Preface
Autonomic dysreflexia (AD) and other autonomic dysfunctions are commonly seen after spinal cord injury (SCI). However, since SCI is relatively uncommon and the stigmata of AD and autonomic dysfunction are relatively unique to this population, few clinicians, except those who treat persons with SCI regularly, have encountered these conditions. As a result, it is thought that AD, which can result in significant morbidity and mortality when unrecognized or poorly managed, is not uncommonly misdiagnosed in the community.
There are 2 reasons for this: First, the people who are most prone to developing AD typically have low baseline blood pressures, and a significant increase in blood pressure may not be appreciated by an evaluating medical provider; second, the most obvious and distressing symptom, a severe headache, is commonly seen in other conditions. Other autonomic dysfunctions, while perhaps less life-threatening than AD, can certainly impact the lives of persons with SCI significantly and likely remain undertreated as well.
This clinical practice guideline (CPG), similar to its preceding versions, is anticipated to remain one of the most important of the Consortium for SCI Medicine CPGs regarding its potential impact on the care of persons with SCI. Not unsurprisingly, the AD CPG and its companion consumer guide, first developed over 2 decades ago, have historically been among the most popular publications of the Consortium for SCI Medicine. This updated CPG has been significantly expanded in scope to address specific scenarios in which AD might be expected to be encountered, including urological procedures, labor and delivery, and self-induced AD (“boosting”). To this end, this CPG includes contributing experts from other fields that were not represented previously, as well as other autonomic dysfunctions that were not previously addressed such as orthostatic hypotension, temperature, and sweating dysregulation.
We are fortunate in the development and peer review of this CPG to be represented by an international team of various stakeholders, including all of the subspecialties who are affected by these recommendations, ranging from experts in urology, sexual medicine, neurology, exercise physiology, and obstetrics to rehabilitation professionals. We hope that this wide-ranging representation will translate into uniform quality practice through the widespread use of this CPG to guide the prevention and treatment of AD and other autonomic dysfunctions in all settings, which can only result in the best outcomes and least amount of morbidity and mortality for those who experience SCI.
On behalf of the consortium steering committee, I want first to acknowledge the leadership of the guideline panel, namely the Co-Chairs, Andrei Krassioukov and Todd Linsenmeyer, in guiding this panel inexorably through the development process. Next to be commended are the panel members themselves for keeping to task and the many reviewers who provided valuable feedback from all areas. All of these people, including the panel Chair and Co-Chair, have volunteered their time to help produce this superb document. In addition, I wish to acknowledge the ongoing support of the Paralyzed Veterans of America, especially President David Zurfluh, Executive Director Carl Blake, and Director of Research and Education Cheryl Vines, as well as the rest of the leadership team without whose support these guidelines would not exist.
Thomas Bryce
Chair
Consortium for Spinal Cord Medicine
Foreword
Autonomic dysreflexia (AD), with its sudden and severe rise in blood pressure, is a potentially life-threatening condition that can occur in anyone with a spinal cord injury (SCI) at or above thoracic level 6 (T6). The resolution of AD requires quick and decisive treatment. Spinal cord medicine health care providers are very familiar with the diagnosis and treatment of AD. However, because of the rapid onset of AD and the potentially severe symptoms, individuals with this condition are often rushed to the nearest health care facility, which may be staffed by health care providers who have little or no experience in the treatment of AD. Therefore, the Spinal Cord Consortium decided to develop a clinical practice guideline (CPG) on the evaluation and management of AD in adults with SCI who present to health care facilities. The first edition was published in 1997. There was a high demand and wide distribution of this CPG, which included 3 printings of the guideline, as well as publication of the guideline in German and Spanish. The steering committee received a report that over 90% of the “hits” to the Paralyzed Veterans of America (PVA) website were for downloads of the AD guideline. In order to make sure the guideline was kept up to date, the SCI consortium steering committee updated the first edition in 2001. The second edition was expanded to include a discussion on AD considerations in children, adolescents, and pregnant women with SCI at T6 and above. In 2017, the SCI consortium again requested an update of the AD guideline, with a mandate to include other autonomic dysfunctions. To meet this challenge, we assembled a panel of some of the top leaders and experts in the field.
The panel decided to add to the AD CPG special AD considerations as they pertain to sexual function, sperm retrieval, pregnancy, lactation, outpatient urological procedures, and sports boosting (induced AD). The sexual function and sperm retrieval section offers guidance to health care providers on advising individuals with SCI about AD considerations during sperm retrieval, pregnancy, and lactation. The lactation section discusses some of the recent evidence that lactation can cause AD. The urology section suggests alternatives to the treatment of AD without sitting the patient up since sitting up would require ending the procedure. Also included in the updated guideline is a considerable expansion of the pharmacological options to treat AD. This expansion was particularly needed because nitropaste, one of the main agents in the prior AD guideline, is no longer available in some countries.
In addition, the panel decided to take on 3 other common autonomic dysfunctions, specifically, orthostatic hypotension, hyperhidrosis, and thermodysregulation, effectively creating 4 stand-alone CPGs under one title: Autonomic Dysreflexia and Other Autonomic Dysfunctions: Preventing the Highs and Lows. Management of Blood Pressure, Sweating, and Temperature Dysfunction.
As with the prior editions, this publication would not have been possible without the strong support and leadership of PVA. We wish to express our heartfelt gratitude particularly to Cheryl Vines for her dedication and the countless hours she spent on this project. We also thank the Chairman of the SCI Consortium, Dr. Thomas Bryce, and the consortium steering committee for their excellent input throughout this process.
We hope that these guidelines will play an important role in the evaluation and management of individuals with SCI who present with signs and symptoms of AD and other autonomic dysfunctions. Moreover, it is the panel’s hope that these guidelines will stimulate further clinical studies in this important area.
Todd A. Linsenmeyer, MD
Co-Chair
Autonomic Dysreflexia and Dysfunctions Panel
Andrei V. Krassioukov, MD, PhD, FRCPC
Co-Chair
Autonomic Dysreflexia and Dysfunctions Panel
Acknowledgments
Paralyzed Veterans of America (PVA) is proud to sponsor the development and dissemination of the spinal cord injury (SCI) clinical practice guidelines (CPGs). For over 25 years, we have partnered with the Consortium of Spinal Cord Medicine in a shared mission to improve the health of individuals living with SCI. Today, hundreds of thousands of copies of the guidelines are used around the world by physicians and other medical professionals who provide care to individuals living with SCI at every level, from the emergency department to acute care, rehabilitation to community services.
We thank Dr. Linsenmeyer and Dr. Krassioukov for their leadership and perseverance in guiding this important new guideline into practice. Sincere thanks is also extended to each of panel members who worked tirelessly, without remuneration, to bring this project to fruition. Dr Thomas Bryce and the members of the SCI Consortium have provided vision, leadership, and support in bringing this and many other CPGs to completion. Their efforts and those of the field reviewers assure the high quality of the recommendations.
As with any project of this magnitude, many were involved in the process. Sincere appreciation goes to Dr. Shelly Selph and her team at the Pacific Northwest Evidence-based Practice Center, Oregon Health and Science University, who conducted the review of the literature and methodology for this guideline.
Within PVA, work on this guideline benefitted from the efforts of nearly every department. But special appreciation goes to medical editor Barbara Every and graphic designers Kevin Johnson and Jonathan Franklin.
Finally, it is only with the significant mission-driven support of PVA, our leadership and our members, that we are able to provide these services. Sincere thanks to PVA President David Zurfluh, Past President Al Kovach, Executive Director Carl Blake, and Deputy Executive Director Shaun Castle for their support.
Grading of the Recommendations
The overall objective of this guideline is to improve the care of individuals with spinal cord injury (SCI) by guiding clinicians and policy makers with its recommendations. The following recommendations use available evidence and—where evidence is limited—panel experience and consensus. The panel based its evidence ratings primarily on research in which the focus of the study was SCI. This information was supplemented by using evidence from trials, guidelines, and expert opinions contained in the scientific literature of non-SCI populations.
For individual patients, decisions are best made by considering these recommendations combined with clinical judgment, the latter based on specific knowledge about each patient’s risk factors, the potential for adverse effects, and the availability of various options within one’s center. The ratings in parentheses below each recommendation in the sections that follow refer to the level of scientific evidence, the strength of the evidence, and the level of panel agreement with the recommendations (Tables 1–3).
Table 1.
Nomenclature for Rating of Evidence and Strength of Panel Agreement
| Level | Description |
|---|---|
| I | Evidence based on randomized controlled clinical trials (or meta-analysis of such trials) of adequate size to ensure a low risk of incorporating false-positive or false-negative results. |
| II | Evidence based on randomized controlled trials that are too small to provide Level I evidence. These may show either positive trends that are not statistically significant or no trends and are associated with a high risk of false-negative results. |
| III | Evidence based on nonrandomized, controlled, or cohort studies; case series; case-controlled studies; or cross-sectional studies. |
| IV | Evidence based on the opinion of respected authorities or expert committees as indicated in published consensus conferences or guidelines. |
| V | Evidence that expresses the opinion of those individuals who have written and reviewed this guideline, based on experience, knowledge of the relevant literature, and discussions with peers. |
Sources: Adapted from “Rules of Evidence and Clinical Recommendation on the Use of Antithrombotic Agents, by D. L. Sackett, 1989, Chest, 95(Suppl. 2), pp. 2S–4S; and Guide to Clinical Preventive Services (2nd ed), by U.S. Preventive Health Services Task Force, 1996, Williams and Wilkins.
Table 3.
Levels of Panel Agreement with the Recommendations
| Level | Mean Agreement Score |
|---|---|
| Low | 1.0 to less than 2.33 |
| Moderate | 2.33 to less than 3.87 |
| Strong | 3.87 to 5.0 |
Table 2.
Categories of the Strength of Evidence Associated with the Recommendations
| Category | Description |
|---|---|
| A | The guideline recommendation is supported by one or more Level I studies. |
| B | The guideline recommendation is supported by one or more Level II studies. |
| C | The guideline recommendation is supported by only one or more Level III, IV or V studies |
Executive Summary of the Recommendations
General Cross-Cutting Recommendations
1. Blood Pressure (BP) Following Spinal Cord Injury (SCI)
-
1.1
Be aware that, compared with the general population, individuals with SCI are likely to have the following systolic BP differences:
In the supine resting position, adults with injuries at or above T1 will likely have low BP (on average systolic BP ~110 mmHg).
In the seated resting position, adults with injuries at or above T6 will likely have low BP (on average systolic BP ~100 mmHg).
-
Age-related changes in BP (i.e., pediatric age group and older individuals) may be different.
(Level - V; Strength - C; Agreement - strong)
2. Autonomic Dysreflexia (AD)
-
2.1
Recognize that those with an SCI at or above T6 may present the signs and symptoms of AD, including:
-
Elevated systolic BP greater than 20 mmHg above their usual baseline in adults and greater than 15 mmHg above their usual baseline in children
Sudden-onset headache
Possible bradycardia or tachycardia
Cardiac arrhythmias, atrial fibrillation, premature ventricular contractions, and atrioventricular conduction abnormalities
Profuse sweating and/or flushing of the skin, typically (face, neck, and shoulders) or possibly below the level of the lesion
Piloerection (goose bumps) above or possibly below the level of the lesion
Blurred vision and/or spots in the individual’s visual fields
Nasal congestion
Feelings of apprehension or anxiety
Few or no symptoms other than elevated BP
(Level - V; Strength - C; Agreement - strong)
-
-
2.2.
Be aware that AD may appear with minimal or no symptoms (silent AD or those with cognitive/verbal communication limitations) despite a significantly elevated BP.
(Level - V; Strength - C; Agreement - strong)
-
2.3
Check the individual’s BP.
(Level - V; Strength - C; Agreement - strong)
-
2.4
If signs or symptoms of AD are present, but BP is not elevated and the cause has not been identified, refer the individual to an appropriate consultant, depending on symptoms.
(Level - V; Strength - C; Agreement - strong)
-
2.5
If AD is diagnosed, identify the trigger(s) in order to manage BP.
(Level - III; Strength - C; Agreement - strong)
-
2.6
If BP is elevated, immediately sit the individual up and lower the legs, if possible.
(Level - III; Strength - C; Agreement - strong)
-
2.7
Monitor BP and pulse frequently (every 1 – 2 minutes) until the individual is stabilized.
(Level - III; Strength - C; Agreement - strong)
-
2.8
Loosen any clothing or constrictive devices.
(Level - III; Strength - C; Agreement - strong)
-
2.9
Determine whether the individual has recently taken a vasopressor or an antihypotensive agent.
(Level - V; Strength - C; Agreement - strong)
-
2.10
Quickly survey the individual for other triggers, beginning with the urinary system.
(Level - III; Strength - C; Agreement - strong)
-
2.11
If an indwelling urinary catheter is not in place, catheterize the individual.
(Level - V; Strength - C; Agreement - strong)
-
2.12
If the elevated BP is at or above 150 mmHg systolic prior to catheterization, consider rapid-onset and short-duration pharmacological management to reduce the systolic BP without causing hypotension.
(Level - V; Strength - C; Agreement - strong)
-
2.13
Consider the use of an antihypertensive agent (such as nitropaste, nifedipine, hydralazine, or sublingual clonidine) with rapid onset and short duration.
(Level - V; Strength - C; Agreement - strong)
-
2.14
Prior to use of nitropaste or any other agent containing nitrate, first inquire about whether the individual has recently taken a phosphodiesterase type 5 inhibitor (PDE5i).
(Level - II; Strength - B; Agreement - strong)
-
2.15
Prior to inserting the catheter, instill lidocaine jelly 2% (if immediately available in the room where the individual is being treated) into the urethra and wait approximately 5 minutes, if possible.
(Level - V; Strength - C; Agreement - strong)
-
2.16
Avoid applying pressure over the bladder (Crede maneuver) or suprapubic tapping, as this may exacerbate AD.
(Level - V; Strength - C; Agreement - strong)
-
2.17
If the individual has an indwelling urinary catheter, check the system along its entire length for kinks, folds, constrictions, or an overfilled drainage bag and for correct catheter placement. If a problem is found, correct it immediately.
(Level - V; Strength - C; Agreement - strong)
-
2.18
If there are no problems with the tubing, drainage bag, or catheter placement and the BP is still elevated, gently irrigate the bladder with a small amount (10–15 cc) of fluid, such as normal saline at body temperature, to determine whether the catheter is blocked. Irrigation should be limited to 5–10 cc for children under 2 years of age. Do not continue to irrigate or attempt to flush the bladder if the fluid is not draining from the catheter, as this will only cause increased bladder distention and increase the BP.
(Level - V; Strength - C; Agreement - strong)
-
2.19
If the catheter is blocked, remove and replace it.
(Level - V; Strength - C; Agreement - strong)
-
2.20
If there is a history of difficulty passing a catheter in a male, consider using a coudé catheter or consult urology.
(Level - V; Strength - C; Agreement - strong)
-
2.21
Prior to replacing the catheter, consider instilling lidocaine jelly 2% (if immediately available) into the urethra or suprapubic tract and wait 3–5 minutes, if possible.
(Level - V; Strength - C; Agreement - strong)
-
2.22
If difficulties arise in removing or replacing the catheter, in addition to instilling lidocaine jelly, consider initiating new or increasing previous pharmacological treatment and an emergency urology consultation.
(Level - V; Strength - C; Agreement - strong)
-
2.23
Monitor the individual’s BP during bladder drainage.
(Level - V; Strength - C; Agreement - strong)
-
2.24
If acute symptoms of AD persist, including sustained elevated BP, suspect fecal impaction.
(Level - II; Strength - B; Agreement - strong)
-
2.25
If the elevated BP persists at or above 150 mmHg systolic, strongly consider pharmacological management prior to laying the individual down to check for fecal impaction.
(Level - V; Strength - C; Agreement - strong)
-
2.26
If fecal impaction is suspected, check the rectum for stool, using the following procedure:
Premedicate with a pharmacological agent as outlined in Recommendation 2.21.
With a gloved hand, generously instill a topical anesthetic agent, such as lidocaine jelly 2%, into the rectum.
Wait 3–5 minutes, if possible, for sensation in the area to decrease.
Then, with a gloved hand, insert a lubricated finger into the rectum and check for the presence of stool. If present, gently remove, if possible.
(Level - II; Strength - B; Agreement - strong)
-
2.27
If AD becomes worse, or stool cannot be removed, stop the manual evacuation and administer pharmacological or additional pharmacological intervention and additional topical anesthetic. When BP is stable below 150 mmHg, proceed with an aggressive bowel evacuation regimen.
(Level - II; Strength - B; Agreement - strong)
-
2.28
If there is no fecal impaction or BP elevation persists despite disimpaction, check for other less frequent causes of AD (SEE LIST OF TRIGGERS ON PAGE 25). If there are no obvious triggers or if the BP cannot be managed locally, the individual must be referred to the hospital emergency department for evaluation and management and possible hospital admission.
(Level - V; Strength - C; Agreement - strong)
-
2.29
While the individual is being evaluated in the emergency department, continue to closely monitor BP to guide pharmacological management of AD and investigate other causes. Consider hospital admission if:
There is poor response to the treatment specified above.
The cause has not been identified.
(Level - V; Strength - C; Agreement - strong)
-
2.30
After successful identification of the trigger and treatment of the elevated BP, monitor the individual for symptomatic hypotension every 2–5 minutes until the BP is stable.
(Level - V; Strength - C; Agreement - strong)
-
2.31
Following an episode of AD, a health care provider should consider the following:
If the individual is an inpatient or in the clinic, monitor closely for at least 2 hours for recurrent AD or hypotension.
If at home, instruct the individual to seek immediate medical attention if AD symptoms reoccur.
Prescribe a BP monitoring device to the individual for home monitoring.
(Level - V; Strength - C; Agreement - strong)
-
2.32
Document the episode of AD and record the effectiveness of the treatment in the individual’s medical record, including the following:
Presenting signs and symptoms and their course
Recordings of BP and pulse
Treatment instituted and response to treatment
Restoration of BP and heart rate to normal levels for the individual
Diagnosis of a history of AD in order to inform future clinicians of the risk in the individual and prior response to treatments initiated
Identification of the cause (trigger) of the AD episode
Whether the individual is comfortable, with no signs or symptoms of AD or secondary complications, such as neurological changes, increased intracranial pressure, or heart failure
(Level - V; Strength - C; Agreement - strong)
-
2.33
After the individual with SCI has been stabilized, review the precipitating cause of the AD episode with the individual, family members, significant others, and caregivers to educate them regarding instigating factors, recognition, management, and prevention of future AD episodes.
Adjust the treatment plan to ensure that future episodes are recognized and treated to prevent a medical crisis or, ideally, are avoided altogether.
Discuss AD during the individual’s education program, so that he or she will be able to minimize the risks known to precipitate AD, solve problems, recognize early onset, and obtain help as quickly as possible.
Have an ongoing conversation and continue education at annual evaluations or clinic appointments.
Give a written wallet card/guide or instruction sheet or consider a medical alert bracelet.
(Level - V; Strength - C; Agreement - strong)
-
2.34
Perform detailed evaluations for individuals with recurrent AD.
(Level - V; Strength - C; Agreement - strong)
3. Autonomic Dysreflexia: Sexuality
Recommendations for Sexual Activity in the Home Setting
-
3.1
Be aware of and educate individuals with SCI at or above T6 that sexual activity may provoke AD.
(Level - V; Strength - C; Agreement - strong)
-
3.2
Be aware that for men and women with SCI at or above T6 who use intense sexual stimulation (including vibratory stimulation), the likelihood of AD is increased.
(Level - V; Strength - C; Agreement - strong)
-
3.3
Encourage individuals with SCI at T6 and above to periodically monitor their BP during sexual activities.
(Level - V; Strength - C; Agreement - strong)
-
3.4
Individuals prone to AD during sexual activity should be encouraged to use a home BP monitor.
(Level - V; Strength - C; Agreement - strong)
-
3.5
If sexual activity causes symptomatic AD, individuals should be encouraged to immediately cease sexual stimulation and follow AD protocol.
(Level - V; Strength - C; Agreement - strong)
-
3.6
Consider instructing and prescribing pharmacological prophylaxis prior to sexual activity in selected individuals who:
Have no history of symptomatic orthostatic hypotension (OH)
Are not taking medication that may potentiate hypotension
Developed AD with systolic BP at or above 150 mm Hg (i.e., during vibratory stimulation, ejaculation, orgasm, sperm retrieval, or urological procedures)
Have symptomatic AD and/or systolic BP greater than 150 mmHg prior to sexual activity or during sperm retrieval
(Level - V; Strength - C; Agreement - strong)
-
3.7
If pharmacological treatment for AD is used in a home setting, instruct individuals on how to recognize, monitor, and treat pharmacologically induced hypotension.
(Level - V; Strength - C; Agreement - strong)
-
3.8
Instruct individuals at risk of AD to recheck BP within 5 minutes of cessation of sexual activity, regardless of symptoms.
(Level - V; Strength - C; Agreement - strong)
-
3.9
If the individual’s high BP does not resolve after 5 minutes, refer to steps for treatment of AD.
(Level - V; Strength - C; Agreement - strong)
-
3.10
Instruct individuals that if all conservative home measures to treat AD or pharmacologically induced hypotension following sexual activity are unsuccessful, an urgent visit to the emergency department is warranted.
(Level - V; Strength - C; Agreement - strong)
4. AD And Cystoscopic (Transurethral And Suprapubic) Urological Procedures And Sperm Retrieval Procedures Performed In The Clinic Setting
-
4.1
Prior to the procedure, counsel the individual to:
Take prescribed medications (such as anticholinergic medications, alpha-blockers)
Have a recent bowel program (within 1–2 days)
Treat urinary tract infection, if present
Hold any as-needed medications that may elevate BP (such as ephedrine, midodrine)
Hold any medications such as phosphodiesterase inhibitors (PDEis), which may not allow nitrates (nitropaste) to be used to treat AD
(Level - V; Strength - C; Agreement - strong)
-
4.2 If prior to the procedure an individual presents with a systolic BP that is greater than 20 mmHg above his or her usual baseline systolic BP, evaluate for possible causes of AD and manage and monitor it.
(Level - V; Strength - C; Agreement - strong)
-
4.3
Consider rescheduling the individual if AD persists despite finding and correcting any obvious reversible causes.
(Level - V; Strength - C; Agreement - strong)
-
4.4
Consider decreasing the risk of AD before urethral instrumentation such as cystoscopy by instilling lidocaine jelly into the urethra at least 3–5 minutes before urethral instrumentation.
