Abstract
BACKGROUND:
Brain metastases are a common occurrence, with literature supporting the treatment of a limited number of brain metastases with stereotactic radiosurgery (SRS), as opposed to whole brain radiotherapy (WBRT). Less well understood is the role of SRS in patients with ≥10 brain metastases.
METHODS:
Patients treated with SRS to ≥10 brain metastases without concurrent WBRT between March 1999 and December 2016 were reviewed. Analysis was performed for overall survival, treated lesion freedom from progression (FFP), freedom from new metastases (FFNMs), and adverse radiation effect. Hippocampal volumes were retrospectively generated in patients treated with up-front SRS for evaluation of dose volume metrics.
RESULTS:
A total of 143 patients were identified with 75 patients having up-front SRS and 68 patients being treated as salvage therapy after prior WBRT. The median number of lesions per patient was 13 (interquartile range [IQR], 11–17). Median total volume of treatment was 4.1 cm3 (IQR, 2.0–9.9 cm3). The median 12-month FFP for up-front and salvage treatment was 96.8% (95% confidence interval [CI], 95.5–98.1) and 83.6% (95% CI, 79.9–87.5), respectively (P < 0.001). Twelve-month FFNMs for up-front and salvage SRS was 18.8% (95% CI, 10.9–32.3) versus 19.2% (95% CI, 9.7–37.8), respectively (P = 0.90). The mean hippocampal dose was 150 cGy (IQR, 100–202 cGy).
CONCLUSIONS:
Excellent rates of local control can be achieved when treating patients with >10 intracranial metastases either in the up-front or salvage setting. Hippocampal sparing is readily achievable with expected high rates of new metastatic lesions in treated patients.
Keywords: Brain metastasis, Hippocampal sparing, SRS
INTRODUCTION
The incidence of brain metastases in patients diagnosed with a malignancy approaches 20%–40%.1–3 Over time, this rate is likely to increase with ongoing improvements in medical imaging and systemic therapies. The typical approach for treatment of brain metastases has been either surgical resection or radiation therapy, with specific indications and rationale helping to determine which treatment modality is preferred.4,5
The paradigm for radiation therapy of brain metastasis ranges from focal approaches, such as stereotactic radiosurgery (SRS), to comprehensive treatments that aim to address both macroscopic and microscopic disease, such as whole brain radiation therapy (WBRT).4,6–8 The choice of these techniques is as much about technical feasibility as it is a trade-off between disease control and toxicity,9–11 with growing evidence supporting SRS alone for a limited number of metastases. In contrast, WBRT is often reserved for greater disease burden, including cases of leptomeningeal disease.7,9 Improved overall survival (OS) is seen for patients treated with SRS to a single lesion compared with multiple lesions,12 with additional prospective trials demonstrating similar outcomes in patients with 2–4 lesions compared with 5–10 lesions when treated with SRS.13,14 Less data exist for the treatment of >10 lesions; however, many reports demonstrate that select patients with numerous brain metastases may have similar outcomes as those with a smaller number of metastases.15–17
One goal of focal radiation treatments of brain metastasis is preservation of neurocognition by sparing of radiation-induced damage to eloquent domains, including the hippocampus.18 However, a concern of using focal approaches for treatment of a large number of brain metastases is the increasing likelihood of additional occult lesions that may be undertreated in the absence of comprehensive brain radiotherapy.19 Moreover, a number of recent trials have reported the results of emerging targeted and immunotherapies for the treatment of brain metastases with encouraging favorable early responses.20–23 It is increasingly important to understand the specific indications, risks, and benefits of each treatment modality, and how they can be used most effectively. Further understanding is needed on the results of SRS in patients with >10 brain metastases, including rates of distant brain failure and the impact on hippocampal dosimetry, as the treatment paradigm for brain metastases broadens.
METHODS
Patients
In an institutional review board–approved retrospective study, institutional databases were reviewed for patients meeting eligibility criteria. Patients included in this analysis were those who underwent SRS to >10 metastatic brain lesions without concurrent WBRT at the University of California San Francisco between March 1999 and December 2016. At the time of treatment, a multidisciplinary team including radiation oncologists, neurosurgeons, and radiologists reviewed each case prior to treatment to assess eligibility for SRS. This assessment included factors such as performance status, number of lesions, systemic disease status, proximity to critical structures, and previous treatments. Patients were not excluded if a lesion was present in or near the hippocampus. Patient were excluded from analysis if the planned SRS was ultimately aborted in favor of WBRT, if the SRS was performed postoperatively, or if the treatment was not delivered in a single session.
