Abstract
A series of diclofenac N-derivatives (2, 4, 6, 8c, 9c, 10a-c) were synthesized in order to test their anti-cancer and anti-inflammatory effects. The anticarcinogen activity has been assayed against three cancer cell lines: HT29, human colon cancer cells; Hep-G2, human hepatic cells; and B16-F10, murine melanoma cells. First, we determined the cytotoxicity of the different compounds, finding that the most effective compound was compound 8c against all cell lines and both compounds 4 and 6 in human Hep-G2 and HT29 cell lines. Compounds 4 and 8c were selected for the percentage of apoptosis determination, cell cycle distribution, and mitochondrial membrane potential measure because these products presented the lowest IC50 values in two of the three cancer cell lines assayed (B16-F10 and HepG2), and were two of the three products with lowest IC50 in HT29 cell line. Moreover, the percentages of apoptosis induction were determined for compounds 4 and 8c, showing that the highest values were between 30 to 60%. Next, the effects of these two compounds were observed on the cellular cycle, resulting in an increase in the cell population in G2/M cell cycle phase after treatment with product 8c, whereas compound 4 increased the cells in phase G0/G1, by possible differentiation process induction. Finally, to determine the possible apoptosis mechanism triggered by these compounds, mitochondrial potential was evaluated, indicating the possible activation of extrinsic apoptotic mechanism. On the other hand, we studied the anti-inflammatory effects of these diclofenac (DCF) derivatives on lipopolysaccharide (LPS) activated RAW 264.7 macrophages-monocytes murine cells by inhibition of nitric oxide (NO) production. As a first step, we determined the cytotoxicity of the synthesized compounds, as well as DCF, against these cells. Then, sub-cytotoxic concentrations were used to determine NO release at different incubation times. The greatest anti-inflammatory effect was observed for products 2, 4, 8c, 10a, 10b, and 9c at 20 µg·mL−1 concentration after 48 h of treatment, with inhibition of produced NO between 60 to 75%, and a concentration that reduces to the 50% the production of NO (IC50 NO) between 2.5 to 25 times lower than that of DCF. In this work, we synthesized and determined for the first time the anti-cancer and anti-inflammatory potential of eight diclofenac N-derivatives. In agreement with the recent evidences suggesting that inflammation may contribute to all states of tumorigenesis, the development of these new derivatives capable of inducing apoptosis and anti-inflammatory effects at very low concentrations represent new effective therapeutic strategies against these diseases.
Keywords: diclofenac, anticancer activity, anti-inflammatory activity, nitric oxide, drug development
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAID) are compounds that produce anti-inflammatory effects, reducing pain, fever, and inflammation. The main mechanism of action of NSAID is the inhibition of cyclooxygenases (COX-1 and -2), enzymes related to the synthesis of key biological mediators (i.e., prostaglandins) involved in inflammation processes [1]. Particularly, diclofenac (DCF) is a well-known and common NSAID used to treat pain and inflammatory diseases, such as rheumatoid arthritis, post-operative traumatic pain, migraine, or fever. Like other NSAIDs, DCF is believed to work via inhibition of prostaglandin synthesis by blocking both COX-1 and -2. Other anti-inflammatory mechanisms proposed for the action of DCF are the inhibition of the thromboxane-prostanoid receptor, reduction of arachidonic acid release and uptake [2], protection on leukocyte-endothelium interactions, inhibition of lipoxygenase enzymes, and activation of nitric oxide-cGMP (3′,5′-cyclic guanosine monophosphate) antinociceptive pathway [1,3]. One example of the activity of DCF is the inhibition of over 93% of the phospholipase A2 enzymes (PLA2) activity, enzyme promoting inflammation via eicosanoids production, and direct activation of pro-inflammatory cells in patients with acute pancreatitis [4].
Recent evidence suggests that inflammatory actions can contribute to all tumorigenesis states [5]. Inflammation is also involved in critical steps of metastasis due to the production of angiogenic factors and cell migration [6]. Particularly, DCF presents an important range of actions, which are of interest in an oncological context, displaying a range of effects on inflammation, immune system response, angiogenic cascade, chemo-sensitivity in tumoral cells, and tumor metabolism. Different molecular mechanisms have been proposed for the anti-cancer action of DCF, some of which are common with other NSAIDs drugs: inhibition of COX-2 and decrease of PGE2 levels. High levels of this PGE2 were found in different types of cancer associated with chronic inflammation, providing a pro-tumor microenvironment [7]. Particularly in processes related to reducing PGE2 synthesis, DCF has shown to delay tumor growth and angiogenesis in BALB/c injected with C-26 adenocarcinoma cells [8] and reduce tumor growth volumes in the murine model [9].
Several in vitro and in vivo studies have demonstrated the antitumor effect of DCF. DCF inhibited cell growth via apoptosis in different cell lines, such as neuroblastoma cells (half maximal inhibitory concentration, IC50 = 30–178 µg·mL−1) [10], ovarian cancer cells SKOV-3, CAOV-3, SW626 and 36M2 (IC50 = 6–60 µg·mL−1) [11], and HEY and OVACAR-5 (IC50 = 15 µg·mL−1) [12], glioblastoma cells HTZ-349, U87MG and A172 (IC50 = 15–60 µg·mL−1) [13], or Hep-G2 cells (IC50 = 50 µg·mL−1) [14]. In vivo studies have demonstrated that DCF promoted a significant growth inhibitory effect on different tumors, such as C57/BL6 mice inoculated with B16 melanoma cells [15], rats carrying neuroblastoma xenografts SH-SY5Y [16], mice SK-N-AS neuroblastoma cells injected [9], mice with implanted fibrosarcoma [17], or BALB/c and CB6F1 mice [18]. When administered combined with other drugs, such as the anti-angiogenic drug TL-118, DCF induces the partial or complete remission of tumors in mice injected with C-26 adenocarcinoma cells [19], or SK-N-AS neuroblastoma cells [20].
Some in vitro reported studies evidenced the pro-apoptotic role of DCF in cancer. For example, it has been demonstrated that DCF induces DNA fragmentation and apoptosis, with caspases activation and cytochrome c release, associated with reactive oxygen species (ROS) increase and inhibition of Akt phosphorylation via phosphoinositide-3-kinase (PI3K) in HL-60 leukemia cells [21]. DCF has been shown to promote DNA fragmentation and caspases activation in neuroblastoma cells [10], increase ROS, activation of caspases-9 and -3, and reduce Bcl-2/Bax ratio and cytochrome c release in A2058 and SAN melanoma cells [22]. In leukemia cells HL-60, THP-1, and samples of acute myeloid leukemia patients, DCF induced apoptosis through activator protein-1 (AP-1) transcription factors (c-Jun, JunB, and Fra-2), induction of growth arrest and DNA damage-45α protein (GADD45α), and activation of c-Jun N-terminal kinase (JNK) [23].
Although DCF has been proved to be an effective analgesic or anti-pyretic in the treatment of cancer-related pain, this drug exhibits limitations and adverse effects towards gastrointestinal irritation, ulceration, platelet dysfunction, and hepatoxicity [24]. In this context, we have synthesized a series of DCF N-derivatives with the final aim of improving the biological properties of DCF as an anti-cancer and anti-inflammatory drug. Cytotoxicity studies using three cancer cell lines, namely B16-F10 murine melanoma cells, Hep-G2 hepatoma cells, and HT29 colon cancer cells, demonstrated that six of the as-synthesized compounds (2, 4, 6, 8c, 9c, and 10c) showed an important improvement in their cytotoxicity activity with regard to DCF, reaching IC50 values between 13 to 48 µg·mL−1. Compounds 4 and 8c were selected, and their apoptosis properties were assayed in Hep-G2 cell line with the percentage of apoptosis between 15 to 60%. In order to determine the plausible apoptotic mechanism of these compounds, the mitochondrial membrane potential was measured. Finally, we investigated the anti-inflammatory properties of synthetized compounds by means of the inflammation process induced in RAW 264.7 macrophages murine cells by bacterial lipopolysaccharide (LPS).
2. Results and Discussion
2.1. Synthesis of DCF Derivatives
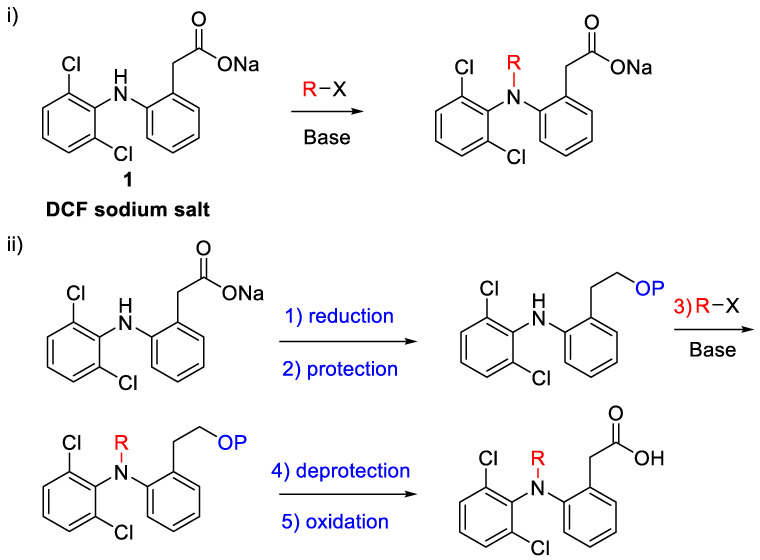
Although the number of DCF analogs synthesized is overwhelming [25,26,27,28], the number of derivatives involving functionalization of the secondary amine of DCF is much more limited [29,30,31,32,33,34,35,36,37]. Scheme 1 shows the synthetic strategies employed for the synthesis of the DCF N-derivatives.
Scheme 1.
Two approaches to DCF N-derivatives.
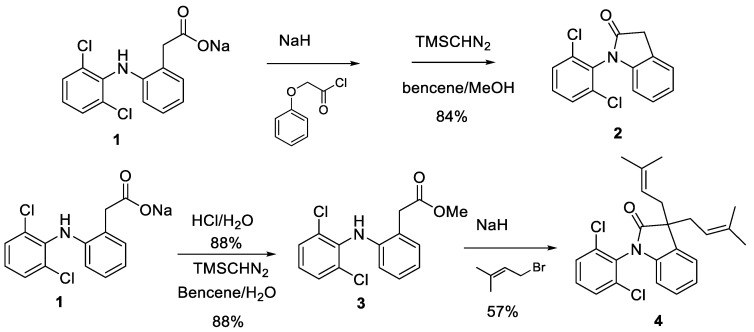
We first tried the direct functionalization of DCF by reacting its sodium salt (1) with different electrophiles in the presence of NaH or K2CO3. Invariably the main reaction product was lactam 2 [38] (Scheme 2). When the corresponding methyl ester (3) was used as a starting material and isopentenyl bromide was used as electrophile, the dialkylated lactam 4 was obtained, again via lactam 2 (Scheme 2).
Scheme 2.
Synthesis of lactam 2 and its diisopentenyl derivative 4.
