Abstract
We performed this study to investigate the diagnostic performance of prostate-specific antigen density (PSAD) in a multicenter cohort of the Chinese Prostate Cancer Consortium. Outpatients with prostate-specific antigen (PSA) levels ≥4.0 ng ml−1 regardless of digital rectal examination (DRE) results or PSA levels <4.0 ng ml−1 and abnormal DRE results were included from 18 large referral hospitals in China. The diagnostic performance of PSAD and the sensitivity and specificity for the diagnosis of prostate cancer (PCa) and high-grade prostate cancer (HGPCa) at different cutoff values were evaluated. A total of 5220 patients were included in the study, and 2014 (38.6%) of them were diagnosed with PCa. In patients with PSA levels ranging from 4.0 to 10.0 ng ml−1, PSAD was associated with PCa and HGPCa in both univariate (odds ratio [OR] = 45.15, P < 0.0001 and OR = 25.38, P < 0.0001, respectively) and multivariate analyses (OR = 52.55, P < 0.0001 and OR = 26.05, P < 0.0001, respectively). The areas under the receiver operating characteristic curves (AUCs) of PSAD in predicting PCa and HGPCa were 0.627 and 0.630, respectively. With the PSAD cutoff of 0.10 ng ml−2, we obtained a sensitivity of 88.7% for PCa, and nearly all (89.9%) HGPCa cases could be detected and biopsies could be avoided in 20.2% of the patients (359/1776 cases). Among these patients who avoided biopsies, only 30 cases had HGPCa. We recommend 0.10 ng ml−2 as the proper cutoff value of PSAD, which will obtain a sensitivity of nearly 90% for both PCa and HGPCa. The results of this study should be validated in prospective, population-based multicenter studies.
Keywords: Chinese, early detection of cancer, prostate cancer, prostate-specific antigen, prostate-specific antigen density
INTRODUCTION
The incidence of prostate cancer (PCa) ranks second among all male malignancies worldwide.1 The incidence of PCa in Asia is much lower than that in Europe and the United States, but it has shown an upward trend in recent years.2 Serum prostate-specific antigen (PSA) is widely applied in the screening and early diagnosis of PCa. However, elevated PSA levels may be due to a series of conditions. Benign prostate hyperplasia (BPH) and histological inflammation of the prostate would cause an increase in PSA level.3,4,5 Therefore, the diagnosis of PCa could not be made based on a slight increase in PSA level alone. The specificity of PSA is low, especially from 4.0 to 10.0 ng ml−1.6
Benson et al.7,8 proposed that prostate-specific antigen density (PSAD) could help improve the detection of PCa and reduce unnecessary biopsies in men with PSA levels ranging from 2.5 ng ml−1 to 10.0 ng ml−1. Some scholars also reported that compared with PSA, PSAD could improve the prediction efficacy of PCa and could reduce unnecessary biopsies.9,10 However, the levels of PSA and prostate volume (PV) in the Chinese population were different from those in Western countries.11 In addition, studies in other Asian countries proposed different cutoff values for the application of PSAD.12,13,14 To date, there have been no large multicenter studies in the Chinese population regarding the efficacy of PSAD and the proper PSAD cutoff value. In this study, we gathered 5220 consecutive biopsies from a multicenter cohort including members of the Chinese Prostate Cancer Consortium to validate the diagnostic performance of PSAD at different cutoff values.
PATIENTS AND METHODS
Inclusion and exclusion criteria
We included outpatients with PSA levels over 4.0 ng ml−1 or less than 4.0 ng ml−1 and abnormal digital rectal examination (DRE) results. We excluded patients suspected of urinary tract infections and urinary retention. Patients who received recent instrumentation or catheterization of the urethra in the past 2 weeks or 5α-reductase inhibitors in the past 2 months were also excluded. A total of 5220 patients who underwent prostate biopsy in 18 different large referral hospitals in China during 2010–2013 were included in this study, and all the hospitals are listed in Supplementary Information (41.8KB, pdf) . The differences in the PCa detection rates for the different regions that contributed to the data are shown in Supplementary Table 1. A total of 10–16 cores from initial transrectal or transperineal biopsies guided with transrectal ultrasound (TRUS) were collected from all patients. This study has passed the ethic review of the Ethics Committee of Shanghai Changhai Hospital (Shanghai, China; No. CHEC2013-149). All patients signed informed consent form for the study.
Supplementary Table 1.
