Abstract
Brain-computer interface and neuromodulation strategies relying on penetrating non-organic electrodes/optrodes are limited by an inflammatory foreign body response that ultimately diminishes performance. A novel “biohybrid” strategy is advanced, whereby living neurons, biomaterials, and microelectrode/optical technology are used together to provide a biologically-based vehicle to probe and modulate nervous-system activity. Microtissue engineering techniques are employed to create axon-based “living electrodes”, which are columnar microstructures comprised of neuronal population(s) projecting long axonal tracts within the lumen of a hydrogel designed to chaperone delivery into the brain. Upon microinjection, the axonal segment penetrates to prescribed depth for synaptic integration with local host neurons, with the perikaryal segment remaining externalized below conforming electrical-optical arrays. In this paradigm, only the biological component ultimately remains in the brain, potentially attenuating a chronic foreign-body response. Axon-based living electrodes are constructed using multiple neuronal subtypes, each with differential capacity to stimulate, inhibit, and/or modulate neural circuitry based on specificity uniquely afforded by synaptic integration, yet ultimately computer controlled by optical/electrical components on the brain surface. Current efforts are assessing the efficacy of this biohybrid interface for targeted, synaptic-based neuromodulation, and the specificity, spatial density and long-term fidelity versus conventional microelectronic or optical substrates alone.
Keywords: biologically-mediated neuromodulation, brain–computer interfaces, living scaffolds, microtissue engineering, tissue engineering
1. Introduction
Brain–computer interface and neuromodulation devices provide a means to record and stimulate the nervous system to mitigate neurological deficits and/or provide a communication platform to drive peripheral devices/prosthetics. Indeed, future initiatives to treat and correct myriad neurological conditions rely on a precise interface with the nervous system for optimal monitoring and modulation. There has been substantial progress using penetrating, inorganic microelectrode arrays and optically based methods to record and stimulate from the central nervous system (CNS). However, conventional microelectrodes produce a chronic foreign body response with concomitant signal degradation over time. Moreover, electrical stimulation and recording currently lack specificity in targeting specific neuronal subtypes (e.g., excitatory versus inhibitory) and/or compartments (e.g., dendritic/somatic versus axonal). Although optogenetics methods can be highly specific, this approach currently requires a viral injection that must diffuse throughout a volume of brain tissue. Also, light can only penetrate a certain depth into tissue, thus limiting the potential range of stimulation. Like microelectrodes, penetrating optical waveguide fibers also result in inflammation and a chronic foreign body response. As such, there is currently a need for a chronically stable and highly specific modality for input to and output from the CNS.
Over the last several years, we have developed microtissue engineered neural networks (micro-TENNs), which are implantable, three-dimensional (3D), anatomically-inspired constructs that replicate the general systems-level anatomy of the nervous system: functionally similar groups of neurons connected by long-spanning axonal tracts.[1–5] Specifically, micro-TENNs are precisely formed, miniature constructs composed of discrete neuronal population(s) connected by long axonal tracts within hydrogel microcolumns, which, to date, have been fabricated using dorsal root ganglia neurons, cerebral cortical neurons (e.g., mixed glutamatergic and GABAergic), and ventral mesencephalic neurons (e.g., dopaminergic).[1–5] Although initially developed to reconstitute degenerated axonal pathways in the brain,[1,5] we have recently been applying the micro-TENN platform as a biologically-based “living electrode” technology to modulate neural circuits.[6] In this radical approach for a neural interface, non-organic components reside on the cortical surface with organic components (i.e., living axon tracts) penetrating the brain. The goal of this interface is to provide high fidelity connectivity via synaptic integration with endogenous neural networks to allow biologically based neuromodulation while mitigating the chronic foreign body response that currently limits conventional penetrating electrodes (Figure 1). If successful, this biohybrid neural interface strategy could open the door for an entirely new platform for the controlled modulation of neural activity to treat neurological disease and injury.
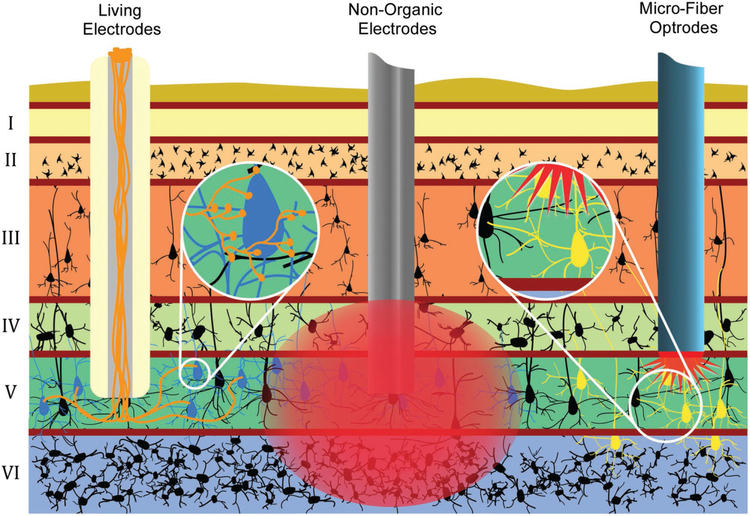
Figure 1.
Advantages of Axon-Based “Living Electrodes” for Neuromodulation: Mechanisms and specificity of neuronal stimulation for “living electrodes” (left) versus conventional electrodes (center) and optrodes (right). Living electrodes provide engineered axonal tracts, fully differentiated neurons, and a controlled 3D cytoarchitecture, potentially improving survival versus delivery of cell suspensions. Construct neurons may be transfected to express opsins in vitro (days prior to implant), thereby avoiding the injection of virus directly into the host while constraining the spatial extent of transfected cells. Living electrodes could offer high specificity, as the constructs can be designed to synapse with specific neuronal subtypes in a given anatomical region (as shown by living electrode axons synapsing with only blue neurons, not black) as opposed to conventional electrodes that inherently stimulate or record from a relatively large 3D volume around the electrode (as shown by large red area of stimulation affecting many layers and neurons). While optrodes can achieve a high level of specificity, the in vivo delivery of opsins generally relies on injection of virus that may diffuse and affect non-target regions (spread of optogenetic transduction is illustrated by yellow neurons in multiple layers). Also, optical methods may have a limited extent due to tissue absorption of light. Finally, living electrodes provide a soft pathway to route signals to/from deep brain structures compared to rigid materials used in electrodes/optrodes, thus potentially minimizing signal loss due to mechanical mismatch/micromotion and glial scarring.
In this article, we outline existing approaches to modulate the CNS and present these in contrast to our living electrode approach, linking preliminary studies to outstanding clinical challenges and mapping a path forward. Specifically, we focus on the capability of axon-based living electrodes to provide targeted, synaptic-based modulation of neuronal circuitry (although their capability to transmit information to the brain surface—in essence a form of “recording”—is briefly considered). For the purposes of this article, we consider the delivery of electricity, light, or chemicals to specific anatomical targets in the brain all as forms of stimulation or modulation. In this sense, we here define neuromodulation as “the intentional modification of the electrophysiological activity of neurons within well-defined anatomical targets within the brain, in order to ameliorate aberrant activity in that target region and compensate for disease and injury in other areas, bias existing endogenous diffuse modulatory systems, or forge alternate connectivity patterns.” We further specify that biologically-mediated neuromodulation refers to approaches that deploy constructs built of living cells to interface and modulate brain activity. As the spatial and temporal scale of such modulation is refined, and as the connectivity becomes more constrained (less divergent: from one-to-many to one-to-one), the modulation can achieve a far more specific effect, and can ultimately input information (such as relaying a receptive field) rather than merely biasing diffuse tone.
2. Overview of Axon-Based “Living Electrodes”
As a component of a biohybrid neural interface, our current-generation living electrodes consist of a precisely formed columnar biomaterial encasement with the internal lumen functionalized via the presence of anatomically constrained living axonal tracts (Figure 2),[5,7] Building on our previous work, we have recently devised methodology to create long-projecting unidirectional axon-based living electrodes for tailored neuromodulation. These consist of excitatory living electrodes built using neurons derived from the cerebral cortex (predominantly glutamatergic), dopaminergic living electrodes built using neurons isolated from the ventral mesencephalon (enriched in dopaminergic neurons), and most recently, inhibitory living electrodes built using neurons isolated from the medial ganglionic eminence (source of GABAergic neurons) (Figure 2). These axon-based living electrode constructs are on the order of several hundred microns in diameter—similar to the diameter of a human hair—yet may extend at least on the order of centimeters to reach deep layers/nuclei in the brain with a relatively small microinjection footprint (Figure 3).[3,4] As such, these engineered living electrodes can be considered a type of composite functionalized biomaterial on multiple levels: 1) the characteristics of the hydrogel microcolumn and extracellular matrix constituents require optimization for each neuronal subtype used to allow for health and long-projecting axonal outgrowth within the lumen prior to implantation; 2) the neuronal-biomaterial encasement scheme allows for controlled functional versatility via the choice of neuron subtype (i.e., to get different neurotransmitters, and hence different excitatory/inhibitory/modulatory effects) and localized drug delivery to foment various implant-host interactions (e.g., pro-survival, controlled outgrowth/plasticity); 3) the protective hydrogel encasement precisely delivers the fundamental integrative units—growth cones from living axonal tracts—to a prescribed location of the brain where they are intrinsically programmed to synaptically integrate with a specific local subpopulation based on phenotype(s) of source axons and target neurons. Indeed, we have previously demonstrated that preformed micro-TENNs may be stereotaxically microinjected into the brain, where they exhibited neuronal survival, maintenance of axonal architecture, and perhaps most importantly, evidence of synaptic integration with host neurons.[1,5] As such, these living axon-based microconstructs may be useful as the biological component of a biohybrid neural-electrical-optical interface, exploiting synaptic integration for target specificity while potentially mitigating biocompatibility and biostability limitations described in other approaches (Figure 3). Although beyond the scope of this article, custom planar optical/electrical arrays are being developed to couple with our axon-based living electrodes on the brain surface, and there has also been previously published technology that may be useful in this regard.[8–13]
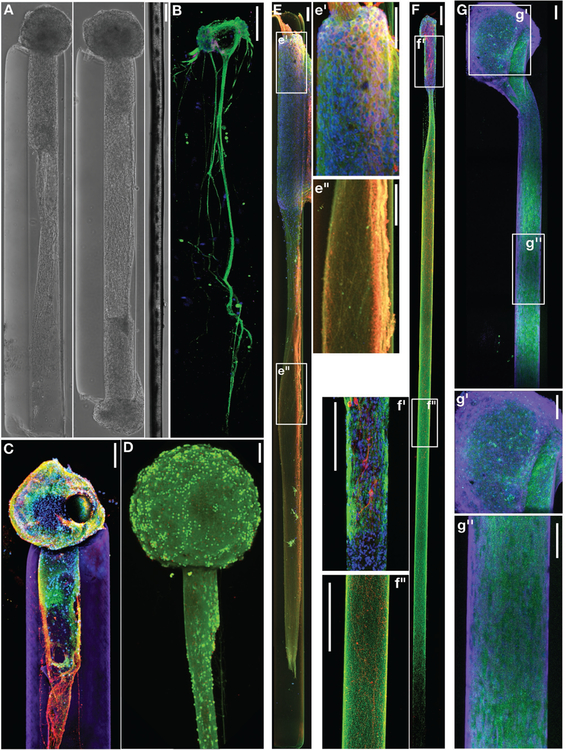
Figure 2.
