Abstract
Objectives
The objective of this study was to use a decision-analytic model to examine the potential economic impact of establishing a remunerated programme for pharmacists prescribing for minor ailments (PPMA) in Ontario, Canada.
Methods
A novel decision tool was developed to assess the economic impact of pharmacists prescribing for upper respiratory tract infections (URTIs), contact dermatitis (CD) and conjunctivitis by performing a cost-minimization analysis from a public payer perspective. Two prescribing strategies were compared: (1) PPMA, where patients may seek care from pharmacists or physicians, and (2) the usual care model (UCM), where all patients receive care from physicians. Two remuneration models for the PPMA strategy were also compared: (1) a prescription-detached scenario (PDS), where pharmacists were remunerated CAD$18.00 for each consultation, and (2) a Prescription-Attached Scenario (PAS), where pharmacists were only remunerated if a decision to prescribe was made.
Key findings
At a service uptake rate of 38% for the PDS, the PPMA model led to savings of $7.51, $4.08 and $5.15 per patient for URTIs, CD and conjunctivitis, respectively. Per 30 000 patients, the PPMA model for these minor ailments was projected to lead to cumulative reductions in visits to the emergency department, family physician and walk-in clinics by 799, 3677 and 5090, respectively.
Conclusions
The results of the study strongly suggest that enabling community pharmacists to assess and prescribe for minor ailments could potentially lead to large savings for the government in Ontario, Canada. In 100% of the PAS scenarios simulated, pharmacists as prescribers led to cost savings.
Keywords: pharmacist, prescribing, minor ailment, expanded scope, economic evaluation
Introduction
In Canada, the role of pharmacists has been rapidly evolving.[1]Many provinces have begun to adopt pharmacist prescribing to varying degrees, where eight provinces currently enable prescribing for minor ailments.[1] Most recently, Ontario has begun developing a pharmacists prescribing for minor ailments (PPMA) programme.[2] Although there is no universally adopted definition for minor ailments, they are often considered as ‘health conditions that can be reliably self-diagnosed by a patient familiar with their condition, and managed with self-care strategies and/or minimal treatment’.[3]
Pharmacists have the potential to address some of the most pressing issues with the Canadian healthcare system. For example, those without a regular primary care provider may need to wait for hours at a walk-in clinic or go to the emergency department (ED) for assessment and treatment of a less urgent issue such as assessment and treatment of a minor ailment or a prescription refill. Even for patients who have a family physician, many are unable to book a same-day appointment. In fact, according to Health Quality Ontario, while 94.1% of Ontario residents have a family physician, only 39.9% of those are able to book a same-day appointment.[4]
Few studies have assessed the economic impact of PPMA models in Canada. Rafferty et al. calculated the cost of this programme in comparison to usual care from public payer and societal perspectives in the province of Saskatchewan.[5] The results of this study illustrated that pharmacists managing minor ailment patients could save Saskatchewan $3 482 660 over 5 years of implementation.[5] However, this analysis was criticized in the literature in terms of design, hypotheses and model inputs.[6] Lathia et al. performed a cost-minimization analysis to evaluate a point-of-care testing programme for strep throat by community pharmacists across five Canadian jurisdictions.[7] However, the input data depended on data from surveys of patients and physicians, in addition to some estimated inputs based on expert opinion.[7]
In the UK, where one of the oldest PPMA programmes exist, Hassell et al. noted that, while PPMA did not demonstrate a significant reduction in physician workload, 37.8% of minor ailment patients chose to seek care from a pharmacist over the study’s 6-month period.[8] A pilot study in two areas of the UK found that the number of physician consultations for minor ailments decreased from 4.1% and 7.9% to 2.6% and 5.3%, respectively.[9] This study also found that the PPMA programme had little impact on physician workload but noted that physicians were supportive of the programme.[9] Lastly, a North East England study concluded that, although physician workload was not impacted, a PPMA programme was expected to shift general physicians’ focus from minor ailments to more complex patients.[10] A systematic review of pharmacy-based minor ailment schemes by Paudyal et al. identified that no study included a full economic evaluation.[11] As such, this study aims to estimate the potential economic impact of a PPMA programme in Ontario, Canada through a decision-analytic model by performing a cost-minimization analysis from a public payer perspective.