The difference in detection rates of prostate cancer and high-grade prostate cancer in different parts of China
| Region | Patients | PCa | HGPCa | Detection rate of PCa (%) | Detection rate of HGPCa (%) | HGPCa/PCa (%) |
|---|---|---|---|---|---|---|
| North China | 1436 | 624 | 519 | 43.5 | 36.1 | 83.2 |
| Yangtze River Delta | 2799 | 1018 | 761 | 36.4 | 27.2 | 74.8 |
| West China | 330 | 112 | 91 | 33.9 | 27.6 | 81.3 |
| South China | 655 | 260 | 211 | 39.7 | 32.2 | 81.2 |
| Total | 5220 | 2014 | 1582 | 38.6 | 30.3 | 78.6 |
PCa: prostate cancer; HGPCa: high-grade prostate cancer
Sample collection
Peripheral blood samples were obtained before DRE and prostate biopsy. PV was calculated using the equation D1 × D2 × D3 × (π/6), with three dimensions measured by TRUS. Three types of electrochemiluminescence immunoassays were used with the Beckman Coulter Access (Beckman Coulter Inc., Brea, CA, USA), Abbott AxSYM (Abbott Laboratories, North Chicago, IL, USA), and Roche Elecsys 2010 systems (F. Hoffmann-La Roche Ltd., Basel, Switzerland). Recalibration was performed according to the WHO standards (PSA-WHO 96/670). All specimens were processed by the Pathology Department of these hospitals. Total PSA (tPSA), free PSA (fPSA), and the ratio of free-to-total PSA (%fPSA) were measured. The results of TRUS (having nodules or not) and other clinical information were collected.
Statistical analyses
We divided the patients into three groups according to PSA levels (4.0–10.0 ng ml−1, 10.1–20.0 ng ml−1, and above 20.0 ng ml−1). Univariate and multivariate stepwise logistic regression analyses were applied to evaluate the prediction efficacy of each variable (age, PSAD, %fPSA, and TRUS results) for predicting PCa and high-grade PCa (HGPCa, Gleason score ≥7). The PCa detection rates of patients in different PSA ranges were calculated. Statistical analyses were performed using R software (R version 3.6.0; The R Foundation for Statistical Computing, Vienna, Austria) and MedCalc Software (MedCalc Software Ltd., Ostend, Belgium). All of the statistical tests were two-sided, and P < 0.05 was considered significantly different.
RESULTS
Patients and biopsies
A total of 5220 patients were included in the study, and 2014 (38.6%) of them were diagnosed with PCa. Among the 2014 patients with PCa, 1582 were diagnosed with HGPCa (78.6%). The characteristics of the cohort are shown in Table 1. The clinical information of these patients was collected prospectively. Age was missing for 84 patients, and the mean age of the cohort was used instead for these patients. The mean age of all the patients was 68.4 (standard deviation [s.d.] = 8.7) years. The mean and median levels of PSA in this cohort were 43.00 (s.d. = 145.61) ng ml−1 and 12.00 (interquartile range [IQR]: 7.50–24.50) ng ml−1, respectively. The mean and median PSAD levels were 1.03 (s.d. = 4.58) ng ml−2 and 0.26 (IQR: 0.14–0.59) ng ml−2, respectively. The mean and median PSA and PSAD levels in patients with PCa were higher than those in patients without PCa. All patients underwent 10- to 16-core prostate biopsies (median 12 cores). Among them, 1589 men underwent 10-core biopsies (30.4%), 101 men underwent 11-core biopsies (1.9%), 2505 men underwent 12-core biopsies (48.0%), and 1025 men underwent 13–16-core biopsies (19.6%). There were 1776, 1566, and 1581 patients with PSA levels of 4.0–10.0 ng ml−1, 10.1–20.0 ng ml−1, and above 20.0 ng ml−1, respectively. The PCa detection rates of these groups were 25.4%, 33.8%, and 62.4%, respectively. The proportions of HGPCa cases in all the PCa cases were 65.6%, 73.9%, and 87.7%, respectively. The distribution of Gleason score in different PSA ranges is detailed in Supplementary Table 2.
Table 1.
