Abstract
Implantable neuroelectronic interfaces have enabled breakthrough advances in the clinical diagnosis and treatment of neurological disorders, as well as in fundamental studies of brain function, behavior, and disease. Intracranial electroencephalography (EEG) mapping with stereo-EEG (sEEG) depth electrodes is routinely adopted for precise epilepsy diagnostics and surgical treatment, while deep brain stimulation has become the standard of care for managing movement disorders. Intracortical microelectrode arrays for high-fidelity recordings of neural spiking activity have led to impressive demonstrations of the power of brain-machine interfaces for motor and sensory functional recovery. Yet, despite the rapid pace of technology development, the issue of establishing a safe, long-term, stable, and functional interface between neuroelectronic devices and the host brain tissue still remains largely unresolved. A body of work spanning at least the last 15 years suggests that safe, chronic integration between invasive electrodes and the brain requires a close match between the mechanical properties of man-made components and the neural tissue. In other words, the next generation of invasive electrodes should be soft and compliant, without sacrificing biological and chemical stability. Soft neuroelectronic interfaces, however, pose a new and significant surgical challenge: bending and buckling during implantation that can preclude accurate and safe device placement. In this topical review, we describe the next generation of soft electrodes and the surgical implantation methods for safe and precise insertion into brain structures. We provide an overview of the most recent innovations in the field of insertion strategies for flexible neural electrodes such as dissolvable or biodegradable carriers, microactuators, biologically-inspired support structures, and electromagnetic drives. In our analysis, we also highlight approaches developed in different fields, such as robotic surgery, which could be potentially adapted and translated to the insertion of flexible neural probes.
Keywords: brain-machine interfaces, intracortical microelectrodes, soft neural electrodes, neurosurgery, neuroelectronic interfaces, brain tissue mechanics, insertion methods
1. Introduction
According to the World Health Organization (WHO), neurological disorders such as epilepsy, Alzheimer’s disease (AD), Parkinsons disease (PD), and stroke are major threats to public health, accounting for 12% of all deaths globally and 6.3% of the global burden of disease [1]. For comparison, HIV/AIDS and malignant neoplasms each constitute 5% of the total global disease burden. Furthermore, neurological disorders alone are responsible for the loss of 92 M years of healthy life (DALYs: disability-adjusted life years) which are projected to grow to 103 M by 2030 [1]. Notably, these figures do not include disorders of the sensory nervous system such as vision and hearing loss, which are responsible for nearly 20% of all neurological impairments [2, 3].
Advances in neural engineering are expanding the treatment options for people affected by neurological diseases. Deep brain stimulation (DBS) therapy in combination with pharmacological therapies has become the standard of care for PD [4] and has recently been approved for a number of other neurological conditions, including epilepsy [5, 6], chronic pain [7], and depression [8]. Neuroelectronic interfaces also offer the unprecedented opportunity to replace missing neural circuits and restore lost functions: vision can be partially restored to the blind through stimulation in the visual cortex [9], and in patients with amputation or limb paralysis from spinal cord injury, neuroprostheses offer promise for restoring tactile sensation, even in prosthetic limbs, through stimulation of intact neural circuitry in the somatosensory cortex [10]. Furthermore, motor intentions can be decoded from the firing patterns of individual neurons during thought or behavior and translated into movement commands for a robotic end-effector [11,12] to perform acitivities of daily living, as well as to control an individual’s own limb via synergistic functional electrical stimulation [13, 14]. While these demonstrations are an excellent proof-of-concept of brain-machine interface (BMI) technology capabilities, they have been thus far implemented only in the short-term and in controlled laboratory environments. In other words, a life-long, ‘take-home’ invasive BMI does not yet exist. Both the software and hardware aspects of BMI are progressing quickly, though the challenge of seamlessly and safely integrating electronic hardware with biological structures remains a critical and largely unsolved problem [15].
In this review, we first provide an overview of the main issues surrounding conventional neuroelectronic probes in terms of biocompatibility, biostability, and long-term performance. Next, we describe new materials and design approaches that are enabling safer and more reliable integration with the nervous system. Then, we will focus on the methods utilized to implant electrodes into brain structures, first by reviewing the more recent experimental approaches such as transient coatings, bioinspired support structures, electromagnetic drives, and both microfluidic and robotic microactuators. We will also discuss a number of methods developed in the fields of robotic surgery and steerable needle development as potential strategies for implantation of flexible neural electrodes.
2. Challenges of the chronic neural interface
A robust BMI which remains functional for extended periods of time would present a significant improvement to both research and clinical care. The ability to control a prosthetic or an individual’s own limbs would greatly enhance quality-of-life and independence for paralyzed patients, while more reliable, long-term stable research tools would enable novel investigations into neural correlates of behavior and disease over the entire lifespan. Current BMI paradigms, however, present a host of challenges, including battery lifetime, reliable wireless telemetry, management of ’big neural data’ [16, 17], and, most critically, the stability of the electrode-tissue interface over chronic implantation periods.
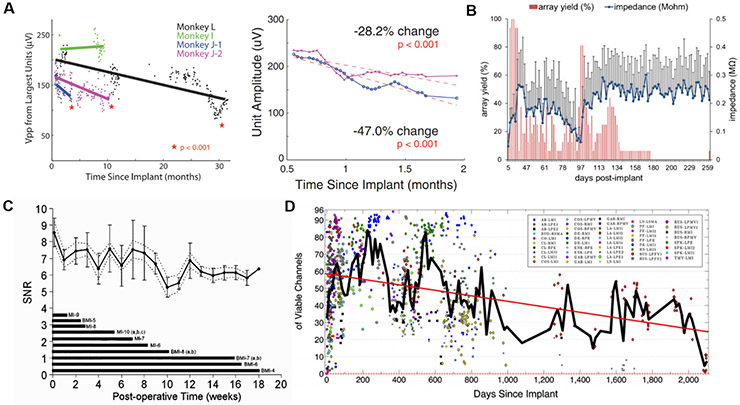
Decades of investigations into the long-term performance of implanted neural electrodes have now established that the quality of signals transduced across this interface considerably decays over time (figure 1). Quantifiable performance metrics indicative of implant failure are crucial for enabling progress in chronic BMI, though a high-fidelity, long-term performing device remains elusive [18]. Below, we provide an overview of the dominant failure pathways underlying these changes in the performance metrics of neural implants, which can be classified into two main categories: biological or structural.
Figure 1.
Performance loss of invasive neural recording electrodes as quantified by various methods, including (A) amplitude of largest recorded units on silicon microelectrode arrays (Utah arrays; reproduced with permission from [19], Copyright 2011); (B) yield and interface impedance of tungsten microwire arrays (reproduced with permission from [20], Copyright 2012); (C) signal-to-noise ratio (SNR) of units recorded on silicon laminar probes (Michigan arrays, reproduced with permission from [21], Copyright 2004), and (D) number of viable channels defined as electrodes on silicon microelectrode arrays (Utah arrays) capable of recording action potentials (reproduced with permission from [22], Copyright 2013).
2.1. Biological failure
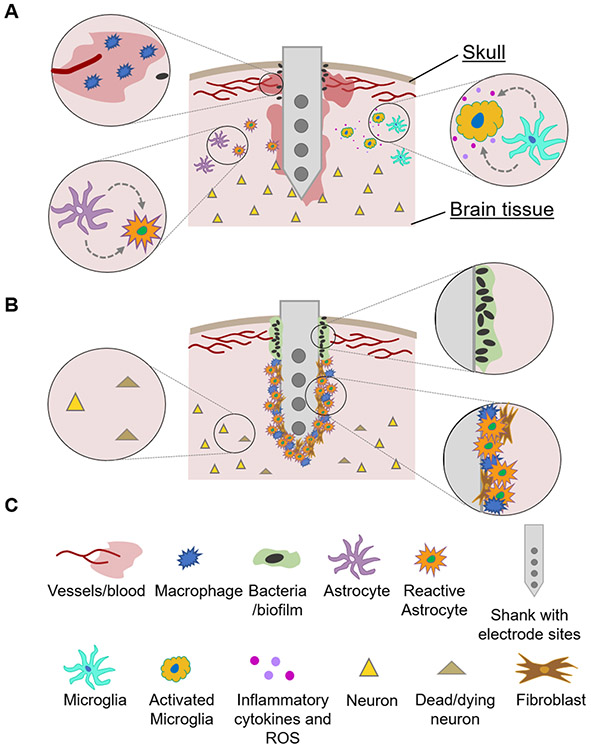
The implantation and long-term indwelling of a stiff probe in the soft brain tissue triggers a cascade of events that are detrimental to the performance of the implant, as well as to the health of the neural structures proximal to it [23-26]. Acutely, the electrode implantation severs capillaries and vessels, the extracellular matrix, and glial and neuronal processes, and pushes aside tissue that had once occupied the electrode space, leading to a local pressure increase [26]. The implantation injury may also damage distant cells and vasculature, especially when it is accompanied by tissue dimpling [27]. The mechanical ‘stab-wound’ trauma initiates the characteristic wound-healing response of the central nervous system (CNS). Blood-borne macrophages enter the wound site through the damaged blood-brain barrier, where they join activated microglia in secreting inflammatory cytokines, reactive oxygen species (ROS), and various cytotoxic and neurotoxic factors [26, 28, 29]. Astrocytes also become activated, transform into a reactive phenotype, and migrate to the wound site [26]. This initial inflammatory response has been associated with a rapid rise in electrode impedance over the first 1–3 weeks post-implantation, caused by the increased density of astrocytes, fibroblasts [30, 31], and microglia surrounding the implant, as well as tissue edema [20, 28]. The acute inflammatory response eventually terminates, and studies have shown that if the electrode is immediately removed after insertion, the signatures of the acute inflammatory response to the stab wound will resolve almost fully to the preinjury state within 16 weeks [32, 33]. However, in the case of a chronically implanted probe, after the initial acute phase the inflammatory reaction to the foreign body turns into a sustained, chronic response. The chronic inflammatory phase is characterized by the presence of reactive astrocytes, activated microglia, and fibroblasts, and oligodendrocyte progenitor cells which form an electrically-insulating glial scar encapsulating the probe [22, 25, 26, 34]. A neurodegenerative zone around the implant also forms, caused by neuronal death and migration [32]. Depending on the severity of injury, a so-called neuronal ‘kill zone’ can extend from tens of microns [35] to more than 500 μm from the electrode site, as evidenced by neuronal cell body loss, as well as decreased expression of neurofilament proteins [32]. Several observations suggest that this neuronal loss is not only of mechanical origin, but is most likely caused by neurotoxic factors in the extracellular space. In addition to neuronal death, the formation of a glial scar primarily composed of reactive astrocytes creates a passivation layer around the electrode. This limits the ionic exchange between the electrode interface and the extracellular space, effectively increasing the distance between the electrode and nearby neurons. Finally, ingress of bacteria, particularly in devices with transcutaneous connections, can result in infections and formation of a bacterial biofilm on the implant [22]. The short- and long-term biological responses to electrode implantation are illustrated in figure 2.
Figure 2.
Illustration of the (A) acute and (B) chronic biological tissue response to microelectrodes implanted into brain tissue. (C) Legend of symbols used in (A) and (B).
2.2. Structural failure
In addition to the biological tissue response, chronically implanted electrodes can lose functionality due to material or structural failures of the device itself. Structural failures can be attributed to the degradation of the conductive/transducing components, or to the breakdown of the insulation/passivation layers around the electrode.
The conductive site of the electrode is susceptible to degradation, even under passive recording operation. Examination of explanted intracortical electrode arrays occasionally reveals broken or bent electrode tips, although it is difficult to assess at which point the damage occurs (i.e. initial implantation or during extraction) [36]. An additional concern is the susceptibility of the conductive elements to corrosion in the warm, saline environment which often contains ROS generated during the neuroinflammatory process. A range of conductive materials have been proposed and evaluated for neural electrodes, including tungsten (W), platinum (Pt), gold (Au), and doped silicon (Si). It has been shown that W microelectrodes experience significant corrosion both under in vivo aging conditions [37] and in vivo during chronic implantation in brain tissue [20], with significant morphological changes to the conductive element observed after only 2 h of implantation. Noble metals, such as Pt and its alloys, are generally considered corrosion-resistant and, thus, are widely implemented as the conductive element in clinical devices such as the electrodes used to record electrocorticography (ECoG) and stereoencephalography (sEEG), as well as for stimulating devices such as DBS systems and cochlear implants [38-40]. However, Pt is susceptible to electrochemical corrosion if operated outside of its charge injection parameters [41-43]. A recent in vivo assay demonstrated that the corrosion products of pulsed Pt electrodes (e.g. nanoparticles) are cytotoxic to fibroblasts and neuroblastomas when at concentrations in excess of 100 μg ml−1, mainly due to the presence of Pt2+ ions [44]. Even passive Pt recording electrodes have been shown to corrode when implanted in non-human primate (NHP) brains for longer that 994 d [45], possibly due to the ROS generated by the immune response. Others, such as Patrick et al [37], have shown that both W and Au-plated W probes are highly susceptible to corrosion and biofouling in vivo, even under passive (i.e. nonstimulating) conditions. To overcome the corrosion instability of metallic electrodes, a number of more electrochemically stable carbon-based materials have been proposed, including graphene [46-49], carbon nanotubes [50-55], conductive diamond [56-60], and carbon fibers [61-64]. For a comprehensive discussion on alternative electrode materials we direct the readers to other extensive reviews [65-68].