(Level - V; Strength - C; Agreement - strong)
-
4.5
In individuals who are prone to AD or have a recent history of AD, consider prophylactic pharmacological treatment to decrease the risk of AD before cystoscopic procedures and sperm retrieval procedures such as:
(Level - V; Strength - C; Agreement - strong)
-
4.6
During sperm retrieval procedures, BP should be monitored at 1-minute intervals.
(Level - V; Strength - C; Agreement - strong)
-
4.7
During cystoscopic and urodynamic procedures, monitor BP in at least 2-minute intervals, preferably with an automatic BP cuff. Perform more frequent BP readings if the patient is developing AD during the procedure.
(Level - V; Strength - C; Agreement - strong)
-
4.8
Rather than immediately sitting an individual up during cystoscopic and urodynamic procedures, attempt to control AD by draining the bladder as needed and, if not resolved, institute a similar pharmacological strategy as that recommended for the management of AD.
(Level - V; Strength - C; Agreement - strong)
-
4.9
During urological cystoscopic and urodynamic procedures, if AD is not controlled by draining the bladder or with pharmacological measures, stop the procedure and sit the individual up.
(Level - V; Strength - C; Agreement - strong)
-
4.10
Monitor BP after cystoscopic or urodynamic procedure or after ejaculation until it subsides to near the individual’s baseline. Monitor for continued elevated BP or OH when the individual is moved to the seated position.
(Level - V; Strength - C; Agreement - strong)
-
4.11
AD prevention and control will be under the direction of the specialist administering anesthesia to individuals who require it while undergoing electroejaculation.
(Level - V; Strength - C; Agreement - strong)
5. AD in Pregnancy, Labor and Delivery, and the Postpartum Period
-
5.1
Instruct health care professionals that women with SCI who have potential of developing AD are at increased risk of severe AD during pregnancy, labor, delivery, and breastfeeding and should be followed by a multidisciplinary team.
(Level - V; Strength - C; Agreement - strong)
-
5.2
An antepartum consultation with an anesthesiologist and the establishment of a plan for induction of epidural or spinal anesthesia at the onset of labor is recommended to assess the risk of AD and to prevent it, in accordance with recommendations of the American College of Obstetricians and Gynecologists.
(Level - V; Strength - C; Agreement - strong)
-
5.3
In pregnant women prone to AD, careful and frequent monitoring of the fetus is recommended, especially during labor and delivery.
(Level - V; Strength - C; Agreement - strong)
-
5.4
AD must be differentiated from preeclampsia during pregnancy and labor to ensure appropriate treatment.
(Level - V; Strength - C; Agreement - strong)
-
5.5
Although individuals with SCI may not perceive pain during labor, anesthesia should be used to prevent AD in women with SCI at T6 and above. Spinal or epidural anesthesia is the most reliable method of preventing AD by blocking stimuli that arise from pelvic organs.
(Level - V; Strength - C; Agreement - strong)
-
5.6
Educate women who have the potential to develop AD that postpartum breastfeeding, breast engorgement, or mastitis may trigger AD.
(Level - V; Strength - C; Agreement - strong)
6. Induced AD (“Boosting”)
-
6.1
Inform individuals with SCI that self-induced AD (e.g., boosting) to benefit daily activities and/or sports performance is a dangerous practice that can result in uncontrollable, life-threatening increases in BP.
(Level - IV; Strength - C; Agreement - strong)
7. Orthostatic Hypotension (OH)
-
7.1
Be aware that OH, defined as a decrease in systolic BP of ≥ 20 mmHg, may occur in individuals with lesions at T6 and above on the assumption of an upright posture from a supine position, regardless of whether symptoms occur.
(Level - V; Strength - C; Agreement - strong)
-
7.2
To accurately diagnose OH in individuals with SCI, perform an orthostatic challenge evaluation (e.g., sit-up test or head-up tilt test).
(Level - V; Strength - C; Agreement - strong)
-
7.3
To prevent or manage OH in individuals with SCI, first consider treating to maintain baseline BP by using nonpharmacological interventions.
(Level - V; Strength - C; Agreement - strong)
-
7.4
Consider pharmacological interventions to treat both symptomatic and asymptomatic OH in individuals with established SCI when nonpharmacological interventions prove to be ineffective.
(Level - V; Strength - C; Agreement - strong)
8. Thermodysregulation
-
8.1
Hypothermia (core temperature less than 35.0°C/95°F)
-
8.1.1
Monitor for signs and symptoms in individuals with SCI at T6 or above who are at risk for developing hypothermia when exposed to a cold environment.
(Level - V; Strength - C; Agreement - strong)
-
8.1.2
If possible, obtain a rectal temperature when evaluating an individual for hypothermia because skin temperate is not accurate for monitoring core body temperature. Oral and tympanic are also acceptable methods of temperature monitoring.
(Level - V; Strength - C; Agreement - strong)
-
8.1.3
Use ambient temperature regulation, insulated clothing, blankets, warm humidified air, and intake of warm fluid into the gastrointestinal (GI) tract to help prevent and manage hypothermia. Heating devices should be used with extreme caution in insensate areas.
(Level - V; Strength - C; Agreement - strong)
-
8.1.4
In cold ambient environments, instruct individuals to consider avoiding alcohol intake, as it causes vasodilation and heat loss.
(Level - V; Strength - C; Agreement - strong)
-
8.1.5
Be aware of and discuss with individuals with SCI that certain medications or substances may disrupt temperature regulation (hypoor hyperthermia), including alpha-agonists (e.g., tizanidine, clonidine), narcotics, oxybutynin, gabapentin, and antidepressants that are norepinephrine and serotonin reuptake inhibitors.
(Level - V; Strength - C; Agreement - strong)
-
8.1.1
-
8.2
Hyperthermia (core temperature more than 37.8°C/100°F)
-
8.2.1
Monitor for signs and symptoms of hyperthermia in individuals with SCI at or above T6 who are at risk for developing hyperthermia when exposed to a hot environment.
(Level - V; Strength - C; Agreement - strong)
-
8.2.2
Treat hyperthermia by decreasing the individual’s core temperature. This includes moving to a cooler environment (preferably an air-conditioned setting), drinking cool liquids, washing with tepid water, and resting.
(Level - V; Strength - C; Agreement - strong)
-
8.2.3
Provide education regarding measures to help prevent neurogenic hyperthermia. Preventative measures include wearing appropriate light-weight and light-colored clothing, maintaining a proper temperature-controlled room (e.g., use of air-conditioning), frequently drinking cold fluids, maintaining appropriate hydration, and having a water spray and/or fan for exposed skin. This is especially important when in a hot environment.
(Level - V; Strength - C; Agreement - strong)
-
8.2.4
Be aware of and discuss with individuals with SCI that certain medications or substances may disrupt temperature regulation (hypoor hyperthermia), including alpha-agonists (e.g., tizanidine, clonidine), narcotics, oxybutynin, gabapentin, and antidepressants that are norepinephrine and serotonin reuptake inhibitors.
(Level - V; Strength - C; Agreement - strong)
-
8.2.5
During exercise, individuals with SCI at T6 or above should be monitored for neurogenic hyperthermia.
(Level - V; Strength - C; Agreement - strong)
-
8.2.1
9. Hyperhidrosis
-
9.1
Evaluation of hyperhidrosis in individuals with SCI at T6 or above T6 should rule out more extensive autonomic dysfunction such as AD.
(Level - V; Strength - C; Agreement - strong)
-
9.2
In the absence of a rise in BP, prevention and management of hyperhidrosis should include identifying other possible triggers.
(Level - V; Strength - C; Agreement - strong)
-
9.3
In those individuals in whom isolated hyperhidrosis is not associated with an identifiable and modifiable cause, consider empirical treatment with anticholinergic medications, unless contraindicated.
(Level - V; Strength - C; Agreement - strong)
-
9.4
If anticholinergic medications do not relieve the hyperhidrosis or are not well tolerated, secondary medications could be considered.
(Level - V; Strength - C; Agreement - low)
Methodology
Literature Search
Researchers from the Pacific Northwest Evidence-based Practice Center, Oregon Health & Science University, Portland, Oregon, conducted the review of the literature. They searched Ovid MEDLINE (1946 through June 6, 2017), the Cochrane Central Register of Controlled Trials (through April 2017), and the Cochrane Database of Systematic Reviews (through June 9, 2017) by using search terms related to SCI and autonomic dysreflexia (AD). See Appendix 1 for complete search strategies. An attempt was also made to identify additional studies through hand searches of reference lists of included studies and reviews. All citations were imported into an electronic database.
Study Selection
Selection of included studies was based on inclusion criteria created in consultation with the PVA. Two reviewers independently assessed titles and abstracts of citations identified through literature searches for inclusion by using the criteria below. Full-text articles of potentially relevant citations were retrieved and assessed for inclusion by both reviewers. Disagreements were resolved by consensus. Results published only in abstract form were not included because inadequate details were available for assessment of risk of bias. The methodologist did consider for inclusion abstracts that had additional information available in slide sets from conference presentations, or those that provided supplemental data from published studies. When the data were sparse, studies with smaller sample sizes (e.g., 10) were included. When a systematic review was included, the individual studies that were addressed within that review were excluded.
Inclusion Criteria
In consultation with the PVA, 8 key questions were formulated. However, it became necessary to combine 2 key questions (prevention and treatment) because of the way the evidence was presented. In addition, a key question (Key Question 6) was added to address non-AD treatments that affect AD. Additional key questions were added later in the project to cover orthostatic hypotension (OH), thermal dysregulation, and hyperhidrosis, which are discussed separately. Key questions and inclusion criteria are presented below.
Key Questions
What is the prevalence of AD in individuals with SCI?
What are the comparative effectiveness and harms of screening to detect AD in asymptomatic individuals with SCI?
What are the comparative effectiveness and harms of different techniques to monitor AD in individuals with SCI with known AD?
What are the comparative benefits and harms of different pharmacological and nonpharmacological methods to prevent and/or treat AD in individuals with SCI?
What are the risk factors for the development of AD after SCI?
What is the evidence that AD improves with treatment of other conditions in individuals with SCI?
What is the evidence that AD influences body function and structures, activities, participation, health, mortality, and quality of life in individuals with SCI?
What are the comparative benefits and harms of screening, monitoring, prevention, and treatment for AD in subgroups of individuals with SCI?
What is the prevalence of hypotension or OH in the spinal cord injured population?
What are the risk factors for hypotension or OH after SCI?
What is the evidence that hypotension or OH after SCI influences body functions and structures, activities, participation, health, mortality, and quality of life in people with SCI?
What are the comparative benefits and harms of different pharmacological and nonpharmacological methods to prevent and/or treat hypotension or OH in patients with SCI?
What is the prevalence of thermodysregulation after SCI?
What are the risk factors for thermodysregulation after SCI?
What is the evidence that thermodysregulation after SCI influences body functions and structures, activities, participation, health, mortality, and quality of life in people with SCI?
What are the comparative benefits and harms of different pharmacological and nonpharmacological treatments for thermodysregulation in patients with SCI?
What is the prevalence of hyperhidrosis after SCI?
What are the risk factors for hyperhidrosis after SCI?
What is the evidence that hyperhidrosis after SCI influences body functions and structures, activities, participation, health, mortality, and quality of life in people with SCI?
What are the comparative benefits and harms of different pharmacological and nonpharmacological treatments for hyperhidrosis in patients with SCI?
Data Abstraction
We abstracted information on population characteristics, interventions, subject enrollment, and prevalence, as well as on the results in terms of efficacy, effectiveness, and harms outcomes for trials, observational studies, and systematic reviews. We recorded intent-to-treat results when reported. Data abstraction was performed by one reviewer and a random sample independently checked by a second reviewer. Differences were resolved by consensus.
Validity Assessment (Risk of Bias)
The research team assessed the internal validity (risk of bias) of randomized trials, observational studies, and systematic reviews by using predefined criteria. These criteria are based on the U.S. Preventive Services Task Force and the National Health Service Centre for Reviews and Dissemination (United Kingdom) criteria (Harris et al., 2001; Khan et al., 2001); the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidelines (Guyatt, Oxman, Vist, et al., 2011); and the Quality in Prognostic Studies (QUIPS) tool (Hayden et al., 2013).
We rated the internal validity of each randomized trial on the basis of the methods used for randomization, allocation concealment, blinding, the similarity of compared groups at baseline, loss to follow-up, and the use of intent-to-treat analysis. Observational studies were rated on nonbiased selection, loss to follow-up, pre-specification of outcomes, well-described and adequate ascertainment techniques, statistical analysis of potential confounders, and adequate duration of follow-up. For studies of risk factors, we used the QUIPS tool (Hayden et al., 2013). The tool includes domains for study participants, attrition, measurement of prognostic factors, statistical adjustment for confounding factors, and appropriate statistical analysis. Systematic reviews were rated on clarity of the review question, specification of inclusion and exclusion criteria, use of multiple databases and search for gray literature, sufficient detail of included studies, adequate assessment of risk of bias of included studies, and adequate summarization of primary studies.
Two reviewers independently assessed the risk of bias of each included study and differences were resolved by consensus. Studies were rated as “low risk of bias,” “medium risk of bias,” or “high risk of bias” from the presence and seriousness of methodological limitations. Studies that had a significant or “fatal” flaw were rated as high risk of bias, studies that met all criteria were rated as low risk of bias, and the remainder were rated as medium risk of bias. Because the medium risk of bias category is broad, studies with this rating vary in their strengths and weaknesses. The results of some studies that were rated as medium risk of bias are likely to be valid, whereas others are only possibly valid. A fatal flaw is reflected in the failure to meet combinations of items from the risk of bias criteria.
An example is a study with high attrition (e.g., 60%) combined with inadequate handling of missing data, or one in which details of randomization and/or allocation concealment were lacking and there were baseline differences in important prognostic characteristics.
PICOTS
Population
Individuals with SCI (adults & children)
Interventions
Screening
Monitoring
-
Prevention
Pharmacological
Nonpharmacological
-
Treatment
Pharmacological
Nonpharmacological
Comparators
Another intervention
Usual care
No intervention
Wait list
Outcomes
Prevalence of AD
-
Frequency or severity of AD
Change in resting blood pressure (BP)/heart rate (HR)
Change in BP/HR with exercise
Change in AD severity scale score
-
Have or developing other symptoms of AD
♦ Headache
♦ Sweating
♦ Piloerection
♦ Visual changes
♦ Other symptoms
Change in function or anatomy
Change in activity or participation
Change in mental health or quality of life
-
Adverse events
Death
Cerebral hemorrhage
Seizures
Retinal hemorrhage
Pulmonary edema
Renal insufficiency
Myocardial infarction
Other serious adverse events
Withdrawal from the study due to adverse events
Setting
Inpatient rehabilitation
Outpatient
Study Design
Epidemiological studies and database studies
-
Clinical trials
Randomized
Nonrandomized
Intervention series (everyone in the study gets the same treatment)
-
Cohort studies
Controlled
Uncontrolled
Case-control studies
Case series
Systematic reviews
Grading the Quality of Evidence
Quality of evidence was graded by using the GRADE approach (Balshem et al., 2011; Guyatt et al., 2013; Guyatt, Oxman, Kunz, et al., 2011; Guyatt, Oxman, Montori, et al., 2011; Guyatt, Oxman, Vist, et al., 2011). Developed to grade the overall quality of a body of evidence, this approach incorporates 4 key domains: risk of bias (including study design and aggregate risk of bias), consistency, directness, and precision of the evidence. It also considers other optional domains that may be relevant for some scenarios, such as a dose-response association, plausible confounding that would decrease the observed effect, strength of association (magnitude of effect), and publication bias.
Table 4 (below) describes the grades of evidence that can be assigned. Grades reflect the quality of the body of evidence to answer key questions. Grades do not refer to the general efficacy or effectiveness of treatments, for example. Two reviewers independently assessed each domain for each outcome and differences were resolved by consensus.
Table 4.
Definitions of the Grades of Overall Quality of Evidence
| Grade | Definition |
|---|---|
| High | High confidence that the true effect lies close to that of the estimate of effect. |
| Moderate | Moderate confidence in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | Limited confidence in the effect estimate. The true effect may be substantially different from the estimate of the effect. |
| Very Low | Very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. |
The quality of the body of evidence was evaluated for each outcome by using a key question.
Data Synthesis
In-text tables were developed to show the study characteristics and results for included studies. Studies were reviewed by using a hierarchy-of evidence approach, in which the best evidence was the focus of our synthesis for each question, population, intervention, and outcome addressed. When possible, we pooled results by using Stata 14. The search and selection of articles is summarized in the literature flow diagram (Figure 1). Database searches resulted in 1,357 potentially relevant articles. After dual review of abstracts and titles, we selected 246 articles for full-text dual review, and 59 studies were determined to meet inclusion criteria and were included in this review.
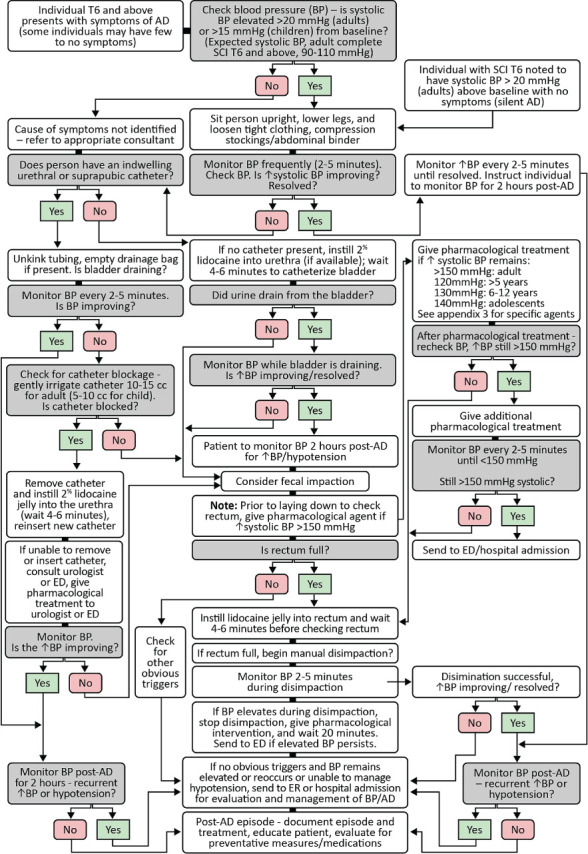
Algorithm for AD Treatment
The Consortium for Spinal Cord Medicine
The Consortium is a collaboration of professional and consumer organizations funded and administered by the Paralyzed Veterans of America (PVA). The Steering Committee, administratively supported by the PVA Research and Education Department, is made up of one representative from each Consortium member organization. The Consortium’s mission is to direct the development and dissemination of evidence-based clinical practice guidelines (CPGs) and companion consumer guides. This mission is solely directed to improving the health care and quality of life for individuals with spinal cord injury (SCI).
Recommendations and Rationales Introduction
General Organization of Autonomic Nervous System
There are 2 components within the autonomic nervous system: sympathetic and parasympathetic (Krassioukov & Weaver, 1996). Most of the visceral organs are innervated by both components of this system, including the heart and bronchopulmonary tree (Krassioukov & Weaver, 1996; Leftkowitz et al., 2007). However, with the exception of the cavernous tissue of the penis and clitoris (which have parasympathetic innervations), the peripheral blood vessels receive only sympathetic innervations. The sympathetic and parasympathetic systems interact with each other within the central nervous system and provide balanced regulation of innervated organs. Central control of autonomic functions occurs via cortical input through the hypothalamus and contributes to coordination of the autonomic circuits with the brain stem, spinal cord, and periphery (See Figure 2).
Figure 2:

Schematic diagram of autonomic efferent innervation of the heart, blood vessels and sweat glands.
Although there are obvious functional differences between the sympathetic and parasympathetic nervous systems, there are also some similarities in their organization. In both systems, 2 neuronal populations are interposed between the central nervous system and target organs. The first neuron is known as the preganglionic neuron, with the cell body within the gray matter of the brain stem or spinal cord. Axons of these neurons, called preganglionic fibers, travel within the ventral roots of the spinal cord or cranial nerves (CNs). Both sympathetic and parasympathetic preganglionic neurons are cholinergic. These fibers release acetylcholine and synapse on the second group of neurons, called postganglionic neurons, located within the autonomic ganglia in the peripheral nervous system. The axons of these neurons are called postganglionic fibers. These fibers have direct connections with the target organs. Sympathetic postganglionic fibers are predominantly adrenergic and release norepinephrine (with the exception of cholinergic sympathetic fibers that innervate sweat glands). All postganglionic parasympathetic ganglionic neurons are cholinergic and release acetylcholine at the target organ.
Sympathetic preganglionic neurons reside in the spinal gray matter in the thoracic (T1–T12) and upper lumbar segments (L1–L2; Figure 2) of the spinal cord (Krassioukov & Weaver, 1996; Schramm et al., 1993). The majority of sympathetic preganglionic neurons are localized within the lateral horns or intermediolateral nucleus of the spinal cord. A small proportion of sympathetic preganglionic neurons are found near the central canal of the spinal cord. Axons of the sympathetic preganglionic neurons exit through the ventral roots and synapse on postganglionic sympathetic neurons located in the spinal paravertebral ganglia (sympathetic chain ganglia) and prevertebral ganglia (the celiac, superior, and inferior mesenteric ganglia). The postganglionic neurons then send their axons via the peripheral nerves to innervate the target organs, including the heart, blood vessels, respiratory tract, sweat glands, sexual organs, and smooth muscles within the gut and bladder. Parasympathetic preganglionic neurons are located within the nuclei of 4 CNs (III, VII, IX, X) in the brain stem and within the sacral spinal segments (S2–S4). Parasympathetic control of the cardiovascular and bronchopulmonary systems and the upper portion of the gastrointestinal (GI) tract occur via the vagus nerve (CN X), which exits from the brain through the base of the skull. There is no parasympathetic innervation of the peripheral vasculature except in the pelvic organs. Parasympathetic innervation of the bladder, reproductive organs, and lower portion of the gut is provided by the sacral portion of the spinal cord (S2–S4).
Autonomic Control of the Cardiovascular System
The heart has a dual innervation from the vagus nerve (CN X: parasympathetic) and upper thoracic segments of the spinal cord (T1–T5: sympathetic). Blood vessels in the upper portion of the body receive sympathetic innervation from the T1–T5 spinal sympathetic neurons (Figure 2), while the major vasculature beds in the gut and lower extremities are under the control of the more caudal T5-L2 spinal sympathetic neurons (Figure 2). The dual innervations of the heart and the segmental differences in sympathetic innervation to a variety of vascular beds are particularly important for the understanding of cardiovascular responses following cervical, mid-thoracic, or lower thoracic spinal cord injury (SCI) (Krassioukov & Claydon, 2006; Mathias & Frankel, 2002).