Characteristics of the study cohort (n=5220)
| Variables | PSA (ng ml−1) | The whole cohort | ||
|---|---|---|---|---|
| 4.0–10.0 | 10.1–20.0 | Above 20.0 | ||
| Age at time of biopsy (year) | ||||
| Mean±s.d. | 66.4±8.5 | 68.9±8.5 | 70.6±8.2 | 68.4±8.7 |
| Median (IQR) | 67.0 (61.0–73.0) | 69.0 (69.0–70.0) | 71.0 (71.0–72.0) | 69.0 (63.0–75.0) |
| PSA at time of biopsy (ng ml−1) | ||||
| Mean±s.d. | 7.18±1.64 | 13.79±2.82 | 119.85±248.01 | 43.00±145.61 |
| Median (IQR) | 7.20 (5.80–8.60) | 13.30 (11.30–15.90) | 41.70 (39.30–44.20) | 12.00 (7.50–24.50) |
| Prostate volume (ml) | ||||
| Mean±s.d. | 51.0±27.2 | 58.7±33.4 | 55.5±37.6 | 54.2±32.7 |
| Median (IQR) | 44.2 (32.1–63.0) | 49.7 (33.9–77.0) | 44.3 (43.5–46.2) | 45.0 (32.2–68.0) |
| PSA density (ng ml−2) | ||||
| Mean±s.d. | 0.18±0.10 | 0.33±0.83 | 2.87±7.98 | 1.03±4.58 |
| Median (IQR) | 0.15 (0.11–0.22) | 0.27 (0.17–0.41) | 1.06 (0.99–1.12) | 0.26 (0.14–0.59) |
| %fPSA | ||||
| Mean±s.d. | 0.16±0.08 | 0.15±0.07 | 0.13±0.09 | 0.15±0.09 |
| Median (IQR) | 0.15 (0.11–0.20) | 0.14 (0.10–0.19) | 0.11 (0.10–0.11) | 0.14 (0.09–0.20) |
| Transrectal ultrasound, n (%) | ||||
| Positive (+) | 244 (13.7) | 220 (14.0) | 340 (21.5) | 902 (17.3) |
| Negative (−) | 1532 (86.3) | 1346 (86.0) | 1241 (78.5) | 4318 (82.7) |
PSA: prostate-specific antigen; %fPSA: the ratio of free-to-total PSA; s.d.: standard deviation; IQR: interquartile range
Supplementary Table 2.
The Gleason score of the patients with different prostate-specific antigen ranges
| PSA ranges | GS <7 | GS ≥7 (HGPCa) | all PCa | HGPCa% |
|---|---|---|---|---|
| Below 4.0 ng ml−1 | 18 | 30 | 48 | 62.5 |
| 4.0–10.0 ng ml−1 | 155 | 296 | 451 | 65.6 |
| 10.0–20.0 ng ml−1 | 138 | 391 | 529 | 73.9 |
| Above 20.0 ng ml−1 | 121 | 865 | 986 | 87.7 |
| The whole cohort | 432 | 1582 | 2014 | 78.6 |
PSA: prostate-specific antigen; GS: Gleason score; PCa: prostate cancer; HGPCa: high-grade prostate cancer
Univariate and multivariate logistic regression analyses
In univariate logistic regression analysis, age, PSAD, %fPSA, and TRUS results were all associated with biopsy results (all P < 0.0001, Table 2)). Multivariate logistic regression analysis indicated these four variables as independent predictors of PCa and HGPCa in all patients.
Table 2.
Univariate and multivariate analyses of variables at the time of biopsy in predicting the risk of prostate cancer and high-grade prostate cancer
| Variables | PCaa | HGPCaa | PCab | HGPCab | ||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Age at biopsy | ||||||||
| Univariate analysis | 1.05 (1.04–1.07) | <0.0001 | 1.06 (1.04–1.08) | <0.0001 | 1.05 (1.05–1.06) | <0.0001 | 1.05 (1.04–1.06) | <0.0001 |
| Multivariate analysis | 1.07 (1.05–1.08) | <0.0001 | 1.07 (1.05–1.09) | <0.0001 | 1.06 (1.05–1.07) | <0.0001 | 1.06 (1.05–1.06) | <0.0001 |
| PSAD | ||||||||
| Univariate analysis | 45.15 (15.70–129.83) | <0.0001 | 25.38 (8.19–78.69) | <0.0001 | 1.15 (1.12–1.19) | <0.0001 | 1.11 (1.09–1.14) | <0.0001 |
| Multivariate analysis | 52.55 (16.42–168.22) | <0.0001 | 26.05 (7.53–90.07) | <0.0001 | 1.10 (1.07–1.14) | <0.0001 | 1.08 (1.05–1.10) | <0.0001 |
| TRUS (nodule), positive versus negative | ||||||||
| Univariate analysis | 1.13 (0.91–1.41) | 0.2728 | 1.02 (0.78–1.33) | 0.8907 | 1.39 (1.25–1.54) | <0.0001 | 1.29 (1.16–1.43) | <0.0001 |