Common failure modes of the insulation materials, as evidenced by scanning electron microscopy (SEM) of explanted Si and W microelectrode arrays, include cracking and delamination, which ultimately lead to current leakage, parasitic capacitances, reductions in electrode impedance (and thus loss of recording ‘specificity’), and accelerated degradation of the underlying conductive layers [36, 45, 69]. Due to its excellent barrier properties and conformal (‘pin-hole free’) deposition, Parylene-C is commonly used as the passivation layer for invasive neural electrodes [70]. The durability of Parlyene-C in chronic neural implants, however, is a subject of debate: while several studies have shown cracking and delamination of micron-thick Parylene-C coatings on rigid microwire electrodes [45, 71, 72], others have reported excellent durability when deposited as a device substrate in both accelerated aging conditions in vivo and chronic implants in vivo [73]. The potential source of these contradictory findings might lie in the mechanical mismatch between the relatively large metal wires and the thin Parylene-C layers, as well as metal-polymer adhesion issues, although a rigorous description of the physical mechanisms and potential material interactions warrants further investigation. Other materials commonly used as passivation layers, such as polyimide (PI), SU-8, and polydimethylsiloxane (PDMS) experience similar failure modes and consequences: insulation breach, impedance drop, and conductor corrosion [20].
It is now widely acknowledged [74, 75] that innovations in materials science are key to realize a stable, long-term integration of man-made electronics with the brain. The failure mechanisms outlined above primarily apply to stiff, metal-based electrode components which are still the mainstay for both basic neuroscience research and FDA-approved clinical applications. A major focus of current neuroengineering research is in the development of electrode technologies that mimic the mechanical properties of the brain tissue, reduce electrode insertion footprint, and improve stability and long-term reliability of the neuroelectronic interface [76]. One effective approach in improving electrode-tissue integration is to engineer electrodes with reduced footprint and/or more mechanically-compliant designs. The following section provides some examples of electrode configurations that exhibit more favorable properties in terms of mechanical compliance, size, or both.
2.3. Softer, smaller, safer
The brain is a soft, viscoelastic material, exhibiting complex nonlinear and hypoelastic mechanical behavior that is challenging to accurately model [77, 78]. Not only does it exhibit spatial and anatomical anisotropy (e.g. white matter versus grey matter versus vasculature), but also a time-dependent response to deformation [78, 79]. These properties have been extensively investigated to create better models of injury-related deformations of brain tissue [80] for simulation and in silico optimization of surgical procedures, including the insertion of electrodes and needles into brain tissue [81].
In contrast with the brain properties, the vast majority of neural electrodes in use for both clinical and research purposes are made of rigid materials such as noble metals or crystalline Si. Electrode arrangements vary considerably based on the intended application [82-84]. For example, single shanks containing multiple electrodes positioned distally along the length are utilized to record simultaneously from several cortical layers or hippocampal structures [69, 85], whereas co-planar arrays and grids are generally used to record from multiple locations within a single layer or depth [86]. To evaluate electrode compatibility, the mechanical properties of specific layouts can be calculated and compared with those of brain tissue. A number of reports have emphasized that reducing the elastic modulus of the electrode materials and the device footprint can significantly improve neuronal survival and reduce glial scarring [82, 83, 87-90]. Additional properties can influence the foreign body response, including density of the constituting conductor [91], and the skull tethering configuration [92]. The complex interaction of many of these variables has been reviewed elsewhere [24, 74, 84] and is beyond the scope of this review.
Though soft and flexible electrodes may significantly improve the long-term electrode-tissue integration, they present a new challenge: surgical implantation. This challenge can be described mathematically as the critical buckling force of a beam hinged at one end [84, 93]:
| (1) |
where Fbuckling is the critical buckling force, I is the geometry-dependent area moment of inertia, E is the elastic (Young’s) modulus, L is the length of the probe, and K is the effective length factor [84]. For symmetrical shapes I of equation (1) is modifed for beams having either square (, where a is the side of the beam section) or cyclindrical (, where r is the radius) cross-sections. For beams with rectangular cross section , where b is the width and h the thickness [51, 75]. The insertion force range for rat cortical tissue is estimated to be between 0.5 and 1 mN [94], though others have measured it at 1.970 mN for a probe having 25 μm thickness and 2.5 mm length [95]. For a neural probe that is undergoing implantation into brain tissue, K is given a value of 0.7, which describes a beam that is translation- and rotation-fixed at the connection side, and rotation-free and translation-fixed at the probe end [96]. It is worth noting that as the glial scar forms, the value would shift towards 0.5, which is the value for a beam that is translation- and rotation-fixed at both ends [97]. Equation (1) describes the property of columnar structures to remain undeformed for loads less than a certain critical threshold. In other words, if the force required to achieve succesful insertion is less than Fbuckling then the electrode will penetrate brain tissue. This buckling force is also used as a measure of the electrode’s mechanical compliance: a strong indicator of its probability to initiate the chronic tissue response [84, 98]. Compliance of neural probes can also be expressed in terms of axial bending stiffness (k), which is defined as [51]:
| (2) |
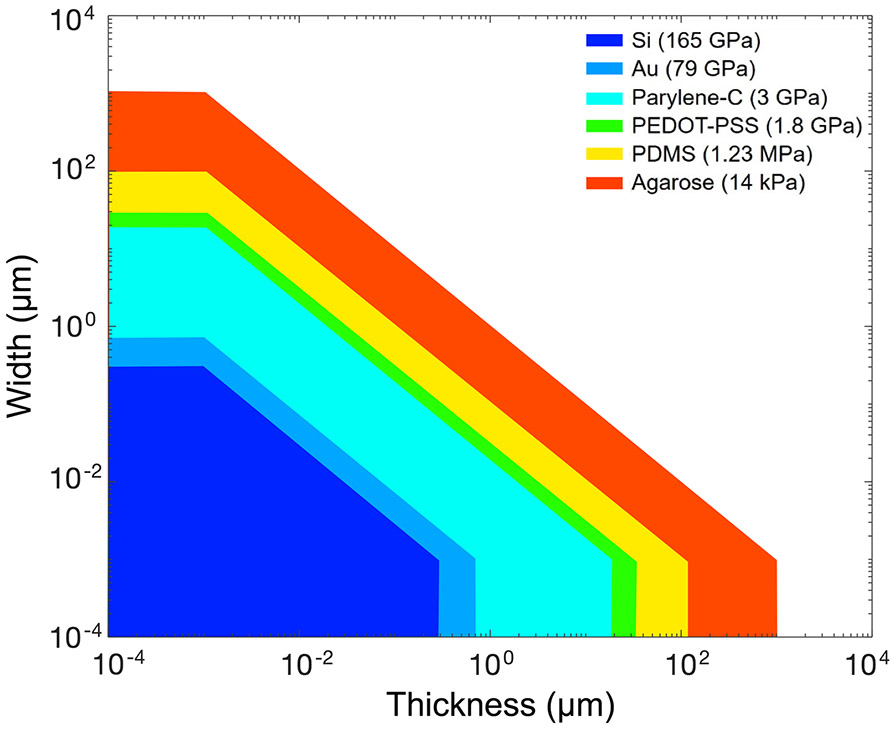
where ACS is the cross-sectional area of the probe. Using equation (2) for an electrodes with rectangular cross-section, the stiffness of a neural probe fabricated from a specific material can be compared to that of a column of brain tissue, as shown in figure 3.
Figure 3.
Design space of several conductor and insulator materials commonly used in neural probes. Colored areas illustrate where device stiffness is less than or equal to the brain axial stiffness (k ⩽ kbrain = 1.4 kPa). Only tethered electrodes with rectangular cross-sections (ACS = b × h) and having a length L = 3 mm were considered. The brain is modeled here as a column with 200 × 200 μm2 cross-section and L = 3 mm. (NB: for a cylindrical beam having the same cross-sectional area as the rectangular beams considered here, the second area moment of inertia is scaled by a factor of 3/π ≈ 95%).
Soft, compliant materials with a low Young’s modulus offer a larger design space in terms of axial bending stiffness, but they are also likely incapable of unassisted surgical insertion. Conversely, probes made out of stiff materials need to approach submicron scale dimensions in order to avoid the mechanical mismatch with brain tissue. Such geometric requirements pose several issues in terms of manufacturing, handling, and surgical insertion, given the brittleness of metals and semiconductors. If metals and Si are deposited as nanometer-thin films onto soft polymeric substrates, though, their contribution to the total device stiffness becomes negligible.
Modeling thin-film neural microelectrodes as ‘sandwich’ structured beams, the total device stiffness is given by [99]:
| (3) |
where EXI is the overall bending stiffness of the structure, and the letters c and f refer to the core material and the coating film, respectively. As the bending stiffness shows a third power dependence on device thickness, the total stiffness of structures comprised of large polymeric substrates and much thinner metallic layers will closely approximate the mechanical properties of the polymeric layer. Furthermore, if the geometry of the electrical contacts is designed carefully, polymeric substrates can provide the additional advantage of strain-relief for thin film metals, thus realizing a device that is both robust and compliant [100-105].
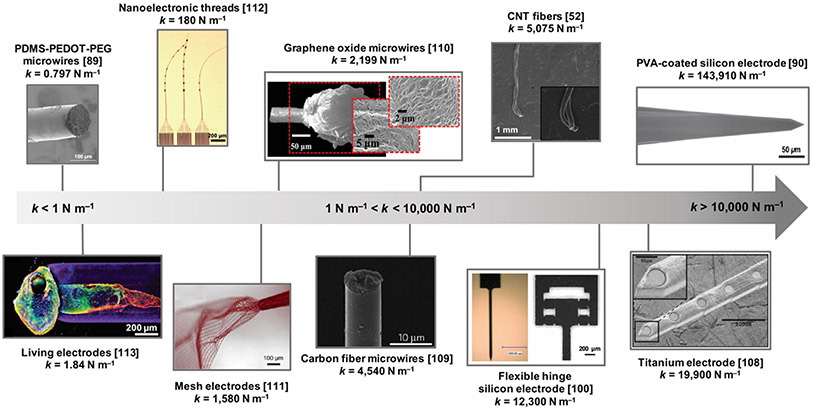
The most promising electrodes for long-term neural integration are either based on softer materials, smaller geometries, or both. Figure 4 includes a range of electrode materials and form factors organized by the mechanical properties of the whole device, rather than simply the intrinsic properties of the materials. The most rigid electrodes are generally made from high elastic modulus crystalline Si, and perform poorly in the long-term compared with more compliant probes, even when surface chemistry is accounted for, as shown by Nguyen et al [89]. While Si probes have been a mainstay for neuroelectronic interfaces, the brittle nature of the material introduces a structrual failure mode. To make a more robust neural probe with slightly lower elastic modulus, McCarthy et al developed a manufacturing process to make laminar neural probes on titanium substrates [106]. Rather than tuning the properties of the probe material itself, Zhang et al developed a compliant hinge system for Si neural probes, significantly reducing the tissue stress and probe micromotion [98]. While alleviating micromotion is extremely important, the introduction of materials with greater inherent flexibility and exceptional electrochemical properties allows for a reduction of the overall device stiffness and footprint. Carbon-based materials have been very popular as free standing neural probes, and authors have utilized carbon nanotube yarns [51], carbon fibers [61], reduced graphene oxide fibers [46], and many other nanocarbon composites to construct neural probes with more favorable mechanical and durability profiles. Exceeding the flexibility of native materials has also been made possible by significantly decreasing the physical size of the device, great examples of which are the mesh electrodes of Dai et al [107] and the nanoelectronic threads of Wei et al [108]. Unsurprisingly, one of the most flexible neural probes to date is the ‘living electrode’, which is comprised of mono- or bi-directional tissue-engineereed neural networks grown through a hydrogel columnar scaffold [109]. The most compliant probe on our list is the PDMS-PEDOT-PEG composite electrode produced by Du et al [88].
Figure 4.
Axial bending stiffness of various intracortical probe designs calculated with equation (2). The examples shown here are representative of different material and design strategies to reduce the effective device stiffness. Probes with k < 10,000 N m −1 are considered compliant, while probes with k > 10,000 N m−1 are considered stiff. Images reproduced with permission from: [88], (Copyright Elesevier, 2017) [108], (Copyright Wiley, 2018) [46], (Copyright Wiley, 2015) [51], (Copyright, American Chemical Society, 2015) [89], (Copyright IOP, 2014) [109], (Copyright, Wiley, 2017) [107], (Copyright, American Chemical Society, 2018) [61], (Copyright, Springer, 2012) [98], (Copyright, Springer, 2018) [106], (Copyright, IEEE, 2009).
In general, the challenges associated with maintaining a functional, invasive neural interface are addressed predominantly by materials selection and probe design. However, electrodes with extremely compliant mechanical properties will not penetrate brain tissue, instead they will buckle under the applied insertion force [84]. Even slightly more rigid materials, such as stainless steel needles, which are capable of penetrating tissue, may not remain straight enough during their trajectory to accurately reach their anatomical destinations [110-112]. The mechanical challenges related to electrode insertion have been reviewed extensively by Lecomte et al [84], as well as others studying the deflection of needles for accurate anatomical targeting during drug injection or radioactive seed placement [113]. As neural interfacing electrodes have become smaller and more flexible to meet the stringent criteria for long-term tissue integration, a number of biomedical engineering and materials science solutions have been developed to enable their accurate surgical implantation into brain tissue.
3. Transient electrode vehicles
For an electrode exhibiting a critical buckling force that is less than that of brain tissue, an insertion guide is required to increase the stiffness [84]. One of the most common approaches is to use a temporary stiffener, either as a coating around the entire probe, or as a fixative to a stiff insertion shuttle. This stiffener generally takes the form of a polymeric coating which provides the probe with temporary rigidity due to either the dissolution or shape memory change of the coating. This section first describes several dissolvable coating materials and concludes with a discussion of shape memory polymers.