Characteristics of the patients, including age at the time of treatment, sex, tumor histology, number of lesions, prior radiation treatments, and performance status, were collected. These data were subsequently used for analysis of treatment-related outcomes of OS, treated lesion freedom from progression (FFP), freedom from new metastases (FFNMs), and freedom from adverse radiation effect (ARE).
Treatment and Follow-Up
Single-session fixed-frame SRS was performed with frame (Gamma Knife Perfexion [Elekta, Stockholm, Sweden]) placement on the day of treatment by a neurosurgeon. Gadolinium contrast-enhanced magnetic resonance imaging (MRI) scan was performed after frame placement with acquisition of 3-dimensional spoiled gradient recalled images for delineation of metastatic lesions and treatment planning. Targets were contoured using Gamma Plan software (Elekta) by the treating physician with planning performed by a departmental physicist. Treatment-specific data were collected including number of lesions treated, target volume, and development of ARE.
Routine follow-up contrast-enhanced MRI scan was recommended to be performed at 3- to 4-month intervals until with-drawal from treatment or death. Follow-up imaging was reviewed by a multidisciplinary radiosurgery tumor board consisting of radiation oncologists, radiologists, and neurosurgeons to evaluate for ARE, local or distance brain failure, and need for additional treatment. The date of last follow-up or death, date of last imaging, recurrence at a treated lesion, distant brain failure, ARE, and need for salvage therapy were recorded for analysis. Hippocampal contours were retrospectively generated for analysis of dosimetric parameters in patients receiving up-front SRS.
To analyze, retrospective hippocampal dose contours were generated on planning 3-dimensional spoiled gradient recalled MRI scan using MIM (MIM Software, Cleveland, Ohio, USA) contouring software to allow for the fusion of multiple MRI sequences. The left and right hippocampal contours were created separately and then averaged to calculate the mean hippocampal dose for each patient. Evaluation of the hippocampal dose was performed using a dose matrix encompassing the entire skull to ensure accurate dose quantification for the hippocampus. Each contour was reviewed by a board-certified radiation oncologist prior to its use in analysis.
Statistical Analysis
Analysis was performed using RStudio (RStudio, Boston, Massachusetts, USA) with the Kaplan-Meier method used for calculation of FFP, FFNMs, OS, and freedom from ARE. This was performed on a per lesion and per patient basis where appropriate, with censoring at the date of progression, last follow-up, or death. Multivariate analysis was performed for assessment of prognostic factors with Cox proportional hazard ratio (HR) being used with a P value of 0.05 for significance. Comparisons of categorical variables were done with the χ2 or Fisher exact test.
RESULTS
From 1999 to 2016, 143 patients were treated to ≥ 10 metastases with fixed-frame Gamma Knife without planned WBRT. Within this cohort, 75 of the patients received SRS without WBRT as up-front treatment for brain metastases, with 68 being treated as salvage therapy after prior WBRT. The overall median age was 57 years, with a significant difference in age (P = 0.011) between patients receiving salvage SRS at 54 years (interquartile range [IQR], 44–61 years) and those receiving up-front SRS at 59 years (IQR, 50–68 years). Median Karnofsky Performance Status Scale (KPS) score in this cohort of patients was 80 (IQR, 70–90) and was not different between the 2 groups. Primary site histology was breast in 52 patients (36.4%), non–small cell lung cancer in 49 patients (34.3%), melanoma in 30 patients (21.0%), and other malignancy in 12 patients (8.3%). A total of 2196 lesions were treated, with a median of 13 lesions (IQR, 11–17) per patient. Median total treatment volume was 4.1 cm3 (IQR, 2.0–9.9 cm3) per patient. The median clinical follow-up time for the entire cohort was 7.4 months (IQR, 2.7–15.9 months), and the median imaging follow-up time was 5.8 months (IQR, 1.2–12.9 months) in those that had evaluable posttreatment MRI scan. Additional patient and treatment characteristics can be found in Table 1.
Table 1.