| Multivariate analysis | 1.06 (0.83–1.35) | 0.6303 | 0.93 (0.70–1.24) | 0.6252 | 1.38 (1.23–1.55) | <0.0001 | 1.28 (1.15–1.43) | 0.0002 |
| %fPSA | ||||||||
| Univariate analysis | 0.07 (0.02–0.30) | 0.0003 | 0.09 (0.02–0.48) | 0.0049 | 0.02 (0.01–0.05) | <0.0001 | 0.02 (0.01–0.04) | <0.0001 |
| Multivariate analysis | 0.07 (0.01–0.34) | 0.0010 | 0.07 (0.01–0.45) | 0.0051 | 0.02 (0.01–0.03) | <0.0001 | 0.01 (0.01–0.03) | <0.0001 |
aPSA 4 ng ml−1–10 ng ml−1; bthe whole cohort. PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; TRUS: transrectal ultrasound; %fPSA: the ratio of free-to-total PSA; PCa: prostate cancer; HGPCa: high-grade prostate cancer; CI: confidence interval
In patients with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1, PSAD was associated with PCa and HGPCa in both univariate and multivariate analyses (all P < 0.0001). PSAD (both P < 0.0001), age (both P < 0.0001), and %fPSA (P = 0.0010 and P = 0.0051, respectively) were all independent predictors of PCa and HGPCa. However, TRUS results were not associated with PCa or HGPCa (P = 0.2728 and P = 0.8907; Table 2)).
In patients with PSA levels ranging from 10.1 ng ml−1 to 20.0 ng ml−1, age and %fPSA were associated with PCa and HGPCa in both univariate and multivariate logistic regression analyses. However, PSAD was not associated with PCa and HGPCa and was not a predictor for either of them in this PSA range (P = 0.2141 and P = 0.1265, respectively). TRUS results were associated with PCa (P = 0.0192) but were not associated with HGPCa (P = 0.0667); however, it was an independent predictor for both PCa and HGPCa (P = 0.0080 and P = 0.0395, respectively).
Diagnostic efficacy
In men with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1, 10.1 ng ml−1 to 20.0 ng ml−1, above 20.0 ng ml−1, and the whole cohort, the areas under the receiver operating characteristic (ROC) curve (AUCs) of PSAD for predicting PCa and HGPCa were all higher than those of PSA (Figure 1 and Table 3). The AUCs of PSAD in predicting PCa and HGPCa in patients with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1 were 0.627 and 0.630, respectively. The AUCs of PSA were 0.526 and 0.524. In patients with PSA levels above 20.0 ng ml−1, although the overall diagnostic performance of PSAD was still higher than that of PSA, the improvement in AUCs in predicting HGPCa was limited (0.664 vs 0.647; P = 0.06).
Figure 1.
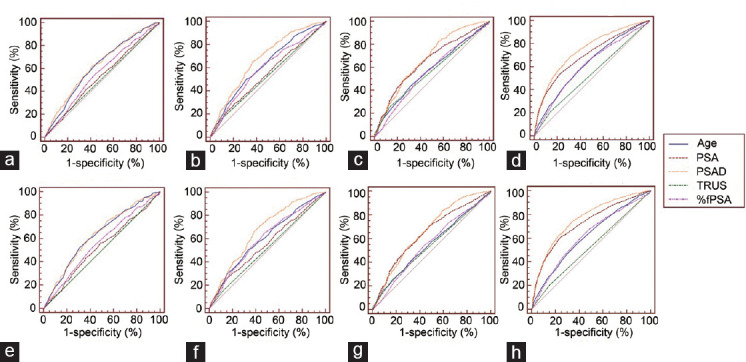
Diagnostic efficacy of PSAD. The comparison of diagnostic efficacy of age, PSA, PASD, TRUS, and %fPSA for PCa in patients (a) with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1, (b) with PSA levels ranging from 10.1 ng ml−1 to 20.0 ng ml−1, (c) with PSA levels above 20.0 ng ml−1, and (d) regardless of PSA levels. The comparison of diagnostic efficacy of age, PSA, PASD, TRUS, and %fPSA for HGPCa in patients (e) with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1, (f) with PSA levels ranging from 10.1 ng ml−1 to 20.0 ng ml−1, (g) with PSA levels above 20.0 ng ml−1, (h) regardless of PSA levels. PCa: prostate cancer; HGPCa: high-grade prostate cancer; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; TRUS: transrectal ultrasound; %fPSA: the ratio of free-to-total PSA.
Table 3.