The first examples of dissolvable coatings in the context of the implantation of fine neural electrodes (25 μm diameter platinum-iridium wires) date back to 1972 [114]. Since this early study, a number of bioresorbable or biodegradable materials such as polyethylene glycol (PEG), sucrose, maltose, silk, gelatin, and carboxymethylcellulose (CMC) have been explored as stiffening coatings for neural probes. Various examples of these coatings are summarized in figure 5. Ultimately, the challenge lies in applying the transient carrier to the electrode or device of interest without damaging the device and excessively increasing its footprint. If the coatings are too thick, this will not only exhacerbate the acute implantation trauma and, potentially, the long-term inflammatory reaction, but also displace tissue and affect electrode-neuron proximity. The importance of electrode-neuron promixity (‘r’) is conveyed by considering that close to the soma the extracellular voltage amplitude decays with distance , where the 1/e falloff distance ‘ro’ has been measured to be ~28 μm in feline cortex [115,116] (further away from the soma the extracellular current becomes indepent from distance and the voltage decay follows a slower 1/r2 dependence [115, 117]). Additionally, the dissolvable or biodegradable coating material itself must exhibit exceptional biocompatibility, and must be compatible with common pre-surgical sterilization procedures. The remainder of this section will specifically focus on examples of applications of biodegradable or dissolvable coatings from PEG, CMC, silk, sucrose, maltose, and others.
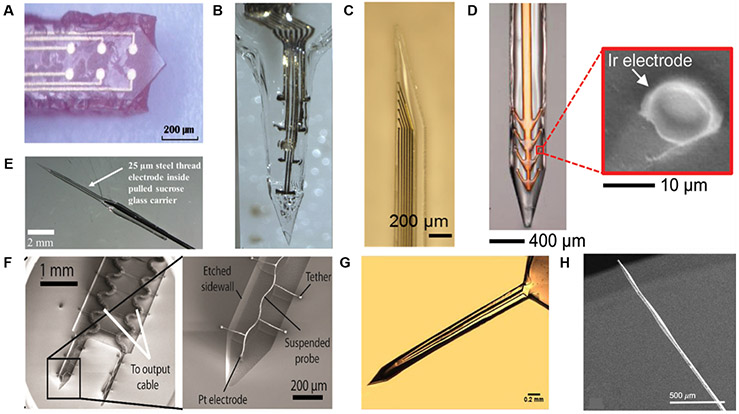
Figure 5.
Transient electrode coatings, including (A) PEG, (B) gelatin, (C) PVA/PLGA, (D) silk, (E) sucrose, (F) CMC, (G) tyrosine-derived polycarbonate E5005(2 K), and (H) maltose. Images reproduced with permission from [118] (Copyright IOP, 2015) [119], (Copyright, Frontiers Media SA, 2015) [120], (Copyright, IOP, 2018) [121], (Copyright, Wiley, 2018) [122], (Copyright, Wiley, 2009) [123], (Copyright, Elsevier, 2016) [124], (Copyright, Springer 2015), and [93] (Copyright, IOP, 2014).
3.1. PEG
PEG-based hydrogels, which are non-ionic and relatively resistant to protein absorption, have been proposed both as biodegradable adhesives for attachment to rigid shanks [51, 125], as well as electrode stiffeners [51, 118, 126, 127]. Gage et al provide detailed instructions for the implantation of Si NeuroNexus electrodes by attaching the probes to insertion rods with PEG, then dissolving the adhesive with saline once the electrode is secured [128]. Similarly, Felix et al designed stiff substrates containing a channel filled with PEG to transiently fasten a flexible probe to a rigid carrier [125]. Du et al demonstrated that PEG adhesives between ultrasoft probes and a stiff shuttle (45 wt% in acetronitrile, MW = 1500 Dalton) quickly dissolve after ~30 s, allowing removal of the shuttle, while leaving the electrode in place at a target depth of 8 mm from the cortical surface [88]. Takeuchi et al used a 10 μm fluidic channel filled with PEG to increase the mechanical stiffness of their flexible parylene probes. After insertion in the brain, the PEG melts, leaving behind a microfluidic channel that can be used for direct drug and fluid delivery [127].
Bjugstad et al studied the tissue response of different PEG formulations engineered to tune the degradation rate in vivo [129]. Specifically, triblock lactic acid-b-PEG-b-lactic acid copolymers showed cotrollable degradation rates, with more lactic acid units leading to faster degradation rates and a lack of lactic acid units leading to a non-degradable formulation. Fast-degrading gels (107 d), slow-degrading gels (140 d), and non-degrading gels were implanted to study the tissue response, in addition to a sham needle insertion in which no hydrogels were injected. Fast-degrading and sham insertions resulted in a sustained astrocyte activation, whereas the slow- and non-degrading sites contained predominantly non-reactive astrocytes. Acutely, a large presence of activated microglia was observed for the sham and fast-degrading groups, whereas the microglial population around the slow- and non-degrading groups were non-reactive, but were present in greater numbers long-term. An NHP trial using identical materials observed a similar microgial response, whereas the astrocyte response to sham and fast-degrading polymers was largely unobservable, likely due to the longer timepoints (4 months Vs. 56 d) [130]. Thus, slow-degrading PEG hydrogels may help reduce the astrocytic response via a mitigation of the vasogenic edema following electrode penetration, while promoting the migration and activation of microglia at the site for the phagocytosis of the degradation products.
3.2. Sugars (sucrose and maltose)
Typical coating-application processes include dipcoating or the manual placement of the polymer onto the probe. A more recent innovation, developed to create arrays of microneedles for drug delivery, is drawing lithography [131, 132]. This approach is especially suited to sugar solutions, which exhibit glass transition temperatures that are tunable by moisture content [122, 133, 134]. An adapted drawing lithography process was used to encapsulate different flexible microelectrodes, including carbon nanotube yarns, graphene yarns, and microscale steel threads, inside rigid sucrose microneedles with a diameter ranging from 0.2 to 0.5 mm [122]. When compared to different microneedle electrode vehicles, including both blunt and sharpened cannulas in agarose brain phantoms, the sucrose microneedles exhibited significantly lower insertion forces due most likely to the sharp, conical tip, as well as the hygroscopic properties of sucrose, which may lubricate the insertion [122]. Minimization of insertion forces can lead to improved anatomical placement and lower strain energy transfer, reducing the amount of tissue trauma. A similar technique was adapted for maltose by Xiang et al, in which thin-film laminar probes were implanted into brain tissue [93]. Sugars such as sucrose and maltose have widespread use in the pharmaceutical industry due to low cost, chemical simplicity, suitability as cell culture substrates, and because of their well-characterized glass transition temperatures and plasticizing effects, which are relevant parameters to control in the manufacturing process [133, 135]. Although a potential concern with using sugars in brain tissue is the generation of hyperosmotic stresses, histological and electrophysiological analysis did not evidence necrosis or inflammatory reaction to sucrose microneedles over a 2-week implantation period [122].
3.3. Silk
Silk, a naturally occuring material comprised of the protein fibroin, has emerged as implantable biomaterial due to its inherent biocompatibility, tunable degradation rate and mechanical properties, and facile processability [121]. A layer-by-layer casting technique was used to coat Pi-based thin-film, flexible cortical electrodes with silk fibroin by Tien et al [121]. The mechanical transition of silk carriers upon hydration in the tissue through controlled crystallization of the protein can also be used for release of therapeutic drugs to the local insertion site. In this work, the method of fabrication involved applying a 15% silk solution to a mold by drawing a bead of solution from the tip of a pin along the probe shank, producing silk coatings on one side of the probe shank, and exposed recording sites on the opposite side. The silk-coated electrodes were characterized with a buckling test comparing uncoated, 1-layer (36.4 ± 5.3 μm), 3-layer (71.3 ± 2.0 μm), and 6-layer silk fibroin coatings (136.7 ± 19.7 μm; Esilk = 1.8 GPa) [121]. The uncoated probe was unable to penetrate the gel phantom, but the 6-layer coated probe was successfully inserted in a straight trajectory, indicating that silk layers allowed the flexible probe to sustain greater forces before buckling. However, it is important to note that the difference in buckling force is attributed to increased thickness of the probe, as discussed in section 2. Immune response to silk coatings evaluated in vivo, in co-cultures of microglia/macrophages and astrocytes, show lower levels of Iba1 and no significant difference in GFAP compared to uncoated probes. Other examples of silk electrode stiffeners are discussed in [84, 118].
3.4. CMC
CMC is a cellulose derivative often used as a thickening agent in the food industry, which has been explored as a dissolving delivery shuttle for neural probes [136]. CMC shuttles have provided mechanical support during high-speed insertion (~80 mm s−1) of ultrasmall Pt microwire electrodes [136]. The stiff CMC shuttles enclosing the electrodes softened at an approximate insertion depth of 1 mm into the brain, due to moisture absorption. Shuttles with a thickness of 0.125 mm were implanted in vivo into the motor cortex, and compared with tapered Parylene-C insulated microwires. Results of immunohistochemical staining showed an increase in neuronal density between weeks 1 and 4, and weeks 4 and 12, for those electrodes implanted using the CMC shuttle. Another study with a dissolvable CMC-based delivery needle that encased an ultra-miniature, ultra-compliant neural probe was shown by Khilwani et al [137]. Once inserted, the needle dissolved and left behind the precisely placed, flexible neural probe in the brain. The capability of these needles to incorporate anti-inflammatory drugs was also demonstrated. A Pt probe insulated in Parylene-C and having a microscale cross section (2.7 μm × 10 μm) was coated with bio-dissolvable coatings prepared as hydrogels from blends of CMC with sugars such as sucrose, glucose, and maltodextrin. At 12 weeks post-implantation, microglia were absent and neural regeneration was observed at the site of insertion. Tissue strain was also reduced when compared to standard Si shank-like probes, with an average Young’s modulus of 5.27 ± 0.22 GPa for the 100% CMC samples. The authors also noted that the fabrication of CMC coatings is advantageous considering that the entire process can be completed at room temperature [137].
3.5. Tyrosine-derived terpolymer
The ultrafast-degrading tyrosine-derived terpolymer, abbreviated E5005(2 k), demonstrates how tuning synthesis enables control of the resulting degradation and erosion properties in dissolvable carriers. In a study by Lewitus et al, when compared to PEG-based terpolymers, poly(trimethylene carconate) (PTMC) did not rapidly erode, which is most likely due to its hydrophobic character in comparison to the hydrophilic character of PEG [138]. Due to the fast resorption and degradation of E5005(2 k), the implant site showed quick recovery, indicating that E5005(2 k) is a strong candidate for localized therapies in the CNS, especially where neuronal survival at the implant site and minimized distance from implant to neuronal somata are crucial [139].
3.6. PVA/PLGA
Hydrolytically degradable polymers such as poly (vinyl alcohol) (PVA) and poly (lactic-co-glycolic acid) (PLGA) have been used to insert thin, flexible Parylene-C probes [120]. Both PVA and PLGA are biocompatible, bioresorbable, and FDA approved; the former is a major component of contact lenses [140], while the latter is known for its excellent, zero-order biodegradative drug-release kinetics [141]. In the work of Pas et al, impedance spectroscopy characterization in vivo highlighted differential effects of the PVA and PLGA degradation kinetics on electrode impedance [120]. The presence of PLGA on the electrode contacts resulted in a >100-fold increase in impedance, followed by a slow recovery of the baseline value after a few weeks post-implant. PVA transiently increased the impedance by only 10-fold, for the initial 10 min. Thus, PLGA appears to be suitable as a stiffening backing, while PVA can be used a transient adhesive. PDMS molding was accordingly used to fabricate a PVA shuttle with a sharp tip that was then dip-coated with PLGA to create a bi-layered shuttle. The PVA/PLGA shuttle was fabricated on the backside of a Parylene-C probe, resulting in a probe with an average thickness of 88 ± 3 μm (n = 3). The PLGA layer dissolved slowly during implantation, thus prolonging degradation and enabling insertion into the hippocampal region CA1 in a mouse brain (>3.5 mm final insertion depth).
3.7. Hybrid coatings
Agorelius et al demonstrated the implantation of electrode arrays of eight individually flexible gold leads embedded within a hard hybrid coating (27.5% gelatin, 1.4% glycerol, 6.9% PEG) that dissolves after implantation into the brain [119]. The authors experimented with a range of thicknesses (75–125 μm), finding that only probes having 125 μm could be implanted successfully without buckling. The hard but dissolvable hybrid encapsulation allowed the conformation and architecture of the array to be retained during implantation—one of the major challenges when integrating flexible electrode arrays with transient carrier systems. In this study, preliminary testing in three animals demonstrated high quality recordings can be obtained using these electrodes. Lind et al demonstrated a similar concept with gelatin, using thin-film electrode arrays [142].
3.8. Shape memory polymers
In delicate physiological environments such as the CNS, the breakdown or dissolution products of degradable stiffeners may present safety and toxicity concerns, especially as the number of implanted electrodes increases. Additionally, the reliable application of a coating may pose significant technical challenges, especially for arrays with high density of small electrodes. Finally, the presence of the coating layer adds to the overall footprint of the device, potentially exacerbating the acute stab injury from implantation. An alternate approach to achieving transiently stiff neural probes without the need for dissolvable or degradable coatings is the use of shape memory polymers (SMPs). Traditional SMPs, by definition, are polymeric smart materials engineered to transition from different shapes upon an external triggering stimulus. In the context of SMP developed for neural interfaces the main property associated with the shape change is softening [143]. By tuning the chemical structure of SMPs, the glass transition temperature (Tg) can be finely modulated over a wide range [144]. For an excellent overview of the mechanics of this change and their relation to chemical structure, readers are directed to the review by Gall et al [144].
