Abstract
In recent years, radionuclide therapy (RT) and targeted radionuclide therapy (TRT) have gained great interest in cancer treatment. This is due to promising results obtained in both preclinical and clinical studies. However, a complete response is achieved in only a small percentage of patients that receive RT or TRT. As a consequence, there have been several strategies to improve RT and TRT outcomes including the combination of these treatments with other well-established anti-cancer therapies, for example, chemotherapy. Combinations of RT and TRT with other therapies with distinct mechanisms of action represent a promising strategy. As for prostate cancer and breast cancer, the two most prevalent cancer types worldwide, several combination-based therapies have been evaluated. In this review, we will provide an overview of the RT and TRT agents currently used or being investigated in combination with hormone therapy, chemotherapy, immunotherapy, and external beam radiation therapy for the treatment of prostate cancer and breast cancer.
Keywords: combination therapy, targeted radionuclide therapy, peptide receptor radionuclide therapy, radionuclide therapy, prostate cancer, breast cancer
1. Introduction
Radionuclide therapy (RT) involves the use of radionuclides that target specific cellular processes or accumulate in cancer cells because of their chemical properties [1]. Targeted radionuclide therapy (TRT) uses radiotracers that target molecules that are overexpressed on cancer cells. These radiotracers roughly consist of two main components, a targeting agent and a radionuclide [2]. The targeting agent can be, for example, an antibody, a natural or synthetic ligand, or a nanobody that can be coupled via a chelator to a radionuclide. There are different types of radionuclides used in medicine and biological research: those who emit α-particles (e.g., 223Ra, 212Bi, 213Bi, 225Ac, and 227Th), β−-particles (e.g., 67Cu, 90Y, 131I, 177Lu, 186Re, and 188Re), β+-particles (e.g., 11C, 13N, 15O, 68Ga, and 18F), auger electrons (e.g., 111In and 125I), and γ-emitters (e.g., 67Cu, 177Lu, and 111In) [3,4,5]. Radionuclides that emit β+-particles and γ-emitters are used in nuclear imaging. Besides, some of these radionuclides can emit both imaging photons and therapeutic particles (e.g., 177Lu and 67Cu). The selection of the optimal radionuclide for both imaging and treatment among other factors depends on the pharmacokinetic properties of the radiotracer. In addition, for treatment options, the type and stage of the disease are also important factors that need to be taken into consideration. In the treatment of large tumors, for example, β−-emitters are considered the ideal radionuclide. These β−-emitters have a long track length and therefore neighboring tumor cells that lack expression of the targeted molecules are also exposed to radiation, the so-called “cross-fire” effect [6,7]. For the treatment of micro-metastasis and small clusters of cancer cells, α-emitters, and auger electron-emitters are considered to be beneficial due to their short range and high-energy which results in a high level of cytotoxicity [8].
The broad option of targeting agents, together with the wide range of radionuclides with unique properties, allows radiotracers to be designed for targeting different types of cancers at different disease stages. Furthermore, a unique feature of RT and TRT is the ability to determine the radiation dose that reaches the tumor and healthy tissues, which can be assessed and correlated with efficacy and toxicity, respectively.
Besides having the most optimal radiopharmaceutical, for the treatment to be effective, several other factors should also be considered, including the cumulative radiation dose delivered, tissue and dose penetration, and the density of the targeted molecule on cancer cells. The delivery of radiopharmaceuticals might not always reach their target as desired due to heterogeneous expressions of the molecular marker that the radiotracer is designed to target and poor vasculature of the tumor [9]. Furthermore, studies have reported changes in radiosensitivity as well as a decrease in expression of the targeted molecule during disease progression and after exposure to other anti-cancer therapies [10,11,12,13,14,15,16]. The above-mentioned factors can greatly impact the success of RT and TRT, and as a consequence, novel strategies are needed to improve the treatment efficacy of RT and TRT. One promising strategy includes the combination of RT and TRT with other anti-cancer therapeutics that have shown promising results in tumor management [17]. This review will provide an overview of RT and TRT agents currently used or being investigated in combination with hormone therapy, chemotherapy, immunotherapy, and local external beam radiation therapy (EBRT) for the treatment of prostate cancer (PCa) and breast cancer (BC).
2. Prostate and Breast Cancer
PCa is the second most diagnosed cancer among men with an estimated 1,276,106 new cases and 358,989 deaths worldwide in 2018 [18]. The estimated PCa incidence and mortality rates per 100,000 males in 2018 were 29.3 and 7.6, respectively. For early detection of PCa, men at risk and between the age of 55–69 are recommended by their physician to be screened for PCa. The prostate-specific antigen (PSA) blood test is currently the first-line screening method used. Abnormal results of this screening are followed by prostate biopsy or multiparametric magnetic resonance imaging for further diagnosis [19,20]. Multiple treatment options exist for men diagnosed with PCa. These treatments are based on the stage and the grade of the tumor. Localized cancers are classified into three risk groups; low, medium, and high risk based on the Gleason score. Men with low-risk PCa are managed by “active surveillance” or “watchful waiting” while high-risk cancers receive more aggressive treatments such as surgery and radiation-based therapy. In advanced metastatic PCa, initial treatments include androgen deprivation therapy (ADT) and in some cases ADT in combination with chemotherapy. Besides these, several RT and TRT agents have been or are being investigated for the treatment of metastatic PCa [20,21,22]. A few RT agents have been accepted for clinical use, for example, 223Ra-dichloride and 153Sm-lexidronam for the treatment of metastatic castration-resistant PCa (mCRPCa) with bone metastases [23,24]. 223Ra-dichloride and 153Sm-lexidronam are both bone targeting agents that accumulate at sites with a high osteoblastic activity where they cause DNA damage in tumor cells [25,26]. Even though no TRT agents have been clinically approved for PCa treatment yet, preclinical and clinical studies show promising results. Among the TRT agents studied for PCa treatment, agents targeting the prostate-specific membrane antigen (PSMA) and the gastrin-releasing peptide receptor (GRPR) are the most well-known.
In females, breast cancer (BC) is the most diagnosed cancer and the leading cause of cancer death. There were an estimated 2,088,849 new cases and 626,679 deaths as a consequence of BC worldwide in 2018 [18]. For the early detection of BC, women at the age of 50 are recommended to be screened. Women at risk or who carry BRCA1 or BRCA2 mutations are advised to be screened as early as 40 years of age. The screening includes both physical breast examination as well as mammographic imaging. Abnormal results of these screenings are followed by biopsy for further diagnosis [27]. Both tumor morphologic characteristics and molecular factors are critical for treatment decisions in BC [28]. The disease is very heterogeneous and consists of multiple biological subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-driven, and basal-like tumors [29,30,31,32]. Among other molecular factors, these subtypes can to some extent be identified by estrogen receptor (ER), progesterone receptor (PR), and HER2 expression, markers that majorly impact the treatment and prognosis of the disease. Luminal A tumors and luminal B tumors are either ER or PR positive, or both ER- and PR-positive. However, luminal A tumors either have higher ER- or PR-expression, or both, compared to luminal B tumors. Furthermore, luminal A tumors are HER2 negative, while luminal B tumors have variable HER2 expression. HER2-driven tumors are usually either ER- or PR-negative, or both ER- and PR-negative, and always HER2-positive. Basal-like tumors are the most aggressive subtype. These tumors are ER-, PR-, and HER2-negative, and are also known as triple-negative BC (TNBC). Tumors that are either ER- or PR-positive, or both ER- and PR-positive, are treated with endocrine therapy alone or in combination with chemotherapy [33]. Patients with either ER- or PR- positive, and HER2-positive tumors benefit more from a combination of hormone and anti-HER2 therapy, while TNBC tumors are treated with chemotherapy [32]. Unfortunately, treatment for metastatic BC remains a challenge. Currently, several markers including HER2, GRPR, and somatostatin receptors (SSTR) are being investigated as possible targets for TRT in the treatment of metastatic BC. Up to now, none of these TRT treatments have been approved for clinical use. Table 1 shows a selection of RT and TRT agents clinically used or preclinically/clinically studied for PCa and BC treatment.
Table 1.