The areas under the receiver operating characteristic curve for prostate-specific antigen density and prostate-specific antigen in predicting the risk of prostate cancer
| Variables | PSA 4.0 ng ml−1–10.0 ng ml−1 | PSA 10 ng ml−1–20 ng ml−1 | PSA above 20 ng ml−1 | All PSA | ||||
|---|---|---|---|---|---|---|---|---|
| PCa | HGPCa | PCa | HGPCa | PCa | HGPCa | PCa | HGPCa | |
| Age | 0.620 | 0.630 | 0.624 | 0.606 | 0.575 | 0.552 | 0.626 | 0.617 |
| PSA | 0.526 | 0.524 | 0.545 | 0.556 | 0.650 | 0.647 | 0.700 | 0.726 |
| PV | 0.623 | 0.625 | 0.666 | 0.655 | 0.580 | 0.568 | 0.600 | 0.588 |
| PSAD | 0.627 | 0.630 | 0.675 | 0.668 | 0.675 | 0.664 | 0.744 | 0.760 |
| TRUS | 0.513 | 0.503 | 0.528 | 0.517 | 0.564 | 0.543 | 0.535 | 0.527 |
| %fPSA | 0.563 | 0.563 | 0.593 | 0.600 | 0.560 | 0.552 | 0.612 | 0.617 |
PSA: prostate-specific antigen; PV: prostate volume; PSAD: prostate-specific antigen density; TRUS: transrectal ultrasound; PCa: prostate cancer; HGPCa: high-grade prostate cancer; %fPSA: the ratio of free-to-total PSA
Optimal PSAD cutoff value for patients with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1
The statistically optimal cutoff value of PSAD in our study was 0.16 ng ml−2, with the highest sum of specificity and sensitivity. This cutoff value would detect 61.6% (278/451 cases) of PCa cases and 63.9% (189/296 cases) of HGPCa cases by performing biopsies in nearly half of the patients (827/1776 cases, 46.6%). At the same time, a total of 173 cases of PCa and 107 cases (36.1% of HGPCa) of HGPCa would be missed. Instead, with the PSAD cutoff value of 0.10 ng ml−2, nearly all (89.9%) HGPCa cases could be detected, and biopsies could be avoided in 20.2% of the patients (359/1776 cases). Among these patients who avoided biopsies, only 30 cases (10.1%) had HGPCa. In contrast, if the 0.16 cutoff was applied, 107 (36.1%) HGPCa cases would be missed (Table 4).
Table 4.
Sensitivity, specificity, positive and negative predict value of the prostate-specific antigen density at different cutoff values in patients with prostate-specific antigen levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1
| Cutoff (ng ml−2) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | HGPCa missed, n/total (%) | Unnecessary biopsies avoided, n/total (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCa | HGPCa | PCa | HGPCa | PCa | HGPCa | PCa | HGPCa | |||
| 0.10 | 88.7 (85.4–91.5) | 89.9 (85.8–93.1) | 23.3 (21.0–25.6) | 22.2 (20.1–24.4) | 28.2 (25.9–30.7) | 18.8 (16.8–20.9) | 85.8 (81.7–89.2) | 91.6 (88.3–94.3) | 30/296 (10.1) | 308/1325 (23.2) |
| 0.11 | 83.8 (80.1–87.1) | 84.5 (79.8–88.4) | 28.5 (26.1–31.0) | 27.4 (25.1–29.7) | 28.5 (26.1–31.0) | 18.9 (16.8–21.1) | 83.8 (80.1–87.1) | 89.8 (86.6–92.4) | 46/296 (15.5) | 378/1325 (28.5) |
| 0.12 | 78.7 (74.6–82.4) | 80.7 (75.8–85.1) | 35.8 (33.2–38.4) | 34.7 (32.2–37.1) | 29.4 (26.9–32.1) | 19.8 (17.6–22.2) | 83.2 (79.8–86.1) | 90.0 (87.2–92.3) | 57/296 (19.3) | 474/1325 (35.8) |
| 0.13 | 73.8 (69.5–77.8) | 77.0 (71.8–81.7) | 42.4 (39.7–45.1) | 41.4 (38.8–43.9) | 30.4 (27.7–33.2) | 20.8 (18.4–23.3) | 82.6 (79.6–85.4) | 90.0 (87.5–92.2) | 68/296 (23.0) | 562/1325 (42.4) |
| 0.14 | 70.7 (66.3–74.9) | 74.0 (68.6–78.9) | 47.0 (44.3–49.7) | 45.8 (43.2–48.4) | 31.2 (28.4–34.2) | 21.4 (19.0–24.1) | 82.5 (79.6–85.2) | 89.8 (87.4–91.9) | 77/296 (26.0) | 623/1325 (47.0) |
