Cardiogenic shock following cardiac surgery is a life-threatening condition characterized by severe myocardial contractile impairment and reduced organ perfusion. In the current era, patient mortality approaches 40%.1 Acute mechanical support therapies are increasingly utilized in patients with progressive organ dysfunction despite optimized management, including mechanical ventilation, blood products, and pharmacotherapy.1 Extracorporeal membrane oxygenation (ECMO) is increasingly used as the first line mechanical circulatory support in patients that are refractory to conventional treatment. ECMO facilitates respiratory gas exchange and provides cardiac output to end-organs, supporting organ recovery, identification of residual lesions and allowing time for “bridge-to-decision” to more durable modes of support.1−6 ECMO is immediately available, is easy to apply, and reliably supports heart and lung functions—these features have made it an attractive option for circulatory support in the post-cardiotomy (PC) setting.1−6 In contrast, implantable or para-corporeal ventricular assist devices (VADs) are more complex, are costly, and do not facilitate respiratory gas exchange. These features have hindered widespread adoption in the immediate PC setting.7−12
Post-cardiotomy ECMO (PC-ECMO) is the most frequent indication for ECMO in the United States.4 The exponential increase in ECMO use has not been accompanied by improved early survival, with some reports of increasing mortality in the last decade.6,13 The published reports of PCECMO are mainly single-center experiences,14−37 frequently combining adult and pediatric patients in the same series, or including PC-ECMO in reports of institutional ECMO experience. 38−55 As a result, the interpretation of PC-ECMO indications, complications, and outcomes can be challenging. PCECMO is increasing, and uniquely complicated, making an analysis of PC-ECMO prevalence, patient selection, in-hospital management, and short and long term outcomes relevant to the multidisciplinary team managing these patients.
PC-ECMO is being increasingly used in both adult and pediatric populations. However, there are distinct differences between PC-ECMO in these groups, and this review will only focus on adult cardiac surgery patients. The authors aim to provide a detailed and comprehensive evaluation of the adult PC-ECMO and present outcomes for veno-arterial (VA) and veno-venous (VV) ECMO. Trends in PC-ECMO use, educational needs, and future perspectives will be addressed to highlight potential applications, required training, and implications for future research. An accompanying review will address PC-ECMO in the pediatric population.
Characteristics of PC-ECMO
Trends in use
The incidence of ECMO implementation in patients after open-heart surgery has been reported between 0.4% and 3.7% (Table 1). PC-ECMO has increased considerably over the past 2 decades.2 Indeed, from 2007 to 2011, non-percutaneous ECMO cannulation increased 2-fold, while the use of percutaneous ECMO increased by more than 15-fold.2 Maxwell and colleagues, evaluating more than 9,000 ECMO patients from the Nationwide Inpatient Sample database in the United States from 1998 to 2009, identified 4,493 patients with approximately 50%, cannulated for cardiogenic shock in the postoperative period.5 Between 2002 and 2011 in the same database, McCarthy and colleagues found that PC-ECMO was the most common indication for ECMO in each year of their study.3 Recently, data from the Extracorporeal Life Support Organization (ELSO) Registry also confirmed a substantial increase in PC-ECMO use over the last 10 years.6 ECMO use in adult populations has also substantially increased in this time, such that the proportion of adults managed on ECMO for PC indication has decremented.3,5
TABLE 1.
Baseline Characteristics of Patients Supported by Extracorporeal Membrane Oxygenation (ECMO)
| Author reference – Year of Publication | Patient N (Male/Female) | Prevalence of ECMO use, % | Patient Age Mean in years±SD (Range) | Pre-ECMO Cardiac Surgery Procedures |
|---|---|---|---|---|
| Magovern14 - 1994 | 21 (17/4) |
0.7 | 61.6 ± 2.2 (33–78) |
CABG 66.6% MV 14.3% Others 19,1% |
| Kodera15 - 1996 | 17 (11/6) |
na | 41.4 (22–64) |
CABG 17.6% Valve 82.4% |
| Fiser16 - 2001 | 51 (29/22) |
0.9 | 61±1.7 (32–77) |
CABG 66.6% Valve 9.8% CABG +Valve 9.8% HTx 13.7% |
| Smedira17 - 2001 | 107 (77/30) |
0.5 | na (18–82) |
CABG 56% Valve 14% CABG+Valve 18% Aorta (desc.) 4% Cong. 7% LV resection 1% |
| Ko18 - 2002 | 76 (48/28) |
2.60 | 56.8±15.9 (na) |
CABG 47.7% Valve 18.4% CABG+Valve 7.9% HTx 15.7% Cong. 3.9% Ao Diss. 2.7% |
| Zhang19 - 2006 | 32 (18/14) |
na | 55.4±11.9 (30–75) |
CABG 15.6% Valve 31.2% CABG+Valve 31.2% CABG+LV An. . 6.2% PEA 6.2 % Others 6.2% |
| Bakhtiary20 - 2007 | 45 (35/10) |
0.8 | 60.1±13.6 (na) |
CABG 44.4% Valve 4.4% CABG+Valve 17.8% CABG+VSD 6.7% HTx 4.4% LVAD 11.1% Others 11,9 |
| Hsu21 - 2010 | 51 (36/15) |
2.9 | 63±15.7 (na) |
CABG 52.9% Valve 21.6% CABG+Valve 13.7% HTx 7.8% Others 3.9% |
| Rastan22 - 2010 | 517 (370/147) |
1.28 | 63.5±11.2 (18–84) |
CABG 37.4% Valve 14.3% CABG+Valve 16.8% Thor. Tx 6.5% Others 25% |
| Elsharkawy23 - 2010 | 239 (163/76) |
0.58 | 59.7 (median) (na) |
CABG 44.3% Valve 33.6% |
| Pokersnik24 - 2012 | 49 (33/16) |
na | 65±13 (na) |
na |
| Mikus25 - 2013 | 14 (9/5) |
0.25 | 53.1±14.3 (25–70) |
CABG 35.7% Valve 42.9% Aortic Root 21.4% |
| Slottosch26 - 2013 | 77 (59/18) |
na | 60±13 (25–83) |
CABG 55.8% Valve 13% CABG +Valve 14.3% Aorta 6.5% HTx 2.6% Others 7.8% (Redo Surgery 19.5%) |
| Unosawa27 - 2013 | 47 (35/12) |
na | 64.4±12.5 (22–83) |
CABG 40.4% Valve 17% Valve+CABG 4.2% Valve+Aorta 2% Aorta 10.6% Aorta+CABG 6.4 % Aortic Root 4.25% VSD 10.6% PE 4.25% |
| Ariyaratnam28 - 2014 | 14 (8/6) |
na | 65.6 (na) |
Valve+CABG 42.8% Aortic Root 14.3% VSD 7% MVR+Maze 7% Ao Diss. 7% Aorta+ AA 7% VSD+CABG7% CABG+Lobectomy 7% |
| Li29 - 2015 | 123 (81/42) |
0.9 | 51.0±12.2 (70–85) |
CABG 35.8% Valve 32.5% CABG+Valve 9.8% CABG+ LVAn. 2.4% HTx 9% Others 10.5% |
| Saxena30 - 2015 a | 45 (31/14) |
na | 76.8±4.6 (70–85) |
na |
| Khorsandi31 - 2016 | 16 (12/4) |
na | 71 (34–83) |
na |
| Distelmaier32 - 2016 b | 385 (271/114) |
3.65 | 65 (median) (55–72) |
CABG 12.5% Valve 28.6% CABG+Valve 21.8% HTx 17.6% LVAD 8% Aorta 6.2% Others 5.2% |
| Mazzeffi33 - 2016 b | 23 (14/9) |
0.42 | 57±15 (34–86) |
CABG 30.4% Valve 52.2% Others 17.4% |
| Chen34 – 2017 | 1,141 (813/328) |
1.91 | 63.8±13.2 (na) |
CABG 63.9% Valve 24.1% CABG+Valve 12% |
| Guihaire35 - 2017 | 92 (53/39) |
0.7 | 64.5 (18–83) |
CABG 8.6% Valve 74.1% Ao Diss. 12.1% LVAD 3.4% Others 1.7% |
| Raffa36 - 2017 | 86 | na | 65±11.2 (31–86) |
CABG 22% CABG+Valve 21% Valve 16% Ao Diss. 4% Others 24% |
| Fux37 – 2018 | 105 (80/25) |
na | 62 (median) (18–77) |
CABG 20% Single, other than -CABG 29% 2 procedures 31% 3 procedures 20% |
AA, ascending aorta; Ao Diss, Aortic dissection; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; Cong, congenital; ECMO, extracorporeal membrane oxygenation; HTx, heart transplant; LV, left ventricle; LVAD, left ventricular assist device; MV, mitral valve; MVR, mitral valve replacement; na, not available; PE, pulmonary embolectomy; PEA, pulmonary endo-arterectomy; Valve, valve replacement or repair; VSD: ventricular septal defect;
Study included only patients over 70 years of age
Study included only extracorporeal cardiopulmonary resuscitation (ECPR) after cardiac surgery
Patient characteristics
The nature of the PC-ECMO indication is that patients invariably require urgent or emergent ECMO support compared with other indications. Baseline characteristics reveal that PC-ECMO patients are older, with a higher incidence of renal insufficiency, prior myocardial infarction, left main coronary artery disease, and left ventricular (LV) dysfunction. They are also more likely to have a prolonged history of coronary artery disease and prior open-heart surgery.17 Age does not appear to be an absolute contraindication, as elderly patients are routinely considered for PC-ECMO. Indeed, most PC-ECMO series include some patients older than 80 years of age (Table 1).56,57
As expected, PC-ECMO is most frequently associated with coronary artery bypass grafting, valve surgery, and a combination of valve or coronary surgery (Table 1). In several series, diagnoses previously considered relative contraindications to ECMO, such as the repair of an acute aortic dissection, have been successfully supported (Table 1). In patients post-heart transplantation, primary graft failure was associated with early post-transplant mortality. The use of ECMO post-heart transplant has been reported to be as high as 10%−15%,58−60 possibly associated with the increased use of marginal donors. Notably, in a series of 124 heart transplants, Listijono and colleagues showed that ECMO was used for 17 (14%) patients with 82% surviving to discharge.61 In patients who received a marginal donor heart, defined by a pre-transplant LV ejection fraction < 45%, 8 out of 9 patients (89%) were managed with ECMO, and of these 88% survived to hospital discharge with normal graft LV function.61 Another indication for ECMO is in patients who develop right ventricular (RV) failure post-left ventricular assist device (LVAD) implantation.62,63 PC-ECMO may be utilized to support the RV, or to bridge to right ventricular assist device.63,64
Indications
The most common indication for PC-ECMO implementation is intraoperative failure to wean from cardiopulmonary bypass (CPB) because of univentricular, biventricular, or respiratory failure. PC-ECMO may also be implemented for delayed refractory cardiogenic shock, postoperative cardiac arrest in the intensive care unit (ICU), respiratory failure, or intractable postoperative ventricular arrhythmias.14−35 Although not all series delineate the specific diagnosis and indication for PC-ECMO, it is evident that the use of ECMO for PC cardiac arrest has been more frequently considered during the last 10 years.65 This is reflected in the 2017 Society of Thoracic Surgeons Expert Consensus for the Resuscitation of Patients Who Arrest After Cardiac Surgery wherein it is recommended that failure to achieve spontaneous circulation is an indication for open cardiac massage and the institution of either central or peripheral ECMO at the bedside.66 The available information with regards to PC-ECMO applied for perioperative cardiac arrest and related outcomes are presented in the Supplementary Materials (Supplementary Table S1).
