Summary
Background
Acalabrutinib is a selective, covalent Bruton tyrosine-kinase inhibitor with activity in chronic lymphocytic leukaemia. We compare the efficacy of acalabrutinib with or without obinutuzumab against chlorambucil with obinutuzumab in patients with treatment-naive chronic lymphocytic leukaemia.
Methods
ELEVATE-TN is a global, phase 3, multicentre, open-label study in patients with treatment-naive chronic lymphocytic leukaemia done at 142 academic and community hospitals in 18 countries. Eligible patients had untreated chronic lymphocytic leukaemia and were aged 65 years or older, or older than 18 years and younger than 65 years with creatinine clearance of 30–69 mL/min (calculated by use of the Cockcroft-Gault equation) or Cumulative Illness Rating Scale for Geriatrics score greater than 6. Additional criteria included an Eastern Cooperative Oncology Group performance status score of 2 or less and adequate haematologic, hepatic, and renal function. Patients with significant cardiovascular disease were excluded, and concomitant treatment with warfarin or equivalent vitamin K antagonists was prohibited. Patients were randomly assigned (1:1:1) centrally via an interactive voice or web response system to receive acalabrutinib and obinutuzumab, acalabrutinib monotherapy, or obinutuzumab and oral chlorambucil. Treatments were administered in 28-day cycles. To reduce infusion-related reactions, acalabrutinib was administered for one cycle before obinutuzumab administration. Oral acalabrutinib was administered (100 mg) twice a day until progressive disease or unacceptable toxic effects occurred. In the acalabrutinib-obinutuzumab group, intravenous obinutuzumab was given on days 1 (100 mg), 2 (900 mg), 8 (1000 mg), and 15 (1000 mg) of cycle 2 and on day 1 (1000 mg) of cycles 3–7. In the obinutuzumab-chlorambucil group, intravenous obinutuzumab was given on days 1 (100 mg), 2 (900 mg), 8 (1000 mg), and 15 (1000 mg) of cycle 1 and on day 1 (1000 mg) of cycles 2–6. Oral chlorambucil was given (0·5 mg/kg) on days 1 and 15 of each cycle, for six cycles. The primary endpoint was progression-free survival between the two combination-therapy groups, assessed by independent review committee. Crossover to acalabrutinib was allowed in patients who progressed on obinutuzumab-chlorambucil. Safety was assessed in all patients who received at least one dose of treatment. Enrolment for this trial is complete, and the study is registered at ClinicalTrials.gov, NCT02475681.
Findings
Between Sept 14, 2015, and Feb 8, 2017, we recruited 675 patients for assessment. 140 patients did not meet eligibility criteria, and 535 patients were randomly assigned to treatment. 179 patients were assigned to receive acalabrutinib-obinutuzumab, 179 patients were assigned to receive acalabrutinib monotherapy, and 177 patients were assigned to receive obinutuzumab-chlorambucil. At median follow-up of 28·3 months (IQR 25·6–33·1), median progression-free survival was longer with acalabrutinib-obinutuzumab and acalabrutinib monotherapy, compared with obinutuzumab-chlorambucil (median not reached with acalabrutinib and obinutuzumab vs 22·6 months with obinutuzumab, hazard ratio [HR] 0·1; 95% CI 0·06–0·17, p<0·0001; and not reached with acalabrutinib monotherapy vs 22·6 months with obinutuzumab, 0·20; 0·13–0·3, p<0·0001). Estimated progression-free survival at 24 months was 93% with acalabrutinib-obinutuzumab (95% CI 87–96%), 87% with acalabrutinib monotherapy (81–92%), and 47% with obinutuzumab-chlorambucil (39–55%). The most common grade 3 or higher adverse event across groups was neutropenia (53 [30%] of 178 patients in the acalabrutinib-obinutuzumab group, 17 [10%] of 179 patients in the acalabrutinib group, and 70 [41%] of 169 patients in the obinutuzumab-chlorambucil group). All-grade infusion reactions were less frequent with acalabrutinib-obinutuzumab (24 [14%] of 178 patients) than obinutuzumab-chlorambucil (67 [40%] of 169 patients). Grade 3 or higher infections occurred in 37 (21%) patients given acalabrutinib-obinutuzumab, 25 (14%) patients given acalabrutinib monotherapy, and 14 (8%) patients given obinutuzumab-chlorambucil. Deaths occurred in eight (5%) patients given acalabrutinib-obinutuzumab, 12 (7%) patients given acalabrutinib, and 15 (9%) patients given obinutuzumab-chlorambucil.
Interpretation
Acalabrutinib with or without obinutuzumab significantly improved progression-free survival over obinutuzumab-chlorambucil chemoimmunotherapy, providing a chemotherapy-free treatment option with an acceptable side-effect profile that was consistent with previous studies. These data support the use of acalabrutinib in combination with obinutuzumab or alone as a new treatment option for patients with treatment-naive symptomatic chronic lymphocytic leukaemia.
Funding
Acerta Pharma, a member of the AstraZeneca Group, and R35 CA198183 (to JCB).
Introduction
Chronic lymphocytic leukaemia is a B-cell malignancy that is usually considered incurable. This disease usually occurs in older patients and has a widely variable disease course. Although chemoimmunotherapy and CD20 antibodies as first-line treatment have greatly improved outcomes,1–5 evidence2,6,7 indicates a benefit of non-chemotherapeutic approaches targeting Bruton tyrosine-kinase (BTK) with ibrutinib, or BCL-2 with venetoclax, reporting superior outcomes compared with chemoimmunotherapy as first-line ther apy. Four randomised studies6–9 assessed ibrutinib alone or with a CD20 antibody in patients with treatment-naive chronic lymphocytic leukaemia, and reported better progression-free survival than with chemoimmunother apy (comparator groups given chlorambucil, chlorambucil-obinutuzumab, bendamustine-rituximab, or fludarabine-cyclophosphamide-rituximab).
Studies combining rituximab with ibrutinib did not report improvement in progression-free survival over ibrutinib monotherapy.6,10 Obinutuzumab mediates superior antibody-dependent cellular cytotoxicity compared with rituximab,11 and was shown to be more efficacious than rituximab among patients with chronic lymphocytic leukaemia in a randomised controlled trial.5 Although obinutuzumab-ibrutinib treatment has shown better efficacy than chlorambucil-obinutuzumab,8 the benefit of adding obinutuzumab to ibrutinib or another BTK inhibitor for patients with untreated chronic lymphocytic leukaemia has not been studied in a trial with BTK inhibitor monotherapy.
Acalabrutinib is a selective, covalent BTK inhibitor with minimal activity against alternative targets, which is approved by the FDA for the treatment of adults with chronic lymphocytic leukaemia.12–14 Acalabrutinib has shown efficacy alone or in combination with obinutuzumab in phase 1–2 studies15,16 in patients with untreated chronic lymphocytic leukaemia (proportion of patients with overall response 95–97%), with durable remissions, favourable long-term tolerability, and low discontinuation rates. The phase 3 ELEVATE-TN study was designed to compare the efficacy and safety of acalabrutinib-obinutuzumab or acalabrutinib monotherapy with obinutuzumab-chlorambucil to ascertain if acalabrutinib with or without obinutuzumab had a therapeutic advantage over chemoimmunotherapy in patients with untreated chronic lymphocytic leukaemia.
Methods
Study design and participants
ELEVATE-TN is a phase 3, randomised, multicentre, open-label study done at 142 academic and community hospitals in 18 countries (appendix p 3). Eligible patients had untreated chronic lymphocytic leukaemia (also called treatment-naive chronic lymphocytic leukaemia) requiring treatment per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria.17 Participants were aged 65 years or older, or older than 18 years and younger than 65 years with comorbidities (creatinine clearance of 30–69 mL/min calculated by use of the Cockcroft-Gault equation or Cumulative Illness Rating Scale for Geriatrics score >6).18 Additional criteria included an Eastern Cooperative Oncology Group performance status (ECOG PS) score 2 or less and adequate haematologic, hepatic, and renal function. Patients with significant cardiovascular disease were excluded, and concomitant treatment with warfarin or equivalent vitamin K antagonists was prohibited (appendix p 10). The independent review and ethics committees of all participating institutions approved the protocol. All patients provided written informed consent. The study was done according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice.
Randomisation and masking
Patients were randomly assigned (1:1:1) via a centralised interactive voice and web response system to receive acalabrutinib-obinutuzumab, acalabrutinib monotherapy, or obinutuzumab-chlorambucil. Patients were stratified based on the presence or absence of del(17)(p13·1), ECOG PS score (0–1 vs 2), and geographic region (North America, western Europe, or other). Patients and investigators were not masked to treatment. An independent data monitoring committee periodically reviewed safety data and efficacy results in the planned interim analysis. A masked independent review committee (IRC) assessed progression and response data. The study sponsor did not do any aggregated analyses by treatment group until after the IRC had been unmasked.
