Abstract
(1) Background: because of close contacts with COVID-19 patients, hospital workers are among the highest risk groups for infection. This study examined the socioeconomic and behavioral correlates of COVID-19 infection among hospital workers in Indonesia, the country hardest-hit by the disease in the Southeast Asia region. (2) Methods: we conducted a cross-sectional study, which collected data from 1397 hospital staff from eight hospitals in the Greater Jakarta area during April–July 2020. The data was collected using an online self-administered questionnaire and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) tests. We employed descriptive statistics and adjusted and unadjusted logistic regressions to analyze the data of hospital workers as well as the subgroups of healthcare and non-healthcare workers. (3) Results: from a total of 1397 hospital staff in the study, 22 (1.6%) were infected. In terms of correlates, being a healthcare worker (adjusted odds ratio (AOR) = 8.31, 95% CI 1.27–54.54) and having a household size of more than five (AOR = 4.09, 1.02–16.43) were significantly associated with a higher risk of infection. On the other hand, those with middle- and upper-expenditure levels were shown to have a lower risk of infection (AOR = 0.06, 0.01–0.66). Behavioral factors associated with COVID-19 infection among healthcare and non-healthcare workers included knowledge of standard personal protective equipment (PPE) (AOR = 0.08, 0.01–0.54) and application of the six-step handwashing technique (AOR = 0.32, 0.12–0.83). (4) Conclusion: among hospital staff, correlates of COVID-19 infection included being a healthcare worker, household size, expenditure level, knowledge and use of PPE, and application of appropriate hand washing techniques.
Keywords: socioeconomic, protective behaviors, COVID-19, healthcare workers, hospital, Indonesia
1. Introduction
Since being officially declared as a global pandemic by the World Health Organization (WHO) in March 2020, coronavirus disease 2019 (COVID-19) has infected over 128.5 million people and has caused more than 2.8 million deaths in 206 countries worldwide by 31 March 2021 [1]. With the burden of the currently existing public health issue, the consequences of this pandemic have been well predicted to be suffered the most by the developing countries compared to their developed counterparts [2]. Despite the implementation of activity restrictions as well as individual and communal protective behaviors at the national and regional levels [3,4], Indonesia has become the country worst-hit by COVID-19 by having the highest number of cases in the South East Asia region in addition to being among the highest mortality rates in the world [5]. As of 31 March 2021, the government has reported over 1.5 million confirmed cases of COVID-19 with 40,858 deaths since the first case was detected on 2 March 2020 [1].
Because of the close contact with COVID-19 patients, those working in healthcare facilities, both healthcare and non-healthcare staff, are among the highest risk groups for infection by COVID-19 [6]. Some studies have found that workers in health facilities have a higher risk of COVID-19 infection than the general population [7,8]. Globally, there were 152,888 healthcare workers recorded as being infected by 8 May 2020 [9]. In Indonesia, a report by the Medical Association revealed that 654 healthcare workers died because of COVID-19 by January 2021 [10]. This has put Indonesia in first and third place in the region and in the world, respectively, in terms of the COVID-19 fatality rate among healthcare workers [11]. With the low healthcare-workers–population ratio, it has been estimated that the country’s healthcare workers have an increased risk of the virus because of high exposure [12]. Considering their critical role in the front line, it is important to understand the correlates of morbidity and mortality among healthcare workers and non-healthcare workers in health facilities in Indonesia.
Previous studies have explored several risk factors related to the previous and current coronavirus infection among hospital staff and/or healthcare workers. Looking back to the SARS-CoV 1 and MERS CoV epidemics, close contact with infected patients, use of PPE, and infection control training turned out to be the predominant risk factors for virus transmission among hospital staff [13,14,15]. In line with the previous epidemics, close contact with infected patients, working in emergency units, overworking, older age, having poor personal protective equipment (PPE), training guidance provision from hospitals, and poor hand hygiene have been found as correlates of COVID-19 infection among healthcare workers [8,9,16,17,18,19,20,21,22]. However, most of these studies were conducted in high-income countries [8,9,17,18,19]. Studies examining the determinants of COVID-19 infections among healthcare workers in low- and middle-income countries (LMICs) were only conducted in China [16,20,21,22]. Thus, our study aims to fill the gap by examining demographic and behavioral correlates of COVID-19 infections among hospital workers in Indonesia, an upper-middle-income country. We hypothesized that COVID-19-related protective behaviors may lower the infection risks, while demographic characteristics may have various significances and relationship directions.
2. Methods
2.1. Study Design and Data
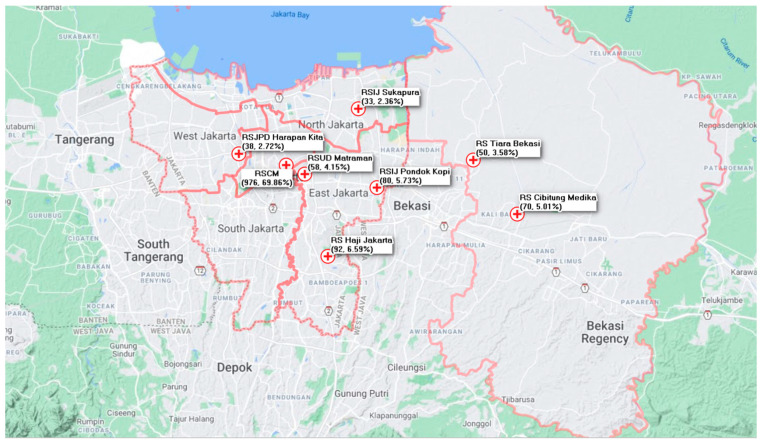
This was a cross-sectional study involving 1397 participants, which included healthcare and non-healthcare hospital workers in eight hospitals in the Greater Jakarta Area (Figure 1), the capital of Indonesia. The area was chosen for several considerations: (1) Jakarta has been one of the epicenters of COVID-19 transmission in Indonesia, which has had relatively high COVID-19 cases since the beginning of the pandemic [1], and (2) as a metropolitan city, Jakarta contains many risk factors for COVID-19 infection, such as poor air pollution [23,24] and severe overcrowding [25].
