Abstract
Background
Diagnosis of COVID-19 infection in cancer patients is critical to co-manage their underlying disease and infection appropriately. Our study aimed at evaluating the sensitivity and specificity of screening patients with cancer for COVID-19 infection.
Methods
All oncology patients receiving care at Department of Oncology at King Abdulaziz Medical City in Riyadh were screened using the acute respiratory infection (ARI) survey. Nasopharyngeal and throat swap for polymerase chain reaction (PCR) testing for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was performed on patients who have high ARI score (i.e. ≥ 4), or any patient requiring elective/emergency hospitalization, undergoing a procedure as well as screening asymptomatic patients receiving chemotherapy between April 1st and July 30, 2020. Institutional Review Board approval was obtained. Descriptive and inferential analyses were done and sensitivity, specificity, positive and negative predictive values (PPV and NPV) were calculated considering the COVID-19 PCR as the gold standard.
Results
During the study period, a total of 473 patients were included with a median age was 56 years (14–104), 51% were female, 73% had solid tumors, and 66% received treatment within the last 3 months. These patients underwent 688 PCR tests along with ARI survey screening. Testing was done in the outpatient, inpatient, and emergency department setting in 41%, 40% and 19% of the patients, respectively. Majority of tests were screening of asymptomatic patients and only 23% were tested for suspected infections with ARI ≥ 4. A total of 54 patients (8%) had positive PCR for COVID-19 infection. The prevalence of infection varied from month to month ranging from 1.09% in April up to 19.70% in June and correlated with the average daily and active case load at a national level. The diagnostic yield of the ARI score also correlated with infection burden nationally. The PPV and NPV of the ARI as a screening tool was 18.24% (0–31.8) and 95.6% (86.36–98.86%) with the PPN fluctuating considerably in parallel with the prevalence of COVID-19 result. Similarly, the sensitivity and specificity of the ARI were 55.77% (0–70.59) and 79.4 (69.19–92), respectively.
Conclusion
The yield of screening asymptomatic patients with cancer varies based on the community burden of COVID-19 infection. As universal screening can cause delays to patient care, it should be tailored based on the individual patient risks and infection burden in the region.
Keywords: COVID-19, RT-PCR, Laboratory testing, Screening, Cancer patients
Background
The 2019 novel coronavirus (COVID-19) pandemic significantly affected cancer patients due to the interruption of their care and their risk of infections [[1], [2], [3], [4], [5]]. Providing optimal care to patients with cancer requires timely delivery of cancer therapy while protecting them from the risk of infection. This approach requires implementations of many interventions to prevent disease transmission such as social distancing, hand hygiene, global masking, and others. One of the most critical measure is early recognition of suspected cases through preemptive screening programs and quarantining them to prevent dissemination of the virus to other patients or staff [6,7]. Suspected cases by clinical screening can undergo laboratory testing by nasal swab and real time-PCR (RT-PCR) test for the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Identifying positive patients will help protecting others form transmission of disease, help in providing close monitoring for any deterioration, and guide in the timing of cancer treatment or procedures by either postponing them or if urgent, performing them under special precautions. The acute respiratory infection (ARI) screening tool is frequently used to identify suspected cases by asking specific questions about symptoms related to COVID-19 or exposure to confirmed cases. Patients with high score of ARI will undergo RT-PCR testing. However, many physicians are screening patients with cancer by RT-PCR routinely irrespective of their symptoms or ARI score especially prior to chemotherapy, radiotherapy, or procedures.
In order to decrease spread of COVID-19 among this vulnerable population, screening, early recognition, and quarantining may be necessary interventions. There are different recommendations on how and when to screen oncology patients for COVID-19 infection including clinical screening and laboratory testing. Multiple groups and organizations including the American Society of Clinical Oncology (ASCO) released guidelines on managing cancer patients during the COVID-19 pandemic [1,[8], [9], [10]]. Among their recommendations, they included screening patients prior to their hospital visit and apply triage protocols if patients have suspicious symptoms and testing asymptomatic patients 48–72 h prior to administration of chemotherapy, stem cell transplantation and other immune-suppressive treatments depending on testing capability. The aim of this study was threefold. First, to assess the yield of COVID-19 RT-PCR testing among oncology patients in different settings. Second, to validate the utility of the locally developed ARI screening tool in predicting the PCR results. Third, to correlate the local incidence and prevalence of infection to the national result.
Methods
Study design
Following due institutional review board approval, this was a retrospective study performed at the oncology department at King Abdulaziz Medical City in Riyadh, Saudi Arabia. The department serves patients with all solid and hematological malignancies and provide all modalities of treatments including surgery, radiation therapy, stem cell transplant and all systemic therapies.