Implementation, management, and complications of PC-ECMO
Cannulation
The location of ECMO cannulation is influenced by the timing and indication, urgency of deployment, cardiocirculatory cardiocirculatory versus respiratory support required, and institutional factors including staff familiarity and availability of ECMO circuits. PC-ECMO is most often utilized for failure to wean from CPB, so operating room cannulation occurs most frequently, followed by the ICU, and rarely on the ward (Table 2). While PC-ECMO can be initiated any time in the postoperative course, the majority of PC-ECMO placements occur within 24−48 hours of procedure (Table 2).
TABLE 2.
Characteristics of Extracorporeal Membrane Oxygenation (ECMO) Placement and Duration of Support in Adult Patients Undergoing Post-Cardiotomy ECMO
| Author reference – Year of Publication | ECMO Implantation Location, % | Time: Surgery-to-ECMO Implant | ECMO Cannulation, % | IABP, % | Left Ventricular Venting, % | Limb Perfusion, % | ECMO Duration, means ± SD (range) |
|---|---|---|---|---|---|---|---|
| Magovern14 - 1994 | OR 54.5 ICU 45.5 |
1–48 hours | Central 76.2 Peripheral 23.8 | 100 | 0 | na | 42.1 hours (19–92 hours) |
| Kodera15 - 1996 | na | Weaned patients 2.85±1.8hrs Unweaned patients 15.8±7.5hrs |
Central and Peripheral | 100 | 100 | na | 11.3±6.0 / 31.0±12.8a hours |
| Fiser16 - 2001 | OR 43.1 ICU 56.8 |
na | Central and Peripheral | na | na | na | 85±12.5 / 64.7±9.2 hours |
| Smedira17 - 2001 | na | na | Central 33 Peripheral 67 | na | na | nab | 48 – 72 hours |
| Ko18 - 2002 | OR 51.3 ICU 48.7 |
na | Central 19.7 Peripheral 80.3 (open 56.4 - percutaneous 21.7) |
69 | 2.6 | 26.3 of femoral cannulation cases | 99±33 hours |
| Zhang19 - 2006 | na | na | Central 40.6 Peripheral 53.1 Subcl. Art.-Fem. Vein 6.3 |
31.2 | na | na | 2.7 ± 1.7 days |
| Bakhtiary20 - 2007 | OR 67 ICU 33 |
24–48 hours (in 6 patients) |
Central 18 Peripheral 64 Subcl. Art.-Fem. Vein 18 |
67 | 0 | nac | 6.4 ± 4.5 days |
| Hsu21 - 2010 | na | na | Central 0 Peripheral 100 |
100 | na | 100d | 7.5 ± 6.7 days |
| Rastan22 - 2010 | OR 41.9 ICU 58.1 |
62.6 hours (in 58.1% of the patients) |
Central 60.8 Peripheral 39.2 | 74.1 | na | 23.4 of femoral cannulation cases | 78.7 ± 68.4 hours |
| Elsharkawy23 – 2010 | OR and ICU | na | Central 33 Peripheral 67 | na | na | na | na |
| Pokersnik24 - 2012 | na | na | Central 35 Peripheral 65 | 59.2 | na | 60 | 3.1 (±2.1)e / 4.1 (±3.7)f / 4.3 (±3.0)g days |
| Mikus25 - 2013 | OR 85.7 ICU 14.3 |
48 hours | Central 57.1 Peripheral 42.9 |
100 | 100h | 100 | 5 days (1–55 days) |
| Slottosch26 - 2013 | OR 44.2 ICU 55.8 |
45±69 hours | Peripheral 100 (open) | 93.5 | 0 | 91 | 79 ± 57 hours |
| Unosawa27 - 2013 | OR 70.2 ICU 29.8 |
na | Central and Peripheral | 83 | 0 | 100i | 63.5 ± 61.5 hours |
| Ariyaratnam28 - 2014 | na | na | Central 100 Peripheral 0 |
na | na | na | 1 – 14 days |
| Li29 - 2015 | OR 49.6 ICU 50.4 |
na | Central 0 Peripheral 100 (open) |
59.3 | na | na | 4.4 ± 3.7 days |
| Saxena30 – 2015j | OR 57.8 ICU 42.2 |
na | Central 66 Peripheral 34 | na | na | na | 103 ±74.3 hours |
| Khorsandi31 - 2016 | na | na | Central 67 Peripheral 33 | na | na | na | > 1 – 33 days |
| Distelmaier32 - 2016k | OR 86 ICU 14 |
na | Aorta-Femoral 10 Subcl-Fem 38 Peripheral 52 |
12 | na | na | 96 hours |
| Mazzeffi33 - 2016 b | OR and ICU | 31 (15–52) min. | Central 60.9 Peripheral 39.1 | 13 | 0 | na | 0 – 14 days |
| Chen34 - 2017 | na | na | na | na | na | na | |
| Guihaire35 - 2017 | OR 86.9 ICU 13.1 |
48 hours | Central 14.7 Peripheral 85.3 |
27.1 | 14.1 | na | 6 days (1–28 days) |
| Raffa36 - 2017 | OR 55.8 ICU 44.2 |
na | Central 17.4 Peripheral 65.1 Central (artery) – Peripheral (vein) 17.4 |
27.1 | na | na | 5 days |
| Fux37 – 2018 | OR 49 | na | Peripheral 76 Central 24 |
na | na | 90% | 7 days (median) (1–55) |
CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; ECPR, extracorporeal cardiopulmonary resuscitation; IABP, intra-aortic balloon pump; ICU, intensive care unit; na, not available; OR, operating room; RVF, right ventricular failure; Subcl-Fem, subclavian artery
Groups related to patients who were weaned and discharged versus patients weaned from ECMO but not discharged and died in hospital
Performed in all patients with femoral cannulation in the most recent series, after an initial experience without it characterized by ischemia episodes of the cannulated limb
Distal limb perfusion performed but no data available
Distal limb perfusion achieved by cannulating the femoral artery with a 8 mm-graft perfusing antegradely and retrogradely
Group of patients receiving Biomedicus (Medtronic Inc, Minneapolis, Minn) pump and Affinity oxygenator (Medtronic Inc. Minneapolis, Minn)
Group of patients receiving Biomedicus (Medtronic Inc, Minneapolis, Minn) and Quadrox D (Getinge, Hirrlingen, Germany) oxygenator
Group of patients receiving Rotaflow (Getinge, Hirrlingen, Germany) pump and Quadrox D (Getinge, Hirrlingen, Germany) oxygenator
Venting was performed in all patients who had central cannulation and no patients with femoral cannulation
Distal limb perfusion was performed in 100% of cases after 1999
Study included only patients over 70 years of age
Study included only extracorporeal cardiopulmonary resuscitation (ECPR) after cardiac surgery
The implementation of intraoperative ECMO generally follows a period of escalating vasoactive support and may be delayed by the placement of an intra-aortic balloon pump (IABP). There is limited data to inform the optimal timing of ECMO cannulation, either in the fail to wean from CPB population, or in those cannulated later in the ICU. Institutional protocols differ with respect to the escalation of vasoactive therapies, optimal pre-load, and acceptable duration of myocardial and systemic exposure to highdose inotropes. In the ICU, the timing of PC-ECMO cannulation may be even more variable, as indications are not well defined, which may contribute to a delay in decision making, resulting in ECMO deployment. Furthermore, there is institutional variability in the logistics for ECMO circuit preparation and implementation.30 ECMO deployed in the setting of extracorporeal cardiopulmonary resuscitation (ECPR) without the return of circulation may present a more straightforward decision but with more complicated logistics and event management.66 Although post-ECMO survival is similar for those cannulated to ECMO in the operation room and the ICU, longer ICU duration prior to ECMO cannulation is associated with poorer survival.22
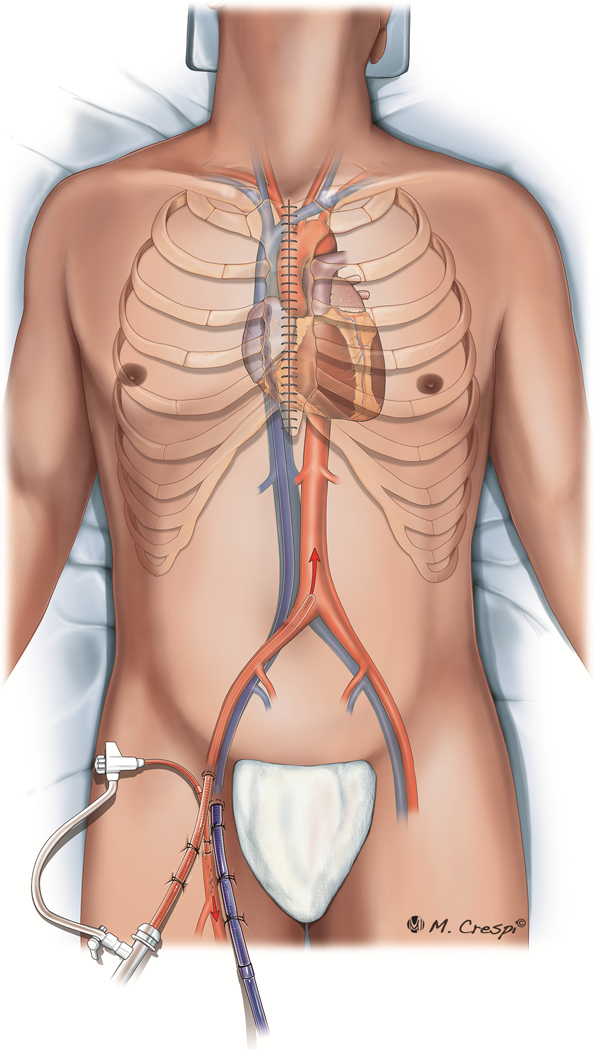
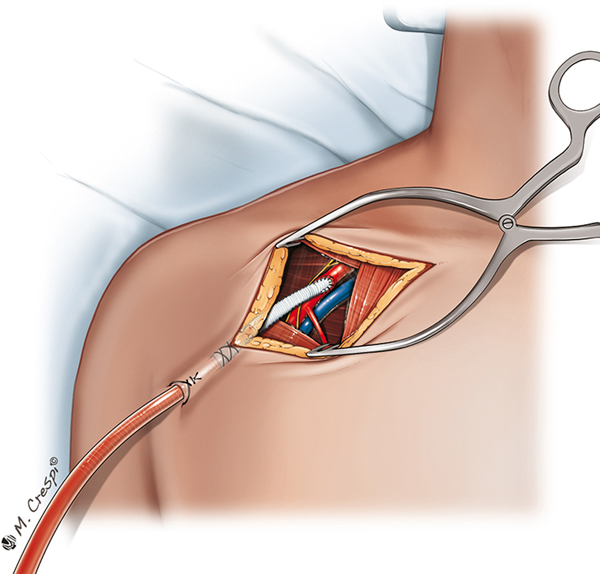
Despite the presence of either a median sternotomy or thoracotomy, which allows central access, peripheral cannulation (Figure 1) is more common than central cannulation for PC-ECMO (Table 2). Interestingly, a peripheral approach was the only access employed in 3 series,21,26,29 whereas a central approach was used exclusively for access in only one center’s experience (Table 2).28 Peripheral cannulation may be preferred to reduce infection risk, avoid resternotomy, and continue uninterrupted chest compressions during ECMO cannulation.