Selection of RT and TRT agents on the market or under development for the treatment of PCa and BC.
| Agent | Type of Cancer | Target | Disease Stage | Development Phase | Trial Name or Registration Number | Ref. |
|---|---|---|---|---|---|---|
| Radionuclide therapy | ||||||
| 223Ra chloride | PCa | Calcium analog | Bone metastasis | Commercially available | - | [34] |
| BC | Clinical study | - | [35] | |||
| 153Sm-lexidronam | PCa/ BC | Binding to hydroxyapatite matrix | Bone metastasis | Commercially available | - | [36] |
| Targeted radionuclide therapy | ||||||
| 177Lu-PSMA-617 | PCa | PSMA | mCRPCa | Phase III; active, not recruiting | NCT03511664 | |
| 227Th- PSMA-TTC | PCa | PSMA | mCRPCa | Phase I; recruiting | NCT03724747 | |
| 177Lu-PSMA/CTT1403 | PCa | PSMA | mCRPCa | Phase I; active, not recruiting | NCT03822871 | |
| 177Lu-PSMA-R2 | PCa | PSMA | mCRPCa | Phase I/II; recruiting | NCT03490838 | |
| 177Lu-J591/TLX591/ 177Lu-DOTA-Rosopatamab | PCa | PSMA | mCRPCa | Clinical study | - | [37] |
| 225Ac-PSMA-617 | PCa | PSMA | mCRPCa | Clinical study | - | [38,39] |
| 177Lu-NeoB, formerly known as 177Lu-NeoBOMB1 | PCa | GRPR | Under investigation | Phase I/II; Recruiting | NCT03872778 | |
| 177Lu-NeoB/NeoBOMB1 | PCa, BC | GRPR | Under investigation | Preclinical study | - | [40,41] |
| 177Lu-RM2 | PCa | GRPR | mCRPCa | Clinical study | - | [42] |
| 177Lu-DOTA0-Tyr3-Octreotate and 177Lu-DOTA-JR11 | BC | SSTR | Under investigation | Preclinical study | - | [43] |
| 188Re-trastuzumab | BC | HER2 | Under investigation | Preclinical study | - | [44] |
RT: radionuclide therapy, TRT: targeted radionuclide therapy, PCa: prostate cancer, BC: breast cancer, PSMA: prostate-specific membrane antigen, GRPR: gastrin releasing peptide receptor, SSTR: somatostatin receptor, HER2: human epidermal growth factor receptor 2, mCRPCa: metastatic castration-resistant prostate cancer.
3. Hormone Therapy-Based Combination Therapy
A key feature of PCa is its hormone responsiveness. The growth of PCa cells is usually dependent on androgens. This was observed first by Huggins and Hodges in 1941 [45]. Since then, ADT has become the primary treatment option for men with advanced metastatic PCa. Several strategies of ADT exist for the treatment of PCa. These strategies are classified into two groups, surgical castration, and medical/chemical castration. Surgical castration is achieved by orchiectomy, a surgical procedure in which one or both testicles are removed. However, its use is not so frequent and declining. Medical castration includes the use of anti-androgens (e.g., bicalutamide, flutamide), androgen synthesis inhibitors (e.g., abiraterone, enzalutamide), and luteinizing hormone-releasing hormone (LHRH) agonists and antagonists (e.g., leuprolide acetate, goserelin). Anti-androgens and drugs that target LHRH receptors represent first and second generations of ADT options. Androgen pathway inhibitors represent the third-generation of ADT drugs [46]. ADT is typically the first systemic treatment after local treatment options have become insufficient, although it is also used as neoadjuvant therapy for local radiation therapy and surgery [47,48,49].
Studies have shown that blockage of androgen can make tumors more radiosensitive. The radiosensitizing effect of ADT can occur through several mechanisms including cell cycle checkpoint inhibition, DNA damage repair pathway, and transcriptional changes in expression of DNA repair genes [50,51,52]. This provides a rationale for combining ADT with RT or TRT.
Once initiated, ADT is generally continued throughout the course of PCa treatment, including during metastatic PCa. Among other sites, bone is one of the most frequent metastatic target sites for PCa. It was reported that 90% of patients with metastatic PCa will develop bone metastasis [53]. The consequences are devastating, and the available treatment options are limited. Currently, 223Ra-dichloride is being used for the treatment of bone metastases [54]. However, patients continue to receive hormone therapy as well. A clinical study showed that 223Ra (50 kBq/kg) can be safely combined with abiraterone or enzalutamide for the treatment of bone metastasis. In this study, the addition of abiraterone or enzalutamide to 223Ra therapy demostrated prolonged median survival in patients with mCRPCa [55]. However, recently one study showed an increased incidence of fractures and deaths in patients who received 223Ra (55 kBq/kg) combined with abiraterone, compared with patients who received abiraterone alone [56]. The toxicity profiles of enzalutamide in combination with 223Ra are consistent with those seen when they are used as single agents. However, skeletal-related events of this combination are still under investigation (NCT02225704).
In a preclinical setting, LNCap cells treated in vitro with abiraterone or enzalutamide showed increased expression of PSMA which was linked to an antiproliferative effect. Interestingly, another cell line that is androgen-independent also increased its PSMA expression after abiraterone and enzalutamide treatment. However, in this case, no antiproliferative effect was observed [57]. Thus, patients especially those with advanced PCa who receive ADT may benefit from a combination of abiraterone or enzalutamide and PSMA-targeting radiotracers. In line with this Moo, T.A., et al., 2017 recently observed a seven-fold increase in 68Ga-PSMA-11 uptake in patients after receiving ADT [58]. Even though the PSMA radiotracer was used for imaging purposes in this study, these findings indicate the high potential of combination therapy when a PSMA radiotracer coupled to a therapeutic radionuclide is applied.
In BC, hormone therapy is given to patients whose tumors express ER, PR, or both. The purpose of hormone therapy is to prevent the interaction between estrogens and estrogen-dependent pathways that stimulate the proliferation of neoplastic cells. This can be performed by blocking the production of estrogens or by blocking the interaction between estrogen and cancer cells. The production of estrogen can be blocked by oophorectomy, aromatase inhibitors (e.g letrozole), or LHRH analogs (e.g., goserelin). The activity of estrogen towards tumor cells can be blocked using a selective estrogen receptor modulator (SERM) or a selective estrogen receptor down regulator (SERD) such as tamoxifen and fulvestrant, respectively [59,60].
Studies suggest that the cross-talk between estrogen and growth-factor signal cascades might inhibit the effects of radiation. Hence blockage of estrogen and thereby its cross-talk with growth-factor signal cascades can potentially make tumors more radiosensitive, providing reasoning for combining anti-estrogen treatment with RT or TRT [61].
Hormonal therapy is the first-line treatment for hormone-positive metastatic BC. As in PCa, in BC bone is also the most preferential metastatic site, and 223Ra in combination with hormone therapy is becoming more apparent in the treatments of bone metastasis in BC as well. A phase II study that evaluated the efficacy of 223Ra in combination with hormone therapy for the treatment of hormone-positive BC with bone metastases found a high disease control rate at nine months (49%) and tumor response rate at six months (54%) [62]. In this study, patients received 223Ra (55 kBq/kg) every four weeks up to six cycles and a hormonal agent, either tamoxifen or fulvestrant. In addition, these patients also received a subcutaneous injection of denosumab, a monoclonal antibody used for the treatment of osteoporosis, every four weeks, which was considered standard of care during the study period.
Interestingly, trastuzumab, an anti-HER2 in combination with 177Lu-DOTAGA-PEG2-RM26, a GRPR TRT, has been shown to prolong overall survival in mice bearing the human PCa PC3 xenograft [63]. This combination therapy strategy might also be interesting for targeting HER2 positive BC that are also ER or PR positive since it is known that this BC subtype often also expresses the GRPR [64,65,66].
4. Chemotherapy-Based Combination Therapy
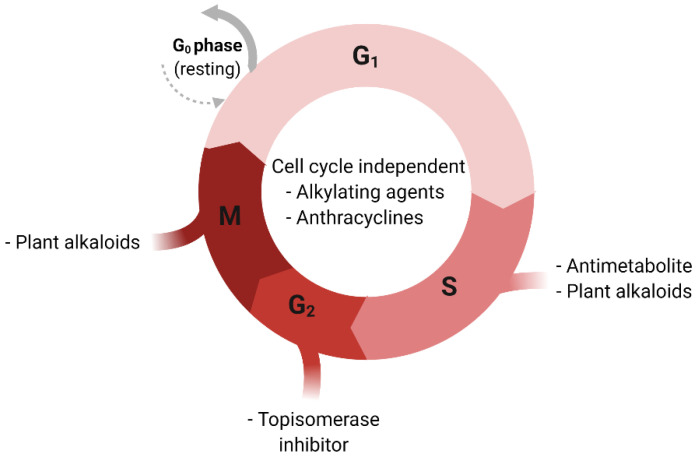
There are several types of chemotherapeutic agents used to treat cancer. These drugs can be categorized based on their chemical structure and their mechanism of action. Some of these drugs target different phases of the cell cycle while others are cell-cycle non-specific (Figure 1) [67]. Alkylating agents and platinum derivatives (e.g., cyclophosphamide and cisplatin, respectively) are active in the resting phase of the cell. These types of agents are therefore considered cell-cycle non-specific. Alkylating agents substitute alkyl groups for hydrogen atoms or bind to DNA causing crosslink formation within DNA double strands and thereby stop tumor growth. Platinum derivatives also cause crosslinking of DNA but do not alkylate [68]. Antimetabolites (e.g., 5-fluorouracil) block cellular metabolism by mimicking normal substances within the cell [69]. Anthracyclines (e.g., doxorubicin) act during multiple phases of the cell-cycle including during DNA replication [70]. Topoisomerase inhibitors (e.g., topotecan) are types of chemotherapy drugs that interfere with the action of topoisomerase enzymes (topoisomerase I and II). These enzymes are involved in the separation of DNA strands before DNA replication [71]. Plant alkaloids such as taxanes (e.g., paclitaxel and docetaxel) act during different phases of the cell cycle and are well-established cancer treatment options for both PCa and BC [71].
Figure 1.
