Abstract
Huggins and Hodges demonstrated the therapeutic effect of gonadal testosterone deprivation in the 1940s and therefore firmly established the concept that prostate cancer is a highly androgen-dependent disease. Since that time, hormonal therapy has undergone iterative advancement, from the types of gonadal testosterone deprivation to modalities that block the generation of adrenal and other extragonadal androgens, to those that directly bind and inhibit the androgen receptor (AR). The clinical states of prostate cancer are the product of a superimposition of these therapies with nonmetastatic advanced prostate cancer, as well as frankly metastatic disease. Today’s standard of care for advanced prostate cancer includes gonadotropin-releasing hormone agonists (e.g., leuprolide), second-generation nonsteroidal AR antagonists (enzalutamide, apalutamide, and darolutamide) and the androgen biosynthesis inhibitor abiraterone. The purpose of this review is to provide an assessment of hormonal therapies for the various clinical states of prostate cancer. The advancement of today’s standard of care will require an accounting of an individual’s androgen physiology that also has recently recognized germline determinants of peripheral androgen metabolism, which include HSD3B1 inheritance.
Keywords: androgens, androgen deprivation therapy, prostate cancer, glucocorticoids, enzalutamide, abiraterone, steroids
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Hormonal therapies that block generation of androgens or directly inhibit the androgen receptor are major therapies for the treatment of advanced prostate cancer.
Indications for various hormonal therapies depend on whether advanced prostate cancer has PSA recurrence only or the presence of metastases based on conventional imaging.
A major therapeutic advance in recent years for metastatic castration-sensitive prostate cancer is the use of next-generation hormonal therapies in addition to medical or surgical castration for frontline therapy.
Mechanisms of resistance to hormonal therapies include various ways in which the tumor enables the regeneration of potent androgens or otherwise reinstates androgen receptor signaling.
Prostate cancer represents a major health problem for men in the United States with over 190 000 expected to be diagnosed in 2020 (1). Although the figure comprises only 10% of all incident cancer cases, prostate cancer is the second leading cause of cancer deaths in men after lung cancer. Five-year survival across stages is highly favorable at 98%, but that figure drops to 30% in patients with metastatic disease (2). The vast majority of men are diagnosed either with localized disease confined to the prostate (76%) or regional disease that has spread to regional lymph nodes (13%) with only 6% of cases having distant metastases (2). Metastatic prostate cancer is not only incurable but also associated with significant morbidity and poor quality of life (3,4). Tumors arise from genomic alterations in epithelial cells of the prostate gland (majority are adenocarcinomas) and present in a multifocal and heterogeneous pattern within the organ. The natural history of the disease is typically long with tumor growth being indolent for decades in many patients.
The prostate gland is dependent on androgens for growth and development with gonadal androgen-deprivation therapy (ADT) serving as the cornerstone of treatment. Standard of care for localized prostate cancer includes surgery, radiation, and active surveillance. Patients with metastatic disease are candidates for medical castration with front-line ADT agents such as long-acting gonadotropin-releasing hormone (GnRH) agonists (goserelin, histrelin, leuprolide, and triptorelin) or GnRH antagonists (degarelix). Patients who undergo ADT achieve castrate levels of circulating testosterone, reduction in tumor burden, improved survival and palliation of symptoms. The majority of patients eventually progress on ADT with progression characterized by rising serum prostate-specific antigen (PSA) levels, incidence of new metastases (most commonly to bone and lymph nodes) or clinical progression (ie, worsening symptoms) despite attaining castrate levels of serum testosterone (<50 ng/dL) (5).
Various clinical states have been described to explain disease biology, inform treatment strategies, and ultimately reflect patient profiles seen in clinical practice (5,6). These include attainment of castration levels of serum testosterone following ADT (castrate vs noncastrate), presence of distant metastases as detected by conventional imaging techniques (metastatic vs nonmetastatic), primary tumor status (localized, locally advanced, or metastatic) and pre- vs post-chemotherapy treatment. Integration of these various schema culminated in the clinical phenotype referred to as castration-resistant prostate cancer (CRPC; previously but inaccurately referred to as “androgen-independent” and “hormone-resistant”), which is responsible for the majority of deaths from prostate cancer and has thus become the focus of intense drug discovery efforts. Early studies, including with murine models, incorrectly assumed that prostate cancer cells achieved androgen independence following surgical castration and did not account for extra-gonadal (ie, adrenal) production of androgen precursors. Subsequent studies identified the presence of intratumoral potent androgens as a driver of tumor growth (7,8). In recent years, there has been a shift away from these traditional clinical states to a more “dynamic” classification system with greater attention toward clinical phenotypes other than metastatic CRPC (9).
Nonmetastatic CRPC as an evolving clinical state
Interest has grown in the treatment of nonmetastatic castration-resistant prostate (nmCRPC), a clinical state defined by rising PSA as evidence of progression despite castrate levels of testosterone and lack of radiologic evidence of metastases upon standard-of-care conventional imaging. Estimates place annual prevalence of nmCRPC at over 100 000 (10). It was not until 2018 that next-generation AR antagonists were approved for this indication; prior to this time, median bone-related metastasis-free survival (MFS) was 25 to 30 months (11,12). Understanding of this clinical state may evolve as more sensitive imaging techniques such as molecularly-targeted positron emission tomography (PET) and whole-body diffusion-weighted magnetic resonance imaging become widely available in clinical practice and are able to detect metastases not previously detected by conventional imaging (13-15). Several studies of these advanced imaging modalities (eg, prostate-specific membrane antigen PET and 18F-fluorocholine PET/computed tomography) detect metastases not seen on conventional techniques (16,17).