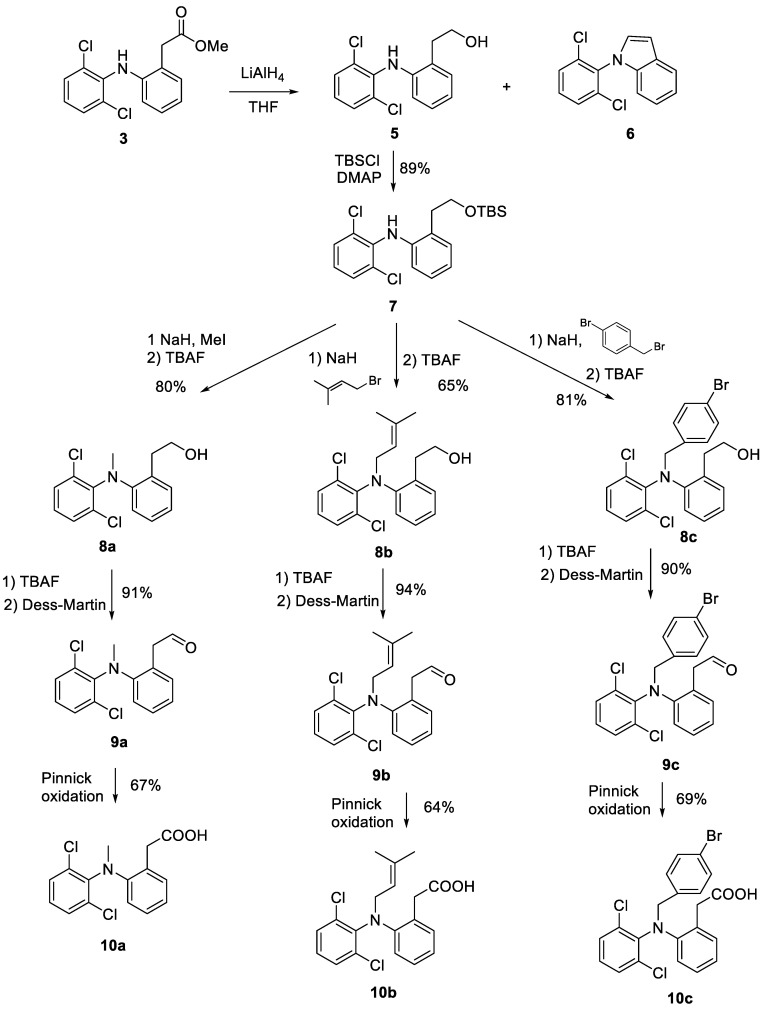
In our second approach, we envisaged that the reduction of the phenylacetic acid of DCF to the corresponding primary alcohol not only would avoid the lactamization side reaction but also would enable the generation of N-functionalized derivatives with different oxidation degree. Following this approach, LAH reduction of methyl ester 3 [39] to obtain alcohol 5 was achieved following the procedure described by Kelly et al. [40]. Together with 5, variable minor amounts of the previously unreported indol derivative 6 were produced in this reduction (Scheme 3). Nucleophilic attack of the secondary amine to the aldehyde intermediate of the reduction process followed by a loss of water was proposed to rationalize the formation of 6.
Scheme 3.
Synthesis of DCF N-derivatives.
Continuing with our synthetic route, treatment of 5 with tert-butyldimethylsilyl chloride (TBSCl) in the presence of 4-dimethylaminopyridine (DMAP) led uneventfully to silyl derivative 7. Gratifyingly the N-derivatization proceeds satisfactorily using NaH as a base and isopentenyl bromide, methyl iodide, and benzyl bromide to produce 8a-8c after tetra-n-butylammonium fluoride (TBAF) deprotection of the silyl protecting group. Soft heating to 50 °C was necessary for the reaction to go to completion after approximately 6 h. Finally, the generation of the corresponding aldehyde derivatives was achieved using Dess-martin oxidations [41], whereas the generation of the targeted phenylacetic acids 10a [37], 10b and 10c involved the oxidation the corresponding aldehydes via Pinnick oxidation [42] (Scheme 3).
2.2. Anti-Carcinogenic Activity
2.2.1. Cancer Cells Proliferation Studies
The eight DCF N-derivatives (2, 4, 6, 8c, 9c, 10a-c) and their precursor (DCF) were assayed on B16-F10 murine melanoma cells, HT29 colon cancer cells, and Hep-G2 hepatoma cells, and IC50 values were determined (Table 1). In addition, we estimated the concentration required for 20 and 80% growth inhibition (IC20 and IC80, respectively). These concentrations were used for the rest of the assays.
Table 1.
Growth-inhibitory effects as IC20, IC50, and IC80 (µg·mL−1) values of compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF on the three cancer cell lines B16-F10, Hep-G2, and HT29. The last column represents the ratio between IC50 values of DCF and the corresponding N-derivative.
| Cell Line | Compound # | IC20 | IC50 | IC80 | IC50 of DCF/ IC50 of Compound |
|---|---|---|---|---|---|
| B16-F10 | DCF | 34.58 ± 8.46 | 52.45 ± 3.58 | 83.75 ± 0.54 | 1.0 |
| 2 | 21.94 ± 3.44 | 40.77 ± 1.33 | 56.59 ± 1.29 | 1.3 | |
| 4 | 37.15 ± 0.07 | 37.79 ± 0.17 | N/A | 1.4 | |
| 6 | 28.22 ± 2.82 | 45.77 ± 6.46 | N/A | 1.1 | |
| 8c | 14.83 ± 3.24 | 25.55 ± 1.19 | 42.01 ± 1.59 | 2.1 | |
| 9c | 21.19 ± 2.00 | 27.35 ± 0.14 | 33.59 ± 1.06 | 1.9 | |
| 10a | 26.79 ± 5.11 | N/A | N/A | N/A | |
| 10b | 6.93 ± 5.48 | 35.45 ± 4.30 | 80.98 ± 0.33 | 1.5 | |
| 10c | 27.42 ± 6.76 | 48.20 ± 3.05 | 63.56 ± 1.19 | 1.1 | |
| Hep-G2 | DCF | 32.48 ± 2.51 | 46.87 ± 0.97 | 76.87 ± 1.60 | 1.0 |
| 2 | 18.45 ± 0.56 | 24.53 ± 1.11 | 35.28 ± 3.91 | 1.9 | |
| 4 | 7.53 ± 3.02 | 14.56 ± 3.07 | 33.06 ± 2.07 | 3.2 | |
| 6 | 12.30 ± 1.00 | 21.58 ± 1.77 | 52.70 ± 7.38 | 2.2 | |
| 8c | 12.83 ± 1.12 | 18.48 ± 1.25 | 29.74 ± 3.03 | 2.5 | |
| 9c | 17.62 ± 1.42 | 28.60 ± 1.75 | 49.21 ± 4.22 | 1.6 | |
| 10a | 23.15 ± 8.17 | 78.93 ± 10.67 | 146.47 ± 13.76 | 0.6 | |
| 10b | 40.63 ± 9.57 | 111.26 ± 16.58 | 184.08 ± 27.89 | 0.4 | |
| 10c | 17.41 ± 1.08 | 21.89 ± 0.90 | 32.33 ± 3.34 | 2.1 | |
| HT29 | DCF | 45.27 ± 0.52 | 52.63 ± 0.54 | 65.90 ± 2.12 | 1.0 |
| 2 | 18.56 ± 1.26 | 28.66 ± 1.91 | 49.14 ± 6.00 | 1.8 | |
| 4 | 3.90 ± 4.74 | 19.80 ± 9.02 | 173.90 ± 25.19 | 2.7 | |
| 6 | 7.70 ± 4.60 | 13.23 ± 4.24 | 30.53 ± 11.87 | 4.0 | |
| 8c | 14.10 ± 1.37 | 20.65 ± 0.76 | 30.48 ± 0.66 | 2.5 | |
| 9c | 17.94 ± 1.06 | 24.93 ± 1.21 | 41.02 ± 3.24 | 2.1 | |
| 10a | 62.79 ± 15.01 | 95.77 ± 7.88 | 113.61 ± 5.75 | 0.5 | |
| 10b | 48.12 ± 2.33 | 69.52 ± 2.86 | 96.54 ± 1.50 | 0.8 | |
| 10c | 31.10 ± 3.38 | 69.30 ± 2.36 | 103.99 ± 0.79 | 0.8 |
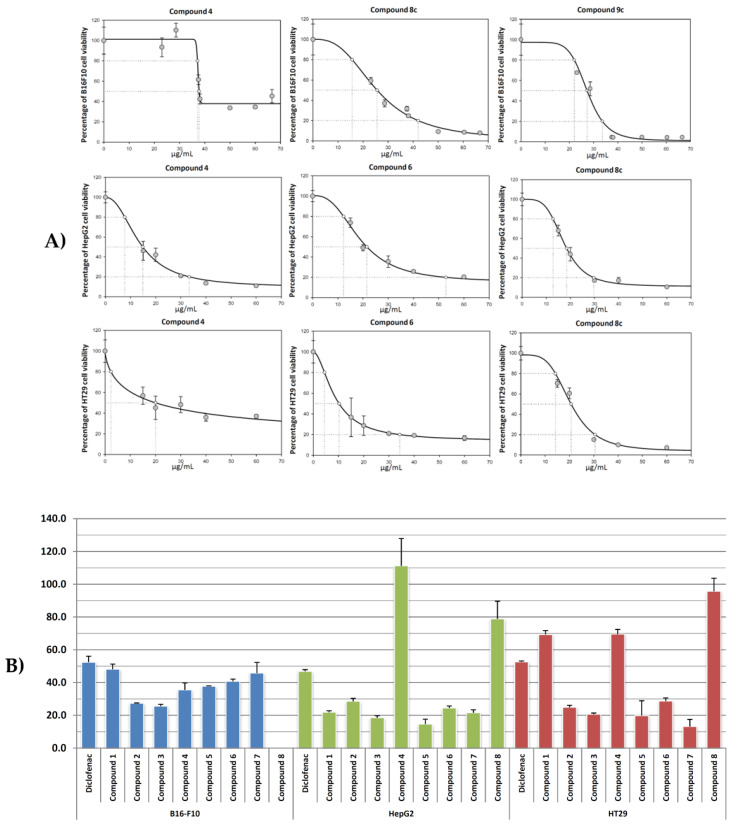
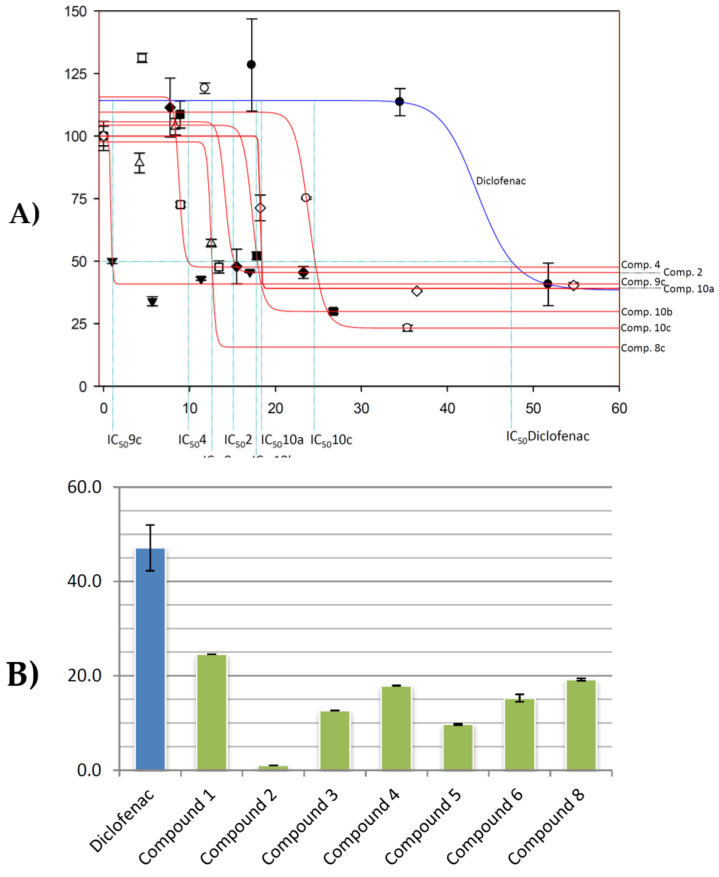
All tested products showed cytotoxic activity in the conditions assayed (Figure 1A), most of the compounds showed IC50 values between 25 to 48 μg·mL−1 in HT29 cells, 14 to 29 μg·mL−1 in Hep-G2 and HT29 cells. Except for compounds 10a, 10b, and 10c, all synthetized compounds displayed lower IC50 values than DCF (IC50 = 52.5, 46.9, and 52.6 μg·mL−1 against B16-F10, Hep-G2, and HT29 cells, respectively). Particularly, derivatives 8c, 9c, 4 and 6 were between two to four times more cytotoxic than their precursor DCF, with IC50 values of 25.6 and 27.4 μg·mL−1 against B16-F10 cells for 8c and 9c, 18.5 and 14.6 μg·mL−1 against Hep-G2 cells for 8c and 4, and 19.8 and 13.2 μg·mL−1 against HT29 cells for 4 and 6, respectively.
