Abstract
Description:
The purpose of this guidance statement is to guide clinicians on colorectal cancer screening in average-risk adults.
Methods:
This guidance statement is derived from a critical appraisal of guidelines on screening for colorectal cancer in average-risk adults and the evidence presented in these guidelines. National guidelines published in English between 1 June 2014 and 28 May 2018 in the National Guideline Clearinghouse or Guidelines International Network library were included. The authors also included 3 guidelines commonly used in clinical practice. Web sites were searched for guideline updates in December 2018. The AGREE II (Appraisal of Guidelines for Research and Evaluation II) instrument was used to evaluate the quality of guidelines.
Target Audience and Patient Population:
The target audience is all clinicians, and the target patient population is adults at average risk for colorectal cancer.
Guidance Statement 1:
Clinicians should screen for colorectal cancer in average-risk adults between the ages of 50 and 75 years.
Guidance Statement 2:
Clinicians should select the colorectal cancer screening test with the patient on the basis of a discussion of benefits, harms, costs, availability, frequency, and patient preferences. Suggested screening tests and intervals are fecal immunochemical testing or high-sensitivity guaiac-based fecal occult blood testing every 2 years, colonoscopy every 10 years, or flexible sigmoidoscopy every 10 years plus fecal immunochemical testing every 2 years.
Guidance Statement 3:
Clinicians should discontinue screening for colorectal cancer in average-risk adults older than 75 years or in adults with a life expectancy of 10 years or less.
Colorectal cancer (CRC) is the second leading cause of cancer-related death in men and women in the United States. The goal of screening is to reduce overall and cancer-specific morbidity and mortality using strategies that have acceptable harms, burden, and costs. The typical pathogenesis of CRC is an adenomatous polyp that slowly increases in size and leads to dysplasia and cancer. Most CRC arises from colonic adenomas. However, 20% to 30% of CRC cases arise through pathways other than the adenoma–carcinoma sequence. Progression from adenoma to invasive cancer varies from 5 years or less to more than 20 years (1). The 10-year cumulative risk for progression to carcinoma is about 10%; some adenomas stabilize and others regress. Progression risk is related to number, size, and histology of adenomatous polyps (1).
Guidelines disagree on the age to start and stop screening, screening interval, and recommended screening method. Strategies differ in the quality of evidence regarding clinical effectiveness, harms, patient burden, recommended frequency of administration, and test accuracy. All strategies require adherence to the complete regimen of screening, follow-up testing, and treatment because benefits are accrued from identification and removal of precancerous lesions and localized cancer.
PURPOSE AND TARGET POPULATION
The goal of this American College of Physicians (ACP) guidance statement is to guide clinicians on age to start and stop CRC screening, frequency of screening, and optimal screening test in asymptomatic, average-risk adults, based on a critical review of existing guidelines and their evidence reviews. This guidance statement does not address surveillance in patients with previously detected adenomatous polyps or diagnosis in persons with signs or symptoms compatible with CRC. Evaluated screening tests include both stool-based tests (guaiac-based fecal occult blood test [gFOBT], fecal immunochemical test [FIT, also called immunochemical-based FOBT], and multitarget stool DNA panel [sDNA]) and direct visualization with endoscopic and radiologic tests (flexible sigmoidoscopy, colonoscopy, and computed tomography [CT] colonography).
The target patient population is asymptomatic, average-risk adults of all sexes, races, and ethnicities. Persons with a family history of CRC; a long-standing history of inflammatory bowel disease; a genetic syndrome, such as familial adenomatous polyposis; or a personal history of CRC or adenomatous polyps are considered to have above-average risk for CRC.
METHODS
The ACP Clinical Guidelines Committee (CGC) develops guidance statements on topics where several guidelines are available but have conflicting recommendations. Guidance statements rely on only evidence referenced in selected guidelines and accompanying evidence reports and do not include de novo reviews or literature searches. The goal of ACP guidance statements is to provide clinicians with a rigorous review of the available guidelines and their cited evidence and to develop subsequent guidance based on an assessment of the benefits and harms reported by the guidelines. Unlike ACP guidelines, guidance statements are not derived from a systematic evidence review and hence do not use the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system to assess quality of evidence or strength of recommendations (2). The CGC’s methods paper provides a more detailed description of the methods and development process for ACP guidance statements (2).
Data Sources and Guideline Selection
We searched the National Guideline Clearinghouse and the Guidelines International Network library for CRC screening guidelines that were developed by national-level organizations and published in English between 1 June 2014 and 28 May 2018; this yielded guidelines from the American College of Radiology (3), Canadian Task Force on Preventive Health Care (CTFPHC) (4), and U.S. Preventive Services Task Force (USPSTF) (5). We included 3 additional guidelines, which were not identified in either database but are commonly used in U.S. clinical practice, from the American Cancer Society (6), Scottish Intercollegiate Guidelines Network (7), and U.S. Multi-Society Task Force on Colorectal Cancer (8). We searched the Web sites of the selected guideline-producing organizations on 1 December 2018 to look for updated versions. We excluded guidelines that were more than 5 years old and thus inactive (American College of Gastroenterology) or that directly endorsed another guideline (American Academy of Family Physicians and Institute for Clinical Systems Improvement, which both endorsed the USPSTF guideline). The Appendix (available at Annals.org) summarizes the recommendations from each evaluated guideline.
Critical Appraisal
Five coauthors independently reviewed, assessed, and scored each guideline using the AGREE II (Appraisal of Guidelines for Research and Evaluation II) instrument (Table 1) (9).
Table 1.
Scaled AGREE II Domain Scores for Each Guideline and Overall Assessment
| Variable | ACR | ACS | CTFPHC | MSTF | SIGN | USPSTF |
|---|---|---|---|---|---|---|
| Scaled domain score, %* | ||||||
| Scope and purpose | 38 | 92 | 96 | 72 | 79 | 92 |
| Stakeholder involvement | 11 | 81 | 79 | 18 | 69 | 62 |
| Rigor of development | 7 | 77 | 83 | 32 | 65 | 82 |
| Clarity of presentation | 71 | 88 | 86 | 76 | 67 | 80 |
| Applicability | 4 | 66 | 86 | 24 | 44 | 49 |
| Editorial independence | 2 | 33 | 80 | 20 | 77 | 88 |
| Overall guideline assessment | ||||||
| Average overall assessment (out of 7)† | 1.6 | 5.0 | 6.4 | 3.2 | 4.2 | 6.0 |
| Would recommend this guideline for use? | 5 no | 1 yes 4 yes with modifications‡ |
3 yes 2 yes with modifications§ |
1 yes 2 yes with modifications∥ 2 no |
3 yes with modifications¶ 2 no |
2 yes 3 yes with modifications** |
ACR = American College of Radiology; ACS = American Cancer Society; AGREE II = Appraisal of Guidelines for Research and Evaluation II; CTFPHC = Canadian Task Force on Preventive Health Care; MSTF = U.S. Multi-Society Task Force on Colorectal Cancer; SIGN = Scottish Intercollegiate Guidelines Network; USPSTF = U.S. Preventive Services Task Force.
Calculated as follows: (obtained score − minimum possible score) ÷ (maximum possible score − minimum possible score).