Figure 1.
Post-cardiotomy ECMO approaches for cannulation: peripheral cannulation with femoral artery and vein access, associated with distal limb arterial perfusion.
In studies of PC-ECMO describing peripheral cannulation, open cannula placement was chosen over a percutaneous approach in the majority of cases.18,26,28 There is evidence that this approach is associated with fewer complications than percutaneous cannulation.27,29,67 A “pseudopercutaneous approach” exposes the femoral vessels, as with an open approach, but tunnels the cannulas through a more inferior thigh incision before inserting them into the vessel, allowing closure of the femoral incision.68−70 This pseudo-percutaneous approach reduces the risk of bleeding and infection post-ECMO implantation and facilitates more straightforward device removal. Although an open surgical closure is still needed, the surgeon has better control of the vascular entry site and can perform embolectomy more easily in patients with distal or proximal thromboses. However, Rastan and colleagues showed that femoral venous drainage was associated with worse prognosis, suggesting that suboptimal right-sided decompression had a negative impact on ECMO flow and management.22
Central PC-ECMO cannulation is associated with higher rates of bleeding and acute renal failure requiring continuous VV hemofiltration when compared with peripheral cannulation, but a recent meta-analysis found no difference in overall survival when compared with peripheral cannulation.44 Technically, emergent central cannulation is associated with a risk of air entrainment and embolization. The benefit of central cannulation lies in improved cardiac decompression and anterograde flow from the proximal aorta, preventing the potential ‘Harlequin syndrome’. Alternative cannulation strategies, that is, arterial inflow via the subclavian artery with either peripheral20,21,33,68−70 or central67 cannulation for venous return have also been employed (Figure 2). Central cannulation generally requires an open sternum to allow the unimpeded exit of atrial and aortic cannulas and to prevent mechanical cardiac compression, which may inhibit venous return.22,27 Innovative strategies to manage the atrial and aortic cannulas may result in sternal closure with tunneled cannula exit in the subxiphoid region (Figure 3). These strategies may facilitate patient extubation, mobilization, and a decreased risk of infection. However, such a configuration may impede adequate cardiac filling during weaning because of RV compression exerted by the cannula course in the mediastinum. In patients who are expected to have an expeditious recovery after short-term support, suprasternal exit sites, by mobilizing the sternocleidomastoid muscle insertions and extending the upper sternal incision with a Y toward the head, may be contemplated (Figure 4). This approach allows the aortic and atrial cannulas to lay vertically and exit cranially, preventing cardiac compression and tamponade during weaning trials. The use of a right anterior minithoracotomy for cannulation of the ascending aorta, right atrium and pulmonary vein (for venting) (Figure 5), has also been reported and may be also useful in avoiding any cardiac compression.70
Figure 2.
Post-cardiotomy ECMO approaches for cannulation: cannulation of the right axillary artery for reperfusion. Femoral vein or right atrial access can be used for venous drainage.
Figure 3.
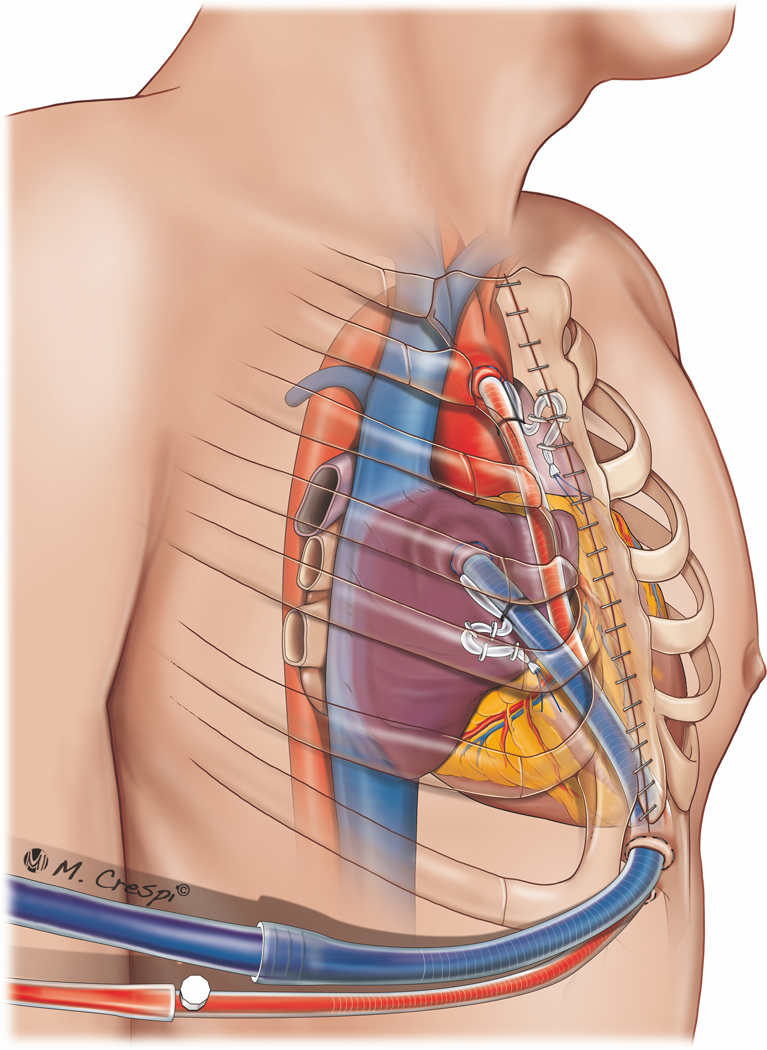
Post-cardiotomy ECMO approaches for cannulation: central cannulation (right atrium and ascending aorta cannulation) with a subxyphoid exit port for the cannulas.
Figure 4.
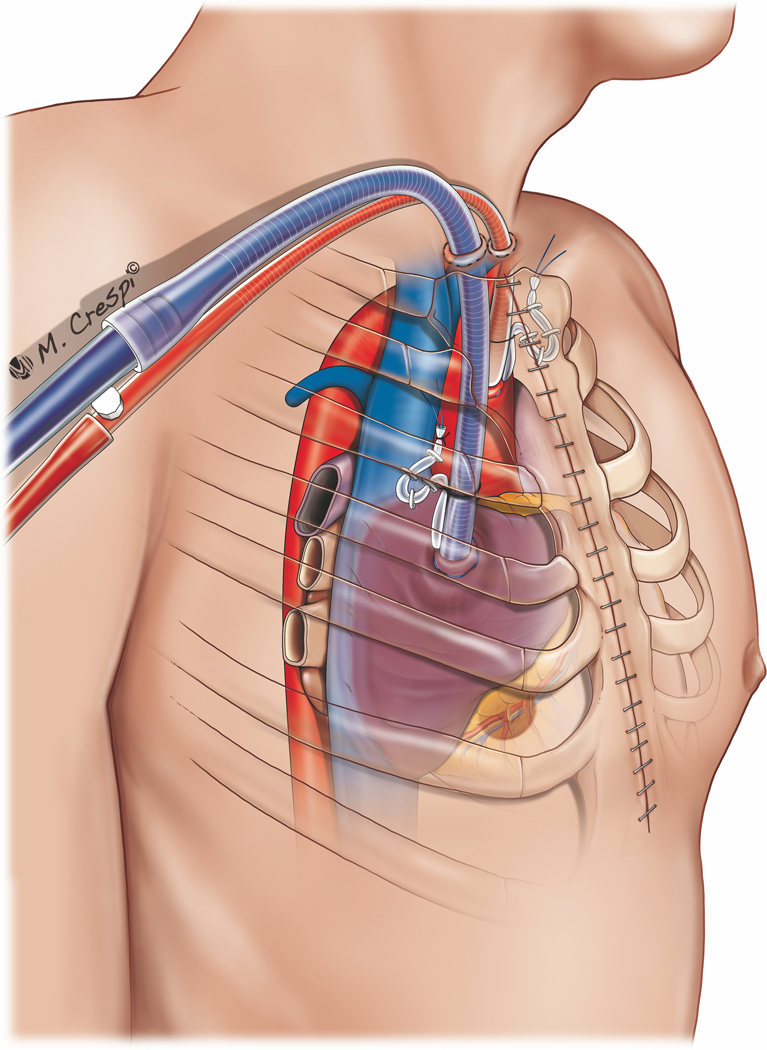
Post-cardiotomy ECMO approaches for cannulation: central cannulation (right atrium and ascending aorta cannulation) with jugular exit port for the cannulas.
Figure 5.
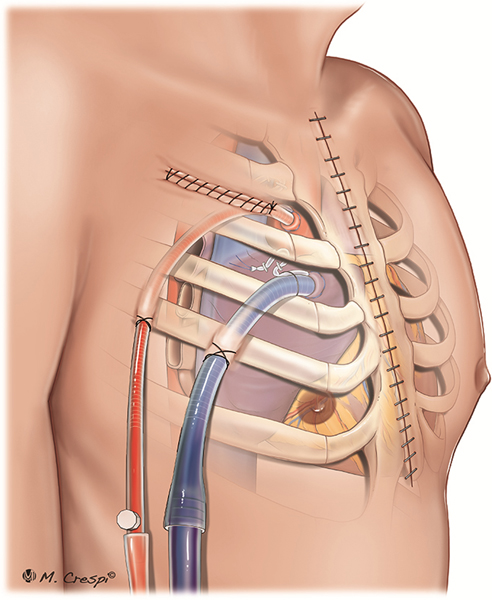
Post-cardiotomy ECMO approaches for cannulation: central cannulation with access through a left mini-thoracotomy, avoiding median sternotomy.
Left ventricular decompression
Veno-arterial ECMO (VA-ECMO), particularly with central cannulation, usually results in effective right-sided cardiac drainage but may not be effective in decompressing the left side of the heart because of the incomplete capture of systemic and bronchopulmonary venous flow. LV distension occurs because of insufficient ejection, either from impaired LV contractility or high afterload, and inevitably raises wall tension. Increased LV and the resulting left atrial pressures result in increased myocardial oxygen consumption and acute pulmonary edema. Furthermore, inadequate LV ejection results in blood stasis in the left cardiac chambers and may lead to clot formation with devastating consequences. In these circumstances, the addition of left-sided catheter drainage may be warranted to fully decompress the left side of the heart.71 In the presence of LV distension and left intraventricular or intra-atrial stasis, LV decompression can be accomplished by a surgically placed catheter in the left atrium or ventricle, or the use of a transaortic valve axial pump device (if no aortic mechanical prosthesis is in place, Supplementary Table S2). In a recent meta-analysis of 17 observational studies, including almost 4,000 VAECMO patients, 42% of the cohort utilized an LV unloading device—IABP in 91.7% of the cases, a percutaneous device in 5.5%, and trans-septal cannulation in 2.8%. There was significantly lower mortality in the combined device group (53%) compared with ECMO without LV unloading (65%).72 Patients with a mechanical prosthesis are at particularly high risk of valve thrombosis and dysfunction with poor LV ejection, increasing the risk of intracavitary clot formation. For these at-risk patients, a higher level of blood anticoagulation may be advisable when unloading the LV.