During the period of March to July 2020, consecutive patients seen at the department whom underwent a COVID-19 RT-PCR test were eligible for inclusion. Nasopharyngeal and throat swabs for COVID-19 real time PCTR were obtained from all patients using appropriate personal protective equipment including N95 respirators within a negative pressure room. All patient and disease related variables were retrospectively collected from the electronic medical records.
Acute respiratory infection (ARI) screening
All patients were contacted via phone call prior to their appointment in the outpatient infusion suite by a health care professional to ensure they were symptom free. In case COVID-19 related symptoms were present, patients were advised not to attend to their scheduled visit and the most responsible physician (MRP) of the patient was contacted to arrange for an alternative and appropriate medical plan. Patients planned for outpatient chemotherapy administration, procedure or an elective admission underwent COVID-19 RT-PCR screening prior. Patients admitted from the emergency department also underwent PCR screening regardless of the reason for admission.
Furthermore, all patients underwent ARI form screening at the time of initial contact with the health care professional (Table 1 ). This is a locally developed screening tool aiming to identify patients with risk factors of COVID-19 infection, such as symptoms of infection and suspected or confirmed exposure to a positive case. In the outpatient setting, patients scoring ≥ 4 points were placed in an isolation room and COVID-19 RT-PCR swab was done, the MRP was contacted for patients scoring >0 but <4 for appropriate disposition whereas patients with score of 0 proceeded to receive their scheduled treatment.
Table 1.
Acute respiratory infection screening tool to identify patients with suspected signs and symptoms of COVID-19 infection and those with exposure risk.
| Question | Point(s) |
|---|---|
| A. Clinical symptoms/signs | |
| Fever (≥38 °C) | 4 |
| Cough (new or worsening) | 4 |
| Shortness of breath (new or worsening) | 2 |
| Sore throat and/or runny nose | 2 |
| Nausea/vomiting AND diarrhea | 2 |
| B. Risk of exposure to COVID-19 | |
| Unprotected exposure to confirmed COVID-19 case in last two weeks | 4 |
| Any health care facilities staff (healthcare worker or supporting services) | 2 |
A score ≥4 → place patient in an isolation room and inform MD for assessment.
Statistical analysis
This was a retrospective study including oncology patients whom were served at our center between March and July 2020. Data were collected retrospectively which included age, gender, diagnosis, systemic therapy used over the last three months, ARI score, indication for RT-PCR testing, location of testing and result. Baseline characteristics were reported using descriptive statistics (numbers, medians and percentage). For inferential statistics, the relationship between the categorical and continuous variables with the study variables will be analyzed using the Pearson’s Chi-square and and Wilcoxon/Kruskal–Wallis as appropriate. Calculation of sensitivity, specificity, positive and negative predictive values were performed considering the COVID-19 RT-PCR as the gold standard.
Results
Baseline patient characteristics & indications for COVID-19 PCR swabs
During the study period, a total of 473 patients were included with a median age of 56 years (14–104), 51% of whom were female. Underlying diagnosis was solid malignancy in 73%, 92 (19%) had a hematological malignancy while 8 (2%) underwent a hematopoietic stem cell transplantation. About two thirds of patients received treatment within the last three months. Most common types of treatment was chemotherapy in 230 (73%) patients followed by targeted therapy (oral or intravenous) in 45 (14%) of patients followed by immunotherapy in 17 (5%) of patients.
A total of 688 COVID-19 PCR tests were done in 473 patients. The indication for testing was for screening purposes in the majority of patients (prior to chemotherapy, a medical procedure or admission to hospital) in 454 (66%) of swabs. Another 159 (23%) swabs were performed in patients suspected to have infection due to symptoms, unprotected exposure to a positive case or a high ARI screen. Finally, 70 (10%) swabs had an unknown indication due to lack of documentation in the medical records. The swabs were taken in the outpatient, inpatient and emergency department in 41%, 40% and 19% of the time, respectively. These results are shown further in Table 2 .
Table 2.
