Abstract
Normal wound healing progresses through inflammatory, proliferative and remodeling phases in response to tissue injury. Collagen, a key component of the extracellular matrix, plays critical roles in the regulation of the phases of wound healing either in its native, fibrillar conformation or as soluble components in the wound milieu. Impairments in any of these phases stall the wound in a chronic, non-healing state that typically requires some form of intervention to guide the process back to completion. Key factors in the hostile environment of a chronic wound are persistent inflammation, increased destruction of ECM components caused by elevated metalloproteinases and other enzymes and improper activation of soluble mediators of the wound healing process. Collagen, being central in the regulation of several of these processes, has been utilized as an adjunct wound therapy to promote healing. In this work the significance of collagen in different biological processes relevant to wound healing are reviewed and a summary of the current literature on the use of collagen-based products in wound care is provided.
Keywords: extracellular matrix, collagen, signaling, inflammation, wound healing, collagen dressings, engineered collagen
1. Introduction
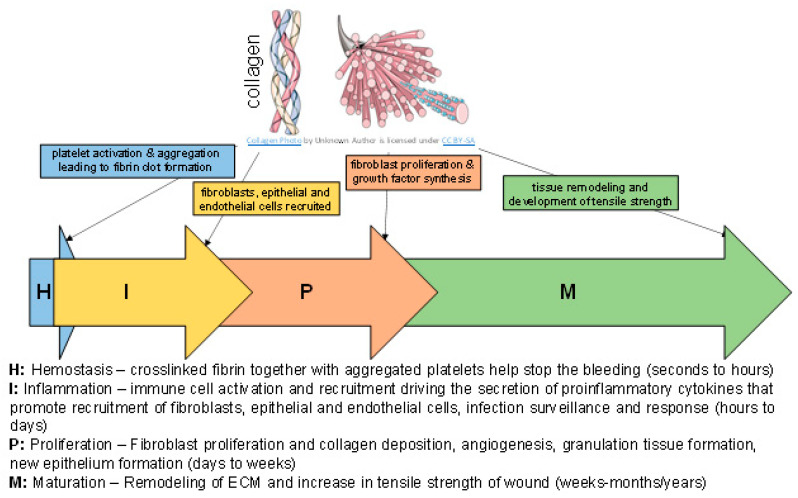
Sophisticated regulation by a number of key factors including the environment of the wound which is rich in extracellular matrix (ECM) drives the process of wound healing [1,2]. The complex macromolecules constituting the ECM include fibrous components (e.g., collagens and elastins) and glycoprotein components (e.g., fibronectin, proteoglycans and laminins). Each of these molecules interact to drive the process of tissue function, growth and repair [3,4,5]. Wound repair is a complex process that is broadly categorized into the following four phases which occur in a temporal sequence but are overlapping: hemostasis, inflammation, proliferation (cellular infiltration, angiogenesis and re-epithelialization) and maturation/remodeling (Figure 1) [1]. Key steps of the wound healing process, such as hemostasis, inflammation and angiogenesis are responsive to the ECM, collagen and its compounds [1,6,7,8,9,10,11,12,13]. In response to injury, collagen induces platelet activation and aggregation resulting in the deposition of a fibrin clot at the injury site. In the inflammatory stage of wound healing, immune cell activation drives the secretion of proinflammatory cytokines which influence migration of fibroblasts, epithelial and endothelial cells. Fibroblasts contribute to collagen deposition. Simultaneously, collagen degradation releases fragments that promote fibroblast proliferation and synthesis of growth factors that lead to angiogenesis and re-epithelialization. Finally, the remodeling of the ECM (balance of new matrix synthesis and matrix metalloproteinase degradative activities) determines the acquisition of tensile strength [14,15,16]. In this work we sought to briefly review the significance of collagen in different biological processes relevant to wound healing. The current literature on the use of collagen-based products in wound care is summarized.
Figure 1.
Brief summary of wound healing phases.
2. Types of Collagens in the Skin and Wound
Collagens are the most abundant protein found throughout the body. In the healing wound, these collagens are synthesized by cells such as fibroblasts and modified into complex morphologies [17,18,19,20,21]. The type, amount and organization of collagen changes in the healing wound and determines the tensile strength of the healed skin. Collagen III is the first to be synthesized in the early stages of wound healing and is replaced by collagen I, the dominant skin collagen. The initial random deposition of collagen during the granulation tissue formation is further enhanced by lysyl oxidase enzyme-induced covalent cross-linking. This process matures the collagen into complex structures that are reoriented for tensile strength restoration. Collagen remodeling continues for months after wound closure and the tensile strength of the repaired tissue increases to about 80–85% of normal tissue if all processes proceed without any perturbations [16].
In the skin, the fibrillar collagens types I, III and V are the most common, followed by fibril-associated collagens type XII, XIV, XVI, and VI. The non-fibrillar collagens type IV, XVIII are found in the basement membrane of the skin [14,18,19,22,23].
3. Processing of Collagen in the Skin and Wound
3.1. Biosynthesis and Cross-Linking
In the healing wound, cells such as fibroblasts (resident, and myeloid cell converted fibroblasts) [24] are the main source of newly synthesized collagen. The biosynthesis activities of fibril-forming collagens are the most extensively studied among all the collagens and involve multiple complex steps requiring the temporal and spatial coordination of several biochemical events [21,25]. Following transcription, the nascent/pre-pro-collagen is post-translationally modified in the endoplasmic reticulum into pro-collagen with the removal of the signal peptide on the N-terminus. Hydroxylation and glycosylation of amino acid residues results in the formation of the triple-helical structure characteristic of collagens. Supported by chaperone proteins, the pro-collagen triple-helical structure is stabilized for further processing and maturation in the Golgi apparatus and assembled into secretory vesicles that are extruded into the extracellular space where the pro-collagen is enzymatically modified into tropocollagen. The final collagen fibril assembly occurs by covalent cross-linking. The mechanical properties (elasticity and reversible deformation) of fibrillar collagens are dependent on this cross-linking process. Some of these cross-links include: (1) disulfide bonds; (2) reducible and mature cross-links produced via the lysyl oxidase pathway; (3) transglutaminase cross-links; and (4) advanced glycation end (AGE) product-mediated cross-links, among others. The nuances of cross-linking vary with the type of collagen and the tissue context and creates a multi-layered hierarchical structure [26]. Mature cross-links add resistance to shear stress. AGE-specific cross-links contribute to increased stiffness of collagens in aged tissues.
3.2. Degradation
Collagen degradation is involved in inflammation, angiogenesis, and re-epithelialization in the wound regulated by complex molecular pathways [27]. During the inflammation phase, soluble fragments from collagen degradation recruit immune cells such as macrophages that patrol the wound for removal of microbes and devitalized tissue. This aids in the transition to the proliferative phase. During this stage, collagen fragments serve as potent angiogenic signals to promote the development of new blood vessels. Keratinocyte migration is also promoted by collagen and contributes to wound re-epithelialization [16,28,29]. Degradation is regulated by extracellular and intracellular pathways. The former involves membrane-bound and secreted proteolytic enzymes. The latter involves internalization of intact collagen fibrils and fragmented collagen (through phagocytosis, macropinocytosis or endocytosis), followed by enzymatic breakdown. Defects in the regulated turnover of collagens results in pathological conditions such as fibrosis [20,30,31,32].
