Abstract
Myogenic differentiation, cell fusion, and myotube formation of skeletal muscle progenitor cells (SMPCs) have key roles during skeletal muscle development and repair. However, after isolation from living tissue and transition to culture dishes, SMPCs gradually lose their function and stop propagating due to the absence of extracellular matrix (ECM). Despite encouraging results of experiments using ECM components in cell culture for maintenance and propagation of some tissue types, the benefits of this approach on SMPC culture are limited, because the bioactive molecules and proteins instantly release and are degraded during culture. In this study, we developed a novel approach to enhance the proliferation and differentiation of human skeletal muscle progenitor cells (hSMPCs) in vitro with skeletal muscle ECM in combination with a modified alginate hydrogel conjugated with gelatin and heparin (Alg-G-H) as a substrate. This Alg-G-H substrate, together with skeletal muscle ECM, significantly enhanced cell expansion, differentiation, and maturation of hSMPCs compared with individual substrata (i.e. gelatin, Matrigel®, or ECM alone). In Western-blot and immunocytochemical analyses, the Alg-G-H-ECM predominantly enhanced expression of skeletal myogenesis markers (MyoD, Myf5, Myogenin, Desmin and Myosin) and myotube formation in hSMPCs. This study demonstrated that combining Alg-G-H substrates with skeletal muscle ECM modulated homeostasis of cell proliferation, differentiation, and maturation of hSMPCs by releasing signaling molecules and growth factors. This technique could be a cost-effective tool for in vitro skeletal muscle cell differentiation and maturation, with potential applications in tissue regeneration and drug development.
Graphical Abstract
1. Introduction
In vitro expansion of human skeletal myocytes has been used to generate 3D tissue models for repairing damaged muscle tissue, and for drug and toxicology screening, disease modeling, and personalized medicine [1, 2]. Although these myocytes are widely cultured in the laboratory, fabrication of functional myocyte-based structures with appropriate properties of contractility remains challenging. Cultured skeletal muscle progenitor cells (SMPCs) rapidly lose myogenic differentiation and cell expansion capability in vitro because myogenesis of SMPCs requires sophisticated bioactive factors, such as tissue-specific extracellular matrix (ECM) and indispensable growth factors, which are lacking in traditional in vitro culture conditions.
Each cell type requires its own substrate to support growth and retain function in vitro. The ECM plays a crucial role in muscle formation and regeneration. Skeletal muscle tissue ECM has a three-dimensional (3D) architecture, primarily comprised of collagen, glycosaminoglycans, proteoglycans (PGs), and bioactive growth factors or cytokines, which combine to form a dynamic network and present tissue-specific cues to support cell proliferation and myotube maturation of SMPCs [3]. In addition, growth factors and cytokines such as IGF1 and VEGF synchronously promote an integrated process of myogenesis with vascularization and innervation [4]. VEGF release from a polymeric scaffold system can be controlled in an extended release manner, to enhance skeletal muscle regeneration and blood vessel formation [5]. Recruitment and presentation of these factors available from the native ECM can promote skeletal muscle regeneration [6, 7]. IGF1, in particular, may be involved in neuroprotective processes [8, 9] and also directly accelerate myogenesis-associated gene and protein expression to regulate MPC proliferation and differentiation [10–12]. The current approach is to consistently add the growth factors and cytokines whenever the medium is changed. However, most growth factors last only a few minutes in the culture medium, because these proteins are quickly degraded by proteinases in vitro. For this and other reasons, it is desirable to have a controlled or slow-release system for protein presentation to cells.
Several biomaterials, including Matrigel®, gelatin, collagen, and other synthetic chemical substrates combined with or without growth factors, have been investigated to mimic the microenvironment for muscle growth, modulate progenitor cell fate, and guide myotube formation [13, 14]. Other hydrogels have been tested in similar tissue engineering-based applications. For example, hyaluronic acid (HA), the most abundant glycosaminoglycan component in the ECM of most tissues, has been widely used in tissue engineering [15, 16], particularly in chemically-modified cross-linkable forms. Further modification of HA with heparin (HA-HP) captures proteins and growth factors, allowing growth factor sequestration and long-term release – useful capabilities for sustained biological effects in vivo and in in vitro 3D cultures [17]. Additionally, the physical parameters of these 3D hydrogels, including topography, porosity, and elastic moduli similar to soft tissues, may constitute a preferable environment for in vitro myogenesis and skeletal muscle regeneration [16].
Alginate is another commonly used material with which hydrogels can be formed readily without any additional chemical modification to establish crosslinking chemistries [18]. Furthermore, it is inexpensive compared to many other hydrogel biomaterials designed for cell culture and tissue engineering, such as Matrigel® and several HA-based products on the market. Consequently, alginate has been widely used for tissue engineering applications, particularly microencapsulation [19, 20]. However, in its native state, alginate does not possess any motifs for cell attachment, nor is it capable of effective growth factor sequestration, limiting its effectiveness.
In the work presented herein, we explore the approach of covalently modifying alginate with gelatin for cell adherence and heparin for growth factor retention. Using this biomaterial, we tested whether incorporating muscle ECM (rich in growth factors) would allow modified alginate to maintain the appropriate exogenous bioactive supplement concentrations to foster myogenesis and myotube formation [21]. This simple, yet novel biomaterial based on alginate, gelatin, and heparin, combined with skeletal muscle tissue-derived ECM, is a potential low-cost tool for achieving effective muscle regeneration. It may have potential for application in clinical settings, as well as formation of muscle constructs to be used in in vitro screening applications.
2. Materials and methods
2.1. Alginate conjugation with gelatin and heparin
To increase the number of available carboxylic acid groups on the alginate polysaccharide chains, carboxymethylation of alginate hydroxyl groups was performed. Alginic acid sodium salt (1 g, Sigma, St. Louis, MO) was mixed into 10 mL 11.25M sodium hydroxide to form a viscous paste and mixed at room temperature on a magnetic stir plate. The alginate paste was then transferred to a 105 mM solution of chloroacetic acid (Sigma) in isopropanol, covered, and stirred for 4 hours at room temperature. The resulting solution was then passed through a Whatman filter paper #2 and a Buechner funnel to collect the insoluble product. The collected product was then dissolved in 100 mL distilled water, and the solution was adjusted to pH 7.0 with 6N HCl. The solution was then transferred to 3,500 Da cutoff dialysis tubing, clamped, and dialyzed against distilled water for 24 hours, during which the water bath was changed 4 times.
Gelatin and heparin were conjugated to the alginate polysaccharide chains through simple EDCI chemistry. First, for each 1 g starting material batch of alginic acid, either 0.5 g, 0.33 g, or 0.25 g gelatin was dissolved in water and added to the alginate solution recovered from the dialysis pouches. pH was adjusted to 4.75 and 0.2 g EDCI (1-ethyl-3-[3-(dimethylamino) propyl]-carbodiimide, Sigma) was added. The solutions were maintained at pH 4.75 using 6N HCl for 2 hours. Next, 0.25 g PEG-diamine (2 kDa, Creative PEG Works, Winston-Salem, NC) and 0.2 g additional EDCI were added to the solution, and maintained at pH 4.75 for 2 hours. Next, 0.25 g heparin (Sigma) and 0.2 g additional EDCI were added to the solution, and maintained at pH 4.75 for 2 hours. The resulting solution was adjusted to pH 7.0 with 6N HCl, transferred to dialysis tubing (3500 Da cutoff), clamped, and transferred to a bath of 0.1M sodium chloride in water. Pouches were dialyzed against the sodium chloride solution for 4 days (3 changes per day), followed by 3 days against distilled water (3 changes per day). Lastly, the product was collected, frozen, and lyophilized to yield alginate-gelatin-heparin (Alg-G-H, Fig. 1). Alg-G-H was stored at −80°C until use.
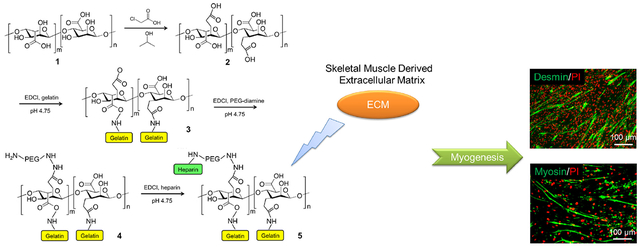
Figure 1. The general chemical synthesis scheme for conjugating alginate with gelatin and heparin.
