Auto-immune disorders have been reported following COVID-19, namely connective tissue disorders [1]. Features of systemic Lupus erythematosus (SLE) in the setting of COVID-19 infection were described [2], [3], [4], although an histopathological proof of SLE related organ damage in this setting is lacking. We report a case of SLE onset in an elderly man followed for quiescent primary Sjögren's syndrome, with evidence of immune complex glomerular disease.
A 76-year-old man, native from Cambodia, was admitted to the emergency department in December 2020 for a 10 day history of asthenia and dyspnea. He had a history of Sjögren's syndrome without systemic complications, diagnosed six years ago with anti-SSA/Ro, anti-SSB/La antibodies positivity and positive salivary gland biopsy. Anti-double stranded DNA (anti-dsDNA) and anti-Sm were negative at the time. Initial evaluation revealed confusion, lower limb edema and hypertension (blood pressure 210/105 mmHg). Nasopharyngeal polymerase chain reaction test was positive for SARS-CoV-2. Blood tests showed acute kidney injury (serum creatinine 339 μmol/L), hemolytic anaemia (hemoglobin 78 g/L, haptoglobin < 0.07 g/L) and thrombocytopenia (platelet count 67 g/L). Urine analysis found gross hematuria and proteinuria (urine protein-to-creatinine ratio 4 g/g, urine albumin-to-creatinine ratio 3.2 g/g). Computed tomography showed consolidations in lower lobes, bilateral pleural effusion, pericardial effusion and non-obstructed normal sized kidneys.
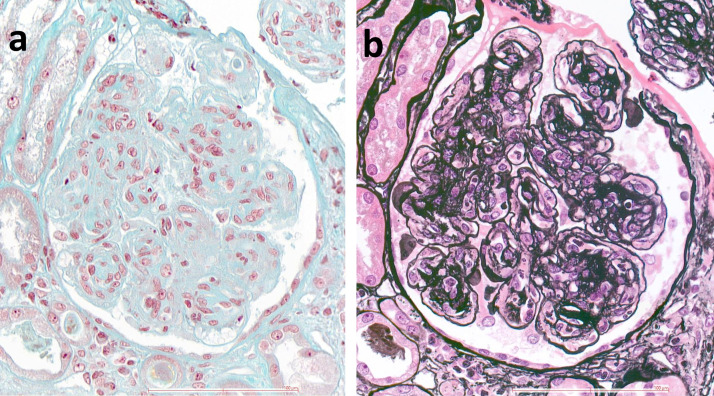
Autoimmune hemolytic anemia was diagnosed (direct Coombs’ test positive for IgG antibody) so 1 mg/kg corticosteroid therapy was started. Blood testing revealed antinuclear antibodies with homogeneous pattern (1:1280), identified as anti-dsDNA antibodies with high titer (343 IU/ml), associated with anti-Sm, anti-SSA/Ro, anti-SSB/La and anti-ribosomal P antibodies. Complement proteins were low (C3 0.23 g/L, C4 < 0.06 g/L). Transjugular renal biopsy showed 12 glomeruli, of which 5 displayed global sclerosis and 7 global lesions characterized by endocapillary and mesangial proliferation (Fig. 1 a) with double contours of the glomerular basement membrane on silver stain (Fig. 1b). No frozen biopsy was available for immunofluorescence study. The patient accumulated 33 points according to the 2019 EULAR/ACR classification criteria [5].
Fig. 1.
Renal biopsy. (a): The glomerulus displays nodular mesangial sclerosis with capillary lumens occluded by the proliferation of mesangial and inflammatory cells (Trichrome stain, × 400); (b): The same glomerulus with extensive duplication of peripheral capillary glomerular basement membrane (silver stain, × 400).
Neurological symptoms went worse and required the patient to be intubated. Magnetic resonance imaging of the brain showed lesions of posterior reversible encephalopathy syndrome so corticosteroid therapy was decreased to 0.5 mg/kg. Cerebrospinal fluid analysis was normal. Cyclophosphamide pulses were introduced. The response to immunosuppressive treatments was poor. He died on day 35 of hospitalization.
SLE onset in elderly men, even with a history of Sjögren's syndrome, is very unusual. Higher serum levels of pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) and decrease of regulatory T cells were found in patients with severe COVID-19. COVID-19 has been shown to trigger anti-phospholipid antibodies which might play a pathogenic role in the thrombotic events frequently found in these patients [6]. Likewise, in our patient with a non-active autoimmune disease, the infection could result in the break of immune homeostasis, the onset of new pathogenic antibodies and in the switch of the phenotype and severity of the underlying auto-immune disease.
Consent
The patient's next of kin provided written informed consent for publication.
Contributors
GR, AP, IB, SF and XM managed the case, designed the work and drafted and revised the paper.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data underlying this article are available in the article
Acknowledgements
None.
References
- 1.Ahmed S., Zimba O., Gasparyan A.Y. COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol. 2021:1–9. doi: 10.1007/s10067-021-05691-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani Cardoso E., Hundal J., Feterman D., et al. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence?. A case report and review of intertwining pathophysiology. Clin Rheumatol. 2020;39:2811–2815. doi: 10.1007/s10067-020-05310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slimani Y., Abbassi R., El Fatoiki F.Z., et al. Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J Med Virol. 2021;93:1184–1187. doi: 10.1002/jmv.26513. [DOI] [PubMed] [Google Scholar]
- 4.Bonometti R., Sacchi M.C., Stobbione P., et al. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci. 2020;24:9695–9697. doi: 10.26355/eurrev_202009_23060. [DOI] [PubMed] [Google Scholar]
- 5.Aringer M., Costenbader K., Daikh D., et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 6.Amezcua-Guerra L.M., Rojas-Velasco G., Brianza-Padilla M., et al. Presence of antiphospholipid antibodies in COVID-19: case series study. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article