The actions of proteolytic enzymes at different stages in the wound healing process guides the remodeling of the repaired tissue. Two important enzyme families are the matrix metalloproteinases (MMPs) and serine proteases. The production and secretion of these enzymes are tightly regulated and are associated with specific cellular subtypes [12,33]. Among the MMPs, collagenases and gelatinases, which degrade intact and damaged fibrillar collagen respectively, are key for collagen turnover during wound healing. Collagens I and III are preferentially cleaved by MMP-1 (also called collagenase-1) and MMP-8 (collagenase-2) while collagen IV is degraded by the gelatinase MMP-9. Extensive research has determined that collagenolytic enzymes can recognize, bind, unwind and cleave the individual strands of the triple helix. It is speculated that this high specificity could be driven by the primary and super-secondary structures of collagen. MMPs drive physiological (development and tissue repair) and pathological (tumorigenesis and metastasis) processes. They also contribute to the release of bioactive fragments (also termed matricryptins) such as endostatin and tumstatin from full-length collagens [34]. These fragments specifically guide blood vessel pruning that in turn enables the re-establishment of the tissue architecture during healing [23,35,36,37,38]. Neutrophil elastase is a serine protease that aids in the same process. A balance of enzyme activity and inhibition is required for normal wound healing and is under tight regulatory control. Imbalances in the levels of these enzymes are a factor in wound chronicity. Wounds infected with microbes that produce these collagen-degrading enzymes add to the imbalance, leading to chronic wounds.
3.3. Receptor-Mediated Signaling
Collagen in all its forms, triple-helical, matrix-incorporated and degraded fragments, are cognate ligands of diverse families of cell surface receptors including integrins, receptor tyrosine kinases and immunoglobulin type receptors [1,39]. In the wound environment, collagens mediate several key steps such as platelet aggregation, inflammation modulation, angiogenesis, granulation tissue formation and re-epithelialization in a integrin signaling-dependent manner [31,32,39]. Receptor tyrosine kinases such as Discoidin Domain Receptors (DDR-1 and DDR-2) bind matrix-incorporated collagen and regulate key wound healing processes. Loss of function of these signaling molecules inhibits keratinocyte proliferation and collagen remodeling during wound healing, resulting in wounds with low tensile strength [40]. Abnormal signaling induced by collagen is observed in pathological conditions such as scar formation [32].
4. Roles for Collagen in the Skin and Wound
Collagen contributes to the mechanical strength and elasticity of tissues and acts as a natural substrate for cellular attachment, proliferation, and differentiation (Figure 1) [16,21,28,29]. Biofilm-mediated upregulation of MMP-2 via microRNAs creates a collagenolytic environment in the wound, sharply decreasing the collagen I/collagen III ratio and compromising the biomechanical properties of the repaired skin, possibly making the repaired skin vulnerable to wound recurrence [41]. A recent mapping study of collagen structure and function suggested that in normal, injured tissue the collagen fibril is in a closed conformation that upon exposure to blood following injury exposes cell- and ligand-binding sites that could promote the wound healing process [20]. Several recent reviews detail roles of collagen in the skin and wounds [1,42,43,44,45,46,47,48,49,50].
4.1. Role in Inflammation
The inflammatory phase of wound healing includes hemostasis and inflammation [51]. Collagen exposure due to injury activates the clotting cascade, resulting in a fibrin clot that stops the initial bleeding. Collagen I and IV fragments can be mediators of inflammation by acting as potent chemoattractants for neutrophils, enhancing phagocytosis and immune responses and modulating gene expression [19,34]. Inflammation is a critical step in the normal process of wound healing and drives the proliferation of fibroblasts which synthesize collagen and ECM [9]. The resolution of inflammation in a timely manner is equally important in normal wound healing. Resolution of inflammation is an active process that is driven by balanced pro and anti-inflammatory responses. A study using a stabilized collagen matrix showed that collagen mounts a robust and sharp inflammatory response that is transient and resolves rapidly to make way for wound healing to advance [6]. Furthermore, an important role for collagen in promoting an anti-inflammatory, pro-angiogenic wound macrophage phenotype via microRNA signaling has also been demonstrated [7,10].
4.2. Role in Angiogenesis
Angiogenesis, a critical component of physiological (development, wound healing) and pathological (cancer) processes, is tightly regulated by the balanced activity of stimulators and inhibitors. ECM remodeling provides critical support for vascular development and collagens play an important role in this process [7,11,13,52,53]. Depending on the type of collagen, the role might be as a promoter or inhibitor of angiogenesis. A live analysis via multiphoton microscopy of neovessel formation in vitro identified a dynamic modulation of collagen I that showed early stage remodeling of collagen fibrils progressing to collagen condensation in later stages of development [54]. Collagen I is known to potently stimulate angiogenesis in vitro and in vivo through engagement of specific integrin receptors. Specifically, the C-propeptide fragment of collagen I recruits endothelial cells, potentially triggering angiogenesis in the healing wound [12]. By contrast, proteolytic collagen fragments of collagen IV and XVIII (e.g., endostatin, arresten, canstatin, tumstatin) show anti-angiogenic properties [23,35]. Studies have shown a role for these fragments in inhibiting proliferation and migration of endothelial cells and inducing endothelial cell apoptosis. These fragments are of interest in curbing angiogenesis in several pathological conditions [12,19,34].
4.3. Role in ECM Remodeling
Collagens are a structural component of the ECM that contribute to skin flexibility in addition to stabilizing growth factors and regulating cell adhesion and signaling between cells and ECM. In the process of wound healing, as the wound tissue undergoes remodeling over years, the adult wound heals with the formation of a ‘normal’ scar. The scar tissue regains anywhere from 50–80% of the original tensile strength of normal skin but may be functionally deficient [55]. The main difference between the scar and unwounded skin appears to be the density, fiber size and orientation of the collagen fibrils [28].
Abnormalities in the ECM reconstitution during wound healing result in hypertrophic and keloid scars. Scarring is a consequence of altered levels of the same molecules that typically make up the ECM, i.e., collagen I and III, fibronectin and laminin are abnormally high in scar tissue [55]. Collagen fiber orientation in scars (normotrophic, hypertrophic and keloid) are parallel to the epithelial surface unlike that of normal skin where the fibers form a three-dimensional basketweave-like network [56]. There are structural and compositional differences between these types of scars. Keloid scars are characterized by abnormally thick bundles of collagen that are poorly organized with fewer cross-links that are found in the deep dermis compared to superficial dermic. Hypertrophic scars have thinner collagen bundles than keloid or normotrophic scars [57,58,59]. The ratio of collagen I to III is higher in keloids than normotrophic scars. Even within the keloid scar, there is a heterogeneity to the collagen I/III ratio [14].
5. Effect of Aging on Collagen in the Skin and Wound
The aged skin has lower collagen density that is increasingly cross-linked and fragmented [60,61]. Together with senescence, collagen fiber remodeling results in increased stiffness. Furthermore, the aging skin has a higher percentage of collagen III [62]. Collagen organization visualized through Fourier transformed infrared imaging, scanning electron microscopy and histological staining showed fragmented, clustered and coarse fiber bundles that are oriented parallel to the skin surface in aging skin [63,64,65]. Age-induced alterations (reduced collagen deposition and increased non-enzymatic cross-linking) in collagen impact the mechanical environment of the skin and predispose it to wound healing impairments [66,67,68].
