Introduction
Since the turn of the millennium, focus has increased substantially with respect to the secondary consequences of autonomic nervous system (ANS) dysfunction in the spinal cord injury (SCI) community. Individuals with SCI rank the restoration of ANS function above regaining the ability to walk.2 Increased attention regarding the impact of ANS impairment on organ system function has improved clinical management and has guided determination of therapeutic treatment efficacy. This effort was supported, in part, by an international collaboration between the Autonomic Standards Committee of the American Spinal Injury Association (ASIA) and the International Spinal Cord Society (ISCoS), which led to the development of the International Standards to document Autonomic Function following SCI (ISAFSCI). Recommendations from this international collaboration were originally published in 20093 and were revised in 2012.4 The ISAFSCI, which is sometimes referred to as the Autonomic Standards, is recommended to be used in conjunction with the full International Standards for Neurological Classification of SCI (ISNCSCI) following the initial injury, and to track the association between changes in ANS function correspondent with changes in the neurological level of injury (NLI) and completeness of injury as classified by the ASIA Injury Severity (AIS) scale. Additionally, the ISAFSCI should be used to track changes in autonomic functions following clinical intervention or during a clinical trial. In addition, a web-based training course is available online (https://asia-spinalinjury.org/learning/) to foster improved understanding regarding the impact of ANS impairment on organ system function and to guide use of the ISAFSCI.
The Autonomic Standards Committee recognizes that, although there is growing attention being paid to ANS impairments following SCI, current use of the ISAFSCI is still limited by a lack of appreciation regarding the dire consequences of ANS impairment to health, vitality, and longevity and by uncertainty regarding the utility of the current assessment tool. Additionally, the complexities of the ANS, the lack of subjective patient-oriented responses, and the fact that the ANS is involved in every organ system in the body renders functional testing difficult following SCI. Nonetheless, it is increasingly apparent that ANS impairment fundamentally underlies many of the functional deficits reported in the SCI population. Therefore, determining the level and severity of injury to the ANS following SCI and tracking changes over time and in response to therapeutic intervention will ultimately lead to improved clinical care, health, and well-being.
Members of the Autonomic Standards Committee created and distributed an international survey to determine the level of awareness and usage of the ISAFSCI and to better understand the needs of the community; the following ISAFSCI revision has incorporated the results of this survey.5–8 This second revision of the ISAFSCI has also incorporated feedback received from the community of SCI clinical healthcare providers and leverages recent novel and relevant literature findings. The ISAFSCI is a living document, and multiple future revisions should be expected as pertinent new information becomes available. The ASIA Autonomic Standards Committee continues to welcome feedback that will facilitate discussion and future refinement of the ISAFSCI assessment.
This revised version of the ISAFSCI provides a format for documenting the impact of SCI on ANS neural control of cardiovascular, thermal and sudomotor, bronchopulmonary, and sacral organ system function, which includes lower urinary tract, gastrointestinal tract, and genitalia and reproductive organs. This document will provide background information and recommendations for ISAFSCI testing of these organ systems pertaining to:
neuroanatomical pathways of control and innervation,
anticipated level of function based on the NLI as obtained on the ISNCSCI,
standardized testing procedures, and
participant preparation.
It should be noted that the purpose of the ISAFSCI is to address the impact of the SCI in and of itself on ANS functioning. Therefore it is vital to acknowledge when other issues are present that may contribute to or impact autonomic function, such as other neuropathies, medications, or co-incident medical conditions.
General Background
Autonomic Nervous System: Anatomy and Function
The ANS is commonly subdivided into the sympathetic nervous system (SNS) and the parasympathetic nervous systems (PNS), and most visceral organs are innervated by both components of the ANS.9 Both the SNS and the PNS are integrated within the central nervous system (CNS) and function to ensure appropriately balanced regulation of innervated organs for a given activity or stressor. Certain cortical structures and the hypothalamus contribute to the regulation of the ANS circuits within the brainstem and spinal cord.
Both divisions of the ANS have two neuronal populations interposed between the CNS and target organs: preganglionic and postganglionic neurons. The cell body of the preganglionic neuron is located within the gray matter of the brain or spinal cord, and the axons of these neurons travel within the ventral roots of the spinal cord or cranial nerves. These fibers synapse on the postganglionic neurons, located within the autonomic ganglia in the peripheral nervous system. The postganglionic axons of the ANS innervate the target organs. Sympathetic preganglionic neurons reside in the spinal gray matter in the thoracic and upper lumbar segments of the spinal cord (T1-L2). The majority of sympathetic preganglionic neurons are localized within the lateral horns or intermediolateral nucleus of the spinal cord. Axons of the sympathetic preganglionic neurons exit through the ventral roots and synapse on sympathetic postganglionic neurons located in the spinal paravertebral ganglia (sympathetic chain ganglia) and prevertebral ganglia (the celiac, superior, and inferior mesenteric ganglia). The postganglionic SNS axons are located within the peripheral nerves and innervate target organ systems such as the cardiovascular, thermoregulatory and sudomotor, bronchopulmonary, lower urinary, gastrointestinal, and genitalia and reproductive organs (Table 1). Parasympathetic preganglionic neurons are located within the brainstem nuclei of four cranial nerves and within the sacral spinal segments (S2–S4). Parasympathetic control of the cardiovascular system and the upper portion of the gastrointestinal tract is through the vagus nerve (CN X), which exits from the medulla through the base of the skull and reaches the sinoatrial (SA) node of the heart, and the nerve cells within the enteric nervous system of the bowel. There is no parasympathetic innervation of the peripheral vasculature except in the pelvic organs and potentially the brain. Parasympathetic innervation of the urinary bladder, reproductive organs, and lower portion of the gut is provided by the sacral portion of the spinal cord (S2–S4) (Table 1).
Table 1.
Organization of ANS innervation of major organ/systems
| Organ/System | SNS (T1-L2) | PNS (CN X, S2–S4) | Somatic/motor |
|---|---|---|---|
| Heart | |||
|
| |||
| T1–T5 | CN X | None | |
| Blood vessels | |||
|
| |||
| Upper body | T1–T5 | CN X | None |
| Lower body | T6-L2 | S2–S4 | |
| Sweat glands | |||
|
| |||
| Face | T1–T4 | None | None |
| Upper limbs | T2–T8 | ||
| Trunk | T4–T12 | ||
| Lower limbs | T12-L2 | ||
| Broncho-pulmonary | |||
|
| |||
| T1–T5 | CN X | C3–C8 | |
| Lower urinary tract | |||
|
| |||
| Detrusor Bladder neck/internal urethral sphincter | T10-L2 | S2–S4 | None |
| External urethral sphinctor | T10–T12 | S2–S4 | S2–S4 |
| Gastro-intestinal tract | |||
|
| |||
| Esophagus to splenic flexure Splenic flexure to rectum/internal anal sphincter | T1-L2 | S2–S4, CN X | None |
| External anal sphincter | T10-L2 | S2–S4 | S2–S4 |
| Genitalia and reproductive | |||
|
| |||
| Vagina | |||
| Female reproductive organs Penis | T10-L2 | S2–S4 | S1–S4 |
| Male reproductive organs | |||
Abbreviations: ANS=autonomic nervous system; SNS=sympathetic nervous system; PNS=parasympathetic nervous system; T=thoracic; CN X=cranial nerve 10; L=lumbar; S=sacral; C-cervical
General Testing Procedures: ANS Function Following SCI
The following are general instructions for conducting the ISAFSCI in patients with SCI. For more details regarding specific organ system testing, please see procedural specifications listed under each organ system below. In general it is recommended that:
-
The ISAFSCI should be administered in conjunction with the full ISNCSCI.
In addition, the ISAFSCI should be conducted anytime there is a change in clinical intervention that may impact autonomic control of any organ system function.
-
The patient must be otherwise healthy, with no current illness or infection, including but not limited to urinary tract infections and pressure wounds.
Document all chronic health conditions.
Document all prescription medications, daily dosing schedule, and time of last dose.
The patient should refrain from caffeine, eating a large meal, heavy exertion, nicotine products, cannabis, and alcohol for a minimum of 4 hours prior to administration of the ISAFSCI.
Upon arrival patients should be asked to empty their bladder and indicate the time of their last bowel movement.
All pressure garments (e.g., abdominal binders, compression stockings) should be removed prior to testing.
The patient should be placed in a quiet, dimly lit, thermo-neutral private room for a period of approximately 10 minutes prior to beginning the ISAFSCI assessment. Optimally, the room temperature should be maintained at 23°C, with relatively humidity between 25% and 35%.10
Autonomic Control: Cardiovascular System
The major organs of the cardiovascular system are the heart and the blood vessels, which are innervated by the ANS.11,12 The heart receives both PNS and SNS innervation,13,14 whereas the vasculature receives only SNS innervation15 (Figure 1). Parasympathetic efferent fibers travel to the heart via the vagus nerve, which exits the CNS at the level of the medulla and includes the dorsal vagal motor nerve (DMNX) and nucleus ambiguous (NA). The vagal nerve innervates the atria, nodes, and Purkinje fibers via local cardiac ganglia. Vagal stimulation to the SA node provides tonic restraint to firing rate, decreases heart rate, conduction velocity, and to a lesser degree, contractility. The primary neurotransmitter of the PNS is acetylcholine, which is released by both preganglionic and postganglionic fibers (vagus nerve).
Figure 1:

Autonomic efferent innervation of the cardiovascular system. RVLM: rostral ventrolateral medulla; DMNX: dorsal vagal motor nerve; NA: nucleus ambiguous. (Modified with permission from Inskip, J. A., et al. Spinal Cord. 2009;47:2–35.)
All tissues of the heart receive SNS innervation from the upper thoracic (T1–T5) cord, and sympathetic stimulation increases heart rate, conduction velocity, and contractility. The peripheral vasculature receives tonic SNS control, which is maintained by catecholaminergic C1 neurons within the medullary cardiovascular center known as rostral ventrolateral medulla (RVLM). Neural control of the vessels in the upper portion of the body receive SNS innervation from the T1–T5 spinal segments, whereas the major vascular beds in the gut and lower extremities are under SNS control from more caudal spinal segments T6-L2. The vessels supplying the splanchnic region are particularly important to blood pressure regulation (i.e., liver, spleen, intestines), as this region contains approximately one-fourth of the total blood volume and is the primary capacitance bed capable of rapid redistribution of blood volume. SNS outflow to the splanchnic bed exits the thoracolumbar cord (T6-L2) and elicits vasoconstriction via alpha and beta adrenoreceptors. With respect to direct SNS control of the heart and peripheral vasculature, the preganglionic neurotransmitter is acetylcholine, and the primary postganglionic sympathetic neurotransmitter is norepinephrine, which is integral to the maintenance of tonic cardiac function, vasomotor tone, and blood pressure.
