Abstract
Learning new motor behaviors or adjusting previously learned actions to account for dynamic changes in our environment requires the operation of multiple distinct motor learning processes, which rely on different neuronal substrates. For instance, humans are capable of acquiring new motor patterns via the formation of internal model representations of the movement dynamics and through positive reinforcement. In this review, we will discuss how changes in human physiological markers, assessed with noninvasive brain stimulation techniques from distinct brain regions, can be utilized to provide insights toward the distinct learning processes underlying motor learning. We will summarize the findings from several behavioral and neurophysiological studies that have made efforts to understand how distinct processes contribute to and interact when learning new motor behaviors. In particular, we will extensively review two types of behavioral processes described in human sensorimotor learning: (1) a recalibration process of a previously learned movement and (2) acquiring an entirely new motor control policy, such as learning to play an instrument. The selected studies will demonstrate in-detail how distinct physiological mechanisms contributions change depending on the time course of learning and the type of behaviors being learned.
Keywords: motor learning, physiology, brain stimulation, TMS, skill learning
Introduction
One of the main features of the animal kingdom is the ability to move. This requires that the nervous system must learn how to move and have the ability to modify appropriate motor commands to lead to the desired outcome. Humans are capable of acquiring the knowledge necessary to perform new motor behaviors through distinct forms of learning. Recent behavioral studies have provided insights into distinct learning processes underlying motor learning, including error-based learning, reinforcement learning, use-dependent learning, and cognitive strategies. Each of these processes is thought to involve different neuronal substrates and computations (Haith and Krakauer 2013; Krakauer and Mazzoni 2011; Shadmehr and Krakauer 2008; Taylor and Ivry 2014; Wolpert and others 1995). Indeed, learning new skills or adjusting previously learned policies require the engagement of several plastic mechanisms in the cerebral cortex, cerebellum, and striatum (Caligiore and others 2017; Dayan and Cohen, 2011; Penhune and Steele, 2012). A current challenge in the motor learning field is to confidently be able to disentangle each of these forms of learning and their respective physiological mechanisms involved in acquiring a new behavior.
To study motor learning, scientists have developed a number of laboratory motor learning tasks and manipulations to weight differently the contributions of error-based learning, reinforcement learning, use-dependent learning, and cognitive strategies. In other words, depending on the task studied, the relative contributions of each of these distinct forms of learning are likely to change due to the different demands each task requires for learning to occur (Fig. 1). Additionally, the relative weights of how much each of these forms of learning contributes to the overall learning process within the same task (i.e., temporal scale of learning) also changes. Importantly though, despite the best efforts to manipulate behavioral tasks to weight one learning process more than the other, it is unlikely that complete, pure isolation occurs. This is because these distinct forms of learning are not functionally independent and their neural substrates cannot get turned off.
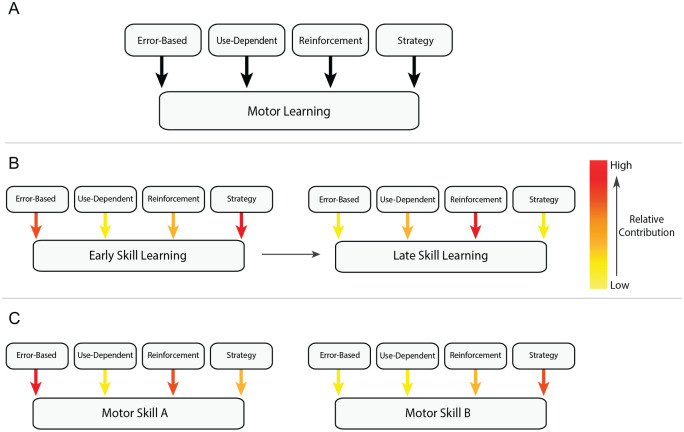
Figure 1.
Processes underlying motor learning. (A) Motor learning is constituted by several different processes all involved in acquiring novel behaviors or calibrating already known ones. This includes error-based, reinforcement, use-dependent plasticity, and strategy-based forms of learning. Error-based is a type of learning based on sensory-predictions errors where the intended movement outcome is compared with the actual executed movement. In other words, a type of learning driven by a mismatch between what you think you are doing and what you perceive you are doing. Reinforcement learning refers to a success-based process in which actions leading to a successful outcome are reinforced, whereas those leading to unsuccessful outcome are avoided. Use-dependent learning is used to describe a phenomenon where behavioral changes are induced through the simple repetition of movements, regardless of whether errors are present or not. Strategy-based learning simply refers to utilizing cognition or explicit knowledge to solve motor problem. Each of these forms of learning are continuously involved in guiding the performance of our movements toward the correct solution. (B) Although these mechanisms work to achieve a common goal (i.e., learning a skill), it is important to consider that the relative contributions of these forms of learning maybe weighed differently throughout the time course of the same motor skill training (i.e., initially picking up a new task vs. after several attempts at the same task; heat map panel: red = more, yellow = less). (C) Similarly, the contributions of these forms of learning may also shift depending on the specific component of the motor task that one is asked to learn. For example, to successfully hit a tennis ball, our brain must develop an understanding of how to interact with a racket/environment/ball (e.g., weight of the racket and ball, type of court), as well as to coordinate an appropriate sequence of movements (i.e., fluid serve).
Using behavioral manipulations to isolate as much as possible specific learning processes, different research teams have used noninvasive brain stimulation techniques to assess their underlying neurophysiological mechanism. In this manner, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have been able to help dissect the neurophysiological mechanisms underlying motor learning, as well as modulate distinct learning processes.
Applying TMS over predetermined areas of the brain we can induce currents, allowing the assessment of brain excitability, as well as disrupt the function of that region in order to explore casual relations to behavior (Box 1). Moreover, TMS paired pulses, where a conditioning pulse is applied over a brain region (e.g., cerebellum; CB) and a test stimulus is delivered over the primary motor cortex (M1), permits assessing the connectivity between two regions, in this case CB-M1 connectivity, critically involved in motor learning. In this manner, TMS, as opposed to imaging methods such as magnetic resonance imaging, has excellent temporal resolution and allows accurate identification of the type of excitability change (i.e., excitatory vs. inhibitory changes). On the other hand, other studies have used tDCS not only to alter learning rates but also combined with TMS before and after motor learning to understand cortical excitability changes resembling long-term plasticity phenomena (Box 2). In this article, we review studies that have integrated these stimulation techniques when humans learn different motor behaviors in order to understand the relative contributions of distinct learning mechanisms and their neurophysiological substrates.
Box 1. Transcranial Magnetic Stimulation to Study Motor Learning.
Transcranial magnetic stimulation (TMS) provides the ability to noninvasively stimulate cortical tissues from various regions of the brain. During a TMS procedure, a short current pulse (~100 µs) applied to the coil of wire induces a magnetic field that passes perpendicular to the current flow in the coil. The magnetic field (~1.5-2 T) penetrates through the scalp and induces an electric field perpendicular to the magnetic field. When applied over the primary motor cortex (M1) representation of a particular muscle, TMS evokes a series of descending corticospinal volleys that activate spinal motor neurons, which in turn activate the desired muscle contralateral to the hemisphere stimulated. The effects induced by TMS are measured in the form of motor-evoked potentials (MEPs), which are easily recorded with electromyography, providing a simple way to investigate changes in the state of M1 before, during and after learning motor tasks. What makes TMS an attractive method for scientific research is that it can be used to either assess or modulate cortical excitability, inhibition, connectivity between distinct brain regions, and plasticity. In particular, connectivity of pathways between different brain areas and M1 has been extensively studied due to the simple readout of MEPs and the critical role M1 plays in a wide-range of motor behaviors (Dayan and Cohen 2011; Penhune and Steele 2012).
To study connectivity with TMS, two coils are required: (1) used to deliver a conditioning pulse of the area of interest and (2) a test pulse applied over M1 (Fig. 3). For instance, measuring the connectivity between the cerebellum and M1 is highly relevant to understand motor learning physiology. Ugawa and others (1995) were the first group to study this connectivity by delivering a conditioning TMS pulse over the cerebellum 5 to 7 ms prior to applying another TMS pulse over M1 resulting in a reduced MEP amplitude relative to trials with no cerebellar stimulation (Daskalakis and others 2004; Pinto and Chen 2001; Ugawa and others 1995). This effect of cerebellar stimulation, known as cerebellar inhibition (CBI), has been suggested to result from TMS activation of Purkinje cells inhibiting the dentate nucleus, which in turn has excitatory projections through ventrolateral thalamus to M1 (Celnik 2015; Daskalakis and others 2004; Pinto and Chen, 2001). This interpretation is supported by work done in patients with lesions in the cerebellar-thalamic-cortical pathway or patients with atrophy of the cerebellar hemisphere showing no CBI (Iwata and Ugawa 2005; Kikuchi and others 2012). A double-cone TMS coil, designed to stimulate deeper tissue, is the most reliable coil to elicit CBI (Hardwick and others 2014; Spampinato and others 2019). Thus, the presence of CBI can reflect cerebellar excitability, at least when M1 excitability does not change or is accounted for during the measurement. Specifically, when measuring CBI at different time-points following behavior, it is critical to adjust the intensity applied over M1 such that the test pulse MEP amplitudes are matched for a fair comparison.
Beyond using TMS to understand the connectivity between two brain regions, previous studies have also made use of paired-pulse techniques to examine excitatory and inhibitory mechanisms within the primary motor cortex during different motor tasks. For instance, short intracortical inhibition (SICI), thought to reflect GABA-A (γ-aminobutyric acid A) receptor neurotransmission has been shown to change in different motor learning tasks (Stagg and others 2011). However, there are some inconsistent results indicating that these changes might not reflect specific motor learning process, but rather the consequence of motor execution (Spampinato and Celnik 2017).