Patient and Treatment Characteristics (N = 143)
| Variable | Value |
|---|---|
| Up-front SRS | 75 |
| Salvage SRS post-WBRT | 68 |
| Age (y) | 57 (46–65) |
| KPS score | 80 (70–90) |
| Histology | |
| Breast | 52 (36.4) |
| Lung | 49 (34.3) |
| Melanoma | 30 (21) |
| Other | 12 (8.3) |
| Number of BMs per patient | 13 (11–17) |
| Prescription dose (Gy) | 19 (18–19) |
| Total treatment volume (cm3) | 4.1 (2.0–9.9) |
Values are number of patients, number of patients (%), or median (interquartile range). SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy; KPS, Karnofsky Performance Status Scale; BM, brain metastasis.
Within this cohort, 112 patients had follow-up MRI scan available for review. Analysis of FFP was performed both on a per lesion basis and a per patient basis. The 12-month per lesion FFPs for up-front SRS versus salvage were 96.8% (95% confidence interval [CI], 95.5–98.1) and 83.6% (95% CI, 79.9–87.5), respectively (P < 0.001), and are demonstrated in Figure 1. On a per patient basis, 12-month FFP for up-front SRS versus salvage was 74.3% (95% CI, 63.0–87.6) compared with 57.1% (95% CI, 40.1–81.4) shown in Figure 2 (P = 0.72). Multivariate analysis (MVA) is shown in Table 2 and demonstrates that salvage SRS was not significantly associated with worse FFP, whereas melanoma histology had significantly worse FFP when compared with breast, lung, or other histology (HR, 4.35; P = 0.003).
Figure 1.

Freedom from progression on a per lesion basis from time of stereotactic radiosurgery (SRS) by up-front SRS versus salvage SRS (P < 0.0001). FFP, freedom from progression; WBRT, whole brain radiotherapy.
Figure 2.
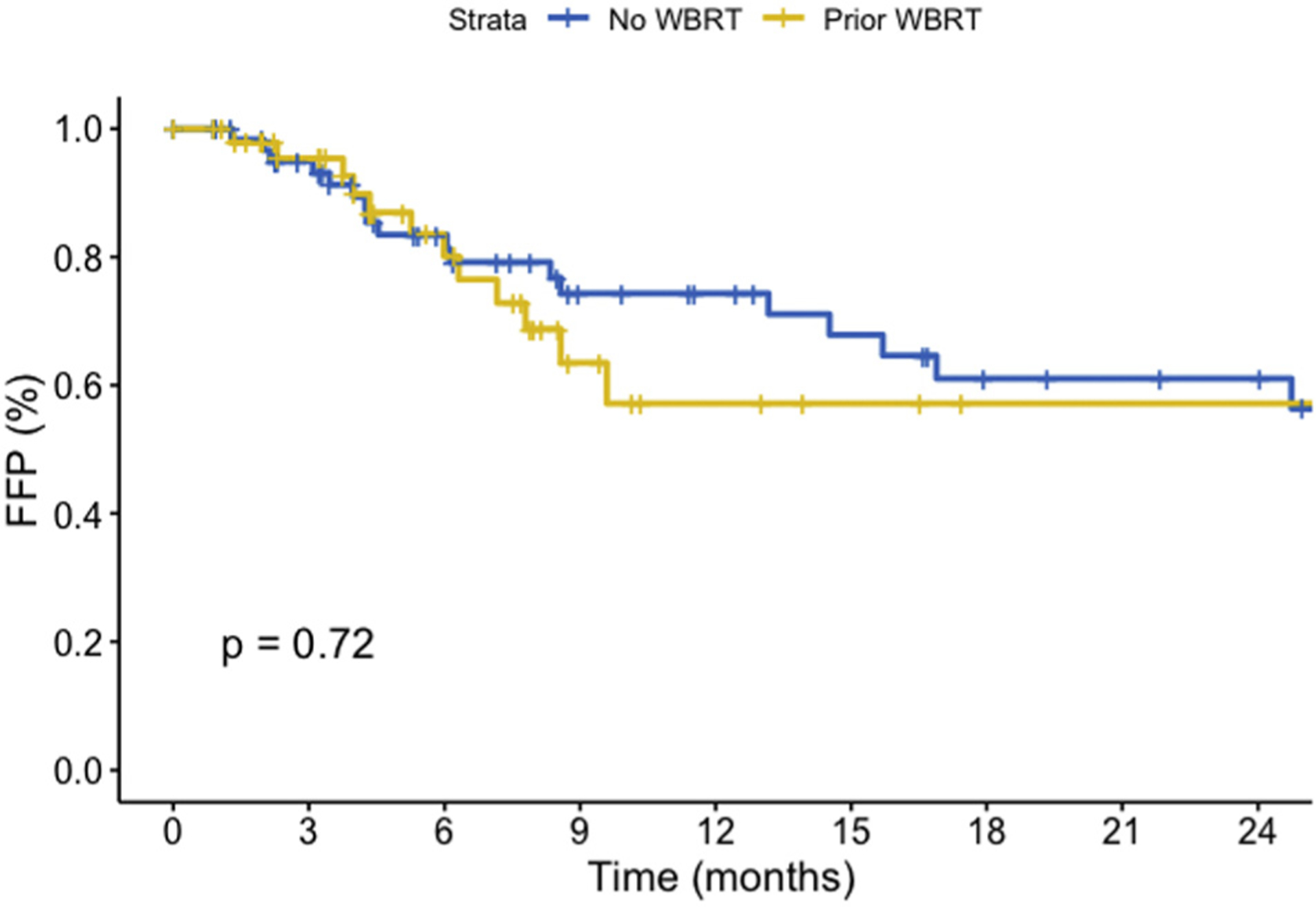
Freedom from progression on a per patient basis from time of stereotactic radiosurgery (SRS) by up-front SRS versus salvage SRS (P = 0.72). FFP, freedom from progression; WBRT, whole brain radiotherapy.
Table 2.
Multivariate Analysis of Factors Associated with Prognosis and Control
| Metric | HR | P value |
|---|---|---|
| FFP (per patient) | ||
| Melanoma | 4.35 | 0.003 |
