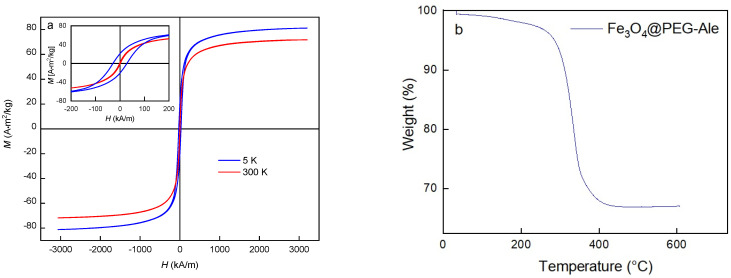
Fe3O4@PEG-Ale nanoparticles (Figure 1b) exhibited the same Dn and size distribution (Figure 3b) as original Fe3O4 particles and slightly increased Dh 110 nm. However, Fe3O4 and Fe3O4@PEG-Ale particles differed in the ζ-potential, amounting to −46 and −28 mV, respectively. Superparamagnetic properties of the pure Fe3O4 nanoparticles were confirmed by measuring of hysteresis loops at 300 and 5 K (Figure 4a). Saturation magnetization at these temperatures was 71 and 81 Am2/kg, respectively, which is close to that of the bulk state [30], and coercivity of the Fe3O4 was 30 kA/m at 5 K and it was negligible at room temperature.
Figure 4.
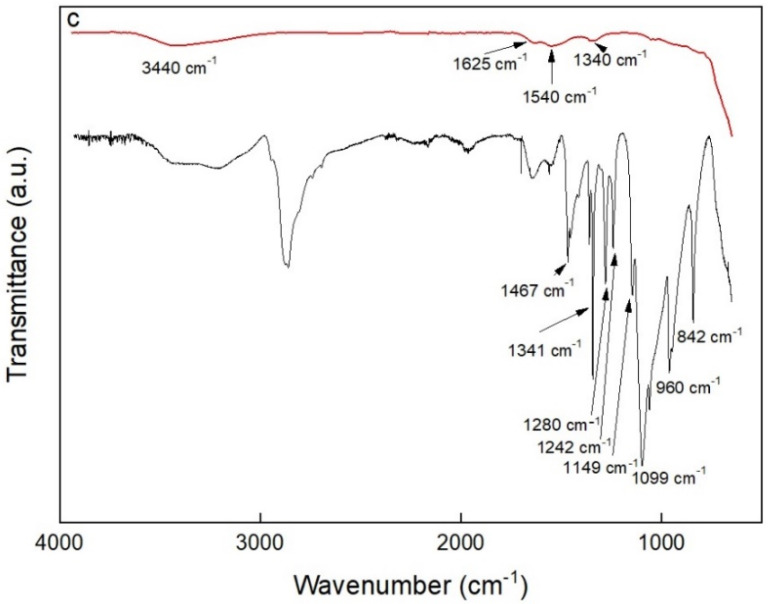
(a) Hysteresis loops of pure Fe3O4 nanoparticles at 5 and 300 K, (b) thermogravimetric analysis of Fe3O4@PEG-Ale, and (c) FTIR spectra of Fe3O4 (red) and Fe3O4@PEG-Ale nanoparticles (black).
Thermogravimetric analysis of Fe3O4@PEG-Ale nanoparticles confirmed the presence of polymer coatings on the iron oxide surface, reaching 33 wt.% (Figure 4b). Moreover, the distance between the attachment points of the PEG-Ale on the particle surface (d) and Flory radius (rf), respectively, were calculated [31,32] from the grafting density of the polymer (0.54 chains/nm2) and Mn of PEG. The rf/d ratio characterized the PEG conformation on particle surface that can be brush-like (rf/d > 1) or mushroom-like (rf/d < 1). As the rf/d ratio of Fe3O4@PEG-Ale particles was 4.45, brush-like conformation of PEG was confirmed. The presence of polymer on surface of nanoparticles was also supported by FTIR (Figure 4c). The spectrum of pure Fe3O4 showed broad band at 3440 and 1625 cm−1 belonging to O–H stretching vibration and O–H deformed vibration, respectively, proving the presence of coordinated OH groups or water on the particle surface [33]. Peaks at 1540 and 1340 cm−1 were probably associated with the presence of ammonium carbonate [34] due to the reaction of air CO2 with ammonia during precipitation of magnetite. The spectrum of PEG-coated nanoparticles showed C–O and C–C stretching and CH2 rocking at 840 cm−1, CH2 rocking and twisting at 960 cm−1, C–O and C–C stretching at 1097 cm−1, C–O stretching and CH2 rocking at 1140 cm−1, CH2 twisting at 1241 and 1278 cm−1, CH2 wagging at 1341 cm−1, and CH2 scissoring at 1466 cm−1.