While conventional polymeric substrate materials, such as polyimide and Parylene-C, undergo glass transition at temperatures which far exceed physiological temperatures [145], SMPs can be engineered to achieve glass transition at or near 37 °C [146-148]. Specifically, these SMPs are rigid at room temperature, but when they are heated above the shape memory transition temperature (Ttrans), they becomes elastomeric [147]. Here, Ttrans can be either a glass transition temperture (Tg) or a melting temperature (Tm), beyond which macroscopic deformations corresponding to increased entropic energy and concurrent changes in molecular chain configuration will occur [147]. Shifts in molecular bonding configuration result in larger changes of the material elastic modulus, allowing for tunable stiffness. Similarly, for SMPs sensititve to hydration, the polymer swells and the free volume of each polymer chain increases, thus causing the Tg to decrease and triggering the macroscopic shape change [146].
Thiol-ene/acrylate SMP microelectrodes were initially developed and demonstrated both for subchronic in vivo studies [149] and chronic intracortical single unit recordings from rat cortex for up to 13 weeks [150]. In these longer chronic in vivo studies, the SMP formulation resulted in a final device with the elastic modulus ranging from ~2 GPa when dry at room temperature, to ~300 MPa under physiological conditions [150] after implantation. Neural probes produced by Simon et al with photolithography and micropatterning of SMPs exhibited a stiffness ranging from 2 GPa at room temperature to 50 MPa slightly above body temperature, which also resulted in the reduction of the frictional strain at the tissue-electrode interface [151]. These devices were capable of acquiring single unit neural activity for up to 77 d in vivo.
While SMPs have traditionally been ‘shape-shifted’ via direct heating [152-154] or hydration, there are other avenues of control, including exposure to light [155, 156], application of electric current [157] or magentic field [158], and pH change [159, 160], all of which can induce changes to the elastic modulus. Much of this control is possible by choosing the correct monomers and crosslinking agents during SMP snythesis, or by experimenting with different shape programming methods [147].
Aside from the modulus reduction, it is of interest to consider how the shape memory properties of SMPs might enable another level of control, including steering neural probes in vivo. Examples of applications include ‘self-tying’ sutures to enable controlled dynamic steering of implanted electrodes [161], or the use of of magnetic or electric fields to drive the electrodes and penetrate tissue safely.
An alternative approach to smart materials for soft electrode microelectrode implantation and, potentially actuation, relies in improved or totally innovative surgical implantation methods and tools. In the next section, we introduce a number of hardware innovations that were developed to not only implant soft electrodes into neural tissue, but also to tune their location within the anatomical region of interest.
4. Microactuation
The transient carriers described in the previous section provide an excellent method for stabilizing flexible electrodes during insertion, but they require exposing the brain to new materials that still increase the device footprint upon implantation and might ultimately affect tissue integrity and electrode biocompatibility [139]. In this section, we describe approaches to electrode delivery that do not require transient vehicles, but instead leverage microactuation strategies to insert flexible microelectrodes into the brain. In microactuation approaches, the electrode length is effectively ‘shortened’ during insertion, so that it is stiff enough to incrementally penetrate into the brain tissue (see the maximal buckling force condition in equation (1)). Microactuation strategies can be divided into two groups: (i) electrically/ultrasonic-driven microactuation and (ii) microfluidic actuation. Since the transient carriers described in the previous section generally begin to soften, swell, or dissolve under physiological conditions, they may not provide a viable approach for steering electrodes into and out of brain tissue, especially to reach deeper structures—another consideration for the use of microactuation as a neural electrode delivery approach.
4.1. Electrically-driven microactuation and ultrasonic insertion techniques
Electronic microactuation of screw-based microdrives was initiallly developed to achieve the goal of repositioning rigid electrodes after initial surgical implantation. For example, Feingold et al developed a ‘reconfigurable chronic electrode array’ in which up to 119 individual 125 μm diameter platinum-iridium wire electrodes were individually repositioned for up to 1 year [162]. However, given the rigidity, density, and size of the electrodes required to achieve driveability, they exhibited poor longevity, with over half of the electrodes losing functionality during the indwelling period. In other words, simply driving the electrode to a new site cannot prevent the immune response. As described above, softer and more flexible probes are required to reduce glial scarring and achieve long-term recordings. In order to achieve controlled insertion of such soft, flexible microelectrodes, several novel electrically-driven microactuation approaches have been developed (figure 6).
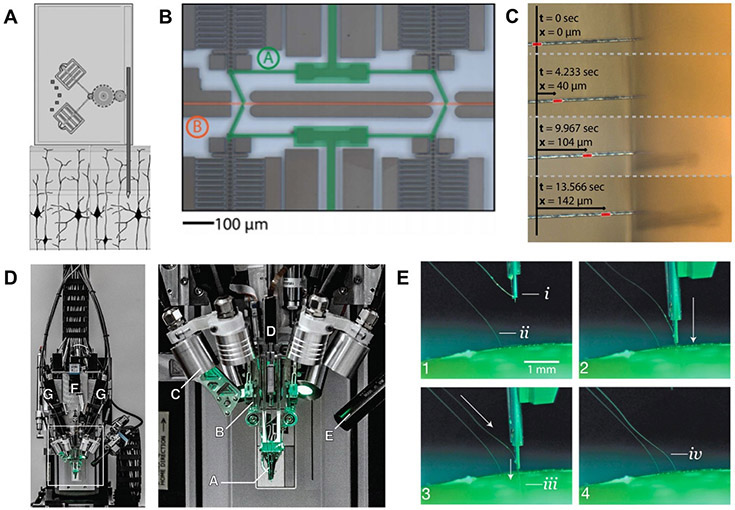
Figure 6.
Electrically-driven microactuated insertion of flexible microelectrodes. (A) Example of the electrostative comb-drive microactuators developed by Muthuswamy et al Reproduced with permission from [163] (Copyright IEEE, 2006). (B) The ‘capacitive finger’ system used to drive (C) carbon fiber microelectrodes into agarose developed by Zoll et al [63]. (D) Surgical electrode stitching robot developed by Neuralinkto implant flexible polyimide multi-channel thread electrodes into brain tissue [164]. (E) Close-up view of electrode stitching process. The threads contain a loop at the end, which provides a handle for the inserting needle to drive them into the brain tissue at a pre-defined depth [164].
One of the earliest examples of microacutationenabled insertion of neural probes was presented by Muthuswamy et al, in which electrostatic comb drives were used to realize 5 mm of bidirectional translation (2 mm if epoxy insulation was used), with 1 μm accuracy [163]. Recently, a system presented by Zoll et al utilized inchworm motors, which are voltage-driven capacitive plates capable of microscale movements [165]. This system was used to deliver single 7 μm carbon fiber microelectrodes in an accurate and controlled fashion into an agar brain phantom (figures 6(B) and (C)). However, it is difficult to assess whether the carbon fiber electrodes remained straight at the target insertion depths (up to 400 μm) without recording from a specific anatomical target or histological assessment. A very recent system presented by Neuralink relies on a robotic inserter (figures 6(D) and (E)) to ‘stitch’ microscale shanks of PI electrode arrays into brain tissue at a rate of 6 threads of 192 electrodes per minute, leading to in vivo recordings from 1020 viable channels with capabilities for up to 3072 channels [164].
Work from Amit Lai’s group has also demonstrated the utility of electrically-driven ultrasonic activation as a means for implanting devices in soft tissue [166, 167]. In these works, thin silicon shank probes were attached to lead zirconate titanate oxide (PZT) piezoelectric plates, and during insertion, the PZT plates were driven to voltages of up to 30 V peak-to-peak. This resulted in high-velocity vibrations at the insertion tip, which cut the tissue ultrasonically while the probe was inserted, providing both stress relief and using less force than would be otherwise required from traditional insertion methods. The authors initially demonstrated a reduction of the insertion force by a factor of 2.6 using this methodology [167]. In a follow-up work, the silicon probes were attached to the PZT plates using dissolvable PEG coatings; upon insertion and dissolution of the PEG coatings, the authors reported reduction of the overal insertion force by a factor of 4.3 [166]. The authors also demonstrated the implantation of ultrasonically-actuated probes in mouse brains. Post mortem histology showed a reduced acute microglial response, suggesting that the ultrasonically-implanted probes caused less damage than traditional implants by a factor of 1.65 [167].
4.2. Microfluidic actuation
A potential alternative to the complex electronic systems described above is microfluidic actuation [168] (figure 7). In this paradigm, microfluidic drives leverage the drag force of a liquid stream to stabilize the electrode during implantation. By controlling the fluid flow rate through the microfluidic drive, the velocity and insertion depth of the microelectrode can be controlled, with larger flow rates corresponding to faster microelectrode velocities and deeper insertion depths. Electrode microactuation and positioning may also be achieved via on-chip flow control systems, such as microfluidic actuation valves. With a microfluidic actuation system, Vitale et al demonstrated feasibility of recording extracellular neuronal spiking activity using flexible carbon nanotube fiber (CNTf) microelectrodes at both cortical (~0.5–1 mm) and subcortical depths (>4 mm). The careful control of insertion depth opens the opportunity to adjust the electrode location relative to the neurons of interest, in order to maximize the signal [169, 170] Compared to syringe injection, the volume of fluid injected into the brain is minimal with microfluidic actuation, and quantification of the ejected fluid volume shows that the volume is well below the level required for safe injection into the brain.
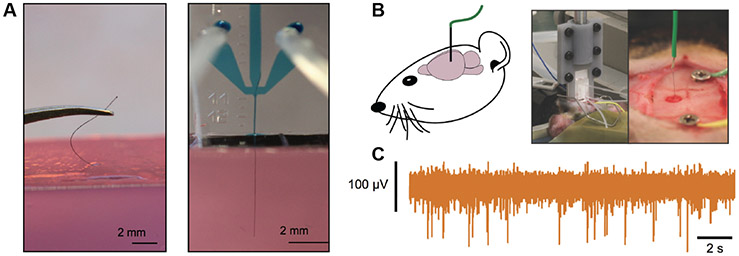
Figure 7.
Microfluidic Assisted Insertion. (A) Manual (left) Vs. microfluidically-assisted (right) insertion of a 12 μm diameter CNTf inside an agar brain phantom. (B) Schematics (left) and photgraphs (right) of microfluidic-assisted insertion of a CNTf microelectrode in the rat brain for neural recording. (C) Representative 2 s of high-pass filterd neural signal recorded in the hippocampus (~4 mm deep) with CNTf microelectrode. Reproduced with permission from [168] (Copyright, American Chemical Society, 2017).
5. Magnetic insertion strategies
Magnetic insertion provides a contactless method to implant flexible probes into brain tissue. This may be particularly advantageous for extremely small or fragile electrodes, as well as a method to reduce exposure and, perhaps, contamination. Furthermore, magnetically-driven systems provide the opportunity to steer in three independent dimensions. There have been several innovations in the ‘smart’ needle insertion and robotic surgery literature which may enable 3-dimensional steering. Tang et al developed an active biopsy needle in which the tip angle relative to the shank can be changed using magnetic forces, enabling an angle change even during driving [171]. Using the same principle, Petrusha et al developed a magnetically-steerable needle designed to correct the implantation trajectory of DBS leads [172].
In the context of flexible neural probe insertion, magnetic insertion has only been investigated in vivo by one research group [173, 174]. In an initial study, Jaroch et al constructed electrodes with sharp ferromagnetic tips attached to flexible shanks that were inserted into tissue via a pulsed magnetic field (figure 8). This system generated a magnetic field via the capacitor’s discharge through inductive coils into an uncharged capacitor (figures 8(A) and (B)). The resulting induced magnetic field surrounded a glass pipette containing the electrode, and accelerated the electrode’s magnetic tip forward, into the neural tissue. Upon exiting the magnetic field, the kinetic energy of the probe tip overcame resistive frictional forces (i.e. insertion and buckling forces) and the momentum of the probe allowed for the sharp tip to penetrate the tissue. The magnetic probe tip was attached to a conductive tether held in tension during acceleration, preventing buckling of the conductive wire throughout insertion. Insertion tests using agar gels showed a linear relation between voltage and penetration depth, with a ratio of input voltage to insertion depth of 39.2 V mm−1 [174]. The authors described the relationship between insertion depth (dp), buckling force (Fb), electrode velocity (ve), and electrode mass (me) using the following equation:
| (4) |
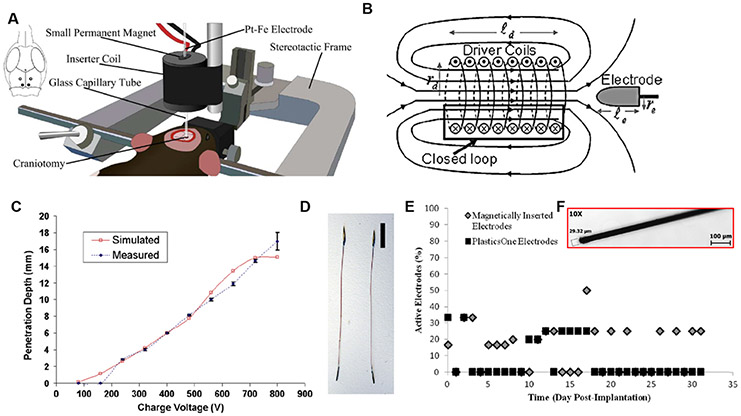
Figure 8.