Procedures
Treatments were administered in 28-day cycles. Oral acalabrutinib was administered (100 mg) twice a day until progressive disease or unacceptable toxic effects occurred. To reduce infusion-related reactions, acalabrutinib was given for one cycle before obinutuzumab.19 Obinutuzumab and chlorambucil were given for six cycles. In the acalabrutinib-obinutuzumab group, intravenous obinutuzumab was given on days 1 (100 mg), 2 (900 mg), 8 (1000 mg), and 15 (1000 mg) of cycle 2 and on day 1 (1000 mg) of cycles 3–7. In the obinutuzumab-chlorambucil group, intravenous obinutuzumab was given on days 1 (100 mg), 2 (900 mg), 8 (1000 mg), and 15 (1000 mg) of cycle 1 and on day 1 (1000 mg) of cycles 2–6. Oral chlorambucil was given (0·5 mg/kg) on days 1 and 15 of each cycle.
If patients in the obinutuzumab-chlorambucil group had IRC-supported disease progression, they were able to cross over to receive acalabrutinib monotherapy. Dose modifications of acalabrutinib and chlorambucil were allowed for management of adverse events (AEs; appendix p 11). The study investigators withdrew patients in the case of consent withdrawal, loss to follow-up, or death.
To track disease progression, study investigators gave patients an MRI or CT scan with contrast dye at baseline, every 12 weeks until cycle 25, and every 24 weeks thereafter until disease progression occurred.
Baseline assessments at screening included central analysis of genomic aberrations with fluorescence in situ hybridisation (FISH), mutational analysis of the immunoglobulin heavy-chain variable-region (IGHV) and cellular antigen tumour protein p53 genes (TP53) by DNA sequencing, and evaluation of lymph-node size by physical assessment and CT or MRI. A central laboratory tested peripheral blood samples obtained from all patients at baseline. FISH probes were used for cytogenetic profiling, and testing assessed abnormalities in chromosomes 13q, 12, 11q, and 17p (Vysis CLL FISH Probe Kit; Abbott Molecular). For IGHV mutation, standard Sanger sequencing was used; assay sensitivity was 10% and the cutoff was 2%. TP53 mutations were analysed by use of the Sanger sequencing methods.20,21 If locally assessed FISH results supporting the status of del(17)(p13·1) before randomisation were available, these could be used only for stratification purposes. Complex karyotype was defined as 3 or more cytogenetic abnormalities based on karyotyping by the central laboratory. Bulky disease was defined as any mass 5 cm or larger. Minimal residual disease was assessed by multicolour flow cytometry in patients with investigator-assessed complete response or complete response with incomplete bone marrow recovery. The European Research Initiative in chronic lymphocytic leukaemia method that was previously described was used for minimal residual disease assessment.22 Peripheral blood or bone marrow samples were collected in sodium heparin tubes and shipped at ambient temperature to the central laboratory for analysis of minimal residual disease with a cutoff of <10–4 (0·01%) for undetectable minimal residual disease.
Outcomes
The primary endpoint was IRC-assessed progression-free survival, defined as the time from randomisation until disease progression by use of iwCLL 2008 criteria, or death; isolated treatment-related lymphocytosis in the absence of other evidence of disease progression was not considered to indicate progressive disease (appendix p 13).17,23 Patients who did not meet these criteria and were alive by the analysis data cutoff date were censored (detailed censoring rules are specified in the statistical analysis plan). Comparison of progression-free survival between acalabrutinib-obinutuzumab and obinutuzumab-chlorambucil was the primary endpoint, and comparison of IRC-assessed progression-free survival between acalabrutinib monotherapy and obinutuzumab-chlorambucil was a secondary endpoint. Additional secondary endpoints included investigator-assessed progression-free survival, IRC and investigator-assessed overall response (the proportion of patients with complete response, complete response with incomplete bone marrow recovery, nodular partial response, or partial response), time to next treatment (defined as time from random assignment to institution of non–protocol-specified treatment for chronic lymphocytic leukaemia, first dose of acalabrutinib monotherapy for patients in the obinutuzumab-chlorambucil group who crossed over, or death), and overall survival (defined as time from random assignment until death due to any cause). Safety was assessed by reported and observed AEs, laboratory measurements, and clinical evaluation across the treatment-emergent period, which was defined as the date of the first dose until 30 days after the date of the last dose of study drug or the date a patient started a new anticancer therapy for chronic lymphocytic leukaemia, whichever was earliest. AEs were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Exploratory outcomes included investigator-assessed progression-free survival and overall response, IRC and investigator-assessed overall response and partial response with lymphocytosis, proportion of patients with undetectable minimal residual disease, improvement in disease-related symptoms, patient-reported outcomes (data not shown), sustained haematological improvement, and medical resource use (data not shown). A post-hoc analysis compared progression-free survival of acalabrutinib-obinutuzumab and acalabrutinib monotherapy.
Statistical analysis
Sample size was calculated assuming a hazard ratio (HR) between the acalabrutinib-obinutuzumab and obinutuzumab-chlorambucil groups of 0·60 for IRC-assessed progression-free survival events. Under the assumption that progression-free survival events would follow exponential distribution, and based on a 2-sided log-rank test (α=0·05), 167 events at final analysis would provide approximately 90% power. One interim analysis was planned when approximately 111 IRC-assessed progression-free survival events had occurred (ie, 67% of the planned events for the final analysis) or when 24 months had elapsed since the last patient was randomly assigned (timed analysis). Based on the timed analysis, the trial met its primary endpoint at the data cutoff (Feb 8, 2019) for the interim analysis, and the independent data monitoring committee recommended the trial be analysed for superior efficacy. Time-to-event endpoints were estimated by use of the Kaplan–Meier method. HRs were calculated by use of Cox proportional modelling, stratified by randomisation stratification factors, and compared by use of a log-rank test. Additional details are provided in the appendix (p 15).
Exploratory prespecified subgroup analyses of efficacy outcomes by baseline and disease characteristics were also done for the primary (acalabrutinib-obinutuzumab vs obinutuzumab-chlorambucil) and secondary (acalabrutinib monotherapy vs obinutuzumab-chlorambucil) comparisons. Comparisons between acalabrutinib-obinutuzumab and acalabrutinib monotherapy were post-hoc analyses. Efficacy was analysed in the intention-to-treat population according to the randomly assigned treatment group. Safety was analysed in all patients who received at least one dose of any study medication. This study is registered with ClinicalTrials.gov, NCT02475681.
Role of the funding source
Acerta Pharma, a member of the AstraZeneca Group, sponsored the study and was involved in the study design and data analyses with the lead investigators. Trial investigator teams collected the data. All authors had full access to the data and analyses and had final responsibility for the decision to submit for publication.
Results
From Sept 14, 2015, to Feb 8, 2017, 675 patients were assessed for eligibility. 140 patients did not meet the eligibility criteria, and 535 eligible patients were randomly assigned to receive acalabrutinib-obinutuzumab (179), acalabrutinib monotherapy (179), or obinutuzumab-chlorambucil (177; figure 1). Baseline demographic and disease characteristics were similar between groups (table 1; appendix p 22). Median age was 70 years (IQR 66–75), and 448 (84%) of 535 patients were aged 65 years or older. Chronic lymphocytic leukaemia international prognostic index score was high risk in 368 (69%) of 535 patients, and very high risk in 66 (12%) of 535 patients.24 Del(17)(p13·1) was present in 49 (9%) patients, del(11)(q22·3) was present in 95 (18%) patients, TP53 mutation was present in 61 (11%) patients, unmutated IGHV was present in 338 (63%) patients, and 92 (17%) patients had complex karyotype.
Figure 1: Trial profile.

The safety population included all randomly assigned patients who received at least one dose of study medication with patients grouped according to the actual treatment received. In the safety population, 178 patients received acalabrutinib-obuinutuzumab, 179 patients received acalabrutinib monotherapy, and 169 patients received obinutuzumab-chlorambucil. 12 patients did not meet eligibility criteria of being younger than 65 years and having a Cumulative Illness Rating Scale for Geriatrics score over 6 or creatine clearance of 30–69 mL/min. *The patient was randomly assigned but subsequently found to have mantle cell lymphoma. †Due to anaemia and pneumonia. ‡Risk of bleeding while taking aspirin and clopidogrel because of a non-ST myocardial infarction requiring a stent. §Due to grade 4 thrombocytopenia, followed by identification of an intestinal mass and subsequent intestinal perforation.