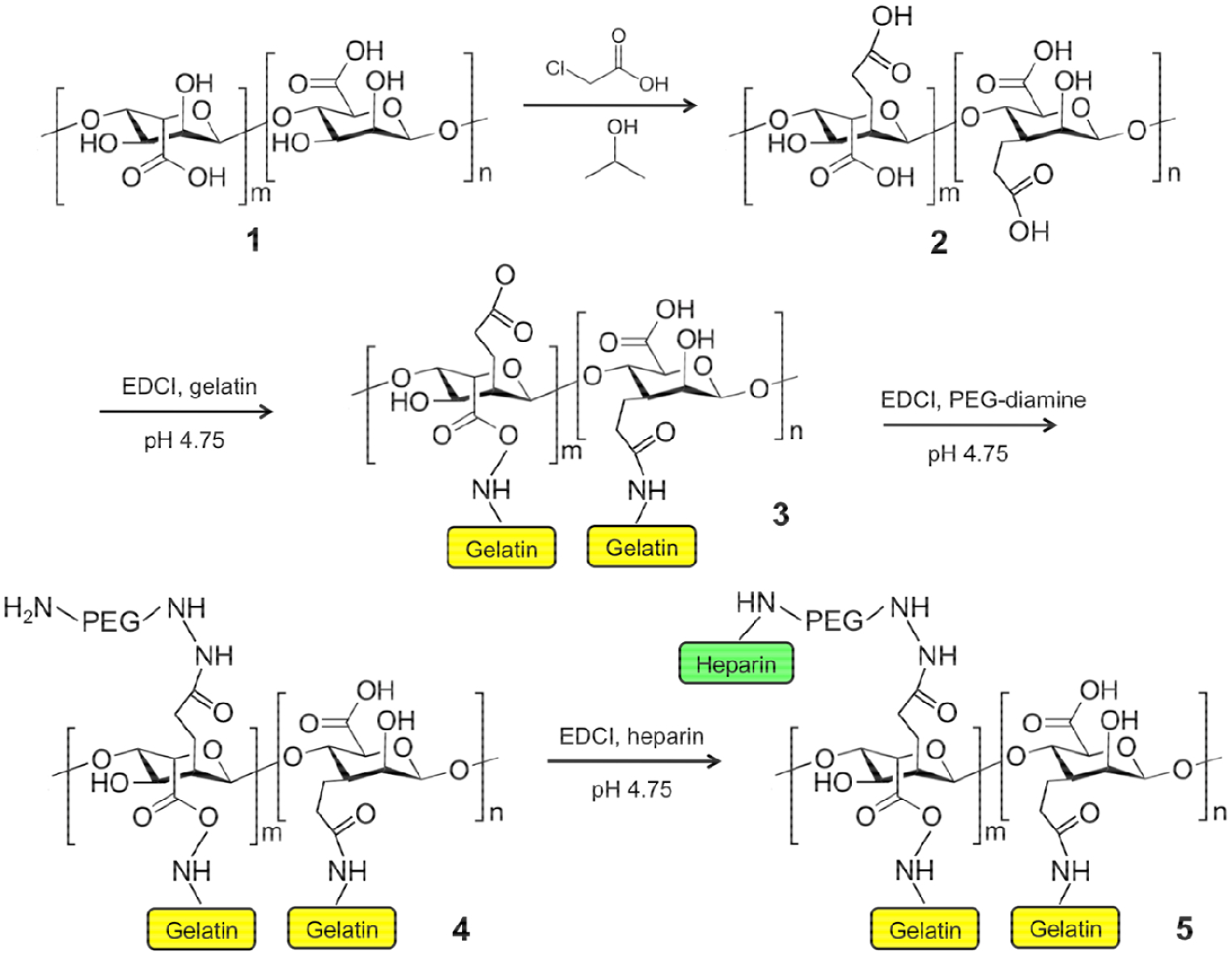
Alcohol groups on the alginate (1) undergo carboxymethylation to provide alginate with increased numbers of carboxylic acid groups (2) for further modification. Gelatin is coupled to the alginate via EDCI chemistry of the gelatin N-terminus amine, resulting in gelatin peptides covalently bound to the alginate (3). Unused carboxylic acid groups on the alginate are then modified with PEG-diamine via EDCI chemistry, (4) after which heparin is bound to the available amine groups of the now pegylated alginate via EDCI chemistry using the carboxylic acid groups on the heparin chains, resulting in alginate covalently modified with both gelatin and heparin (5).
2.2. Hydrogel formation
Alg-G-H and traditional alginate hydrogels (Alg) were formed using the standard sodium-to-calcium ion exchange method [21–24]. Alg-G-H dry product or alginic acid sodium salt was dissolved in distilled water at 1.5% w/v. In parallel, calcium chloride was dissolved at 500 mM in distilled water. To form hydrogels, the alginate solutions were introduced dropwise into the CaCl2 solution, as per the commonly employed microencapsulation approach. We also formed Alg-G-H and Alg substrates in well plates and chamber slides by first adding the alginate solutions to the wells, after which the CaCl2 solution was carefully added over the alginate solutions. The resulting supernatant was aspirated after 5 minutes of crosslinking.
2.3. Rheological assessment
Alg-G-H and Alg hydrogel shear elastic moduli were assessed via rheology. Hydrogels were prepared as described above in 20 mm by 20 mm custom wells (3 mm deep), which were held in place on the stage by double-sided tape. Measurements of the shear elastic modulus G’ of the hydrogel materials were performed on the rheometer (HR-2 Discovery Rheometer, TA Instruments, Newcastle, DE), using a 12-mm diameter parallel plate steel geometry at room temperature. To initiate each test, the 12-mm steel plate geometry was gradually lowered until it first made contact at the surface of the sample. To normalize measurements across all samples, the geometry was then lowered further at a constant rate until the axial force measured by the rheometer (normal force acting upwards on the geometry from the hydrogel) reached 0.4 N or greater. Shear elastic modulus G’ values were then determined for each sample by applying a shear stress sweep protocol ranging from 0.6 to 10 Pa at an oscillation frequency of 1 Hz. This protocol was modified from a protocol employed in several earlier studies [25–27].
2.4. Initial cell-based material characterization: cell adherence and morphology
To test the effects of gelatin modification on cell adherence capability, the 3 formulations of Alg-G-H described above were tested. The starting material ratios to alginate were 20%, 25%, and 33% gelatin, respectively. Bone marrow-derived mesenchymal stem cells (Lonza, Allendale, NJ), which require adherence to a substrate for isolation and culture, were seeded on the hydrogels in 24-well plates at 50,000 cells per well. After 24 hours, cells were examined under light microscopy with a Zeiss Axiovert microscope (Carl Zeiss, Dublin, CA) and assessed for adherence, cell spreading, and presence of filopodia.
Three characteristics were assessed to determine the effects of gelatin modification on cell cultures. These included cell adherence, cell eccentricity (a measure of roundness or uniformly spread cells), and length of cell filopodia. Cell morphologic characteristics were characterized by evaluating representative images of cells on the 4 different alginate substrates at 0%, 20%, 25%, and 33% gelatin (n = 10 fields of view for adherence; n = 25 for eccentricity; and n = 40 for filopodia length). Adherence was determined by counting cells after a media wash. For eccentricity e calculations, cells were generally considered to be roughly elliptical in shape. Images of the cells captured on the microscope were first calibrated and scaled. Next, a semi-major axis (a) and semi-minor axis (b) were determined for each ellipse and their lengths were measured. Eccentricity e was then calculated by solving for e using b2 − a2(1-e2). Filopodia lengths were measured by defining them as extensions of the cell that were connected to, but oriented away from the main cell body. Calibration and quantification were performed using ImageJ software (NIH).
Finally, to further verify the time-dependent cell adherence and proliferation on 4 substrates, cell growth curves wer calculated by MTS assay (CellTiter 96 One Solution Reagent Promega, Madison, WI) and absorbance determined on a Molecular Devices SpectrumMax M5 (Molecular Devices) tunable plate reader system at a wavelength of 490 nm.
2.5. Preparation of skeletal muscle ECM solution
Porcine-derived skeletal muscle ECM digest solutions were prepared as originally described for other tissue types [28, 29]. First, porcine skeletal muscle tissue was thoroughly washed with Dulbecco’s phosphate buffered saline (DPBS) to remove blood or contaminants for the exterior of the tissue. The tissues were next minced into thin strips (approximately 3 cm by 0.5 cm) with surgical scalpels. Samples were further minced into mm-size pieces to increase the total surface area of the tissue and improve efficiency of the decellularization steps. The minced tissue pieces were then placed in 1 L plastic bottles with 500 mL distilled water and agitated using a rotary shaker at 200 rpm for 3 days at 4°C in the cold room; water times daily. At the end of the 3-day period, was discarded and replaced with fresh distilled water 3 water was replaced with 2% Triton X-100 distilled water and the tissue pieces were agitated for 4 days. Next the tissue pieces were agitated in 2% TX-100 in distilled water with 0.1% NH4OH for 24 h. During these two TX-100-containing rinse periods, the solutions were discarded and replaced with fresh solutions twice daily. At the end of these wash periods, the resulting translucent tissues were washed for an additional 2 days in fresh distilled water, during which water was discarded and replaced with fresh distilled water 3 times per day, in order to remove any traces of TX-100 or NH4OH. The resulting decellularized tissue pieces were frozen and stored at −80°C until further use.
Next, the frozen decellularized tissue (primarily extracellular matrix material) was lyophilized for at least 48 h. The resulting dehydrated samples were pulverized into a powder with a cryomill. One gram of powder was weighed out and mixed with 100 mg pepsin (porcine gastric mucosa, Sigma, St. Louis, MO) and sterilized by gamma irradiation at 1 Mrad. The remaining ECM preparation steps were then performed under sterile conditions.
Hydrochloric acid (0.1 N, 100 mL) was added to the sterilized dry ECM and pepsin in a conical tube and agitated for 2 days at 37° on a rotational shaker. The resulting mixture was transferred to several 50 ml conical tubes and centrifuged at 3000 rpm for 15 min. The supernatant was transferred to a fresh 50 mL conical and the pellet of insoluble material was discarded as waste. This was repeated two more times until the ECM solution supernatant liquid was clear. Sodium hydroxide was then added incrementally to raise the pH of the solutions to 7.0. Lastly, to remove any remaining particulate matter that could be left, the solution was passed through a 0.45 mm syringe tip filter (Fisher Scientific). The resulting skeletal muscle ECM solutions were frozen and stored at −80°C until further use.
2.6. Analyses of ECM solutions
ECM solutions were first analyzed quantitatively for levels of collagen, elastin, and glycosaminoglycans (GAG). To evaluate each type of ECM component, 25 mg of skeletal muscle ECM solution was removed from storage and used for the assays (n = 3 samples). The samples underwent further chemical digestion specific to each particular assay. For collagen samples were degraded with HCL; For GAGs, samples were degraded with papain; and for elastin, samples were degraded with oxalic acid. Degraded samples were then analyzed according to the manufacturer’a guidelines using Sircol Collagen, Blyscan GAG, and Fastin Elastin assay kits (Biocolor Life Sciences Assays, Carrickfergus, UK).
Next, ECM solutions were analyzed for a panel of potent growth factors using a Human Growth Factor Array (RayBiotech, Norcross, GA). One mL aliquots of ECM solution were prepared as described above for analysis and sent on dry ice to the company for growth factor and cytokine quantification.
ECM solution samples with 10 μg total protein content were used for Western blot analyses to assess the content of elastin and three types of collagen. The ECM solution samples were loaded on 10% precast gel (Bio-Rad Laboratories, Hercules, CA), and following Western blots (see section 2.12) were processed with the primary antibodies collagen I (1:5000), collagen III (1:5000), collagen IV (1:1000), and elastin (1:100; all from Abcam). Chemiluminescent images were captured with a Fujifilm LAS-3000 Luminescent Image Analyzer system.