Figure 1.
Participating hospitals in the Greater Jakarta Area.
The primary data collection was conducted from 9 April–1 July 2020. The participants were selected through two channels: partnership agreement and online recruitment. Participants from five hospitals were recruited through a partnership agreement with the Center for Indonesia’s Strategic Development Initiatives (CISDI), whereas the rest were recruited online. An online recruitment was posted on social media to attract hospitals interested in getting free Reverse Transcription-Polymerase Chain Reaction (RT-PCR) tests for their staff. The inclusion criteria for the hospitals included being a COVID-19 referral hospital, having staff with confirmed COVID-19 cases for the past 14 days, and not receiving any access to regular RT-PCR tests from the government. In the recruitment process of participants in each hospital, we suggested including healthcare and non-healthcare workers with the following criteria: (1) had close contact with at least one COVID-19 patient and (2) developed COVID-19 related symptoms. However, in practice, we had minimum control to select the participants based on those criteria.
Participants were asked to fill out a self-administered questionnaire to collect information regarding demographic characteristics and protective behaviors. The data on SARS-CoV-2 infection were obtained based on oropharyngeal and nasopharyngeal swab specimens by trained healthcare workers at participating hospitals. All specimens were sent for RT-PCR testing to the University of Indonesia Clinical Microbiology Lab, which is among the first few laboratories appointed as a COVID-19 laboratory in Indonesia. Results of the self-assessed questionnaire and the tests were matched and analyzed.
2.2. Study Variables
The primary dependent variable was COVID-19 infection (1 = positive, 0 = otherwise). An additional dependent variable was having at least one of the main COVID-19 symptoms. The UK National Health Service recommends anyone who experiences one of these main symptoms to get an immediate COVID-19 test: a high temperature, a continuous cough, partial/complete loss of the sense of smell, or partial/complete loss of the sense of taste [26].
The independent variables included two groups: sociodemographic characteristics and protective behaviors. First, sociodemographic variables included sex, being a healthcare worker, age, household size, expenditure level, and smoking status. Under National Law 36/2014, healthcare workers include doctors, dentists, nurses, pharmacists, laboratory staff, and medical interns/residents. The age groups included young adults (19–24 years of age), adults (25–44 years of age), and those middle-aged and over (>44 years of age). The expenditure (expenditure was used as a proxy of income since the data of self-stated income tends to be undervalued) levels included poor, vulnerable, aspiring middle class, middle class, and upper class [27]. The cut-off for each expenditure group was updated using 2019 data from the Bureau of Statistics and was converted into household levels in our study questionnaire [28]. Smoking status indicated whether a person actively smoked cigarettes within the past month.
Second, variables related to protective behaviors included knowledge of PPE standards, application of the six steps of handwashing, the use of PPE when in contact with suspected or positive COVID-19 patients, physical distancing, the use of a mask outside of the home, and the index of handwashing frequency. Regarding the knowledge of PPE standards, we asked whether the respondents knew about the minimum PPE requirement for their jobs at healthcare facilities based on the recommendation of the Ministry of Health 2020. We also asked whether a person always applies the six-step hand washing technique recommended by the WHO, maintains physical distancing, and uses a mask outside of the home. Additionally, a handwashing index was created as a proxy of handwashing behaviors, using a weighted factor analysis based on 4-point-Likert-scale questions, which asked whether respondents use hand sanitizer or wash their hands using soap on several essential occasions. These occasions included: (1) after being in a public place, (2) before eating, (3) after using the toilet, and (4) after touching animals or taking out trash. The designated occasions were developed based on the Center for Disease Control’s ten critical handwashing times [29]. In the analysis, we used a dummy variable indicating whether a person’s handwashing index was above or equal to the median value.
2.3. Data Analysis
We employed three statistical analyses: descriptive analysis, bivariate analysis, and multivariate logistic regressions. We conducted data analyses for hospital staff (healthcare and non-healthcare workers), healthcare workers, and non-healthcare workers. We conducted bivariate analyses to assess the correlation between each independent variable and COVID-19 infection, and we performed multivariate logistic regressions to assess the socioeconomic and behavioral correlates of infection. We reported odds ratios (ORs), adjusted odds ratios (AORs), confidence intervals, and p-values. All analyses were performed in STATA 15 and used a 5% level of statistical significance.
3. Results
Table 1 provides the sample characteristics. In sociodemographic terms, 82.6% of the sample were healthcare workers and 17.9% were non-healthcare workers, 62.2% were female, 77.6% were 25–44 years old, 54.5% had a 3–4 household size, 35.9% were poor or vulnerable, and 10.2% actively smoked. In terms of protective behavior (Table 1B), among all samples, 98.4% knew of PPE standards, 79.0% reported doing the six-step handwashing technique, 55% reported always using PPE when in contact with actual or suspected COVID-19 cases. Additionally, 61.7% had a high index of handwashing frequency, 41.7% reported always keeping physical distance, and 92.3% reported always using masks outside of the home. In terms of dependent variables, 1.57% of the samples had confirmed COVID-19. In terms of COVID-19 symptoms, 4.2%, 16.9%, 14.2%, and 1.7% of the samples had a fever, cough, sore throat, and shortness of breath, respectively.
Table 1.