3.2. Blood Pressure, Heart Rate and Red Blood Cell Parameters
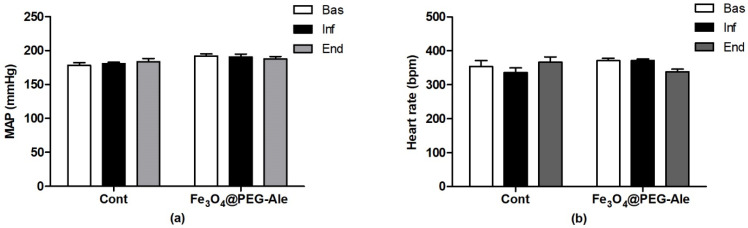
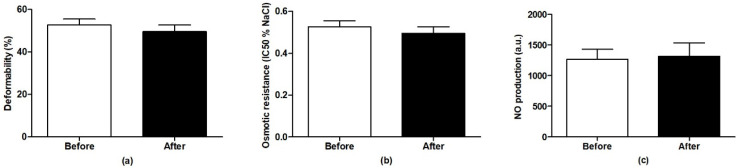
MAP and HR of all rats at the beginning of the experiment were 184 ± 3 mmHg and 361 ± 10 bpm (n = 6–7 per group) and no significant differences between the groups were observed. Administration of Fe3O4@PEG-Ale did not alter BP and HR, neither during the infusion nor at the end of the experiment compared to the corresponding time-point in the control group (Figure 5a,b). There were no differences in RBC deformability (n = 3), osmotic resistance (n = 3) and NO production (n = 5) by RBCs in rats determined 100 min after administration of Fe3O4@PEG-Ale nanoparticles, compared to basal levels of these rats (Figure 6).
Figure 5.
(a) Mean arterial pressure and (b) heart rate of rats 10–14 min before infusion, during infusion, and 100 min after infusion of nanoparticles. The values represent the mean ± SEM, n = 6–7 per group. Fe3O4@PEG-Ale, Fe3O4@PEG-alendronate nanoparticles; MAP, mean arterial pressure; HR, heart rate; Bas, basal value; Inf, value determined during infusion; End, value at the end of the experiment; bpm, beats per minute.
Figure 6.
(a) Red blood cell deformability, (b) osmotic resistance, and (c) nitric oxide production in spontaneously hypertensive rats before and 100 min after Fe3O4@PEG-Ale nanoparticle infusion. The values represent the mean ± SEM, n = 3 (deformability and osmotic resistance), n = 5 (NO production).
3.3. Determination of Nitric Oxide Synthase Activity
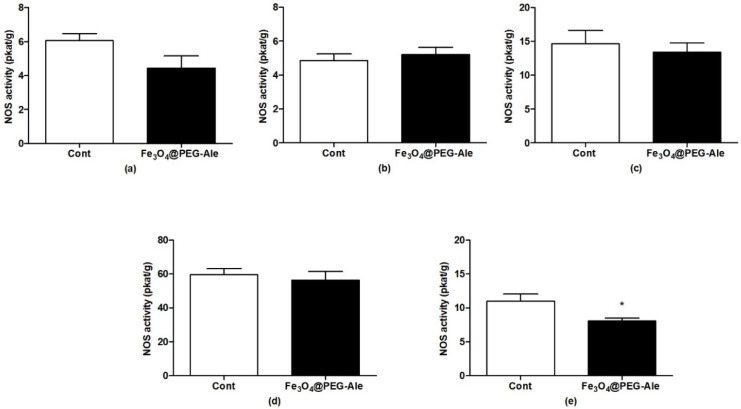
To determine the effect of Fe3O4@PEG-Ale nanoparticles on NO production, activity of NOS was determined in the rat aorta, left heart ventricle, liver, hypothalamus and kidney (n = 6–7 per group). Nanoparticles had no significant effect on NOS activity in the aorta, left heart ventricle, liver, and hypothalamus (Figure 7a–d). Significant reduction of NOS activity was observed in the kidneys by ~26%, (p < 0.05) vs. the control group (Figure 7e).
Figure 7.
Nitric oxide synthase activities in (a) aorta, (b) left heart ventricle, (c) liver, (d) hypothalamus, and (e) kidney after IV administration of Fe3O4@PEG-Ale nanoparticles in rats. The values represent the mean ± SEM. * p < 0.05 vs. control group, n = 6–7 per group. Cont, control; NOS, nitric oxide synthase; Fe3O4@PEG-Ale, Fe3O4@PEG-alendronate nanoparticles.