Baroreceptor afferents are not shown in Figure 1; however, they play an important role in sensing blood pressure and transmitting this information through the glossopharyngeal and vagus nerves to the medulla for appropriate ANS responses.16 These afferent pathways remain anatomically intact after SCI.
To understand cardiovascular responses after SCI and how they differ depending on neurological level of injury (NLI), two key elements need to be considered. First, there is dual innervation of the heart from both the SNS and PNS. Second, specific SNS spinal segments are responsible for controlling vascular tone in particular vascular beds, without PNS influence. These considerations can explain why cardiovascular orthostatic responses and plasma catecholamine concentrations at rest and in response to exercise17,18 may be normal or abnormal following cervical, mid-thoracic, or lower thoracic SCI.
Current Definitions and Anticipated Level of Function: Cardiovascular System
Similar to the current form,4 it is recommended in the revised edition that a description of remaining general autonomic function be documented for heart rate and blood pressure (see Tables 2 and 3). Information regarding cardiovascular autonomic control should be determined based on a combination of neurological examination, clinical history, and most current ISNCSCI evaluation. Definitions of cardiovascular autonomic abnormalities are presented as part of the recording table (Table 2).
Table 2.
General autonomic function
| Cardiovascular | Scoring | Condition | Definitions | Score |
|---|---|---|---|---|
| Heart Rate | Normal (2) | 61–99 bpm | ||
| Supine _______mmHg | Altered (1) | Bradycardia | ≤60 bpm | |
| Tachycardia | ≥100 bpm | |||
| Arrhythmias § | ||||
| Seated _______mmHg | Not Tested (NT): indicate reason | |||
| Systolic BP | Normal (2) | 91–139 mmHg | ||
| Supine _______mmHg | Altered (1) | Supine Hypotension | SBP ≤ 90 mmHg | |
| Orthostatic Hypotension | Fall ≥ 20 mmHg within 10 minutes* | |||
| Neurogenic Shock | within 30 days of injury; heart rate ≤ 60 bpm; SBP ≤ 90 mmHg | |||
| Autonomic Dysreflexia** | increase in SBP > 20 mmHg above baseline | |||
| Supine Hypertension | ≥140 mmHg | |||
| Seated _______mmHg | Not Tested (NT): indicate reason | |||
| Diastolic BP | Normal (2) | 61–89 mmHg | ||
| Supine _______mmHg | Altered (1) | Supine Hypotension | ≤60 mmHg | |
| Orthostatic Hypotension | Fall ≥ 10 mmHg within 10 minutes* | |||
| Supine Hypertension | ≥90 mmHg | |||
| Seated_______mmHg | Not Tested (NT): indicate reason | |||
| § define the arrhythmia; * within 3 minutes, but delayed orthostatic hypotension; within 10 minutes, may be more common in the SCI population. ** self-report “within the past 7-days”. *** unanticipated i.e., not in response to known intervention (e.g., medication). | ||||
|
| ||||
| Thermoregulation Core Body Temp | Scoring | Conditions | Definitions | Score |
| Normal (2) | Normal | 36.4–37.6 °C (97.5–99.7 °F) | ||
| Altered (1) | Subnormal | 35.1–36.3 °C (95.1–97.4 °F) | ||
| Elevated | 37.7–37.9°C (99.8–100.3 °F) | |||
| Hypothermia | ≤35°C (≤95°F) | |||
| Hyperthermia | ≥38.0°C (≥100.4°F) | |||
| Not Tested (NT): indicate reason | ||||
| ** Under ambient conditions: 20–25°C (68–77°F); 30–50% relative humidity; wearing single-layer, indoor garments; after 10-minutes of rest; no acute illness or infection | ||||
| Sudomotor* | Scoring | Conditions | Definitions | Score |
| Normal (2) | Normal sweating | Sweating on all skin surfaces | ||
| Altered (1) | Hypohidrosis | Diminished sweating above NLI | ||
| Diminished sweating below NLI | ||||
| Hyperhidrosis | Excessive sweating above NLI | |||
| Excessive sweating below NLI | ||||
| Absent (0) | Anhidrosis | No sweating above or below NLI | ||
| Not Tested (NT): indicate reason | ||||
|
| ||||
| * Record sweating responses to high ambient heat or exercise only. Do not record sweating associated with AD, OH, or mental stress. | ||||
|
| ||||
| Broncho-pulmonary System | Findings | Conditions | Definition | Score |
| Normal (2) | ||||
| Altered (1) | Invasive ventilation | 24 hours/day | ||
| Partial invasive ventilatory support | < 24 hours/day | |||
| Impaired voluntary respiration not requiring ventilatory support | Continuous Positive Airway Pressure (CPAP) for sleep apnea | |||
| Not Tested (NT): indicate reason | ||||
|
| ||||
| Forced Vital Capacity (FVC) * | supine _____ seated _____ abdominal binder: YES_____ NO_____ mL ____; kg ____; mL/kg ______ | |||
Table 2.
General autonomic function
| Cardiovascular | Scoring | Condition | Definitions | Score |
|---|---|---|---|---|
| Heart Rate | Normal (2) | 61–99 bpm | ||
| Supine _______mmHg | Altered (1) | Bradycardia | ≤60 bpm | |
| Tachycardia | ≥100 bpm | |||
| Arrhythmias § | ||||
| Seated _______mmHg | Not Tested (NT): indicate reason | |||
| Systolic BP | Normal (2) | 91–139 mmHg | ||
| Supine _______mmHg | Altered (1) | Supine Hypotension | SBP ≤ 90 mmHg | |
| Orthostatic Hypotension | Fall ≥ 20 mmHg within 10 minutes* | |||
| Neurogenic Shock | within 30 days of injury; heart rate ≤ 60 bpm; SBP ≤ 90 mmHg | |||
| Autonomic Dysreflexia** | increase in SBP > 20 mmHg above baseline | |||
| Supine Hypertension | ≥140 mmHg | |||
| Seated _______mmHg | Not Tested (NT): indicate reason | |||
| Diastolic BP | Normal (2) | 61–89 mmHg | ||
| Supine _______mmHg | Altered (1) | Supine Hypotension | ≤60 mmHg | |
| Orthostatic Hypotension | Fall ≥ 10 mmHg within 10 minutes* | |||
| Supine Hypertension | ≥90 mmHg | |||
| Seated_______mmHg | Not Tested (NT): indicate reason | |||
| § define the arrhythmia; * within 3 minutes, but delayed orthostatic hypotension; within 10 minutes, may be more common in the SCI population. ** self-report “within the past 7-days”. *** unanticipated i.e., not in response to known intervention (e.g., medication). | ||||
|
| ||||
| Thermoregulation Core Body Temp | Scoring | Conditions | Definitions | Score |
| Normal (2) | Normal | 36.4–37.6 °C (97.5–99.7 °F) | ||
| Altered (1) | Subnormal | 35.1–36.3 °C (95.1–97.4 °F) | ||
| Elevated | 37.7–37.9°C (99.8–100.3 °F) | |||
| Hypothermia | ≤35°C (≤95°F) | |||
| Hyperthermia | ≥38.0°C (≥100.4°F) | |||
| Not Tested (NT): indicate reason | ||||
| ** Under ambient conditions: 20–25°C (68–77°F); 30–50% relative humidity; wearing single-layer, indoor garments; after 10-minutes of rest; no acute illness or infection | ||||
| Sudomotor* | Scoring | Conditions | Definitions | Score |
| Normal (2) | Normal sweating | Sweating on all skin surfaces | ||
| Altered (1) | Hypohidrosis | Diminished sweating above NLI | ||
| Diminished sweating below NLI | ||||
| Hyperhidrosis | Excessive sweating above NLI | |||
| Excessive sweating below NLI | ||||
| Absent (0) | Anhidrosis | No sweating above or below NLI | ||
| Not Tested (NT): indicate reason | ||||
|
| ||||
| * Record sweating responses to high ambient heat or exercise only. Do not record sweating associated with AD, OH, or mental stress. | ||||
|
| ||||
| Broncho-pulmonary System | Findings | Conditions | Definition | Score |
| Normal (2) | ||||
| Altered (1) | Invasive ventilation | 24 hours/day | ||
| Partial invasive ventilatory support | < 24 hours/day | |||
| Impaired voluntary respiration not requiring ventilatory support | Continuous Positive Airway Pressure (CPAP) for sleep apnea | |||
| Not Tested (NT): indicate reason | ||||
|
| ||||
| Forced Vital Capacity (FVC) * | supine _____ seated _____ abdominal binder: YES_____ NO_____ mL ____; kg ____; mL/kg ______ | |||
Table 3.
Sacral autonomic function
| Bladder Emptying | Method Frequency Timing Voluntarily | Yes ______ No ______ | ||
|---|---|---|---|---|
| System/Organ | Scoring | Anticipated Function (based on ISNCSCI) | Anticipated Functional Score | Patient Reported Score |
| Awareness bladder fullness | Normal (2) | Any level injury with normal sensation in the T11-L2 and S3–S5 dermatomes | ||
| Altered (1) | Any level injury with partial preservation of sensation in the T11-L2 and/or S3–S5 dermatomes | |||
| Absent (0) | NLI at or above T9 no sensation below | |||
| Not Tested (NT): indicate reason | ||||
| Ability to prevent bladder leakage | Normal (2) | Individuals with normal sensation and motor function in the S3-S5 dermatomes | ||
| Altered (1) | Individuals with partial sensation and motor function in the S3–S5 dermatomes | |||
| Absent (0) | No motor function at the S3–5 dermatomes | |||
| Not Tested (NT): indicate reason | ||||
| Bowel Emptying | Method Frequency Timing Voluntarily | Yes ______ No ______ | ||
| System/Organ | Scoring | Anticipated Function (based on ISNCSCI) | Anticipated Functional Score | Patient Reported Score |
| Awareness of bowel fullness | Normal (2) | Normal sensation and motor function in the S3–S5 dermatomes | ||
| Altered (1) | Partial preservation of sensation or motor function in the S3–S5 dermatomes | |||
| Absent (0) | No sensation or motor function in the S3–5 dermatomes | |||
| Not Tested (NT): indicate reason |
Table 3.