Cell-cycle and respective sensitivity to chemotherapeutic agents. G0; Gap 0 phase, G1: Gap 1 phase, G2: Gap 2 phase, S: Synthesis phase, M: Mitosis phase.
Understanding how chemotherapies work has helped scientists predict which drugs or therapies may be combined successfully. The combination of chemotherapy and radiotherapy has long been used in the treatment of several types of cancers [72,73,74,75,76]. The effect of this combination occurs through several mechanisms [67]. Chemotherapy and radiation can both induce DNA damage. Single strand breaks (SSBs) in the DNA caused by these therapies individually can normally be repaired rapidly. However, radiation-induced SSBs in the DNA can become more difficult to repair with the addition of chemotherapeutic agents such as alkylating agents and platinum derivatives, because DNA crosslinking caused by these chemotherapeutics hinder DNA damage repair. Furthermore, chemotherapeutic agents that effect nucleoside and nucleotide metabolism can inhibit post-radiation DNA damage repair. Moreover, agents such as taxanes exert their cytotoxic activity by preventing microtubule disassembly during the G2/M phase of the cell cycle, causing cell arrest. During these phases, cells are more radiosensitive because endogenous radioprotectors molecules are at their lowest. In addition, chemotherapies that target the S phase of the cell cycle and those that inhibit either proliferation, growth factor pathways, or both, may be effective in preventing tumor cell repopulation after radiotherapy, which is one of the crucial factors determining cure probability in radiotherapy [77,78]. Another benefit that chemotherapy brings when used in combination with radiotherapy is its ability to make hypoxic cells more radiosensitive. Tumor radiosensitivity rapidly decreases in hypoxic environments. The presence of oxygen can prevent repair of the DNA damage (oxygen makes the DNA damage permanent), the so-called “oxygen-enhancement effect”. This means that in hypoxic conditions, higher radiation doses are required to achieve the same biologic effect as in the presence of oxygen [79]. Chemotherapy can reoxygenate tumors by removing cells from the tumor allowing hypoxic cell to have more access to oxygen, and thus making them more sensitive to radiation [80,81].
As for RT and TRT, similar interactions to the above mentioned are expected. However, these might vary since the characteristics of RT and TRT (heterogeneous irradiation, long exposure, and low absorbed dose rate) differ from those of conventional radiotherapy (homogeneous irradiation, short exposure, and high absorbed dose rate) [82]. Nevertheless, there is growing evidence that supports the benefits of chemotherapy combined with RT or TRT. In an in vitro study, the combination of cisplatin and 186Re-HEDP (1.84 or 3.69 MBq/mL), a bone targeting agent, showed a synergetic effect in the treatment of R3327-MATLyLu cells [83]. These are metastatic anaplastic tumor (MAT) cells with high metastatic potential to the lymph nodes and lungs (LyLu) derived from the Dunning rat R3327 [84]. 186Re-HEDP and cisplatin alone both cause a decrease in the number of surviving tumor cells, and the combination of the two showed a greater effect in a dose dependent manner. The benefits of cisplatin in combination with bone targeting RT were later confirmed by a randomized clinical trial using 89Sr instead of 186Re-HEDP. They found that low-doses of cisplatin combined with 89Sr (148 MBq) significantly improved the quality of life and the duration of metastatic bone pain reduction in patients with CRPCa [85]. Pain relief was observed in 91% of the patients who received combination therapy and 63% in the placebo group with a median duration of 120 and 60 days, respectively. Furthermore, fewer new painful sites and less bone disease progression were observed when the combination therapy was administered.
Another study investigated the efficacy of docetaxel in combination with 177Lu-radiolabeled anti-Lewis Y (LeY) human monoclonal antibody hu3S193 in mice bearing LeY positive DU145 PCa xenografts [86]. The LeY antigen is a blood group-related antigen that is overexpressed in over 60–90% of human epithelial cancers [87]. Treatment of DU145 xenografts with 177Lu-hu3S193 (3.7 MBq) alone caused a moderate delay in tumor growth of about 80 days. However, mice treated with the combination of 177Lu-hu3S193 and docetaxel survived until the end of the study and had a tumor growth delay of about 140 days. The authors also investigated the effect of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor AG1478 in combination with 177Lu-hu3S193. EGFR is involved in regulating cellular proliferation, differentiation, and survival, thus blockage of the EGFR is expected to cause inhibition of cell growth [88]. This combination has shown to reduce the rate of tumor growth, however, to a lower extent compared to 177Lu-hu3S193 plus docetaxel. Furthermore, in other studies, docetaxel has shown to have a radiosensitizing effect in vitro in human colon, lung, head, neck, cervical, and several PCa cell lines [89,90,91,92,93].
In BC, chemotherapy is generally recommended as the first-line treatment for patients with TNBC. Patient-related factors such as tumor biology, disease growth rate, presence of metastases, menopausal status, and comorbidities should also be considered in the decision on a specific chemotherapy [94]. Anthracyclines alone, or in combination with other agents, are among the most active chemotherapies for the treatment of BC. One study investigated the effect of a monoclonal antibody targeting the EGFR, 177Lu-anti-EGFR in combination with docetaxel, doxorubicin plus the poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor rucaparib (PARP inhibitors work by inhibiting DNA repair), for the treatment of TNBC cells [95]. 177Lu-anti-EGFR (300 MBq/kg) in combination with docetaxel and doxorubicin reduced the growth rate of MDA-MB-231 and HCI-002 BC xenografts by 83 days vs. 52 days when the BC xenografts were treated with 177Lu-anti-EGFR alone. The triple-agent combination therapy resulted in full eradication of the established xenograft. Furthermore, no recurrences were observed up to 120 days for MDA-MB-231 xenografts and >195 days for HCI-002 xenografts after the triple-agent combination treatment. Further analysis suggested that inhibition of central DNA repair proteins was crucial to the efficacy of the triple-agent combination.
Recently, 177Lu-Bombesin-PLGA (paclitaxel) nanomedicine has been investigated as a potential combination therapy for BC [96]. Here the radionuclide 177Lu was coupled to the bombesin (BN) peptide which is known to have a high affinity for the GRPR. High expression of GRPR is seen in several types of cancers including BC [97]. Paclitaxel (PTX) and, 177Lu-Bombesin were both loaded onto the poly lactic-co-glycolic acid (PLGA) nanobody and studied on MDA-MB-BC cells. The chemotherapeutic effect of PTX and the radiation of 177Lu-BN (5 MBq) produced by the 177Lu-BN-PLGA(PTX) nanosystem had a synergistic effect on MDA-MB-231 cell viability compared to BN-PLGA(PTX) alone. Interestingly, internalization with regard to total uptake was higher for 177Lu-BN-PLGA(PTX) compared to 177Lu-BN alone.
Furthermore, there is an ongoing clinical trial investigating the effect of 223Ra-dichloride (55 kBq/kg) in HER2 negative, hormone-positive BC patients with bone metastases treated with exemestane and everolimus (NCT02258451). The results of this study have not been published yet.
5. Immunotherapy-Based Combination Therapy
In recent years, immunotherapy has become an important cancer treatment. Several types of immunotherapy for cancer are known including monoclonal antibodies (e.g., anti-cytotoxic T-lymphocyte-associated protein 4), checkpoint inhibitors (e.g., anti-programmed cell death protein 1 (anti-PD-1) and its ligand programmed death-ligand 1 (anti-PD-L1)), cytokines, T-cell therapy, and vaccines. Infiltration into the tumor microenvironment and successful activation of effector T lymphocytes are crucial for the success of immunotherapy. However, tumor cells have developed a variety of mechanisms to reduce anti-cancer immunity [98]. A strategy to overcome this is by combining immunotherapy with other types of therapies that make tumors more T-cell inflamed, especially in PCa and BC since the majority of these tumors are not inflamed [99,100]. Several studies have shown that depending on the type of radiotherapy, radiation can induce a tumor-specific immune response or reprogram the tumor microenvironment in a way that facilitates both immune cell recognition and immune-mediated killing [101,102,103,104]. Furthermore, radiation can also induce systemic antitumor effects in unirradiated tumors/metastases after local radiation, known as the abscopal effect [105,106,107]. The radiation-induced abscopal effect occurs through several mechanism which involves the release of a number of endogenous damage-associated molecular patterns (DAMPs) by apoptotic cells via a process termed “immunogenic cell death” (ICD). Subsequently, DAMPs triggers dendritic cells (DCs) resulting in an enhanced tumor antigen presentation, and stimulates cytotoxic T lymphocyte and the release of cytokines and chemokines which all facilitates immune response to cancer cells [107,108].