Table 1:
Characteristics of the patients at baseline, intention-to-treat population
| Acalabrutinib-obinutuzumab (n=179) | Acalabrutinib monotherapy (n=179) | Obinutuzumab-chlorambucil (n=177) | |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 70·0 (65·0–75·0) | 70·0 (66·0–75·0) | 71·0 (67·0–76·0) |
| ≥75 | 53 (29·6%) | 50 (27·9%) | 52 (29·4%) |
| ≥65 | 144 (80·4%) | 151 (84·4%) | 153 (86·4%) |
| <65* | 35 (19·6%) | 28 (15·6%) | 24 (13·6%) |
| Creatinine clearance 30–69 mL/min† | 2 (1·1%) | 4 (2·2%) | 7 (4·0%) |
| CIRS-G >6† | 30 (16·8%) | 21 (11·7%) | 15 (8·5%) |
| Any of the above† | 31 (17·3%) | 24 (13·4%) | 20 (11·3%) |
| Sex | |||
| Female | 68 (38·0%) | 68 (38·0%) | 71 (40·1%) |
| Male | 111 (62·0%) | 111 (62·0%) | 106 (59·9%) |
| ECOG PS | |||
| 0–1 | 169 (94·4%) | 165 (92·2%) | 167 (94·4%) |
| 2 | 10 (5·6%) | 14 (7·8%) | 10 (5·6%) |
| CLL-IPI score | |||
| 0–1 (low risk) | 9 (5·0%) | 4 (2·2%) | 5 (2·8%) |
| 2–3 (intermediate risk) | 27 (15·1%) | 18 (10·1%) | 25 (14·1%) |
| 4–6 (high risk) | 115 (64·2%) | 134 (74·9%) | 119 (67·2%) |
| 7–10 (very high risk) | 23 (12·8%) | 20 (11·2%) | 23 (13·0%) |
| Rai stage | |||
| 0 | 3 (1·7%) | 0 | 1 (0·6%) |
| I | 54 (30·2%) | 48 (26·8%) | 50 (28·2%) |
| II | 36 (20·1%) | 44 (24·6%) | 48 (27·1%) |
| III | 48 (26·8%) | 50 (27·9%) | 40 (22·6%) |
| IV | 38 (21·2%) | 37 (20·7%) | 38 (21·5) |
| High-risk features | |||
| Chromosome 17p13·1 deletion | 17 (9·5%) | 16 (8·9%) | 16 (9·0%) |
| Chromosome 11q22·3 deletion | 31 (17·3%) | 31 (17·3%) | 33 (18·6%) |
| Unmutated IGHV | 103 (57·5%) | 119 (66·5%) | 116 (65·5%) |
| Mutated TP53 | 21 (11·7%) | 19 (10·6%) | 21 (11·9%) |
| Complex karyotype | 29 (16·2%) | 31 (17·3%) | 32 (18·1%) |
| Including chromosome 17p13·1 deletion | 8 (4·5%) | 8 (4·5%) | 7 (4·0%) |
| Without chromosome 17p13·1 deletion | 21 (11·7%) | 23 (12·8%) | 25 (14·1%) |
| Chromosome 17p13·1 deletion and/or mutated TP53 | 25 (14·0%) | 23 (12·8%) | 25 (14·1%) |
| Chromosome 17p13·1 deletion and mutated TP53 | 13 (7·3%) | 12 (6·7%) | 12 (6·8%) |
| Any cytopenia at baseline | 93 (52·0%) | 85 (47·5%) | 77 (43·5%) |
| Haemoglobin ≤11·0 g/dL | 67 (37·4%) | 68 (38·0%) | 69 (39·0%) |
| Platelet count ≤100 000/μL | 44 (24·6%) | 33 (18·4%) | 34 (19·2%) |
| Absolute neutrophil count ≤1500 μL | 9 (5·0%) | 10 (5·6%) | 5 (2·8%) |
| CIRS-G score‡ | |||
| n, median (IQR) | 117, 6·0 (3·0–8·0) | 115, 6·0 (3·0–8·0) | 118, 5·5 (4·0–8·0) |
| Creatinine clearance (mL/min) | |||
| Median (IQR) | 76·5 (59·0–92·5) | 75·0 (58·0–98·0) | 70·0 (55·0–90·0) |
| <60 mL/min | 45 (25·1%) | 48 (26·8%) | 56 (31·6%) |
| Time from initial diagnosis (months) | |||
| Median (IQR) | 30·5 (9·4–70·7) | 24·4 (7·0–70·3) | 30·7 (9·4–64·2) |
Data are n (%) unless otherwise specified. CIRS-G=Cumulative Illness Rating Scale for Geriatrics. CLL-IPI=chronic lymphocytic leukaemia international prognostic index. ECOG PS=Eastern Cooperative Oncology Group performance status. IGHV=immunoglobulin heavy-chain variable gene. TP53=cellular tumour antigen p53 gene.
Twelve patients did not meet eligibility criteria of being younger than 65 years and having a CIRS-G score higher than 6 or creatinine clearance of 30–69 mL/min.
Percentages are for the total population in the treatment group.
CIRS-G reporting was not required for all patients.
At data cutoff for the interim analysis on Feb 8, 2019 (based on a timed analysis after 24 months, as the required number of events was not met [107 of 111]), treatment was ongoing in 142 (79%) of 179 patients in each of the acalabrutinib-containing groups, with a median relative dose intensity of over 98% (figure 1 and appendix p 23). 137 (77%) of 177 patients completed six cycles of obinutuzumab-chlorambucil, and 163 (91%) patients completed six cycles of obinutuzumab in the acalabrutinib-obinutuzumab group. 55 (31%) of 177 patients assigned to obinutuzumab-chlorambucil required additional therapy, with 45 (82%) of 55 patients crossing over to receive acalabrutinib monotherapy.
After a median follow-up of 28·3 months (IQR 25·6–33·1), median progression-free survival was significantly longer with acalabrutinib-obinutuzumab (not reached, 95% CI not evaluable–not evaluable) than with obinutuzumab-chlorambucil (22·6 months, 20·2–27·6), with a 90% reduction in relative risk of progression or death with acalabrutinib-obinutuzumab (HR 0·10, 0·06–0·17; p<0·0001, figure 2A). Median progression-free survival was significantly longer with acalabrutinib monotherapy (not reached, range 34·2–not evaluable) versus obinutuzumab-chlorambucil (22·6, 95% CI 20·2–27·6) HR 0·20, 95% CI 0·13–0·30; p<0·0001). Investigator-assessed progression-free survival is shown in appendix (p 16). Estimated progression-free survival at 24 months was 93% with acalabrutinib-obinutuzumab (95% CI 87–96%) and 87% with acalabrutinib monotherapy (81–92%) versus 47% with obinutuzumab-chlorambucil (39–55%). The HR for progression-free survival between acalabrutinib-obinutuzumab and acalabrutinib monotherapy was 0·49 (95% CI 0·26–0·95, post-hoc analysis). Among the patients with disease progression or death, the median time to event was 12·7 months (IQR 8·3–20·2) for 14 patients receiving acalabrutinib-obinutuzumab, 13·9 months (5·7–23·4) for 26 patients receiving acalabrutinib monotherapy and 16·4 months (11·8–21·0) for 93 patients receiving obinutuzumab-chlorambucil.
Figure 2: Progression-free survival, response rate, and overall survival per independent review committee assessment.

(A) Kaplan–Meier estimates of progression-free survival (primary endpoint). (B) Best response. (C) Kaplan–Meier estimates of overall survival. All assessments were done by independent review committee and all analyses were done in the intention-to-treat population. NE=not evaluable. NR=not reached. *Six (3%) patients had unknown response, and one (1%) patient had a response of non-progressive disease, defined as not having adequate CT or MRI data and not meeting criteria for progressive disease by physical examination. †Two (1%) patients had partial response with lymphocytosis, three (2%) patients had progressive disease, 12 (7%) patients had unknown response, and one patient’s response was not evaluable. ‡Two (1%) patients had non-progressive disease, 12 (7%) patients had an unknown response, one (1%) patient had no evaluable disease, and eight (5%) patients’ responses were not evaluable.
In prespecified subgroup analyses, there was a consistent benefit in favour of acalabrutinib-obinutuzumab or acalabrutinib monotherapy over obinutuzumab-chlorambucil (figure 3). Estimated median 24-month progression-free survival was longer in patients treated with acalabrutinib-obinutuzumab than in patients treated with obinutuzumab-chlorambucil in patients with unmutated IGHV (acalabrutinib-obinutuzumab 91%, 95% CI 83–95%; 103 patients vs obinutuzumab-chlorambucil 31%, 22–40%; 116 patients), in patients with mutated IGHV (acalabrutinib-obinutuzumab 96%, 87–99%; 74 patients vs obinutuzumab-chlorambucil 76%, 61–86%; 59 patients), in patients with del(17)(p13·1) (acalabrutinib-obinutuzumab 88%, 61–97%; 17 patients vs obinutuzumab-chlorambucil 22%, 5–45%; 16 patients), in patients with del(11)(q22·3) (acalabrutinib-obinutuzumab 87%, 68–95%; 31 patients vs obinutuzumab-chlorambucil 24%, 11–41%; 33 patients), in patients with bulky disease (acalabrutinib-obinutuzumab 90%, 76–96%; 46 patients vs obinutuzumab-chlorambucil 28%, 16–42%; 55 patients), and in patients with mutated TP53 (acalabrutinib-obinutuzumab 95%, 70–99%; 21 patients vs obinutuzumab-chlorambucil 19%, 5–41%; 21 patients, figure 3 and figure 4).
Figure 3: Prespecified subgroup analyses of progression-free survival for acalabrutinib and obinutuzumab versus obinutuzumab-chlorambucil and acalabrutinib monotherapy versus obinutuzumab-chlorambucil.

