Abstract
Polymyxins are an important class of antibiotics for the treatment of bacterial infections due to multi-drug resistant Gram-negative pathogens. However, their clinical utility is limited by nephrotoxicity. Here we report a series of promising next-generation polymyxin nonapeptides identified based on our understanding of the relationship of structure with activity, cytotoxicity, and kidney compartment accumulation. We demonstrate that nonapeptides with an amine-containing N-terminal moiety of specific regio- and stereochemistry possess superior in vitro activity, together with lower cytotoxicity compared to polymyxin B. We further demonstrate that compounds with a beta-branched aminobutyrate N-terminus with an aryl substituent offer a promising combination of low cytotoxicity and kidney exposure, leading to low toxicity in the mouse. From this series, SPR206 has been selected as a development candidate.
Keywords: polymyxin, antimicrobial resistance, multidrug-resistant bacteria, nephrotoxicity, antibacterials, Gram-negative
The rise of multi-drug resistant (MDR) pathogens, particularly Gram-negative bacteria has resulted in much concern around the world. For example, in the EU/European Economic Area alone, the European Centre for Disease Control estimates that 33,000 deaths resulted from antibiotic-resistant bacterial infections in 2015.1 The toll in the US has been estimated at 23,000,2 however, this has recently been suggested to be a significant underestimate.3 To address this global crisis, the World Health Organisation (WHO) recommends that carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant and 3rd generation cephalosporin-resistant Enterobacteriacae are pathogens of critical priority for research and development of new antibiotics.4 Polymyxins remain the only antibiotic class available for treatment of many MDR Gram-negative bacteria, and their clinical use has seen a resurgence in recent years, with polymyxin B (PMB, 1a, Figure 1) and polymyxin E (1b, Colistin, available as the prodrug colistin methane sulphonate, CMS) both in clinical use. However, therapeutic utility of both drugs is limited by toxicities, particularly nephrotoxicity (renal toxicity).5,6 There is therefore a strong need for new polymyxin derivatives with improved therapeutic index.
Figure 1.

Structure of Polymyxin B1 and Polymyxin E1 showing the polymyxin numbering system
1a. Polymyxin B1 R = Ph
1b. Polymyxin E1 (Colistin) R = 2-propyl
Polymyxins are cyclic decapeptides characterised by the presence of five positively charged amine residues from the amino acid 2,4-diamino butyric acid (Dab), and a lipophilic fatty-acyl N-terminus. The major constituents of the natural products, PMB and colistin are shown in Figure 1, using the conventional polymyxin numbering system. The mechanism of action of polymyxins is not fully understood, however it is clear that an initial, specific interaction with the lipid A component of lipopolysaccharide (LPS), in the outer membrane (OM) of Gram-negative bacteria, is crucial for antimicrobial activity. Following this initial interaction, polymyxins go on to disrupt the cytoplasmic membrane, ultimately leading to bacterial cell death. The positively charged amine residues and lipophilic N-terminal chain play a key role in the interaction of polymyxins with LPS.7,8 Nmr studies of PMB with LPS indicate that in addition to the electrostatic interactions of the positively charged Dab residues with the negatively charged phosphate and carboxylate groups of lipid A, the hydrophobic residues of PMB (phenylalanine, leucine, and fatty acyl group) align with the lipid chains of the LPS. The exact mechanism of renal toxicity of polymyxins is unknown, but PMB has been shown to be extensively transported into the kidney of rat and mouse, where it accumulates within renal proximal tubular cells.9,10 The accumulation of PMB and colistin in rat kidney has recently been investigated by mass-spectrometry imaging,11 and a fluorescence-labelled polymyxin derivative has been used to study renal disposition in single renal tubular cells.12,13
Over the past ten years, a number of groups have sought to prepare polymyxin analogues with lower nephrotoxicity, and these have been extensively reviewed.14-18 Specifically, these efforts produced compounds with reduced positive charge (Northern Antibiotics),19 modification of the N-terminus (Cubist, originally from BioSource Pharm),20 and substitution of the Dab residue at position 3 with diaminopropionic acid (Dap) together with N-terminal modification (Pfizer).21 Additionally, modification of the central heptapeptide core has recently been described and shown to be associated with lower in vitro toxicity.22,23 Finally, polymyxins with varying total positive charge and lacking an N-terminal lipid chain have been shown to be devoid of meaningful antibacterial activity, yet capable of potentiating the activity of co-administered antibiotics while showing significantly reduced cytotoxicity.24, 25 The archetypal compound in this arena is polymyxin B nonapeptide (PMBN), although more recently, SPR741 (discovered by Northern Antibiotics and developed by Spero Therapeutics) has completed phase 1 clinic studies.26
Here we report the investigation of the structure-activity relationships (SAR) of a new class of polymyxin antibiotics, derived from PMBN. We chose to start with a nonapeptide scaffold since certain polymyxin nonapeptide derivatives for example compound (2), (Table 1), had been reported to have potent antibacterial activity.14 The importance of the amino acid as position 1 for activity (and toxicity) was at this stage not clearly defined and it was an early target for us to understand this relationship further.
Table 1.
MIC (μg/mL), in vitro cytotoxicity, and kidney exposure of compounds of general structure (Va) n=1 showing the importance of position of the amino group on the N-terminal chain
 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R1C(O)- | diastereomera | E.coli IHMA 558090 |
E. coli ATCC 25922 |
K. pneumoniae ATCC 4352 |
P. aeruginosa ATCC 27853 |
A. baumannii NCTC13424 |
Cytotoxicity (HK-2 IC50 rel to PMB) |
4h kidney level (μg/g) | Kidney AUC4-16 (μg.h/g) |
|
| 1a |  |
- | 4 | 0.25 | 0.125 | 0.5 | 0.25 | 1 | 126 | 688 |
| 2 | - | 16 | 0.5 | 0.5 | 0.5 | 4 | 3.0 | 110 | 574 | |
| 3 |  |
- | ND | 0.25 | 0.25 | 0.5 | 1 | ND | ND | ND |
| 4 | - | ND | 8 | 8 | 1 | >8 | ND | ND | ND | |
| 5 |  |
- | ND | 4 | 4 | 1 | 4 | ND | ND | ND |
| 6 |  |
D1 | 16 | 0.125 | 0.06 | 0.25 | 0.25 | ND | ND | ND |
| 7 | D2 | 2 | 0.06 | 0.06 | 0.125 | 0.25 | 1.1 | 212 | 982 | |
| 8 |  |
D1 | 16 | 0.125 | ND | 0.125 | 0.06 | ND | 242 | ND |
| 9 | D2 | 8 | 0.125 | ND | 0.125 | 0.125 | ND | ND | ND | |
| 10 |  |
D1 | 64 | 2 | 0.5 | 0.5 | >8 | ND | ND | ND |
| 11 | D2 | 2 | 0.125 | 0.06 | 0.125 | 0.125 | 4.2 | 289 | 3320 | |
| 12 |  |
- | ND | 0.5 | 0.5 | 0.5 | 2 | ND | ND | ND |
| 13 |  |
- | ND | >8 | >8 | 4 | >8 | ND | ND | ND |
ND=not determined.
Where compounds were prepared from racemic starting material at the acyl terminus, the resulting diastereomers are denoted D1 or D2 referring to faster-eluting and slower-eluting diastereomers by reverse-phase HPLC respectively. If D1 or D2 are not specified, the material was prepared from starting material of known absolute stereochemistry.
Developing structural relationships for antimicrobial activity, cytotoxicity and kidney drug exposure has uncovered analogues with improved in vitro and in vivo activity and reduced in vivo toxicity, compared to PMB. These novel polymyxin nonapeptides contain an N-terminal acyl moiety bearing an amine-containing substituent with specific regio- and stereochemistry.
Results and Discussion.