| 0.15 | 64.8 (60.1–69.2) | 67.2 (61.6–72.5) | 52.5 (49.8–55.2) | 51.2 (48.6–53.8) | 31.7 (28.7–34.8) | 21.6 (19.0–24.4) | 81.4 (78.6–84.0) | 88.7 (86.3–90.7) | 97/296 (32.8) | 696/1325 (52.5) |
| 0.16 | 61.6 (57.0–66.2) | 63.9 (58.1–69.3) | 58.6 (55.9–61.2) | 56.9 (54.3–59.4) | 33.6 (30.4–36.9) | 22.9 (20.0–25.9) | 81.8 (79.2–84.2) | 88.7 (86.5–90.7) | 107/296 (36.1) | 776/1325 (58.6) |
PPV: positive predict value; NPV: negative predict value; 95% CI: 95% confidence interval; PCa: prostate cancer; HGPCa: high-grade prostate cancer
A total of 432 patients had PCa with Gleason score of 6 (432/2014 cases, 21.4%) in the whole cohort. When we took 0.10 ng ml−2 as the PSAD cutoff value, 395 patients would be diagnosed with PCa, and 37 (37/432 cases, 8.6%) Gleason 6 patients would avoid biopsies (Table 5).
Table 5.
Gleason 6 prostate cancer that can be avoided from biopsies at different prostate-specific antigen density cutoff values
| Cutoffs (ng ml-2) | Gleason score=6 | Avoided, n/total (%) |
|---|---|---|
| 0.10 | 395 | 37/432 (8.6) |
| 0.11 | 387 | 45/432 (10.4) |
| 0.12 | 373 | 59/432 (13.7) |
| 0.13 | 359 | 73/432 (16.9) |
| 0.14 | 352 | 80/432 (18.5) |
| 0.15 | 341 | 91/432 (21.1) |
| 0.16 | 335 | 97/432 (22.5) |
DISCUSSION
To the best of our knowledge, this multicenter study is the largest and only nationwide study of PSAD in a Chinese population with patients from 18 hospitals across China. Although PSAD is a predictor that has been extensively studied in Western populations, it was shown in previous studies that substantial differences existed between Chinese and Western populations in terms of PSA and its derivatives.11 At the same time, there were no large-scale multicenter studies in the Chinese population that could provide convincing evidence on the national scale, and the diagnostic efficacy of PSAD was still based on studies from the Western populations.
PSAD was confirmed as an independent predicator for PCa and HGPCa in patients with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1 and the whole cohort. In patients with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1, the PSAD cutoff value of 0.10 ng ml−2 could detect the majority of clinically significant HGPCa cases (sensitivity of 89.9% for HGPCa). The cutoff value of 0.15 ng ml−2 proposed by previous studies would miss 33.8% of the HGPCa cases in our study. Clearly, this cutoff led to too many missed HGPCa cases. Compared with 0.15 ng ml−2, the cutoff value of 0.10 ng ml−2 would only miss approximately 10% of HGPCa cases; thus, we suggest the application of the cutoff value of 0.10 ng ml−2 in the Chinese population.
Many Asian scholars have proposed new cutoff values for PSAD, including Saema A in Thailand and Patil SR in India.12,15 The proposed PSAD cutoff value varied among studies in Asian populations. A study in Chinese patients suggested that a PSAD level of 0.15 ng ml−2 was the optimal cutoff for patients with PSA levels ranging from 2.5 ng ml−1 to 10.0 ng ml−1, and the sensitivity and specificity were 64.4% and 64.6%, respectively.16 However, this cutoff would miss about 30% of PCa patients who may progress to advanced stages if not detected.