IGHV=immunoglobulin heavy-chain variable gene. NE=not evaluable. TP53=cellular tumour antigen p53 gene. ECOG PS=Eastern Cooperative Oncology Group performance status. Forest plot showing progression-free survival as assessed by an independent review committee analysed by prespecified subgroups according to baseline demographic and clinical characteristics. The 95% CI was based on exact binomial distribution. For bulky disease, the prespecified subgroup of 5 cm or larger is presented. For 10 cm or larger (the iwCLL definition) the hazard ratio for acalabrutinib-obinutuzumab versus obinutuzumab-chlorambucil was 0·11 (95% CI 0·06–0·19) for smaller than 10 cm and NE for 10 cm or larger, and for acalabrutinib monotherapy versus obinutuzumab-chlorambucil was 0·18 (0·11–0·30) for smaller than 10 cm and 0·22 (0·07–0·21) for 10 cm or larger.
Figure 4: Kaplan–Meier curves of independent review committee–assessed progression-free survival for patients with high-risk genomic features and high-risk features.
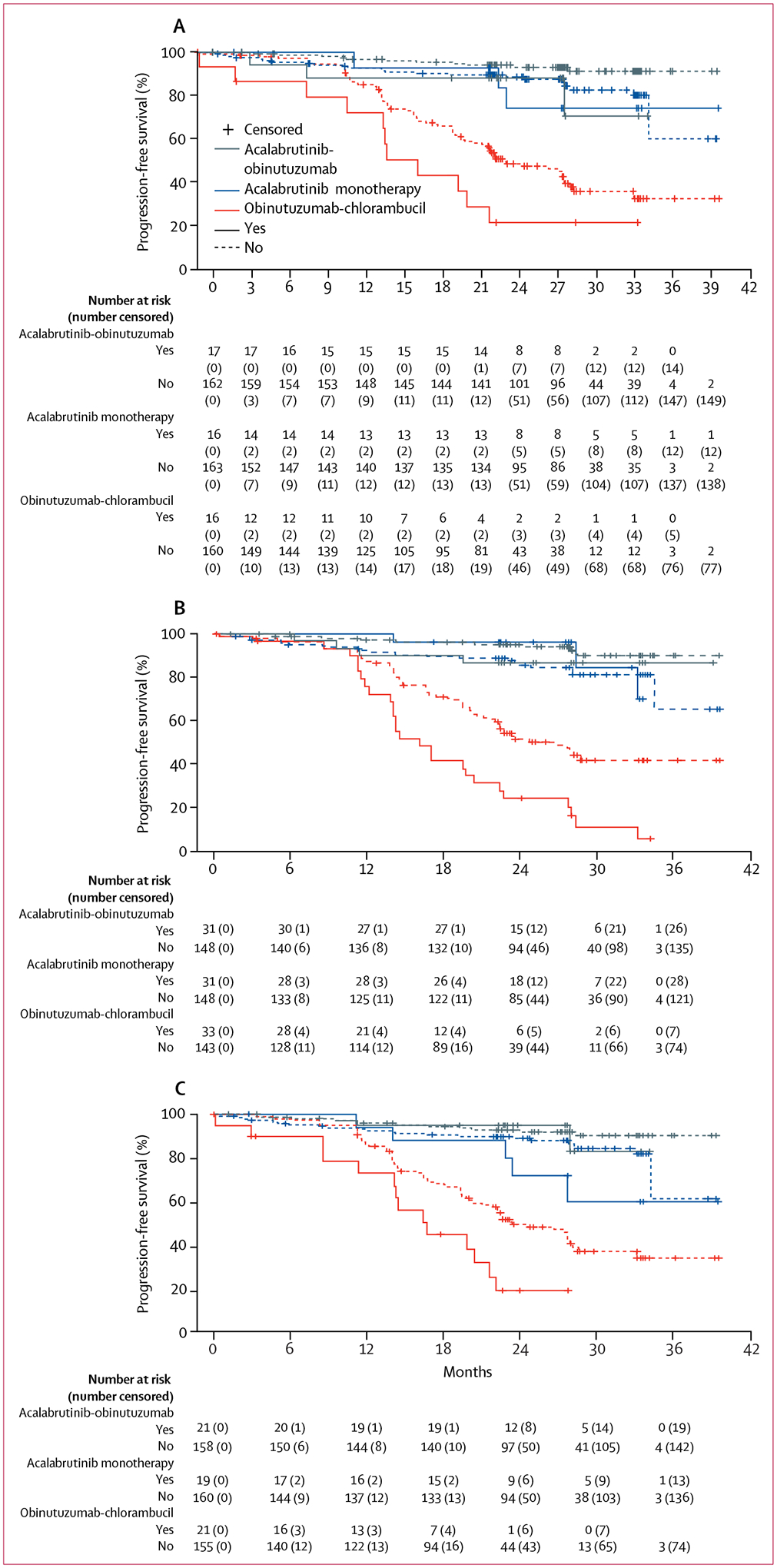
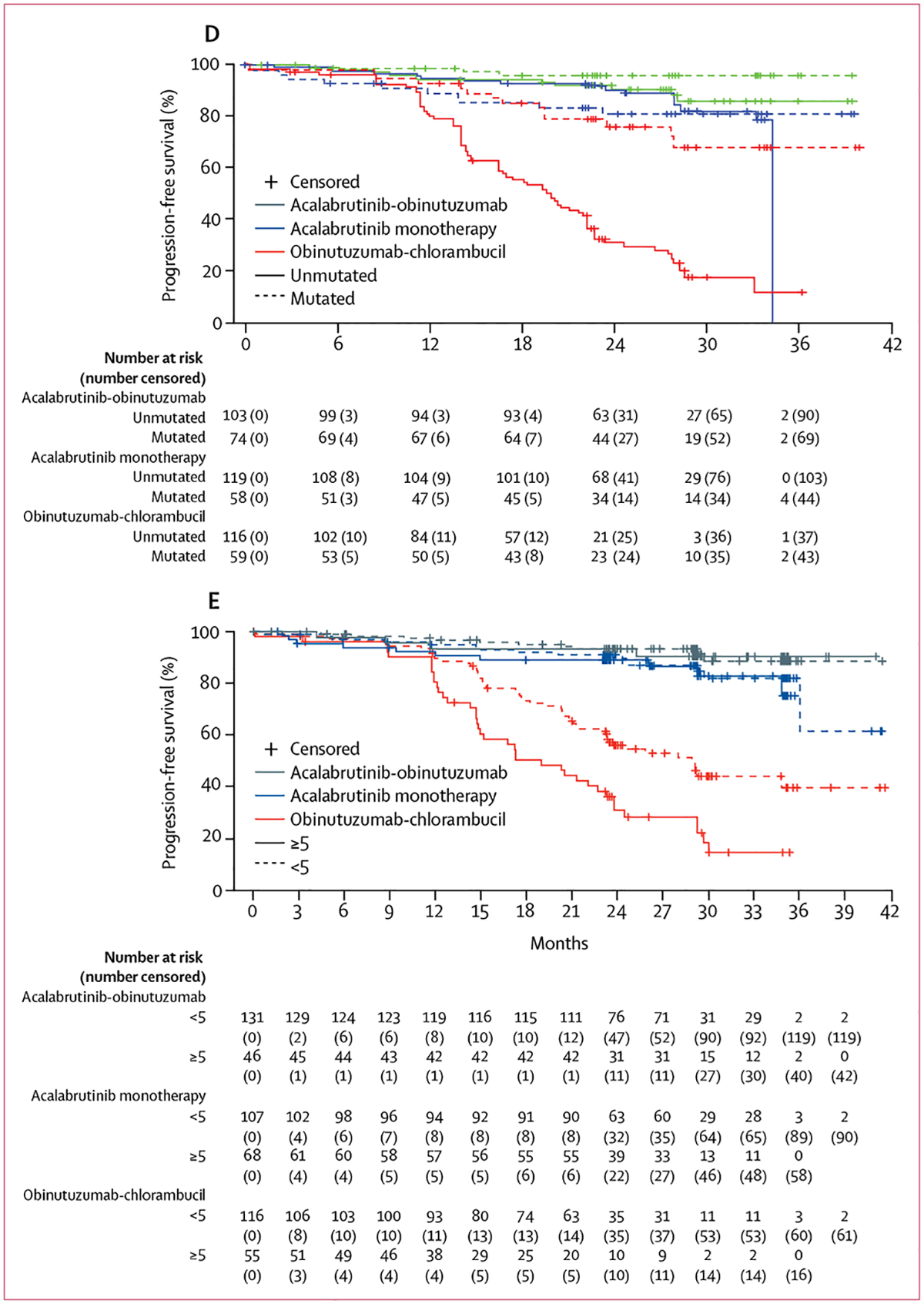
(A) Del(17)(p13·1). (B) Del(11)(q22·3). (C) Cellular tumour antigen p53 gene mutation. (D) Unmutated immunoglobulin heavy-chain variable gene. (E) Bulky disease.
