Abstract
Toll-like receptor 4 (TLR4) contributes to the pathophysiology of diabetes. This happens, at least in part, because TLR4 modulates the enzyme NADPH oxidase, a primary source of ROS in vascular structures. Increased oxidative stress disrupts key vascular signaling mechanisms and drives the progression of diabetes, elevating the likelihood of cardiovascular diseases. Recently, it has been shown that patients with diabetes are also at a higher risk of developing severe coronavirus disease 2019 (COVID-19). Given the importance of the interaction between TLR4 and NADPH oxidase to the disrupted diabetic vascular system, we put forward the hypothesis that TLR4-mediated NADPH oxidase-derived ROS might be a critical mechanism to help explain why this disparity appears in diabetic patients, but unfortunately, conclusive experimental evidence still lacks in the literature. Herein, we focus on discussing the pathological implications of this signaling communication in the diabetic vasculature and exploring this crosstalk in the context of diabetes-associated severe COVID-19.
Keywords: TLR4, NADPH oxidase, ROS, Diabetes, COVID-19
Graphical abstract
1. Introduction
The innate immune receptor, Toll-like receptor 4 (TLR4), exerts critical roles in acute and chronic diseased states [1]. The former occurs as this receptor recognizes lipopolysaccharide (LPS), a key component of Gram-negative bacteria's outer membrane [2], and the latter as the activation of TLR4 by endogenous ligands also induces low-grade inflammation and oxidative stress. These downstream effects of TLR4 mainly result from its signaling communication with the transcriptional factor Nuclear factor (NF)-κB and the enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, respectively. Furthermore, a feed-forward interplay exists between NF-κB and reactive oxygen species (ROS), the byproduct of NADPH oxidase [3]. The link between TLR4 and NF-κB has been extensively explored in the literature [4]; consequently, we will concentrate our efforts in disclosing the other pathological arm of this receptor, the NADPH oxidase, which has also been reported to modulate NF-κB activation. While a direct connection between TLR4 and this enzyme was described more than fifteen years ago [5], there is still debate about its underlying consequences in chronic diseases, such as diabetes. Vascular oxidative stress plays a causal role in the pathophysiology of diabetes, driving morbidity and mortality in diabetic patients; therefore, the understanding of these mechanisms is of utmost importance for this disease. Furthermore, diabetic patients are at a greater risk of requiring intensive care following a diagnostic of coronavirus disease 2019 (COVID-19) [6,7], the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus [8], in the ongoing pandemic, which already claimed more than three million lives worldwide [9]. Interestingly, previous studies have independently linked TLR4 and NADPH oxidase to COVID-19, but whether there is a direct connection between these mechanisms and COVID-19 outcomes in diabetic patients is still elusive. Herein, we focus on discussing the pathological implications of this signaling interaction in the diabetic vasculature and exploring this crosstalk in the context of diabetes-associated severe COVID-19.
2. TLR4-mediated activation of vascular NADPH oxidase in diabetes
NADPH oxidase is a main source of ROS [i.e., superoxide (O2 •−) and hydrogen peroxide (H2O2)] in biological systems, especially because its pathological activation also modulates other ROS-generating enzymes, such as uncoupled endothelial nitric oxide synthase (eNOS) and xanthine oxidase (for review, [10]). Mammalian organisms express seven isoforms of this enzyme; however, only four variants are commonly found in the vascular wall. These isoforms – NOX1, NOX2, NOX4, and NOX5 – are asymmetrically distributed in the layers of blood vessels playing an essential part in maintaining vascular homeostasis. In diabetes, however, perturbations in the redox balance of vascular NADPH oxidase trigger an exacerbation in ROS production, which has severe pathological implications for the disease mechanisms [11]. While hyperglycemia, a hallmark of both types of diabetes, can increase this enzyme's expression and activity in the vasculature, evidence also points to TLR4 as an additional regulator of NADPH oxidase-mediated ROS in this disease.
It is well accepted that oxidative stress plays a major role in the pathophysiology of diabetes and that NADPH oxidase is a core cause of ROS in the vasculature of diabetic animals; however, there is still limited data from human studies. Narrowing this gap, we previously demonstrated that human type 1 diabetic and hypertensive donors have increased levels of p22phox and NOX1, a tendency towards higher levels of NOX2, p47phox, and p67phox, as well as reduced expression of NOX4. Interestingly, in this population, it is also possible to establish a strong positive correlation between p22phox and TLR4 as well as p22phox and its co-adaptor MD2 [12], but whether targeting TLR4 could avert alterations in the levels of p22phox in the human diseased aorta is still unknown. Nevertheless, further studies are warranted to answer this and other questions in this context, which will hopefully diminish the toll NADPH oxidative-induced ROS production exerts in the diabetic vasculature.
In VSMCs challenged with high glucose, there is a drastic increase in O2 •− and H2O2 production, and TLR4 inhibition with CLI-095, which targets TLR4 via the TIR domain, prevents these alterations [12,13]. Pharmacological blockade of TLR4 with this inhibitor is also able to avoid increased levels of O2 •− in endothelial cells exposed to palmitate [14]. In human macrovascular endothelial cells, high glucose stimulates TLR4 triggering inflammatory mediators and ROS [15]. Interestingly, the authors showed that high glucose was capable of modulating both MyD88-dependent and TRIF-dependent pathways. Furthermore, in type 1 diabetic rats, acute inhibition of TLR4 attenuates O2 •− generation in isolated aortas, which ultimately improves vascular function in these animals [12]. Similarly, chronic blockade of this receptor with an anti-TLR4 neutralizing antibody reduces ROS levels in mesenteric arteries [14], and even lowers systemic blood pressure [12]. Unfortunately, although it seems that these outcomes were produced following the downregulation of NADPH oxidase, these studies were unable to pinpoint the specific source of ROS affected by TLR4. Insights into this interplay were demonstrated in a previous study, which specifically showed that type 2 diabetic mice with a mutation in TLR4 were protected against endothelial dysfunction as they had reduced induction of NOX1 and NOX4 [16].
Together, these data corroborate the notion that TLR4 contributes to the activation of NADPH oxidase during diabetes, and that this crosstalk might be a core mechanism driving the pathophysiology of diabetic vascular complications.