Sacral autonomic function
| Ability to prevent bowel leakage | Normal (2) | Individuals with normal sensation and motor function in the S3–S5 dermatomes | ||
| Altered (1) | Individuals with partial sensation and motor function in the S3–S5 dermatomes | |||
| Absent (0) | No motor function at the S3–5 dermatomes | |||
| Not Tested (NT): indicate reason | ||||
| System/Organ | Scoring | Anticipated Function (based on ISNCSCI) | Anticipated Functional Score | Patient Reported Score |
| Psychogenic arousal | Normal (2) | Normal sensation and reflex motor function at T11-L2 | ||
| Altered (1) | Partial sensation and motor reflex function at T11-L2 | |||
| Absent (0) | No sensation or reflex motor function at T11-L2 | |||
| Not Tested (NT): indicate reason | ||||
| Reflex genital arousal | Normal (2) | Normal sensation and reflex function at S3–5 | ||
| Altered (1) | Partial sensation and motor reflex function at S3–5 | |||
| Absent (0) | No sensation or motor function at S3–5 | |||
| Not Tested (NT): indicate reason | ||||
| Orgasm | Normal (2) | Intact S3–5 sensation and or motor function with any degree of preserved sacral reflexes | ||
| Altered (1) | No S3–5 sensation or motor function and preserved sacral reflexes | |||
| Absent (0) | No S3–5 sensation or motor function and absent sacral reflexes | |||
| Not Tested (NT): indicate reason | ||||
| Ejaculation | Normal (2) | Normal T11–12 sensation and sacral reflexes | ||
| Altered (1) | Diminished sensation at T11–12 dermatomes and normal sacral reflexes | |||
| Absent (0) | No sensation at T11–12 dermatomes and absent sacral reflexes | |||
| Not Tested (NT): indicate reason |
The recognition and assessment of cardiac dysrhythmias includes documentation of bradycardia, defined as a heart rate ≤ 60 beats per minute (bpm), and tachycardia, defined as a heart rate ≥ 100 bpm. It should be noted that bradycardic heart rates of ≤ 50 bpm have been reported in more than one-third of newly injured patients with cervical and thoracic injuries above T6.19 Other dysrhythmias should also be documented on the assessment form. Abnormalities of arterial blood pressure include supine hypertension, defined as a supine systolic blood pressure ≥ 140 mmHg and/or a supine diastolic blood pressure ≥ 90 mmHg, and supine hypotension, defined as a systolic blood pressure ≤ 90 mmHg and/or a diastolic blood pressure ≤ 60 mmHg.
Orthostatic hypotension (OH) is defined as a symptomatic or asymptomatic decrease in blood pressure of ≥ 20 mmHg systolic and/or ≥ 10 mmHg diastolic within 3 minutes of moving from supine to an upright position.20 It should also be noted that a delayed decrease in blood pressure that meets these criteria may also occur21,22 and if time allows, monitoring of blood pressure response for 10 minutes after transferring to the seated position is recommended. Symptoms of OH include, but are not limited to dizziness, lightheadedness, blurred vision, coat-hanger neck pain, and fatigue, which should be documented on the assessment form.
Autonomic dysreflexia (AD) is a constellation of signs and/or symptoms most commonly occurring in people with SCI at or above T6, which occurs in response to noxious or non-noxious stimuli below the NLI23 in particular, bladder stimulation.24 The predominant sign of AD is an increase in systolic blood pressure > 20 mmHg above baseline, which may or may not be associated with symptoms including headache, flushing, piloerection, stuffy nose, sweating above the level of the lesion, and dysrhythmia. It is important to note that many people with SCI cannot detect when they are having an episode of AD,25 a term known as “silent” AD, which can have fatal consequences.26,27 Finally, athletes with SCI may induce AD to increase blood pressure and improve sports performance, a practice known as “boosting”, which has been banned by the International Paralympic Committee.28–32
Neurogenic shock is a syndrome that may or may not be symptomatic and can occur at any period following SCI, although it is most common in newly injured patients within the first 30 days of injury. Neurogenic shock is associated with reduced vascular tone below the NLI due to disrupted descending supra-spinal sympathetic control.24–26 This condition is characterized primarily by supine systolic blood pressure ≤ 90 mmHg, which cannot be attributed to low intravascular volume (e.g., blood loss, dehydration, sepsis, and cardiac disorders) and a heart rate ≤ 60 bpm. Whereas neurogenic shock is characterized by changes in blood pressure and heart rate (autonomic), spinal shock is characterized by marked reductions in spinal reflex (motor) activity below the NLI.
The anticipated degree of cardiovascular ANS impairment can be based on the NLI as evaluated on the ISNCSCI exam. In general it is expected that during the clinical evaluation of cardiovascular autonomic function, individuals with complete lesions at or above T6 are more likely to have resting bradycardia and hypotension in the supine position and will experience OH during clinical evaluation (Table 2). In addition, these individuals are expected to report episodic bouts of AD. Individuals with lesions below T6 will typically demonstrate normal to high heart rates and blood pressures in the supine position upon clinical evaluation but may not display OH and are not likely to report bouts of AD. However, the ISAFSCI should be conducted in conjunction with performing the ISNCSCI to verify and document cardiovascular ANS function, regardless of the level or completeness of injury.33–36
Procedural Specifications: Cardiovascular System
Equipment recommendations:
Recommended: Manual auscultation of brachial artery pressure with heart rate monitoring at 1-minute intervals
Alternative: Continuous beat-to-beat heart rate and blood pressure monitoring
Assessment recommendations:
-
Upon arrival, the patient should be placed in the supine position to rest and the time of day should be recorded.
While resting in the supine position apply three ECG electrodes to the chest and abdomen for recording of heart rate. Place a brachial cuff around the left arm for monitoring of blood pressure.
After instrumentation is applied, allow the patient at least 10 minutes of quiet rest before recording supine heart rate and blood pressure at 1-minute intervals for 10 minutes*.
While resting in the supine position, patients should be asked if they are experiencing any of the following symptoms: lightheadedness, dizziness, blurred vision, tingling in the ears, pounding in the head, goosebumps, nausea, or other (ask to name and describe).
Ask the patient to describe activities that cause the above symptoms and any preventative or corrective actions they take to prevent or remedy these symptoms.
-
Following the 10-minute supine recording of heart rate and blood pressure, passively move the patient into the seated position with the hip and knees at a 90° angle or, if possible, perform a head-up-tilt maneuver to 60°. Take care that the participant does not exert effort during the position change.
Monitor and record seated heart rate and blood pressure at 1-minute intervals for 10 minutes*.
While they are resting quietly in the seated position, ask the patients, at 1-minute intervals, if they are experiencing any of the following symptoms: lightheadedness, dizziness, blurred vision, tingling in the ears, pounding in the head, goose-bumps, nausea, or other (ask to name and describe).
*While 10-minute observations of heart rate and blood pressure in the supine and seated position are optimal, 3 minutes is adequate if time is limited.
Autonomic Control: Thermoregulatory System
Thermoregulation is the ability to precisely control core body temperature within a relatively narrow range despite exposure to a wide range of environmental temperature challenges. Effective thermoregulation relies on appropriate hypothalamic integration of peripheral vasomotor status, involuntary shivering, and sweating (sudomotor control) for maintenance of core temperature.37,38 Thermoregulatory vasomotor and sudomotor functions are both innervated by autonomic nerves; functional changes after SCI are assessed on the ISAFSCI.
Sensory input from warm- and cold-sensitive transient receptor potential cation channels in the periphery allow skin temperatures to be transmitted as temperature information by second order ascending neurons to both the thalamus and lateral parabrachial nucleus. Third-order neurons transmit warm and cold temperature information from the thalamus to the cortex for temperature awareness, which drives behavioral response (i.e., add or remove clothing, seek cooler or warmer environments), and from the parabrachial nucleus to the preoptic area of the hypothalamus (POAH), which orchestrates the appropriate thermo-effector response.39,40 The POAH processes skin temperature along with core and hypothalamic temperatures. If those temperatures exceed the upper or lower limits of the narrow set-point range (or neutral zone), the POAH will coordinate activation of heat dissipation or heat retention and thermogenesis mechanisms, respectively.41
Warm information from the skin and spinal cord to the POAH inhibits cold-sensitive neurons from activating SNS preganglionic neurons in the RVLM, which prevents peripheral cutaneous vasoconstriction and decreases heat retention. Warm information also inhibits activation of shivering-producing neurons in the dorsomedial hypothalamus, which prevents shivering and thermogenesis. This same information will simultaneously excite warm-sensitive neurons to activate peripheral cutaneous vasodilation and sweating to increase heat loss utilizing radiation and evaporation, respectively. Cold information from the skin and spinal cord to the POAH has the opposite effect; it inhibits warm-sensitive neurons to prevent heat loss from radiation and sweating and excites cold sensitive neurons to increase cutaneous vasoconstriction and shivering to increase insulation and thermogenesis, respectively.39,40
POAH regulation of cutaneous vasoconstriction is mediated solely by sympathetic adrenergic pathways in glabrous areas (e.g., palms, soles, areas of the face) for active constriction to conserve body heat and passive dilation to dissipate body heat. Non-glabrous areas (e.g., head, trunk, extremities) receive dual innervation by SNS adrenergic-mediated vasoconstriction to conserve body heat and SNS cholinergic-mediated vasodilation to dissipate body heat.42,43
Current Definitions and Anticipated Level of Function: Thermoregulatory System
Temperature dysregulation is defined as a person’s inability to maintain core body temperature within the normal range (37 ± 0.6°C) despite having no signs of illness or infection.42,44–47 Thus, in persons with SCI, core body temperature outside of the normal range may result from exposure to hot or cold environmental temperatures, which should be documented on the ISAFSCI assessment form. Core temperatures below the normal range, but above the threshold of hypothermia (35°C) may be documented as subnormal. Whereas core temperatures above the normal range, but below the threshold for hyperthermia (38°C) may be documented as elevated.