Acute Period Following SCI
Initially following the injury, there is a marked reduction or abolition of sensory, motor, and reflex function of the spinal cord below the level of injury known as spinal shock (Ditunno et al., 2004). This condition is also commonly accompanied by severe cardiovascular dysfunctions, especially with an injury at the cervical level. Individuals with this SCI typically present with severe hypotension and persistent bradycardia, common components of the phenomenon known as neurogenic shock (Krassioukov et al., 2007). Clinical observations strongly suggest that prolonged and severe hypotension that requires vasopressive therapy correlates well with the severity of the SCI and with cervical or high-thoracic injury, and it can last up to 5 weeks after injury (Atkinson & Atkinson, 1996; Hadley et al., 2002; Mathias & Frankel, 2002; Nacimiento & Noth, 1999; Vale et al., 1997). The detailed management of cardiovascular dysfunctions following SCI were described in the previously published Paralyzed Veterans of America Early Acute Management clinical practice guideline (Consortium of Spinal Cord Medicine, 2008).
The major organs of the cardiovascular system are the heart and the blood vessels. The heart receives both parasympathetic and sympathetic innervation. Parasympathetic efferents travel to the heart in the vagus nerve (CN X), which exits the CNS at the level of the medulla. At the level of medullar are forming vagus efferent output including dorsal vagal motor nerve (DMNX) and nucleus and nucleus ambigus (NA). The vagus nerve innervates the atria, nodes, and Purkinje fibers via local cardiac ganglia, and vagal activity decreases heart rate, contractility, and conduction velocity. Primary neurotransmitter released by pre-ganglionic and postganglionic parasympathetic fibers (CN X) is acetylcholine. Sympathetic activity has an opposite, stimulatory effect on the heart. All tissues of the heart receive sympathetic input from the upper thoracic (T1–T5) cord.
Peripheral vasculature receives tonic sympathetic control, that provided by the medullar cardiovascular center known as rostroventrolateral medulla (RVLM). The vessels supplying the splanchnic region – the liver, spleen, and intestines – are most important in cardiovascular control. The splanchnic bed is densely-innervated, highly compliant, and contains approximately one-fourth of the total blood volume in humans at rest. As such, it is the primary capacitance bed in the body. Sympathetic outflow to the splanchnic bed exits the thoracolumbar cord (T5-L2) and provides tonic vasoconstriction via alpha adrenoreceptors (αAR). Primary neurotransmitter released by sympathetic postganglionic fibers at the target organs is norepinephrine. However, similar to parasympathetic nervous system at the level of ganglion pre-ganglionic fibers release acetylcholine.
Similar to blood vessels, the sweat glands are predominantly under sympathetic control. Sweat glands in the upper portion of the body receive sympathetic innervation from T1–T5 spinal sympathetic neurons, whereas the glands of the lower part of the body are under the control of the T5–L2 spinal sympathetic neurons. In contract to blood vessels, the eccrine sweat glands involved in temperature regulation are innervated by sympathetic postganglionic cholinergic fibers with acetylcholine as a primary neurotransmitter (muscarinic receptors, mAChR). Supraspinal control of sweating, by regions in the hypothalamus and amygdala is now better defined in humans using neuroimaging studies. (Modified from Inskip) (Inskip et al. 2009).
Abbreviations: AR, adrenergic receptors; CVLM, caudal ventrolateral medulla; DMNX, dorsal vagal motor nerve; g, ganglion; mAChR, muscarinic cholinergic receptors; NA, nucleus ambiguous; n, nerve; NTS, nucleus of the solitary tract; RVLM, rostral ventrolateral medulla; (+) denotes excitatory synapses; (−) denotes inhibitory synapses.
Inskip, J.A., L.M. Ramer, M.S. Ramer, and A.V. Krassioukov. 2009. ‘Autonomic assessment of animals with spinal cord injury: tools, techniques and translation’, Spinal Cord, 47: 2–35.
Overview
In general, the resting arterial blood pressure (BP) in individuals with cervical and high-thoracic SCI is lower than that in able-bodied subjects. However, most of these individuals also experience life-threatening episodes of hypertension, known as autonomic dysreflexia (AD), on a daily basis (Krassioukov & Weaver, 1996). This condition is characterized by hypertension, with systolic BP reaching up to 300 mmHg, and can be accompanied by a pounding headache, slow heart rate (HR), and upper body flushing (Elliott & Krassioukov, 2006; Mathias & Frankel, 2002). Untreated episodes of AD may have serious consequences, including intracranial hemorrhage, retinal detachment, seizures, cardiac arrhythmia, and death (Eltorai et al., 1992; Pine et al., 1991; Yarkony et al., 1986).2,3,4 These sudden increases in arterial BP can be provoked by a range of different noxious and non-noxious stimuli, including bowel and bladder distension, spasms, pressure sores, urinary bladder catheterization, or even tight shoelaces (Teasell et al., 2000).5 Furthermore, there are numerous reports of iatrogenic triggering factors such as cystoscopy, cystometry, penile vibratory stimulation and electroejaculation (EEJ) for sperm retrieval, electrical stimulation of muscles, and use of tight suspension systems during locomotor training (Chang et al., 1991; Geigle et al., 2013; Giannantoni et al., 1998; Sheel et al., 2005).
Most commonly, AD is observed in individuals with cervical and high-thoracic (above T6) SCI (Krassioukov & Weaver, 1996). However, even in tetraplegics, AD is not always severe and may be asymptomatic or characterized simply by sweating and piloerection (Kirshblum et al., 2002). The level and completeness of injury are also important in the prevalence of AD, as dysreflexia is 3 times more common in tetraplegics with complete injury than in individuals with incomplete injury (Curt et al., 1997).
The mechanisms underlying the development of AD are still poorly understood. However, some experimental animal and clinical data suggest that plastic changes within the central nervous system following SCI are among the contributing factors in the development of this condition (Krassioukov & Weaver, 1996).
An important consideration, however, is that although AD occurs more often in the chronic stage of SCI at or above the 6th thoracic segment, studies have shown clinical evidence of early episodes of AD in the first days and weeks after the injury (Krassioukov et al., 2003; Silver, 2000). In fact, it seems likely that AD is underrecognized in the acute phase of SCI (Krassioukov et al., 2003).
Another important matter is that, despite the fact that AD is unpleasant (Elliott & Krassioukov, 2006) and a life-threatening emergency (Eltorai et al., 1992), some wheelchair athletes with SCI voluntarily induce it before competition in order to enhance their performance (Harris, 1994). Self-induced AD, commonly referred as “boosting,” is considered unethical and illegal by the International Paralympic Committee Medical Commission (IPC; 2016).
Special Considerations of AD and this CPG
AD is characterized by a sudden, significant increase in both systolic and diastolic BP above the usual levels and may be accompanied by either bradycardia or tachycardia in individuals with SCI at or above T6 in response to noxious and non-noxious stimuli below the level of injury.
Systolic and diastolic BPs are known to fluctuate during AD. However, for the purposes of this CPG, management strategies are focused on systolic BP. Therefore, individuals will be considered to have AD if their BP is greater than 20 mmHg above their usual baseline in adults and greater than 15 mmHg above their baseline in children. AD may include, in addition to increased BP, a constellation of other signs and symptoms, including headache, flushing, piloerection, stuffy nose, sweating above the level of the lesion, vasoconstriction below the level of the lesion, and dysrhythmias. This syndrome may or may not be symptomatic and may occur at any period following SCI (Krassioukov et al., 2012).
Special Considerations When Taking BP Measurements in Children and Adolescents
For children and adolescents, age and body size are determinants of normal BPs, with BPs increasing with advancing age and approximating adult norms in older teenagers (“Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents,” 1996). Similar to adults with SCI, children and adolescents with cervical and upper thoracic SCI are expected to have lower baseline BPs than those in the general population. Therefore, it is important to determine and document baseline BPs annually, or as needed as the child or adolescent with SCI ages. For these guidelines, the panel agreed that systolic BPs at or above 150 mmHg in adults, 120 mmHg in children under 5 years old, 130 mmHg in children 6–12 years old, and 140 mmHg in adolescents are the thresholds above which pharmacological agents should be considered.
It is important that health care providers be calm and maintain a reassuring environment in the presence of the child’s parents or caregiver when obtaining BPs. Any anxiety associated with obtaining BPs in children and adolescents may make it difficult to obtain accurate measurements, both for baseline determinations and during an episode of AD. Teaching parents how to obtain BPs or having school nurses obtain baseline BPs may be beneficial.
It is important to use appropriately sized BP cuffs when measuring BP in children and adolescents. The width of the BP cuff should be approximately 40% of the arm circumference, measured midway between the olecranon and the acromion (Perloff et al., 1993). The cuff bladder should cover 80% to 100% of the circumference of the arm. A BP cuff that is too small may result in overestimation of the individual’s BP. In contrast, a BP cuff that is too large may result in underestimation of the BP, which is less than the error of overestimation with a cuff that is too small. If an appropriately sized BP cuff is not available, interpretation of the BP reading is complicated.
Pathophysiology of Autonomic Dysreflexia
Triggers
AD has many potential causes. It is essential that the specific cause be identified and treated in order to resolve an episode of AD and to prevent recurrence. Any painful or irritating stimuli below the level of injury may cause AD. Bladder and bowel problems are the most common causes of AD. The following are some of the more common potential AD triggers:
Urinary System
Bladder distention
Bladder or kidney stones
Blocked catheter
Catheterization
Detrusor sphincter dyssynergia
Shock wave lithotripsy
Urinary tract infection
Urological instrumentation, such as cystoscopy or testing requiring catheterization
GI System
Appendicitis
Bowel distention
Bowel impaction
Gallstones
Gastric ulcers or gastritis
GI instrumentation
Hemorrhoids
Integumentary System
Constrictive clothing, shoes, or appliances
Contact with hard or sharp objects
Blisters
Burns, sunburn, or frostbite
Ingrown toenail
Insect bites
Pressure injuries
Reproductive System
Sexual activity, including sexual intercourse
Sexually transmitted diseases
High sexual arousal and/or orgasmic release
A second orgasmic release or ejaculation soon after first orgasm will likely provoke more severe AD
Male
Ejaculation
Epididymitis
High-intensity vibrators used to induce ejaculation
Priapism (especially from intracavernosal injection)
Prostatitis
Scrotal compression (sitting on scrotum)
Sperm retrieval (EEJ and vibratory stimulation)
Female
Lactation, breastfeeding, mastitis
Menstruation
Painful intercourse and/or friction
Pregnancy, especially labor and delivery, including ectopic pregnancy
Vaginitis
Other Systemic Causes
Boosting (an episode of AD intentionally caused by an athlete with SCI in an attempt to enhance physical performance)
Deep vein thrombosis
Excessive alcohol intake
Excessive caffeine or other diuretic intake
Fractures or other trauma below the level of injury
Functional electrical stimulation
Heterotopic bone
Over-the-counter or prescribed stimulants
Pulmonary emboli
Substance abuse
Sunburn
Syringomyelia
Surgical or invasive diagnostic procedures
1. Blood Pressure Following Spinal Cord Injury
1.1 Be aware that, compared with the general population, individuals with SCI are likely to have the following systolic BP differences:
In the supine resting position, adults with injuries at or above T6 will likely have low BP (on average systolic BP ~110 mmHg).
In the seated resting position, adults with injuries at or above T6 will likely have low BP (on average systolic BP ~100 mmHg).
-
Age-related changes in BP (i.e., pediatric age group and older individuals) may be different.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Because of autonomic nervous system impairment and loss of descending sympathetic cardiovascular control, individuals with SCI, particularly those with lesions above T6, have low resting BP (Phillips & Krassioukov, 2015). The prevalence of systolic hypotension (~110 mmHg) as identified in the medical record was 39% in veterans with tetraplegia (C1–C8) and 23.5% in veterans with high thoracic lesions (T1–T6) (Zhu et al., 2013). Of note, the prevalence of systolic hypotension was lower (10%) in veterans with lesions at T7 and below (Zhu et al., 2013). Although the impact of systolic hypotension in the chronic SCI population has not been fully identified, emerging evidence suggests adverse effects on cerebral circulation and cognition (Saleem et al., 2018; Wecht et al., 2018; Phillips et al., 2014).
2. Autonomic Dysreflexia
An individual with an SCI at or above T6 presents with an acute onset of signs and symptoms of AD.
2.1 Recognize that those with an SCI at or above T6 may present the signs and symptoms of AD, including:
-
Elevated systolic BP greater than 20 mmHg above their usual baseline in adults and greater than 15 mmHg above their usual baseline in children
Sudden-onset headache
Possible bradycardia or tachycardia
Cardiac arrhythmias, atrial fibrillation, premature ventricular contractions, and atrioventricular conduction abnormalities
Profuse sweating and/or flushing of the skin, typically (face, neck, and shoulders) or possibly below the level of the lesion
Piloerection (goose bumps) above or possibly below the level of the lesion
Blurred vision and/or spots in the individual’s visual fields
Nasal congestion
Feelings of apprehension or anxiety
-
Few or no symptoms other than elevated BP
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
In addition to a sudden onset of hypertension, an individual may have one or more of these signs and symptoms when experiencing an episode of AD. Symptoms may be minimal or even absent, despite an elevated BP. See Figure 3.
2.2 Be aware that AD may appear with minimal or no symptoms (silent AD or those with cognitive/verbal communication issues) despite a significantly elevated BP.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Health care providers should be aware that, in addition to a minimal or lack of symptoms of AD (silent AD), the varying cognitive and verbal communication abilities of children, adolescents, and adults can cause the symptoms of AD to be absent, subtle, vague, or expressed imperfectly. This is especially true for children and adolescents. For instance, preschool-aged children, even though they are verbal, may present with vague complaints such as irritability because they may not be able to accurately articulate that they are experiencing a pounding headache—a cardinal feature of AD.
2.3 Check the individual’s BP.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Elevated BPs can be life-threatening and need immediate investigation and treatment.
An individual with SCI above T6 often has a normal systolic BP in the 90–110 mmHg range. Therefore, a systolic BP of greater than 20 mmHg above baseline may be a sign of AD that may or may not need treatment (Krassioukov et al., 2012).
Systolic BP elevations of more than 20 mmHg above baseline in adolescents with SCI or more than 15 mmHg above baseline in children with SCI may be a sign of AD (Hickey et al., 2004).
2.4 If signs or symptoms of AD are present, but BP is not elevated and the cause has not been identified, refer the individual to an appropriate consultant, depending on signs and symptoms.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
AD is characterized by a rise in systolic BP greater than 20 mmHg above an individual’s baseline BP. Symptoms such as sudden onset of a severe headache or blurry vision can be a manifestation of other potentially life-threating conditions besides AD, and an immediate referral to another consultant, depending on the signs and symptoms, is necessary.
2.5 If AD is diagnosed, identify the trigger(s) in order to manage BP.
(Level - III; Strength - C; Panel Agreement -strong)
Rationale
See Triggers subsection under Pathophysiology of AD for a list of common causes of AD (Page 18). In general, the most common triggers of AD are genitourinary or GI related.
2.6 If BP is elevated, immediately sit the individual up and lower the legs if possible.
(Level - III; Strength - C; Panel Agreement -strong)
Rationale
Performing this maneuver may allow pooling of blood in the lower extremities and may reduce BP. If possible, in addition to sitting the individual up, lower his or her legs (Claydon, Hoi, et al., 2006, V. E. Claydon et al., 2006)
2.7 Monitor BP and pulse frequently (every 1–2 minutes) until the individual is stabilized.
(Level - III; Strength - C; Panel Agreement -strong)
Rationale
BPs can rise and fall quickly in relation to persistence of AD triggers and or increasing effectiveness of treatment. Ideally use a continuous BP monitor if available.
2.8 Loosen any clothing or constrictive devices.
(Level - III; Strength - C; Panel Agreement -strong)
Rationale
Loosening of any clothing and removal of constrictive devices (abdominal binders and compression stockings) may allow pooling of blood in the abdomen and lower extremities and reduce BP (Smit et al., 1999).
2.9 Determine whether the individual has recently taken a vasopressor or an antihypotensive agent.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Midodrine (Proamatine) (10 mg) increases standing systolic BP by 22 mmHg (28%) versus placebo (Jankovic et al., 1993). A reported side effect is supine hypertension (8%). The recent use of a vasopressive agent, such as pseudoephedrine (Sudafed), fludrocortisone (Fiorinef), ephedrine, midodrine (Proamatine), or droxidopa (Northera), may help explain the elevation in BP or additive effect of another instigating cause. Because these medications may exacerbate another trigger for AD, other causes still need to be evaluated and treated. Knowing that there has been a recent intake of a vasopressor or antihypotensive agent may also explain a less pronounced response of another pharmacological agent used to treat the hypertension. It may also require closer follow-up monitoring for a reoccurrence of the hypertension after the other pharmacological agent has worn off.
2.10 Quickly survey the individual for other triggers, beginning with the urinary system.
(Level - III; Strength - C; Panel Agreement -strong)
Rationale
The most common cause of AD is bladder distention. Bladder distention in itself causes a noxious stimulus that triggers AD, or, more commonly, it results in involuntary bladder contractions that cause sphincter contractions (detrusor sphincter dyssynergia), which, in turn, cause AD (Guttmann & Whitteridge, 1947; Lee et al., 1995; Trop & Bennett, 1991).
2.11 If an indwelling urinary catheter is not in place, catheterize the individual.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
If the bladder is overdistended, draining it will decrease the noxious stimuli and bladder and sphincter overactivity, resulting in a resolution of the AD.
2.12 If the elevated BP is at or above 150 mmHg systolic prior to catheterization, consider rapid-onset and short-duration pharmacological management to reduce the systolic BP without causing hypotension
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Removing and replacing a catheter can exacerbate BP. The use of a short-acting agent such as topical nitropaste will help prevent further increases in BP during the catheter change. Be aware that after the bladder is drained, BP monitoring is important since there may be persistent effects from the short-acting pharmacological agent.
There are no studies showing the exact point at which BP becomes dangerous. For this recommendation, the panel decided to adopt 150 mmHg systolic BP as the value at which pharmacological treatment should be considered for adults (Krassioukov et al., 2012).
In the pediatric age group, knowing the child’s baseline BP is crucial when deciding whether to intervene with antihypertensive medications. Indications for pharmacological intervention may include a systolic BP of 120 mmHg in infants and younger children (under 5 years old), 130 mmHg in older children (6–12 years old), and 140 mmHg in adolescents (Hickey et al., 2004).
See Appendix 2: Medications Used for Autonomic Dysreflexia.
2.13 Consider the use of an antihypertensive agent (such as nitropaste, nifedipine, hydralazine, or sublingual clonidine) with rapid onset and short duration.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
A number of short-acting antihypertensive agents have been described in treating elevated BP caused by AD. See Appendix 2 for more details on medications, their onset, and their duration of action (Krassioukov et al., 2009; Solinsky et al., 2017). There are 2 important considerations for use of an antihypertensive agent: rapid onset and short duration of action. A long-acting antihypertensive agent may cause hypotension after the instigating cause of AD has been found and corrected.
Note: Nitropaste is not available in some countries. If nitropaste or nitrates are not available or their use is contraindicated, consider administering another short-acting, rapid-onset antihypertensive agent.
2.14 Prior to use of nitropaste or any other agent containing nitrate, first inquire about whether the individual has recently taken a phosphodiesterase type 5 inhibitor (PDE5i).
(Level - II; Strength - B; Panel Agreement -strong)
Rationale
Use of a nitrate-containing medication such as nitropaste is contraindicated when an individual has recently taken a PDE5i because the combination of the 2 drugs can cause a sudden severe drop in BP. Be aware that the length of time of potential interaction between PDE5i and nitrate varies depending on which PDE5i was used.
Note: Nitropaste is not available in some countries. If nitropaste or nitrates are not available or their use is contraindicated, consider administering another short-acting, rapid-onset antihypertensive agent.
2.15 Prior to inserting the catheter, instill lidocaine jelly 2% (if immediately available in the room where the individual is being treated) into the urethra and wait approximately 5 minutes, if possible.
(Level - II; Strength - B; Panel Agreement -strong)
Rationale
Catheterization can exacerbate AD as a result of triggering sphincter overactivity as the catheter is being passed. The use of lidocaine jelly may decrease sensory input and relax the sphincter, thereby facilitating catheterization (Lidocaine Hydrochloride Jelly USP, 2%,(package insert), 2012).
With the possible exception of topical nitropaste that can be removed immediately, pharmacological agents are generally not recommended at this point for reducing the risk of hypotension because draining of the bladder is expected to immediately lower the BP. Exercise clinical judgment regarding elevated BP; immediate catheterization may be necessary. Note: When passing the catheter, some of the lidocaine jelly may pass into the “eye holes” at the end of the catheter. The lidocaine jelly may be viscous enough that it may transiently block the catheter and there may not be an immediate flow of urine out of the catheter. A 5-cc bolus flush of air, sterile water, or saline through the newly inserted catheter may be needed to begin the flow through the catheter.
2.16 Avoid applying pressure over the bladder (Crede maneuver) or suprapubic tapping, as this may exacerbate AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
It is very important to avoid any bladder pressure (pushing down on the bladder), bladder tapping, or use of large volumes of bladder irrigation, as this may also cause bladder contraction and sphincter dyssynergia, which could exacerbate the AD (Lee et al., 2017).
2.17 If the individual has an indwelling urinary catheter, check the system along its entire length for kinks, folds, constrictions, or an overfilled drainage bag and for correct catheter placement. If a problem is found, correct it immediately.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Any of the above-listed problems can cause bladder distention and AD and is easily correctable.
2.18 If there are no problems with the tubing, drainage bag, or catheter placement and the BP is still elevated, gently irrigate the bladder with a small amount (10–15 cc) of fluid, such as normal saline at body temperature, to determine whether the catheter is blocked. Irrigation should be limited to 5–10 cc for children under 2 years of age. Do not continue to irrigate or attempt to flush the bladder if the fluid is not draining from the catheter, as this will only cause increased bladder distention and increase the BP.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Use of a larger volume or of a cold solution may irritate the bladder and exacerbate AD. If a lidocaine solution is readily available, irrigation with it may be beneficial by decreasing sensory input from the bladder (Solinsky et al., 2020). Bladder pressure or tapping may also cause bladder contractions and sphincter dyssynergia and exacerbate AD.
2.19 If the catheter is blocked, remove and replace it.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Confirming catheter blockage and changing the catheter to allow bladder drainage should be performed as quickly as possible to allow resolution of AD.