Methods
Study design
A decision tree was developed to perform a cost-minimization analysis of pharmacist prescribing, designed to assess a proposed PPMA programme in Ontario, Canada, and compare it to the current prescribing strategy to identify the least costly method. In the context of this study, minor ailments were defined as self-limiting conditions that may be managed with or without therapeutic intervention. Three conditions were evaluated: upper respiratory tract infections (URTIs), contact dermatitis (CD) and conjunctivitis. CD and conjunctivitis were chosen based on previous work which found that they are among the most common minor ailments presenting to the ED in Ontario.[12] On the other hand, URTI was chosen based on a 2012 Canadian study which stated that, if 16% of people who saw a physician for cold/flu symptoms practiced self-care, it would save the Canadian healthcare system $98 million annually or allow nearly half a million Canadians to have access to a family physician.[13] The analyses were performed following the guidelines for economic evaluation by the Canadian Agency for Drugs and Technologies in Health (CADTH).[14] The difference in the cost to treat each case of minor ailment and the number of family physician visits, walk-in clinic visits and ED visits avoided were the primary outcomes of interest. The study was performed from a public payer perspective with the costs adjusted to 2019 Canadian dollars.
Strategies
Two prescribing strategies were considered in this study:
(1) PPMA: In this strategy, pharmacists have the authority to assess and prescribe for minor ailments. Therefore, patients seeking care for a minor ailment have the option of going to either a community pharmacist or a physician (walk-in clinic, ED or family physician office). In either case, unless there is a need for an urgent referral to the ED, the prescriber has the option of recommending a prescription drug, a non-prescription drug or not recommending any drug therapy. If the ailment does not resolve following the first encounter, the patient returns for a second round of assessment and treatment. Those who initially received care from a pharmacist have the choice to go back to a pharmacist or to a physician, while those who initially received care from a physician were assumed to seek care from a physician again. In either case, a prescription drug was assumed to have been recommended in all cases for the second round of therapy.
(2) Usual care model (UCM): This model represents the current prescribing strategy for minor ailments, where pharmacists are unable to prescribe for minor ailments. Therefore, all patients in this model receive care from a family physician office, walk-in clinic or the ED. Similar to the PPMA model, the prescriber has the options of recommending a prescription drug, a non-prescription drug or not recommending any drug therapy. If the ailment does not resolve following the first encounter, all patients were assumed to seek care from a physician for the second time, following the same care-seeking probabilities as the initial visit, where a prescription drug was assumed to have been recommended in all cases.
Two billing scenarios were considered for the PPMA model:
(1) Prescription-detached scenario (PDS): In this scenario, it was assumed that pharmacists would be financially compensated by the government through a consultation fee for assessing patients regardless of the outcome of the assessment and their treatment decision.
(2) Prescription-attached scenario (PAS): In this scenario, it was assumed that pharmacists would be compensated only if the assessment resulted in the provision of a prescription.
Decision model
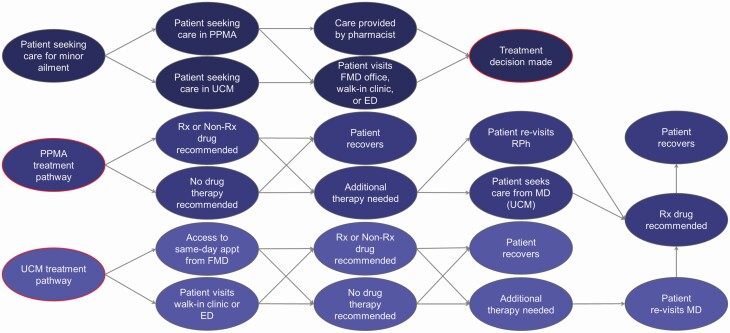
A decision tree was developed using TreeAge Pro 2019 decision analysis software to model patient movement through the two prescribing strategies.[15] A conceptual diagram of the model is illustrated in Figure 1.
Figure 1.
Patient progression through the PPMA and the UCM models. Patients could choose to seek care from either the PPMA model or the UCM. In the PPMA model, patients are assessed by either a pharmacist or a physician (walk-in, ED or family physician). The prescriber has the option of recommending a prescription product, over the counter (OTC) product or not recommend anything. If the ailment does not resolve following the first round of treatment, the patient returns for a second round of assessment, either from a pharmacist or a physician. Alternatively, in the UCM, patients are assessed only by a physician (walk-in, ED or family physician). All patients were assumed to recover after one or two rounds of treatment. Abbreviations: PPMA, pharmacists prescribing for minor ailment; UCM, usual care model; RPh, registered pharmacist; FMD, family physician; ED, emergency department; URTI, upper respiratory tract infection; CD, contact dermatitis.
Model inputs
Probabilities and costs
Most of the probabilities and costs used in the model were derived partially based on the literature and expert opinion, or modelling assumptions when there was insufficient literature. Notably, out-of-pocket costs such as non-prescription drugs and other patient-specific costs were not considered as the model was assessed from a public payer perspective. The prescription drug costs are reimbursed by the provincial government for residents ≤24 years or ≥65 years of age, or for patients 24–65 who are on social assistance. For those not meeting the eligibility, drug costs are charged out-of-pocket or covered through private insurance and thus not accounted for in the model. A full list of the probability and cost parameters is provided in Table 1. Detailed descriptions of the sources of the model parameters may be found in Supplementary Appendix 1.
