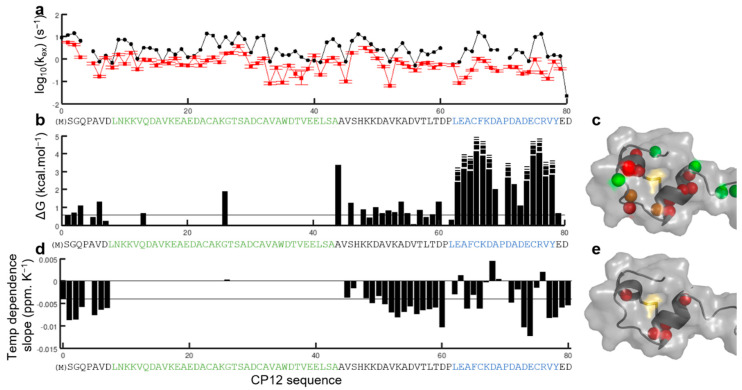
Figure 2.
Labile amide protons in CP12red and CP12ox. (a) Solvent-amine intrinsic proton exchange rates calculated using SPHERE [44] (black) and solvent–amide proton exchange rates measured using CLEANEX-PM experiment [39,43] on CP12red (red). The similarity between both values indicated that CP12red is intrinsically disordered. The signal intensity as a function of the spin-lock delay in the CLEANEX-PM experiments is shown in Figure S1. (b) Gibbs free energy of CP12ox backbone amides derived from the measured solvent–amine proton exchange using CLEANEX-PM on CP12ox versus CP12red (refer to Material and Methods for details), assuming that the exchange falls into the EX2 regime that is highly probable at this low pH (7). The measured rates of exchange for CP12ox amide at pH 7 and pH 6 are shown Figure S2. In (b,d), the stars indicate residues for which the resonances are broadened beyond detection in CP12ox [31], and the disulfide bridges are indicated above the sequence. (d) Value of the slope of the temperature dependence of the proton chemical shift for CP12ox residues. The line at −4.6 ppm K−1 indicates the threshold below which the data indicate the absence of a hydrogen bond [50]. The temperature dependence curves of the chemical shift of the amine proton for all CP12ox residues are shown Figure S4. (c) Structure of the stable C-terminal helical turn with the amide protons protected from the exchange with water highlighted in red, and those exposed to exchange in green. (e) On the same structure, the amide protons for which the temperature dependence of the chemical shift has a positive slope are highlighted in red. The CP12 sequence is indicated below the graphs, with the following color coding: the residues that fold in the stable helical turn in the C-terminal region of CP12ox are in blue, and the residues that undergo chemical exchange in CP12ox are in green [31].