Abstract
TREM2 is known for its role in microglial phagocytosis and in neurodegenerative diseases. In this issue of Immunity, Filipello et al. (2018) show that microglial TREM2 is required for synaptic pruning in early development. TREM2-deficient mice show altered social behavior in adulthood, linking TREM2 to neurodevelopmental disease.
Microglial cells have traditionally been regarded as the tissue-resident macrophages of the central nervous system (CNS). However, in recent years, they have emerged as critical players in multiple brain functions in health and disease. Under physiologic conditions, microglia dynamically patrol the brain parenchyma and perform a range of cellular functions such as maturation of neuronal circuits in development, remodeling of synaptic structural plasticity, and removal of apoptotic neurons and glia. Programmed neuronal cell death, or apoptosis, during early brain development is essential for proper maturation of the CNS, leading to the refinement of neuronal network and synaptic connectivity. Microglia have recently been shown to efficiently phagocytose apoptotic cells and synapses during CNS development (Paolicelli et al., 2011). Additionally, microglia actively support neuronal survival and differentiation by secretion of trophic factors and guide CNS architecture. These microglial functions support normal brain development and maintain brain homeostasis. Triggering receptor expressed on myeloid cells 2 (TREM2) is a type I transmembrane receptor that is uniquely expressed in the brain by microglia. Filipello et al. (2018) investigated TREM2-mediated microglial synaptic pruning during development and its implication in autism spectrum disorders (ASDs).
Neurodevelopmental disorders such as ASD have a multifactorial origin (both genetic and environmental). ASDs are characterized by analtered social communication and repetitive patterns of behavior. The cause of ASD is unknown but is associated with an abnormal brain shaping and maturation of neuronal connectivity during the postnatal period (axonal sprouting alterations and/or deficiency in elimination of neurons and synapses). There is an impairment in the processing and integration of several sensory and emotional inputs. This has been suggested to be a consequence of neuronal network deficits observed in sensory, social, or emotional cerebral regions of ASD patients (Park et al., 2016).
TREM2 has been associated with a number of neurodegenerative diseases. Several studies show that a genetic variant of TREM2, namely R47H, enhances the risk of Alzheimer’s disease (AD) by 3-fold, making it one of the major risk factors for developing the disease (Guerreiro et al., 2013). More recently, TREM2 variants have also been linked to other neurologic diseases such as Parkinson’s disease, amyotrophic lateral sclerosis, and fronto-temporal dementia (Colonna and Butovsky, 2017). These recent genetic findings have focused the attention of the neurodegenerative diseases field on microglia and specifically the role of TREM2 in regulating microglial functions. Indeed, TREM2 acts as a key regulator of microglial activation and response to injury, and recent evidence revealed a crucial role of TREM2 in maintaining the survival and metabolic fitness of microglia (Ulland et al., 2017). Functionally, TREM2 is involved in phagocytosis, proliferation, pro-survival signaling, and lipid sensing. Phospholipids such as phosphatidylserine, lipoproteins including APOE (apolipoprotein E), and β-amyloid have been identified as endogenous extracellular ligands for TREM2. Through these ligands, TREM2 senses the microenvironment and mediates the phagocytosis of dead neurons, myelin, and amyloid plaques. These findings support the critical role for this receptor in modulating microglial changes in pathological conditions in the CNS (Ulrich et al., 2017). Several groups have shed light onto the role of TREM2 in disease, with most findings being consistent with the hypothesis that TREM2 mutations lead to a reduction in the microglial response to neuronal injury. Recently, it has been shown that TREM2 plays a critical role in microglial phenotype switch from homeostatic to neurodegenerative upon phagocytosis of apoptotic neurons (Krasemann et al., 2017). Consistent with these findings, TREM2 deficiency in a mouse model of AD led to a reduced number of microglia around the plaques (Ulrich et al., 2017). While loss of TREM2 is associated with significant reduction in phagocytosis, its haplo-deficiency reduces plaque compaction and axonal dystrophy without impairing amyloid phagocytosis in a mouse model of AD (Yuan et al., 2016).
