Abstract
Objectives:
To analyze symptomatic and asymptomatic COVID-19 patients in Saudi Arabia in terms of initial presentation, risk factors, laboratory findings, clinical outcomes and healthcare utilization.
Methods:
All laboratory-confirmed reverse transcription–polymerase chain reaction positive COVID-19 patients who had been tested at three governmental hospitals in Saudi Arabia (two in Riyadh and one in Makkah) between March 8 and May 18, 2020 were included. Demographics, COVID-19 variables, clinical characteristics and healthcare utilization variables were extracted and combined, and a descriptive analysis was conducted. Symptomatic and asymptomatic (on presentation) patients' data were compared.
Results:
Eighty percent of the patients were males (81.4% of symptomatic and 73.2% of asymptomatic patients, P = 0.02). Moreover, 47.6% and 38.4% of symptomatic and asymptomatic patients were aged 40–64 years, respectively. Fever, cough and breathing difficulties were frequent presenting symptoms. Overall, diabetes (16.4%), hypertension (11.7%), chronic respiratory disease (7.1%) were the most frequent comorbidities, with no differences between the two groups. Symptomatic patients had higher C-reactive protein levels (3.55 vs. 0.30 mg/L; P < 0.0001) and lower total lymphocytes (1.41 vs. 1.70; P = 0.02). ICU admission and mortality were 12.1% and 4.1% in symptomatic, compared to 6.0% and 2.9% in asymptomatic patients, respectively.
Conclusion:
In the studied COVID-19 cohort, symptomatic patients tended to be older, had higher C-reactive protein and more lymphopenia with worse outcome than asymptomatic patients. This granular analysis of COVID-19 cohorts enables identification of at-risk cohorts in future waves, optimizing development of patient pathways and public health interventions.
Keywords: Coronavirus, epidemiology, public health surveillance, SARS-CoV-2, Saudi Arabia, symptoms
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), remains one of the most challenging zoonotic infectious disease faced worldwide. As of February 10, 2021, over 106 million cases of COVID-19 had been reported worldwide that have resulted in > 2 million deaths.[1] In the Kingdom of Saudi Arabia (KSA), which is the largest country in the Arabian Peninsula with a population of 34 million, 370,987 COVID-19 cases had been reported with 6410 deaths as of March 2nd, 2021.[2]
A large proportion of those testing positive for COVID-19 are pauci-symptomatic or asymptomatic.[3] In symptomatic patients, the clinical presentation of COVID-19 varies markedly and is impacted by risk factor groups, including older age and patients with comorbidities.[4] The clinical features of COVID-19 appears to differ in prevalence in different clinical populations.[5]
In the Middle East, few studies have reported the course and clinical presentation of COVID-19 and compared these between symptomatic and asymptomatic patients. Therefore, this study aims to compare the initial presentation, risk factors, laboratory findings and healthcare utilization of symptomatic and asymptomatic COVID-19 patients from KSA.
METHODS
This retrospective study analyzed the clinical data of all laboratory-confirmed reverse transcription–polymerase chain reaction-positive COVID-19 patients who had been tested at three governmental hospitals in KSA (two in Riyadh and one in Makkah) between March 8 and May 18, 2020. This study was approved by the Ethics Committee of Ministry of Health (MoH) (Ref no.: 20-63E) and permissions were obtained from the concerned departments of all three hospitals.
Participants and setting
In KSA, healthcare is available for free at governmental hospitals for nationals, and expatriates typically access the private healthcare system through insurance supplied by their employer. Nonetheless, the Saudi government had passed an order for free COVID-19 treatment across the country to ensure larger accessibility.[6] In addition, the testing facility was freely made available by the MoH at healthcare facilities, all individuals with suspected COVID-19 – either based on symptoms or exposure to those with confirmed COVID-19 – can be tested at these sites. If positive, they are immediately enrolled in the MoH testing and contact tracing program. In the early phase of the pandemic, all patients who tested positive were admitted to hospitals, which was later changed to only include symptomatic patients. To provide data for comparison between symptomatic and asymptomatic patients, this study retrospectively analyzed the data of the initial phase.