Impaired ability to redistribute blood from peripheral to central compartments (and vice versa), due to altered vasomotor control of peripheral and splanchnic vascular beds is prevalent in persons with SCI at or above T648,49 and limits the ability to increase or decrease insulation in cold and hot environments, respectively. Hence, in persons with cervical and upper thoracic SCI, core body temperature is anticipated to be below the normal range (36.4–37.6°C) when exposed to ambient temperatures less than thermo-neutral (~25–27°C) and above the normal range when exposed to ambient temperatures above thermo-neutral.47 This direct rise and fall of core body temperature with change in ambient temperature is defined as poikilothermy and is more evident in SCI at or above T1 since both cardiac and upper body vasomotor SNS control (T1–T5) are interrupted. Therefore at room temperature (20–25°C), it is anticipated that persons with cervical SCI (tetraplegia) will have subnormal core body temperature (<36.4°C),50 persons with high thoracic SCI (high paraplegia T1–T6) will have core body temperature in the lower range of normal, and persons with SCI below T6 will have core body temperature in the normal range.
Procedural Specifications: Thermoregulatory System
Equipment recommendations
Recommended: oral temperature measurement
Alternative: rectal temperature measurement or ingestible telemetry capsule
Assessment recommendations
The exam room temperature should be within the range of 20–25°C (68–77°F) with 30–50% relative humidity. To account for circadian variability, multiple core body temperature assessments should be taken and time of day recorded.51
To assess core body temperature, the patient should rest quietly for at least 10 minutes to acclimate to room temperature and no liquids should be consumed by mouth for at least 10 minutes prior to oral temperature measurement. During the final two minutes prior to measurement, the patient’s mouth should be kept closed, with minimal talking, and the oral thermometer should be inserted into either the right or left sublingual area (under the tongue) and should not be clenched between the teeth.47 Two measurements should be performed and if they are within 0.2°C, the average of the two readings is recorded. If they are not within 0.2°C, assess a third time and average the two closest readings.
Autonomic Control: Sudomotor System
Normal sudomotor responses occur during whole body heat stress conditions from either passive ambient heat exposure and/or internal metabolic heat production during exercise, which causes core temperature to rise above the POAH thermoregulatory set point (e.g., 37.0°C). When the POAH senses a rise in core body temperature, SNS efferent sudomotor fibers are activated and synapse in the brainstem. Efferent brainstem fibers travel to the intermediolateral cell column within the lateral horn of the thoracolumbar spinal cord.39, 40 The preganglionic fibers then pass into the sympathetic chain that lies just outside the spinal column at T1-L2. Within the sympathetic chain, the neurons may synapse in the ganglion at the same level or travel up or down to synapse at another ganglion. Preganglionic SNS neurons arising from T1–T4 innervate sweat glands of the face, T2–T8 innervate sweat glands of the upper body (upper limbs and upper trunk), and T9-L2 innervate sweat glands of the lower body (lower trunk and lower limbs).52 Afterwards, these postganglionic neurons travel to their target organs, which include the sweat glands and blood vessels. During heat stress in able-bodied persons, acetylcholine release from the sympathetic cholinergic nerve terminal stimulates sweating for evaporative cooling.53,54
Current Definitions and Anticipated Level of Function: Sudomotor System
Given the anatomic location of the SNS within the thoracolumbar spinal cord, it follows that the degree of remaining SNS control would be proportional to the NLI as determined by the ISNCSCI exam. More specifically, minimal to no sweating is anticipated in those with cervical cord injuries as their injury is above the most cephalad SNS level (T1), and partial sweating is anticipated in those with injuries in the thoracic or lumbar cord.55 Studies quantifying core body temperature and heat storage during heat stress conditions consistently demonstrate this pattern.55–59 Thus, the pattern of core body temperature rise is proportionate to the NLI under conditions of internal heat production (exercise) and in response to ambient heat exposure.
We expect persons with motor complete cervical SCI to be unable to sweat (anhidrosis) both above and below the NLI in response to heat stress. For persons with motor complete thoracic injuries (T1–T6), hypohidrosis (less sweating than normal) or normal sweating is anticipated on skin surfaces above the NLI with hypohidrosis or anhidrosis seen below the NLI. For persons with injuries below T6, we anticipate normal sudomotor responses above the NLI and either hypohidrosis or anhidrosis below the NLI.
It should be noted that hyperhidrosis on the face and upper body may occur secondary to a massive SNS reflex (i.e., AD), but this is sympathetic adrenergic activation of sweat glands and not a hypothalamic-driven sympathetic cholinergic sudomotor response to heat stress. Thus, this hyperhidrosis is not a thermoregulatory function and should not be documented in the sudomotor section of the ISAFSCI.
Procedural Specifications: Sudomotor System
Equipment recommendations
General recommendations
Document the patient’s subjective response to standardized questions regarding the ability to sweat (intact, altered, or absent) on skin surface areas relative to the NLI in response to a heat challenge. If > 40% of total body surface area (SCI at or below T8–10) has intact sudomotor activity, thermoregulation will be similar to persons without SCI.55 Thus, thermoregulatory function can be predicted based on self-report of total body surface area with intact sweating.
Although numerous techniques are available to precisely quantify sudomotor function.60 (e.g., TST, quantitative sudomotor axon reflex test/QSART, quantitative direct and indirect axon reflex testing/QDIRT, and silicone impressions), these techniques require specialized equipment and can be extremely time consuming for both the clinician and patient; thus they are primarily used in non-clinical or research settings. Furthermore, QSART, QDIRT, and silicone impressions only measure postganglionic function in a small (<1 cm2 skin) surface area so these tests are of little use in defining the loss of sudomotor activity due to SCI/preganglionic pathology and whole body thermoregulatory capacity (given small surface area tested). Starch iodine test is the simplest bedside test to measure preganglionic sudomotor activity (assuming no underlying postganglionic disorder in addition to SCI, which is rare) in persons with SCI over a large skin surface area, but it does require heat stress of at least a 1°C rise in core body temperature. While optimal, the starch iodine test is not clinically practical as most clinics will not have access to the required heat chamber or water immersion equipment necessary to conduct the test. While the ISAFSCI can be used in clinical trials, it is also designed to be conducted efficiently in a typical (non-research) clinical setting without specialized equipment.
Assessment recommendations
Ask the patient if they are able to sweat during exercise or when exposed to hot environments. If yes, continue to ask if they sweat above or below their NLI. If sweating occurs as a result of another condition (e.g., AD, full bladder, OH, mental stress, or illness), do not record in the sudomotor section of the ISAFSCI, but you may document this in their clinical record and in the appropriate cardiovascular section of the ISAFSCI. Also, document activities that cause sweating in the individual patient.
Autonomic Control: Bronchopulmonary System
Similar to other organ systems, impairment in respiratory function is directly related to the NLI and may be associated with the AIS completeness of injury following SCI.3,4 In individuals with an intact CNS, respiration occurs through coordinated activity of the somatic nervous system (control of inspiratory and expiratory muscles) and the ANS (bronchial tone and secretions). The diaphragm is the major muscle of inspiration and is innervated by the phrenic nerve (C3–C5). Persons with mid-cervical SCI usually maintain the ability to breathe spontaneously (see section on Anticipated Level of Function), but they experience reduced forced vital capacity (FVC) and more severe restrictive impairment compared to persons with thoracolumbar injury. Expiratory muscle function, especially the ability to generate forceful cough for airway clearance, may be severely impaired in persons with cervical SCI and to a lesser extent in those with lower levels of injury because of paralysis of abdominal muscles and the lateral internal intercostal muscles (T1–T12).
Autonomic control of the bronchopulmonary system (Figure 2) is principally governed by the PNS. Whereas SNS neurotransmission to the bronchopulmonary system, which arises from spinal segments T1–T6, has historically been thought to have little functional significance in human airways. However, studies in persons with cervical SCI and interrupted SNS control of the bronchopulmonary system indicate reduced airway caliber and exaggerated bronchoconstriction.61,62
Figure 2:
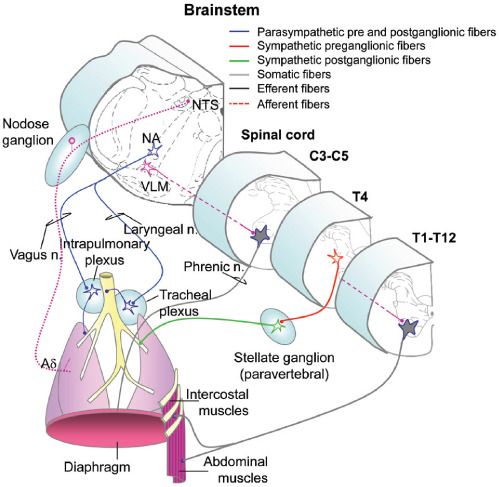
Innervation of the respiratory system. NTS: nucleus of the solitary tract; NA: nucleus ambiguous; VLM: ventrolateral medulla. (Modified with permission from Inskip, J. A., et al. Spinal Cord. 2009;47:2–35.)
Neural innervation of the muscles of respiration (diaphragm, intercostal and abdominal muscles) exit the thoracolumbar spinal cord from T1–T12 and are modulated, in part, by autonomic pre motor neurons in the ventrolateral medulla (VLM), which project to motor neurons in the spinal cord. The airways receive both PNS and SNS input. The PNS provides the innervation to the smooth muscle of the airways and is thus most important in controlling airway diameter. Preganglionic PNS neurons originate in the nucleus ambiguous (NA) and innervate the trachea and the bronchi via the laryngeal and vagus nerves, respectively. PNS control of the airways is conveyed via acetylcholine; its action is excitatory resulting in bronchoconstriction. SNS innervation of smooth muscle is comparatively scant. Preganglionic SNS neurons exit at T4 and travel to paravertebral ganglia, and postganglionic neurons elicit bronchodilation, acting through β-adrenergic receptors. The airways also have extensive afferent innervation. The most important afferents regulating respiration are vagal mechanoreceptors, with cell bodies in the nodose ganglia and central axons projecting to the nucleus of the solitary tract (NTS).1
Current Definitions and Anticipated Level of Function: Bronchopulmonary System
Invasive ventilatory support includes, but is not limited to, mechanical ventilators, phrenic nerve stimulators, diaphragmatic pacers, external negative pressure devices, and bi-level positive airway pressure (BiPAP). These devices do not include routine administration of oxygen, intermittent positive pressure breathing, or continuous positive airway pressure (CPAP). Ventilatory assistance does not include “limited, short-term use,” which is defined as respiratory support used as part of the medical treatment for other pulmonary complications, emergency mouth-to-mouth or machine resuscitation, emergency “bagging,” or operative/post-operative ventilator support used for less than 7 days.
