Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for acute myeloid leukemia (AML). However, most patients experience relapse after allo-HSCT, with a poor prognosis, and treatment options are limited. The lack of an ideal targetable antigen is a major obstacle for treating patients with relapsed AML. CD38 is known to be expressed on most AML and myeloma cells, and its lack of expression on hematopoietic stem cells (HSCs) renders it a potential therapeutic target for relapsed AML. To investigate the clinical therapeutic efficacy and safety of CD38-targeted chimeric antigen receptor T (CAR-T-38) cells, we enrolled 6 AML patients who experienced relapse post-allo-HSCT (clinicaltrials.gov: NCT04351022). Prior to CAR-T-38 treatment, the blasts in the bone marrow of these patients exhibited a median of 95% (92–99%) CD38 positivity. Four weeks after the initial infusion of CAR-T-38 cells, four of six (66.7%) patients achieved complete remission (CR) or CR with incomplete count recovery (CRi); the median CR or CRi time was 191 (range 117–261) days. The cumulative relapse rate at 6 months was 50%. The median overall survival (OS) and leukemia-free survival (LFS) times were 7.9 and 6.4 months, respectively. One case relapsed 117 days after the first CAR-T-38 cell infusion, with remission achieved after the second CAR-T-38 cell infusion. All six patients experienced clinically manageable side effects. In addition, multiparameter flow cytometry (FCM) revealed that CAR-T-38 cells eliminated CD38 positive blasts without off-target effects on monocytes and lymphocytes. Although this prospective study has a limited number of cases and a relatively short follow-up time, our preliminary data highlight the clinical utility and safety of CAR-T-38 cell therapy in treating relapsed AML post-allo-HSCT.
Keywords: Chimeric antigen receptor T cells, CAR-T-38, Relapsed acute myeloid leukemia, Allogeneic hematopoietic stem cell transplantation, Cytokine release syndrome
To the Editor,
Acute myeloid leukemia (AML) patients who experience relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) have a dismal prognosis, with a one-year overall survival (OS) rate of only 20% [1, 2]. Recently, chimeric antigen receptor T (CAR-T) cell therapy has emerged as one of the most promising therapeutic approaches for those with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) [3, 4]. Although numerous tumor antigens, such as CD33, CD123 and CLL1, have been explored as potential target antigens for AML treatment in the past few decades [5–7], CAR-T cell therapy in AML remains challenging due to the lack of an ideal specific antigen target and the risk of fatal “off-tumor, on-target” side effects [8].
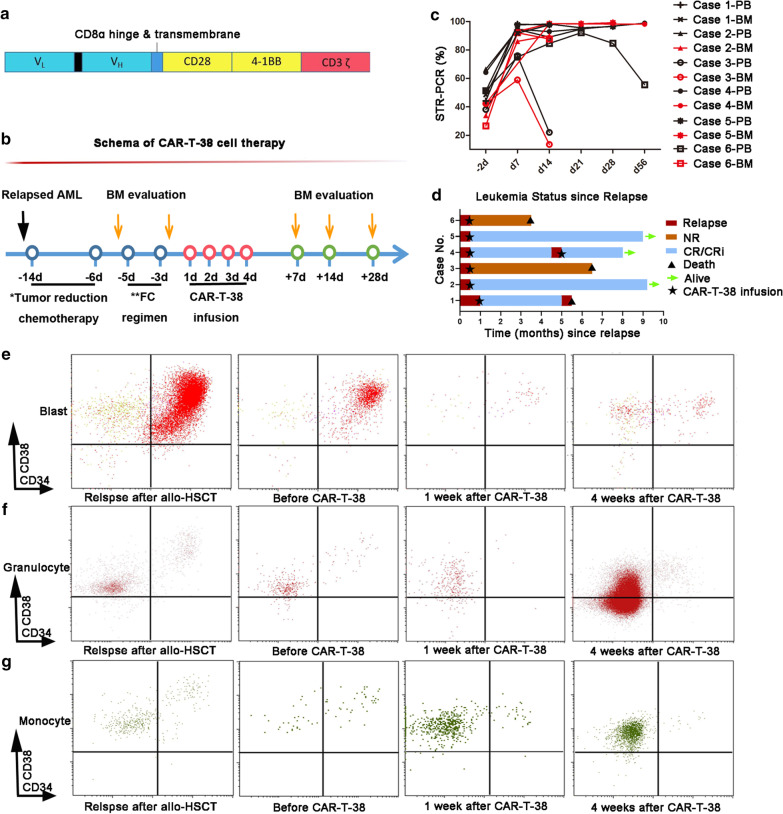
CD38 is known to be expressed on most AML blast cells or plasma cells in multiple myeloma but not on healthy hematopoietic stem cells (HSCs) [9]. Studies of daratumumab monotherapy targeting CD38 report encouraging clinical efficacy, favorable safety profiles and mild hematologic toxicities in multiple myeloma patients [10]. To investigate whether a similar immunotherapeutic approach can be applied for the treatment of AML, we selected the same CD38 epitope (CD38 scFv) as daratumumab for our CD38-CAR-T cells (CAR-T-38) (Fig. 1a). Theoretically, CAR-T-38 cells should exhibit anticancer effects and safety profiles similar to those of daratumumab; moreover, CAR-T-38 cells possess innovative “live-drug” functionality. In this prospective study (NCT04351022), we evaluated the clinical therapeutic efficacy and safety of CAR-T-38 cell therapy in six patients with relapsed AML post-allo-HSCT. None of the cases responded to multiple lines of salvage treatments, and all had CD38-positive blasts in their bone marrow at the time of relapse. The patients’ clinical characteristics are summarized in Table 1. For the initial tumor-reduction chemotherapy (Fig. 1b), only patient 1 achieved blast reduction from 15.5% to 5%, whereas the remaining five showed no response. All patients were pretreated with fludarabine and cyclophosphamide (FC) regimens prior to CAR-T-38 immunotherapy. CAR-T-38 cells (4 from autologous and 2 from donors) at a median dose of 8.05 (6.1–10) × 10 [6] cells per kilogram of body weight were administered after the FC regimens. The median marrow donor