Neuronal-Axonal Living Electrodes: (A) Phase contrast images of unidirectional (left) and bidirectional (middle) “living electrodes” built using cerebral cortical neurons, each at 5 days in vitro (DIV), next to a single human hair (right). (B) Confocal reconstruction of a living electrode built using dorsal root ganglia neurons showing unidirectional axonal tracts immunolabeled to denote neuronal somata (MAP-2; purple) and axons (tau; green), with nuclear counterstain (blue). (C) Confocal reconstruction of a unidirectional, cerebral cortical neuronal living electrode at 11 DIV, immunolabeled for axons (β-tubulin-III; red) and synapses (synapsin; green), with a nuclear counterstain (Hoechst; blue). The surrounding hydrogel micro-column is shown in purple. (D) Confocal reconstruction of a unidirectional cortical neuronal living electrode stained for viability at 10 DIV (green: live cells via calcein-AM; red: nuclei of dead cells via ethidium homodimer-1). Scale bars A-D: 100 μm. (E-G) Long-projecting unidirectional axon-based living electrodes for tailored neuromodulation. (E) Confocal reconstruction of an excitatory living electrode built using neurons derived from the cerebral cortex (predominantly glutamatergic), immunolabeled at 28 DIV for axons (β-tubulin-III; red) and neuronal somata/dendrites (MAP-2; green), with nuclear counterstain (Hoechst; blue). Insets of the aggregate (e’) and axonal (e”) regions are outlined and shown to the right. Scale bars: 100 μm. (F) Confocal reconstruction of a dopaminergic living electrode built using neurons isolated from the ventral mesencephalon (enriched in dopaminergic neurons), immunolabeled at 28 DIV for axons (β-tubulin-III; green) and tyrosine hydroxylase (dopaminergic neurons/axons; red), with nuclear counterstain (Hoechst; blue). Insets of the aggregate (f’) and axonal (f”) regions are outlined and shown to the left. Scale bars: 250 μm. (G) Confocal reconstruction of an inhibitory living electrode built using neurons isolated from the medial ganglionic eminence (source of GABAergic neurons), immunolabeled at 14 DIV for axons (β-tubulin-III; purple) and GABA (inhibitory neurons/axons; green), with nuclear counterstain (Hoechst; blue). Insets of the aggregate (g’) and axonal (g”) regions are outlined and shown below. Scale bars: 100 μm.
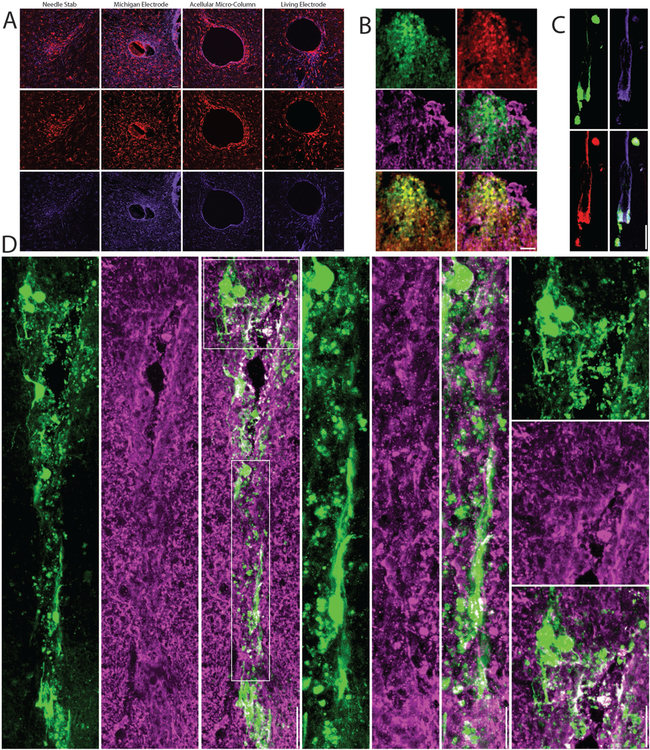
Figure 3.
Neuronal Survival, Synaptic Integration, and Host Response Following Living Electrode Implantation In Vivo: (A) Host response to living electrodes versus conventional microelectrodes. Representative confocal micrographs at 1-month post-implant of brain sections orthogonal to a needle stab (negative control), a Michigan microelectrode (positive control), acellular hydrogel micro-column, or a living electrode (hydrogel micro-column encasing neurons + axonal tracts) immunolabeled for microglia/macrophages (IBA-1; red) and astrocytes (GFAP; purple). Peri-electrode host reactivity was reduced around living electrodes, even though current-generation living electrodes have a larger footprint than Michigan microelectrodes. (B-D) Confocal reconstructions showing survival and integration of living electrode neurons/axons at 1-week or 1-month post-implant. (B) Superficial (dorsal) living electrode neurons on the brain surface transduced to express GFP (on the synapsin promoter; green) and immunolabeled for the neuronal marker NeuN (red) and the synaptic marker synapsin (purple) with various dual- and tri-channel combinations. (C) Living electrode neurons and aligned axons (GFP+) within the lumen of the micro-column stained to identify neuronal somata and dendrites (MAP-2; red) and axons (β-tubulin-III; purple). (D) Neurons and neurites projecting in the cerebral cortex from the deep end of the living electrode, with callout boxes showing putative synapses (synapsin+ puncta; purple) between host and living electrode neurons/neurites (GFP+). Scale bars: 50 μm.
2.1. Theoretical Advantages of Axon-Based “Living Electrodes”
Axon-based living electrodes have the potential to exploit biological mechanisms-of-action to achieve an unparalleled combination of specificity, spatial density, and long-term fidelity in neural stimulation (Figure 4). The biologically-mediated neuromodulation theoretically attained by axon-based living electrodes offers the following attributes: 1) Target specificity and synaptic integration: based on intrinsic programming, implanted axons should preferentially integrate with specific neuronal subtype(s) and form synapses, which are the natural vehicle for inter-neuron communication and offer nuanced inputs not possible with standard approaches; 2) High spatial density of inputs via biological multiplexing: hundreds to thousands of synapses are possible per implanted axon, thus a robust effect may be elicited by relatively few axons; 3) Long-term stability/tolerance: as the columnar hydrogel encasement is gradually resorbed, only living axonal tracts remain that by then would have integrated with host neurons via synapses, which theoretically can last the lifetime of an organism; these biological components are far less likely to evoke a chronic foreign body response than non-organic electrodes. Although micro-TENNs are currently created from allogeneic neurons that have yet to evoke an immune response, the more likely choice for clinical deployment are autologous cells, such as patient-specific neurons derived from induced pluripotent stem cells (iPSCs), because implanted “host” axons are even less likely to provoke an immune response.[14–16] Moreover, in ongoing efforts we are employing computational modeling to further our understanding of specificity, biological multiplexing, and stability as related to the living electrodes, and these functional simulations also serve as a platform for the design and optimization of living electrodes in the future. In principle, the living electrode strategy addresses key challenges in the neural interface field, although these putative advantages need to be validated experimentally in comparison to conventional approaches.
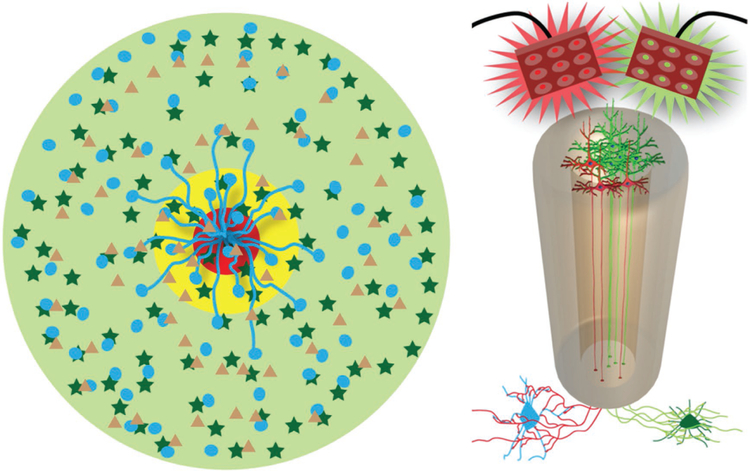
Figure 4.
Mechanisms-of-Action for Axon-Based Living Electrodes: Synaptic Specificity, Biological Multiplexing, and Stability. “Living electrodes” may offer high specificity, as the constructs can be designed to synapse with specific neuronal subtypes, as demonstrated conceptually by living electrode axons synapsing with only circle neurons, not star neurons (left cartoon). This may be exploited in mixed neuron living electrodes where a subpopulation (blue cells) is excited with red light while another subpopulation (dark green cells) could be inhibited by green light (right cartoon). Multiplexing: one living electrode axon can (in theory) synapse with hundreds to thousands of host neurons – creating a significant amplification effect. We currently build living electrodes with 5000–50 000 neurons within a column less than twice the diameter of a human hair. Moreover, living electrodes may offer stability as synaptic integration offers permanence not possible with standard approaches while the biological nature of the constructs may mitigate the chronic foreign body response.
Although explored in detail later in this article, applications of axon-based living electrodes include nuanced control of specific facets of a neural circuit-of-interest, for instance increasing synaptic input to directly strengthen/augment a pathway (inhibitory or excitatory), or even indirect inhibition via excitation of inhibitory neurons. Also, living electrodes may provide highly localized delivery of modulatory neurotransmitters, for instance reward/arousal circuitry using dopamine or other modulatory neurotransmitters. As specific examples, our existing repertoire of living electrodes provide an opportunity to target specific neural circuitry: motor control in Parkinson’s Disease (using dopaminergic neurons),[3] sensory input/feedback to deep nuclei (using excitatory neurons), inputs to visual cortex for visual prosthetics (using excitatory neurons), learning and memory (using hippocampal neurons), and inhibition of seizure foci (using inhibitory neurons) (Figure 5). First, we will put our living electrode strategy in context with existing approaches for brain-machine interface and neuromodulation.
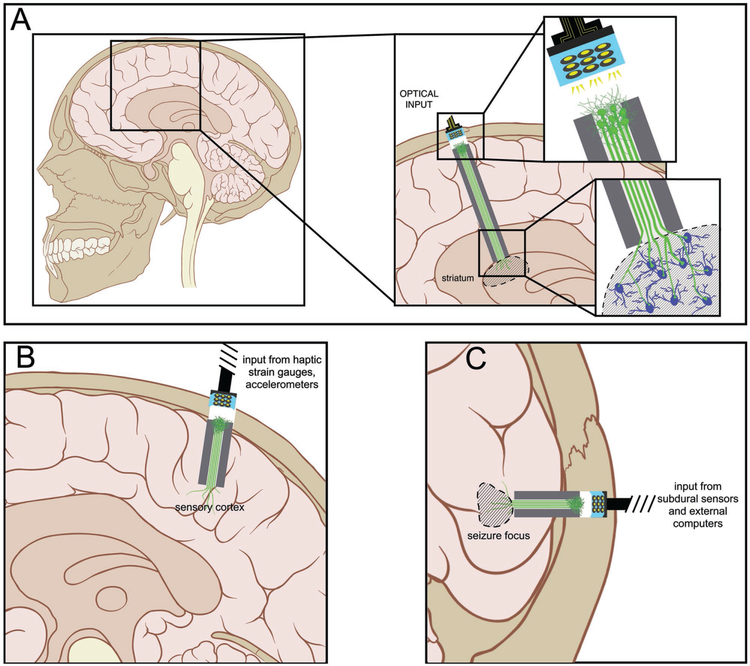
Figure 5.
Potential Applications of Axon-Based Living Electrodes: Custom engineered living electrodes consisting of a phenotypically-controlled populations of neurons extending long axonal tracts through a biocompatible micro-column may be stereotactically transplanted to span various regions to treat particular disease processes. (A) Axons projecting from dopaminergic living electrodes may form synapses within local striatal architecture, and, due to in vitro functionalization with channelrhodopsins, may release dopamine upon optical stimulation of the perikaryal segment at the brain surface. This mimics the substantia nigra pars compacta input to the striatum in a manner that can be externally controlled. (B) Axons from glutamatergic living electrodes may preferentially synapse onto layer IV neurons within primary sensory cortex to convey illusory haptic feedback via surface optical stimulation to achieve closed-loop control of neuromotor prosthetics in patients with paralysis. (C) Axons from GABAergic living electrodes could be implanted to oppose seizure foci such that optical stimulation would cause net suppression of seizure activity in patients with lesional epilepsy.