2.7. Growth factor release capability of Alg-G-H
To test the ability to release growth factors in the Alg-G-H gel, we chose the crucial cytokines IGF1 and VEGF and whole ECM, encapsulated them in Alg-G-H or an alginate control, and compared release profiles. Following gel preparation (100 μL gels in 96-well plates), 100 μL PBS was placed above the gels. The liquid supernatant from the wells were collected every 2 days and stored at −80°C until quantification by ELISA. For ELISA analyses, VEGF samples were diluted 4 times by volume and IGF1 samples were diluted twice by volume with PBS. VEGF and IGF1 were measured with human VEGF and IGF1 ELISA kits (Abcam). Absorbance values were determined using a wavelength of 450 nm on the plate reader (Molecular Devices SpectrumMax M5, Molecular Devices, Sunnyvale, CA).
2.8. Human SMPCs culture and cell propagation
The protocol for obtaining human skeletal muscle samples was approved by the Wake Forest University Institutional Review Board [30]. Surgical waste material was obtained during plastic surgery on the gracilis muscle from the normal inner thigh tissue from the donor. Human skeletal muscle progenitor cells (hSMPCs) at passage 4 (p4) were seeded on uncoated tissue culture dishes for expansion. We used muscle progenitor cells growth medium with supplement (PeproTech, Rocky Hill, NJ), and refreshed the medium every 2 days for cell propagation.
2.9. hSMPCs proliferation on Alg-G-H
The Alg-G-H lyophilized product was dissolved in hSMPCs culture medium at a concentration of 2% (w/v), or at a concentration of 4% w/v followed by a 1:1 dilution with ECM solution, bringing the final Alg-G-H concentration to 2% to form the Alg-G-H+ECM mixture. To coat 48-well plates, Alg-G-H solution, ECM only solution, or Alg-G-H-ECM solution were added to each well in volumes of 200 μl, and incubated at 37°C for 3 hours. Superfluous solutions were carefully aspirated, after which the 5% CaCl2 were added carefully on top to crosslink the Alg-G-H and Alg-G-H-ECM gel for another 10 min at −80°C, After 3 rinses, the plates were dried in a biosafety cabinet for 15 minutes at room temperature. Then MPCs were seeded on the coated plates at a density of 3,000 cells per well (n = 3). Every 2 days the supernatant was collected. Additionally, every 2 days, relative cell number was detected by MTS assay (CellTiter 96 One Solution Reagent, Promega, Madison, WI) and absorbance determined on a Molecular Devices SpectrumMax M5 (Molecular Devices) tunable plate reader system at a wavelength of 490 nm.
2.10. hSMPCs differentiation on different matrix substrates
Media for skeletal muscle differentiation was prepared with high glucose Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, Waltham, MA) containing 5% fetal bovine serum (FBS, GE Healthcare, Logan, UT), 2% horse serum (GE Healthcare), 1% insulin-transferrin-selenium solution (ITS, Lonza, Allendale, NJ), 250 nM dexamethasone (Lonza), and 1% antibiotic antimycotic solution (AA, Hyclone, Logan, UT).
100 mm dishes and 4-well chamber slides were coated prior to cell seeding and induction of differentiation with the following materials: 0.1% gelatin (Sigma), 1% collagen (Corning, Corning, NY), Matrigel® (Corning), hyaluronic acid gel with heparin (HA-HP, HyStem-HP, ESI-BIO, Alameda, CA), and Alg-G-H. Skeletal muscle ECM was used to coat plastic surfaces, or combined with the HA and Alg-G-H materials. Chamber slides and dishes in each coating condition were kept at 37°C for 3 hours, followed by aspirating the superfluous fluid and drying slides at room temperature before use.
hSMPCs were seeded on the aforementioned substrates, as well as uncoated plastic controls, at 3,000 cells per well of the 4-well chamber slides and 105 cells on 100 mm dishes. Cells were cultured for 2 weeks to assess skeletal muscle differentiation. A subset of the most successful differentiation substrates underwent a repeated differentiation study using nearly serum-free media (1% FBS, 0% horse serum), to assess the capability of the ECM to retain differentiation potential.
2.11. Immunofluorescent staining for myoblast and myotube formation
After the 2-week differentiation period, cells were fixed with 4% paraformaldehyde before immunofluorescent staining for 15 minutes, rinsed 3 times with PBS solution, permeabilized with 0.2% TritonX-100 in PBS at 4°C for 10 minutes, and rinsed 3 times with PBS. Cells were then incubated with protein block solution (Dako, Carpinteria, CA) at room temperature for 1 hour. Primary antibodies were incubated overnight at 4°C: MyoD (Santa Cruz, 1:100), Myf5 (Santa Cruz, 1:100), Myogenin (Abcam, 1:250), Desmin (Abcam, 1:250) and Myosin (Abcam, 1:250), after which they were rinsed 3 times with PBS. Secondary antibodies (anti-Mouse/Rabbit, Invitrogen, 1:300) were incubated with the cells at room temperature for 2 hours, rinsed with PBS, and mounted with mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Stained samples were observed and imaged via fluorescence on a Leica DM4000B microscope system. Positively stained myotubes and fused syncytial cells with 3 nuclei were counted per field of view for statistical analyses. We choose four visual fields in each well of chamberslides with myogenic proteins staining, percentages of nuclei in syncytia that had positive staining for myogenic proteins were calculated versus overall nuclei assessed in different coating conditions.
2.12. Western blot analyses for expression of myogenesis proteins
For further analyses of myogenesis-related protein expression, Western blots were performed. Following the 2-week differentiation period, total proteins were collected using a RIPA reagent (Pierce, Rockford, IL) with a 1% protease/ phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA). Protein samples with 10 μg total protein content were loaded on 10% precast gels (Bio-Rad Laboratories, Hercules, CA), run at 100 V for 1 hour, followed by 12 V and transferred for 1 hour onto a nitrocellulose membrane. Samples were blocked with 5% skim milk at room temperature for 1 hour, and then incubated with 1% skim milk containing the following primary antibodies at room temperature for 2 hours or 4°C overnight: MyoD (Santa Cruz, 1:100), Myf5 (Santa Cruz, 1:100), Myogenin (Abcam, 1:1000), Desmin (Abcam, 1:1000) and slow skeletal Myosin (abcam, 1:1000). Samples were rinsed 3 times with PBS solution containing 0.1% Tween20 (PBST), and secondary antibodies (anti-Mouse/Rabbit, Abcam, 1:5000) were then added and incubated for 1 hour at room temperature. Finally the samples were rinsed 3 times with PBST, then developed with Supersignal® West Femto Maximum Sensitive Substrate (Thermo) for 1 min at room temperature. Chemiluminescent images were analyzed with a Fujifilm LAS-3000 Luminescent Image Analyzer system.
2.13. Statistical analyses
Percentage of nuclei in syncytia was calculated as the ratio of nuclei contained in myogenesis proteins positively stained myotubes grew on different substrates. Cellular structures must contain more than 2 nuclei (i.e. number of nuclei 3) to be considered a myotube. Four visual fields per chamberslide well were checked for the percentages of nuclei in syncytia. In addition, all analyses were based on 3 independent experiments with more than 3 replicates. Data were analyzed by the one-way analysis of variance (ANOVA) with GraphPad Prism5 for all groups, and then used the Student’s t-test for comparison of two groups. Results are presented as mean ± standard error of the mean, p values were calculated for significant differences either as * p< 0.05 or ** p< 0.01.
3. Results
3.1. Alg-G-H characterization
Various formulations of Alg and Alg-G-H hydrogels (1%, 2%, and 3%) were prepared and characterized by rheologic testing (Fig. 2A). Surprisingly, Alg hydrogels did not appear to increase in shear elastic modulus (G’), with increasing concentration. Conversely, the G’ of Alg-G-H hydrogels did increase with increasing concentration, but not significantly. The loss modulus, G”, was significantly reduced compared to G’ (p < 0.01), as expected with most viscoelastic materials (Fig. 2A).
Figure 2. Material characterization of Alg-G-H.
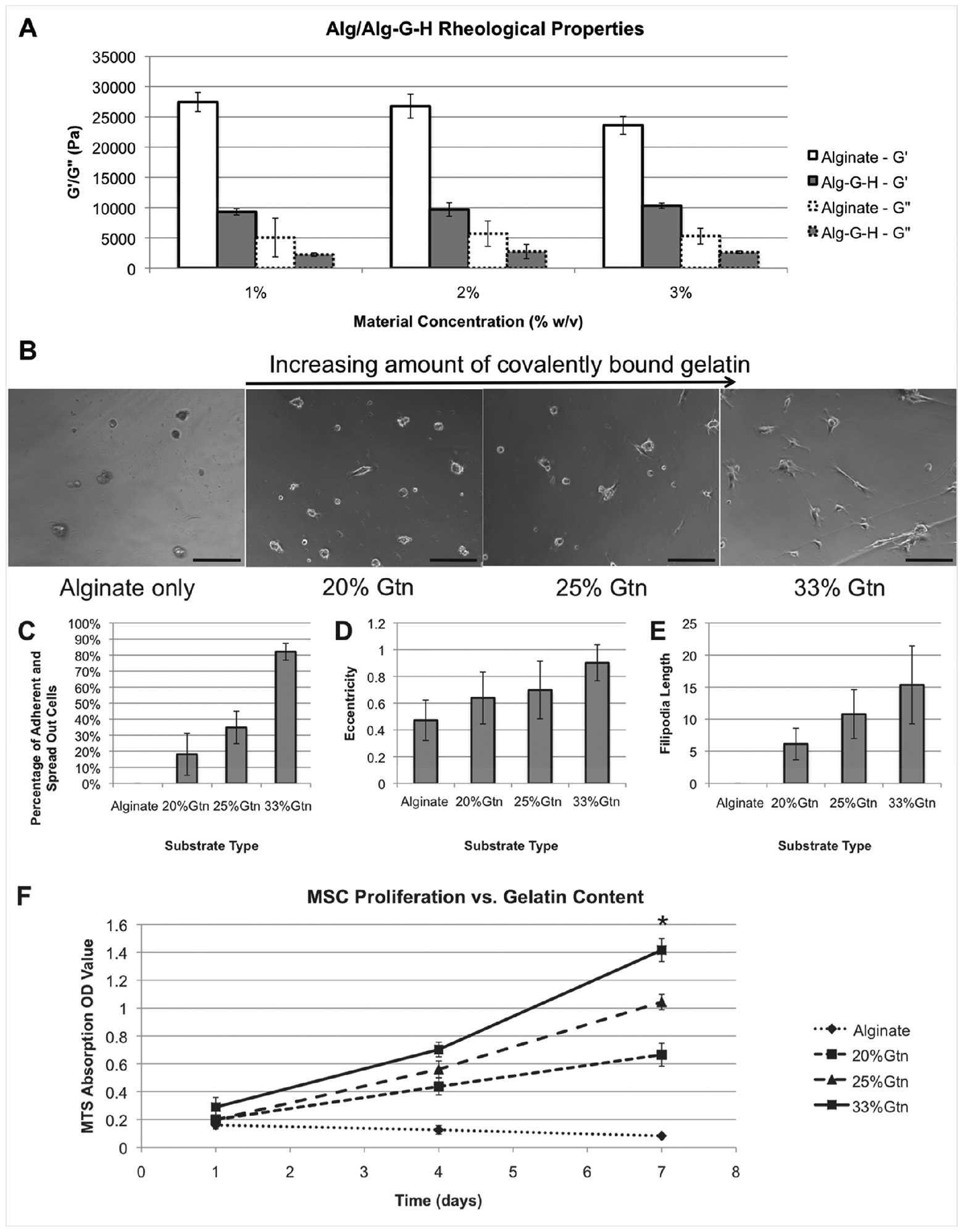
A) Rheological properties of unmodified alginate and Alg-G-H. G’ (storage modulus) and G” (loss modulus) are shown for 3 concentrations of alginate and Alg-G-H hydrogels. B) Images of MSCs seeded on alginate or Alg-G-H hydrogels, verified successful incorporation of the gelatin component in Alg-G-H material. Because an increasing relative ratio of gelatin-to-alginate is used in the chemical synthesis, the resulting hydrogel material is more successful at supporting cell adhesion. Scale bar=100 μm. C-E) Morphological assessment of cells seeded on alginate and Alg-G-H hydrogels. Cultures were assessed for C) percentage of adherent and spreading cells, D) eccentricity, and E) filopodia length. In each condition, a higher relative value indicates increased ability to attach and interact with the hydrogel. F) Proliferation of MSCs on alginate and different gelatin concentrations of Alg-G-H hydrogels up to 7 days. Data were shown based on 3 independent experiments with 3 replicates as mean ± SEM, One-way analysis of variance (ANOVA) and Student’s t-test were used for statistical calculation: * p < 0.01 in comparisons between Alg-G-H 33% gelatin versus all other substrates.