Baseline characteristics of the patient cohort that underwent COVID-19 swab along with its indication.
| Characteristic | N (%) |
|---|---|
| Female gender, n (%) | 243 (51) |
| Age, median (range) | 56 (14−106) |
| Area, n (%) | |
| Outpatient | 282 (41) |
| Inpatient | 271 (40) |
| Emergency | 130 (19) |
| Reason for testing, n (%) | |
| Screening (chemo or procedure) | 219 (32) |
| Screening (pre-admission) | 235 (34) |
| Suspected | 159 (23) |
| Unknown | 70 (10) |
| Diagnosis, n (%) | |
| Medical oncology | 344 (73) |
| Malignant hematology | 92 (19) |
| Other hematology disorder | 26 (6) |
| Stem cell transplant | 8 (2) |
| Other | 3 (<1) |
| Oncology treatment within three months, n (%) | |
| Yes | 311 (66) |
| No | 162 (34) |
| Oncology treatment type, n (%) | |
| Chemotherapy | 230 (73) |
| Targeted oral/intravenous | 45 (14) |
| Hormonal | 12 (4) |
| Immunotherapy | 17 (5) |
| Radiotherapy | 9 (3) |
Utility of acute respiratory infection screening
The median ARI score was 2 (0−10) with 314 (46%) swabs having a score of 0 while 159 (23%) swabs having an ARI score ≥4. Overall, a total of 54 (8%) of swabs were positive for a total of 43 patients. There were a total of 215 repeated swabs, 11 of whom were repeat positive. These results are shown further in Table 3 .
Table 3.
Result of all COVID-19 polymerase chain reaction swabs and acute respiratory infection score.
| Characteristic | N (%) |
|---|---|
| ARI score, median (range) | 2 (0−10) |
| ARI score, n (%) | |
| 0 | 314 (46) |
| 1–<4 | 210 (31) |
| ≥4 | 159 (23) |
| Unknown | 5 (<1) |
| Result of testing, n (%) | |
| Positive | 54 (8) |
| Negative | 634 (92) |
Stratified by ARI score (high vs. low), patient gender, underlying diagnosis and treatment received were similar between the two cohorts. High ARI score was significantly associated with swabs taken in the emergency department (39% vs. 13%, p < 0.0001), having a suspected case (53% vs. 14%, p < 0.0001) and having a positive COVID-19 PCR result (18% vs. 4%, p < 0.0001). These results are shown further in Table 4 .
Table 4.
Relationship between characteristics of cancer patients and their results of PCR test for COVID-19 according to acute respiratory infection score value.
| Characteristic | ARI < 4 | ARI ≥ 4 | p Value |
|---|---|---|---|
| Female gender, n = 473 (%) | 195 (52) | 180 (48) | 0.66 |
| Age n = 473, median (range) | 55 (14−103) | 61 (14−106) | 0.0003 |
| Area, n = 688 (%) | <0.0001 | ||
| Outpatient | 244 (47) | 38 (24) | |
| Inpatient | 212 (40) | 59 (37) | |
| Emergency | 68 (13) | 62 (39) | |
| Reason for testing, n = 688 (%) | <0.0001 | ||
| Screening (chemo or procedure) | 193 (37) | 26 (16) | |
| Screening (pre-admission) | 209 (40) | 26 (16) | |
| Suspected | 75 (14) | 84 (53) | |
| Unknown | 47 (9) | 23 (14) | |
| Diagnosis, n = 473 (%) | 0.49 | ||
| Medical oncology | 270 (72) | 74 (76) | |
| Malignant hematology | 72 (19) | 19 (20) | |
| Other hematology disorder | 23 (6) | 3 (3) | |
| Stem cell transplant | 7 (2) | 1 (1) | |
| Other | 3 (1) | 0 | |
| Treatment within three months, n = 688 (%) | 0.25 | ||
| Yes | 375 (72) | 121 (76) | |
| No | 121 (28) | 38 (24) | |
| Result of testing, n = 688 (%) | <0.0001 | ||
| Positive | 23 (4) | 29 (18) | |
| Negative | 501 (96) | 130 (82) |
a5 pts with unknown ARI were excluded.
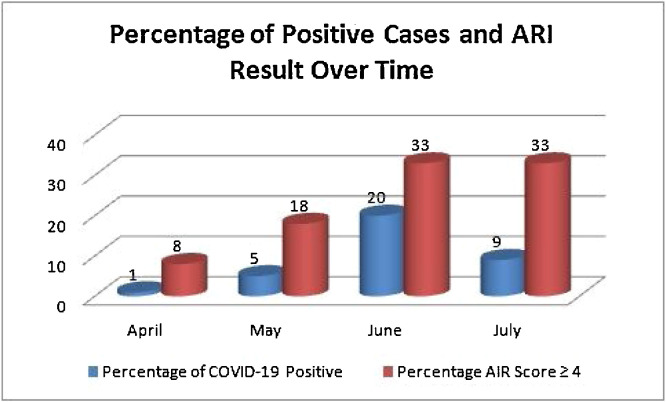
Over the study period, the performance of the ARI as a screening tool was examined on a monthly basis. Overall, the prevalence of COVID-19 infection was 7.61% ranging from 1% in April to 19.7% in at its peak in June (Fig. 1 ). Considering COVID-19 PCR as the gold standard for diagnosis, the PPN and NPV of the ARI as a screening tool was 18.24% (0–31.8) and 95.6% (86.36–98.86% with the PPN fluctuating considerably in parallel with the prevalence of COVID-19 result. Similarly, the sensitivity and specificity of the ARI were 55.77% (0–70.59) and 79.4 (69.19–92), respectively. Furthermore, the prevalence of COVID-19 infection locally correlated with the infection burden on a national level measured as average daily new infections and daily active infections as shown in Table 5 .