2.2. Overview of Existing Approaches
Several prominent neural interface strategies have been developed to modulate nervous system activity, toward the goal of mitigating deficits associated with neurological injury and/or improving our understanding of CNS function. These techniques include non-invasive electromagnetic stimulation (such as repetitive transcranial magnetic stimulation,[17,18] and transcranial current stimulation,[17,19] electrical macrostimulation using low-impedance electrodes at the cortical surface or in deep brain structures,[20–24] electrical microstimulation using high-impedance microelectrodes implanted into the cortex or deeper via microwire,[25–28] microfluidic approaches that deliver chemicals from a reservoir to be infused at targeted areas,[29–31] and optogenetic approaches that leverage light delivered by waveguides to neural tissue that has been genetically transformed to respond to light.[32,33] Beyond light, neurons can be genetically engineered to respond to ultrasound, magnetic fields and other stimuli; each such approach would require a device to deliver the stimuli into the brain.[34,20] The two most successful neurotechnologies to date have been cochlear implants to restore auditory perception following sensorineural hearing loss, and deep brain stimulation (DBS) to treat movement disorders. More recently, DBS has been used successfully to arrest seizures in patients with epilepsy, and to improve certain types of medically refractory depression.[35,36] While cochlear implants (and auditory brainstem implants used when cochlear stimulation is compromised by afferent dysfunction in neurofibromatosis) operate by “playing” the tonotopy of the spiral ganglion (or cochlear nuclei in the medulla), the mechanism by which DBS quenches tremors, dystonias, seizures and other aberrant activity has not been fully elucidated.[37]
The dominant modalities of neuromodulation are electrical stimulation (the mechanism utilized in cochlear implants and DBS) and, more recently, optogenetic methods. Electrical stimulation can nonspecifically activate a large population of cells while optogenetic methods allow for more spatially selective stimulation via genetic transduction with opsins and activation using optical fibers. Each current technology has advantages and inherent limitations in stability, selectivity, or spatial density that limit usage for large-scale network integration. Electrical microstimulation with microelectrode interfaces suffer from a lack of specificity: Even in the best case scenario, electrical micro-stimulation is spatially non-specific, given that current alters the potential of a large volume of tissue, changing the membrane potential of neurons/dendritic fields adjacent to the electrodes (excitatory as well as inhibitory interneurons) as well as axonal fibers of passage.[38,39] The specificity can deteriorate further with neuronal loss in the vicinity of the electrodes or electrode movement.[40] Gliosis can lead to increasing impedance requiring increased current levels,[41,42] thus leading to more frequent battery changes and the risk of diathermy as higher currents are needed to achieve adequate electrophysiological stimulation levels.[43] Chronic electrical stimulation appears to accelerate the degradation of microelectrode arrays in a manner that both reduces their functionality to stimulate and record, and poses risks to the patient as device materials break down into the brain and circulate in physiological fluid.[44] Optogenetic strategies have the distinct disadvantage of needing to deliver (e.g., microinject) viruses into the brain to transduce neurons to express opsins; however, with the use of phenotype-specific expression promoters this can be highly specific. Gliosis and loss of neurons in the vicinity of micro-fiber optical probes would also detrimentally affect optical stimulation, although less is understood about these potential effects.
2.2.1. Existing Approaches: Invasive Electrical Stimulation
DBS is a clinically established and approved treatment for essential tremor, dystonia and Parkinson’s disease.[45] DBS may be considered an electrical macrostimulation approach in which current is passed across two or more low-impedance macro-electrode (generally 1–2 mm in diameter) contacts in a target nucleus within the brain. Despites its approval, use, and efficacy over the past several decades, the exact mechanism by which DBS achieves its therapeutic benefit remains controversial. While electrical macrostimulation oriented in parallel with the long axis of a neuronal soma and axon tends to depolarize a neuron and increase firing rate, the effects of electrical stimulation within the brain may be far more complex based on the position and geometry of the electrode, neighboring neurons, and fibers of passage. Whether a given electrical stimulation event tends to depolarize and hence increase the firing rate of target neurons, hyperpolarize and hence decrease the firing rate of target neurons, affect excitatory or inhibitory neurons, or simply disrupt the fine timing of neural activity (hence “releasing” circuits from pathologic hypersynchronous resonant activity) is not well understood.[37] Although DBS has become a key treatment option for certain types of essential tremor and Parkinson’s disease, it has proven unexpectedly difficult to treat other conditions with this technology. Except for the recently approved application of DBS for certain types of medically refractory epilepsy (either open-loop, e.g., anterior nucleus of the thalamus;[35] or closed-loop, e.g., Neuropace),[46] and despite numerous small trials in human patients with chronic pain syndromes, refractory depression, refractory obsessive-compulsive disorder, and Alzheimer’s disease, DBS has not been shown to be consistently effective for these conditions or numerous other neurological conditions.[47–50] Part of the success of DBS for certain movement disorders likely relates to the extremely stereotyped anatomy and pathophysiology of these conditions such that an electrical “reversible lesion” in the globus pallidus pars internus or subthalamic nucleus can normalize activity in well-characterized basal ganglia-corticothalamic circuits; other conditions may not offer this discrete anatomical simplicity in targeting and thus may require technologies that better take into account global network activity and are able to target specific neuronal types within target areas.
While DBS deploys larger, low-impedance macro-electrodes, electrical micro-stimulation can be achieved by using microwires or machined arrays of rigid microelectrodes. These microstimulation approaches can precisely target an anatomic site with greater spatial specificity and more controlled current spread than macro-DBS; however, like all electrical stimulation in the brain, there is no way to target particular neurons and exclude others, or avoid modulating non-target fibers of passage. Electrical microstimulation has attracted interest as a mode to provide sensory feedback in patients implanted with neuromotor prosthetic systems to restore movement following paralysis from spinal cord injury, amyotrophic lateral sclerosis or brainstem stroke.[51–56] In this setting, electrical microstimulation of sensory cortex could provide somatosensory haptic feedback from robotic arms or peripheral nerve recordings, to allow the paralyzed patient to “feel” the position of the device and the texture and sensation of objects the effector would contact.[57–59] A major confound of both DBS and microstimulation approaches is that the stimulation itself introduces a large artifact that precludes simultaneous recording and this in turn limits the fidelity of closed-loop systems modulating a target brain area.
2.2.2. Existing Approaches: Optogenetics
Optogenetics comprises an approach in which a targeted set of cells, such as neurons of a particular phenotype, are transduced with viruses (usually an adeno-associated virus or a lentivirus) to express photosensitive ion channels (i.e., opsins) or G-protein coupled receptor components.[33,60–64] Microbe-derived proteins can be tailored to respond to particular wavelengths of light and can be coupled to different types of channels with permeability to different ions and different membrane kinetic properties to achieve unprecedented specificity in achieving targeted effects, either inhibition or excitation. However, simply rendering target neurons photosensitive will not suffice: light must be delivered to the tissue. In vivo, this is usually done with implantable waveguides that deliver light generated by an external laser. There are also approaches in which other sets of neurons are rendered capable of luminescing, and hence they can optically stimulate optogentically-modified neighbors.[62,65] Unlike electrical macro- and microstimulation, where thousands to millions of neurons are activated, optogenetic approaches can selectively modulate the activity of single neurons or even single neurites on a target neuron. This spatial selectivity is both the strength and weakness of optogenetic approaches compared to electrical stimulation approaches: the same extraordinary specificity in spatial precision also renders the approach vastly underpowered to drive large populations of neurons, which appears necessary in DBS applications, such as disrupting aberrant basal ganglia activity in tremor or dystonia.
While optogenetics have proven an extraordinary basic neuroscience tool in culture and non-human animal models, and are presently explored in clinical trials for retinal disease,[66] thus far this approach has not yet been introduced into the human brain itself. Optogenetic stimulation faces clinical challenges of how to transduce cells without resulting in the vector straying from the desired site and hence limiting spatial selectivity and potentially evoking an immune response. Moreover, the optical properties of the brain curtail consistent, reliable transmission of light, thereby limiting the spatial density and extent.[67,68] While opsin proteins can be engineered to respond only to specific wavelengths of light, there do not yet exist techniques to ensure that the photons emitted from a waveguide target a particular neuron in the spherical volume within which photons would diffract. Implantation of additional optrodes, with the goal of enhancing spatial coverage, risks causing additional disruptive trauma.[69–73]
Both electrical and optogenetic methods generally rely on relatively stiff inorganic electrodes/optrodes to interface with the CNS. For microstimulation/activation, these relatively rigid, inorganic electrodes must be inserted into the brain, which inevitably leads to an eventual astrogliotic inflammatory response that diminishes the robustness and consistency of recordings.[74–76] An implanted intracortical interface impacts the tissue response and affects neural recordings through many pathways. For instance, microelectrode interfaces suffer from a lack of specificity and signal drift, possibly due to neuronal loss in the vicinity of the electrodes or electrode movement (from motion of pulmonary or cardiac sources, or from movement of the head itself). Inflammatory gliosis can also ultimately compromise electrical stimulation by driving up impedance, or optical stimulation by physically blocking the path of light. An implanted electrode causes damage, initiates an acute response, and continually agitates a chronic response. This chronic response has many feedback loops and mechanical factors that limit and prevent restoration of the tissue to native levels. The chronic response is considered the leading cause of the general degeneration of signals over time. Interactions between many complex factors contribute to the chronic response to an implant including size, shape, material stiffness, surface roughness, porosity, and chemical modification; however, in general stiff inorganic electrodes/optrodes result in decreased performance over time. Although significant improvements have been made using more mechanically compliant electrodes or co-factors to modulate the inflammatory response,[77–80] to date there is no reliable strategy to prevent a chronic foreign body response to inorganic electrodes.
2.2.3. Existing Approaches: Biomaterials Functionalized Via Living Cells
The use of decellularized tissue subsequently populated with living cells is already part of human clinical care and comprises an area of intensive research to repair organs and structures throughout the body.[81–83] In the nervous system, inert scaffolds seeded with neural progenitors have thus far only been used as conduits to accelerate peripheral and cranial nerve regeneration.[84–87] Embryonic and other progenitor neural cells have been implanted into the brain (including in humans), and these serve more as microscopic “drug factories” than functional components capable of interfacing with external devices.[88–90] There has been tremendous effort to add bioactive molecules to otherwise inert electrodes and other devices implanted into the brain,[80,91–97] Beyond coating electrodes with peptides and other bioactive molecules, certain groups have seeded living neurons and other support cells directly onto electrodes and have shown survival upon implantation.[98,99] While these approaches may enhance biostability and biocompatibility of the electrodes, they do not completely ameliorate the foreign body response and the cell-seeding techniques do not appear to fully leverage the information processing capabilities of the neurons.
2.3. Our Approach: Biologically-Based Neuromodulation Using “Living Electrodes”
2.3.1. Basis for Axon-Based Living Electrodes
As noted previously, our recently developed neuron/axon-based “living electrodes” have built on our previously established micro-TENN platform that was developed for the targeted neurosurgical reconstruction of long-distance axonal pathways in the brain.[1,2,5] Indeed, a common goal in deploying preformed neural constructs, which we often refer to as “living scaffolds”, is to mimic specific neuroanatomical and functional features to allow for direct integration with the nervous system to facilitate targeted axonal pathfinding, drive endogenous stem cell migration, or assume a functional role in neural circuitry.[1,5,7,14,100–102] By appropriately leveraging these reparative mechanisms, and in particular the ability of our various tissue engineered constructs to structurally and functionally integrate with host cells, we can create new methods to interface devices (e.g., electronic, optical, and/or mechanical) with the nervous system.[103–106] While the development of novel electrodes/optrodes could lead to a capture of more single neurons, our living electrode strategy presents another solution. For brain–machine interfaces (BMIs; also called brain–computer interfaces, or BCIs) our tissue engineered living electrodes serve as a biological intermediary between the host nervous system and devices, theoretically providing the ability to input information (i.e., neuromodulation), output information (i.e., recording neural activity), or both simultaneously.