Table 1.
Model parameters
| Parameter | URTI point estimate (±25%) | CD point estimate (±25%) | Conjunctivitis point estimate (±25%) |
|---|---|---|---|
| Prescribing probabilities (%) | |||
| Prescription drug | 30.20[16] (22.65–37.75) | 78.753,[17] (59.06–98.44) | 46.40[18, 19] (34.80–58.00) |
| Non-prescription drug | 59.801,[16] (44.85–74.75) | 8.753,[17] (6.56–10.94) | 33.60[18, 19] (25.20–42.00) |
| No drug therapy | 10.001 (7.50–12.50) | 12.503,[17] (9.38–15.63) | 20.00[18] (0.00–40.00) |
| Prescription drug – PAS pharmacist | 40.201 (30.15–37.75) | 88.751 (66.56–100.00) | 56.401 (42.30–70.50) |
| Non-prescription drug – PAS pharmacist | 54.801 (41.10–68.50) | 3.751 (2.81–4.69) | 28.601 (21.45–35.75) |
| No drug therapy – PAS pharmacist | 5.001 (3.75–6.25) | 7.501 (5.63–9.38) | 15.001 (11.25–18.75) |
| Care-seeking probabilities (%) | |||
| Seeking care from pharmacist | 38.00[8] (0.00–100.00) | ||
| Seeking care from physician – ED | 10.001 (7.50–12.50) | 3.20[20] (2.40–4.00) | 13.00[21] (9.75–16.25) |
| Seeking care from physician – Walk-in clinic | 52.501 (39.38–65.63) | 59.301 (44.48–74.13) | 49.501 (37.10–61.90) |
| Seeking care from physician – FMD office | 37.504 (28.13–46.88) | ||
| Seeking care from pharmacist for a second visit | 10.001 (7.50–12.50) | ||
| Treatment outcome probabilities (%) | |||
| Getting better after prescription drug | 90.00[16] (67.50–100.00) | 50.003 (37.50–62.50) | 63.10[16] (47.30–78.90) |
| Getting better after non-prescription drug | 75.001 (56.25–93.75) | 50.003 (37.50–62.50) | 56.401 (42.30–70.50) |
| Getting better after no drug therapy | 60.001 (45.00–75.00) | 50.003 (37.50–62.50) | 49.70[22] (37.30–62.10) |
| Miscellaneous probabilities (%) | |||
| FMD billing by fee-for-service | 72.10[23] (54.08–90.13) | ||
| FMD billing by capitation | 27.90[23] (20.93–34.88) | ||
| Drug coverage by government | 26.002 (–) | ||
| Costs ($CAD) | |||
| Pharmacist consultation fee | 18.001 (13.50–22.50) | ||
| Prescription medication4 | 7.803 (5.85–9.75) | ||
| ED visit5 | 172.94[24] (129.71–216.18) | ||
| Walk-in clinic visit6 | 27.70[25] (20.78–34.63) | ||
| FMD billing by FFS6 | 27.70[25] (20.78–34.63) | ||
| FMD billing by CAP7 | 0.001 (–) |
$CAD, Canadian dollar; PAS, prescription-attached scenario; ED, emergency department; FMD, family physician; FDS, fee-detached scenario; FAS, fee-attached scenario; MA, modelling assumption; CIHI, Canadian Institute for Health Information; URTI, upper respiratory tract infection; CD, contact dermatitis.
1Modelling assumption; 2Unpublished data; 3Expert opinion; 4Based on 26% of Ontarians receive drug coverage through the government (unpublished work by Alsabbagh et al.); 5Cost adjusted for inflation to 2019; 6Assumed 50% minor assessment and 50% intermediate assessment; 7CAP: Capitation payment, where physicians are paid a set amount per registered person per time unit to provide care when needed (i.e. regardless if the person seeks care or not).
Modelling assumptions
A number of modelling assumptions were applied in the development of the model. They include physician billing, prescribing decision and care-seeking behaviour. Notably, it was assumed that the treatment decisions and the outcomes between physicians and pharmacists would be identical – an assumption that forms the basis of conducting a cost-minimization analysis. A detailed description may be found in Supplementary Appendix 1.
Analytic strategy
A base-case analysis was performed at 20% increments of the PPMA service uptake rate and at 38%[8] to determine the difference in treatment cost per patient between the PPMA model and the UCM based on probabilistic sensitivity analysis (PSA). A deterministic sensitivity analysis (DSA) was also performed to delineate the impact of each model parameter on the overall outcome of the study.