2.20 If there is history of difficulty passing a catheter in a male, consider using a coudé catheter or consult urology.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
A coudé catheter may be useful at passing the catheter into the bladder if there is significant detrusor sphincter dyssynergia or a high median lobe of the prostate. This catheter is designed to more easily pass over the bladder neck with less trauma than occurs with a straight catheter. It is especially useful in those with a history of sphincterotomy and in older men, in particular those with a history of prostatic hypertrophy or transurethral resection of the prostate. When passing a coudé catheter, the tip of the catheter should be pointed upwards.
2.21 Prior to replacing the catheter, consider instilling lidocaine jelly 2% (if immediately available) into the urethra or suprapubic tract and wait 3–5 minutes, if possible.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
As discussed in Recommendation 2.15, catheterization can exacerbate AD. The use of lidocaine jelly may decrease sensory input and relax the sphincter to facilitate catheterization. Administration of 10 cc of 2% lidocaine into the existing catheter 3–5 minutes prior to catheter change demonstrated a significantly attenuated rise in systolic BP immediately after the catheter change (Solinsky & Linsenmeyer, 2019). Note: The lidocaine may be viscous enough that it could transiently block the catheter and there may not be an immediate flow of urine out of the catheter. A 5-cc bolus flush of air, sterile water, or saline instilled through the newly inserted catheter may be helpful to begin the flow through the catheter.
2.22 If difficulties arise in removing or replacing the catheter, in addition to instilling lidocaine jelly, consider initiating new or increasing previous pharmacological treatment and an emergency urology consultation.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Attempts to remove an encrusted catheter and pulling it through the urethra will likely exacerbate AD, and therefore beginning pharmacological treatment should be strongly considered before attempting to remove a catheter that is not easy to remove. The catheter could potentially become lodged in the urethra when attempts are made to remove it, and so an emergency urology consult should also be considered.
2.23 Monitor individual’s BP during bladder drainage.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Sudden decompression of a large volume of urine would be expected to normalize BP. However, this may cause hypotension if the individual has already been given pharmacological agents to decrease BP. The individual’s BP should continue to be monitored until it is noted to have stabilized (see Section 7, Orthostatic Hypotension).
2.24 If acute symptoms of AD persist, including a sustained elevation in BP, suspect fecal impaction.
(Level - II; Strength - B; Panel Agreement -strong)
Rationale
Fecal impaction is the second most common cause of AD (Colachis, 1992; Lee et al., 1995).
2.25 If the elevated BP persists at or above 150 mmHg systolic, strongly consider pharmacological management prior to laying the individual down to check for fecal impaction.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Laying an individual down and performing digital stimulation would be expected to exacerbate AD. The control of hypertension may need to be addressed prior to digital stimulation or other diagnostic maneuvers, which may exacerbate AD.
2.26 If fecal impaction is suspected, check the rectum for stool, using the following procedure:
Premedicate with a pharmacological agent as outlined in Recommendation 2.21.
With a gloved hand, generously instill a topical anesthetic agent, such as lidocaine jelly 2%, into the rectum.
Wait 4–6 minutes, if possible, for sensation in the area to decrease.
Then, with a gloved hand, insert a lubricated finger into the rectum and check for the presence of stool. If present, gently remove, if possible.
(Level - II; Strength - B; Panel Agreement -strong)
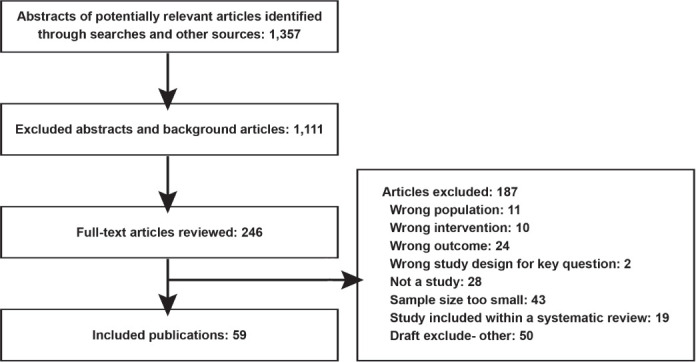
Rationale
A rectal examination may exacerbate AD (Furusawa et al., 2007).
For additional information about neurogenic bowel, refer to Management of the Neurogenic Bowel in Adults with Spinal Cord Injury (Consortium for Spinal Cord Medicine, 1998).
2.27 If AD becomes worse, or stool cannot be removed, stop the manual evacuation and administer pharmacological or additional pharmacological intervention and additional topical anesthetic. When BP is stable below 150 mmHg, proceed with an aggressive bowel evacuation regimen.
(Level - II; Strength - B; Panel Agreement -strong)
Rationale
Continued attempts at removing stool will likely worsen the individual’s BP. Therefore, it is best to wait approximately 20 minutes to allow the pharmacological agents to have an effect before proceeding with further disimpaction.
2.28 If there is no fecal impaction or BP elevation persists despite disimpaction, check for other less frequent causes of AD (see list of triggers page 18). If there are no obvious triggers or if the BP cannot be managed locally, the individual must be referred to the hospital emergency department for evaluation and management and possible hospital admission.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
While performing the above recommendations, evaluation of other possible triggers should also be undertaken, such as checking skin for pressure injuries, checking for ingrown toenails, etc. Other common potential triggers of AD are listed in the Triggers subsection under Pathophysiology of AD.
If pharmacological treatment is not able to control BP, further assessment may need to include more advanced diagnostic procedures. The scope of additional diagnostic procedures should be based on individual clinical presentation and medical judgment.
In addition, more extensive treatment that requires emergency department and hospital personnel and equipment may be needed.
2.29 While the individual is being evaluated in the emergency department, continue to closely monitor BP to guide pharmacological management of AD and investigate other causes. Consider hospital admission if:
There is poor response to the treatment specified above
-
The cause has not been identified
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Because of the loss of sensation, individuals with SCI can have significant pathology with minimal symptoms. These symptoms may include problems such as acute abdominal pathology, long bone fractures, and ingrown toenails (Braddom & Rocco, 1991). Individuals with SCI frequently have positive urine culture results. However, this may not be the precipitating cause for AD; therefore, other causes of AD should also be investigated.
2.30 After successful identification of the trigger and treatment of the elevated BP, monitor the individual for symptomatic hypotension every 2–5 minutes until the BP is stable.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
After treatment of the precipitating cause of AD, the individual’s BP may continue to drop lower than baseline because of the persistent effect of the medications used to treat the AD. After the trigger is identified and treated, wipe off the nitropaste (if used) when BP is less than 20 mmHg above the individual’s baseline BP.
Treat severe (symptomatic) hypotension by laying the individual down and elevating his or her legs. Additional corrective measures are not usually required. However, if indicated, consider intravenous fluids and adrenergic agonists (i.e., in a monitored setting, intravenous norepinephrine for reversal of severe hypotensive events). See Section 7, Orthostatic Hypotension, for more information.
2.31 Following an episode of AD, a health care provider should consider the following:
If the individual is an inpatient or in the clinic, monitor closely for at least 2 hours for recurrent AD or hypotension.
If at home, instruct the individual to seek immediate medical attention if AD symptoms reoccur.
-
Prescribe a BP monitoring device to the individual for home monitoring.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
The hypertension and symptoms may have resolved because of the medication management rather than treatment of the cause. Symptoms managed by pharmacological treatment may begin to reverse themselves within this time frame.
2.32 Document the episode of AD and record the effectiveness of the treatment in the individual’s medical record, including the following:
Presenting signs and symptoms and their course
Recordings of BP and pulse
Treatment instituted and response to treatment
Restoration of BP and HR to normal levels for the individual
Diagnosis of a history of AD in order to inform future clinicians of the risk in the individual and prior response to treatments initiated
Identification of the cause (trigger) of the AD episode
Whether the individual is comfortable, with no signs or symptoms of AD or secondary complications, such as neurological changes, increased intracranial pressure, or heart failure
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Good documentation will help future evaluation of triggers and treatment that may be helpful if the individual presents with another episode of AD.
2.33 After the individual with SCI has been stabilized, review the precipitating cause of the AD episode with the individual, family members, significant others, and caregivers to educate them regarding instigating factors, recognition, management, and prevention of future AD episodes.
Adjust the treatment plan to ensure that future episodes are recognized and treated to prevent a medical crisis or, ideally, are avoided altogether.
Discuss AD during the individual’s education program, so that he or she will be able to minimize the risks known to precipitate AD, solve problems, recognize early onset, and obtain help as quickly as possible.
Have an ongoing conversation and continue education at annual evaluations or clinic appointments.
Give a written wallet card/guide or instruction sheet or consider a medical alert bracelet.
(Level - V; Strength - C; Panel Agreement -strong)
See Appendix 3: Education: Health Care Professionals, Individuals with Spinal Cord Injury, Family, and Caregivers
Rationale
A written guide or alert, such as a wallet card, may help individuals with SCI to communicate with their health care providers, parents, and guardians. Such an alert system is especially needed when concomitant injuries that have resulted in reduced or limited cognition and verbal skills cause language barriers that may hinder the individual’s ability to communicate that he or she is experiencing AD.
A written treatment plan for AD prepared for adults, children, and adolescents with SCI should include:
The individual’s normal BP, which is updated annually or more frequently as needed
Diagnostic criteria
An emergency management plan
Limited cognition, language barriers, and verbal skills hinder the ability of younger children to communicate that they are experiencing AD with health care providers, teachers, and other adults who are responsible for supervising their activities.
Parents of young children should consider using some form of medical alert identification and ensuring that appropriate education is provided to adults who have significant interactions with and responsibility for their child with SCI, such as teachers, school nurses, coaches, and community-based health care providers.
2.34 Perform detailed evaluations for individuals with recurrent AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
See the list of potential triggers in the Triggers subsection under Pathophysiology of AD. There may be subtle changes in an individual’s medical condition, such as a worsening of detrusor sphincter dyssynergia or an expanding syrinx that is causing recurrent AD. Therefore, a detailed medical evaluation is warranted.
3. Autonomic Dysreflexia: Sexuality
After SCI, individuals retain the capacity for sexual desire and mental arousal, but will have various physical pelvic arousal manifestations, depending on their level of injury and completeness. Individuals with SCI are fully capable of sexual response and enjoyment, and they often rate sexual functioning as their highest health priority after SCI (Anderson, 2004; New, 2016; Simpson et al., 2012).
AD is likely to be more severe the stronger and longer the genital stimulation is, but often symptomatic AD decreases over time (months and years). However, there is no assurance that BP elevations will be reduced, as “silent AD” can still occur (Ekland et al., 2008; Kirshblum et al., 2002).
Sexual activity after SCI is encouraged and is associated with an improved quality of life for men and women (Anderson et al., 2007a, 2007b). The individual with SCI must balance symptoms and risks of sexually induced AD with the benefits of sexual activity and the rewards of intimacy.
Recommendations for Sexual Activity in the Home Setting
3.1 Be aware of and educate individuals with SCI at or above T6 that sexual activity may provoke AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
In individuals with SCI at T6 and above, systolic BP is known to increase more during sexual activity than it does in able-bodied individuals (Davidson et al., 2016).
3.2 Be aware that for men and women with SCI at or above T6 who use intense sexual stimulation (including vibratory stimulation), the likelihood of AD is increased.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Intense sexual stimulation has been noted to cause significant elevations in systolic BP in individuals with injuries at T6 and above (Davidson et al., 2016). A second orgasm can cause even higher BP.
3.3 Encourage individuals with SCI at T6 and above to periodically monitor their BP during sexual activities.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Some symptoms of AD may become less severe or bothersome over time with sexual activities (or with penile vibratory stimulation [PVS]) without a corresponding reduction in BP. In some individuals following SCI, high BPs may even be asymptomatic (silent AD). Therefore, it should not be presumed that during sexual activities or PVS, diminution or lack of AD symptoms means lower BP or that AD is not occurring. If possible, attempts at ejaculation or orgasmic release should be done in a clinic with continuous BP readings so that a decision on safety and the need for prophylaxis in the private home setting can be made.
3.4 Individuals prone to AD during sexual activity should be encouraged to use a home BP monitor.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Symptoms of elevated BP may be unreliable or silent.
3.5 If sexual activity causes symptomatic AD, individuals should be encouraged to immediately cease sexual stimulation and follow AD protocol.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
The triggering event should be stopped immediately. The individual should sit up with his or her feet lowered, any restrictive clothing or bedding removed, and any kinking of catheter tubing corrected. If AD persists, consider going to the emergency department for further evaluation and management.
3.6 Consider instructing and prescribing pharmacological prophylaxis prior to sexual activity in selected individuals who:
Have no history of symptomatic orthostatic hypotension (OH)
Are not taking medication that may potentiate hypotension
Developed AD with systolic BP at or above 150 mm Hg (i.e., during vibratory stimulation, ejaculation, orgasm, sperm retrieval, or urological procedures)
-
Have symptomatic AD and/or systolic BP greater than 150 mmHg prior to sexual activity
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Pharmacological medications that may be useful for AD prophylaxis include the following:
Prazosin 1 mg: 1–3 hours prior to PVS or 1 mg twice daily if AD with sexual activity is a consistent problem.
Nitropaste 1–2 inches (2.5–5 cm): Apply to the forehead or non-hairy portions of the body 5 minutes prior to sexual activity. This can be wiped off with an alcohol swab with cessation of sexual stimulation. Nitropaste is contraindicated with the use of a PDE5i for erection enhancement (Sinha et al., 2017). Be careful to avoid inadvertent transference to a partner.
Nifedipine 10 mg (puncture capsule with needle; administer under tongue and instruct to then swallow capsule with water): Should be reserved for men and women who are familiar with the effects of the medication prior to sexual stimulation. This medication can cause a significant drop in BP (OH).
PDE5 is taken for improvement of erection are safe in men with SCI unless they cause undue hypotension in an individual. Headache and facial flushing are common side effects of PDE5is (drugs for erection enhancement) and can be misinterpreted as AD and erroneously treated with nitrates, a contraindication.
Note: Nitropaste is not available in some countries. If nitropaste or nitrates are not available or their use is contraindicated, consider administering 25 mg captopril sublingually or another short-acting, rapid-onset antihypertensive agent (see Recommendation 2.21).
3.7 If pharmacological treatment for AD is used in a home setting, instruct individuals on how to recognize, monitor, and treat pharmacologically induced hypotension.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
OH frequently occurs following pharmacological management of AD (see Section 7, Orthostatic Hypotension).
3.8 Instruct individuals at risk of AD to recheck BP within 5 minutes of cessation of sexual activity, regardless of symptoms.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Note that BP may remain elevated for longer than 5 minutes after cessation of sexual activity. With cessation of ejaculation or arousal, elevated BP should resolve within 5 minutes (Sinha et al., 2017).
3.9 If the individual’s high BP does not resolve after 5 minutes, refer to steps for treatment of AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
An individual’s BP should decrease close to baseline after 5 minutes of cessation of sexual activity.
3.10 Instruct individuals that if all conservative home measures to treat AD or pharmacologically induced hypotension following sexual activity are unsuccessful, an urgent visit to the emergency department is warranted.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
More extensive diagnostic testing and treatment requiring an emergency department or hospital setting may be needed (see Recommendation 2.28).
4. Autonomic Dysreflexia: Cystoscopic (Transurethral and Suprapubic) Urological Procedures and Sperm Retrieval Procedures
Overview
Cystoscopic (transurethral and suprapubic), urodynamic, and sperm retrieval procedures represent unique challenges in managing AD in individuals with SCI at T6 and above. Urodynamics and many cystoscopic procedures can generally be done in a clinic setting without general or spinal anesthesia or intravenous sedation (e.g., cystoscopy, bladder biopsies, simple removal of foreign bodies, and treatment of bladder stones). Note that when an individual is transferred from a wheelchair to the urology table, frequently the BP transiently elevates but then decreases after a few minutes. These procedures involve bladder and urethral instrumentation, as well as bladder distention, which are triggers for AD. The first step to sit an individual up, as it is not practical if a person develops AD in the middle of the urodynamics or urological cystoscopic procedure unless the AD is severe and the procedure needs to be terminated immediately.
Sperm retrieval procedures include the use of penile vibratory stimulation (PVS) and electroejaculation (EEJ). The unique challenge with PVS or EEJ sperm retrieval procedures is that ejaculation is a potent trigger for AD and there can be a sudden severe elevation in BP during ejaculation. The health care provider needs to carefully monitor and anticipate a BP elevation and know the best strategies to manage AD in these situations.
Recommendations
4.1 Prior to the procedure, counsel the individual to:
Take prescribed medications (such as anticholinergic medications, alpha-blockers)
Have a recent bowel program (within 1–2 days)
Treat urinary tract infection, if present
Hold any as-needed medications that may elevate BP (such as ephedrine, midodrine)
-
Hold any medications such as PDEi5s, which may not allow nitrates (nitropaste) to be used to treat AD
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Attempt to eliminate factors that may augment AD during a procedure. One of the most common causes of AD is constipation, making it imperative that the individual has had a recent bowel program. Anticholinergic medications help to decrease involuntary bladder contractions and alpha-blockers help decrease detrusor sphincter dyssynergia. In cases of symptomatic urinary tract infection, it sometimes takes several days of antibiotics for bladder wall inflammation to resolve. See Triggers subsection under Pathophysiology of AD for other possible causes. PDE5is should be withheld because nitropaste is frequently used to treat AD, and the concurrent use of PDE5is and nitrates is contraindicated due to the risk of profound hypotension.
4.2 If prior to the procedure an individual presents with a systolic BP that is greater than 20 mmHg above their usual baseline systolic BP, evaluate for possible causes of AD and manage and monitor it.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Reversible causes include kinked catheter tubing, an overfull leg bag, tight clothing or the presence of an abdominal binder, or sitting on a pressure injury. These causes can be easily corrected and the individual can then be set up for the procedure if the high BP resolves with corrective actions.
4.3 Consider rescheduling the individual if AD persists despite finding and correcting any obvious reversible causes.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
The individual needs a more extensive evaluation and management if reversible causes are either not found or persist despite treatment in order to avoid severe AD if a procedure is undertaken.
4.4 Consider decreasing the risk of AD before urethral instrumentation such as cystoscopy by instilling lidocaine jelly into the urethra at least 3–5 minutes before urethral instrumentation.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
While lidocaine jelly is useful with cystoscopy, these measures are not as helpful for urodynamic evaluation because the lidocaine jelly decreases sensory afferents and can affect the outcome of the study.
4.5 In individuals who are prone to AD or have a recent history of AD, consider prophylactic pharmacological treatment to decrease the risk of AD before cystoscopic procedures and sperm retrieval procedures.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Induced ejaculation from PVS or EEJ usually causes AD. Prophylactic pharmacological management helps to blunt this response.
Prophylactic pharmacological medication should not be given before urodynamics, since one of the important aspects of the urodynamics is to determine if and how much the BP elevates with bladder filling and voiding and if there are any symptoms. It is important to have pharmacological agents at the bedside, as they may be urgently needed to treat AD.
For prevention of AD prior to cystoscopic procedures, consider anticholinergic medication at least 1 hour prior to the procedure in individuals with neurogenic detrusor overactivity. Consider an alpha-blocker the night before or the morning of the procedure to help decrease detrusor sphincter dyssynergia (and AD).
In individuals with known episodes of AD, consider applying nitropaste 0.5–1 inch (1.25–2.5 cm) prior to starting the procedure. Apply to the forehead or non-hairy portions of the body 4–6 minutes prior to urological or sperm retrieval procedures. This can be wiped off at cessation of the procedure. DO NOT use if there is recent history of PDEi use, i.e., sildenafil [Viagra], if vardenafil [Levitra] has been used in the last 24 hours, or if tadalafil [Cialis] has been taken within the last 4 days.
Note: Nitropaste is not available in some countries. If nitropaste or nitrates are not available or their use is contraindicated, consider administering another short-acting, rapid-onset antihypertensive agent. For example, if nitropaste is not available, try nitrospray 400 mcg/spray, 1 spray q 5–10 minutes, maximal 3 sprays in 30 minutes.
For prevention of AD prior to sperm retrieval procedures, nifedipine 10 mg bite and chew should be reserved for those men familiar with the effects of the medication prior to sexual stimulation, or who are monitored in these clinic procedures to assess and manage the potential for a significant drop in BP (OH).
4.6 During sperm retrieval procedures, BP should be monitored at 1-minute intervals.
Rationale
Systolic BP needs to be monitored very closely during sperm retrieval since it can elevate and change rapidly when ejaculation occurs (Krassioukov et al., 2009). If AD develops during vibratory stimulation or ejaculation, monitor for the absolute level of systolic BP and symptoms and decide whether to continue or stop if ejaculation has not been reached. A well-functioning automated BP machine is suggested.
(Level - V; Strength - C; Panel Agreement -strong)
4.7 During cystoscopic and urodynamic procedures, monitor BP in at least 2-minute intervals, preferably with an automatic BP cuff. Perform more frequent BP readings if the patient is developing AD during the procedure.
Rationale
BP may initially not elevate rapidly during the beginning of the urodynamic or cystoscopic procedure but can elevate and change rapidly (Krassioukov et al., 2009). However, BP changes are usually less acute than during sperm retrieval procedures because the stimulus is more amenable to control by draining the bladder.
4.8 Rather than immediately sitting an individual up during cystoscopic and urodynamic procedures, attempt to control AD by draining the bladder as needed and, if not resolved, institute a similar pharmacological strategy as that recommended for the management of AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
It is not practical to tilt the urology table up 60–70 degrees during a procedure. A sudden change in position will usually produce artifact and affect the urodynamic recording of the intravesical pressures. Since bladder distention is a major trigger for AD, draining the bladder and pharmacological treatment as needed while remaining in a supine position is usually effective. In severe cases in which draining the bladder and pharmacological treatment is not effective, an individual will need to be sat up. However, in most cases, AD can be controlled by maintaining the individual in a lithotomy position.
4.9 During cystoscopic and urodynamic procedures, if AD is not controlled by draining the bladder or with pharmacological measures, stop the procedure and sit the individual up.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Draining the bladder helps to control the AD since bladder distention is a trigger for AD. Sitting the individual up allows pooling of blood into the lower extremities to decrease BP.
4.10 Monitor BP after cystoscopic or urodynamic procedure or after ejaculation until it subsides to near the individual’s baseline. Monitor for continued elevated BP or OH when the individual is moved to the seated position.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
In general, BP should return to baseline levels within 5–20 minutes after ejaculation. There is normally no need for additional medications or treatment. However, be aware of potential malignant AD following ejaculation (protracted AD after ejaculation is over or more severe AD with usual triggers for days or months) (Elliott & Krassioukov, 2006). Be aware that BP treatments used to prevent or treat AD may have a persistent effect and cause OH.