A series of studies suggested cutoff values lower than 0.15 ng ml−2, which were similar to our findings. Zheng et al.14 suggested the cutoff value of 0.134 ng ml−2, with a sensitivity and specificity of 90% and 33.7%, respectively. Their strategy was similar to ours, which is to obtain 90% sensitivity. In a study of 2606 patients in the Chinese population, Teoh et al.17 suggested that the cutoff value of 0.12 ng ml−2 had a sensitivity of 94.5% for PCa. In our study, the cutoff of 0.12 ng ml−2 could only detect 78.7% of PCa cases and 80.7% of HGPCa cases. This did not meet our target of the detection rate of 90%. However, there were studies that recommended higher cutoff values. Liu et al.18 conducted a study on 197 men with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1 and identified 0.25 ng ml−2 as the optimal cutoff value for PCa and 0.20 ng ml−2 for HGPCa. The sensitivity and specificity were 75.4% and 75.8% for PCa and 76.7% and 80.1% for HGPCa, respectively. In another study by Arai et al.19 in Japanese patients, the higher cutoff value of 0.19 ng ml−2 was proposed with high sensitivity (75%) and high specificity (87%). We found that although the cutoff values they suggested were higher, the diagnostic efficacy of PSAD was still higher than that of the abovementioned studies. PSAD had higher diagnostic efficacy in both studies than in our study (sensitivity 61.6% and specificity 58.6%) at the statistically optimal cutoff.
We suggest that the differences in selecting cutoff values depend on both the diagnostic efficacy of PSAD and the diagnostic strategy. In situ ations with poor diagnostic efficacy of PSAD, the cutoff should be lower to obtain a higher sensitivity to reduce the number of missed HGPCa cases. However, in situ ations with robust diagnostic efficacy, it is practical to choose a higher cutoff value that could maintain a higher detection rate and avoid unnecessary biopsies at the same time.
The lower diagnostic efficacy of PSAD in this study may be due to the multicenter study design with PV measured in different hospitals by different physicians. However, we consider this study to be consistent with the current clinical scenarios, and thus, we suggest that the cutoff value in this study, with the largest number of cases, should be applied in Chinese and other East Asian populations. Interestingly, the latest guidelines of the European Association of Urology (EAU) suggested no optimal PSAD cutoff value. However, they stated that with the increase in PSAD, the possibility of clinically significant PCa increases. This highlights that personalized management should always be considered for every patient and decisions should be made according to the clinical scenario.
In addition, we paid special attention to patients with Gleason score 6 (3+3) because of the mild nature of this type of malignancy.20 In our study, 432 patients had a Gleason score of 6. When we took the cutoff from 0.10 to 0.16 serially, 8.6%, 10.4%, 13.7%, 16.9%, 18.5%, 21.1%, and 22.5% of Gleason 6 patients would avoid biopsies, respectively. In general, we found that PSAD could not effectively distinguish HGPCa from low-grade PCa (LGPCa); thus, we do not recommend this cutoff to be effective in reducing the number of LGPCa cases detected.
There are some limitations in this study. First, this is a retrospective study; however, the data were prospectively collected. Second, multicenter research also has inherent limitations. Biopsies were performed by different urologists and were read by different pathologists. However, this factor was unlikely to cause large systematic differences that could be observed. The multicenter research design had some flaws. The patients in this study underwent biopsies from 2010 to 2013, and prebiopsy MRI was not routinely suggested in major guidelines. In addition, the recording of DRE is not standardized among the centers for patients with PSA levels over 4.0 ng ml−1; thus, we were unable to analyze the DRE results. Finally, this study was not population based, and as such, it is possible that our results overestimate the rate of PCa.
CONCLUSION
The diagnostic performance of PSAD was higher than PSA in all PSA ranges. In patients with PSA levels ranging from 4.0 ng ml−1 to 10.0 ng ml−1, PSAD could be an independent predictor of PCa and HGPCa. We recommend 0.10 ng ml−2 as the proper cutoff value of PSAD, which will obtain a sensitivity of nearly 90% for both PCa and HGPCa. The results of this study should be validated in prospective, population-based multicenter studies.
AUTHOR CONTRIBUTIONS
ZJS, RC, JKQ, and WZ made substantial contributions to collecting the dataset, conceiving the study idea, participating in data analyses, conducting statistical analyses, manuscript preparation, and manuscript editing. YY, FBW, and HXW collected the dataset and participated in data analyses. MYW and SYJ participated in clinical studies and data analyses. ZJS and RC participated in the study design and coordination. RC has reviewed and polished the manuscript. The CPCC guided us in designing this multi-center study and collecting the dataset. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study is supported by the Fund for National Natural Science Foundation Youth Project (No. 81702514 to RC). Some members of the Chinese Prostate Cancer Consortium provided assistance in data collection. The 18 large referral hospitals across China helped us collect the data and design this study. All of these hospitals are listed in Supplementary Information (41.8KB, pdf) .