These living electrodes are preformed 3D constructs consisting of neural cells and biomaterial matrices in a defined cytoarchitecture, and primarily function as axon/synaptic-based inputs for controlled neurophysiological stimulation. The living electrodes are anisotropic, consisting of long, aligned axonal tracts extending from discrete neuronal population(s).[2,7] To enable precise control of neuronal phenotypic composition, axonal architecture, and functional attributes, these constructs are generated in vitro prior to delivery in vivo.[1,2,5] Axon-based living electrodes can achieve biologically mediated neuromodulation with control from external devices, e.g., driven by externalized microelectrodes and/or optrodes coupled to microprocessors.[1,2,5,7,100,103,105,107,108] This neuromodulatory design would be comprised of unidirectional living electrodes, i.e., only possessing a neuronal population at one of the ends. However, bidirectional living electrodes would, theoretically, be able to transmit information both to and from the brain. In this case, because neural populations within the living electrodes, and between the living electrodes and the host tissue, couple reciprocally, these constructs may also be used to facilitate a sort of “recording”, such that a facsimile of neural activity within the brain is synaptically relayed to neurons within the living electrode. This activity could be reflected on the aspect of the construct externalized to the surface of the brain where non-penetrating subdural, epidural or subgaleal multielectrode arrays could record and transmit these signals to either external computers or microprocessors implanted elsewhere in the body.
2.3.2. Engineered Neuronal/Axonal “Living Electrodes” as a Functionalized Composite Biomaterial
We have pioneered microtissue engineering techniques to create preformed, injectable constructs containing discrete neuronal populations spanned by long axonal tracts within miniature tubular hydrogels (microscale diameter and extending up to several centimeters) (see Figure 2).[1,2,107] Hydrogel microcolumns were optimized in vitro to support neuronal survival and directed axon growth. Microcolumns are generally 5–30 mm in length with an outer diameter of 350–500 μm, and are fabricated using agarose alone or with a carboxymethylcellulose outer shell to permit needleless injection into the brain.[1,2,5] The central lumen (150–400 μm inner diameter) contains the neuronal somata at one or both ends, and contains an optimized extracellular matrix cocktail to direct axonal outgrowth longitudinally.
We assert that these neuronal/axonal-based constructs with controlled architecture within a custom biomaterial encasement may collectively be considered as a functionalized composite biomaterial. Indeed, the principal components of this system are each precisely engineered and are crucial for the overall functionality: 1) the outer hydrogel shell, 2) the inner ECM lumen, and 3) the specialized neuronal populations with prescribed architecture of axonal tracts. For instance, the outer agarose shell aids in the generation of the ideal neuronal cytoarchitecture, ensures biocompatibility within the brain, and serves to protect the construct following transplantation. Agarose is stable in vitro, and the pore size is large enough to allow for lateral diffusion of oxygen and nutrients from media, but small enough to prevent the escape of neurite growth cones (e.g., the pore size of 3% agarose is <100 nm).[109] Therefore, the outer agarose shell directs the longitudinal outgrowth of the neurites by constraining their growth to the tube interior. Also, this hydrogel encasement protects the construct throughout the transplantation process and acts as a physical barrier between the construct neurons/axons and the potentially “hostile environment” of the micro-stab wound (e.g., blood, immune cells) that is inevitable for virtually all approaches that deliver exogenous cells into the brain. Once in vivo, the agarose is relatively inert and, based on the concentration, the stiffness may match that of the brain for mechanical parity.[110] This mechanical parity is of the utmost importance as it has been shown that mechanical mismatch within the brain exacerbates fibrosis.[111,112] The ends of the agarose tube are open to allow for neuronal integration with the brain immediately, but the vast majority of the surface area of the construct only interacts directly with brain tissue after the hydrogel encasement degrades over a period of several weeks.[2] This controlled degradation gradually introduces the cells into the brain (ideally after the “hostile” environment has subsided), and is not believed to create by-products that exacerbate an inflammatory response. Moreover, the transparency of agarose enables straightforward imaging of the neuronal aggregates and axons throughout their growth, as well as during histological assessment following transplantation (only relevant at relatively short-term time points). This preformed hydrogel encasement also gives us the ability to readily tune the dimensions of the living electrodes. We can alter the inner and outer diameters of the agarose tubes, as well as the wall thickness, stiffness, and length in order to select specific bulk mechanical properties, degradation times, and the “dose” of neurons/axons that can be delivered per living electrode. Bioactive ligands (e.g., collagen IV and laminin) may be conjugated to agarose through controlled chemical coupling.[113,114] Collectively, this approach allows us to create an optimal/reproducible microenvironment as a vehicle to deliver axonal tracts to precise locations in the brain for local synaptic integration—effectively bringing the local microenvironment along with the preformed neuronal networks. Lastly, we can apply multiple living electrodes in an array to achieve a multifasciculated structure. Due to this multitude of attractive properties, we have primarily utilized agarose for the outer shell of our biomaterial encasement scheme; however, it is noteworthy that there are several other materials that may fit these criteria, including hyaluronic acid and alginate.[115,116]
In addition to the outer hydrogel encasement, the characteristics of the inner lumen are also precisely engineered to contribute to the overall functionality of the living electrodes. For instance, we developed the inner ECM cocktails (either 1 mg mL−1 collagen or 1 mg mL−1 collagen + 1 mg mL−1 laminin) over extensive work in both 2D and 3D culture systems as well as within the microcolumns in order to optimize neuronal adhesion, cell health/viability, axonal outgrowth, and axonal tract cytoarchitecture.[1,107,114,117–121] We established these final ECM cocktails for our current living electrodes after testing the use of various densities and mixtures of laminin, collagen I, Matrigel, fibrin, hyaluronic acid, and collagen IV. The densities of our current ECM cocktails provide structural support for the neuronal aggregates without inhibiting axonal penetration and outgrowth through the ECM. Moreover, we have found that it is generally necessary to optimize the individual ECM cocktails for each neuronal subtype utilized. Of note, this hydrogel-ECM encasement scheme is a versatile biomaterial platform that can be further “functionalized” by providing a vehicle for controlled release of compounds to mitigate acute host inflammation, improve implant survival, and both control and facilitate axonal outgrowth and synaptic integration.[122–126]
As described above, to date we have generated these constructs using multiple neuronal subtypes, including primary dorsal root ganglion neurons, cerebral cortical neurons (predominantly glutamatergic), ventral mesencephalic neurons (enriched in dopaminergic), and medial ganglionic eminence neurons (predominantly GABAergic). Aggregates of neurons precisely delivered within the proteinaceous lumen at one or both ends of the microcolumns can be cultured for weeks to months in vitro based on the desired length of axonal outgrowth. These constructs exhibit robust neuronal survival with the majority of the somata remaining in a tight cluster at the seeding site(s). Electrical stimulation of one population of neurons causes action potentials to travel across the axonal region to the other population as measured by real-time calcium fluctuations.[127] By adjusting culture conditions and days in vitro, living electrodes can be made at lengths of at least several centimeters to reach deep brain structures or can be tailored to the scale of hundreds of microns to millimeters to penetrate specific layers in the cortex.[1,5,107,127] For in vivo delivery, the hydrogel casing provides structural support to protect the microtissue during transportation and transplantation.[1,5] Cultured living electrodes can be drawn into a needle, slowly inserted into the cortex, and expelled using a plunger. We previously reported that following micro-TENN delivery into the brain, immunohistochemistry and fluorescent microscopy revealed surviving neurons in the construct interior, which maintained a tight cluster with axonal fascicles extending parallel to the axis of implantation.[1,2,4] The neurons within the construct survived, maintained their axonal architecture, and integrated with the surrounding cortex as dendrites from implanted neurons gave rise to synapses with host neurons.[1,2] We have recently adapted techniques for transducing light-activated opsins using cortical neurons in vitro that will allow for precise activation of the transplanted living electrodes in vivo. This approach allows for activation of the construct neurons without activating the host cells, allowing for a clear understanding of the role of the transplanted construct in affecting host circuitry. Thus, to date we have demonstrated electrophysiological activity and information flow across the living electrodes as well as implant survival and synaptic integration with host neurons. In total, these living electrodes should be considered as a functionalized composite biomaterial, where the combination of the hydrogel encasement, ECM lumen, and optically-active neurons/axonal tracts work synergistically towards the ultimate goal of enabling biologically-relevant, synaptic-based augmentation of deep neural circuitry with accessibility/control from the brain surface.
2.3.3. Attributes of Axon-Based Living Electrodes
With further development, these axon-based living electrodes may effectively serve as a functionalized material to allow for biologically-based neuromodulation that embodies three features impossible to achieve with any existing electrical, optical, microfluidic-chemical, genetic or pharmaceutical approach: stability, specificity, and biological multiplexing. Because these constructs are made of engineered living neurons and axonal tracts, they are biocompatible (allogeneic neurons alone appear to not elicit a foreign body response and inflammation; and living electrodes could be made using autologous sources of neurons as noted previously), biostable (they are themselves not damaged by the brain and should not be affected by micromotion), and can generate synaptic contacts onto host parenchyma neurons that in principle could last the lifetime of the organism. These features can thus achieve a notable stability: the constructs are stable in space, physically integrating into the brain parenchyma, and stable in time, able to achieve their modulation-stimulation and relay-recording functions. By virtue of being forged in vitro of preselected neuron types, cell/genetic engineering, and biomaterial/matrix chemistry, the constructs can also achieve unprecedented specificity. Neuron phenotypes can be selected to release/secrete certain specific neurotransmitters to restore levels relevant to particular disease processes. Constructs can be tailored to specific lengths to achieve connectivity at specific anatomical targets and synapse with specific neuronal subtypes within those regions. The proteinacious matrix and co-delivered factors can be altered to haptotactically and chemotactically attract specific host neuron-types to be targeted by the modulation. Unlike electrical stimulation that non-specifically activates a somewhat indiscriminate 3D volume of tissue, or optical stimulation limited by diffraction and waveguide placement, the construct neurons can target specific neurons without any extraneous, artifactual activation (provided that synaptic specificity is achieved). Finally, because the constructs can be seeded with one or more populations of neurons, these living neural networks can perform multiplexing operations both within themselves and by achieving high-information targeted output to parenchyma. This biological multiplexing can be defined broadly to encompass biological versions of the types of channel selection, multiplexing and demultiplexing used in telecommunications. Such biological multiplexing comprises both convergent and divergent signaling: signal processing within many neurons of the construct can converge on to single host parenchyma targets, and one construct neuron can have axons divergently branching to target many host parenchyma neurons (Figure 6). Likewise, because neurons in the host brain are themselves embedded in endogenous neural networks, the ability of the living construct to send axonal outputs to one of these host neurons allows a specific, stable, activation of that endogenous neural network. Because one axon can in principle synapse onto thousands of target neurons, a relatively small population of neurons within the construct could achieve a widespread effect. By deploying micropatterning techniques, living electrodes can be forged in vitro to enable fine-grained time-division multiplexing when implanted in vivo (Figure 7).
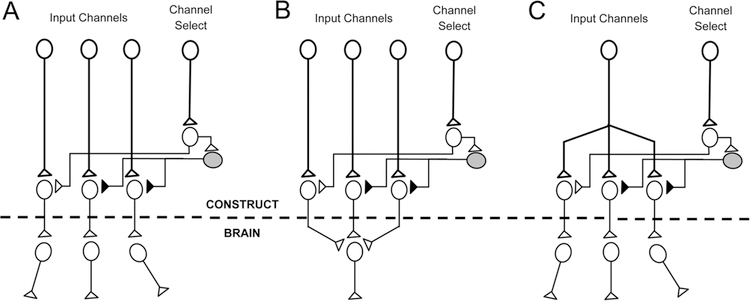
Figure 6.
Exploiting “Biological Multiplexing” in Living Electrodes. More sophisticated living electrodes may be developed to further exploit so-called biological multiplexing. By fabricating the constructs in vitro using microprinting and micropatterning techniques, specific synaptic architectures can be achieved to yield certain fine-grained signal manipulations linking the construct to the brain. (A) In the simplest form, “channel select” bundles of axons can transmit signals to select which other bundles transmit signals into the brain, and which are silenced. (B) Multiple channels that converge on to one final common output can likewise be toggled by the “channel select” in a biological instantiation that most resembles the kind of multiplexing used in telecommunications. (C) Likewise, a single input channel can be selected and diverted to one or more parallel outputs to “demultiplex” that signal.
Figure 7.