In order to investigate the SAR of the N-terminus of polymyxin nonapeptides, derivatives were synthesised from commercially available PMB according to Scheme 1. BOC protection followed by cleavage of the exocyclic peptide chain with the commercially-available protease Savinase® afforded the intermediate BOC-protected heptapeptide (II).27 Introduction of a suitably protected dipeptide unit afforded (III), and deprotection delivered nonapeptide (IV). Finally, acylation of (IV) with the novel N-terminal groups followed by deprotection, afforded compounds of general structure (V). In instances where the N-terminal group contained a chiral centre, separation of the diastereomers could be readily achieved employing preparative HPLC. Tables 1, 2, 3, and 4 show the minimum inhibitory concentration (MIC) of compounds 2 - 47 of general structure (V) against strains of Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 4352), Pseudomonas aeruginosa (ATCC 27853), and Acinetobacter baumannii (NCTC 13424), as well as a strain of E. coli with reduced susceptibility to PMB (IHMA 558090). Additionally, in vitro cytotoxicity against a human kidney proximal tubule epithelial cell line (HK-2) and exposure of each compound in the kidney of mouse following subcutaneous dosing is presented. Polymyxin B (1a) is included for comparison.
Scheme 1. General synthesis of acylated polymyxin nonapeptides Reagents and conditions:

(i)BOC2O, NEt3, CH3CN, H2O; (ii) Savinase®, CH3CN, H2O; (iii) CBZThr (O-tBu)-Dap(BOC)OH, or CBZThr (O-tBu)-Dab(BOC)OH, HATU, NEtiPr2, DCM, DMF; (iv) NH3+HCOO−, 10% Pd/C, MeOH; (v) R1COOH, HATU, NEtiPr2, DCM, DMF ; (vi) TFA, DCM, followed by prep HPLC.
Table 2.
MIC (μg/mL), in vitro cytotoxicity, and kidney exposure of compounds of general structure (V) containing a piperidine or piperazine in the N-terminal group
 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1C(O)- | diastereomera | n |
E.coli IHMA 558090 |
E. coli ATCC 25922 |
K. pneumoniae ATCC 4352 |
P. aeruginosa ATCC 27853 |
A. baumannii NCTC13424 |
Cytotoxicity (HK-2 IC50 rel to PMB) |
4h kidney level (μg/g) | Kidney AUC4-16 (μg.h/g) | |
| 14 |  |
- | 1 | ND | 0.25 | 0.5 | 0.5 | 1 | 20.8 | ND | ND |
| 15 |  |
- | 1 | ND | >8 | >8 | 1 | >8 | ND | ND | ND |
| 16 |  |
- | 1 | ND | 8 | 2 | 1 | 4 | ND | ND | ND |
| 17 |  |
D1 | 1 | ND | 4 | 8 | 1 | 8 | 3.7 | ND | ND |
| 18 | D2 | 1 | ND | 0.25 | 0.125 | 0.25 | 0.125 | 1.9 | ND | ND | |
| 19 |  |
D2 | 1 | ND | 0.25 | 0.125 | 0.5 | 0.25 | 4.1 | ND | ND |
| 20 |  |
D2 | 1 | 4 | 0.06 | 0.06 | 0.25 | 0.125 | 3.6 | 516 | 5462 |
| 21 |  |
D2 | 1 | 8 | 0.25 | 0.125 | 0.25 | 0.5 | 4.6 | ND | ND |
| 22 |  |
- | 1 | 16 | 0.25 | 0.125 | 0.5 | 0.5 | 9.1 | 357 | 3406 |
| 23 |  |
- | 1 | ND | 0.125 | 0.125 | 0.25 | 0.25 | ND | ND | ND |
| 24 |  |
D2 | 0 | 1 | 0.03 | 0.03 | 0.25 | 0.125 | 8.8 | 469 | 5161 |
| 25 |  |
D2 | 0 | 0.5 | 0.125 | 0.06 | 0.5 | 0.125 | 5 | 603 | 4430 |
| 26 |  |
D2 | 0 | 2 | 0.06 | 0.06 | 0.125 | 0.06 | 7.3 | ND | ND |
| 27 |  |
- | 0 | 8 | 0.25 | 0.125 | 0.5 | 0.5 | 16.6 | 510 | 4374 |
| 28 |  |
- | 0 | 8 | 0.125 | 0.06 | 0.25 | 0.125 | 10.0 | ND | ND |
ND=not determined.
Where compounds were prepared from racemic starting material at the acyl terminus, the resulting diastereomers are denoted D1 or D2 referring to faster-eluting and slower-eluting diastereomers by reverse-phase HPLC respectively. If D1 or D2 are not specified, the material was prepared from starting material of known absolute stereochemistry. Solid and dashed lines represent relative stereochemistry. Solid and dashed wedges represent absolute stereochemistry.
Table 3.
MIC (μg/mL), in vitro cytotoxicity and kidney exposure of alpha substituted aminobutyrates and aminopropionates
 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | n | diastereomera |
E. coli IHMA 558090 |
E. coli ATCC 25922 |
K. pneumoniae ATCC 13882 |
P. aeruginosa ATCC 27853 |
A. baumannii NCTC13424 |
A. baumannii ATCC 19003 |
Cytotoxicity HK-2 Rel to PMB |
4h kidney level (μg/g) |
Kidney AUC4-16 (μg.h/g) |
|
| 29 | n-hexyl | 0 | D2 | 1 | 0.06 | ND | 0.125 | 0.06 | 0.125 | 4.2 | 473 | 4896 |
| 30 | n-hexyl | 1 | D1 | 8 | 0.25 | 0.25b | 0.5 | 0.125 | 1 | ND | ND | ND |
| 31 | n-hexyl | 1 | D2 | 1 | 0.125 | 0.125b | 0.125 | 0.03 | 0.125 | 2.3 | 224 | 1390 |
| 32 | 3-methyl butyl | 1 | D2 | 4 | 0.06 | 0.03 | 0.125 | 0.06 | 0.06 | 5.5 | 331 | 1682 |
| 33 | cyclohexyl | 1 | D2 | 4 | 0.06 | 0.125 | 0.125 | 0.06 | 0.125 | 3.8 | 309 | 1276 |
| 34 | cyclohexyl methyl | 1 | D2 | 4 | 0.06 | 0.25 | 0.25 | 0.125 | 0.25 | 3.3 | 430 | 3522 |
| 35 | 3-chlorophenyl | 1 | D2 | 2 | 0.125 | 0.125 | 0.03 | 0.125 | 0.125 | 5 | 589 | ND |
| 36 | 4-chlorophenyl | 1 | D2 | 2 | 0.06 | 0.125 | 0.125 | 0.06 | 0.125 | 4.8 | 533 | ND |
| 37 | 3-naphthyl | 1 | D2 | 2 | 0.03 | 0.125 | 0.125 | 0.125 | ND | 3.5 | 548 | 7568 |
| 38 | 4-chlorobenzyl | 1 | D2 | 2 | ND | 0.25 | 0.125 | 1 | 2.0 | ND | 176 | 1194 |
| 39 | 3-naphthyl methyl | 1 | D2 | 2 | 0.25 | 0.25 | 0.25 | 0.5 | 4.0 | ND | ND | ND |
ND=not determined.
Where compounds were prepared from racemic starting material at the acyl terminus, the resulting diastereomers are denoted D1 or D2 referring to faster-eluting and slower-eluting diastereomers by reverse-phase HPLC respectively. If D1 or D2 are not specified, the material was prepared from starting material of known absolute stereochemistry.
K.pneumoniae ATCC10031.
Table 4.