Figure 1.
(A) Effects of compounds 4, 8c, and 9c, on the viability of cell B16-F10, and compounds 4, 6, and 8c on cells HT29 and Hep-G2, after treatment for 72 h in a range of 0 to 100 μg/mL. Each point represents the mean value ± S.D. of at least two independent experiments performed in triplicate. (B) IC50 values (μg·mL−1) of compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF on B16-F10, Hep-G2 and HT29 cancer cells.
In general terms, the most effective compound was compound 8c in all cell lines, and compounds 4 and 6 in Hep-G2 and HT29 cells lines, with IC20 values between 4 to 21 μg·mL−1, IC50 values between 13 to 27 μg·mL−1, and IC80 values between 30 to 42 μg·mL−1. These compounds were more effective than DCF in the three lines assayed, being between 1.4 to 2.1 times more cytotoxic than DCF in B16-F10 cells, between 2.2 to 3.2 times in Hep-G2 cells, and between 2.5 to 4.0 times in HT29 cells (Figure 1B). The lowest IC50 values were reached by compound 8c in B16-F10 cells, compound 4 in Hep-G2 cells, and compound 6 in HT29 colon cancer cells.
Compounds possessing a 2-(2-((4-bromobenzyl)(2,6-dichlorophenyl)amino)phenyl moiety, namely compounds 8c, 9c and 10c, showed good results in the cytotoxicity induced in the three cell lines, except for compound 10c in HT29 cells. Compound 10b resulted in being the least cytotoxic compound in the three cell lines assayed. Compounds with indolin substituent, 2, 4, and 6, also presented good results in all cell lines assayed. Finally, compound 10a did not induce cytotoxicity in the lines assayed. These effects could be related to a rise of the polarity of the complete molecule, without increasing the molecular weight or molecular volume, since probably most of these products performed their biological activity through the cellular membrane, which is prevented by excessively large molecular volumes.
A limited number of articles have been found describing the anti-cancer potential of DCF N derivatives. Thus, the cytotoxicity of the several oxadiazole DCF derivatives was analyzed in NIH 3T3 fibroblast cells. The oxadiazole derivative of diclofenac showed a value of IC50 = 32.0 ± 0.6 µg·mL−1, whereas the phenacyl derivative of 1,3,4-oxadiazole diclofenac showed an IC50 = 76.7 ± 6.0 µg·mL−1 [43].
Compounds 4 and 8c were selected since they showed the lowest IC50 values in two out of the three cancer cell lines assayed (B16-F10 and HepG2), and were two of the three products with lowest IC50 in HT29 cell line. Compound 6 was not chosen because it showed a low IC50 value in HT29 cell line, and this value is very similar to that found for compound 4 in HepG2 cells. These products were selected for the percentage of apoptosis determination, cell cycle distribution, and mitochondrial membrane potential measure. All these assays were realized in HepG2 hepatoma cell line by flow cytometry analysis in a fluorescence-activated cell sorter.
2.2.2. Characterization of Apoptotic Effects
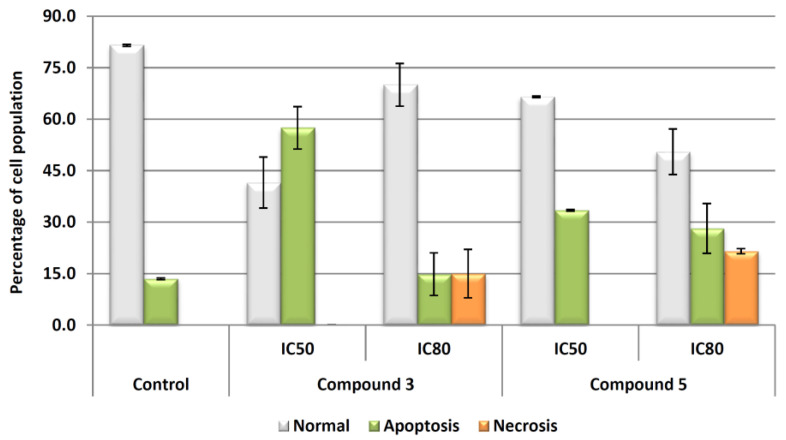
The two compounds previously selected, 4 and 8c, showed clearly apoptotic effects in treated cells, with total apoptosis between 30 and 57% at IC50 concentrations and between 15 to 35% at IC80 concentrations for products 8c and 4, respectively (Figure 2). The high percentage of necrosis (15%) for compound 8c could be explained by a possible faster induction of apoptosis in response to product 8c, and it could be related to the increase of the cell population in G2/M phase, as a consequence of apoptosis mechanism activation.
Figure 2.
Flow cytometry results after exposure of Hep-G2 cells to DCF and the derivatives 4 and 8c for 72 h at IC50 and IC80 concentrations. Apoptotic cells (green bars) were positive in annexin V, necrotic cells (orange bars) were positive in propidium iodide. Values are expressed as means ± S.E.M. of at least two experiments in duplicate.
2.2.3. Cell Cycle Arrest and Distribution
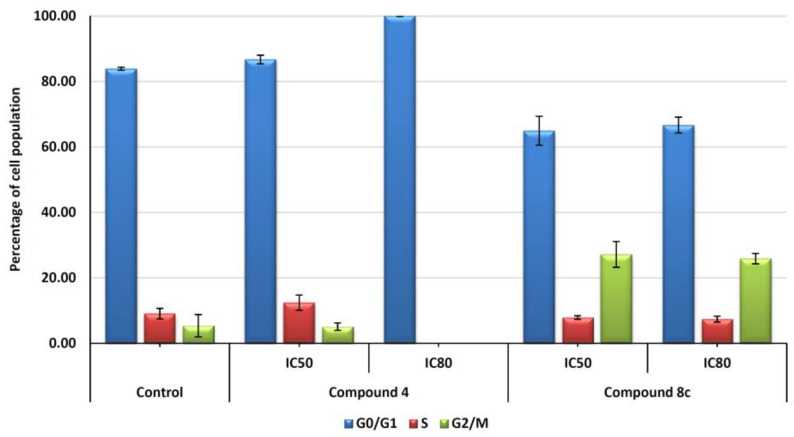
Further, we have investigated the effect of compounds 4 and 8c on cell-cycle distribution in order to determine the possible cytostatic effects related to the cytotoxic response. Hep-G2 cells were treated with the selected products 4 and 8c at both IC50 and IC80 concentrations. Flow cytometry was used to measure DNA ploidy as well as alterations in cell-cycle profiles (see the experimental section for further details). DNA histogram analysis (Figure 3) revealed that treatment with compound 8c with both concentrations (IC50 and IC80) increase cell population at the G2/M phase compared with untreated control cells. This phenomenon might be a consequence of apoptosis induction, with an increase of 21% of cells in this cell cycle phase. On the other hand, treatment with product 4 produced an important increase in G0/G1 phase at IC80 concentration, reaching 100% of cells in this cell cycle phase. In this case, this high value could be due to a possible differentiation process activated in response to product 4. Future assays will be necessary to confirm this point.
Figure 3.
Cell-cycle phase representation (%) with respect to untreated control cells. Hep-G2 cells were treated with compounds 4 and 8c for 72 h at IC50 and IC80 concentrations. Cells in the G0/G1 phase (blue bars), S phase (orange bars), and G2/M phase (green bars) were counted. Values represent means ± S.E.M. of at least two independent experiments performed in triplicate.
2.3. Effects on Changes in Mitochondrial Membrane Potential
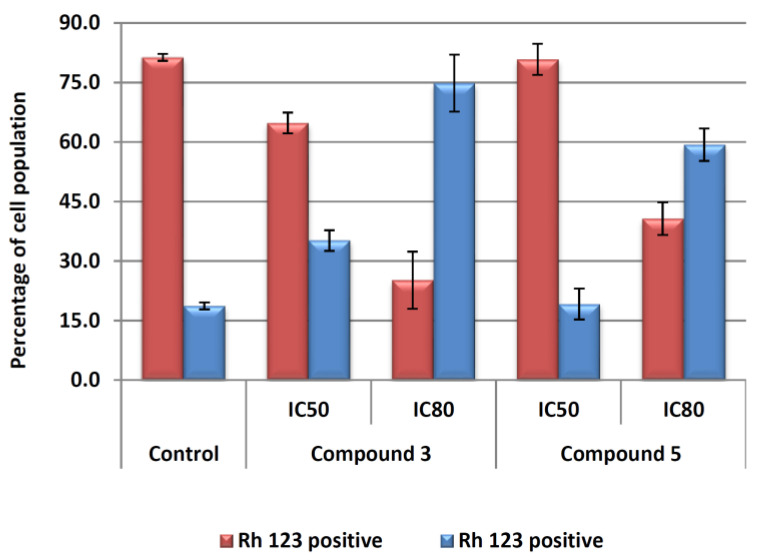
The treatment with both compounds 4 and 8c produced changes in the mitochondrial membrane potential (MMP, Figure 4). For compound 8c at concentrations IC50 and IC80, 35 and 75% of cells lost MMP (Rh123 negative). Regarding compound 4, there were practically no changes with respect to untreated control cells at IC50 concentration. However, at IC80 concentration, the percentage of cells with loss of MMP was about 60%.
Figure 4.
Flow-cytometry analysis of Rh123 staining after exposure of Hep-G2 cells to compounds 4 and 8c for 72 h at IC50 and IC80 concentrations. Rh123 positive cells (red bars) and Rh123 negative cells (blue bars) were counted. Values are expressed as means ± S.E.M. of at least two experiments in duplicate.
These results are in agreement with those found in the apoptosis and cell cycle analysis. In both cases, the treatment produced apoptosis without loss of MMP at IC50 concentration, probably due to the activation of the extrinsic apoptosis mechanism. At IC80 concentration, both products produced the loss of MMP, probably because of the secondary activation of the intrinsic apoptotic pathway. The loss of MMP was faster in response to compound 8c than to compound 4, as showed the percentage of cells with loss of MMP at IC50 and IC80 concentrations, which agreed with the results of apoptosis and cell cycle obtained previously.
2.4. Effects DCF N-Derivatives in the Inflammatory Proces
Nitric oxide (NO), a molecule mediator in acute or chronic inflammation, is produced by constitutive or inducible nitric oxide synthases (iNOS). Its production is activated in response to pro-inflammatory signals as cytokines such as TNFα (tumor necrosis factor α) or INF-γ (interferon-γ), enterotoxin, or LPS (either bacterial lipopolysaccharide), in macrophages [44]. Here, the decrease of NO concentration has been used as an indirect marker of the inflammatory process [44,45]. In the inflammatory response process, NO is released as an intermediate or second messenger. RAW 264.7 murine macrophage cells are an especially indicated model in anti-inflammatory compounds screening studies since they produce the highest release of NO during the inflammatory response. In this study, the anti-inflammatory potential of DCF N-derivatives was analyzed by measuring nitrites in a cell culture medium since the nitrites concentration is proportional to the NO release. With this purpose, we measured the inhibition in NO production in activated LPS RAW 264.7 macrophage cells.
2.4.1. Raw 264.7 Cell Viability
First, RAW 264.7 cell viability was assayed with compounds 2 [46], 4, 6, 8c, 9c, 10a-c, and DCF to establish sub-cytotoxic concentrations and, thus, to assure that anti-inflammatory effects are a consequence of the anti-inflammatory activity of the compounds rather than their cytotoxicity. The concentration of products required for 50% growth inhibition was determined as previously described for B16-F10, Hep-G2, and HT29 cells. (see the experimental section for further details). The results showed similar cytotoxicity for all compounds at the conditions assayed (Table 2). Based on these results, sub-cytotoxic concentrations used in the determination of anti-inflammatory response were set at 5, 10, and 20 µg·mL−1.