3.4. Production of Superoxide
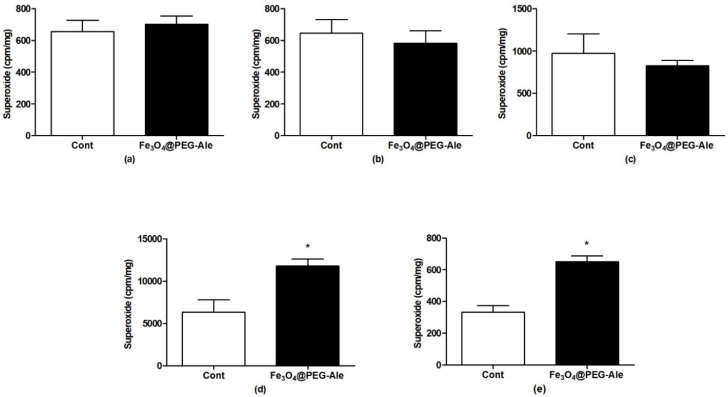
NPs did not elevate superoxide production in the aorta, left heart ventricle and liver, (Figure 8a–c). The highest levels of superoxide in control conditions were found in the spleen, in which NPs elevated the superoxide level approximately by 86% (p < 0.05) vs. control (Figure 8d). Superoxide production was also significantly elevated in the kidney by ~96% (p < 0.05) vs. the control group (Figure 8e).
Figure 8.
Superoxide production in (a) aorta, (b) left heart ventricle, (c) liver, (d) spleen, and (e) kidney after IV administration of Fe3O4@PEG-Ale nanoparticles in rats. The values represent the mean ± SEM * p < 0.05 vs. control group, n = 5–6 per group. Cont, control; Fe3O4@PEG-Ale, Fe3O4@PEG-alendronate nanoparticles.
3.5. Examination of Contractions of the Femoral Artery
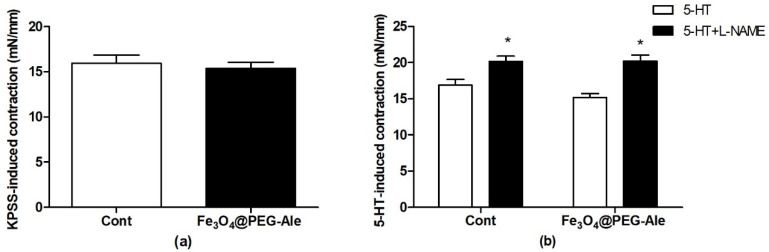
The mean internal diameters of all arterial segments of control and Fe3O4@PEG-Ale-treated rats were 682 ± 4 and 680 ± 8 µm, respectively, and they did not differ significantly. The maximal depolarization-induced contractions produced by high concentration of potassium (125 mmol/L K+) in the control and Fe3O4@PEG-Ale groups did not differ significantly (Figure 9a). 5-HT-induced contractions in the absence of L-NAME were similar in the control and Fe3O4@PEG-Ale groups (Figure 9b). Pre-incubation of the arteries with L-NAME significantly enhanced the 5-HT-induced contraction of the femoral arteries in both groups investigated (p < 0.05 for both groups) and Fe3O4@PEG-Ale did not alter this parameter (Figure 9b).
Figure 9.
Maximal depolarization-induced contractions produced by (a) KPSS and (b) serotonin (5-hydroxytryptamine)-induced contractions in the absence and presence of NO synthase inhibitor L-NAME. The values represent mean ± SEM. * p < 0.05 vs. 5-HT, n = 12 per group. Cont, control; Fe3O4@PEG-Ale, Fe3O4@PEG-alendronate nanoparticles; KPSS, high concentration of potassium (125 mmol/L)-containing physiological saline solution; 5-HT, serotonin; L-NAME, N(ω)-nitro-L-arginine methyl ester.
3.6. Examination of Relaxations of the Femoral Artery
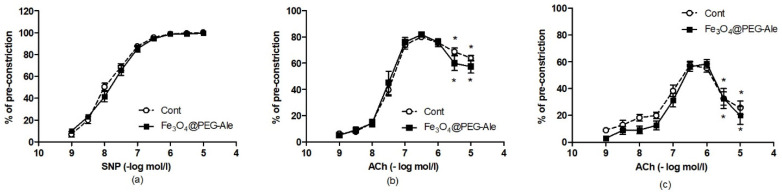
Sodium nitroprusside-induced and acetylcholine-induced concentration-response curves of 5-HT-precontracted femoral arteries are shown in Figure 10. SNP-induced relaxations were similar in the control and Fe3O4@PEG-Ale groups (Figure 10a). ACh-induced relaxations in the absence (Figure 10b) and presence (Figure 10c) of L-NAME were not altered by Fe3O4@PEG-Ale. Pre-treatment of the arteries with L-NAME led to significant reduction of relaxations at the highest Ach concentration in both groups investigated vs. the maximal relaxation in the given curve, suggesting no differences in the release of endothelium-derived contracting factors between control and NP-treated rats.