All cases presented in this study are from three governmental hospitals, that are part of the Saudi MoH: Al Noor Specialist Hospital, a 500-bed specialized teaching hospital located centrally in Makkah;[7] Prince Mohammed bin Abdulaziz Hospital, a 500-bed hospital in Riyadh that is one of the major hospitals for COVID-19 case referrals;[8] and Al-Imam Abdulrahman Al Faisal Hospital, Riyadh.
Grouping of patients was based on their initial presentation, with those presenting with at least one of the following symptoms were considered symptomatic: fever, cough, shortness of breath (SOB), sore throat, digestive symptoms, rhinorrhea, headache, or other symptoms. In the absence of any of the mentioned symptoms, patients were considered asymptomatic.
Data collection
The following data were collected: demographics (gender, weight, and height), symptoms at presentation, reasons for testing (suspected as per case definition, contact of a positive case or travel history), baseline vitals and laboratory results, complete blood count, liver function, C-reactive protein, lactate dehydrogenase (LDH), D-dimer, alkaline phosphatase, albumin and total bilirubin. Moreover, information regarding treatment received, intensive care unit (ICU) admission, discharge date and survival (i e., death date) were also extracted. Data extraction was done by a single author and was quality checked by another author to ensure accuracy and quality of data.
Statistical analysis
The data were manually entered into a Microsoft Excel (Microsoft Corp, Redmond, WA), and then transferred to SPSS version 25 (SPSS Inc., Chicago, IL, USA) for data cleaning, management and analysis. The collected information is presented as total numbers and valid percentage for categorical variables, and as median and interquartile range (IQR) for continuous variables. Differences between variables for symptomatic and asymptomatic patients were assessed with a Chi-square test or independent t-test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Demographic characteristics
A total of 1051 patients tested positive for COVID-19 in the three hospitals during the included period. Table 1 details the demographic characteristics of these patients. The sample consisted of 80.3% males, with 2.0% being aged ≤18 years and 8.9% aged ≥65 years. Further, 24.7% of the patients were Saudi, 3.1% were healthcare workers, 5.9% were tested due to travel and 16.4% were linked epidemiologically to a confirmed case. In total, 913 (86.9%) patients were symptomatic. Significantly more males were symptomatic than asymptomatic (81.4% vs. 73.2%, respectively; P = 0.02). Moreover, symptomatic patients were more likely to be non-Saudi (P < 0.0001), and were less likely to be linked epidemiologically to a confirmed case (13.9%) compared to asymptomatic patients (32.6%, P < 0.0001).
Table 1.
Demographic, occupation, and reason for testing for patients infected with severe acute respiratory syndrome coronavirus-2 as well as distribution according to symptoms
| Category | Level | All (n=1051), n (%) | Symptomatic (n=913), n (%) | Asymptomatic (n=138), n (%) | P |
|---|---|---|---|---|---|
| Hospital | Riyadh 1 | 136 (12.9) | 104 (11.4) | 32 (23.2) | <0.0001 |
| Riyadh 2 | 597 (56.8) | 504 (55.2) | 93 (67.4) | ||
| Makkah 1 | 318 (30.3) | 305 (33.4) | 13 (9.4) | ||
| Gender | Male | 844 (80.3) | 743 (81.4) | 101 (73.2) | 0.02 |
| Female | 207 (19.7) | 170 (18.6) | 37 (26.8) | ||
| Age (years) | ≤18 | 21 (2.0) | 13 (1.4) | 8 (5.8) | 0.01 |
| 19-39 | 446 (42.7) | 383 (42.2) | 63 (45.7) | ||
| 40-64 | 485 (46.4) | 432 (47.6) | 53 (38.4) | ||
| ≥65 | 93 (8.9) | 79 (8.7) | 14 (10.1) | ||
| Nationality | Saudi | 260 (24.7) | 201 (22.0) | 59 (42.8) | <0.0001 |
| Non-Saudi | 791 (75.3) | 712 (78.0) | 79 (57.2) | ||
| Healthcare worker | 28 (3.1) | 22 (2.7) | 6 (5.7) | 0.12 | |
| Reason for testing | Linked to travel | 62 (5.9) | 49 (5.4) | 13 (9.4) | <0.0001 |
| Linked to confirmed case | 172 (16.4) | 127 (13.9) | 45 (32.6) |
Comorbidities and symptomatology of the studied patients
The most common comorbidities were diabetes (16.4%), hypertension (11.7%) and chronic respiratory disease (7.1%). Diabetics were more likely to be symptomatic (17.5% vs. 12.0% among non-symptomatic), although the association was not statistically significant (P = 0.15). No significant differences were found in other comorbidities between the two groups. For BMI, data on weight was only available for 551 patients (52.4%), and symptomatic patients were found to more likely be obese than asymptomatic patients (26.6% and 18.8%, respectively, P = 0.09) was observed [Table 2].