Finally, TMS has been used to disrupt neural processes in a time specific manner during a behavioral performance. In this manner, TMS permits the assessment of causality between specific brain activity (or region) and behavior, complimenting the correlative nature of imaging (e.g., magnetic resonance imaging), magnetoencephalography or electroencephalography methods.
Box 2. Transcranial Direct Current Stimulation to Study Motor Learning.
Transcranial direct current stimulation (tDCS) is another commonly used noninvasive brain stimulation technique, which involves a weak current (~1-2 mA) delivered through the skull via two small electrodes. Unlike the application of TMS, tDCS does not directly induce neuronal firing of action potentials; however, it is capable of modulating cortical excitability in a relatively short bout (>10 minutes). Animal works have demonstrated that tDCS can alter the resting membrane potential of neurons and induce excitability changes in spontaneous neuronal discharges and evoked potential amplitudes for up to 5 hours (Creutzfeldt and others 1962; Purpura and McMurtry 1965). In humans, current-induced excitability changes in M1 are dependent on stimulation intensity and duration, but have been reported to last for up to 90 minutes post-stimulation (Liebetanz and others 2002; Nitsche and Paulus 2000, 2001;).
The lasting effects on cortical excitability changes following tDCS application are thought to involve synaptic plasticity mechanisms similar to long-term potentiation (LTP). Indeed, a recent study demonstrated that in vivo tDCS induced a robust enhancement in synaptic plasticity, including LTP changes in the hippocampus (Rohan and others 2015). In humans, antagonizing N-methyl-d-asparate (NMDA) receptors prevents the induction of long-lasting after-effects (Nitsche and others 2003), while agonists increase the duration of the aftereffects (Nitsche and others 2004). Animal studies have confirmed the dependency of NDMA receptors (Rohan and others 2015) and extended this knowledge by showing that direct current stimulation LTP was absent in BDNF and TrkB mutant mice (Fritsch and others 2010). Although it is assumed that anodal tDCS can effectively produce changes in cortical excitability, it is important to note that evidence of large inter- and intraindividual variability responses to stimulation (Ammann and others 2017; Guerra and others 2017), which can be attributed to many factors (Polanía and others 2018), including biological (i.e., age, anatomy, brain state, time of day, etc.) and methodological factors (i.e., stimulation protocols and outcome measures). Nevertheless, numerous studies in humans have demonstrated that combining motor practice with anodal tDCS (AtDCS) over M1 augments learning (Cantarero and others 2015; Reis and others 2009; for review, see Ammann and others 2016) and the amount of GABA reduction induced by M1 tDCS has been found to correlate to the degree of motor learning (Stagg and others 2011). Of note, while other brain regions have been targeted with anodal tDCS to enhance motor learning, a similar response cannot be assumed to what has been described with M1 stimulation. For example, the mechanisms of action of cerebellar tDCS are different from those described for M1 (Grimaldi and others 2016). Together, these findings provide a strong rationale for using tDCS over M1 as an LTP-like inducing protocol, with the potential to either assess LTP-like plasticity changes or modulate motor behavior (Fig. 4).
Error-Based Learning
One of the most basic forms of learning is the ability to perform accurate movements by accounting for changes to our body or environment through predictive mechanisms. For instance, the ability of a tennis player to adjust to different surfaces, the tension of the rackets, or the weight of the tennis balls (e.g., when raining) requires a feedforward flexible control process to hit the next ball. This short-term form of learning (i.e., within minutes to hours) known as error-based learning refers to the modification of behavior based on an error signal that compares the sensory outcomes of expected and realized movements (Mazzoni and Krakauer 2006; Tseng and others 2007). Instead of providing information about movement success, sensory prediction errors provide details as to how the past movement has failed (Bastian 2011). Thus, sensory prediction errors are vectorial as they indicate details (e.g., direction, force, etc.) about how subsequent movements should be modified to result in a successful action. Sensory prediction errors can be used to calibrate the internal representations of body dynamics and the environment (i.e., developing forward models) and recalibrate for changes in either (Shadmehr and Krakauer, 2008; Schlerf and others 2012b). Therefore, this type of learning consists of developing sensory-motor maps (forward internal models) to reduce sensory prediction errors.
Error-based processes are known to heavily contribute to learning the many well-studied adaptation behavioral tasks. In these paradigms, participants gradually account for a perturbation (e.g., modification of sensory feedback; see Fig. 2A) introduced to the task workspace. Several studies have shown that learning these paradigms are mainly driven by the reduction of sensory prediction errors caused by perturbations via trial-by-trial modifications of a forward model (Bastian 2011; Tseng and others 2007). Behaviorally, this is characterized by a gradual improvement in performance to an altered condition (e.g., perturbation) of a previously known behavior, where performance returns to baseline levels. Importantly, learning from an error can occur on a single trial, thus movement adaptation is evident even when perturbations are introduced in a random fashion (Donchin and others 2003; Thoroughman and Shadmehr 2000). Several theoretical and experiential studies have highlighted a critical role of the cerebellum in error-driven, forward internal models, including the generation of predictions (Miall and others 2007) and encoding of sensory prediction errors for updating forward models (Blakemore and others 2001; Diedrichsen and others 2005b; Wolpert and others 1998). Indeed, individuals with cerebellar pathology exhibit significant impairments in a wide range of sensorimotor adaptation tasks which can be attributed to a failure of learning via sensory prediction errors (Martin and others 1996; Maschke and others 2004; Smith and Shadmehr 2005; Tseng and others 2007). This reveals that the activity of the cerebellum is critical during the feedforward process that is needed for successful motor adaptation.
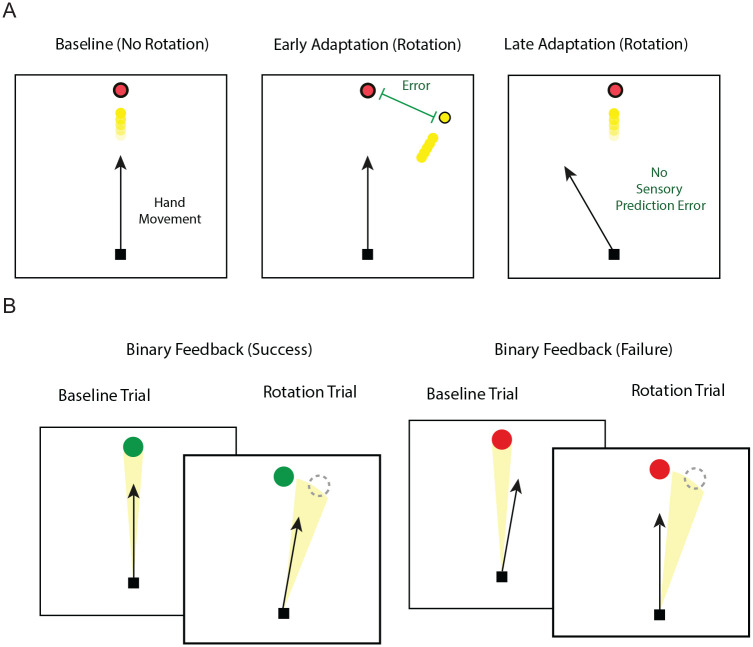
Figure 2.
Behavioral studies over the past several years have used several forms of motor adaptation tasks (e.g., rotations, prisms, force-field, split-belt walking) to elucidate the learning processes responsible for calibrating the mapping between desired outcomes and motor commands. This process is critical for our ability to adjust our daily movements to environment demands, such as walking over different surfaces (i.e., concrete, sand, ice). In adaptation tasks, a perturbation is suddenly introduced, altering the relationship between a movement and its resulting sensory feedback in the task workspace. (A) This figure depicts a commonly used visuomotor reaching adaptation task. In this setup, participants are asked to make “shooting” reaches toward a visual target by moving an on-screen cursor (yellow dot). The cursor represents the position of their hand (i.e., participants vision of the arm is blocked), therefore perturbations can be applied to the cursor (i.e., visuomotor transformations). The “shooting” action does not allow participants to correct movements within a trial, but rather only for learning via through endpoint feedback error to adjust movements on the next trial (i.e., feed-forward sensory prediction errors). Participants are capable of performing accurate reaches to targets within the workspace when there is no visuomotor manipulation (baseline). When a rotation is applied to the cursor unknowingly to the participants, they initially execute and observe large errors (early adaptation). The goal of the task for the participant then becomes to minimize the movement error between the cursor and target (i.e., minimize sensory prediction errors). This can be accomplished over multiple reaches. If the cursor perturbation is then removed, participants execute errors in the opposite direction, depicting the retention of the previously acquired sensory-motor map. (B) By providing only binary feedback about task performance (i.e., success or failure), participants can also learn a cursor rotation using reinforcement learning. In this example, if the participants land in the correct zone (highlighted in yellow) a “success” visual feedback is provided (green). However, if the participant does not land in this zone, they are given a “failure” feedback (red). Unlike learning from sensory prediction errors, learning via reinforcement does not lead to sensorimotor recalibration. Explicit processes (i.e., aiming strategies; not shown) also influences visuomotor learning, in particular when large rotations are introduced.
Animal works have also provided evidence that the activity of Purkinje cells in the cerebellar cortex is engaged during forward model learning utilizing sensory prediction (Herzfeld and others 2018; Nixon and Passingham 2000; Pasalar and others 2006; Roitman and others 2005). Of particular interest, Purkinje cell simple spike activity recorded in monkeys were found related to the kinematics of the arm movement rather than the motor command responsible for the actual arm movement kinematics (Pasalar and others 2006), suggesting that the output of the cerebellum is more linked to predictions about the consequences of movement, rather contributing directly to the motor command.