In an in vitro study, 223Ra-dichloride has been shown to enhance T cell-mediated killing of PCa (LNCap and PC3), BC (MDA-MB-231 and ZR75-1), and lung cancer (H1703 and H441) cells [109]. These cell lines were first exposed to 4 or 10 gray (Gy) of 223Ra, then co-cultured with CD8+ effector T cells specific for carcinoembryonic antigen (CEA, HLA-A2-restricted), mucin-1 (MUC-1, HLA-A2-restricted), and brachyury (HLA-A2/A24-restricted) epitopes. This resulted in a significant increase in T cell-mediated killing after both 4 and 10 Gy of radiation. Furthermore, exposure to 223Ra significantly increased the expression of calreticulin and MHC-I, both molecules that facilitate and support the initiation of anti-cancer immunity [109,110,111]. Several clinical studies have/are investigating the combination of immunotherapy with 223Ra for the treatment of PCa. These combinations are mainly focused on abrogating immunosuppression using immune checkpoint inhibitors. A phase-I randomized clinical trial evaluated the safety and tolerability of the PD-L1 checkpoint inhibitor atezolizumab when given in combination with 223Ra-dichloride (55 kBq/kg) in patients with mCRPCa (NCT02814669). Although this study is completed, no results have been published yet. In addition, a phase-II study is assessing the antigen-specific immune response of sipuleucel-T vaccine therapy with or without 223Ra (50 kBq/kg) (NCT02463799), whereas another phase-II study is investigating the combination of 223Ra plus the anti-PD-1 checkpoint inhibitor pembrolizumab as a possible treatment for castration-resistant PCa (NCT03093428).
In a preclinical study, the checkpoint inhibitor anti-PD-1 in combination with a PSMA TRT in mice bearing PCa tumor was investigated [112]. Anti-PD-1 in combination with 225Ac-PSMA-617 (30 kBq) demonstrated improved tumor control compared to the monotherapies. The time to tumor progression was 47.5 days in the combined therapy group vs. 33.5 days with anti-PD-1 or 30 days with 225Ac-PSMA-617 alone. In line with this, survival was extended to 51.5 days when animals received combination therapy vs. 37 and 32 days, respectively, when animals were treated with anti-PD-1 or 225Ac-PSMA-617 alone.
To our knowledge, there are no studies that have reported on the combination of immunotherapy with RT or TRT in BC. However, the findings gained from the studies with PCa could provide crucial insight for the development of these kinds of combination therapies for BC as well.
6. External Beam Radiation Therapy-Based Combination Therapy
Besides hormone therapy, chemotherapy, and immunotherapy, radiation therapy or radiotherapy remains an important therapeutic option for cancer treatment. About 50% of all cancer patients will receive radiotherapy of some kind throughout the period of their illness [113]. There are several types of EBRT including 3D conformal radiotherapy, intensity-modulated radiation therapy, image-guided radiotherapy, stereotactic body radiation therapy, photons radiation, and particle radiations [114]. EBRT is commonly used in everyday radiation therapy treatment. The biological effect of radiation, in general, is DNA damage. If the radiation-induced DNA damage is irreparable, the cell will eventually die during cell division. Thus, the higher the dose the higher is the chance of irreversible DNA double-strand breaks (DSBs). However, the applicable dose of EBRT is limited by the radiosensitivity of the surrounding healthy tissue. The addition of RT or TRT to EBRT offers the possibility to increase the dose applied to the solid tumor without exceeding the limitations of the surrounding normal tissues. Another advantage of this combination is that it can be directed against both localized tumors and metastases [115].
With regard to the literature, reports about EBRT in combination with RT or TRT for the treatment of PCa are rare. A phase one study investigated EBRT (8, 20, or 30 Gy) in combination with 223Ra (50 kBq/kg) in patients with hormone-refractory PCa needing EBRT due to bone pain [116]. EBRT in combination with 223Ra caused a significant reduction in bone-alkaline phosphatase concentration compared to EBRT alone. The median time to first skeletal-related events such as bone pain and bone fractures was 14 weeks in the combined treatment and 11 weeks for EBRT alone. Furthermore, overall survival was higher for patients who received EBRT in combination with 223Ra (65 weeks) compared to EBRT alone (46 weeks). Another group also observed a reduction in pain intensity in patients with mCRPCa after being treated with EBRT (8–30 Gy) in combination with another bone targeting radionuclide, 153Sm (37 MBq/kg) [117]. Toxicity profiles of this combination therapy were acceptable and fully reversible. Hematological toxicity analysis demonstrated no statistically significant differences between the combination therapy and the monotherapy in hemoglobin concentration and the number of blood cells. Other adverse events such as hypercalcemia were reported among a few candidates in both study arms, but no pathological fractures or spinal cord compression was observed in these patients.
As for BC, Cornelissen, B., et al., 2004 were able to increase the efficacy of EBRT by combining it with a TRT targeting the DNA damage response protein, γH2AX, using anti-γH2AX antibodies conjugated to 111In [118]. Here, MDA-MB-468 BC cells were exposed first to 111In-DTPA-anti-γH2AX (6 MBq/μg) after which EBRT (10 Gy) was applied. Immunostaining for γH2AX showed an increase in the number of γH2AX foci/cells in the combination therapy compared to EBRT alone, which is an indication of more DNA DSBs. This was confirmed with a clonogenic assay which showed a reduction in the survival rate of MDA-MB-468 BC cells. Furthermore, the combination EBRT plus 111In-DTPA-anti-γH2AX significantly decreased the growth rate of MDA-MB-231 xenografts in vivo compared to the single agents. However, this strategy may not be effective in metastatic disease, because only cells in the radiation field will have increased γH2AX after EBRT.
7. Discussion and Conclusions
The rising interest in TRT has led to the evaluation of several combination strategies. Besides the above-mentioned combination therapies for PCa and BC, other interesting combinations of TRT with other anti-cancer drugs have been studied for targeting of other cancer types. The knowledge gained from these studies can potentially be used to develop promising combination treatments for PCa and BC in the future. Well-known TRT agents that have been widely studied in combination are those that target the SSTR. SSTRs are G protein-coupled receptors with five known subtypes (SSTR1–5). They are found in several tissues including neuroendocrine tumors (NETs) and to a lesser extent in BC. Because of their high expression in NETs, several radiolabeled SST analogs have been developed over the years, with octreotate having the highest uptake. 177Lu-DOTA0-Tyr3-Octreotate also known as 177Lu-octreotate is the first TRT approved by the EMA and the FDA for the treatment of SSTR-positive gastroenteropancreatic NETs [119]. Even though 177Lu-octreotate has proven successful in the treatment of several NETs [120,121,122], only a limited number of patients show complete remission after the treatment [123,124]. Therefore, many approaches that combine SSTR TRT with other therapies are being explored to improve its therapeutic efficacy. Capecitabine (CAP) and temozolomide (TEM) in combination with 177Lu-octreotate have been investigated by several groups [125,126,127,128,129,130,131,132] with some showing tumor response rates that are higher compared to 177Lu-octreotate alone, the latter reported by Kwekkeboom, D.J., et al., 2008 [120]. A pre-clinical study evaluated what would be the most effective treatment schedule for TEM and SSTR TRT combination therapy [133]. In this study, treatment with TEM resulted in increased tumor perfusion which reached its peak after 14 days. At this time point, the uptake of 177Lu-octreotate was also at its peak in the combined therapy group. The peak of radioactivity uptake was not influenced by tumor volume since the average tumor size was in the same range at day 0 and day 13. An increased level of SSTR2 expression was not the reason for the increased uptake of 177Lu-octreotate as no difference in SSTR2 expression prior to vs. after TEM treatment was observed. However, the cell line (H69) used in this study already expresses high levels of SSTR2 which may not increase further after TEM treatment. In line with this, another group did show a positive correlation between SSTR2 expression and 177Lu-octreotate uptake in low SSTR2 expressing cells after TEM treatment [134].
Several studies have examined the influence of other chemotherapies on SSTR expression and uptake of radiotracers. However, the results of these studies are variable. One study showed that several pancreatic tumor cell lines reduced the expression of high-affinity binding sites for 111In-labeled lanreotide derivative DOTA-LAN, another radiolabeled SST analog, after being treated with gemcitabine, 5-fluorouracil, cisplatin, camptothecin, mitomycin C, and doxorubicin [135]. In a different study, upregulation of SSTR2 expression in three different NET cell lines was demonstrated after treatment with 5-fluorouracil, however, in combination with epigenetic modifiers such as decitabine or tacedinaline [136].
The above-mentioned chemotherapeutic agents investigated in combination with radiolabeled SST analogs are used to treat different types of cancers, including PCa and BC. To the best of our knowledge, no studies have investigated whether these specific chemotherapeutic agents can cause similar effects in regard to the upregulation of SSTR2 expression in BC. Furthermore, it would be very interesting to know what the effects of these agents are on other receptors that are expressed by PCa (e.g., PSMA and GRPR) and BC cells (e.g., GRPR). Tumor perfusion should also be taken into consideration since this can potentially lead to higher doses of radiopharmaceuticals at the tumor site, even when target expression levels remain the same.
Radiation can also increase SSTR expression. Two studies demonstrated up-regulation of SSTR1, SSTR2, and SSTR5 expressions on NET cells after being treated with ionizing radiation in vitro [137,138]. Thus, irradiation of tumor tissue could be used to increase the specific binding of subsequent administrated 177Lu-octreotate, resulting in an increased absorbed dose to the tumor cells. A few studies investigated whether pre-treatment or priming with 177Lu-octreotate could induce similar effects. One group demonstrated higher uptake of 111In-octreotate following an injection of a low non-curative amount of 177Lu-octreotate. Priming with a high 177Lu-octreotate concentration resulted in lower uptake of 111In-octreotate [139]. However, a follow-up study found no statistically significant difference in SSTR expression between treatment with and without priming [140]. This indicates that the increase in tumor uptake here might not be due to increased levels of SSTR but rather another mechanism. Nevertheless, a potential approach to increase the efficacy of TRT might involve treatment with EBRT followed by fractionated treatment with TRT.