Regardless, patients with nmCRPC are recognized as a discrete population with PSA doubling times used to enroll them in trials that continue to use standardized imaging techniques such as computed tomography, magnetic resonance imaging, and bone scans. Formal recognition of nmCRPC as a clinical state that could be amenable to therapeutic intervention led to consensus by the Oncologic Drugs Advisory Committee on the use of MFS as a valid clinical endpoint in clinical trials. This led to 2 pivotal phase 3 trials (PROSPER and SPARTAN) that demonstrated both delayed time to metastases and overall survival (OS) benefit in nmCRPC patients treated with next-generation androgen receptor (AR) antagonists (enzalutamide and apalutamide, respectively) in combination with ADT (18-20). A meta-analysis done by the ICECaP Working Group included over 33 000 patients from 43 clinical trials and found MFS to be a strong surrogate for OS in patients with high-risk localized prostate cancer (21). A retrospective analysis of 1207 high-risk nmCRPC patients enrolled in the SPARTAN trial also found a positive correlation between MFS and OS [R = 0.69; hazard ratio (HR) 0.69; 95% confidence interval (CI) 0.69-0.70; P < 0.0001] (22).
Metastatic castration-sensitive prostate cancer
Several established agents (docetaxel, abiraterone, apalutamide, and enzalutamide) have either received approval for or are currently being tested in metastatic castration sensitive prostate cancer (mCSPC; known historically as “androgen dependent” or “hormone-naïve”). This clinical state encompasses men with radiologically confirmed metastases who have not previously received ADT. Incidence of mCSPC has increased significantly in the past 2 decades even as overall incidence of prostate cancer has declined with a dismal 5-year survival of 30% (23,24). In the past 5 years, a series of phase 3 clinical trials accompanied by US Food and Drug Administration (FDA) approvals have redefined the standard of care for mCSPC to include docetaxel, abiraterone + prednisone, or the next-generation AR antagonists apalutamide or enzalutamide in addition to the established “backbone” of medical or surgical ADT. Key trials that established efficacy of these various agents in combination with traditional ADT include STAMPEDE, GETUG-AFU 15, and CHAARTED (docetaxel) (25-27), LATITUDE and STAMPEDE (abiraterone) (25,28), ARCHES and ENZAMET (enzalutamide) (29,30), and TITAN (apalutamide) (31). Several ongoing phase 3 trials are testing novel combinations across therapeutic classes (eg, ADT + chemotherapy + next-generation AR antagonists) and treatment modalities (eg, radiation therapy) (32). The key question for clinicians will be how to select the most appropriate combination for a particular mCSPC patient, especially given lack of head-to-head studies or predictive biomarkers. Several patient- and tumor-associated variables have been proposed to help guide therapy, including tumor volume, toxicity profile, cost, and treatment duration (32,33).
Androgen Physiology
HPG axis
In both males and females, sex hormone signaling is regulated by the hypothalamic-pituitary-gonadal axis. This system of regulation was proposed in pioneering work by Harris (34,35); years later, Schally and Guillemin shared the Nobel Prize (36) after independently isolating the responsible substance secreted from the hypothalamus of the brain (37,38). In the hypothalamus, GnRH is secreted by GnRH neurons into circulation in the hypophysial portal where it reaches the anterior pituitary and binds to GnRH receptors. This GnRH secretion occurs in a pulsatile manner (~1 pulse/h) and begins in puberty. After activation of GnRH receptors, the anterior pituitary secretes 2 other key hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In males, LH acts in the testes and stimulates Leydig cells to produce testosterone, the primary male sex hormone. Testosterone negatively feeds back on the hypothalamic-pituitary-gonadal axis, inhibiting GnRH secretion to further regulate hormone levels (36,39,40)
The androgen receptor
Androgens regulate many crucial aspects of male development and reproductive functions via action of the AR (41). Transcription of AR-regulated genes is induced after AR binding by testosterone and especially by the most potent androgen, 5α-dihydrotestosterone (DHT), which is produced in peripheral tissues (including the prostate) from testosterone by activity of the enzyme 5α-reductase (42,43). Recognition that prostate cancer is driven by androgen action came nearly 80 years ago with the finding by Huggins and Hodges that medical or surgical castration causes regression of prostate tumors (44). Normal prostate development is driven by androgens. However, AR signaling is altered in prostate cancer such that androgen signaling in prostate cancer promotes tumor proliferation and progression (8).
Androgen-responsive genes in CRPC
Studies in the early 2000s identified numerous genes with expression that was suppressed during the tumor regression phase of ADT and then returned to prior or greater expression levels following development of tumor resistance. Although this expression pattern alone is not in and of itself proof of the restoration of androgen signaling, the fact that well-known AR target genes such as KLK2 and KLK3 were present in this pattern pointed in the direction of restored androgen signaling. Cell line experiments further supported the link with androgen signaling for a subset of the differentially expressed genes (45,46).