MIC (μg/mL), in vitro cytotoxicity and kidney exposure of beta substituted aminobutyrates
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | Diastereomera |
E. coli IHMA558090 |
E. coli ATCC 25922 |
K. pneumoniae ATCC 13882 |
P. aeruginosa ATCC 27853 |
A. baumannii NCTC13424 |
A. baumannii ATCC 19003 |
Cytotoxicity HK-2 Rel to PMB |
4h kidney level (μg/g) |
Kidney AUC4-16 (μg.h/g) |
|
| 40 | n-pentyl | D1 | 4 | 0.06 | 0.06 | 0.125 | 0.06 | 0.25 | 5.9 | 224 | 1435 |
| 41 | n-pentyl | D2 | 4 | 0.06 | 0.06 | 0.125 | 0.06 | 0.5 | 6.6 | 470 | 3058 |
| 42 | cyclohexyl | D1 | 4 | 0.06 | 0.125 | 0.06 | 0.25 | ND | 6.9 | 198 | ND |
| 43 | cyclohexyl | D2 | 8 | 0.25 | 0.25 | 0.125 | 0.25 | 0.25 | 7.9 | ND | ND |
| 44 | 3-chlorophenyl | D1 | 8 | 0.125 | 0.125 | 0.25 | 0.06 | 0.125 | 11.6 | 170 | 850 |
| 45 | 3-chlorophenyl | D2 | 8 | 0.125 | 0.125 | 0.125 | 0.5 | ND | 7.8 | 381 | ND |
| 46 | benzyl | D1 | 8 | 0.25 | 0.25 | 0.125 | 0.125 | 2.0 | 12.0 | 159 | 847 |
| 47 | benzyl | D2 | 8 | 0.125 | 0.5 | 0.125 | 0.25 | ND | 8 | 346 | ND |
ND=not determined
Compounds structures are denoted D1 or D2 on the basis of elution order and by comparison with the NMR of (44) and (45) of known absolute stereochemistry (supplementary data).
Contribution of Dab-1 to antimicrobial activity.
It has been suggested,8,14 based on the activity of the octyl nonapeptide, 228 (Table 1), that the Dab residue at position 1 of PMB (1a) is not essential for in vitro antimicrobial activity. Using the N-terminal octanoyl decapeptide 329 (Table 1) as baseline SAR surrogate for PMB we directly explored the contribution of the amine of Dab-1 to the antimicrobial activity of PMB with the synthesis of the glycine analogue 4. MIC values for compound 4 are significantly elevated (except for P. aeruginosa) compared to PMB, indicating that the amine of Dab-1 plays a significant role in antimicrobial activity. This is consistent with structural studies7 in which Dab-1 of PMB appears to make an interaction with a phosphate group of LPS. Similarly, the importance of stereochemical orientation at amino acid-1 (recently also noted by Cooper et al)22 was readily demonstrated by the d-Dab-1 analogue 5, in which antimicrobial activity also degrades compared to PMB. The MIC values for nonapeptide 2 in which the entire Dab-1 residue is missing, do not differ much from compound 3. We hypothesise that since polymyxins rely on both electrostatic and hydrophobic interactions, the absence of the amine interaction in 2 is compensated by increased hydrophobicity in the N-terminal region resulting from loss of the peptide linker. Similar findings on the importance of the amino acid residue Dab-1 in polymyxins have recently been reported.30
Effect of amine positioning in aminoacyl nonapeptides
Having established the importance of the amine side-chain of Dab-1 for antimicrobial activity of polymyxins, we investigated the impact of the length and position of the amine branch, the stereochemistry at the branch point and the nature of the terminal moiety beyond the amine branch on antimicrobial activity and kidney cytotoxicity. Diastereomers 6 and 7 containing an ethylamine branch positioned in exactly the same space as the side-chain of Dab-1 in PMB, both demonstrated antibacterial activity comparable to PMB, but with 7 exhibiting better activity than 6 against the less susceptible strain of E.coli. Interestingly, there is less difference in antimicrobial activity between these diastereomers than was observed between polymyxin decapeptide diastereomers 3 and 5. This could be attributed to greater flexibility in the alkyl substituted aminobutyryl chain (6, 7) than in the corresponding acylated amino acid (3, 5). Moving the terminal alkyl substituent to the beta position of the aminobutyryl group gave rise to diastereomers 8 and 9, which exhibited no significant difference in antimicrobial activity relative to each other and were similar in antimicrobial activity to the alpha-substituted analogues 6 and 7. Shortening the amine branch to the methylamine (compounds 10—11), also resulted in promising activity, though with greater differences in antimicrobial activity between the diastereomers, particularly against A. baumannii and E. coli IHMA 558090. Encouragingly, the more active diastereomer (11) also exhibited a fourfold reduction in HK-2 cytotoxicity compared to PMB. The alpha-amino acids 12 and 13, prepared from d- and l-cyclohexylglycine, respectively, were less potent than compounds with a methylamine or ethylamine branch and also showed a strong diastereomer preference in favour of compound 12. It should be noted that the compound with the N-terminal natural l-amino acid, 13 demonstrated antimicrobial activity against only P. aeruginosa, in agreement with previous findings for des-fatty-acyl polymyxins by Katsuma et al.31 Interestingly, the more active analogue, 12, possesses a d-configuration amino substituent, which is the same orientation of the side chain amino group as l-Dab, i.e. that of the natural polymyxins. The same stereochemical preference (d-amino acids) also forms the basis of a recent patent application from Xellia Pharmaceuticals.32
Exploration of nonapeptides containing a saturated heterocycle in the N-terminal moiety
As demonstrated with compounds 14-28 (Table 2), we found that a primary amine in the N-terminal chain was not essential for antimicrobial activity, and that conformational restriction of the amine into a heterocycle was well-tolerated. Heterocyclic N-terminal groups demonstrated strict regiochemical and stereochemical requirements. The diastereomeric 3-piperidines 14 and 15 highlighted the strong diastereomeric preference for the (S) diastereomer, i.e. the same orientation as the side chain amine in d-Dab. Furthermore, fixing the amine group into the 4-piperidine (16) was not tolerated. The antimicrobial activity of the 3-piperidine terminal group was further improved with the introduction of an alkyl or aryl substituent as demonstrated with 17-21. As described above, a strong diastereomeric preference for the amino-bearing substituent was observed as exemplified by the cis pair 17 and 18. (Going forward, only the active diastereomer is shown). Conversely, cis and trans isomers 18 and 19 exhibited near identical antimicrobial activity, indicating less demand on the stereochemical orientation of the terminal alkyl group compared to the amino-bearing stereocenter. The trans-isobutyl (20) and trans-aryl (21) substituted compounds exhibited comparable antimicrobial activity together with reduced kidney cytotoxicity compared to PMB. The addition of a second amine group in the side chain in the form of substituted piperazines 22 and 23 led to retention of antimicrobial potency and analogue 22 exhibited significantly lower cytotoxicity compared to PMB.
As previously noted by Magee et al,21 substitution of Dab with Dap at the position adjacent to the cyclic core of polymyxin derivatives (position 3, Figure 1) can enhance antimicrobial activity, while simultaneously reducing kidney cytotoxicity. We probed this observation with piperidines 24-26, which generally exhibited improved antimicrobial activity, especially against E. coli IHMA558090, and kidney cytotoxicity compared to the corresponding Dab-3 analogues (19-21). Similarly, N-alkyl and N-aryl piperazines 27 and 28, respectively retained antimicrobial activity and demonstrated improved kidney cytotoxicity compared to Dab-3 counterparts 22 and 23.
Relationship between kidney exposure and in vivo nephrotoxicity
The accumulation of polymyxin B within kidney proximal tubular cells10 is consistent with extensive renal reabsorption and likely a contributing factor in the observed nephrotoxicity of the class. We investigated the exposure in kidney of some of our novel derivatives by measuring drug levels in the kidney at 4, 8 and 16 h after a single subcutaneous dose of 17.2 mg/kg to mouse. The area under the concentration-time curve (AUC) from 4-16 hours was calculated and used to determine the extent of accumulation and persistence of the compounds in the kidney. Disappointingly, and despite improved kidney cell cytotoxicity compared to PMB, promising heterocyclic analogues 20 and 24 demonstrated stronger renal toxicity than polymyxin B based on renal biomarkers of kidney injury in a mouse model of renal toxicity (albeit not statistically significant in the case of 20; supplementary data). This is likely explained by the fact that these analogues exhibited much higher exposure in the kidney than PMB (Table 2). Similarly, piperazine 22 showed no reduction in in vivo renal toxicity compared to PMB despite exhibiting a 9-fold reduction in kidney cytotoxicity. Again, the exposure of 22 in kidney far exceeded that of PMB.
These results directed our search for less toxic analogues on achieving both low kidney exposure and low kidney cytotoxicity, while simultaneously retaining good antibacterial activity.