Magnetic insertion strategies. (A), (B) Insertion schematics and principle of operation. (C) Penetration depth of the electrode as a function of the aplied voltage. (D) A spear-like, magnetically-tipped electrode used in the initial demonstration of magnetic insertion. (E) In vivo longevity of magnetically-inserted 25 μm platinum-iron wires (F) compared with commercially-available, 150 μm stainless steel (304) electrodes. The platinum-iron microwires were ~25% viable 31 d post-implantation. Reproduced with permission [173] (Copyright IEEE, 2105) and [174] (Copyright Elsevier, 2009).
The velocity (ve) of the electrode was controlled by the applied current, given that current dictates electric field strength and, thus, force and acceleration of the probe by equation (4) [173]. While this insertion method was successful, the millimeter-scale of the recording tip limits spatial resolution in vivo. To address the issue of size and probe compliance, Dryg et al presented a thorough characterization of magnetically-inserted microwires (25 μm diameter) fabricated from a platinum-iron alloy [173] (figure 8(C)). The authors optimized the surgical procedure and examined the electrode performance over a 31-day period in vivo, compared with a much larger and stiffer, commercially-available electrode, which was implanted using standard methods (figure 8(D)). The results of their study showed that the magnetically-inserted platinum-iron wires retained approximately 25% more functionality than the commercially-available electrodes. However, this outcome was likely due to the differences in electrode properties (e.g. stiffness and diameter, figure 8) rather than the surgical insertion procedure used. Nevertheless, the use of magnetic insertion provides an interesting alternative to the other methodologies described above, although there may be some concerns about the neurotoxicity of iron-containing alloys [175]. Furthermore, all of these ferromagnetic electrodes are not compatible with MRI, and perhaps preclude the possibility of implanting multiple probes within close proximity, if a magnetized material is utilized. One possible solution maybe the use of a biodegradable magnetic component. Though this has not yet been explored for neural implants, Yan et al have presented a magnetite-hydrogel composite, which may enable the creation of magnetically-steerable, biodegradable microbots [176].
6. Bio-inspiration
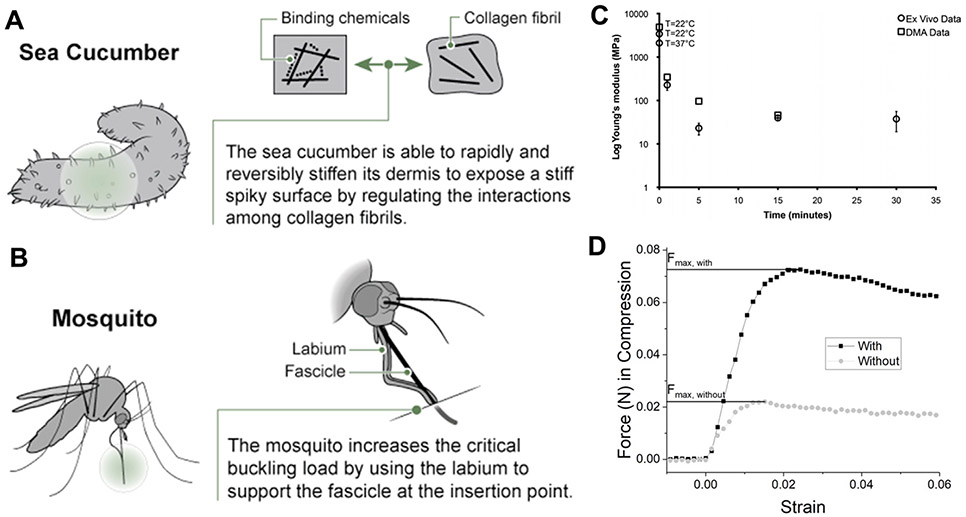
Taking inspiration from nature for the design of modern technology is not a novel concept, and has great precedence in materials science [177], engineering [178], and manufacturing [179]. In Biomimicry, author and environmental scientist Janine Benyus describes a number of nature-inspired solutions to human problems, ranging from water-proof adhesives inspired by mussels, to solar cells designed to emulate photosynthesis [180]. A recent review builds on the concept of studying nature to inform neural interface technologies in particular, in which both bioinspiration and biomimicry are used to improve the implementation of brain-contacting devices [181]. To apply this concept to the surgical implantation of electrodes into brain tissue, neural engineers have found inspiration from the study of several species, including the sea cucumber [182], squid [183], and mosquito [184]. In addition to these methods, the steerable needle community has found inspiration from parasitic wasps [185].
A new class of responsive polymers was discovered by studying the dermis architecture of the echinoderm Holothuroidea (sea cucumber), an invertebrate that can rapidly and reversibly switch the stiffness of its skin [90] (figures 9(A) and (B)). This initial breakthrough has led to a new class of bioinspired, chemo-responsive, mechanically-adaptive polymer nanocomposites that are initially rigid, but capable of softening upon exposure to in vivo conditions. This may enable controllably and selectively switching between stiff and compliant states. The design of these nanocomposites mimics the sea cucumber dermis by utilizing a soft polymer matrix (PVA) that is reinforced with a controllable structure of rigid cellulose nanofibers, which interact with each other via hydrogen bonding, forming a load-bearing network with high stiffness. Upon introduction of a compound which can compete for hydrogen bonds (e.g. water), the load bearing network is disassembled, and the stiffness is reduced.
Figure 9.
Surgical implantation approaches inspired by nature. (A) Mechanically-adaptive polymers, such as those found in the sea cucumber dermis, can be rigid during implantation and soften following implantation due to disruption of hydrogen bonds in an aqueous environment. (B) Mosquito prey biting strategy. Reproduced with permission from [181] (copyright Elsevier, 2018). (C) Decrease in Young’s modulus of the nanocomposite in response to physiological conditions. Reproduced with permission from [186] (copyright IOP, 2011). (D) Example of the increase in buckling force possible using a mosquito-inspired support structure. Reproduced with permission from [184].
A comparison between the sea cucumber-inspired nanocomposite-based and neat polymer microprobes showed that the nanocomposites have a higher storage modulus of (E’ ~ 5 GPa) compared to neat polymers (E’ ~ 2 GPa) [186]. The polymer system underwent a phase transition at physiologically relevant temperatures (37–40 °C), allowing the tensile storage modulus to decrease from ~5 GPa to 12 MPa within 15 min of exposure to physiological conditions. The nanocomposite microprobes were implanted in vivo for up to 8 weeks, resulting in increased cell density along with a lack of necrosis or extensive gliosis at the insertion site. Using this biologically-inspired adaptive substrate for intracortical microelectrodes could be useful in that it may decrease the size of the necessary implant to minimize surgical trauma.
To bite prey, a mosquito uses an insertion guide to enable its proboscis to penetrate through the skin, to reach vascular structures and feed off other animals. Shoffstall et al drew inspiration from the mosquito to build intracortical microelectrode insertion guides (figures 9(C) and (D)) [184]. In nature the mosquito can increase the critical buckling force of each fascicle, or blood-sucking microneedle, by reducing the effective length of the fascicles through a secondary structure, the labium, as an insertion guide. This strategy reduces the force required to penetrate the skin, which results in critical loads greater than the penetration load, therefore preventing buckling. The mosquito’s labium is able to brace the fascicles during insertion, which mechanically changes the end condition of the fascicles from a free to a fixed end, thus reducing the effective length factor, K. To mimic the mosquito’s insertion technique, a lateral support containing a narrow slit was designed to enable robust insertion of novel microprobes while preventing buckling. Insertion tests in agarose phantoms of 75 μm-thick, 13.5 mm-long polyethylene probes (PE, E = 300 MPa) resulted in a 92.3% insertion success rate with the mosquito-inspired guide, compared to 23.1% without it. Using the guide, buckling occurred in 19.2% of the trials, while without the guide the buckling rate was 84.6%. This system was also tested in vivo, where the insertion guide enabled 100% successful insertions, compared to only 37.5% without the guide (n = 8 trials). A similar strategy for reducing the effective probe insertion length was recently presented by Arafat et al [187]. This insertion guide contains 4 pairs of arms which support the electrode during implantation. Pt microwires having diameters of 25 μm were inserted with a force of 0.5087 mN, if dura mater was removed first. This method also includes an innovative ‘drilling’ option to penetrate the dura mater, which requires insertion forces up to 10 mN [187].
A similar natural structure worth considering for bioinspired surgical implantation strategies is the stinging element, called the ovipositor, used by wasps. These ovipositors can, in some cases, even penetrate wood. The wasp ovipositor has previously inspired a surgical solution in which a microtextured locking system is applied to DBS leads to mitigate electrode migration [188]. A microtextured surface serves to lock into the tissue without damaging it; this is realted to the fact that the ovipositors of parasitic wasps are not inteded to severely injure their hosts, but to merely allow them to attach to and remain attached to their host. Perhaps this concept could be applied to the problem of electrode micromotion during implantation, which can further exacerbate the foreign body response [189, 190]. The ovipositor has also inspired the design of steerable surgical needles [191, 192], such as the Soft Tissue Intervention and Neurosurgical Guide system (STING), which has been used to access deep brain areas [185]. With some adaptation, the STING approach appears to be potentially promising for the implantation and long-term integration of neural electrodes. Taking further inspiration from nature may inspire solutions to a number of challenges of sustainable neural interfacing hardware, a few of which have been highlighted recently by Shoffstall et al [181].
7. Conclusions
Implantable neuroelectronic interfaces for probing and manipulating the dynamics of neural circuits have advanced our understanding on the fundamental mechanisms governing thoughts, actions, cognition, perception, and disease, as well as the implementation of therapeutic neuromodulation protocols for a number of neurological and neuromuscular disorders. Furthermore, BMI systems integrating high-fidelity recordings up to single cell resolution with sophisticated robotic and artificial neural network technologies, offer the unprecedented opportunity to restore motor control in locked-in and paralyzed individuals. Yet, the issues of longevity, reliability, and biocompatibility of the implanted probes still critically hamper the translational potential of many of these technologies.
The intense research effort dedicated in the last decade to enhance the integration between the host neural tissue and man-made electrodes, has evidenced that complex interactions between biological and structural factors determines the failure modalities of the implanted probes. In addition to the critically pressing issues of long-term material biostability and packaging, the material and mechanical mismatch between the tissue and the electrodes are key determinants of the ultimate probe integration and long-term performance. To address this fundamental mismatch, smaller, softer, more compliant microelectrode designs have been proposed and, with them, sophisticated and ingenious strategies for implanting these soft structures into the brain tissue.
A number of these strategies involve the deposition of hydrogels and biodegradable polymers, to confer temporary rigidity to the electrode during the implantation procedure. In addition to enhancing stiffness, these coatings have been shown to contribute to probe integration, both by reducing friction forces at the electrode-tissue interface and by actively mitigating the response of the immune cells. The main drawback associated to stiffening coatings is the increase in electrode footprint, which exacerbates the acute implantation trauma and severely affects the integrity of the surrounding cells.
The recent push towards microactuation, magnetic, and bio-inspired insertion strategies offers the opportunity to address the implantation challenge, while minimally perturbing the brain natural environment. Regardless of the specific mechanism of insertion, the common approach in all of these strategies relies on minimizing the effective length of the electrode, thereby maximizing the critical buckling force. Leveraging advanced microfabrication and robotic technologies, the validity of these ‘active’ insertion strategies has been demonstrated in large scale implantation of >100 flexible microelectrodes. Finally, innovations from the robotic surgery and steerable needle fields appear to be easily adaptable to the insertion of soft neural probes.
However, despite the successful demonstration of the feasibility of these novel insertion approaches into cortical structures in rodent brains, the gold standards for clinical electrodes have essentially remained unchanged and, with them, the protocols for surgical implantation.
In addition to addressing the structural and material longevity of soft electrode technologies, a viable microelectrode-insertion strategy solution that allows precisely targeting deeper regions in larger species is still missing. This significantly limits the translational potential of soft electrode technologies to clinical care, where typical targets are in subcortical regions. Furthermore, many of the implantation methodologies described here have been developed to implant single lead configurations, either individual microwires or laminar probes with multiple contact sites arranged longitudinally along the shank. As large scale, high density, ultra-high channel count electrode arrays (>1000 contacts) based on soft materials are being developed with the ultimate goal of human translation, there will come the inevitable challenge of engineering a viable strategy for targeted, accurate, and repeatable insertion of these neuroelectronic interfaces.
Acknowledgments
The authors wish to acknowledge the support from the McCabe Fellow Award, the Mirowski Family Foundation, Neil and Barbara Smit (F V), the National Institute of Health (1R21NS106434-01 from NINDS to F V, T32 Training Grant in Neuroenginering to N V A, and R01NS107550 from NINDS to T H L and A G R), and the National Science Foundation Graduate Research Fellowship Program (DGE-1S4529S to N D and B M).