In an important finding for the prostate cancer field, chromosomal rearrangements resulting in fusions between the androgen-regulated gene TMPRSS2 and the ETS transcription factors ERG and ETV1 were found to be strikingly common in patient tumor samples (47). It was initially not known whether expression of these fusions, common early events in prostate tumor development, was restored in CRPC. Findings from xenograft studies and analysis of patient samples showing ERG expression in CRPC provided additional evidence for the continued importance of androgen signaling in what was once thought to be an androgen-independent disease (48,49). Prostate-specific antigen, a protein encoded by the androgen-regulated KLK3 gene, is a well-known biomarker for prostate cancer. Serum PSA screening for early detection of prostate cancer has been a common clinical practice since the 1990s (50). The recognition that not only does the initiation of ADT lead to declines in serum PSA levels but also that subsequent rebounds of PSA levels are an indication of treatment failure is another sign of the frequently androgen-dependent nature of CRPC (51).
Extragonadal precursor steroids in CRPC
The increasingly strong evidence of an important role for androgen signaling in the progression of CRPC raised the question of how this androgen signaling is mediated. That this progression happens despite continuing suppression of circulating testosterone during ADT led researchers to look for other potential sources of androgens. Multiple studies have demonstrated several key points (52-54). Namely, xenograft studies as well as studies of patient primary tumors and CRPC metastases demonstrated that CRPC tumors have elevated potent androgen levels sufficient to maintain AR signaling. Moreover, expression of steroidogenic enzymes responsible for the production of potent androgens appears to be upregulated in CRPC tumors (55,56).
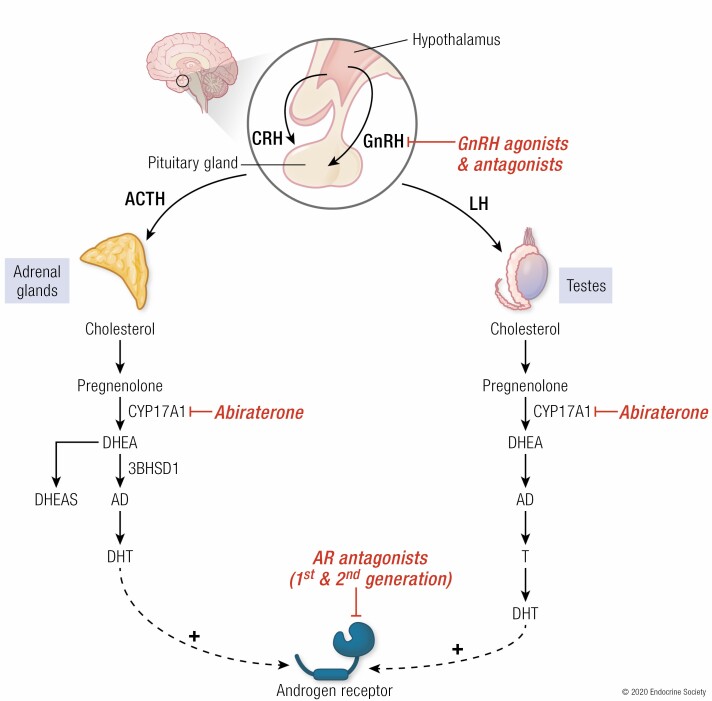
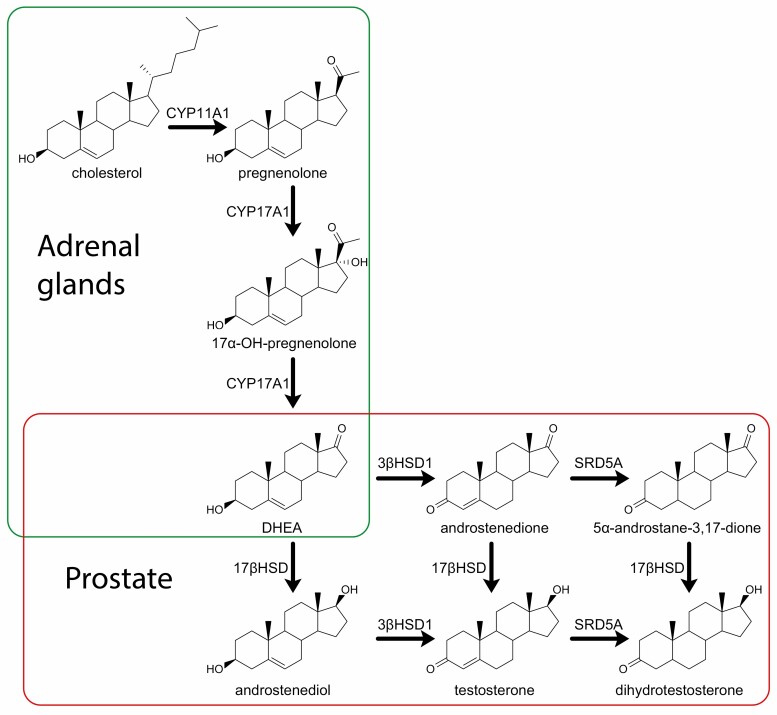
The gonads are not the only source of circulating androgens (Fig. 1). Dehydroepiandrosterone (DHEA) is an androgen precursor produced in the adrenal glands that is one of the most abundant circulating steroids; its sulfated form DHEA-S is the most abundant steroid in circulation (57). Adrenal production of DHEA from cholesterol requires the enzyme CYP17A1. Conversion in peripheral tissues such as the prostate of DHEA to DHT requires 3 enzymes: 3β-hydroxysteroid dehydrogenase (3βHSD1), 17β-hydroxysteroid dehydrogenase, and 5α-reductase (SRD5A) (58,59). DHEA remains abundant in the circulation of men undergoing ADT. Demonstration of the activity of these 3 enzymes in CRPC cell lines and therefore of conversion from DHEA to the most potent androgen DHT showed the potential for intratumoral androgen synthesis from adrenal precursors, indicating a mechanism by which prostate cancer could continue to be androgen-driven despite ADT (60,61).