6. Collagen Formats and Applications in Wound Healing
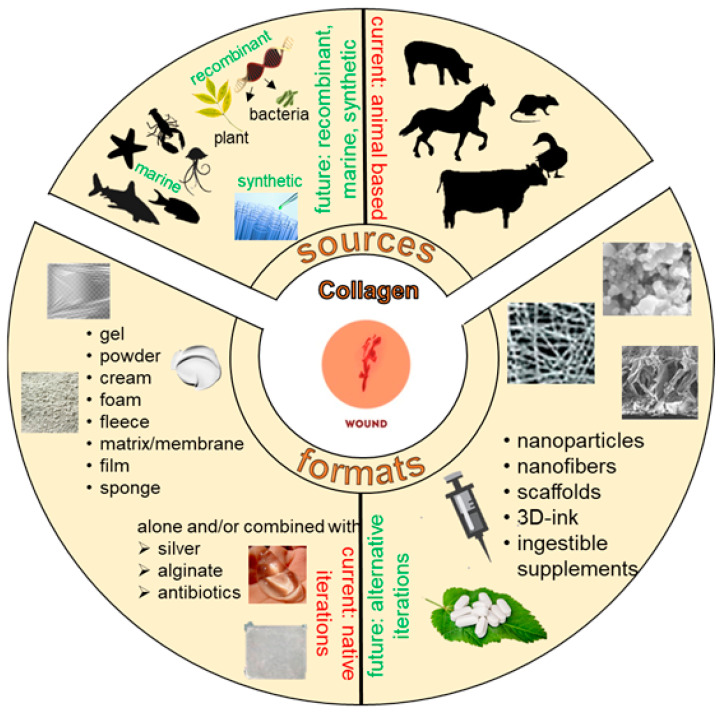
Aberrations in the normal progression through the wound healing phases results in the development of chronic wounds that need to be managed appropriately for healing to complete. Key factors in the hostile environment of a chronic wound are persistent inflammation, increased destruction of ECM components due to elevated MMPs and improper activation of soluble mediators of the wound healing process. Because collagen is an important regulator of several of these processes, it has been utilized as an adjunct wound therapy to promote healing. Biocompatibility, low immunogenicity, ability to recruit wound healing responsive cells (macrophages, fibroblasts etc.) and ease of application are some of the reasons why collagen-based biomaterials have been used for wound dressings. Standard collagen sources are typically bovine, equine, avian or porcine in origin (Figure 2) [69]. There are significant disadvantages associated with the use of animal-based collagen products including development of allergic reactions, transmission of prion diseases (e.g., bovine spongiform encephalopathy) and microbial contamination [70,71]. Furthermore, in some communities there are religious constraints associated with use of bovine- and porcine-derived tissue. Therefore, alternative natural (marine) or engineered (recombinant human collagen from bacterial or plant material) sources of collagen have been considered.
Figure 2.
Sources and formats of collagen for wound healing applications.
Collagen applied as adjunct therapy in wound healing could promote healing potentially by acting as: (i) a decoy/sink for the raging MMPs and other enzymes in the wound thereby abating inflammation and restoring progression into the reparative stages; (ii) a substrate aiding in the migration of key cellular components of wound healing; or (iii) a promoter of a proangiogenic, anti-inflammatory environment to resolve the injury towards healing [6,7,8,10,17,53].
6.1. Collagen Wound Dressings
Collagen applications in wound healing have been tested in numerous ways. They have been used as matrices/scaffolds for tissue engineering, hemostatics, soft tissue repair and more recently as a drug delivery system [6,10,11,26,53,72,73,74,75,76,77,78,79,80]. Collagen wound dressings contain collagen blended with natural and synthetic polymers such as polyethylene oxide, poly (L-lactic acid), hyaluronic acid, elastin and silk fibroin, alginate, chitosan, etc [73,81,82,83,84,85]. These blended fabrications have incorporated other additives such as insulin [86], antibiotics [87,88,89,90,91,92] or gold nanoparticles [93,94,95] and have been tested mostly in in vitro studies or small animal models of wound healing. An evidence-based review of clinical studies on antibacterial integrated collagen wound dressings indicated that most studies were limited by small sample sizes and mixed chronic wound etiologies [46]. Therefore, although positive outcomes are reported, robust evaluation of the specific value of these integrated wound dressings as it related to clinical diabetic foot ulcers remains promising but inconclusive. The need for larger, standardized clinical studies to claim treatment efficacy thus arises.
A plethora of formulations of collagen as amorphous gels, sheet or powder forms, combined with other agents (e.g., silver, for the antimicrobial properties, or ethylenediaminetetraacetic acid (EDTA), carboxymethyl cellulose (CMC) or alginate, i.e., collagen enhancers) are available as wound dressings in the market (Figure 2) [6,7,8,10,46,53,77,96]. The sponge/fleece version of collagen has been tested as a cell-free matrix that promoted new tissue formation in a limited study [47,75]. Particulate or powdered collagen have minimal covalent cross-linking and are active upon administration as signaling molecules [77].
Collagen is also used as a surface coating to enhance moisture retention and promote cell adhesion within scaffolds/matrices [73,81,82,83,84,86,87,88,89,90,91,92,93,94,95]. Water retention is important to keep the wound bed moist. The arginine-glycine-aspartic acid (RGD) sites on collagen binds integrins of cells and promotes cell adhesion and migration of fibroblasts and keratinocytes in in vitro studies. A collagen-coated scaffold implanted in a rat burn wound model showed faster wound re-epithelialization and healing compared to standard of care dressing [97].
The technological processing of collagen tissues to hydrolysates and subsequent rebuilding into formats used for clinical applications was recently reviewed [98]. Several publications show the potential for collagen as a flexible biomaterial for wound healing. However, there is still a need for high-quality studies and randomized control trials to support their clinical applications [46,75].
In recent years interest in collagen nanostructures has surged [78]. Nano collagen is a relatively new material and is made of collagen reduced to a nanoparticulate size. This nano-size provides a higher surface area-to-volume ratio. Electrospinning is the primary technique used to produce biocompatible nano collagen fibers. Collagen nanoparticles have been tested for their application as therapeutic drug delivery systems. For example, a gold-loaded hydroxyapatite collagen nanostructure was tested in vitro and was shown to promote cell adhesion, growth and proliferation [99]. The localized delivery of therapeutic factors using a material that is stable and compatible with the tissue microenvironment of the wound is a key advantage of nano collagens [78]. Insufficient knowledge and research of these nano particles is a limiting factor and requires additional in-depth study.
6.1.1. Recombinant Human Collagen
The risks associated with the use of animal sources of collagen can be overcome by the use of alternatively sourced collagen. Plant-derived human collagen (PDHC; typically recombinant human collagen engineered in plants like tobacco) have similar scaffold properties to wild-type human collagen [100,101,102,103,104]. Various formulations (gel, matrix, electrospun scaffolds and lyophilized sponges) of PDHC have been experimentally derived and tested (Figure 2) [102]. A recent pilot study used PDHC on chronic ulcers of various etiologies and demonstrated that the product was safe for use and promoted faster wound closure [105].
A potential problem with recombinant human collagens from non-animal sources is the requirement for post-translational proline hydroxylation that potentially limits large scale production [106]. The discovery of collagen-like proteins, Scl1 and Scl2 from Streptococcus pyogenes led to the generation of constructs in a recombinant E. coli system in an effort to establish large-scale production methods. Bacterial-derived collagens serve as a biosynthetic ground-up approach, where non-animal collagen with no specific bioactivity can be manipulated for desired interactions. A test of this system in the context of human mesenchymal stem cells was shown to have chondrogenic potential [107].
6.1.2. Marine Collagen
Collagen I has been extracted from various marine sources such as fish skin, jellyfish, sponges and squid (Figure 2) [70,71,108,109,110,111,112,113,114,115,116]. Marine-derived collagen I was shown to promote wound healing in experimental (rodent models) and clinical studies [117,118,119]. The marine source of collagen is beneficial because the abundance of material that would typically be considered ‘waste products’ in the fish processing industry can be recycled into collagen-based wound dressings as well as derivatized into dietary supplements for weight management and sugar control [108].
Marine-derived collagens are chemically and mechanically different from mammalian collagen but are considered favorable for biomedical applications due to high biodegradability and biocompatibility and low immunogenicity [70]. Marine-derived collagen I thermostability has been tested by several groups and found to be lower than mammalian-derived collagen suggesting a need for engineering additional cross-links prior to use in wound healing or other biological applications. These differences have been attributed to the amino acid (particularly glycine, proline and hydroxyproline) content of the collagen I [69]. A systematic review of collagen from marine sources in skin wound healing described studies performed in animal models and highlighted the potential for wound healing applications [70]. The field is now ready for clinical trials.