Figure 10.
(a) Sodium nitroprusside-induced relaxation and acetylcholine-induced relaxations (b) in the absence and (c) presence of L-NAME in the femoral arteries. The values represent the mean ± SEM, n = 12 per group. * p < 0.05 vs. the maximal relaxation in the same group. Cont, control; Fe3O4@PEG-Ale, Fe3O4@PEG-alendronate nanoparticles, ACh, acetylcholine; SNP, sodium nitroprusside.
4. Discussion
In this study, superparamagnetic PEG-Ale-covered magnetite nanoparticles were synthetized. Magnetite was preferred to maghemite not only due to a one-step synthesis, but also because Fe(III) is metabolized to Fe(II) in the living organism. We also hypothesized that difference in cytotoxicity between both iron oxides is negligible. The prepared magnetite nanoparticles were coated with PEG-based polymer, that is considered to be biocompatible and bioinert and able to temporarily mask the nanoparticles against immune system and prolong their circulation in the blood stream [35]. The polymer was designed to be terminated by functional end-groups to allow a solid attachment to the iron oxide surface. In this respect, Ale appeared to be especially convenient, as its bisphosphonate groups readily conjugate to the iron oxide surface forming stable complexes [35]. Moreover, both iron oxides and Ale, as well as PEG, are FDA-approved for using in human medicine [21,22,36]. This is the advantage of Ale compared to previously used neridronate that is still waiting for final approval. Besides, Ale is easily commercially available and reasonably priced.
The increase in Dn of neat Fe3O4 and Fe3O4@PEG-Ale nanoparticles was not detected by TEM, and Dh was only slightly raised, probably due to the presence of poly(ethylene glycol) shell. Difference between the hydrodynamic diameter Dh and Dn, was observed, with Dh being naturally higher than the number-average diameter Dn due to several reasons. First, hydrodynamic diameter can be approximated as Dh = ∑Di6/∑Di5, which provides larger numbers than Dn = ∑Di/N [37]. Second, DLS method is very sensitive to the presence of large particles, which also exponentially increases intensity of scattered light [38]. As a result, even a small fraction of large particles can dramatically increase the hydrodynamic diameter. The third reason is that DLS measures objects in solution, where they can aggregate and scatter light more intensively than the individual particles, while TEM determines individual particles.
Absolute value of ζ-potential decreased after PEG coating, which suggests that electroneutral PEG coating was bound to the iron oxide surface. The presence of polymer was also quantified by thermogravimetric analysis and confirmed by FTIR spectroscopy. The rf/d ratio > 1 confirmed that PEG-Ale was densely packed on the particle surface in a brush-like conformation. Magnetic measurement exhibited typical behavior for superparamagnetic materials; that means the nanoparticles are magnetic in an external magnetic field, but in its absence, they do not exhibit magnetism and do not aggregate as, e.g., ferromagnetic nanoparticles.
We also investigated biological effect of these NPs in hypertensive rats. The main findings are that Fe3O4@PEG-Ale (i) did not alter BP and HR, (ii) had no negative effects on fundamental RBC properties, and (iii) did not affect vascular function after acute intravenous administration. In addition, Fe3O4@PEG-Ale did not induce increase of superoxide and reduction in NO production in the tissue of the aorta, left heart ventricle, and liver.
As already mentioned in the introduction, the use of various types of NP in biomedical and medical applications depends on their biocompatibility, as well as stability. In human studies, vasodilatation associated with hypotension has been observed, when certain iron oxide NPs were administered as contrast agents to improve magnetic resonance imaging [21]. Similarly, a transient decrease of BP was observed after application of poly(acrylic acid)-coated γ-Fe2O3 nanoparticles in mice [39]. No effect of PEG-coated iron oxide NPs on BP and HR was found on normotensive rats using the same experimental protocol, which is in agreement with our current finding using different NPs in SHR [8]. However, PEG-coated iron oxide NPs altered vascular function of normotensive Wistar–Kyoto (WKY) rats in terms of elevation of endothelium-dependent NO-mediated components of vasorelaxation, and partially reduced 5-HT-induced contraction. In addition, PEG-coated NPs reduced the sensitivity of VSMCs to NO in WKY rats [8]. Similar vascular changes were not present in SHR rats after application of Fe3O4@PEG-Ale in this study. Researchers also showed that iron oxide NP accumulation in endothelial cells can modify vascular function, NO bioavailability, and/or induce oxidative stress [40,41,42]. In this study, NO synthase activity and superoxide production were not changed significantly in the aorta of SHR. These findings, together with no changes in vascular functions, suggested that Fe3O4@PEG-Ale do not affect negatively the endothelium and vascular smooth muscle cells of the femoral artery in rats with high BP. Similarly, Fe3O4@PEG-Ale had no negative effects in the human umbilical vein endothelial cell cultures [43].