Sample characteristics.
| Variables | (1) | (2) | (3) | |||
|---|---|---|---|---|---|---|
| Hospital Workers (N = 1397) |
Healthcare Workers (N = 1154) |
Non-Healthcare Workers (N = 243) |
||||
| n | % | n | % | n | % | |
| (A) Demographics | ||||||
| Sex | ||||||
| Female | 869 | 62.2 | 762 | 66.03 | 107 | 44.03 |
| Male | 528 | 37.8 | 392 | 33.97 | 136 | 55.97 |
| Age group | ||||||
| 19–24 years | 126 | 9.020 | 83 | 7.190 | 43 | 17.700 |
| 25–44 years | 1084 | 77.59 | 921 | 79.81 | 163 | 67.08 |
| >44 years | 187 | 13.39 | 150 | 13 | 37 | 15.23 |
| Household size | ||||||
| 1–2 | 268 | 19.18 | 229 | 19.84 | 39 | 16.05 |
| 3–4 | 761 | 54.47 | 636 | 55.11 | 125 | 51.44 |
| ≥5 | 368 | 26.34 | 289 | 25.04 | 79 | 32.51 |
| Expenditure class | ||||||
| Poor | 202 | 14.46 | 163 | 14.12 | 39 | 16.05 |
| Vulnerable | 299 | 21.4 | 226 | 19.58 | 73 | 30.04 |
| Aspiring middle class | 600 | 42.95 | 502 | 43.5 | 98 | 40.33 |
| Middle and upper class | 296 | 21.19 | 263 | 22.79 | 33 | 13.58 |
| Active smoking status | ||||||
| No | 1254 | 89.76 | 1087 | 94.19 | 167 | 68.72 |
| Yes | 143 | 10.24 | 67 | 5.81 | 76 | 31.28 |
| (B) Protective behavior | ||||||
| Knowledge of PPE standards | ||||||
| No | 22 | 1.570 | 8 | 0.690 | 14 | 5.760 |
| Yes | 1375 | 98.43 | 1146 | 99.31 | 229 | 94.24 |
| Application of the six-step hand washing technique | ||||||
| Otherwise | 294 | 21.050 | 238 | 20.620 | 56 | 23.050 |
| Always | 1103 | 78.95 | 916 | 79.38 | 187 | 76.95 |
| The use of PPEs when in contact with suspected/positive COVID-19 patients | ||||||
| Otherwise | 627 | 44.880 | 479 | 41.510 | 148 | 60.910 |
| Always | 770 | 55.12 | 675 | 58.49 | 95 | 39.09 |
| Index of hand-washing frequency | ||||||
| Low | 535 | 38.300 | 441 | 38.210 | 94 | 38.680 |
| High | 862 | 61.7 | 713 | 61.79 | 149 | 61.32 |
| Physical distancing | ||||||
| Otherwise | 814 | 58.27 | 698 | 60.49 | 116 | 47.74 |
| Always | 583 | 41.73 | 456 | 39.51 | 127 | 60.49 |
| The use of a mask outside of the home | ||||||
| Otherwise | 108 | 7.73 | 91 | 7.89 | 17 | 7 |
| Always | 1289 | 92.27 | 1063 | 92.11 | 226 | 93 |
| (C) Signs and symptoms | ||||||
| Fever | 58 | 4.15 | 47 | 4.07 | 11 | 4.53 |
| Cough | 236 | 16.89 | 197 | 17.07 | 39 | 16.05 |
| Runny nose | 198 | 14.17 | 175 | 15.16 | 23 | 9.47 |
| Sore throat | 198 | 14.17 | 175 | 15.16 | 23 | 9.47 |
| Shortness of breath | 24 | 1.72 | 18 | 1.56 | 6 | 2.47 |
| Common cold | 58 | 4.15 | 51 | 4.42 | 7 | 2.88 |
| Headache | 171 | 12.24 | 139 | 12.05 | 32 | 13.17 |
| Muscle ache | 129 | 9.23 | 109 | 9.45 | 20 | 8.23 |
| Nausea | 70 | 5.01 | 59 | 5.11 | 11 | 4.53 |
| Watery eyes | 22 | 1.57 | 20 | 1.73 | 2 | 0.82 |
| Sputum production | 125 | 8.95 | 102 | 8.84 | 23 | 9.47 |
| Dizziness | 79 | 5.65 | 61 | 5.29 | 18 | 7.41 |
| Rash on skin | 20 | 1.43 | 18 | 1.56 | 2 | 0.82 |
| Loss of appetite | 41 | 2.93 | 33 | 2.86 | 8 | 3.29 |
| Anosmia | 12 | 0.86 | 11 | 0.95 | 1 | 0.41 |
| Ageusia | 12 | 0.86 | 11 | 0.95 | 1 | 0.41 |
| Tingling sensation | 26 | 1.86 | 20 | 1.73 | 6 | 2.47 |
| Delirium | 6 | 0.43 | 1 | 0.09 | 5 | 2.06 |
| (D) Dependent variables | ||||||
| RT-PCR result | ||||||
| Negative | 1375 | 98.43 | 1134 | 98.27 | 241 | 99.18 |
| Positive | 22 | 1.57 | 20 | 1.73 | 2 | 0.82 |
| Having at least one main symptom | ||||||
| No | 1124 | 80.46 | 923 | 79.98 | 201 | 82.72 |
| Yes | 273 | 19.54 | 231 | 20.02 | 42 | 17.28 |
| Data are n/N (%) if not specified | ||||||
By subgroup, the characteristics of healthcare workers and non-healthcare workers varied. Healthcare worker samples were primarily female (66%), and non-healthcare worker samples were mainly males (56%). Additionally, 79.8% vs. 67.1% of healthcare workers and non-healthcare workers were 25–44 years old, 33.7% vs. 46.1% of healthcare workers and non-healthcare workers were poor or vulnerable, and 5.8% vs. 31.3% of healthcare workers and non-healthcare workers were smokers. Furthermore, healthcare workers were shown to have higher infection rates, at 1.73%, than non-healthcare workers, at 0.82%. Healthcare workers reported higher rates of application of the six-step handwashing technique, knowledge of PPE standards, PPE usage when in contact with suspected/positive patients, and handwashing frequency.