CPAP is coded when a mechanical device is used for chronic or obstructive sleep apnea. Mechanical devices include CPAP, adaptive servo ventilation, or BiPAP when used specifically for sleep apnea. Forced vital capacity (FVC) is defined as the total volume of air that a person can forcibly exhale during a maximal expiratory effort measured by simple spirometry.63
Individuals with AIS grade A or B at C2 or above may expect to require full ventilator support. Individuals with C3-C5 AIS A or B may expect to achieve partial ventilator independence. Individuals with injuries below C5 are not expected to require invasive ventilatory support. Expected outcomes may be reduced based on comorbidities that impair bronchopulmonary system function (e.g., asthma, smoking, obstructive sleep apnea, and chronic obstructive pulmonary disease). The prevalence of sleep apnea in people with SCI is 63–72% compared to 25–33% in the non-SCI population.64 The prevalence of sleep-disordered breathing among individuals with complete tetraplegia has been estimated at 60%. Individuals with incomplete tetraplegia and paraplegia appear to be at lower risk.65,66
Procedural Specifications: Bronchopulmonary System
Equipment recommendations
Recommended: A handheld Wright spirometer or simple digital spirometer
Alternative: Any commercially available diagnostic spirometer that meets the American Thoracic Society/European Respiratory Society guidelines.67
Assessment recommendations
Correct posture for assessment of the bronchopulmonary system comes from the standards published for the uninjured population67,68 and the SCI population.69,70
The patient should be seated erect, with shoulders slightly back and chin slightly elevated.
Feet should be flat on floor (or footrest) with legs uncrossed.
Well-fitting dentures should be left in place.
A chair with arms is preferable to prevent patient from falling sideways should syncope occur.
For the maneuvers described, a nose clip or manual occlusion of the nostrils should be used.
If testing is undertaken with the patient in a different position, this must be documented in the report.
Autonomic and Somatic Control: Sacral Function
Sacral Function
Restoration of sacral function is a high priority for neurologic recovery in persons with SCI.2 However, the ASIA Autonomic Standards committee recognizes that restoration of sacral function is not solely related to recovery of ANS function, as sacral function is also governed by somatic nervous system control. Quantifying the retention and recovery of sacral function after SCI is important and must be based on both neurologic and subjective responses when using the ISAFSCI. Based on these points, the recommendation is that the sacral standards must specifically include:
Determination of voluntary anal contraction and sensation.
Determination of the anal wink and/or bulbocavernosus reflexes.
Assessment of remaining sensation and motor function at T11-L2 and S3 to S5 spinal segments.
Current Definitions and Anticipated Level of Function: Sacral Function
Voluntary sphincter contraction (contraction of the external anal sphincter): Insert the tip of the index finger in the anus and ask the person to squeeze the finger. Then grade the person’s ability to voluntarily squeeze the finger: 2 = normal squeeze; 1 = partial squeeze; 0 = no squeeze.
Reflex function: This can be tested in a number of ways, depending on the purpose of the exam. At a minimum, it is recommended that the anal wink reflex is tested and if no response is noted, the bulbocavernosus reflex is tested.
Anal wink: Perform the anal wink reflex by scratching the skin with a pin on both sides of the anus and observing whether there are subsequent contractions and whether the tone is normal or hyperactive, or stretch the anal sphincter with the fingertip inserted and feel for a reflex contraction.
Bulbocavernosus: Perform the bulbocavernosus reflex by placing the tip of the index finger in the anus and squeeze the penis or clitoris, or pull on pubic hairs, which can be done in the presence of an indwelling urethral catheter, to determine if there is a reflex anal sphincter contraction.
Anatomic diagnosis: Based upon whether the sacral reflexes listed above are an upper motor neuron (UMN) or lower motor neuron (LMN) lesion, and the NLI for motor and sensory function:
Supraconal: Supraconal, or suprasacral, lesions refer to lesions above the sacral spinal cord. Usually the sacral spinal cord is intact below the injury and will develop a UMN type of lesion with hyperactive sacral reflexes, allowing reflex activity for erection, bladder, or rectal contraction. More rarely, the lesion will spread to the sacral spinal cord below and create a LMN type of lesion with absent sacral reflexes.
Conal: Conus medullaris syndrome involves injury to the more rostral part of the spinal cord, most commonly related to a thoracolumbar bony injury. It typically manifests itself with a mixture of UMN and LMN symptoms.
Cauda equina: Cauda equina syndrome involves the lumbosacral nerve roots of the cauda equina and may spare the spinal cord itself. Injury to the nerve roots will produce a LMN type of lesion, with absent or diminished sacral reflexes, areflexic bowel and bladder, and absent reflexogenic erection.
Procedural Specifications: Sacral Function
General Recommendations
When performing the sacral ISAFSCI examination, it is important that there is privacy for the patient and the examiner.
Reflex Sacral Control
1. Results may be listed separately or in combined score to determine overall sacral function.
2. The sacral section of the ISAFSCI should be completed first, based upon the examiner’s best anticipated judgement of the patient’s remaining responses in light of the physical examination, recognizing that these responses can change over time.
Example: if an individual with a complete injury at the level of C5 reports control of bowel function despite the fact that they have no anal sensation (none in response to deep anal pressure* and no S4-5 sensation) and no voluntary anal sphincter control, then the examiner should still list the individual as having no voluntary control.
Example: if an individual with a complete injury at the level of C6 with an AIS A lesion and no sensation below the NLI has a suprapubic tube, the examiner should indicate the individual as having no voluntary control.
*It is important to test deep anal pressure and not rectal pressure. This is performed by pressing against the anal sphincter and not by performing a deep rectal examination as is traditionally done in general medicine.
-
3.
Subjective sacral responses should be recorded to reflect the reported abilities of the individual based only on the impact of SCI (i.e., not those of medications, etc.), with the recorded level of function to determine differences that can be used in the development of treatment strategies. If a patient is known to have preexisting arousal or orgasmic dysfunction, the proper response should be not or unable to be tested for these concerns.
-
4.
Sacral reflexes such as the anal wink and/or bulbocavernosus reflex should be tested.
Assessment of functioning at the T11-L2 spinal segment is important to determine the presence of psychogenic genital arousal. The more sparing of sensation in these areas, the more likely that psychogenic genital arousal will be present. Additionally for males, scrotal reflexes (cremaster or dartos reflexes) and testes sensation give additional information on specific T11-L2 spinal sympathetic functioning.
Determination of the sacral reflex status such as the anal wink71 and/or bulbocavernosus reflex72–74 is necessary to help define the type of neurologic lesion affecting the sacral spinal segments. Presence of hyperactive or asymmetric reflexes below the level of lesion is anticipated in an UMN injury or partial LMN injury, while the absence of both reflexes is expected in a complete LMN injury.
-
5.
Scoring for all sacral autonomic function conforms to the other ISAFSCI sections as:
2 = normal function – the same as prior to injury
1 = altered function – different from prior to injury
0 = absent function – no function
NT = Not or unable to be tested
Lower Urinary Tract
Lower urinary tract (LUT) function is controlled by neural circuits in the brain and spinal cord that coordinate the activity of visceral smooth muscle in the urinary bladder and urethra with activity of striated muscle in the external urethral sphincter (Figure 3).3,75 LUT function involves central pathways (supraspinal and spinal) and peripheral pathways (pelvic parasympathetic, lumbar sympathetic, and somatic pudendal nerve). Axons of Onuf ’s nucleus in sacral cord segments innervate the external urethral sphincter through the pudendal nerve. The bladder receives SNS innervation through hypogastric nerves and sacral PNS innervation through pelvic nerves. Afferent information from the LUT enters the spinal segments and synapses on interneurons that either make local segmental connections with motor pathways or send their axons to the brain. Ascending pathways connect with structures in the brainstem, including the pons and periaqueductal gray matter of the midbrain to execute reflex functions. In conjunction, with higher centers of the brain (cingulate and frontal gyri), these reflexes mediate storage and conscious perception of sensations arising from the LUT.3,76 Activation of sympathetic circuits through spinal reflexes mediates detrusor muscle relaxation and bladder neck contraction, resulting in storage of urine. Activation of sacral parasympathetic efferent neurons results in detrusor contraction, which promotes voiding.
Figure 3:
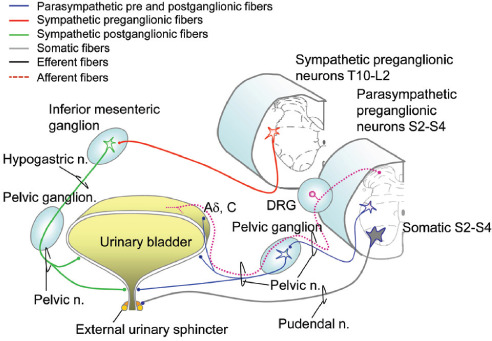
Innervation of the lower urinary tract. DRG: dorsal root ganglion; Aδ, C fibers: mechanosensitive afferents. (Modified with permission from Inskip, J. A., et al. Spinal Cord. 2009;47:2–35.)
The LUT receives the bulk of its innervation from three nerves: (1) the hypogastric nerve carries SNS innervation to the bladder and is responsible for urinary storage that is mediated by α-adrenergic receptors expressed in the trigone, bladder neck, and urethra (excitatory) and by β-adrenergic receptors expressed in the bladder dome (inhibitory); (2) the pelvic nerve contains PNS input originating in the sacral cord (S2–S4) and controls micturition via cholinergic muscarinic receptors; and (3) the pudendal nerve exits the sacral spinal cord and provides somatic innervation to the striated muscles of the external urethral sphincter.4
In addition to their efferent function, each of these nerves carries afferent input from the LUT via the dorsal root ganglion (DRG), and information about bladder distention is carried by mechanosensitive afferents (Aδ, C fibers) found primarily in the pelvic nerve. These afferents signal the coordinated switch between storage and micturition. The pudendal and hypogastric nerves are not depicted in Figure 3 but mostly contain nociceptive afferents.
Normal volitional voiding is achieved with voluntary relaxation of the external urethral sphincter. The LUT micturition reflex pathway has two modes of operation: storage and elimination. In infants these mechanisms function in a reflex manner to produce involuntary voiding. In adults, urine storage and elimination are subject to voluntary control as a result of connections between the forebrain and brainstem. SCI damages the spinal tracts involved in central control of the LUT, often leading to simultaneous activation of PNS neurons innervating the detrusor and somatic neurons innervating the external urethral sphincter. This conflicting abnormality causes varying degrees of loss of synergy or detrusor-sphincter dyssynergia.4
Current Definitions and Anticipated Level of Function: Lower Urinary Tract Function
Awareness of the need to empty the bladder is defined as a feeling in the lower abdomen that makes the person feel a need to empty their bladder. This is not to be confused with sweating or headache or other bodily symptoms that may occur with AD. Regarding anticipated anatomic responses, any NLI with normal sensation corresponding to T11-L2 and S3–S5 is scored as 2. NLI with preservation of partial sensation corresponding to T11-L2 and/or S3-S5 is a score of 1. Any NLI with no sacral sensation or motor function in the S3–S5 dermatomes is a score of 0.