| Salvage post-WBRT | – | ns |
| FFNMs | ||
| Number of BMs, prior WBRT, histology, volume | – | ns |
| Overall survival | ||
| Salvage post-WBRT | 1.61 | 0.019 |
| KPS score | 0.98 | 0.017 |
| Total volume | 1.03 | 0.031 |
| Number of BMs, age, histology | – | ns |
| FFARE | ||
| Melanoma | 2.13 | 0.002 |
| Salvage post-WBRT | 1.63 | 0.005 |
| Number of BMs, volume, age | – | ns |
HR, hazard ratio; FFP, freedom from progression; WBRT, whole brain radiotherapy; ns, not significant; FFNM, freedom from new metastasis; BM, brain metastasis; KPS, Karnofsky Performance Status Scale; FFARE, freedom from adverse radiation effect.
The 12-month FFNM for up-front SRS compared with salvage was 18.8% (95% CI, 10.9–32.3) versus 19.2% (95% CI, 9.7–37.8), respectively (P = 0.90), shown in Figure 3. The median time to FFNMs was 4.0 months for up-front treatment and 4.7 months for salvage treatment. On MVA, there were no significant factors, including, number of metastases, age, prior treatment, or histology, that were associated with improved FFNMs. Salvage therapies were not specifically analyzed, but included additional SRS, partial brain radiotherapy, or WBRT depending on the clinical scenario.
Figure 3.

Freedom from new metastases per patient from time of stereotactic radiosurgery (SRS) by up-front SRS versus salvage SRS (P = 0.90). FFNM, freedom from new metastasis; WBRT, whole brain radiotherapy.
Over the period of review, a total of 137 patients had died and 6 patients remained alive. The median OS for up-front SRS and salvage treatment was 11.7 and 7.4 months, respectively (P < 0.001), with results demonstrated in Figure 4. Analysis of the OS from time of initial diagnosis of brain metastases revealed no significant difference (P = 0.34) in OS between up-front or salvage SRS and is demonstrated in Supplementary Figure 1. On MVA, there was worse OS associated with salvage SRS after prior WBRT (HR, 1.61; P = 0.019) and with total treated volume (HR, 1.03; P = 0.031), and lower KPS score (HR, 0.98; P = 0.017) demonstrated significantly worse outcomes.
Figure 4.