Another important finding suggesting good biocompatibility of Fe3O4@PEG-Ale is the fact that these NPs did not modify RBC deformability, which represents the crucial characteristic allowing RBC passage through the narrow capillaries in the microcirculation and is also a determinant of whole blood viscosity. RBC deformability is maintained by various regulatory mechanisms, among which NO production by RBCs plays an important role. In this study, NO production by RBCs was not affected by infusion of Fe3O4@PEG-Ale. In addition, Fe3O4@PEG-Ale did not modify the RBC properties to challenge the changes in osmotic pressure. Thus, the Fe3O4@PEG-Ale NPs seem to be RBC-biocompatible during in vivo conditions in SHR. In addition to the aorta, Fe3O4@PEG-Ale did not induce elevation in superoxide production in the tissues of the left heart ventricle and liver. On the other hand, Fe3O4@PEG-Ale elevated superoxide production in the kidney and spleen. This may be related to the fact that NPs are excreted by the kidneys and/or internalized in the spleen. Our findings are in contrast to elevated superoxide production in the liver, aorta and left heart ventricle found in normotensive rats using PEG-coated NPs [44]. We assume that the differences can result mainly from different hemodynamic situation in SHR, as well as from size and different physicochemical properties of NPs.
NO is the main vasorelaxant molecule in the cardiovascular system, but serves as a neurotransmitter and neuromodulator in organisms. In this study, reduced NO production was found only in the kidneys, together with elevated superoxide production. This finding is similar to findings in the kidney of WKY rats using PEG-coated NPs without Ale [8,44]. On the other hand, our findings suggested that Fe3O4@PEG-Ale NPs produced less changes in the cardiovascular tissue, liver and hypothalamus than previously used PEGylated NPs, which may result from different physicochemical properties, size and/or modified coating. In hypertensive rats, an important role may be played by altered hemodynamic state (blood pressure and blood flow), which may accelerate NPs clearance from circulation. However, independently of differences in above mentioned factors (hemodynamic situation, physicochemical properties, and coating of NPs), reduction of NO and elevated superoxide in the kidneys, might suggest at least partial NOS uncoupling resulting in oxidative stress. As oxidative damage might later be followed by functional and/or structural changes in the tissues, attention should be paid to the possible harmful effect of NPs to kidneys.
In conclusion, we prepared superparamagnetic magnetite NPs with Dn = 11 nm covered with PEG-Ale coating, and moderately narrow size distribution, for possible use as an agent increasing MRI contrast. Determination of biological influences of Fe3O4@PEG-Ale NPs did not show negative effects on the cardiovascular system and fundamental RBC parameters after acute intravenous administration in SHR. Fe3O4@PEG-Ale NPs induced increase in superoxide and reduction in NO production in the kidney. Thus, despite that there were no significant effects of Fe3O4@PEG-Ale on the cardiovascular system and RBCs, further studies are needed to evaluate their effect in the kidneys. These findings contribute to complex knowledge about behavior of magnetic nanoparticles in in vivo animal models, considering also the influence of high BP, which makes this paper valuable in terms of nanotoxicology research.
Acknowledgments
The authors thank J. Petova, and B. Bolgacova for their excellent technical assistance.