6.2. Percutaneous Collagen Induction
In 1995, a method called subcision was introduced as a minimally invasive way to treat scars using extremely fine needles to disrupt dermal collagen to trigger dermal remodeling and skin resurfacing [120,121,122]. This method is now known as microneedling or percutaneous collagen induction (PCI). The microneedles puncture the outer layers of the skin into the papillary dermis to initiate the release of growth factors that trigger collagen I and elastin formation. This method has been applied to the treatment of acne, scars, facial rejuvenation, alopecia, pigmentation issues, etc. in small cohort clinical studies. A recent review of the evidence available on PCI techniques for scar treatment identified benefits such as little to no side effects and versatility of application [120]. However, this and other reviews have pointed out the lack of high-quality studies with sufficient numbers of patients following a standardized outcome protocol [121,122].
6.3. Hydrolyzed Collagen
Native collagen can be denatured and hydrolyzed with acids, alkali or thermal treatment (with enzymatic digestion) to produce low molecular weight (3–6 KDa) peptides with unique physicochemical and biological properties compared to the native form [123]. The advantages of hydrolyzed collagen (HC) are that it is highly soluble, easily absorbed and distributed in the human body, cost effective, easily emulsified and stabilized for use. However, a disadvantage is that unlike the native form, HC needs to be combined with other biopolymers (cellulose or chitosan) to form scaffolds or films. Interestingly, HC has antioxidant and antimicrobial activities. Hydrogel preparations of HC were shown to have antibacterial activity against Escherichia coli and Staphylococcus aureus. These preparations were also shown to promote cell proliferation and migration and burn wound healing. HC in electrospun nanofibrous scaffolds was shown to have biomechanical and antimicrobial properties [81,110]. A recent review on the different types of HC, sources and applications as biomaterials provides additional details on this form of collagen [124]. Hydrolyzed collagen powder is available in the market as a dressing for moderate to heavily exudative wounds. A small randomized clinical trial on patients with burn wounds that used a hydrolyzed collagen supplement suggested a promising role for HC in wound healing [125].
6.4. Collagen Bioink
Three dimensional (3D) bioprinting is an evolving adaptive manufacturing technique that offers the development of wound treatments that can deal with the issues presented by traditional wound dressings (i.e., need for frequent dressing change, adherence to wound tissue making dressing changes painful) [126]. The bioprinting process integrates cell-laden hydrogels, also called bioinks, together with motorized systems to create complex structures that can be catered precisely to the patient or situation in question [79,80,126,127,128]. In 2009, a 3D-printed human skin construct incorporating collagen I together with fibroblasts and keratinocytes was the first successful attempt at creating a skin implant. This was followed by other studies that have improved on this attempt and even tested them in situ in a porcine model. The porcine studies showed that the collagen–fibrinogen bioink-printed skin implants incorporating cells significantly enhanced wound re-epithelialization compared to control treatments. Further developments in this line include a laser-assisted bioprinting and robotic solid freeform fabrications to provide contactless, automated solutions using collagen bioinks [129]. As of 2020, there are only about 30 published research articles on this topic.
Collagen bioinks are currently the most popular material for 3D engineering, primarily because of the history of their use in clinical practice, biocompatibility and low immunogenicity. Collagen I is the most common type used for bioink manufacturing and has been used in the laboratory-based bioprinting of skin, bone and cartilage, cardiovascular tissues, liver, nerve regeneration and cornea with limited testing conducted in vitro and in vivo (small animal models) [79,80,126].
A key issue with collagen bioinks is the need for specific pH and temperatures to initiate matrix gelation that could be toxic to cells [130]. Additional challenges include precise tissue detailing (including development of structures like sweat glands and hair follicles) and the need for improved cross-linking techniques for structural control of the bioink-generated product [126]. The production of constructs for large wound areas is a challenge. Furthermore, the search for a 3D printed human skin graft that can support functions such as thermoregulation, touch or sweat is still unfulfilled [129,131].
7. Clinical Studies
A search of clinicaltrials.gov using the criteria (collagen + skin + wound) identified 45 clinical studies. This may be viewed as early phase in clinical development. Various formats of collagen dressings have been included in these studies (Table 1). Among the top three formats used in the context of skin health and wound healing are matrices, dietary supplements and sponges with antimicrobials incorporated. Composites of collagen with silk fibroin, alginate and other polymers were also noted. Some of these studies were terminated or withdrawn (~11%) either due to funding limitations, change in study prioritization or undisclosed reasons. Several studies are in the actively recruiting stage. The lack of results associated with the completed studies makes the evaluation of the impact of these collagen applications for clinical use difficult at this time.
Table 1.
Collagen dressing formats for wound healing clinical studies.
| Collagen Format | Number of Studies |
|---|---|
| Matrix/scaffold/mesh | 15 |
| Dietary supplement | 8 |
| Sponge | 7 |
| Percutaneous induction/microneedling | 5 |
| Composites (e.g., silk fibroin, alginate) | 5 |
| Gel | 3 |
| Paste/powder | 2 |
8. Collagen Wound Dressing Market
In 2019, the global collagen dressings market was valued at approximately USD 926 million and is projected to expand at a compound annual growth rate (CAGR) of ~5% from 2020 to 2030 [132,133]. North America is projected to dominate the global collagen dressings market. Key drivers of the market are collagen composites that include an antimicrobial principle. Despite the rising interest in alternative (and less immunogenic) sources of collagen, bovine origin collagen dominates the markets. Tissue engineering advances such as the electrospinning and 3D bioprinting methods of producing collagen composites are expected to positively impact the market [132,133].
9. Closing Remarks
A Pubmed search using the keywords collagen and wound healing lists >10,000 publications promoting various collagen formats as being ideal for wound-healing applications. The poor mechanical and thermal properties of collagen are moving the field towards the use of blends with other materials such as alginate, chitosan and cellulose. However, depending on the ratio of mixtures in these blends, it may not be possible to clearly delineate the exact impact of the collagen component on the treatment outcomes. Most of the published studies are based on in vitro or small animal models. Preclinical porcine studies are necessary to test the translational value of the reported basic science investigations. Collagens can directly modulate the wound microenvironment, serve as a scaffold for cellular attachment and function or deliver biologically active principles or antimicrobials to aid in wound healing. Therefore, for effective translational value, personalization or tailoring collagen biomaterials to the therapeutic need is critical. This nuance is not captured well in current research. While technology is evolving rapidly to produce customized collagen composite scaffolds or nanoparticles incorporating stem cells and other bioactive molecules, the research that can bring these products to clinical practice is limited. The leap from bench to bedside requires rigorous preclinical and clinical testing with demonstrable beneficial outcomes and that is a gap in current collagen biomaterials translational research.