Human Neurophysiological Studies of Error-Based Learning
Studies using TMS have provided neurophysiological evidence for the involvement of the cerebellum when learning tasks rely on error-based learning. To study physiological contributions of the cerebellum, researchers have used a paired-pulse TMS protocol to measure changes in the connectivity between the CB-M1 connectivity (also known as “cerebellar inhibition” or CBI) before and after learning (Fig. 3A). In this protocol, a TMS pulse is applied over a cerebellar hemisphere (3 cm from the inion). This stimulation is thought to activate Purkinje cells in lobules VII and VIII of the targeted cerebellar cortex, which in turn inhibits the deep cerebellar nuclei which have excitatory connections to M1. Thus, if a second TMS pulse is delivered over M1 5-7ms following the cerebellar stimulation the MEP amplitude will be reduced. Therefore, it is thought that CBI reflects the normal inhibitory tone the cerebellum exerts over M1 via the thalamus (Celnik 2015). This interpretation is supported by patient work, as no CBI is found when there are lesions to the cerebellar-thalamic-cortical pathway or atrophy of the cerebellar hemisphere (Shirota and others 2010; Ugawa and others 1997).
Figure 3.
(A) Paired-pulse transcranial magnetic stimulation (TMS) configuration for assessing cerebellar-M1 connectivity (cerebellar-inhibition or CBI). A figure-of-eight coil is placed over the left M1 (test stimulus; TS), whereas a double-cone coil is placed over the right cerebellum, 3 cm inferior and lateral to the inion (conditioning stimulus; CS). CBI refers to the ratio between the motor-evoked potential (MEP) amplitudes after applying the conditioned test stimulus (CS + TS; magenta) and the MEP amplitudes produced following the unconditioned test stimulus (TS alone; dark blue). (B) Schematic representation of the interpretation illustrating how cerebellar-M1 connectivity changes following learning. Although the physiology of stimulating this region are not completely understood, double cone coil stimulation is thought to result in the parallel-fiber-mediated activation of Purkinje cells, which in turn inhibit the deep cerebellar nuclei (Celnik 2015) that have a disynaptic excitatory connection to M1 via the thalamus. Prior to learning, a test pulse over M1 combined with a cerebellar conditioning pulse on highly active Purkinje cells (marked in red) results in a decreased activation of M1. This is depicting by smaller MEP amplitudes as a result of combined stimulation (magenta trace) when compared to the MEPs elicited by just stimulating over M1 (blue trace). CBI has been found to reduce following a variety of motor learning tasks (Jayaram and others 2011; Schlerf and others 2012a; Schlerf and others 2015; Spampinato and Celnik 2017; Spampinato and others 2017), which has been interpreted as resulting from long-term depression like changes in the activity of parallel fiber–Purkinje cell synapses, as shown in animal studies (Medina and Lisberger 2008). Thus, following learning, stimulation of less active Purkinje cells (marked in yellow) is not as likely to inhibit M1. Further evidence of this interpretation has been provided by studies utilizing transcranial direct current stimulation (tDCS) to modulate cerebellar activity. One study showed that anodal cerebellar tDCS effects were consistent with the concept of increased excitability of Purkinje cells, since the authors were able to elicit a stronger CBI effect at low conditioned stimulation intensity following tDCS stimulation (Galea and others 2009). Following this logic, the authors also showed that cathodal tDCS resulted in a reduced CBI effect. Together, this collection of results indicates that cerebellar stimulation, to a degree, reflects the state of Purkinje cells and how they respond to both behavioral and brain stimulation interventions.
Neurophysiological studies in animal models have shown a link between the amount of error-based learning and the subsequent activity in Purkinje cell firing (Gilbert and Thach 1977; Medina and Lisberger 2008; Yang and Lisberger 2014). Mechanisms resembling long-term depression (LTD), in which the response of Purkinje cells to sensory information (mossy fiber inputs) is decreased following an error signal (climbing fibers), appears as a dominant neurophysiological process that underlies cerebellar contributions to learning (Yang and Lisberger 2014). Thus, assessing CBI changes following human motor learning presents as a potential marker for this physiological process. Specifically, if CBI were to reflect aspects of Purkinje cell activity, one would predict the overall CBI effect to be reduced in humans following error-based learning (Fig. 3B). A reduction of CBI is precisely what occurs following several error-based dependent tasks, including split-belt adaptation (Jayaram and others 2011), visuomotor adaptation (Schlerf and others 2012a; Uehara and others 2018) and in recalibrating new visual-force mappings (Spampinato and Celnik, 2017, 2018; Spampinato and others 2020). The reduced CBI response is most prominently found at the initial phase of learning, where sensory prediction errors are largest. Interestingly, Jayaram and colleagues found that participants experiencing a larger magnitude of adaptation (i.e., a greater degree of learning) expressed a greater reduction in CBI, therefore suggesting a link between error-based motor learning and changes in human cerebellar excitability. As such, changes in CBI following learning have been interpreted as LTD-like changes occurring in the Purkinje cell–parallel fiber synapses (Celnik 2015) consistent with the neurophysiological studies of animal models (Gilbert and Thach 1977; Medina and Lisberger 2008; Yang and Lisberger, 2014). This is supported by recent work in humans showing that modulation of cerebellar activity by means of theta-burst stimulation, accelerates the error reduction process in visuomotor adaptation (Koch and others 2020). Interestingly, theta-burst patterns in animal models have shown to induce plasticity changes in both mossy fiber–granule cells synapses and at Purkinje cell–parallel fiber synapses (D’Angelo 2014). Together, the evidence from human studies suggests that changes in CB-M1 connectivity following learning represents the involvement of the cerebellar-dependent, error-based form of learning.
Reinforcement
The central nervous system is capable of learning and selecting appropriate motor actions based on simple feedback of whether a movement was successful or a failure. This behavioral process termed reinforcement learning (RL), explains how we learn to choose and repeat actions that maximize reward (i.e., lead to success). RL requires individuals to “explore” which actions lead to successful outcomes without caring about how the movement was done. For instance, the tennis player explores different grips, different body postures or different wrist motions to hit the ball more successfully. Thus, learning behaviors via reinforcement rely on exploration of different actions which are reinforced (either repeated or avoided) based on the outcome.
Rather than using a prediction error that arises from the differences between the actual versus predicted sensory consequences of a movement as a learning signal, reinforcement learning is driven by a prediction error that encodes the probability of an action resulting in a success (Sutton and Barto 1998). That is, if an individual’s outcome of particular action resulted in something they did not expect (i.e., reward or punishment), an error signal (positive or negative, respectively) will update expectations about that action. This will lead to either repeating or avoiding the prior action. Unlike the vector feedback a sensory prediction error provides, a reinforcement signal failure does not provide information about how (directionally) a behavioral should be adjusted (Izawa and Shadmehr 2011; Therrien and others 2016). In other words, in contrast to supervised learning, the reward we see only tells us whether the action we chose was good or bad, not what would have been the “correct” action.
Several studies have highlighted key differences between learning via reinforcement versus error-based that yield different behavioral outcomes. For instance, via reward prediction errors it is possible to learn a new motor behavior that is identical to actions learned via error-based learning in a motor adaptation task presenting a perturbation (Galea and others 2015; Izawa and Shadmehr 2011; Wu and others 2004). Interestingly, when a motor action is learned via success-based feedback, participants do not realign their proprioceptive estimates of limb position to match vision (i.e., no recalibration of proprioception), a phenomenon that is present when learning via sensory-prediction errors (Izawa and Shadmehr 2011). Although learning via reinforcement is a slower process than error-based learning, participants exhibit longer retention with success-based feedback (Abe and others 2011; Izawa and Shadmehr 2011; Therrien and others 2016).
It is widely accepted that the basal ganglia plays a key role in selecting appropriate actions and in reinforcement learning. Both action selection and reinforcement are facilitated by dopaminergic neuron activity, which encodes reward prediction errors when reward outcomes are higher or lower than expected (Schultz 2016). The basal ganglia are thought to use reward prediction errors in order to encourage the selection of wanted movements and inhibition of unwanted ones (Albin and others 1989). This indicates that connections between the basal ganglia and M1 are critical for motor-related reinforcement learning (Doyon and others 2009). M1 is a primary output target of basal ganglia–thalamo-cortical circuits that shows degeneration after diseases of basal ganglia (Dumas and others 2012) and also receives dopaminergic inputs that are capable of modulating M1 synaptic plasticity (Hosp and Luft 2013; Hosp and others 2011; Molina-Luna and others 2009). Stimulation of ventral tegmental area dopaminergic cells enhanced motor-map reorganization and recovery following motor cortex lesions (Castro-Alamancos and Borrel 1995). Blocking M1 dopamine receptors or lesioning cortical dopaminergic pathways impairs both LTP (Guo and others 2015; Molina-Luna and others 2009) and motor skill acquisition (Hosp and Luft 2013; Hosp and others 2011), indicating the role of dopamine receptors in learning skilled movements. Interestingly, human studies on Parkinson’s patients have also described impaired M1 plasticity due to massive loss of dopaminergic neurons, including a loss of LTP indirect pathways and a replacement of LTD with LTP on the indirect pathway, which are only capable of producing aftereffects if patients are given l-dopa (Kishore and others 2012; Kishore and others 2014; Morgante and others 2006; Suppa and others 2011). Together these results suggest that dopamine-driven reinforcement mechanisms might be mediate, in-part, by the expression of learning-related M1 LTP-like plasticity.