Table 2.
Comorbidities and body mass index among patients infected with severe acute respiratory syndrome coronavirus-2 as well as distribution according to symptoms
| Category | Level | All (n=1051), n (%) | Symptomatic (n=913), n (%) | Asymptomatic (n=138), n (%) | P |
|---|---|---|---|---|---|
| Comorbidities | Any comorbidity | 218 (29.7) | 183 (30.1) | 35 (28.0) | 0.64 |
| Diabetes | 120 (16.4) | 105 (17.5) | 15 (12.0) | 0.15 | |
| Hypertension | 86 (11.7) | 70 (11.5) | 16 (12.8) | 0.68 | |
| Cancer | 6 (0.8) | 5 (0.8) | 1 (0.8) | 0.98 | |
| Respiratory disease | 52 (7.1) | 42 (6.9) | 10 (8.0) | 0.67 | |
| Cardiovascular disease | 30 (4.1) | 25 (4.1) | 5 (4.0) | 0.95 | |
| Kidney disease | 16 (2.2) | 12 (2.0) | 4 (3.2) | 0.39 | |
| Other | 33 (4.5) | 23 (3.8) | 10 (8.0) | 0.04 | |
| BMI (kg/m2) | Underweight (<18.5) | 10 (1.8) | 6 (1.3) | 4 (4.7) | 0.09 |
| Normal (18.5-24.9) | 206 (37.4) | 173 (37.1) | 33 (38.8) | ||
| Overweight (25-29.9) | 195 (35.4) | 163 (35.0) | 32 (37.6) | ||
| Obese (≥30) | 140 (25.4) | 124 (26.6) | 16 (18.8) |
BMI – Body mass index
In the symptomatic group, the most common symptoms were fever and cough (66.0% and 64.5%, respectively). SOB, sore throat, and digestive symptoms were also frequently reported, although at a lower frequency (26.2%, 20.3% and 6.4%, respectively) [Figure 1]. Headache, fatigue, rhinorrhea or any other symptom were rarely reported.
Figure 1.
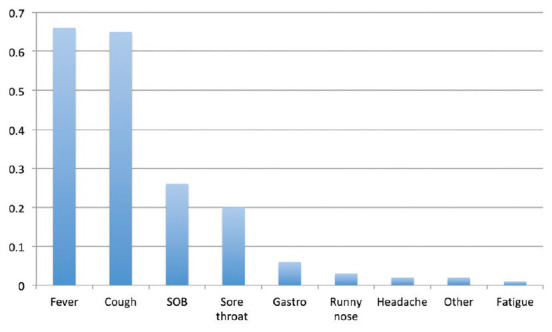
Prevalence of symptoms among patients infected with SARS-CoV-2 who were reported to be symptomatic
Laboratory findings
Overall, no medians were found to be outside the reference ranges. When comparing the two groups, symptomatic patients were found to have significantly higher C-reactive protein levels (3.55 vs. 0.30 mg/L; P = <0.0001) and lower total lymphocyte levels (1.41 vs. 1.70; P = 0.02). Significant difference was not noted in any other studied parameters. Regarding vital signs, symptomatic patients had higher heart rate (95.0 beats/min) than asymptomatic patients (86.0 beats/min) (P < 0.0001) [Table 3].
Table 3.