The best overall response rate was significantly better with acalabrutinib-obinutuzumab (94%, 95% CI 89–97%) versus obinutuzumab-chlorambucil (79%, 72–84%; p<0·0001, figure 2B). Overall response was 86% for acalabrutinib monotherapy (95% CI 80–90%, p=0·08 vs obinutuzumab-chlorambucil). More patients had IRC-assessed complete response (including complete response with incomplete bone marrow recovery) with acalabrutinib-obinutuzumab (24 [13%] of 179 patients) than with obinutuzumab-chlorambucil (8 [5%] of 177 patients). One (1%) of 179 patients had complete response with acalabrutinib monotherapy. Results of investigator-assessed overall response were similar to IRC-assessed overall responses (appendix p 16). In patients who had investigator-assessed complete response or complete response with incomplete bone marrow recovery, rates of undetectable minimal residual disease were similar between the acalabrutinib-obinutuzumab and obinutuzumab-chlorambucil groups in peripheral blood (21 [49%] of 43 patients in the acalabrutinib-obinutuzumab group vs 14 [61%] of 23 patients in the obinutuzumab-chlorambucil group) or bone marrow (11 [26%] of 43 patients in the acalabrutinib-obinutuzumab group vs 5 [22%] of 23 patients in the obinutuzumab-chlorambucil group), and only one (7%) of 14 patients in the acalabrutinib monotherapy group had undetectable minimal residual disease in peripheral blood by flow cytometry (appendix pp 17, 24). Although minimal residual disease samples were collected only for patients with complete response or complete response with incomplete bone marrow recovery, 24 (13%) of 179 patients in the acalabrutinib-obinutuzumab group versus 14 (8%) of 177 patients in the obinutuzumab-chlorambucil group had undetectable minimal residual disease and a complete response with or without incomplete bone marrow recovery. Improvements in haematologic parameters from baseline (haemoglobin, absolute neutrophil count, and platelet count) were greater in the acalabrutinib-containing groups than in the obinutuzumab-chlorambucil group (appendix p 18).
The median overall survival was not reached in any group (acalabrutinib-obinutuzumab vs obinutuzumab-chlorambucil HR 0·47, 95% CI 0·21–1·06; p=0·06, acalabrutinib monotherapy vs obinutuzumab-chlorambucil 0·60; 0·28–1·27, p=0·16; figure 2C); estimated overall survival at 24 months was 95% with acalabrutinib-obinutuzumab (95% CI 91–97%), 95% with acalabrutinib monotherapy (90–97%), and 92% with obinutuzumab-chlorambucil (86–95%).
Five (3%) of 179 patients in the acalabrutinib-obinutuzumab group, 11 (6%) of 179 patients in the acalabrutinib monotherapy group, and 55 (31%) of 177 patients in the obinutuzumab-chlorambucil group had initiated subsequent chronic lymphocytic leukaemia therapy at time of analysis (appendix p 25). Median time to next treatment was not reached in any group (appendix p 19); the risk of needing a subsequent therapy was reduced with acalabrutinib-obinutuzumab (HR 0·14, 95% CI 0·08–0·26; p<0·0001) and acalabrutinib monotherapy (0·24, 0·15–0·40; p<0·0001) compared with obinutuzumab-chlorambucil. Progression with Richter’s transformation occurred in one (1%) of 179 patients in the acalabrutinib-obinutuzumab group, five (3%) of 179 patients in the acalabrutinib mono therapy group, and one (1%) of 177 patients in the obinutuzumab-chlorambucil group, which occurred after 7·9 months of treatment with acalabrutinib monotherapy after cross over. Median time to onset of Richter’s transformation was 4·7 months (IQR 1·3–11·7) across groups.
The median duration of exposure was 27·7 months in the acalabrutinib-obinutuzumab group (IQR 25·0–32·8), 27·7 months in the acalabrutinib monotherapy group (24·8–33·0), and 5·6 months in the obinutuzumab-chlorambucil group (5·5–5·9; appendix p 23). The most common any-grade and grade 3 or higher AEs are summarised in table 2 (also see appendix p 26 for AEs during the first 6 months of treatment). The most common grade 3 or higher AE across groups was neutropenia (acalabrutinib-obinutuzumab 53 [30%] of 178 patients, acalabrutinib monotherapy 17 [10%] of 179 patients, and obinutuzumab-chlorambucil 70 [41%] of 169 patients). Grade 4–5 neutropenia occurred in 36 (20%) patients receiving acalabrutinib-obinutuzumab (12 patients required granulocyte-colony stimulating factor [G-CSF], one patient required repeated G-CSF), 10 (6%) patients receiving acalabrutinib monotherapy (three patients required G-CSF, one patient required repeated G-CSF), and 26 (15%) patients receiving chlorambucil-obinutuzumab (seven patients required G-CSF, one patient required repeated G-CSF). Grade 3 or higher infusion-related reactions and tumour lysis syndrome were more frequent in the obinutuzumab-chlorambucil group (nine [5%] higher infusion-related reactions and 13 [8%] tumour lysis syndromes) than in the acalabrutinib-obinutuzumab group (four [2%] higher infusion-related reactions and two [1%] tumour lysis syndromes). Serious AEs were reported in 69 (39%) of 178 patients in the acalabrutinib-obinutuzumab group, 57 (32%) of 179 patients in the acalabrutinib monotherapy group, and 37 (22%) of 169 patients in the obinutuzumab-chlorambucil group (appendix p 27). Despite the longer treatment duration in the acalabrutinib-containing groups, the frequency of discontinuations due to AEs was not higher (20 [11%] in the acalabrutinib-obinutuzumab group, 16 [9%] in the acalabrutinib monotherapy group, and 25 [14%] in the obinutuzumab-chlorambucil group; figure 1, appendix p 28).
Table 2:
Adverse events occurring in at least 10% of patients in any treatment group
| Acalabrutinib-obinutuzumab (n=178) | Acalabrutinib monotherapy (n=179) | Obinutuzumab-chlorambucil (n=169) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 1–2 | Grade ≥3 | Any grade | Grade 1–2 | Grade ≥3 | Any grade | Grade 1–2 | Grade ≥3 | |
| Summary of adverse events | |||||||||
| Any | 171 (96·1%) | 46 (25·8%) | 125 (70·2%) | 170 (95·0%) | 81 (45·3%) | 89 (49·7%) | 167 (98·8%) | 49 (29·0%) | 118 (69·8%) |
| Serious | 69 (38·8%) | 11 (6·2%) | 58 (32·6%) | 57 (31·8%) | 4 (2·2%) | 53 (29·6%) | 37 (21·9%) | 4 (2·4%) | 33 (19·5%) |
| Led to drug discontinuation (any grade) | 20 (11·2%) | ‥ | ‥ | 16 (8·9%) | ‥ | ‥ | 25 (14·1%) | ‥ | ‥ |
| Most common adverse events | |||||||||
| Headache | 71 (39·9%) | 69 (38·8%) | 2 (1·1%) | 66 (36·9%) | 64 (35·8%) | 2 (1·1%) | 20 (11·8%) | 20 (11·8%) | 0 |
| Diarrhoea | 69 (38·8%) | 61 (34·3%) | 8 (4·5%) | 62 (34·6%) | 61 (34·1%) | 1 (0·6%) | 36 (21·3%) | 33 (19·5%) | 3 (1·8%) |
| Neutropenia | 56 (31·5%) | 3 (1·7%) | 53 (29·8%) | 19 (10·6%) | 2 (1·1%) | 17 (9·5%) | 76 (45·0%) | 6 (3·6%) | 70 (41·4%) |
| Fatigue | 50 (28·4%) | 47 (26·4%) | 3 (1·7%) | 33 (18·4%) | 31 (17·3%) | 2 (1·1%) | 29 (17·2%) | 28 (16·6%) | 1 (0·6%) |
| Contusion | 42 (23·6%) | 42 (23·6%) | 0 | 27 (15·1%) | 27 (15·1%) | 0 | 7 (4·1%) | 7 (4·1%) | 0 |
| Arthralgia | 39 (21·9%) | 37 (20·8%) | 2 (1·1%) | 28 (15·6%) | 27 (15·1%) | 1 (0·6%) | 8 (4·7%) | 6 (3·6%) | 2 (1·7%) |
| Cough | 39 (21·9%) | 39 (21·9%) | 0 | 33 (18·4%) | 32 (17·9%) | 1 (0·6%) | 15 (8·9%) | 15 (8·9%) | 0 |
| Upper respiratory tract infection | 38 (21·3%) | 34 (19·1%) | 4 (2·2%) | 33 (18·4%) | 33 (18·4%) | 0 | 14 (8·3%) | 13 (7·7%) | 1 (0·6%) |
| Nausea | 36 (20·2%) | 36 (20·2%) | 0 | 40 (22·3%) | 40 (22·3%) | 0 | 53 (31·4%) | 53 (31·4%) | 0 |
| Dizziness | 32 (18·0%) | 32 (18·0%) | 0 | 21 (11·7%) | 21 (11·7%) | 0 | 10 (5·9%) | 10 (5·9%) | 0 |
| Back pain | 25 (14·0%) | 24 (13·5%) | 1 (0·6%) | 25 (14·0%) | 23 (12·8%) | 2 (1·1%) | 14 (8·3%) | 13 (7·7%) | 1 (0·6%) |
| Constipation | 25 (14·0%) | 25 (14·0%) | 0 | 20 (11·2%) | 20 (11·2%) | 0 | 17 (10·1%) | 16 (9·5%) | 1 (0·6%) |
| Infusion-related reaction | 24 (13·5%) | 20 (11·2%) | 4 (2·2%) | 0 | 0 | 0 | 67 (39·6%) | 58 (34·3%) | 9 (5·3%) |
| Vomiting | 24 (13·5%) | 23 (12·9%) | 1 (0·6%) | 22 (12·3%) | 21 (11·7%) | 1 (0·6%) | 19 (11·2%) | 18 (10·7%) | 1 (0·6%) |
| Pyrexia | 23 (12·9%) | 23 (12·9%) | 0 | 12 (6·7%) | 11 (6·1%) | 1 (0·6%) | 35 (20·7%) | 34 (20·4%) | 1 (0·6%) |
| Thrombocytopenia | 23 (12·9%) | 8 (4·5%) | 15 (8·4%) | 13 (7·3%) | 8 (4·5%) | 5 (2·8%) | 24 (14·2%) | 4 (2·4%) | 20 (11·8%) |
| Oedema peripheral | 22 (12·4%) | 21 (11·8%) | 1 (0·6%) | 16 (8·9%) | 15 (8·4%) | 1 (0·6%) | 12 (7·1%) | 12 (7·1%) | 0 |