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Umbehr MH, Gurel B, Murtola TJ, Sutcliffe S, Peskoe SB, et al. Intraprostatic inflammation is positively associated with serum PSA in men with PSA <4 ng ml−1, normal DRE and negative for prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:264–9. doi: 10.1038/pcan.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simardi LH, Tobias-MacHado M, Kappaz GT, Taschner Goldenstein P, Potts JM, et al. Influence of asymptomatic histologic prostatitis on serum prostate-specific antigen: a prospective study. Urology. 2004;64:1098–101. doi: 10.1016/j.urology.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 5.Thorpe A, Neal D. Benign prostatic hyperplasia. Lancet. 2003;361:1359–67. doi: 10.1016/S0140-6736(03)13073-5. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida K, Honda M, Sumi S, Arai K, Suzuki S, et al. Levels of free prostate-specific antigen (PSA) can be selectively measured by heat treatment of serum: free/total-PSA ratios improve detection of prostate carcinoma. Clin Chim Acta. 1999;280:195–203. doi: 10.1016/s0009-8981(98)00189-2. [DOI] [PubMed] [Google Scholar]
- 7.Benson MC, Whang IS, Pantuck A, Ring K, Kaplan SA, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–6. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 8.Benson MC, Whang IS, Olsson CA, McMahon DJ, Cooner WH. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol. 1992;147:817–21. doi: 10.1016/s0022-5347(17)37394-9. [DOI] [PubMed] [Google Scholar]
- 9.Verma A, St Onge J, Dhillon K, Chorneyko A. PSA density improves prediction of prostate cancer. Can J Urol. 2014;21:7312–21. [PubMed] [Google Scholar]
- 10.Jue JS, Barboza MP, Prakash NS, Venkatramani V, Sinha VR, et al. Re-examining prostate-specific antigen (PSA) density: defining the optimal PSA range and patients for using PSA density to predict prostate cancer using extended template biopsy. Urology. 2017;105:123–8. doi: 10.1016/j.urology.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Sjoberg DD, Huang Y, Xie L, Zhou L, et al. Prostate specific antigen and prostate cancer in Chinese men undergoing initial prostate biopsies compared with Western cohorts. J Urol. 2017;197:90–6. doi: 10.1016/j.juro.2016.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saema A, Kochakarn W, Lertsithichai P. PSA density and prostate cancer detection. J Med Assoc Thai. 2012;95:661–6. [PubMed] [Google Scholar]
- 13.Kosaka T, Mizuno R, Shinojima T, Miyajima A, Kikuchi E, et al. The implications of prostate-specific antigen density to predict clinically significant prostate cancer in men ≤50 years. Am J Clin Exp Urol. 2014;2:332–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng XY, Xie LP, Wang YY, Ding W, Yang K, et al. The use of prostate specific antigen (PSA) density in detecting prostate cancer in Chinese men with PSA levels of 4-10 ng/mL. J Cancer Res Clin Oncol. 2008;134:1207–10. doi: 10.1007/s00432-008-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil SR, Pawar PW, Sawant AS, Patil AV, Narwade SS, et al. TRUS biopsy yield in Indian population: a retrospective analysis. J Clin Diagn Res. 2017;11:PC01–5. doi: 10.7860/JCDR/2017/25473.9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YR, Wei XH, Uhlman M, Lin XT, Wu SF, et al. PSA density improves the rate of prostate cancer detection in Chinese men with a PSA between 2.5-10.0 ng ml-1 and 10.1-20.0 ng ml-1: a multicenter study. Asian J Androl. 2015;17:503–7. doi: 10.4103/1008-682X.142129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teoh JY, Yuen SK, Tsu JH, Wong CK, Ho BS, et al. The performance characteristics of prostate-specific antigen and prostate-specific antigen density in Chinese men. Asian J Androl. 2017;19:113–6. doi: 10.4103/1008-682X.167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wang ZQ, Li M, Zhou MY, Yu YF, et al. Establishment of two new predictive models for prostate cancer to determine whether to require prostate biopsy when the PSA level is in the diagnostic gray zone (4-10 ng ml−1) Asian J Androl. 2020;22:213–6. doi: 10.4103/aja.aja_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai Y, Maeda H, Ishitoya S, Okubo K, Okada T, et al. Prospective evaluation of prostate specific antigen density and systematic biopsy for detecting prostate cancer in Japanese patients with normal rectal examinations and intermediate prostate specific antigen levels. J Urol. 1997;158:861–4. doi: 10.1097/00005392-199709000-00046. [DOI] [PubMed] [Google Scholar]
- 20.Lavery HJ, Droller MJ. Do Gleason patterns 3 and 4 prostate cancer represent separate disease states? J Urol. 2012;188:1667–75. doi: 10.1016/j.juro.2012.07.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