Key findings
Base-case analysis
In the base-case analysis, we compared the PPMA model and the UCM for URTIs, CD and conjunctivitis in Ontario, Canada. At a PPMA service uptake rate of 38%, in the PDS, the PPMA saved $7.51, $4.08 and $5.15 per patient for URTIs, CD and conjunctivitis, respectively, compared with the UCM. On the contrary, in the PAS, the PPMA was projected to have greater savings of $12.26, $4.89 and $9.27 for URTIs, CD and conjunctivitis, respectively. Per 30 000 patients at the same service uptake rate, the PPMA model was projected to lead to cumulative reductions of 799 ED visits, 3677 family physician visits and 5090 walk-in clinic visits. The full results of the base-case analysis are summarized in Table 2.
Table 2.
Base-case analysis by PPMA uptake rate
| Prescribing strategy (uptake rate) | PDS: Cost ($CAD)1,2 | PDS: ΔCost ($CAD)1,2 | PAS: Cost ($CAD)1,2 | PAS: ΔCost ($CAD)1,2 | ΔED visits3 | ΔFMD visits3 | ΔWalk-in clinic visits3 |
|---|---|---|---|---|---|---|---|
| (A) Upper respiratory tract infections | |||||||
| UCM | 52.21 | n/a | 52.40 | n/a | n/a | n/a | n/a |
| PPMA (0%) | 52.21 | 0 | 52.40 | 0 | –20 | –85 | +11 |
| PPMA (20%) | 48.26 | –3.95 | 45.85 | –6.36 | –194 | –815 | –1021 |
| PPMA (38%) | 44.70 | –7.51 | 39.95 | –12.26 | –366 | –1463 | –1968 |
| PPMA (60%) | 40.35 | –11.86 | 32.74 | –19.47 | –593 | –2238 | –3101 |
| PPMA (80%) | 36.40 | –15.81 | 26.18 | –26.03 | –799 | –2923 | –4132 |
| PPMA (100%) | 32.45 | –19.76 | 19.63 | –32.58 | –1000 | –3589 | –5147 |
| (B) Contact dermatitis | |||||||
| UCM | 54.45 | n/a | 54.45 | n/a | n/a | n/a | n/a |
| PPMA (0%) | 54.45 | 0 | 54.45 | 0 | +3 | –16 | –39 |
| PPMA (20%) | 52.31 | –2.15 | 51.87 | –2.58 | –65 | –581 | –930 |
| PPMA (38%) | 50.37 | –4.08 | 49.56 | –4.89 | –127 | –1111 | –1663 |
| PPMA (60%) | 48.02 | –6.43 | 46.72 | –7.73 | –196 | –1738 | –2630 |
| PPMA (80%) | 45.87 | –8.58 | 44.14 | –10.31 | –285 | –2302 | –3468 |
| PPMA (100%) | 43.72 | –10.73 | 41.57 | –12.88 | –342 | –2868 | –4361 |
| (C) Conjunctivitis | |||||||
| UCM | 69.23 | n/a | 69.40 | n/a | n/a | n/a | n/a |
| PPMA (0%) | 69.23 | 0 | 69.40 | 0 | –3 | –29 | –12 |
| PPMA (20%) | 66.52 | –2.17 | 64.52 | –4.88 | –159 | –596 | –735 |
| PPMA (38%) | 64.08 | –5.15 | 60.13 | –9.27 | –306 | –1103 | –1459 |
| PPMA (60%) | 61.09 | –8.14 | 54.77 | –14.63 | –563 | –1688 | –2240 |
| PPMA (80%) | 58.38 | –10.85 | 49.90 | –19.50 | –736 | –2174 | –2957 |
| PPMA (100%) | 55.67 | –13.56 | 45.03 | –24.37 | –935 | –2653 | –3688 |
Abbreviations: UCM, usual care model; RM, RPh model; PDS, prescription-detached scenario; PAS, prescription-attached scenario; ED, emergency department; FMD, family physician; n/a, not applicable; PSA, probabilistic sensitivity analysis; $CAD, Canadian dollars.
All costs are shown in 2019 Canadian dollars. 1Per patient; 2Based on PSA of 10 000 replications; 3Per 10 000 patients.
Probabilistic sensitivity analysis
A PSA using 10 000 Monte Carlo simulations was conducted to assess the uncertainties in all model parameters and to increase confidence in the results of the base-case analysis. In the PAS, the PSA indicated that the PPMA model was cost saving 100% of the time for all three minor ailments. In the PDS, the PPMA model was cost saving in 100%, 96.6% and 99.9% of the simulations for URTIs, CD and conjunctivitis, respectively. The results of the PSA are illustrated in Supplementary Figures S1 and S2.