Author Contributions
All authors (S.S.M.-S., S.R., C.K.S.) contributed to writing the manuscript draft. S.S.M.-S. and C.K.S. writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Wound healing and tissue injury/repair research in the authors’ labs include the following from both the National Institutes of Health and Department of Defense: 1R01DK128845, 1R01DK125835, R01NR015676, R01GM077185, R01GM108014, R01DK114718, R01NR013898, U01DK119090, 5R01AI138981 W81XWH-21-1-0033, W81XWH-17-2-0069, MTEC-17-02-PA-18; W81XWH-19-1-0120.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy P.S., Evans G.R.D. Advances in Wound Healing: A Review of Current Wound Healing Products. Plast. Surg. Int. 2012;2012:190436. doi: 10.1155/2012/190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouw J.K., Ou G., Weaver V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes R.O. Stretching the boundaries of extracellular matrix research. Nat. Rev. Mol. Cell Biol. 2014;15:761–763. doi: 10.1038/nrm3908. [DOI] [PubMed] [Google Scholar]
- 5.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Masry M.S., Chaffee S., Das Ghatak P., Mathew-Steiner S.S., Das A., Higuita-Castro N., Roy S., Anani R.A., Sen C.K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019;33:2144–2155. doi: 10.1096/fj.201800352R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A., Abas M., Biswas N., Banerjee P., Ghosh N., Rawat A., Khanna S., Roy S., Sen C.K. A Modified Collagen Dressing Induces Transition of Inflammatory to Reparative Phenotype of Wound Macrophages. Sci. Rep. 2019;9:14293. doi: 10.1038/s41598-019-49435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A., Datta S., Roche E., Chaffee S., Jose E., Shi L., Grover K., Khanna S., Sen C.K., Roy S. Novel mechanisms of Collagenase Santyl Ointment (CSO) in wound macrophage polarization and resolution of wound inflammation. Sci. Rep. 2018;8:1696. doi: 10.1038/s41598-018-19879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosique R.G., Rosique M.J., Farina J.A., Jr. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflamm. 2015;2015:316235. doi: 10.1155/2015/316235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elgharably H., Roy S., Khanna S., Abas M., Dasghatak P., Das A., Mohammed K., Sen C.K. A modified collagen gel enhances healing outcome in a preclinical swine model of excisional wounds. Wound Repair Regen. 2013;21:473–481. doi: 10.1111/wrr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchand M., Monnot C., Muller L., Germain S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 2019;89:147–156. doi: 10.1016/j.semcdb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kisling A., Lust R.M., Katwa L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019;228:30–34. doi: 10.1016/j.lfs.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Twardowski T., Fertala A., Orgel J.P., San Antonio J.D. Type I collagen and collagen mimetics as angiogenesis promoting superpolymers. Curr. Pharm. Des. 2007;13:3608–3621. doi: 10.2174/138161207782794176. [DOI] [PubMed] [Google Scholar]
- 14.Xue M., Jackson C.J. Extracellular Matrix Reorganization during Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care. 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinke J.M., Sorg H. Wound repair and regeneration. Eur. Surg. Res. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 16.Schultz G., Chin G., Moldawer L., Diegelmann R. Principles of Wound Healing. Volume 23. University of Adelaide Press; Adelaide, Australia: 2011. [PubMed] [Google Scholar]
- 17.Barnes M. Update on Collagens: What You Need to Know and Consider. Plast. Surg. Nurs. 2019;39:112–115. doi: 10.1097/PSN.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 18.Reilly D.M., Lozano J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthetic Res. 2021;8:2. doi: 10.20517/2347-9264.2020.153. [DOI] [Google Scholar]
- 19.Ricard-Blum S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San Antonio J.D., Jacenko O., Fertala A., Orgel J. Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine. Bioengineering. 2020;8:3. doi: 10.3390/bioengineering8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorushanova A., Delgado L.M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., Mullen A.M., Bayon Y., Pandit A., Raghunath M., et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019;31:1801651. doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 22.Gould L.J. Topical Collagen-Based Biomaterials for Chronic Wounds: Rationale and Clinical Application. Adv. Wound Care. 2016;5:19–31. doi: 10.1089/wound.2014.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenzel D., Schmidt A., Reimann K., Hescheler J., Pfitzer G., Bloch W., Fleischmann B.K. Endostatin, the proteolytic fragment of collagen XVIII, induces vasorelaxation. Circ. Res. 2006;98:1203–1211. doi: 10.1161/01.RES.0000219899.93384.ed. [DOI] [PubMed] [Google Scholar]
- 24.Sinha M., Sen C.K., Singh K., Das A., Ghatak S., Rhea B., Blackstone B., Powell H.M., Khanna S., Roy S. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat. Commun. 2018;9:936. doi: 10.1038/s41467-018-03208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M., Cronin K., Crane J.S. Biochemistry, Collagen Synthesis. StatPearls Publishing; Treasure Island, FL, USA: 2020. [(accessed on 10 March 2021)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507709/ [PubMed] [Google Scholar]
- 26.Liu X., Zheng C., Luo X., Wang X., Jiang H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;99:1509–1522. doi: 10.1016/j.msec.2019.02.070. [DOI] [PubMed] [Google Scholar]
- 27.Sprangers S., Everts V. Molecular pathways of cell-mediated degradation of fibrillar collagen. Matrix Biol. 2019;75–76:190–200. doi: 10.1016/j.matbio.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Guo S., Dipietro L.A. Factors affecting wound healing. J. Dent. Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 30.Govindaraju P., Todd L., Shetye S., Monslow J., Puré E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix Biol. 2019;75–76:314–330. doi: 10.1016/j.matbio.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koivisto L., Heino J., Häkkinen L., Larjava H. Integrins in Wound Healing. Adv. Wound Care. 2014;3:762–783. doi: 10.1089/wound.2013.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeltz C., Gullberg D. The integrin–collagen connection—A glue for tissue repair? J. Cell Sci. 2016;129:653–664. doi: 10.1242/jcs.180992. [DOI] [PubMed] [Google Scholar]
- 33.Klein T., Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricard-Blum S., Ballut L. Matricryptins derived from collagens and proteoglycans. Front. Biosci. 2011;16:674–697. doi: 10.2741/3712. [DOI] [PubMed] [Google Scholar]
- 35.Kareva I., Abou-Slaybi A., Dodd O., Dashevsky O., Klement G.L. Normal Wound Healing and Tumor Angiogenesis as a Game of Competitive Inhibition. PLoS ONE. 2016;11:e0166655. doi: 10.1371/journal.pone.0166655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wietecha M.S., Cerny W.L., DiPietro L.A. Mechanisms of vessel regression: Toward an understanding of the resolution of angiogenesis. Curr. Top. Microbiol. Immunol. 2013;367:3–32. doi: 10.1007/82_2012_287. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt A., Wenzel D., Thorey I., Sasaki T., Hescheler J., Timpl R., Addicks K., Werner S., Fleischmann B.K., Bloch W. Endostatin influences endothelial morphology via the activated ERK1/2-kinase endothelial morphology and signal transduction. Microvasc. Res. 2006;71:152–162. doi: 10.1016/j.mvr.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt A., Wenzel D., Thorey I., Werner S., Fleischmann B.K., Bloch W. Endostatin down-regulates soluble guanylate cyclase (sGC) in endothelial cells in vivo: Influence of endostatin on vascular endothelial growth factor (VEGF) signaling. Endothelium. 2005;12:251–257. doi: 10.1080/10623320500476690. [DOI] [PubMed] [Google Scholar]