Figure 1.
Pathways and enzymes to production of DHT. Steroid metabolism pathways and key enzymes for production of DHEA in the adrenal glands and conversion of DHEA to the potent androgen DHT in the prostate. Note that there are numerous isoforms of 17β-hydroxysteroid dehydrogenase; types 3 and 5 in particular are thought to be the important enzymes with reductive preference in prostate cancer (62).
Evolution of Treatment
A fundamental understanding of androgen physiology led to pioneering efforts by Charles Huggins and Clarence Hodges in the 1940s (44,63) toward defining ADT. They found that levels of an acid phosphatase [now known as prostatic acid phosphatase (PAP)] overexpressed in prostate cancer dropped sharply in 8 patients who underwent surgical castration (ie, bilateral orchiectomy) and 5 patients who underwent chemical castration (ie, estrogen injections). Conversely, testosterone administration increased PAP levels in these same men to above precastration levels. Importantly, these early experiments made the link between suppression of hormone production and reduction in a biomarker (PAP) associated with disease activity. In effect, their experiments demonstrated the androgen-dependent nature of prostate cancer. Huggins and Hodges went on to win the Nobel Prize in Physiology in 1966 and their work laid the foundation for novel therapies over the coming decades.
Subsequent therapeutic strategies focused on dysregulation of the hypothalamic-pituitary-testicular axis to shut down testosterone production. These included synthetic versions of estrogen such as diethylstilbestrol, which suppressed serum testosterone levels by augmenting negative feedback on the hypothalamus, thereby reducing release of LH (64,65). Use of estrogens however was limited due to significant side effects such as gynecomastia, sexual dysfunction, and venous thromboembolic and cardiovascular events (66,67). The 1960s and 1970s saw development of both steroidal (megestrol acetate, cyproterone acetate, medroxyprogesterone) and nonsteroidal antiandrogen therapies such as first-generation AR antagonists (bicalutamide, flutamide, and nilutamide). Cyproterone acetate is a steroidal antiandrogen that works by directly inhibiting the AR (68). While it has a cleaner side effect profile than estrogen therapy, chronic use is associated with liver toxicity and venous thromboembolism (69,70).
First-generation selective AR antagonists such as bicalutamide work by inhibiting binding of testosterone and DHT to the AR and are often given in combination with GnRH agonists in metastatic disease to reduce effects of the initial clinical flare (ie, bone pain, spinal cord compression, and obstructive uropathy) (71,72) and were thought to provide maximum androgen blockade. Efficacy of these agents has been limited by significantly lower binding affinities to AR (eg, 30-fold weaker than DHT) compared to next-generation AR antagonists (73-75). Survival benefit atop conventional ADT is limited; an extensive meta-analysis of 27 trials and 8275 patients that examined outcomes of maximum androgen blockade (with nilutamide, flutamide or cyproterone acetate) vs androgen suppression with orchiectomy or GnRH agonists found a modest 5-year incremental survival benefit of 2% to 3% with the prior (76).
It was proposed in the 1990s that SRD5A inhibitors (finasteride and dutasteride) that reduce conversion of testosterone to DHT could prevent the development of prostate cancer. To that end, 2 multiyear, placebo-controlled, randomized prevention trials (PCPT and REDUCE) were conducted in the early 2000s (77,78). Both trials showed a >20% risk reduction of prostate cancer incidence over several years but an increased risk of high-grade tumors. A subsequent analysis of data from these trials by the FDA concluded that dutasteride and finasteride increased the incidence of high-grade tumors by a modest 0.5% and 0.7%, respectively (79). However, hormonal interventions are also known to perturb prostate cancer pathologic grading. Nevertheless, analyses of these trials precluded approval of these agents in prevention of prostate cancer and led to warnings being placed in their labels.