Table 2 provides the bivariate (OR) and multivariate (AOR) analyses of all samples and healthcare workers. Note that the results for non-healthcare workers were not reported here because most independent variables were omitted in the regressions (potentially because the number of infections was very low). In the multivariable analysis, among all samples, higher risks of COVID-19 infection were significantly associated with the status of being healthcare workers (AOR = 8.31, 95% CI 1.27–54.54). In terms of socioeconomic correlates, the results show that the male sex, a larger household size, a higher expenditure level, and not smoking were associated with higher risks of infection. However, only a household size of more than five (AOR = 4.09, 95% CI 1.02–16.43) was statistically significant at a 5% level. In terms of protective behaviors, the results show that knowledge of PPE standards, always applying handwashing techniques, always using PPEs when in contact with suspects or cases, always applying physical distancing, and always using a mask outside of the home were associated with lower risks of infection. However, only knowledge of PPE standards (AOR = 0.08, 95% CI 0.01–0.54) and applying the six steps of handwashing (AOR = 0.32, 95% CI 0.12–0.83) were statistically significant at a 5% level.
Table 2.
Unadjusted and adjusted odds ratios of factors associated with COVID-19 infection.
| Variables | (1) | (2) | (3) | (4) | ||||
|---|---|---|---|---|---|---|---|---|
| Healthcare Workers | Healthcare Workers | Hospital Workers | Hospital Workers | |||||
| (N = 1154) | (N = 1007) | (N = 1397) | (N = 1397) | |||||
| OR (CI 95%) | p-Value | AOR (CI 95%) | p-Value | OR (CI 95%) | p-Value | AOR (CI 95%) | p-Value | |
| (A) Demographics | ||||||||
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.05 (0.41–2.65) | 0.922 | 1.90 (0.68–5.29) | 0.222 | 1.14 (0.48–2.69) | 0.762 | 1.91 (0.71–5.16) | 0.201 |
| Age group | ||||||||
| 19–24 years | Ref | Ref | Ref | Ref | ||||
| 25–44 years | 0.58 (0.13–2.62) | 0.478 | 0.75 (0.16–3.63) | 0.723 | 0.54 (0.15–1.89) | 0.333 | 0.66 (0.19–2.32) | 0.513 |
| >44 years | 1.40 (0.26–7.37) | 0.694 | 2.31 (0.40–13.38) | 0.351 | 1.13 (0.26–4.80) | 0.872 | 2.16 (0.50–9.35) | 0.301 |
| Status of being a healthcare worker | ||||||||
| No | Ref | Ref | ||||||
| Yes | NA | NA | NA | NA | 2.13 (0.49–9.16) | 0.312 | 8.31 (1.27–54.54) | 0.027 |
| Household size | ||||||||
| 1–2 | Ref | Ref | Ref | Ref | ||||
| 3–4 | 2.00 (0.44–9.09) | 0.371 | 2.94 (0.76–11.42) | 0.12 | 2.13 (0.47–9.59) | 0.324 | 3.03 (0.75–12.15) | 0.118 |
| ≥5 | 2.82 (0.58–13.70) | 0.199 | 3.69 (0.92–14.84) | 0.066 | 2.96 (0.62–14.04) | 0.173 | 4.09 (1.02–16.43) | 0.047 |
| Expenditure class | ||||||||
| Poor | Ref | Ref | Ref | Ref | ||||
| Vulnerable | 1.21 (0.28–5.13) | 0.799 | 0.79 (0.17–3.70) | 0.768 | 0.67 (0.19–2.35) | 0.531 | 0.50 (0.14–1.76) | 0.282 |
| Aspiring middle class | 1.19 (0.33–4.34) | 0.787 | 0.68 (0.16–2.99) | 0.613 | 0.74 (0.25–2.14) | 0.574 | 0.44 (0.13–1.45) | 0.175 |
| Middle and upper class | 0.20 (0.02–1.98) | 0.17 | 0.084 (0.01–1.21) | 0.069 | 0.13 (0.02–1.15) | 0.067 | 0.06 (0.01–0.66) | 0.022 |
| Active smoking status | ||||||||
| No | Ref | Ref | ||||||
| Yes | NA | NA | NA | NA | 0.41 (0.06–3.10) | 0.39 | 0.43 (0.07–2.58) | 0.355 |
| (B) Protective behavior | ||||||||
| Knowledge of PPE standards | ||||||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes | 0.12 (0.01–1.01) | 0.051 | 0.06 (0.00–0.63) | 0.02 | 0.15 (0.03–0.67) | 0.014 | 0.08 (0.01–0.54) | 0.01 |
| Application of the six-step hand washing technique | ||||||||
| Otherwise | Ref | Ref | Ref | Ref | ||||
| Always | 0.48 (0.19–1.20) | 0.117 | 0.30 (0.11–0.83) | 0.02 | 0.46 (0.19–1.11) | 0.083 | 0.32 (0.12–0.83) | 0.019 |
| The use of PPEs when in contact with suspected/positive COVID-19 patients | ||||||||
| Otherwise | Ref | Ref | Ref | Ref | ||||
| Always | 0.47 (0.19–1.15) | 0.098 | 0.38 (0.13–1.09) | 0.073 | 0.46 (0.19–1.10) | 0.082 | 0.37 (0.13–1.02) | 0.055 |
| Index of hand-washing frequency | ||||||||
| Low | Ref | Ref | Ref | Ref | ||||
| High | 0.75 (0.31–1.83) | 0.53 | 0.75 (0.26–2.12) | 0.587 | 0.62 (0.27–1.43) | 0.26 | 0.61 (0.23–1.60) | 0.317 |
| Physical distancing | ||||||||
| Otherwise | Ref | Ref | Ref | Ref | ||||
| Always | 1.54 (0.64–3.74) | 0.337 | 2.42 (0.81–7.22) | 0.114 | 1.40 (0.60–3.26) | 0.43 | 2.52 (0.6–7.42) | 0.092 |
| The use of a mask outside of the home | ||||||||
| Otherwise | Ref | Ref | ||||||
| Always | NA | NA | NA | NA | 1.77 (0.24–13.31) | 0.578 | 3.44 (0.42–27.99) | 0.248 |
Note: OR = odds ratio; AOR = adjusted odds ratio; Ref = reference group; NA = not applicable. We also performed bivariate and multivariable analyses among non-healthcare workers, but most independent variables were omitted potentially because the number of COVID-19 infections was very low.