3. TLR4 and vascular NADPH oxidase in diabetes: Pathological implications
Excessive ROS production, especially O2 •− triggers vascular dysfunction as it hampers the availability of nitric oxide (NO), a short-lived gaseous transmitter [17]. In a healthy vasculature, there is a balance between the levels of ROS and NO, but unfortunately, during diabetes, this equilibrium is lost, which favors oxidative stress, and ultimately, diabetic vascular disease. O2 •− directly interacts with NO to form peroxynitrite, a highly toxic molecule capable of oxidizing tetrahydrobiopterin, an eNOS cofactor. Reduced NO bioavailability can also happen following eNOS uncoupling, a mechanism mediated by activated NADPH oxidase during pathological conditions. Nevertheless, whether direct or indirect, lower levels of NO compromises the vessel's integrity and it is a hallmark for developing complications in the vascular system, particularly because NO contributes to a plethora of intrinsic mechanisms, such as regulation of vascular tone and maintenance of blood pressure [18]. Interestingly, in diabetic animals, the blockade of TLR4 with an anti-TLR4 antibody enhances nitrite levels in the corpus cavernosum, a vascular-like structure [19]. Similarly, it increases NO content in aortas isolated from SHR [20], and in animals lacking TLR4, HMGB1 negative effects on eNOS are reduced, improving endothelial-dependent relaxation [21]. In summary, it seems that preventing TLR4 activation in diabetic vascular tissues ameliorates vascular function not only by reducing NADPH oxidase activity but also by ultimately restoring NO levels.
Another key pathological mechanism linked to elevated ROS levels is the subsequent low-grade sterile inflammation. TLR4 is a critical regulator of NF-κB, a transcriptional factor that plays regulatory roles in the secretion of pro-inflammatory cytokines, including IL-6 and TNF-α [22]. Blockade of TLR4 reduces the circulating levels of these inflammatory mediators in murine models of hypertension [23,24] as well as their local expression in vascular and vascular-like structures [24,25]. These effects are attributed to the fact that NF-κB is downstream TLR4, but it is also possible that reduced NADPH oxidase activity, and consequently, ROS levels contribute to this process. Crosstalk exists between NF-κB and ROS [3] and between ROS and pro-inflammatory cytokines [26]. Strengthening this argument, the ability of TLR4 to elicit pro-inflammatory responses is hampered in cells lacking the NOX4 isoform [5,27,28], which might indicate that this enzyme also modulates sterile inflammation following TLR4 activation. It is undeniable that further studies are needed to dissect the interplay between TLR4, vascular NADPH oxidase, and inflammatory cytokines in diabetes, which will shed light on diabetes' underlying pathophysiological mechanisms.
In a murine model of type 1 diabetes, chronic inhibition of TLR4 and its co-adaptor MD2, lowers systemic blood pressure [12]. Hyperglycemia-induced renin-angiotensin system (RAS) activation in kidneys involves the MD2–TLR4–MAPK pathway [29]. The RAS system is a crucial regulator of this hemodynamic parameter, and angiotensin II (AngII), its main vasoconstrictor peptide, directly modulates NADPH oxidase activity [30]. Likewise, AngII is a potent activator of the TLR4-MD2 complex [31,32]. While the role of the RAS system in blood pressure control is undeniable, TLR4-mediated effects in this parameter are less clear and somewhat controversial [33,34]. However, it has been proposed to be a unifying mechanism in hypertension with emergent – yet not fully understood – roles in the kidneys, central nervous system, and vasculature [35]. In the context of diabetes, we argue that reduced levels of ROS and pro-inflammatory cytokines as well as downregulation of the RAS system are sufficient mechanisms to positively impact blood pressure, which is extremely important for this disease since 20–60% of diabetic patients subsequently develop hypertension [36], increasing the risk of cardiovascular events.
The RAS is multifaceted, and consequently, it is also critical to consider a potential impact of TLR4-mediated NADPH oxidase-derived ROS in the ACE2/Ang(1–7) axis. Activation of ACE2 is capable of improving endothelium-dependent relaxation in diabetic and hypertensive animals and the mechanism involves reduction in vascular ROS production [37]. Building into these findings, a previous study demonstrated that in endothelial cells, ACE2 prevents increased ROS generation in response to AngII by regulating p22phox [38]. Moreover, ACE2 knockout vascular smooth muscle cells display higher ROS production and NADPH oxidase activity upon AngII stimulation [39]. While the literature lacks a report examining a direct role of TLR4-derived ROS and ACE2 in the vasculature, it is possible that increased oxidative stress, downstream TLR4, is also a detrimental factor for ACE2 expression/activity. It has been demonstrated that AngII-derived NOX2-dependent ROS acts as a negative regulator of ACE2 [40,41]. Increased ROS phosphorylates p38 MAPK, leading to the activation of TACE (a.k.a, ADAM17) and cleavage of ACE2 active ectodomain [[40], [41], [42]]. Nevertheless, it is unknown if increased ROS levels mediated by TLR4 in diabetes would also impact ACE2.
The interplay between TLR4 and NADPH oxidase can also result in the activation of MAPK. While it has been described that MAPKs are recruited downstream TLR4, ROS are also well-known mediators of MAPK [43]; therefore, elevated oxidative stress can act as a second TLR4 signal pathway to induce MAPK during diabetes. In type 1 diabetic rats, stimulation of TLR4 contributes to diabetic cardiomyopathy [44], and renal fibrosis and dysfunction [29] via modulation of MAPK-induced activation of the transcription factor AP-1 and MAPK-mediated RAS system, respectively. Even though these studies did not specifically evaluated levels of ROS, increased oxidative stress is present in both the heart and kidneys during diabetes [45]. Furthermore, elevated levels of MAPK direct impact the functionality of vascular structures. Blockade of MAPK, ERK1/2 and p38, attenuates high glucose-induced vascular endothelial dysfunction [46]. Similar results were also observed in the corpus cavernosum of murine diabetic animals following the inhibition of ERK1/2 [47] and p38 [48]. In this sense, the TLR4-ROS-MAPK pathway has, at least, twofold importance acting via indirect and direct mechanisms during diabetes; nevertheless, in both cases, the vessel's integrity will likely be compromised, and vascular dysfunction will prevail.