Deterministic sensitivity analysis
A DSA was performed to delineate the effects of model parameters on the results of both the PDS and PAS remuneration models. The results of the 10 most impactful parameters are summarized in Supplementary Figures S3 and S4. Of the 10 parameters for URTIs and conjunctivitis, five were cost-associated, three were healthcare-seeking behaviour-associated probabilities, and two were probabilities associated with the availability of family physician appointments. Overall, the probability of patients choosing to receive care from a pharmacist over a physician (service uptake rate) had the greatest impact on the outcome for both pharmacist reimbursement scenarios, while the rest of the parameters had minimal impact on the results. In all cases that were considered, the PPMA model was found to be cost minimizing for the government. Our analyses indicated that most of the modelling assumptions that were made had negligible impact on the overall outcome of the study.
Discussion
We performed a cost-minimization analysis from a public payer perspective. The results of our analyses suggest that by enabling pharmacists to prescribe for URTIs, CD and conjunctivitis in Ontario, the government may save a significant amount of money and potentially improve system efficiency. Of the 10 000 simulations of the model that were run, the PPMA model was projected to be cost saving in 100% of the simulations in the PAS and cost saving in at least 96% of the simulations in the PDS billing strategy. Across the ailments, cost savings were the largest for URTIs, followed by conjunctivitis and CD.
Our findings must be considered in the context of the following limitations. First, a high number of modelling assumptions had to be made in our model or had to extrapolate input parameters from sources based on other population settings. This was difficult to avoid due to an overall lack of literature available on PPMA. However, sensitivity analyses showed that most of the parameters with modelling assumptions had negligible effect on the overall outcome of the study – cost savings is highly likely with PPMA. Secondly, our study was unable to assess for the differences in the clinical impact of the PPMA. Due to the lack of real-world evidence, it was assumed that the clinical outcome and prescribing habits would be identical between pharmacists and physicians. Such assumption formed the basis of conducting a cost-minimization analysis. While this type of analysis provides important insight into policy makers, re-analysis following PPMA implementation using real-world evidence of clinical effectiveness is necessary. Third, with a sizeable province like Ontario, geographical differences in the accessibility of various healthcare providers may affect care-seeking behaviours; and this would affect the model parameters. However, modelling the province to the fine details in the absence of literature pertaining to the availability of pharmacist prescribing in comparison to other providers is a considerably challenging task. Lastly, our current model was unable to assess for the investment costs associated with implementing a successful prescribing model for pharmacists. While the exact components and the costs associated with the implementation in Ontario are unclear, such amount can be expected to be greatest at the beginning, with lower costs over subsequent years. Training of pharmacists is not anticipated to be a significant barrier in Ontario where pharmacists graduate with highly clinically focused Doctor of Pharmacy degrees and obtain a practice certificate through a national examination. However, potential costs associated with additional training for regulatory purposes may add to the implementation cost. Further assessment of the impact of implementation costs on economic outcomes is warranted.
While it is difficult to postulate what the service uptake rate would be for such a novel service, it is reasonable to anticipate the uptake of novel services within pharmacy practice to be slow and gradual.[26] For example, in the first 2 years of the implementation of the Ontario Pharmacy Smoking Cessation Program, only a third of pharmacies were noted to have participated.[27] The uptake among patients also needs time, in spite of the societal positive attitude among patients.[28–30] It is still a relatively new concept and patients need time to perceive their pharmacists as professionals who assess and prescribe for minor ailments. If the trend in service uptake rate follows that of immunization by pharmacists, one may expect to see increasing service uptake and savings over time.[31]
Even at an assumed 38% service uptake of the PPMA, the saving of the healthcare system from a public payer perspective is still significant. The Institute for Clinical Evaluative Sciences (ICES) estimated that the annual burden for URTIs and conjunctivitis averages at 3 197 886 and 408 064 healthcare utilization episodes per year in Ontario, whereas the Canadian Center of Occupational Health estimates 1000 occupation-related CD cases annually.[32, 33] Therefore, at a service uptake rate of 38% for the PDS, one may expect annual savings of $24 016 123, $2 101 529 and $4080 for URTIs, CD and conjunctivitis, respectively. Alternatively, for the PAS, savings of $38 985 402, $3 782 753 and $4890 may be expected. The government’s spending on the Ontario Health Insurance Program (OHIP) between 2016–17 and 2017–18 increased by $949 285 300.[34] Thus, enabling pharmacists to care for patients with URTIs and CD may make this annual growth in OHIP expenditure decrease by approximately 2.7% (PDS) to 4.5% (PAS).