Abbreviations
| 5-HT | 5-hydroxytryptamine (serotonin) |
| ACh | acetylcholine |
| Ale | alendronate |
| ANOVA | analysis of variance |
| Bas | baseline |
| BP | arterial blood pressure |
| bpm | beats per minute |
| Cont | control |
| Ð | dispersity |
| D h | hydrodynamic diameter |
| DLS | dynamic light scattering |
| D n | number-average diameter |
| D w | weight-average diameter |
| FDA | Food and Drug Administration |
| Fe3O4@PEG-Ale | magnetite coated with poly(ethylene glycol)-alendronate |
| FTIR | Fourier-transform infrared |
| HR | heart rate |
| i.v. | intravenous |
| KPSS | high concentration of potassium (125 mmol/l)-containing physiological saline solution |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| MAP | mean arterial pressure |
| M n | number-average molar mass |
| M w | weight-average molar mass |
| MRI | magnetic resonance imaging |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NPs | nanoparticles |
| PB | phosphate buffer |
| PD | polydispersity |
| PEG | poly(ethylene glycol) |
| PEG-NHS | α-methoxy poly(ethylene glycol) succinimidyl ester |
| PSS | physiological saline solution |
| RBC | red blood cell |
| SEM | standard error of mean |
| SHR | spontaneously hypertensive rat |
| SNP | sodium nitroprusside |
| TEM | transmission electron microscope |
| TGA | thermogravimetric analysis |
| WKY | Wistar-Kyoto rat |
| ZFC-FC | zero-field-cooled and field-cooled |
Author Contributions
Conceptualization, D.H. and I.B.; data curation, V.O., V.P., I.B., formal analysis, H.M., I.B., S.L. and J.R., funding acquisition, D.H. and I.B.; investigation, V.P., V.O., H.M., M.K., P.B., A.M., M.K., S.L., J.R., D.R. and T.J.; methodology, V.O., V.P., H.M., M.K., P.B., S.L., J.R. and I.B.; project administration, D.H. and I.B.; visualization, D.H., S.L. and I.B.; writing—original draft, H.M., D.H., J.R. and I.B.; writing—review and editing, H.M., D.H., J.R., S.L., P.B., M.K., A.M., and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech Science Foundation (No. 20-02177J), the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic (No. 2/0160/17 and 2/0157/21] and by the Slovak Research and Development Agency (No. APVV-16-0263).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the State Veterinary and Food Administration of the Slovak Republic (protocol code 3619/16-221, date of approval 28 October 2016 and protocol code 3052/2021-220, date of approval 26 February 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Y., Lu Z., Li Y., Yang J., Zhang X. Surface modification of iron oxide-based magnetic nanoparticles for cerebral theranostics: Application and prospection. Nanomaterials. 2020;10:1441. doi: 10.3390/nano10081441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Gómez M.A., Belderbos S., Yañez-Vilar S., Piñeiro Y., Cleeren F., Bormans G., Deroose C.M., Gsell W., Himmelreich U., Rivas J. Development of superparamagnetic nanoparticles coated with polyacrylic acid and aluminum hydroxide as an efficient contrast agent for multimodal imaging. Nanomaterials. 2019;9:1626. doi: 10.3390/nano9111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephen Z.R., Kievit F.M., Zhang M. Magnetite nanoparticles for medical MR imaging. Mater. Today. 2011;14:330–338. doi: 10.1016/S1369-7021(11)70163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fütterer S., Andrusenko I., Kolb U., Hofmeister W., Langguth P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD) J. Pharm. Biomed. 2013;86:151–160. doi: 10.1016/j.jpba.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Wáng Y.X.J., Idée J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017;7:88–122. doi: 10.21037/qims.2017.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y.N., Poon W., Tavares A.J., McGilvray I.D., Chan W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release. 2016;240:332–348. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Arami H., Khandhar A., Liggitt D., Krishnan K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015;44:8576–8607. doi: 10.1039/C5CS00541H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Líšková S., Bališ P., Mičurová A., Kluknavský M., Okuliarová M., Puzserová A., Škrátek M., Sekaj I., Maňka J., Valovič P., et al. Effect of iron oxide nanoparticles on vascular function and nitric oxide production in acute stress-exposed rats. Physiol. Res. 2020;69:1067–1083. doi: 10.33549/physiolres.934567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolenko Y.V., Bañobre-López M., Rodríguez-Abreu C., Carbó-Argibay E., Sailsman A., Piñeiro-Redondo Y., Cerqueira M.F., Petrovykh D.Y., Kovni K., Lebedev O.I., et al. Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J. Phys. Chem. C. 2014;118:8691–8701. doi: 10.1021/jp500816u. [DOI] [Google Scholar]