- 39.Boraschi-Diaz I., Wang J., Mort J.S., Komarova S.V. Collagen Type I as a Ligand for Receptor-Mediated Signaling. Front. Phys. 2017;5:12. doi: 10.3389/fphy.2017.00012. [DOI] [Google Scholar]
- 40.Müller A.K., Meyer M., Werner S. The roles of receptor tyrosine kinases and their ligands in the wound repair process. Semin. Cell Dev. Biol. 2012;23:963–970. doi: 10.1016/j.semcdb.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Roy S., Santra S., Das A., Dixith S., Sinha M., Ghatak S., Ghosh N., Banerjee P., Khanna S., Mathew-Steiner S., et al. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann. Surg. 2020;271:1174–1185. doi: 10.1097/SLA.0000000000003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naomi R., Fauzi M.B. Cellulose/Collagen Dressings for Diabetic Foot Ulcer: A Review. Pharmaceutics. 2020;12:881. doi: 10.3390/pharmaceutics12090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naomi R., Ratanavaraporn J., Fauzi M.B. Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing. Materials. 2020;13:3097. doi: 10.3390/ma13143097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felician F.F., Xia C., Qi W., Xu H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018;15:e1700557. doi: 10.1002/cbdv.201700557. [DOI] [PubMed] [Google Scholar]
- 45.Gaspar-Pintiliescu A., Stanciuc A.M., Craciunescu O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019;138:854–865. doi: 10.1016/j.ijbiomac.2019.07.155. [DOI] [PubMed] [Google Scholar]
- 46.Amirrah I.N., Mohd Razip Wee M.F., Tabata Y., Bt Hj Idrus R., Nordin A., Fauzi M.B. Antibacterial-Integrated Collagen Wound Dressing for Diabetes-Related Foot Ulcers: An Evidence-Based Review of Clinical Studies. Polymers. 2020;12:2168. doi: 10.3390/polym12092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallaske F., Pallaske A., Herklotz K., Boese-Landgraf J. The significance of collagen dressings in wound management: A review. J. Wound Care. 2018;27:692–702. doi: 10.12968/jowc.2018.27.10.692. [DOI] [PubMed] [Google Scholar]
- 48.Sahana T.G., Rekha P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018;45:2857–2867. doi: 10.1007/s11033-018-4296-3. [DOI] [PubMed] [Google Scholar]
- 49.Steen E.H., Wang X., Boochoon K.S., Ewing D.C., Strang H.E., Kaul A., Masri L., Balaji S., Hollier L.H., Jr., Keswani S. Wound Healing and Wound Care in Neonates: Current Therapies and Novel Options. Adv. Ski. Wound Care. 2020;33:294–300. doi: 10.1097/01.ASW.0000661804.09496.8c. [DOI] [PubMed] [Google Scholar]
- 50.Yeung D.A., Kelly N.H. The Role of Collagen-Based Biomaterials in Chronic Wound Healing and Sports Medicine Applications. Bioengineering. 2021;8:8. doi: 10.3390/bioengineering8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinno H., Prakash S. Complements and the Wound Healing Cascade: An Updated Review. Plast. Surg. Int. 2013;2013:146764. doi: 10.1155/2013/146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng X., Tonnesen M.G., Mousa S.A., Clark R.A.F. Fibrin and Collagen Differentially but Synergistically Regulate Sprout Angiogenesis of Human Dermal Microvascular Endothelial Cells in 3-Dimensional Matrix. Int. J. Cell Biol. 2013;2013:231279. doi: 10.1155/2013/231279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elgharably H., Ganesh K., Dickerson J., Khanna S., Abas M., Ghatak P.D., Dixit S., Bergdall V., Roy S., Sen C.K. A modified collagen gel dressing promotes angiogenesis in a preclinical swine model of chronic ischemic wounds. Wound Repair Regen. 2014;22:720–729. doi: 10.1111/wrr.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkpatrick N.D., Andreou S., Hoying J.B., Utzinger U. Live imaging of collagen remodeling during angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H3198–H3206. doi: 10.1152/ajpheart.01234.2006. [DOI] [PubMed] [Google Scholar]
- 55.Profyris C., Tziotzios C., Do Vale I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012;66:1–10. doi: 10.1016/j.jaad.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 56.Ehrlich H.P., Desmoulière A., Diegelmann R.F., Cohen I.K., Compton C.C., Garner W.L., Kapanci Y., Gabbiani G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am. J. Pathol. 1994;145:105–113. [PMC free article] [PubMed] [Google Scholar]
- 57.Gauglitz G.G., Korting H.C., Pavicic T., Ruzicka T., Jeschke M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shih B., Garside E., McGrouther D.A., Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010;18:139–153. doi: 10.1111/j.1524-475X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 59.Verhaegen P.D., van Zuijlen P.P., Pennings N.M., van Marle J., Niessen F.B., van der Horst C.M., Middelkoop E. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen. 2009;17:649–656. doi: 10.1111/j.1524-475X.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 60.Marcos-Garcés V., Molina Aguilar P., Bea Serrano C., García Bustos V., Benavent Seguí J., Ferrández Izquierdo A., Ruiz-Saurí A. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014;225:98–108. doi: 10.1111/joa.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blair M.J., Jones J.D., Woessner A.E., Quinn K.P. Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care. 2020;9:127–143. doi: 10.1089/wound.2019.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lovell C.R., Smolenski K.A., Duance V.C., Light N.D., Young S., Dyson M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987;117:419–428. doi: 10.1111/j.1365-2133.1987.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 63.Fisher G.J., Quan T., Purohit T., Shao Y., Cho M.K., He T., Varani J., Kang S., Voorhees J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yasui T., Yonetsu M., Tanaka R., Tanaka Y., Fukushima S., Yamashita T., Ogura Y., Hirao T., Murota H., Araki T. In vivo observation of age-related structural changes of dermal collagen in human facial skin using collagen-sensitive second harmonic generation microscope equipped with 1250-nm mode-locked Cr:Forsterite laser. J. Biomed. Opt. 2013;18:31108. doi: 10.1117/1.JBO.18.3.031108. [DOI] [PubMed] [Google Scholar]
- 65.Wu S., Li H., Yang H., Zhang X., Li Z., Xu S. Quantitative analysis on collagen morphology in aging skin based on multiphoton microscopy. J. Biomed. Opt. 2011;16:040502. doi: 10.1117/1.3565439. [DOI] [PubMed] [Google Scholar]
- 66.Szauter K.M., Cao T., Boyd C.D., Csiszar K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol. Biol. 2005;53:448–456. doi: 10.1016/j.patbio.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 67.Bailey A.J., Paul R.G., Knott L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998;106:1–56. doi: 10.1016/S0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed T., Nash A., Clark K.E., Ghibaudo M., de Leeuw N.H., Potter A., Stratton R., Birch H.L., Enea Casse R., Bozec L. Combining nano-physical and computational investigations to understand the nature of “aging” in dermal collagen. Int. J. Nanomed. 2017;12:3303–3314. doi: 10.2147/IJN.S121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davison-Kotler E., Marshall W.S., García-Gareta E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering. 2019;6:56. doi: 10.3390/bioengineering6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cruz M.A., Araujo T.A., Avanzi I.R., Parisi J.R., de Andrade A.L.M., Rennó A.C.M. Collagen from Marine Sources and Skin Wound Healing in Animal Experimental Studies: A Systematic Review. Mar. Biotechnol. 2021;23:1–11. doi: 10.1007/s10126-020-10011-6. [DOI] [PubMed] [Google Scholar]
- 71.Silva T.H., Moreira-Silva J., Marques A.L., Domingues A., Bayon Y., Reis R.L. Marine origin collagens and its potential applications. Mar. Drugs. 2014;12:5881–5901. doi: 10.3390/md12125881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chouhan D., Dey N., Bhardwaj N., Mandal B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials. 2019;216:119267. doi: 10.1016/j.biomaterials.2019.119267. [DOI] [PubMed] [Google Scholar]