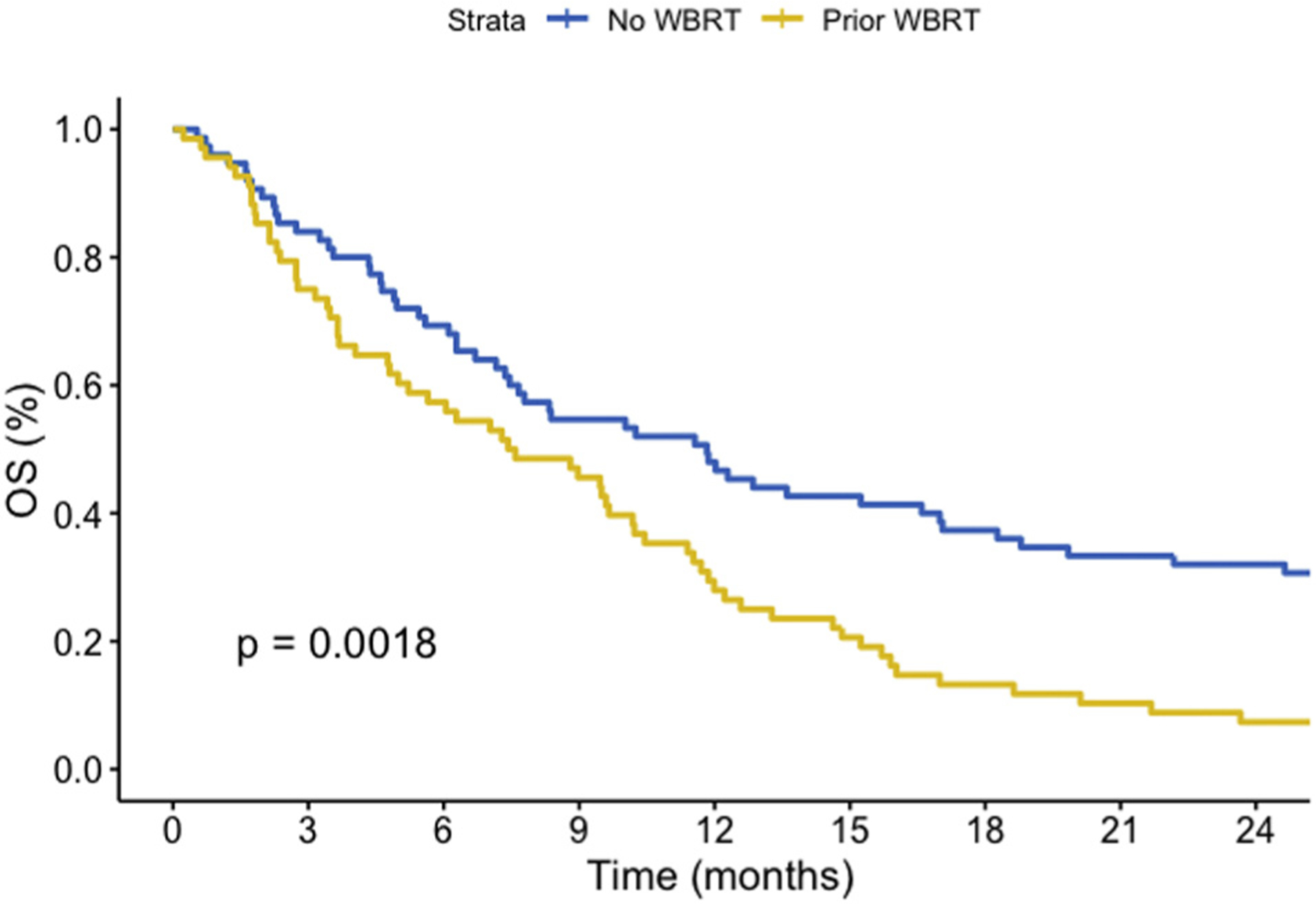
Overall survival from time of stereotactic radiosurgery (SRS) by up-front SRS versus salvage SRS (P = 0.0018). OS, overall survival; WBRT, whole brain radiotherapy.
ARE was noted on follow-up imaging for 16 patients (11%), and was symptomatic in 3 patients (2%). In total, symptomatic ARE was seen in <1% of the total treated 2196 lesions. The 12-month ARE rate for up-front SRS and salvage patients was 10.6% (95% CI, 1.1–19.3) and 36.0 (95% CI, 11.2–53.9) on a per patient basis (P = 0.11) and can be seen in Figure 5. On MVA analysis, rates of ARE were significantly associated with salvage SRS after WBRT (HR, 1.62; P = 0.005) and melanoma histology (HR, 2.13; P = 0.002).
Figure 5.