An intriguing aspect of TLR4 is the fact that the impact of its activation appears to be greater in inducing inflammation in cultured microvascular than in macrovascular endothelial cells [49], but whether a similar pattern also occurs for oxidative stress is unknown. This could be of utmost importance for the vasculature in the brain, which contains arteries that branches extensively into smaller arteries [50]. A recent study demonstrated that (a) ischemic stroke not only increases TLR4 expression in total brain homogenates but that it also substantially affects TLR4 expression in the brain microvasculature and (b) the blockade of TLR4 ameliorates neurovascular injury [51]. Furthermore, in brain microvascular endothelial cells challenged with the conditions observed in post stroke bleeding, TLR4 inhibition attenuated NF-κB expression and IL-6, further improving membrane permeability and elevating tight junction proteins, survival, and angiogenesis [51], which highlights a role for TLR4 in the brain vasculature under diabetes. Cerebral microvascular NADPH oxidase is intrinsically linked to a healthy cerebrovascular environment, but it can also participate in disease mechanisms [52]. While it is still elusive if TLR4 directly modulates cerebral microvascular NADPH oxidase during diabetes leading to inflammation, its role in mediating the systemic form of this enzyme is more established. For example, in human microvascular endothelial cells, NADPH oxidase-derived ROS is critical for palmitate to activate TLR4-induced NF-κB [53], which calls for additional investigation in the context of the complex cerebrovascular environment.
While it is critical to disentangle these unwanted transfers of signals (Fig. 1 ), it is unquestionable that TLR4-mediated NADPH oxidase activation is a key target-mechanism in diabetes, especially because as we discussed it crosstalk with major signaling pathways that are well-accepted to drive the pathophysiology of diabetic vascular disease, and it might be a candidate pathway to help explain COVID-19 severity in diabetic patients.
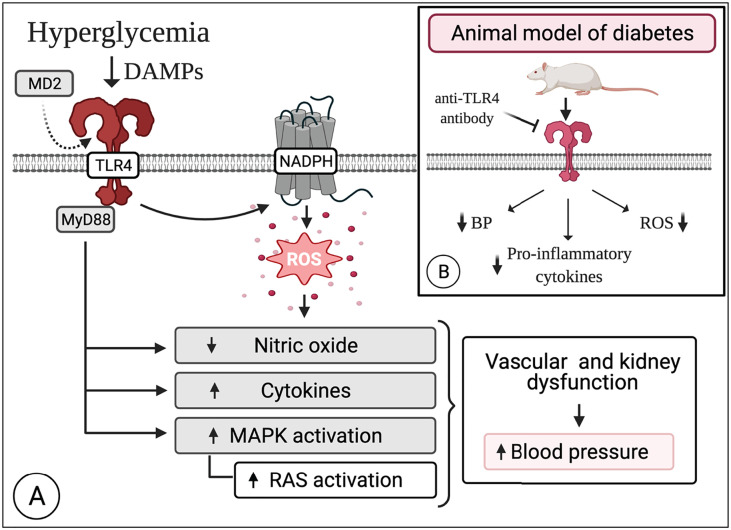
Fig. 1.
Overview of the mechanisms affected by TLR4 following stimulation of vascular NADPH oxidase in diabetes. Hyperglycemia itself or in combination with other DAMPs modulates the expression of the TLR4-MD2 complex. TLR4 induces the activation of NADPH oxidase leading to exacerbated production of ROS in the vascular system. Excessive levels of ROS disrupt key signaling pathways, ultimately reducing the availability of NO, increasing the production of pro-inflammatory cytokines and the activation status of MAPKs, which in turn impacts the RAS system in the kidneys. Together, these alterations might account for an increase in blood pressure. Excitingly, blockade of the TLR4-MD2 complex lowers blood pressure, reduces low-grade inflammation, and attenuates ROS levels, improving vascular function in murine animals. Created with BioRender.com.
4. COVID-19 in diabetic patients: Is there a role for TLR4-mediated NADPH oxidase activation?
Severe COVID-19 mainly occurs in patients with pre-existing conditions [54], including diabetes [6]. While the knowledge about the mechanisms involved in the pathogenesis and pathophysiology of SARS-CoV-2 infection has grown exponentially in the last year, there are still many missing pieces in this puzzle. In diabetes, both TLR4 and NADPH oxidase levels/activity are altered, and it seems that these mechanisms, whether together or independently, might play a role in diabetes-associated severe COVID-19. It has been proposed that increased oxidative stress occurs in response to the impact of SARS-CoV-2 [55], and recently, it was reported that COVID-19 patients receiving intensive care have disrupted systemic oxidative stress status [56]. NADPH oxidase is involved in the pathogenesis of viral infections [57], and it might be a critical player during the course of COVID-19 [58], mainly because it has been demonstrated that, compared with controls, COVID-19 patients have higher circulating levels of soluble NOX2-derived peptide (s-NOX2-dp) [59]. Furthermore, s-NOX2-dp levels are even higher in COVID-19 patients receiving intensive care, similarly to what happened to patients with or without thrombotic events [59]. As discussed, TLR4 activates NADPH oxidase in the vasculature, and not surprisingly, this receptor also responds to viral infection [60], including to SARS-CoV-2 [61,62] contributing to inflammation [63]. Briefly speaking, stimulation of TLR4 can lead to the activation of MyD88-dependent (i.e., canonical) and TRIF-dependent (i.e., alternative) pathways to induce inflammatory genes and type I interferon (for review, please see [64]). Therefore, with the ongoing pandemic, the interaction between TLR4 and NADPH oxidase under diabetes has gained an additional layer of importance, particularly because it may help explain why the diabetic population has increased susceptibility for developing the serious form of this disease, which is associated with higher average-case mortality. Still, to the best of our knowledge, at this point, experimental evidence demonstrating a direct and causative role for TLR4 and NADPH oxidase in diabetic patients suffering from concomitant COVID-19 lacks in the literature.