As was also previously mentioned, radiation can facilitate anti-cancer immunity via a range of cellular mechanisms of both tumor and immune cells [101,102,103,104]. It has been demonstrated, that in contrast to high radiation doses, lower doses of radiation induce several changes within tumor cells that ultimately facilitate immune cell recognition and immune-mediated tumor killing [141,142]. These results are promising and offer a great opportunity for the use of RT or TRT in combination with immune therapy. However, studies showed that radiation can also lead to immunosuppression. It is therefore very important to take the immunosuppressive mechanisms of radiation into account when designing combination therapies. For example, radiation has been shown to activate the transforming growth factor β and promotes the accumulation of regulatory T cells and pro-tumorigenic macrophages [143,144,145]. Fortunately, there is a promising approach to overcome these immunosuppressive mechanisms of radiation. This approach involves the use of the before-mentioned immune checkpoint inhibitors such as the anti-PD-1 in combination with radiation. Clinical trials have shown safety and impressive anti-tumor responses of antibodies blocking the activity of PD-1 or PD-L1 [146,147], and others have shown increased levels of PD-L1 in tumors after local irradiation [148]. This provides a clear rationale for combining RT or TRT with immune checkpoint inhibitors, for example, as was done for the combination of 225Ac-PSMA-617 with anti-PD1 [112]. However, a considerable amount of work is still required to define the optimal dose and radionuclide that can create maximal interactions with the immune system and to identify the type and schedule of immunomodulatory drugs that are best suitable for combination treatment.
Besides, the development and application of RT and TRT itself face many challenges as well. For example, a short half-life of radiotracers in the blood circulation can be a major obstacle since this will lead to deterioration of radiotracers before reaching the intended target. The loss or altering of binding affinity to the target due to in vivo metabolism are other potential challenges. Specific uptake of radiotracers in healthy organs expressing the target can be dose-limiting and reduce effectiveness. Finally, small radiolabeled molecules such as peptides can cause renal toxicity due to uptake and retention of radionuclides in the kidneys as a consequence of renal clearance and partial reabsorption, while larger radiolabeled molecules such as antibodies can cause hematological toxicity because of their long circulation half-life
In conclusion, there is still a significant amount of work that needs to be done in order to fully benefit from combination therapies with RT and TRT for PCa and BC. Nevertheless, the knowledge gained from current studies can help identify promising combination therapy strategies that can be further explored to improve the efficacy of RT or TRT and thus clinical outcomes of PCa and BC in the future.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kassis A.I., Adelstein S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005;46:4S–12S. [PubMed] [Google Scholar]
- 2.Yeong C.H., Cheng M.H., Ng K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B. 2014;15:845–863. doi: 10.1631/jzus.B1400131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ersahin D., Doddamane I., Cheng D. Targeted radionuclide therapy. Cancers. 2011;3:3838–3855. doi: 10.3390/cancers3043838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dash A., Chakraborty S., Pillai M.R., Knapp F.F., Jr. Peptide receptor radionuclide therapy: An overview. CancerBiother. Radiopharm. 2015;30:47–71. doi: 10.1089/cbr.2014.1741. [DOI] [PubMed] [Google Scholar]
- 5.Nayak T.K., Brechbiel M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009;20:825–841. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcu L., Bezak E., Allen B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018;123:7–20. doi: 10.1016/j.critrevonc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Pouget J.P., Navarro-Teulon I., Bardiès M., Chouin N., Cartron G., Pèlegrin A., Azria D. Clinical radioimmunotherapy--the role of radiobiology. Nat. Rev. Clin. Oncol. 2011;8:720–734. doi: 10.1038/nrclinonc.2011.160. [DOI] [PubMed] [Google Scholar]
- 8.Makvandi M., Dupis E., Engle J.W., Nortier F.M., Fassbender M.E., Simon S., Birnbaum E.R., Atcher R.W., John K.D., Rixe O., et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018;13:189–203. doi: 10.1007/s11523-018-0550-9. [DOI] [PubMed] [Google Scholar]
- 9.Siemann D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mengdi Q., Almasan A., Gurkan-Cavusoglu E. Computational analysis of androgen receptor dependent radiosensitivity in prostate cancer. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016;2016:1426–1429. doi: 10.1109/EMBC.2016.7590976. [DOI] [PubMed] [Google Scholar]
- 11.Rong C., Meinert E., Hess J. Estrogen Receptor Signaling in Radiotherapy: From Molecular Mechanisms to Clinical Studies. Int. J. Mol. Sci. 2018;19:713. doi: 10.3390/ijms19030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klintman M., Buus R., Cheang M.C., Sheri A., Smith I.E., Dowsett M. Changes in Expression of Genes Representing Key Biologic Processes after Neoadjuvant Chemotherapy in Breast Cancer, and Prognostic Implications in Residual Disease. Clin. Cancer Res. 2016;22:2405–2416. doi: 10.1158/1078-0432.CCR-15-1488. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz T.A., Stivers D.N., Stec J., Ayers M., Clark E., Bolt A., Sahin A.A., Symmans W.F., Hess K.R., Kuerer H.M., et al. Global gene expression changes during neoadjuvant chemotherapy for human breast cancer. Cancer J. 2002;8:461–468. doi: 10.1097/00130404-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Beltran H., Wyatt A.W., Chedgy E.C., Donoghue A., Annala M., Warner E.W., Beja K., Sigouros M., Mo F., Fazli L., et al. Impact of Therapy on Genomics and Transcriptomics in High-Risk Prostate Cancer Treated with Neoadjuvant Docetaxel and Androgen Deprivation Therapy. Clin. Cancer Res. 2017;23:6802–6811. doi: 10.1158/1078-0432.CCR-17-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmusvaara S., Erkkilä T., Urbanucci A., Waltering K., Seppälä J., Larjo A., Tuominen V.J., Isola J., Kujala P., Lähdesmäki H., et al. Chemical castration and anti-androgens induce differential gene expression in prostate cancer. J. Pathol. 2012;227:336–345. doi: 10.1002/path.4027. [DOI] [PubMed] [Google Scholar]
- 16.Severson T.M., Nevedomskaya E., Peeters J., Kuilman T., Krijgsman O., van Rossum A., Droog M., Kim Y., Koornstra R., Beumer I., et al. Neoadjuvant tamoxifen synchronizes ERalpha binding and gene expression profiles related to outcome and proliferation. Oncotarget. 2016;7:33901–33918. doi: 10.18632/oncotarget.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill M.R., Falzone N., Du Y., Vallis K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017;18:e414–e423. doi: 10.1016/S1470-2045(17)30379-0. [DOI] [PubMed] [Google Scholar]
- 18.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 19.Catalona W.J. Prostate Cancer Screening. Med. Clin. N. Am. 2018;102:199–214. doi: 10.1016/j.mcna.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M., Fossati N., Gross T., Henry A.M., Joniau S., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part. 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Litwin M.S., Tan H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 22.Jadvar H. Targeted Radionuclide Therapy: An Evolution toward Precision Cancer Treatment. AJR Am. J. Roentgenol. 2017;209:277–288. doi: 10.2214/AJR.17.18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluetz P.G., Pierce W., Maher V.E., Zhang H., Tang S., Song P., Liu Q., Haber M.T., Leutzinger E.E., Al-Hakim A., et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2014;20:9–14. doi: 10.1158/1078-0432.CCR-13-2665. [DOI] [PubMed] [Google Scholar]
- 24.Anderson P. Samarium for osteoblastic bone metastases and osteosarcoma. Expert Opin. Pharmacother. 2006;7:1475–1486. doi: 10.1517/14656566.7.11.1475. [DOI] [PubMed] [Google Scholar]
- 25.Deshayes E., Roumiguie M., Thibault C., Beuzeboc P., Cachin F., Hennequin C., Huglo D., Rozet F., Kassab-Chahmi D., Rebillard X., et al. Radium 223 dichloride for prostate cancer treatment. Drug Des. Dev. Ther. 2017;11:2643–2651. doi: 10.2147/DDDT.S122417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X., He G., Liu J., Luo F., Peng X., Tang S., Gao Z., Lin Q., Keller J.M., Yang T., et al. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40:730–738. doi: 10.1016/j.ctrv.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller M.S., Lee C.I., Elmore J.G. Breast cancer screening: An evidence-based update. Med. Clin. N. Am. 2015;99:451–468. doi: 10.1016/j.mcna.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald E.S., Clark A.S., Tchou J., Zhang P., Freedman G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016;57:9S–16S. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 29.Perou C.M., Sørlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 30.Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørlie T., Tibshirani R., Parker J., Hastie T., Marron J.S., Nobel A., Deng S., Johnsen H., Pesich R., Geisler S., et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makki J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015;8:23–31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumachi F., Brunello A., Maruzzo M., Basso U., Basso S.M. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013;20:596–604. doi: 10.2174/092986713804999303. [DOI] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations, (Radium RA-223 Dichloride) [(accessed on 19 April 2021)]; Available online: https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=N&Appl_No=203971#29574.