Table 3 provides additional results for multivariate (AOR) analyses using at least one main symptom as the outcome variable. Among all samples, in terms of socioeconomic correlates, the results show that the female sex, a younger age group (19–24 years), a smaller household size, a higher expenditure level, and smoking were associated with a higher rate of at least one main symptom. However, only the expenditure level showed statistical significance. In terms of protective behaviors, knowledge of PPE standards, always applying handwashing techniques, using PPE when in contact with suspected or known cases, applying physical distancing, and using a mask outside of the home were associated with a lower rate of at least one main symptom. However, only always using PPE when in contact with suspected or known cases showed statistical significance.
Table 3.
Unadjusted and adjusted odds ratios of factors associated with experiencing at least one of COVID-19’s main symptoms.
| Variables | (1) | (2) | (3) | |||
|---|---|---|---|---|---|---|
| Healthcare Workers | Non-Healthcare Workers | All Samples | ||||
| N = 1154 | N = 243 | (N = 1397) | ||||
| AOR (CI 95%) | p-Value | AOR (CI 95%) | p-Value | AOR (CI 95%) | p-Value | |
| (A) Demographics | ||||||
| Sex | ||||||
| Female | Ref | Ref | Ref | |||
| Male | 0.84 (0.60–1.19) | 0.329 | 1.01 (0.43–2.37) | 0.974 | 0.84 (0.61–1.14) | 0.26 |
| Age group | ||||||
| 19–24 years | Ref | Ref | Ref | |||
| 25–44 years | 0.58 (0.33–1.00) | 0.051 | 1.58 (0.40–5.01) | 0.438 | 0.73 (0.45–1.19) | 0.213 |
| >44 years | 0.68 (0.34–1.35) | 0.267 | 2.03 (0.51–8.10) | 0.313 | 0.87 (0.47–1.62) | 0.671 |
| Status of being a healthcare worker | ||||||
| No | Ref | |||||
| Yes | NA | NA | NA | NA | 1.36 (0.89–2.08) | 0.153 |
| Household size | ||||||
| 1–2 | Ref | Ref | Ref | |||
| 3–4 | 0.91 (0.62–1.34) | 0.637 | 0.57 (0.23–1.40) | 0.219 | 0.84 (0.59–1.19) | 0.332 |
| ≥5 | 0.79 (0.50–1.25) | 0.316 | 0.78 (0.27–2.29) | 0.656 | 0.78 (0.52–1.17) | 0.232 |
| Expenditure class | ||||||
| Poor | Ref | Ref | Ref | |||
| Vulnerable | 1.38 (0.81–2.37) | 0.239 | 2.48 (0.69–9.96) | 0.201 | 1.46 (0.90–2.36) | 0.127 |
| Aspiring middle class | 1.56 (0.95–2.55) | 0.076 | 2.94 (0.71–12.16) | 0.136 | 1.66 (1.06–2.59) | 0.027 |
| Middle and upper class | 1.13 (0.64–2.00) | 0.664 | 2.16 (0.42–11.06) | 0.353 | 1.20 (0.71–2.02) | 0.489 |
| Active smoking status | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.40 (0.73–2.65) | 0.31 | 0.78 (0.28–2.16) | 0.63 | 1.13 (0.66–1.93) | 0.658 |
| (B) Protective behavior | ||||||
| Knowledge of PPE standards | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 0.27 (0.07–1.07) | 0.063 | 1.35 (0.24–7.72) | 0.735 | 0.63 (0.24–1.66) | 0.348 |
| Application of WHO hand-washing steps | ||||||
| Otherwise | Ref | Ref | Ref | |||
| Always | 0.85 (0.58–1.23) | 0.386 | 0.63 (0.30–1.33) | 0.224 | 0.82 (0.59–1.15) | 0.258 |
| The use of PPE when in contact with suspected/positive COVID-19 patients | ||||||
| Otherwise | Ref | Ref | Ref | |||
| Always | 0.61 (0.45–0.83) | 0.002 | 0.64 (0.30–1.38) | 0.254 | 0.63 (0.47–0.83) | 0.001 |
| Index of hand-washing frequency | ||||||
| Low | Ref | Ref | Ref | |||
| High | 0.73 (0.53–1.01) | 0.06 | 1.60 (0.71–3.61) | 0.254 | 0.81 (0.6–1.10) | 0.178 |
| Physical distancing | ||||||
| Otherwise | Ref | Ref | Ref | |||
| Always | 1.00 (0.71–1.42) | 0.993 | 0.64 (0.29–1.40) | 0.264 | 0.93 (0.68–1.27) | 0.646 |
| The use of a mask outside of the home | ||||||
| Otherwise | Ref | Ref | Ref | |||
| Always | 0.68 (0.41–1.14) | 0.142 | 0.76 (0.22–2.70) | 0.676 | 0.67 (0.42–1.07) | 0.095 |
Note: dependent variable = dummy, having at least one main symptom; OR = odds ratio; AOR = adjusted odds ratio; ref = reference group; NA = not applicable.
4. Discussion
Our findings show that larger household sizes and middle to upper expenditure levels were significantly associated with higher risks of COVID-19 infection among hospital workers. Additionally, knowledge of PPE standards and use of PPE and frequency of application of the six-step handwashing technique were significant correlates of lower risks of infection. Our results also showed that sociodemographic variables (e.g., sex and age) and behavioral variables (e.g., physical distancing, the use of a mask, and the index of handwashing frequency) were associated with higher or lower risks of infection but were not statistically significant. This may be due to not having a large enough sample, given the very low infection rates in the sample (1.57%). Note that the results for all samples may be mainly driven by the characteristics of the healthcare workers.