Table 2.
Growth-inhibitory effects as IC20, IC50, and IC80 (µg·mL−1) values of compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF against RAW 264.7 monocyte/macrophage murine cells. The last column represents the ratio between IC50 of DCF and the IC50 of the derivatives.
| Cell line | Comp. | IC20 | IC50 | IC80 | IC50 of DCF/ IC50 of Comp. |
|---|---|---|---|---|---|
| RAW 264.7 | DCF | 51.91 ± 0.89 | 69.00 ± 1.13 | 90.52 ± 1.85 | 1.0 |
| 2 | 26.43 ± 3.68 | 30.99 ± 0.77 | 36.36 ± 3.44 | 2.2 | |
| 4 | 13.87 ± 0.89 | 17.90 ± 1.31 | 23.29 ± 2.41 | 3.9 | |
| 6 | 14.68 ± 1.30 | 19.08 ± 1.47 | 25.21 ± 2.27 | 3.6 | |
| 8c | 14.01 ± 0.14 | 16.71 ± 0.47 | 20.07 ± 1.27 | 4.1 | |
| 9c | 20.30 ± 0.83 | 22.70 ± 1.03 | 25.50 ± 1.51 | 3.0 | |
| 10a | 41.42 ± 7.87 | 72.92 ± 3.57 | 95.37 ± 1.23 | 0.9 | |
| 10b | 28.56 ± 1.10 | 35.64 ± 4.83 | 45.64 ± 12.53 | 1.9 | |
| 10c | 35.29 ± 2.83 | 47.17 ± 3.66 | 64.92 ± 6.97 | 1.5 |
2.4.2. Nitric Oxide Production
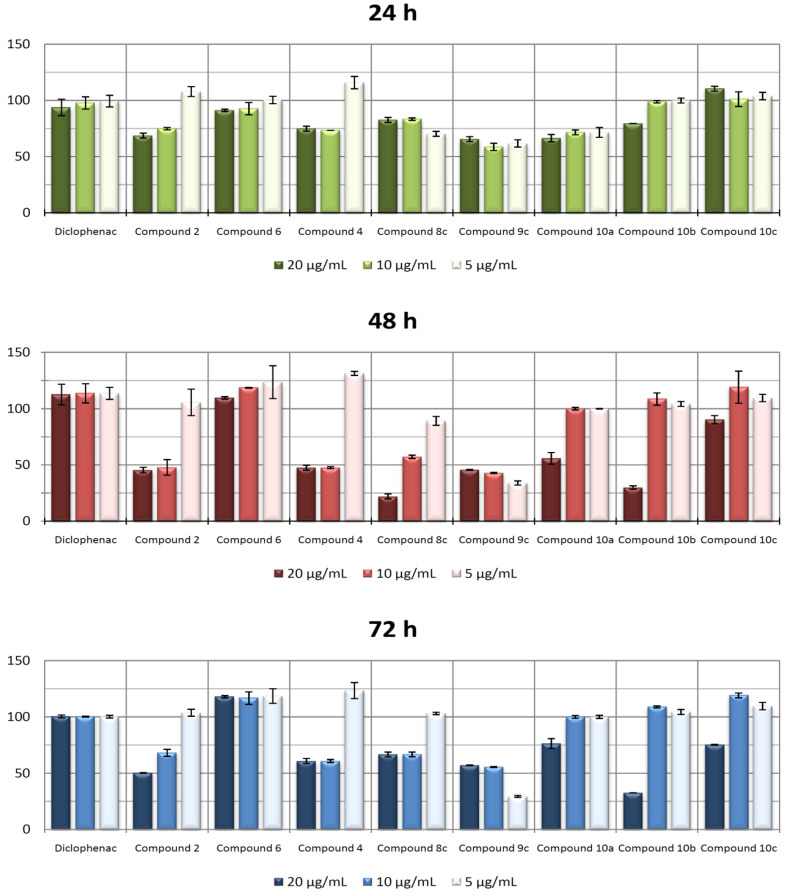
Firstly, macrophages RAW 264.7 were activated with LPS for 24 h. After this stimulation period, cells were incubated with compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF for 72 h. Aliquots at different incubation times were taken, and nitrite concentration was determined (see the experimental section for further details). Our results showed that at the concentrations assayed (5, 10, and 20 µg·mL−1), all compounds produced inhibition of NO release higher than DCF with respect to the positive control (only LPS treated control cells, 100% release), except for compounds 6 and 10c (Figure 5). After 24 h, NO release inhibition between 25 to 30% was achieved when using between 10 and 20 µg·mL−1 of products 2, 4, 8c, 9c, 10a, and 10b, and 5 µg·mL−1 of products 8c, 9c, and 10a. The highest anti-inflammatory effect was reached with 20 µg·mL−1 of compounds 8c and 10b at 48 h, with 75% of NO inhibition. Compound 9c showed the highest NO inhibition (60%) at the lower concentration (5 µg·mL−1). Products 2, 4, and 10a showed an inhibition close to 50% at 10 and 20 µg·mL−1 concentrations. The results obtained at 72 h of incubation were similar to those found at 48 h.
Figure 5.
Effect of compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF on the release of nitrites in RAW 264.7 macrophage murine cells. After activation of the inflammatory process, compounds were incubated for 24 h, 48 h, and 72 h at 5, 10, and 20 µg·mL−1 concentrations. The data represent the mean ± S.D. of at least two independent experiments performed in triplicate.
For a complete anti-inflammatory characterization of compounds, we calculated the concentration that reduces to 50% the production of NO (IC50 NO) at 48 h of cell incubation (Figure 6A). Our data showed that the IC50 NO concentration of compound 9c (IC50 NO = 1.89 ± 0.11 µg·mL−1) was lower than those found for the rest of the compounds. This value was 25 times less than that found for DCF (IC50 NO = 47.12 ± 4.85 µg·mL−1). Products 2, 4, 8c, 10a, and 10b showed IC50 NO values between 10 to 20 µg·mL−1. These data were between 2.5 to 4.5 times more effective than DCF. The IC50 NO for compound 10c was 24.57 ± 0.3 µg·mL−1, the highest found. In summary, all products assayed, except 6, inhibited the inflammation process produced by LPS in RAW 264.7 murine macrophage-monocyte cells. The greatest anti-inflammatory effects were observed at 48 h of incubation. At 20 µg·mL−1, the products that showed greater inhibition of NO release were compounds 8c, 9c, and 10b. However, according to IC50 NO values, the products with greater effectiveness were products 4, 8c, and 9c (Figure 6B).
Figure 6.
(A). Sigmoidal curves of the effect of compounds 2, 4, 6, 8c, 9c, 10a-c (red) and DCF (blue) on the release of nitrites in RAW 264.7 monocyte/macrophage murine cells activated with LPS. The values corresponding to IC50 NO for each compound can be seen in the curves. The data represent the mean ± S.D. of at least two independent experiments performed in triplicate. (B). NO release-inhibitory effects. IC50 NO μg·mL−1 concentrations for compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF in RAW 264.7 monocyte/macrophage murine cells activated with LPS.
Other DCF derivatives with potent inhibition of NO production have been synthesized, such as a number of oxadiazole derivatives, which presented IC50 NO values ranging from 7.13 ± 1.0 µg·mL−1 to 14.8 ± 2.0 µg·mL−1. These results were obtained in LPS stimulated mouse macrophage J774.2 cell line [43]. Additionally, different DCF-thiadiazole, DCF -triazole, and DCF oxadiazole hybrids proved to show moderate to strong anti-inflammatory activity in acute carrageenan-induced paw edema in male adult albino rats, in comparison with DFC [47]. Furthermore, a series S-substituted phenacyl 1,3,4-oxadiazoles and Schiff bases derived from DCF were tested in vivo for their anti-inflammatory activity [48]. Eight of these derivatives showed significant anti-inflammatory activity in the carrageenan-induced rat paw edema model.
2.5. Conclusions
The design of new drugs based on proven bioactive products, as DCF, is a new and promising strategy to find new compounds with high and enhanced biochemical properties. Particularly, the functionalization of the secondary amine in the development of new derivatives has demonstrated to be an efficient strategy to achieve several interesting bioactive compounds. N-derivatives 2, 4, 6, 8c, 9c, and 10c showed an improved cytotoxic effect against B16-F10, Hep-G2, and HT29 cancer cells than pristine DCF. Particularly, compounds 4 and 8c were found to be the most effective against all cell lines, with IC50 values between 13 to 27 μg·mL−1. The selected compounds, 4 and 8c, showed clearly apoptotic effects in treated cells, with a total apoptosis between 30 and 57% at IC50 concentrations. DNA histogram analysis revealed that treatment with IC50 concentration of compound 8c produced an increase in the cell population at the G2/M phase compared with untreated control cells. On the contrary, when using IC80 concentration of product 4, an important increase of in G0/G1 phase. Further, 8c was able to produce changes in MMP (35% at IC50). Finally, the results obtained in the anti-inflammatory test showed that the anti-inflammatory response of 2, 4, 8c, 10a, and 10b is from 2.5 to 4.5-fold better than DCF. Particularly, among all the tested compounds, after 48 h 4, 8c and 9c (20 µg·mL−1) showed the greater inhibition of NO release. In conclusion, 8c shows the most encouraging results and could be further exploited for developing new, improved anti-inflammatory and anti-cancer agents.
3. Materials and Methods
3.1. Chemistry
Silica gel 60 (35-70 μm) was used for flash column chromatography. NMR spectra were obtained in a Varian Direct-Drive 600 (1H 600 MHz/13C 150 MHz), Varian Direct-Drive 500 (1H 500 MHz/13C 125 MHz), and Varian Direct-Drive 400 (1H 400 MHz/13C 100 MHz). Accurate mass determinations were performed on a SYNAPT G2-Si Q-TOF mass spectrometer (Waters, Milford, MA, USA) equipped with high-efficiency T-Wave ion mobility and an orthogonal Z–spray™ electrospray ionization (ESI) source. MassLynx v.4.1 software was used for HRMS instrument control, peak detection, and integration. Reactions were monitored by TLC on 0.25 mm E. Merck silica gel plates (60F-254) visualized under UV light and by applying a phosphomolybdic acid solution in EtOH followed by heat. High-quality reagents were purchased at the highest quality that was commercially available and was used without further purification.
3.2. Synthesis of DCF N-Derivatives
3.2.1. Preparation of DCF from Its Sodium Salt (1)
To a heated (50 °C) solution of DCF sodium salt (2 g, 6.76 mmol) in 500 mL of water, 2N HCl solution was added under stirring till pH = 6. The resulting precipitated was filtered to afford an 88% yield of DCF (1.75 g, 5.95 mmol).
3.2.2. Treatment of 1 with Phenoxyacetyl Chloride in the Presence of NaH
To a solution of 1 (200 mg, 0.63 mmol) in 17 mL THF, under an argon atmosphere, was added 50.3 mg of NaH (1.26 mmol). Then, 0.17 mL of phenoxyacetyl chloride (1.26 mmol) were added dropwise. The resulting solution was stirred for 18 h, and then 25 mL of EtOAc were added, followed by 5 mL of saturated NH4Cl. The aqueous layer was diluted with water (15 mL) and extracted with MTBE (3 × 15 mL). The combined organic layers were washed with brine (3 × 25 mL) dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (H:MTBE, 1:1) to give a 84% yield of compound 2 (151 mg, 0.54 mmol) [38].
Compound 2. 1H NMR (500 MHz, CDCl3) δ 7.53 (d, J = 8.1 Hz, 2H), 7.39 (dd, J = 8.7, 7.6 Hz, 1H), 7.36 (bd, J = 7.4 Hz, 1H), 7.23 (td, J = 7.7, 1.2 Hz, 1H), 7.12 (td, J = 7.5, 1.1 Hz, 1H), 6.43 (dt, J = 8.0, 1.0 Hz, 1H), 3.80 (s, 2H) (Figure S1a). 13C NMR (125.1 MHz, CDCl3) δ 173.65 (C), 143.34 (C), 135.52 (2C), 130.87 (CH), 130.48 (C), 129.08 (2CH), 127.97 (CH), 124.87 (CH), 124.32 (C), 123.11 (CH), 109.16 (CH), 35.77 (CH2) (Figure S1b).