Unfortunately, all compounds from the heterocyclic series examined showed high kidney exposure compared to PMB (Table 2). Compounds with an acyclic N-terminal moiety showed generally lower kidney exposure than the compounds with N-terminal heterocycles. Additionally, aminobutyryl compounds 7 (Table 1) and 31 (Table 3) showed lower kidney exposure than their aminopropionyl counterparts, 11 (Table 1) and 29 (Table 3), respectively. These results led us to concentrate efforts on substituted aminobutyrates for further optimisation.
Optimisation of alpha and beta-substituted aminobutyrates
Table 3 presents results from efforts to optimise the alpha-substituted acyclic series for antimicrobial activity, kidney cytotoxicity and mouse kidney exposure. In all cases, the amino acid, Dap was utilised at the position 3 (adjacent to the cyclic core). Of note, where compounds contained an N-terminus which was prepared from racemic starting material, the resulting diastereomers were separated and denoted D1 or D2, referring to faster-eluting and slower-eluting diastereomers by reverse-phase HPLC, respectively. Interestingly, we observed that any difference in antimicrobial activity between diastereomers consistently favoured the slower-eluting diastereomer, especially for activity against A. baumannii and E. coli IHMA 558090 (see for example, diastereomers 30 and 31, and the previously described 6 and 7). Thus for all other compounds in Table 3, only the more active diastereomer (D2) is shown. Replacement of the linear alkyl chain in 31 with branched or cycloalkyl groups (32-34) retained excellent in vitro activity, with the possible exception of E. coli IHMA 558090. Similarly, aryl substitution (35-37) maintained parity with compound 31 with respect to antimicrobial activity and kidney cytotoxicity, however, a trend toward higher kidney exposure at the 4-hour time-point was evident. Replacement of the phenyl substituent with a benzylic group (38) corrected the high kidney exposure, however, the benzylic compounds (38, 39) exhibited reduced activity against strains of A. baumanii.
Beta-substituted aminobutyrates with Dap-3 (40-47, Table 4) exhibited an encouraging balance of antimicrobial activity and kidney cytotoxicity. Here, however, an important distinction between the diastereomers (D1 and D2) was noted. While each diastereomer demonstrated a similar antibacterial profile, and similar kidney cytotoxicity, kidney exposure was notably lower for D1, the faster eluting diastereomer, than for D2, the slower eluting diastereomer (compare 40 with 41, 44 with 45, 46 with 47). In particular, compounds 44 and 46 (both D1 diastereomers), exhibit significantly improved kidney cytotoxicity, both >10-fold less cytotoxic than PMB, together with relatively low kidney exposure compared to other aminoacylnonapeptides.
The racemic acyl side-chain of compounds 44 and 45 was separated by chiral HPLC (see Supplementary Information for details), and the absolute stereochemistry of each enantiomer determined by small molecule X-ray crystallography. The side-chain with (3S)-stereochemistry led to compound 44.
As is evident from above, aryl-substitution is advantageous, offering a good balance of antimicrobial activity, kidney cytotoxicity and mouse kidney exposure. The 3-chlorophenyl substituted compound, 44 demonstrates the best overall spectrum of activities and is the subject of further investigations below.
In vivo efficacy.
The efficacy of compound 44 was assessed and compared to PMB employing the neutropenic mouse thigh and lung infection model, Figures 2 and 3, respectively. In the neutropenic mouse thigh model (Figure 2), a carbapenem-resistant strain of A. baumannii, NCTC13301 was employed as the infecting organism. The infection developed well in this model, increasing from 6.1 log10 cfu/g pre-treatment to 9.2 log10 cfu/g over the 16htime course in the untreated control arm. Compound 44 and PMB were administered i.v. three times, at 4-hour intervals, across the same dose range (0.11, 0.43, 0.86, and 3.44 mg/kg/dose). The dose response profiles for compound 44 and PMB were almost identical, with maximum reductions in bacterial burden of 4.3 log10 cfu/g and 3.4 log10 cfu/g relative to the pre-treatment level, respectively.
Figure 2.
Efficacy of compound 44 (SPR206) and PMB against A baumannii NCTC13301 in a neutropenic mouse thigh model.
Both thighs were treated independently to generate two data points per animal. Horizontal bars show the geometric mean. * indicates significant difference from vehicle control (p ≤ 0.05). MIC (μg mL): PMB, 0.25; (44), 0.06.
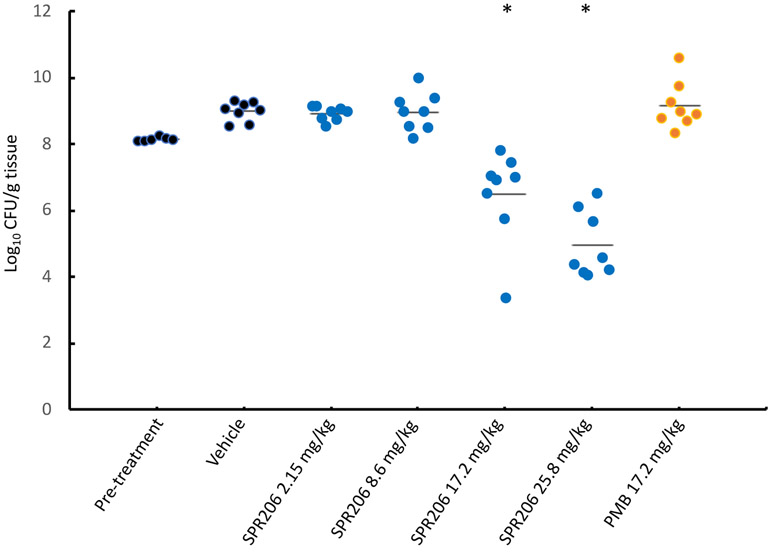
Figure 3.
Efficacy of compound 44 (SPR206) and PMB against A baumannii NCTC13301 in a neutropenic mouse pneumonia model.
Each point represents the bacterial count from one pair of lungs. Horizontal bars show the geometric mean. * indicates significant difference from vehicle control (p ≤ 0.05). MIC (μg mL): PMB, 0.25; (44), 0.06.
In the neutropenic mouse lung infection model utilizing the same infecting organism, A. baumannii NCTC13301 (Figure 3), a higher inoculum (8.1 log10cfu/g) was required to establish robust infection (developed to 9.0 log10cfu/g over the 16htime course). Compound 44 and PMB were administered subcutaneously three times, at 4-hour intervals over the duration of the experiment. In this instance however, compound 44 was administered over a dose range of 2.15, 8.6, 17.2, and 25.8 mg/kg/dose, whereas PMB was administered at only 17.2 mg/kg/dose. In this model, the dose of 17.2 mg/kg PMB, which represents the maximum tolerated dose, had no effect on development of the infection. In contrast, compound 44 at the same dose reduced bacterial burden in the lungs by 1.6 log10cfu/g compared to the pre-treatment level and by 3.2 log10cfu/g compared to the pre-treatment level at 25.8 mg/kg/dose. Notably, compound 44 dosed at 25.8 mg/kg/dose was well tolerated. The poor performance of PMB in models of neutropenic mouse lung infection has previously been noted33 which could perhaps be attributed to relatively low concentrations of PMB in epithelial lining fluid 34
In vivo nephrotoxicity
A model was established to facilitate medium throughput assessment of nephrotoxicity of novel polymyxin derivatives in mice. The model allows the determination of biomarkers for renal injury and corresponding histopathology. Specifically, mice were dosed subcutaneously 4 times at 8-hour intervals. Immediately after the last dose, urine was collected for 24 hours (for assessment of renal biomarkers, KIM-1, cystatin-C, and albumin), after which the mice were sacrificed and kidneys examined for macroscopic and microscopic changes. Results for PMB and novel compounds, 44 and 46, are shown in Table 5. No histopathological changes were noted in kidneys of animals treated with compounds 44 or 46 at 25 mg/kg/dose. In contrast, all animals treated with PMB at 25 mg/kg/dose, and five out of six animals treated with PMB at 12.5 mg/kg/dose, showed signs of tubular degeneration/regeneration, albeit minimal in nature. The levels of KIM-1, cystatin-C, and albumin in urine were statistically significantly higher for PMB than compounds 44 and 46 when dosed at the same 25 mg/kg/dose level. Indeed, the biomarker levels recorded for compound 44 after a 25 mg/kg/dose regimen are more similar to levels recorded for PMB at 12.5 mg/kg/dose. While this model is only an acute assessment of the potential for nephrotoxicity of these novel derivatives, it allows for prioritisation of new polymyxins for additional, longer duration and higher order species evaluation, which has been undertaken for compound 44.35
Table 5.