The analysis of all samples revealed that being a healthcare worker was positively correlated with COVID-19 status. In other words, the infection rates were significantly higher among healthcare workers compared to non-healthcare workers, which was similar to a study in China, which showed that the infection rates were 2.10% and 0.43% among healthcare and non-healthcare workers, respectively [30]. The results also corroborate findings from previous studies, which discovered that the infection risk of healthcare workers was significantly higher than that of non-healthcare workers [8,22,30]. The positive association between being a healthcare worker and COVID-19 status may be explained by several factors experienced particularly by healthcare workers, such as performing certain medical procedures, prolonged contact with infected patients, and working pressures during the pandemic period [14,16,31].
We also found a significant association between larger household size and infection. This result is consistent with previous studies indicating positive relationships between household size and COVID-19 infection in the general population [32,33,34]. A possible link between the two indicators is that the within-household infection rate is higher than the non-household one, so that the larger household size may increase contacts and spread of SARS-CoV-2 [35]. In terms of expenditure levels, we found that being in the middle and upper expenditure levels was protective of contracting COVID-19, which supports evidence from previous studies that low socioeconomic status and expenditure may increase the risk of COVID-19 infection [17,36]. A potential explanation may be the lower compliance of lower-expenditure people in applying protective measures, such as wearing masks, physical distancing, and washing hands [37,38] and the lower immune system of those with a lower socioeconomic status due to higher stress levels and a higher allostatic load, which makes them more susceptible to COVID-19 [39,40,41,42].
In our study, knowledge of standard PPE and use of PPE when in contact with suspects or patients showed protective effects of COVID-19 infections among all samples and healthcare samples. However, the effect of the latter was only significant at the 10% level. Similarly, previous studies have shown that knowledge of the disease and proper use of PPE have an inverse association with being infected with SARS-CoV-1 [43], another coronavirus type that previously caused an epidemic. It has been suggested that the proper use of various types of PPE, adequate provision of PPE, and sufficient access to PPE may protect healthcare workers from contracting COVID-19 [14,18,19]. Although the negligible effect of the use of PPE in this study was unexpected, the direction of the correlation is still consistent with earlier studies.
To our knowledge, there is currently no study evaluating the effect of the six-step hand washing technique on COVID-19 status among healthcare workers. Our finding is supportive of other studies showing that handwashing frequency, especially in contact with patients, may protect healthcare workers from being infected by SARS-CoV-2 and SARS-CoV-1 [21,39,44,45]. The significant correlation of the indicator may also stem from the hypothesis that applying the six-step hand washing technique is biologically more effective than implementing non-six-step handwashing techniques [46].
Our study had several limitations. First, we used self-administered questionnaires for sample characteristics and behaviors. This may pose risks of under- or over-reporting. Second, this was a cross-sectional study, which may be improved in future investigations by applying cohort studies to draw statistical inferences. Despite the limitations, this study provides further evidence that hospital workers face challenges in combating COVID-19 at work. Besides the higher infection risk of the healthcare workers, as found in the current study, previous research also discovered overwhelming workload burdens of healthcare workers that may lead to some health and psychological problems such as greater sleep disorders and headache episodes [47] and more depressive, anxiety, and burnout symptoms [48]. To ensure that healthcare and non-healthcare workers, particularly those in LMICs, can make significant contributions to combat the pandemic and indirectly generate potential economic impacts for the country [49,50], further efforts are needed to provide adequate knowledge and training of proper PPE use and to supply sufficient standardized PPE in contact with patients.
5. Conclusions
Our study assessed the socioeconomic and behavioral correlates of COVID-19 infections among healthcare workers at eight hospitals in the Greater Jakarta Area, the capital of Indonesia. We found that healthcare workers were at significantly higher risks of contracting COVID-19 compared to non-healthcare workers at hospitals. We also found that socioeconomic correlates such as a larger household size and middle and upper expenditure levels were significantly associated with higher risks of infection. Moreover, protective behaviors such as knowledge and use of PPE and frequency of applying the six-step handwashing technique were significantly associated with lower risks among hospital workers. These findings add to the evidence of the determinants of COVID-19 infections of healthcare and non-healthcare workers at hospitals in LMICs.
Author Contributions
A.B. and M.T.A. conceptualized the study, applied for ethics, and prepared the study instrument. O.H. and P.A.R. conducted data collection. A.B., M.T.A., and D.K. conducted data cleaning and analyses. A.B., M.T.A., G.K., and D.K. drafted the manuscript, while O.H. and P.A.R. provided input. All authors have read and agreed to the published version of the manuscript.
Funding
The project is partly funded and supported by Solidaritas Berantas COVID-19 (solidarity for COVID-19 response in Indonesia) by providing free RT-PCR tests for hospital workers.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Atma Jaya Catholic University (Number: 768A/III/LPPM.PM.10.05/07/2020, Date: 13 July 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 17 February 2021)]; Available online: https://covid19.who.int/
- 2.COVID-19 and the Least Developed Countries. [(accessed on 10 May 2021)]; Available online: https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/publication/PB_66.pdf.
- 3.Minister of Health of the Republic of Indonesia. [(accessed on 10 May 2021)]; Available online: https://app.glueup.com/resources/protected/edm/48950/73317dab-06cf-45b5-a602-cc915fc87504.pdf.
- 4.Jakarta GoSCRo . Regional Regulations of the Special Capital Region of Jakarta. Jakarta GoSCRo; Jakarta, Indonesia: 2020. [Google Scholar]
- 5.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Prevention, Identification and Management of Health Worker Infection in the Context of COVID-19. [(accessed on 23 January 2020)]; Available online: https://www.who.int/publications/i/item/10665-336265.