While both the PDS and PAS were able to demonstrate savings in costs, the magnitude of savings was greater for the PAS. This is likely driven by the fact that, in the PAS, there are fewer opportunities for pharmacists to get financially compensated for consulting patients. It is important to be cognizant of the potential clinical implications of the PAS. In our model, it was assumed that pharmacists will have a slightly higher probability of providing prescription drugs than physicians in the PAS due to the financial incentive to issue a prescription. While there is no firm evidence to suggest pharmacists are more likely to prescribe within the PAS, it is likely a reasonable assumption to make.[35, 36] A Swiss study found that higher drug costs are observed where physicians assume dispensing responsibilities in addition to their traditional prescribing role.[37] Such differences may potentially lead to over-prescribing of medications and lead to unnecessary therapy for patients and costs incurred to the government. As such, one must consider whether eliminating such an incentive from the system may lead to better care for the patients, albeit at a lower savings for the government. However, a Canadian study (RxOUTMAP) that considered the outcomes of community pharmacists prescribing for uncomplicated urinary tract infections showed that when enabled to prescribe, pharmacists provide care that is, from a therapeutic point of view, appropriate and clinically effective.[38] But it should be noted that the RxOUTMAP outcomes were observed within the context of a research study, which may result in different outcomes than in real-life practice and considered a different condition than those in our study.
In addition to the financial savings of the PPMA model, our study demonstrated that a notable number of physician and ED visits could be saved. This has a number of important implications for the healthcare system. Most importantly, by relieving pressure from the physicians’ offices and re-routing some patients to community pharmacies, physicians may have more time to focus on cases with greater severity and urgency. While previously published studies have found that overall physician workload is not reduced by PPMA programmes,[8–10] one may extrapolate that their workload specifically for minor ailment assessments may decrease.
The results of this model were consistent with those of previously reported studies both in Canada and internationally. Rafferty et al., in Saskatchewan, Canada, considered the cost savings associated with pharmacists prescribing for allergic rhinitis, gastroesophageal reflux disease, headache, cold sore and musculoskeletal issues, found that this service saved approximately $801 347 and $201 552 in 2014 from societal and public payer perspectives, respectively.[5] Additionally, a U.K. prospective cohort study of minor ailment consultation in ED, general practice offices and community pharmacies demonstrated similar results.[39] Based on survey data of patients presenting with musculoskeletal pain, eye discomfort, gastrointestinal disturbance or upper respiratory tract problems, the authors found that care provided in community pharmacies resulted in significantly lower costs while maintaining similar health-related outcomes.[39] While the overall conclusion of cost saving is consistent with our study, the difference in magnitude of cost savings may be attributable to a number of factors including differences in the included minor ailments, different number of cases seen in Ontario (recognizing the differences in population),[40] and other methodological differences. As our model only examined three conditions, one may also expect overall cost savings to increase as the number of included ailments within the PPMA programme increases.
Conclusion
By enabling pharmacists to prescribe for URTIs, CD and conjunctivitis, the Ontario government could potentially save large amounts of money, and prevent thousands of physician visits for minor ailments depending on the rate of service uptake. The study explored two unique reimbursement strategies that may provide an early insight into policy makers on the potential impact of PPMA and the associated cost savings depending on the reimbursement strategy.
Funding
This study was funded by the University of Waterloo School of Pharmacy Interdisciplinary Seed Fund. Dr. Wong’s research programme was supported by the Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council (NSERC) and Ontario Ministry of Research, Innovation, and Science Early Researcher Award. Dr. Alsabbagh’s research programme was supported by the Canadian Society of Hospital Pharmacists (CSHP). The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author Contributions
J.J.K.: methodology, data collection, data analysis, writing – original draft, writing – review and editing; A.H.T.: methodology, data collection, data analysis, writing – review and editing; L.P.: methodology, data collection, data analysis, writing – review and editing; N.N.: writing – review and editing; S.K.D.H.: writing – review and editing; W.W.L.W.: conceptualization, methodology, data analysis, funding acquisition, writing – review and editing, supervision; M.W.A.: conceptualization, methodology, data analysis, funding acquisition, writing – review and editing, supervision.
Conflict of Interest
All authors declared no conflicts of interest.
Supplementary Material
References
- 1. Canadian Pharmacists Association. Pharmacists’ Expanded Scope of Practice. CPhA. https://www.pharmacists.ca/pharmacy-in-canada/scope-of-practice-canada/ (21 March 2020, date last accessed).
- 2. Ontario College of Pharmacists. Expanded Scope of Practice. OCP. https://www.ocpinfo.com/about/key-initiatives/expanded-scope-of-practice/ (21 March 2020, date last accessed).