- 73.Hernández-Rangel A., Martin-Martinez E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. A. 2021 doi: 10.1002/jbm.a.37154. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Bhardwaj N., Chouhan D., Mandal B.B. 14—3D Functional Scaffolds for Skin Tissue Engineering. In: Deng Y., Kuiper J., editors. Functional 3D Tissue Engineering Scaffolds. Woodhead Publishing; Cambridge, UK: 2018. pp. 345–365. [Google Scholar]
- 75.Constantin V., Carâp A., Bobic S., Budu V., Kaya M., Marin S., Marin M.M., Socea B. Tissue Engineering—Collagen Sponge Dressing for Chronic Wounds; Proceedings of the 7th International Conference on Advanced Materials and Systems (ICAMS); Bucharest, Romania. 18–20 October 2018; pp. 63–68. [DOI] [Google Scholar]
- 76.Dhaliwal K., Lopez N. Hydrogel dressings and their application in burn wound care. Br. J. Community Nurs. 2018;23:S24–S27. doi: 10.12968/bjcn.2018.23.Sup9.S24. [DOI] [PubMed] [Google Scholar]
- 77.Kallis P.J., Friedman A.J. Collagen Powder in Wound Healing. J. Drugs Dermatol. 2018;17:403–408. [PubMed] [Google Scholar]
- 78.Lo S., Fauzi M.B. Current Update of Collagen Nanomaterials-Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics. 2021;13:316. doi: 10.3390/pharmaceutics13030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marques C.F., Diogo G.S., Pina S., Oliveira J.M., Silva T.H., Reis R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019;30:32. doi: 10.1007/s10856-019-6234-x. [DOI] [PubMed] [Google Scholar]
- 80.Osidak E.O., Kozhukhov V.I., Osidak M.S., Domogatsky S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprinting. 2020;6:270. doi: 10.18063/ijb.v6i3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramadass S.K., Nazir L.S., Thangam R., Perumal R.K., Manjubala I., Madhan B., Seetharaman S. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds. Colloids Surf. B Biointerfaces. 2019;175:636–643. doi: 10.1016/j.colsurfb.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 82.Chong C., Wang Y., Fathi A., Parungao R., Maitz P.K., Li Z. Skin wound repair: Results of a pre-clinical study to evaluate electropsun collagen-elastin-PCL scaffolds as dermal substitutes. Burns. 2019;45:1639–1648. doi: 10.1016/j.burns.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Maitz J., Wang Y., Fathi A., Ximena Escobar F., Parungao R., van Zuijlen P., Maitz P., Li Z. The effects of cross-linking a collagen-elastin dermal template on scaffold bio-stability and degradation. J. Tissue Eng. Regen. Med. 2020;14:1189–1200. doi: 10.1002/term.3082. [DOI] [PubMed] [Google Scholar]
- 84.Vonbrunn E., Mueller M., Pichlsberger M., Sundl M., Helmer A., Wallner S.A., Rinner B., Tuca A.C., Kamolz L.P., Brislinger D., et al. Electrospun PCL/PLA Scaffolds Are More Suitable Carriers of Placental Mesenchymal Stromal Cells Than Collagen/Elastin Scaffolds and Prevent Wound Contraction in a Mouse Model of Wound Healing. Front. Bioeng. Biotechnol. 2020;8:604123. doi: 10.3389/fbioe.2020.604123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geanaliu-Nicolae R.-E., Andronescu E. Blended Natural Support Materials-Collagen Based Hydrogels Used in Biomedicine. Materials. 2020;13:5641. doi: 10.3390/ma13245641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ehterami A., Salehi M., Farzamfar S., Vaez A., Samadian H., Sahrapeyma H., Mirzaii M., Ghorbani S., Goodarzi A. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018;117:601–609. doi: 10.1016/j.ijbiomac.2018.05.184. [DOI] [PubMed] [Google Scholar]
- 87.Ahmadian S., Ghorbani M., Mahmoodzadeh F. Silver sulfadiazine-loaded electrospun ethyl cellulose/polylactic acid/collagen nanofibrous mats with antibacterial properties for wound healing. Int. J. Biol. Macromol. 2020;162:1555–1565. doi: 10.1016/j.ijbiomac.2020.08.059. [DOI] [PubMed] [Google Scholar]
- 88.Hajikhani M., Emam-Djomeh Z., Askari G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int. J. Biol. Macromol. 2021;172:143–153. doi: 10.1016/j.ijbiomac.2021.01.051. [DOI] [PubMed] [Google Scholar]
- 89.Marson B.A., Deshmukh S.R., Grindlay D.J.C., Ollivere B.J., Scammell B.E. A systematic review of local antibiotic devices used to improve wound healing following the surgical management of foot infections in diabetics. Bone Jt. J. 2018;100-b:1409–1415. doi: 10.1302/0301-620X.100B11.BJJ-2018-0720. [DOI] [PubMed] [Google Scholar]
- 90.Min J.G., Sanchez Rangel U.J., Franklin A., Oda H., Wang Z., Chang J., Fox P.M. Topical Antibiotic Elution in a Collagen-Rich Hydrogel Successfully Inhibits Bacterial Growth and Biofilm Formation In Vitro. Antimicrob. Agents Chemother. 2020;64:e00136-20. doi: 10.1128/AAC.00136-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qian Z., Bai Y., Zhou J., Li L., Na J., Fan Y., Guo X., Liu H. A moisturizing chitosan-silk fibroin dressing with silver nanoparticles-adsorbed exosomes for repairing infected wounds. J. Mater. Chem. B. 2020;8:7197–7212. doi: 10.1039/D0TB01100B. [DOI] [PubMed] [Google Scholar]
- 92.Shi L., Lin F., Zhou M., Li Y., Li W., Shan G., Xu Y., Xu J., Yang J. Preparation of biocompatible wound dressings with dual release of antibiotic and platelet-rich plasma for enhancing infected wound healing. J. Biomater. Appl. 2021:885328221996013. doi: 10.1177/0885328221996013. [DOI] [PubMed] [Google Scholar]
- 93.Bai M.Y., Ku F.Y., Shyu J.F., Hayashi T., Wu C.C. Evaluation of Polyacrylonitrile Nonwoven Mats and Silver-Gold Bimetallic Nanoparticle-Decorated Nonwoven Mats for Potential Promotion of Wound Healing In Vitro and In Vivo and Bone Growth In Vitro. Polymers. 2021;13:516. doi: 10.3390/polym13040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmad T., McGrath S., Sirafim C., do Amaral R., Soong S.L., Sitram R., Turkistani S., Santarella F., Kearney C.J. Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles. Biomater. Sci. 2020 doi: 10.1039/D0BM01277G. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 95.Boomi P., Ganesan R., Prabu Poorani G., Jegatheeswaran S., Balakumar C., Gurumallesh Prabu H., Anand K., Marimuthu Prabhu N., Jeyakanthan J., Saravanan M. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in vitro and in vivo Conditions. Int. J. Nanomed. 2020;15:7553–7568. doi: 10.2147/IJN.S257499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu S., Applewhite A.J., Niezgoda J., Snyder R., Shah J., Cullen B., Schultz G., Harrison J., Hill R., Howell M., et al. Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Adv. Ski. Wound Care. 2017;30:S1–S18. doi: 10.1097/01.ASW.0000525951.20270.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pal P., Dadhich P., Srivas P.K., Das B., Maulik D., Dhara S. Bilayered nanofibrous 3D hierarchy as skin rudiment by emulsion electrospinning for burn wound management. Biomater. Sci. 2017;5:1786–1799. doi: 10.1039/C7BM00174F. [DOI] [PubMed] [Google Scholar]
- 98.Meyer M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online. 2019;18:24. doi: 10.1186/s12938-019-0647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mondal S., Hoang G., Manivasagan P., Moorthy M.S., Phan T.T.V., Kim H.H., Nguyen T.P., Oh J. Rapid microwave-assisted synthesis of gold loaded hydroxyapatite collagen nano-bio materials for drug delivery and tissue engineering application. Ceram. Int. 2019;45:2977–2988. doi: 10.1016/j.ceramint.2018.10.016. [DOI] [Google Scholar]
- 100.Shoseyov O., Posen Y., Grynspan F. Human collagen produced in plants: More than just another molecule. Bioengineered. 2014;5:49–52. doi: 10.4161/bioe.26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu W., Burdick J.A., van Osch G.J. Plant-derived recombinant human collagen: A strategic approach for generating safe human ECM-based scaffold. Tissue Eng. Part A. 2013;19:1489–1490. doi: 10.1089/ten.tea.2013.0139. [DOI] [PubMed] [Google Scholar]