4.11 AD prevention and control will be under the direction of the specialist administering anesthesia to individuals who require it while undergoing electroejaculation.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Spinal or epidural anesthesia blocks the afferent stimuli causing AD.
5. Autonomic Dysreflexia in Pregnancy, Labor and Delivery, and the Postpartum Period
Overview
Women with SCI should discuss with their obstetrician exactly how labor and delivery will be managed and have a multidisciplinary care plan. Since AD can interfere with uteroplacental blood flow, a pregnant woman with SCI should be checked weekly for effacement and dilation of the cervix beginning at 28 weeks of gestation. In some cases, early hospitalization may be necessary (Jackson & Wadley, 1999).
Diminished sensation and absence of pain may result in unrecognized conventional labor symptoms or atypical labor symptoms, especially in women whose injury levels are T10 and above (Jackson & Wadley, 1999). The health care provider should explain these phenomena (e.g., increase in spasm, presentation of intravaginal bleeding, referred pain to the shoulder, or AD) to the woman so that she can recognize them and avoid unmonitored or precipitous labor.
For women with injuries above T6 who present with hypertension during pregnancy, labor, or delivery, it is critical to differentiate preeclampsia from AD (preeclampsia occurs with the same frequency in able-bodied women and in women with disabilities) so that it is not misdiagnosed. Use of general or epidural anesthetics may assist in reducing AD risks. Delivery in women with SCI can also be complicated by hip disarticulation, contractures, heterotrophic ossification, and severe spasticity.
Recommendations
5.1 Instruct health care professionals that women with SCI who have the potential of developing AD are at increased risk of severe AD during pregnancy, labor, delivery, and breastfeeding and should be followed by a multidisciplinary team.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
AD can be unrecognized in pregnancy and delivery because BP within the normal range in an able-bodied woman may represent a large increase in a woman with a high-level injury who was significantly hypotensive to begin with. Cases of AD have occurred in the antepartum, intrapartum, and postpartum periods. Many health care providers will need education on the causes, signs, and symptoms of AD in women with SCI. Since AD can interfere with uteroplacental blood flow, careful monitoring of the fetus is recommended when dysreflexia is severe or frequent during pregnancy (Skowronski & Hartman, 2008). AD must especially be watched for during the third trimester. During labor, AD must be distinguished from preeclampsia. After delivery, breastfeeding can also be a trigger for AD. Decisions on pharmacological treatment of hypertension and long-term medication use must be made in the context of drug safety during pregnancy and breastfeeding.
5.2 An antepartum consultation with an anesthesiologist and the establishment of a plan for induction of epidural or spinal anesthesia at the onset of labor is recommended to assess the risk of AD and to prevent it, in accordance with recommendations of the American College of Obstetricians and Gynecologists.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Although individuals with SCI may perceive no pain during labor, anesthesia should be used to prevent AD. Spinal or epidural anesthesia extending to the T10 level is the most reliable method of preventing AD by blocking stimuli that arise from pelvic organs (American College of Obstetricians and Gynecologists, 2002). A consultation with the anesthesiologist should also consider the safe use of antihypertensives during pregnancy and labor, as well as those recommended for AD long-term use. It should also be used as an educational experience for the mother and support persons to recognize not only typical signs of labor, but also atypical, including AD.
5.3 In pregnant women prone to AD, careful and frequent monitoring of the fetus is recommended, especially during labor and delivery.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Fetal monitoring and maternal BP measurements should be done at weekly intervals starting at 28 weeks in women with SCI, especially in individuals prone to AD. Care of pregnant women with AD should take into account that other problems such as preeclampsia may be mistaken for AD. When considering treatment of AD, remember that a woman’s position can affect it. For example, hypotension due to compression of the vena cava may occur if the woman is in the supine position. Therefore, although a lateral tilt or upright position is important to improve uterine blood flow, it is expected to also increase BP, which may require further treatment (Skowronski & Hartman, 2008).
5.4 AD must be differentiated from preeclampsia during pregnancy and labor to ensure appropriate treatment.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Some clinical features make it difficult to distinguish preeclampsia from AD. Preeclampsia usually begins after 20 weeks of pregnancy in women whose BP had been normal. Both preeclampsia and AD are often diagnosed while the woman is in labor, but the clinical presentation of preeclampsia may vary from the typical triad of hypertension, proteinuria, and edema. Preeclampsia occurs with the same frequency in able-bodied women and women with disabilities. It is critical to distinguish between AD and preeclampsia of pregnancy, since the treatments are different and unrecognized AD can lead to serious consequences. Both AD and preeclampsia can present with hypertension, headache, clonus, and edema, but during labor, AD occurs during the contractions and resolves between them, whereas in preeclampsia, hypertension is constant, unaffected by the contractions, and accompanied by proteinuria, which should not occur in a woman with SCI who has normal kidney function (Signore et al., 2011).
5.5 Although individuals with SCI may not perceive pain during labor, anesthesia should be used to prevent AD in women with SCI above T6. Spinal or epidural anesthesia is the most reliable method of preventing AD by blocking stimuli that arise from pelvic organs.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Spinal anesthesia is similar to epidural anesthesia in that it is placed, with a needle, in the same approximate location of the lower spine. However, with spinal anesthesia, the needle is passed beyond the epidural space into the subdural space, allowing an even deeper intensity of regional anesthesia in a distribution that is similar to that achieved with epidural anesthesia. Spinal or epidural anesthesia extending to the T10 level is the most reliable method of preventing AD by blocking stimuli that arise from the pelvic organs.
See Appendix 4: Pregnancy and Breastfeeding Precautions with Autonomic Dysreflexia Medications
5.6 Educate women who have the potential to develop AD that postpartum breastfeeding, breast engorgement, or mastitis may trigger AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
AD was experienced by 38.9% of women with a high-level injury who were breastfeeding (Holmgren et al., 2018). Because AD is typically initiated by any stimulus below the injury level, it is plausible that an infant’s suckling, breast engorgement, or mastitis is a sufficient trigger (see Appendix 4 for pregnancy and breastfeeding precautions for AD medications).
6. Induced Autonomic Dysreflexia (Boosting)
Overview
The important components of cardiovascular control needed for exercise performance include the ability of the arterial BP and HR to respond appropriately to the increased demands of the body during exercise. Unfortunately, in individuals with SCI, all of the systems that are crucial for exercise performance, including cardiovascular, are affected by the SCI, and the extent of dysfunction and ability of the cardiovascular system to respond to exercise depends on the level and severity of the injury (Phillips, Squair, & Krassioukov, 2017; West et al., 2013).
With respect to exercise and cardiovascular system adaptation after SCI, studies have consistently shown that, relative to athletes with lower levels of SCI and athletes with an intact autonomic nervous system, athletes with SCI who have injuries above T6 have a low resting BP and episodes of OH (Phillips, Squair, Currie, et al., 2017), lower maximal HR (due to altered sympathetic tone and reduced catecholamine release) (Currie, West, et al., 2015; Gee et al., 2020), lower maximal oxygen uptake, and lower peak power in response to submaximal and maximal exercise. All of these factors contribute to fatigue and alter athletic performance (Harris, 1994). This altered autonomic control of the cardiovascular system that can affect exercise performance creates the potential for an uneven playing field between wheelchair athletes who practice inappropriate strategies to accommodate for their loss of (physiological) function. Boosting, or voluntarily induced AD, is a practice unique to athletes with a high-level SCI and is sometimes used by this population to improve exercise performance (Bhambhani et al., 2010; Schmid et al., 2001). Boosting may not only improve performance by increasing an athlete’s BP, but it may also do so by preventing the common symptoms of low arterial BP such as reduced physical capacity, fatigue, dizziness, and syncope (Phillips, Squair, & Krassioukov, 2017). There are various anecdotal reports of different methods of boosting during competition, including sitting on one’s scrotum, clamping one’s Foley catheter, and even breaking one’s big toe.
Boosting was first prohibited by the IPC in 1994 because of concerns surrounding the ethics and performance-enhancing properties of the practice. The IPC, which has a duty of care to ensure that athletes are competing in a safe manner at sanctioned events, has since recognized that AD is also a safety risk to athletes with SCI and prohibits them from competing in a dysreflexic state whether intentional or not (Van De Vliet, 2012).
The latest IPC “Position Statement on Autonomic Dysreflexia and Boosting” states that “a hazardous dysreflexic state is considered to be present when the systolic blood pressure is 160 mmHg or above” (2016). With this in mind, the current approach of the IPC is to test an athlete’s BP before competition; if the athlete’s systolic BP is found to be at or above the threshold of 160 mmHg, he or she will be reexamined approximately 10 minutes later.
Recommendations
6.1 Inform individuals with SCI that self-induced AD (e.g., boosting) to benefit daily activities and/or sports performance is a dangerous practice that can result in uncontrollable, life-threatening increases in BP.
(Level - IV; Strength - C; Panel Agreement -strong)
Rationale
Individuals with injuries at T6 and above need to be aware of possible serious consequences of self-induced AD.
7. Orthostatic Hypotension (OH)
Overview
In addition to low resting arterial BP, many individuals with high SCI also experience a further drop in BP when they assume an upright position. This is particularly common in the acute phase of injury (Sidorov et al., 2008). The Consensus Committee of the American Autonomic Society and the American Academy of Neurology define OH as a decrease in systolic BP of 20 mmHg or more, or in diastolic BP of 10 mmHg or more, on the assumption of an upright posture from a supine position, regardless of whether symptoms occur (1996). The symptoms of OH in individuals with SCI are similar to those in able-bodied individuals (Cleophas et al., 1986) and include fatigue or weakness, light-headedness, dizziness, blurred vision, dyspnea, and restlessness (Frisbie & Steele, 1997; Sclater & Alagiakrishnan, 2004). However, OH can also be asymptomatic; Illman and colleagues (Illman et al., 2000) reported that 41.1% of individuals with SCI who developed OH were asymptomatic, despite significant falls in BP. Claydon and Krassioukov (2006) also reported that OH persists asymptomatically in the chronic stage of SCI, despite a marked decrease in arterial BP. Furthermore, in the same study, the presence of symptomatic and asymptomatic OH was dependent on the neurological level of injury. BP decreases indicative of OH were observed in 7 of 14 (50%) subjects with cervical SCI and in 2 of 11 (18%) subjects with thoracic SCI. Symptomatic OH was present in 5 (36%) subjects with cervical SCI and in 2 (18%) subjects with thoracic SCI and required early termination of the test in 2 of the subjects with cervical SCI (Claydon & Krassioukov, 2006). Asymptomatic OH has also been reported in other able-bodied populations with autonomic disturbances and is likely a result of protective alterations in cerebral autoregulation despite cerebral hypoperfusion (Gonzalez et al., 1991; Houtman et al., 2000; Mathias et al., 1999).
Several mechanisms have been proposed for the development of OH in individuals with SCI. Interruption of sympatho-excitatory efferent pathways from the brain stem to the spinal sympathetic preganglionic neurons involved in vasoconstriction causes failure of short-term reflex BP regulation (Blackmer, 2003; Claydon, Hol, et al., 2006). This leads to pooling of blood in the viscera and dependent vasculature below the level of injury. Resting catecholamine levels are also lower in individuals with cervical SCI compared with those with paraplegia and able-bodied individuals, and there is no significant increase in epinephrine or norepinephrine levels when individuals with tetraplegia undergo a head-up tilt (Claydon, Steeves, et al., 2006; Claydon & Krassioukov, 2006; Mathias et al., 1980). Individuals with SCI are also reported to have impaired baroreflex function (Wecht et al., 2003), smaller plasma volumes due to hyponatremia (Frisbie & Steele, 1997), and possible cardiovascular deconditioning, at least in the early period following SCI, as a result of prolonged periods of bed rest (Vaziri, 2003). Any combination of these factors can further increase the likelihood and severity of OH. On the other hand, a number of changes occur after SCI that can mitigate the severity of OH, including the recovery of spinal sympathetic reflexes, development of spasticity and increased muscle tone, and changes in the reninangiotensin system (Mathias et al., 1975; Wecht et al., 2005). Although these changes have the potential to reduce the severity of OH, the reality is that OH remains a problem for a significant majority of individuals with SCI.
Special considerations of OH and this CPG
For the purposes of this CPG, systolic BP is used to evaluate and treat OH. However, note that the Consensus Committee of the American Autonomic Society and the American Academy of Neurology have defined OH as a decrease in systolic BP of ≥ 20 mmHg and/or a decrease in diastolic BP of ≥ 10 mmHg within 3 minutes upon assumption of an upright posture from a supine position, regardless of whether symptoms occur (Freeman et al., 2011). However, delayed onset of OH beyond 3 minutes of assuming the upright position should be considered, which may be relevant in individuals with SCI (Gibbons & Freeman, 2006). Symptoms of OH in individuals with SCI are similar to those in the uninjured population (Cleophas et al., 1986) and include fatigue or weakness, light-headedness, dizziness, blurred vision, dyspnea, and restlessness (Frisbie & Steele, 1997; Sclater & Alagiakrishnan, 2004). Note that BP monitoring is essential to differentiate OH from AD because AD may have similar symptoms. OH, like AD, can also be asymptomatic; 41.1% of individuals with SCI who developed OH were asymptomatic, despite significant falls in BP (Illman et al., 2000). Therefore, development of symptoms on assuming an upright position may not be a reliable estimate of OH in individuals with SCI.
Recommendations
7.1 Be aware that OH, defined as a decrease in systolic BP of ≥ 20 mmHg, may occur in individuals with lesions at T6 and above on assumption of an upright posture from a supine position, regardless of whether symptoms occur.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Delayed onset of pharmacological agents, prolonged effect of pharmacological agents, or correction of the cause of the AD episode may cause delayed onset or worsening of OH beyond the classic description of “3 minutes of assuming the upright position” in individuals with SCI. Symptoms of OH in individuals with SCI are similar to those in the uninjured population (Cleophas et al., 1986). Therefore, the presence or absence of symptoms on assuming an upright position or being treated for AD is not a reliable estimate of OH in individuals with SCI.
7.2 To accurately diagnose OH in individuals with SCI, perform an orthostatic challenge evaluation (e.g., sit-up test or head-up tilt test).
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
The orthostatic challenge test is particularly helpful to diagnosis BP instability and OH in individuals with SCI. The test involves passive movement of the patient from the supine to the seated or head-up tilt position. It is important to make sure individuals with lesions above T6 are not experiencing AD in that many, if not most, individuals may have asymptomatic OH. The recommended technique: Review and document BP management medications, which should be held for at least 5 half-lives, and remove compression garments/stockings prior to testing. After at least 5 minutes of quiet supine rest, begin to monitor and record brachial BP at 1-minute intervals in the supine position for at least 5 minutes. Passively move the individual to the seated or head-up tilt position. Passive movement means that the patient does not provide any voluntary muscle contraction to facilitate the positional changes (Currie, Wong, et al., 2015). The sit-up test involves moving the patient to the seated position with hips and knees at 90-degree angles. It is recommended that the head-up tilt maneuver be performed on a motorized tilt table, such that the upright position is attained within 10 seconds, and that the angle of tilt be at least 45°, which should be recorded (Shen et al., 2017). Brachial BP should be monitored and recorded at 1-minute intervals in the seated or head-up tilt position for at least 10 minutes.
7.3 To prevent or manage OH in individuals with SCI, first consider treating to maintain baseline BP by using nonpharmacological interventions.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Low-level evidence supports the use of nonpharmacological remedies for treatment of OH, including the following: thromboembolic compressive stockings, abdominal binders, functional electrical stimulation, increasing salt and water intake, sitting the individual in a reclined position, and slow transition from a supine to a seated position (Claydon, Steeves, et al., 2006; Helmi et al., 2013; Smit et al., 1999).
7.4 Consider pharmacological interventions to treat both symptomatic and asymptomatic OH in individuals with established SCI when nonpharmacological interventions prove to be ineffective.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Low-quality evidence supports the use of midodrine hydrochloride in the management of hypotension and OH after SCI (doses of 2.5 to 10 mg once or twice daily while upright) (Krassioukov et al., 2009; Nieshoff et al., 2004; Phillips et al., 2014; Wecht et al., 2010, 2020). There is also low-quality evidence on the use of fludrocortisone, droxidopa (Northera), for treatment of OH in individuals with SCI (Krassioukov et al., 2009). Case reports indicate that ephedrine and fludrocortisone have been used to help maintain an individual’s BP near baseline (Groomes & Huang, 1991). With each pharmacological intervention, monitor BP and symptoms daily.
8. Thermodysregulation
Overview
Poikilothermia refers to the inability to regulate core body temperature (responding to changes in the environment by sweating to cool off or shivering to warm up) and is found in particular in individuals with SCI lesions above T6. Some sources state that this effect occurs as low as T8. Clinically, poikilothermia can be manifested by hypothermia or hyperthermia (Krassioukov et al., 2007). Hypothermia is more commonly seen because damage to the descending sympathetic pathways reduces the ability to regulate vascular tone and blood flow.
To document thermodysregulation after SCI, clinicians and researchers are encouraged to use the International SCI Skin and Thermoregulation Function Basic Data Set that documents thermoregulation history, including hyperthermia, hypothermia, and history of hyperhidrosis or hypohidrosis above or below the level of the lesion (Karlsson et al., 2012).
Recommendations
8.1 Hypothermia (core temperature less than 35°C/95°F)
Hypothermia, defined as a core temperature of less than 35°C/95°F, is a recognized hazard for people with SCI, primarily during exposure to a cold environment (Menard & Hahn, 1991). Rarely, it presents from the presence of a syrinx (Gray et al., 1986) or brain injuries (Nussey et al., 1986) or is induced by medications (Berard et al., 1989).
Recommendations
-
8.1.1 Monitor for signs and symptoms in individuals with SCI at or above T6 who are at risk for developing hypothermia when exposed to a cold environment.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
As a result of autonomic dysfunction, individuals with SCI at T6 and above often have episodes of subnormal body temperature in a normal ambient environment and may not present with any symptoms. When individuals with SCI are exposed to a cold environment, they are prone to developing hypothermia. The skin can be cold to the touch, and the individual can appear drowsy, confused, irritable, or combative and may lose consciousness. Hypothermia, if severe, can cause significant complications, including compromise of respiration and cardiac arrhythmias (Khan et al., 2007).
-
8.1.2 If possible, obtain a rectal temperature when evaluating an individual for hypothermia because skin temperature is not as accurate for monitoring core body temperature. Oral and tympanic are also acceptable methods of temperature monitoring.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
After SCI, rectal temperature is the most accurate available method (Moran & Mendal, 2002). If a rectal thermometer is not available, oral and tympanic are acceptable methods of temperature monitoring.
-
8.1.3 Use ambient temperature regulation, insulated clothing, blankets, warm humidified air, and intake of warm fluid into the gastrointestinal (GI) tract to help prevent and manage hypothermia. Heating devices should be used with extreme caution in insensate areas.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Individuals with SCI at T6 and above have a poor ability to regulate core body temperature. The measures listed above have been documented as being helpful in preventing and managing hypothermia. Dressing in layers of clothing may be helpful, as they can be added or removed as needed.
-
8.1.4 In cold ambient environments, instruct individuals to consider avoiding alcohol intake, as it causes vasodilation and heat loss.
(Level - V; Strength - C; Panel Agreement -strong)
Alcohol intake can cause peripheral vasodilation and heat loss and should thus be avoided when cold conditions are anticipated.
-
8.1.5 Be aware of and discuss with individuals with SCI that certain medications or substances may disrupt temperature regulation (hypo- or hyperthermia), including alpha-agonists (e.g., tizanidine, clonidine), narcotics, oxybutynin, gabapentin, and antidepressants that are norepinephrine and serotonin reuptake inhibitors.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
These medications include alpha-agonists (e.g., tizanidine, clonidine), narcotics, oxybutynin, gabapentin, and antidepressants that are norepinephrine and serotonin reuptake inhibitors (Adubofour et al., 1996; Cuddy, 2004; Kameyama et al., 1986; Menard & Hahn, 1991; Westaway et al., 2015). Although the effects of these medications are not well understood, their effects on thermoregulation are usually dose dependent.
Overview
8.2 Hyperthermia (core temperature > 37.8°C /100°F)
Hyperthermia, a core temperature of more than 37.8°C/100°F, is a diagnosis of exclusion, unrelated to infection or other identifiable causes, and is due to extensive interruption of the autonomic thermoregulatory mechanisms. Neurogenic fever, also known as “quad fever,” has been reported to occur in acute SCI in approximately 1 in every 20–25 individuals (Colachis & Otis, 1995; McKinley et al., 2006; Schmidt & Chan, 1992). A complete evaluation and workup should be initiated as opposed to assuming that all fevers in individuals with SCI are neurological in nature (Colachis & Otis, 1995; McKinley et al., 2006; Savage et al., 2016). For the athlete with SCI, the inability to acclimate to warm environments can impair performance and endurance. Neurogenic fever is more commonly seen in individuals with neurological complete injuries and with higher levels of injury (Handrakis et al., 2017).
Recommendations
-
8.2.1 Monitor for signs and symptoms of hyperthermia in individuals with SCI at or above T6 who are at risk for developing hyperthermia when exposed to a hot environment.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Hyperthermia may occur during hospitalization if the room is hot or the individual is covered with blankets, or on a hot day if the individual is outside or sitting in a hot car. Signs and symptoms include an elevated temperature; feelings of being weak, dizzy, hot, and dry; and with possible headache, nausea, and visual disturbances, although individuals may not present with any symptoms.
-
8.2.2 Treat hyperthermia by decreasing the individual’s core temperature. This includes moving to a cooler environment (preferably an air-conditioned setting), drinking cool liquids, washing with tepid water, and resting.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Individuals with SCI at T6 and above have a limited ability to regulate core body temperature.
-
8.2.3 Provide education regarding measures to help prevent neurogenic hyperthermia. Preventative measures include wearing appropriate light-weight and light-colored clothing, maintaining a proper temperature-controlled room (e.g., use of air-conditioning), frequently drinking cold fluids and maintaining appropriate hydration, and having a water spray and/or fan for exposed skin. This is especially important when in a hot environment.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Individuals with SCI at T6 and above are predisposed to poor temperature regulation and need to be aware that they can develop neurogenic hyperthermia.