Final overall assessment questions on AGREE II.
Individual reviewer suggested modifications: “Solid methods, but overreliance on modeling overestimating benefits. Overreliance on modeling and any [colorectal cancer] mortality reduction despite lack of overall mortality. Minimizes harms info including nothing related to overdiagnosis or overtreatment. Rising incidence indicates very small in absolute terms (2/100,000) and likely at least partially accounted for by detection issues.”
Individual reviewer suggested modifications: “Limited access to flex sig/colonoscopy may make this guideline less generalizable to U.S.”
Individual reviewer suggested modifications: “Age to stop and rationale for tier 1 screening that does not include [fecal occult blood testing] is not clear. May involve societal conflicts (all [gastrointestinal]).”
Individual reviewer suggested modifications: “It is vague and does not provide useful implementation for type, who, when, how often. Does not assess harms or costs including opportunity. Little data provided. Scope extends beyond screening, methods for making recommendations weren’t described.”
Individual reviewer suggested modifications: “Overreliance on modeling, assumption of 100% adherence in models biases towards greater net benefit. Overreliance on [colorectal cancer] mortality reduction. Little info on advanced cancer incidence. No breakout by sex especially for younger or to look at finer gradation of net benefit by age (findings from [randomized controlled trials] and absolute risk show very little to no net benefit).”
Clinician Peer Review
The guidance statement was peer-reviewed through Annals of Internal Medicine and by ACP Regents and Governors, who represent ACP members at the regional and international level.
Public Member Review
The development process for the guidance statement included participation by public members (2 members of the CGC and 7 members of the CGC’s Public Panel) to share their perspectives, values, and preferences (2).
CRITICAL APPRAISAL OF EVALUATED GUIDELINES
Evaluated guidelines addressed both stool-based and direct visualization screening methods (Table 2). Differences between high- and low-scoring guidelines were mostly due to methodological rigor, applicability, and editorial independence. The CTFPHC and USPSTF guidelines scored highest overall (>6 out of 7); the American College of Radiology guideline scored lowest (1.6); and guidelines from the American Cancer Society, U.S. Multi-Society Task Force on Colorectal Cancer, and Scottish Intercollegiate Guidelines Network had moderate AGREE II scores (between 3 and 5) (Table 1).
Table 2.
Summary of Included Recommendations for CRC Screening in Average-Risk Adults From Assessed Guidelines*
| Guideline, Year (Reference) | Age-Based Screening Recommendations | Screening Methods and Intervals |
|---|---|---|
| ACR, 2018 (3) | ≥50 y: screen | CT colonography (usually appropriate): every 5 y Double-contrast barium enema radiography (may be appropriate): every 5 y MR colonography (may be appropriate): every 5 y |
| ACS, 2018 (6) | 45–75 y (if good health and life expectancy ≥10 y): screen 76–85 y: individualize decision on the basis of patient preferences, life expectancy, health status, and screening history ≥85 y: discourage screening |
FIT: annual HSgFOBT: annual Multitarget sDNA: every 3 y Colonoscopy: every 10 y CT colonography: every 5 y Flexible sigmoidoscopy: every 5 y |
| CTFPHC, 2016 (4) | 50–74 y: screen ≥75 y: do not screen |
FOBT (either gFOBT or FIT): every 2 y Flexible sigmoidoscopy: every 10 y Colonoscopy: not recommended as a CRC screening test |
| MSTF, 2017 (8) | ≥50 y: screen ≥45 y (if African American): screen ≥75 y or life expectancy <10 y (if up to date on screening and have negative results on prior screening tests): consider not screening Individuals with no prior screening should be considered for screening up to age 85 y, depending on consideration of their age and comorbid conditions |
Colonoscopy (tier 1): every 10 y FIT (tier 1): annual CT colonography (tier 2): every 5 y† FIT plus sDNA (tier 2): every 3 y Flexible sigmoidoscopy (tier 2): every 10 y Capsule colonoscopy (tier 3): every 5 y Septin 9: not recommended as a CRC screening test |
| SIGN, 2016 (7) | Population-based screening | Quantitative FIT set at a fecal hemoglobin concentration cutoff that is appropriate for investigative capacity; screening interval NA |
| USPSTF, 2016 (5) | 50–75 y: screen 76–85 y: individualize decision, taking into account the patient’s overall health and screening history |
gFOBT: annual FIT: annual FIT plus sDNA: every 1 to 3 y Colonoscopy: every 10 y CT colonography: every 5 y Flexible sigmoidoscopy: every 5 y Flexible sigmoidoscopy with FIT: flexible sigmoidoscopy every 10 y plus FIT every year |
ACR = American College of Radiology; ACS = American Cancer Society; CRC = colorectal cancer; CT = computed tomography; CTFPHC = Canadian Task Force on Preventive Health Care; FIT = fecal immunochemical test; FOBT = fecal occult blood test; gFOBT = guaiac-based FOBT; HSgFOBT = high-sensitivity gFOBT; MR = magnetic resonance; MSTF = U.S. Multi-Society Task Force on Colorectal Cancer; NA = not available; sDNA = stool DNA panel; SIGN = Scottish Intercollegiate Guidelines Network; USPSTF = U.S. Preventive Services Task Force.
The Appendix (available at Annals.org) gives full recommendations and details about strength of the recommendations.
Tier 2 is for patients who decline colonoscopy and FIT, and tier 3 is for patients who decline tier 1 and 2 screening methods.
For our guidance statement, we considered recommendations for adoption or adaptation and examined evidence reviews from the 2 highest-scoring guidelines (CTFPHC and USPSTF) (Table 1). All 5 reviewers concluded that they would recommend or recommend with modification the CTFPHC and USPSTF guidelines. Unlike the USPSTF guideline, the CTFPHC guideline did not include modeling data, and polyp detection and prevention were not considered in its development. Instead, the authors relied on results of randomized controlled trials (RCTs) reporting all-cause mortality, CRC-specific mortality, and incidence of late-stage cancer, as well as population-based estimates of CRC incidence and mortality by age and sex. When evaluating evidence in the guidelines, we prioritized direct evidence from research studies over modeling data; in the absence of direct evidence, however, we included evidence from modeling studies. The Supplement Table (available at Annals.org) summarizes the evidence.
STOOL-BASED TESTS
The CTFPHC recommends biennial screening with FIT or FOBT, whereas the USPSTF recommends annual FIT or FOBT screening and FIT plus sDNA every 1 to 3 years. Any positive result from a stool-based test should be followed up with a diagnostic colonoscopy.
gFOBT
Benefits
The CTFPHC and USPSTF evidence reviews found a reduction in CRC mortality with gFOBT screening (4, 10). Evidence from RCTs showed that CRC screening using gFOBT reduced CRC-specific mortality in adults aged 45 to 80 years (relative risk [RR], 0.82 [95% CI, 0.73 to 0.92]; number needed to screen [NNS], 377 [CI, 249 to 887]; median follow-up, 18.2 years) (4). All-cause mortality was not reduced in any trial or in pooled results. No studies assessed clinical outcomes using currently available high-sensitivity gFOBT (HSgFOBT), although most modeling studies use diagnostic accuracy data from HSgFOBT to assess long-term benefits and harms of gFOBT.