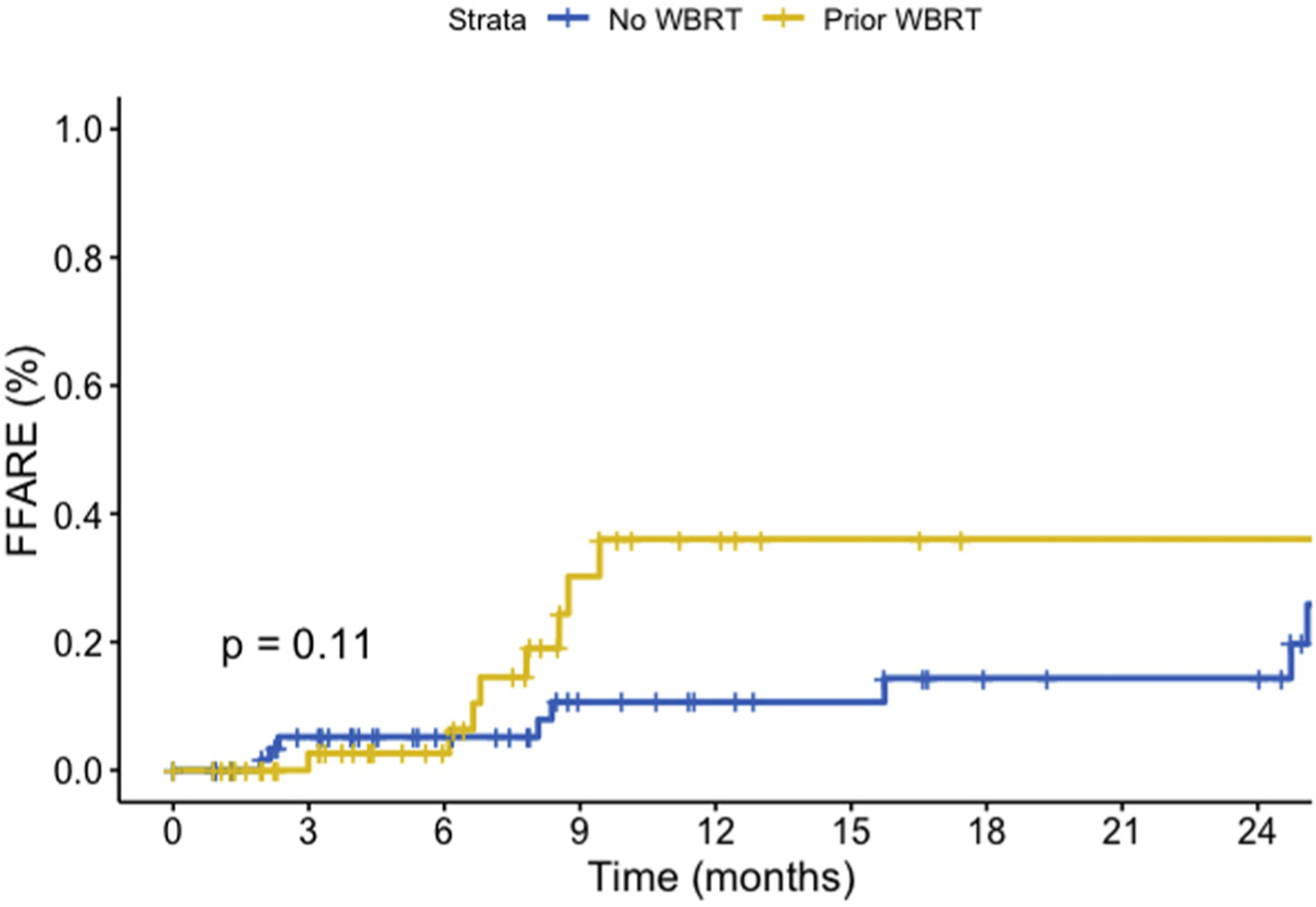
Rates of adverse radiation effect from time of stereotactic radiosurgery demonstrated on a per patient basis (P = 0.11). FFARE, freedom from adverse radiation effect; WBRT, whole brain radiotherapy.
Retrospective contours were created of the brain and hippocampus for 62 of the 75 patients who received up-front SRS. Mean hippocampal dose demonstrated a median value of 161 cGy (IQR, 94–258 cGy). This cohort of patients was treated to a median of 12 lesions each (IQR, 11–17), with a median dose of 1900 cGy per lesion. In a subset of patients (n = 36), the mean brain dose was calculated and found to be 150 cGy (IQR, 100–202 cGy).