-
8.2.4 Be aware of and discuss with individuals with SCI that certain medications or substances may disrupt temperature regulation (hypo- or hyperthermia), including alpha-agonists (e.g., tizanidine, clonidine), narcotics, oxybutynin, gabapentin, and antidepressants that are norepinephrine and serotonin reuptake inhibitors.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
These medications include alpha-agonists (e.g., tizanidine, clonidine), narcotics, oxybutynin, gabapentin, and antidepressants that are norepinephrine and serotonin reuptake inhibitors (Adubofour et al., 1996; Cuddy, 2004; Kameyama et al., 1986; Menard & Hahn, 1991; Westaway et al., 2015). Although the effects of these medications are not well understood, their effects on thermoregulation are usually dose dependent.
-
8.2.5 During exercise, individuals with SCI at or above T6 should be monitored for neurogenic hyperthermia.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
Individuals with cervical or high-thoracic SCI are predisposed to developing hyperthermia during exercise (Petrofsky, 1992; Price, 2006), athletes with tetraplegia demonstrating greater and continual increases in core temperature than athletes with paraplegia do, which may impact performance (Trbovich et al., 2016). The use of a cooling vest, however, has not been shown to be helpful (Trbovich et al., 2014).
9. Hyperhidrosis Overview
Hyperhidrosis, or excessive sweating, is commonly seen in individuals with SCI. The condition can be seen independently or it can be associated with AD (Krassioukov et al., 2009). The estimated prevalence of the condition has been reported as 27% in a single European center from the results of a mailed-in questionnaire (Andersen et al., 1992). In 32% of those cases, a somatic cause of the excessive sweating was identified (e.g., infections, dyspepsia, climacteric sweating, autonomic hyperreflexia), leaving 68% of cases in which no contributing somatic cause was identified. The sweating was present both above and below the level of the SCI and occurred in those with tetraplegia and with paraplegia.
Sweating is a normal mechanism of thermal regulation aimed at cooling the body. Hyperhidrosis may be operationally defined as sweating that occurs in excess of that required for the normal thermoregulatory function. In SCI, this criterion might be difficult to determine objectively, as areas of hyperhidrosis may occur in an individual as a thermoregulatory response to compensate for other body surface areas that are anhidrotic. A more useful clinical definition would be that hyperhidrosis is excessive sweating that is annoying to the individual. Hyperhidrosis can present concurrently with other autonomic dysregulation phenomena after acute SCI, or it can start later (weeks, months, or years). Symptoms of excessive sweating on one or both sides of the face and body can involve areas above and below the level of the SCI.
Triggers
Specific underlying triggers of hyperhidrosis have included syringomyelia (Glasauer & Czyrny, 1994), spinal cord tethering (Falci et al., 2009), fullness of the bladder or bowels (Haas & Geng, 2008), renal calculi (Borawski et al., 2006), and contralateral irritation of the ischial skin (Gorman, 2010). In many situations, individuals report that positioning makes a difference in sweating severity. This was evident in the ischial skin case report: When the individual sat up, the irritation caused by the presence of heterotopic bone around the ischium triggered the sweating.
Treatment and management
The main initial management approach for individuals presenting with hyperhidrosis is to evaluate for underlying sympathetic triggers, much as one would do in AD. This includes evaluating for bladder or bowel fullness and removing pressure or other nociceptive stimuli. If no clear cause can be determined, then direct treatment of the sympathetically mediated sweating reflex is necessary.
Recommendations
9.1 Evaluation of hyperhidrosis in individuals with SCI at or above T6 should rule out a more extensive autonomic dysfunction such as AD.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
In one European study, 32% of cases of hyperhidrosis and its underlying cause were identified and therefore treatable (Andersen et al., 1992).
9.2 In the absence of a rise in BP, prevention and management of hyperhidrosis should include identifying other possible triggers.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
The following possible triggers for hyperhidrosis were identified fullness of the bladder or bowel, urinary tract infection or renal stones, skin lesions or irritations, or particular exacerbating positions in the bed or wheelchair (Andersen et al., 1992).
9.3 In those individuals in whom isolated hyperhidrosis is not associated with an identifiable and modifiable cause, consider empirical treatment with anticholinergic medications, unless contraindicated.
(Level - V; Strength - C; Panel Agreement -strong)
Rationale
There have been only a few case reports on medications useful for treatment of hyperhidrosis. The one most studied, propoxyphene (Darvon), is no longer available, and the results were not overly convincing (Andersen et al., 1992; Tashjian & Richter, 1985). The standard anticholinergic medications have not been specifically reported as treatments in hyperhidrosis related to SCI, but make intuitive sense because acetylcholine is a neurotransmitter in eccrine sweat glands (Fast, 1977). One case report on a synthetic anticholinergic medication, bornaprine, is available (Sergi et al., 2008).
9.4 If anticholinergic medications do not relieve the hyperhidrosis or are not well tolerated, secondary medications could be considered.
(Level - V; Strength - C; Panel Agreement - low)
Rationale
There have been case reports on the use of phenoxybenzamine (Shessel et al., 1978) and gabapentin (Adams et al., 2002) in individuals with SCI. A case report exists for indomethacin in generalized hyperhidrosis (Tkach, 1982), and clonidine has been also discussed in some non-SCI-related recommendations and case reports (Albadrani, 2017; Walling & Swick, 2011). Other possible medications include oxybutynin, scopolamine or amitriptyline, propantheline, phenoxybenzamine, gabapentin, indomethacin, and clonidine.
Future Research Recommendations
Overall, the panel noted from the methodological review that information is limited in most areas dealing with AD and autonomic dysfunctions.
Therefore, while there was good consensus within the panel, the majority of the CPG recommendations were formulated by using panel expert opinions and field reviews. It was clear to the panel that numerous areas that concern AD and autonomic dysfunctions could benefit from more studies. A few of the potential topics are listed below.
Cognition and Abnormal BP Control after SCI
Current evidence suggests that cognitive dysfunction in individuals with SCI may be partially attributed to unstable BP control (Phillips et al., 2014; Sachdeva et al., 2018; Wecht & Bauman, 2013). Specifically, low arterial BP and episodic OH have been implicated in immediate and inappropriate changes in cerebral blood flow velocity with an adverse impact on memory/cognition. Prolonged exposure to episodes of AD (transient hypertension) has been shown to have a negative impact on the cerebral vasculature, which may result in diverse cognitive dysfunction (Nightingale et al., 2020). It also should be acknowledged that factors other than BP dysregulation may contribute to cognitive dysfunction after SCI and there is an urgent need for further research in this area.
Autonomic Dysreflexia
There is an urgent need for exploration of new pharmacological agents for the management and/or prophylactic treatment of AD (e.g., short onset of action and short half-life) for children, adolescents, and adults with SCI who are prone to AD.
There is a need for exploration of potential nonpharmacological measures to manage aberrant BP alterations following SCI (e.g., epidural or transcutaneous spinal cord stimulations) for children, adolescents, and adults with SCI who are prone to AD.
There is a need for development and testing of new technology for continuous noninvasive BP monitoring of cardiovascular functions during routine activities of daily living in individuals with SCI.
What are the comparative benefits and harms of methods to prevent AD for children, adolescents, and adults with SCI who are prone to AD?
What are the comparative benefits and harms of different pharmacological treatments for AD for children, adolescents, and adults with SCI who are prone to AD? When should pharmacological treatment be started for children, adolescents, and adults with SCI who are prone to AD?
What are the benefits and harms of screening, prevention, monitoring, and treatment for children, adolescents, and adults with SCI who are prone to AD?
What prevention and treatment information is available on AD in individuals with neurodegenerative spinal cord conditions (e.g., multiple sclerosis, cancer)?
Is there evidence to support one BP cutoff versus another for children, adolescents, and adults with SCI who are prone to AD?
When and how often should BP be measured for children, adolescents, and adults with SCI who are prone to AD?
AD Special Considerations
What are the most effective steps that can reduce the incidence of AD prior to sexual activity, sperm retrieval, and outpatient cystoscopic or urodynamic procedures (i.e., time, duration and intensity of stimulation, medications, bladder or bowel fullness)?
What are the risk factors and BP parameters that should alert health care professionals or individuals to avoid sexual activity, sperm retrieval, and outpatient cystoscopic or urodynamic procedures until the risk factors are treated?
Parameters need to be developed to reduce the incidence of AD with pregnancy, labor, and breastfeeding (i.e., a list of triggers to avoid).
What is the neurophysiology of AD in individuals who develop AD who have neurodegenerative disorders or SCI below T6?
Does boosting (self-induced AD) increase athletic performance?
Orthostatic Hypotension
There is a need for exploration and expansion of our pharmacological arsenal for the management of OH for children, adolescents, and adults with SCI who are prone to AD (we currently have only 2 agents).
Thermal Dysregulation
What are the best ways to differentiate and evaluate fever caused by thermal dysregulation versus fever caused by infection (an ASAP evaluation)?
What are the most efficient practical strategies of “staying warm”?
Hyperhidrosis
There is a need for exploration and expansion of our pharmacological arsenal for management of hyperhidrosis for children, adolescents, and adults with SCI who are prone to AD.
Other
What is the role of spinal cord electrical stimulation in enhancing autonomic function?
Education
What are the most effective ways to disseminate information about AD and other autonomic dysfunctions following SCI to those at risk of AD and to health care providers?
What is the role and what are the most effective and helpful educational materials (websites, printed AD card, alert wrist band, instruction sheet, etc.) for education about AD and other autonomic dysfunctions?
Appendices
Appendix 1 Search Strategies
General Searches Database: Ovid MEDLINE
spinal cord injury.mp. or Spinal Cord Injuries/
Fever/or Hypothermia/or thermodysregulation.mp.
thermoregulation.mp. or Body Temperature Regulation/
Hypothermia, Induced/or Hypothermia/or Body Temperature Regulation/or poikilothermia.mp. or Body Temperature/
2 or 3 or 4
1 and 5
hypotension.mp. or Hypotension/or Hypotension, Orthostatic/or Post-Exercise Hypotension/
Hypertension/or hypertension.mp. or Hypertension, Malignant/
orthostatic hypotension.mp. or Hypotension, Orthostatic/
7 or 8 or 9
1 and 10
autonomic dysreflexia.mp. or Autonomic Dysreflexia
6 or 11 or 12
limit 13 to English language
limit 14 to animals
14 not 15
Database: EBM Reviews - Cochrane Database of Systematic Reviews
spinal cord injury.mp. or Spinal Cord Injuries/
Fever/or Hypothermia/or thermodysregulation.mp.
thermoregulation.mp. or Body Temperature Regulation/
Hypothermia, Induced/or Hypothermia/or Body Temperature Regulation/or poikilothermia.mp. or Body Temperature/
2 or 3 or 4
1 and 5
hypotension.mp. or Hypotension/or Hypotension, Orthostatic/or Post-Exercise Hypotension/
Hypertension/or hypertension.mp. or Hypertension, Malignant/
orthostatic hypotension.mp. or Hypotension, Orthostatic/
7 or 8 or 9
1 and 10
autonomic dysreflexia.mp. or Autonomic Dysreflexia
6 or 11 or 12
Database: EBM Reviews - Cochrane Central Register of Controlled Trials
spinal cord injury.mp. or Spinal Cord Injuries/
Fever/or Hypothermia/or thermodysregulation.mp.
thermoregulation.mp. or Body Temperature Regulation/
Hypothermia, Induced/or Hypothermia/or Body Temperature Regulation/or poikilothermia.mp. or Body Temperature/
2 or 3 or 4
1 and 5
hypotension.mp. or Hypotension/or Hypotension, Orthostatic/or Post-Exercise Hypotension/
Hypertension/or hypertension.mp. or Hypertension, Malignant/
orthostatic hypotension.mp. or Hypotension, Orthostatic/
7 or 8 or 9
1 and 10
autonomic dysreflexia.mp. or Autonomic Dysreflexia
6 or 11 or 12
limit 13 to English language
Hyperhidrosis Database: Ovid MEDLINE
hyperhidrosis.mp. or Hyperhidrosis/
Sweat/or sweat$.mp.
Sweat/or Hyperhidrosis/or excessive sweat$.mp.
1 or 2 or 3
spinal cord injury.mp. or Spinal Cord Injuries
4 and 5
limit 6 to English language
limit 7 to animals
7 not 8
Database: EBM Reviews - Cochrane Database of Systematic Reviews
spinal cord injury.mp. or Spinal Cord Injuries/
Fever/or Hypothermia/or thermodysregulation.mp.
thermoregulation.mp. or Body Temperature Regulation/
Hypothermia, Induced/or Hypothermia/or Body Temperature Regulation/or poikilothermia.mp. or Body Temperature/
2 or 3 or 4
1 and 5
hypotension.mp. or Hypotension/or Hypotension, Orthostatic/or Post-Exercise Hypotension/
Hypertension/or hypertension.mp. or Hypertension, Malignant/
orthostatic hypotension.mp. or Hypotension, Orthostatic/
7 or 8 or 9
1 and 10
autonomic dysreflexia.mp. or Autonomic Dysreflexia
6 or 11 or 12
hyperhidrosis.mp. or Hyperhidrosis/
Sweat/or sweat$.mp.
Sweat/or Hyperhidrosis/or excessive sweat$.mp.
14 or 15 or 16
spinal cord injury.mp. or Spinal Cord Injuries/
17 and 18
Database: EBM Reviews - Cochrane Central Register of Controlled Trials
spinal cord injury.mp. or Spinal Cord Injuries/
Fever/or Hypothermia/or thermodysregulation.mp.
thermoregulation.mp. or Body Temperature Regulation/
Hypothermia, Induced/or Hypothermia/or Body Temperature Regulation/or poikilothermia.mp. or Body Temperature/
2 or 3 or 4
1 and 5
hypotension.mp. or Hypotension/or Hypotension, Orthostatic/or Post-Exercise Hypotension/
Hypertension/or hypertension.mp. or Hypertension, Malignant/
orthostatic hypotension.mp. or Hypotension, Orthostatic/
7 or 8 or 9
1 and 10
autonomic dysreflexia.mp. or Autonomic Dysreflexia/
6 or 11 or 12
limit 13 to English language
hyperhidrosis.mp. or Hyperhidrosis/
Sweat/or sweat$.mp.
Sweat/or Hyperhidrosis/or excessive sweat$.mp.
15 or 16 or 17
spinal cord injury.mp. or Spinal Cord Injuries/
18 and 19
limit 20 to English language
Appendix 2
Medications Used for Autonomic Dysreflexia
| Name of Drug | Dose and Route of Administration | Onset of Action | Duration of Action | Precautions4 |
|---|---|---|---|---|
2% Nitroglycerin ointment1,2
|
Spread thin layer of 0.5 to 2 inches (1.25 to 5 cm) topically on clean, dry, hair-free site on chest or back | 15 to 30 minutes | 7 hours | Do not administer within 24 hours of sildenafil or vardenafil or within 48 hours of tadalafil
|
| Captopril | 25 mg sublingually once | Within 15 minutes (peaks at 1 to 2 hours) | 2 to 6 hours (prolonged in renal impairment) |
|
| Clonidine | 0.1 – 0.2 mg orally | 30 to 60 minutes (oral) (peaks at 1 to 3 hours) |
|
|
| Hydralazine | 10 – 20 mg orally once | 20 to 30 minutes (peaks at 1 to 2 hours)
|
2 to 4 hours, although some sources state up to 12 hours (hypotension may last longer) |
|
| Nifedipine IR3 | 10 mg orally or sublingually* once | ~20 minutes (oral) ~10 minutes (sublingual) (peaks at 0.5 to 2 hours) | 8 hours |
|
Note: These drugs are not presented in the recommended order in which they should be used; rather, they have all been effective in treating increased blood pressure (BP) due to autonomic dysreflexia.
Sources:Clinical Pharmacology and Lexicomp monographs for each of the drugs in the table.
1. Nitropaste is not available in some countries.
2. Prior to the use of nitropaste or any other agent containing nitrate, first inquire whether the individual has recently taken a phosphodiesterase inhibitor (PDEi). Use of nitropaste when an individual has recently taken a PDEi is contraindicated because a combination of the 2 drugs can cause a sudden severe drop in BP.
3. Sublingual nifedipine has variable and unpredictable absorption and is not recommended because there is little difference in bioavailability when compared with swallowing nifedipine whole.
4. During post-treatment with pharmacological agents, it is important to monitor BP for possible hypotension (if this occurs, see Section 7, Orthostatic Hypotension) or reoccurrence of hypertension as the pharmacological agent wears off.’
Appendix 3
Education: Health Care Professionals, Individuals with Spinal Cord Injury, Family, and Caregivers
Educational programs for autonomic dysreflexia (AD) should be structured and comprehensive; should consider the home and clinic setting; and should be directed at all levels of health care professionals, patients, and caregivers. The content and timing will depend on medical stability, readiness to learn, safety, and related factors. An educational program for AD should include the following:
Common triggers of AD
Signs and symptoms of AD
Pathophysiology of AD with concurrent signs and symptoms
Importance of knowing baseline blood pressure (BP)
Consideration of an AD kit
Measures for treatment in the home setting
Identification of point of care when transitioning to a local emergency department and what resources to bring
Use of antihypertensive medications to reduce BP while evaluating AD triggers
Monitoring patient BP during procedures, including wound debridement and bowel care
Education of the patient, family, and caregivers is critical in the prevention and treatment of AD. Hospital-based education programs during the rehabilitation stay include a wide variety of need-to-know information, which is now delivered over a short length of stay. In fact, the individual at risk of AD may not even experience an episode while participating in rehabilitation and thus will need to recall the information in the home setting. Patient education may be better understood if a variety of education modalities are used (paper, online, peer mentor). An AD kit, including a BP monitor, catheters, and antihypertensive medication, could be developed for use on discharge from the rehabilitation facility. Individuals at risk for AD should carry a wallet reference card to provide to ongoing providers and emergency personnel if they are unable to treat AD in the home setting. They should also consider obtaining a Medic Alert bracelet.
McGillivray et al. reported that 41% of individuals who are at high risk of AD had not heard of AD. This is concerning, as AD can be prevented, and if episodes occur, they can commonly be treated by individuals with spinal cord injury (SCI) or their caregiver in the home setting. Many of the individuals were unaware of their baseline BP while lying and sitting.
Individuals with SCI at risk for AD and caregivers should be able to articulate the following:
Triggers of AD
Baseline BP
Signs and symptoms, what they experience as AD
Steps to take to treat
When to use the antihypertensive medications
When to transition to the emergency department
SCI providers should assess, at least annually, patient experiences with AD, triggers, signs and symptoms, and treatment management. Providers should also assess bladder, bowel, and insensate skin management. For example, some individuals with SCI may catheterize their bladder when they experience a tingling sensation in their head (AD symptoms). Education regarding AD should be reviewed annually and possibly more frequently in those who sustain a concurrent traumatic brain injury.
Additional health care providers should also be provided with SCI-related education about AD, as secondary complications can trigger it. If an individual who is experiencing AD is suspected to be triggered by constipation or impaction, an aggressive bowel program is needed. If the stool impaction is proximal, oral stimulants should be given 8 hours prior to the bowel program (see the CPG Management of the Neurogenic Bowel in Adults with Spinal Cord Injury; Consortium for Spinal Cord Medicine, 1998). BP should be managed with an antihypertensive agent while waiting for the effects of the oral stimulant and bowel program.
If the individual at risk of AD has a pressure injury:
Avoid direct pressure over the wound
Reposition as needed
Assess for appropriate pressure relief support systems
Consider pain medication or lidocaine prior to wound debridement or dressing changes (see the CPG Pressure Ulcer Prevention and Treatment Following Injury; Consortium for Spinal Cord Medicine, 2014).
Appendix 4
Pregnancy and Breastfeeding Precautions with Autonomic Dysreflexia Medications
| Name of Medication | Pregnancy Risk | Breastfeeding Risk | Other Risks |
|---|---|---|---|
| Clonidine | Crosses placenta and umbilical concentrations are similar to maternal serum; amniotic fluid up to 4x maternal serum concentration | Do not use in breastfeeding women; concentration in milk is up to 2x that of maternal serum | Apathy syndrome, hypoglycemia, hypotonia, drowsiness, feeding difficulties, and hyperexcitability |
| Nitroglycerin ointment | Crosses placenta, but data show that concentrations at birth in umbilical cord are low | Limited data; no adverse events noted in breastfeeding infants of mothers using topical nitroglycerin for anal fissures | Unknown |
| Hydralazine | Crosses placenta; IV hydralazine is the recommended agent for use in acute hypertension of systolic BP >160 mmHg in women with preeclampsia or during the postpartum period | Present in breast milk; however, the relative infant dosage (RID) is 0.3% to 3%, which is under the acceptable concentration of < 10% | Unknown; adverse events have been reported in animal studies |
| Nifedipine IR | Crosses the placenta | Present in breast milk; RID of 0.27% to 3.2% is less than the 10% considered acceptable; use has been recommended for Raynaud phenomenon of nipple in breastfeeding women | Increase in perinatal asphyxia, cesarean delivery, prematurity, and intrauterine growth retardation |
| Captopril | Crosses placenta; contraindicated in pregnancy | Present in breast milk; RID of 0.01% to 0.02% is under acceptable concentration of <10% | Decreased fetal renal function, fetal lung hypoplasia and skeletal hypoplasia, hypotension, and death of neonate/fetus |
Sources: Lexicomp medication databases and monographs for each medication.
Appendix 5
Panel Conflict of Interest Statement
Consortium for Spinal Cord Medicine Policy on Conflicts of Interest
The Consortium for Spinal Cord Medicine (hereafter referred to as “the Consortium”) is a collaboration of professional and consumer organizations funded and administered through Paralyzed Veterans of America (hereafter referred to as “PVA”). PVA wants to ensure that regular business of the Consortium’s Steering Committee and the guideline development process are free from conflicts of interest. PVA recognizes that those on the Steering Committee and Guidelines Development Panels are involved in a variety of organizations and projects, and may hold financial investments which might create actual or potential conflicts of interest or the appearance of a conflict (each a “conflict” or “conflict of interest”).
To achieve that result, the following policy is adopted:
1. Applicability.
This Policy applies to the Consortium’s Steering Committee Members, including the Chair and Vice-Chair, in addition to those members on the Guideline Development Panels (collectively, “Covered Persons”).
2. Term.
This agreement is effective for the term the Covered Person is a member of the Steering Committee and/or a Guideline Development Panel, notwithstanding how active or passive a role he or she may play as a member of the Steering Committee or a Guideline Development Panel.
3. Determining the Existence of a Conflict.
The guidelines set forth below shall be used to determine the existence of a conflict. The guidelines are meant to be illustrative and not exclusive; a conflict may exist even though the situation in question is not included below. Each Covered Person bears the personal responsibility for initially determining if a conflict of interest exists with respect to such Covered Person. If a Covered Person has any questions regarding the existence of a conflict, such Covered Person should promptly contact the Steering Committee Chair.