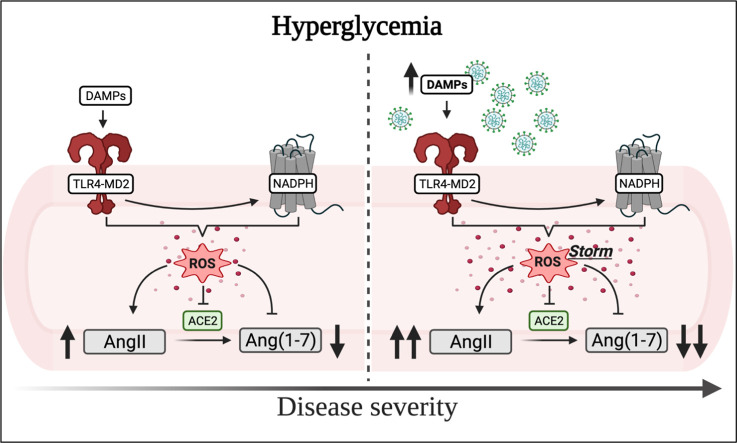
The severity of COVID-19 in diabetic patients might occur because the SARS-CoV-2 virus crosstalk with one or more crucial pathway(s) [6], and it is likely to involve the activation of TLR4 and NADPH oxidase. The internalization of SARS-CoV-2 occurs via ACE2 [65], an enzyme with counterregulatory actions in the cardiovascular system, that is affected during diabetes [66]. The binding of this virus to ACE2 reduces its availability, negatively impacting the functionality of the biological systems where this enzyme exerts critical physiological functions [67], including in the diabetic vasculature [66]. Reduced levels of ACE2 associate with higher levels of AngII (vasoconstrictor) and lower expression of Ang(1–7) (vasodilator), which in the context of diabetes contributes to the pathophysiology of vascular complications. It is well accepted that, in diseased states, augmented NADPH oxidase-derived ROS can impact ACE2 levels. Likewise, ACE2 deficiency increases the generation of NADPH oxidase-derived ROS [68]. Therefore, we hypothesized that since diabetic patients already have disturbance in these mechanisms, partly caused by the interaction between TLR4 and NADPH oxidase, subsequent exposure to the SARS-CoV-2 virus, leading to a diagnostic of COVID-19, will most likely amplify this disruption, contributing to, among others, a ROS storm, consequently leaving these patients vulnerable to the complications that arise with the more serious form of this disease.
TLR4 levels are upregulated in COVID-19 patients, and its activation contributes to disease progression [69]. While a conclusive mechanism of activation for TLR4 during SARS-CoV-2 infection is yet-to-be-confirmed, it is possible that this receptor is answering to both exogenous (PAMPs) and endogenous (DAMPs) ligands. Diabetic patients already have higher circulating levels of PAMPs [[70], [71], [72]] and DAMPs [73], which are potentially exacerbated following a diagnostic of COVID-19. The upregulation in DAMP production might lead to an intensified response by immune receptors, such as TLR4, and could be a mechanism driving dysregulated immune activation in diabetic patients, including in the vascular system as it innermost layer (i.e., endothelium) constantly interacts with these circulating agents. A potential outcome of increased TLR4 activation, besides higher production of inflammatory mediators through NF-κB, is the over-production of ROS via NADPH oxidase, which in a positive feedback loop is also capable of inducing inflammation. As highlighted, augmented ROS levels damage the vasculature, and since the vascular system is in direct contact with all systems in the human body, it likely contributes to systemic tissue damage, which is observed in many COVID-19 patients receiving intensive care.
There is growing evidence that COVID-19 acts as a vascular disease [[74], [75], [76]]. In fact, the pathways derangements caused by SARS-CoV-2 are a hallmark for the development of vascular complications. In general, endothelial cells, which forms the inner layer of blood vessels, abundantly express ACE2; therefore, they can be a target of the virus, which, as discussed by others, could lead to immune activation, RAS alterations, and pro-thrombotic imbalance in vascular endothelial structures (for review, please see [75]), which, accordingly, would be potential mechanisms exacerbated by the pre-existence of diabetes. Moreover, endothelial cells-associated therapies have been proposed to counterbalance the impact of SARS-CoV-2 infection [77]. While the mechanisms of COVID-19-associated damage are still under extensive investigation, post-mortem analysis of COVID-19 patients revealed involvement of endothelial cells in multiple vascular beds [78]. The observed endotheliitis appeared to be the result of the interplay between viral infection and host-response, and it could help explain the systemic impairment of microcirculatory function [78]. Immune activation via TLR4 in the vasculature contributes to low-grade inflammation and ROS generation [12,13,20,23,25]. Likewise, AngII is a potent pro-inflammatory and ROS modulator acting via the AT1r and TLR4 [79], and reported data show that COVID-19 patients have a remarkable increase in the circulating levels of this RAS mediator [80], potentially as a consequence of ACE2 downregulation. Finally, ROS crosstalk with NF-κB, reinforcing the vicious cycle. Nevertheless, it is yet-to-be-established if host-derived DAMPs are activating TLR4 during COVID-19, which would contribute to inflammation and oxidative stress, as well as whether this process is amplified by the presence of diabetes.
While no studies have specifically investigated DAMP alteration in diabetic patients with COVID-19, there are many other DAMP molecules, besides AngII [80], that could be altered in the course of the disease, such as S100A9 [69] and HSP70 [81], to name a few. For example, evidence suggests that HSP70 might be another prime DAMP candidate, since it answers to viral infections and its expression levels are modulated by fever [82,83], a common symptom of COVID-19. More compelling, it has been demonstrated that the circulating levels of HSP70 were remarkably elevated in COVID-19 patients receiving intensive care [81]. During diabetes, HSP70 co-exist in the intra- and extra-cellular (i.e., iHSP70 and eHSP70, respectively) environment [84]. eHSP70 interacts with the TLR4-MD2 complex [85,86], triggering its downstream mediators, potentially in diabetes [87], and both fractions of this protein have an intricate relationship with the NADPH oxidase enzyme [88]. The induction of iHSP70 might bring beneficial effects to the host organism, including increased capacity of re-fold damaged proteins and inhibition of pro-apoptotic pathways. iHSP70 also crosstalks with Ca2+ handling mechanisms in the vasculature [89,90] in a sex-dependent manner [91], which could also be an affected mechanism in diabetic patients suffering from COVID-19, a disease where the variable sex influences its outcome [92]. Not surprising, it has been hypothesized that the controlled induction of iHSP70 could be a therapeutical approach against SARS-CoV-2, including in diabetic patients [93]. Nevertheless, there are still many unanswered questions that must be addressed to fully expose these mechanisms, which will eventually allow researchers to determine if they are suited targets to manage COVID-19 in diabetic patients.