In general, a clear trend in both attachment and morphology was seen relative to gelatin percentages in the hydrogels (Fig. 2B). Surfaces without gelatin showed poor adherence – no visible cells were attached to the hydrogel. Upon rocking of the well plate, cells would roll or float away. However, as gelatin content increased, more and more cells adhered to hydrogels (Fig. 2C). At the highest level of gelatin composition (33%), over 80% of cells were firmly attached to the Alg-G-H surface.
Eccentricity (e) was used to determine proclivity of cells to become spindle-shaped, or spread linearly, characteristics common to cells of certain tissue types such as muscle or some mesenchymal lineages. e values close to 0.5 indicate round cells, generally observed in a nonadherent state on Alg-only hydrogels. Conversely, e values almost equal to 1 indicate a more linear, mesenchymal, and not evenly rounded morphology. Notably, the value of e increased steadily as the percentage of gelatin in the substrate increased (Fig. 2D).
Similar to the previous metrics, the presence of gelatin clearly affected filopodia presence and length (Fig. 2E). On Alg-only hydrogels, no filopodia were observed. Filopodia length increased with increasing percentages of gelatin in the hydrogel substrates. Based on these results, we used the 33% gelatin Alg-G-H substrates for the remaining studies.
To test effect of 4 substrates on cell viability and growth, proliferation rates were quantified by MTS assays (Fig. 2F). On Alg-only hydrogels, absorbance rates of MSCs decreased slightly over time, indicating that cells did not attach and proliferate well. In contrast, the absorbance rates increased with time and the rates raised with increasing gelatin concentrations when cells seeded on Alg-G-H hydrogels, suggesting that incorporation of the covalently-bound gelatin enhances cell adhesion, help cells to form the spindle-shape and proliferation. In addition, 33% gelatin of Alg-G-H substrates supported significantly higher adhesion and proliferation than all other substrates (p < 0.01).
3.2. Composition of porcine-derived skeletal muscle ECM
Biocolor ECM component assays revealed that collagen and elastin were present in the ECM solutions at much higher levels than GAGs. The total collagen content of skeletal muscle ECM solutions was 62.00 mg/mL and the elastin content was 56.10 mg/mL. However, GAGs were only present at 0.24 mg/ mL (Fig. 3A). Western blot results showed that collagen type I was by far the primary collagen type present in the skeletal muscle ECM, at 20 μg total protein for each well. Collagen types III and IV were present in relatively low percentages (Fig. 3B).
Figure 3. Components of skeletal muscle ECM.
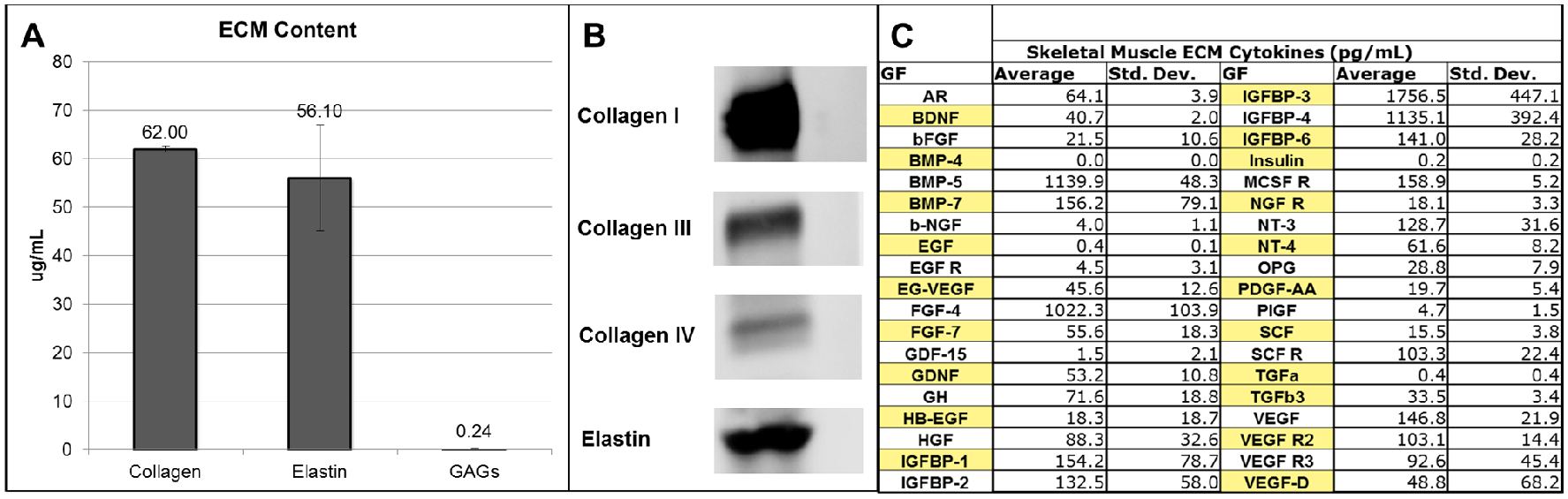
A) Collagen, elastin and glycosaminoglycans (GAGs) in content of skeletal muscle ECM. B) Western blot analysis for protein content of collagen I, III and IV in skeletal muscle ECM. C) Concentrations of cytokines in skeletal muscle ECM.
The growth factor and cytokine proteomics array results showed that the ECM solutions included a range of potent growth factors and cytokines (shown in pg/mL, Fig 3C). Present in relatively high levels were BMP-5 (1139.9 pg/mL), FGF-4 (1022.3 pg/mL), IGFBP-3 (1756.5 pg/mL), and IGFBP-4 (1135.1 pg/mL). Other important cytokines were also present, including bFGF (21.5 pg/mL), HGF (88.3 pg/mL), SCF receptor (103.3 pg/mL), TGF-3 (33.5 pg/mL), and VEGF (146.8 pg/mL), but at relatively lower concentrations.
3.3. VEGF and IGF1 release
Analyses of VEGF release from Alg-G-H and Alg hydrogels are shown in Fig. 4A–B. VEGF release was constant at each time point form Alg-G-H hydrogels, while release from Alg hydrogels decreased over time (Fig. 4A). Fig. 4B shows a similar release curve as Fig. 4A with ECM for both hydrogels, but VEGF content in ECM was present in low concentrations. These data suggest that heparin binding of VEGF successfully increased the effective duration of growth factor release. Conversely, IGF1, which does not bind heparin, showed no significant difference in release profiles between Alg-G-H and Alg hydrogels (Fig. 4C). Likewise, when the release study was repeated using the ECM solution rather than IGF, nearly the same release behavior of IGF was observed (Fig. 4D).
Figure 4. Growth factor release profiles of Alg-G-H gel with ECM compared to Alg gel alone after 2 weeks of induction.