References
- [1].World Health Organization 2006. Neurological Disorders: Public Health Challenges, (Geneva: WHO Press; ) [Google Scholar]
- [2].Agrawal Y. Prevalence of hearing loss and differences by demographic characteristics among us adults: data from the national health and nutrition examination survey, 1999-2004. Arch. Intern. Med. 2008;168:1522. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- [3].Group E D P R. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- [4].Benabid A, Chabardes S, Mitrofanis J and Poliak P 2009. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease Lancet Neurol. 8 67–81 [DOI] [PubMed] [Google Scholar]
- [5].Lega B, Halpern C, Jaggi J and Baltuch G 2010. Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions Neurobiol. Dis 38 354–60 [DOI] [PubMed] [Google Scholar]
- [6].Cukiert A and Lehtimäki K 2017. Deep brain stimulation targeting in refractory epilepsy Epilepsia 58 80–84 [DOI] [PubMed] [Google Scholar]
- [7].Rasche D, Rinaldi P, Young R and Tronnier V 2006. Deep brain stimulation for the treatment of various chronic pain syndromes Neurosurg. Focus 21 E8. [DOI] [PubMed] [Google Scholar]
- [8].Mayberg H, Lozano A, Voon V, McNeely H, Seminowicz D, Hamani C, Schwalb J and Kennedy S 2005. Deep brain stimulation for treatment-resistant depression Neuron 45 651–60 [DOI] [PubMed] [Google Scholar]
- [9].Dobelle WH, Mladejovsky MG and Girvin JP 1974. Artificial vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis Science 183 440–4 [DOI] [PubMed] [Google Scholar]
- [10].Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML and Gaunt RA 2016. Intracortical microstimulation of human somatosensory cortex Sci. Transl. Med 8 361ra141. [DOI] [PubMed] [Google Scholar]
- [11].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS and van der Smagt P 2012. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm Nature 485 372–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJ, Velliste M, Boninger ML and Schwartz AB 2013. High-performance neuroprosthetic control by an individual with tetraplegia Lancet 381 557–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, Sharma G, Sederberg PB, Glenn BC and Mysiw WJ 2016. Restoring cortical control of functional movement in a human with quadriplegia Nature 533 247. [DOI] [PubMed] [Google Scholar]
- [14].Ajiboye AB, Willett FR, Young DR, Memberg WD, Murphy BA, Miller JP, Walter BL, Sweet JA, Hoyen HA and Keith MW 2017. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration Lancet 389 1821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gunasekera B, Gunasekera T, Saxena R, Bellamkonda L and Karumbaiah L 2015. Intracortical recording interfaces: current challenges to chronic recording function ACS Chem. Neurosci 6 68–83 [DOI] [PubMed] [Google Scholar]
- [16].Neely RM, Piech DK, Santacruz SR, Maharbiz MM and Carmena JM 2018. Recent advances in neural dust: towards a neural interface platform Curr. Opin. Neurobiol 50 64–71 [DOI] [PubMed] [Google Scholar]
- [17].Chen D, Hu Y, Cai C, Zeng K and Li X 2017. Brain big data processing with massively parallel computing technology: challenges and opportunities Softw. Pract. Exp 47 405–20 [Google Scholar]
- [18].Ward M, Rajdev P, Ellison C and Irazoqui P 2009. Toward a comparison of microelectrodes for acute and chronic recordings Brain Res. 1282 183–200 [DOI] [PubMed] [Google Scholar]
- [19].Chestek C. et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural. Eng. 2011;8:045005. doi: 10.1088/1741-2560/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Prasad A, Xue Q-S, Sankar V, Nishida T, Shaw G, Streit WJ and Sanchez JC 2012. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants J. Neural. Eng 9 056015. [DOI] [PubMed] [Google Scholar]
- [21].Vetter RJ, Williams JC, Hetke JF, Nunamaker EA and Kipke DR 2004. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex IEEE Trans. Biomed. Eng 51 896–904 [DOI] [PubMed] [Google Scholar]
- [22].Barrese J, Rao N, Paroo K, Triebwasser C, Vargas Irwin C, Franquemont L and Donoghue J 2013. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates J. Neural. Eng 10 066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Michelson NJ, Vazquez AL, Eles JR, Salatino JW, Purcell EK, Williams JJ, Cui XT and Kozai TD 2018. Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface J. Neural. Eng 15 033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wellman SM and Kozai TDY 2017. Understanding the inflammatory tissue reaction to brain implants to improve neurochemical sensing performance ACS Chem. Neurosci 8 2578–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kozai TDY, Jaquins Gerstl A, Vazquez A, Michael A and Cui X T 2015. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies ACS Chem. Neurosci 6 48–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Polikov V, Tresco P and Reichert W 2005. Response of brain tissue to chronically implanted neural electrodes J. Neurosci. Methods 148 1–18 [DOI] [PubMed] [Google Scholar]
- [27].Bjornsson C, Oh SJ, Al-Kofahi Y, Lim Y, Smith K, Turner J, De S, Roysam B, Shain W and Kim SJ 2006. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion J. Neural. Eng 3 196. [DOI] [PubMed] [Google Scholar]
- [28].Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S and Kurihara T 2001. Neurotrophin secretion from cultured microglia J. Neurosci. Res 65 322–31 [DOI] [PubMed] [Google Scholar]
- [29].Salatino JW, Ludwig KA, Kozai TD and Purcell EK 2017. Glial responses to implanted electrodes in the brain Nat. Biomed. Eng 1 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fawcett JW and Asher RA 1999. The glial scar and central nervous system repair Brain Res. Bull 49 377–91 [DOI] [PubMed] [Google Scholar]
- [31].Shearer MC and Fawcett JW 2001. The astrocyte/meningeal cell interface–a barrier to successful nerve regeneration? Cell Tissue Res. 305 267–73 [DOI] [PubMed] [Google Scholar]
- [32].Biran R, Martin DC and Tresco PA 2005. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays Exp. Neurol 195 115–26 [DOI] [PubMed] [Google Scholar]
- [33].Potter K, Buck A, Self W and Capadona J 2012. Stab injury and device implantation within the brain results in inversely multiphasic neuroinflammatory and neurodegenerative responses J. Neural. Eng 9 046020. [DOI] [PubMed] [Google Scholar]
- [34].Silver J 2016. The glial scar is more than just astrocytes Exp. Neurol 286 147–9 [DOI] [PubMed] [Google Scholar]
- [35].Edell DJ, Toi V, McNeil VM and Clark L 1992. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex IEEE Trans. Biomed. Eng 39 635–43 [DOI] [PubMed] [Google Scholar]
- [36].Prasad A, Xue Q-S, Dieme R, Sankar V, Mayrand R, Nishida T, Streit WJ and Sanchez JC 2014. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants Frontiers Neuroeng. 7 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patrick E, Orazem ME, Sanchez JC and Nishida T 2011. Corrosion of tungsten microelectrodes used in neural recording applications J. Neurosci. Methods 198 158–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Coffey RJ 2009. Deep brain stimulation devices: a brief technical history and review Artif. Organs 33 208–20 [DOI] [PubMed] [Google Scholar]
- [39].Zeng S, Zeng F-G, Harrison WR, Xiaoan S and Haihong F 2008. Cochlear implants: system design, integration, and evaluation IEEE Rev. Biomed. Eng 1 115–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Clark GM and Hallworth RJ 1976. A multiple-electrode array for a cochlear implant J. Laryngol. Otology 90 623–7 [DOI] [PubMed] [Google Scholar]
- [41].Shepherd R, Murray M, Hougiton M and Clark GM 1985. Scanning electron microscopy of chronically stimulated platinum intracochlear electrodes Biomaterials 6 237–42 [DOI] [PubMed] [Google Scholar]
- [42].Hudak E, Mortimer J and Martin H 2010. Platinum for neural stimulation: voltammetry considerations J. Neural. Eng 7 026005. [DOI] [PubMed] [Google Scholar]
- [43].Green RA, Matteucci PB, Dodds CWD, Palmer J, Dueck WF, Lovell NH, Hassarati RT, Byrnes Preston PJ and Suaning GJ 2014. Laser patterning of platinum electrodes for safe neurostimulation J. Neural. Eng 11 056017. [DOI] [PubMed] [Google Scholar]
- [44].Wissel K, Brandes G, Pütz N, Angrisani G L, Thieleke J, Lenarz T and Durisin M 2018. Platinum corrosion products from electrode contacts of human cochlear implants induce cell death in cell culture models PloS One 13 e0196649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barrese JC, Aceros J and Donoghue JP 2016. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates J. Neural. Eng 13 026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Apollo NV, Maturana MI, Tong W, Nayagam DAX, Shivdasani MN, Foroughi J, Wallace GG, Prawer S, Ibbotson MR and Garrett DJ 2015. Soft, flexible freestanding neural stimulation and recording electrodes fabricated from reduced graphene oxide Adv. Funct. Mater 25 3551–9 [Google Scholar]
- [47].Chen C-H, Lin C-T, Hsu W-L, Chang Y-C, Yeh S-R, Li L-J and Yao D-J 2013. A flexible hydrophilic-modified graphene microprobe for neural and cardiac recording Nanomedicine 9 600–4 [DOI] [PubMed] [Google Scholar]
- [48].Liu T-C, Chuang M-C, Chu C-Y, Huang W-C, Lai H-Y, Wang C-T, Chu W-L, Chen S-Y and Chen Y-Y 2015. Implantable graphene-based neural electrode interfaces for electrophysiology and neurochemistry in in vivo hyperacute stroke model ACS Appl. Mater. Interfaces 8 187–96 [DOI] [PubMed] [Google Scholar]
- [49].Zhao S, Liu X, Xu Z, Ren H, Deng B, Tang M, Lu L, Fu X, Peng H and Liu Z 2016. Graphene encapsulated copper microwires as highly MRI compatible neural electrodes Nano Lett. 16 7731–8 [DOI] [PubMed] [Google Scholar]
- [50].Chen G, Dodson B, Johnson F, Hancu I, Fiveland E, Zhang W, Galligan C, Puleo C, Davis RC and Ashe J 2018. Tissue-susceptibility matched carbon nanotube electrodes for magnetic resonance imaging J. Magn. Reson 295 72–79 [DOI] [PubMed] [Google Scholar]
- [51].Vitale F, Summer son SR, Aazhang B, Kemere C and Pasquali M 2015. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes ACS Nano 9 4465–74 [DOI] [PubMed] [Google Scholar]
- [52].David Pur M, Bareket Keren L, Beit Yaakov G, Raz Prag D and Hanein Y 2014. All-carbon-nanotube flexible multi-electrode array for neuronal recording and stimulation Biomed. Microdevices 16 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Foroughi J, Spinks G M, Antiohos D, Mirabedini A, Gambhir S, Wallace GG, Ghorbani S R, Peleckis G, Kozlov M E and Lima MD 2014. Highly conductive carbon nanotube-graphene hybrid yarn Adv. Funct. Mater 24 5859–65 [Google Scholar]
- [54].Zhang H, Patel PR, Xie Z, Swanson SD, Wang X and Kotov NA 2013. Tissue-compliant neural implants from microfabricated carbon nanotube multilayer composite ACS Nano 7 7619–29 [DOI] [PubMed] [Google Scholar]
- [55].Keefer E, Botterman B, Romero M, Rossi A and Gross G 2008. Carbon nanotube coating improves neuronal recordings Nat. Nanotechnol 3 434–9 [DOI] [PubMed] [Google Scholar]
- [56].Ahnood A, Meffin H, Garrett DJ, Fox K, Ganesan K, Stacey A, Apollo NV, Wong YT, Lichter SG and Rentier W 2017. Diamond devices for high acuity prosthetic vision Adv. Biosyst 1 1600003. [DOI] [PubMed] [Google Scholar]
- [57].Garrett DJ, Saunders AL, McGowan C, Specks J, Ganesan K, Meffin H, Williams RA and Nayagam DA 2015. In vivo biocompatibility of boron doped and nitrogen included conductive-diamond for use in medical implants J. Biomed. Mater. Res 104 19–26 [DOI] [PubMed] [Google Scholar]
- [58].Ganesan K, Garrett DJ, Ahnood A, Shivdasani MN, Tong W, Turnley AM, Fox K, Meffin H and Prawer S 2014. An all-diamond, hermetic electrical feedthrough array for a retinal prosthesis Biomaterials 35 908–15 [DOI] [PubMed] [Google Scholar]
- [59].Hadjinicolaou A et al. 2012. Electrical stimulation of retinal ganglion cells with diamond and the development of an all diamond retinal prosthesis Biomaterials 33 5812–20 [DOI] [PubMed] [Google Scholar]
- [60].Garrett D, Ganesan K, Stacey A, Fox K, Meffin H and Prawer S 2012. Ultra-nanocrystalline diamond electrodes: optimization towards neural stimulation applications J. Neural. Eng 9 016002. [DOI] [PubMed] [Google Scholar]
- [61].Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA and Kipke DR 2012. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces Nat Mater. 11 1065–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Massey TL, Santacruz SR, Hou JF, Pister KS, Carmena JM and Maharbiz MM 2018. A high-density carbon fiber neural recording array technology J. Neural Eng 16 016024. [DOI] [PubMed] [Google Scholar]
- [63].Zoll RS, Schindler CB, Massey TL, Drew DS, Maharbiz MM and Pister KSJ 2019. MEMS-actuated carbon fiber microelectrode for neural recording IEEE Trans. Nanobiosci 18 234–9 [DOI] [PubMed] [Google Scholar]
- [64].Guitchounts G, Markowitz J, Liberti W and Gardner T 2013. A carbon-fiber electrode array for long-term neural recording J. Neural. Eng 10 046016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cogan S 2008. Neural stimulation and recording electrodes Annu. Rev. Biomed. Eng 10 275–309 [DOI] [PubMed] [Google Scholar]