- 35.Coleman R., Aksnes A.K., Naume B., Garcia C., Jerusalem G., Piccart M., Vobecky N., Thuresson M., Flamen P. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res. Treat. 2014;145:411–418. doi: 10.1007/s10549-014-2939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Food and Drug Administration Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations, (Samarium Sm-153 Lexidronam Pentasodium) [(accessed on 19 April 2021)]; Available online: https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=N&Appl_No=020570#20000.
- 37.Niaz M.J., Batra J.S., Walsh R.D., Ramirez-Fort M.K., Vallabhajosula S., Jhanwar Y.S., Molina A.M., Nanus D.M., Osborne J.R., Bander N.H., et al. Pilot Study of Hyperfractionated Dosing of Lutetium-177-Labeled Antiprostate-Specific Membrane Antigen Monoclonal Antibody J591 ((177) Lu-J591) for Metastatic Castration-Resistant Prostate Cancer. Oncologist. 2020;25:e477–e895. doi: 10.1634/theoncologist.2020-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerecker B., Tauber R., Knorr K., Heck M., Beheshti A., Seidl C., Bruchertseifer F., Pickhard A., Gafita A., Kratochwil C., et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021;79:343–350. doi: 10.1016/j.eururo.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Kratochwil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 40.Dalm S., de Blois E., Haeck J., Doeswijk G., Orlandi F., BARBATO D., Tedesco M., Konijnenberg M., De Jong M. 177Lu-NeoBOMB1 for GRPR-mediated cancer treatment: The effect of peptide mass on efficacy and safety. J. Nucl. Med. 2018;59:534. [Google Scholar]
- 41.Kaloudi A., Lymperis E., Giarika A., Dalm S., Orlandi F., Barbato D., Tedesco M., Maina T., de Jong M., Nock B.A. NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [(67)Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice. Molecules. 2017;22:1950. doi: 10.3390/molecules22111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurth J., Krause B.J., Schwarzenböck S.M., Bergner C., Hakenberg O.W., Heuschkel M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [(177)Lu]Lu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:123–135. doi: 10.1007/s00259-019-04504-3. [DOI] [PubMed] [Google Scholar]
- 43.Dalm S.U., Nonnekens J., Doeswijk G.N., de Blois E., van Gent D.C., Konijnenberg M.W., de Jong M. Comparison of the Therapeutic Response to Treatment with a 177Lu-Labeled Somatostatin Receptor Agonist and Antagonist in Preclinical Models. J. Nucl. Med. 2016;57:260–265. doi: 10.2967/jnumed.115.167007. [DOI] [PubMed] [Google Scholar]
- 44.Luo T.Y., Cheng P.C., Chiang P.F., Chuang T.W., Yeh C.H., Lin W.J. 188Re-HYNIC-trastuzumab enhances the effect of apoptosis induced by trastuzumab in HER2-overexpressing breast cancer cells. Ann. Nucl. Med. 2014;29:52–62. doi: 10.1007/s12149-014-0908-8. [DOI] [PubMed] [Google Scholar]
- 45.Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 46.Crawford E.D., Heidenreich A., Lawrentschuk N., Tombal B., Pompeo A., Mendoza-Valdes A., Miller K., Debruyne F., Klotz L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. doi: 10.1038/s41391-018-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S., Shelley M., Harrison C., Coles B., Wilt T.J., Mason M.D. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst. Rev. 2006;18:CD006019. doi: 10.1002/14651858.CD006019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKay R.R., Choueiri T.K., Taplin M.E. Rationale for and review of neoadjuvant therapy prior to radical prostatectomy for patients with high-risk prostate cancer. Drugs. 2013;73:1417–1430. doi: 10.1007/s40265-013-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones C.U., Hunt D., McGowan D.G., Amin M.B., Chetner M.P., Bruner D.W., Leibenhaut M.H., Husain S.M., Rotman M., Souhami L., et al. Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer. N. Engl. J. Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 50.Polkinghorn W.R., Parker J.S., Lee M.X., Kass E.M., Spratt D.E., Iaquinta P.J., Arora V.K., Yen W.F., Cai L., Zheng D., et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghashghaei M., Niazi T.M., Heravi M., Bekerat H., Trifiro M., Paliouras M., Muanza T. Enhanced radiosensitization of enzalutamide via schedule dependent administration to androgen-sensitive prostate cancer cells. Prostate. 2018;78:64–75. doi: 10.1002/pros.23445. [DOI] [PubMed] [Google Scholar]
- 52.Palacios D.A., Miyake M., Rosser C.J. Radiosensitization in prostate cancer: Mechanisms and targets. BMC Urol. 2013;13:4. doi: 10.1186/1471-2490-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong S.K., Mohamad N.V., Giaze T.R., Chin K.Y., Mohamed N., Ima-Nirwana S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019;20:2587. doi: 10.3390/ijms20102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nevedomskaya E., Baumgart S.J., Haendler B. Recent Advances in Prostate Cancer Treatment and Drug Discovery. Int. J. Mol. Sci. 2018;19:1359. doi: 10.3390/ijms19051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saad F., Carles J., Gillessen S., Heidenreich A., Heinrich D., Gratt J., Lévy J., Miller K., Nilsson S., Petrenciuc O., et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: An international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–1316. doi: 10.1016/S1470-2045(16)30173-5. [DOI] [PubMed] [Google Scholar]
- 56.Smith M., Parker C., Saad F., Miller K., Tombal B., Ng Q.S., Boegemann M., Matveev V., Piulats J.M., Zucca L.E., et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–419. doi: 10.1016/S1470-2045(18)30860-X. [DOI] [PubMed] [Google Scholar]
- 57.Murga J.D., Moorji S.M., Han A.Q., Magargal W.W., DiPippo V.A., Olson W.C. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate. 2015;75:242–254. doi: 10.1002/pros.22910. [DOI] [PubMed] [Google Scholar]
- 58.Hope T.A., Truillet C., Ehman E.C., Afshar-Oromieh A., Aggarwal R., Ryan C.J., Carroll P.R., Small E.J., Evans M.J. Ga-68-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. 2017;58:81–84. doi: 10.2967/jnumed.116.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moo T.A., Sanford R., Dang C., Morrow M. Overview of Breast Cancer Therapy. PET Clin. 2018;13:339–354. doi: 10.1016/j.cpet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Draganescu M., Carmocan C. Hormone Therapy in Breast Cancer. Chirurgia. 2017;112:413–417. doi: 10.21614/chirurgia.112.4.413. [DOI] [PubMed] [Google Scholar]
- 61.Chargari C., Toillon R.A., Macdermed D., Castadot P., Magné N. Concurrent hormone and radiation therapy in patients with breast cancer: What is the rationale? Lancet Oncol. 2009;10:53–60. doi: 10.1016/S1470-2045(08)70333-4. [DOI] [PubMed] [Google Scholar]
- 62.Ueno N.T., Tahara R.K., Saigal B., Fujii T., Reuben J.M., Gao H., Lucci A., Ibrahim N.K., Damodaran S., Shen Y., et al. Phase II study of Ra-223 combined with hormonal therapy and denosumab for treatment of hormone receptor-positive breast cancer with bone-dominant metastasis. J. Clin. Oncol. 2018;36:1065. doi: 10.1200/JCO.2018.36.15_suppl.1065. [DOI] [Google Scholar]
- 63.Mitran B., Rinne S.S., Konijnenberg M.W., Maina T., Nock B.A., Altai M., Vorobyeva A., Larhed M., Tolmachev V., de Jong M., et al. Trastuzumab cotreatment improves survival of mice with PC-3 prostate cancer xenografts treated with the GRPR antagonist (177) Lu-DOTAGA-PEG2 -RM26. Int. J. Cancer. 2019;145:3347–3358. doi: 10.1002/ijc.32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morgat C., Schollhammer R., Macgrogan G., Barthe N., Vélasco V., Vimont D., Cazeau A.L., Fernandez P., Hindié E. Comparison of the binding of the gastrin-releasing peptide receptor (GRP-R) antagonist 68Ga-RM2 and 18F-FDG in breast cancer samples. PLoS ONE. 2019;14:e0210905. doi: 10.1371/journal.pone.0210905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morgat C., MacGrogan G., Brouste V., Vélasco V., Sévenet N., Bonnefoi H., Fernandez P., Debled M., Hindié E. Expression of Gastrin-Releasing Peptide Receptor in Breast Cancer and Its Association with Pathologic, Biologic, and Clinical Parameters: A Study of 1432 Primary Tumors. J. Nucl. Med. 2017;58:1401–1407. doi: 10.2967/jnumed.116.188011. [DOI] [PubMed] [Google Scholar]
- 66.Dalm S.U., Sieuwerts A.M., Look M.P., Melis M., van Deurzen C.H., Foekens J.A., de Jong M., Martens J.W. Clinical Relevance of Targeting the Gastrin-Releasing Peptide Receptor, Somatostatin Receptor 2, or Chemokine C-X-C Motif Receptor 4 in Breast Cancer for Imaging and Therapy. J. Nucl. Med. 2015;56:1487–1493. doi: 10.2967/jnumed.115.160739. [DOI] [PubMed] [Google Scholar]
- 67.Seiwert T.Y., Salama J.K., Vokes E.E. The concurrent chemoradiation paradigm--general principles. Nat. Clin. Pract. Oncol. 2007;4:86–100. doi: 10.1038/ncponc0714. [DOI] [PubMed] [Google Scholar]