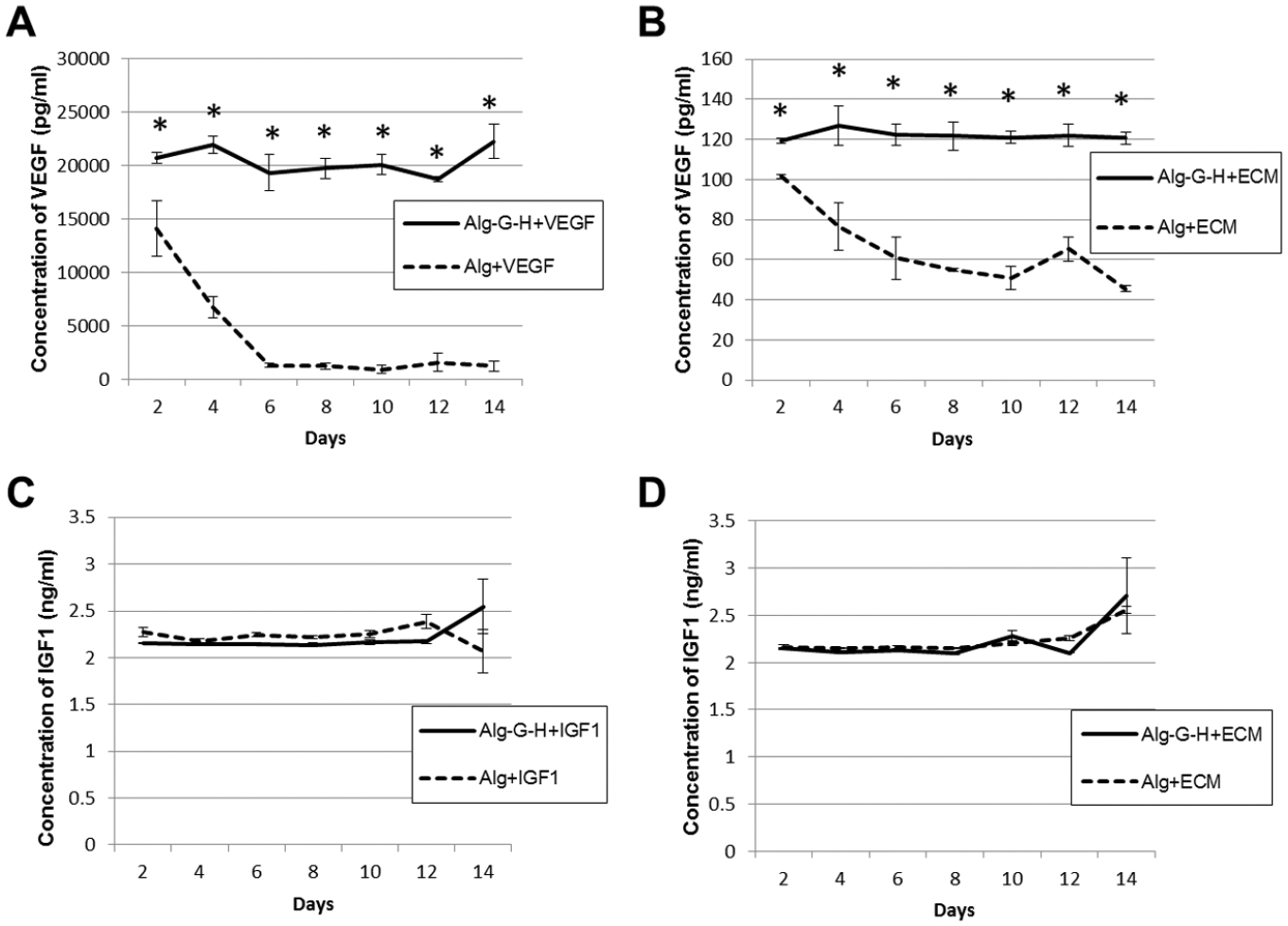
A) VEGF release in Alg alone and Alg-G-H conditions. B) VEGF release in Alg+ECM and Alg-G-H+ECM conditions. C) IGF1 release in Alg alone and Alg-G-H conditions. D) IGF1 release in Alg+ECM and Alg-G-H+ECM conditions. Data are shown for 3 independent experiments with 3 replicates as mean ± SEM. Student’s t test was used for calculation of the statistical significance between different release conditions at each time point, * p<0.05.
3.4. hSMPC proliferation in Alg-G-H and ECM conditions
Compared with hSMPCs on uncoated plates as controls, hSMPCs had similar proliferation profiles when seeded on Alg-G-H gel, ECM solution, and Alg-G-H+ECM gel mixture-coated plates within 7 days (Fig. 5). hSMPCs reached the logarithmic phase of growth in 3 to 5 days and kept stable without distinct decrease in cell populations. This indicated the effective support of Alg-G-H hydrogel with or without ECM for hSMPC proliferation, serving as reliable substrates for skeletal muscle cell differentiation and myotube maturation.
Figure 5. Growth curves of hSMPCs on different substrates.
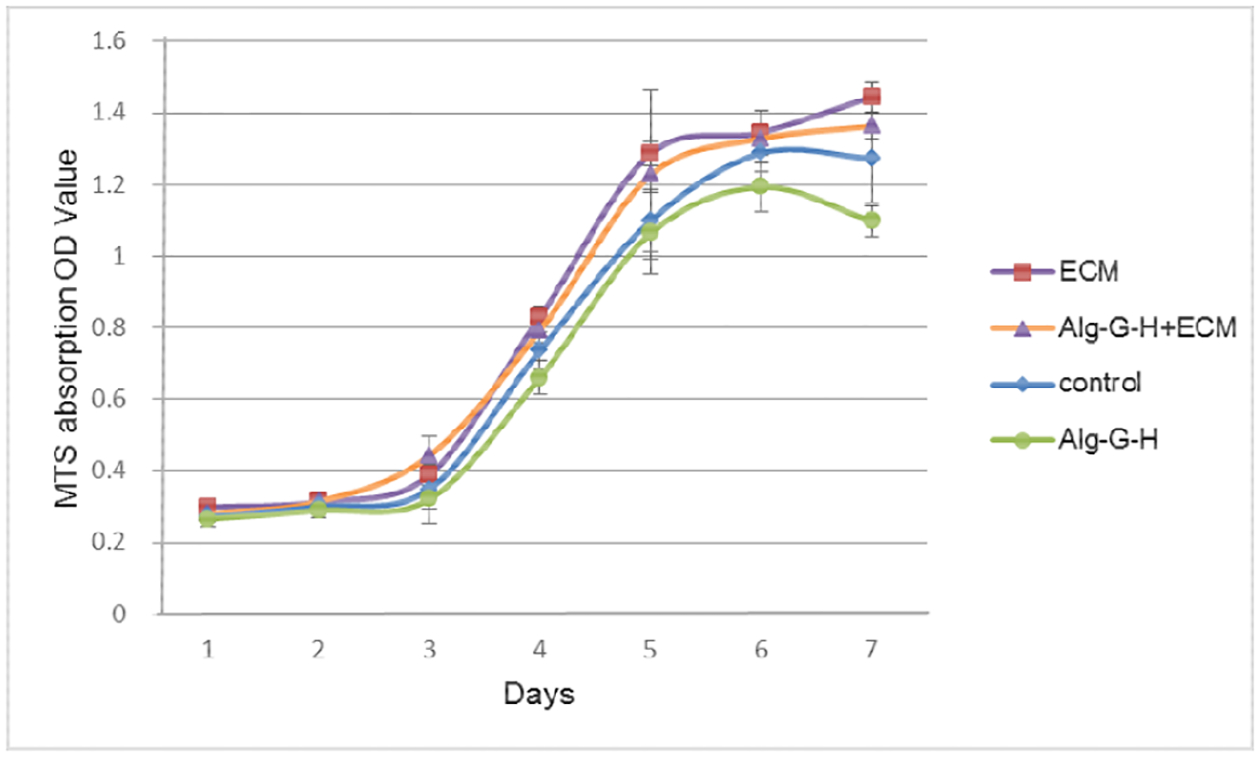
Agl-G-H, skeletal muscle ECM alone, and Alg-G-H+ECM gel solution coated conditions after 7 days of induction; cells on uncoated plates were the controls. Data were shown on 3 independent experiments with 3 replicates as mean ± SEM.
3.5. hSMPC differentiation and myotube formation on different matrices
When hSMPCs were maintained in skeletal muscle differentiation medium for 2 weeks, in many substrate conditions, myoblasts fused to form myotube structures with multiple nuclei arranged along the center of the tubes or gathered in one end. These could be positively stained with myogenesis-specific proteins in different stages, such as MyoD (details in Supplementary Figure S. 1) and Myf5 (S. 2) in the early myoblast period, Myogenin (S. 3) in the intermediate period, and Desmin (S. 4), and Myosin (S. 5) in the more mature period. Fig. 6 shows results for the control, Matrigel®, HA-HP-ECM, and Alg-G-H-ECM conditions; results for the remaining conditions are shown in Supplementary Figs. 1–5. However, the relative numbers of myotubes and multi-nuclei cell fusion rates (percentage of nuclei in positively stained syncytial cells versus total cell nuclei assessed) differed among conditions. Compared with uncoated conditions with few myotubes, hSMPCs had more myotubes formed separately in gelatin, Matrigel®, ECM solution and Alg-G-H gel-coated conditions (especially in HA-HP conditions, used as a positive control). In addition, the ECM solution combined coating conditions with Alg-G-H and HA-HP gel increased the quantity of myotubes and fused nuclei compared with individual gel conditions. Also, there were significantly more cells expressing Myf5, Myogenin, Desmin, and Myosin on Alg-G-H-ECM than those on Alg-G-H (p<0.05). Similarly, significantly more cells displayed MyoD, Myogenin and Myosin on HA-HP-ECM than on HA-HP (p<0.01) (Fig. 7 A–E).
Figure 6. Myogenic differentiation of hSMPCs on different substrates within 2 weeks.