GnRH agonists such as histrelin, goserelin, leuprolide, and triptorelin were approved in the 1980s and continue to be the mainstay of ADT today. These long-acting agents work by activating GnRH receptors, which leads to an initial “flare” of increased FSH and LH and, in turn, testosterone production, which can be especially problematic in patients with metastatic disease who are at risk for an acute increase in pain and other potential adverse consequences of an increase in tumor volume (eg, spinal cord compression or urinary obstruction). The majority of patients do not experience clinically significant effects of the flare phenomenon with incidence of symptoms estimated at ~10% and possibly lower in more contemporary practice (72). For such patients, treatment guidelines recommend using an antiandrogen such as a first-generation AR antagonist for the initial few weeks following the start of GnRH agonist therapy (80,81). Continuous stimulation, as opposed to the physiologic pulsatile pattern of GnRH receptors, leads to their downregulation and subsequent desensitization of the pituitary gland to effects of GnRH (82). This is followed by suppression of FSH and LH production and a dramatic drop in gonadal testosterone production to castrate levels over 3 to 4 weeks (83). These agents may be associated with increased likelihood of adverse events compared to orchiectomy including cardiovascular events, fractures, and peripheral arterial disease (84,85).
GnRH antagonists offered an alternative route to testosterone suppression via direct inhibition of GnRH receptors found in the anterior pituitary gland. This mechanism of action avoided the initial surge of FSH and LH and thus the initial testosterone rise and clinical flare associated with GnRH agonists. These agents are analogs of GnRH that competitively inhibit GnRH receptors (86). However, clinical adoption of GnRH antagonists has historically been limited due to low solubility, poor potency, and adverse events. Abarelix, an injectable GnRH antagonist, showed no testosterone surge and shortened time to medical castration compared to GnRH agonists in multiple pivotal phase 3 trials but was withdrawn from the US market in 2006 due to safety concerns (87). Degarelix is another injectable GnRH antagonist that was approved by the FDA in 2009 on the basis of a phase 3 noninferiority trial that showed more rapid reduction in testosterone levels and improved PSA control compared to leuprolide (88,89). However, more patients who received degarelix (40%) reported reactions at the injection site compared to leuprolide (<1%; P < 0.001); the incidence of adverse events was otherwise similar between treatments.
A novel oral GnRH antagonist, relugolix, has recently been developed for treatment of advanced prostate cancer, including with the HERO trial (90). Several phase 1 and 2 trials have demonstrated that this drug rapidly and sustainably reduces (within 3 days and up to 24 weeks) levels of FSH, LH, testosterone, and PSA compared to both leuprolide and degarelix (91-94). The randomized, open-label phase 3 HERO trial compared once-daily relugolix (N = 622) to leuprolide acetate injections every 3 months for 48 weeks (N = 308) in men with advanced prostate cancer (90). The primary endpoint of sustained castration (ie, testosterone level <50 ng/dL) at 48 weeks of treatment was reached in the relugolix arm compared to leuprolide (96.7% vs 88.8% respectively; P < 0.001). Analysis of key secondary endpoints also showed relugolix to be superior, including testosterone suppression at day 4 (56.0% vs 0%; P < 0.001), PSA response at day 15 followed by confirmation at day 29 (79.4% vs 19.8%; P < 0.001), and mean FSH level at end of week 24 (1.72 vs 5.95 IU/L P < 0.001). An initial testosterone surge was not seen in the relugolix arm, with testosterone level returning to the normal range in more patients at 90 days after discontinuation of treatment (54% vs 3%; P = 0.002). Notably, patients in the relugolix arm had a lower cumulative incidence of a major adverse cardiovascular event at the end of the 48-week treatment course (2.8% vs 5.6%; HR 0.46, 95% CI, 0.24-0.88). Subgroup analysis showed that patients with a prior cardiovascular history experienced fewer major adverse cardiovascular events on relugolix than leuprolide (3.6% vs 17.8%). This finding supports prior studies that have demonstrated lower risk of cardiovascular events in men taking GnRH antagonists compared to those taking GnRH agonists (95,96). The FDA granted the drug priority review in June 2020 with approval granted in December 2020 for patients with advanced prostate cancer.
In the early 1980s, the imidazole antifungal agent ketoconazole was shown at high doses in in vitro and in vivo models to rapidly induce castrate levels of serum testosterone through inhibition of cytochrome P450 enzymes (including CYP17A1) required for both adrenal and gonadal steroidogenesis (97-99). Ketoconazole was used early on as adjunctive therapy to long-acting GnRH agonists to reduce the initial flare of disease and has limited clinical efficacy (with no improvement in OS) as second-line therapy for CRPC in the pre-, post-, and combination chemotherapy settings (100).
The role of chemotherapy has evolved considerably since the early 2000s when the first agents were approved for mCRPC. Until then, patients who had no response to ADT with GnRH agonists were treated with one or more secondary agents, including corticosteroids and ketoconazole. The first chemotherapy agent to receive FDA approval for mCRPC was mitoxantrone in 1996, but its adoption was limited because it did not improve OS despite palliating pain in ~30% of patients (101,102). Docetaxel was the first chemotherapy agent to provide incremental survival benefit in patients when compared to mitoxantrone and prednisone in 2 pivotal phase 3 trials (103,104) and was approved in 2004. Docetaxel became the preferred chemotherapy agent for mCRPC with a 3- to 4-month increase in OS and thus led to the conception of the pre- vs post-chemotherapy stratification of mCRPC patients. This framework informed clinical trial and approval strategies for novel therapies such as abiraterone and enzalutamide, both of which were initially approved for mCRPC in the post-chemotherapy setting (COU-AA-301 and AFFIRM trials, respectively) (105,106).