Cumulative reduction in CRC mortality did not statistically significantly differ between annual and biennial gFOBT screening after more than 30 years of follow-up (annual screening: RR, 0.68 [CI, 0.56 to 0.82]; biennial screening: RR, 0.78 [CI, 0.65 to 0.93]) (10). Screening with gFOBT compared with no screening reduced the incidence of late-stage CRC (RR, 0.92 [CI, 0.85 to 0.99]; absolute risk reduction, 1.1 cases [CI, 0.20 to 2.02 cases] per 1000 persons screened; NNS, 876 [CI, 496 to 5051]) (11).
No evidence shows that relative benefits of gFOBT will differ in patients younger than 60 years. However, because CRC incidence increases with age, the absolute benefit is higher in older persons than in those younger than 60 years. The CTFPHC estimated that the NNS with biennial gFOBT is 2655 (CI, 1757 to 6244) for adults aged 45 to 59 years and 492 (CI, 326 to 1157) for adults aged 60 to 80 years (4). Neither guideline reported adherence for gFOBT.
Harms
The CTFPHC found a rate of false-positive results of 12.2 (CI, 10.7 to 13.7) per 1000 persons screened and a rate of false-negative results of 5.5 (CI, 2.8 to 8.2) per 1000 persons screened (11). The USPSTF notes another potential harm: injury to the colon or other complications related to colonoscopy after a positive result on a stool-based test (5).
Diagnostic Accuracy
The sensitivity of HSgFOBT for detecting CRC ranges from 62% to 79%; specificity ranges from 87% to 96% (5). High-sensitivity gFOBTs are currently the predominant form of gFOBT in terms of availability and recommendation.
FIT
Sensitivity and specificity of the many available FITs vary considerably. Generalizations about FIT should be considered with this important caveat.
Benefits
The CTFPHC evidence review included 1 RCT that found no significant reduction in CRC mortality (RR, 0.88 [CI, 0.72 to 1.07]; 0.28 fewer deaths [CI, 0.63 fewer to 0.15 more deaths] per 1000 persons screened) with a single FIT (4), although the RCT was based on a short follow-up (8 years) in a young population (majority aged 30 to 49 years). Compared with gFOBT, FIT is associated with increased patient adherence because it requires no dietary restrictions and only 1 sample (vs. 3 samples for gFOBT) (4, 12). No data were available regarding associations between FIT and all-cause mortality or late-stage CRC.
Harms
The CTFPHC found an overall rate of false-positive results of 87.9 (CI, 52.4 to 123.4) per 1000 persons screened and an overall rate of false-negative results of 0.69 (CI, −0.02 to 1.4) per 1000 persons screened (11). These rates vary because of differing cut points used in clinical practice and in laboratories. We identified no other harms except for those associated with follow-up colonoscopy after positive results on stool-based tests (5).
Diagnostic Accuracy
The USPSTF reported that the sensitivity of FIT for detecting CRC ranges from 73% to 88% and specificity from 91% to 96%, and the CTFPHC found a median sensitivity of 81.5% (range, 53.3% to 100%) and median specificity of 95% (range, 87.2% to 96.9%) (4, 10). Several FITs are available, and sensitivity and specificity are highly variable among the different tests and cut points used.
FIT Plus sDNA
Benefits
No RCT data were available to determine the clinical benefits, including effects on CRC incidence or CRC-related and all-cause mortality.
Harms
Data on harms are limited because most information comes from a single diagnostic accuracy study in which the authors had potentially important conflicts of interest (13). Additional harms unique to FIT plus sDNA (vs. HSgFOBT or FIT) arise from the sDNA component of the test, which lowers its specificity for CRC screening. A positive result despite negative findings on colonoscopy may be due to neoplastic changes not visible on colonoscopy or the presence of noncolonic aerodigestive or supracolonic neoplasms. Patients with positive sDNA results and negative findings on a follow-up colonoscopy may have more aggressive short-term surveillance because of heightened concerns related to unresolved false-positive findings. Uncertainty remains as to the net benefit of additional evaluations after negative colonoscopy findings for a positive result on FIT plus sDNA.
Diagnostic Accuracy
The USPSTF reported that the sensitivity of FIT plus sDNA for detecting CRC was 92% (CI, 84% to 97%) and specificity was 84% (CI, 84% to 85%) (10). No other RCT data on benefits or harms were available.
Overdiagnosis and Overtreatment Due to Positive Results on Stool-Based Screening Tests
No data were available. However, because the 10year cumulative risk for progression from polyps to clinically detectable colon cancer is about 10% (1), the risk for overdiagnosis and overtreatment are likely to be substantially higher in persons with limited life expectancy due to age or comorbid conditions.
DIRECT VISUALIZATION TESTS
The CTFPHC recommends flexible sigmoidoscopy every 10 years and recommends against colonoscopy as a screening test. The USPSTF recommends screening colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, flexible sigmoidoscopy every 10 years with annual FIT, or CT colonography every 5 years.
Flexible Sigmoidoscopy
Benefits
The CTFPHC and USPSTF evidence reviews found that flexible sigmoidoscopy reduced CRC in adults aged 55 to 74 years after a median follow-up of 11 years (CRC mortality RR, 0.72 [CI, 0.65 to 0.81]; NNS, 850 [CI, 673 to 1205] based on CTFPHC [11]; incidence rate ratio, 0.73 [CI, 0.66 to 0.82] based on USPSTF [10]). Three of the included trials offered 1-time flexible sigmoidoscopy, and the fourth offered a second screen at 3 to 5 years. Adherence ranged from 58% to 84%, and rates of diagnostic colonoscopy ranged from 5% to 33% because of differences in referral criteria (10). The USPSTF found that the CRC mortality benefit was limited to distal CRC (incidence rate ratio, 0.63 [CI, 0.49 to 0.84]) (10). Flexible sigmoidoscopy reduced the incidence of late-stage cancer (RR, 0.75 [CI, 0.66 to 0.86]; absolute risk reduction, 1.7 cases [CI, 1.0 to 2.4 cases] per 1000 persons screened) (4). It did not lead to a statistically significant reduction in all-cause mortality compared with no screening. One included trial found lower CRC mortality with flexible sigmoidoscopy plus a single FIT test than with flexible sigmoidoscopy alone (10).
The CTFPHC review found that the absolute effect on CRC mortality of flexible sigmoidoscopy was greater in adults aged 60 years or older than in those younger than 60 years (absolute reductions in CRC mortality were 5 deaths per 10 000 persons screened for ages 45 to 59 years and 29 deaths per 10 000 persons screened for ages 60 to 80 years) (4). Evidence stratified by age showed a CRC-specific mortality benefit for ages 65 to 74 years but not for ages 55 to 64 years (4). No evidence suggests that the relative benefits of flexible sigmoidoscopy are lower in patients younger than 65 years, but because CRC incidence increases with age, the absolute benefit also increases with age and will be lower in younger adults. Adherence to flexible sigmoidoscopy in trials ranged from 58% to 84% (10).