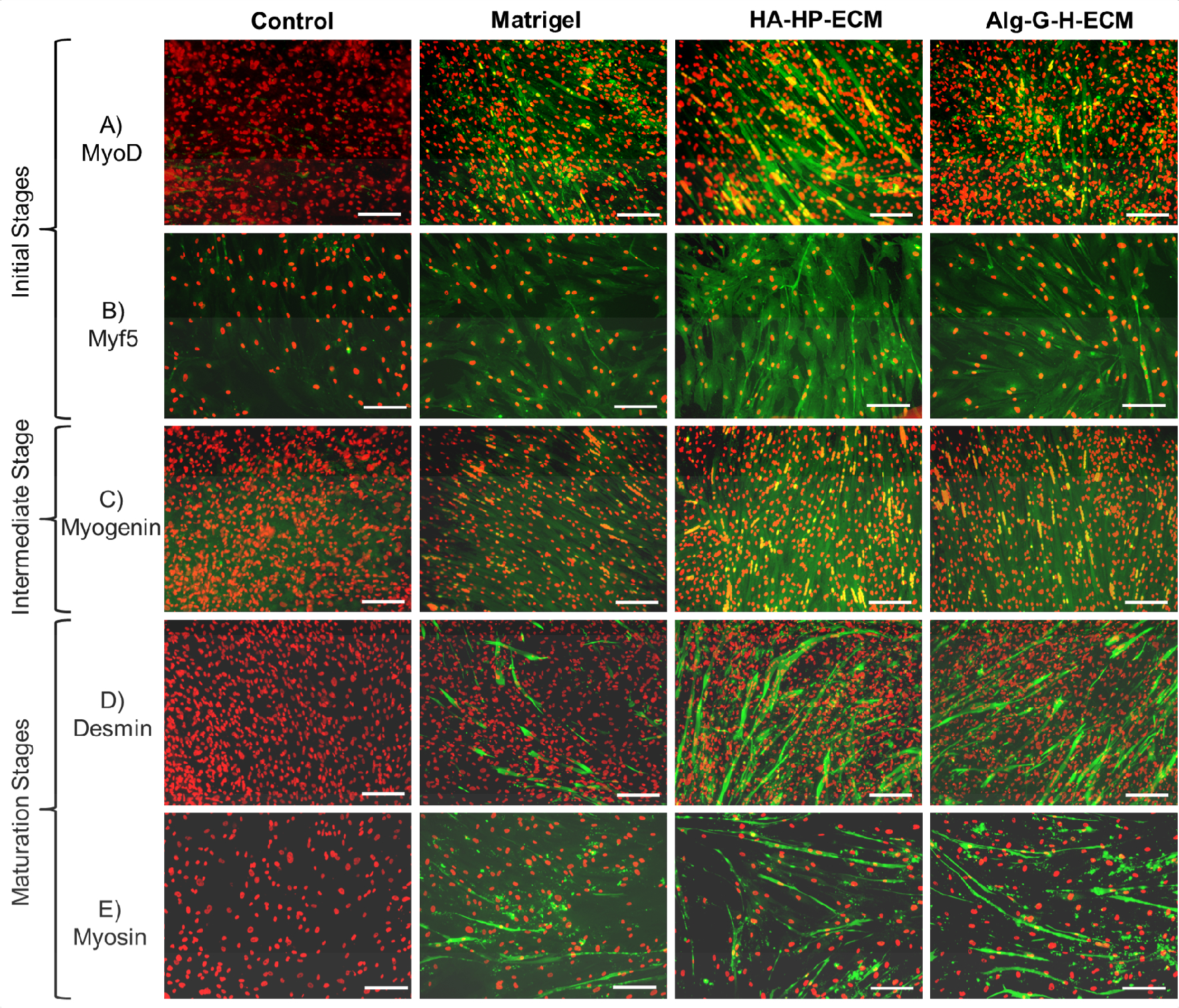
Myotube formation and multinuclear fusion on Matrigel®, HA-HP-ECM, and Alg-G-H-ECM coating conditions, compared with the gel with no ECM or uncoated condition as control, detected by immunofluorescent staining after 2-week differentiation with A) MyoD, B) Myf5 in the initial stage of myogenesis, C) Myogenin in the intermediate stage of myogenesis, D) Desmin, and E) Myosin in the maturation stage of myogenesis. All the differentiated cells positively stained with myogenesis markers performed green fluorescent, nuclei stained with propidium iodide (PI, red). Representative images are shown from 3 independent experiments with at least 3 replicates. Scale bar=100 μm.
Figure 7. Multinuclear cell fusion rate with expression of myogenesis markers in 2-week differentiation.
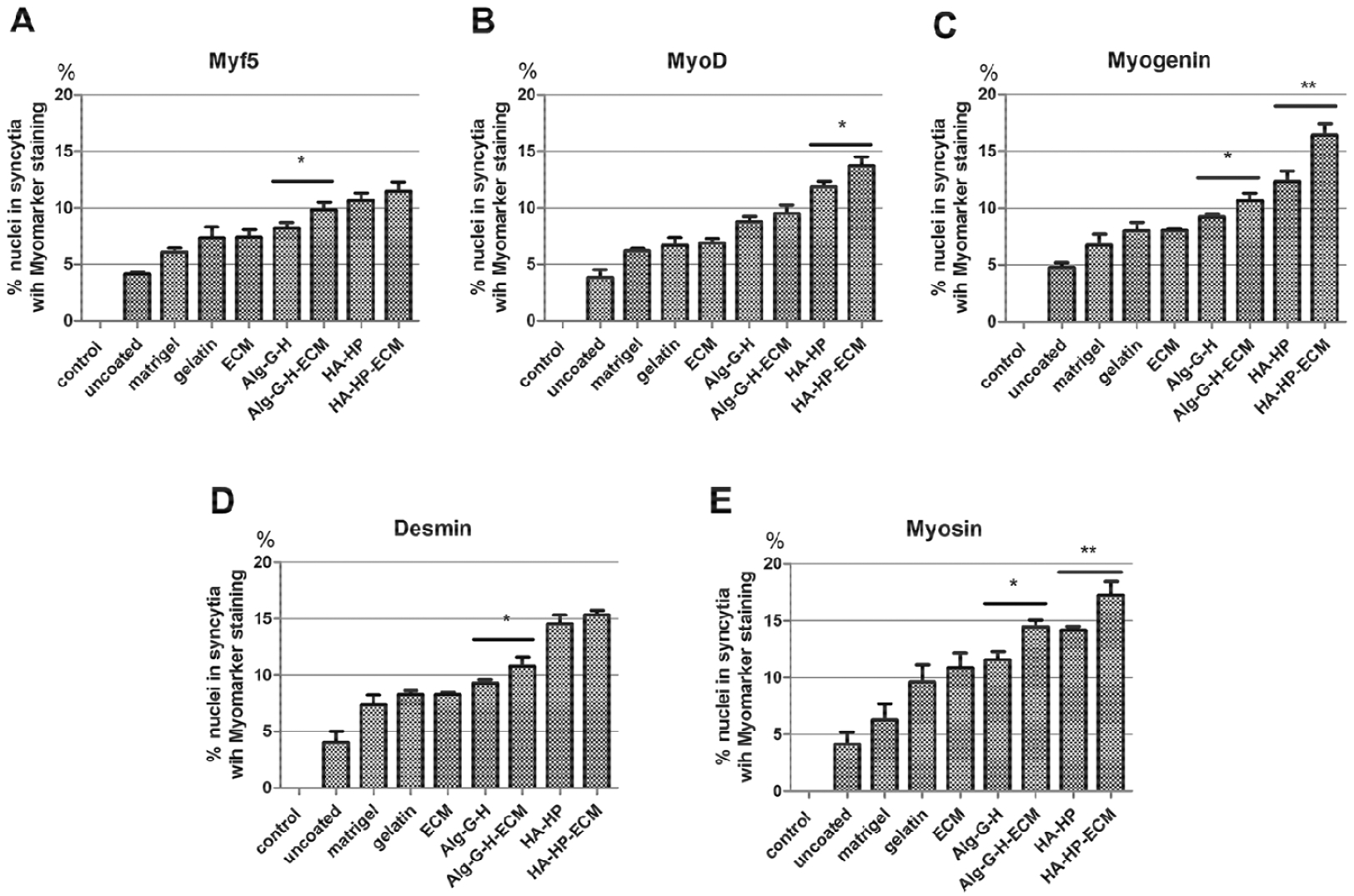
Percentage of nuclei in syncytia with positive staining for A) MyoD, B) Myf5, C) Myogenin, D) Desmin, and E) Myosin versus overall nuclei assessed, in different coating conditions such as Matrigel®, gelatin, ECM, Alg-G-H, Alg-G-H-ECM, HA-HP, HA-HP-ECM, compared with uncoated conditions and normal hSMPCs as control. Histograms are means ± SEM of four experiments on each independent culture condition. Data were analyzed by the one-way analysis of variance (ANOVA) for all groups, and then Student’s t-test for comparison of two groups. Results are presented as mean ± SEM, * p<0.05, ** p<0.01.
3.6. Protein expression of hSMPCs in differentiation periods
Different matrices may provide discrepant microenvironments for differentiation of hSMPCs. Protein expression of MyoD, Myf5, Myogenin, Desmin, and Myosin on Western blots was generally consistent with immunostaining observations (Fig. 8 A). The Alg-G-H and HA-HP gel solution mixed with ECM showed stronger protein expression compared with the other conditions.
Figure 8. Myogenesis protein expression profiles of hSMPCs in different coating conditions.
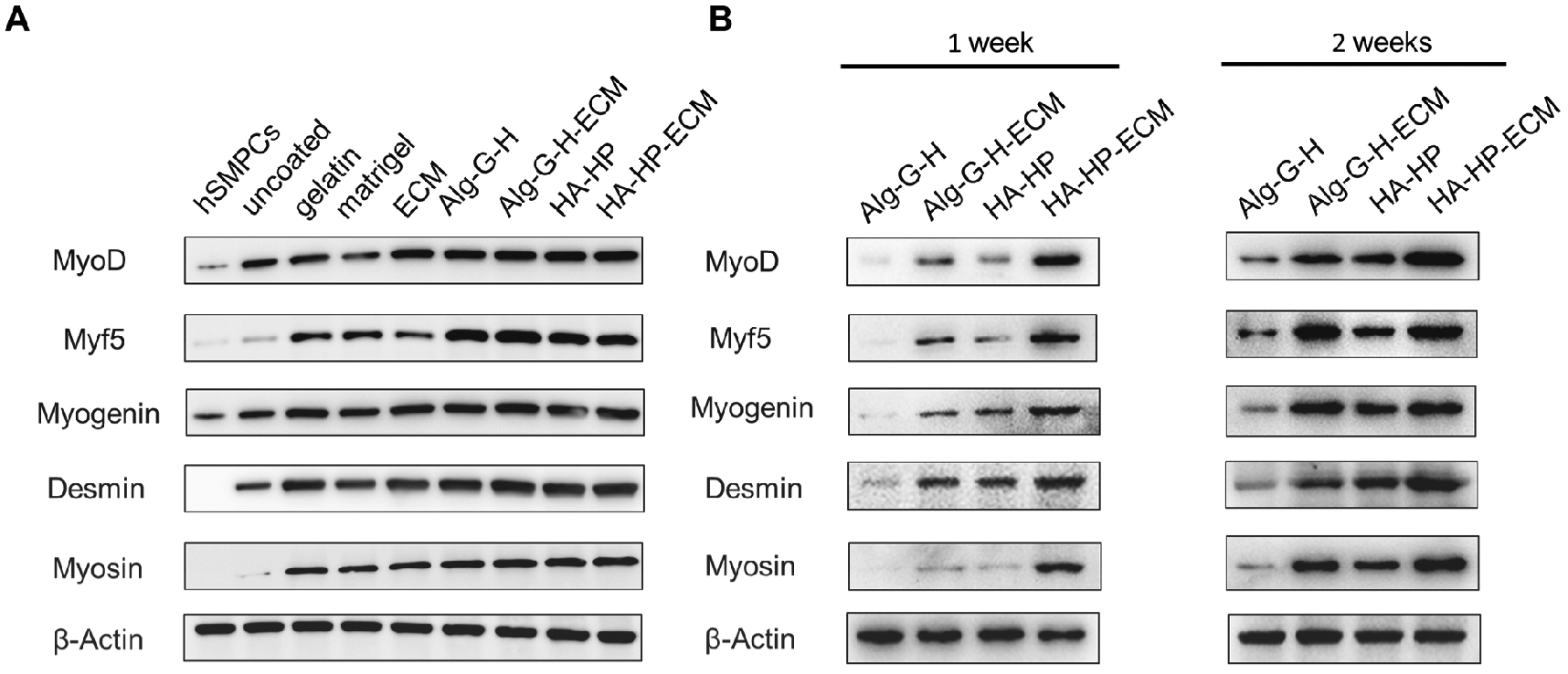
A) Overall myogenesis protein expression profiles detected by Western blot after 2-week differentiation in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA-HP and HA-HP-ECM coating conditions. B) Myogenesis protein expression of hSMPCs induced with 1% FBS after 1 week and 2 weeks in Alg-G-H, Alg-G-H-ECM, HA-HP and HA-HP-ECM coating conditions. Representative images are shown from experiments performed at least three times.