- [66].Fattahi P, Yang G, Kim G and Abidian MR 2014. A review of organic and inorganic biomaterials for neural interfaces Adv. Mater 26 1846–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Harris AR and Wallace GG 2018. Organic electrodes and communications with excitable cells Adv. Funct. Mater 28 1700587 [Google Scholar]
- [68].Rastogi SK, Kalmykov A, Johnson N and Cohen-Karni T 2018. Bioelectronics with nanocarbons J. Mater. Chem. B 6 7159–78 [DOI] [PubMed] [Google Scholar]
- [69].Kozai TD, Catt K, Li X, Gugel ZV, Olafsson VT, Vazquez AL and Cui XT 2015. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording Biomaterials 37 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Teo AJT, Mishra A, Park I, Kim YJ, Park WT and Yoon YJ 2016. Polymeric biomaterials for medical implants and devices ACS Biomater. Sci. Eng 2 454–72 [DOI] [PubMed] [Google Scholar]
- [71].Loeb GE, Bak M, Salcman M and Schmidt E 1977. Parylene as a chronically stable, reproducible microelectrode insulator IEEE Trans. Biomed. Eng 24 121–8 [DOI] [PubMed] [Google Scholar]
- [72].Schmidt E, McIntosh J and Bak M 1988. Long-term implants of Parylene-C coated microelectrodes Med. Biol. Eng. Comput 26 96–101 [DOI] [PubMed] [Google Scholar]
- [73].Lecomte A, Degache A, Descamps E, Dahan L and Bergaud C 2017. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors Sensors Actuators B 251 1001–8 [Google Scholar]
- [74].Wellman SM, Eles JR, Ludwig KA, Seymour JP, Michelson NJ, McFadden WE, Vazquez AL and Kozai TD 2018. A materials roadmap to functional neural interface design Adv. Funct. Mater 28 1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chen R, Canales A and Anikeeva P 2017. Neural recording and modulation technologies Nat. Rev. Mater 2 16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Takmakov P, Ruda K, Phillips KS, Isayeva IS, Krauthamer V and Welle CG 2015. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species J. Neural. Eng 12 026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Samadi-Dooki A and Voyiadjis GZ 2019. A fully nonlinear viscohyperelastic model for the brain tissue applicable to dynamic rates J. Biomech 84 211–7 [DOI] [PubMed] [Google Scholar]
- [78].Budday S, Sommer G, Holzapfel GA, Steinmann P and Kuhl E 2017. Viscoelastic parameter identification of human brain tissue J. Mech. Behav. Biomed. Mater 74 463–76 [DOI] [PubMed] [Google Scholar]
- [79].Sack I, Beierbach B, Hamhaber U, Klatt D and Braun J 2008. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography NMR Biomed. 21 265–71 [DOI] [PubMed] [Google Scholar]
- [80].Cloots RJ, Van Dommelen J, Kleiven S and Geers M 2013. Multi-scale mechanics of traumatic brain injury: predicting axonal strains from head loads Biomech. Model Mechanobiol 12 137–50 [DOI] [PubMed] [Google Scholar]
- [81].Lehocky CA, Fellows-Mayle W, Engh JA and Riviere CN 2017. Tip design for safety of steerable needles for robot-controlled brain insertion Robot. Surg. Res. Rev 4 107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jeong JW, Shin G, Park SI, Yu KJ, Xu L and Rogers JA 2015. Soft materials in neuroengineering for hard problems in neuroscience Neuron 86 175–86 [DOI] [PubMed] [Google Scholar]
- [83].Lee JH, Kim H, Kim JH and Lee SH 2016. Soft implantable microelectrodes for future medicine: prosthetics, neural signal recording and neuromodulation Lab. Chip 16 959–76 [DOI] [PubMed] [Google Scholar]
- [84].Lecomte A, Descamps E and Bergaud C 2018. A review on mechanical considerations for chronically-implanted neural probes J. Neural. Eng 15 031001. [DOI] [PubMed] [Google Scholar]
- [85].Drake KL, Wise K, Farraye J, Anderson D and BeMent S 1988. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity IEEE Trans. Biomed. Eng 35 719–32 [DOI] [PubMed] [Google Scholar]
- [86].Muller R, Le H-P, Li W, Ledochowitsch P, Gambini S, Bjorninen T, Koralek A, Carmena JM, Maharbiz MM and Alon E 2015. A minimally invasive 64-channel wireless μECoG implant IEEE J. Solid-State Circuits 50 344–59 [Google Scholar]
- [87].Spencer KC, Sy JC, Ramadi KB, Graybiel AM, Langer R and Cima MJ 2017. Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants Sci. Rep 7 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Du ZJ, Kolarcik CL, Kozai TDY, Luebben SD, Sapp SA, Zheng XS, Nabity JA and Cui XT 2017. Ultrasoft microwire neural electrodes improve chronic tissue integration Acta Biomater. 53 46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nguyen JK, Park DJ, Skousen JL, Hess-Dunning AE, Tyler DJ, Rowan S J, Weder C and Capadona JR 2014. Mechanically-compliant intracortical implants reduce the neuroinflammatory response J. Neural. Eng 11 056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Capadona J, Tyler D, Zorman C, Rowan S and Weder C 2012. Mechanically adaptive nanocomposites for neural interfacing MRS Bull. 37 581–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lind G, Linsmeier CE and Schouenborg J 2013. The density difference between tissue and neural probes is a key factor for glial scarring Sci. Rep 3 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thelin J, Jörntell H, Psouni E, Garwicz M, Schouenborg J, Danielsen N and Linsmeier CE 2011. Implant size and fixation mode strongly influence tissue reactions in the CNS PLoS ONE 6 e16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xiang Z, Yen SC, Xue N, Sun T, Tsang WM, Zhang S,, Liao LD, Thakor NV and Lee C 2014. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle J. Micromech. Microeng 24 065015 [Google Scholar]
- [94].Jensen W, Hofmann UG and Yoshida K 2003. Assessment of subdural insertion force of single-tine microelectrodes in rat cerebral cortex Proc. of the 25th Annual Int. Conf. of the IEEE Eng. Med. Biol. Soc. pp 2168–71 [Google Scholar]
- [95].Wester B, Lee R and LaPlaca M 2009. Development and characterization of in vivo flexible electrodes compatible with large tissue displacements J. Neural. Eng 6 024002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chen W-F and Duan L 2014. Bridge Engineering Handbook: Construction and Maintenance (Boca Raton, FL: CRC press; ) [Google Scholar]
- [97].Salcman M and Bak MJ 1973. Design, fabrication, and in vivo behavior of chronic recording intracortical microelectrodes IEEE Trans. Biomed. Eng 20 253–60 [DOI] [PubMed] [Google Scholar]
- [98].Zhang W, Tang J, Li Z and Ma Y 2018. A novel neural electrode with micro-motion-attenuation capability based on compliant mechanisms—physical design concepts and evaluations Med. Biol. Eng. Comput 56 1911–23 [DOI] [PubMed] [Google Scholar]
- [99].Carlsson LA and Kardomateas GA 2011. Structural and Failure Mechanics of Sandwich Composites vol 121 (Berlin: Springer; ) [Google Scholar]
- [100].Lacour SP, Courtine G and Guck J 2016. Materials and technologies for soft implantable neuroprostheses Nat. Rev. Mater 1 16063 [Google Scholar]
- [101].Bloch J, Lacour SP and Courtine G 2017. Electronic dura mater meddling in the central nervous system JAMA Neurol 74 470–5 [DOI] [PubMed] [Google Scholar]
- [102].Kim D-H, Ghaffari R, Lu N and Rogers J 2012. Flexible and stretchable electronics for biointegrated devices Annu. Rev. Biomed. Eng 14 113–28 [DOI] [PubMed] [Google Scholar]
- [103].Lacour SP, Benmerah S, Tarte E, FitzGerald J, Serra J, McMahon S, Fawcett J, Graudejus O, Yu Z and Morrison B 2010. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces Med. Biol. Eng. Comput 48 945–54 [DOI] [PubMed] [Google Scholar]
- [104].Rogers JA, Someya T and Huang Y 2010. Materials and mechanics for stretchable electronics Science 327 1603–7 [DOI] [PubMed] [Google Scholar]
- [105].Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang S-W and Vanleer AC 2011. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo Nat. Neurosci 14 1599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].McCarthy PT, Madangopal R, Otto KJ and Rao MP 2009. Titanium-based multi-channel, micro-electrode array for recording neural signals 2009 Annual Int. Conf. of the IEEE Eng. Med. Biol. Soc. pp 2062–5 [DOI] [PubMed] [Google Scholar]
- [107].Dai X, Hong G, Gao T and Lieber CM 2018. Mesh nanoelectronics: seamless integration of electronics with tissues Acc. Chem. Res 51 309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wei X, Luan L, Zhao Z, Li X, Zhu H, Potnis O and Xie C 2018. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording Adv. Set 5 1700625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Serruya MD et al. 2018. Engineered axonal tracts as “living electrodes” for synaptic-based modulation of neural circuitry Adv. Funct. Mater 28 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Abolhassani N and Patel R 2006. Deflection of a flexible needle during insertion into soft tissue Engineering in Medicine and Biology Society, 2006. EMBS’06. 28th Annual Int. Conf. of the IEEE pp 3858–61 [DOI] [PubMed] [Google Scholar]
- [111].Alterovitz R, Goldberg K, Pouliot J, Taschereau R and and Hsu I-C 2003. Needle insertion and radioactive seed implantation in human tissues: simulation and sensitivity analysis 2003 Proc. ICRA’03. IEEE Int. Conf. on Robotics and Automation pp 1793–9 [Google Scholar]
- [112].Taschereau R, Pouliot J, Roy J and Tremblay D 2000. Seed misplacement and stabilizing needles in transperineal permanent prostate implants Radiother. Oncol 55 59–63 [DOI] [PubMed] [Google Scholar]
- [113].Abolhassani N, Patel R and Moallem M 2007. Needle insertion into soft tissue: a survey Med. Eng. Phys 29 413–31 [DOI] [PubMed] [Google Scholar]
- [114].Chorover SL and Deluca A-M 1972. A sweet new multiple electrode for chronic single unit recording in moving animals Physiol. Behav 9 671–4 [DOI] [PubMed] [Google Scholar]
- [115].Maguire YG, Shapiro MG, Cybulski TR, Glaser JI, Amodei D, Stranges PB, Kalhor R, Dalrymple DA, Seo D and Alon E 2013. Physical principles for scalable neural recording Frontiers Comput. Neurosci 7 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gray CM, Maldonado PE, Wilson M and McNaughton B 1995. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex J. Neurosci. Methods 63 43–54 [DOI] [PubMed] [Google Scholar]
- [117].Rail W 1962. Electrophysiology of a dendritic neuron model Biophys. J 2 145–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lecomte A, Castagnola V, Descamps E, Dahan L, Blatché MC, Dinis TM, Leclerc E, Egles C and Bergaud C 2015. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: towarda double time-scale tool for local drug delivery J. Micromech. Microeng 25 125003 [Google Scholar]
- [119].Agorelius J, Tsanakalis F, Friberg A, Thorbergsson P, Pettersson LME and Schouenborg J 2015. An array of highly flexible electrodes with a tailored configuration locked by gelatin during implantation—initial evaluation in cortex cerebri of awake rats Frontiers Neurosci. 9 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Pas J, Rutz AL, Quilichini P, Slézia A, Ghestem A, Kaszas A, Donahue M, Curto V, O’Connor RP and Williamson A 2018. A bilayered PVA/PLGA-bioresorbable shuttle to improve the implantation of flexible neural probes J. Neural. Eng 15 065001. [DOI] [PubMed] [Google Scholar]
- [121].Tien LW, Wu F, Tang-Schomer MD, Yoon E, Omenetto FG and Kaplan DL 2013. Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes Adv. Funct. Mater 23 3185–93 [Google Scholar]
- [122].Apollo NV, Jiang J, Cheung W, Baquier S, Lai A, Mirebedini A, Foroughi J, Wallace GG, Shivdasani MN and Prawer S 2018. Development and characterization of a sucrose microneedle neural electrode delivery system Adv. Biosyst 2 1700187 [Google Scholar]
- [123].Kozai TDY and Kipke DR 2009. Insertion shuttle withcarboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain J. Neurosci. Methods 184 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lo M-C, Wang S, Singh S, Kohn J, Shreiber DI and and Zahn JD 2015. Coating flexible probes with an ultra fast degrading polymer to aid in tissue insertion Biomed. Microdevices 17 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Felix S, Shah K, George D, Tolosa V, Tooker A, Sheth H, Delima T and Pannu S 2012. Removable silicon insertion stiffeners for neural probes using polyethylene glycol as a biodissolvable adhesive Conf. Proc. IEEE Eng. Med. Biol. Soc pp 871–4 [DOI] [PubMed] [Google Scholar]
- [126].Patel PR, Na K, Zhang H, Kozai TDY, Kotov NA, Yoon E and Chestek CA 2015. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings J. Neural. Eng 12 046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Takeuchi S, Ziegler D, Yoshida Y, Mabuchi K and Suzuki T 2005. Parylene flexible neural probes integrated with microfluidic channels Lab. Chip 5 519–23 [DOI] [PubMed] [Google Scholar]
- [128].Gage GJ, Stoetzner CR, Richner T, Brodnick SK, Williams JC and Kipke DR 2012. Surgical implantation of chronic neural electrodes for recording single unit activity and electrocorticographic signals J. Vis. Exp 60 3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Bjugstad KB, Lampe K, Kern DS and Mahoney M 2010. Biocompatibility of poly(ethylene glycol)-based hydrogels in the brain: an analysis of the glial response across space and time J. Biomed. Mater. Res. A 95 79–91 [DOI] [PubMed] [Google Scholar]