Pulmonary artery cannulation represents an alternative to direct LV unloading but is not available to all patients. The use of a transaortic valve axial pump device has been reported to be beneficial in ECMO patients.73,74 Patel and associates showed improved in-hospital survival (43% vs. 22%) with the use of a transaortic device in combination with VA-ECMO.75 Significantly higher than anticipated risk-adjusted survival was reported in 106 patients undergoing combined VA-ECMO with the use of a transaortic valve device (Impella, Abiomed).76 Despite these data, there are no reports of transaortic valve axial pump device utilization in the PC-ECMO setting. The benefits include LV unloading with theoretically improved LV recovery, while complications such as hemolysis with renal failure, and an increase in surgical complexity (Supplementary Table S2) warrant further investigation to evaluate the impact of this configuration.71
The concomitant use of IABP in patients on ECMO support for cardiogenic shock remains controversial. IABP remains the first line of support used in the operating room for cardiovascular failure.2,8 Combined with VA-ECMO, the IABP is used to improve coronary perfusion and support LV ejection and blood flow pulsatility by decreasing the afterload, decreasing LV wall tension, and thereby decreasing blood stasis in the left heart.20,21,71,77,78 In multiple PCECMO series that we have reviewed, an IABP was used concomitantly in 12%−100% of patients (Table 2). Although this institutional variability may represent patient heterogeneity, it is more likely thst these differences in utilization reflect a lack of consensus regarding the relative benefit of IABP in ECMO-supported patients.32,33,77,78 Consistent with this is the fact that in some PC-ECMO series, IABP use was associated with improved survival,17,22 while in others, no survival differences were found.19,23,24,26,27,40,47 Reassuringly, although the IABP competes for femoral arterial access, conceivably problematic in peripheral ECMO, there does not appear to be an increase in the risk of limb ischemia, as those authors uniformly using an IABP in patients on PC-ECMO showed very low rates of limb-related complications.14,21,25 Despite the physiologically justification and early reports of results, the clinical benefits of LV decompression remain unproven.
Management of patients on PC-ECMO
The management of patients supported by PC-ECMO differs substantially from the management of patients on ECMO support for other indications. First, adult cardiac surgery patients often have significant comorbidities which may impact the outcomes of ECMO. Second, ECMO may be used as a bridge-to-recovery and also as bridge-to-advanced mechanical circulatory support. PC- ECMO is only rarely used as a bridge-to-decision, as the typical cardiac surgery patient has been well characterized clinically during the preoperative work up, and the goals of therapy are clear. Optimal ECMO flow remains unclear, as some argue that allowing LV ejection might be superior to full support, taking into account the magnitude of heart damage, while assessing the need for adequate peripheral perfusion.79 As previously described, facilitating an injured LV to eject adds additional myocardial work and energy consumption to an already compromised heart. Finally, bleeding and coagulopathy are major issues in this setting.
In some patients, the need for perioperative ECMO may be predictable, based on severe clinical or cardiocirculatory-pulmonary conditions, and assessed preoperatively or intraoperatively, thereby pre-empting cardiac failure post-CPB. In these patients, prophylactic ECMO initiation may be of benefit, despite the associated risk of complications, as it allows a smoother perioperative course with the reduced use of vasopressors and the avoidance of severe hemodynamic compromise.80
The use of vasoactive medications remains another controversial issue in patients supported with ECMO, particularly PC-ECMO. As stated above, the support of cardiac contractility may improve LV unloading and prevent intracardiac hemostasis, as well as to support ECMO weaning,80 at the expense of increasing myocardial work, which may impede recovery. Recently, it was shown that pre-treatment with levosimendan (Symdax, Abbot), a calcium-sensitizing inodilator, seems to facilitate weaning from VA-ECMO, reducing the use of high-dose inotropes.81 The weaning rates were 83.3% with versus 27.3% without levosimendan infusion.81 However, these data are preliminary and require confirmation.81,82 The degree of contractility necessary to protect the unvented heart in the setting of ECMO is unclear, and thus, there is no definable goal for inotropic support. This aspect of ECMO management requires investigation.
Duration of PC-ECMO support
The duration of the ECMO support varies between reports, but patients are rarely supported longer than 15 days for PC indication (Table 2). Several investigators advocate short (48–72 hours) support times and, if insufficient recovery is observed, implementing more advanced mechanical support.16,21,26 This reflects 2 important aspects of PC-ECMO in adult patients: 1) if recovery occurs, it is usually observed early after surgery,83 and 2) severe and irreversible complications are frequent and typically occur shortly after ECMO initiation, resulting in high mortality.6,22
A longer duration of ECMO support may be required and effective in specific patients, such as patients in need of circulatory support after heart transplantation, to allow myocardial recovery or the resolution of refractory pulmonary hypertension.16 However, for those patients, post-cardiac surgery, the lack of early recovery should precipitate a transition to more durable forms of support, that is, LVAD or heart transplant listing. Unfortunately, these therapies are not universally available. This speaks to the need for a relationship between all cardiac programs and a sophisticated heart failure center (with VAD and heart transplant expertise) to facilitate patient transfers when indicated.
Complications associated with PC-ECMO
Complications in ECMO patients are common and frequently determine a patient’s final outcome.84 Table 3 shows the most common adverse events associated with PC-ECMO. In published reports, limited information is often provided regarding the specific complications encountered, rather they are categorized in broad terms (e.g., clot, hemorrhage, stroke), hampering any in-depth analysis. However, bleeding is the most frequent complication encountered, occurring in up to 90% of patients in some series.14,20 PC-ECMO patients are at high risk for hemorrhagic events, as typical postoperative surgery-related bleeding is magnified by the early need for anticoagulation required for the ECMO circuit. Furthermore, the coagulopathy and bleeding encountered may be exacerbated by a recently highlighted, heparin-like effect induced by ECMO, even when direct thrombin inhibitors are employed as alternatives to heparin. Ranucci and colleagues detected a heparin-like effect in 23 of 41 patients (56%), most likely owing to a release of heparinoids from the glycocalyx or mast cells, as the result of the systemic inflammatory response to the ECMO circuit or sepsis.39 To avoid exacerbating the coagulopathy that is already present postbypass, many investigators advocate avoiding heparin infusion for the first 12–48 hours, provided that high flows are maintained to prevent clot formation.18,21 Heparin can be withheld for even longer periods in patients with continued postoperative bleeding.16,18,21,22,24,30,47
TABLE 3.
Complication Rates in Adult Patients Undergoing Post-Cardiotomy Extracorporeal Membrane Oxygenation (ECMO)
| Authorreference – Year of Publication | Bleeding, % | ECMO System Failure, % | Liver Failure, % | Sepsis/ Infection/ Bacteremia, % | CNS Events, % | Kidney Failure, % | Limb Ischemia (including amputation or fasciotomy), % |
|---|---|---|---|---|---|---|---|
| Magovern14 - 1994 | 91 | 33 (oxygenator change) | na | 33 | 28.5 | 19 | 4.7 |
| Kodera15 - 1996 | na | na | na | na | na | na | na |
| Fiser16 - 2001 | na | na | na | 7 | 28 | 4 | na |
| Smedira17 - 2001 | na | 5 | na | 48 | 29 | 39 | 27 |
| Ko18 - 2002 | 46 | na | na | 26 | 11.8 | na | 17.1 |
| Zhang19 - 2006 | na | na | na | na | na | na | na |
| Bakhtiary20 - 2007 | 87 | 9 | na | 58 | 9 | 87 | 22 |
| Hsu21 - 2010 | 2.9 | na | na | 34 | 29 | 75 | 5.9 |
| Rastan22 - 2010 | 58 | na | na | 24.8 | 17.4 | 65 | 19.9 |
| Elsharkawy23 – 2010 | na | na | na | 36 | na | na | na |
| Pokersnik24 - 2012 | 71 | 63.6a 0b 7.4c |
na | 32.6 | 6.1 | 32.6 | na |
| Mikus25 - 2013 | 64.3 | 7.1 | na | 42.8 | 0 | 50 | 0 |
| Slottosch26 - 2013 | 29.9 | na | 3.9 | 29 | 22.1 | 68.8 | 20.8 |
| Unosawa27 - 2013 | na | na | na | na | 12.7 | 17 | 10.6 |
| Ariyaratnam28 - 2014 | na | na | na | na | na | na | na |
| Li29 - 2015 | na | na | na | 22.3 | 6.2d | 39.2 | 29.9 |
| Saxena30 - 2015e | na | na | na | 24.4 | 4.4 | 44.4 | 13.3 |
| Khorsandi31 - 2016 | na | na | na | 12.5 | 18.7 | 18.7 | 12.5 |
| Distelmaier32 - 2016f | na | na | na | na | na | na | na |
| Mazzeffi33 – 2016f | 6.2 | na | na | 18.8 | 25 | na | na |
| Chen34 - 2017 | 11.3 | na | na | 13.2 | 4.3 | 32.9 | 2.3 |
| Guihaire35 - 2017 | 19.5 | na | na | 52.1 | 3.2 | na | 9.8 |
| Raffa36 - 2017 | 46.4 | na | na | 21.4 | 29.7 | 29.8 | 10.7 |
| Fux37 – 2018 | 68 | na | na | 24 | intracranial hemorrhage 7 stroke 16 brain death 8 |
70 | leg fasciotomy 7 leg amputation 1 |
Abbreviations: ARF, acute renal failure; AST, aspartate transaminase; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CNS, central nervous system; CPB, cardiopulmonary bypass; CPK, creatinephosphokinase; CPR, cardiopulmonary resuscitation; CRF, chronic renal failure; CVVH, continuous veno-venous hemofiltration; ECMO, extracorporeal membrane oxygenation; HTx, heart transplant; IABP, intra-aortic balloon pump; LV, left ventricle; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MOF, multiorgan failure; na, not available; PA, pulmonary artery; VA, veno-arterial; VAD, ventricular assist device; CNS, central nervous system
Group of patients receiving Biomedicus (Medtronic Inc, Minneapolis, Minn) pump and Affinity oxygenator (Medtronic Inc. Minneapolis, Minn)
Group of patients receiving Biomedicus (Medtronic Inc, Minneapolis, Minn) and Quadrox D (Getinge, Hirrlingen, Germany) oxygenator
Group of patients receiving Rotaflow (Getinge, Hirrlingen, Germany) pump and Quadrox D (Getinge, Hirrlingen, Germany) oxygenator
Only stroke reported
Study included only patients over 70 years of age
Study included only extracorporeal cardiopulmonary resuscitation (ECPR) after cardiac surgery
There are additional adverse events, similar to those seen in other ECMO settings, but exacerbated as a result of the preceding CPB run and cardiac surgery. Central nervous system complications have recently been shown to occur in 15% of the adult patients supported with VA-ECMO and 7% of the adult patients supported with VV-ECMO.85,86 In PCECMO, these rates may be much higher, occurring in up to 30% of the patients.14,16,17,21 The caregiver must remain cognizant of the high rate of neuro-complications. A high level of suspicion, appropriate neuro-monitoring, and the prompt execution of ECMO management modifications, including a low threshold for neuroimaging, all play an important role in the care of the PC-ECMO patient.87 Interestingly, it appears that elderly patients are not at higher risk for neurological adverse events when compared with younger patients in the PC-ECMO setting.30 A study of the ELSO registry showed equal rates of cerebral injury in patients older than 70 years of age who underwent ECMO for cardiogenic shock.84
In contrast to the higher rates of neurological adverse events in PC-ECMO, renal failure and limb ischemia occur at rates similar to other indications for ECMO. Several investigators have consistently reported reduced limb ischemia with the routine use of ipsilateral distal perfusion.22 A delay in cannula placement, triggered by signs of ischemia, may lead to a reperfusion injury with a poor outcome28 (Table 3). Although distal femoral artery cannulation placed directly into the vessel is easy and effective, femoral artery access can also be achieved by placing a femoral artery chimney graft (a Dacron or Hemashield prosthetic graft) of 6–8-mm diameter, anastomosed end-to-side to the femoral artery, thus maintaining antegrade and retrograde arterial flow to the ipsilateral lower limb.21 In PC-ECMO, continuous near-infrared spectroscopy monitoring of the adequacy of limb perfusion is no different from the management applied in other ECMO settings with peripheral arterial access.70
Reassuringly, ECMO circuit failure occurs at a very low rate (Table 3). Although circuit failure may be underreported, the general consensus is that the current ECMO technology is safe with a low incidence of catastrophic complications owing to component failure or dysfunction. Most of the time, these events are foreseeable and involve the pump-head or oxygenator. Even so, further improvements in circuit components, including greater biocompatibility, are expected in the coming years.