- 7.Martin C., Montesinos I., Dauby N., Gilles C., Dahma H., Van Den Wijngaert S., De Wit S., Delforge M., Clumeck N., Vandenberg O. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J. Hosp. Infect. 2020;106:102–106. doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.-G., Ma W., Mehta R.S., Warner E.T., Sikavi D.R., Lo C.-H., et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health. 2020;5:475–483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandyopadhyay S., Baticulon R.E., Kadhum M., Alser M., Ojuka D.K., Badereddin Y., Kamath A., Parepalli S.A., Brown G., Iharchane S., et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Glob. Health. 2020;5:003097. doi: 10.1136/bmjgh-2020-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrea L. 654 Tenaga Kesehatan Gugur Lawan Pandemi Covid-19 di Indonesia. [(accessed on 19 February 2021)]; Available online: https://databoks.katadata.co.id/datapublish/2021/01/28/654-tenaga-kesehatan-gugur-lawan-pandemi-covid-19-di-indonesia.
- 11.Coronavirus Kills 647 Health Workers in Indonesia. [(accessed on 10 May 2021)]; Available online: https://www.aa.com.tr/en/asia-pacific/coronavirus-kills-647-health-workers-in-indonesia/2125642.
- 12.PAIR Occupational Health and Safety (OHS) Risks among Indonesian Healthcare Workers during the COVID-19 Pandemic. [(accessed on 10 January 2020)]; Available online: https://pair.australiaindonesiacentre.org/news/occupational-health-and-safety-ohs-risks-among-indonesian-healthcare-workers-during-the-covid-19-pandemic/
- 13.Alraddadi B.M., Al-Salmi H.S., Jacobs-Slifka K., Slayton R.B., Estivariz C.F., Geller A.I., Al-Turkistani H.H., Al-Rehily S.S., Alserehi H.A., Wali G.Y., et al. Risk Factors for Middle East Respiratory Syndrome Coronavirus Infection among Healthcare Personnel. Emerg. Infect. Dis. 2016;22:1915–1920. doi: 10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R., Dana T., Buckley D.I., Selph S., Fu R., Totten A.M. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Ann. Intern. Med. 2020;173:120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W., Tang F., Fang L.-Q., De Vlas S.J., Ma H.-J., Zhou J.-P., Looman C.W.N., Richardus J.H., Cao W.-C. Risk factors for SARS infection among hospital healthcare workers in Beijing: A case control study. Trop. Med. Int. Health. 2009;14:52–59. doi: 10.1111/j.1365-3156.2009.02255.x. [DOI] [Google Scholar]
- 16.Bai Y., Wang X., Huang Q., Wang H., Gurarie D., Ndeffo-Mbah M., Fan F., Fu P., Horn M.A., Xu S., et al. SARS-CoV-2 infection in health care workers: A retrospective analysis and model study. MedRxiv. 2020 doi: 10.1101/2020.03.29.20047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firew T., Sano E.D., Lee J.W., Flores S., Lang K., Salman K., Greene M.C., Chang B.P. Protecting the front line: A cross-sectional survey analysis of the occupational factors contributing to healthcare workers’ infection and psychological distress during the COVID-19 pandemic in the USA. BMJ Open. 2020;10:042752. doi: 10.1136/bmjopen-2020-042752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoernke K., Djellouli N., Andrews L., Lewis-Jackson S., Manby L., Martin S., Vanderslott S., Vindrola-Padros C. Frontline healthcare workers’ experiences with personal protective equipment during the COVID-19 pandemic in the UK: A rapid qualitative appraisal. BMJ Open. 2021;11:046199. doi: 10.1136/bmjopen-2020-046199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H., Hegde S., LaFiura C., Raghavan M., Sun N., Cheng S., Rebholz C.M., Seidelmann S.B. Access to personal protective equipment in exposed healthcare workers and COVID-19 illness, severity, symptoms and duration: A population-based case-control study in six countries. BMJ Glob. Health. 2021;6:004611. doi: 10.1136/bmjgh-2020-004611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M., He P., Liu H.G., Wang X.J., Li F.J., Chen S., Lin J., Chen P., Liu J.H., Li C.H. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. 2020;43:209–214. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk Factors of Healthcare Workers with Corona Virus Disease 2019: A Retrospective Cohort Study in a Designated Hospital of Wuhan in China. Clin. Infect. Dis. 2020;71:2218–2221. doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J.T., Liu Z.D., Fan Z.W., Zhao L., Cao W.C. Epidemiology of and Risk Factors for COVID-19 Infection among Health Care Workers: A Multi-Centre Comparative Study. Int. J. Environ. Res. Public Health. 2020;17:7149. doi: 10.3390/ijerph17197149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali N., Islam F. The Effects of Air Pollution on COVID-19 Infection and Mortality—A Review on Recent Evidence. Front. Public Health. 2020;8:580057. doi: 10.3389/fpubh.2020.580057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coker E.S., Cavalli L., Fabrizi E., Guastella G., Lippo E., Parisi M.L., Pontarollo N., Rizzati M., Varacca A., Vergalli S. The Effects of Air Pollution on COVID-19 Related Mortality in Northern Italy. Environ. Resour. Econ. 2020;76:611–634. doi: 10.1007/s10640-020-00486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlem G. Corrigendum to: Descriptive analysis of social determinant factors in urban communities affected by COVID-19. J. Public Health. 2020;43:112. doi: 10.1093/pubmed/fdaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Health Service Symptoms of Coronavirus. [(accessed on 10 May 2021)]; Available online: https://www.nhs.uk/conditions/coronavirus-covid-19/symptoms/
- 27.The World Bank Aspiring Indonesia-Ecpnading the Middle Class. [(accessed on 10 May 2021)]; Available online: https://www.worldbank.org/en/country/indonesia/publication/aspiring-indonesia-expanding-the-middle-class.
- 28.Central Bureau of Statistics Persentase Penduduk Miskin Maret 2019 Sebesar 9,41 Persen. [(accessed on 10 May 2021)]; Available online: https://www.bps.go.id/pressrelease/2019/07/15/1629/persentase-penduduk-miskin-maret-2019-sebesar-9-41-persen.html.