- 10.Roca A.G., Marco J.F., Morales M.P., Serna C.J. Effect of nature and particle size on properties of uniform magnetite and maghemite nanoparticles. J. Phys. Chem. C. 2007;111:18577–18584. doi: 10.1021/jp075133m. [DOI] [Google Scholar]
- 11.Barrow M., Taylor A., Fuentes-Caparrós A.M., Sharkey J., Daniels L.M., Mandal P., Park B.K., Murray P., Rosseinsky M.J., Adams D.J. SPIONs for cell labelling and tracking using MRI: Magnetite or maghemite? Biomater. Sci. 2018;6:101–106. doi: 10.1039/C7BM00515F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luong D., Sau S., Kesharwani P., Iyer A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules. 2017;18:1197–1209. doi: 10.1021/acs.biomac.6b01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NDong C., Tate J.A., Kett W.C., Batra J., Demidenko E., Lewis L.D., Hoopes P.J., Gerngross T.U., Griswold K.E. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE. 2015;10:e0115636. doi: 10.1371/journal.pone.0115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrade R.G.D., Veloso S.R.S., Castanheira E.M.S. Shape anisotropic iron oxide-based magnetic nanoparticles: Synthesis and biomedical applications. Int. J. Mol. Sci. 2020;21:2455. doi: 10.3390/ijms21072455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalzon B., Torres A., Reymond S., Gallet B., Saint-Antonin F., Collin-Faure V., Moriscot C., Fenel D., Schoehn G., Aude-Garcia C., et al. Influences of nanoparticles characteristics on the cellular responses: The example of iron oxide and macrophages. Nanomaterials. 2020;10:266. doi: 10.3390/nano10020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez I., Mestach D., Leiza J.R., Asua J.M. Adhesion enhancement in waterborne acrylic latex binders synthesized with phosphate methacrylate monomers. Prog. Org. Coat. 2008;61:38–44. doi: 10.1016/j.porgcoat.2007.09.012. [DOI] [Google Scholar]
- 17.Guénin E., Hardouin J., Lalatonne Y., Motte L. Bivalent alkyne-bisphosphonate as clickable and solid anchor to elaborate multifunctional iron oxide nanoparticles with microwave enhancement. J. Nanopart. Res. 2012;14:965. doi: 10.1007/s11051-012-0965-7. [DOI] [Google Scholar]
- 18.Chen B.W., He Y.C., Sung S.Y., Le T.T.H., Hsieh C.L., Chen J.Y., Wei Z.H., Yao D.J. Synthesis and characterization of magnetic nanoparticles coated with polystyrene sulfonic acid for biomedical applications. Sci. Technol. Adv. Mater. 2020;21:471–481. doi: 10.1080/14686996.2020.1790032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guibert C., Dupuis V., Peyre V., Fresnais J. Hyperthermia of magnetic nanoparticles: Experimental study of the role of aggregation. J. Phys. Chem. C. 2015;119:28148–28154. doi: 10.1021/acs.jpcc.5b07796. [DOI] [Google Scholar]
- 20.Turcheniuk K., Tarasevych A.V., Kukhar V.P., Boukherroub R., Szunerits S. Recent advances in surface chemistry strategies for the fabrication of functional iron oxide based magnetic nanoparticles. Nanoscale. 2013;5:10729–10752. doi: 10.1039/c3nr04131j. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011;1:35–40. doi: 10.3978/j.issn.2223-4292.2011.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen E.K.U., Nielsen T., Wittenborn T., Rydtoft L.M., Lokanathan A.R., Hansen L., Østergaard L., Kingshott P., Howard K.A., Besenbacher F., et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale. 2012;4:2352–2361. doi: 10.1039/c2nr11554a. [DOI] [PubMed] [Google Scholar]
- 23.Ronnander P.L., Simon H., Spilgies A., Koch A., Scherr S. Dissolving polyvinylpyrrolidone-based microneedle systems for in-vitro delivery of sumatriptan succinate. Eur. J. Pharm. Sci. 2018;114:84–92. doi: 10.1016/j.ejps.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 24.Boda S.K., Wang H., John J.V., Reinhardt R.A., Xie J. Dual delivery of alendronate and E7-BMP-2 peptide via calcium chelation to mineralized nanofiber fragments for alveolar bone regeneration. ACS Biomater. Sci. Eng. 2020;6:2368–2375. doi: 10.1021/acsbiomaterials.0c00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernatova I. Endothelial dysfunction in experimental models of arterial hypertension: Cause or consequence? Biomed. Res. Int. 2014;2014:598271. doi: 10.1155/2014/598271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumienna-Kontecka E., Silvagni R., Lipinski R., Lecouvey M., Marincola F.C., Crisponi G., Nurchib V.M., Leroux Y., Kozlowski H. Bisphosphonate chelating agents: Complexation of Fe(III) and Al(III) by 1-phenyl-1-hydroxymethylene bisphosphonate and its analogues. Inorg. Chim. Acta. 2002;339:111–118. doi: 10.1016/S0020-1693(02)00918-0. [DOI] [Google Scholar]