Human Neurophysiological Studies of M1 Plasticity and Reinforcement Learning
Combining the application of TMS with tDCS (see Box 2) can also provide scientists with a powerful approach to study physiological mechanisms contributing to human motor learning. Classical animal studies have demonstrated that motor learning leads to LTP in M1 and results in a reduced capacity to artificially induce more LTP-like changes (Rioult-Pedotti and others 1998; Rioult-Pedotti and others 2000; Rioult-Pedotti and others 2007). Evidence for this phenomenon—termed occlusion—is consistent with homeostatic mechanisms of plasticity, which is thought to stabilize the overall synaptic weight and firing rates of a neuronal network within a physiologically reasonable dynamic range (Karabanov and others 2015). Homeostatic plasticity can be assessed in humans by applying interventions such as paired associative stimulation (PAS; Rosenkranz and others 2007; Stefan and others 2006; Ziemann and others 2004) and anodal tDCS (Cantarero and others 2013; Siebner and others 2004) following motor learning (Fig. 4). In this technique, TMS is applied prior to and after the application of these protocols, to assess whether M1 is capable of responding and the magnitude of change following PAS or tDCS. This quantification is done twice, before and after a motor task is trained. The rationale for this is that if the training leads to learning using LTP resources, then introducing a plasticity protocol that relies (at least in part) on LTP-like mechanisms (i.e., tDCS; Fritsch and others 2010; Nitsche and others 2003) should result in a reduced response after learning relative to a baseline state, consistent with principles of homeostatic metaplasticity.
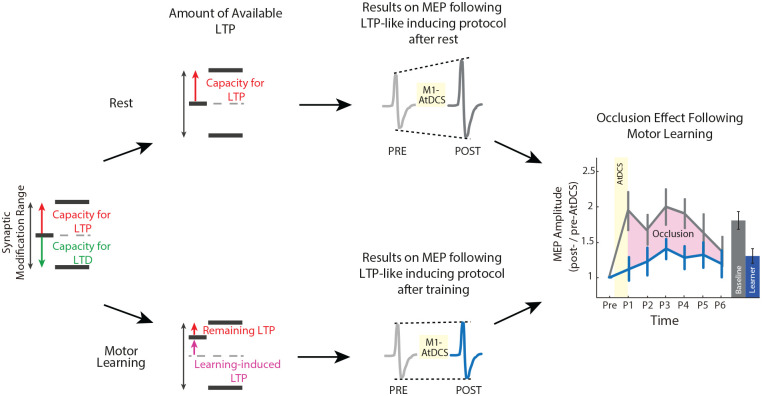
Figure 4.
Schematic representation of the interpretation of occlusion of M1 long-term potentiation (LTP)-like plasticity after motor learning. The concept of occlusion relates to the idea that induction of LTP and long-term depression (LTD) within M1 reach a saturation point. This is consistent with a homeostatic plasticity mechanism that acts to control neuronal activity and excitability levels within a physiologically useful modification range. Thus, there is a limited capacity or a particular synaptic modification range for how much potentiation M1 can undergo. At rest, M1 excitability is at the center of the synaptic modification range and has a certain capacity for LTP-like plasticity (red arrow). In this scenario, when anodal transcranial direct current stimulation (tDCS; i.e., LTP-like inducing protocol) is applied to M1, that facilitates excitability via LTP-like plasticity. This is evident by comparing motor-evoked potential (MEP) amplitudes via transcranial amgnetic stimulation (TMS) over M1 before (light gray) and after (dark gray) anodal tDCS application, which can modulate excitability for ~30 minutes. However, since motor learning induces LTP-like plasticity in M1, as shown in animal studies, then there is a reduced range for further LTP-like plasticity. Thus, the effect of anodal tDCS on M1 excitability facilitation following learning (blue) can be smaller than that in baseline condition. This phenomenon is referred to as occlusion of LTP-like plasticity and can be seen as a signature indicating the presence of homeostatic M1 LTP-like plasticity.
Consistent with animal research, humans show occlusion to plasticity-inducing protocols following motor learning tasks, in particular to those tasks in which successful actions were reinforced (Cantarero and others 2013; Spampinato and Celnik 2017, 2018; Uehara and others 2018). Interestingly, occlusion of M1-LTP like plasticity has been associated with the amount of motor skill learning retention (Cantarero and others 2013) and is only prominent when a substantial amount of successful actions are performed (Spampinato and Celnik 2017). The link of occlusion and reinforcement learning is supported by recent behavioral studies showing that providing additional positive feedback during learning trials enhances motor retention (Galea and others 2015; Shmuelof and others 2012; Spampinato and others 2019). Moreover, as animal models have shown that successful motor learning and M1 synaptic plasticity in M1 requires dopaminergic signaling (Rioult-Pedotti and others 2015), the occlusion effect seen in humans likely reflects the influences of the basal ganglia on M1 plasticity during motor learning.
Disentangling Error-Based and Reinforcement Learning
It could be argued that attributing the occlusion effect to a particular form of learning is difficult as skill tasks by nature are complex and thus require the engagement of multiple forms of learning. However, recent behavioral experiments have been able to dissect error-based and reinforcement learning components within a visuomotor adaptation task (Izawa and Shadmehr 2011; Therrien and others 2016). This was achieved by giving participants success-based binary feedback instead of vector error feedback during perturbation trails (see Fig. 2B). More recently, Uehara and colleagues designed a study that utilized this manipulation of feedback presented to participants when learning the same motor actions to understand the distinct physiological markers associated with reinforcement and error-based learning (Uehara and others 2018). In one experiment, participants were given vector feedback in order to adapt motor commands (i.e., stronger reliance on error-based learning; Fig. 5A), while in another experiment only binary feedback (i.e., weighing more reinforcement) was provided (Fig. 5B). The authors showed that learning the motor behavior relying on binary feedback led to occlusion of M1 LTP-like plasticity, but not cerebellar excitability changes. On the other hand, when participants relied on error-based mechanisms to learn the same motor behavior the investigators found cerebellar excitability changes (modulation of CBI), but not for M1 LTP-like plasticity, especially early on during the training. In this double dissociation, the authors elegantly showed that learning-induced changes in M1 LTP-like plasticity are a marker of the involvement of reinforcement form of learning, while also providing further evidence that cerebellar excitability changes are associated to error-based learning processes.
Figure 5.
Learning a visuomotor cursor rotation via different forms of learning (i.e., via sensory prediction errors vs. reinforcement) relies on different physiological mechanisms. (A) Visuomotor task version relying on error-based learning. Participants performed a center-out reaching task in which they controlled the movement of a yellow computer-cursor from a central starting position to a target (eight possible locations) while receiving online and endpoint cursor feedback (light blue dot). (B) Behavioral reach angle data. Positive values indicate counterclockwise deviation. Solid lines and shaded areas show the mean and standard errors of the mean (SEM) for each 8-trial epoch for the constant perturbation (blue; i.e., a constant 30° rotation was administered in “Perturb” section) and the random perturbation sessions (gray). Only the Constant group shifted the reaching direction when exposed to the constant perturbation, but not when exposed to the random perturbation. (C) Cerebellar inhibition (CBI) results. Bar graphs and vertical error bars indicate the mean CBI ratio (the ratio of the conditioned/unconditioned test stimulus [TS] motor-evoked potential [MEP] amplitude) for the constant and the random sessions at each time point. The authors found that CBI selectively reduced after participants of the Constant group accounted for the perturbation (i.e., reduced CBI at “Post” time-point). (D) The AtDCS effects on M1 excitability for the Constant and Random groups. MEP amplitudes normalized to that of preAtDCS are presented for each time point (Pre, P0, . . ., P15). No significant occlusion was found for either group after training and adapting via sensory-motor prediction errors. (E) Motor task version relying on reinforcement learning. Here, participants reached from a central starting position to one target. Only binary feedback (success or failure) was presented (in the form of target colors) instead of vector cursor feedback. (F) Reach angle. Solid lines and shaded areas indicate the mean and SEM of each 8-trial epoch for the Learner (light blue) and the Non-Learner groups (gray). (G) The AtDCS effects on M1 excitability and (H) CBI results for the Learner and the Non-Learner groups. Here, the authors showed learning via binary feedback elicited changes in M1 LTP-like plasticity (i.e., occlusion), but did not modulate cerebellar excitability changes. This study elegantly showed that we can learn the same motor behavior via different forms of motor learning engaging distinct neurophysiological mechanisms (Uehara and others 2018). Figure used with permission from Oxford University Press https://doi.org/10.1093/cercor/bhx214).
Use-Dependent Learning
The ability for the world’s top athletes to consistently execute quality movements surely relates to the countless hours of practice they perform. However, repeating movements does not necessarily lead to desirable outcomes. This is because motor behaviors are shaped not only by sensory signals (error and reward) but also by the history of previous motor actions. The tennis player who practices the motions of a swing multiple times right before the actual play is in part invoking use-dependent learning mechanisms that have been shown to reduce reaction time and variability. In other words, the repetition of the same movement is capable of altering performance, even when no information about the movement outcome is provided. This form of learning, known as use-dependent learning (UDL), is a simple and goal-independent process that forms motor memories by changing movements to become more similar to the previous movement (Reinkensmeyer and others 2016; Wolpert and others 2011). For example, repeating point-to-point curved movements around an obstacle led to a distinct curved trajectory of movements on subsequent trials, even when the obstacle was removed (Jax and Rosenbaum 2007). This also illustrates that UDL can lead to nonoptimal or energetically favorable solutions when adapting to environmental changes in redundant tasks (Diedrichsen and others 2010). While UDL results in directional biases in subsequent movements, behavioral studies have demonstrated that repetition reduces the variability of movements (Huang and others 2011; Verstynen and Sabes, 2011). Furthermore, recent studies showed that repetition also produces faster movements (Hammerbeck and others 2014) by facilitation of movement planning (Mawase and others 2018).