Current Treatment Strategies for Advanced Prostate Cancer
The current hormonal therapeutic armamentarium for advanced prostate cancer is informed by 2 broad approaches: inhibition of conversion of extragonadal precursor steroids to testosterone and DHT with abiraterone and direct blockade of the AR to prevent binding to its ligands, testosterone, and DHT with next-generation AR antagonists such as apalutamide, darolutamide, or enzalutamide (Table 1).
Table 1.
Therapeutic classes and agents approved for prostate cancer, October 2020
| Class | Target | Agent | Mechanism of action | Indication(s) | Notable side effects |
|---|---|---|---|---|---|
| Nonsteroidal androgen receptor antagonist (first generation) | Androgen receptor | Bicalutamide, flutamide, nilutamide | Competitively and reversibly inhibit binding of testosterone and DHT to ligand binding domain of androgen receptor | In combination with GnRH agonists in metastatic disease | Hot flashes, pain, infection, abdominal pain |
| Nonsteroidal androgen receptor antagonist (second generation) | Androgen receptor | Apalutamide, darolutamide, enzalutamide | Competitively and reversibly inhibit binding of testosterone and DHT to ligand binding domain of androgen receptor & downstream inhibition of AR translocation to nucleus from cytoplasm, recruitment of coactivators and binding to DNA |
CRPC,a mCSPC,b nmCRPC,c (in combination with ADTc) | Fatigue, hypertension, seizures (enzalutamide), arthralgia, nausea, hot flashes |
| Androgen biosynthesis inhibitor | Steroidal enzyme CYP17A1 (17 alpha-hydroxylase/C17,20 lyase) | Abiraterone | Abiraterone acetate (prodrug) converted in vivo to abiraterone which inhibits CYP17A1 expressed in adrenal, testicles and prostate tumor | mCRPC, mCSPC (in combination with prednisone and ADT) | Hypokalemia, hypertension, edema, adrenal insufficiency, hepatotoxicity |
| GnRH antagonists | GnRH receptor | Degarelix, relugolix | Competitively and reversibly inhibit GnRH receptors in pituitary gland which blocks release of FSH and LH | Advanced prostate cancer | Injection site reaction (degarelix), hot flashes, fatigue, weight gain, hepatotoxicity |
| GnRH agonists | GnRH receptor | Histrelin, goserelin, leuprolide, triptorelin | Continuous stimulation of GnRH receptor that leads to initial surge in FSH, LH, testosterone, and DHT followed by reductions | Advanced prostate cancer (including mCRPC) | General pain, hot flashes and sweating, gastrointestinal disorders |
Note that not all agents in a given class are approved for all indications of their class. Castration
a Resistant prostate cancer.
b Metastatic castration sensitive prostate cancer.
c Nonmetastatic castration resistant prostate cancer.
d Androgen deprivation therapy.
Inhibition of extragonadal precursor steroids: Abiraterone
Abiraterone acetate is an oral prodrug that is deacetylated to its active metabolite abiraterone which inhibits the steroidogenic CYP17A1 enzyme, a member of the cytochrome P450 family. Abiraterone is currently indicated in the United States for treatment of mCRPC and mCSPC in combination with prednisone. CYP17A1 acts primarily in the adrenal gland to catalyze 2 steps that convert precursor steroids to testosterone, DHT, and DHEA (107). Early phase 1 studies of abiraterone showed increased levels of ACTH (due to decreased production of cortisol from CYP17A1 inhibition) and thus led to mineralocorticoid excess in treated patients; this can lead to adverse events such as fluid retention, hypertension, and hypokalemia (108,109). Patients in subsequent trials received glucocorticoids (eg, oral prednisone) in combination with abiraterone to mitigate risk of such adverse events. Notably, abiraterone is a steroidal drug that shares structural similarity with DHEA and, as a result, is metabolized by various enzymes of the steroidogenic machinery such as 3βHSD1 (encoded by HSD3B1) and SRD5A. This process leads to downstream production of various metabolites that can act as both AR antagonists (∆ 4-abiraterone) and partial AR agonists (3-keto-5α-abiraterone) (110,111). The missense-encoding HSD3B1(1245C) variant has been shown to modulate production of 3-keto-5α-abiraterone, with metabolite concentration increasing with increasing number of HSD3B1(1245C) alleles inherited (0, 1, or 2) (112).
Clinical use of abiraterone has expanded since initial approval as successive trials demonstrated efficacy and safety for various indications, initially in the mCRPC post-chemotherapy (ie, docetaxel) setting followed by the mCRPC pre-chemotherapy setting and ultimately in combination with upfront ADT. COU-AA-301 was published in 2011 and served as the pivotal trial for initial approval of abiraterone in the United States (105). Men whose disease had not responded to docetaxel were randomly assigned (N = 1195) on a 2:1 basis to abiraterone + prednisone (N = 797) or placebo + prednisone (N = 398) treatment, respectively. Overall survival was superior in the abiraterone + prednisone arm compared to the placebo + prednisone arm (14.8 months vs 10.9 months, respectively; HR 0.65; 95% CI 0.54-0.77; P < 0.0001) with an associated 35% absolute risk reduction of death. The study was unblinded at interim analysis given this favorable difference in survival. Secondary endpoints in the abiraterone arm were also superior, including progression-free survival (PFS) and PSA response rate (P < 0.001 for all secondary endpoints). Perhaps not unexpectedly, patients in the abiraterone + prednisone arm experienced a higher incidence of fluid retention, hypertension, and hypokalemia compared to the placebo + prednisone arm but most events were low-grade in severity.