Baseline laboratory results and vital signs for patients infected with severe acute respiratory syndrome coronavirus-2 as well as distribution according to symptoms
| Findings | Normal value | Median (IQR) | P | ||
|---|---|---|---|---|---|
| All (n=1051) | Symptomatic (n=913) | Asymptomatic (n=138) | |||
| WBCs (×109/L) | 3.5-9.5 | 6.6 (3.6) | 6.5 (3.6) | 6.9 (3.8) | 0.91 |
| Hb (g/L) | 130-175 | 143.0 (25.0) | 143.0 (25.0) | 144.0 (27.0) | 0.26 |
| Platelets (×109/L) | 150-350 | 231.0 (106.0) | 231.0 (109.0) | 230.0 (95.0) | 0.89 |
| Neutrophils (×109/L) | 1.8-6.3 | 3.89 (3.16) | 3.92 (3.21) | 3.66 (3.06) | 0.31 |
| Lymphocytes (×109/L) | 1-3.3 | 1.45 (0.85) | 1.41 (0.86) | 1.70 (0.71) | 0.01 |
| CK (U/L) | 25-200 | 123.0 (195.8) | 130.5 (211.8) | 96.0 (86.0) | <0.0001 |
| ALT (U/L) | 10-45 | 36.0 (34.0) | 36.0 (35.0) | 31.0 (30.0) | 0.20 |
| AST (U/L) | 10-40 | 36.0 (31.0) | 37.0 (33.0) | 29.0 (17.0) | 0.050 |
| Creatinine (µmol/L) | 57-111 | 79.0 (26.1) | 79.5 (26.9) | 74.5 (24.8) | 0.31 |
| Albumin (g/L) | 40-55 | 40.0 (10.0) | 39.3 (11.0) | 42.0 (8.0) | 0.047 |
| Bilirubin (µmol/L) | 0-21 | 9.4 (6.6) | 9.6 (6.5) | 8.7 (6.2) | 0.30 |
| PT (s) | 9.5-13.5 | 13.6 (1.3) | 13.6 (1.3) | 13.7 (1.1) | 0.23 |
| PTT (s) | 25-37 | 35.8 (7.2) | 35.8 (7.3) | 35.4 (5.1) | 0.65 |
| INR | 0.8-1.2 | 1.02 (0.11) | 1.02 (0.11) | 1.03 (0.11) | 0.86 |
| CRP (mg/dL) | 0.0-0.40 | 2.78 (9.08) | 3.55 (9.52) | 0.30 (1.23) | <0.0001 |
| Erythrocytes sedimentation rate (mm/h) | 0-15 | 26.5 (59.9) | 35.0 (66.7) | 5.34 (10.9) | <0.0001 |
| LDH (U/L) | 50-242 | 265.0 (196.5) | 273.5 (203.0) | 209.0 (101.0) | <0.0001 |
| D-Dimer (ng/mL) | 0-229 | 0.91 (1.70) | 0.91 (1.50) | 0.79 (3.3) | 0.65 |
| Ferritin (ng/mL) | 15-400 | 629.1 (1006.1) | 629.1 (1005.2) | 597.3 (695.0) | 0.88 |
| Lactate (mmol/L) | 0.5-1 | 1.66 (0.66) | 1.68 (0.70) | - | - |
| Temperature (°C) | 35.5-37.5 | 37.3 (1.0) | 37.4 (1.0) | 37.0 (0.5) | <0.0001 |
| HR (beats/min) | 60-100 | 93.0 (23.0) | 95.0 (23.0) | 86.0 (17.0) | <0.0001 |
| RR (breaths/min) | 12-20 | 20.0 (1.0) | 20.0 (1.0) | 20.0 (1.0) | <0.0001 |
| SBP (mmHg) | 90-120 | 125.0 (20.0) | 125.0 (20.0) | 129.0 (20.0) | 0.10 |
| DBP (mmHg) | 60-90 | 78.0 (14.0) | 78.0 (13.0) | 75.0 (15.0) | 0.20 |
AST – Aspartate aminotransferase; ALT – Alanine aminotransferase; CK – Creatine kinase; CRP – C-reactive protein; DBP – Diastolic blood pressure; Hb – Hemoglobin; HR – Heart rate; INR – International normalized ratio; LDH – Lactate dehydrogenase; NLR – Neutrophil-lymphocyte ratio; PT – Prothrombin time; PTT – Partial thromboplastin time; RR – Respiratory rate; SBP – Systolic blood pressure – WBCs – White blood cells; IQR – Interquartile range
Healthcare utilization and outcome
Antibiotics and hydroxychloroquine were frequently administered (73.9% and 32.0%, respectively), while steroids and antiviral drugs were less common (14.8% and 20.5%, respectively) [Table 4]. Antibiotics were more frequently administered to symptomatic patients (78.6% vs. 34.5%, P < 0.0001). Similarly, hydroxychloroquine and corticosteroids were more frequently administered to symptomatic patients (34.7% and 14.8%, respectively) than asymptomatic patients (9.2% and 2.5%, respectively) (P < 0.0001 and 0.001, respectively). Oseltamivir use was not significantly different between the two groups.