chimerism recovered from 46.3% (26.6–66.4%) to 92.5% (58.9–98.1%) at one week and to 97.5% (13.5–98.4%) at two weeks post-CAR-T-38 infusion (Fig. 1c). Moreover, at four weeks after CAR-T-38 infusion, 66.7% of patients (4/6; patients 1, 2, 4 and 5) achieved complete remission (CR) (including 1 with CR and 3 with CR with incomplete count recovery (CRi)) and full donor chimerism. In contrast, patient 6 exhibited a 44% reduction in bone marrow blasts; patient 3 showed no response (NR). Interestingly, patient 4 experienced relapse 117 days after the first CAR-T-38 infusion but did achieve remission after a second CAR-T-38 treatment (Fig. 1d). OS was calculated from the date of CAR-T-38 infusion to the date of death. Leukemia-free survival (LFS) was calculated from the date of CR or CRi post-CAR-T-38 infusion to the date of relapse or death or the last follow-up. The 6-month OS and LFS rates were both 50%, and the median OS and LFS were 7.9 and 6.4 months, respectively (Fig. 1d). Multiparameter flow cytometry (FCM) revealed that CD38-positive blasts were effectively eliminated after CAR-T-38 infusion (Fig. 1e). However, CD38-positive granulocytes and monocytes increased gradually after CAR-T-38 infusion (Fig. 1f, g).
Fig. 1.
CAR-T-38 therapy regimen and treatment response among 6 relapsed AML patients post-allo-HSCT. a Schematic of the CAR-T-38 structure. b Schematic of the CAR-T-38 therapy regimen (* and ** supplementary file). c Variation in donor chimerism in the bone marrow (BM) and peripheral blood (PB) was measured by short tandem repeat-polymerase chain reaction (STR-PCR). d Patients 1, 2, 4, and 5 achieved complete remission (CR) or CR with incomplete count recovery (CRi) at 4 weeks after CAR-T-38 infusion. Patients 1 and 4 relapsed within six months. Patient 4 experienced relapse 117 days after the first CAR-T-38 infusion but exhibited remission after the second CAR-T-38 treatment. The 6-month OS and LFS rates were both 50%. CD38-positive blasts were reduced, and CD38-positive granulocytes and monocytes gradually increased at 1 and 4 weeks after CAR-T-38 infusion. e The CD38-positive blast population is shown in the upper right (red) of the CD34/CD38 plot. f, g CD38-positive granulocytes and monocytes, based on forward versus side scatter and CD38 expression, are shown in the upper left (crimson) and the lower left (green), respectively
Table 1.
Clinical characteristics of patients
| Patient characteristics | N = 6 (%) |
|---|---|
| Median age (range) years | 34.5 (7–52) |
| Female sex | 1 (16.7%) |
| ECOG performance status ≤ 2 | 6 (100%) |
| Disease | |
| AML | 5 (83.3%) |
| AML evolving from MDS | 1 (16.7%) |
| Median percentage of CD38 positive blasts (%) | 95% (92–99%) |
| Allo-HSCT donor | |
| Unrelated donor (10/10) | 2 (33.3%) |
| Haplo donor (5/10) | 4 (66.7%) |
| Conditioning regimen | |
| Modified BuCy | 5 (83.3%) |
| Decitabine + TBI-Cy | 1 (16.7%) |
| Median time to recurrence post-allo-HSCT (months) | 7.5 (5–17) |
| Treatment for relapsed AML post-allo-HSCT | |
| Withdrawal of immunosuppression | 5 (83.3%) |
| Donor lymphocyte infusion | 2 (33.3%) |
| Azacitidine + Venetoclax | 2 (33.3%) |
| Lenalidomide | 1 (16.7%) |
| Interferon-α | 1 (16.7%) |
| Tumor reduction chemotherapy before CAR-T-38 infusion | |
| Decitabine + HAAG | 5 (83.3%) |
| Decitabine + EAAG | 1 (16.7%) |
| Source of CAR-T-38 | |
| Autologous | 4 (66.7%) |
| Donor | 2 (33.3%) |
| Median CAR-T-38 dose (range) | 8.05 (6.1–10) × 106/kg |
| Treatment response | |
| 1 week after CAR-T-38 infusion | 2 CRi (33.3%) |
| 2 weeks after CAR-T-38 infusion | 4 CRi (66.7%) |
| 4 weeks after CAR-T-38 infusion | 4 (3 CR and 1 CRi) (66.7%) |
| 6-month cumulative recurrence | 2 (50%) |
| Adverse events | |
| GvHD | 0 (0.0%) |
| CRS grade 1–2 | 5 (83.3%) |
| CRS grade 3 (hematological toxicity) | 1 (16.7%) |
| ICANS | 0 (0.0%) |
| Neutropenia (< 500/μl) | 6 (100.0%) |
| Thrombocytopenia (< 10,000/μl) | 6 (100.0%) |
| Documented infection | 1(16.7%) |
ECOG: Eastern Cooperative Oncology Group; AML: Acute myeloid leukemia; MDS: Myelodysplastic syndrome; allo-HSCT: Allogeneic hematopoietic stem cell transplantation; Haplo donor: Haploidentical donor; BuCy: High-dose busulfan and cyclophosphamide; TBI: Total body irradiation. HAAG: Homoharringtonine (H), Cytarabine (A), Aclarubicin (A), Granulocyte colony stimulating factor (G); ECAG: Etoposide (E), Cytarabine (C), Aclarubicin (A), Granulocyte colony stimulating factor (G); CRS: Cytokine release syndrome; ICANS: Immune effector cell-associated neurotoxicity syndrome
Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are well-known major adverse events that limit the clinical application of CAR-T cell therapy. In our study, five patients presented mild CRS (Grade I–II), and only one experienced grade III hepatotoxicity with elevated serum transaminase and bilirubin levels according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. All of these events were transient and clinically manageable. All patients had pancytopenia before CAR-T-38 infusion, and neutropenia appeared to persist during CAR-T-38 therapy. The median duration of neutropenia (absolute neutrophil count (ANC) of < 500/μl) was 22 (7–35) days. The median duration of platelet recovery was 25 (18–123) days. No patients manifested neurological toxicities or experienced severe infections.
Although the sample size was small and the follow-up time was limited, our preliminary prospective study highlights that the clinical utility and safety of CAR-T-38 therapy for treating AML patients with relapse post-allo-HSCT.
Acknowledgements
We thank all of the patients who consented to this study.
Abbreviations
- AML
Acute myeloid leukemia
- CAR-T
Chimeric antigen receptor T cell
- CAR-T-38
CD38-CAR-T cell
- Allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- HSCs
Hematopoietic stem cells
- CR
Complete remission
- CRi
CR with incomplete count recovery
- NR
No response
- OS
Overall survival
- LFS
Leukemia-free survival
- CRS
Cytokine release syndrome
- ICANS
Immune effector cell-associated neurotoxicity syndrome
- FCM
Multiparameter flow cytometry
- BuCy
High-dose busulfan and cyclophosphamide
- TBI
Total body radiation
- FC
Fludarabine and cyclophosphamide
- CTCAE
Common Terminology Criteria for Adverse Events
- ANC
Absolute neutrophil count
Authors' contributions
DW, XT, LY, NX, LK, TL, and XZ were responsible for the study concept and design. QC and CQ collected and analyzed the data and wrote the first draft of the manuscript. XT, HD, JY, ZL, QC, BS, and WC treated the patients and assisted in the data collection. QC, CQ, NX, LK, WS, and MZ provided input for the figures and table. XT, QC, CC, and CQ wrote the final draft of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (81873443, 82070162, 81900175, 81400155, 81700139, 81730003), the National Science and Technology Major Project (2017ZX09304021), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Provincial Medical Talent (ZDRCA2016045), the Major Natural Science Research Projects in Institutions of Higher Education of Jiangsu Province (19KJA210002), the Key Science Research Project of Jiangsu Commission of Health (K2019022), the Translational Research Grant of NCRCH (2020ZKZC04), the Natural Science Foundation of Jiangsu Province (BK20190181, BK20201169, BK20170360), the National Key R&D Program of China (2019YFC0840604, 2017YFA0104502), the Key R&D Program of Jiangsu Province (BE2019798), Jiangsu Medical Outstanding Talents Project (JCRCA2016002) and Jiangsu Provincial Key Medical Center (YXZXA2016002).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This clinical trial was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qingya Cui and Chongsheng Qian contributed equally to this work
Contributor Information
Lei Yu, Email: ylyh188@163.com.
Depei Wu, Email: drwudepei@163.com.
Xiaowen Tang, Email: tangxiaowen@suda.edu.cn.
References
- 1.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 2.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:454–459. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119:6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Wei J, Han X, Bo J, Han W. Target selection for CAR-T therapy. J Hematol Oncol. 2019;12:62. doi: 10.1186/s13045-019-0758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13:86. doi: 10.1186/s13045-020-00910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konopleva M, Rissling I, Andreeff M. CD38 in hematopoietic malignancies. Chem Immunol. 2000;75:189–206. doi: 10.1159/000058769. [DOI] [PubMed] [Google Scholar]
- 10.Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–321. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.