DISCUSSION
The use of SRS for the treatment of brain metastases has evolved over time, from its beginnings as an adjunct to WBRT to playing a primary role as the treatment of choice in many patients. Prospectively, it has been demonstrated that WBRT plus SRS, when compared with SRS alone, in patients with 1–4 brain metastases does not improve OS, and can be successfully salvaged.7,24 Further trials have investigated the feasibility of treating up to 10 metastatic lesions in a single session,14 but little data exist regarding the treatment of >10 brain metastases with SRS alone.
An early retrospective analysis of 26 patients for the treatment of ≥ 10 brain metastases demonstrated a median OS of 7.8 months, and patients with a KPS score >80 or controlled primary disease had significantly better survival.15 Additional data demonstrate lower OS of 4–6 months; however, most series represent a heterogeneous cohort of patients with some including only 11.5% of patients being treated up-front with SRS.16,17 Within our series of 143 patients, those receiving WBRT and SRS boost were excluded to thoroughly examine the role of SRS in patient with >10 brain metastases, making it one of the largest series reported.
With the reasonable number of patients receiving SRS alone for their central nervous system metastatic disease, further analysis could be done regarding prognosis and disease control. Up-front SRS in our series demonstrated a 12-month FFNMs rate of 18.8%. This is lower than prior prospective series which demonstrated rates of 30%–35%. However, prior prospective series did not include patients with ≥ 10 metastases.7,14 In analyses comparing FFNMs with increasing number of metastases treated, patients with between 3 and 10 metastases treated had statistically insignificant differences in FFNMs.25 Likely, the low FFNMs in our series demonstrate an increasing continuum of risk in patients with a greater baseline intracranial metastatic burden. This in itself should not be seen as a rationale to avoid up-front SRS in patients with ≥ 10 brain metastases, especially with the understanding that neither WBRT or SRS provide a significant survival advantage from the time of initial diagnosis of brain metastases.
An ongoing challenge in treatment of numerous intracranial metastases is the association of WBRT with changes in cognition over time.9,10 Unique to this series is the quantification of the dosimetric effect of SRS on the hippocampus when treating >10 metastases. The use of linear accelerator-based planning for hippocampal sparing can decrease the median hippocampal dose to between 550 and 780 cGy, or the equivalent of 49–73 cGy per 200 cGy fractions received.26 We reveal that SRS would deliver a mean dose of 150 cGy in a single session of treatment, much lower than can be achieved using linear accelerator-based hippocampal sparing during WBRT. Although only the preliminary results of NRG-CC01, a phase III study evaluating the neurocognitive outcomes after hippocampal avoidance, have been published, they do note a significant benefit in neurocognitive outcomes with hippocampal avoidance.11,27 Our cohort demonstrates that the treatment of ≥ 10 metastases with SRS provides expectedly low hippocampal doses, and it is reasonable to expect this provides at least equivalent neurocognitive benefit to patients while providing good rates of FFP and FFNMs.
Additionally, the increasing use of targeted and immunotherapies in the treatment of brain metastases offers an opportunity for further understanding of the role of SRS. A large retrospective study has demonstrated an OS advantage to SRS over early generation tyrosine kinase inhibitors.28 However, ongoing reports demonstrate increasing intracranial efficacy and control in the treatment of brain metastases with these agents.20–23 The combination of targeted systemic or immunotherapy plus SRS may be able to combine the strengths of both treatments. There is potential for improved local control and durability of treatment for lesions targeted during SRS, with systemic therapies improving FFNMs to levels comparable with WBRT.29,30
A final consideration in the use of SRS is the development of ARE in patients who may have otherwise been asymptomatic. Only 1% of the lesions treated in this series developed ARE; however, given the high burden of lesions per patient, this amounts to a modest rate of 11% of patients, with only 2% of patients having symptomatic ARE. This is similar to reported results of ARE in other studies of this nature, with between 13% and 18% of patients having ARE on follow-up imaging, with 0%–2% of all patients being symptomatic.16,17 A point of active research is if this risk is enough to warrant delaying radiation therapy while trialing targeted systemic agents. Considering the risks of progressive disease, or toxicities of WBRT, a thoughtful discussion of the risk of SRS should be undertaken with patients given its efficacy and low rates of toxicity.