Harms
Flexible sigmoidoscopy is an invasive procedure that requires some bowel preparation and time spent attending an outpatient examination. Major bleeding that required hospitalization occurred in 0.09 patients (CI, 0.04 to 0.15 patients) per 1000 and minor bleeding occurred in 0.36 patients (CI, 0.16 to 0.56 patients) per 1000 (11). The USPSTF results showed that risk for major bleeding was 2 events (CI, 0.7 to 4.41 events) in 10 000 procedures (10). Flexible sigmoidoscopy may require a follow-up diagnostic or therapeutic colonoscopy.
Diagnostic Accuracy
No data are available.
Colonoscopy
Benefits
No RCT data were available to determine the clinical benefits, including effects on CRC incidence or CRC-related and all-cause mortality. Indirect evidence from RCTs of flexible sigmoidoscopy, which allows direct visualization of the descending colon, suggests a CRC-specific mortality benefit. Modeling studies used in the USPSTF guideline also suggest such a benefit.
Harms
Colonoscopy is an invasive procedure that requires bowel preparation and time spent attending an outpatient examination, and it is typically done using moderate sedation. The CTFPHC found 0.49 perforations (CI, 0.36 to 0.62 perforations) per 1000 colonoscopies (11), and the USPSTF-estimated rate was similar at 4 perforations (CI, 2 to 5 perforations) in 10 000 procedures (10). Follow-up colonoscopy after positive findings on flexible sigmoidoscopy screening resulted in 14 perforations (CI, 9 to 26 perforations) per 10 000 procedures and 34 major bleeding events (CI, 5 to 63 events) per 10 000 procedures (10). The risk for major bleeding requiring hospitalization was estimated as 1.08 events (CI, 0.85 to 1.32 events) per 1000 procedures by the CTFPHC (11) and 8.21 events (CI, 4.98 to 13.51 events) per 10 000 procedures by the USPSTF (10). The USPSTF notes that cardiopulmonary adverse events may occur with colonoscopy if sedation is used but that the frequency is unknown (5).
Diagnostic Accuracy
Diagnostic accuracy studies showed that screening colonoscopy had a sensitivity ranging from 89% to 98% for detecting adenomas measuring at least 10 mm and 75% to 93% for detecting adenomas measuring at least 6 mm (10).
CT Colonography
Benefits
No RCTs assessed clinical benefits, including reduction in CRC-related or all-cause mortality.
Harms
Computed tomography colonography generally requires bowel preparation and time to attend a radiologic evaluation. Any positive finding requires additional follow-up by colonoscopy. Few studies evaluated or reported harms. Studies did not show major adverse events, although CT colonography commonly resulted in extracolonic findings (27% to 69% of examinations), which led to diagnostic follow-ups (5% to 37%) and treatments (3%). The estimated radiation dose for screening ranged from 1 to 7 mSv across studies (10).
Diagnostic Accuracy
None of the studies were statistically powered to estimate the performance of CT colonography in detecting CRC. Instead, studies provide information on diagnostic accuracy for adenoma detection. For detecting adenomas measuring at least 10 mm, the sensitivity of screening CT colonography ranges from 67% to 94% and specificity from 86% to 98% (5). For detecting adenomas measuring at least 6 mm, sensitivity ranges from 73% to 98% and specificity from 89% to 91% (10).
Overdiagnosis and Overtreatment Due to Direct Visualization Screening Tests
No data are available.
COMPARISON OF SCREENING STRATEGIES
Evidence from RCTs directly comparing screening methods was lacking. Most information used to inform guideline recommendations about screening strategies comes from modeling based on findings from RCTs of gFOBT and flexible sigmoidoscopy, diagnostic accuracy studies of all screening methods, and observational studies of clinical benefits and harms. The USPSTF (but not the CTFPHC) relied heavily on modeling studies to inform its screening strategy recommendations. A survey of 1047 primary care patients in Canada included by the CTFPHC found that respondents preferred screening tests that were noninvasive, required no preparation, and involved no pain (14). Another included survey found that avoiding test side effects; minimizing false-positive results; and the combined priority of screening frequency, test preparation, and test procedure were important (15). Evidence from models conducted for the USPSTF generally supports the use of any of several screening methods, including gFOBT, FIT, FIT plus sDNA, colonoscopy, and CT colonography. However, output from modeling studies has limitations. Many models assume 100% adherence to a screening strategy; such models would overestimate the benefits and underestimate the harms seen in clinical practice. Models are most useful when they are based on strong primary evidence, ideally from RCTs, for the main outcome of interest (in this case, overall and disease-specific mortality) (16). For many CRC screening strategies, RCTs have not been done on screening method; time to initiate and end screening; frequency of screening; or effects according to patient characteristics, including sex, comorbid conditions, and race/ethnicity. A more recent review reported findings largely consistent with the USPSTF and CTFPHC reports: Although evidence is sufficient for each individual test (gFOBT, FIT, sigmoidoscopy, and colonoscopy), comparative evidence is lacking (17).
TIME OF INITIATION AND FREQUENCY OF SCREENING
Data from RCTs were limited regarding age to initiate screening and frequency of screening. The CTFPHC guideline indicates that the absolute reduction in CRC mortality due to screening is much smaller in adults aged younger than 60 years than in those aged 60 to 80 years, primarily because of much lower CRC incidence. The CTFPHC recommends screening with FOBT every 2 years on the basis of the interval used in most of the gFOBT RCTs. In 1 U.S. study that compared gFOBT screening every year or every 2 years versus no screening, both intervals reduced CRC mortality among persons aged 60 to 69 years, and evidence did not indicate that effectiveness varied by annual versus biennial screening (18). The CTFPHC also recommends screening with flexible sigmoidoscopy every 10 years because 3 of the 4 screening trials examined 1-time screening and found a reduction in CRC mortality and incidence through more than 10 years of follow-up. The fourth trial found that the magnitude of mortality benefit for screening at baseline followed by 1 screening at 3 or 5 years was similar to that of 1-time screening. The modeling study to support the USPSTF guideline found similar benefits and harms among the following screening strategies for adults aged 50 to 75 years: annual FIT, sigmoidoscopy every 10 years plus annual FIT, colonoscopy every 10 years, and CT colonography every 5 years (19). The CTFPHC provides a weak recommendation for screening adults aged 50 to 59 years because of the lower absolute benefit in this age group, recognizing that the desirable effects probably outweigh the undesirable effects but that appreciable uncertainty exists.
DISCONTINUATION OF SCREENING
Little information from RCTs exists on when to discontinue screening, and most studies did not include persons older than 75 years. These decisions require balancing increased risk for CRC incidence and mortality with advancing age and the increased screening harms, screening burden, and competing causes of death that come with advanced age and comorbid conditions. Benefits from continued screening are likely to be lower among persons who have had multiple rounds of screening with negative results than among unscreened individuals. Modeling studies for the USPSTF indicate that for adults aged 75 years and older, especially for those who have had prior screening, mortality benefits are at best small and incremental but harms are increased. Natural history and modeling studies suggest that the time to progress from an adenomatous polyp to cancer is about 10 years or longer and that the time to prevent 1 CRC death per 1000 persons screened is about 10 years (20). Furthermore, the harms and burden of direct visualization screening methods (especially colonoscopy) increase in adults older than 75 years and in patients with serious comorbid conditions (21, 22).