The ability to prevent leakage is defined as the ability to voluntarily prevent voiding through external urethral sphincter contraction. Regarding anticipated anatomic responses, a score of 2 refers to any NLI with normal sensation corresponding to S3–S5. NLI with preservation of partial sensation corresponding to S3–S5 is a score of 1, and any NLI with no motor function corresponding to S3–S5 is a score of 0.
Procedural Specifications: Lower Urinary Tract Function
Equipment recommendations
No equipment is needed
Assessment recommendations
-
Awareness of the need to empty the bladder- the feeling in the lower abdomen that makes the person feel a need to empty their bladder, not to be confused with sweating or headache or other bodily symptoms that may occur with AD.
-
Subjective Responses: Ask the patient: “Do you have lower abdominal sensation of bladder filling?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
-
Ability to prevent leakage- the ability to voluntarily prevent voiding through external urethral sphincter contraction.
-
Subjective Responses: Ask the patient: “Can you hold your urine if your bladder is full and you need to urinate?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
Gastrointestinal Tract
Bowel function requires coordinated activity among the somatic, autonomic, and enteric nervous systems. Colonic peristalsis is coordinated by a network of neurons linking the brain to the colonic mucosa (Figure 4). The vagus nerve courses from the brainstem and innervates the gut down to the splenic flexure of the colon. The inferior splanchnic nerve carries pelvic parasympathetic fibers from S2–4 of the spinal cord to the distal bowel (splenic flexure, the left colon, and rectum). The enteric nervous system consists of two neuronal networks: Auerbach’s intramuscular myenteric plexus and Meissner’s submucosal plexus. The myenteric plexus with unmyelinated fibers and postganglionic parasympathetic cell bodies mainly coordinates motility, while the submucosal plexus relays sensory and local motor responses. The internal anal sphincter is a continuation of the circular muscle layer of the rectum under reflex control by the enteric nervous system and S2–4 spinal segments of the spinal cord. The external anal sphincter and pelvic floor are supplied by the mixed motor and sensory somatic pudendal nerve providing voluntary control. There is also evidence of sympathetic control of the external anal sphincter. The bowel functions of storage, stool propulsion, and defecation are dependent on coordinated control from the two components of the ANS: the enteric nervous system and voluntary motor control of the skeletal muscles of the pelvic floor and external anal sphincter.
Figure 4:
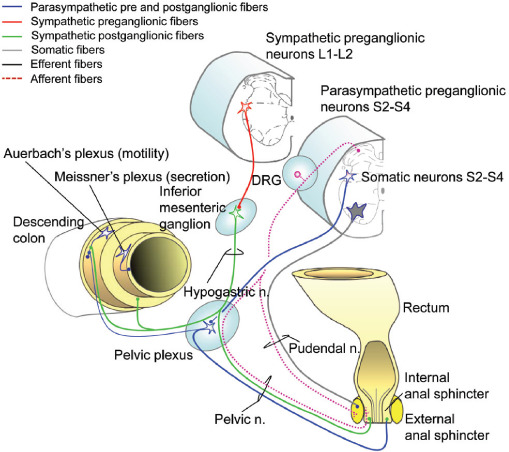
Innervation of the distal gastrointestinal track. (Modified with permission from Inskip, J. A., et al. Spinal Cord. 2009;47:2–35.)
Autonomic innervation of the gastrointestinal tract is required to modulate the intrinsic activity of the enteric nervous system. In the distal gastrointestinal tract as shown in Figure 4, the ANS coordinates storage and evacuation by regulating colon motility and anal sphincter tone. Parasympathetic innervation of the distal colon and rectum originates in the sacral cord (S2–S4), while the upper gastrointestinal tract (to the level of the splenic flexure) is innervated by the vagus nerve, which is not depicted in Figure 4. Postganglionic PNS neurons enhance smooth muscle activity (via acetylcholine). Sympathetic innervation originates from spinal segments T10-L1, which is mainly postganglionic, and inhibits muscle and secretory activity indirectly by noradrenergic modulation of activity in both Meissner’s and Auerbach’s plexuses.
The internal anal sphincter receives both SNS and PNS innervation, while the external anal sphincter is innervated by somatic fibers traveling in the pudendal nerve (S2–S4). Afferent information from this area travels in both the pelvic and pudendal nerves via the DRG.
Current Definitions and Anticipated Level of Function: Gastrointestinal Tract Function
Sensation of the need to empty the bowels is defined as a feeling in the lower abdomen that makes the person feel a need to move their bowels. This is not to be confused with sweating or headache which may occur with symptoms of autonomic dysreflexia. Regarding anticipated anatomic responses, any NLI with normal sensation corresponding to S3–S5 refers to a score of 2. Any NLI with partial preservation of sensation corresponding to S3–S5 scores as a 1 and injury with no sensation or motor function in the S3–S5 dermatomes scores as a 0. If the patient has a preexisting or current colostomy and or ileostomy, the answer will be NT for all questions.
Ability prevent leakage is defined as the ability to voluntarily prevent a bowel movement through anal sphincter contraction. Any NLI with normal sensation and normal motor function corresponding to S3–S5 refers to a score of 2 on the anticipated anatomic responses scale. Any NLI with partial preservation of sensation or motor function corresponding to S3–S5 scores as a 1 and no sensation or motor function corresponding to S3–S5 as a 0.
Procedural Specifications: Gastrointestinal Tract Function
Equipment recommendations
No equipment is needed
Assessment recommendations
-
Sensation of the need to empty the bowels: a feeling in the lower abdomen that makes the person feel a need to move their bowels, not to be confused with sweating or headache which may occur with symptoms of autonomic dysreflexia.
-
Subjective Responses: Ask the patient: “Do you have sensation in your abdominal and/or pelvic area of the need to move your bowels?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
-
Ability to prevent leakage: the ability to voluntarily prevent a bowel movement through anal sphincter contraction.
-
Subjective Responses: Ask the patient: “Can you hold your bowels if you feel the need to have a bowel movement?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
Genitalia and Reproductive Organs
Pelvic organs and sexual genitalia receive innervation from both components of the ANS as depicted for male (Figure 5). Similar to the LUT, sympathetic innervation is provided through the hypogastric nerve and parasympathetic innervation through the pelvic nerve.77 Psychogenic erection and vaginal lubrication are thought to be regulated by the SNS in conjunction with the PNS. In contrast, reflex erection and vaginal lubrication are thought to be activated by the PNS alone.3,78 Ejaculation is a neurologically more complicated phenomenon and relies on the coordination of the SNS (T11-L2) and PNS (S2–4) in addition to the somatic nervous system through the pudendal nerve (S2–5). Ejaculation and orgasm do not always coincide and neurologic control of orgasm is less well understood.79,80 However, studies in SCI suggest that an intact sacral reflex arc is necessary for orgasm to occur.3,79
Figure 5:
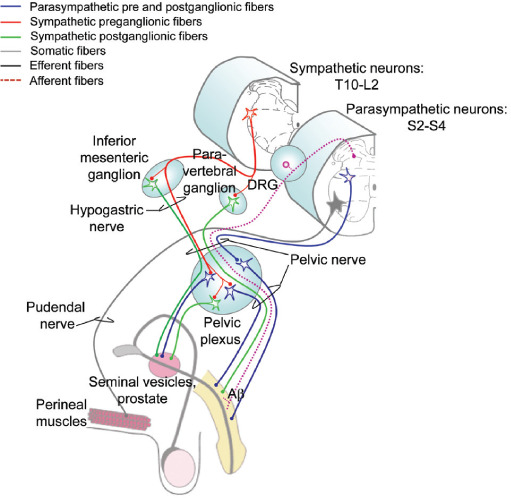
Innervation of the pelvic organs and male genitalia. DRG: dorsal root ganglion. (Modified with permission from Inskip, J. A., et al. Spinal Cord. 2009;47:2–35.)
Innervation of the Reproductive Organs and Genitalia
In the pelvic organs and genitalia there are three main tissue types: secretory, erectile, and striated muscle.77 The majority of the autonomic innervation to these tissues comes from the bilateral pelvic ganglia, which contains both sympathetic and parasympathetic neurons. Parasympathetic preganglionic neurons originate in the sacral cord (S2–S4) and travel in the pelvic nerve to the pelvic ganglia. Sympathetic innervation originates in the lower thoracic and lumbar cord (T10-L2) and travels via the hypogastric nerves to innervate the pelvic ganglia; sympathetic nerves also travel to the pelvic plexus via the pelvic nerve, which is mixed sympathetic and parasympathetic innervation. These plexuses form a diffuse neural network on either side of the prostate (males) or cervix (females). In both sexes, the largest nerve exiting from the pelvic plexus is the cavernous nerve (also called the penile nerve in males). For simplicity, only innervation of the seminal vesicles is illustrated here. Somatic innervation of the striated perineal muscles, which include the ischiocavernosus, bulbocavernosus, and levator ani, originates in the sacral cord (S2–4). Afferent information from the pelvic organs is relayed to the spinal cord via the genito-spinal nerves (pelvic, hypogastric, and pudendal; for simplification, only the pelvic nerve is illustrated here) and sensory pathways ascend bilaterally in the dorsal quadrant of the spinal cord.
Current Definitions and Anticipated Level of Function: Genitalia and Reproductive Organs
Psychogenic genital arousal is defined as signs of genital vasocongestion that occur from mental/visual sexual stimulation, most commonly known as erection in males and lubrication in females. Regarding anticipated anatomic responses, any NLI with normal sensation corresponding to T11-L2 refers to a score of 2. Any NLI with partial preservation of sensation corresponding to T11-L2 is a score of 1 and any NLI with no sensation or motor function corresponding to T11-L2 is a score of 0.
Reflex genital arousal is defined as signs of genital vasocongestion that occur from local stimulation in the perineal area, most commonly known as erection in males and lubrication in females. Any NLI with normal sensation or motor function and intact reflex function corresponding to S3–S5 refers to a score of 2 on the anticipated anatomic responses scale. Partial preservation of sensation or motor or reflex function corresponding to S3–S5 is score of 1, while no sensation or motor function and absent sacral reflexes corresponding to S3–S5 is a score of 0.