5. Final considerations
The current literature supports the notion that TLR4-mediated NADPH oxidase activation is a key pathological mechanism in the diabetic vasculature. Increased ROS production in diabetes is a risk factor for the appearance of complications in the vascular system as it impacts a myriad of mechanisms, including the availability of NO, levels of pro-inflammatory cytokines, the phosphorylation status of MAPK, and even blood pressure. Furthermore, in diabetic patients, the previous disruption of these signaling mechanisms, favoring increased oxidative stress, might be a contributing factor for the outcome of an infection by SARS-CoV-2 especially because ROS crosstalk with ACE2 impacting both arms of the RAS system (Fig. 2 ), and therefore this topic deserves further investigation. In fact, a very throbbing problem at this point is to determine if the mechanism by which diabetic patients infected with the SARS-CoV-2 virus elicit a more pathological response resulting in severe COVID-19 involves this candidate pathway. This is important since, overall, following modulation of TLR4, reduced oxidative stress is observed in animal models of diabetes, which is due, at least in part, to decreased NADPH oxidase activity. Even though there is mounting evidence that the activation of TLR4 has pathological consequences in the diabetic vasculature, one might argue that there is still a long road to fully uncover the contributions of this receptor in this condition, particularly in the context of COVID-19. Equally important is our lacking understanding of the mid- to long-term implications of targeting this receptor in chronic diseases. TLR4 has a myriad of functions in mammalian organisms, from recognizing invading pathogens to orchestrating immune responses. Thus, it has been proven difficult to devise strategies to modulate this receptor without compromising the host's ability to fight infections, and with the ongoing pandemic caution should be exercised when drawing conclusions from animal reports.
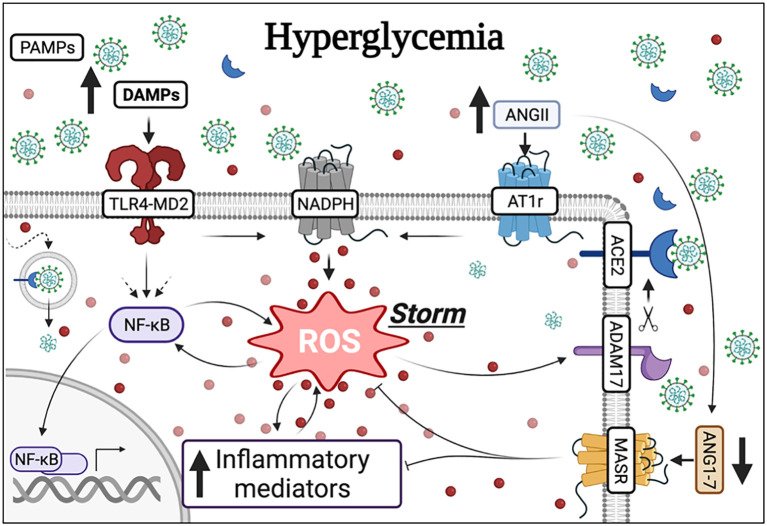
Fig. 2.
The hypothesis that TLR4-mediated NADPH oxidase derived ROS contributes to COVID-19 severity in diabetic patients. Hyperglycemia, a hallmark of both types of diabetes, modulates the TLR4-MD2 complex as well as the activity of the enzyme NADPH oxidase. The TLR4-MD2 complex also independently stimulates NADPH oxidase-induced ROS as well as the transcriptional factor NF-κB, leading to the release of pro-inflammatory mediators. NF-κB can also be recruited by other pathways (dashed arrows). There is an interplay between ROS and NF-κB and ROS and inflammatory mediators. Elevated levels of ROS, mediated by the AT1r, also impact the availability of ACE2 via ADAM17, which ultimately favors AngII in detriment of Ang(1–7). Additionally, Ang(1–7) acting via the MASR impairs ROS and inflammatory mediators. Diabetic patients infected by the SARS-CoV-2 virus, which binds to ACE2, are at a greater risk of developing severe COVID-19. Both TLR4 and NADPH oxidase are involved in the response orchestrated by infected organisms against virus and are potential candidates in the ongoing pandemic. In this context, it is possible that, since these mechanisms are already activated in diabetic patients, the response against the SARS-CoV-2 virus is exacerbated triggering a ROS storm in this population. Additionally, these mechanisms can also trigger over-production of DAMPs to sustain a vicious cycle. These endogenous signals created at disproportional levels could be, in turn, responsible for dysregulated immune responses in diabetic patients. Created with BioRender.com.
There are, however, many other pressing queries that should be taken into perspective when one analyzes the interplay between TLR4 and vascular NADPH oxidase during diabetes in the context of COVID-19. Here, we would like to briefly share some of these ideas.
-
•
There is growing concern with the fact that COVID-19 could be precipitating/inducing new onset diabetes [[94], [95], [96], [97]], as the SARS-CoV-2 virus might damage the pancreatic islets [98]. Consequently, elevating the widespread damage, and ultimately, increasing the circulation of glucose and glucose-related DAMPs. If this is the case, would these DAMPs also amplify the TLR4-mediated vascular NADPH oxidase response?
-
•
COVID-19 patients are at a higher risk of developing neurologic complications, including stroke [99], a diabetic complication in which TLR4 was previously implicated [51], but whether this is exacerbated by the presence of diabetes remains unknown, and if TLR4 is involved in this process, at this point, is even less clear.
-
•
It has been shown that ACE2 is an interferon-stimulated gene [100]. TLR4 activates interferon regulatory factor 3 via TRIF pathway [101]. Therefore, it has been proposed that SARS-CoV-2 could be stimulating TLR4 to induce ACE2 expression [102]. In this case, could TLR4 be playing a dual role (i.e., both its arms are activated)?
-
•
Considering the involvement of TLR4 [69] and NADPH oxidase [59] in the pathophysiology of SARS-CoV-2, could these mechanisms offer new therapeutical strategies to manage COVID-19?
Lastly, we would like to leave the reader with a final question. What are the mid- to long-term implications of severe COVID-19 to the vasculature, especially the diabetic one?