Post-cardiotomy ECMO outcomes
Weaning from PC-ECMO and survival to hospital discharge
As expected, successful weaning from PC-ECMO varies greatly within published series, ranging from 31% to 76%, with almost half of the published experiences showing a weaning rate at or slightly above 50% (Table 4). Survival to hospital discharge rates are far less, ranging from 16% to 52%, with fewer than 30% of the centers reporting survival-to-discharge above 40% (Table 4). Of note, even in the face of considerably improved technology and increased experience in managing ECMO care, survival has not improved in the last 20 years.24 In fact, based on the most recent report from the ELSO registry, there has been a gradual decline in the survival after PC-ECMO, as low as 15% survival in a recent analysis.6 This may be owing, at least in part, to more widespread application of this technology to higher risk patients, but it remains a sobering observation.
TABLE 4.
Outcome and predictors of in-hospital mortality of adult patients undergoing post-cardiotomy ECMO
| Authorreference – Year of Publication | Weaning, % | In-Hospital Survival, % | Bridge to LVAD/HTx, n/total patients % | In-Hospital Survival LVAD/HTx after ECMO, n/total patients % | 1-Year Survival, % | Predictors of In-Hospital Mortality |
|---|---|---|---|---|---|---|
| Magovern14 - 1994 | 76 | 52 | None | - | 47.6 | Mitral valve surgery Non-convertible ventricular fibrillation |
| Kodera15 - 1996 | 70.6 | 47.1 | None | - | na (30.9 at 5 years) |
ECMO duration > 60 hours |
| Fiser16 - 2001 | 31 | 16 | na | na | na | Age >65 yrs., LVEF <30% after 48 hours on ECMO Prolonged CPR (> 5 minutes) prior to ECMO implant |
| Smedira17 - 2001 | 39.2 | 33,3 | HTx: 0 (0%) LVAD:18/107 (16.8%) |
HTx: - LVAD: 13/18 (72%) at 1-year follow-up |
na | na |
| Ko18 - 2002 | 60.5 | 26.3 | HTx: 2/76 (2.6%) LVAD: 2/76 (2.6%) |
HTx: 0/2 (0%) LVAD: 1/2 (50%) |
45.4 | ARF requiring dialysis on ECMO Peak CPK (U/L)a |
| Zhang19 - 2006 | 43.7 | 25 | na | na | na | Blood lactate level 48 hours after ECMO initiation MB--isoenzyme 48 hours after ECMO initiation CK-MB relative index (CK-MB/total CK) 48 hours after ECMO initiation |
| Bakhtiary20 - 2007 | 55.5 | 29 | HTx: 2/45 (4%) LVAD: 5/45 (11%) |
HTx: 1/2 (50%) LVAD: 3/5 (60%) at 30-day follow-up |
29 | PA hypertension Diabetes Lack of IABP |
| Hsu21 - 2010 | 53 | 24.8 | HTx: 4/51 (8%) LVAD: - |
HTx: 3/4 (75%) at 30-day follow-up; 1/4 (25%) at 1-year follow-up. LVAD:- |
16.5 | Low peri-op serum albumin level Platelet count Oxygen pressure of the venous tube of the ECMO Poor cardiac systolic function |
| Rastan22 - 2010 | 63.3 | 30,4 | HTx: 5/517 (1%) LVAD: 15/517 (3%) |
HTx: 2/5 (40%) LVAD: 3/15 (20%) |
na | Age >70, Diabetes, Obesity, Preoperative CRF, Operative lactate >4 mmol/l, Logistic Euroscore >20 |
| Elsharkawy23 – 2010 | na | 16 | na | na | na | Older age Higher preoperative albumin Diabetes CABG surgery Longer CPB time Cardiogenic shock at time of VA-ECMO implant |
| Pokersnik24 - 2012 | 54.9 (63.6b/ 45.5c/ 55.6d) | 36 | HTx: - LVAD: 2/33 (6%) |
HTx: - LVAD: 0/2 (0%) |
na | Older age |
| Mikus25 - 2013 | 50 | 42.8 | None | - | na | High transfusion rate |
| Slottosch26 - 2013 | 62 | 32.6 | HTx: 2/77 (2.6%) VAD: -- |
HTx: 0/2 (0%) VAD:-- |
na | Older age Greater lactate after 24hours of ECMO Longer ECMO duration ECMO related or gastrointestinal complications |
| Unosawa27 - 2013 | 61.7 | 40.3 | na | - | 24.5 | Older age Incomplete sternal closure ECMO support >48 hours Dialysis for ARF MOF Brain injury Peak Cr (mg/dl) during ECMO |
| Ariyaratnam28 - 2014 | 50 | 31.2 | na | - | na | Increase requirements for vasoconstrictors |
| Li29 - 2015 | 56 | 34.1 | na | - | na | Age Gender (Female) Mean lactate concentration (at 6 and 12 hours from ECMO initiation) Lactate clearance (at 6 and 12 hours from ECMO initiation) |
| Saxena30 – 2015e | 53.4 | 24.4 | None | - | na | Preoperative atrial fibrillation CVVH Lactic acidosis on ECMO Persistent coagulopathy on ECMO Delayed ECMO implant MOF in patients 50–69 High postop AST |
| Khorsandi31 - 2016 | na | 51 | na | - | 29.8 | Older age Emergent nature of surgery Pre-existing preoperative severe LV impairment |
| Distelmaier32 – 2016f | na | 32 | na | - | na | na |
| Mazzeffi33 - 2016 f | na | 30.1 | HTx: - LVAD: 2/23 (8.7%) |
HTx: - LVAD: na |
na | Age >50 years, Cause of cardiac arrest except tamponade |
| Chen34 - 2017 | na | 38.3 | na | na | 24.1 | ARF Massive blood transfusion |
| Guihaire35 - 2017 | 48 | 42 | HTx: 2/92 (2.17%) LVAD: 2/92 (2.17%) |
HTx: 2/2 (100%) LVAD: 1/2 (50%) | 39 | Age Valvular surgery Peak lactate level at 24 hrs |
| Raffa36 - 2017 | 49.4 | 37 | None | - | na | Age >65 years Postoperative Arrhythmias |
| Fux37 – 2018 | 51 | 56 | HTx: 1/105 (0.9%) LVAD: 1/105 (0.9%) |
na | na | Arterial lactate Ischemic heart disease |
AST: aspartate transaminase; ARF: acute renal failure; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CPB: cardio-pulmonary bypass; CPK: creatine-phosphokinase; CPR: cardiopulmonary resuscitation; CRF: chronic renal failure; CVVH: continuous veno-venous hemofiltration; ECMO: extracorporeal membrane oxygenation; HTx: heart transplant; IABP: intra-aortic balloon pump; LV; left ventricle; LVAD; left ventricular assist device; LVEF: left ventricular ejection fraction; MOF: multiorgan failure; na: not available; PA: pulmonary artery; VA: veno-arterial; VAD: ventricular assist device
Values in the first 3 days of ECMO initiation
Group of patients receiving Biomedicus (Medtronic Inc, Minneapolis, Minn) pump and Affinity oxygenator (Medtronic Inc. Minneapolis, Minn)
Group of patients receiving Biomedicus (Medtronic Inc, Minneapolis, Minn) and Quadrox D (Getinge, Hirrlingen, Germany) oxygenator
Group of patients receiving Rotaflow (Getinge, Hirrlingen, Germany) pump and Quadrox D (Getinge, Hirrlingen, Germany) oxygenator
Study included only patients over 70 years of age
Study included only extracorporeal cardiopulmonary resuscitation (ECPR) after cardiac surgery
The presence of cardiac arrest as indication for PC-ECMO is understandably a negative predictor of weaning or survival to-discharge in all series examined (Table 1); however, this must be viewed as preliminary data, as only 2 papers specifically address ECPR in this setting.32,33 Survival in patients with cardiac arrest ranged from 0% to 48%, but factors such as quality of cardiopulmonary resuscitation (CPR), that is, open versus closed chest, the location of arrest, that is, in the operating room or in the ICU, and time to ECMO institution, are all likely to be of paramount importance. An intraoperative onset arrest might have a better prognosis owing to more rapid ECMO cannulation and limited hypoperfusion-based organ injury, but this hypothesis has not been explored.
Multiorgan system failure, despite recovery from myocardial failure, is an important contributor to mortality, but granular data to inform this observation are lacking.20,21 In fact, the actual cause of death may be interpreted in a misleading fashion in ECMO patients, as reported by Rastan and associates who showed that in almost 30% of autopsies, an unexpected cause of death was found, and in 80% of the patients, an unrecognized concomitant illness was present.89 Overall, that study showed mortality owing to a cardiac etiology in > 60% of patients, multiorgan system failure in 10%, and neurological complications in 5%.89
The survival of patients who received PC-ECMO for post-heart transplant graft dysfunction is approximately 45%, better than typically seen in PC-ECMO, as post-graft dysfunction is frequently reversible.16,18,29,90 Takeda and colleagues recently compared patient outcomes after primary graft failure with PC-ECMO support versus VAD support, and showed that PC-ECMO had significantly better outcomes, including bleeding after post-implant rethoracotomy (30% vs 70%, p < 0.01), renal replacement therapy (11% vs 53%, p < 0.01), duration of support (5.2 ± 3.9 days vs 14 § 17 days, p = 0.011), and weaning rates (89% vs 59%, p = 0.03), with a trend toward better survival to hospital discharge (41% vs 19%).91 The favorable impact of ECMO on patients post-heart transplant when compared with other settings has been highlighted by other investigators, showing significantly better in-hospital and post-discharge survivals.92,93
Outcomes in elderly patients
The effect of patient age on survival requires highlighting in the context of PC-ECMO, as many eligible patients in this setting are elderly. In an analysis of 131 adult patients (28% ≥ 65 years of age) with refractory cardiopulmonary failure supported by ECMO, Narotsky and colleagues observed 48% survival at one year.51 Age over 65 years was associated with an ~2-fold higher risk of death.51 However, when the analysis was adjusted for confounders, age was not a statistically significant predictor for in-hospital death, although a trend for increased risk remained.51 Saito and colleagues analyzed 91 patients who required emergency ECMO for a variety of reasons including PC, and found that age was not a predictor of mortality, concluding that elderly patients can benefit from ECMO, although they require more time to recover.41 However, Elshakarwy et al found a linear relationship between mortality after PC-ECMO support and age, but there was no identified age specific cut off after which ECMO was futile.23 Consistent with this, a recent large database evaluation demonstrated that older patients who require ECMO support for cardiac or respiratory indications have a significantly worse prognosis; however, the data does not appear to support older age as an absolute contraindication to PC-ECMO.88 These findings create a conundrum for surgeons and patients’ relatives when considering ECMO support for an elderly patient. Survival rates in elderly patients who undergo ECMO for cardiogenic shock may range between 25% and 30%.30,88 These survival rates may be influenced by selection bias, but it is encouraging that well-selected elderly patients managed on ECMO may do well. Additional investigation is needed to elucidate which older patients have the best survival after PC-ECMO. At this time, limited information is available on medium and long term patient disability after PC-ECMO, and one must conclude that further experience is needed to best determine in whom it is contraindicated.