MULTIPLE CHRONIC CONDITIONS
Serious comorbid conditions include chronic obstructive pulmonary disease, diabetes, heart failure, moderate to severe liver disease, chronic hepatitis, advanced chronic kidney disease or end-stage kidney disease, and dementia. Persons with limited life expectancy (<10 years) would likely not benefit from CRC screening because it takes at least 10 years for benefits to accrue (20, 23). In addition, harms include having unnecessary, burdensome, potentially harmful, and costly screening tests.
SUBPOPULATIONS
Few trials have assessed CRC screening methods according to sex or race. Long-term follow-up data suggest that the benefit of screening in women may be limited to those older than 60 years. Incidence and mortality of CRC are slightly higher in men than women, higher in black than white persons, and lower in Asians and Pacific Islanders than white persons (24). The difference in incidence rate between men and women has been attributed to lifestyle variables, which highlights the importance of reducing risk by modifying these factors (6).
COSTS OF SCREENING INTERVENTIONS
Table 3 summarizes costs for the various screening methods in the United States. The CTFPHC looked at resources as a factor in developing their recommendations, whereas the USPSTF did not take costs into account. Colonoscopies for follow-up of a positive result on an alternative screening test and for removal of polyps found at screening (that is, follow-up colonoscopies) are often considered diagnostic rather than screening procedures, and thus the patient may be billed for procedure-related fees.
Table 3.
Summary of Costs Associated With CRC Screening Tests in the United States
| Screening Strategy | Unit Cost, $ | Frequency | 10-Year Cost, $* |
|---|---|---|---|
| gFOBT† | 6–28 | Annual | 60–280 |
| gFOBT† | 6–28 | Every 2 y | 30–140 |
| FIT‡ | 20 | Annual | 200 |
| FIT‡ | 20 | Every 2 y | 100 |
| Sigmoidoscopy† without biopsy | 715–3384 | Every 5 y | 1430–6768 |
| Colonoscopy (screening)† | 911–6946+ | Every 10 y | 911–6946+ |
| CT colonography (no contrast)† | 337–1538+ | Every 5 y | 674–3076+ |
| sDNA‡ | 509 | Every 3 y | 1527 (3 screenings) |
CRC = colorectal cancer; CT = computed tomography; FIT = fecal immunochemical test; gFOBT = guaiac-based fecal occult blood test; sDNA = stool DNA panel.
For the screening test only; does not account for follow-up testing after a positive initial result.
From reference 25, for ZIP code 19106. Bluebook reports that its costs are paid insurance claims for the locality. Minimum and maximum payments are provided. For some maximum values, Bluebook adds a “+”, suggesting that some payments exceed the maximum value provided.
From reference 26.
Screening more frequently than recommended results in little additional reduction in cancer-related deaths but large avoidable health care costs, more false-positive results, and the harms and burden of the screening procedure. As with other cancer screening tests, more frequent and more sensitive screening strategies can lead to overdiagnosis and overtreatment, although the extent to which this exists in CRC is unknown.
AREAS OF INSUFFICIENT EVIDENCE
Head-to-head trials assessing the comparative effectiveness and harms of screening methods would be valuable to help clinicians and patients understand the relative benefits and harms of these tests. Results from 2 ongoing RCTs comparing colonoscopy versus stool-based examinations are anticipated (10). More research beyond diagnostic accuracy assessment is needed to evaluate the clinical benefits and harms of FIT plus sDNA and especially CT colonography. Until then, other screening methods have stronger direct and indirect evidence of clinical effectiveness in reducing CRC mortality. Racial and ethnic disparities, as well as sex differences related to CRC screening and mortality, have not been sufficiently studied.
Cancer overdiagnosis is defined as the detection of histologically confirmed cancer through screening that would not otherwise have manifested clinically or been diagnosed in a person’s lifetime (27). Although data exist for overdiagnosis and overtreatment of other types of screen-detected cancer and their precursors, such data do not exist for screen-detected colon polyps and CRC. However, natural history studies indicate, and most modeling studies assume, that many polyps and some cancer cases progress slowly. These polyps and cancer cases may never cause symptoms in an individual’s lifetime, especially in adults with limited life expectancy due to age or comorbid conditions. Because almost all polyps and cancer are treated, there is risk for overtreatment (28). Detection and treatment increase with more intensive screening strategies. However, risk for underdetection and undertreatment also exists because CRC remains a leading cause of cancer-related morbidity and mortality, and many persons who may be eligible and interested do not have screening.
Few data are available on the harms associated with the preparation required for colonoscopy—particularly in older, sicker adults—including inconvenience; burden; and the harms and costs of conscious sedation, which is used in most colonoscopies in the United States. For all tests, psychological harms, including distress and worry, have not been reliably reported.
Identifying and optimizing the balance of benefits and harms to achieve high-value care for many persons are important. Although intense research and efforts have focused on increasing CRC screening and enhancing adherence, research is needed to understand the implications for well-informed individuals who decline recommended strategies for CRC screening (for example, rather than starting at age 50 years they want to begin at a later age, or they want to increase intervals between flexible sigmoidoscopy or colonoscopy tests from 10 to 15 years). Screening adherence is problematic: Findings from RCTs indicate that adherence ranges from 58% to 84% and rates of diagnostic colonoscopy from 5% to 33% because of differences in referral criteria. Information is insufficient regarding patient preferences about balancing the risks and benefits of CRC screening strategies.
ACPGUIDANCE STATEMENTS
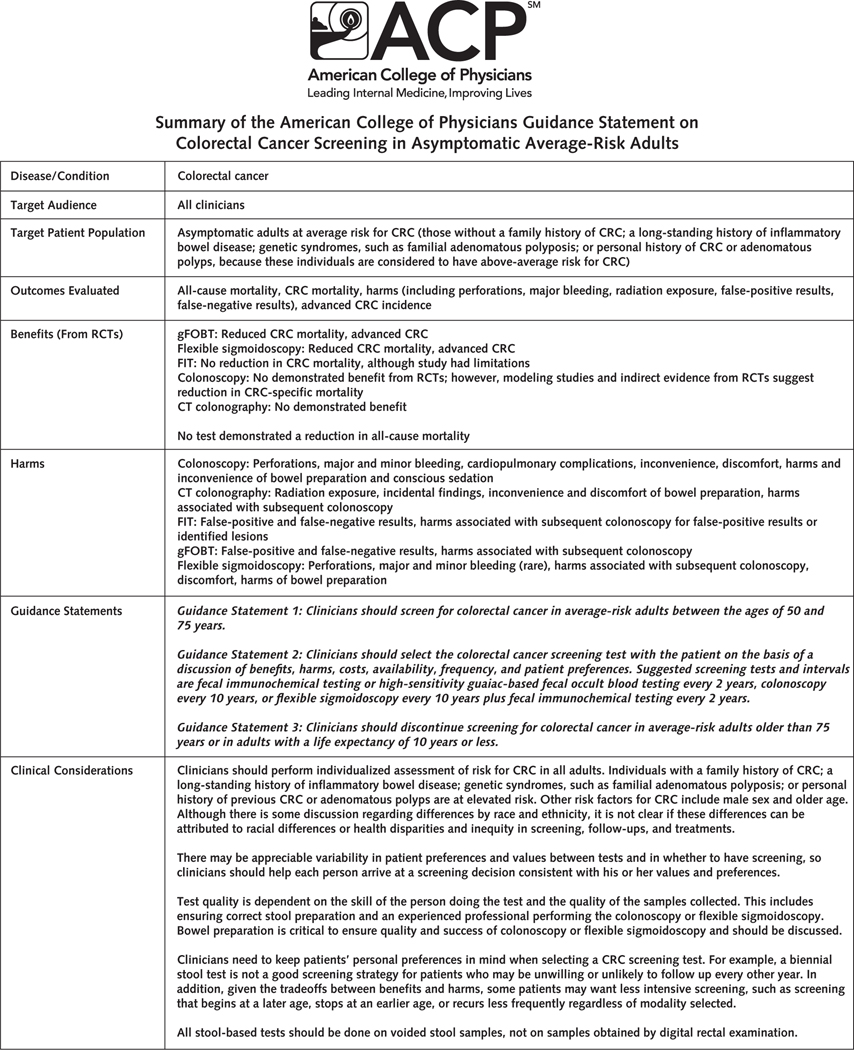
The Figure summarizes the guidance statements, clinical considerations, and talking points for patients.