- 102.Shilo S., Roth S., Amzel T., Harel-Adar T., Tamir E., Grynspan F., Shoseyov O. Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng. Part A. 2013;19:1519–1526. doi: 10.1089/ten.tea.2012.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Willard J.J., Drexler J.W., Das A., Roy S., Shilo S., Shoseyov O., Powell H.M. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part A. 2013;19:1507–1518. doi: 10.1089/ten.tea.2012.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brodsky B., Kaplan D.L. Shining light on collagen: Expressing collagen in plants. Tissue Eng. Part A. 2013;19:1499–1501. doi: 10.1089/ten.tea.2013.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiser I., Tamir E., Kaufman H., Keren E., Avshalom S., Klein D., Heller L., Shapira E. A Novel Recombinant Human Collagen-based Flowable Matrix for Chronic Lower Limb Wound Management: First Results of a Clinical Trial. Wounds. 2019;31:103–107. [PubMed] [Google Scholar]
- 106.An B., Kaplan D.L., Brodsky B. Engineered recombinant bacterial collagen as an alternative collagen-based biomaterial for tissue engineering. Front. Chem. 2014;2:40. doi: 10.3389/fchem.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parmar P.A., St-Pierre J.-P., Chow L.W., Puetzer J.L., Stoichevska V., Peng Y.Y., Werkmeister J.A., Ramshaw J.A.M., Stevens M.M. Harnessing the Versatility of Bacterial Collagen to Improve the Chondrogenic Potential of Porous Collagen Scaffolds. Adv. Healthc. Mater. 2016;5:1656–1666. doi: 10.1002/adhm.201600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Salvatore L., Gallo N., Natali M.L., Campa L., Lunetti P., Madaghiele M., Blasi F.S., Corallo A., Capobianco L., Sannino A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;113:110963. doi: 10.1016/j.msec.2020.110963. [DOI] [PubMed] [Google Scholar]
- 109.Lim Y.S., Ok Y.J., Hwang S.Y., Kwak J.Y., Yoon S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs. 2019;17:467. doi: 10.3390/md17080467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ouyang Q.Q., Hu Z., Lin Z.P., Quan W.Y., Deng Y.F., Li S.D., Li P.W., Chen Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018;112:1191–1198. doi: 10.1016/j.ijbiomac.2018.01.217. [DOI] [PubMed] [Google Scholar]
- 111.Song E., Kim S.Y., Chun T., Byun H.J., Lee Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials. 2006;27:2951–2961. doi: 10.1016/j.biomaterials.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 112.Felician F.F., Yu R.H., Li M.Z., Li C.J., Chen H.Q., Jiang Y., Tang T., Qi W.Y., Xu H.M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019;22:12–20. doi: 10.1016/j.cjtee.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pozzolini M., Millo E., Oliveri C., Mirata S., Salis A., Damonte G., Arkel M., Scarfì S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs. 2018;16:465. doi: 10.3390/md16120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dang Q.F., Liu H., Yan J.Q., Liu C.S., Liu Y., Li J., Li J.J. Characterization of collagen from haddock skin and wound healing properties of its hydrolysates. Biomed. Mater. 2015;10:015022. doi: 10.1088/1748-6041/10/1/015022. [DOI] [PubMed] [Google Scholar]
- 115.Hoyer B., Bernhardt A., Heinemann S., Stachel I., Meyer M., Gelinsky M. Biomimetically mineralized salmon collagen scaffolds for application in bone tissue engineering. Biomacromolecules. 2012;13:1059–1066. doi: 10.1021/bm201776r. [DOI] [PubMed] [Google Scholar]
- 116.Shiratsuchi E., Nakaba M., Yamada M. Elastin hydrolysate derived from fish enhances proliferation of human skin fibroblasts and elastin synthesis in human skin fibroblasts and improves the skin conditions. J. Sci. Food Agric. 2016;96:1672–1677. doi: 10.1002/jsfa.7270. [DOI] [PubMed] [Google Scholar]
- 117.Baldursson B.T., Kjartansson H., Konrádsdóttir F., Gudnason P., Sigurjonsson G.F., Lund S.H. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: A noninferiority study. Int. J. Low. Extrem. Wounds. 2015;14:37–43. doi: 10.1177/1534734615573661. [DOI] [PubMed] [Google Scholar]
- 118.Badois N., Bauër P., Cheron M., Hoffmann C., Nicodeme M., Choussy O., Lesnik M., Poitrine F.C., Fromantin I. Acellular fish skin matrix on thin-skin graft donor sites: A preliminary study. J. Wound Care. 2019;28:624–628. doi: 10.12968/jowc.2019.28.9.624. [DOI] [PubMed] [Google Scholar]
- 119.Woodrow T., Chant T., Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: A prospective evaluation. J. Wound Care. 2019;28:76–80. doi: 10.12968/jowc.2019.28.2.76. [DOI] [PubMed] [Google Scholar]
- 120.Atiyeh B.S., Abou Ghanem O., Chahine F. Microneedling: Percutaneous Collagen Induction (PCI) Therapy for Management of Scars and Photoaged Skin-Scientific Evidence and Review of the Literature. Aesthetic Plast. Surg. 2021;45:296–308. doi: 10.1007/s00266-020-01927-4. [DOI] [PubMed] [Google Scholar]
- 121.Iosifidis C., Goutos I. Percutaneous collagen induction (microneedling) for the management of non-atrophic scars: Literature review. Scars Burn Health. 2019;5:2059513119880301. doi: 10.1177/2059513119880301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hou A., Cohen B., Haimovic A., Elbuluk N. Microneedling: A Comprehensive Review. Dermatol. Surg. 2017;43:321–339. doi: 10.1097/DSS.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 123.Hong H., Fan H., Chalamaiah M., Wu J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019;301:125222. doi: 10.1016/j.foodchem.2019.125222. [DOI] [PubMed] [Google Scholar]
- 124.León-López A., Morales-Peñaloza A., Martínez-Juárez V.M., Vargas-Torres A., Zeugolis D.I., Aguirre-Álvarez G. Hydrolyzed Collagen-Sources and Applications. Molecules. 2019;24:4031. doi: 10.3390/molecules24224031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bagheri Miyab K., Alipoor E., Vaghardoost R., Saberi Isfeedvajani M., Yaseri M., Djafarian K., Hosseinzadeh-Attar M.J. The effect of a hydrolyzed collagen-based supplement on wound healing in patients with burn: A randomized double-blind pilot clinical trial. Burns. 2020;46:156–163. doi: 10.1016/j.burns.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 126.Smandri A., Nordin A., Hwei N.M., Chin K.Y., Abd Aziz I., Fauzi M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers. 2020;12:1782. doi: 10.3390/polym12081782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pahlevanzadeh F., Mokhtari H., Bakhsheshi-Rad H.R., Emadi R., Kharaziha M., Valiani A., Poursamar S.A., Ismail A.F., RamaKrishna S., Berto F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials. 2020;13:3980. doi: 10.3390/ma13183980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gopinathan J., Noh I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018;22:11. doi: 10.1186/s40824-018-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaur A., Midha S., Giri S., Mohanty S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019;2019:1286054. doi: 10.1155/2019/1286054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee V., Singh G., Trasatti J.P., Bjornsson C., Xu X., Tran T.N., Yoo S.S., Dai G., Karande P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods. 2014;20:473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vig K., Chaudhari A., Tripathi S., Dixit S., Sahu R., Pillai S., Dennis V.A., Singh S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017;18:789. doi: 10.3390/ijms18040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Biospace Collagen Dressings Market: Exceptional Biological Properties of Collagen Dressings to Fuel Market Growth. [(accessed on 20 April 2021)]; Available online: https://www.biospace.com/article/collagen-dressings-market-exceptional-biological-properties-of-collagen-dressings-to-fuel-market-growth/
- 133.MarketsandMarkets Wound Dressings Market by Type (Traditional, Advanced (Alginate, Collagen, Hydrogel, Foam, Hydrocolloid, Film)), Wound Type (Traumatic, Surgical, Diabetic Foot, Venous Leg Ulcer & Burns), End User (Hospital, ASCs, Homecare)—Global Forecast to 2025. [(accessed on 24 April 2021)]; Available online: https://www.marketsandmarkets.com/Market-Reports/wound-dressings-market-123903496.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.