Histopathology score based on tubular degeneration/regeneration and urinary biomarkers of kidney injury following subcutaneous dosing of PMB, compounds 44 and 46 in the mouse
| PMB 12.5 mg/kg |
PMB 25 mg/kg |
44 25 mg/kg |
46 25 mg/kg |
|
|---|---|---|---|---|
| Kidney Histopathology Score: | ||||
| Zero | 1 | 0 | 6 | 6 |
| Minimal | 5 | 6 | 0 | 0 |
| Urinary Biomarkers: | ||||
| KIM-1 (ng/24h) | 1.6* | ≥58.2 | 0.9* | 2.3* |
| Cystatin C (ng/24h) | 591* | 1425 | 754* | 893* |
| Albumin (μg/24h) | 284* | 1147 | 343* | 749 |
For urinary biomarkers, indicates significantly different (p ≤ 0.05) to PMB at 25mg/kg
Conclusion
A series of novel polymyxin B derivatives with improved antibacterial potency and reduced kidney cytotoxicity compared to polymyxin B has been discovered and described herein. The key to achieving this differentiated profile was replacing the amino acid at position 1 and the fatty acyl chain present in PMB with appropriate branched amine-containing acyl moieties of specific regio- and stereochemistry. We have demonstrated that balancing kidney cytotoxicity and kidney exposure of polymyxin derivatives plays a key role in a preclinical animal model of nephrotoxicity, and that structure-kidney exposure relationships exist for this class and can be optimized. Compounds with an aminobutyrate N-terminus, substituted at the β-position with an aryl-containing moiety, exemplified by compound 44 (denoted SPR206), show reduced cytotoxicity in a renal proximal tubule epithelial cell line and do not show the elevated kidney drug exposure seen with some other aminoacylnonapeptides. This combination of factors leads to low in vivo nephrotoxicity. Compound 44 achieves this while retaining excellent broad-spectrum antibacterial activity that has been shown to translate to excellent in vivo efficacy in models of mouse thigh and lung infection. These promising attributes, and others that have been disclosed elsewhere,35-37 have resulted in the declaration of SPR206 as a drug candidate that is currently undergoing clinical evaluation by Spero Therapeutics.
Experimental Section
For in vitro work, test compounds were supplied as either TFA or acetate salts, and no correction was made for salt form. For all in vivo work test compounds were supplied as acetate salts, and all weights refer to free base equivalent.
Antimicrobial susceptibility testing.
The in vitro antimicrobial activity (MIC) of compounds 1a, and 2 - 47 was determined against Escherichia coli CA058 (IHMA 558090; reduced susceptibility to polymyxins), Escherichia coli ATCC25922, Pseudomonas aeruginosa ATCC27853, Klebsiella pneumoniae ATCC4352, Acinetobacter baumanii NCTC13424 (an OXA-23 carbapenemase producer) and A.baumanii ATCC19003 by broth microdilution using cation-adjusted Mueller-Hinton broth (Oxoid, CM0405) according to CLSI guidelines.38
In vitro cytotoxicity.
Mammalian cell toxicity was measured using confluent monolayers of the human HK-2 proximal tubule epithelial cell line. Compounds were incubated with cells for 24h at 37°C in 5% CO2 using a top concentration of 1,000 or 3,000 μg/mL with semi-log dilutions to give a 9-point concentration range. Cell viability was measured using resazurin blue. Compound concentration values were plotted as log values to enable a dose-response curve to be fitted. The bottom of the curve was constrained to zero and IC50 values were determined using GraphPad Prism. The relative cytotoxicity is reported as the ratio of the IC50 of test compound to that of PMB in the same experiment. (Horizon Discovery Ltd.).
In vivo PK.
Single timepoint (4h) kidney exposure: Drug solutions were prepared at 1.72 mg/mL in water for injection. Groups of male CD-1 mice (n = 2) were dosed by single subcutaneous administration at 17.2mg/kg. Kidneys were collected at 4h post dose and stored immediately at −80°C. On the day of analysis, the kidneys were thawed to room temperature. Each pair of kidneys was weighed and treated with 2 mL/g 9:1 aqueous 0.15% TFA:MeOH. Homogenisation was carried out using ZrO2 beads (ca. 1 g beads/g tissue, Next Advance Bullet Blender Blue CE, BBX24B-CE). Homogenised kidneys were further diluted (30 μl + 970 μl 9:1 aqueous 0.15% TFA:MeOH). Samples were mixed (900 rpm, 20 minutes) then centrifuged (3000 rpm, 15 min). The resulting supernatant was diluted and analysed by LCMS/MS by comparison to standards spiked into blank matrix.
Kidney time course study: Drug solutions were prepared at 1.72 mg/mL in water for injection. Groups of CD-1 mice (n = 3 per timepoint) were dosed by single subcutaneous administration at 17.2mg/kg. Kidneys were collected at 4h, 8h, and 16 h post dose. Kidney preparation was carried out as described above. Analysis of kidney drug levels was carried out by LCMS/MS by comparison to standards spiked into blank matrix. The area under the concentration-time curve was determined over the 4 to 16 hour range.
In vivo efficacy.
For efficacy determination in the neutropenic mouse thigh model male CD-1 mice (n=5) were rendered neutropenic (cyclophosphamide i.p. 150mg/kg d-4, 100mg/kg d-1) then inoculated in each thigh with 1.5 x 105 cfu of A. baumannii NCTC13301. Mice were dosed i.v. with PMB sulphate or test compounds at 2, 6 and 10h post inoculation. One group was sacrificed just prior to dosing and the remainder were harvested after 16h and thighs prepared for colony counts. Both thighs were treated separately to generate two data points per animal (Evotec Ltd.).
For the neutropenic mouse lung model male CD-1 mice (n=8) were rendered neutropenic (cyclophosphamide i.p. 200mg/kg d-4, 150mg/kg d-1) then inoculated intranasally with 3.3 x 107 cfu of A. baumannii NCTC 13301 per lung pair. Mice were dosed s.c. with PMB sulphate or test compounds at 2, 6 and 10h post inoculation. One group was sacrificed just prior to dosing and the remainder were harvested after 16h and lungs prepared for colony counts (Evotec Ltd.). Significance with respect to vehicle control was determined by Kruskal-Wallis test. All in vivo efficacy experiments were performed under UK Home Office Licence (PA67E0BAA) and with local ethical committee clearance (Alderley Park, Agenda AWERB Committee).
In vivo nephrotoxicity.
Renal toxicity in male CD-1 mice (n = 6) was determined for selected compounds after dosing s.c three times per day at 17.2 mg/kg (at 4h intervals) for 4 consecutive days, or after dosing s.c. at 25mg/kg at 8h intervals for 24 hours, with urine collection over the following 24 hours for determination of biomarker levels. Levels of urinary biomarkers KIM-1, cystatin-C and albumin in the urine were measured by ELISA. After urine collection mice were sacrificed for kidney histopathology. Experiments were conducted in accordance with the Animals (Scientific Procedures) Act (1986) Amendment Regulations 2012 with UK Home Office Guidance on the implementation of the Act with all applicable Codes of Practice for the care and housing of laboratory animals.