Table 4.
Intensive care unit admission, management, and outcome of patients infected with severe acute respiratory syndrome coronavirus-2 as well as distribution according to symptoms
| Category | Level | All, n (%) | Symptomatic (n=913), n (%) | Asymptomatic (n=138), n (%) | P |
|---|---|---|---|---|---|
| Medication | Antibiotic | 596 (73.9) | 566 (78.6) | 30 (34.5) | <0.0001 |
| Hydroxychloroquine | 258 (32.0) | 250 (34.7) | 8 (9.2) | <0.0001 | |
| Steroid | 94 (14.8) | 92 (14.8) | 2 (2.5) | 0.001 | |
| Oseltamivir | 130 (20.5) | 117 (21.1) | 13 (16.5) | 0.34 | |
| ICU admission | 115 (11.3) | 107 (12.1) | 8 (6.0) | 0.04 | |
| Hospital length of stay (days)* | 0-1 | 82 (10.2) | 73 (10.4) | 9 (8.7) | 0.19 |
| 2-7 | 423 (52.5) | 362 (51.6) | 61 (58.7) | ||
| 8-14 | 216 (26.8) | 187 (26.7) | 29 (27.9) | ||
| >14 | 84 (10.4) | 79 (11.3) | 5 (4.8) | ||
| Outcome | Remained admitted at the time of data collection | 240 (22.8) | 207 (22.7) | 33 (23.9) | 0.78 |
| Discharged | 770 (73.3) | 669 (73.3) | 101 (73.2) | ||
| Died | 41 (3.9) | 37 (4.1) | 4 (2.9) |
*Among discharged patients. ICU – Intensive care unit
A total of 11.3% of the patients were admitted to the ICU, with symptomatic patients more likely to be admitted (12.1% vs. 6.0%, P = 0.04). Hospital stay of less than 2 days was rare (10.2%); most were admitted for 2–7 days (52.5%%), followed by 8–14 days (26.8%) and >2 weeks (10.4%). No differences were found between the two groups in terms of lengths of hospital stay. The vast majority (73.3%) of the patients had been discharged at the time of data collection, but about one-fifth (22.8%) remained admitted, while 3.9% had died.
DISCUSSION
The clinical features of patients hospitalized for COVID-19 in the KSA were similar to patients in studies from other parts of the world: a significant number of our patients had underlying medical conditions, and a considerable proportion of patients were asymptomatic.