Bridging to other therapies
Transitioning to VAD support or heart transplantation listing from PC-ECMO appears to improve in-hospital survival, as shown by Smedira and coworkers.17 They found a 75% early survival rate in ECMO patients bridged to a VAD after a short ECMO run.17 Despite this apparent effectiveness, < 20% of patients supported with PC-ECMO transition to other support, that is, VAD or heart transplantation (range 0%−20%) (Table 4), totally dependent upon the suitability of the candidate for more durable therapies and the availability of hub-and-spoke networks allowing patient transfers to centers capable of these advanced heart failure options.
Predictors of outcomes after PC-ECMO
The prediction of ECMO weaning and survival after PCECMO has been addressed by many investigators.14−35 Pre- and intraoperative factors, post-ECMO events, and the patient’s physiological response to circulatory support, all play a critical role (Table 4). Among pre-ECMO factors, ECPR was a strong negative predictor of survival in several experiences.14,16,30 In 2 series, no post-cardiotomy patient who underwent ECPR and was elderly or who had prolonged CPR prior to establishment of ECMO support survived.16,30 However, other investigators have found that neither CPR nor the time spent in resuscitation had a significant effect on survival.18,32,33 Currently, the following factors negatively influence survival after PC-ECMO support: lactate concentration immediately prior to ECMO initiation, as well as its highest level 12–48 hours post-ECMO initiation,19,22,26,29,30,35,37,43,83 renal and liver failure,94−96 respiratory failure,97,98 and the duration of ECMO support.16,18,26,27 Regarding pre-ECMO lactates, Fux and collaborators have recently shown that a value above 10 mmol/l at implant is associated with a 90% in-hospital mortality, with no survival when lactate levels were >15 mmol/l.37 Surprisingly, neither ECMO cannulation location (intraoperative, ICU, or ward)27 nor the duration of ECMO support has been shown to impact survival.19,22,24,30 Other predictors of success in weaning or survival after PCECMO are reported in Table 4.
Long term outcomes after PC-ECMO
Despite the complex perioperative course of PC-ECMO patients, the long term survival of patients who survive to discharge appears favorable, with the vast majority of patients still alive at 1-year follow-up (Table 4). Saxena and colleagues showed that even in a high-risk cohort of patients over 70 years of age, survival was 69% at 3 years, and 51% at 5 years, confirming good post-discharge prognosis of PC-ECMO patients, even in the elderly.30
Post-cardiotomy veno-venous ECMO for respiratory dysfunction
Respiratory insufficiency is a common complication after cardiac surgery and an independent predictor of in-hospital mortality.97,98 Despite respiratory complications in 7% to 30% of patients,97,98 there is a paucity of published reports on ECMO therapy when this complication is severe, treated either with VA- or veno-venous ECMO (VV-ECMO). VVECMO has been increasingly employed as therapy for primary, refractory respiratory failure as a result of the outcomes seen in conventional ventilatory support versus ECMO for severe adult respiratory failure in the CESAR trial.99 There are very few reports, however, specifically addressing the use of VV-ECMO in PC patients. Nakamura and colleagues explored the outcomes of VV-ECMO in 11 PC patients, ranging in age from 35 to 83 years, after various cardiac surgery procedures.56 This series showed favorable overall outcomes (7 patients were discharged, 64%), and age was the only predictor of a poor outcome.56 The mean age of the survivors was 54 years, while non-survivors were mean 80 years old.56 Interestingly, Song and coworkers recently reported their experience in 13 PC patients supported with VV-ECMO for acute respiratory distress syndrome, and age did not predict survival (overall in-hospital survival 69%).57 In this series, 7 patients received VV-ECMO within 24 hours of surgery and the remainder (6 patients) at a median of 8.5 days from surgery.57 They showed that the Respiratory ECMO Survival Prediction score100 proved to be a good predictor of survival (100% in class III, 50% in class IV, and 20% in Class V).57 Noteworthy, in this experience, septic shock was responsible for all the deaths during ECMO support.57 Further research is needed to understand the indications for VV-PC-ECMO and to systematically minimize pre- and intraoperative risk factors for PC respiratory failure requiring VV-ECMO, that is, smoking cessation, avoiding ventilation-induced lung injury, decreasing blood exposure, and minimizing CPB times.
Controversial issues and future perspectives on PC-ECMO
ECMO technology has undergone remarkable progress in the last 20 years. More advanced, user-friendly, miniaturized technology has rendered the wider application of this temporary support possible, in many instances for conditions once viewed as contraindications.12,101 Obvious targets to improve effectiveness include biocompatible circuitry (e.g., the pump, oxygenator, and tubing design), more reliable anticoagulation, rational vasoactive or inotropic support, a better understanding of the most effective ways to achieve temporary cardiopulmonary support, more effective monitoring, and increasing provider skill and education. Given the technical aspects of ECMO implementation, the peculiarities of ECMO management, and the expense and ethical implications of ECMO use, universal provider education is a crucial target if outcomes are to improve.102,103 Available ECMO courses, case-presentations and simulation training with a multidisciplinary target audience may mitigate the lack of high volume ECMO use at most centers and improve standardization and evidence based care for all.103,104
As we witness an exponential increase in ECMO use, we must question in whom is it truly beneficial, determining in whom it provides time to recover as opposed to simply prolonging death.103 ECMO enthusiasts are particularly vulnerable to criticism.6 Because ECMO is easy to institute, it is being used in patients with increasing disease severity, more complex procedures with acute, post-bypass myocardial dysfunction, and in patients who heretofore were viewed as unsalvageable.30,39,40,50,87,101 Reoperations, advanced age, surgical urgency, and poor cardiac reserve are being seen more commonly and contribute to cardiopulmonary insufficiency post-CPB, with subsequent pressure on the caregiving team to institute PC-ECMO. It is also possible that PC-ECMO is being overutilized to avoid intraoperative deaths, a rarity these days, moving the inevitable from the operating room to the ICU.11,102 However, PC-ECMO is still in its infancy and suffers from a lack of sufficient evidence to inform optimal use, that is, patient selection, ECMO configuration, prevention or management of complications, and decision making for recovery or exit strategies of more durable therapies. To this end, the authors propose concentrating resources to address the following areas to improve PC-ECMO effectiveness: 1) the underlying pathophysiology of PC cardiopulmonary failure and the impact of ECMO on those processes, 2) the development of a standardized, evidence-based, structured approach to monitoring ECMO delivery and patient outcomes, 3) the identification of both patient- and population specific predictors of outcomes, 4) the education and training of ECMO caregivers, and, finally, 5) the ethical and economic implications of ECMO utilization. For sure, the dilemmas associated with ECMO are all part of a complex scenario, but, to a large degree, providers must accept responsibility for shepherding their use, despite the lack of data to inform them.6,103 Preoperative discussion with highrisk patients and their families about the use of PC-ECMO should be encouraged to minimize ethical issues that may arise post-operatively. The futility of ECMO in complex patients with limited or no indication for more advanced treatment should be considered in a timely manner.
Limitations of the review
This review has some limitations, as it encompasses different patient conditions and different ECMO approaches in its attempt to be comprehensive. However, underlying cardiopulmonary insufficiency and a lack of response to conservative, conventional treatments after cardiac surgery are common to all the studies included. The scenarios for PC-ECMO include conditions ranging from failure to wean from CPB, to cardiogenic shock hours to days after cardiac surgery, to cardiac arrest in the ICU or on the ward. PCECMO in heart transplant recipients is usually included in PC-ECMO series, as we have done, but this specific patient population tends to have better outcomes when compared with non-transplant patients.47,53 In addition, the studies reviewed include a wide range of resuscitation times prior to ECMO initiation, and in some circumstances, include patients who need both cardiac and respiratory support post-operatively. This highlights the extreme variability in the post-cardiotomy patients who received PC-ECMO. Finally, institutional variability in ECMO management is high, and may impact the results of studies and how the associated data has been interpreted.47,53
Conclusions
PC-ECMO represents the most frequent indication for temporary mechanical circulatory support with increasing use expected in the future. Considerable variability regarding surgical access and cannula placement still exists, apparently without major differences in outcomes regardless of the technique used. Although PC-ECMO can be life-saving and is only employed when there are few alternatives, mortality and morbidity remain high, reflecting underlying disease severity and an imperfect solution. When PC-ECMO is determined to be the best option for patient care, its deployment should be rapid, as delay, with a resultant longer duration of circulatory hypoperfusion and hypoxia, is related to increasingly poor outcomes. Survivors of PC-ECMO have favorable early outcomes. Long term survival has not been as well-studied, although some small series show good 1- and 3-year survival. ECMO-specific educational training programs focusing on patient selection, cannulation techniques, ECMO management, and ethical considerations, along with industry-driven refinements to circuit components, will almost certainly improve the effectiveness of this powerful technology.
Supplementary Material
Footnotes
Disclosure statement
R.L. is a consultant and conducts clinical trial for LivaNova (London, UK), is a consultant for Medtronic (Minneapolis, MN), and an advisory board member of PulseCath (Arnhem, The Netherlands). D.B. is on the medical advisory board for Baxter, a past medical advisory board member for ALung Technologies, and an anticipated future medical advisory board member for BREETHE. D.B. is on the Trial Steering Committee for the VENT-AVOID trial sponsored by ALung Technologies. The other authors have no conflicts to disclose.
Supplementary data
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2019.08.014.