Synthesis
Synthesis of intermediates IVa and IVb, and final compounds 44 and 45 is given below. Synthesis and characterisation of all non-commercially available carboxylic acids used in the preparation of final compounds, together with the dipeptides CBZThr (O-tBu)-Dab(BOC)OH and CBZThr (O-tBu)-Dap(BOC)OH is given in the supplementary information. Analytical HPLC was performed on all final compounds on an Agilent 1100 System with a Phenomenex Hyperclone C18 BDS 5 μm (4.6 mm x 150 mm) column, eluted with appropriate water/acetonitrile gradients containing 0.15% TFA, with detection at 210 and 254 nm. All final compounds had purity >90% by HPLC. All reagents used for chemical synthesis were purchased from commercially available sources and used without further purification. Preparative HPLC was performed on a Gilson preparative HPLC system using a Waters Sunfire C18 OBD 5 μm (19 mm x 150 mm) column eluted with appropriate water/acetonitrile gradients containing 0.15% TFA, with detection at 210 nm. 1H NMR spectra were recorded at 400 MHz on a Mercury 400 NMR spectrometer (Agilent Technologies) or at 300 MHz on a DPX300 spectrometer (Bruker). Chemicals shifts (δ) are reported in ppm downfield from TMS. Coupling constants J are recorded in Hertz (Hz). Mass spectra were recorded on an LCQ DecaXP mass spectrometer with +ve ion electrospray ionisation. High resolution mass spectral analysis was performed on the Agilent 6200 series time of flight (ToF) system (compound 44). High resolution nanoelectrospray analysis (HRMS (+ve nESI)) was performed on the Thermo LTQ Orbitrap XL using the Advion TriVersa NanoMate (compound 45).
H-Thr(O-tBu)-Dab(BOC)-Cyclo[Dab-Dab(BOC)-DPhe-Leu-Dab(BOC)-Dab(BOC)-Thr] (IVa).
(i) CBZ-Thr(O-tBu)-Dab(BOC)-Cyclo[Dab-Dab(BOC)-DPhe-Leu-Dab(BOC)-Dab(BOC)-Thr] (IIIa)
CBZThr (O-tBu)-Dab(BOC)OH (1.73g, 3.39mmol) and Intermediate II (BOC protected polymyxin heptapeptide)25 (3.0g, 2.8mmol) were charged to a flask to which dry DCM (85mL) and dry DMF (17mL) were added with stirring. To the stirred solution was added N,N-diisopropylethylamine (1.46mL, 8.4mmol) and after stirring for 5 minutes, O-(7-azabenzotriazol −1-yl)-N,N,N’N’-tetramethyluronium hexafluorophosphate (1.29g, 3.39mmol) was added in a single portion. The mixture was sonicated for 2 minutes then left to stir at ambient temperature for 18h. The reaction mixture was then evaporated and the residue azeotroped with toluene (3 x 100mL) to remove residual DMF. The residue was dried under vacuum for 3h to ensure complete removal of toluene. Water (50mL) was added to this material and the mixture was rapidly stirred for 3h with occasional sonication. The title compound was collected by suction filtration as a fine, white solid and washed with water (2 x 25mL) then dried under vacuum for 15h to obtain the protected coupled product as a white solid (4.6g, 3.0mmol, 100% yield), m/z 1554 [M+H]+.
(ii) Title Compound
Intermediate IIIa (CBZ-Thr(O-tBu)-Dab(BOC)-Cyclo[Dab-Dab(BOC)-DPhe-Leu-Dab(BOC)-Dab(BOC)-Thr], 5.41g, 3.48mmol), ammonium formate (6.6g, 104.4mmol) and 10% Pd-C (2.0g) were charged to a flask under N2. MeOH (270ml) was added and the mixture was stirred under N2 for 4.5h. LCMS showed MH+ for product and loss of starting material. The mixture was filtered under suction through a pad of celite and washed with MeOH (50mL). The filtrate and washings were evaporated to a colourless oil which was partitioned between a solvent mixture of EtOAc/MeOH (4:1; 250mL) and water (250mL). The aqueous phase was further extracted with the same, fresh solvent mixture (2 x 100mL). The combined organic extracts were dried (Na2SO4) and evaporated to a colourless oil. This material was purified by chromatography on silica gel (100g SepPak column) eluting with a gradient of 0 – 4% MeOH in EtOAc (0-4%). Fractions containing the product (Rf 0.30 in EtOAc/MeOH/NH4OH880 95:5:1, visualized with KMnO4 spray) were pooled and evaporated to give the title compound as a crisp foam (4.0g, 2.8mmol, 81% yield), m/z 1418.8 [M+H]+, 660.5 [M-BOC+2H]2+. 1H NMR (300 MHz, CD3OD) 0.68 – 0.76 (7H, m), 1.12 – 1.25 (17H, m, incl 1.20, s, O-tBu), 1.48 (36H, s, O-tBu), 1.78 – 2.05 (11H, m), 2.21 – 2.32 (1H, m), 2.94 – 3.30 (11H, m), 3.50 – 3.60 ( 1H, m), 4.01 – 4.18 (4H, m), 4.26 – 4.50 (6H, m), 7.22 – 7.38 (5H, m).
H-Thr(O-tBu)-Dap(BOC)-Cyclo[Dab-Dab(BOC)-DPhe-Leu-Dab(BOC)-Dab(BOC)-Thr] (IVb)
Intermediate II (BOC protected polymyxin heptapeptide)25 was coupled to the dipeptide CBZThr (O-tBu)-Dap(BOC)OH on a 6.23 mmol scale as described for intermediate IIIa. The CBZ protected derivative was deprotected without further purification, as described for Intermediate IVa. After work-up, the crude deprotected product was chromatographed in 2 batches each on a 340g silica column with slow gradient elution of 0 – 100% ethyl acetate to solvent A (where solvent A = ethyl acetate: methanol: concentrated ammonium hydroxide (SG.880)) to remove a closely-running unidentified by-product. Product-containing fractions were combined and evaporated to give the desired material as a white foam (3.1g, 35%). m/z 1406 [M+H]. 1H NMR (300 MHz, CD3OD) 0.70-0.76 (7H, m), 1.19 – 1.28 (16H, m), 1.46 (36H, s, O-tBu), 1.82 – 2.04 (11H, m), 2.21 – 2.35 (2H, m), 3.01 – 3.20 (6H, m), 3.22 – 3.35 (4H, m), 3.48 – 3.70 (3H, m), 4.05 – 4.18 (4H, m), 4.26 – 4.50 (6H, m), 7.22 – 7.38 (5H, m).
[4-amino-3-(3-chlorophenyl)butanoyl]-Thr-Dap-Cyclo[Dab-Dab-DPhe-Leu-Dab-Dab-Thr] (44) and (45).
Intermediate IVb (249 mg, 0.177 mmol) was dissolved in dichloromethane (30 mL), and treated with 4 ((tert-butoxycarbonyl)amino)-3-(3-chlorophenyl)butanoic acid (67 mg, 1.2 equiv) and N,N-diisopropylethylamine (0.087 mL, 3.0 equiv.), followed by HATU (135 mg, 2.0 equivalent). After 16 h completion of the reaction was confirmed by LCMS and the reaction mixture was evaporated to dryness. Water (30 mL) was added and the mixture triturated then stirred vigorously for 3 h. The resultant precipitate was collected by filtration and dried in vacuo overnight. (292 mg obtained). This Boc-protected material was dissolved in dichloromethane (4.5mL) and treated with TFA (1.5 mL). The reaction mixture was stirred at room temperature for 5h until LCMS confirmed complete deprotection. The solvent was evaporated and the residue chromatographed by preparative HPLC. Fractions containing the early-running diastereomer were combined, evaporated to low volume, and lyophilised to afford 44 as the TFA salt as a white solid (48mg). Fractions containing the later-running diastereomer were combined, evaporated to low volume, and lyophilised to afford 45 as the TFA salt as a white solid (48 mg). Each compound was converted to the corresponding acetate salt as follows: AG1-X2 resin (Bio-Rad Laboratories Ltd) acetate form 200-400 mesh, (3g) was placed in a fritted cartridge and conditioned by washing with 10% aqueous acetic acid followed by 1% aqueous acetic acid. A solution of the compound 44 TFA salt (48mg) in water (1mL) was applied to the column, and the column allowed to drip under gravity, eluting with water. Product-containing fractions were combined and lyophilised to a white solid (40 mg).