Because the differentiation medium contains horse and fetal bovine serum – which may contain undefined growth factors and cytokines that could also aid in directing skeletal muscle differentiation and maturation – we reduced the serum concentration to the minimum level of 1% and once again prepared substrates. We limited the conditions to Alg-G-H, Alg-G-H-ECM, HA-HP, and HA-HP-ECM based on their performance in the previous experiment, and induced differentiation of hSMPCs for 1 week and 2 weeks. MyoD, Myf5, Myogenin, Desmin, and Myosin were all expressed in the 4 coating conditions, but appeared more strongly expressed in coating conditions with ECM (Fig. 8 B). Expression levels of Desmin and Myosin in later stages of myotube formation were higher after 2 weeks of induction than after 1 week, as would be expected.
4. Discussion
Tissue regeneration offers great therapeutic potential for muscle damage due to congenital defects, severe trauma, and aging-associated or degenerative disorders. While progress has been made in harnessing biological approaches for skeletal muscle regeneration, there are still challenges in propagating muscle cells in vitro and retaining skeletal muscle cell function with physiological contraction forces [31, 32]. In this study, we confirmed that by incorporating growth factor-rich muscle ECM into alginate hydrogels covalently modified with gelatin and heparin, we could provide the critical sites for cell adherence and maintain the appropriate concentrations of bioactive factors and growth factor retention, thus creating appropriate culture conditions for hSMPC cell growth and myogenesis.
To construct skeletal muscle units, stimulating growth factors, cytokines, chemokines are required to mimic the in vivo environment for muscle cell differentiation and maturation [33]. Gelatin, Matrigel®, and hyaluronic acid biomaterials were tested alongside our novel modified alginate biomaterial as basal substrates for skeletal muscle growth and maturation. Hyaluronic acid hydrogels combined with heparin (HA-HP) can successfully integrate ECM components for creating in vivo-like environments [28, 29]. We hypothesized these would be an effective positive control condition, along with Matrigel®, which possesses adequate cytokines and growth factors. However, Matrigel® originates from mouse sarcoma, which restricts its applicability for future use in clinical applications [34].
Other researchers have used synthetic polymers to fabricate biocompatible materials that are combined with skeletal muscle ECM molecules physically or covalently, promoting viability, adhesion, proliferation, and differentiation of skeletal muscle cells. These substrates with typical ECM components were engineered to resemble the native skeletal muscle tissue structures that facilitate applications in tissue engineering and regenerative medicine [35–37]. While this concept of integrating ECM materials with other biomaterials is not new, we identified a potential new avenue for this approach using alginate. As described above, alginate is used very widely in tissue engineering research, despite being inert, and not supporting cell adherence or cytokine loading. As such, we aimed to modify this inexpensive and widely employed material to have more biologically active properties. Our study showed that a novel biomaterial of alginate conjugated with gelatin and heparin (Alg-G-H) possessed functions similar to the HA-HP hydrogels. With the combination of skeletal muscle-specific ECM gel solution as a coating substrate, Alg-G-H supplied a requisite niche for hSMPC proliferation and later myotube maturation. Notably, Alg-G-H generally matched the more expensive HA-HP in these outcomes, and significantly outperformed Matrigel®.
Within the niche provided by skeletal muscle-specific ECM, multipotent stem cells such as satellite cells or hSMPCs can self-renew, proliferate, and differentiate when necessary. Without the necessary bioactive agents provided by the ECM, these cells can lose these capabilities [33, 38, 39]. Our data demonstrated that crucial growth factors and cytokines in the ECM (including HGF, IGF1, FGF2, EGF, and TGF 1) together help to regulate myogenesis in developing or regenerating stages in muscle tissue [40].
Protein expression profiles of cells from Alg-G-H and HA-HP gel coatings after 2 weeks compared with 1 week of differentiation demonstrate that ECM integration supports differentiation, likely through sustained protein release, through its continuous supply of indispensable growth factors and cytokines sequestered by the Alg-G-H gel. In response to cues from the microenvironment, myogenesis starts in satellite cells or muscle progenitor cells (Pax7 expression) [41], enters the myoblast stage (MyoD and Myf5 expression) [42, 43], develops multi-cell fusion and further maturation of muscle fibers (Myogenic and Desmin expression) [44], and finally reaches the maturation stage of myotubes (Myosin expression). In our study, on this ECM and hydrogel substrate, hSMPCs underwent differentiation to form myoblasts, followed by myotube maturation. In many of the other comparison substrates, these endpoints were not consistently observed, suggesting that the ECM plays an integral role in modulating the fate of hSMPCs. It appears that components of muscle ECM combined with the alginate and heparin niche facilitate activation of differentiation mechanisms by supplying physical and chemical cues via cell-cell and cell-matrix communication [45].
In these experiments, Alg-G-H-based substrates with VEGF could sequester the requisite proteins and chemical compounds and support sustained release for 2 weeks sufficient for myogenesis. On the other hand, IGF1 had release kinetics similar to the alginate-only hydrogels, which did not contain heparin. Nevertheless, the lack of heparin-modulated release of IGF1 did not prevent successful myogenesis and maturation [46]. It is also possible that physical components from the ECM (e.g. collagens, elastin, GAGs) immobilized within the cross-linked hydrogels may be important in coordinating requisite conditions for skeletal muscle regeneration [37]. These components of the ECM can certainly be important in creating an environment for cell function, and we will explore the respective roles of these and other ECM components in subsequent studies.
Recently, tissue engineering has advanced to include 3D in vitro cell culture techniques [47, 48]. The ultimate goal is to create and sustain tissue that most closely mimics in vivo tissue [49]. To do so, it is important to recapitulate environments of actual tissue that support cellular function and phenotype, which is generally not possible using traditional 2D culture methods on plastic substrates [50]. Alg-G-H as a hydrogel material has advantages in terms of flexible manipulation and low cost. Alginate can be sourced and chemically modified in the lab for significantly less than modified hyaluronic acid kits and Matrigel®. However, alginate gels were not completely transparent, which could limit their applicability for true 3-D encapsulation cultures. Although growth kinetics and differentiation of hSMPCs may not be negatively affected, microscopy (and associated visual assays focused on morphology, biomarker expression, and other visual characteristics) become difficult in conditions of opacity. We are currently working to remedy this issue to achieve a more broadly applicable biomaterial complete with a variety of uses in 3D culture. As 3D cultures are significantly better representations of native tissues in the human body, skeletal muscle ECM can be added as an optimal substrate into Alg-G-H hydrogels to form 3D tissue constructs in vivo. In future experiments, we will study whether Alg-G-H hydrogel with muscle ECM can improve skeletal muscle tissue integrity, functionality, and structure In vivo.
5. Conclusions
Alginate, covalently modified with gelatin and heparin, was developed as a novel yet simple variation of alginate, to generate a more bioactive material with potential for application in regenerative medicine and tissue engineering. This material, combined with ECM solution prepared from skeletal muscle, was advantageous for skeletal muscle progenitor cell maintenance and differentiation into mature muscle fibers compared to other hydrogel conditions. The incorporation of gelatin and heparin allows cell adherence, and supports sequestration of heparin-binding cytokines to sustain the required biochemical niche characteristics for cell survival and differentiation. The alginate-based material was an effective platform for skeletal muscle cell proliferation and differentiation to form mature myotubes. This combination could be an economical alternative for skeletal muscle regeneration and tissue engineering of skeletal muscle constructs, compared to more expensive biomaterials commercially available. This improved preparation has potential application for clinical use as a therapeutic treatment for muscular injuries and volumetric muscle loss.
Supplementary Material
S. 1. MyD staining (green) in the initial stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA and HA-ECM coating conditions, compared with uncoated condition and noninduced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S. 2. Myf5 staining (green) in the initial stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA and HA-ECM coating conditions, compared with uncoated condition and non-induced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S. 3. Myogenin staining (green) in the intermediate stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA and HA-ECM coating conditions, compared with uncoated condition and noninduced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S 4. Desmin staining (green) in the maturation stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA, HA-ECM coating conditions, compared with uncoated condition and noninduced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S 5. Myosin staining (green) in the maturation stage of myogenesis in 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA, HA-ECM coating conditions, compared with uncoated condition and non-induced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
Significance Statement.
Alginate based biomaterials are commonly used in tissue engineering and regenerative medicine field, however, the inefficient sequestration of growth factors restricted its utilization. In this study, a novel alginate based substrates was produced covalently modified with gelatin and heparin, in order to capture more effective cytokines and proteins in the culture milieu, keep homeostasis for cell survival and tissue regeneration with growth factor sequestration and long-term release capacities. Combining with skeletal muscle derived ECM, the modified Alginate-Gelatin-Heparin gel could most effectively mimic the tissue specific microenvironment to support skeletal muscle progenitor cells proliferation, differentiation and myotube formation. Additionally, the economical and practical features will make it more promising in high-throughput application for regenerative medicine research.