- 68.Lajous H., Lelièvre B., Vauléon E., Lecomte P., Garcion E. Rethinking Alkylating(-Like) Agents for Solid Tumor Management. Trends Pharmacol. Sci. 2019;40:342–357. doi: 10.1016/j.tips.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Peters G.J. Novel Developments in the Use of Antimetabolites. Nucleosides Nucleotides Nucleic Acids. 2014;33:358–374. doi: 10.1080/15257770.2014.894197. [DOI] [PubMed] [Google Scholar]
- 70.Kizek R., Adam V., Hrabeta J., Eckschlager T., Smutny S., Burda J.V., Frei E., Stiborova M. Anthracyclines and ellipticines as DNA-damaging anticancer drugs: Recent advances. Pharmacol. Ther. 2012;133:26–39. doi: 10.1016/j.pharmthera.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Mihlon F.T., Ray C.E., Jr., Messersmith W. Chemotherapy agents: A primer for the interventional radiologist. Semin. Interv. Radiol. 2010;27:384–390. doi: 10.1055/s-0030-1267852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durand R.E., LePard N.E. Effects of mitomycin C on the oxygenation and radiosensitivity of murine and human tumours in mice. Radiother. Oncol. 2000;56:245–252. doi: 10.1016/S0167-8140(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 73.Bartelink H., Kallman R.F., Rapacchietta D., Hart G.A. Therapeutic enhancement in mice by clinically relevant dose and fractionation schedules of cis-diamminedichloroplatinum (II) and irradiation. Radiother. Oncol. 1986;6:61–74. doi: 10.1016/S0167-8140(86)80110-4. [DOI] [PubMed] [Google Scholar]
- 74.Nylén U., Cekan E., Jonasson G.B., Lewin F., Skog S. Effects of 5-fluorouracil on cell cycle arrest and toxicity induced by X-irradiation in normal mammalian cells. Cell Prolif. 2001;34:85–98. doi: 10.1046/j.1365-2184.2001.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seiwert T.Y., Salama J.K., Vokes E.E. The chemoradiation paradigm in head and neck cancer. Nat. Clin. Pract. Oncol. 2007;4:156–171. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- 76.O’Rourke N., Roqué I Figuls M., Farré Bernadó N., Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst. Rev. 2010;18:CD002140. doi: 10.1002/14651858.CD002140.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J.J., Tannock I.F. Repopulation of cancer cells during therapy: An. important cause of treatment failure. Nat. Rev. Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 78.Shapiro G.I., Harper J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Invest. 1999;104:1645–1653. doi: 10.1172/JCI9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Saggar J.K., Tannock I.F. Chemotherapy Rescues Hypoxic Tumor Cells and Induces Their Reoxygenation and Repopulation-An Effect That Is Inhibited by the Hypoxia-Activated Prodrug TH-302. Clin. Cancer Res. 2015;21:2107–2114. doi: 10.1158/1078-0432.CCR-14-2298. [DOI] [PubMed] [Google Scholar]
- 81.Milas L., Hunter N.R., Mason K.A., Milross C.G., Saito Y., Peters L.J. Role of reoxygenation in induction of enhancement of tumor radioresponse by paclitaxel. Cancer Res. 1995;55:3564–3568. [PubMed] [Google Scholar]
- 82.Pouget J.P., Lozza C., Deshayes E., Boudousq V., Navarro-Teulon I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015;2:12. doi: 10.3389/fmed.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geldof A.A., de Rooij L., Versteegh R.T., Newling D.W., Teule G.J. Combination 186Re-HEDP and cisplatin supra-additive treatment effects in prostate cancer cells. J. Nucl. Med. 1999;40:667–671. [PubMed] [Google Scholar]
- 84.Isaacs J.T., Yu G.W., Coffey D.S. The characterization of a newly identified, highly metastatic variety of Dunning R 3327 rat prostatic adenocarcinoma system: The MAT LyLu tumor. Invest. Urol. 1981;19:20–23. [PubMed] [Google Scholar]
- 85.Sciuto R., Festa A., Rea S., Pasqualoni R., Bergomi S., Petrilli G., Maini C.L. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: A randomized clinical trial. J. Nucl. Med. 2002;43:79–86. [PubMed] [Google Scholar]
- 86.Kelly M.P., Lee S.T., Lee F.T., Smyth F.E., Davis I.D., Brechbiel M.W., Scott A.M. Therapeutic Efficacy of Lu-177-CHX-A ‘‘-DTPA-hu3S193 Radioimmunotherapy in Prostate Cancer Is Enhanced by EGFR Inhibition or Docetaxel Chemotherapy. Prostate. 2009;69:92–104. doi: 10.1002/pros.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hellström I., Garrigues H.J., Garrigues U., Hellström K.E. Highly Tumor-Reactive, Internalizing, Mouse Monoclonal-Antibodies to Ley-Related Cell-Surface Antigens. Cancer Res. 1990;50:2183–2190. [PubMed] [Google Scholar]
- 88.Herbst R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 89.Dunne A.L., Mothersill C., Robson T., Wilson G.D., Hirst D.G. Radiosensitization of colon cancer cell lines by docetaxel: Mechanisms of action. Oncol. Res. 2004;14:447–454. doi: 10.3727/0965040041791455. [DOI] [PubMed] [Google Scholar]
- 90.Milas L. Docetaxel/radiation combinations: Rationale and preclinical findings. Clin. Lung Cancer. 2002;3:S29–S36. doi: 10.3816/CLC.2002.s.011. [DOI] [PubMed] [Google Scholar]
- 91.Pradier O., Rave-Fränk M., Lehmann J., Lücke E., Boghun O., Hess C.F., Schmidberger H. Effects of docetaxel in combination with radiation on human head and neck cancer cells (ZMK-1) and cervical squamous cell carcinoma cells (CaSki ) Int. J. Cancer. 2001;91:840–845. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1142>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 92.Hennequin C., Giocanti N., Favaudon V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 1996;56:1842–1850. [PubMed] [Google Scholar]
- 93.Lange R., ter Heine R., van Wieringen W.N., Tromp A.M., Paap M., Bloemendal H.J., de Klerk J.M., Hendrikse N.H., Geldof A.A. Cytotoxic effects of the therapeutic radionuclide rhenium-188 combined with taxanes in human prostate carcinoma cell lines. Cancer Biother. Radiopharm. 2017;32:16–23. doi: 10.1089/cbr.2016.2129. [DOI] [PubMed] [Google Scholar]
- 94.Andre F., Zielinski C.C. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann. Oncol. 2012;23:46–51. doi: 10.1093/annonc/mds195. [DOI] [PubMed] [Google Scholar]
- 95.Al-Ejeh F., Shi W., Miranda M., Simpson P.T., Vargas A.C., Song S., Wiegmans A.P., Swarbrick A., Welm A.L., Brown M.P., et al. Treatment of triple-negative breast cancer using anti-EGFR-directed radioimmunotherapy combined with radiosensitizing chemotherapy and PARP inhibitor. J. Nucl. Med. 2013;54:913–921. doi: 10.2967/jnumed.112.111534. [DOI] [PubMed] [Google Scholar]
- 96.Gibbens-Bandala B., Morales-Avila E., Ferro-Flores G., Santos-Cuevas C., Meléndez-Alafort L., Trujillo-Nolasco M., Ocampo-García B. (177)Lu-Bombesin-PLGA (paclitaxel): A targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;105:110043. doi: 10.1016/j.msec.2019.110043. [DOI] [PubMed] [Google Scholar]
- 97.Reubi J.C., Wenger S., Schmuckli-Maurer J., Schaer J.C., Gugger M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6-14) Clin. Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 98.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13:394. doi: 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drake C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Emens L.A. Breast cancer immunobiology driving immunotherapy: Vaccines and immune checkpoint blockade. Expert Rev. Anticancer Ther. 2012;12:1597–1611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chakraborty M., Wansley E.K., Carrasquillo J.A., Yu S., Paik C.H., Camphausen K., Becker M.D., Goeckeler W.F., Schlom J., Hodge J.W. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin. Cancer Res. 2008;14:4241–4249. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gulley J.L., Madan R.A., Tsang K.Y., Arlen P.M., Camphausen K., Mohebtash M., Kamrava M., Schlom J., Citrin D. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin. Biol. Ther. 2011;11:1409–1418. doi: 10.1517/14712598.2011.615741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chi K.H., Liu S.J., Li C.P., Kuo H.P., Wang Y.S., Chao Y., Hsieh S.L. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 104.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N., Pfirschke C., Voss R.H., Timke C., Umansky L., et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 105.Ukidve A., Cu K., Kumbhojkar N., Lahann J., Mitragotri S. Overcoming biological barriers to improve solid tumor immunotherapy. Drug Deliv. Transl. Res. 2021:1–26. doi: 10.1007/s13346-021-00923-8. [DOI] [PubMed] [Google Scholar]