Figure.
Summary of the ACP guidance statement on CRC screening in asymptomatic average-risk adults.
ACP = American College of Physicians; CRC = colorectal cancer; CT = computed tomography; FIT = fecal immunochemical test; gFOBT = guaiac-based fecal occult blood test; RCT = randomized controlled trial.
Guidance Statement 1: Clinicians should screen for colorectal cancer in average-risk adults between the ages of 50 and 75 years.
Current evidence suggests that regular screening for CRC in average-risk adults reduces CRC-specific mortality but not all-cause mortality (Supplement Table). Although the median age for CRC diagnosis is 67 years and persons aged 65 to 75 years derive the most direct benefit from screening for CRC, screening in adults aged 50 to 75 years also has benefit. Both the CTFPHC and USPSTF guidelines recommend screening persons aged 50 to 75 years. The absolute risk reduction in CRC mortality from screening increases with age, from 0.037% in those younger than 60 years (45 to 59 years) to 0.20% in those aged 60 years or older (60 to 80 years) for biennial gFOBT, and from 0.05% in those younger than 60 years (45 to 59 years) to 0.29% in those aged 60 years or older (60 to 80 years) for flexible sigmoidoscopy (4). The CTFPHC guideline showed that the net benefit of screening in adults aged 50 to 59 years is small, and this finding may influence some individuals’ decision whether to have screening before age 60 years. Patient values and preferences may influence a person’s decision to not undergo screening regardless of age.
Guidance Statement 2: Clinicians should select the colorectal cancer screening test with the patient on the basis of a discussion of benefits, harms, costs, availability, frequency, and patient preferences. Suggested screening tests and intervals are fecal immunochemical testing or high-sensitivity guaiac-based fecal occult blood testing every 2 years, colonoscopy every 10 years, or flexible sigmoidoscopy every 10 years plus fecal immunochemical testing every 2 years.
No evidence from the assessed guidelines and their evidence reviews directly compares various CRC screening interventions. All screening tests are associated with potential benefits as well as harms. Clinical decisions need to be individualized using patient clinical characteristics, patient preferences, and screening test frequency and availability. Because many eligible patients have never been screened and some may not adhere to recommendations about subsequent screening or follow-up of positive findings on screening tests (such as colonoscopy after a positive result on a stool-based screening test), patient informed decision making and adherence are important factors in selection of a CRC screening test. Discussion should include such topics as suggested frequency, bowel preparation, anesthesia, transportation to and from the examination site, time commitments, and the necessary steps if a test result is positive.
Suggested CRC screening tests and intervals are FIT or HSgFOBT every 2 years, colonoscopy every 10 years, or flexible sigmoidoscopy every 10 years plus FIT every 2 years (Figure). The CTFPHC and USPSTF both recommend FIT, gFOBT, and flexible sigmoidoscopy. The CTFPHC did not recommend colonoscopy as a screening test in part because of a lack of access in Canada. We include colonoscopy as an option for screening, as does the USPSTF, because indirect evidence (not from RCTs) suggests an association between reduced CRC mortality and colonoscopy compared with other options (5). Furthermore, access is less of an issue in the United States, and direct visualization rules out many false-negative results. Colonoscopy is also necessary to confirm any positive findings on stool-based tests.
Fecal immunochemical testing is associated with better sensitivity and specificity than gFOBT, and both tests need to be repeated. However, test accuracy varies by type of FIT. The CTFPHC recommends a 2-year interval because most RCT data are from trials that examined biennial screening with gFOBT. Furthermore, a single large U.S. study found no significant difference between annual and biennial gFOBT in overall or cumulative CRC mortality through 30 years of follow-up (18). Thus, screening biennially rather than annually with gFOBT (and likely other stool-based screening strategies) would result in similar reductions in CRC mortality while decreasing the harms and burden of screening. Only HSgFOBT is recommended because standard gFOBT is associated with lower diagnostic accuracy. Positive findings on stool-based tests should be confirmed with direct visualization tests; thus, stool-based testing is sometimes a 2-step process.
The combination of flexible sigmoidoscopy with FIT is more beneficial than flexible sigmoidoscopy alone as a screening test (5, 19). Although the USPSTF recommends flexible sigmoidoscopy every 10 years plus FIT every year on the basis of modeling studies, most RCT data were from trials of biennial screening with gFOBT (11). In addition, the CRC mortality reduction did not statistically significantly differ between annual and biennial gFOBT screening after more than 30 years of follow-up (18). Therefore, we suggest FIT every 2 years in combination with flexible sigmoidoscopy every 10 years.
Effectiveness of colonoscopy has not been evaluated in RCTs, but it is associated with the best sensitivity (89% to 98%) for adenomas measuring at least 10 mm and has been widely used for CRC screening on the basis of observational and modeling data. In addition, CRC mortality benefits associated with flexible sigmoidoscopy can be considered strong indirect evidence for colonoscopy benefits because both screening tests use direct visualization. Screening colonoscopy is currently recommended every 10 years (if results are normal). Modeling data suggest that screening every 15 years, rather than every 10 years, preserves most of the benefit in CRC mortality and life-years gained while reducing colonoscopy harms, burden, and costs. Flexible sigmoidoscopy and colonoscopy are expensive and invasive, require preparation, and are associated with harms (major bleeding and perforation). Flexible sigmoidoscopy is associated with lower event rates for harms than colonoscopy but does not evaluate the entire colon. Both require attending an endoscopy appointment, and colonoscopy is typically done using moderate (conscious) sedation that adds costs and requires another person to drive the patient after the procedure.
No RCTs have been done to assess the effects on morbidity and mortality of FIT plus sDNA or CT colonography, and indirect evidence is largely derived from a limited number of diagnostic test accuracy and modeling studies. Both tests are limited by reduced specificity and higher false-positive rates than other screening options with higher evidence of net clinical benefit. Although FIT plus sDNA is associated with higher sensitivity than FIT (92% for CRC and 42% for adenomas), it has lower specificity (84% for CRC and 87% for advanced adenomas) and increased harms associated with colonoscopy follow-ups due to more false-positive results. Uncertainty exists in follow-up of patients with a negative colonoscopy finding after positive results on an FIT plus sDNA test. Because a positive sDNA result may also be caused by noncolonic aerodigestive or supracolonic neoplasms, additional evaluation may be necessary. The net benefit of additional evaluation for suspected noncolorectal malignant tumors in the gastrointestinal system is unknown, but this is not the intent of CRC screening. Also, FIT plus sDNA is more expensive than FIT.