| Pain in extremity | 22 (12·4%) | 21 (11·8%) | 1 (0·6%) | 11 (6·1%) | 11 (6·1%) | 0 | 7 (4·1%) | 7 (4·1%) | 0 |
| Urinary tract infection | 22 (12·4%) | 21 (11·8%) | 1 (0·6%) | 22 (12·3%) | 19 (10·6%) | 3 (1·7%) | 8 (4·7%) | 8 (4·7%) | 0 |
| Anaemia | 21 (11·8%) | 11 (6·2%) | 10 (5·6%) | 25 (14·0%) | 13 (7·3%) | 12 (6·7%) | 20 (11·8%) | 8 (4·7%) | 12 (7·1%) |
| Rash | 21 (11·8%) | 20 (11·2%) | 1 (0·6%) | 25 (14·0%) | 24 (13·4%) | 1 (0·6%) | 8 (4·7%) | 8 (4·7%) | 0 |
| Chills | 20 (11·2%) | 20 (11·2%) | 0 | 8 (4·5%) | 8 (4·5%) | 0 | 14 (8·3%) | 13 (7·7%) | 1 (0·6%) |
| Nasopharyngitis | 20 (11·2%) | 19 (10·7%) | 1 (0·6%) | 17 (9·5%) | 17 (9·5%) | 0 | 7 (4·1%) | 7 (4·1%) | 0 |
| Pneumonia | 19 (10·7%) | 9 (5·1%) | 10 (5·6%) | 13 (7·3%) | 9 (5·0%) | 4 (2·2%) | 5 (3·0%) | 2 (1·7%) | 3 (1·8%) |
| Decreased appetite | 18 (10·1%) | 18 (10·1%) | 0 | 10 (5·6%) | 10 (5·6%) | 0 | 13 (7·7%) | 12 (7·1%) | 1 (0·6%) |
| Dyspnoea | 15 (8·4%) | 15 (8·4%) | 0 | 12 (6·7%) | 9 (5·0%) | 3 (1·7%) | 17 (10·1%) | 14 (8·3%) | 3 (1·8%) |
Data are n (%).
Predefined events of clinical interest for acalabrutinib are summarised in appendix (p 30). Atrial fibrillation was observed in six (3%) of 178 patients receiving acalabrutinib-obinutuzumab, seven (4%) of 179 patients receiving acalabrutinib monotherapy, and one (1%) of 169 patients receiving obinutuzumab-chlorambucil (appendix p 31). No ventricular tachyarrhythmias were reported. Grade 3 or higher hypertension occurred in five (3%) patients in the acalabrutinib-obinutuzumab group, four (2%) patients in the acalabrutinib monotherapy group, and five (3%) patients in the obinutuzumab-chlorambucil group. Bleeding events occurred at any grade in 76 (43%, 73 [41%] grade 1–2) patients in the acalabrutinib-obinutuzumab group, 70 (39%, 67 [37%] grade 1–2) patients in the acalabrutinib monotherapy group, and 20 (12%, all grade 1–2) patients in the obinutuzumab-chlorambucil group. The most common bleeding events were contusion (42 [24%] patients in the acalabrutinib-obinutuzumab group, 27 [15%] patients in the acalabrutinib monotherapy group, and 7 [4%] patients in the obinutuzumab-chlorambucil group) and petechiae (14 [8%] patients in the acalabrutinib-obinutuzumab group, 16 [9%] patients in the acalabrutinib monotherapy group, no patients in the obinutuzumab-chlorambucil group). The incidence of grade 3 or higher bleeding events was the same with acalabrutinib-obinutuzumab and acalabrutinib monotherapy (three patients [2%]) compared with no events in the obinutuzumab-chlorambucil group (appendix p 33). Four of the six patients with grade 3 or higher bleeding events were also given antithrombotic agents (two patients received aspirin, one patient received clopidogrel, two patients enoxaparin, and one patient received heparin). Two patients had subdural bleeding events: one patient had two events (grade 1 and grade 2) in the acalabrutinib-obinutuzumab group, and one patient had a grade 1 event in the obinutuzumab-chlorambucil group. Grade 3 or higher infection occurred in 37 (21%) patients given acalabrutinib-obinutuzumab, 25 (14%) patients given acalabrutinib monotherapy, and 14 (8%) patients given obinutuzumab-chlorambucil (appendix p 34). One patient in the acalabrutinib monotherapy group died from an aspergillosis infection. Second primary malignancies were observed in 19 (11%) patients in the acalabrutinib-obinutuzumab group, 16 (9%) patients in the acalabrutinib monotherapy group, and 13 (8%) patients in the chlorambucil-obinutuzumab group (appendix pp 20, 21). Across all groups, 22 (55%) of the 40 second primary malignancies were nonmelanoma skin cancers.
Death from any cause was recorded in eight (5%) patients in the acalabrutinib-obinutuzumab group, 12 (7%) patients in the acalabrutinib monotherapy group, and 15 (9%) patients in the obinutuzumab-chlorambucil group (appendix p 36). These included four deaths from chronic lymphocytic leukaemia disease progression (two in the acalabrutinib-obiutuzumab group, one in the acalabrutinib monotherapy group, one in the obinutuzumab-chlorambucil group), two deaths due to Richter’s transformation (one in the acalabrutinib monotherapy group, one in the obinutuzumab-chlorambucil group), and 21 deaths due to AEs (four in the acalabrutinib-obinutuzumab group, six in the acalabrutinib monotherapy group, and 11 in the obinutuzumab-chlorambucil group; appendix p 36).
Discussion
Acalabrutinib is a selective, covalent BTK inhibitor developed as a potential alternative to other standard therapies in chronic lymphocytic leukaemia. To our knowledge, ELEVATE-TN is the first randomised, phase 3 study examining the efficacy and safety of acalabrutinib in the first-line treatment setting in chronic lymphocytic leukaemia. In ELEVATE-TN, acalabrutinib plus obinutuzumab or acalabrutinib monotherapy were associated with improved efficacy over obinutuzumab-chlorambucil. After a median follow-up of 28·3 months, the primary endpoint was met at the interim analysis, with significantly longer IRC-assessed progression-free survival with acalabrutinib-obinutuzumab versus obinutuzumab-chlorambucil in patients with treatment-naive chronic lymphocytic leukaemia.
Although a secondary endpoint, acalabrutinib monotherapy was also associated with statistically improved progression-free survival over obinutuzumab-chlorambucil. Several randomised studies have evaluated ibrutinib alone or with a CD20 antibody in treatment-naive chronic lymphocytic leukaemia, showing progression-free survival improvements compared with chemoimmunotherapy;6–9 however, real-world evidence suggests that discontinuation due to AEs is more common in clinical practice compared with randomised studies.25,26 Although our study stands as an independent validation of acalabrutinib benefit as initial chronic lymphocytic leukaemia therapy, the results are similar to those from iLLUMINATE, in which median progression-free survival for ibrutinib-obinutuzumab was not reached after a median follow-up of 31·3 months and the 30-month progression-free survival was 79%, while we report an estimated 24-month progression-free survival of 93% with acalabrutinib-obinutuzumab and 87% with acalabrutinib monotherapy in a similar patient population.8 Median progression-free survival with obinutuzumab-chlorambucil (22·6 months) was similar to that in iLLUMINATE (19·0 months),8 again suggesting similar patient populations.Like the iLLUMINATE and Alliance trials, most patients in ELEVATE-TN were older (median age 70 years) but physically fit (median CIRS-G score 6, median creatinine clearance 75 mL/min, 94% ECOG PS score 0–1).6,8 In contrast, median progression-free survival was shorter than that observed in the CLL11 study (26·7 months), which might have been because of the inclusion of a greater proportion of patients with high-risk features in this study, or a difference in the frequency of CT scans that often identifies early, asymptomatic progressive disease.5 Longer remission might be expected in real-world practice, in which CT surveillance is not recommended by the iwCLL 2018 response criteria, published after this study was fully enrolled.17
Efficacy improvements were consistently observed across prespecified patient subgroups with acalabrutinib-obinutuzumab and acalabrutinib monotherapy versus obinutuzumab-chlorambucil, including age and high-risk genomic features such as del(17)(p13·1), unmutated IGHV, and complex karyotype, in which outcomes are often worse with chemoimmunotherapy.27 This study substantiates and extends results from previous trials showing that BTK inhibitors are efficacious in patients with both high-risk and low-risk disease characteristics and are associated with better outcomes than with chemoimmunotherapy.8,28 After a median 28 months of follow-up, only 3% of patients in the acalabrutinib-obinutuzumab group and 6% of patients in the acalabrutinib monotherapy group had initiated subsequent chronic lymphocytic leukaemia treatment. Furthermore, fewer patient deaths had occurred at the time of analysis in the acalabrutinib groups than in the chemoimmunotherapy group. However, these data are immature, and longer follow-up of survival with continuous therapy is required.