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Both authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Molteni M., Gemma S., Rossetti C. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediat. Inflamm. 2016;2016:6978936. doi: 10.1155/2016/6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park B.S., Song D.H., Kim H.M., Choi B.-S., Lee H., Lee J.-O. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 3.Morgan M.J., Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T., Akira S. Signaling to NF-kappaB by toll-like receptors. Trends Mol. Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Park H.S., Jung H.Y., Park E.Y., Kim J., Lee W.J., Bae Y.S. Cutting edge: direct interaction of TLR4 with NAD (P) H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-κB. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 6.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrera F.J., Shekhar S., Wurth R., Moreno-Pena P.J., Ponce O.J., Hajdenberg M., Alvarez-Villalobos N.A., Hall J.E., Schiffrin E.L., Eisenhofer G., Porter F., Brito J.P., Bornstein S.R., Stratakis C.A., González-González J.G., Rodíguez-Gutiérrez R., Hannah-Shmouni F. Prevalence of diabetes and hypertension and their associated risks for poor outcomes in Covid-19 patients. J. Endocr. Soc. 2020;4 doi: 10.1210/jendso/bvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konior A., Schramm A., Czesnikiewicz-Guzik M., Guzik T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014;20:2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira A.A., Faustino J., Webb R.C., Nunes K.P. Blockade of the TLR4–MD2 complex lowers blood pressure and improves vascular function in a murine model of type 1 diabetes. Sci. Rep. 2020 doi: 10.1038/s41598-020-68919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrillo-Sepulveda M.A., Spitler K., Pandey D., Berkowitz D.E., Matsumoto T. Inhibition of TLR4 attenuates vascular dysfunction and oxidative stress in diabetic rats. J. Mol. Med. 2015;93:1341–1354. doi: 10.1007/s00109-015-1318-7. [DOI] [PubMed] [Google Scholar]
- 14.Kramer B., França L.M., Zhang Y., de Paes A.M., Gerdes A. Martin, Carrillo-Sepulveda M.A. Western diet triggers toll-like receptor 4 signaling-induced endothelial dysfunction in female wistar rats. Am. J. Physiol. Heart Circ. Physiol. 2018 doi: 10.1152/ajpheart.00218.2018. [DOI] [PubMed] [Google Scholar]
- 15.Pahwa R., Nallasamy P., Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J. Diabetes Complicat. 2016;30:563–572. doi: 10.1016/j.jdiacomp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Liang C.-F., Liu J.T.C., Wang Y., Xu A., Vanhoutte P.M. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4Significance. Arterioscler. Thromb. Vasc. Biol. 2013;33:777–784. doi: 10.1161/ATVBAHA.112.301087. [DOI] [PubMed] [Google Scholar]
- 17.Sena C.M., Leandro A., Azul L., Seiça R., Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front. Physiol. 2018 doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K., Pittman R.N., Popel A.S. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008;10:1185–1198. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes K.P., de Oliveira A.A., Szasz T., Biancardi V.C., Webb R.C. Blockade of toll-like receptor 4 attenuates erectile dysfunction in diabetic rats. J. Sex. Med. 2018;15:1235–1245. doi: 10.1016/j.jsxm.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Hernanz R., Martinez-Revelles S., Palacios R., Martin A., Cachofeiro V., Aguado A., Garcia-Redondo L., Barrus M.T., d Batista P.R., Briones A.M. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br. J. Pharmacol. 2015;172:3159–3176. doi: 10.1111/bph.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z., Peng X., Li X., Tu T., Yang H., Teng S., Zhang W., Xing Z., Tang J., Hu X., Fang Z., Zhou S. HMGB1 impairs endothelium-dependent relaxation in diabetes through TLR4/eNOS pathway. FASEB J. 2020 doi: 10.1096/fj.202000242R. [DOI] [PubMed] [Google Scholar]
- 22.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Therapy. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bomfim G.F., Dos Santos R.A., Oliveira M.A., Giachini F.R., Akamine E.H., Tostes R.C., Fortes Z.B., Webb R.C., Carvalho M.H.C. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin. Sci. 2012;122:535–543. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes K.P., Bomfim G.F., Toque H.A., Szasz T., Clinton Webb R. Toll-like receptor 4 (TLR4) impairs nitric oxide contributing to angiotensin II-induced cavernosal dysfunction. Life Sci. 2017;15:219–226. doi: 10.1016/j.lfs.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Bomfim G.F., Echem C., Martins C.B., Costa T.J., Sartoretto S.M., Dos Santos R.A., Oliveira M.A., Akamine E.H., Fortes Z.B., Tostes R.C., Webb R.C., Carvalho M.H. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 2015;122:1–7. doi: 10.1016/j.lfs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., Andresen B.T., Hill M., Zhang J., Booth F., Zhang C. Role of reactive oxygen species in tumor necrosis factor-alpha induced endothelial dysfunction. Curr. Hypertens. Rev. 2008;4:245–255. doi: 10.2174/157340208786241336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H.S., Chun J.N., Jung H.Y., Choi C., Bae Y.S. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 2006 doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Asehnoune K., Strassheim D., Mitra S., Kim J.Y., Abraham E. Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NF-κB. J. Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Fang Q., Jin Y., Liu Z., Zou C., Yu W., Li W., Shan X., Chen R., Khan Z., Liang G. Blockade of myeloid differentiation 2 attenuates diabetic nephropathy by reducing activation of the renin-angiotensin system in mouse kidneys. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cat A. Nguyen Dinh, Montezano A.C., Burger D., Touyz R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J., Zou C., Mei L., Zhang Y., Qian Y., You S., Pan Y., Xu Z., Bai B., Huang W. MD2 mediates angiotensin II-induced cardiac inflammation and remodeling via directly binding to Ang II and activating TLR4/NF-κB signaling pathway. Basic Res. Cardiol. 2017;112:9. doi: 10.1007/s00395-016-0599-5. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z., Li W., Han J., Zou C., Huang W., Yu W., Shan X., Lum H., Li X., Liang G. Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2) Sci. Rep. 2017;7 doi: 10.1038/srep44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes K.P., De Oliveira A.A., Lima V.V., Clinton Webb R. Toll-like receptor 4 and blood pressure: lessons from animal studies. Front. Physiol. 2019;10:655. doi: 10.3389/fphys.2019.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunes K.P., de Oliveira A.A., Mowry F.E., Biancardi V.C. Targeting toll-like receptor 4 signalling pathways: can therapeutics pay the toll for hypertension? Br. J. Pharmacol. 2018;176:1864–1879. doi: 10.1111/bph.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goulopoulou S., Webb R.C. Symphony of vascular contraction how smooth muscle cells lose harmony to signal increased vascular resistance in hypertension. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.113.02444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arauz-Pacheco C., Parrott M., Raskin P. Treatment of hypertension in adults with diabetes-American diabetes association. Diabetes Care. 2003;26:80S–82. doi: 10.2337/diacare.26.2007.S80. [DOI] [PubMed] [Google Scholar]