Human Neurophysiological Studies of Use-Dependent Learning
Using TMS, past studies demonstrated that the repetition-dependent biases originate in M1 since transcranial electrical stimulation (TES) cannot elicit biased-evoked movements (Classen and others 1998) and movement repetition leads to plastic reorganizational changes in M1 (Butefisch and others 2000; Dayan and Cohen 2011). For instance, when individuals repeatedly perform thumb movements in a particular direction, TMS of M1 following training is more likely to elicit responses in the trained direction (Classen and others 1998). This classic study was the first to show that a brief amount of repetitive training alters the TMS responses, indicating that even a small bout of repetition is capable of enhancing the excitability of cortical representations of particular movements. The short-term changes in representations have been interpreted to be mediated by Hebbian-like plasticity or synaptic alterations within M1 (Butefisch and others 2000; Orban de Xivry and others 2011), as these changes by TMS are disrupted when individuals are given lorazepam, a GABA agonist (Butefisch and others 2000). This concept is supported by animal literature, where repetitive movements (especially when rewarded) strengthen horizontal cortical connections through LTP mechanisms (Rioult-Pedotti and others 1998), induces functional map reorganization (Kleim and others 1998), and leads to the formation of postsynaptic dendritic spines (Fu and others 2012; Xu and others 2009; Yang and others 2009). Interestingly in humans, atDCS applied over M1, which has been shown to modulate excitability via NMDA-related activity (Fritsch and others 2010) and decrease GABA (Stagg and others 2011) enhances the initial formation and retention of new motor memories assimilated via repetitive training (Galea and Celnik, 2009; Koyama and others 2015; Rroji and others 2015). However, it is important to note that plastic changes observed during these TMS studies, do not necessarily mean motor learning has occurred since there is no behavioral expression of the learning; the new directional biases are elicited by TMS-evoked movements. Because of this subtle difference, the TMS literature has referred to this TMS paradigm as use-dependent plasticity (UDP), rather than UDL.
The long-term involvement of UDL that is necessary for executing complex motor skills can also be realized with TMS. Indeed, TMS can show functional map reorganization following substantial training (Pascual-Leone and others 1995). This is done by conducting corticomotor mapping, which consists of delivering TMS pulses with the same intensity to different sites over M1. The resulting MEPS responses and direction of hand movements induced by TMS can be compared before and after extensive motor training. For example, the unimanual practice of a piano piece of a naïve individual enlarges specifically the hand representation of the trained motor cortex, whereas expert piano players do not enlarge their motor cortical representations due to years of training on this skill (Pascual-Leone and others 1995). Rather, TMS applied to well-trained musicians is more likely to evoke muscle activity patterns that resemble features of trained movements (i.e., piano playing–like movements, violin grasping; Gentner and others 2010). Moreover, action observation via the engagement of mirror neuron activity has been shown to facilitate motor learning (Mattar and Gribble 2005). Interestingly, these effects might be mediated by UDP-related mechanism, as prior studies have shown action observation can elicit directional changes in the likelihood of TMS-evoked movements to follow the observed movements (Stefan and others 2005). This effect of action observation on UDP has also been shown to enhance the effects of motor training in healthy older adults and stroke patients (Celnik and others 2006; Celnik and others 2008).
Given that motor behaviors in daily life are complex and not constrained by laboratory manipulations it is likely that the different forms of learning interact with each other when acquiring these skills. In this manner, it is likely that when training a motor task over time, performance repetition is facilitated by UDL mechanisms resulting in less variability and reduction of the motor planning time. While this occurs, motor errors become smaller increasing the likelihood of engaging reinforcement learning mechanisms (Diedrichsen and others 2010). Indeed, animal works have shown the existence of dopamine receptors in M1, suggesting a candidate mechanism for reward-based modulation of use-dependent plasticity (Hosp and others 2011; Huntley and others 1992; Luft and Schwarz 2009). The interaction between RL and UDL was investigated by a recent study. Movement repetition leading to the correct solution of a visuomotor motor transformation resulted in an increased use-depending learning rate, but only if the repetitions were associated with successfully achieving the target (Mawase and others 2017). Although previous behavioral studies have demonstrated that successful repeated movements elicit larger changes in movement direction biases than non-reward repetition (Huang and others 2011), the physiological effects of learning (i.e., improved performance) on UDP have only recently been investigated. In a series of experiments, Mawase and others used TMS to assess plasticity changes in M1 after individuals learned a repetitive motor skill with reinforced and nonreinforced movements (Fig. 6). Here they assessed UDP, TMS applied over M1 representation of the trained muscle (flexor brevis polis) before and after the behavioral intervention (see Fig. 6 caption for further details). They found that movement repetition in the context of learning a motor skill enhanced UDP (i.e., a greater shift of involuntary TMS-induced responses of the thumb after training). This effect was mediated by success-based reinforcement of the trained skill. The results of this study indicated that the performance of motor tasks likely engages different learning mechanisms that interact with each other leading to faster learning, as tested in this study, but theoretically, they could also interfere or compliment across mechanisms.
Figure 6.
The role of positive-feedback reward signals to learning via use-dependent plasticity (UDP). (A) Schematic of an experimental task that assess UDP. Transcranial magnetic stimulation (TMS) is delivered over the left M1 to elicit thumb movements before and after training. The direction of TMS-evoked thumb movements was recorded and the proportion of TMS-evoked thumb movements falling in the training direction zone (TDZ; magenta) are measured. (B) Representative subject data displaying TMS-evoked thumb movements before (gray, left) and after (blue, middle) training. Mawase and others calculated the group average depicting the probability distribution of the thumb directions before and after task performance. Individuals who trained on this paradigm showed a significant increase in the proportion of TMS-evoked thumb movements within the TDZ. (C) The study design and setup of experiment 2 used in Mawase and others (2018) in which the authors showed that explicit rewards modulate UDP. In short, participants were randomly assigned to either a reward-group or random-reward group. Importantly, only the reward-group (blue) received explicit reward coinciding with task success, whereas the random reward group (red) received an explicit reward randomly throughout performance, independent of task success. TMS-evoked thumb movements were assessed before and after training for both groups. (D) A 2-dimensional histogram showing the total number of TMS-evoked movements for all participants, separated by group. Here, only the reward-group significantly benefited from receiving meaningful reward as they showed a dramatic change in TMS-evoked thumb movement direction, whereas the random-reward group did not show a significant change from baseline. Figures adapted from Mawase and others 2017; https://dx.doi.org/10.1523/jneurosci.3303-16.2017.
Cognitive Strategies
Recent behavioral works have reinvigorated interest in the role of cognitive strategy during motor learning (Mazzoni and Krakauer 2006; Taylor and Ivry 2014; Taylor and others 2014). Indeed, although often overlooked as motor learning mechanism, cognition plays an undeniable role in motor learning as ability to follow instruction and to develop new strategies is critical for individuals to execute successful actions. For example, the tennis player will listen to instructive strategies from a coach to improve the serve.
Classic models of motor learning proposed an initial learning phase that required considerable cognitive demands before new behaviors becoming fluid and autonomous (Fitts and Posner 1967). During the cognitive phase, individuals begin to develop an understanding of the task goals, including the objective of the task and the environmental factors that may influence their ability to perform. Learning these components likely requires the use of explicit knowledge, working memory and strategic implementation. While cognition plays an important role in learning new tasks, behavioral studies have only recently begun to address how employing cognitive strategies affect motor learning.
One behavioral study was able to disentangle the distinct roles for implicit error-based learning and explicit strategy to account for a perturbation (Taylor and others 2014). The authors developed a method to assess the explicit process by having participants report their reach aiming direction prior to executing them. They found that explicit learning fluctuated early in training (i.e., variability in the first few movements). In contrast, implicit learning (i.e., learning via sensory prediction errors, or error-based learning) occurred slowly throughout learning and was not sensitive to changing task instructions. The results from this study, and others, critically demonstrates that overall performance reflects the joint operation of both processes (Mazzoni and Krakauer 2006).
The prefrontal cortex has been associated with cognitive processes, including planning, goal representation, and performance monitoring (Miller and Cohen 2001). The dorsal lateral prefrontal cortex (dlPFC) is thought to play an important role in explicit learning, especially in scenarios that require participants to develop a cognitive strategy to resolve task demands (Taylor and Ivry 2014). Indeed, several motor adaptation studies have consistently shown the activation of dlPFC during visuomotor learning tasks (Anguera and others 2010; Floyer-Lea and Matthews 2005; Shadmehr and Holcomb 1997). The contributions of the dlPFC appear critical to the initial stages of learning given that greater activation levels in dlPFC are proportional to initial adaptation rates (Anguera and others 2010) and patients with prefrontal damage are particularly impaired during the early stages of adaptation, often showing lack awareness of the perturbations imposed (Slachevsky and others 2001).
To date, there are no studies that have directly tested neurophysiological mechanisms related to strategy in the context of motor learning. However, there are a few investigations that have attempted to use brain stimulation to modulate cognitive performance during simple procedural tasks. For example, 5-Hz rTMS administered over the dlPFC impaired performance of serial reaction time task (SRTT) (Pascual-Leone and others 1996), whereas silencing dlPFC with intermittent theta-burst stimulation can disrupt the negative influence of declarative memory processes during motor learning (Galea and others 2010), thus overall facilitating offline motor sequence learning in humans. Furthermore, cerebellar-cortical interactions also appear to play an important role in this type of learning. Indeed, prominent changes in CBI are found following the observation and execution of the SRTT (Torriero and others 2011) and cerebellar rTMS has been shown to interfere with procedural skill acquisition (Torriero and others 2004). One study has successfully studied the interactions between the dlPFC and M1 using paired-pulse TMS, which changes depending on the task demands (Hasan and others 2013). Future studies could utilize this paired-pulse technique to further understand the connectivity between frontal brain regions and M1 during learning. Additionally, future studies could study the interactions between dlPFC and the cerebellum given the reciprocal connections between these areas (Kelly and Strick 2003) and the recently highlighted importance of the cerebellum in cognition (Stoodley and Schmahmann 2011).