4. Guidelines for Determining Existence of Conflict.
A conflict may exist if the Covered Person is unduly influenced by others (i.e. his/her spouse, parent, child, or other individual with whom such Covered Person has a close personal, business or professional relationship (including persons with whom such Covered Person is a partner, shareholder in a closely held corporation, coauthor or other close professional coworker or colleague) to the detriment of and against the mission of the Consortium, the Steering Committee, the Guideline Development Panels, and PVA.
5. Disclosure of Conflict: Recusal.
If a Covered Person determines that a conflict exists, then he or she shall notify immediately the Steering Committee Chair or the Director of PVA’s Research and Education Department. The Chair, with input from the Director of Research and Education, shall determine whether a conflict exists (except that in cases of conflicts involving the Chair, the Vice Chair shall decide). The decision on conflicts and the basis of that decision shall be reported to the Steering Committee and recorded in the minutes. Unless otherwise determined by the Chair (or, as appropriate, the Vice Chair) in individual cases, if a conflict is found to exist, the affected person shall recuse himself/herself from all discussions, determinations and votes with respect to the matter with which the conflict exists, and shall excuse him/herself from all meetings at which any discussions regarding the matter take place. Following the termination of such determinations and discussions involving the conflict, such Covered Person may rejoin the meeting.
Policy on Confidentiality
In the course of conducting regular business for the Consortium and/or Guideline Development Panel(s), Steering Committee Members and Panel Members may receive and be given access to confidential information concerning PVA or another entity working with the Consortium. To ensure that the confidentiality of the information will be maintained, the following Policy on Confidentiality is adopted.
1. Applicability.
This Policy applies to the Consortium’s Steering Committee Members, including the Chair and Vice-Chair, in addition to those members on the Guideline Development Panels (collectively, “Covered Persons”).
2. Term.
This agreement is effective for the term the Covered Person is a member of the Steering Committee and/or a Guideline Development Panel, notwithstanding how active or passive a role they may play as a member of the Steering Committee or a Guideline Development Panel.
3. Definition of Confidential Information.
“Confidential Information” means (i) all written business, financial, technical and scientific information relating to the Consortium and which PVA has marked conspicuously “CONFIDENTIAL,” “PROPRIETARY,” or similar marking; or (ii) oral information which is specified as confidential by the Steering Committee and/or PVA. All documents derived during the guideline development process are confidential, and they remain so until 1) the document has been approved for publication by a vote of the Steering Committee and 2) the document is released by PVA as a printed document.
“Confidential Information” shall exclude information which (a) is in the public domain at the time of disclosure; (b) is in the possession of the Consortium (including any Covered Person) free of any obligation of confidence prior to the time of disclosure; (c) though originally within the definition of “Confidential Information”, subsequently becomes part of the public knowledge through no fault of the Consortium (including any Covered Person), as of the date of its becoming part of the public knowledge; (d) though originally within the definition of “Confidential Information”, subsequently is received by the Consortium (including any Covered Person) without any obligation of confidentiality from a third party who is free to disclose the information, as of the date of such third-party disclosure; or (e) is independently developed by the Consortium without the use of any Confidential Information.
4. Nondisclosure of Confidential Information.
Each Covered Person agrees not to disclose to any person outside the Consortium or its affiliates (including for these purposes Chapters and International Affiliates) any Confidential Information, except as provided below. Each Covered Person agrees that he/she will use the Confidential Information only for the purpose of Consortium business. Notwithstanding the foregoing, a Covered Person may disclose the Confidential Information (i) to employees, professional advisors, volunteer scientists and other Covered Persons asked to participate in Consortium business, consultants and agents of the Consortium who have a need to know and who have been informed of this Policy on Confidentiality; or (ii) to the extent required by a court order or by law. Each Covered Person shall use the same degree of care, but not less than a reasonable degree of care, that he/she uses to protect the Consortium’s own most highly confidential information to prevent any unauthorized or inadvertent disclosure of Confidential Information.
Any individual having question(s) concerning this policy or its applicability in a given situation(s) should address those question(s) to the Director of Research and Education (PVA).
5. Return of Confidential Information.
Each Covered Person agrees to return to the Chair of the Steering Committee or the Director of Research and Education, all tangible materials incorporating Confidential Information made available or supplied to such Covered Person and all copies and reproductions thereof upon request of the Chair of the Committee and/or the Director of Research and Education (PVA).
Certification Regarding Conflicts of Interest and Confidentiality of Information
Each Covered Person agrees to comply with the provisions of these Policies so long as he/she is a Covered Person. By signing, you are confirming that you have read and understand the above Policy on Conflicts of Interest and Confidentiality and agree to abide by same during all times that you are a Covered Person, as defined in the Policy
Signed:

Certification Regarding Consortium Policies and Procedures
Each Covered Person agrees to comply with the provisions of the policies and procedures outlined in the Clinical Practice Guideline Orientation Manual so long as he/she is a Covered Person. By signing, you are confirming that you have read and understand the Clinical Practice Guidelines Orientation Manual Policies and Procedures and agree to abide by same during all times that you are a Covered Person.
Signed:

References
- 1.Adams B. B, Vargus-Adams J. N, Franz D. N, Kinnett D. G. Hyperhidrosis in pediatric spinal cord injury: A case report and gabapentin therapy. Journal of the American Academy of Dermatology. 2002;46(3):444–446. doi: 10.1067/mjd.2002.113681. [DOI] [PubMed] [Google Scholar]
- 2.Adubofour K. O, Kajiwara G. T, Goldberg C. M, King-Angell J. L. Oxybutynin-induced heatstroke in an elderly patient. Annals of Pharmacotherapy. 1996;30(2):144–147. doi: 10.1177/106002809603000207. [DOI] [PubMed] [Google Scholar]
- 3.Albadrani A. Clonidine is effective for the treatment of primary idiopathic hyperhidrosis and hot flushes: A case report. Journal of Medical Case Reports. 2017;11(1):16. doi: 10.1186/s13256-016-1174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Obstetric management of patients with spinal cord injuries. Obstetrics and Gynecology. 2002;100(3):625–627. doi: 10.1016/s0029-7844(02)02261-5. [DOI] [PubMed] [Google Scholar]
- 5.Andersen L. S, Biering-Sørensen F, Müller P. G, Jensen I. L, Aggerbeck B. The prevalence of hyperhidrosis in patients with spinal cord injuries and an evaluation of the effect of dextropropoxyphene hydrochloride in therapy. Paraplegia. 1992;30(3):184–191. doi: 10.1038/sc.1992.53. [DOI] [PubMed] [Google Scholar]
- 6.Anderson K. D. Targeting recovery: Priorities of the spinal cord-injured population. Journal of Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 7.Anderson K. D, Borisoff J. F, Johnson R. D, Stiens S. A, Elliott S. L. The impact of spinal cord injury on sexual function: Concerns of the general population. Spinal Cord. 2007a;45(5):328–337. doi: 10.1038/sj.sc.3101977. [DOI] [PubMed] [Google Scholar]
- 8.Anderson K. D, Borisoff J. F, Johnson R. D, Stiens S. A, Elliott S. L. Spinal cord injury influences psychogenic as well as physical components of female sexual ability. Spinal Cord. 2007b;45(5):349–359. doi: 10.1038/sj.sc.3101979. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson P. P, Atkinson J. L. Spinal shock. Mayo Clinic Proceedings. 1996;71(4):384–389. doi: 10.4065/71.4.384. [DOI] [PubMed] [Google Scholar]
- 10.Balshem H, Helfand M, Schünemann H. J, Oxman A. D, Kunz R, Brozek J, Vist G. E, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt G. H. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Berard E. J, Boucand M. H, Depassio J, Fyon J. P. Effects of bethanechol and adreno-blockers on thermoregulation in spinal cord injury. Paraplegia. 1989;27(1):46–50. doi: 10.1038/sc.1989.7. [DOI] [PubMed] [Google Scholar]
- 12.Bhambhani Y, MacTavish J, Warren S, Thompson W. R, Webborn A, Bressan E, De Mello M. T, Tweedy S, Malone L, Frojd K, Van De Vliet P, Vanlandewijck Y. Boosting in athletes with high-level spinal cord injury: Knowledge, incidence and attitudes of athletes in paralympic sport. Disability and Rehabilitation. 2010;32(26):2172–2190. doi: 10.3109/09638288.2010.505678. [DOI] [PubMed] [Google Scholar]
- 13.Blackmer J. Rehabilitation medicine: 1. Autonomic dysreflexia. CMAJ Canadian Medical Association Journal = Journal de l’Association Medicale Canadienne. 2003;169(9):931–935. [PMC free article] [PubMed] [Google Scholar]
- 14.Borawski K. M, Sur R. L, Preminger G. M. Renal calculi presenting as hyperhidrosis in patient with spinal cord injury. Urology. 2006;67(5):1084, e13-1084.e14. doi: 10.1016/j.urology.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Braddom R. L, Rocco J. F. Autonomic dysreflexia. A survey of current treatment. American Journal of Physical Medicine & Rehabilitation. 1991;70(5):234–241. [PubMed] [Google Scholar]
- 16.Chang C. P, Chen M. T, Chang L. S. Autonomic hyperreflexia in spinal cord injury patient during percutaneous nephrolithotomy for renal stone: A case report. Journal of Urology. 1991;146(6):1601–1602. doi: 10.1016/S0022-5347(17)38180-6. [DOI] [PubMed] [Google Scholar]
- 17.Claydon V. E, Hol A. T, Eng J. J, Krassioukov A.V. Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2006;87(8):1106–1114. doi: 10.1016/j.apmr.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Claydon V. E, Krassioukov A. V. Orthostatic hypotension and autonomic pathways after spinal cord injury. Journal of Neurotrauma. 2006;23(12):1713–1725. doi: 10.1089/neu.2006.23.1713. [DOI] [PubMed] [Google Scholar]
- 19.Claydon V. E, Steeves J. D, Krassioukov A. Orthostatic hypotension following spinal cord injury: Understanding clinical pathophysiology. Spinal Cord. 2006;44(6):341–351. doi: 10.1038/sj.sc.3101855. [DOI] [PubMed] [Google Scholar]
- 20.Cleophas T. J. M, Kauw F. H.W, Bijl C, Meijers J, Stapper G. Effects of beta adrenergic receptor agonists and antagonists in diabetics with symptoms of postural hypotension: a double-blind, placebo-controlled study. Angiology. 1986;37(11):855–862. doi: 10.1177/000331978603701110. [DOI] [PubMed] [Google Scholar]
- 21.Colachis S. C. Autonomic hyperreflexia with spinal cord injury. The Journal of the American Paraplegia Society. 1992;15(3):171–186. doi: 10.1080/01952307.1992.11735871. [DOI] [PubMed] [Google Scholar]
- 22.Colachis S. C, Otis S. M. Occurrence of fever associated with thermoregulatory dysfunction after acute traumatic spinal cord injury. American Journal of Physical Medicine & Rehabilitation. 1995;74(2):114–119. doi: 10.1097/00002060-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Consortium for Spinal Cord Medicine Neurogenic bowel dysfunction in adults after spinal cord injury. Paralyzed Veterans of America. 2020 https://www.pva.org/research-resources/publications/clinical-practice-guidelines/ [Google Scholar]
- 24.Consortium of Spinal Cord Medicine. Early acute management in adults with spinal cord injury: A clinical practice guideline for health-care professionals. Paralyzed Veterans of America. 2008 doi: 10.1043/1079-0268-31.4.408. https://www.pva.org/research-resources/publications/clinical-practice-guidelines/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Consortium for Spinal Cord Medicine. Pressure ulcer prevention and treatment following injury: a clinical practice guideline for healthcare providers. 2014 doi: 10.1080/10790268.2001.11753592. Paralyzed Veterans of America. https://www.pva.org/research-resources/publications/clinical-practice-guidelines/ [DOI] [PubMed]
- 26.Cuddy M. L. S. The effects of drugs on thermoregulation. AACN Clinical Issues. 2004;15(2):238–253. doi: 10.1097/00044067-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Currie K. D, West C. R, Hubli M, Gee C. M, Krassioukov A. V. Peak heart rates and sympathetic function in tetraplegic nonathletes and athletes. Medicine and Science in Sports and Exercise. 2015;47(6):1259–1264. doi: 10.1249/MSS.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 28.Currie K. D, Wong S. C, Warburton D. E, Krassioukov A. V. Reliability of the sit-up test in individuals with spinal cord injury. The Journal of Spinal Cord Medicine. 2015;38(4):563–566. doi: 10.1179/2045772315Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curt A, Nitsche B, Rodic B, Schurch B, Dietz V. Assessment of autonomic dysreflexia in patients with spinal cord injury. Journal of Neurology Neurosurgery and Psychiatry. 1997;62(5):473–477. doi: 10.1136/jnnp.62.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson R, Elliott S, Krassioukov A. Cardiovascular responses to sexual activity in able-bodied individuals and those living with spinal cord injury. Journal of Neurotrauma. 2016;33(24):2161–2174. doi: 10.1089/neu.2015.4143. [DOI] [PubMed] [Google Scholar]
- 31.Ditunno J. F, Little J. W, Tessler A, Burns A. S. Spinal shock revisited: A four-phase model. Spinal Cord. 2004;42(7):383–395. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- 32.Ekland M. B, Krassioukov A. V, McBride K. E, Elliott S. L. Incidence of autonomic dysreflexia and silent autonomic dysreflexia in men with spinal cord injury undergoing sperm retrieval: Implications for clinical practice. Journal of Spinal Cord Medicine. 2008;31(1):33–39. doi: 10.1080/10790268.2008.11753978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott S, Krassioukov A. V. Malignant autonomic dysreflexia in spinal cord injured men. Spinal Cord. 2006;44(6):386–392. doi: 10.1038/sj.sc.3101847. [DOI] [PubMed] [Google Scholar]
- 34.Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: Case report and review. Paraplegia. 1992;30(5):355–360. doi: 10.1038/sc.1992.82. [DOI] [PubMed] [Google Scholar]
- 35.Falci S. P, Indeck C, Lammertse D. P. Posttraumatic spinal cord tethering and syringomyelia: Surgical treatment and long-term outcome. Journal of Neurosurgery Spine. 2009;11(4):445–460. doi: 10.3171/2009.4.SPINE09333. [DOI] [PubMed] [Google Scholar]
- 36.Fast A. Reflex sweating in patients with spinal cord injury: A review. Archives of Physical Medicine and Rehabilitation. 1977;58(10):435–437. [PubMed] [Google Scholar]
- 37.Freeman R, Wieling W, Axelrod F. B, Benditt D. G, Benarroch E, Biaggioni I, Cheshire W. P, Chelimsky T, Cortelli P, Gibbons C. H, Goldstein D. S, Hainsworth R, Hilz M. J, Jacob G, Kaufmann H, Jordan J, Lipsitz L. A, Levine B. D, Low P. A, van Dijk J. G. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Autonomic Neuroscience Basic and Clinical. 2011;161(1–2):46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Frisbie J. H, Steele D. J. R. Postural hypotension and abnormalities of salt and water metabolism in myelopathy patients. Spinal Cord. 1997;35(5):303–307. doi: 10.1038/sj.sc.3100436. [DOI] [PubMed] [Google Scholar]
- 39.Furusawa K, Sugiyama H, Ikeda A, Tokuhiro A, Koyoshi H, Takahashi M, Tajima F. Autonomic dysreflexia during a bowel program in patients with cervical spinal cord injury. Acta Medica Okayama. 2007;61(4):221–227. doi: 10.18926/AMO/32867. [DOI] [PubMed] [Google Scholar]
- 40.Gee C. M, Currie K. D, Phillips A. A, Squair J. W, Krassioukov A. V. Spinal cord injury impairs cardiovascular capacity in elite wheelchair rugby athletes. Clinical Journal of Sport Medicine Official Journal of the Canadian Academy of Sport Medicine. 2020;30(1):33–39. doi: 10.1097/JSM.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 41.Geigle P. R, Frye S. K, Perreault J, Scott W. H, Gorman P. H. Atypical autonomic dysreflexia during robotic-assisted body weight supported treadmill training in an individual with motor incomplete spinal cord injury. Journal of Spinal Cord Medicine. 2013;36(2):153–156. doi: 10.1179/2045772312Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannantoni A, Di Stasi S. M, Scivoletto G, Mollo A, Silecchia A, Fuoco U, Vespasiani G. Autonomic dysreflexia during urodynamics. Spinal Cord. 1998;36(11):756–760. doi: 10.1038/sj.sc.3100684. [DOI] [PubMed] [Google Scholar]
- 43.Gibbons C. H, Freeman R. Delayed orthostatic hypotension: A frequent cause of orthostatic intolerance. Neurology. 2006;67(1):28–32. doi: 10.1212/01.wnl.0000223828.28215.0b. [DOI] [PubMed] [Google Scholar]
- 44.Glasauer F. E, Czyrny J. J. Hyperhidrosis as the presenting symptom in post-traumatic syringomyelia. Paraplegia. 1994;32(6):423–429. doi: 10.1038/sc.1994.69. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez F, Chang J. Y, Banovac K, Messina D, Martinez Arizala A, Kelley R. E. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia. 1991;29(1):37–42. doi: 10.1038/sc.1991.1. [DOI] [PubMed] [Google Scholar]
- 46.Gorman P. H. Unilateral hyperhidrosis from a contralateral source in an individual with C4 complete tetraplegia. Journal of Spinal Cord Medicine. 2010;33(4):428–430. doi: 10.1080/10790268.2010.11689723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray H. H, Smith L. D, Moore R. H. Idiopathic periodic hypothermia and bizarre behaviour in the presence of occult syringomyelia. Postgraduate Medical Journal. 1986;62(726):289–290. doi: 10.1136/pgmj.62.726.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groomes T. E, Huang C. T. Orthostatic hypotension after spinal cord injury: Treatment with fludrocortisone and ergotamine. Archives of Physical Medicine and Rehabilitation. 1991;72(1):56–58. [PubMed] [Google Scholar]
- 49.Guttmann L, Whitteridge D. Effects of bladder distension on autonomic mechanisms after spinal cord injuries. Brain A Journal of Neurology. 1947;70(Pt 4):361–404. doi: 10.1093/brain/70.4.361. [DOI] [PubMed] [Google Scholar]
- 50.Guyatt G. H, Oxman A. D, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl E. A, Norris S, Vist G, Dahm P, Shukla V. K, Higgins J, Falck-Ytter Y, Schünemann H. J. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. Journal of Clinical Epidemiology. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Guyatt G. H, Oxman A. D, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams J. W, Meerpohl J, Norris S. L, Akl E. A, Schünemann H. J. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. Journal of Clinical Epidemiology. 2011;64(12):1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Guyatt G. H, Oxman A. D, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke R, Rind D, Dahm P, Akl E. A, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Schünemann H. J. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology. 2013;66(2):151–157. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Guyatt G. H, Oxman A. D, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl E. A, Djulbegovic B, Falck-Ytter Y, Norris S. L, Williams J. W, Atkins D, Meerpohl J, Schünemann H. J. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias) Journal of Clinical Epidemiology. 2011;64(4):407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Haas U, Geng V. Sensation of defecation in patients with spinal cord injury. Spinal Cord. 2008;46(2):107–112. doi: 10.1038/sj.sc.3102067. [DOI] [PubMed] [Google Scholar]
- 55.Hadley M. N, Walters B. C, Grabb P. A, Oyesiku N. M, Przybylski G. J, Resnick D. K, Ryken T. C. Blood pressure management after acute spinal cord injury. Neurosurgery. 2002;50(3 Suppl):S58–62. doi: 10.1097/00006123-200203001-00012. [DOI] [PubMed] [Google Scholar]
- 56.Handrakis J. P, Trbovich M, Hagen E. M, Price M. Thermodysregulation in persons with spinal cord injury: Case series on use of the autonomic standards. Spinal Cord Series and Cases. 2017;3:17086. doi: 10.1038/s41394-017-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris P. Self-induced autonomic dysreflexia (‘boosting’) practised by some tetraplegic athletes to enhance their athletic performance. Paraplegia. 1994;32(5):289–291. doi: 10.1038/sc.1994.50. [DOI] [PubMed] [Google Scholar]
- 58.Harris R. P, Helfand M, Woolf S. H, Lohr K. N, Mulrow C. D, Teutsch S. M, Atkins D, Methods Work Group, Third US Preventive Services Task Force Current methods of the US Preventive Services Task Force: A review of the process. American Journal of Preventive Medicine. 2001;20(3 Suppl):21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 59.Hayden J. A, van der Windt D. A, Cartwright J. L, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Annals of Internal Medicine. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 60.Helmi M, Lima A, Gommers D, van Bommel J, Bakker J. Inflatable external leg compression prevents orthostatic hypotension in a patient with a traumatic cervical spinal cord injury. Future Cardiology. 2013;9(5):645–648. doi: 10.2217/fca.13.60. [DOI] [PubMed] [Google Scholar]
- 61.Hickey K. J, Vogel L. C, Willis K. M, Anderson C. J. Prevalence and etiology of autonomic dysreflexia in children with spinal cord injuries. The Journal of Spinal Cord Medicine. 2004;27(Suppl 1):S54–60. doi: 10.1080/10790268.2004.11753786. [DOI] [PubMed] [Google Scholar]
- 62.Holmgren T, Lee A. H. X, Hocaloski S, Hamilton L. J, Hellsing I, Elliott S, Hultling C, Krassioukov A. V. The influence of spinal cord injury on breastfeeding ability and behavior. Journal of Human Lactation Official Journal of International Lactation Consultant Association. 2018;34(3):556–565. doi: 10.1177/0890334418774014. [DOI] [PubMed] [Google Scholar]
- 63.Houtman S, Colier W. N. J. M, Oeseburg B, Hopman M. T. E. Systemic circulation and cerebral oxygenation during head-up tilt in spinal cord injured individuals. Spinal Cord. 2000;38(3):158–163. doi: 10.1038/sj.sc.3100970. [DOI] [PubMed] [Google Scholar]
- 64.Illman A, Stiller K, Williams M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord. 2000;38(12):741–747. doi: 10.1038/sj.sc.3101089. [DOI] [PubMed] [Google Scholar]
- 65.International Paralympic Committee 2016. Position statement on autonomic dysreflexia and boosting; pp. 1–3. In IPC Handbook, 4.2. [Google Scholar]
- 66.Jackson A. B, Wadley V. A multicenter study of women’s self-reported reproductive health after spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1999;80(11):1420–1428. doi: 10.1016/S0003-9993(99)90253-8. [DOI] [PubMed] [Google Scholar]
- 67.Jankovic J, Gilden J. L, Hiner B. C, Kaufmann H, Brown D. C, Coghlan C. H, Rubin M, Fouad-Tarazi F. M. Neurogenic orthostatic hypotension: A double-blind, placebo-controlled study with midodrine. The American Journal of Medicine. 1993;95(1):38–48. doi: 10.1016/0002-9343(93)90230-m. [DOI] [PubMed] [Google Scholar]
- 68.Kameyama T, Nabeshima T, Matsuno K. Effect of tizanidine on body temperature in the mouse. Research Communications in Chemical Pathology and Pharmacology. 1986;52(2):225–235. [PubMed] [Google Scholar]
- 69.Karlsson A. K, Krassioukov A. V, AlexanDer M. S, Donovan W, Biering-Sørensen F. International spinal cord injury skin and thermoregulation function basic data set. Spinal Cord. 2012;50(7):512–516. doi: 10.1038/sc.2011.167. [DOI] [PubMed] [Google Scholar]
- 70.Khan K. S, ter Riet G, Glanville J, Sowden A. J, Kleijnen J. Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews (No. 4, second edition; CRD Report) 2001. University of York.