References
- 1.Bellumkonda L, Gul B, Masri SC. Evolving concepts in diagnosis and management of cardiogenic shock. Am J Cardiol 2018;122:1104–10 [DOI] [PubMed] [Google Scholar]
- 2.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support. J Am Coll Cardiol 2014;64:1407–15 [DOI] [PubMed] [Google Scholar]
- 3.McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcome: 2002–2012. Sem Thorac Surg 2015;27:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use increased 433% in adults in the United States from 2006 to 2011. ASAIO J 2015:61:31–36 [DOI] [PubMed] [Google Scholar]
- 5.Maxwell BG, Powers AJ, Sheikh AY, Lee PHU, Lobato RL, Wong JK. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg 2014;148;416–21 [DOI] [PubMed] [Google Scholar]
- 6.Whitman GJR. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J Thorac Cardiovasc Surg 2016;153:95–101 [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DJ, Oz MC. Mechanical support for postcardiotomy cardiogenic shock. Sem Thorac Cardiovasc Surg 2000;12:220–8 [DOI] [PubMed] [Google Scholar]
- 8.Curtis JJ, McKenney-Knox CA, Wagner-Mann CC. Postcardiotomy centrifugal assist: a single surgeon’s experience. Artif Organs 2002;26:994–7 [DOI] [PubMed] [Google Scholar]
- 9.Jurmann MJ, Siniawski H, Erb M, Drews T, Hetzer R. Initial experience with miniature axial flow ventricular assist devices for postcardiotomy heart failure. Ann Thorac Surg 2004;77:1642–7 [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AF, Grab JD, Gammie JS, et al. A decade of short-term outcomes in post-cardiac surgery ventricular assist device implantation. Circulation 2007;116:606–12 [DOI] [PubMed] [Google Scholar]
- 11.Akay MH, Gregoric ID, Radovancevic R, Cohn WE, Frazier OH. Timely use of a CentriMag heart assist device improves survival in postcardiotomy cardiogenic shock. J Card Surg 2011;26:548–52 [DOI] [PubMed] [Google Scholar]
- 12.Sylvin EA, Stern DR, Goldstein DJ. Mechanical support for postcardiotomy cardiogenic shock: has progress been made? J Card Surg 2010;25:442–54 [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara S, Takeda K, Garan A, et al. Contemporary mechanical circulatory support therapy for postcardiotomy shock. Gen Thorac Cardiovasc Surg 2016;64:183–91 [DOI] [PubMed] [Google Scholar]
- 14.Magovern GJ Jr, Magovern JA, Benckart DH, et al. Extracorporeal membrane oxygenation: preliminary results in patients with postcardiotomy cardiogenic shock. Ann Thorac Surg 1994;57:1462–71 [DOI] [PubMed] [Google Scholar]
- 15.Kodera K, Kitamura M, Hachida M, Endo M, Hashimoto A, Koyanagi H. Biventricular bypass with oxygenation for postcardiotomy ventricular failure. Artif Organs 1996;20:724–7 [PubMed] [Google Scholar]
- 16.Fiser SM, Tribble CG, Kaza AK, et al. When to discontinue extracorporeal membrane oxygenation for postcardiotomy support. Ann Thorac Surg 2001;71:210–4 [DOI] [PubMed] [Google Scholar]
- 17.Smedira NG, Blackstone EH. Postcardiotomy mechanical support: risk factors and outcomes. Ann Thorac Surg 2001;71:S60–6 [DOI] [PubMed] [Google Scholar]
- 18.Ko WJ, Lin CY, Chen RJ, Wang SS, Lin FY, Chen YS. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg 2002;73:538–45 [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Kofidis T, Kamiya H, et al. Creatine kinase isoenzyme MB relative index as predictor of mortality on extracorporeal membrane oxygenation support for postcardiotomy cardiogenic shock in adult patients. Eur J Cardio-Thorac Surg 2006;30:617–20 [DOI] [PubMed] [Google Scholar]
- 20.Bakhtiary F, Keller H, Dogan S, et al. Venoarterial extracorporeal membrane oxygenation for treatment of cardiogenic shock : clinical experiences in 45 adult patients. J Thorac Cardiovasc Surg 2008;135 :382–8 [DOI] [PubMed] [Google Scholar]
- 21.Hsu PS, Chen JL, Hong GJ, et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 patients. Eur J Cardio-Thorac Surg 2010;37:328–33 [DOI] [PubMed] [Google Scholar]
- 22.Rastan AJ, Dege A, Mohr M, et al. Early and late outcome of 517 consecutive patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302–11 [DOI] [PubMed] [Google Scholar]
- 23.Elsharkawy HA, Li L, Skr Esa WA, Sessler D, Bashour CA. Outcome in patients who require venoarterial extracorporeal membrane oxygenation support after cardiac surgery. J Cardio-Thorac Vasc Anesth 2010;24:946–51 [DOI] [PubMed] [Google Scholar]
- 24.Pokersnik JA, Buda T, Bashour A, Gonzalez-Stawinski GV. Have changes in ECMO technology impacted outcomes in adult patients developing postcardiotomy cardiogenic shock? J Card Surg 2012;27:246–52 [DOI] [PubMed] [Google Scholar]
- 25.Mikus E, Tripodi A, Calvi S, Del Giglio M, Cavallucci A, Lamarra M. CentriMag venoarterial extracorporeal membrane oxygenation supporta s treatment for patients with refractory postcardiotomy cardiogenic shock. ASAIO J 2013:59:18–23 [DOI] [PubMed] [Google Scholar]
- 26.Slottosch I, Liakopoulos O, Kuhn E, et al. Outcomes after peripheral extracorporeal membrane oxygenation therapy for postcardiotomy cardiogenic shock: a single centre experience. J Surg Res 2013;181:47–55 [DOI] [PubMed] [Google Scholar]
- 27.Unosawa S, Sezai A, Hata M, et al. Long-term outcomes of patients undergoing ECMO for refractory postcardiotomy cardiogenic shock. Surg Today 2013;43:264–70 [DOI] [PubMed] [Google Scholar]
- 28.Ariyaratnam P, McLean LA, Cale ARJ, Loubani M. Extracorporeal membrane oxygenation for the post-cardiotomy patient. Heart Fail Rev 2014;19:717–25 [DOI] [PubMed] [Google Scholar]
- 29.Li CL, Wang H, Jia M, Ma N, Meng X, Hou XT. The early dynamic behaviour of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: a retrospective observational study. J Thorac Cardiovasc Surg 2015;149:1445–50 [DOI] [PubMed] [Google Scholar]
- 30.Saxena P, Neal J, Joyce LD, et al. Extracorporeal membrane oxygenation support in postcardiotomy elderly patients: the Mayo Clinic experience. Ann Thorac Surg 2015;99:2053–60 [DOI] [PubMed] [Google Scholar]
- 31.Khorsandi M, Shaikhrezai K, Prasad S, et al. Advanced mechanical circulatory support for post-cardiotomy cardiogenic shock: a 20-year outcome analysis in a non-transplant unit. J Cardio-Thorac Surg 2016;11:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dilstelmaier K, Schrutka L, Binder C, et al. Cardiac arrest does not affect survival in post-operative cardiovascular surgery patients undergoing extracorporeal membrane oxygenation. Resuscitation 2016;104:24–7 [DOI] [PubMed] [Google Scholar]
- 33.Mazzeffi MA, Sanchez PG, Herr D, et al. Outcomes of extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest in adult cardiac surgery patients. J Thorac Cardiovasc Surg 2016;152:1133–9 [DOI] [PubMed] [Google Scholar]
- 34.Chen SW, Tsai FC, Lin YS, et al. Long-term outcomes of extracorporeal membrane oxygenation support for postcardiotomy shock. J Thorac Cardiovasc Surg 2017;154:469–77 [DOI] [PubMed] [Google Scholar]
- 35.Guihaire J, Dang Van S, Rouze S, et al. Clinical outcomes in patients after extracorporeal membrane oxygenation support for post-cardiotomy cardiogenic shock: a single-centre experience. Interact Cardiovasc Thorac Surg 2017, doi: 10.1093/icvts/ivx155. [DOI] [PubMed] [Google Scholar]
- 36.Raffa GM, Gelsomino S, Sluijpers N, et al. In-hospital outcome of post-cardiotomy extracorporeal life support in adult patients: the 2007–2017 Maastricht experience. Crit Care Resusc. 2017;19:53–61. [PubMed] [Google Scholar]
- 37.Fux T, Holm M, Corbascio M, Lund LH H, van der Linden JsVenoarterial Extracorporeal Membrane Oxygenation for Postcardiotomy Shock: Risk Factors for Mortality. J Thorac Cardiovasc Surg 2018;156:1894–1902.e3, 10.1016/j.jtcvs.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 38.Ranucci M, Ballotta A, Kandil H, et al. Bivalirudin-based versus heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Critical Care 2011;15:275–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranucci M, Barushnikova E, Isgrò G, et al. Heparin-like effect in post-cardiotomy extracorporeal membrane oxygenation patients. Critical Care 2014;18:504–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith C, Bellomo R, Raman JS, et al. An extracorporeal membrane oxygenation-based approach to cardiogenic shock in an older population. Ann Thorac Surg 2001;71:1421–7 [DOI] [PubMed] [Google Scholar]
- 41.Saito S, Nakatani T, Kobayashi J, et al. Is extracorporeal life support contraindicated in elderly patients? Ann Thorac Surg 2007;83:140–5 [DOI] [PubMed] [Google Scholar]
- 42.Lan C, Tsai PR, Chen YS, Ko WJ. Prognostic factors for adult patients receiving extracorporeal membrane oxygenation as mechanical circulatory support – A 14-year experience at a medical center. Artif Organs 2010;34:59–64 [DOI] [PubMed] [Google Scholar]
- 43.Hei F, Lou S, Li J, et al. Five-year results of 121 consecutive patients treated with extracorporeal membrane oxygenation at Fu Wai hospital. Artif Organs 2011;35:572–8 [DOI] [PubMed] [Google Scholar]
- 44.Raffa GM, Kowalewski M, Brodie D, et al. Meta-Analysis of Peripheral or Central ECMO in Postcardiotomy and Non-Postcardiotomy Shock. Ann Thorac Surg. 2018, doi: 10.1016/j.athoracsur.2018.05.063. [DOI] [PubMed] [Google Scholar]
- 45.LoforteA, Marinelli G, Musumeci F, et al. Extracorporeal membrane oxygenation support in refractory cardiogenic shock: treatment strategies and analysis of risk factors. Artif Organs 2014;38:129–41 [DOI] [PubMed] [Google Scholar]
- 46.Saed D, Stosik H, Islamovic M, et al. Femoro-femoral versus atrio-aortic ECMO: selecting the ideal cannulation technique. Artif Organ 2014;38:549–55 [DOI] [PubMed] [Google Scholar]
- 47.Carroll BJ, Shah RV, Murthy V, et al. Clinical features and outcomes in adults with cardiogenic shock supported with extracorporeal membrane oxygenation. Am J Cardiol 2015;116:1624–30 [DOI] [PubMed] [Google Scholar]
- 48.Truby L, Mundy L, Kalesan B, et al. Contemporary outcomes of venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock at a large tertiary care center. ASAIO J 2015;61:403–9 [DOI] [PubMed] [Google Scholar]
- 49.Brunet J, Valette X, Ivascau C, et al. Extracorporeal life support for refractory cardiac arrest or shock: a 10-year study. ASAIO J 2015;61:676–81 [DOI] [PubMed] [Google Scholar]
- 50.Tarzia V, Bertolussi G, Bianco R, et al. Extracorporeal life support in cardiogenic shock: impacts of acute versus chronic etiology on outcome. J Thorac Cardiovasc Surg 2015;150:333–40 [DOI] [PubMed] [Google Scholar]
- 51.Narotsky DL, Mosca MS, Mochari-Greenberger H, et al. Short-term and long-term survival of veno-arterial extracorporeal membrane oxygenation in an adult patient population: does older age matter? Perfusion 2016;31:366–75 [DOI] [PubMed] [Google Scholar]