About 80% of COVID-19 patients in this study were male; similar proportions have been observed in China, Italy and the United States.[9,10,11] It is currently unclear why COVID-19 infects males at a higher rate, and further research will be needed to understand this epidemiologic trend. The median age in this study (41 years) was noticeably lower than that in other countries. For example, the median age of patients with COVID-19 reported from other countries was between 62 − and 66 − years old in the United States,[9,12,13] 63 − years old in Italy,[10] and ranged from 49 − to 56 − years old in Wuhan, China.[11,14,15] This could be attributed to the fact that that KSA has a younger population compared to European and Asian countries.[16,17]
Diabetes, hypertension, and chronic lung disease were common comorbidities, similar to previous reports of patients hospitalized for COVID-19,[18,19,20,21] It is well established that patients with diabetes are at increased risk of viral infections and related complications,[22] due to impaired immune responses against viral pathogens. Although not yet fully established, it has been postulated that the weakened immune system expedites the progression of COVID-19 in patients with diabetes.[23] Accordingly, those with diabetes are at higher risk of severe disease, need for mechanical ventilation and admission to ICU; these factors likely contribute to the overall poor survival in this subset of patients.[24] Although the pathogenesis of COVID-19 in patients with hypertension or COPD remains poorly understood, both comorbidities are independently associated with a 2.5-and a 5.7-fold risk of COVID-19 disease severity and death, respectively.[4,25,26]
In our cohort, the majority of the patients were symptomatic. Among asymptomatic patients, some developed symptoms during the hospital stay. We believe the percentage of positive asymptomatic is underestimated, as one of the criteria for undertaking a COVID-19 test in KSA is the presence of symptoms or known exposure to a confirmed case. A random community wide testing would provide a more robust understanding of the percentage of asymptomatic patients. Further studies focused on random, unbiased testing of individuals would be necessary to accurately determine the exact percentage of patients who remain asymptomatic. Understanding this proportion is of great importance, as asymptomatic patients are at risk of transmitting the virus.[27,28] In fact, it is believed that the spread of the infection by asymptomatic individuals, combined with the relatively high basic reproduction number (R0) of 5.7[29] hastened the global pandemic and hindered public health efforts to effectively prevent and control the pandemic. Several studies have attempted to estimate the rate of asymptomatic COVID-19 infection, but the estimates vary widely (from 2% to 52%) and depend on the geographic location and setting.[29,30,31,32] Irrespective of the actual value, our results support a low threshold for testing of COVID-19 and stress the need for testing availability to diagnose COVID-19, particularly in asymptomatic patients.
Interestingly, most of the laboratory values were within normal ranges. The degree of abnormalities in the laboratory findings of patients with COVID-19 varies across different studies,[11,12,13,14,15] but the levels of LDH and total lymphocytes are consistently reported as elevated in these patients. In our study, both the LDH and lymphocytes level were within the normal range. It must be noted that the patients in this study were younger and had fewer comorbidities; presumably, they were healthier and more able to mount an adequate immune response against the virus, which would minimize the degree of laboratory abnormalities. To fully understand the link between age and degree of laboratory abnormalities, future large-scale studies are needed to compare patients with and without laboratory abnormalities, particularly LDH, lymphocyte, ferritin, D-dimer, procalcitonin and interleukin 6 levels. Similarly, the lack of laboratory abnormalities combined with fewer comorbidities and younger age may have contributed to the study's favorable composite endpoints. Overall, 10% of our cohort was admitted to the ICU, nearly 73% of patients were discharged and <4% died. These rates compared favorably to previously reported values for ICU admission (14%−39%), discharge (26%−66%) and mortality (7%−26%).[9,10,11,12,13,14,15,33]
The study had some noteworthy limitations and strengths. The study was a retrospective, cross-sectional analysis, and no patient data were collected after hospital discharge. Further, we were unable to collect the data for several different parameters for a significant proportion of patients. Notwithstanding, the study included COVID-19 patients from hospitals in two large metropolitan cities in KSA. To our knowledge, this is only the third study that describes the characteristics of patients with confirmed COVID-19 hospitalized in the KSA.[34,35] Therefore, this study adds to the growing body of literature characterizing the clinical features of COVID-19. The majority of currently available studies are retrospective in design, limited to single centers and carried out during short time periods. In the future, larger prospective, multi-center studies are required to be conducted over longer observation periods and with a larger sample size. Likewise, more studies are necessary to adequately examine and compare the clinical features and outcomes of patients with COVID-19 and MERS, to facilitate the implementation of optimal prevention and control strategies not only for COVID-19 but also in the event of any future coronavirus outbreaks.