Orgasm is defined as an overall subjective sensation perceived as a climax in women and men. In men this sensation is often, but not always, accompanied by ejaculation, and in women multiple episodes of this sensation can occur. Regarding anticipated anatomic responses, intact sacral reflexes with normal sensation corresponding to S3–S5 is a score of 2. A score of 1 refers to either no sensation or motor function with preserved sacral reflexes corresponding to S3–S5 or intact S3–5 sensation or motor function with any degree of preserved sacral reflexes. No S3–S5 sensation or motor function and absent sacral reflexes corresponds to a score of 0.
Ejaculation is defined as an external propulsion of seminal fluid from the urethra which occurs through genital stimulation with or without vibratory stimulation. Any NLI with normal sensation and sacral reflexes corresponding to T11-L2 is a score of 2 on the anticipated anatomic responses scale. A score of 1 refers to any NLI with diminished sensation corresponding to T11-L2 with presence of sacral reflexes. It should be noted that dribbling ejaculation or minimal ejaculation is often a sign of retrograde ejaculation and this should be graded as a 1. Any NLI with abolished sensation corresponding to T11-L2 with absent sacral reflexes is a score of 0.
Procedural Specifications: Genitalia and Reproductive Organs Function
Equipment recommendations
No equipment is needed.
Assessment recommendations
-
Psychogenic genital arousal: signs of genital vasocongestion that occur from mental/visual sexual stimulation, most commonly known as erection in males and lubrication in females.
-
Subjective Responses: Ask the patient: “Do you have genital arousal, e.g., erections or lubrication when are psychologically sexually aroused?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
-
Reflex genital arousal: signs of genital vasocongestion that occur from local stimulation in the perineal area, most commonly known as erection in males and lubrication in females.
-
Subjective Responses: Ask the patient: “Do you have genital arousal, i.e., erections or lubrication, when your genitals are touched?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
-
Orgasm: an overall subjective sensation perceived as a climax in women and men. In men this sensation is often, but not always, accompanied by ejaculation, and in women multiple episodes of this sensation can occur.
-
Subjective Responses: Ask the patient: “Are you able to achieve orgasm with sexual stimulation?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
-
Ejaculation: external propulsion of seminal fluid from the urethra which occurs through genital stimulation with or without vibratory stimulation.
-
Subjective Responses: Ask the patient: “Are you able to achieve ejaculation with sexual stimulation?”
Score as: NT, 0, 1, 2 according recommendations (see Page 19)
-
For additional reference and data collection procedures, see the International Datasets at the following link: https://www.iscos.org.uk/international-sci-data-sets
Acknowledgment
We would like to thank the American Spinal Injury Association and the International Spinal Cord Society for their support of the Autonomic Standards Committee. Additionally, we would like to acknowledge Jennifer Coker, PHD, MPH for her roll in editing and reorganizing the manuscript and Caroline Miller, BS, for her role in the preparation of the manuscript.
References
- 1.Inskip J. A, Ramer L. M, Ramer M. S, Krassioukov A. V. Autonomic assessment of animals with spinal cord injury: tools, techniques and translation. Spinal Cord . 2009;47:2–35. doi: 10.1038/sc.2008.61. doi. [DOI] [PubMed] [Google Scholar]
- 2.Anderson K. D. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma . 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. doi. [DOI] [PubMed] [Google Scholar]
- 3.Alexander M. S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord . 2009;47:36–43. doi: 10.1038/sc.2008.121. doi. [DOI] [PubMed] [Google Scholar]
- 4.Krassioukov A, et al. International standards to document remaining autonomic function after spinal cord injury. J Spinal Cord Med . 2012;35:201–210. doi: 10.1179/1079026812Z.00000000053. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander M, Wecht J, Krassioukov A, International Autonomic Standards, C Pulse article: Survey on the current usage of the International Standards for the Assessment of Autonomic Function after Spinal Cord Injury (ISAFSCI) Spinal Cord Ser Cases . 2017;3:17100. doi: 10.1038/s41394-017-0025-8. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson R. A, et al. Inter-Rater Reliability of the International Standards to Document Remaining Autonomic Function after Spinal Cord Injury. J Neurotrauma . 2017;34:552–558. doi: 10.1089/neu.2016.4489. doi. [DOI] [PubMed] [Google Scholar]
- 7.Round A. M, et al. An evaluation of the International Standards to Document Remaining Autonomic Function after Spinal Cord Injury: input from the international community. Spinal Cord . 2017;55:198–203. doi: 10.1038/sc.2016.152. doi. [DOI] [PubMed] [Google Scholar]
- 8.Squair J. W, le Nobel G, Noonan V. K, Raina G, Krassioukov A. V. Assessment of clinical adherence to the international autonomic standards following spinal cord injury. Spinal Cord . 2015;53:668–672. doi: 10.1038/sc.2015.54. doi. [DOI] [PubMed] [Google Scholar]
- 9.Krassioukov A. V, Weaver L. C. Morphological changes in sympathetic preganglionic neurons after spinal cord injury in rats. Neuroscience . 1996;70:211–225. doi: 10.1016/0306-4522(95)00294-s. doi. [DOI] [PubMed] [Google Scholar]
- 10.Low PA S. D. Laboratory Evaluation of Autonomic Failure. In: Low PA, Benarroch EE, editors. Clinical Autonomic Disorders 3 ed . Baltimore: Lippincott Williams and Wilkins; 2003. [Google Scholar]
- 11.Wecht J. M, Bauman W. A. Implication of altered autonomic control for orthostatic tolerance in SCI. Auton Neurosci . 2018;209:51–58. doi: 10.1016/j.autneu.2017.04.004. doi. [DOI] [PubMed] [Google Scholar]
- 12.Biering-Sorensen F, et al. Alterations in cardiac autonomic control in spinal cord injury. Auton Neurosci . 2018;209:4–18. doi: 10.1016/j.autneu.2017.02.004. doi. [DOI] [PubMed] [Google Scholar]
- 13.Calaresu F. R, Yardley C. P. Medullary basal sympathetic tone. Annu Rev Physiol . 1988;50:511–524. doi: 10.1146/annurev.ph.50.030188.002455. doi. [DOI] [PubMed] [Google Scholar]
- 14.Coote J. H. Myths and realities of the cardiac vagus. J Physiol . 2013;591:4073–4085. doi: 10.1113/jphysiol.2013.257758. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claydon V. E, Krassioukov A. V. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma . 2006;23:1713–1725. doi: 10.1089/neu.2006.23.1713. doi. [DOI] [PubMed] [Google Scholar]
- 16.Draghici A. E, Taylor J. A. Baroreflex autonomic control in human spinal cord injury: Physiology, measurement, and potential alterations. Auton Neurosci . 2018;209:37–42. doi: 10.1016/j.autneu.2017.08.007. doi. [DOI] [PubMed] [Google Scholar]
- 17.Schmid A, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol (1985) 1998;85:635, 641. doi: 10.1152/jappl.1998.85.2.635. doi. [DOI] [PubMed] [Google Scholar]
- 18.Schmid A, et al. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. J Auton Nerv Syst . 1998;68:96–100. doi: 10.1016/s0165-1838(97)00127-6. [DOI] [PubMed] [Google Scholar]
- 19.Bartholdy K, et al. Cardiac arrhythmias the first month after acute traumatic spinal cord injury. J Spinal Cord Med . 2014;37:162–170. doi: 10.1179/2045772313Y.0000000181. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman R, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci . 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. doi. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons C. H, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology . 2006;67:28–32. doi: 10.1212/01.wnl.0000223828.28215.0b. doi 67/1/28[pii] [DOI] [PubMed] [Google Scholar]
- 22.Byun J. I, et al. Delayed orthostatic hypotension: Severity of clinical symptoms and response to medical treatment. Auton Neurosci . 2018;213:81–85. doi: 10.1016/j.autneu.2018.06.005. doi. [DOI] [PubMed] [Google Scholar]
- 23.Krassioukov A, et al. “The ABCs of AD”: A prospective evaluation of the efficacy of an educational intervention to increase knowledge of autonomic dysreflexia management among emergency health care professionals. J Spinal Cord Med . 2016;39:190–196. doi: 10.1179/2045772315Y.0000000037. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter M, et al. Prediction of autonomic dysreflexia during urodynamics: a prospective cohort study. BMC Med . 2018;16:53. doi: 10.1186/s12916-018-1040-8. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirshblum S. C, House J. G, O’Connor K, C. Silent autonomic dysreflexia during a routine bowel program in persons with traumatic spinal cord injury: a preliminary study. Arch Phys Med Rehabil . 2002;83:1774–1776. doi: 10.1053/apmr.2002.36070S0003999302006019. doi. [pii] [DOI] [PubMed] [Google Scholar]
- 26.Wan D, Krassioukov A. V. Life-threatening outcomes associated with autonomic dysreflexia: a clinical review. J Spinal Cord Med . 2014;37:2–10. doi: 10.1179/2045772313Y.0000000098. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaidyanathan S, Soni B. M, Mansour P, Oo T. Fatal collapse due to autonomic dysreflexia during manual self-evacuation of bowel in a tetraplegic patient living alone: lessons to learn. Int Med Case Rep J . 2017;10:361–365. doi: 10.2147/IMCRJ.S135586. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzeo F, Santamaria S, Iavarone A. “Boosting” in Paralympic athletes with spinal cord injury: doping without drugs. Funct Neurol . 2015;30:91–98. doi: 10.11138/fneur/2015.30.2.091. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blauwet C. A, et al. Testing for boosting at the Paralympic games: policies, results and future directions. Br J Sports Med . 2013;47:832–837. doi: 10.1136/bjsports-2012-092103. doi. [DOI] [PubMed] [Google Scholar]
- 30.Bhambhani Y, et al. Boosting in athletes with high-level spinal cord injury: knowledge, incidence and attitudes of athletes in paralympic sport. Disabil Rehabil . 2010;32:2172–2190. doi: 10.3109/09638288.2010.505678. doi. [DOI] [PubMed] [Google Scholar]