- 37.Fraga-Silva R.A., Costa-Fraga F.P., Murça T.M., Moraes P.L., Martins Lima A., Lautner R.Q., Castro C.H., Soares C.M.A., Borges C.L., Nadu A.P., Oliveira M.L., Shenoy V., Katovich M.J., Santos R.A.S., Raizada M.K., Ferreira A.J. Angiotensin-converting enzyme 2 activation improves endothelial function. Hypertension. 2013;61:1233–1238. doi: 10.1161/HYPERTENSIONAHA.111.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J., Slutsky A.S., Peterson M.D., Backx P.H., Penninger J.M., Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 39.Vb P., Jc Z., D F., R B., Js M., N P., Ms M., St D., Z K., Gy O. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension (Dallas, Tex.: 1979). 2014;64 doi: 10.1161/HYPERTENSIONAHA.114.03388. [DOI] [PubMed] [Google Scholar]
- 40.Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu P., Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol. Cell. 2010;37:551–566. doi: 10.1016/j.molcel.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCubrey J.A., LaHair M.M., Franklin R.A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 2006 doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Luo W., Han J., Khan Z.A., Fang Q., Jin Y., Chen X., Zhang Y., Wang M., Qian J., Huang W., Lum H., Wu G., Liang G. MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat. Commun. 2020 doi: 10.1038/s41467-020-15978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kayama Y., Raaz U., Jagger A., Adam M., Schellinger I.N., Sakamoto M., Suzuki H., Toyama K., Spin J.M., Tsao P.S. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015;16:25234–25263. doi: 10.3390/ijms161025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandra S., Fulton D.J.R., Caldwell R.B., Caldwell R.W., Toque H.A. Hyperglycemia-impaired aortic vasorelaxation mediated through arginase elevation: role of stress kinase pathways. Eur. J. Pharmacol. 2019 doi: 10.1016/j.ejphar.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunes K.P., Toque H.A., Caldwell R.B., Caldwell R.W., Webb R.C. Extracellular signal-regulated kinase (ERK) inhibition decreases arginase activity and improves corpora cavernosal relaxation in streptozotocin (STZ)-induced diabetic mice. J. Sex. Med. 2011 doi: 10.1111/j.1743-6109.2011.02499.x. [DOI] [PubMed] [Google Scholar]
- 48.Toque H.A., Nunes K.P., Yao L., Liao J.K., Webb R.C., Caldwell R.B., Caldwell R.W. Activated rho kinase mediates diabetes-induced elevation of vascular arginase activation and contributes to impaired corpora cavernosa relaxation: possible involvement of p38 MAPK activation. J. Sex. Med. 2013;10:1502–1515. doi: 10.1111/jsm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Z., Li Y., Jin J., Zhang X., Lopes-Virella M.F., Huang Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler. Thromb. Vasc. Biol. 2012;32:1696–1706. doi: 10.1161/ATVBAHA.112.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joutel A., Faraci F.M. Cerebral small vessel disease: insights and opportunities from mouse models of collagen IV-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2014;45:1215–1221. doi: 10.1161/STROKEAHA.113.002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdul Y., Abdelsaid M., Li W., Webb R.C., Sullivan J.C., Dong G., Ergul A. Inhibition of toll like Receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: possible role of vascular endothelial TLR-4. Mol. Neurobiol. 2019;56:1607–1617. doi: 10.1007/s12035-018-1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y., Pagano P.J. Microvascular NADPH oxidase in health and disease. Free Radic. Biol. Med. 2017;109:33–47. doi: 10.1016/j.freeradbiomed.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maloney E., Sweet I.R., Hockenbery D.M., Pham M., Rizzo N.O., Tateya S., Handa P., Schwartz M.W., Kim F. Activation of NF-kB by palmitate in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009;29(9):1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pincemail J., Cavalier E., Charlier C., Cheramy-Bien J.-P., Brevers E., Courtois A., Fadeur M., Meziane S., Goff C.L., Misset B., Albert A., Defraigne J.-O., Rousseau A.-F. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A pilot study. Antioxidants (Basel). 2021;10 doi: 10.3390/antiox10020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Damiano S., Sozio C., La Rosa G., Santillo M. NOX-dependent signaling dysregulation in severe COVID-19: clues to effective treatments. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.608435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., Cammisotto V., Lichtner M., Alessandri F., De Angelis M., Miele M.C., D’Ettorre G., Ruberto F., Venditti M., Pugliese F., Mastroianni C.M. Nox2 activation in Covid-19. Redox Biol. 2020;36:101655. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olejnik J., Hume A.J., Mühlberger E. Toll-like receptor 4 in acute viral infection: too much of a good thing. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandão S.C.S., de Ramos J.O.X., Dompieri L.T., Godoi E.T.A.M., Figueiredo J.L., Sarinho E.S.C., Chelvanambi S., Aikawa M. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2021;58:102–110. doi: 10.1016/j.cytogfr.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choudhury A., Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020;92:2105–2113. doi: 10.1002/jmv.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shirato K., Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. PNAS. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel V.B., Parajuli N., Oudit G.Y. Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications. Clin. Sci. (Lond.) 2014;126:471–482. doi: 10.1042/CS20130344. [DOI] [PubMed] [Google Scholar]