3.2.3. Treatment of DCF (5) with Diazomethane
To a stirred solution of DCF 5 (296 mg, 1.00 mmol) in 4.1 mL of benzene and 1 mL of MeOH, 0.6 mL (1.2 mmol) of a 2 M solution of TMSCH2N2 in hexanes were added dropwise. After consumption of the starting product (30 min), the mixture was concentrated under reduced pressure. Purification by flash chromatography (H:MTBE, 4:1) afforded 278 mg (88% yield) of ester 3 (278 mg, 0.9 mmol). The spectroscopic data of compound 3 coincide with those reported in the literature [39].
3.2.4. Treatment of Compound 3 with Isopentenyl Bromide in the Presence of NaH
To a stirred solution of ester 3 (165 mg, 0.53 mmol) in 5 mL THF, under an argon atmosphere, was added 21.2 mg of NaH (0.53 mmol). Then, 0.1 mL of isopentenyl bromide were added dropwise. The resulting solution was stirred for 30 min, and then 15 mL of MTBE were added, followed by 3 mL of saturated NH4Cl. The aqueous layer was diluted with water (15 mL) and extracted with MTBE (3 × 15 mL). The combined organic layers were washed with brine (3 × 20 mL) dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (H:MTBE, 6:1) to afford 125 mg of compound 4 (0.30 mmol, 57%).
Compound 4. 1H NMR (500 MHz, CDCl3) δ 7.50 (d, J = 8.1 Hz, 2H), 7.36 (dd, J = 8.6, 7.6 Hz, 1H), 7.30 (dd, J = 7.4, 0.8 Hz, 1H), 7.19 (td, J = 7.7, 1.3 Hz, 1H), 7.10 (td, J = 7.5, 1.1 Hz, 1H), 6.37 (dt, J = 7.7, 0.8 Hz, 1H), 5.03 (thept, J = 7.6, 1.5 Hz, 2H), 2.72 (dd, J = 14.2, 8.0 Hz, 2H), 2.64 (dd, J = 14.1, 7.0 Hz, 2H), 1,57 (bs, 6H), 1,57 (bs, 6H) (Figure S1d). 13C NMR (125.1 MHz, CDCl3) δ 178.35 (C), 142.01 (C), 135.59 (2C), 134.85 (2C), 131.93 (C), 130.73 (C), 130.41 (CH), 128.99 (2CH), 127.49 (CH), 123.88 (CH), 122.53 (CH), 118.53 (2CH), 108.58 (CH), 53.64 (C), 36.02 (2CH2), 25.94 (2CH3), 18.06 (2CH3) (Figure S1e). HRMS TOF (ESI+) m/z calculated for C24H26ClNO [M + H]+ 414.1391, found 414.1399.
3.2.5. Reduction of DCF Methyl Ester 3 with Lithium Aluminum Hydride (LAH)
To a solution of DCF methyl ester (3) (1 g, 3.4 mmol) in 25 mL THF, cooled at 0 °C and under an argon atmosphere, was added 384 mg of LAH (10 mmol). The resulting solution was stirred for 1 h and then diluted with methyl tert-butyl ether (MTBE) (100 mL) and washed with saturated NH4Cl (15 mL). The aqueous layer was extracted with MTBE (3 × 30 mL). The combined organic layers were washed with brine (3 × 25 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. Purification by flash chromatography (H:MTBE, 1:1) afforded 190 mg (0.68 mmol, 20%) of compound 6 and 550 mg (1.97 mmol, 58%) of compound 5 [40].
Compound 5: 1H NMR (600 MHz, CDCl3) δ = 7.36 (d, J = 8.1 Hz, 2H), 7.21 (dd, J = 7.4, 1.5 Hz, 1H), 7.10 (td, J = 7.7, 1.7 Hz, 1H), 7.00 (t, J = 8.1 Hz, 1H), 6.96 (td, J = 7.4, 1.1 Hz, 1H), 6.51 (dd, J = 8.0, 1.1 Hz, 1H), 4.04 (t, J = 5.9 Hz, 2H), 3.04 (t, J = 5.9 Hz, 2H) (Figure S1g). 13C NMR (151 MHz, CDCl3) δ = 142.64 (C), 137.74 (C), 130.63 (CH), 129.84 (CH), 128.88 (CH), 128.71 (C), 126.99 (CH), 124.03 (CH), 121.62 (C), 116.88 (CH), 64.17 (CH2), 34.85 (CH2) (Figure S1h).
Compound 6: 1H NMR (500 MHz, CDCl3) δ 7.80 (m, 1H), 7.56 (d, J = 8.1 Hz, 2H), 7.40 (t, J = 8.1 Hz, 1H), 7.28 (m, 2H), 7.19 (d, J = 3.3 Hz, 1H), 7.06–7.01 (m, 1H), 6.84 (d, J = 3.4 Hz, 1H) (Figure S1j). 13C NMR (125.1 MHz, CDCl3) δ 136.21 (C), 135.64 (2C), 134.86 (C), 130.09 (CH), 128.95 (2CH), 128.33 (C), 128.06 (CH), 122.60 (CH), 121.15 (CH), 120.58 (CH), 110.29 (CH), 103.87 (CH). HRMS TOF (ESI+) m/z calculated for C14H10Cl2N [M + H]+ 262.0190, found 262.0177 (Figure S1k).
3.2.6. Treatment of Alcohol 6 with TBSCl
To a stirred solution of compound 6 (337 mg, 1.2 mmol) in 20 mL of dry dichloromethane (DCM) were added under argon atmosphere 500 mg (4.1 mmol) of 4-(dimethylamino)pyridine (DMAP) and tert-butyldimethylsilyl chloride (TBSCl) (328 mg, 2.2 mmol). After consumption of the starting product (45 min), the mixture was diluted with 150 mL of DCM and washed with 1 N HCl (3 × 20 mL), Na2CO3 (3 × 30 mL) and brine (3 × 30 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The resulting reaction crude was flash chromatographed (H:MTBE, 5:1) to afford 423 mg (1.1 mmol) of compound 7 (89%).
Compound 7: 1H NMR (500 MHz, CDCl3) δ 7.36 (d, J = 8.1 Hz, 2H), 7.20 (dd, J = 7.4, 1.5 Hz, 1H), 7.07 (td, J = 7.7, 1.6 Hz, 1H), 7.01 (t, J = 8.1 Hz, 1H), 6.94 (td, J = 7.4, 1.2 Hz, 1H), 6.49 (dd, J = 8.0, 1.0 Hz, 1H), 3.99 (t, J = 6.7 Hz, 2H), 3.01 (t, J = 6.7 Hz, 2H), 0.88 (s, 9H), 0.06 (s, 6H) (Figure S1l). 13C NMR (125.1 MHz, CDCl3) δ 142.21 (C), 137.80 (C), 130.56 (CH), 130.09 (2C), 128.86 (2C), 128.59 (C), 126.78 (CH), 124.17 (CH), 121.60 (CH), 117.04 (CH), 63.91 (CH2), 35.32 (CH2), 26.04 (3CH3), 18.60 (C), -5.22 (2CH3). HRMS TOF (ESI+) m/z calculated for C20H28Cl2NOSi [M + H]+ 397.1317, found 397.1308 (Figure S1m).
3.2.7. Treatment of Compound 7 with Methyl Iodide in the Presence of NaH: Synthesis of 8a
To a stirred solution of compound 7 (395 mg, 1 mmol) in 18 mL THF, under an argon atmosphere, was added 75 mg of NaH (3.1 mmol). After stirring for 30 min, 0.15 mL (2.4 mmol) of methyl iodide was added dropwise, and the resulting solution was stirred for 1 h. The mixture was then cooled at 0 °C and diluted with 150 mL of MTBE. Then 5 mL of saturated NH4Cl were added dropwise. The aqueous layer was diluted with water (15 mL) and extracted with MTBE (3 × 20 mL). The combined organic layers were washed with brine (3 × 40 mL) dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resulting crude product was dissolved in 8 mL of dry THF, and 700 mg of TBAF was added to the resulting solution at room temperature and under argon atmosphere. After stirring for 30 min, the reaction mixture was diluted with 150 mL of EtOAc, washed with brine (3 × 35 mL), dried over anhydrous Na2SO4 and concentrated in vacuo. The reaction crude was purified by flash chromatography (H:MTBE, 1:1) to afford 238 mg of compound 8a (0.8 mmol, 80%).
Compound 8a. 1H NMR (600 MHz, CDCl3) δ 7.34 (d, J = 8.0 Hz, 2H), 7.25 (td, J = 7.4, 1.4 Hz, 1H), 7.21 (dd, J = 8.2, 1.4 Hz, 1H), 7.13 (dd, J = 7.5, 1.7 Hz, 1H), 7.10 (t, J = 8.0 Hz 1H), 6.98 (td, J = 7.4, 1.4 Hz, 1H), 3.55 (t, J = 6.9 Hz, 2H), 3.32 (s, 3H), 2.45 (t, J = 6.9 Hz, 2H) (Figure S1o). 13C NMR (151 MHz, CDCl3) δ 147.95 (C), 147.95 (C), 135.14 (2C), 130.78 (C), 129.78 (2CH), 129.05 (CH), 127.05 (CH), 126.93 (CH), 121.83 (CH), 120.39 (CH), 62.33 (CH2), 40.49 (CH), 35.06 (CH2). HRMS TOF (ESI+) m/z calculated for C15H16NOCl2 [M + H]+ 296.0699, found 296.0691 (Figure S1p).
3.2.8. Dess-Martin Oxidation of 8a
To a stirred solution of 8a (100 mg, 0.34 mmol) in 15 mL of DCM under argon was added the Dess−Martin reagent (305 mg, 0.72 mmol). After stirring for 40 min at room temperature, the reaction was quenched with 10 mL of a saturated solution of Na2S2O3−NaHCO3 that was added slowly to the mixture. The organic layer was then washed with brine (3 × 20 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by flash chromatography (H:MTBE, 2:1) to obtain 91 mg of 9a (0.31 mmol, 91%).
Compound 9a. 1H NMR (600 MHz, CDCl3) δ 9.17 (t, J = 1.9 Hz, 1H), 7.36–7.33 (m, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.26 (d, J = 8.1 Hz, 1H), 7.10 (t, J = 8.1 Hz, 1H), 7.05–7.00 (m, 1.0 Hz, 2H), 3.31 (d, J = 1.9 Hz, 2H), 3.30 (s, 3H) (Figure S1y). 13C NMR (151 MHz, CDCl3) δ 199.56 (CHO), 148.57 (C), 143.59 (C), 135.35 (2C), 132.19 (CH), 129.93 (2CH), 128.20 (CH), 127.48 (CH), 123.41 (C), 121.98 (CH), 120.26 (CH), 46.65 (CH2), 40.27 (CH) (Figure S1z). HRMS TOF (ESI+) m/z calculated for C15H14NOCl2 [M + H]+ 294.0452, found 294.0442.
3.2.9. Pinnick Oxidation of 9a
Aldehyde 9a (98 mg, 0.33 mmol) was dissolved in 7 mL of tert-butyl alcohol and 1.2 mL of 2-methyl-2-butene. A solution of sodium chlorite (85 mg, 0.94 mmol) and sodium dihydrogenphosphate dihydrate (95 mg, 0.61 mmol) in 1.5 mL of water was added dropwise over a 10 min period. The yellow reaction mixture was stirred at room temperature for 40 min. Volatile components were then removed under vacuum. The residue was dissolved in 30 mL of water and extracted with two 25 mL portions of MTBE. The combined organic layers were washed with brine, dried, and concentrated under reduced pressure. Purification by flash chromatography (H:MTBE, 10:1) afforded 69 mg of compound 10a [37] (0.22 mmol, 67%).