The limitations of this study include its retrospective nature and the length of the time period analyzed. Of the patients selected for up-front SRS, there may be bias toward higher performance status and lower total intracranial volume of disease. This could have affected disease progression and hippocampal dose received. Additionally, because this experience spans multiple decades, the outcomes may reflect evolving treatment paradigms and provider preferences. We also lack detailed cause of death data; however, for most patients in this series, mortality was linked to extracranial disease burden. To more fully understand these results, patients with >10 intracranial lesions should be included in future prospective studies involving SRS.
Overall, SRS for patients with ≥ 10 brain metastases as up-front therapy or at recurrence after prior WBRT can provide excellent rates of local control in properly selected patients. Hippocampal avoidance can be readily achieved with hippocampal doses lower than those that are feasible for linear accelerator-based WBRT. Although rates of ARE were modest, this was consistent with prior studies, and given the potential benefit in cognitive outcomes when avoiding WBRT, a thorough discussion with patients should be had about the risks and benefits of each approach.
Supplementary Material
Abbreviations and Acronyms
- ARE
Adverse radiation effect
- CI
Confidence interval
- FFNM
Freedom from new metastasis
- FFP
Treated lesion freedom from progression
- HR
Hazard ratio
- IQR
Interquartile range
- KPS
Karnofsky Performance Status Scale
- MRI
Magnetic resonance imaging
- MVA
Multivariate analysis
- OS
Overall survival
- SRS
Stereotactic radiosurgery
- WBRT
Whole brain radiation therapy
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. [DOI] [PubMed] [Google Scholar]
- 3.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 4.Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322:494–500. [DOI] [PubMed] [Google Scholar]
- 6.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363: 1665–1672. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. [DOI] [PubMed] [Google Scholar]
- 8.Lindquist C Gamma knife surgery for recurrent solitary metastasis of a cerebral hypernephroma: case report. Neurosurgery. 1989;25:802–804. [DOI] [PubMed] [Google Scholar]
- 9.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases. JAMA. 2016; 316:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. [DOI] [PubMed] [Google Scholar]
- 11.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson B, Hanssens P, Wolff R, Soderman M, Lindquist C, Beute G. Thirty years’ experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009;111:449–457. [DOI] [PubMed] [Google Scholar]
- 13.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15: 387–395. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH, Im YS, Nam DH, Park K, Kim JH, Lee JI. Gamma Knife radiosurgery for ten or more brain metastases. J Korean Neurosurg Soc. 2008;44:358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandhi R, Kondziolka D, Panczykowski D, et al. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg. 2012;117:237–245. [DOI] [PubMed] [Google Scholar]
- 17.Rava P, Leonard K, Sioshansi S, et al. Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2013;119:457–462. [DOI] [PubMed] [Google Scholar]
- 18.Gondi V, Deshmukh S, Brown PD, et al. A phase III trial of hippocampal avoidance (HA) in addition to whole-brain radiotherapy (WBRT) plus memantine to preserve neurocognitive function (NCF) in patients with brain metastases (BM). J Clin Oncol. 2019;37(suppl 15);2009. [Google Scholar]
- 19.Garcia MA, Anwar M, Yu Y, et al. Brain metastasis growth on preradiosurgical magnetic resonance imaging. Pract Radiat Oncol. 2018;8:e369–e376. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung Cancer. J Clin Oncol. 2018;36:3290–3297. [DOI] [PubMed] [Google Scholar]
- 23.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549–558. [DOI] [PubMed] [Google Scholar]
- 25.Serizawa T, Hirai T, Nagano O, et al. Gamma knife surgery for 1–10 brain metastases without prophylactic whole-brain radiation therapy: analysis of cases meeting the Japanese prospective multi-institute study (JLGK0901) inclusion criteria. J Neurooncol. 2010;98:163–167. [DOI] [PubMed] [Google Scholar]
- 26.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique, utilizing helical tomotherapy and linear accelerator-based intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;15: 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondi V, Pugh S, Brown DP, et al. NCOG-01. Preservation of neurocognitive function (NCF) with hippocampal avoidance during whole-brain radiotherapy (WBRT) for brain metastases: preliminary results of phase III trial NRG ONCOLOGY CC001. Neuro Oncol. 2018;20. vi172. [Google Scholar]
- 28.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. [DOI] [PubMed] [Google Scholar]
- 29.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JJ, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5:707–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