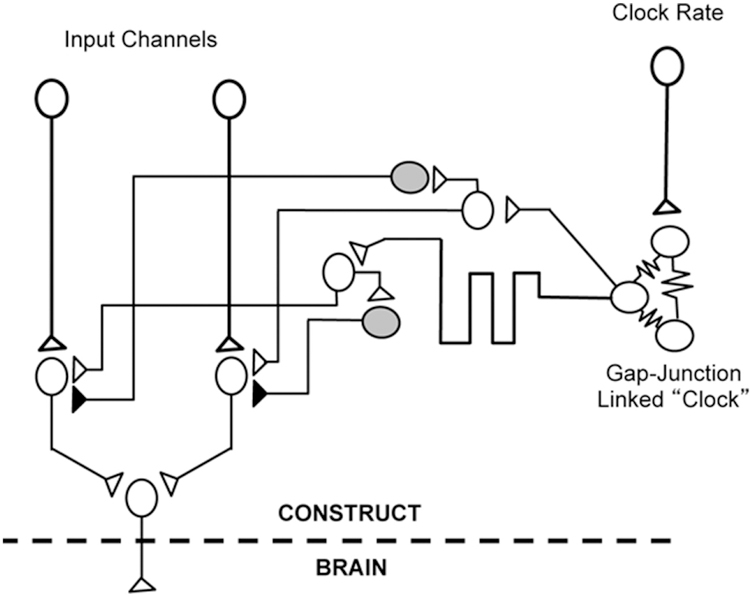
Potential for Time-Division “Biological Multiplexing” in Living Electrodes. Future iterations of living electrodes may exploit delay lines emanating from a single “clock” circuit formed by a cluster of neurons linked by gap junctions (coupled damped oscillators) and micropatterned inhibitory and excitatory connections. Thus, multiple parallel input channels can be multiplexed serially with each clock cycle to a single target output neuron that in turn links to the brain. The rate of the clock (and hence the multiplexing sampling duration) can be altered by driving the clock circuit directly.
2.3.4. Potential Applications for Axon-Based Living Electrodes
Current clinical and experimental applications of DBS are the most likely initial applications for axon-based living electrodes; however, given the potential for increased specificity and reduced footprint of living electrodes, the investigation of this technology to mitigate other disorders may be warranted. Indeed, living electrodes may be deployed to augment or replace traditional forms of neuromodulation, or may be applied for more far-reaching drug delivery applications. For instance, living electrodes may be precisely delivered to key locations to influence the strength of specific connections. Here, inhibitory (e.g., GABAergic) living electrodes may be designed to form synapses to modulate pathways that are exerting too much influence and causing detrimental functional effects, for example to dampen hypersynchronous activity in a circuitry exhibiting epileptiform activity (described in more detail below). Conversely, excitatory (e.g., glutamatergic) living electrodes may form synapses to augment weak pathways, for example with axons from the construct releasing glutamate at the target of a degenerating pathway. Living electrodes may also act by bulk release of neurotransmitters at the axonal terminal, either via tonic (self pacing/continuous) activity, by responding to inputs from the host to the living electrode neuronal somata/dendrites, or controlled from externalized hardware or computer. This type of biological neuromodulation can provide direct (i.e., synaptically-mediated) excitatory or inhibitory inputs, or both simultaneously, or can provide controlled release of diffuse modulatory neurotransmitters (e.g., dopamine) to augment circuit function. Axon-based living electrodes can uniquely fulfill this role—over more common neuronal transplants for instance—by acting based on network feedback relayed and processed reciprocally between the construct and the surrounding brain with the potential for computer-controlled regulation/feedback. There are no current technologies that could exceed the theoretical targeted connectivity and potential lifetime stability of biological synapses, underscoring the promise of this micro-tissue engineering based approach to neural interface. A sample of such future applications is detailed below:
Parkinson’s disease:
We are developing micro-TENNs grown using dopaminergic neurons to be implanted into the degenerating nigrostriatal pathway in Parkinson’s disease.[3,5] These dopaminergic micro-TENNs exhibiting both neuronal somata and axonal components are uniquely suitable to simultaneously replace the lost dopaminergic neurons within the substantia nigra pars compacta (SNpc) and recapitulate the entire nigrostriatal pathway spanning many centimeters to provide dopaminergic inputs into striatum. Dopaminergic output of the micro-TENN would be continuously modulated by striatal feedback and SNpc input to alleviate potential runaway dopamine excess and dystonia, a potential side effect from mesencephalic dopaminergic cell transplants into the striatum. Unlike DBS, which attempts to disrupt pathologic activity in the indirect pathway, micro-TENNs are themselves an auxiliary pathway. This engineered circuit is unique in that it is mimicking the function of dopaminergic axons projecting from the SNpc to the striatum and seeks to provide dopaminergic inputs that can be tuned and controlled. In addition to direct circuit reconstruction, optogenetically active micro-TENNs may also be deployed as dopaminergic living electrodes to provide controlled neuromodulatory input via engineered axonal tracts (see Figure 5a). Here, the neuronal somata population is left quasi-externalized on the brain surface to allow for controlled interface with a sub cranial micro-LED array. The interface beyond the nigrostriatal tract would provide a mechanism whereby information from other brain areas (e.g., beta oscillations recorded from primary motor cortex), external sensors (e.g., gyroscopes and accelerometers both within the battery case in the chest wall or streamed from implanted or externally worn sensors in the hands or feet), and external computers (e.g., processing 3D motion capture and force sensors embedded in the shoes, treadmill and gait analyzer surfaces), could modulate the basal ganglionic circuitry into a healthier activity pattern.
Friedrich’s ataxia:
In most cases of Friedrich’s ataxia, the expansion of the trinucleotide (GAA) repeat in intron 1 of both alleles of the frataxin gene on chromosome 9q13 leads to reduced transcription of the gene (i.e., silencing), decreased expression of the gene product frataxin, and ultimate destruction of the dorsal column pathways. Patients consequently develop severe motor impairments in the absence of proprioceptive and epicritic signals from the periphery. Living electrodes could provide an artificial sensory arc: by tapping into signals from periphery (such as strain gauges, accelerometers and gyroscopes worn at joints in all four limbs, or from implanted cuff recordings of peripheral nerves), living electrodes implanted into primary sensory cortices could provide sensory feedback and allow improved voluntary movement and functional independence. Grown with glutamatergic neurons, these living electrodes could be implanted to terminate in layer IV of the post-central gyrus; because living electrodes are themselves quite small, multiple constructs could be implanted corresponding to different joints (e.g., gyros from the left knee driving a living electrode implanted in the right medial sensory cortex, left elbow and shoulder to right lateral sensory cortex, and vice versa for the right extremities and left hemisphere).
Severe motor impairment and sensory feedback:
In brainstem stroke, spinal cord injury, muscular dystrophy and amyotrophic lateral sclerosis, people are rendered paralyzed because the substrate of voluntary motor control (primary motor cortex) is functionally disconnected from the skeletal muscles (and in certain cases bulbar-pharyngeal muscles also). Neuromotor prosthetics comprise a class of brain-computer or brain-machine interfaces that seek to overcome this paralysis by recording directly from the brain and decoding this recorded activity to control devices in the environment, trigger robotic actuators, or drive implanted neuromuscular stimulators. While several human trials have shown the safety and efficacy of this approach, patients achieve control purely by visual feedback. While the sensory arc may be retained in certain patients with motor neuron or muscular disease, it is lost in complete spinal cord transection and is unavailable in all patients when using external robotics. Several groups have attempted to provide haptic feedback by linking tactile signals to electrical stimulation provided by macro- and microelectrodes implanted into primary sensory cortex.[51,128] This type of artificial haptic feedback appears to be effective in non-human primates and has not yet been tested in humans. As with children and adults with Friedrich’s ataxia, living electrodes offer the promise of recapitulating and expanding the sensory arc by being implanted directly into sensory cortex (see Figure 5b). In addition to being driven by externally worn sensors, living electrode activity could be triggered by sensors mounted on robotic arms, powered robotic exoskeletal braces, wheelchair components and other assistive devices. In this way, a paralyzed patient could literally “feel” their own limbs and the “limbs” of these devices to facilitate enhanced voluntary control. Additionally, by providing a bidirectional interface to both motor and sensory cortex that is routed through internally implanted microprocessors, the living electrodes could modulate inter-cortical communication in a real-time closed-loop to restore motor function and sensory/proprioceptive feedback.
Chronic pain:
Tailored living electrodes may be useful to modulate inputs to a pain-dampening circuit. Living electrodes could be created using peptidergic neurons secreting endorphins or enkephalins and then implanted in the substantia gelatinosa of the spinal cord, the periaqueductal gray, ventroposterior thalamus or the anterior cingulate cortex. This would replace the non-specific approaches of spinal and brain electrical stimulators. Control of this neuromodulation could be user-dependent (e.g., analogous to a systemic pharmaceutical pump) and, unlike microfluidics that would directly inject opiates or other peptides, and unlike electrodes that would nonspecifically modulate a target volume, living electrodes comprised of neurons would themselves undergo up- and down-regulation hence providing additional prophylaxis against the development of tolerance, abuse or withdrawal.
Alzheimer’s disease and Dementia with Lewy Bodies:
A hallmark of both Alzheimer’s disease (an amyloid-tauopathy) and dementia with Lewy bodies (an alpha-synucleinopathy), is loss of cholinergic neurons in the basal forebrain. These neurons are reciprocally linked to medial temporal lobe structures, including the hippocampal formation, and are necessary to form episodic memories. Living electrodes built using cholinergic neurons could be implanted into the septal nuclei or other adjacent basal forebrain nuclei such as the nucleus basalis of Meynert or the diagonal band of Broca. A living electrode stereotactically implanted in the basal forebrain and semi-externalized to the brain service (following the path of the columns of the fornix) could allow closed-loop control with external computers: different subpopulations of neurons within the living electrode (cholinergic, GABAergic, glutamatergic) could be triggered differentially via optogenetics and intraosseous anchored waveguides, depending on detection of memory interference local field potential signatures decoded from the activity of separate living electrodes implanted into the temporal lobe to enhance episodic encoding. Likewise external cues (e.g., reminders on a smart phone, and user-triggered push button flagging) could be used to modulate basal forebrain activity to enhance storage and recall. A second living electrode could be implanted into entorhinal cortex and the hippocampus and then linked, via external computers, to the living electrode implanted into the basal forebrain to functionally re-instantiate the bidirectional fornix septohippocampal pathway.
Frontotemporal dementia and autism spectrum disorder:
For agrammatic primary progressive aphasia, a frontotemporal dementia (FTD) tauopathy affecting the dominant inferior frontal gyrus, living electrodes could be implanted both to link Broca’s area to premotor and primary motor cortices (to compensate for aphemia and allow motor substitution gestures) and to link Broca’s area to Wernicke’s area as an artificial arcuate fasciculus. In behavioral variant frontotemporal dementia (a TDP-43opathy and sometimes tauopathy), constructs linking degenerating orbitofrontal cortices to intact dorsolateral prefrontal, frontopolar, and anterior cingulate cortices could reinstantiate behavioral inhibition and self-regulation. In the semantic dementia variant of FTD (TDP-43 or tau), degeneration of the fronto-ventral aspects of the temporal lobe may occur leading to loss of semantic knowledge stores and a variety of reading and perceptual disturbances. An excitatory glutamatergic living electrode implanted into the visual word form area of the fusiform gyrus could conceivably boost residual function in this area, and the living electrode could be crafted as an auxiliary axonal bundle linking primary and secondary visual cortical areas to the ventral temporal lobe to recreate the lost “ventral-what” pathway and restore semantic processing. In both autism-spectrum disorder and behavioral variant frontotemporal dementia, social perception and interaction are compromised. A living electrode built with glutamatergic neurons at the surface and within left dorsolateral prefrontal cortex[129] and oxytocinergic neurons apposed to supraoptic and paraventricular nuclei in the hypothalamus[130] could quench behavioral disinhibition and recover social behavior;[131] the surface cortical population could be triggered by external computers tracking social cues decoded from microphones and micro-cameras mounted unobtrusively in the frames of glasses, hearing aids, bracelets or other apparel.[132]
Stroke and cerebral palsy:
Both ischemic and hemorrhagic stroke result in focal brain tissue destruction and varying degrees of inflammation. In ischemic stroke, a surrounding penumbra of tissue may remain functional and simultaneously metabolically vulnerable to further insult (such as from decreased blood pressure or hypoxia). When occurring in utero or in the perinatal period, stroke (e.g., germinal matrix hemorrhage) can lead to a static insult around which the rest of the brain attempts to develop normally, in certain cases leading to cerebral palsy with varying degrees of motor and cognitive impairment. When an area of the brain is damaged, two aspects of function are lost: the local gray matter “computation” and also the axonal (both focal intrinsic and also crossing fibers of passage) “connectivity.” Micro-TENNs could directly restore both computation and connectivity and serve as “replacement parts” for the irreversibly damaged piece of the brain and to metabolically, electrically and functionally revive and support the surrounding penumbra. In an animal model of stroke with middle cerebral artery occlusion, optogenetic grafts were shown to restore functional mobility. Whereas this graft was “driven” by an external laser, a functionalized living electrode could allow both intact areas of the brain, and external modulation triggered by body sensors or computer-driven rehabilitation, to do the “driving” to restore activity within the penumbra and restore functional mobility and behavior following stroke.[133]
Refractory depression:
Severe clinical depression that is refractory to pharmacotherapy, psychotherapy and electroconvulsive therapy, is characterized by neurometabolic derangements including disrupted glucose uptake in limbic structures including the cingulate gyrus. Micro-TENNs could be implanted to enhance connectivity between frontopolar cortex and the anterior cingulate, or to link supragenual to subgenual anterior cingulate cortices so that the former modulates the latter to restore normal metabolic activity and relieve symptoms. Likewise, if seeded with dopaminergic neurons, living electrodes implanted into the nucleus accumbens could be deployed to provide dynamic, phasic alteration of catecholamine tone and hence alter mood salience labeling of thoughts and perception to relieve depressive symptoms without causing rebound dysphoria or tolerance post-synaptic upregulation.