- [130].Bjugstad K, Redmond D Jr, Lampe K, Kern D, Sladek J Jr and Mahoney M 2008. Biocompatibility of PEG-based hydrogels in primate brain Cell Transplant. 17 409–15 [PubMed] [Google Scholar]
- [131].Lee K and Jung H 2012. Drawing lithography for microneedles: a review of fundamentals and biomedical applications Biomaterials 33 7309–26 [DOI] [PubMed] [Google Scholar]
- [132].Lee K, Lee C and Jung H 2011. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose Biomaterials 32 3134–40 [DOI] [PubMed] [Google Scholar]
- [133].Starzak M and Mathlouthi M 2006. Temperature dependence of water activity in aqueous solutions of sucrose Food Chem. 96 346–70 [Google Scholar]
- [134].Hancock BC and Zografi G 1994. The relationship between the glass transition temperature and the water content of amorphous pharmaceutical solids Pharm. Res 11 471–7 [DOI] [PubMed] [Google Scholar]
- [135].Mathlouthi M and Reiser P 1995. Sucrose: Properties and Applications (New York: Springer Science & Business Media; ) [Google Scholar]
- [136].Kozai TD, Gugel Z, Li X, Gilgunn PJ, Khilwani R, Ozdoganlar OB, Fedder GK, Weber DJ and Cui XT 2014. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes Biomaterials 35 9255–68 [DOI] [PubMed] [Google Scholar]
- [137].Khilwani R, Gilgunn PJ, Kozai TD, Ong XC, Korkmaz E, Gunalan PK, Cui XT, Fedder GK and Ozdoganlar OB 2016. Ultra-miniature ultra-compliant neural probes with dissolvable delivery needles: design, fabrication and characterization Biomed. Micro devices 18 97. [DOI] [PubMed] [Google Scholar]
- [138].Lewitus D, Smith K, Shain W and Kohn J 2011. Ultrafast resorbing polymers for use as carriers for cortical neural probes Acta Biomater. 7 2483–91 [DOI] [PubMed] [Google Scholar]
- [139].Lewitus D, Smith K, Shain W, Bolikal D and Kohn J 2011. The fate of ultrafast degrading polymeric implants in the brain Biomaterials 32 5543–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hyon S-H, Cha W-I, Ikada Y, Kita M, Ogura Y and Honda Y 1994. Poly (vinyl alcohol) hydrogels as soft contact lens material J. Biomater. Sci. Polym. Ed 5 397–406 [DOI] [PubMed] [Google Scholar]
- [141].Maulvi FA, Lakdawala DH, Shaikh AA, Desai AR, Choksi HH, Vaidya RJ, Ranch KM, Koli AR, Vyas BA and Shah DO 2016. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery J. Control. Release 226 47–56 [DOI] [PubMed] [Google Scholar]
- [142].Lind G, Linsmeier C, Thelin J and Schouenborg J 2010. Gelatine-embedded electrodes–a novel biocompatible vehicle allowing implantation of highly flexible microelectrodes J. Neural. Eng 7 046005. [DOI] [PubMed] [Google Scholar]
- [143].Ware T et al. 2013. Smart polymers for neural interfaces smart polymers for neural interfaces Polym. Rev 3724 108–29 [Google Scholar]
- [144].Gall K, Yakacki CM, Liu Y, Shandas R, Willett N and Anseth KS 2005. Thermomechanics of the shape memory effect in polymers for biomedical applications J. Biomed. Mater. Res 73A 339–48 [DOI] [PubMed] [Google Scholar]
- [145].Ecker M, Joshi-Imre A, Modi R, Frewin C, Sandoval AG, Maeng J, Gutierrez-Heredia G, Pancrazio JJ and Voit W 2019. From softening polymers to multi-material based bioelectronic devices Multifunct. Mater 2 012001 [Google Scholar]
- [146].Ware T, Simon D, Arreaga-salas DE, Reeder J, Rennaker R, Keefer EW and Voit W 2012. Fabrication of responsive, softening neural interfaces Adv. Funct. Mater 22 3470–9 [Google Scholar]
- [147].Zhao Q, Qi HJ and Xie T 2015. Progress in polymer science recent progress in shape memory polymer: new behavior, enabling materials, and mechanistic understanding Prog. Polym. Sci 49-50 79–120 [Google Scholar]
- [148].Meng Q and Hu J 2009. Composites: part A a review ofshape memory polymer composites and blends Composites Part A 40 1661–72 [Google Scholar]
- [149].Ware T, Simon D, Liu C, Musa T, Vasudevan S, Sloan A, Keefer EW, Ii RLR and Voit W 2013. Thiol-ene/acrylate substrates for softening intracortical electrodes J. Biomed. Mater. Res. B: Appl. Biomater 102B 1–11 [DOI] [PubMed] [Google Scholar]
- [150].Stiller AM. et al. Chronic intracortical recording and electrochemical stability of thiol-ene/acrylate shape memory polymer electrode arrays. Macromachines. 2018;9:500. doi: 10.3390/mi9100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Cabanlit M, Maitland D, Wilson T, Simon S, Wun T, Gershwin ME and Water JVD 2007. Polyurethane shape-memory polymers demonstrate functional biocompatibility in vitro Macromol. Biosci 1 48–55 [DOI] [PubMed] [Google Scholar]
- [152].Voit BW, Ware T, Dasari RR, Smith P, Danz L, Simon D, Barlow S, Marder SR and Gall K 2010. High-strain shape-memory polymers Adv. Funct. Mater 20 162–71 [Google Scholar]
- [153].Ware T, Hearon K, Lonnecker A, Wooley KL, Maitland DJ and Voit W 2012. Triple-shape memory polymers based on self-complementary hydrogen bonding Macromolecules 45 1062–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ware T et al. 2012. Three-dimensional flexible electronics enabled by shape memory polymer substrates for responsive neural interfaces Macromol. Mater. Eng 297 1193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Maitland DJ, Metzger MF, Schumann D, Lee A and Wilson TS 2002. Photothermal properties of shape memory polymer micro-actuators for treating stroke Lasers Surg. Med 11 1–11 [DOI] [PubMed] [Google Scholar]
- [156].Jiang BH, Kelch S and Lendlein A 2006. Polymers move in response to light Adv. Mater 18 1471–5 [Google Scholar]
- [157].Lu H and Gou J 2011. Nanopaper enabled shape-memory nanocomposite with vertically aligned nickel nanostrand: controlled synthesis and electrical actuation Soft Matter. 7 7416–23 [Google Scholar]
- [158].Vialle G, Prima MD, Hocking E, Gall K, Garmestani H, Sanderson T and Arzberger SC 2009. Remote activation of nanomagnetite reinforced shape memory polymer foam Smart Mater. Struct 18 115014 [Google Scholar]
- [159].Chen H, Li Y, Liu Y, Gong T, Wang L and Zhou S 2014. Polymer chemistry memory and drug release † Polym. Chem 5 5168–74 [Google Scholar]
- [160].Song Q, Chen H, Zhou S, Zhao K, Wang B and Hu P 2016. Polymer Chemistry Polymer Chemistry pp 1739–46 [Google Scholar]
- [161].Lendlein A and Langer R 2002. Biodegradable, elastic shape-memory polymers for potential biomedical applications Science 296 1673–7 [DOI] [PubMed] [Google Scholar]
- [162].Feingold J, Desrochers TM, Fujii N, Harlan R, Tierney PL, Shimazu H, Amemori K and Graybiel AM 2011. A system for recording neural activity chronically and simultaneously from multiple cortical and subcortical regions in non-human primates J. Neurophysiol 107 1979–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Muthuswamy J, Okandan M, Jain T and Gilletti A 2005. Electrostatic microactuators for precise positioning of neural microelectrodes IEEE Trans. Biomed. Eng 52 1748–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Musk E 2019. An integrated brain-machine interface platform with thousands of channels (submitted) ( 10.1101/703801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Penskiy I and Bergbreiter S 2012. Optimized electrostatic inchworm motors using a flexible driving arm J. Micromech. Microeng 23 015018 [Google Scholar]
- [166].Chen P and Lai A 2015. Detachable ultrasonic enabled inserter for neural probe insertion using biodissolvable Polyethylene Glycol 2015 Transducers - 2015 18th Int. Conf. on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) pp 125–8 [Google Scholar]
- [167].Chen P, Clark C, Shen CJ, Schaffer C and Lai A 2013. Ultrasonically actuated inserted neural probes for increased recording reliability 2013 Transducers & Eurosensors XXVII: The 17th Int. Conf. on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII) pp 872–5 [Google Scholar]
- [168].Vitale F, Vercosa DG, Rodriguez AV, Pamulapati SS, Seibt F, Lewis E, Yan JS, Badhiwala K, Adnan M and Royer-Carfagni G 2017. Fluidic microactuation of flexible electrodes for neural recording Nano Lett. 18 326–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Pesaran B 2009. Uncovering the mysterious origins of local field potentials Neuron 61 1–2 [DOI] [PubMed] [Google Scholar]
- [170].Buzsã¡ki GR, Anastassiou C and Koch C 2012. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes Nat. Rev. Neurosci 13 407–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tang L, Chen Y and He X 2007. Magnetic force aided compliant needle navigation and needle performance analysis ROBIO 2007 IEEE Int. Conf. on Robotics and Biomimetics pp 612–6 [Google Scholar]
- [172].Petruska A J, Ruetz F, Hong A, Regli L, Sürücü O, Zemmar A and Nelson BJ 2016. Magnetic needle guidance for neurosurgery: initial design and proof of concept 2016 IEEE Int. Conf. on Robotics and Automation (ICRA) pp 4392–7 [Google Scholar]
- [173].Dryg ID, Ward MP, Qing KY, Mei H, Schaffer JE and Irazoqui PP 2015. Magnetically inserted neural electrodes: tissue response and functional lifetime IEEE Trans. Neural. Syst. Rehabil. Eng 23 562–71 [DOI] [PubMed] [Google Scholar]
- [174].Jaroch DB, Ward MP, Chow EY, Rickus JL and Irazoqui PP 2009. Magnetic insertion system for flexible electrode implantation J. Neurosci. Methods 183 213–22 [DOI] [PubMed] [Google Scholar]
- [175].Selim MH and Ratan RR 2004. The role of iron neurotoxicity in ischemic stroke Ageing Res. Rev 3 345–53 [DOI] [PubMed] [Google Scholar]
- [176].Yan X, Zhou Q, Vincent M, Deng Y, Yu J, Xu J, Xu T, Tang T, Bian L and Wang YXJ 2017. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy Sci. Robot 2 eaaq1155. [DOI] [PubMed] [Google Scholar]
- [177].Mohammed JS and Murphy WL 2009. Bioinspired design of dynamic materials Adv. Mater 21 2361–74 [Google Scholar]
- [178].Zhang Q, Yang X, Li P, Huang G, Feng S, Shen C, Han B, Zhang X, Jin F and Xu F 2015. Bioinspired engineering of honeycomb structure–Using nature to inspire human innovation Prog. Mater. Sci 74 332–400 [Google Scholar]
- [179].Leitão P 2008. A bio-inspired solution for manufacturing control systems Int. Conf. on Information Technology for Balanced Automation Systems (Berlin: Springer; ) pp 303–14 [Google Scholar]
- [180].Benyus JM 1997. Biomimicry: Innovation Inspired by Nature (New York: Harper Perenial; ) [Google Scholar]
- [181].Shoffstall AJ and Capadona JR 2018. Bioinspired materials and systems for neural interfacing Current Opin. Biomed. Eng 6 110–119 [Google Scholar]
- [182].Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ and Weder C 2008. Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis Science 319 1370–4 [DOI] [PubMed] [Google Scholar]
- [183].Fox JD, Capadona JR, Marasco PD and Rowan SJ 2013. Bioinspired water-enhanced mechanical gradient nanocomposite films that mimic the architecture and properties of the squid beak J. Am. Chem. Soc 135 5167–74 [DOI] [PubMed] [Google Scholar]
- [184].Shoffstall AJ, Srinivasan S, Willis M, Stiller AM, Ecker M, Vo it WE, Pancrazio JJ and Capadona JR 2018. A mosquito inspired strategy to implant microprobes into the brain Sci. Rep 8 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Frasson L, Ko S, Turner A, Parittotokkaporn T, Vincent J F and Rodriguez Y Baena F 2010. STING: a soft-tissue intervention and neurosurgical guide to access deep brain lesions through curved trajectories Proc. Inst. Mech. Eng. H 224 775–88 [DOI] [PubMed] [Google Scholar]
- [186].Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ and Capadona JR 2011. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes J. Neural. Eng 8 046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Arafat MA, Rubin LN, Jefferys JGR and Irazoqui PP 2019. A method of flexible micro-wire electrode insertion in rodent for chronic neural recording and a device for electrode insertion IEEE Trans. Neural. Syst. Rehabil. Eng 27 1724–31 [DOI] [PubMed] [Google Scholar]
- [188].Parittotokkaporn T, Thomas DG, Schneider A, Huq E, Davies BL, Degenaar P and Baena FR 2012. Microtextured surfaces for deep-brain stimulation electrodes: a biologically inspired design to reduce lead migration World Neurosurg. 77 569–76 [DOI] [PubMed] [Google Scholar]
- [189].Polanco M, Yoon H and Bawab S 2014. Micromotion-induced dynamic effects from a neural probe and brain tissue interface J. Micro/ Nanolithography MEMS MOEMS 13 023009 [Google Scholar]
- [190].Gilletti A and Muthuswamy J 2006. Brain micromotion around implants in the rodent somatosensory cortex J. Neural. Eng 3 189–95 [DOI] [PubMed] [Google Scholar]
- [191].Ko SY and Baena FR 2013. Toward a miniaturized needle steering system with path planning for obstacle avoidance IEEE Trans. Biomed. Eng 60 910–7 [DOI] [PubMed] [Google Scholar]
- [192].Oldfield M, Burrows C, Kerl J, Frasson L, Parittotokkaporn T, Beyrau F and Baena FR 2014. Highly resolved strain imaging during needle insertion: results with a novel biologically inspired device J. Mech. Behav. Biomed. Mater 30 50–60 [DOI] [PubMed] [Google Scholar]