CONCLUSION
In this studied COVID-19 cohort in KSA, about 80% of the patients were male. Symptomatic patients tended to be older, had higher C-reactive protein and more lymphopenia with worse outcome than asymptomatic patients. This granular analysis of COVID-19 cohorts enables identification of at-risk cohorts in future waves, optimizing development of patient pathways and public health interventions.
Ethical considerations
Ethical approval for this study was obtained from the Ethics Committee of Saudi Ministry of Health (Ref no.: 20-63E) on April 22, 2020.
Peer review
This article was peer-reviewed by three independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.WHO. WHO Health Emergency Dashboard. 2021. [[Last accessed on 2021 Feb 10]]. WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int .
- 2.WorldOMeter. Saudi Arabia Coronavirus. 2021. [[Last accessed on 2021 Feb 10]]. 98,869 Cases and 676 Deaths – Worldometer. Available from: https://www.worldometers.info/coronavirus/country/saudi-arabia/
- 3.Day M. Covid-19: Four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375. doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Centers for Disease Control and Prevention. 2020. [[Last accessed on 2020 Apr 06]]. Coronavirus Disease 2019 (COVID-19) Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html .
- 5.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1–5. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alshammari TM, Altebainawi AF, Alenzi KA. Importance of early precautionary actions in avoiding the spread of COVID-19: Saudi Arabia as an Example. Saudi Pharm J. 2020;28:898–902. doi: 10.1016/j.jsps.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Noor Specialist Hospital n.d. [[Last accessed on 2021 Mar 21]]. Available from: http://nsh.med.sa/Pages/Home.aspx .
- 8.Prince Mohammed Bin Abdulaziz Hospital. EyeofriyadhCom n.d. [[Last accessed on 2021 Mar 21]]. Available from: https://www.eyeofriyadh.com/directory/details/2754_prince-mohammed-bin-abdulaziz-hospital .
- 9.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382:2372–4. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl) 2020;7:91–6. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 13.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saudi Arabia General Authority for Statistics. General Authority for Statistics. General Authority for Statistics n.d. [[Last accessed on 2020 Jan 06]]. Available from: https://www.stats.gov.sa/en .
- 17.Alyami MH, Naser AY, Orabi MA, Alwafi H, Alyami HS. Epidemiology of COVID-19 in the Kingdom of Saudi Arabia: An ecological study. Front Public Health. 2020;8:506. doi: 10.3389/fpubh.2020.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report (MMWR) | MMWR n.d. [[Last accessed on 2020 Jun 18]]. Available from: https://www.cdc.gov/mmwr/index.html .
- 19.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: A systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 20.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21 doi: 10.1177/1470320320926899. 1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronsteiner B, Chaichana P, Sumonwiriya M, Jenjaroen K, Chowdhury FR, Chumseng S, et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol. 2019;49:1092–106. doi: 10.1002/eji.201848037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe COVID-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): A pooled analysis. Pol Arch Intern Med. 2020;130:304–9. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 27.Almadi MA, Aljebreen AM, Azzam N, Alammar N, Aljahdli ES, Alsohaibani FI, et al. COVID-19 and endoscopy services in intermediately affected countries: A position statement from the Saudi Gastroenterology Association. Saudi J Gastroenterol. 2020;26:240–8. doi: 10.4103/sjg.SJG_161_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54:12–16. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishiura H, Kobayashi T, Miyama T, Suzuki A, Jung SM, Hayashi K, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–5. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ki M; Task Force for 2019-nCoV. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020;42:e2020007. doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimball A. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–81. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold JA, Wong KK, Szablewski CM, Patel PP, Rossow J, da Silva J, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19 — Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545–550. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsofayan YM, Althunayyan SM, Khan AA, Hakawi AM, Assiri AM. Clinical characteristics of COVID-19 in Saudi Arabia: A national retrospective study. J Infect Public Health. 2020;13:920–5. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alahmari AA, Khan AA, Elganainy A, Almohammadi EL, AM Hakawi, Assiri AM, et al. Epidemiological and clinical features of COVID-19 patients in Saudi Arabia. J Infect Public Health. 2021;14:437–43. doi: 10.1016/j.jiph.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