Although a post-hoc analysis for which the study was not statistically powered to examine, the three-arm design is an advantage because it provides important insights regarding the therapeutic value of adding a third-generation CD20 antibody to a BTK inhibitor. The addition of obinutuzumab to acalabrutinib could improve rates and depths of responses. Although our study was not powered to detect a difference in progression-free survival between the two acalabrutinib groups, we observed better progression-free survival with obinutuzumab addition. Because this was a post-hoc analysis, we are unable to state whether the difference was statistically significant. Future trials should address the difference in progression-free survival between acalabrutinib groups in a definitive manner, and explore whether a fixed-duration regimen of acalabrutinib-obinutuzumab could lead to significant response. Cytopenia, infusion-related reactions, and grade 3 or higher infections were more common with acalabrutinib-obinutuzumab compared with acalabrutinib monotherapy. Therefore, the addition of obinutuzumab to BTK therapy has risks and benefits that might depend on the patient’s individual circumstances and guideline recommendations. Our data support either acalabrutinib plus obinutuzumab or acalabrutinib monotherapy as an acceptable therapy for chronic lymphocytic leukaemia that is superior to chlorambucil-obinutuzumab. The benefit of adding obinutuzumab to acalabrutinib differ from results of two randomised trials6,10 that showed no progression-free survival, overall response, or complete response benefit of adding the less efficacious CD20 antibody, rituximab, to ibrutinib. Therefore, we cannot presume that all BTK inhibitor and anti-CD20 combinations will provide similar benefits.
Chemoimmunotherapy remains a frequently used first-line treatment option for patients with chronic lymphocytic leukaemia.1,3 Despite the associated toxic effects, chemoimmunotherapy offers the advantages of a fixed treatment duration compared with agents used for continuous treatment. Because BTK inhibitors are administered until disease progression, tolerability and long-term safety are crucial, especially in chronic lymphocytic leukaemia, which tends to affect an older population with comorbidities. Safety findings were consistent with those previously reported for each regimen, with no new safety signals identified.5,15,16 79% of patients given acalabrutinib in our study remain on treatment after a median of 28 months follow-up. All-cause mortality favoured acalabrutinib-obinutuzumab over obinutuzumab-chlorambucil, but SAEs, including grade 3 and higher infection, were more common with acalabrutinib-obinutuzumab than with obinutuzumab-chlorambucil. Frequency of progression with Richter’s transformation was consistent with the available literature on patients with treatment-naive chronic lymphocytic leukaemia29,30 (<1% of patients in the acalabrutinib-obinutuzumab group, 3% of patients in the acalabrutinib monotherapy group, and <1% of patients in the obinutuzumab-chlorambucil group [7·9 months after crossover to acalabrutinib monotherapy]). Most AEs were observed during the first 6 months of treatment and the rate of discontinuation due to AEs was similar with acalabrutinib-obinutuzumab and acalabrutinib monotherapy, as were the rates of cardiac, bleeding, and infection events.
Although this was a large, randomised controlled trial with IRC assessment, it had some limitations. Patients and investigators were not masked to study treatments, and a 6-month fixed duration regimen is compared with continuous therapy. This study did not include ibrutinib, which would have facilitated a direct comparison with acalabrutinib and will be addressed in an ongoing direct comparison of acalabrutinib to ibrutinib in relapsed patients with chronic lymphocytic leukaemia at high risk (NCT02477696). In addition, future real-world studies are needed to assess whether the predicted better tolerability of acalabrutinib versus ibrutinib will translate into an observed difference in clinical practice. Given the short follow-up period and the paucity of events in this study, extended follow-up will be needed to detect any differences in overall survival; however, the ability of patients to cross over and receive acalabrutinib could preclude detection. It should be noted that, at the time of study design, ibrutinib was approved by FDA and EMA but was not globally approved or reimbursed for first-line therapy of patients with chronic lymphocytic leukaemia and del(17)(p13·1) or TP53 mutations. Although obinutuzumab-chlorambucil might now be used less frequently in treatment-naive chronic lymphocytic leukaemia, it was an approved regimen at the time of the ELEVATE-TN study design, and was also used as the control in the iLLUMINATE, CLL14, and Unity-CLL studies, which enrolled patients concurrently with our trial.
In summary, in patients with treatment-naive chronic lymphocytic leukaemia, acalabrutinib with or without obinutuzumab improved progression-free survival over chemoimmunotherapy, and had a manageable safety profile, with 79% of patients remaining on therapy after a median follow-up of 28·3 months. The phase 3 ELEVATE-TN study substantiates that acalabrutinib with or without obinutuzumab is an efficacious and well tolerated treatment for patients with treatment-naive chronic lymphocytic leukaemia.
Supplementary Material
Research in context.
Evidence before this study
We searched the PubMed database (using the terms “treatment-naive” OR “treatment naive” OR “untreated” AND “Bruton” OR “Bruton’s” OR “ibrutinib” OR “acalabrutinib” OR “zanubrutinib” AND “chronic lymphocytic leukemia” OR “chronic lymphocytic leukaemia”) to find research published between Jan 1, 2000, and Oct 3, 2019. No published randomised studies have analysed the efficacy of the Bruton tyrosine-kinase (BTK) inhibitor acalabrutinib either as monotherapy or in combination with an anti-CD20 monoclonal antibody in patients with untreated chronic lymphocytic leukaemia, or compared acalabrutinib (with or without an anti-CD20 antibody) with chemoimmunotherapy. Previous randomised, controlled studies have shown the superior efficacy of the BTK inhibitor ibrutinib with or without an anti-CD20 monoclonal antibody compared with chemoimmunotherapy in chronic lymphocytic leukaemia. These studies reported that adding rituximab to ibrutinib treatment did not increase the likelihood for progression-free survival versus ibrutinib monotherapy in patients with chronic lymphocytic leukaemia. Obinutuzumab, which is a more potent and efficacious anti-CD20 monoclonal antibody compared with rituximab, used in combination with ibrutinib, has been shown to provide greater efficacy than chemoimmunotherapy.
Added value of this study
The results of this study provide new evidence for therapy in patients with treatment-naive chronic lymphocytic leukaemia by showing the efficacy of acalabrutinib used with or without obinutuzumab compared with chemoimmunotherapy. Acalabrutinib with or without obinutuzumab was associated with improved efficacy over obinutuzumab-chlorambucil with a manageable safety profile. After a median follow-up of 28·3 months, the primary endpoint was met at the interim analysis, showing significantly longer progression-free survival with acalabrutinib-obinutuzumab versus obinutuzumab-chlorambucil. Similarly, acalabrutinib monotherapy was associated with statistically improved progression-free survival versus obinutuzumab-chlorambucil therapy. Improvements in progression-free survival were consistently observed across prespecified patient subgroups, including age and high-risk genomic features such as del(17)(p13.1), unmutated immunoglobulin heavy-chain variable-region (IGHV), and complex karyotype. A post hoc analysis showed the benefit of adding obinutuzumab therapy to acalabrutinib in treatment-naive patients who have chronic lymphocytic leukaemia, which had not been previously reported in a randomised trial. Indeed, this is the first study to provide insight into the value of a third-generation anti-CD20 antibody plus a BTK inhibitor over a BTK inhibitor alone in chronic lymphocytic leukaemia.
Implications of all the available evidence
These results show the clinical potential of acalabrutinib as first-line therapy, either in combination with obinutuzumab or as monotherapy, to improve clinical outcomes in patients with chronic lymphocytic leukaemia.
Acknowledgments
The study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. The authors thank the investigators and coordinators at each of the clinical sites; the patients who participated in this trial and their families; and Allison Cherry PhD (Team 9 Science), and Adele Buss PhD (Oxford PharmaGenesis Inc, Newtown, PA, USA), for their medical writing support (funded by Acerta Pharma, a member of the AstraZeneca Group). JCB was supported by the National Cancer Institute (R35 CA198183) in the USA.