Computed tomography colonography is associated with extracolonic findings (40% to 70% of screening examinations), many of which are not clinically important but lead to additional evaluation (10). These incidental findings can lead to overtreatment: 5% to 37% of findings require diagnostic follow-up, and only 3% of those patients will require treatment (5). Screening intervals, based on modeling studies, are more frequent than for colonoscopy (5, 6). In addition, suspected positive findings on CT colonography still require follow-up colonoscopy, thereby reducing advantages of CT colonography as a “less invasive” direct visualization test.
Guidance Statement 3: Clinicians should discontinue screening for colorectal cancer in average-risk adults older than 75 years or in adults with a life expectancy of 10 years or less.
Risk for harm from screening, especially serious harm, increases with age (10). When to discontinue screening is important for older adults because the harms of screening tests outweigh the benefits in most adults aged 75 years or older. Persons with no history of CRC screening may benefit from screening after age 75 years, whereas those who have received regular screening with negative results may not. Screening in average-risk individuals may pick up aberrant findings that warrant further surveillance; this guidance statement covers screening only, not surveillance or diagnosis.
It is important to consider the time from screening to potential CRC mortality benefit, risk for other causes of death, and harms of screening tests. Based on pooled results of gFOBT RCTs, the average time to prevent 1 death from CRC for 1000 patients screened was 10.3 years (20). Modeling studies done for the USPSTF show that any incremental benefit is at most small and unlikely to outweigh harms, especially among those who have had prior screening (5, 6).
Accurate prediction of individual life expectancy is difficult. However, among 75-year-old men and women in the United States, average life expectancy is 9.9 and 12 years, respectively. Among men and women aged 70 years with serious comorbid conditions, life expectancy is 8.9 and 10.8 years, respectively (23). Therefore, most persons aged 75 years or older, as well as most adults who are younger than 75 years but have serious comorbid conditions (such as chronic renal failure), are unlikely to benefit from screening but would undergo unnecessary, burdensome, potentially harmful, and costly screening tests.
Supplementary Material
Acknowledgment:
The CGC thanks the following members of the ACP CGC Public Panel for their review and comments on the paper from a nonclinician, public perspective: Cynthia Appley, Larry Curley, Ray Haeme, Billy Oglesby, James Pantelas, Missy Carson Smith, and Lelis Vernon.
Financial Support: Financial support for the development of this guidance statement comes exclusively from the ACP operating budget.
Note: Guidance statements are “guides” only and may not apply to all patients and all clinical situations. Thus, they are not intended to override clinicians’ judgment. All ACP guidance statements are considered automatically withdrawn or invalid 5 years after publication, or once an update has been issued.
APPENDIX: SUMMARY OF EVALUATED GUIDELINES USING THE AGREEII INSTRUMENT
American College of Radiology Recommendations (2018)
“For average-risk individuals, CT colonography is usually appropriate for colorectal cancer screening” (3).
American Cancer Society Recommendations (2018)
“The ACS [American Cancer Society] recommends that adults aged 45 years and older with an average risk of CRC undergo regular screening with either a high-sensitivity stool-based test or a structural (visual) examination, depending on patient preference and test availability. As a part of the screening process, all positive results on noncolonoscopy screening tests should be followed up with timely colonoscopy. The recommendation to begin screening at age 45 years is a qualified recommendation. The recommendation for regular screening in adults aged 50 years and older is a strong recommendation” (6).
“The ACS recommends that average-risk adults in good health with a life expectancy of greater than 10 [years] continue CRC screening through the age of 75 [years] (qualified recommendation)” (6).
“The ACS recommends that clinicians individualize CRC screening decisions for individuals aged 76 through 85 [years] based on patient preferences, life expectancy, health status, and prior screening history (qualified recommendation)” (6).
“The ACS recommends that clinicians discourage individuals over age 85 [years] from continuing CRC screening (qualified recommendation)” (6).
CTFPHC Recommendations (2016)
“We recommend screening adults aged 60 to 74 years for colorectal cancer with FOBT (either gFOBT or FIT) every two years or flexible sigmoidoscopy every 10 years. (Strong recommendation; moderate-quality evidence)” (4).
“We recommend screening adults aged 50 to 59 years for colorectal cancer with FOBT (either gFOBT or FIT) every two years or flexible sigmoidoscopy every 10 years. (Weak recommendation; moderate-quality evidence)” (4).
“We recommend not screening adults aged 75 years and older for colorectal cancer. (Weak recommendation; low-quality evidence)” (4).
“We recommend not using colonoscopy as a screening test for colorectal cancer. (Weak recommendation; low-quality evidence)” (4).
U.S. Multi-Society Task Force on Colorectal Cancer Recommendations (2017)
“We recommend that clinicians offer CRC screening beginning at age 50 (strong recommendation, high-quality evidence)” (8).
“We suggest that sequential offers of screening tests, offering multiple screening options, and risk-stratified screening are all reasonable approaches to offering screening (weak recommendation, low-quality evidence)” (8).
“We recommend CT colonography every 5 years or FIT-fecal DNA every 3 years (strong recommendation, low-quality evidence) or flexible sigmoidoscopy every 5 to 10 years (strong recommendation, high-quality evidence) in patients who refuse colonoscopy and FIT” (8).
“We suggest that capsule colonoscopy (if available) is an appropriate screening test when patients decline colonoscopy, FIT, FIT-fecal DNA, CT colonography, and flexible sigmoidoscopy (weak recommendation, low-quality evidence)” (8).
“We suggest against Septin9 for CRC screening (weak recommendation, low-quality evidence)” (8).
“We recommend colonoscopy every 10 years or annual FIT as first-tier options for screening the average-risk persons for colorectal neoplasia (strong recommendation; moderate-quality evidence)” (8).
“We recommend that physicians performing screening colonoscopy measure quality, including the adenoma detection rate (strong recommendation, high-quality evidence)” (8).
“We recommend that physicians performing FIT monitor quality (strong recommendation, low-quality evidence)” (8).
“We recommend that screening begin in nonAfrican American average-risk persons at age 50 years (strong recommendation; moderate-quality evidence)” (8).
“We suggest that screening begin in African Americans at age 45 years (weak recommendation, very-low-quality evidence)” (8).
“We suggest that persons who are up to date with screening and have negative prior screening tests, particularly colonoscopy, consider stopping screening at age 75 years or when life expectancy is less than 10 years (weak recommendation, low-quality evidence)”
(8).
“We suggest that persons without prior screening should be considered for screening up to age 85, depending on consideration of their age and comorbidities (weak recommendation, low-quality evidence)” (8).
Scottish Intercollegiate Guidelines Network Recommendations (2016)
“Population screening for colorectal cancer should continue in the Scottish population using quantitative FIT set at a faecal haemoglobin concentration cut-off that is appropriate for investigative capacity, but no lower than the analytical sensitivity of the FOBT guaiac test” (7) (A recommendation).