- 3. Canadian Pharmacists Association. Minor Ailments. CPhA. https://www.pharmacists.ca/advocacy/advocacy-government-relations-initiatives/value-for-services/minor-ailments/ (21 March 2020, date last accessed).
- 4. Health Quality Ontario. Primary Care Performance in Ontario. HQO. https://www.hqontario.ca/System-Performance/Primary-Care-Performance (1 October 2019, date last accessed).
- 5. Rafferty E, Yaghoubi M, Taylor Jet al.. Costs and savings associated with a pharmacists prescribing for minor ailments program in Saskatchewan. Cost Eff Resour Alloc 2017; 15: 3. 10.1186/s12962-017-0066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmer R. Minor ailments, major problems: a critical appraisal of Rafferty et al. (2017). Cost Eff Resour Alloc. 2018; 16: 57. 10.1186/s12962-018-0160-5. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lathia N, Sullivan K, Tam Ket al.. Cost-minimization analysis of community pharmacy-based point-of-care testing for strep throat in 5 Canadian provinces. Can Pharm J (Ott) 2018; 151: 322–31. 10.1177/1715163518790993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassell K, Whittington Z, Cantrill Jet al.. Managing demand: transfer of management of self limiting conditions from general practice to community pharmacies. BMJ 2001; 323: 146–7. 10.1136/bmj.323.7305.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellingham C. How the minor ailments service works. Pharm J 2004; 272: 115–6. [Google Scholar]
- 10. Baqir W, Leoroyd T, Sim Aet al.. Cost analysis of a community pharmacy ‘minor ailment scheme’ across three primary care trusts in the North East of England. J Public Health 2011; 33: 551–5. 10.1093/pubmed/fdr012 [DOI] [PubMed] [Google Scholar]
- 11. Paudyal V, Watson MC, Sach Tet al.. Are pharmacy-based minor ailment schemes a substitute for other service providers? A systematic review. Br J Gen Pract 2013; 63: e472–81. 10.3399/bjgp13X669194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alsabbagh MW, Houle SKD. The proportion, conditions, and predictors of emergency department visits that can be potentially managed by pharmacists with expanded scope of practice. Res Social Adm Pharm 2019; 15: 1289–97. 10.1016/j.sapharm.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 13. Willemsen KR, Harrington G. From patient to resource: the role of self-care in patient-centered care of minor ailments. Selfcare 2012; 3: 43–55. [Google Scholar]
- 14. Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Published March 2017. (21 March 2020, date last accessed).
- 15. TreeAge Software Inc. TreeAge Pro 2019.Williamstown, MA. [Google Scholar]
- 16. Vandepitte WP, Ponthong R, Srisarang S. Treatment outcomes of the uncomplicated upper respiratory tract infection and acute diarrhea in preschool children comparing those with and without antibiotic prescription. J Med Assoc Thai 2015; 98: 974–84. [PubMed] [Google Scholar]
- 17. Statescu L, Branisteanu D, Dobre Cet al.. Contact dermatitis – epidemiological study. Mædica J Clin Med 2011; 6: 277–81. [PMC free article] [PubMed] [Google Scholar]
- 18. van Weert HCPM, Tellegen E, Te Riet G. A new diagnostic index for bacterial conjunctivitis in primary care. A re-derivation study. Eur J Gen Pract 2014; 20: 202–8. 10.3109/13814788.2013.842970 [DOI] [PubMed] [Google Scholar]
- 19. Shekhawat NS, Shtein RM, Blackley TSet al.. Antibiotic prescription fills for acute conjunctivitis among enrollees in a large United States managed care network. Ophthalmology 2017; 124: 1099–107. 10.1016/j.ophtha.2017.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol 2011; 147: 118. 10.1001/archdermatol.2010.246 [DOI] [PubMed] [Google Scholar]
- 21. Taylor JG, Joubert R. Pharmacist-led minor ailment programs: a Canadian perspective. Int J Gen Med 2016; 9: 291–302. 10.2147/IJGM.S99540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickstrom K. Acute bacterial conjunctivitis – benefits versus risks with antibiotic treatment. Act Ophthalmol 2008; 86: 2–4. 10.1111/j.1600-0420.2007.01110.x [DOI] [PubMed] [Google Scholar]
- 23. Canadian Institute for Health Information. Physicians in Canada 2016 Summary Report. https://secure.cihi.ca/free_products/Physicians_in_Canada_2016.pdf. Published 2016. (12 December 2019, date last accessed).
- 24. Ontario Ministry of Health and Long-Term Care. Schedule of Benefits Physician Services under the Health Insurance Act. http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20191001.pdf. Published September 6, 2019. (12 December 2019, date last accessed).