44: (faster isomer) 1H NMR (400 MHz, D2O): δ (ppm) 0.70 (3 H, d, J 6.1 Hz), 0.77 (3 H, d, J 6.3 Hz), 0.78-0.90 (1H, m), 1.13 (3H, d, J 6.3 Hz), 1.17 (3H, d, J 6.4Hz), 1.36-1.52 (2H, m), 1.75-2.06 (17 H, m, includes 1.91, s, OAc), 2.10-2.30 (4H, m), 2.72-2.91 (4H, m), 3.02-3.49 (15H, m), 4.12-4.32 (8 H, m), 4.48 (1 H, dd, J 5.6, 9.0 Hz), 4.54-4.60 (1H, m), 4.63-4.68 (1H, m), 7.25-7.41(9H, m). 13C NMR (100 MHz, D2O): δ (ppm) 17.7, 18.4, 20.8, 22.1 (AcOH), 21.9, 22.7, 27.3, 27.2, 29.7, 30.1, 34.6, 35.1, 35.6, 35.8, 36.1, 37.8, 38.4, 39.2, 39.6, 42.7, 49.6, 49.8, 50.0, 50.3, 50.8, 51.5, 53.1, 55.3, 59.2, 59.6, 66.3, 67.1, 127.0, 128.2, 128.6, 129.4, 129.8, 129.9, 131.8, 134.9, 136.1, 140.8, 170.0, 170.3, 171.8, 172.0, 172.3, 172.6, 172.8, 173.0, 173.2, 174.6, 178.1 (AcOH). m/z 1145 [M+H]+, 573[M+2H]2+. HRMS (+ve ESI) (m/z): [M + H]+ calcd for C52H83ClN15O12, 1144.6029; found,1144.5973.
In a similar way, compound 45 as the TFA salt (44mg) was converted to the corresponding acetate salt (33mg). 1H NMR (400 MHz, D2O): δ (ppm) 0.60- 0.67 (6 H, m), 0.69 – 0.84 (4 H, m), 1.16 (3H, d, J 6.4Hz), 1.33-1.50 (2 H, m), 1.76-2.04 (14 H, m, includes 1.88, s, OAc), 2.06-2.26 (4 H, m), 2.67-2.86 (4 H, m), 3.00-3.16 (8 H, m), 3.23 – 3.48 (7H, m), 3.98-4.04 (1 H, m) 4.14-4.30 (7H, m), 4.45 (1 H, dd, J 5.6, 9.0 Hz), 4.54 (1 H, appears as t, J 8.3 Hz), 4.72 (1 H, dd, J 5.0, 8.9 Hz), 7.20-7.40 (9 H, m). m/z 1145 [M+H]+, 573[M+2H]2+. HRMS (+ve nESI) [M+H]+ C52H83N15O12Cl, 1144.6029; found,1144.6013; [M+2H]2+ calcd for C52H84N15O12Cl, 572.8051; found, 572.8043; [M+3H]3+ calcd for C52H85N15O12Cl, 382.2058; found, 382.2058.
[(3S)-4-amino-3-(3-chlorophenyl)butanoyl]-Thr-Dap-Cyclo[Dab-Dab-DPhe-Leu-Dab-Dab-Thr] (44) Alternative synthesis:
Compound 44 was also synthesised from Intermediate IVb and (3S)-4-{(tert-butoxycarbonyl)amino}-3-(3-chlorophenyl)butanoic acid under the conditions described above, to obtain material identical to that obtained by separation of the diastereomers.
Supplementary Material
Acknowledgements:
This work was partially supported by a grant awarded to Cantab Anti-infectives Ltd by Innovate UK under the Biomedical Catalyst scheme, award reference 101357. This work has been funded in part with US Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract HHSN272201500014C. In-life pharmacokinetics and renal toxicity studies were carried out at Pharmidex Pharmaceutical Services, Ltd, UK. Histopathology was performed by Charles River Laboratories, Edinburgh, UK. In vivo efficacy experiments were performed at Evotec (UK) Ltd, Alderley Park, Macclesfield, Cheshire, UK. Cytotoxicity assays were performed at Horizon Discovery Ltd, Cambridge, UK. Scale-up of compound 44, chiral separation and small-molecule X-ray structure determination of intermediates was carried out at WuXi Apptec (Shanghai) Ltd, P.R.China. Strain of E.coli with reduced susceptibility to Polymyxin B was obtained from IHMA Europe Sarl, Monthey, Switzerland. High resolution mass spectrometry was carried out at the National Mass Spectrometry Facility at Swansea University, UK.
Footnotes
Supporting Information
Syntheses of carboxylic acids, chiral separation of 3-{[(tert-butoxycarbonyl)amino]methyl}-4-phenylbutanoic acid, confirmation of stereochemistry by small molecule X-ray crystallography, syntheses of dipeptides, characterisation of final compounds 2 - 43 and 46 - 47, mouse renal toxicity of compounds 20, 22 and 24.
References
- (1).Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, and Monnet DL (2018) Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis (18)30605–4. DOI: 10.1016/S1473-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: 2013. [Google Scholar]
- (3).Burnham JP, Olsen MA, and Kollef MH (2018) Re-estimating annual deaths due to multidrug-resistant organism infections. Infection Control & Hospital Epidemiology 0, 1–2 DOI: 10.1017/ice.2018.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Prioritisation of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections including tuberculosis. Geneva: World Health Organisation; 2017(WHO/EKP/IAU/2017.12). [Google Scholar]
- (5).Elias LS, Konzen D, Krebs JM, and Zavascki AP (2010) The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J. Antimicrob. Chemother 65, 2231–2237. DOI: 10.1093/jac/dkq285 [DOI] [PubMed] [Google Scholar]
- (6).Justo JA and Bosso JA (2015) Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy 35 (1), 28–33. DOI: 10.1002/phar.1493 [DOI] [PubMed] [Google Scholar]
- (7).Mares J, Kumaran S, Gobbo M, and Zerbe O (2009) Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J. Biol. Chem 284, 11498–11506. DOI: 10.1074/jbc.M806587200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kanazawa K, Sato Y, Ohki K, Okimura K, Uchida Y, Shindo M, and Sakura N (2009) Contribution of each amino acid residue in polymyxin B to antimicrobial and lipopolysaccharide binding activity. Chem. Pharm. Bull. (Tokyo), 57, 240–244. DOI: 10.1248/cpb.57.240 [DOI] [PubMed] [Google Scholar]
- (9).Abdelrauf K, He J, Ledesma KR, Hu M, and Tam V (2012) Pharmacokinetics and Renal Disposition of Polymyxin B in an Animal Model. Antimicrob. Agents Chemother 56 (11), 5724–5727. DOI: 10.1128/AAC.01333-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Abdelrauf K, Chang K-T, Yin T Hu M, and Tam VH (2014) Uptake of Polymyxin B into Renal Cells. Antimicrob. Agents Chemother, 58, 4200–4202. DOI: 10.1128/AAC.02557-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nilsson A, Goodwin RJA, Swales JG, Gallagher R, Shankaran H, Sathe A, Selvi Pradeepan S, Xue A, Keirstead N, Sasaki JC, Andren PE, and Gupta A (2015) Investigating Nephrotoxicity of Polymyxin Derivatives by Mapping Renal Distribution Using Mass Spectrometry Imaging. Chem. Res. Toxicol 28, 1823–1830. DOI: 10.1021/acs.chemrestox.5b00262. [DOI] [PubMed] [Google Scholar]
- (12).Yun B, Azad MAK, Wang J, Nation RL, Thompson PE, Roberts KD, Velkov T, and Li J (2015) Imaging the distribution of polymyxins in the kidney. J. Antimicrob. Chemother 70, 827–829. DOI: 10.1093/jac/dku441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yun B, Azad MAK, Nowell CJ, Nation RL, Thompson PE, Roberts KD, Velkov T, and Li J (2015) Cellular Uptake and Localization of Polymyxins in Renal Tubular Cells Using Rationally Designed Fluorescent Probes. Antimicrob. Agents Chemother 59, 7489–7496. DOI: 10.1128/AAC.01216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Velkov T, Thompson PE, Nation RL and Li J (2010) Structure-activity relationships of Polymyxin Antibiotics. (2010) J. Med. Chem, 53, 1898–1916. DOI: 10.1021/jm900999h [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Vaara M (2013) Novel derivatives of polymyxins. J. Antimicrob. Chemother 68, 1213–1219. DOI: 10.1093/jac/dkt039 [DOI] [PubMed] [Google Scholar]