Fig. 1.
Percentage of COVID-19 positive cases and acute respiratory infection score on a monthly basis.
Table 5.
Performance and utility of the ARI score locally compared with the infection burden on a national level.
| April | May | June | July | Cumulative | |
|---|---|---|---|---|---|
| Average daily COVID-19 cases at national level, n | 711 | 2016 | 3519 | 2745 | 2250 |
| Average daily active COVID-19 cases at national level, n | 390 | 7970 | 26,396 | 53,570 | 26,333 |
| Total number of PCR tests done, n | 196 | 147 | 134 | 204 | 681a |
| PCR positive tests, n (%) | 2 (1) | 7 (5) | 26 (20) | 17 (8) | 52 (8) |
| Percentage of positive PCR tests in ARI ≥ 4, n (%) | 0 | 3 (43) | 14 (54) | 12 (71) | 29 (56) |
| Sensitivity | 0.00% | 42.86% | 53.85% | 70.59% | 55.77% |
| Specificity | 92.02% | 82.86% | 71.70% | 69.19% | 79.40% |
| Positive predictive value | 0 | 11.11% | 31.82% | 17.39% | 18.24% |
| Negative predictive value | 98.86% | 96.67% | 86.36% | 96.24% | 95.61% |
| Prevalence | 1.05% | 4.76% | 19.70% | 8.42% | 7.61% |
An additional 7 PCR swabs were done in March 2020 but not included herein.
Discussion
The COVID-19 pandemic presented considerable challenges in delivering optimal cancer care to patients. A number of societies including the European Society of Medical Oncology (ESMO) as well as ASCO recommended to screen patients prior to cancer treatment but additional factors such as laboratory capacity with PCR turnaround time must be considered at each institution as it can further cause delays to care delivery. The ESMO reported the utility of a pre-screening tool for COVID-19 infection using a patient-reported platform [11]. Using this tool, COVID-19 related symptoms are monitored and uploaded in the patient’s electronic records, with an alarm triggered in such cases to advise the patient to report for PCR testing.
The issue of screening asymptomatic cancer patients is subject to debate. Lee et al., reported on 1989 tests in 1226 patients at the UK Birmingham chemotherapy cancer center and observed an infection prevalence of 0.6% among asymptomatic patients [12]. On the other hand, Al-Shamsi et al., 109 asymptomatic cancer patients underwent serial screening swabs totaling 384 specimens and noted that 25 out of 32 patients (78.1%) were diagnosed while asymptomatic [13]. From these reports and our own observations, such numerical quantitation is variable based on the community status of the pandemic at a given time point. Therefore, giving a percentage of prevalence among this population is inaccurate as it is time-bound and correlates with external variables. Herein, we noted that the overall prevalence of infection during the study period was 7.61%, however, it varied considerably with time correlating with the proportion of active cases at a national level. The predictive value of the ARI screening tool varied with the prevalence of the infection over time. Specifically, the PPV performance of the ARI was poor ranging from 0 to 31% indicating that this screening tool is not ideal for ruling in infection. On the other hand, the NPV ranged from 86 to 98% indicating that it would be a helpful tool in ruling out infection. Furthermore, even the value of ARI screening correlated with the disease burden in the community and when the case load was low, there was an overestimation of the suspected cases among cancer patients leading to performing of more tests. Collectively, the ARI would be most helpful to rule out infection in a time of low infection burden nationally.
This analysis carries a number of limitations that should be highlighted. First, the attributes of the ARI screening tool were compared to the PCR test which is considered to be the gold standard. However, false negative PCR samples are possible particular due to the nasopharyngeal swab acquisition technique and other limitations of the techniques itself may lead to underestimation of the ARI value [[14], [15], [16], [17], [18]]. Second, approximately 10% of the cohort did not have ARI results and it was unclear whether it was due to lack of administration or documentation in the medical records. Lastly, it is possible but less likely that patients may not report their infection symptoms due to fears of delaying their planned oncology treatments.