- 52.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from veno-arterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Critical Care 2016;20–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demodion P, Fournel L, Golmard JL, Niculescu M, Pavie A, Leprince P. Predictors of 30-day mortality and outcome in cases of myocardial infarction with cardiogenic shock treated by extracorporeal membrane oxygenation. Eur J Cardio-Thorac Surg 2014;45:47–54 [DOI] [PubMed] [Google Scholar]
- 54.Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant 2013;32:106–11 [DOI] [PubMed] [Google Scholar]
- 55.Xie A, Phan K, Tsai YC, Yan TD, Forrest P. venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: a metanalysis. J Cardio-Thorac Vasc Anesth 2015;29:637–45 [DOI] [PubMed] [Google Scholar]
- 56.Nakamura H, Yamaguchi H, Amano A, Nakao T. Veno-venous extracorporeal membrane oxygenation is effective against post-cardiotomy acute respiratory failure in adults. Gen Thorac Cardiovasc Surg 2013;61:402–8 [DOI] [PubMed] [Google Scholar]
- 57.Song JH, Woo WK, Song SH, et al. Outcome of veno-venous extracorporeal membrane oxygenation use in acute respiratory distress syndrome after cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2016;8:1804–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou NK, Chi NH, Wu IW, et al. Extracorporeal membrane oxygenation to rescue cardiopulmonary failure after heart transplantation: a single-centre experience. Transplant Proceed 2010;42:943–5 [DOI] [PubMed] [Google Scholar]
- 59.Borges Lima E, da Cunha CR, Barzilai VS, et al. Experience of ECMO in primary graft dysfunction after orthotopic heart transplantation. Arq Bras Cardiol 2015;105:285–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Alessandro C, Aubert S, Golmard JL, et al. ECMO temporary support for early graft failure after cardiac transplantation. Eur J Cardio-Thorac Surg 2010;37:343–9 [DOI] [PubMed] [Google Scholar]
- 61.Kittleson MM, Patel J, Moriguchi J, et al. Heart transplant recipients supported with ECMO: outcomes from a single-centre experience. J Heart Lung Transplant 2011;30:1250–6 [DOI] [PubMed] [Google Scholar]
- 62.Listijono D, Watson A, Pye R, et al. Usefulness of ECMO for early cardiac allograft dysfunction. J Heart Lung Tranplant 2011;30:783–9 [DOI] [PubMed] [Google Scholar]
- 63.Dandel M, Krabatsch T, Valk F. Left ventricular versus biventricular mechanical support: decision making and strategies for avoidance of right heart failure after left ventricular assist device implantation. Int J Cardiol 2015;198:241–50 [DOI] [PubMed] [Google Scholar]
- 64.Haneya A, Philipp A, Puehle T, et al. Temporary percutaneous right ventricular support using a centrifugal pump in patients with postoperative acute refractory right ventricular failure a er le ventricular assist device implantation. Eur J Cardiothorac Surg 2012;41:219–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6:552–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y, Xing J, Du Z, Liu F, Hou X. Extracorporeal cardiopulmonary resuscitation for adult patients who underwent post-cardiac surgery. Eur J Med Res 2016;20:83–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Society of Thoracic Surgeons Task Force on Resuscitation After Cardiac S. The Society of Thoracic Surgeons Expert Consensus for the Resuscitation of Patients Who Arrest After Cardiac Surgery. Annals Thorac Surg 2017;103:1005–20 [DOI] [PubMed] [Google Scholar]
- 68.Ranney DN, Benrashid E, Meza JM, et al. Central cannulation as a viable alternative to peripheral cannulation in extracorporeal membrane oxygenation. Sem Thorac Cardiovasc Surg 2017;29:188–95 [DOI] [PubMed] [Google Scholar]
- 69.Navia J, Atik FA, Beyer EA, Ruda Vega P. Extracorporeal membrane oxygenation with right axillary artery perfusion. Ann Thorac Surg 2005;79:2163–5 [DOI] [PubMed] [Google Scholar]
- 70.Biscotti M, Bacchetta M. The “Sport Model”: extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg 2014;98:1487–9 [DOI] [PubMed] [Google Scholar]
- 71.Babu A. Techniques of venoarterial ECMO support and conversion to temporary LVAD. Op Tech Thorac Cardiovasc Surg 2014;19:365–79 [Google Scholar]
- 72.Meani P, Gelsomino S, Natour E, et al. Modalities and effects of left ventricular unloading on extracorporeal life support: a review of the literature. Eur J Heart Fal 2017;19:81–88 [DOI] [PubMed] [Google Scholar]
- 73.Russo JJ, Aleksova N, Pitcher I, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol 2019;73:654–62 [DOI] [PubMed] [Google Scholar]
- 74.Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella on top of VA-ECMO may improves survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404–12 [DOI] [PubMed] [Google Scholar]
- 75.Tepper S, Faraz Masood M, Baltazar Garcia M, et al. Left ventricular unloading by Impella device versus surgical vent during extracorporeal life support. Ann Thorac Surg 2017;104:861–7 [DOI] [PubMed] [Google Scholar]
- 76.Patel SM, Lipinski J, Al-Kindi SG, et al. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J 2019;65:21–8 [DOI] [PubMed] [Google Scholar]
- 77.Schrage B, Burkhoff D, Rubsamen N, et al. Unloading of the left ventricle during venoarterial extracorporeal membrane oxygenation therapy in cardiogenic shock. J Am Coll Cardiol HF 2018;6:1035–43 [DOI] [PubMed] [Google Scholar]
- 78.Cheng R, Hachamovitch R, Makkar R, et al. Lack of survival benefit found with the use of intra-aortic balloon pump in ECMO: a pooled experience of 1517 patients. J Invas Cardiol 2015;27:453–8 [PubMed] [Google Scholar]
- 79.Nuding S, Werdan K. IABP plus ECMO – Is one and one more than two? J Thorac Dis 2017;9:961–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pappalardo F, Montisci A. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) in post-cardiotomy cardiogenic shock: how much pump flow is enough? J Thorac Dis 2016;8:1444–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haft JW. Temporary mechanical circulatory support for post-cardiotomy shock: don’t come late to the party. J Thorac Cardiovasc Surg 2015;149:1451–2 [DOI] [PubMed] [Google Scholar]
- 82.Dilstelmaier K, Roth C, Schrutka L, et al. Beneficial effects of levosimendan on survival in patients undergoing extracorporeal membrane oxygenation after cardiovascular surgery. Brit J Anesth 2016;117:52–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Affronti A, di Bella I, Carino D, Ragni T. Levosimendan may improve weaning outcomes in venoarterial ECMO patients. ASAIO J. 2013;59:554–7 [DOI] [PubMed] [Google Scholar]
- 84.Distelmaier K, Wiedemann D, Binder C, et al. Duration of extracorporeal membrane oxygenation support and survival in cardiovascular surgery patients. J Thorac Cardiovasc Surg 2018;155;2471–6 [DOI] [PubMed] [Google Scholar]
- 85.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610–6 [DOI] [PubMed] [Google Scholar]
- 86.Lorusso R, Barili F, Di Mauro M, et al. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: Results from the Extracorporeal Life Support Organization Registry. Crit Care Med 2016;44:e964–e972 [DOI] [PubMed] [Google Scholar]
- 87.Lorusso R, Gelsomino S, Parise O, et al. Neurologic injury in adults supported with veno-venous ECMO for respiratory failure: findings from the Extracorporeal Life Support Organization Registry. Crit Care Med 2017;45:1389–97 [DOI] [PubMed] [Google Scholar]
- 88.Wong JK, Smith TN, Pitcher HT, Hirose H, Cavarocchi NC. Cerebral and lower limb near infra-red spectroscopy in adults on extracorporeal membrane oxygenation. Artif Organs. 2012;36:659–67 [DOI] [PubMed] [Google Scholar]
- 89.Lorusso R, Gelsomino S, Parise O, et al. Veno-Arterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock in Elderly Patients: Trends in Application and Outcome from the Extracorporeal Life Support Organization (ELSO) Registry. Ann Thorac Surg 2017;104:62–9 [DOI] [PubMed] [Google Scholar]
- 90.Rastan AJ, Lachmann N, Walther T, et al. Autopsy findings in patients on postcardiotomy ECMO. Int J Artif Organs 2006;29:1121–31 [DOI] [PubMed] [Google Scholar]
- 91.Marasco SF, Vale M, Pellegrino V, et al. Extracorporeal membrane oxygenation in primary graft failure after heart transplant. Ann Thorac Surg 2010;90:1541–7 [DOI] [PubMed] [Google Scholar]
- 92.Takeda K, Li B, Garan AR, et al. Improved outcome from ECMO versus ventricular assist device temporay support of primary graft dysfunction after heart transplant. Heart Lung 2017;36:640–6 [DOI] [PubMed] [Google Scholar]
- 93.Rubino A, Costanzo D, Stanszus D, et al. Central veno-arterial extracorporeal membrane oxygenation (C-VA-ECMO) after cardio-thoracic surgery: a single-center experience. J Cardio-Thoraxc Vasc Anesth 2018;32:1169–74 [DOI] [PubMed] [Google Scholar]
- 94.Chen K, Hou J, Tang H, Hu S. Concurrent implantation of intra-aortic balloon pump and extracorporeal membrane oxygenation improved survival of patients with postcardiotomy shock. Artif Organs 2019;43:142–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan X, Jia S, Meng X, et al. Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria. Eur J Cardio-Thorac Surg 2010;37:334–8 [DOI] [PubMed] [Google Scholar]
- 96.Roth C, Schrutka L, Binder C, et al. Liver function predicts survival in patients undergoing extracorporeal membrane oxygenation following cardiovascular surgery. Crit Care 2016;20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dilstelmaier K, Winter MP, Rutzler K, et al. Serum butyrylcholiesterase predicts survival after extracorporeal membrane oxygenation after cardiovascular surgery. Crit Care 2014;18:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rahmanian PB, Adams DH, Castillo JG, Carpentier A, Filsoufi F. Predicting hospital mortality and analysis of long-term survival after major non-cardiac complications in cardiac surgery patients. Ann Thorac Surg 2010;90:1221–9 [DOI] [PubMed] [Google Scholar]
- 99.Stephens RS, Shah AS, Whitman GJR. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg 2013;95:1122–9 [DOI] [PubMed] [Google Scholar]
- 100.Peek G, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351–63 [DOI] [PubMed] [Google Scholar]
- 101.Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after ECMO for severe acute respiratory failure. Am J Respir Crit Care Med 2014;189:1374–8 [DOI] [PubMed] [Google Scholar]
- 102.Lin TW, Tsai MT, Hu YN, et al. Postoperative ECMO support for acute type A aortic dissection. Ann Thorac Surg 2017;104:827–33 [DOI] [PubMed] [Google Scholar]
- 103.Rao V. Defying death: can new ECMO technology improve the outcome of postcardiotomy shock? J Card Surg 2012;27:253–4 [DOI] [PubMed] [Google Scholar]
- 104.Arora RC, Wischmeyer PE, Singal RK. Extracorporeal membrane oxygenation postcardiotomy: “With great power come great responsibility”. J Thorac Cardiovasc Surg 2017;153:102–3 [DOI] [PubMed] [Google Scholar]
- 105.Burkhart HM, Riley JB, Lynch JJ, et al. Simulation-based postcardiotomy extracorporeal membrane oxygenation crisis training for thoracic surgery residents. Ann Thorac Surg 2013;95:901–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.