Declaration of interests
JPS reports personal fees from AbbVie, Acerta Pharma (a member of the AstraZeneca Group), AstraZeneca, Genentech, Pharmacyclics, Sunesis, and TG Therapeutics, during the conduct of this study. WJ reports grant support from AstraZeneca, Janssen, and Roche during the conduct of this study. AS reports research support from Acerta Pharma (a member of the AstraZeneca Group) during the conduct of this study; research support and personal fees from AbbVie, AstraZeneca, Celegene, Janssen, Pharmacyclics, and Verastem; personal fees from Genentech, Gilead, Kite Pharma, Jazz Pharmaceuticals, Novartis, Beigene, and Seattle Genetics; received a drug for an investigator-initiated trial from Bristol-Myers Squibb, outside of the submitted work; and owns stock in COTA Healthcare. JMP reports personal fees from AstraZeneca, Gilead, and Pharmacyclics, outside the submitted work. IWF reports fees to his institution for conducting clinical trials or consulting fees from AbbVie, Seattle Genetics, TG Therapeutics, Verastem, Roche, Gilead Sciences, Kite Pharma, Acerta Pharma (a member of the AstraZeneca Group), Agios, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Genentech, Incyte, Infinity Pharmaceuticals, Janssen, Karyopharm Therapeutics, Novartis, Pharmacyclics, Portola Pharmaceuticals, Trillium Therapeutics, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, AstraZeneca, Juno Therapeutics, Unum Therapeutics, MorphoSys, Great Point Partners LLC, Nurix Therapeutics, and Yingli Pharmaceuticals. MK reports Advisory Board membership for AstraZeneca, Celgene, and Pharmacyclics; and Speaker Bureau participation for Seattle Genetics, outside the submitted work. TM reports personal fees from AbbVie, Alexion, Gilead, Janssen, Leeds Teaching hospitals (UK), Morphosys, Novartis, and Sunesis, outside the submitted work. RW reports travel grants and personal fees from AbbVie, Gilead, and Janssen; and travel grants from Takeda and Chugai, outside the submitted work. YH reports personal fees from AbbVie, Janssen, and Roche, outside the submitted work.VB reports grant funding and personal fees from Gilead and Janssen; research funding from Research Manitoba and the CancerCare Manitoba Foundation; personal fees from AbbVie, AstraZeneca, and the CancerCare Manitoba Foundation, University of Manitoba, outside the submitted work; and licensing fees from Biogen and a US patent entitled “Kinase inhibitors and methods use thereof”, 14/052,661, issued 2014/02/13. SC reports grants and personal fees from AbbVie, Celgene, Janssen, and Pharmacyclics; and personal fees from Astellas. AstraZeneca, Beigene, and Novartis; grant support from Acerta Pharma (a member of the AstraZeneca Group), Gilead, and Takeda, outside the submitted work. GF reports personal fees from AbbVie, AstraZeneca, Janssen, and Roche, outside the submitted work. PW reports personal fees from Alfred Health (Public Hospital) and Peninsula Health (Public Hospital) outside the submitted work.
KK reports personal fees from AbbVie and Janssen, outside the submitted work. PG reports grants and personal fees from AbbVie, Gilead, Janssen, Pharmacyclics, and Sunesis; personal fees from Acerta Pharma (a member of the AstraZeneca Group), Adaptive, Arqule, BeiGene; and grant support from Novartis, outside the submitted work. AJ reports personal fees from AbbVie, Amgen, Celgene, Gilead, Janssen, Novartis, Roche, Sanofi, and Idem Consultancy, outside the submitted work. FC reports non-financial support from Gilead, Roche; personal fees and non-financial support from AbbVie and Janssen; grant funding and personal fees from Sunesis; and personal fees from AstraZeneca. JAW reports grant support and personal fees from Pharmacyclics; personal fees from Janssen; and grant funding from AbbVie, Karyopharm, Loxo, Morphosys, and Verastem outside the submitted work. GS reports personal fees from Acerta Pharma (a member of the AstraZeneca Group) during the conduct of this study; and personal fees from AbbVie, Amgen, Autolous, Bristol-Myers Squibb, Celgene, Epizyme, Gilead, Kite, Janssen, Merck, Morphosys, Novartis, Pfizer, Roche, Servier, and Takeda, outside the submitted work. RI is an employee of Acerta Pharma (a member of the AstraZeneca Group). VM is an employee of Acerta Pharma (a member of the AstraZeneca Group), and owns stock in AstraZeneca and Gilead Sciences. PP is an employee of Acerta Pharma (a member of the AstraZeneca Group), and holds equity in Acerta Pharma and AstraZeneca. MHW reports personal fees from Acerta Pharma (a member of the AstraZeneca Group) and AstraZeneca. SW was an employee of Acerta Pharma (a member of the AstraZeneca Group) at the time the study was conducted. JCB reports personal fees from Acerta Pharma (a member of the AstraZeneca Group), Genentech, Janssen, and Pharmacyclics, outside the submitted work. ME, GC, LMF and WGW report no competing interests.
Footnotes
See Online for appendix
Data sharing
Acerta Pharma, a member of the AstraZeneca Group, is committed to data transparency and will consider data sharing requests on a case-by-case basis. Any requests for de-identified patient data can be submitted to Acerta Pharma 3 months post-publication and ending 5 years after Article publication with the intent-to-achieve aims of the original proposal. In addition, Acerta Pharma will provide the study protocol, statistical analysis plan, and informed consent form, and will post results on ClinicalTrials.gov as required.
References
- 1.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016; 17: 928–42. [DOI] [PubMed] [Google Scholar]
- 2.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019; 380: 2225–36. [DOI] [PubMed] [Google Scholar]
- 3.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 2016; 127: 208–15. [DOI] [PubMed] [Google Scholar]
- 4.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–74. [DOI] [PubMed] [Google Scholar]
- 5.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–10. [DOI] [PubMed] [Google Scholar]
- 6.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018; 379: 2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 2019; 381: 432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 43–56. [DOI] [PubMed] [Google Scholar]
- 9.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373: 2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood 2019; 133: 1011–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol 2013; 190: 2702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golay J, Ubiali G, Introna M. The specific Bruton tyrosine kinase inhibitor acalabrutinib (ACP-196) shows favorable in vitro activity against chronic lymphocytic leukemia B cells with CD20 antibodies. Haematologica 2017; 102: e400–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA takes second action under international collaboration, approves new treatment option for patients with chronic lymphocytic leukemia. [news release]. Food and Drug Administration: November 21, 2019. https://www.fda.gov/news-events/press-announcements/fda-takes-second-action-under-international-collaboration-approves-new-treatment-option-patients (accessed Dec 10, 2019). [Google Scholar]
- 14.Hassenruck F, Knodgen E, Gockeritz E, et al. Sensitive detection of the natural killer cell-mediated cytotoxicity of anti-CD20 antibodies and its impairment by B-cell receptor pathway inhibitors. Biomed Res Int 2018; 2018: 1023490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd JC, Woyach JA, Furman RR, et al. Acalabrutinib in treatment-naive (TN) chronic lymphocytic leukemia (CLL): updated results from the phase 1/2 ACE-CL-001 study. Blood 2018; 132 (suppl 1): 692. [Google Scholar]
- 16.Woyach JA, Rogers KA, Bhat SA, et al. Acalabrutinib with obinutuzumab (Ob) in treatment-naive (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL): three-year follow-up. J Clinical Oncol 2019; 37 (suppl 15): 7500. [Google Scholar]
- 17.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41: 237–48. [DOI] [PubMed] [Google Scholar]
- 19.Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood 2015; 126: 842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malcikova J, Tausch E, Rossi D, et al. ERIC recommendations for TP53 mutation analysis in chronic lymphocytic leukemia-update on methodological approaches and results interpretation. Leukemia 2018; 32: 1070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez D, Martinez P, Wade R, et al. Mutational status of the TP53 gene as a predictor of response and survival in patients with chronic lymphocytic leukemia: results from the LRF CLL4 trial. J Clin Oncol 2011; 29: 2223–29. [DOI] [PubMed] [Google Scholar]
- 22.Rawstron AC, Bottcher S, Letestu R, et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 2013; 27: 142–49. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol 2012; 30: 2820–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International CLLIPIwg. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol 2016; 17: 779–90. [DOI] [PubMed] [Google Scholar]
- 25.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018; 103: 874–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol 2018; 93: 1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallek M Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol 2019; 94: 1266–87. [DOI] [PubMed] [Google Scholar]
- 28.Kipps TJ, Fraser G, Coutre SE, et al. Long-term studies assessing outcomes of ibrutinib therapy in patients with del(11q) chronic lymphocytic leukemia. Clin Lymphoma, Myeloma Leuk 2019; 19: 715–22. [DOI] [PubMed] [Google Scholar]
- 29.Parikh SA, Rabe KG, Call TG, et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol 2013; 162: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi D, Cerri M, Capello D, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol 2008; 142: 202–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