Epilepsy:
The application for epilepsy exhibits two ways in which the advanced functionality of living electrodes could achieve treatment goals in a manner impossible with existing approaches. In the first, living electrodes could be forged such that the population of neurons closest to the target area secreted the inhibitory neurotransmitter GABA diffusely to the target region, either constitutively or evoked from the brain surface based on measurements of early epileptiform activity (as described below). In this approach, the living electrode effectively serves as a GABA reservoir and delivery system (see Figure 5c). Alternatively, the living electrode could be seeded with excitatory glutamatergic neurons in an extracellular matrix decorated with neuroligins to coax synaptogenesis with local endogenous GABAergic neurons. Either approach could achieve disruption of hypersynchronous activity and hence arrest generation or transmission of pathological seizures from a target region in the brain, and further experimental work will be needed to identify which approach, or a hybrid of both, would be most effective. Because neurons in the epileptiform network within the brain may form synapses onto dendrites extending out from neurons within the construct, the construct could achieve focal, closed-loop self-attenuating circuits such that focal epileptiform activity would quench itself via this auto-inhibitory loop mediated by the inhibitory living electrode. For multi-focal epilepsy, living electrodes could be implanted at two or more epileptigenic foci (e.g., identified by intracranial surface and depth recording). Sensors (e.g., intraosseous or subgaleal leads capturing ongoing local field potentials) could be used to pick up signatures of pre-seizure or seizure activity to trigger photostimulation of optogenetically modified surface externalized micro-TENNs to pre-emptively arrest seizure propagation in a manner impossible with conventional electrodes.
2.4. Challenges in Deploying Living Electrodes
While living electrode strategies provide key advantages for restoring aspects of nervous system structure and function, they are in an early stage of development and present several formidable hurdles. Living cells may induce an immune response from host tissue leading to inflammation or rejection of the graft.[90] This immune response differs depending on the cell type transplanted: while glial cells elicit a vigorous response and generally show poor attrition upon transplantation, constructs consisting of pure neurons appear to be well tolerated by the host with increased survival.[100,134–137] A deleterious immune response may also be mitigated through the use of autologous cells from patients. Neurons, oligodendrocytes, astrocytes, and Schwann cells can be differentiated from human embryonic stem cells, induced pluripotent stem cells, and adipose-derived stem cells,[15,137–140] Although direct in vivo delivery of stem cells may replace lost cells and encourage neural regeneration through the release of trophic factors, the mechanism by which they stimulate the nervous system remains unclear, and they have the potential to differentiate into undesirable phenotypes and/or result in tumorigenesis.[138] In comparison, there are notable advantages to the use of differentiated neurons within living electrodes. Existing protocols to differentiate stem cells into specific neuronal sub-types—such as cortical projection neurons, interneurons, dopaminergic A9 neurons, spinal motor neurons—can be used to engineer living electrodes with specific neuronal compositions. Because neurons are both terminally differentiated and physically constrained by the 3D architecture of the engineered construct, this approach likely carries less risk for tumorigenesis, but more carefully conducted studies are needed to prove this supposition. Tantalizingly, differentiated neurons can be genetically modified to enhance regenerative responses. Prior studies suggest that the low survival of transplanted cells can be due to delivery into a degenerating or “hostile” injured environment. Using transfection techniques or viral transduction, the durability and regenerative potential of differentiated neurons could be augmented through the overexpression of trophic factors.[141–143] This approach could make engineered tissue resistant to the underlying pathophysiology of neurodegenerative disease.
There may also be issues with either insufficient or extraneous axonal integration with the host. We have found that the maturation and source of the living electrode neurons influence the promiscuity for integration with the host, and thus may be varied based on application. Moreover, exogenous factors may be co-delivered as a component of the biomaterial encasement of the living electrodes to enhance outgrowth and plasticity. If excessive and/or non-target axonal outgrowth is observed—recently seen in neuronal transplants derived from certain stem cells—then inhibitory “barriers” can be delivered along anatomical borders.[144] However, if living electrodes malfunction and/or fail to elicit the desired effect, they will be inherently difficult to remove due to likely significant integration with host tissue, as opposed to an electrode or optrode, which may simply be pulled out. Here, living electrodes may be engineered to contain a controlled “kill switch” driving programmed cell death of living electrode neurons and hence axons. Indeed, the ability to employ different strategies to induce programmed cell death in transplanted constructs is a potentially important method to enhance the safety of living electrodes.[145] Hughes et al. recently developed an optogenetic protein Bax designed to induce apoptosis upon exposure to 488 nm light.[145] As an alternative strategy, there are multiple suicide-gene technologies in development that can be embedded into living electrode constructs. These suicide genes are biologically inert until activated by the introduction of a prodrug, and two clinically validated constructs, iCasp9 and HSV-TK, are well-suited for different situations based on rapid versus gradual apoptosis, respectively.[145–148] Despite these challenges, our proposed living electrodes may prove to be a highly effective strategy to naturally affect deep neural circuitry from the brain surface with a degree of specificity and permanence not possible with alternative approaches.
2.5. Conclusion
Current brain-machine interface and neuromodulatory device strategies suffer from impermanence, non-specificity, and a significant foreign-body response upon implantation. We are developing a novel strategy based on so-called “living electrodes”—a micro-columnar biomaterial scheme functionalized with preformed, anatomically-constrained living neurons and axonal tracts. This approach represents a blend of tissue engineering and micro-electrical techniques to facilitate host-device integration, including axonal, dendritic, and synaptic integration to/from host. This biohybrid neural interface via living electrodes offers several notable advantages over traditional electrical and/or optical stimulation methods, and therefore may yield a more robust interface. Synaptic integration via engineered axonal tracts offers a permanence and target specificity not possible with conventional approaches. A robust effect may be driven via a novel mechanism that we refer to as “biological multiplexing”, whereby the recruitment of numerous host neurons may be elicited using constructs with relatively few axons. Moreover, purely biological living electrodes may mitigate the foreign body response inherent in non-organic electrodes/optrodes. Collectively, these mechanisms may enable prosthetic/device control, sensory or proprioceptive feedback, and/or neuromodulation using living electrodes. This biological “wet” interface with transplanted living neurons/axons shows promise by acting as a natural intermediary between host and electronic device(s). If successful, this potentially transformative technology at the interface of neuroscience and engineering will lay the foundation for preformed implantable neural networks as a viable alternative to conventional electrodes to treat a range of nervous system disorders.
Acknowledgements
Financial support provided by the National Institutes of Health (U01-NS094340 (D.K.C.) & T32-NS043126 (J.P.H.)), Michael J. Fox Foundation (Therapeutic Pipeline Program #9998 (D.K.C.)), Penn Medicine Neuroscience Center Pilot Award (D.K.C.), Department of Veterans Affairs (Merit Review #B1097-I (D.K.C.), Career Development Award #IK2-RX001479 (J.A.W.), and Career Development Award #IK2-RX002013 (H.I.C.)), National Science Foundation (Graduate Research Fellowships DGE-1321851 (L.A.S. & D.O.A.)), and American Association of Neurological Surgeons and Congress of Neurological Surgeons (2015–2016 Codman Fellowship in Neurotrauma and Critical Care (D.P.)).
Biography

Dr. Mijail “Misha” Serruya is a neuroscientist-neurologist with expertise in direct brain–computer interfaces. Dr. Serruya earned his undergraduate neuroscience, medical and graduate neuroscience degrees at Brown University. He helped co-found and launch Cyberkinetics, a neurotechnology startup company that ran the first pilot clinical trial of a chronically implantable multielectrode array-based brain–computer interface for people paralyzed with traumatic and degenerative neurological conditions. Dr. Serruya completed his clinical training in neurology at the University of Pennsylvania, with rotations at Children’s Hospital of Philadelphia, followed by a fellowship in cognitive neurology. He is currently on the faculty of Thomas Jefferson University.

Dayo Adewole is a third-year doctoral Bioengineering student in the Cullen Lab at the University of Pennsylvania (Penn). In 2015, he earned a Bachelor’s and Master’s of engineering from Penn in Bioengineering and Robotics, respectively. He was awarded a pre-doctoral Graduate Research Fellowship from the National Science Foundation in 2015, which he has used to further the design and development of a biohybrid neural interface using neural tissue engineering and optogenetics.

D. Kacy Cullen is an Associate Professor of Neurosurgery and Bioengineering at the University of Pennsylvania, and he serves as Co-Director of the Center for Neurotrauma, Neurodegeneration & Restoration at the Corporal Michael J. Crescenz VA Medical Center in Philadelphia, PA. He received a Ph.D. in Biomedical Engineering from the Georgia Institute of Technology (Georgia Tech) in 2005, followed by postdoctoral fellowships in Neuroengineering at Georgia Tech, and then at the Center for Brain Injury & Repair at the University of Pennsylvania. Dr. Cullen’s current research program operates at the intersection of Neural Engineering, Neurotrauma, and Regenerative Medicine (http://www.med.upenn.edu/cullenlab/).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Mijail D. Serruya, Department of Neurology, Thomas Jefferson University, Philadelphia, PA 19107, USA
James P. Harris, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA
Dayo O. Adewole, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA; Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, PA 19104, USA
Laura A. Struzyna, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA; Department of Bioengineering, School of Engineering and Applied Science, University of Pennsylvania, Philadelphia, PA 19104, USA
Justin C. Burrell, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA
Ashley Nemes, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA.
Dmitriy Petrov, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA.
Reuben H. Kraft, Computational Biomechanics Group, Department of Mechanical & Nuclear Engineering, Department of Biomedical Engineering, The Pennsylvania State University, University Park, PA 16801, USA
H. Isaac Chen, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA.
John A. Wolf, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA
D. Kacy Cullen, Center for Brain Injury & Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Neurotrauma, Neurodegeneration & Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA 19104, USA.