Following demonstration of efficacy in the post-chemotherapy setting, abiraterone’s label was further expanded to include chemotherapy-naïve mCRPC patients based on results of the pivotal phase 3 COU-AA-302 trial (113). In this trial, 1088 patients with mCRPC who had not previously received chemotherapy were randomly allocated to receive abiraterone + prednisone (N = 546) or placebo + prednisone (N = 542). This trial was similarly unblinded at interim analysis given favorable efficacy, including a strong trend toward OS benefit. Abiraterone + prednisone treatment had superior efficacy as evaluated by median PFS (16.5 vs 8.4 months; HR 0.53; 95% CI 0.45-0.62; P < 0.001) and median OS (not reached vs 27.2 months; HR 0.75; 95% CI 0.61-0.93; P = 0.01) with median follow-up of 22.2 months. Various quality-of-life metrics were also improved in the treatment arm (eg, onset of pain, time to opioid initiation, performance status, etc). The toxicity profile was in line with COU-AA-301 despite a follow-up period that was nearly twice as long (22.2 vs 12.8 months). An extended analysis at median follow-up of 49.2 months showed median OS at 34.7 months compared to 30.3 months in the placebo arm (HR 0.81; 95% CI 0.70-0.93; P = 0.0033) (114). Notably, the treatment arm had a higher rate of grade 3 to 5 adverse events, including cardiac disorders (8% vs 4%) and elevated liver function tests (8% vs <3%). The incidence of grade 3 to 5 mineralocorticoid-excess-related symptoms were not notably higher in the treatment arm.
Subsequent trials established the role of abiraterone in the upfront treatment of mCSPC in combination with ADT (ie, LATITUDE and STAMPEDE) (25,28). LATITUDE was a multi-institutional, randomized, double-blinded, placebo-controlled phase 3 trial that allocated 1199 newly diagnosed mCSPC patients to receive either ADT + abiraterone + prednisone (N = 597) or ADT + dual placebos (N = 602) (28). Like prior trials, LATITUDE was also stopped at interim analysis after a median follow-up period of 30.4 months due to a favorable survival benefit with ADT + abiraterone + prednisone treatment. Median OS was not reached in the treatment arm compared to 34.7 months in the ADT + dual placebo arm (HR of death 0.2; 95% CI 0.51-0.76; P < 0.001). Median PFS was superior in the treatment arm, at 33.0 months vs 14.8 months (HR of progression or death 0.47, 95% CI 0.39-0.55; P < 0.001) as were several secondary end points. Rates of mineralocorticoid-related adverse events (hypertension and hypokalemia) were higher than in prior trials; the authors attribute these to the lower prednisone dose used in this trial (5 mg vs 10 mg), longer treatment duration with abiraterone and stricter criteria used to define hypertension. Ultimately, these adverse events were medically managed and rarely led to discontinuation of therapy.
The STAMPEDE trial is a multi-institutional phase 2 and 3 trial that adopted a unique multiarm, multistage platform design to enroll men with newly diagnosed high-risk locally advanced or metastatic prostate cancer who were initiating long-term ADT for the first time (25). A total of 1917 patients were allocated on a randomized 1:1 basis to receive either ADT (N = 957) or ADT + abiraterone + prednisone (N = 960) as first-line therapy. The multiarm, multistage design allowed investigators to administer various treatments to participants following progression in either arm. An intermediate analysis used failure-free survival as a criterion to decide whether randomization to these arms should continue. There were fewer deaths in the combination arm (184 vs 262; HR 0.63; 95% CI 0.52-0.76; P < 0.001) as well as fewer treatment failures (248 vs 535; HR 0.29; 95% CI 0.25-0.34; P < 0.001). Survival was more favorable in the combination arm with 83% alive at 3 years compared to 76% in the ADT monotherapy arm (HR 0.63; 95% CI 0.52-0.76; P < 0.001). Benefit in terms of longer failure-free survival was more pronounced in the combination arm at 75% after 3 years vs 45% in the ADT monotherapy arm (HR for treatment failure 0.29; 95% CI 0.25-0.34; P < 0.001). More men in the ADT monotherapy were treated with second-line therapies compared to combination therapy [477 of 957 (50%) vs 196 of 960 (20%), respectively]. The incidence of grade 3 to 5 adverse events was higher in the combination arm (47% vs 33%) with 9 of 15 grade 5 events occurring in the combination arm. Notably, fewer patients (12%) in the combination arm reported symptomatic skeletal events over 3 years compared to the ADT monotherapy arm (22%; HR for symptomatic skeletal events 0.46; 95% CI 0.37-0.58; P < 0.001). Overall, STAMPEDE confirmed a role for abiraterone as first-line therapy in combination with ADT in men with locally advanced or metastatic prostate cancer.