- 106.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu Z.I., McArthur H.L., Ho A.Y. The Abscopal Effect of Radiation Therapy: What Is It and How Can. We Use It in Breast Cancer? Curr. Breast Cancer Rep. 2017;9:45–51. doi: 10.1007/s12609-017-0234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou J., Wang G., Chen Y., Wang H., Hua Y., Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019;23:4854–4865. doi: 10.1111/jcmm.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malamas A.S., Gameiro S.R., Knudson K.M., Hodge J.W. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget. 2016;7:86937–86947. doi: 10.18632/oncotarget.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Y.C., Weng W.C., Lee H. Functional roles of calreticulin in cancer biology. Biomed. Res. Int. 2015;2015:526524. doi: 10.1155/2015/526524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wieczorek M., Abualrous E.T., Sticht J., Álvaro-Benito M., Stolzenberg S., Noé F., Freund C. Major Histocompatibility Complex. (MHC) Class. I and MHC Class. II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Czernin J., Current K., Mona C.E., Nyiranshuti L., Hikmat F., Radu C.G., Lückerath K. Immune-Checkpoint Blockade Enhances (225)Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2021;62:228–231. doi: 10.2967/jnumed.120.246041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Begg A.C., Stewart F.A., Vens C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 114.Baskar R., Lee K.A., Yeo R., Yeoh K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dietrich A., Koi L., Zöphel K., Sihver W., Kotzerke J., Baumann M., Krause M. Improving external beam radiotherapy by combination with internal irradiation. Br. J. Radiol. 2015;88:20150042. doi: 10.1259/bjr.20150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nilsson S., Franzén L., Parker C., Tyrrell C., Blom R., Tennvall J., Lennernäs B., Petersson U., Johannessen D.C., Sokal M., et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 117.Baczyk M., Milecki P., Pisarek M., Gut P., Antczak A., Hrab M. A prospective randomized trial: A comparison of the analgesic effect and toxicity of 153Sm radioisotope treatment in monotherapy and combined therapy including local external beam radiotherapy (EBRT) among metastatic castrate resistance prostate cancer (mCRPC) patients with painful bone metastases. Neoplasma. 2013;60:328–333. doi: 10.4149/neo_2013_044. [DOI] [PubMed] [Google Scholar]
- 118.Cornelissen B., Darbar S., Kersemans V., Allen D., Falzone N., Barbeau J., Smart S., Vallis K.A. Amplification of DNA damage by a gammaH2AX-targeted radiopharmaceutical. Nucl. Med. Biol. 2012;39:1142–1151. doi: 10.1016/j.nucmedbio.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 119.Hennrich U., Kopka K. Lutathera((R)): The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals. 2019;12:114. doi: 10.3390/ph12030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwekkeboom D.J., de Herder W.W., Kam B.L., van Eijck C.H., van Essen M., Kooij P.P., Feelders R.A., van Aken M.O., Krenning E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 121.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strosberg J., Wolin E., Chasen B., Kulke M., Bushnell D., Caplin M., Baum R.P., Kunz P., Hobday T., Hendifar A., et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018;36:2578–2584. doi: 10.1200/JCO.2018.78.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bodei L., Kidd M., Paganelli G., Grana C.M., Drozdov I., Cremonesi M., Lepensky C., Kwekkeboom D.J., Baum R.P., Krenning E.P., et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 124.Bergsma H., van Lom K., Raaijmakers M., Konijnenberg M., Kam B., Teunissen J., de Herder W.W., Krenning E.P., Kwekkeboom D.J. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2018;59:452–458. doi: 10.2967/jnumed.117.189712. [DOI] [PubMed] [Google Scholar]
- 125.Claringbold P.G., Turner J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology. 2016;103:432–439. doi: 10.1159/000434723. [DOI] [PubMed] [Google Scholar]
- 126.Kesavan M., Claringbold P.G., Turner J.H. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology. 2014;99:108–117. doi: 10.1159/000362558. [DOI] [PubMed] [Google Scholar]
- 127.Claringbold P.G., Price R.A., Turner J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012;27:561–569. doi: 10.1089/cbr.2012.1276. [DOI] [PubMed] [Google Scholar]
- 128.Strosberg J.R., Fine R.L., Choi J., Nasir A., Coppola D., Chen D.T., Helm J., Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peixoto R.D., Noonan K.L., Pavlovich P., Kennecke H.F., Lim H.J. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J. Gastrointest. Oncol. 2014;5:247–252. doi: 10.1200/jco.2014.32.3_suppl.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saif M.W., Kaley K., Brennan M., Garcon M.C., Rodriguez G., Rodriguez T. A retrospective study of capecitabine/temozolomide (CAPTEM) regimen in the treatment of metastatic pancreatic neuroendocrine tumors (pNETs) after failing previous therapy. JOP. 2013;14:498–501. doi: 10.6092/1590-8577/1589. [DOI] [PubMed] [Google Scholar]
- 131.Abbasi S., Kashashna A., Albaba H. Efficacy of capecitabine and temozolomide combination in well-differentiated neuroendocrine tumors: Jordan experience. Pancreas. 2014;43:1303–1305. doi: 10.1097/MPA.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 132.Kotteas E.A., Syrigos K.N., Saif M.W. Profile of capecitabine/temozolomide combination in the treatment of well-differentiated neuroendocrine tumors. Onco Targets Ther. 2016;9:699–704. doi: 10.2147/OTT.S72155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bison S.M., Haeck J.C., Bol K., Koelewijn S.J., Groen H.C., Melis M., Veenland J.F., Bernsen M.R., de Jong M. Optimization of combined temozolomide and peptide receptor radionuclide therapy (PRRT) in mice after multimodality molecular imaging studies. EJNMMI Res. 2015;5:62. doi: 10.1186/s13550-015-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shah R.G., Merlin M.A., Adant S., Zine-Eddine F., Beauregard J.M., Shah G.M. Chemotherapy-Induced Upregulation of Somatostatin Receptor-2 Increases the Uptake and Efficacy of (177)Lu-DOTA-Octreotate in Neuroendocrine Tumor Cells. Cancers. 2021;13:232. doi: 10.3390/cancers13020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fueger B.J., Hamilton G., Raderer M., Pangerl T., Traub T., Angelberger P., Baumgartner G., Dudczak R., Virgolini I. Effects of chemotherapeutic agents on expression of somatostatin receptors in pancreatic tumor cells. J. Nucl. Med. 2001;42:1856–1862. [PubMed] [Google Scholar]
- 136.Jin X.F., Auernhammer C.J., Ilhan H., Lindner S., Nölting S., Maurer J., Spöttl G., Orth M. Combination of 5-Fluorouracil with Epigenetic Modifiers Induces Radiosensitization, Somatostatin Receptor 2 Expression, and Radioligand Binding in Neuroendocrine Tumor Cells In Vitro. J. Nucl. Med. 2019;60:1240–1246. doi: 10.2967/jnumed.118.224048. [DOI] [PubMed] [Google Scholar]
- 137.Oddstig J., Bernhardt P., Nilsson O., Ahlman H., Forssell-Aronsson E. Radiation induces up-regulation of somatostatin receptors 1, 2, and 5 in small cell lung cancer in vitro also at low absorbed doses. Cancer Biother. Radiopharm. 2011;26:759–765. doi: 10.1089/cbr.2010.0921. [DOI] [PubMed] [Google Scholar]
- 138.Oddstig J., Bernhardt P., Nilsson O., Ahlman H., Forssell-Aronsson E. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl. Med. Biol. 2006;33:841–846. doi: 10.1016/j.nucmedbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 139.Bernhardt P., Oddstig J., Kölby L., Nilsson O., Ahlman H., Forssell-Aronsson E. Effects of treatment with (177)Lu-DOTA-Tyr(3)-octreotate on uptake of subsequent injection in carcinoid-bearing nude mice. Cancer Biother. Radiopharm. 2007;22:644–653. doi: 10.1089/cbr.2007.333. [DOI] [PubMed] [Google Scholar]
- 140.Dalmo J., Spetz J., Montelius M., Langen B., Arvidsson Y., Johansson H., Parris T.Z., Helou K., Wängberg B., Nilsson O., et al. Priming increases the anti-tumor effect and therapeutic window of (177)Lu-octreotate in nude mice bearing human small intestine neuroendocrine tumor GOT1. EJNMMI Res. 2017;7:6. doi: 10.1186/s13550-016-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ifeadi V., Garnett-Benson C. Sub-lethal irradiation of human colorectal tumor cells imparts enhanced and sustained susceptibility to multiple death receptor signaling pathways. PLoS ONE. 2012;7:e31762. doi: 10.1371/journal.pone.0031762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., Camphausen K., Luiten R.M., de Ru A.H., Neijssen J., et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kachikwu E.L., Iwamoto K.S., Liao Y.P., DeMarco J.J., Agazaryan N., Economou J.S., McBride W.H., Schaue D. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chiang C.S., Fu S.Y., Wang S.C., Yu C.F., Chen F.H., Lin C.M., Hong J.H. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barcellos-Hoff M.H., Derynck R., Tsang M.L., Weatherbee J.A. Transforming growth factor-beta activation in irradiated murine mammary gland. J. Clin. Investig. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R., Fu Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.