References
- [1].Struzyna LA, Wolf JA, Mietus CJ, Adewole DO, Chen HI, Smith DH, Cullen DK, Tissue Eng. Part A 2015, 21, 2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Struzyna LA, Harris JP, Katiyar KS, Isaac Chen H, Cullen DK, Neural Regen. Res 2015, 10, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duda JE, Struzyna LA, Browne KD, Chen HI, Wolf JA, Cullen DK, Proceedings of 21st International Congress of Parkinson’s Disease and Movement Disorders 2017. [Google Scholar]
- [4].Struzyna LA, Adewole DO, Gordián-Vélez WJ, Grovola MR, Burrell JC, Katiyar KS, Petrov D, Harris JP, Cullen DK,J. Vis. Exp 2017, 123, e55609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harris JP, Struzyna LA, Murphy PL, Adewole DO, Kuo E, Cullen DK, J. Neural Eng 2016, 13, 16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adewole DO, Serruya MD, Harris JP, Burrell JC, Petrov D, Chen HI, Wolf JA, Cullen DK, Crit. Rev. Biomed. Eng 2016, 44, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Struzyna LA, Katiyar K, Cullen DK, Curr. Opin. Solid State Mater. Sci 201418, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu KJ, Kuzum D, Hwang SW, Kim BH, Juul H, Kim NH, Won SM, Chiang K, Trumpis M, Richardson AGK, Cheng H, Fang H, Thompson M, Bink H, Talos D, Seo KJ, Lee HN, SK LB, Kim JH, Lee JY, Huang Y, Jensen FE, Dichter MA, Lucas TH, Viventi J, Litt B, Rogers JA, Nat Mater 2016, 15, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco J. a, Kim Y-S, Avrin AE, Tiruvadi VR, Hwang S-W, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers J. a, Litt B, Nat. Neurosci 2011, 14, 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Richner TJ, Thongpang S, Brodnick SK, Schendel AA, Falk RW, Krugner-Higby LA, Pashaie R, Williams JC, J. Neural Eng 2014, 11, 16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams JC and Denison T, Sci. Transl. Med 2013, 5, 177ps6. [DOI] [PubMed] [Google Scholar]
- [12].Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR, IEEE Trans. Biomed. Eng 2001, 48, 361. [DOI] [PubMed] [Google Scholar]
- [13].Pashaie R, Anikeeva P, Lee JH, Prakash R, Yizhar O, Prigge M, Chander D, Richner TJ, Williams J, IEEE Rev. Biomed. Eng 2014, 7, 3. [DOI] [PubMed] [Google Scholar]
- [14].Chen HI, Jgamadze D, Serruya MD, Cullen DK, Wolf JA, Smith DH, Front. Syst. Neurosci 2016, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tornero D, Wattananit S, Grønning Madsen M, Koch P, Wood J, Tatarishvili J, Mine Y, Ge R, Monni E, Devaraju K, Hevner RF, Brüstle O, Lindvall O, Kokaia Z, Brain 2013, 136, 3561. [DOI] [PubMed] [Google Scholar]
- [16].Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB, Biomaterials 2015, 37, 242. [DOI] [PubMed] [Google Scholar]
- [17].Wang W, Collinger JL, Perez MA, Tyler-Kabara EC, Cohen LG, Birbaumer N, Brose SW, Schwartz AB, Boninger LL, Weber DJ, Phys. Med. Rehabil. Clin. North Amer 2010, 21, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M, J. Neural Transm 2012, 120, 813. [DOI] [PubMed] [Google Scholar]
- [19].Chi RP, Fregni F, Snyder AW, Brain Res 2010, 1353, 168. [DOI] [PubMed] [Google Scholar]
- [20].Lewis PM, Thomson RH, Rosenfeld JV, Fitzgerald PB, Neuroscientist 2016, 22, 406. [DOI] [PubMed] [Google Scholar]
- [21].Mayberg HS, Riva-Posse P, Crowell AL, JAMA Psychiatry 2016, 73, 6. [DOI] [PubMed] [Google Scholar]
- [22].Krishna V, King NKK, Sammartino F, Strauss I, Andrade DM, Wennberg RM, Lozano AM, Neurosurgery 2016, 0, 1. [DOI] [PubMed] [Google Scholar]
- [23].Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, Cramer SC, Venkatesan L, Neurorehabil. Neural Repair 2016, 30, 107. [DOI] [PubMed] [Google Scholar]
- [24].Brown JA, Lutsep HL, Weinand M, Cramer SC, Neurosurgery 2006, 58, 464. [DOI] [PubMed] [Google Scholar]
- [25].Collinger JL, Foldes S, Bruns TM, Wodlinger B, Gaunt R, Weber DJ, J. Spinal Cord Med 2013, 36, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, Bensmaia SJ, Schwartz AB, Boninger ML, GauntSci RA Transl. Med 2016, 8, 361ra141. [DOI] [PubMed] [Google Scholar]
- [27].Tabot GA, Kim SS, Winberry JE, Bensmaia SJ, Neurobiol. Dis 2015, 83, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lehew G, Nicolelis M, Meth. Neural Ensemble Record 2008, 1. [Google Scholar]
- [29].Ziegler D, Suzuki T, Takeuchi S, J. Microelectromechanical Syst 2006, 15, 1477. [Google Scholar]
- [30].Wise KD, Anderson DJ, Hetke JF, Kipke DR, Najafi K, Proc. IEEE 2004, 92, 76. [Google Scholar]
- [31].Son Y, Lee HJ, Kim J, Lee CJ, Yoon ES, Kim TG, Cho IJ, Proc. IEEE Int. Conf. Micro Electro Mech. Syst. (MEMS) 2015, 2015, 158. [Google Scholar]
- [32].Yazdan-Shahmorad A, Diaz-Botia C, Hanson TL, Kharazia V, Ledochowitsch P, Maharbiz MM, Sabes PN, Neuron 2016, 89, 927. [DOI] [PubMed] [Google Scholar]
- [33].Grosenick L, Marshel JH, Deisseroth K, Neuron 2015, 86, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Long X, Ye J, Zhao D, Zhang SJ, Sci. Bull 2015, 60, 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Length F, Research O, Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, Desalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, Epilepsia 2010, 51, 899.20331461 [Google Scholar]
- [36].Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhe HG, Notten P, van Laarhoven J, Visser I, Figee M, de Kwaasteniet BP, Horst F, Schene AH, van den Munckhof P, Beute G, Schuurman R, Denys D, JAMA Psych 2016, 73, 456. [DOI] [PubMed] [Google Scholar]
- [37].Goodman WK, Insel TR, Biol. Psychiatry 2009, 65, 263. [DOI] [PubMed] [Google Scholar]
- [38].Cusin C, Evans KC, Carpenter LL, Greenberg BD, Malone DAJ, Eskandar E, Dougherty DD, Psychiatric Annals 2010, 40, 477. [Google Scholar]
- [39].Galvan A, Hu X, Smith Y, Wichmann T, PLoS One 2012, 7, e50808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP, IEEE Trans. Neural Syst. Rehabil. Eng 2005, 13, 524. [DOI] [PubMed] [Google Scholar]
- [41].Schmidt S, Horch K, Normann R, J. Biomed. Mater. Res 1993, 27, 1393. [DOI] [PubMed] [Google Scholar]
- [42].Biran R, Martin DC, Tresco PA, Exp. Neurol 2005, 195, 115. [DOI] [PubMed] [Google Scholar]
- [43].Henderson JM, Henderson JM, Tkach J, Tkach J, Phillips M, Phillips M, Baker K, Baker K, Shellock FG, Shellock FG, Rezai AR, Rezai AR, Neurosurgery 2005, 57, E1063. [DOI] [PubMed] [Google Scholar]
- [44].Negi S, Bhandari R, Rieth L, Van Wagenen R, Solzbacher F, Neurosci J Methods 2010, 186, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Umemura A, Oyama G, Shimo Y, Nakajima M, Nakajima A, Jo T, Sekimoto S, Ito M, Mitsuhashi T, Hattori N, and Arai H, Neurol. Med. Chir. (Tokyo) 2016, 56, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morrell MJ, Neurology 2011, 77, 1295. [DOI] [PubMed] [Google Scholar]
- [47].Ovadia D, Bottini G, Curr. Opin. Neurol 2015, 28, 598. [DOI] [PubMed] [Google Scholar]
- [48].Cleary DR, Ozpinar A, Raslan AM, Ko AL, Neurosurg. Focus 2015, 38, E2. [DOI] [PubMed] [Google Scholar]
- [49].De Jesus S, Almeida L, Peng-Chen Z, Okun MS, Hess CW, Expert Rev. Neurother 2015, 15, 1067. [DOI] [PubMed] [Google Scholar]
- [50].Lozano AM, Lipsman N, Neuron 2013, 77, 406. [DOI] [PubMed] [Google Scholar]
- [51].Kim S, Callier T, Tabot GA, Tenore FV, Bensmaia SJ, Front Syst Neurosci 2015, 9, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Song W, Kerr CC, Lytton WW, Francis JT, PLoS One 2013, 8, e57453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tabot GA, Dammann JF, Berg J. a, Tenore FV, Boback JL, Vogelstein RJ, Bensmaia SJ, Proc. Natl. Acad. Sci. USA 2013, 110, 18279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Callier T, Schluter EW, Tabot G. a, Miller LE, Tenore FV, Bensmaia SJ, J. Neural Eng 2015, 12, 56010. [DOI] [PubMed] [Google Scholar]
- [55].Hiremath SV, Chen W, Wang W, Foldes S, Yang Y, Tyler-Kabara EC, Collinger JL, Boninger ML, Front. Integr. Neurosci 2015, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Klaes C, Shi Y, Kellis S, Minxha J, Revechkis B, Andersen RA, J. Neural Eng 2014, 11, 56024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tan D, Tyler D, Sweet J, Miller J, Neuromodulation 2016, 19, 254. [DOI] [PubMed] [Google Scholar]
- [58].Tyler DJ, Curr. Opin. Neurol 2015, 28, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schiefer M, Tan D, Sidek SM, Tyler DJ, J. Neural Eng 2015, 13, 16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jarvis S, Schultz SR, Front. Syst. Neurosci 2015, 9, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Häusser M, Nat. Methods 2014, 11, 1012. [DOI] [PubMed] [Google Scholar]
- [62].Kravitz AV, Kreitzer AC, Curr. Opin. Neurobiol 2011, 21, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Miesenböck G, Annu. Rev. Cell Dev. Biol 2011, 27, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Boyden ES, F1000 Biol. Rep 2011, 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bernstein JG, Boyden ES, Trends Cognitive Sci 2011, 15, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].RST-001 Phase I/II Trial for Advanced Retinitis Pigmentosa (NCT02556736), https://clinicaltrials.gov/ct2/show/NCT02556736 (accessed: July 2017).