USPSTF Recommendations (2016)
“The USPSTF recommends screening for colorectal cancer starting at age 50 years and continuing until age 75 years (A recommendation). … The risks and benefits of different screening methods vary” (5).
“The decision to screen for colorectal cancer in adults aged 76 to 85 years should be an individual one, taking into account the patient’s overall health and prior screening history (C recommendation)” (5). “Adults in this age group who have never been screened for colorectal cancer are more likely to benefit” (5).
“Screening would be most appropriate among adults who (1) are healthy enough to undergo treatment if colorectal cancer is detected and (2) do not have comorbid conditions that would substantially limit their life expectancy” (5).
Footnotes
Disclaimer: The authors of this article are responsible for its contents, including any clinical or treatment recommendations. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-0642. All financial and intellectual disclosures of interest were declared, and potential conflicts were discussed and managed according to CGC policy (29). Two committee members were recused from voting on recommendations because of moderate-level conflicts of interest: Dr. Lin (authored USPSTF systematic review) and Dr. Vijan (authored recent relevant publications). A record of disclosures of interest and management of conflicts of interest is kept for each CGC meeting and conference call and can be viewed at www.acponline.org/clinical_information/guidelines/guidelines/conflicts_cgc.htm.
Contributor Information
Amir Qaseem, American College of Physicians, Philadelphia, Pennsylvania.
Carolyn J. Crandall, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, California.
Reem A. Mustafa, University of Kansas Medical Center, Kansas City, Kansas.
Lauri A. Hicks, Centers for Disease Control and Prevention, Atlanta, Georgia.
Timothy J. Wilt, Minneapolis Veterans Affairs Medical Center, Minneapolis, Minnesota..
References
- 1.Bonnington SN, Rutter MD. Surveillance of colonic polyps: are we getting it right? [Editorial]. World J Gastroenterol. 2016;22:1925–34. [PMID: 26877600] doi: 10.3748/wjg.v22.i6.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qaseem A, Kansagara D, Lin JS, et al. ; Clinical Guidelines Committee of the American College of Physicians. The development of clinical guidelines and guidance statements by the Clinical Guidelines Committee of the American College of Physicians: update of methods. Ann Intern Med. 2019;170:863–70. [PMID: 31181568] doi: 10.7326/M18-3290 [DOI] [PubMed] [Google Scholar]
- 3.Moreno C, Kim DH, Bartel TB, et al. ; Expert Panel on Gastrointestinal Imaging. ACR appropriateness criteria colorectal cancer screening. J Am Coll Radiol. 2018;15:S56–68. [PMID: 29724427] doi: 10.1016/j.jacr.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Canadian Task Force on Preventive Health Care. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188:340–8. [PMID: 26903355] doi: 10.1503/cmaj.151125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315: 2564–75. [PMID: 27304597] doi: 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 6.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–81. [PMID: 29846947] doi: 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 7.Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of colorectal cancer. SIGN publication no. 126. Edinburgh: SIGN; 2011. Revised August 2016. Accessed at www.sign.ac.uk/assets/sign126.pdf on 31 July 2018. [Google Scholar]
- 8.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112:1016–30. [PMID: 28555630] doi: 10.1038/ajg.2017.174 [DOI] [PubMed] [Google Scholar]
- 9.Brouwers MC, Kho ME, Browman GP, et al. ; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–42. [PMID: 20603348] doi: 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:2576–94. [PMID: 27305422] doi: 10.1001/jama.2016.3332 [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick-Lewis D, Usman A, Warren R, et al. Screening for Colorectal Cancer. Ottawa: Canadian Task Force on Preventive Health Care; 2014. Accessed at http://canadiantaskforce.ca/wp-content/uploads/2016/03/crc-screeningfinal031216.pdf on 30 May 2019. [Google Scholar]
- 12.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 135. AHRQ publication 14–05203-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 13.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014; 370:1287–97. [PMID: 24645800] doi: 10.1056/NEJMoa1311194 [DOI] [PubMed] [Google Scholar]
- 14.Marshall DA, Johnson FR, Phillips KA, et al. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health. 2007;10:415–30. [PMID: 17888107] [DOI] [PubMed] [Google Scholar]
- 15.Dolan JG, Boohaker E, Allison J, et al. Patients’ preferences and priorities regarding colorectal cancer screening. Med Decis Making. 2013;33:59–70. [PMID: 22895558] doi: 10.1177/0272989X12453502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habbema JD, Wilt TJ, Etzioni R, et al. Models in the development of clinical practice guidelines. Ann Intern Med. 2014;161:812–8. [PMID: 25437409] doi: 10.7326/M14-0845 [DOI] [PubMed] [Google Scholar]
- 17.Lauby-Secretan B, Vilahur N, Bianchini F, et al. ; International Agency for Research on Cancer Handbook Working Group. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378:1734–40. [PMID: 29580179] doi: 10.1056/NEJMsr1714643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–14. [PMID: 24047060] doi: 10.1056/NEJMoa1300720 [DOI] [PubMed] [Google Scholar]
- 19.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016; 315:2595–609. [PMID: 27305518] doi: 10.1001/jama.2016.6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, et al. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. [PMID: 23299842] doi: 10.1136/bmj.e8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Albéniz X, Hsu J, Bretthauer M, et al. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70 to 79 years: a prospective observational study. Ann Intern Med. 2017;166:18–26. [PMID: 27669524] doi: 10.7326/M16-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran AH, Man Ngor EW, Wu BU. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA Intern Med. 2014;174:1675–82. [PMID: 25111954] doi: 10.1001/jamainternmed.2014.3746 [DOI] [PubMed] [Google Scholar]
- 23.Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013;159:667–76. [PMID: 24247672] doi: 10.7326/0003-4819-159-10-201311190-00005 [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. SEER Cancer Stat Facts: Colorectal Cancer. Accessed at https://seer.cancer.gov/statfacts/html/colorect.html on 8 August 2018.
- 25.Healthcare Bluebook. Accessed at www.healthcarebluebook.com/ui/consumerfront on 21 August 2018. [Google Scholar]
- 26.Medicare 2018 Clinical Laboratory Fee Schedule (Q3). Accessed at www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/18CLABQ3.zip on 22 August 2018.
- 27.Davies L, Petitti DB, Martin L, et al. Defining, estimating, and communicating overdiagnosis in cancer screening. Ann Intern Med. 2018;169:36–43. [PMID: 29946705] doi: 10.7326/M18-0694 [DOI] [PubMed] [Google Scholar]
- 28.Kalager M, Wieszczy P, Lansdorp-Vogelaar I, et al. Overdiagnosis in colorectal cancer screening: time to acknowledge a blind spot [Editorial]. Gastroenterology. 2018;155:592–5. [PMID: 30076834] doi: 10.1053/j.gastro.2018.07.037 [DOI] [PubMed] [Google Scholar]
- 29.Qaseem A, Wilt TJ; Clinical Guidelines Committee of the American College of Physicians. Disclosure of interests and management of conflicts of interest in clinical guidelines and guidance statements: methods from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2019;171:354–61. [PMID: 31426089] doi: 10.7326/M18-3279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.