- 31.Gee C. M, Nightingale T. E, West C. R, Krassioukov A. V. Infographic. Doping without drugs: how para-athletes may self-harm to boost performance. Br J Sports Med . 2020 doi: 10.1136/bjsports-2020-101980. doi. [DOI] [PubMed] [Google Scholar]
- 32.Squair J. W, Phillips A. A, Currie K. D, Gee C, Krassioukov A. V. Autonomic testing for prediction of competition performance in Paralympic athletes. Scand J Med Sci Sports . 2018;28:311–318. doi: 10.1111/sms.12900. doi. [DOI] [PubMed] [Google Scholar]
- 33.Currie K. D, Krassioukov A. V. A walking disaster: a case of incomplete spinal cord injury with symptomatic orthostatic hypotension. Clin Auton Res . 2015;25:335–337. doi: 10.1007/s10286-015-0309-7. doi. [DOI] [PubMed] [Google Scholar]
- 34.Hubli M, Gee C. M, Krassioukov A. V. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens . 2015;28:173–181. doi: 10.1093/ajh/hpu122. doi. [DOI] [PubMed] [Google Scholar]
- 35.Hubli M, Krassioukov A. V. Ambulatory blood pressure monitoring in spinal cord injury: clinical practicability. J Neurotrauma . 2014;31:789–797. doi: 10.1089/neu.2013.3148. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzelnick C. G, et al. Blood Pressure Instability in Persons With SCI: Evidence From a 30-Day Home Monitoring Observation. Am J Hypertens . 2019;32:938–944. doi: 10.1093/ajh/hpz089. doi. [DOI] [PubMed] [Google Scholar]
- 37.Fealey R. D. Interoception and autonomic nervous system reflexes thermoregulation. Handb Clin Neurol . 2013;117:79–88. doi: 10.1016/B978-0-444-53491-0.00007-9. doi. [DOI] [PubMed] [Google Scholar]
- 38.Greaney J. L, Kenney W. L, Alexander L. M. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci . 2016;196:81–90. doi: 10.1016/j.autneu.2015.11.002. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison S. F. Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci . 2016;196:14–24. doi: 10.1016/j.autneu.2016.02.010. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison S. F, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) . 2011;16:74–104. doi: 10.2741/3677. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pallubinsky H, Schellen L, van Marken Lichtenbelt W. D. Exploring the human thermoneutral zone -A dynamic approach. J Therm Biol . 2019;79:199–208. doi: 10.1016/j.jtherbio.2018.12.014. doi. [DOI] [PubMed] [Google Scholar]
- 42.Johnson J. M, Minson C. T, Kellogg D. L., Jr Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol . 2014;4:33–89. doi: 10.1002/cphy.c130015. doi. [DOI] [PubMed] [Google Scholar]
- 43.Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol (1985) . 2010;109:1221–1228. doi: 10.1152/japplphysiol.00298.2010. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sessler D. I. Thermoregulatory defense mechanisms. Crit Care Med . 2009;37:S203–210. doi: 10.1097/CCM.0b013e3181aa5568. doi. [DOI] [PubMed] [Google Scholar]
- 45.Jurkovich G. J. Environmental cold-induced injury. Surg Clin North Am . 2007;87:247–267. doi: 10.1016/j.suc.2006.10.003. viii doi: [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y. Thermoregulation following Spinal Cord Injury: Theory and Fact. Med Sci Sports Exerc . 2019;51:2425. doi: 10.1249/MSS.0000000000002086. doi. [DOI] [PubMed] [Google Scholar]
- 47.Price M. J, Trbovich M. Thermoregulation following spinal cord injury. Handb Clin Neurol . 2018;157:799–820. doi: 10.1016/B978-0-444-64074-1.00050-1. doi. [DOI] [PubMed] [Google Scholar]
- 48.Wecht J. M, La Fountaine Michael F, Handrakis John P, West Christopher R, Phillips Aaron, Ditor David S, Sharif Hisham, Bauman William A, Krassioukov Andrei V. Autonomic Nervous System Dysfunction Following Spinal Cord Injury: Cardiovascular, Cerebrovascular, and Thermoregulatory Effects. Current Physical Medicine and Rehabilitation Reports . 2015;3:197–205. [Google Scholar]
- 49.Krassioukov A, Warburton D. E, Teasell R, Eng J. J. A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil . 2009;90:682–695. doi: 10.1016/j.apmr.2008.10.017. doi S0003-9993(09)00058-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan S, Plummer M, Martinez-Arizala A, Banovac K. Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med . 2007;30:27–30. doi: 10.1080/10790268.2007.11753910. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thijssen D. H, et al. The effects of thoracic and cervical spinal cord lesions on the circadian rhythm of core body temperature. Chronobiol Int . 2011;28:146–154. doi: 10.3109/07420528.2010.540364. doi. [DOI] [PubMed] [Google Scholar]
- 52.Granstein R.D, Luger T.A, editors. Neuroimmunology of the Skin . Springer; 2009. [Google Scholar]
- 53.Wong B. J, Hollowed C. G. Current concepts of active vasodilation in human skin. Temperature (Austin) . 2017;4:41–59. doi: 10.1080/23328940.2016.1200203. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson J. M, Kellogg D. L., Jr Thermoregulatory and thermal control in the human cutaneous circulation. Front Biosci (Schol Ed) 2010;2:825–853. doi: 10.2741/s105. doi. [DOI] [PubMed] [Google Scholar]
- 55.Trbovich M, et al. Correlation of neurological level and sweating level of injury in persons with spinal cord injury. J Spinal Cord Med . 2020:1–8. doi: 10.1080/10790268.2020.1751489. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guttmann L, Silver J, Wyndham C. H. Thermoregulation in spinal man. J Physiol . 1958;142:406–419. doi: 10.1113/jphysiol.1958.sp006026. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West C. R, Alyahya A, Laher I, Krassioukov A. Peripheral vascular function in spinal cord injury: a systematic review. Spinal Cord . 2013;51:10–19. doi: 10.1038/sc.2012.136. doi. [DOI] [PubMed] [Google Scholar]
- 58.Price M. J, Campbell I. G. Effects of spinal cord lesion level upon thermoregulation during exercise in the heat. Med Sci Sports Exerc . 2003;35:1100–1107. doi: 10.1249/01.MSS.0000074655.76321.D7. doi. [DOI] [PubMed] [Google Scholar]
- 59.Trbovich M, Ortega C, Schroeder J, Fredrickson M. Effect of a cooling vest on core temperature in athletes with and without spinal cord injury. Top Spinal Cord Inj Rehabil . 2014;20:70–80. doi: 10.1310/sci2001-70. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Illigens B. M, Gibbons C. H. Sweat testing to evaluate autonomic function. Clin Auton Res . 2009;19:79–87. doi: 10.1007/s10286-008-0506-8. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dicpinigaitis P. V, Spungen A. M, Bauman W. A, Absgarten A, Almenoff P. L. Bronchial hyperresponsiveness after cervical spinal cord injury. Chest . 1994;105:1073–1076. doi: 10.1378/chest.105.4.1073. doi. [DOI] [PubMed] [Google Scholar]
- 62.Schilero G. J, Spungen A. M, Bauman W. A, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol . 2009;166:129–141. doi: 10.1016/j.resp.2009.04.002. doi. [DOI] [PubMed] [Google Scholar]
- 63.Biering-Sorensen F, et al. International spinal cord injury pulmonary function basic data set. Spinal Cord . 2012;50:418–421. doi: 10.1038/sc.2011.183. doi. [DOI] [PubMed] [Google Scholar]
- 64.Squair J. W, et al. Sleep-disordered breathing is associated with brain vascular reactivity in spinal cord injury. Neurology . 2019;93:e2181–e2191. doi: 10.1212/WNL.0000000000008619. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zakrasek E. C, et al. Pulmonary outcomes following specialized respiratory management for acute cervical spinal cord injury: a retrospective analysis. Spinal Cord . 2017;55:559–565. doi: 10.1038/sc.2017.10. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiodo A. E, Sitrin R. G, Bauman K. A. Sleep disordered breathing in spinal cord injury: A systematic review. J Spinal Cord Med . 2016;39:374–382. doi: 10.1080/10790268.2015.1126449. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller M. R, et al. Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. doi. [DOI] [PubMed] [Google Scholar]
- 68.Graham B. L, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelley A, et al. Spirometry testing standards in spinal cord injury. Chest . 2003;123:725–730. doi: 10.1378/chest.123.3.725. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cosortium for Spinal Cord, M. Respiratory management following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med . 2005;28:259–293. doi: 10.1080/10790268.2005.11753821. doi. [DOI] [PubMed] [Google Scholar]
- 71.Kirshblum S, Eren F. Anal reflex versus bulbocavernosus reflex in evaluation of patients with spinal cord injury. Spinal Cord Ser Cases . 2020;6:2. doi: 10.1038/s41394-019-0251-3. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Previnaire J. G, Alexander M. The sacral exam-what is needed to best care for our patients? Spinal Cord Ser Cases . 2020;6:3. doi: 10.1038/s41394-019-0252-2. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Previnaire J. G. The importance of the bulbocavernosus reflex. Spinal Cord Ser Cases . 2018;4:2. doi: 10.1038/s41394-017-0012-0. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graves D. E. Bulbocavernosus or anal reflex, one or both should be tested after spinal cord injury. Spinal Cord Ser Cases . 2020;6:4. doi: 10.1038/s41394-019-0253-1. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshimura N, de Groat W. C. Neural control of the lower urinary tract. Int J Urol . 1997;4:111–125. doi: 10.1111/j.1442-2042.1997.tb00156.x. doi. [DOI] [PubMed] [Google Scholar]
- 76.Mehnert U, et al. Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve--an fMRI study in healthy women. Neuroimage . 2008;41:682–689. doi: 10.1016/j.neuroimage.2008.03.006. doi. [DOI] [PubMed] [Google Scholar]
- 77.Krassioukov A, Elliott S. Neural Control and Physiology of Sexual Function: Effect of Spinal Cord Injury. Top Spinal Cord Inj Rehabil . 2017;23:1–10. doi: 10.1310/sci2301-1. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alwaal A, Breyer B. N, Lue T. F. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril . 2015;104:1051–1060. doi: 10.1016/j.fertnstert.2015.08.033. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sipski M, Alexander C. J, Gomez-Marin O. Effects of level and degree of spinal cord injury on male orgasm. Spinal Cord . 2006;44:798–804. doi: 10.1038/sj.sc.3101954. doi. [DOI] [PubMed] [Google Scholar]
- 80.Elliott S. Ejaculation and Orgasm: Sexuality in Men with SCI. Topics in Spinal Cord Injury Rehabilitation . 2002;8:1–15. [Google Scholar]