6. Acknowledgements
The authors acknowledge funding support from NIH grant R56 DK100669 (Y Zhang), Wake Forest Institute for Regenerative Medicine Promoting Discoveries Award (A Skardal) and Singapore Ministry of Health’s National Medical Research Council under its CS Individual Research Grant scheme NMRC/CIRG/1356/2013. We also acknowledge the editorial assistance of Karen Klein, MA, in the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: McClain).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nature Cell Biology, 18 (2016), pp. 246–254. [DOI] [PubMed] [Google Scholar]
- [2].Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science, 345 (2014), p. 1247125. [DOI] [PubMed] [Google Scholar]
- [3].Langer R, Vacanti JP. Tissue engineering. Science, 260 (1993), pp. 920–926. [DOI] [PubMed] [Google Scholar]
- [4].Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, and Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proceedings of the National Academy of Sciences of the United States of America, 107 (2010), pp. 3287–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature Biotechnology, 19 (2001), pp. 1029–1034. [DOI] [PubMed] [Google Scholar]
- [6].Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nature Cell Biology, 9 (2007), pp. 255–267. [DOI] [PubMed] [Google Scholar]
- [7].Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. Journal of Thrombosis and Haemostasis, 5 (2007), pp. 590–598. [DOI] [PubMed] [Google Scholar]
- [8].Coleman MP, Ribchester RR. Programmed axon death, synaptic dysfunction and the ubiquitin proteasome system. Current Drug Targets CNS and Neurological Disorders, 3 (2004), pp. 227–238. [DOI] [PubMed] [Google Scholar]
- [9].Musaro A, Dobrowolny G, Rosenthal N. The neuroprotective effects of a locally acting IGF-1 isoform. Experimental Gerontology, 42 (2007), pp. 76–80. [DOI] [PubMed] [Google Scholar]
- [10].Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature, 400 (1999), pp. 581–585. [DOI] [PubMed] [Google Scholar]
- [11].Bark TH, McNurlan MA, Lang CH, Garlick PJ. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. The American Journal of Physiology, 275 (1998), pp. E118–123. [DOI] [PubMed] [Google Scholar]
- [12].Shansky J, Creswick B, Lee P, Wang X, Vandenburgh H. Paracrine release of insulin-like growth factor 1 from a bioengineered tissue stimulates skeletal muscle growth in vitro. Tissue Engineering, 12 (2006), pp. 1833–1841. [DOI] [PubMed] [Google Scholar]
- [13].Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, Ramalingam M, Khademhosseini A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Engineering Part A, 18 (2012), pp. 2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. Journal of Cellular and Molecular Medicine, 12 (2008), pp. 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Current Opinion in Biotechnology, 24 (2013), pp. 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Markert CD, Guo X, Skardal A, Wang Z, Bharadwaj S, Zhang Y, Bonin K, Guthold M. Characterizing the micro-scale elastic modulus of hydrogels for use in regenerative medicine. Journal of the Mechanical Behavior of Biomedical Materials, 27 (2013), pp. 115–127. [DOI] [PubMed] [Google Scholar]
- [17].Kiick KL. Peptide- and protein-mediated assembly of heparinized hydrogels. Soft Matter, 4 (2008), pp. 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giovagnoli S, Luca G, Blasi P, Mancuso F, Schoubben A, Arato I, Calvitti M, Falabella G, Basta G, Bodo M, Calafiore R, Ricci M. Alginates in Pharmaceutics and Biomedicine: Is the Future so Bright? Current Pharmaceutical Design, 21 (2015), pp. 4917–4935. [DOI] [PubMed] [Google Scholar]
- [19].Khanna O, Moya ML, Greisler HP, Opara EC, Brey EM. Multilayered microcapsules for the sustained-release of angiogenic proteins from encapsulated cells. American Journal of Surgery, 200 (2010), pp. 655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu G, Pareta RA, Wu R, Shi Y, Zhou X, Liu H, Deng C, Sun X, Atala A, Opara EC, Zhang Y. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials, 34 (2013), pp. 1311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Molecular therapy : the journal of the American Society of Gene Therapy, 22 (2014), pp. 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromolecular Bioscience, 6 (2006), pp. 623–633. [DOI] [PubMed] [Google Scholar]
- [23].Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials, 20 (1999), pp. 45–53. [DOI] [PubMed] [Google Scholar]
- [24].Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on microbead swelling. Journal of Microencapsulation, 23 (2006), pp. 613–621. [DOI] [PubMed] [Google Scholar]
- [25].Skardal A, Zhang J, McCoard L, Oottamasathien S, Prestwich GD. Dynamically crosslinked gold nanoparticle - hyaluronan hydrogels. Advanced Materials, 22 (2010), pp. 4736–4740. [DOI] [PubMed] [Google Scholar]
- [26].Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Engineering Part A, 16 (2010), pp. 2675–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials, 31 (2010), pp. 6173–6181. [DOI] [PubMed] [Google Scholar]
- [28].Skardal A, Smith L, Bharadwaj S, Atala A, Soker S, Zhang Y. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials, 33 (2012), pp. 4565–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Skardal A, Devarasetty M, Kang HW, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomaterialia, 25 (2015), pp. 24–34. [DOI] [PubMed] [Google Scholar]
- [30].Eberli D, Soker S, Atala A, Yoo JJ. Optimization of human skeletal muscle precursor cell culture and myofiber formation in vitro. Methods 47 (2009), pp. 98–103. [DOI] [PubMed] [Google Scholar]
- [31].Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials, 26 (2005), pp. 1857–1875. [DOI] [PubMed] [Google Scholar]
- [32].Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Engineering in Medicine and Biology Magazine : the Quarterly Magazine of the Engineering in Medicine & Biology Society, 27 (2008), pp. 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GN. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials, 53 (2015), pp. 502–521. [DOI] [PubMed] [Google Scholar]
- [34].Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry, 25 (1986), pp. 312–318. [DOI] [PubMed] [Google Scholar]
- [35].Jana S, Levengood SK, and Zhang M, Anisotropic Materials for Skeletal-Muscle-Tissue Engineering. Advanced Materials, 28 (2016), pp. 10588–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ma Z, Mao Z, and Gao C, Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids and Surfaces B Biointerfaces, 60 (2007), pp. 137–157. [DOI] [PubMed] [Google Scholar]
- [37].Jun I, Kim SJ, Choi E, Park KM, Rhim T, Park J, Park KD, and Shin H, Preparation of biomimetic hydrogels with controlled cell adhesive properties and topographical features for the study of muscle cell adhesion and proliferation. Macromolecular Bioscience, 12 (2012), pp. 1502–1513. [DOI] [PubMed] [Google Scholar]
- [38].Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiological Reviews, 84 (2004), pp. 209–238. [DOI] [PubMed] [Google Scholar]
- [39].Velleman SG. The role of the extracellular matrix in skeletal muscle development. Poultry Science, 78 (1999), pp. 778–784. [DOI] [PubMed] [Google Scholar]
- [40].Velleman SG. Meat Science and Muscle Biology Symposium: extracellular matrix regulation of skeletal muscle formation. Journal of Animal Science, 90 (2012), pp. 936–941. [DOI] [PubMed] [Google Scholar]
- [41].Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature, 435 (2005), pp. 948–953. [DOI] [PubMed] [Google Scholar]
- [42].Tajbakhsh S. Skeletal muscle stem and progenitor cells: reconciling genetics and lineage. Experimental Cell Research, 306 (2005), pp. 364–372. [DOI] [PubMed] [Google Scholar]
- [43].Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell, 75 (1993), pp. 1351–1359. [DOI] [PubMed] [Google Scholar]
- [44].Pownall ME, Gustafsson MK, Emerson CJ. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annual Review of Cell and Developmental Biology, 18 (2002), pp. 747–783. [DOI] [PubMed] [Google Scholar]
- [45].Gilbert TW, Stewart-Akers AM, Sydeski J, Nguyen TD, Badylak SF, Woo SL. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Engineering, 13 (2007), pp. 1313–1323. [DOI] [PubMed] [Google Scholar]
- [46].Mullen LM, Best SM, Ghose S, Wardale J, Rushton N, Cameron RE. Bioactive IGF-1 release from collagen-GAG scaffold to enhance cartilage repair in vitro. Journal of Materials Science Materials in Medicine, 26 (2015), p. 5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kim MJ, Shin YC, Lee JH, Jun SW, Kim CS, Lee Y, Park JC, Lee SH, Park KD, Han DW. Multiphoton imaging of myogenic differentiation in gelatin-based hydrogels as tissue engineering scaffolds. Biomaterials Research, 20 (2016), p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yan W, George S, Fotadar U, Tyhovych N, Kamer A, Yost MJ, Price RL, Haggart CR, Holmes JW, Terracio L. Tissue engineering of skeletal muscle. Tissue Engineering, 13 (2007), pp. 2781–2790. [DOI] [PubMed] [Google Scholar]
- [49].Lee N, Robinson J, Lu H. Biomimetic strategies for engineering composite tissues. Current Opinion in Biotechnology, 40 (2016), pp. 64–74. [DOI] [PubMed] [Google Scholar]
- [50].Choi J, Lee EK, Choo J, Yuh J, Hong JW. Micro 3D cell culture systems for cellular behavior studies: Culture matrices, devices, substrates, and in-situ sensing methods. Biotechnology Journal, 10 (2015), pp. 1682–1688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S. 1. MyD staining (green) in the initial stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA and HA-ECM coating conditions, compared with uncoated condition and noninduced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S. 2. Myf5 staining (green) in the initial stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA and HA-ECM coating conditions, compared with uncoated condition and non-induced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S. 3. Myogenin staining (green) in the intermediate stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA and HA-ECM coating conditions, compared with uncoated condition and noninduced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S 4. Desmin staining (green) in the maturation stage of myogenesis after 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA, HA-ECM coating conditions, compared with uncoated condition and noninduced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.
S 5. Myosin staining (green) in the maturation stage of myogenesis in 2 weeks induction in gelatin, Matrigel®, ECM, Alg-G-H, Alg-G-H-ECM, HA, HA-ECM coating conditions, compared with uncoated condition and non-induced SMPCs as control. Nuclei marked with propidium iodide. Representative images are shown from experiments performed at least three times. Scale bar=100 μm.