Compound 10a: 1H NMR (600 MHz, CDCl3) δ 7.31 (ta, J = 8,1 Hz, 1H), 7.29 (d, J = 8.1 Hz, 2H), 7.20 (d, J = 8.1 Hz, 1H), 7.13 (td, J = 6.8 Hz, 1H), 7.03 (t, J = 8.1 Hz, 1H), 7.01 (t, J = 7.4 Hz, 1H), 3.42 (s, 2H), 3.30 (s, 3H) (Figure S1h2). 13C NMR (151 MHz, CDCl3) δ 175.94 (COOH), 148.14 (C), 143.39 (C), 135.62 (2C), 132.09 (CH), 129.76 (2CH), 128.05 (CH), 127.28 (CH), 124.47 (C), 121.90 (CH), 120.47 (CH), 40.47 (CH), 36.85 (CH2) (Figure S1i2). HRMS TOF (ES+) m/z calculated for C15H14NO2Cl2 [M + H]+ 310.0402, found 310.0390.
3.2.10. Treatment of Compound 7 with Isopentenyl Bromide in the Presence of NaH: Synthesis of 8b
To a stirred solution of compound 7 (675 mg, 1.65 mmol) in 25 mL THF, under argon atmosphere, was added 123 mg of NaH (5.1 mmol). After stirring for 30 min, 0.7 mL (0.95 mmol) of isopentenyl bromide were added dropwise, and the resulting solution was stirred for 6 h at 50 °C. The mixture was then cooled at 0 °C and diluted with 150 mL of MTBE. Then 15 mL of saturated NH4Cl were added dropwise. The aqueous layer was extracted with MTBE (3 × 30 mL). The combined organic layers were washed with brine (3 × 40 mL) dried over anhydrous Na2SO4 and concentrated under reduced pressure. The resulting crude product was dissolved in 25 mL of dry THF, and 1.8 mL of 1M TBAF solution in THF was added to the resulting solution at room temperature and under argon atmosphere. After stirring for 3 h the reaction mixture was diluted with 200 mL of EtOAc, washed with brine (3 × 50 mL), dried over anhydrous Na2SO4 and concentrated in vacuo. The reaction crude was purified by flash chromatography (H:MTBE, 9:1) to afford 384 mg of compound 8b (1.1 mmol, 65%).
Compound 8b. 1H NMR (500 MHz, CDCl3) δ 7.31 (d, J = 8.0 Hz, 2H), 7.23–7.17 (m, 2H), 7.10 (dd, J = 7.5, 1.5 Hz, 1H), 7.05 (dd, J = 8.3, 7.7 Hz, 1H), 6.95 (ddd, J = 7.6, 6.1, 2.3 Hz, 1H), 5.39 (thept, J = 6.13, 1.4 Hz, 1H), 4.32 (d, J = 6.2 Hz, 2H), 3.52 (t, J = 6.9 Hz, 2H), 2.41 (t, J = 6.9 Hz, 2H), 1.67 (bd, J = 1.4 Hz, 3H), 1.63 (bd, J = 1.4 Hz, 3H) (Figure S1q). 13C NMR (126 MHz, CDCl3) δ 146.70 (C), 143.49 (C), 135.31 (2C), 134.25 (C), 130.70 (CH), 129.89 (C), 129.77 (2CH), 126.72 (CH), 126.55 (CH), 121.97 (CH), 121.94 (CH), 121.55 (CH), 62.39 (CH2), 51.06 (CH2), 35.03 (CH2), 25.75 (CH3), 17.90 (CH3) (Figure S1r). HRMS TOF (ES+) m/z calculated for C19H22NOCl2 [M + H]+ 350.1078, found 350.1062.
3.2.11. Dess-Martin Oxidation of 8b
To a stirred solution of 8b (60 mg, 0.17 mmol) in 5 mL of DCM under argon was added the Dess−Martin reagent (144 mg, 0.34 mmol). After stirring for 20 min at room temperature, the reaction was diluted with DCM (20 mL) and quenched with 2 mL of a saturated solution of Na2S2O3−NaHCO3 that was added slowly to the mixture. The organic layer was then washed with brine (3 × 10 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (H:MTBE, 2:1) to obtain 58 mg of 9b (0.16 mmol, 94%).
Compound 9b. 1H NMR (500 MHz, CDCl3) δ 9.18 (t, J = 2.0 Hz, 1H), 7.34–7.28 (m, 3H), 7.24 (d, J = 7.7 Hz, 1H), 7.08 (t, J = 8.1 Hz, 1H), 7.05–6.98 (m, 2H), 5.41 (bt, J = 6.3 Hz, 1H), 4.32 (d, J = 6.4 Hz, 2H), 3.30 (d, J = 2.1 Hz, 2H), 1.70 (bs, 3H), 1.64 (bs, 3H) (Figure S1b2). 13C NMR (126 MHz, CDCl3) δ 199.67 (C), 147.44 (C), 142.94 (C), 135.58 (2C), 134.66 (C), 132.12 (CH), 129.94 (2CH), 127.90 (CH), 127.16 (CH), 124.26 (C), 122.10 (CH), 121.88 (CH), 121.21 (CH), 51.02 (CH2), 46.64 (CH2), 25.76 (CH3), 17.91 (CH3) (Figure S1c2). HRMS TOF (ES+) m/z calculated for C19H20NOCl2 [M + H]+ 348.0922, found 348.0901.
3.2.12. Pinnick Oxidation of 9b
Aldehyde 9b (243 mg, 0.71 mmol) was dissolved in 20 mL of tert-butyl alcohol and 3 mL of 2-methyl-2-butene. A solution of sodium chlorite (260 mg, 2.87 mmol) and sodium dihydrogenphosphate dihydrate (295 mg, 1.89 mmol) in 5 mL of water was added dropwise over a 20 min period. The yellow reaction mixture was stirred at room temperature for 50 min. Volatile components were then removed under vacuum. The residue was dissolved in 30 mL of water and extracted with two 50 mL portions of MTBE. The combined organic layers were washed with brine, dried, and concentrated under reduced pressure. Purification by flash chromatography (H:MTBE, 10:1) afforded 164 mg of compound 10b (0.45 mol, 64%).
Compound 10b: 1H NMR (400 MHz, CDCl3) δ 7.24 (d, J = 8.0 Hz, 1H), 7.27–7.24 (m, 1H), 7.19 (dd, J = 8.3, 1.3 Hz, 1H), 7.12 (dd, J = 7.6, 1.7 Hz, 1H), 7.02–6.96 (m, 2H), 5.40 (thep, J = 6.4, 1.4 Hz, 1H), 4.32 (dt, J = 6.4, 1.3 Hz, 2H), 3.40 (s, 2H), 1.68 (d, J = 1.4 Hz, 3H), 1.62 (d, J = 1.4 Hz, 3H) (Figure S1k2). 13C NMR (101 MHz CDCl3) δ 176.83 (C), 147.11 (C), 142.60 (C), 135.90 (2C), 134.47 (C), 131.97 (CH), 129.73 (2CH), 127.70 (CH), 126.95 (CH), 125.30 (C), 121.99 (CH), 121.98 (CH), 121.38 (CH), 51.15 (CH2), 36.93 (CH2), 25.74 (CH3), 17.86 (CH3) (Figure S1l2). HRMS TOF (ES+) m/z calculated for C19H20NO2Cl2 [M + H]+ 364.0871, found 364.0862.
3.2.13. Treatment of Compound 7 with 4-Bromobenzyl Bromide in the Presence of NaH: Synthesis of 8c
To a stirred solution of compound 7 (854 mg, 2.16 mmol) in 30 mL THF, under argon atmosphere, were added 500 mg of NaH (21 mmol). After stirring for 30 min, 1350 mg (5.4 mmol) of 4-bromobenzyl bromide were added portion wise, and the resulting solution was stirred for 8 h at 50 °C. The mixture was then cooled at 0 °C and diluted with 150 mL of MTBE. Then 15 mL of saturated NH4Cl were added dropwise. The aqueous layer was extracted with MTBE (3 × 30 mL). The combined organic layers were washed with brine (3 × 40 mL) dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resulting crude product was dissolved in 50 mL of dry THF and 128 mL of 1M TBAF solution in THF was added to the resulting solution at room temperature and under argon atmosphere. After stirring for 4 h the reaction mixture was diluted with 200 mL of EtOAc, washed with brine (3 × 50 mL), dried over anhydrous Na2SO4 and concentrated in vacuo. The reaction crude was purified by flash chromatography (H:MTBE, 9:1) to afford 786 mg of compound 8c (1.75 mmol, 81%).
Compound 8c. 1H NMR (600 MHz, CDCl3) δ 7.37 (d, J = 8.6 Hz, 2H), 7.33 (d, J = 8.6 Hz, 2H), 7.33–7.29 (m, 1H), 7.31 (d, J = 8.0 Hz, 2H), 7.12 (d, J = 7.3 Hz, 1H), 7.08 (dd, J = 8.4, 7.7 Hz, 1H), 7.05–7.01 (m, 1H), 6.95–6.90 (m, 1H), 4.86 (s, 2H), 3.55 (t, J = 7.0 Hz, 2H), 2.58 (t, J = 7.0 Hz, 2H) (Figure S1t). 13C NMR (151 MHz, CDCl3) δ 145.16 (C), 143.87 (C), 136.74 (C), 135.21 (2C), 131.45 (2CH), 130.57 (C), 130.55 (CH), 130.01 (2CH), 129.35 (2CH), 127.04 (CH), 126.46 (C), 123.21 (CH), 122.68 (CH), 120.68 (C), 62.24 (CH2), 55.46 (CH2), 34.55 (CH2) (Figure S1u). HRMS TOF (ES+) m/z calculated for C21H19NOCl2Br [M + H]+ 450.0027, found 450.0021.
3.2.14. Dess-Martin Oxidation of 8c
To a stirred solution of 8c (375 mg, 0.80 mmol) in 25 mL of DCM under argon was added the Dess−Martin reagent (677 mg, 1.6 mmol). After stirring for 50 min at room temperature, the reaction was diluted with DCM (20 mL) and quenched with 10 mL of a saturated solution of Na2S2O3−NaHCO3 that was added slowly to the mixture. The organic layer was then washed with brine (3 × 20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (H:MTBE, 2:1) to obtain 325 mg of 9c (0.72 mmol, 90%).
Compound 9c. 1H NMR (600 MHz, CDCl3) δ 9.15 (t, J = 1.8 Hz, 1H), 7.38 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.1 Hz, 4H), 7.14–7.04 (m, 3H), 7.02 (dd, J = 7.6, 1.7 Hz, 1H), 6.96 (td, J = 7.3, 1.3 Hz, 1H), 4.82 (s, 2H), 3.44 (d, J = 1.8 Hz, 2H) (Figure S1e2). 13C NMR (151 MHz, CDCl3) δ 199.04 (CHO), 145.66 (C), 143.37 (C), 136.34 (C), 135.37 (2C), 132.22 (CH), 131.51 (2CH), 130.17 (2CH), 129.28 (2CH), 127.62 (CH), 127.60 (CH), 124.93 (C), 123.03 (CH), 122.77 (CH), 120.79 (C), 55.27 (CH2), 46.50 (CH2) (Figure S1f2). HRMS TOF (ESI+) m/z calculated for C21H17NOCl2Br [M + H]+ 447.9871, found 447.9837.
3.2.15. Pinnick Oxidation of 9c
Aldehyde 9c (335 mg, 0.72 mmol) was dissolved in 20 mL of tert-butyl alcohol and 3 mL of 2-methyl-2-butene. A solution of sodium chlorite (266 mg, 2.94 mmol) and sodium dihydrogenphosphate dihydrate (298 mg, 1.91 mmol) in 5 mL of water was added dropwise over a 20 min period. The yellow reaction mixture was stirred at room temperature for 50 min. Volatile components were then removed under vacuum. The residue was dissolved in 30 mL of water and extracted with 2 50 mL portions of MTBE. The combined organic layers were washed with brine, dried, and concentrated under reduced pressure. Purification by flash chromatography (H:MTBE, 10:1) afforded 131 mg of compound 10c (0.5 mmol, 69%).