- 27.Waterborg J.H. The Lowry method for protein quantitation. In: Walker J.M., editor. The Protein Protocols Handbook. 3rd ed. Humana Press; Totowa, NJ, USA: 2009. pp. 7–10. [Google Scholar]
- 28.Kluknavsky M., Balis P., Skratek M., Manka J., Bernatova I. (–)-Epicatechin reduces the blood pressure of young borderline hypertensive rats during the post-treatment period. Antioxidants. 2020;9:96. doi: 10.3390/antiox9020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radosinska J., Jasenovec T., Radosinska D., Balis P., Puzserova A., Skratek M., Manka J., Bernatova I. Ultra-small superparamagnetic iron-oxide nanoparticles exert different effects on erythrocytes in normotensive and hypertensive rats. Biomedicines. 2021;9:377. doi: 10.3390/biomedicines9040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornell R.M., Schwertmann U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses. John Wiley; Weinheim, Germany: 2003. [DOI] [Google Scholar]
- 31.Labouta H.I., Gomez-Garcia M., Sarsons C.D., Nguyen T., Kennard J., Ngo W., Terefe K., Iragorri N., Lai P., Rinker K.D., et al. Surface-grafted polyethylene glycol conformation impacts the transport of PEG-functionalized liposomes through a tumour extracellular matrix model. RSC Adv. 2018;8:7697–7708. doi: 10.1039/C7RA13438J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahme K., Chen L., Hobbs R.G., Morris M.A., O’Driscoll C., Holmes J.D. PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2013;3:6085–6094. doi: 10.1039/C3RA22739A. [DOI] [Google Scholar]
- 33.Iyengar S.J., Joy M., Ghosh C.K., Dey S., Kotnala R.K., Ghosh S. Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core-shell nanostructures fabricated through an aqueous route. RSC Adv. 2014;4:64919–64929. doi: 10.1039/C4RA11283K. [DOI] [Google Scholar]
- 34.Khanna R.K., Moore M.H. Carbamic acid: Molecular structure and IR spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1999;55:961–967. doi: 10.1016/S1386-1425(98)00228-5. [DOI] [PubMed] [Google Scholar]
- 35.Patsula V., Horák D., Kučka J., Macková H., Lobaz V., Francová P., Herynek V., Heizer T., Páral P., Šefc L. Synthesis and modification of uniform PEG-neridronate-modified magnetic nanoparticles determines prolonged blood circulation and biodistribution in a mouse preclinical model. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-47262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings S.R., Santora A.C., Black D.M., Russell R.G.G. History of alendronate. Bone. 2020;137:115411. doi: 10.1016/j.bone.2020.115411. [DOI] [PubMed] [Google Scholar]
- 37.Thomas J.C. The determination of log normal particle size distributions by dynamic light scattering. J. Colloid Interface Sci. 1987;117:187–192. doi: 10.1016/0021-9797(87)90182-2. [DOI] [Google Scholar]
- 38.Fischer K., Schmidt M. Pitfalls and novel applications of particle sizing by dynamic light scattering. Biomaterials. 2016;98:79–91. doi: 10.1016/j.biomaterials.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Iversen N.K., Frische S., Thomsen K., Laustsen C., Pedersen M., Hansen P.B., Bie P., Fresnais J., Berret J.F., Baatrup E., et al. Superparamagnetic iron oxide polyacrylic acid coated γ-Fe2O3 nanoparticles do not affect kidney function but cause acute effect on the cardiovascular function in healthy mice. Toxicol. Appl. Pharmacol. 2013;266:276–288. doi: 10.1016/j.taap.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Zhu M.T., Wang Y., Feng W.Y., Wang B., Wang M., Ouyang H., Chai Z.F. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J. Nanosci. Nanotechnol. 2010;10:8584–8590. doi: 10.1166/jnn.2010.2488. [DOI] [PubMed] [Google Scholar]
- 41.Astanina K., Simon Y., Cavelius C., Petry S., Kraegeloh A., Kiemer A.K. Superparamagnetic iron oxide nanoparticles impair endothelial integrity and inhibit nitric oxide production. Acta. Biomater. 2014;10:4896–4911. doi: 10.1016/j.actbio.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Yarjanli Z., Ghaedi K., Esmaeili A., Rahgozar S., Zarrabi A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017;18:51. doi: 10.1186/s12868-017-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benyettou F., Hardouin J., Lecouvey M., Jouni H., Motte L. PEGylated versus non-PEGylated γ-Fe2O3@alendronate nanoparticles. J. Bioanal. Biomed. 2012;4:39–45. doi: 10.4172/1948-593X.1000062. [DOI] [Google Scholar]
- 44.Škrátek M., Dvurečenskij A., Kluknavský M., Barta A., Bališ P., Mičurová A., Cigáň A., Eckstein-Andicsová A., Maňka J., Bernátová I. Sensitive SQUID bio-magnetometry for determination and differentiation of biogenic iron and iron oxide nanoparticles in the biological samples. Nanomaterials. 2020;10:1993. doi: 10.3390/nano10101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are contained within the article.