Neurophysiological Changes throughout the Time Course of Learning and Distinct Components of a Motor Skill
As evident from the previous section, different processes contribute to the overall learning of a new motor behavior. For instance, error-based, reinforcement, use-dependent learning and strategic learning are all capable of affecting how individuals perform motor adaptation tasks (Haith and Krakauer 2013; Krakauer and Mazzoni 2011; Shmuelof and others 2012; Taylor and Ivry 2014). However, in this section, we will argue that the relative weights of how these forms of learning (and their associated brain regions) contribute to the overall learning changes throughout the course of the acquisition of a new behavior. For instance, disrupting M1 with TMS only impairs a motor adaptation task once performance plateaus (Orban de Xivry and others 2011), indicating that repetition of the same motor command plays a significant role in how much M1 contributes to motor adaptation.
Two recent studies in humans have investigated neurophysiological changes throughout the time course of learning. Spampinato and Celnik (2017) assessed cerebellar excitability and M1 LTP-like plasticity changes before, during and after healthy individuals learned a new skill, the sequential visual isometric pinch task or SVIPT (Fig. 7). The SVIPT requires participants to simultaneously learn how to control a new device in a novel environment (sensorimotor map), along with performing a sequence of isometric movements. The authors reasoned that learning this skill would rely more heavily on a cerebellar-dependent error-based learning mechanism early on, to learn the dynamics of the skill task, prior to shifting the weights to M1 (and basal ganglia), which can incorporate other forms of motor learning such as reinforcement and use-dependent (Spampinato and Celnik 2017). Consistent with this hypothesis, the authors found changes in cerebellar excitability early, but not late in skill learning, whereas M1 LTP-like plasticity was present only during the later stages of motor skill learning. These results were the first to demonstrate a temporal dissociation in the neurophysiological role the cerebellum and M1 play during skill learning, suggesting that the relative contributions of the different forms of motor learning may shift as learning occurs. Similarly, Uehara and others (2018) used the same physiological markers as the previous study, but while healthy participants performed a reaching visuomotor adaptation task known to engage error-based learning early on and reinforcement mechanisms as participants account for the perturbation (Shmuelof and others 2012). The authors showed that CBI changed early, but not in the later phase of adaptation, whereas occlusion of M1 LTP-like plasticity was present only late, but not early in learning. Collectively, the results from these studies using very different motsor tasks indicate that early on in learning, the cerebellar-dependent error-based process is weighted strongly to learn the task dynamics before the weight shifts to M1, incorporating other forms of learning (i.e., reward-based or use-dependent).
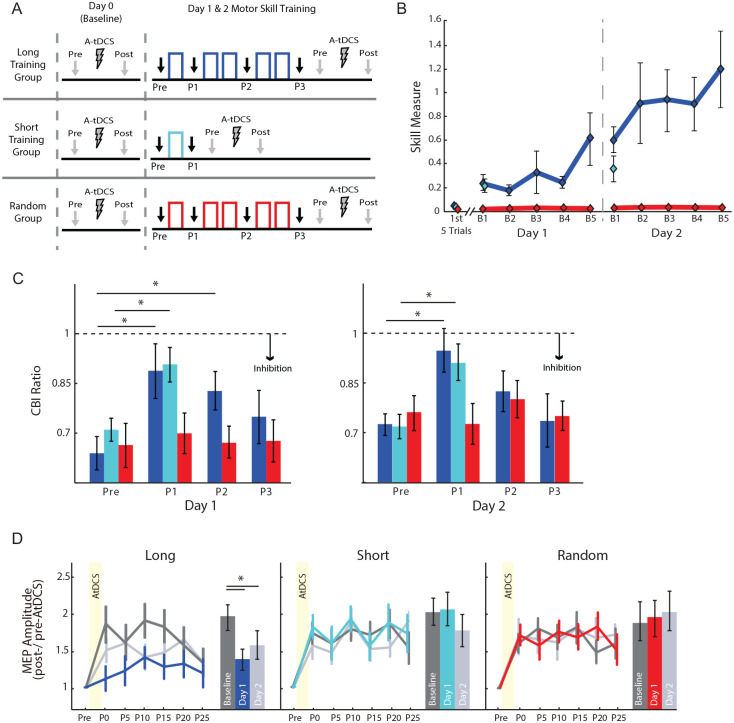
Figure 7.
Results from Spampinato and Celnik (2017), which investigated the temporal dynamics of two physiological mechanisms during motor skill learning. (A) The experimental design where individuals performed a sequence of movements by squeezing a pinch-force transducer to control the movement of an on-screen computer cursor. Individuals were split into three separate groups: Long (blue), Short (light blue), and Random (red). The Long and Random groups completed a total of 150 trials (5 blocks), whereas the Short group completed 1 block of 30 trials. Importantly, only the Long and Short groups had a consistent sensorimotor mapping between cursor movements and pinch-force production. Cerebellar inhibition (CBI) was assessed throughout training (Black arrows; Pre, P1, P2, P3), while occlusion was measured at the end of each day. (B) The skill performances for the Long (blue), Short (light blue), and Random (red) groups are presented for each block and day of training. Importantly, no learning is present in the random group (C) Cerebellar-M1 connectivity (CBI) changes throughout learning a de novo skill. The bar graphs show the mean CBI ratio on the y-axis for groups where learning occurred (blue and light blue) and for a random movement scenario (red). The x-axis represents different stimulation time points: before training (Pre), during (P1, P2) after the training session (P3). The authors show that only the training group (individuals capable of learning a new sensorimotor relationship) had a reduction in CBI (i.e., less inhibition). Specifically, the cerebellar physiological changes were prominent at the beginning of learning, which progressively returned toward baseline levels despite further skill improvement. (D) The AtDCS effects on M1 excitability for the long (blue), short (light blue) and random groups (red). The average MEP amplitude (standardized to the pretDCS MEP amplitude) is depicted on the y-axis and the x-axis represents successive TMS measurements taken prior to application of AtDCS (Pre), immediately after AtDCS (Post 1, P1) and repeated every 5 minutes up to 25 minutes post-AtDCS (P2, . . ., P6). Here, the authors show that in comparison to baseline (dark gray) occlusion only occurs after significant amount of learning occurs following a training session (i.e., Long group, blue and light gray). Overall, these results indicate that learning a new skill involves cerebellar-dependent processes early in learning; and as learning proceeds, M1-LTP-like plasticity can be observed, suggesting that other forms of learning are incorporated (e.g., reinforcement or use-dependent). Figures adapted from Spampinato and Celnik (2017); https://doi.org/10.1038/srep40715.
Interestingly, the neurophysiological findings follow similar results from several neuroimaging studies. For instance, changes in cerebellar activity with learning were described in the early stages of learning (Diedrichsen and others 2005a; Doyon and others 2003; Floyer-Lea and Matthews 2005; Hardwick and others 2013; Steele and Penhune 2010), whereas other studies have documented increases in the activity of striatum and motor cortex with extended training (Dayan and Cohen 2011; Floyer-Lea and Matthews 2005; Penhune and Steele 2012; Steele and Penhune 2010; Wiestler and Diedrichsen 2013). However, while examining the role and interactions of distinct brain regions throughout the different stages of motor skill learning is of great relevance, it is important to consider that many of the imaging studies mentioned above may have engaged many motor learning processes in order to master the skill. In other words, beyond considering the different stages of learning a motor skill, decomposing a particular skill into its different subcomponents (i.e., sensorimotor map and sequence) is critically important to disentangle the role of different motor learning processes and their respective neurophysiological mechanisms.
Consider the scenario of learning how to shoot a basketball. A coach may first have a trainee grip and hold the ball in a shooting position (i.e., understand how to control the ball/learn the weight of the ball) before instructing the sequence of movements to shoot the ball (e.g., the entire shooting motion and follow-through stroke). One recent study assessed the distinct physiological contributions of the cerebellum and M1 when participants learned distinct components of a motor skill. Here the investigators broke down the SVIPT into its two components: learning the sensorimotor map (or internal model) versus learning a sequence of movements (Spampinato and Celnik 2018). The authors found that acquiring the de novo map only modulated cerebellar excitability and not in M1. In contrast, learning a sequence of movements elicited changes in both CBI and LTP-like plasticity in M1; however, the cerebellar changes were only found during the early stages of learning (Fig. 8). These results suggest that the makeup of different motor skills and its components determine what the relative contributions of different brain regions are when learning the new skill. Moreover, the combined results of this section clearly show that the cerebellum, striatum, and motor cortical regions are all engaged in motor sequence learning, but that their contributions are not always confined to particular stages of learning depending on what specifically participants are asked to learn.
Figure 8.