- 29.Centers for Disease Control and Prevention When and How to Wash Your Hands. [(accessed on 10 May 2021)]; Available online: https://www.cdc.gov/handwashing/when-how-handwashing.html.
- 30.Zheng L., Wang X., Zhou C., Liu Q., Li S., Sun Q., Wang M., Zhou Q., Wang W. Analysis of the Infection Status of Healthcare Workers in Wuhan During the COVID-19 Outbreak: A Cross-sectional Study. Clin. Infect. Dis. 2020;71:2109–2113. doi: 10.1093/cid/ciaa588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Wang X., Jia X., Li J., Hu K., Chen G., Wei J., Gong Z., Zhou C., Yu H., et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. 2020;26:767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borjas G.J. Demographic Determinants of Testing Incidence and Covid-19 Infections in New York City Neighborhoods. [(accessed on 10 May 2021)]; Available online: https://ssrn.com/abstract=3574417.
- 33.Figueroa J.F., Wadhera R.K., Mehtsun W.T., Riley K., Phelan J., Jha A.K. Association of race, ethnicity, and community-level factors with COVID-19 cases and deaths across U.S. counties. Healthcare. 2021;9:100495. doi: 10.1016/j.hjdsi.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin C.A., Jenkins D.R., Minhas J.S., Gray L.J., Tang J., Williams C., Sze S., Pan D., Jones W., Verma R., et al. Socio-demographic heterogeneity in the prevalence of COVID-19 during lockdown is associated with ethnicity and household size: Results from an observational cohort study. EClinicalMedicine. 2020;25:100466. doi: 10.1016/j.eclinm.2020.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng H.-Y., Jian S.-W., Liu D.-P., Ng T.-C., Huang W.-T., Lin H.-H. High transmissibility of COVID-19 near symptom onset. MedRxiv. 2020 doi: 10.1101/2020.03.18.20034561. [DOI] [Google Scholar]
- 36.Smith S., Morbey R., de Lusignan S., Pebody R.G., Smith G.E., Elliot A.J. Investigating regional variation of respiratory infections in a general practice syndromic surveillance system. J. Public Health. 2020 doi: 10.1093/pubmed/fdaa014. [DOI] [PubMed] [Google Scholar]
- 37.Papageorge N.W., Zahn M.V., Belot M., van den Broek-Altenburg E., Choi S., Jamison J.C., Tripodi E. Socio-demographic factors associated with self-protecting behavior during the Covid-19 pandemic. J. Popul. Econ. 2021;34:691–738. doi: 10.1007/s00148-020-00818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peltzer K., Pengpid S. Oral and hand hygiene behaviour and risk factors among in-school adolescents in four Southeast Asian countries. Int. J. Environ. Res. Public Health. 2014;11:2780–2792. doi: 10.3390/ijerph110302780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen E., Fisher E.B., Bacharier L.B., Strunk R.C. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosom. Med. 2003;65:984–992. doi: 10.1097/01.PSY.0000097340.54195.3C. [DOI] [PubMed] [Google Scholar]
- 40.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y., Zilioli S., Rodriguez-Stanley J., Peek K.M., Cutchin M.P. Socioeconomic status and differential psychological and immune responses to a human-caused disaster. Brain Behav. Immun. 2020;88:935–939. doi: 10.1016/j.bbi.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz A.J., Mentz G., Lachance L., Johnson J., Gaines C., Israel B.A. Associations between socioeconomic status and allostatic load: Effects of neighborhood poverty and tests of mediating pathways. Am. J. Public Health. 2012;102:1706–1714. doi: 10.2105/AJPH.2011.300412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeb M., McGeer A., Henry B., Ofner M., Rose D., Hlywka T., Levie J., McQueen J., Smith S., Moss L., et al. SARS among critical care nurses, Toronto. Emerg. Infect. Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau J.T., Fung K.S., Wong T.W., Kim J.H., Wong E., Chung S., Ho D., Chan L.Y., Lui S.F., Cheng A. SARS transmission among hospital workers in Hong Kong. Emerg. Infect. Dis. 2004;10:280–286. doi: 10.3201/eid1002.030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishiyama A., Wakasugi N., Kirikae T., Quy T., Ha L.D., Ban V.V., Long H.T., Keicho N., Sasazuki T., Kuratsuji T. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn. J. Infect. Dis. 2008;61:388–390. [PubMed] [Google Scholar]
- 46.Reilly J.S., Price L., Lang S., Robertson C., Cheater F., Skinner K., Chow A. A Pragmatic Randomized Controlled Trial of 6-Step vs. 3-Step Hand Hygiene Technique in Acute Hospital Care in the United Kingdom. Infect. Control Hosp. Epidemiol. 2016;37:661–666. doi: 10.1017/ice.2016.51. [DOI] [PubMed] [Google Scholar]
- 47.Dalewski B., Palka L., Kiczmer P., Sobolewska E. The Impact of SARS-CoV-2 Outbreak on the Polish Dental Community’s Standards of Care—A Six-Month Retrospective Survey-Based Study. Int. J. Environ. Res. Public Health. 2021;18:1281. doi: 10.3390/ijerph18031281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sunjaya D.K., Herawati D.M.D., Siregar A.Y.M. Depressive, anxiety, and burnout symptoms on health care personnel at a month after COVID-19 outbreak in Indonesia. BMC Public Health. 2021;21:227. doi: 10.1186/s12889-021-10299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters A., Lotfinejad N., Simniceanu A., Pittet D. The economics of infection prevention: Why it is crucial to invest in hand hygiene and nurses during the novel coronavirus pandemic. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone P.W. Economic burden of healthcare-associated infections: An American perspective. Expert Rev. Pharmacoecon. Outcomes Res. 2009;9:417–422. doi: 10.1586/erp.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon reasonable request.