In conclusion, these findings challenge the application of universal recommendations of screening all Oncology patients prior to treatment without consideration of the status of the pandemic in the community. Furthermore, there are variations in the type of treatments, procedures, type of underlying malignancies, patients’ general condition and underlining comorbidities [19]. Screening at the surge of the pandemic for patients with certain malignancies (hematologic malignancies, lung cancer or metastatic cancers), or procedure (particularly aerosol generating procedures) is probably justifiable. However, universal screening when the prevalence in the community is low may not be cost effective or practical as it may cause unnecessary delays to treatments and inconvenience to the patients. Adapting the recommendations for local setting and situations is required similar to what was suggested for testing asymptomatic healthcare workers [20].
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgments
We would like to acknowledge all members of the health care team of physicians, nurses, nurse specialists, coordinators and administrators who provide excellent care to our patients. We would also acknowledge our clinic nurse manager Ms. Noridayu Rashid and nurse specialist Nadine Mabsout for data collection.
References
- 1.Curigliano G., Banerjee S., Cervantes A., Garassino M.C., Garrido P., Girard N., et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31(10):1320–1335. doi: 10.1016/j.annonc.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessy S.A., Davies E.A., Jazieh A.-R. Cancer care during the COVID-19 pandemic: a perspective from Saudi Arabia. Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martei Y.M., Rick T.J., Fadelu T., Ezzi M.S., Hammad N., Quadri N.S., et al. Impact of COVID-19 on cancer care delivery in Africa: a cross-sectional survey of oncology providers in Africa. JCO Glob Oncol. 2021;(7):368–377. doi: 10.1200/go.20.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards M., Anderson M., Carter P., Ebert B.L., Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1(6):565–567. doi: 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jazieh A.R., Akbulut H., Curigliano G., Rogado A., Alsharm A.A., Razis E.D., et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. 2020;(6):1428–1438. doi: 10.1200/go.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutikov A., Weinberg D.S., Edelman M.J., Horwitz E.M., Uzzo R.G., Fisher R.I. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172(11):756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabiana G., Caliandro M., Surgo A., Carbonara R., Bonaparte I., Fiorentino A. Cancer patients in covid-19 era: swimming against the tide. Radiother Oncol. 2020;149:109–110. doi: 10.1016/j.radonc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ASCO Special Report: a Guide to Cancer Care Delivery During the COVID-19 Pandemic. Dec 2020. https://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-https://www.asco.org/sites/new-.
- 9.You B., Ravaud A., Canivet A., Ganem G., Giraud P., Guimbaud R., et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21(5):619–621. doi: 10.1016/S1470-2045(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Shamsi H.O., Alhazzani W., Alhuraiji A., Coomes E.A., Chemaly R.F., Almuhanna M. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters M., van Dam P., Rasschaert M.A., Vulsteke C., De Keersmaecker S., Croes L., et al. Prescreening for COVID-19 in patients receiving cancer treatment using a patient-reported outcome platform. ESMO Open. 2020;5(3) doi: 10.1136/esmoopen-2020-000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y.W., Hill T., Topping O., Tilby M., Baker M., Greig J., et al. Utility of COVID-19 screening in cancer patients. Cancer Cell. 2020;38(3):306–307. doi: 10.1016/j.ccell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Shamsi H.O., Coomes E.A., Aldhaheri K., Alrawi S. Serial screening for COVID-19 in asymptomatic patients receiving anticancer therapy in the United Arab Emirates. JAMA Oncol. 2021;7(1):129–131. doi: 10.1001/jamaoncol.2020.5745. Published online 5 November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296(2):E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dramé M., Tabue T., Proye E., Hequet F., Hentzien M., Kanagaratnam L., et al. Should RT-PCR be considered a gold standard in the diagnosis of COVID-19? J Med Virol. 2020;92(11):2312–2313. doi: 10.1002/jmv.25996. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Yao L., Li J., Chen L., Song Y., Cai Z., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92(7):903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinh D.B., Zhao X., Kiong K.L., Guo T., Jozaghi Y., Yao C., et al. vol. 42. John Wiley and Sons Inc.; 2020. Overview of COVID-19 testing and implications for otolaryngologists; pp. 1629–1633. (Head and neck). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madariaga A., McMullen M., Sheikh S., Kumar R., Liu F.F., Zimmermann C., et al. COVID-19 testing in patients with cancer: does one size fit all? Clin Cancer Res. 2020;26(18):4737–4742. doi: 10.1158/1078-0432.ccr-20-2224. [DOI] [PubMed] [Google Scholar]
- 20.Treibel T.A., Manisty C., Burton M., McKnight Á., Lambourne J., Augusto J.B., et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(10237):1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]