Compound 10c: 1H NMR (600 MHz, CDCl3) δ 7.37 (d, J = 8.5 Hz, 2H), 7.32 (d, J = 8.4 Hz, 1H), 7.27 (d, J = 7.6 Hz, 3H), 7.15–7.06 (m, 2H), 7.03 (d, J = 8.1 Hz, 1H), 7.01 (t, J = 8.1 Hz, 1H), 6.96 (td, J = 7.1, 0.8 Hz, 1H), 4.86 (s, 2H), 3.50 (d, J = 4.3 Hz, 2H) (Figure S1l2). 13C NMR (101 MHz, CDCl3) δ 174.54 (C), 145.26 (C), 143.04 (C), 136.46 (C), 135.53 (2C), 132.06 (CH), 131.46 (2CH), 130.03 (2CH), 129.30 (2CH), 127.56 (CH), 127.33 (CH), 126.03 (C), 123.15 (CH), 122.79 (CH), 120.73 (C), 55.49 (CH2), 36.43 (CH2) (Figure S1m2). HRMS TOF (ESI+) m/z calculated for C21H17NO2Cl2Br [M + H]+ 463.9820, found 463.9829.
3.3. Cell Viability Assays
All cell lines used in this article were provided by the cell bank of the University of Granada, Spain: HT29 human colorectal adenocarcinoma cell line (ECACC no. 9172201; ATCC no. HTB-38); Hep-G2 human hepatocarcinoma cell line (ECACC no. 85011430; B16-F10 mouse melanoma cell line (ATCC no. CRL-6475), and RAW 264.7 monocyte/macrophage murine cell line (ATCC no. TIB-71). All cell lines were cultured in DMEM supplemented with 2 mM glutamine, 10% heat-inactivated FCS, 50 µg·mL−1 of gentamicin, being incubated at 37 °C, in an atmosphere of 5% CO2 and 95% humidity. Subconfluent monolayer cells were used in all experiments.
DCF and its 8 different derivatives were dissolved at 5 mg·mL−1 in DMSO, and stored at 4 °C. Before use in cell treatment, these compounds were diluted in cell culture medium at adequate concentrations for each experiment. Apoptosis, mitochondrial-membrane potential and cell-cycle analysis were measured at IC50 and IC80 in HT29, Hep-G2 and B16-F10. For nitrite concentration, ¾·IC50, ½·IC50 and ¼·IC50 concentrations were used to ensure sub-cytotoxic concentration against RAW 264.7 cells.
The effect of each treatment with compounds 2, 4, 6, 8c, 9c, 10a-c, and DCF on cell cytotoxicity were determined in all cell lines described before. The cells viability was determined by using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) proliferation assay (Sigma, St. Louis, MO, USA), which is based on the ability of live cells to cleave the tetrazolium ring, thus producing formazan, which absorbs at 570 nm.
For this assay, 6 × 103 HT29 cells, 15 × 103 Hep-G2 cells, 5 × 103 B16-F10 cells and 6 × 103 RAW 264 cells per well were grown on a 96-well plate and incubated with the different products (0–200 μg·mL−1). After 72 h, 100 µL of MTT solution (0.5 mg·mL−1) in a mixture of 50% of PBS (Phosphate-Buffered Saline) and 50% of cell medium was added to each well. After 1.5 h of incubation, cells were washed twice with PBS, and formazan was resuspended in 100 μL DMSO. Relative cell viability, with respect to untreated control cells, was measured by absorbance at 570 nm on an ELISA plate reader (Tecan Sunrise MR20-301, Männedorf, Switzerland, Männedorf, Switzerland).
3.4. Anexin V-FICT/Propidium Iodide Flow-Cytometric Analysis
Apoptosis was analyzed with flow-cytometry by using a FACScan flow-cytometer (fluorescence-activated cell sorter) (Coulter Corporation, Hialeah, FL, USA). In brief, 15 × 104 Hep-G2 cells per well were plated in 24-well plates with 1.5 mL of medium, following treatment with the compounds for 72 h at the IC50 and IC80 concentrations, calculated previously. The cells were collected and resuspended in binding buffer (10 mM HEPES/NaOH, pH = 7.4, 140 mM NaCl, 2.5 mM CaCl2). Annexin V-FITC conjugate (1 mg·mL−1) was added and incubated for 30 min at room temperature in darkness. Just before FACS analysis, cells were stained with 20 µL of 1 mg·mL−1 propidium iodide (PI) solution. In each experiment, approximately 10 × 103 cells were analyzed, and the experiment was duplicated 3 times. The percentages of apoptosis were determined with annexin V-FICT/PI by FACS (flow-activated cell sorter) cytometric analysis, differentiating early apoptotic cells (An-V+ and PI-) from late apoptotic (An-V+ and PI+), necrotic (An-V- and PI+) or normal cells (An-V- and PI-).
3.5. Cell-Cycle Analysis
The cell-cycle was analyzed again with flow cytometry by using a fluorescence-activated cell sorter (FACS) at 488 nm in an Epics XL flow cytometer (Coulter Corporation, Hialeah, FL, USA). For this assay, 15 × 104 Hep-G2 cells per well were plated in 24-well plates with 1.5 mL of medium, following treatment with the compounds for 72 h at the IC50 and IC80 concentrations. After treatment, cells were washed twice with PBS and harvested by trypsinization, then were resuspended in 1X TBS (10 Mm Tris, 150 Mm NaCl), followed by the addition of Vindelov Buffer (100 mM Tris, 100 Mm NaCl, 10 mg/mL RNase, 1 mg/mL PI, pH 8). Samples were allowed to stand for 15 min on ice. Just before FACS analysis, cells were stained with 20 µL of 1 mg·mL−1 PI solution. DNA content is directly proportional to the PI fluorescence, allowing to determine the percentage of cells in each cell-cycle phase. In this way, we are able to visualize cell subpopulations with differing DNA contents. Changes in DNA concentrations are characteristic of apoptosis and cell-cycle arrest. The data were analyzed to determine the percentage of cells at each phase of the cell cycle (G0/G1, S, and G2/M).
3.6. Flow-Cytometry Analysis of the Mitochondrial-Membrane Potential
The loss of mitochondrial membrane potential (MMP) during the apoptotic process has been related to the intrinsic mechanism of apoptosis activation. To approach the mechanism of apoptosis implicated in the apoptotic response of Hep-G2 cells, we analyzed the MMP with rhodamine 123 (Rh123) stained. This compound is a membrane-permeable fluorescent cationic dye selectively taken up by mitochondrial, and its fluorescence is proportional to the mitochondrial membrane potential. Oxidative damage was studied by flow-cytometry analysis of the mitochondrial membrane potential, using dihydrorhodamine 123 (DHR) oxidized to the highly fluorescent product rhodamine 123 (Rh123). The formation of Rh123 can be monitored by fluorescence spectroscopy using excitation and emission wavelengths at 500 and 536 nm, respectively. The intracellular measurement of the mitochondrial membrane potential was made by cytometry determination of Rh123. In the same way as in the apoptosis assays, 15 × 104 Hep-G2 cells per well were plated in 24-well plates with 1.5 mL of medium, following treatment with the compounds for 72 h at the IC50 and IC80 concentrations. After treatment, the medium was removed and a fresh medium with DHR was added at a final concentration of 5 µg/mL. After 30 min of incubation, the medium was removed, and the cells were washed and resuspended in PBS with 5 µg·mL−1 of PI. The intensity of fluorescence from Rh123 and PI was determined using a FACScan flow-cytometer (Coulter Corporation, Hialeah, FL, USA).
3.7. Determination of Nitrite Concentration
The concentration of nitrites was determined in assay based on the Griess reaction. This nitrite concentration was used as an indicator of NO production. RAW 264.7 cells were plated at 6 × 104 cells/well in 24-well cell culture plates, supplemented with 10 µg/mL of LPS, except untreated negative control cells. After 24 h of plating, the cells were incubated for 24 h with the test compounds at ¾·IC50, ½·IC50, and ¼·IC50 concentrations determined by MTT proliferation assay. The supernatants were collected at 24, 48, and 72 h to determine nitrite concentration and/or stored at −80 °C for further use.
For the Griess reaction, 150 µL of supernatant test samples or sodium nitrite standard (0–120 µM) were taken and mixed with 25 µL of the Griess reagent A (0.1% N-N-(1-naphthyl)-ethylenediamine dihydrochloride) and 25 µL of the Griess reagent B (1% sulfanilamide in 5% of phosphoric acid), in a 96-well plate. After 15 min of incubation at room temperature, the absorbance was measure at 540 nm on an ELISA plate reader (Tecan Sunrise MR20-301, Männedorf, Switzerland, Männedorf, Switzerland). The absorbance was referred to nitrite standard curve to determine the concentration of nitrite in the supernatant. The percentage of NO production was determined, assigning 100% at the increase between negative control (untreated cells) and positive control (cells only treated with 10 µg·mL−1 of LPS).
3.8. Statistics
Statistical and non-linear sigmoidal regression analyses were performed with the SigmaPlot 12.5 software (Systat Softwre Inc. San Jose, CA, USA). All quantitative data were summarized as the means ± standard deviation (SD). All data shown here were representative of at least 2 independent experiments performed in triplicate.
Acknowledgments
José Luis Gómez Navas is thanked for his contribution in the early stages of this work.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22105067/s1, Figure S1. copies of NMR spectra of the synthesized compounds.