- (16).Velkov T, Roberts KD, Thompson PE and Li J (2016) Polymyxins: a new hope in combating Gram-negative superbugs. Future Med. Chem 8 1017–1025. DOI: 10.4155/fmc-2016-0091 [DOI] [PubMed] [Google Scholar]
- (17).Brown P and Dawson MJ (2017) Development of new polymyxin derivatives for multi-drug resistant Gram-negative infections. J. Antibiotics, 70, 386–394. DOI: 10.1038/ja.2016.146 [DOI] [PubMed] [Google Scholar]
- (18).Rabanal F and Cajal Y (2017) Recent advances and perspectives in the design and development of polymyxins. Nat. Prod. Rep 34, 886–908. DOI: 10.1039/c7np00023e [DOI] [PubMed] [Google Scholar]
- (19).Vaara M, Fox J, Loidl G, Siikanen O, Apajalahti J, Hansen F, Frimodt-Møller N, Nagai J, Takano M, and Vaara T (2008) Novel polymyxin derivatives carrying only three positive charges are effective antibacterial agents. Antimicrob. Agents Chemother 52, 3229–3236. DOI: 10.1128/AAC.00405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Leese RA, Biosource Pharm, Inc. Antibiotic compositions for the treatment of Gram Negative Infections. International Patent Application WO2010075416, July 1st 2010. [Google Scholar]
- (21).Magee TV, Brown MF, Starr JT, Ackley DC; Abramite JA, Aubrecht J, Butler A, Crandon JL, Dib-Hajj F, Flanagan ME, Granskog K, Hardink JR, Huband MD, Irvine R, Kuhn M, Leach KL, Li B, Lin J, Luke DR, MacVane SH, Miller AA, McCurdy S, McKim JM Jr., Nicolau DP, Nguyen TT, Noe MC, O'Donnell JP, Seibel SB, Shen Y, Stepan AF, Tomaras AP, Wilga PC, Zhang L, Xu J, Chen JM (2013) Discovery of Dap-3 polymyxin analogues for the treatment of multidrug-resistant Gram-negative nosocomial infections. J. Med. Chem 56, 5079–5093. DOI: 10.1021/jm400416u [DOI] [PubMed] [Google Scholar]
- (22).Gallardo-Godoy A, Muldoon C, Becker B, Elliott AG, Lash LH Huang JX, Butler M, Pelingon R, Kavanagh AM, Ramu S, Phetsang W, Blaskovitch MAT, and Cooper M (2016) Activity and predicted nephrotoxicity of synthetic analogues based on Polymyxin B. J. Med. Chem 59 (3) 1068–1077. DOI: 10.1021/acs.jmedchem.5b01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Vaara M, Vaara T and Tyrrell JM (2017) Structure–activity studies on polymyxin derivatives carrying three positive charges only reveal a new class of compounds with strong antibacterial activity. Peptides 91, 8–12. DOI: 10.1016/j.peptides.2017.03.002 [DOI] [PubMed] [Google Scholar]
- (24).Vaara M (2019) Polymyxin Derivatives that Sensitize Gram-Negative Bacteria to Other Antibiotics. Molecules 24, 249–264. DOI: 10.3390/molecules24020249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Vaara M, Siikanen O, Apajalahti J, Fox J, Frimodt-Møller N, He H, Poudyal A, Li J, Nation RL, and Vaara T (2010) A novel Polymyxin derivative that lacks the fatty acyl tail and carries only three positive charges has strong synergism with agents excluded by he intact outer membrane. Antimicrob. Agents Chemother 54, 3341–3346. DOI: 10.1128/AAC.01439-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Eckburg P, Farinola N, Utley L, Walpole S, T Keutzer T, Kopp E, Coleman S, Tomayko J (2018) Safety of SPR741, a novel polymyxin potentiator, in healthy adults receiving single- and multiple-dose intravenous administrations. Poster 2206, ECCMID., Madrid. [Google Scholar]
- (27).Li B, Akin A, Magee TV, Martinez C, Szeliga J Vuong DV (2015) Syntheses of Dap-3 Polymyxin Analogues via a Tris-Boc-Protected Polymyxin B Heptapeptide. Synthesis 47, 2088–92. DOI: 10.1055/s-0034-1380549 [DOI] [Google Scholar]
- (28).Okimura K; Ohki K; Sato Y; Ohnishi K; Uchida Y; Sakura N (2007) Chemical conversion of natural polymyxin B and colistin to their N-terminal derivatives. Bull. Chem. Soc. Jpn 80, 543–55 [Google Scholar]
- (29).Sakura N; Itoh T; Uchida Y; Ohki K; Okimura K; Chiba K; Sato Y; Sawanishi H (2004) The contribution of the N-terminal structure of polymyxin B peptides to antimicrobial and lipopolysaccharide binding activity. Bull. Chem. Soc. Jpn 77, 1915–1924. [Google Scholar]
- (30).Gallardo-Godoy A, Hansford KA, Muldoon C, Becker B, Elliott AG, Huang JX, Pelingon R, Butler MS, Blaskovich MAT, and Cooper MA (2019) Structure-Function Studies of Polymyxin B Lipononapeptides. Molecules 24, 553–566. doi: 10.3390/molecules24030553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Katsuma N, Sato Y, Ohki K, Okimura K, Ohnishi K, and Sakura N (2009) Development of des-fatty acyl-Polymyxin B decapeptide analogs with Pseudomonas aeruginosa-specific antimicrobial activity. Chem. Pharm. Bull 57, 332–336. DOI: 10.1248/cpb.57.332 [DOI] [PubMed] [Google Scholar]
- (32).Apeland IM, Bjørnstad V, Budnjo A, Gunnes S, Kenyon RF, and Larsson CAR, Xellia Pharmaceuticals APS. Polymyxin Derivatives. International Patent Application WO2016166103, October 20th 2016. [Google Scholar]
- (33).Bowers DR, Cao H, Zhou J, Ledesma KR, Sun D, Lomovskaya O, and Tam V (2015). Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob. Agents Chemother 59, 2720–2725. DOI: 10.1128/AAC.04110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).He J, Gao S, Hu M, Chow DS-L, and Tam VH (2013). A validated ultra-performance liquid chromatography–tandem mass spectrometry method for the quantification of polymyxin B in mouse serum and epithelial lining fluid: application to pharmacokinetic studies. J. Antimicrob. Chemother 68, 1104–1110. DOI: 10.1093/jac/dks536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lister T, Utley L, and Bleavins M (2018). A GLP 14 Day Repeat Dose Toxicology Study of SPR206 in Monkeys. Poster 146, ASM-ESCMID, Lisbon, Portugal. [Google Scholar]
- (36).Grosser L, Heang K, Rubio A (2018). In Vivo Efficacy of SPR206 in an Immunocompetent Murine Ascending Urinary Tract Infection Model Caused by Escherichia coli. Poster 144, ASM-ESCMID, Lisbon, Portugal. [Google Scholar]
- (37).Grosser L, Heang K, Teague J, Warn P, Corbett D, Dawson MJ, and Rubio A, (2018). In Vivo Efficacy of SPR206 in Murine Lung and Thigh Infection Models Caused by Multidrug Resistant Pathogens Pseudomonas aeruginosa and Acinetobacter baumanii . Poster 143, ASM-ESCMID, Lisbon, Portugal. [Google Scholar]
- (38).Clinical and Laboratory Standards Institute (2012). Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically: approved standard, 9th ed. CLSI document M07-A9. CLSI, Wayne, PA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.