- 25. Dawson H, Zinck G. CIHI survey: ED spending in Canada: a focus on the cost of patients waiting for access to an in-patient bed in Ontario. Healthc Q 2009; 12: 25–8. 10.12927/hcq.2009.20411 [DOI] [PubMed] [Google Scholar]
- 26. Weiss MC, Sutton J. The changing nature of prescribing: pharmacists as prescribers and challenges to medical dominance. Sociol Health Illn 2009; 31: 406–21. 10.1111/j.1467-9566.2008.01142.x [DOI] [PubMed] [Google Scholar]
- 27. Wong L, Burden AM, Liu YYet al.. Initial uptake of the Ontario Pharmacy Smoking Cessation Program: descriptive analysis over 2 years. Can Pharm J 2015; 148: 29–40. 10.1177/1715163514562038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aronson JK, Henderson G, Webb DJet al.. A prescription for better prescribing. BMJ 2006; 333: 459–60. 10.1136/bmj.38946.491829.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Twisselmann B. A prescription for better prescribing: summary of responses. BMJ 2006; 333: 601. 10.1136/bmj.333.7568.601-a [DOI] [Google Scholar]
- 30. Bishop AC, Boyle TA, Morrison Bet al.. Public perception of pharmacist expanded scope of practice services in Nova Scotia. Can Pharm J 2015; 148: 274–83. 10.1177/1715163515596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waite NM, Cadarette SM, Campitelli MAet al.. Characteristics of patients vaccinated against influenza in physician offices versus pharmacies and predictors of vaccination location: a cross-sectional study. CMAJ Open 2019; 7: E421–9. 10.9778/cmajo.20180189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Institute for Clinical Evaluative Sciences. Ontario Burden of Infectious Disease Study. ICES. https://www.ices.on.ca/flip-publication/ontario-burden-of-infectionus-disease/index.html#1/z. Published December 2010. (17 March 2020, date last accessed).
- 33. Canadian Centre for Occupational Health. Dermatitis, Irritant Contact. Canadian Centre for Occupational Health and Safety. https://www.ccohs.ca/oshanswers/diseases/dermatitis.html (2 December 2019, date last accessed).
- 34. Ontario Ministry of Health and Long Term Care. Archived – Expenditure Estimates for the Ministry of Health and Long Term Care (2017–18). https://www.ontario.ca/page/expenditure-estimates-ministry-health-and-long-term-care-2017–18 (4 April 2020, date last accessed).
- 35. Chou YJ, Yip WC, Lee CHet al.. Impact of separating drug prescribing and dispensing on provider behaviour: Taiwan’s experience. Health Policy Plan 2003; 18: 316–29. 10.1093/heapol/czg038 [DOI] [PubMed] [Google Scholar]
- 36. Nguyen H. The principal-agent problems in health care: evidence from prescribing patterns of private providers in Vietnam. Health Policy Plan 2011; 26(suppl 1): i53–62. 10.1093/heapol/czr028. [DOI] [PubMed] [Google Scholar]
- 37. Kaier B, Schmid C. Does physician dispensing increase drug expenditures? Empirical evidence from Switzerland. Health Econ 2016; 25: 71–90. 10.1002/hec.3124 [DOI] [PubMed] [Google Scholar]
- 38. Beahm NP, Smyth DJ, Tsuyuki RT. Outcomes of urinary tract infection management by pharmacists (RxOUTMAP): a study of pharmacist prescribing and care in patients with uncomplicated urinary tract infections in the community. Can Pharm J 2018; 151: 305–14. 10.1177/1715163518781175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watson MC, Ferguson J, Barton GRet al.. A cohort study of influences, health outcomes and costs of patients’ health seeking behaviour for minor ailments from primary and emergency care settings. BMJ Open 2015; 5: e006261. 10.1136/bmjopen-2014-006261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Statistics Canada. Population Estimates, Quarterly. Statistics Canada. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901.Updated April 24, 2020 (24 April 2020, date last accessed).
- 41. Severens JL. Discounting health outcomes in economic evaluation: the ongoing debate. Value Health 2004; 7: 397–401. 10.1111/j.1524-4733.2004.74002.xw [DOI] [PubMed] [Google Scholar]
- 42. Harrison JA, Mullen PD, Green LW. A meta-analysis of studies of the health belief model with adults. Health Educ Res 1992; 7: 107–16. 10.1093/her/7.1.107 [DOI] [PubMed] [Google Scholar]
- 43. Bank of Canada. Inflation Calculator. https://www.bankofcanada.ca/rates/related/inflation-calculator/ (12 December 2019, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.