- 71.Khan S, Plummer M, Martinez-Arizala A, Banovac K. Hypothermia in patients with chronic spinal cord injury. Journal of Spinal Cord Medicine. 2007;30(1):27–30. doi: 10.1080/10790268.2007.11753910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirshblum S. C, House J. G, O’Connor K. C. Silent autonomic dysreflexia during a routine bowel program in persons with traumatic spinal cord injury: A preliminary study. Archives of Physical Medicine and Rehabilitation. 2002;83(12):1774–1776. doi: 10.1053/apmr.2002.36070. [DOI] [PubMed] [Google Scholar]
- 73.Krassioukov A, Biering-Sørensen F, Donovan W, Kennelly M, Kirshblum S, Krogh K, Alexander M. S, Vogel L, Wecht J, Autonomic Standards Committee of the American Spinal Injury Association/International Spinal Cord Society International standards to document remaining autonomic function after spinal cord injury. The Journal of Spinal Cord Medicine. 2012;35(4):201–210. doi: 10.1179/1079026812Z.00000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krassioukov A, Claydon V. E. The clinical problems in cardiovascular control following spinal cord injury: An overview. Progress in Brain Research. 2006;152:223–229. doi: 10.1016/S0079-6123(05)52014-4. [DOI] [PubMed] [Google Scholar]
- 75.Krassioukov A. V, Furlan J. C, Fehlings M. G. Autonomic dysreflexia in acute spinal cord injury: An under-recognized clinical entity. Journal of Neurotrauma. 2003;20(8):707–716. doi: 10.1089/089771503767869944. [DOI] [PubMed] [Google Scholar]
- 76.Krassioukov A. V, Karlsson A.-K, Wecht J. M, Wuermser L.-A, Mathias C. J, Marino R. J, Joint Committee of American Spinal Injury Association and International Spinal Cord Society Assessment of autonomic dysfunction following spinal cord injury: Rationale for additions to International Standards for Neurological Assessment. Journal of Rehabilitation Research and Development. 2007;44(1):103–112. doi: 10.1682/jrrd.2005.10.0159. [DOI] [PubMed] [Google Scholar]
- 77.Krassioukov A. V, Warburton D. E, Teasell R, Eng J. J, Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of the management of autonomic dysreflexia after spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2009;90(4):682–695. doi: 10.1016/j.apmr.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krassioukov A. V, Weaver L. C. Physical medicine and rehabilitation: State of the art reviews. In: Teasell R, Baskerville VB, editors. Anatomy of the autonomic nervous system. Hanley & Belfus; 1996. pp. 1–14. [Google Scholar]
- 79.Lee A. H. X, Phillips A. A, Squair J. W, Barak O. F, Coombs G. B, Ainslie P. N, Sarafis Z. K, Mijacika T, Vucina D, Dujic Z, Krassioukov A. V. Alarming blood pressure changes during routine bladder emptying in a woman with cervical spinal cord injury. Spinal Cord Series and Cases. 2017;3:17101. doi: 10.1038/s41394-017-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee B. Y, Karmakar M. G, Herz B. L, Sturgill R. A. Autonomic dysreflexia revisited. The Journal of Spinal Cord Medicine. 1995;18(2):75–87. doi: 10.1080/10790268.1995.11719383. [DOI] [PubMed] [Google Scholar]
- 81.Leftkowitz R. J, Hoffman B. B, Taylor P. Neurohumoral transmission: The autonomic and somatic motor nervous system. In: Gilman A. G, Rall T. W, Nies A. S, Taylor R, editors. The pharmacological basis of therapeutics. Pergamon Press; 2007. pp. 84–121. [Google Scholar]
- 82.Lidocaine Hydrochloride Jelly USP, 2% 2011. Food and Drug Administration.
- 83.Mathias C. J, Christensen N. J, Corbett J. L, Frankel H. L, Goodwin T. J, Peart W. S. Plasma catecholamines, plasma renin activity and plasma aldosterone in tetraplegic man, horizontal and tilted. Clinical Science and Molecular Medicine. 1975;49(4):291–299. doi: 10.1042/cs0490291. [DOI] [PubMed] [Google Scholar]
- 84.Mathias C. J, Christensen N. J, Frankel H. L, Peart W. S. Renin release during head-up tilt occurs independently of sympathetic nervous activity in tetraplegic man. Clinical Science. 1980;59(4):251–256. doi: 10.1042/cs0590251. [DOI] [PubMed] [Google Scholar]
- 85.Mathias C. J, Frankel H. L. Autonomic disturbances in spinal cord lesions. In: Mathias C. J, Bannister R, editors. Autonomic failure a textbook of clinical disorders of the autonomic nervous system. 4th ed. Oxford University Press; 2002. pp. 839–881. [Google Scholar]
- 86.Mathias C. J, Mallipeddi R, Bleasdale-Barr K. Symptoms associated with orthostatic hypotension in pure autonomic failure and multiple system atrophy. Journal of Neurology. 1999;246(10):893–898. doi: 10.1007/s004150050479. [DOI] [PubMed] [Google Scholar]
- 87.McGillivray CF, Hitzig SL, Craven BC, Tonack MI, Krassioukov AV. Evaluating knowledge of autonomic dysreflexia among individuals with spinal cord injury and their familires. JSpinal Cord Med. 2009;32(1):54–62. doi: 10.1080/107902268.2009.11760753. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKinley W, McNamee S, Meade M, Kandra K, Abdul N. Incidence, etiology, and risk factors for fever following acute spinal cord injury. The Journal of Spinal Cord Medicine. 2006;29(5):501–506. doi: 10.1080/10790268.2006.11753899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Menard M. R, Hahn G. Acute and chronic hypothermia in a man with spinal cord injury: Environmental and pharmacologic causes. Archives of Physical Medicine and Rehabilitation. 1991 https://pubmed.ncbi.nlm.nih.gov/2059112/ [PubMed] [Google Scholar]
- 90.Moran D. S, Mendal L. Core temperature measurement: Methods and current insights. Sports Medicine (Auckland NZ) 2002;32(14):879–885. doi: 10.2165/00007256-200232140-00001. [DOI] [PubMed] [Google Scholar]
- 91.Nacimiento W, Noth J. What, if anything, is spinal shock? Archives of Neurology. 1999;56(8):1033–1035. doi: 10.1001/archneur.56.8.1033. [DOI] [PubMed] [Google Scholar]
- 92.New P. W. Secondary conditions in a community sample of people with spinal cord damage. Journal of Spinal Cord Medicine. 2016;39(6):665–670. doi: 10.1080/10790268.2016.1138600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nieshoff E. C, Birk T. J, Birk C. A, Hinderer S. R, Yavuzer G. Double-blinded, placebo-controlled trial of midodrine for exercise performance enhancement in tetraplegia: A pilot study. The Journal of Spinal Cord Medicine. 2004;27(3):219–225. doi: 10.1080/10790268.2004.11753752. [DOI] [PubMed] [Google Scholar]
- 94.Nightingale T. E, Zheng M. M. Z, Sachdeva R, Phillips A. A, Krassioukov A. V. Diverse cognitive impairment after spinal cord injury is associated with orthostatic hypotension symptom burden. Physiology & Behavior. 2020;213:112742. doi: 10.1016/j.physbeh.2019.112742. [DOI] [PubMed] [Google Scholar]
- 95.Nussey S. S, Ang V. T, Jenkins J. S. Chronic hypernatraemia & hypothermia following subarachnoid haemorrhage. Postgraduate Medical Journal. 1986;62(728):467–471. doi: 10.1136/pgmj.62.728.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perloff D, Grim C, Flack J, Frohlich E. D, Hill M, McDonald M, Morgenstern B. Z. Human blood pressure: Determination by sphygmomanometry. Circulation. 1993;88(5 I):2460. doi: 10.1161/01.CIR.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 97.Petrofsky J. S. Thermoregulatory stress during rest and exercise in heat in patients with a spinal cord injury. European Journal of Applied Physiology and Occupational Physiology. 1992;64(6):503–507. doi: 10.1007/BF00843758. [DOI] [PubMed] [Google Scholar]
- 98.Phillips A. A, Krassioukov A. V. Contemporary cardiovascular concerns after spinal cord injury: mechanisms, maladaptations, and management. Journal of Neurotrauma. 2015;32(24):1927–1942. doi: 10.1089/neu.2015.3903. [DOI] [PubMed] [Google Scholar]
- 99.Phillips A. A, Squair J. R, Currie K. D, Tzeng Y. C, Ainslie P. N, Krassioukov A. V. 2015 ParaPan American Games: Autonomic function, but not physical activity, is associated with vascular-cognitive impairment in spinal cord injury. Journal of Neurotrauma. 2017;34(6):1283–1288. doi: 10.1089/neu.2016.4751. [DOI] [PubMed] [Google Scholar]
- 100.Phillips A. A, Squair J. W, Krassioukov A. V. Paralympic medicine: the road to rio. Journal of Neurotrauma. 2017;34(11):2001–2005. doi: 10.1089/neu.2016.4715. [DOI] [PubMed] [Google Scholar]
- 101.Phillips A. A, Warburton D. E. R, Ainslie P. N, Krassioukov A. V. Regional neurovascular coupling and cognitive performance in those with low blood pressure secondary to high-level spinal cord injury: Improved by alpha-1 agonist midodrine hydrochloride. Journal of Cerebral Blood Flow and Metabolism Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(5):794–801. doi: 10.1038/jcbfm.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pine Z. M, Miller S. D, Alonso J. A. Atrial fibrillation associated with autonomic dysreflexia. American Journal of Physical Medicine & Rehabilitation. 1991;70(5):271–273. doi: 10.1097/00002060-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 103.Price M. J. Thermoregulation during exercise in individuals with spinal cord injuries. Sports Medicine (Auckland NZ) 2006;36(10):863–879. doi: 10.2165/00007256-200636100-00005. [DOI] [PubMed] [Google Scholar]
- 104.Sachdeva R, Gao F, Chan C. C. H, Krassioukov A. V. Cognitive function after spinal cord injury: A systematic review. Neurology. 2018;91(13):611–621. doi: 10.1212/WNL.0000000000006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saleem S, Vucina D, Sarafis Z, Lee A. H. X, Squair J. W, Barak O. F, Coombs G. B, Mijacika T, Krassioukov A. V, Ainslie P. N, Dujic Z, Tzeng Y.-C, Phillips A. A. Wavelet decomposition analysis is a clinically relevant strategy to evaluate cerebrovascular buffering of blood pressure after spinal cord injury. American Journal of Physiology Heart and Circulatory Physiology. 2018;314(5):H1108–H1114. doi: 10.1152/ajpheart.00152.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Savage K. E, Oleson C. V, Schroeder G. D, Sidhu G. S, Vaccaro A. R. Neurogenic fever after acute traumatic spinal cord injury: a qualitative systematic review. Global Spine Journal. 2016;6(6):607–614. doi: 10.1055/s-0035-1570751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmid A, Schmidt-Trucksäß A, Huonker M, König D, Eisenbarth I, Sauerwein H, Brunner C, Storch M. J, Lehmann M, Keul J. Catecholamines response of high performance wheelchair athletes at rest and during exercise with autonomic dysreflexia. International Journal of Sports Medicine. 2001;22(1):2–7. doi: 10.1055/s-2001-11330. [DOI] [PubMed] [Google Scholar]
- 108.Schmidt K. D, Chan C. W. Thermoregulation and fever in normal persons and in those with spinal cord injuries. Mayo Clinic Proceedings. 1992;67(5):469–475. doi: 10.1016/s0025-6196(12)60394-2. [DOI] [PubMed] [Google Scholar]
- 109.Schramm L. P, Strack A. M, Platt K. B, Loewy A. D. Peripheral and central pathways regulating the kidney: A study using pseudorabies virus. Brain Research. 1993;616(1–2):251–262. doi: 10.1016/0006-8993(93)90216-A. [DOI] [PubMed] [Google Scholar]
- 110.Sclater A, Alagiakrishnan K. Orthostatic hypotension. A primary care primer for assessment and treatment. Geriatrics. 2004;59(8):22–27. [PubMed] [Google Scholar]
- 111.Sergi R, Massone A, Moretto S, Oggerino C, Bertolotto F, Losio L, Ottonello M. Hyper-hidrosis treatment with bornaprine in the acute phase of spinal cord-injured patients. Spinal Cord. 2008;46(8):571–573. doi: 10.1038/sc.2008.12. [DOI] [PubMed] [Google Scholar]
- 112.Sheel A. W, Krassioukov A. V, Inglis J. T, Elliott S. L. Autonomic dysreflexia during sperm retrieval in spinal cord injury: Influence of lesion level and sildenafil citrate. Journal of Applied Physiology. 2005;99(1):53–58. doi: 10.1152/japplphysiol.00154.2005. [DOI] [PubMed] [Google Scholar]
- 113.Shen W.-K, Sheldon R. S, Benditt D. G, Cohen M. I, Forman D. E, Goldberger Z. D, Grubb B. P, Hamdan M. H, Krahn A. D, Link M. S, Olshansky B, Raj S. R, Sandhu R. K, Sorajja D, Sun B. C, Yancy C. W. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2017;136(5):e60–e122. doi: 10.1161/CIR.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 114.Shessel F. S, Carrion H. M, Politano V. A. Phenoxybenzamine and sweating in the spinal cord injury patient. Journal of Urology. 1978;120(1):60–61. doi: 10.1016/S0022-5347(17)57039-1. [DOI] [PubMed] [Google Scholar]
- 115.Sidorov E. V, Townson A. F, Dvorak M. F, Kwon B. K, Steeves J, Krassioukov A. V. Orthostatic hypotension in the first month following acute spinal cord injury. Spinal Cord. 2008;46(1):65–69. doi: 10.1038/sj.sc.3102064. [DOI] [PubMed] [Google Scholar]
- 116.Signore C, Spong C. Y, Krotoski D, Shinowara N. L, Blackwell S. C. Pregnancy in women with physical disabilities. Obstetrics and Gynecology. 2011;117(4):935–947. doi: 10.1097/AOG.0b013e3182118d59. [DOI] [PubMed] [Google Scholar]
- 117.Silver J. R. Early autonomic dysreflexia. Spinal Cord. 2000;38(4):229–233. doi: 10.1038/sj.sc.3100996. [DOI] [PubMed] [Google Scholar]
- 118.Simpson L. A, Eng J. J, Hsieh J. T. C, Wolfe D. L. The health and life priorities of individuals with spinal cord injury: A systematic review. Journal of Neurotrauma. 2012;29(8):1548–1555. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinha V, Elliott S, Ibrahim E, Lynne C. M, Brackett N. L. Reproductive health of men with spinal cord injury. Topics in Spinal Cord Injury Rehabilitation. 2017;23(1):31–41. doi: 10.1310/sci2301-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Skowronski E, Hartman K. Obstetric management following traumatic tetraplegia: Case series and literature review. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;48(5):485–491. doi: 10.1111/j.1479-828X.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 121.Smit A. A. J, Halliwill J. R, Low P. A, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. The Journal of Physiology. 1999;519(1):1–10. doi: 10.1111/j.1469-7793.1999.0001o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Solinsky R, Bunnell A. E, Linsenmeyer T. A, Svircev J. N, Engle A, Burns S. P. Pharmacodynamics and effectiveness of topical nitroglycerin at lowering blood pressure during autonomic dysreflexia. Spinal Cord. 2017;55(10):911–914. doi: 10.1038/sc.2017.58. [DOI] [PubMed] [Google Scholar]
- 123.Solinsky R, Linsenmeyer T. A. Intravesical lidocaine decreases autonomic dysreflexia when administered prior to catheter change. The Journal of Spinal Cord Medicine. 2019;42(5):557–561. doi: 10.1080/10790268.2018.1518764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solinsky R, Tam K, Linsenmeyer T. A. Onset of the action of intravesical lidocaine after spinal cord injury. Neurourology and Urodynamics. 2020;39(1):376–381. doi: 10.1002/nau.24216. [DOI] [PubMed] [Google Scholar]
- 125.Tashjian E. A, Richter K. J. The value of propoxyphene hydrochloride (Darvon) for the treatment of hyperhidrosis in the spinal cord injured patient: An anecdotal experience and case reports. Paraplegia. 1985;23(6):349–353. doi: 10.1038/sc.1985.55. [DOI] [PubMed] [Google Scholar]
- 126.Teasell R. W, Arnold J. M. O, Krassioukov A. V, Delaney G. A. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2000;81(4):506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- 127.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46(5):1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 128.Tkach J. R. Indomethacin treatment of generalized hyperhidrosis. Journal of the American Academy of Dermatology. 1982;6(4 Pt 1):545. doi: 10.1016/s0190-9622(82)80371-x. [DOI] [PubMed] [Google Scholar]
- 129.Trbovich M. B, Kiratli J. B, Price M. J. The effects of a heat acclimation protocol in persons with spinal cord injury. Journal of Thermal Biology. 2016;62(Pt A):56–62. doi: 10.1016/j.jtherbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 130.Trbovich M, Ortega C, Schroeder J, Fredrickson M. Effect of a cooling vest on core temperature in athletes with and without spinal cord injury. Topics in Spinal Cord Injury Rehabilitation. 2014;20(1):70–80. doi: 10.1310/sci2001-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trop C. S, Bennett C. J. Autonomic dysreflexia and its urological implications: A review. The Journal of Urology. 1991;146(6):1461–1469. doi: 10.1016/s0022-5347(17)38140-5. [DOI] [PubMed] [Google Scholar]
- 132.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98(4 Pt 1):649–658. [PubMed] [Google Scholar]
- 133.Vale F. L, Burns J, Jackson A. B, Hadley M. N. Combined medical and surgical treatment after acute spinal cord injury: Results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. Journal of Neurosurgery. 1997;87(2):239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- 134.Van De Vliet P. Antidoping in paralympic sport. Clinical Journal of Sport Medicine. 2012;22(1):21–25. doi: 10.1097/JSM.0b013e31824206af. [DOI] [PubMed] [Google Scholar]
- 135.Vaziri N. D. Nitric oxide in microgravity-induced orthostatic intolerance. Relevance to spinal cord injury. 2003;26(5)(Pt.1) doi: 10.1080/10790268.2003.11753653. [DOI] [PubMed] [Google Scholar]
- 136.Walling H. W, Swick B. L. Treatment options for hyperhidrosis. American Journal of Clinical Dermatology. 2011;12(5):285–295. doi: 10.2165/11587870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 137.Wecht J. M, Bauman W. A. Decentralized cardiovascular autonomic control and cognitive deficits in persons with spinal cord injury. The Journal of Spinal Cord Medicine. 2013;36(2):74–81. doi: 10.1179/2045772312Y.0000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wecht J. M, De Meersman R. E, Weir J. P, Spungen A. M, Bauman W. A. Cardiac autonomic responses to progressive head-up tilt in individuals with paraplegia. Clinical Autonomic Research. 2003;13(6):433–438. doi: 10.1007/s10286-003-0115-5. [DOI] [PubMed] [Google Scholar]
- 139.Wecht J. M, Radulovic M, Weir J. P, Lessey J, Spungen A. M, Bauman W. A. Partial angiotensin-converting enzyme inhibition during acute orthostatic stress in persons with tetraplegia. The Journal of Spinal Cord Medicine. 2005;28(2):103–108. doi: 10.1080/10790268.2005.11753806. [DOI] [PubMed] [Google Scholar]
- 140.Wecht J. M, Rosado-Rivera D, Handrakis J. P, Radulovic M, Bauman W. A. Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Archives of Physical Medicine and Rehabilitation. 2010;91(9):1429–1435. doi: 10.1016/j.apmr.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 141.Wecht J. M, Weir J. P, Katzelnick C. G, Chiaravalloti N. D, Kirshblum S. C, Dyson-Hudson T. A, Weber E, Bauman W. A. Double-blinded, placebo-controlled crossover trial to determine the effects of midodrine on blood pressure during cognitive testing in persons with SCI. Spinal Cord. 2020 doi: 10.1038/s41393-020-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wecht J. M, Weir J. P, Katzelnick C. G, Wylie G, Eraifej M, Nguyen N, Dyson-Hudson T, Bauman W. A, Chiaravalloti N. Systemic and cerebral hemodynamic contribution to cognitive performance in spinal cord injury. Journal of Neurotrauma. 2018;35(24):2957–2964. doi: 10.1089/neu.2018.5760. [DOI] [PubMed] [Google Scholar]
- 143.West C. R, Romer L. M, Krassioukov A. V. Autonomic function and exercise performance in elite athletes with cervical spinal cord injury. Medicine and Science in Sports and Exercise. 2013;45(2):261–267. doi: 10.1249/MSS.0b013e31826f5099. [DOI] [PubMed] [Google Scholar]
- 144.Westaway K, Frank O, Husband A, McClure A, Shute R, Edwards S, Curtis J, Rowett D. Medicines can affect thermoregulation and accentuate the risk of dehydration and heat-related illness during hot weather. Journal of Clinical Pharmacy and Therapeutics. 2015;40(4):363–367. doi: 10.1111/jcpt.12294. [DOI] [PubMed] [Google Scholar]
- 145.Yarkony G. M, Katz R. T, Wu Y. C. Seizures secondary to autonomic dysreflexia. Archives of Physical Medicine and Rehabilitation. 1986;67(11):834–835. [PubMed] [Google Scholar]
- 146.Zhu C, Galea M, Livote E, Signor D, Wecht J. M. A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. Journal of Spinal Cord Medicine. 2013;36(5):463–475. doi: 10.1179/20457. [DOI] [PMC free article] [PubMed] [Google Scholar]