Results from a study in which a motor skill task was broken down into distinct motor components (a sensorimotor mapping vs. sequence) while physiological changes were assessed. (A) The experimental design where individuals learned a logarithmic mapping between the movement of an onscreen cursor and pinch-force production. Participants were trained on this mapping for three days prior to introducing a skill version (i.e., integration of the logarithmic map and a sequence of movements). Cerebellar inhibition (CBI) was assessed throughout the map and skill training, while occlusion was measured at the end of each day. (B) Behavioral map and skill data. Overall, participants were able to learn the sensorimotor map as shown by a reduction in the time to reach successfully a target and improve their skill score with training. (C) CBI results. The y-axis depicts the CBI ratio and the x-axis represents different time points of map and skill learning. First, the authors show that CBI significantly reduces when learning the sensorimotor map. Interestingly, despite having knowledge of the mapping, CBI still reduces early on during skill learning. (D) The AtDCS effects on M1 excitability recorded at rest (Baseline Day) and following map and skill training. Here, when compared to baseline responses, significant occlusion was found only after skill training and not following sensorimotor map learning. (E) In another experiment, participants were placed in either Training or Random groups. The Training group was exposed to the same nine-element sequence throughout training, whereas the Random group received a randomized nine-element sequence order on each trial. (F) Sequence behavioral data. Only the participants from the Training group were able to learn as shown by a reduction of both sequence movement-time and response time to execute the sequence. (G) CBI results and (H) the AtDCS effects on M1 excitability for the Training and Random groups. The authors found that only learning a consistent sequence elicited changes in cerebellar excitability changes and resulted in occlusion of M1 LTP-like plasticity. Altogether these results indicate that a complex skill is learned by different forms of learning engaging distinct and specific neurophysiological mechanisms (Spampinato and Celnik 2018). Figure used with permission from Elsevier; https://doi.org/10.1016/j.cortex.2018.03.017.
Can We Use These Different Forms of Learning to Enhance Motor Recovery in Patients with Neurological Disease?
As previously discussed, learning and performing everyday tasks likely involves all the forms of learning discussed in this review. Therefore, if any of these forms of learning and their neural substrates remain intact after illness or neurological damage, they could be targeted to facilitate motor relearning in patients. For example, patients with cerebral stroke maintain the ability to adapt to environmental changes, likely through error-based learning, and are capable of showing aftereffects from adaptation leading to improved gait step-symmetry (Reisman and others 2007). Similarly, providing success- and failure-based reinforcement feedback to stroke patients enhances the rate of motor learning (Quattrocchi and others 2017). Specifically, patients who learned via reinforcement conditions were also faster to adapt their movements when exposed to a similar perturbation on a subsequent day, suggesting that reinforcement can have lasting beneficial effects on the rate of motor learning after stroke. Results from studies utilizing tDCS to modulate specific forms of learning have also provided some promising evidence for this technique to be implemented as a rehabilitation co-adjuvant aimed at enhancing motor function following cerebral stroke. For example, depending on the polarity applied to the cerebellar cortex, tDCS leads to changes in the rate of motor adaptation in locomotor (Jayaram and others 2012), visuomotor (Galea and others 2011), and force-related perturbations tasks (Herzfeld and others 2014). Importantly, these effects have also been described in older adults (Hardwick and Celnik 2014). Recent clinical studies have shown that targeting the cerebellum with brain stimulation improves gait and balance control in both stroke and ataxic patients (Benussi and others 2015; Koch and others 2019), providing an encouraging outlook for future translational research. While these results provide promising evidence for modulating specific brain regions, or learning mechanisms, during the training of clinically relevant tasks, future studies will need to address whether this approach results in substantial, meaningful gains. For example, while numerous studies have shown that it is possible to augment a specific learning process of constrained, reductionist tasks, this effect might have little impact when modulating a complex motor behavior that engages multiple forms or learning.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Danny Spampinato  https://orcid.org/0000-0001-8471-0859
https://orcid.org/0000-0001-8471-0859
References
- Abe M, Schambra H, Wassermann EM, Luckenbaugh D, Schweighofer N, Cohen LG. 2011. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr Biol 21:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–75. [DOI] [PubMed] [Google Scholar]
- Ammann C, Spampinato D, Márquez-Ruiz J. 2016. Modulating motor learning through transcranial direct-current stimulation: an integrative view. Front Psychol 7:1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann C, Lindquist MA, Celnik PA. 2017. Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimul 10:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. 2010. Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22:1917–30. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. 2011. Moving, sensing and learning with cerebellar damage. Curr Opin Neurobiol 21:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Koch G, Cotelli M, Padovani A, Borroni B. 2015. Cerebellar transcranial direct current stimulation in patients with ataxia: a double-blind, randomized, sham-controlled study. Mov Disord 12:1701–5. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. 2001. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport 12:1879–84. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, and others. 2000. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A 97:3661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, Doya K, and others. 2017. Consensus paper. Towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum 16:203–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero G, Tang B, O’Malley R, Salas R, Celnik P. 2013. Motor learning interference is proportional to occlusion of LTP-like plasticity. J Neurosci. 33:4634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero G, Spampinato D, Reis J, Ajagbe L, Thompson T, Kopal Kulkarni, and others. 2015. J Neurosci. 35:3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Borrel J. 1995. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 68:793–805. [DOI] [PubMed] [Google Scholar]
- Celnik P. 2015. Understanding and modulating motor learning with cerebellar stimulation. Cerebellum 14:171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. 2006. Encoding a motor memory in the older adult by action observation. Neuroimage 29:677–84. [DOI] [PubMed] [Google Scholar]
- Celnik P, Wbester B, Glasser D, Cohen LG. 2008. Effects of aciton observation on physical training after stroke. Stroke 39:1814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallet M. 1998. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–23. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. 1962. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol 5:436–52. [DOI] [PubMed] [Google Scholar]
- D’Angelo E. 2014. The organization of plasticity in the cerebellar cortex: from synapses to control. Prog Brain Res 210:31–58. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. 2004. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. 2011. Neuroplasticity subserving motor skill learning. Neuron 72:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. 2005. a. Neural correlates of reach errors. J Neurosci 25:9919–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Lehman SL, Ivry RB. 2005. b. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. J Neurophysiol 93:801–12. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. 2010. Use-dependent and error-based learning of motor behaviors. J Neurosci. 30:5159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Francis JT, Shadmehr R. 2003. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J Neurosci 23:9032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, and others. 2009. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. 2003. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41:252–62. [DOI] [PubMed] [Google Scholar]
- Dumas EM, Van den Bogaard SJ, Ruber ME, Reilman RR, Stout JC, Craufurd D, and others. 2012. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington’s disease. Hum Brain Mapp 33:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts PM, Posner MI. 1967. Human Performance. Brooks/Cole. [Google Scholar]
- Floyer-Lea A, Matthews PM. 2005. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol 94:512–8. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, and others. 2010. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. 2012. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Albert NB, Ditye T, Miall RC. 2010. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J Cogn Neurosci 22:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Celnik P. 2009. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol 102:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. 2009. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29:9115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Mallia E, Rothwell JC, Diedrichsen J. 2015. The dissociable effects of punishment and reward on motor learning. Nat Neurosci 18:597–602. [DOI] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. 2011. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21:1761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. 2010. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol 20:1869–74. [DOI] [PubMed] [Google Scholar]
- Gilbert PF, Thach WT. 1977. Purkinje cell activity during motor learning. Brain Res 128:309–28. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos, Bastian A, Cortes M, Davis NJ, Edwards DJ, and others 2016. Cerebellar transcranial direct current stimulation (ctDCS): a novel approach to understanding cerebellar function in health and disease. Nueroscientist 22:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, López-Alonso V, Cheeran B, Suppa A. 2017. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett 719:133330. [DOI] [PubMed] [Google Scholar]
- Guo L, Xiong H, Kim JI, Wu YW, Lalchandani RR, Cui Y, and others. 2015. Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson’s disease. Nat Neurosci 18:1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Krakauer JW. 2013. Model-based and model-free mechanisms of human motor learning. Adv Exp Med Biol 782:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerbeck U, Yousif N, Greenwood R, Rothwell JC, Diedrichsen J. 2014. Movement speed is biased by prior experience. J Neurophysiol 111:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Celnik PA. 2014. Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging 35:2217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Lesage E, Miall RC. 2014. Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimul 7:643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. 2013. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67:283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Galea JM, Casula EP, Falkai P, Bestmann S, Rothwell JC. 2013. Muscle and timing-specific functional connectivity between the dorsolateral prefrontal cortex and the primary motor cortex. J Cogn Neurosci 25:558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. 2018. Encoding of error and learning to correct that errorby the Purkinje cells of the cerebellum. Nat Neurosci 21:736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O’Shea J. 2014. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage 98:147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Luft AR. 2013. Dopaminergic meso-cortical projections to M1: role in motor learning and motor cortex plasticity Front Neurol 4:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. 2011. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci 31:2481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakaeur JW. 2011. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70:787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW, Morrison JH, Prikhozhan A, Sealfon SC. 1992. Localization of multiple dopamine receptor subtype mRNAs in human and monkey motor cortex and striatum. Brain Res Mol Brain Res 15:181–8. [DOI] [PubMed] [Google Scholar]
- Iwata NK, Ugawa Y. 2005. The effects of cerebellar stimulation on the motor cortical excitability in neurological disorders: a review. Cerebellum 4:218–23. [DOI] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. 2011. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol 7:e1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jax SA, Rosenbaum DA. 2007. Hand path priming in manual obstacle avoidance: evidence that the dorsal stream does not only control visually guided actions in real time. J Exp Psychol Hum Percept Perform 33:425–41. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik PA. 2011. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 21:1901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. 2012. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol 107:2950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Ziemann U, Hamada M, George MS, Quartarone A, Classen J, and others. 2015. Consensus paper. Probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul 8:442–54. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. 2003. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S, Mochizuki H, Moriya A, Nakatani-Enomoto S, Nakamura K, Hanajima R, and others. 2012. Ataxic hemiparesis: neurophysiological analysis by cerebellar transcranial magnetic stimulation. Cerebellum 11:259–63. [DOI] [PubMed] [Google Scholar]