- [67].Azimipour M, Baumgartner R, Liu Y, Jacques SL, Eliceiri K, Pashaie R, J. Biomed. Opt 2014, 19, 75001. [DOI] [PubMed] [Google Scholar]
- [68].Al-Juboori SI, Dondzillo A, Stubblefield EA, Felsen G, Lei TC, Klug A, PLoS One 2013, 8, e67626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pashaie R, Baumgartner R, Richner TJ, Brodnick SK, Azimipour M, Eliceiri KW, Williams JC, IEEE Trans. Biomed. Eng 2015, 62, 2327. [DOI] [PubMed] [Google Scholar]
- [70].Ozden I, Wang J, Lu Y, May T, Lee J, Goo W, O’Shea DJ, Kalanithi P, Diester I, Diagne M, Deisseroth K, Shenoy KV, Nurmikko AV, J. Neurosci. Methods 2013, 219, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kanno S, Lee S, Harashima T, Kuki T, Kino H, Mushiake H, Yao H, Tanaka T, in Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual Int. Conf. of the IEEE, IEEE, Piscataway, NJ, USA, 2013, pp. 253–256. [DOI] [PubMed] [Google Scholar]
- [72].Welkenhuysen M, Hoffman L, Luo Z, De Proft A, Van den Haute C, Baekelandt V, Debyser Z, Gielen G, Puers R, Braeken D, Sci. Rep 2016, 6, 20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chamanzar M, Borysov M, Maharbiz MM, Blanche TJ, Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual Int. Conf. of the IEEE, IEEE, Piscataway, NJ, USA, 2014, pp. 6838–6841. [DOI] [PubMed] [Google Scholar]
- [74].Harris JP, Tyler DJ, Crit. Rev. Biomed. Eng 2013, 41, 435. [PubMed] [Google Scholar]
- [75].Tresco PA, Winslow BD, Crit. Rev. Biomed. Eng 2011, 39, 29. [DOI] [PubMed] [Google Scholar]
- [76].Rennaker RL, Miller J, Tang H, Wilson DA, J. Neural Eng 2007, 4, L1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Harris JP, Capadona JR, Miller RH, Healy BC, Shanmuganathan K, Rowan SJ, Weder C, Tyler DJ, J. Neural Eng 2011, 8, 66011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhong Y, Bellamkonda RV, Soc JR Interface 2008, 5, 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR, Nat. Mater 2012, 11, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Winslow BD, Christensen MB, Yang WK, Solzbacher F, Tresco PA, Biomaterials 2010, 31, 9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Klinger A, Kawata M, Villalobos M, Jones RB, Pike S, Wu N, Chang S, Zhang P, DiMuzio P, Vernengo J, Benvenuto P, Goldfarb RD, Hunter K, Liu Y, Carpenter JP, Tulenko TN, Hernia 2016, 20, 161. [DOI] [PubMed] [Google Scholar]
- [82].Koenig F, Lee JS, Akra B, Hollweck T, Wintermantel E, Hagl C, Thierfelder N, Artif. Organs 2016, 40, 727. [DOI] [PubMed] [Google Scholar]
- [83].Hamilton NJ, Kanani M, Roebuck DJ, Hewitt RJ, Cetto R, Culme-Seymour EJ, Toll E, Bates AJ, Comerford AP, McLaren CA, Butler CR, Crowley C, McIntyre D, Sebire NJ, Janes SM, O’Callaghan C, Mason C, De Coppi P, Lowdell MW, Elliott MJ, Birchall MA, Am. J. Transplant 2015, 15, 2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Z. J. Ma F, Zhu T, Xu F, Wang Z, Zheng Y, Tang Q, Chen L, Shen Y, Acta Biomater 2017, 1, 188. [DOI] [PubMed] [Google Scholar]
- [85].Gu X, Ding F, Yang Y, Liu J, Prog. Neurobiol 2011, 93, 204. [DOI] [PubMed] [Google Scholar]
- [86].Hassan NH, Sulong AF, Ng MH, Htwe O, Idrus RBH, Roohi S, Naicker AS, Abdullah S, J. Orthop. Res 2012, 30, 1674. [DOI] [PubMed] [Google Scholar]
- [87].Lokanathan Y, Ng MH, Hasan S, Ali A, Mahmod M, Htwe O, Roohi SA, Bt Hj Idrus R, Abdullah S, Naicker AS, J. Biosci. Bioeng 2014, 118, 231. [DOI] [PubMed] [Google Scholar]
- [88].Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J, Eur. J. Neurosci 2009, 29, 562. [DOI] [PubMed] [Google Scholar]
- [89].Kim SU, de Vellis J, J. Neurosci. Res 2009, 87, 2183. [DOI] [PubMed] [Google Scholar]
- [90].Barker RA, Widner H, NeuroRx 2004, 1, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC, Mater. Sci 2003, 24, 777. [DOI] [PubMed] [Google Scholar]
- [92].He W, McConnell GC, Bellamkonda RV, J. Neural Eng 2006, 3, 316. [DOI] [PubMed] [Google Scholar]
- [93].Gutowski SM, Templeman KL, South AB, Gaulding JC, Shoemaker JT, LaPlaca MC, Bellamkonda RV, Lyon LA, Garca AJ, J. Biomed. Mater. Res. – Part A 2014, 102, 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gutowski SM, Shoemaker JT, Templeman KL, Wei Y, Latour RA, Bellamkonda RV, LaPlaca MC, García AJ, Biomaterials 2015, 44, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhong Y, McConnell GC, Ross JD, Deweerth SP, Bellamkonda RV, in 2nd International IEEE EMBS Conference on Neural Engineering, IEEE, Piscataway, NJ, USA, 2005, pp. 522–525. [Google Scholar]
- [96].Abidian MR, Martin DC, Adv. Funct. Mater 2009, 19, 573. [Google Scholar]
- [97].Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC, J. Biomed. Mater. Res 2001, 56, 261. [DOI] [PubMed] [Google Scholar]
- [98].Azemi E, Gobbel GT, Cui XT, J. Neurosurg 2010, 113, 673. [DOI] [PubMed] [Google Scholar]
- [99].De Faveri S, Maggiolini E, Miele E, De Angelis F, Cesca F, Benfenati F, Fadiga L, Front. Neuroeng 2014, 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Huang JH, Cullen DK, Browne KD, Groff R, Zhang J, Pfister BJ, Zager EL, Smith DH, Tissue Eng. Part A 2009, 15, 1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Winter CC, Katiyar KS, Hernandez NS, Song YJ, Struzyna LA, Harris JP, Cullen DK, Acta Biomater 2016, 38, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Katiyar KS, Winter CC, Struzyna LA, Harris JP, Cullen DK, J. Tissue Eng. Regen. Med 2016. [DOI] [PMC free article] [PubMed]
- [103].Kameswaran N, Cullen DK, Pfister BJ, Ranalli NJ, Huang JH, Zager EL, Smith DH, Neurol. Res 2008, 30, 1063. [DOI] [PubMed] [Google Scholar]
- [104].Cullen DK, Patel AR, Doorish JF, Smith DH, Pfister BJ, J. Neural Eng 2008, 5, 374. [DOI] [PubMed] [Google Scholar]
- [105].Cullen DK, Wolf JA, Smith DH, Pfister BJ, Crit. Rev. Biomed. Eng 2011, 39, 243. [DOI] [PubMed] [Google Scholar]
- [106].Cullen DK, Smith DH, Sci. Am 2013, 308, 52. [DOI] [PubMed] [Google Scholar]
- [107].Cullen DK, Tang-Schomer MD, Struzyna LA, Patel AR, Johnson VE, Wolf JA, Smith DH, Tissue Eng. Part A 2012, 18, 2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Cullen DK, Wolf J. a, Vernekar VN, Vukasinovic J, LaPlaca MC, Crit. Rev. Biomed. Eng 2011, 39, 201. [DOI] [PubMed] [Google Scholar]
- [109].Pluen A, Netti PA, Jain RK, Berk DA, Biophys. J 1999, 77, 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lewitus DY, Landers J, Branch JR, Smith KL, Callegari G, Kohn J, Neimark AV, Adv. Funct. Mater 2011, 21, 2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Moshayedi P, Ng G, Kwok JCF, Yeo GSH, Bryant CE, Fawcett JW, Franze K, Guck J, Biomaterials 2014, 35, 3919. [DOI] [PubMed] [Google Scholar]
- [112].Polikov VS, Tresco PA, Reichert WM, Neurosci J Methods 2005, 148, 1. [DOI] [PubMed] [Google Scholar]
- [113].Bellamkonda R, Ranieri JP, Aebischer P, J. Neurosci. Res 1995, 41, 501. [DOI] [PubMed] [Google Scholar]
- [114].Cullen DK, LaPlaca MC, J. Neurotrauma 2006, 23, 1304. [DOI] [PubMed] [Google Scholar]
- [115].Collins MN, Birkinshaw C, Carbohydrate Polymers 2013, 92, 1262. [DOI] [PubMed] [Google Scholar]
- [116].Lee KY, Mooney DJ, Prog. Polym. Sci 2012, 37, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cullen DK, Lessing MC, Laplaca MC, Ann. Biomed. Eng 2007, 35, 835. [DOI] [PubMed] [Google Scholar]
- [118].Cullen DK, Vukasinovic J, Glezer A, LaPlaca MC, in Annual International Conference of the IEEE Engineering in Medicine and Biology – Proceedings, New York, NY, USA, 2006, pp. 636–639. [DOI] [PubMed] [Google Scholar]
- [119].Cullen DK, Vukasinovic J, Glezer A, Laplaca MC, J. Neural Eng 2007, 4, 159. [DOI] [PubMed] [Google Scholar]
- [120].Irons HR, Cullen DK, Shapiro NP, Lambert NA, Lee RH, Laplaca MC, J. Neural Eng 2008, 5, 333. [DOI] [PubMed] [Google Scholar]
- [121].LaPlaca MC, Cullen DK, McLoughlin JJ, Cargill RS, J. Biomech 2005, 38, 1093. [DOI] [PubMed] [Google Scholar]
- [122].Adams CS, Antoci V, Harrison G, Patal P, Freeman TA, Shapiro IM, Parvizi J, Hickok NJ, Radin S, Ducheyne P, J. Orthop. Res 2009, 27, 701. [DOI] [PubMed] [Google Scholar]
- [123].Ducheyne P, Mauck RL, Smith DH, Nat. Mater 2012, 11, 652. [DOI] [PubMed] [Google Scholar]
- [124].Giacoia GP, Taylor-Zapata P, Mattison D, Clin. Ther 2008, 30, 2097. [DOI] [PubMed] [Google Scholar]
- [125].Macarthur JW, Purcell BP, Shudo Y, Cohen JE, Fairman A, Trubelja A, Patel J, Hsiao P, Yang E, Lloyd K, Hiesinger W, Atluri P, Burdick JA, Woo YJ, Circulation 2013, 128, S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mealy JE, Rodell CB, Burdick JA, J. Mater. Chem. B 2015, 8010, 8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chan TM, Chan MSA, Dhobale A, Adewole DO, Cullen DK, Kraft R, in IEEE Signal Processing in Medicine and Biology Symposium (SPMB16), Philadelphia, PA, USA, 2016. [Google Scholar]
- [128].Fridman GY, Blair HT, Blaisdell AP, Judy JW, Exp. Brain Res 2010, 203, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Amatachaya A, Jensen MP, Patjanasoontorn N, Auvichayapat N, Suphakunpinyo C, Janjarasjitt S, Ngernyam N, Aree-Uea B, Auvichayapat P, Behav. Neurol 2015, 2015. [DOI] [PMC free article] [PubMed]
- [130].Semeniken K, Merchenthaler I, Hu W, Dudas B, J. Chem. Neuroanat 2009, 37, 229. [DOI] [PubMed] [Google Scholar]
- [131].Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, Borrie M, Wells J, Dziobek I, Pasternak S, Mitchell DGV, Rankin K, Kertesz A, Boxer A, Neurology 2015, 84, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Duda M, Daniels J, Wall DP, Autism Dev J. Disorders 2016, 46, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Daadi MM, Klausner JQ, Bajar B, Goshen I, Lee-Messer C, Lee SY, Winge MCG, Ramakrishnan C, Lo M, Sun G, Deisseroth K, Steinberg GK, Cell Transplant 2015, 25, 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Dayawansa S, Wang EW, Liu W, Markman JD, Gelbard H. a, Huang JH, Neurol. Res 2014, 36, 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Cristofanilli M, Harris VK, Zigelbaum A, Goossens AM, Lu A, Rosenthal H, Sadiq SA, Stem Cells Dev 2011, 20, 2065. [DOI] [PubMed] [Google Scholar]
- [136].Iwata A, Browne KD, Pfister BJ, Gruner JA, Smith DH, Tissue Eng 2006, 12, 101. [DOI] [PubMed] [Google Scholar]
- [137].Kim SU, de Vellis J, J. Neurosci. Res 2009, 87, 2183. [DOI] [PubMed] [Google Scholar]
- [138].Faroni A, Terenghi G, Reid AJ, Int. Rev. Neurobiol 2013, 108, 121. [DOI] [PubMed] [Google Scholar]
- [139].Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA, Cell Stem Cell 2013, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hu B-Y, Weick JP, Yu J, Ma L-X, Zhang X-Q, Thomson JA, Zhang S-C, Proc. Natl. Acad. Sci. USA 2010, 107, 4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Choi SS, Lee S-R, Kim SU, Lee HJ, Exp. Neurobiol 2014, 23, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Chiu AY, Rao MS, Neurotherapeutics 2011, 8, 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Korecka JA, Verhaagen J, Hol EM, Regen. Med 2007, 2, 425. [DOI] [PubMed] [Google Scholar]
- [144].Liu Y, Kelamangalath L, Kim H, Han SB, Tang X, Zhai J, Hong JW, Lin S, Son YJ, Smith GM, Exp. Neurol 2016, 283, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hughes RM, Freeman DJ, Lamb KN, Pollet RM, Smith WJ, Lawrence DS, Angew. Chem. Int. Ed 2015, 54, 12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Jones BS, Lamb LS, Goldman F, Di Stasi A, Front. Pharmacol 2014, 5, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Gargett T, Brown MP, Front. Pharmacol 2014, 5, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Eissenberg LG, Rettig M, Dehdashti F, Piwnica-Worms D, DiPersio JF, Front. Pharmacol 2014, 5, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]