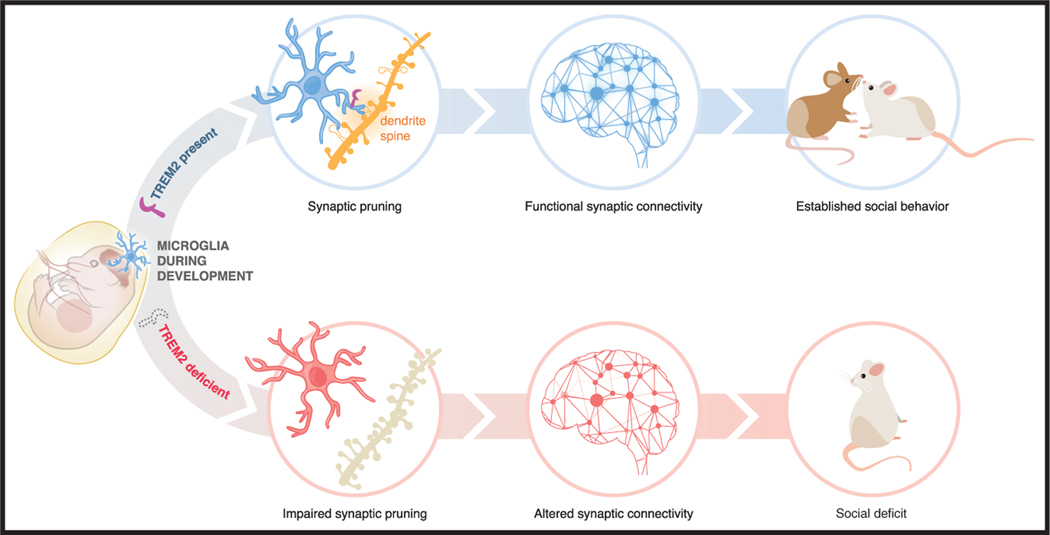
Here, Filipello et al. (2018) find that TREM2 regulates synapse phagocytosis during development, and its absence leads to impaired synaptic refinement, associated with social behavioral defects that are typically altered in ASD (Figure 1). First, the authors analyzed microglial density and morphology in Trem2–/– animals at postnatal day 18–20. At this time point, they observed a significant decrease in microglial density in the hippocampal CA1 region. The remaining microglia showed a more ramified morphology than wild-type microglia, which suggests that they are less inflammatory. This phenotype was no longer observed in P90 (3-month-old) animals, indicating a specific effect of microglial TREM2 in young mice. Next, the authors analyzed whether these early changes in microglial density and morphology translate into neuronal deficits. Indeed, lack of TREM2 led to increased synapse density and higher frequency of excitatory post-synaptic currents in the CA1 region of the hippocampus at the early stage of neuronal development. The impaired clearance of excess synapses by microglia during brain development resulted in altered functional brain connectivity in adult mice. These findings show that TREM2 deficiency and concomitant impairment of synapse elimination during early stages of development have a strong effect on functional brain connectivity in adulthood. Finally, loss of TREM2 results in an altered social behavior phenotype, which is a feature of neurodevelopmental disorders, such as ASD. In support of these findings, Filipello et al. (2018) present evidence that TREM2 protein levels are reduced in post-mortem brains of patients affected by idiopathic ASD and are negatively correlated with the severity of the symptoms.
Figure 1. TREM2 Is Required for Synaptic Refinement, Brain Connectivity, and Social Behavior.
TREM2 is expressed by microglia in the CNS. During development, microglia engulf excessive spines and neurons in order to shape normal brain connectivity. The lack of TREM2 is shown here to impair synapse elimination, which is accompanied by a reduction of functional brain connectivity. In adult mice, this leads to alterations in social behavior. These findings demonstrate a novel role of TREM2 in neurodevelopment.
In summary, the authors show a new function of TREM2 in modulation of microglial activity to eliminate synapses and sculpt neuronal circuits during early stages of brain development. In light of the known role of microglia in synaptic refinement and the current knowledge about the function of TREM2, Filipello et al. (2018) describe a novel aspect of the function of the immune molecule TREM2 in regulation of normal brain development. This adds to previous studies that have described immune molecules involved in synaptic pruning during development (Schafer et al., 2012).
Further studies will be necessary to investigate whether TREM2 mutations are causative of neurodevelopmental diseases and how they may increase the risk for neurodegenerative diseases by impacting neuronal development. Thus, it would be of interest to study the role of human TREM2 mutations in the context of brain development and how this impacts synapse elimination by microglia. New tools like microglia derived from induced pluripotent stem cells with human TREM2 mutations could be valuable for such future studies. Finally, this study opens up the possible link of ASD to microglia-mediated neurodevelopmental processes, which will help to develop novel therapies.
REFERENCES
- Colonna M., and Butovsky O. (2017). Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello F., Morini R., Corradini I., Zerbi V., Canzi A., Michalski B., Erreni M., Markicevic M., Starvaggi-Cucuzza C., Otero K., et al. (2018). TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48, this issue, 979–991. [DOI] [PubMed] [Google Scholar]
- Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe JS, Younkin S., et al. ; Alzheimer Genetic Analysis Group (2013). TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O’Loughlin E., Xu Y., Fanek Z., et al. (2017). The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira TA, Guiducci E., Dumas L., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Park HR, Lee JM, Moon HE, Lee DS, Kim BN, Kim J., Kim DG, and Paek SH (2016). A short review on the current understanding of Autism spectrum disorders. Exp. Neurobiol. 25, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R., Mardinly AR, Yamasaki R., Ransohoff RM, Greenberg ME, Barres BA, and Stevens B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A., Beatty WL, Loboda AA, Zhou Y., Cairns NJ, Kambal A., et al. (2017). TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JD, Ulland TK, Colonna M., and Holtzman DM (2017). Elucidating the role of TREM2 in Alzheimer’s disease. Neuron 94, 237–248. [DOI] [PubMed] [Google Scholar]
- Yuan P., Condello C., Keene CD, Wang Y., Bird TD, Paul SM, Luo W., Colonna M., Baddeley D., and Grutzendler J. (2016). TREM2 haplodeficiency in mice and humans impairs the microglia barrier function leading to decreased amyloid compaction and severe axonal dystrophy. Neuron 90, 724–739. [DOI] [PMC free article] [PubMed] [Google Scholar]