- Kishore A, Joseph T, Velayudhan B, Popa T, Meunier S. 2012. Early, severe and bilateral loss of LTP and LTD-like plasticity in motor cortex (M1) in de novo Parkinson’s disease. Clin Neurophysiol 123:822–8. [DOI] [PubMed] [Google Scholar]
- Kishore A, Popa T, Balachandran A, Chandran S, Pradeep S, Backer F, and others. 2014. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson’s disease: clues from dyskinetic patients. Cereb Cortex 24:2055–67. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. 1998. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol 80:3321–5. [DOI] [PubMed] [Google Scholar]
- Koch G, Bonnì S, Casula EP, Iosa M, Paolucci S, Pellicciari MC, and others 2019. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol 2:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Esposito R, Motta C, Casula EP, Di Lorenzo F, Bonnì S, and others 2020. Improving visuo-motor learning with cerebellar theta burst stimulation: behavioral and neurophysiological evidence. Neuroimage 208:116424. [DOI] [PubMed] [Google Scholar]
- Koyama S, Tanaka S, Tanabe S, Sadato N. 2015. Dual-hemisphere transcranial direct current stimulation over primary motor cortex enhances consolidation of a ballistic thumb movement. Neurosci Lett 588:49–53. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P. 2011. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21:636–44. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. 2002. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125:2238–47. [DOI] [PubMed] [Google Scholar]
- Luft AR, Schwarz S. 2009. Dopaminergic signals in primary motor cortex. Int J Dev Neurosci 27:415–21. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. 1996. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119:1183–98. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. 2004. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol 91:230–8. [DOI] [PubMed] [Google Scholar]
- Mattar AA, Gribble PL. 2005. Motor learning by observing. Neuron 46:153–60. [DOI] [PubMed] [Google Scholar]
- Mawase F, Lopez D, Celnik PA, Haith AM. 2018. Movement repetition facilitate response preparation. Cell Rep 4: 801–8. [DOI] [PubMed] [Google Scholar]
- Mawase F, Uehara S, Bastian AJ, Celnik PA. 2017. Motor learning enhances use-dependent plasticity. J Neurosci 37:2673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. 2006. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. 2008. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci 11:1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Christensen LOD, Cain O, Sanley J. 2007. Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5:e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. [DOI] [PubMed] [Google Scholar]
- Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, and others. 2009. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 4:e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. 2006. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 129:1059–69. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, and others. 2003. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke F, Frommann K, and others. 2004. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci 19:2720–6. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3): 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. 2001. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–901. [DOI] [PubMed] [Google Scholar]
- Nixon PD, Passingham RE. 2000. The cerebellum and cognition: cerebellar lesions impair sequence learning but not conditional visuomotor learning in monkeys. Neuropsychologia 38:1054–72. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Criscimagna-Hemminger SE, Shadmehr R. 2011. Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cereb Cortex 21:1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ. 2006. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9:1404–11. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallet M. 1995. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acuisition of new fine motor skills. J Neurophsiol 74:1037–45. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Wassermann EM, Grafman J, Hallet M. 1996. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res 107:479–85. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Steele CJ. 2012. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav Brain Res 226:579–91. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Chen R. 2001. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res 140:505–10. [DOI] [PubMed] [Google Scholar]
- Polanía R, Nitsche MA, Ruff CC. 2018. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci 21:174–87. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. 1965. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28:166–85. [DOI] [PubMed] [Google Scholar]
- Quattrocchi G, Greenwood R, Rothwell JC, Galea JM, Bestmann S. 2017. Reward and punishment enhance motor adaptation in stroke. J Neurol Neurosurg Psychiatry 88:730–6. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, and others. 2009. Noninvasive cortical stimulation acquisition over multiple days through an effect on consolidation. PNAS 106:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Burdet E, Casadio M, Krakauer JW, Kwakkel G, Lang CE, and others. 2016. Computational neurorehabilitation: modeling plasticity and learning to predict recovery. J Neuroeng Rehabil 13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K. 2007. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130:1861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Donoghue JP, Dunaevsky A. 2007. Plasticity of the synaptic modification range. J Neurophysiol 98:3688–95. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. 2000. Learning-induced LTP in neocortex. Science. 290:533–6. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. 1998. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1:230–4. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Pekanovic A, Atiemo CO, Marshall J, Luft AR. 2015. Dopamine promotes motor cortex plasticity and motor skill learning via plc activation. Plos One 10:e0124986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan JG, Carhuatanta KA, McInturf SM, Jankord R. 2015. Modulating hippocampal plasticity with in vivo brain stimulation. J Neurosci 35:12824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman AV, Pasalar S, Johnson MT, Ebner TJ. 2005. Position, direction of movement, and speed tuning of cerebellar Purkinje cells during circular manual tracking in monkey. J Neurosci 25:9244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell JC. 2007. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci 27:12058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rroji O, van Kuyck K, Nuttin B, Wenderoth N. 2015. Anodal tDCS over the primary motor cortex facilitates long-term memory formation reflecting use-dependent plasticity. PLoS One 10:e0127270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Bastian AJ, Celnik PA. 2012. a. Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci. 32:11610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Ivry RB, Diedrichsen J. 2012. b. Encoding of sensory prediction errors in the human cerebellum. J Neurosci 32:4913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlerf JE, Galea JM, Spampinato D, Celnik PA. 2015. Laterality differences in cerebellar-motor cortex connectivity. Cereb Cortex 25:1827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. 2016. Dopamine reward prediction error coding. Dialog Clin Neurosci 18:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. 1997. Neural correlates of motor memory consolidation. Science 277:821–5. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. 2008. A computational neuroanatomy for motor control. Exp Brain Res 185:359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y, Hamada M, Hanajima R, Terao Y, Matsumoto H, Ohinami S, and others. 2010. Cerebellar dysfunction in progressive supranuclear palsy: a transcranial magnetic stimulation study. Mov Disord 14:2413–9. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW, Mazzoni P. 2012. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol 108:578–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, and others. 2004.Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24:3379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slachevsky A, Pillon B, Fourneret P, Pradat-Diehl P, Jeannerod M, Dubois B. 2001. Preserved adjustment but impaired awareness in a sensory-motor conflict following prefrontal lesions. J Cogn Neurosci 13:332–40. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. 2005. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol 93:2809–21. [DOI] [PubMed] [Google Scholar]
- Spampinato D, Celnik P. 2017. Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Sci Rep 7:40715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato D, Celnik P. (2018) Deconstructing skill learning and its physiological mechanisms. Cortex 104:90–102. [DOI] [PubMed] [Google Scholar]
- Spampinato DA, Block HJ, Celnik PA. 2017. Cerebellar-M1 connectivity changes associated with motor learning are somatotopic specific. J Neurosci 37:2377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato DA, Satar Z, Rothwell JC. 2019. Combining reward and M1 transcranial direct current stimulation enhances the rentention of newly learnt sensorimotor mappings. Brain Stimul 5:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato DA, Celnik PA, Rothwell JC. 2020. Cerebellar-motor cortex connectivity: one or two networks. J Neurosci 40:4230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. 2011. The role of GABA in human motor learning. Curr Biol 6:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CJ, Penhune VB. 2010. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci 30:8332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, and others. 2005. Formation of a motor memory by action observation. J Neurosci 25:9339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, and others. 2006. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex 16:376–85. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2011. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppa A, Marsili L, Belvisi D, Conte A, Lezzi E, Modugno N, and others. 2011. Lack of LTP-like plasticity in primary motor cortex in Parkinson’s disease. Exp Neurol 227: 296–301. [DOI] [PubMed] [Google Scholar]
- Sutton RG, Barto AG. 1998. An introduction to reinforcement learning. Cambridge, MA: MIT Press. [Google Scholar]
- Taylor JA, Ivry RB. 2014. Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog Brain Res. 210:217–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. 2014. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci. 34:3023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien AS, Wolpert DM, Bastian AJ. 2016. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain. 139:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. 2000. Learning of action through adaptive combination of motor primitives. Nature 407:742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. 2004. Interference of left and right cerebellar rTMS with procedural learning. J Cogn Neurosci 9:1605–11. [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Ferlazzo F, and others. 2011. Changes in cerebello-motor connectivity during procedural learning by actual execution and observation. J Cogn Neurosci 2:338–48. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. 2007. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98:54–62. [DOI] [PubMed] [Google Scholar]
- Uehara S, Mawase F, Celnik P. 2018. Learning similar actions by reinforcement or sensory-prediction errors rely on distinct physiological mechanisms. Cereb Cortex 28:3478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R. 1997. Magnetic stimulation over the cerbellum in patients with ataxia. Electroencephalogr Clin Neurophysiol 104:453–8. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. 1995. Magnetic stimulation over the cerebellum in humans. Ann Neurol 37:703–13. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Sabes PN. 2011. How each movement changes the next: an experimental and theoretical study of fast adaptive priors in reaching. J Neurosci 31:10050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestler T, Diedrichsen J. 2013. Skill learning strengthens cortical representations of motor sequences. Elife 9:00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. 2011. Principles of sensorimotor learning. Nat Rev Neurosci 12:739–51. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. 1995. An internal model for sensorimotor integration. Science 269:1880–2. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. 1998. Internal models in the cerebellum. Trends Cogn Sci 2:338–47. [DOI] [PubMed] [Google Scholar]
- Wu T, Kansaku K, Hallett M. 2004. How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol 91:1690–8. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, and others. 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462:915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. 2009. Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lisberger SG. 2014. Role of plasticity at different sites across the time course of cerebellar motor learning. J Neurosci 34:7077–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D. 2004. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci 24:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]