- 67.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wysocki J., Ortiz-Melo D.I., Mattocks N.K., Xu K., Prescott J., Evora K., Ye M., Sparks M.A., Haque S.K., Batlle D., Gurley S.B. ACE2 deficiency increases NADPH-mediated oxidative stress in the kidney. Phys. Rep. 2014;2 doi: 10.1002/phy2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sohn K.M., Lee S.-G., Kim H.J., Cheon S., Jeong H., Lee J., Kim I.S., Silwal P., Kim Y.J., Paik S., Chung C., Park C., Kim Y.-S., Jo E.-K. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling That mimics bacterial sepsis. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayashree B., Bibin Y.S., Prabhu D., Shanthirani C.S., Gokulakrishnan K., Lakshmi B.S., Mohan V., Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014 doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 71.Gomes J.M.G., de Costa J.A., de Alfenas R.C.G. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metab. Clin. Exp. 2017 doi: 10.1016/j.metabol.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 72.Trøseid M., Nestvold T.K., Rudi K., Thoresen H., Nielsen E.W., Lappegård K.T. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: Evidence from bariatric surgery. Diabetes Care. 2013 doi: 10.2337/dc13-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin J.J., Lee E.K., Park T.J., Kim W. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res. Rev. 2015;24:66–76. doi: 10.1016/j.arr.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Becker R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis. 2020;50:499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siddiqi H.K., Libby P., Ridker P.M. COVID-19 – A vascular disease. Trends Cardiovasc. Med. 2021;31:1–5. doi: 10.1016/j.tcm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barbosa L.C., Gonçalves T.L., de Araujo L.P., de Rosario L.V.O., Ferrer V.P. Endothelial cells and SARS-CoV-2: an intimate relationship. Vasc. Pharmacol. 2021;137:106829. doi: 10.1016/j.vph.2021.106829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biancardi V.C., Bomfim G.F., Reis W.L., Al-Gassimi S., Nunes K.P. The interplay between angiotensin II, TLR4 and hypertension. Pharmacol. Res. 2017;120:88–96. doi: 10.1016/j.phrs.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fraser D.D., Cepinskas G., Slessarev M., Martin C., Daley M., Miller M.R., O’Gorman D.B., Gill S.E., Patterson E.K., dos Santos C.C. Inflammation profiling of critically Ill coronavirus disease 2019 patients. Crit. Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim M.Y., Oglesbee M. Virus-heat shock protein interaction and a novel Axis for innate antiviral immunity. Cells. 2012;1:646–666. doi: 10.3390/cells1030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guihur A., Rebeaud M.E., Fauvet B., Tiwari S., Weiss Y.G., Goloubinoff P. Moderate fever cycles as a potential mechanism to protect the respiratory system in COVID-19 patients. Front. Med. 2020;7 doi: 10.3389/fmed.2020.564170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krause M., Heck T.G., Bittencourt A., Scomazzon S.P., Newsholme P., Curi R., Homem de Bittencourt P.I. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/249205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Oliveira A.A., Faustino J., de Lima M.E., Menezes R., Nunes K.P. Unveiling the interplay between the TLR4/MD2 complex and HSP70 in the human cardiovascular system: a computational approach. Int. J. Mol. Sci. 2019;20:3121. doi: 10.3390/ijms20133121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asea A., Rehli M., Kabingu E., Boch J.A., Baré O., Auron P.E., Stevenson M.A., Calderwood S.K. Novel signal transduction pathway utilized by extracellular HSP70 role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 87.de Oliveira A.A., Webb R.C., Nunes K.P. Toll-like receptor 4 and heat-shock protein 70: is it a new target pathway for diabetic vasculopathies? Curr. Drug Targets. 2018;20:51–59. doi: 10.2174/1389450119666180821105544. [DOI] [PubMed] [Google Scholar]
- 88.Krause M., Bock P.M., Takahashi H.K., De Bittencourt P.I.H., Newsholme P. The regulatory roles of NADPH oxidase, intra-and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin. Sci. 2015;128:789–803. doi: 10.1042/CS20140695. [DOI] [PubMed] [Google Scholar]
- 89.de Oliveira A.A., Priviero F., Tostes R.C., Webb R.C., Nunes K.P. Dissecting the interaction between HSP70 and vascular contraction: role of Ca 2 + handling mechanisms. Sci. Rep. 2021;11:1420. doi: 10.1038/s41598-021-80966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Oliveira A.A., Nunes K.P. An additional physiological role for HSP70: assistance of vascular reactivity. Life Sci. 2020;256:117986. doi: 10.1016/j.lfs.2020.117986. [DOI] [PubMed] [Google Scholar]
- 91.De Oliveira A.A., Priviero F., Webb R.C., Nunes K.P. Impaired HSP70 expression in the aorta of female rats: a novel insight into sex-specific differences in vascular function. Front. Physiol. 2021;12:666696. doi: 10.3389/fphys.2021.666696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viveiros A., Rasmuson J., Vu J., Mulvagh S.L., Yip C.Y.Y., Norris C.M., Oudit G.Y. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am. J. Phys. Heart Circ. Phys. 2020;320:H296–H304. doi: 10.1152/ajpheart.00755.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krause M., Gerchman F., Friedman R. Coronavirus infection (SARS-CoV-2) in obesity and diabetes comorbidities: is heat shock response determinant for the disease complications? Diabetol. Metab. Syndr. 2020;12:63. doi: 10.1186/s13098-020-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., Mingrone G., Boehm B., Cooper M.E., Chai Z., Del Prato S., Ji L., Hopkins D., Herman W.H., Khunti K., Mbanya J.-C., Renard E. New-onset diabetes in Covid-19. N. Engl. J. Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J.-K., Jin J.-M., Liu S., Bai P., He W., Wu F., Liu X.-F., Chai Z.-L., Han D.-M. New onset COVID-19–related diabetes: an indicator of mortality. MedRxiv. 2020 doi: 10.1101/2020.04.08.20058040. 2020.04.08.20058040. [DOI] [Google Scholar]
- 96.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020;22:1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassett C.E., Gedansky A., Migdady I., Bhimraj A., Uchino K., Cho S.-M. Neurologic complications of COVID-19. Cleve. Clin. J. Med. 2020;87:729–734. doi: 10.3949/ccjm.87a.ccc058. [DOI] [PubMed] [Google Scholar]
- 100.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.-E., Barbry P., Leslie A., Kiem H.-P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.org, HCA Lung Biological Network SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.035. 1016–1035.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doyle S., Vaidya S., O’Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 102.Aboudounya M.M., Heads R.J. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediat. Inflamm. 2021;2021:8874339. doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]