Author Contributions
Conceptualization, F.J.R.-Z., J.A.L., A.R.-D. and J.F.Q.d.M.; investigation, A.G., F.J., A.G.-G., H.A., S.R.; writing—original draft preparation, F.J.R.-Z., A.R.-D. and J.F.Q.d.M.; writing—review and editing, F.J.R.-Z., S.R. and J.F.Q.d.M.; funding acquisition, F.J.R.-Z. and J.F.Q.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by grant MINISTERIO DE ECONOMÍA Y COMPETITIVIDAD, PID2019-106222RB-C32/SRA (State Research Agency, 10.13039/501100011033) and by the “Consejería de Economía, Conocimiento, Empresas y Universidad. Junta de Andalucía”, grant number B1-BIO-281-UGR18.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gan T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010;26:1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- 2.Orido T., Fujino H., Hasegawa Y., Toyomura K., Kawashima T., Murayama T. Indomethacin decreases arachidonic acid uptake in HCA-7 human colon cancer cells. J. Pharmacol. Sci. 2008;108:389–392. doi: 10.1254/jphs.08167SC. [DOI] [PubMed] [Google Scholar]
- 3.Triggiani M., Granata F., Frattini A., Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2006;1761:1289–1300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Mäkelä A., Kuusi T., Schröder T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand. J. Clin. Lab. Investig. 1997;57:401–407. doi: 10.3109/00365519709084587. [DOI] [PubMed] [Google Scholar]
- 5.Bondar T., Medzhitov R. The origins of tumor-promoting inflammation. Cancer Cell. 2013;24:143–144. doi: 10.1016/j.ccr.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakanishi M., Rosenberg D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seed M.P., Brown J.R., Freemantle C.N., Papworth J.L., Colville-Nash P.R., Willis D., Somerville K.W., Asculai S., Willoughby D.A. The inhibition of colon-26 adenocarcinoma development and angiogenesis by topical diclofenac in 2.5% hyaluronan. Cancer Res. 1997;57:1625–1629. [PubMed] [Google Scholar]
- 9.Larsson K., Kock A., Idborg H., Arsenian Henriksson M., Martinsson T., Johnsen J.I., Korotkova M., Kogner P., Jakobsson P.-J. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc. Natl. Acad. Sci. USA. 2015;112:8070–8075. doi: 10.1073/pnas.1424355112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnsen J.I., Lindskog M., Ponthan F., Pettersen I., Elfman L., Orrego A., Sveinbjörnsson B., Kogner P. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 11.Zerbini L.F., Czibere A., Wang Y., Correa R.G., Otu H., Joseph M., Takayasu Y., Silver M., Gu X., Ruchusatsawat K., et al. A novel pathway involving melanoma differentiation associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory grug–induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006;66:11922–11931. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]
- 12.Valle B.L., D’Souza T., Becker K.G., Wood III W.H., Zhang Y., Wersto R.P., Morin P.J. Non-steroidal anti-inflammatory drugs decrease E2F1 expression and inhibit cell growth in ovarian cancer cells. PLoS ONE. 2013;8:e61836. doi: 10.1371/journal.pone.0061836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leidgens V., Seliger C., Jachnik B., Welz T., Leukel P., Vollmann-Zwerenz A., Bogdahn U., Kreutz M., Grauer O.M., Hau P. Ibuprofen and diclofenac restrict migration and proliferation of human glioma cells by distinct molecular mechanisms. PLoS ONE. 2015;10:e0140613. doi: 10.1371/journal.pone.0140613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagi K., Kawasaki Y., Nakamura H., Miura T., Takeda T., Esumi S., Matsunaga H., Kitamura Y., Sendo T. Differential combined effect of COX inhibitors on cell survival suppressed by sorafenib in the HepG2 cell line. Biol. Pharm. Bull. 2014;37:1234–1240. doi: 10.1248/bpb.b13-00963. [DOI] [PubMed] [Google Scholar]
- 15.Gottfried E., Lang S.A., Renner K., Bosserhoff A., Gronwald W., Rehli M., Einhell S., Gedig I., Singer K., Seilbeck A., et al. New Aspects of an Old Drug—Diclofenac targets MYC and glucose metabolism in tumor cells. PLoS ONE. 2013;8:e66987. doi: 10.1371/journal.pone.0066987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsen J.I., Lindskog M., Ponthan F., Pettersen I., Elfman L., Orrego A., Sveinbjörnsson B., Kogner P. NSAIDs in neuroblastoma therapy. Cancer Lett. 2005;228:195–201. doi: 10.1016/j.canlet.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 17.Hoferová Z., Fedorocko P., Hofmanová J., Hofer M., Znojil V., Minksová K., Soucek K., Egyed A., Kozubík A. The effect of nonsteroidal anti-inflammatory drugs ibuprofen, flurbiprofen, and diclofenac on in vitro and in vivo growth of mouse fibrosarcoma. Cancer Investig. 2002;20:490–498. doi: 10.1081/CNV-120002149. [DOI] [PubMed] [Google Scholar]
- 18.Falkowski M., Skogstad S., Shahzidi S., Smedsröd B., Sveinbjörnsson B. The effect of cyclooxygenase inhibitor diclofenac on experimental murine colon carcinoma. Anticancer Res. 2003;23:2303–2308. [PubMed] [Google Scholar]
- 19.Edrei Y., Gross E., Corchia N., Abramovitch R. Improved efficacy of a novel anti-angiogenic drug combination (TL-118) against colorectal-cancer liver metastases; MRI monitoring in mice. Br. J. Cancer. 2012;107:658–666. doi: 10.1038/bjc.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komar-Stossel C., Gross E., Dery E., Corchia N., Meir K., Fried I., Abramovitch R. TL-118 and gemcitabine drug combination display therapeutic efficacy in a MYCN amplified orthotopic neuroblastoma murine model—Evaluation by MRI. PLoS ONE. 2014;9:e90224. doi: 10.1371/journal.pone.0090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue A., Muranaka S., Fujita H., Kanno T., Tamai H., Utsumi K. Molecular mechanism of diclofenac-induced apoptosis of promyelocytic leukemia: Dependency on reactive oxygen species, akt, bid, cytochrome and caspase pathway. Free Radic. Biol. Med. 2004;37:1290–1299. doi: 10.1016/j.freeradbiomed.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Albano F., Arcucci A., Granato G., Romano S., Montagnani S., De Vendittis E., Ruocco M.R. Markers of mitochondrial dysfunction during the diclofenac-induced apoptosis in melanoma cell lines. Biochimie. 2013;95:934–945. doi: 10.1016/j.biochi.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki R., Moon Y., Norimura T., Eling T. Ionizing radiation enhances the expression of the nonsteroidal anti-inflammatory drug-activated gene (NAG1) by increasing the expression of TP53 in human colon cancer cells. Radiat. Res. 2006;165:125–130. doi: 10.1667/RR3492.1. [DOI] [PubMed] [Google Scholar]
- 24.Cooper D.L., Harirforoosh S. Design and optimization of PLGA-based diclofenac loaded nanoparticles. PLoS ONE. 2014;9:e87326. doi: 10.1371/journal.pone.0087326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafeez F., Zahoor A.F., Ahmad S., Ahmad M., Faiz S. Recent progress in the synthesis of diclofenac based NSAIDs analogs/derivatives. Synth. Commun. 2019;49:325–350. doi: 10.1080/00397911.2018.1515367. [DOI] [Google Scholar]
- 26.Miyamoto K., Yasuda Y., Yoshioka K. Glycosaminoglycan Derivative and Method for Producing Same. US20190184023A1. U.S. Patent. 2019 Jun 20;
- 27.Laskin J.D., Heck D.E., Lacey C.J., Heindel N.D., Young S.C. Augmenting moieties for anti-inflammatory compounds. US10752582B2. U.S. Patent. 2020 Aug 25;
- 28.Akgul O., Di Cesare Mannelli L., Vullo D., Angeli A., Ghelardini C., Bartolucci G., Alfawaz Altamimi A.S., Scozzafava A., Supuran C.T., Carta F. Discovery of novel nonsteroidal anti-inflammatory drugs and carbonic anhydrase inhibitors hybrids (NSAIDs–CAIs) for the management of rheumatoid arthritis. J. Med. Chem. 2018;61:4961–4977. doi: 10.1021/acs.jmedchem.8b00420. [DOI] [PubMed] [Google Scholar]
- 29.Osman H.A., Nazeruddin G.M. Design, synthesis, biological evaluation and docking studies of some new diclofenac analogues. Br. J. Pharm. Res. 2014;4:770–777. doi: 10.9734/BJPR/2014/5030. [DOI] [Google Scholar]
- 30.Ravichandran V., Mohan S., Kumar K.S. Synthesis and antimicrobial activity of Mannich bases of isatin and its derivatives with 2-[(2,6-dichlorophenyl)amino]phenylacetic acid. Arkivoc. 2007;14:51–57. doi: 10.3998/ark.5550190.0008.e07. [DOI] [Google Scholar]
- 31.Shah B., Patil P., Shah H. Chemical modification of paracetamol and their antimicrobial and pharmacological evaluation. Int. J. Pharm. Res. Bio-Sci. 2014;3:12–31. [Google Scholar]
- 32.Blumberg L.C., Lowe J.A., Almarsson O., Alvarez J., Zeidan T.A. Prodrugs of secondary amine compounds. US8969337B2. U.S. Patent. 2015 Mar 03;
- 33.Chen C.-H. Novel composition for treating metabolic syndrome and other conditions. US20120183600A1. U.S. Patent. 2012 Jul 19;
- 34.Biere H., Rufer C., Boettcher I. Diphosphonic acid derivatives and pharmaceutical preparations containing them. DE3225469A1. Germany Patent. 1984 Jan 05;
- 35.Tsuchihashi G., Ogura K., Sakota R., Hashiba I., Fukushima S. O-(N-Allyl-2,6-dichloroanilino)phenylacetic acid Derivative and a Process for Preparing the Same. US4242522A. U.S. Patent. 1980 Dec 30;
- 36.Sakota R., Nagano K., Ando Y., Tsuchihashi G., Ogura K. [N-Benzyl-O-(2,6-dichloroanilino)phenyl]acetic acid Derivatives. CA1113484A. Canada Patent. 1981 Dec 01;
- 37.Sallmann A., Pfister R. Substituted derivatives of 2-anilinophenylacetic acids and a process of preparation. US3558690A. U.S. Patent. 1971 Jan 26;
- 38.Virsodia V., Manvar A., Upadhyay K., Loriya R., Karia D., Jaggi M., Singh A., Mukherjee R., Shaikh M.S., Coutinho E.C., et al. Synthesis of 1-(2,6-dichlorophenyl)-3-methylene-1,3-dihydro-indol-2-one derivatives and in vitro anticancer evaluation against SW620 colon cancer cell line. Eur. J. Med. Chem. 2009;44:1355–1362. doi: 10.1016/j.ejmech.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Palkar M.B., Singhai A.S., Ronad P.M., Vishwanathswamy A.H.M., Boreddy T.S., Veerapur V.P., Shaikh M.S., Rane R.A., Karpoormath R. Synthesis, pharmacological screening and in silico studies of new class of Diclofenac analogues as a promising anti-inflammatory agents. Bioorg. Med. Chem. 2014;22:2855–2866. doi: 10.1016/j.bmc.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Oza V.B., Smith C., Raman P., Koepf E.K., Lashuel H.A., Petrassi H.M., Chiang K.P., Powers E.T., Sachettinni J., Kelly J.W. Synthesis, structure, and activity of diclofenac analogues as transthyretin amyloid fibril formation inhibitors. J. Med. Chem. 2002;45:321–332. doi: 10.1021/jm010257n. [DOI] [PubMed] [Google Scholar]
- 41.Dess D.B., Martin J.C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983;48:4155–4156. doi: 10.1021/jo00170a070. [DOI] [Google Scholar]
- 42.Bal B.S., Childers W.E., Pinnick H.W. Oxidation of α,β-un saturated aldehydes. Tetrahedron. 1981;37:2091–2096. doi: 10.1016/S0040-4020(01)97963-3. [DOI] [Google Scholar]
- 43.Shah S., Arshia, Kazmi N.S., Jabeen A., Faheem A., Dastagir N., Ahmed T., Khan K.M., Ahmed S., Raza A., et al. Diclofenac 1,3,4-oxadiazole derivatives; biology-oriented drug synthesis (BIODS) in search of better non-steroidal, non-acid antiinflammatory agents. Med. Chem. 2018;14:674–687. doi: 10.2174/1573406414666180321141555. [DOI] [PubMed] [Google Scholar]
- 44.Navas A., Jannus F., Fernandez B., Cepeda J., O’donnell M.M., Diaz-Ruiz L., Sanchez-Gonzalez C., Llopis J., Seco J.M., Rufino-Palomares E., et al. Designing single-molecule magnets as drugs with dual anti-inflammatory and anti-diabetic effects. Int. J. Mol. Sci. 2020;21:3146. doi: 10.3390/ijms21093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Valdivia A.A., García-García A., Jannus F., Zabala-Lekuona A., Méndez-Arriaga J.M., Fernández B., Medina-O’donnell M., Ramírez-Rodríguez G.B., Delgado-López J.M., Pastrana-Martínez L.M., et al. Antiparasitic, anti-inflammatory and cytotoxic activities of 2D coordination polymers based on 1H-indazole-5-carboxylic acid. J. Inorg. Biochem. 2020;208:111098. doi: 10.1016/j.jinorgbio.2020.111098. [DOI] [PubMed] [Google Scholar]
- 46.Santos J.L., Moreira V., Campos M.L., Chelucci R.C., Barbieri K.P., Souto P.C., Matsubara M.H., Teixeira C., Bosquesi P.L., Peccinini R.G., et al. Pharmacological evaluation and preliminary pharmacokinetics studies of a new diclofenac prodrug without gastric ulceration effect. Int. J. Mol. Sci. 2012;13:15305–15320. doi: 10.3390/ijms131115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahmoud M.H., El-Dean A.M.K., Abdel-Mohsen S.A., Tolba M.S. New diclofenac derivatives as anti-microbial, anti-inflammatory agents: Design, synthesis, biological screening, and molecular docking study. Russ. J. Bioorg. Chem. 2021;47:208–220. doi: 10.1134/S1068162021010088. [DOI] [Google Scholar]
- 48.Bhandari S.V., Bothara K.G., Raut M.K., Patil A.A., Sarkate A.P., Mokale V.J. Design, synthesis and evaluation of antiinflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorganic Med. Chem. 2008;16:1822–1831. doi: 10.1016/j.bmc.2007.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability upon request.