Androgen receptor blockade
The second broad strategy for treatment of advanced prostate cancer is blockade of the ligand-binding domain of the AR to prevent binding of testosterone and DHT. The AR binding affinity of first-generation AR antagonists such as bicalutamide, flutamide, and nilutamide is relatively modest (76). In the past decade, several next-generation AR antagonists have been approved (enzalutamide, apalutamide, daralutamide) for increasingly expansive clinical indications, evolving from treatment in the post-chemotherapy setting to chemotherapy-naïve to the nmCRPC and mCSPC settings.
Enzalutamide
Enzalutamide is a potent inhibitor of the AR with a 5- to 8-fold greater binding affinity than bicalutamide (115,116). The drug also inhibits AR translocation to the nucleus and binding to DNA and coactivators. Current FDA-approved indications for enzalutamide include treatment of CRPC and mCSPC, either as first- or second-line therapy depending on the clinical scenario. Enzalutamide was initially approved in the postdocetaxel setting based on the pivotal AFFRIM trial (106). This was a randomized, placebo-controlled, double-blinded phase 3 trial that allocated 1199 men with post-chemotherapy CRPC on a 2:1 basis to enzalutamide or placebo. The trial was unblinded after a planned interim analysis (upon 520 deaths) showed favorable OS benefit in the treatment arm of 18.4 months vs 13.6 months in the placebo arm (HR 0.63; 95% CI 0.53-0.75; P < 0.001). Superior efficacy of enzalutamide was also noted on a statistically significant basis for all secondary endpoints including PFS, PSA reduction >50%, quality of life, time to PSA progression, and time to first skeletal-related event. Men in the treatment arm reported higher rates of diarrhea, hot flashes, fatigue, hypertension, and a small percentage of men who received enzalutamide (0.6%) experienced seizures.
AFFRIM laid the foundation for the next pivotal trial, PREVAIL, which demonstrated superior efficacy of enzalutamide compared to placebo in patients with CRPC who had not previously received chemotherapy (117). This global, multi-institutional, double-blind phase 3 trial randomly allocated 1717 patients to either enzalutamide (N = 872) or matched placebo (N = 845). Similar to AFFIRM, this trial was stopped after interim analysis, which showed markedly improved PFS and OS with enzalutamide. Progression-free survival at 12 months was 65% in the treatment arm compared to 14% in the placebo arm, with an associated risk reduction of 81% (HR 0.19; 95% CI 0.15-0.23; P < 0.001). At a median follow-up of 22 months, OS was 72% in the enzalutamide arm compared to 65% in the placebo arm, which was a 29% reduction in risk of death (HR 0.71; 95% CI 0.60-0.84; P < 0.001). Investigators estimated median OS to be 32.4 months with enzalutamide vs 30.2 months for placebo. All secondary endpoints were improved with enzalutamide treatment including time to initiation of chemotherapy (HR 0.35) and time to first serious reportable event (HR 0.72). Surprisingly, hypertension was a frequent adverse effect of enzalutamide, with a mechanism that was unknown. Subsequently, it was found that potent AR antagonists can induce loss of the enzyme 11β-HSD2, which appears to biochemically phenocopy loss-of-function germline mutations in 11β-HSD2 and result in hypertension (118).
Following the clinical success of enzalutamide in the docetaxel-naïve setting, investigators completed the PROSPER trial to demonstrate its efficacy in delaying metastases in patients with nmCRPC (119). This randomized, double-blinded phase 3 trial recruited 1401 men with nmCRPC and a PSA doubling time of ≤10 months (median 3.7 months) who were continued on ADT; subjects received either enzalutamide (N = 933) or placebo (N = 468). Metastasis-free survival was the primary clinical endpoint. Patients in the enzalutamide arm had a significantly higher median MFS of 36.6 months compared to 14.6 months in the placebo arm (HR for metastasis or death 0.29; 95% CI 0.24-0.35; P < 0.001) with an associated 71% reduction in risk of metastasis or death. Use of enzalutamide also prolonged time to first use of antineoplastic agents (39.6 vs 17.7 months; HR 0.21; P < 0.001). Death from any cause was lower in the treatment arm compared to placebo (103 out of 933 patients [11%] vs 62 out of 468 [13%] respectively) with no difference in median OS.
With the success of two subsequent phase 3 trials, ENZAMET and ARCHES (29,30), the number of clinical indications for enzalutamide further expanded to include mCSPC. ENZAMET was a large, open-label phase 3 trial that randomized 1125 men with mCSPC to ADT in combination with either enzalutamide (N = 563) or a standard nonsteroidal antiandrogen agent (bicalutamide, flutamide, or nilutamide; N = 562) until progression or unacceptable toxicity. At a median follow-up period of 34 months, the enzalutamide arm had fewer deaths than the standard-care group (102 vs 143; HR 0.67; 95% CI 0.52-0.86; P = 0.002) and 3-year OS estimated at 80% (based on 94 events) vs 72% (based on 130 events), respectively. Secondary endpoints were superior with enzalutamide treatment, including PSA PFS (P < 0.001) and clinical PFS (P < 0.001). However, the incidence of certain adverse events was higher in the enzalutamide arm, including fatigue (6% vs 1%) and seizures (1% vs 0%). Seizures are a previously documented side effect of enzalutamide; 6 of 7 patients who experienced them while on enzalutamide in this trial discontinued the drug, and the remaining patients had already progressed prior to seizure onset. Clinicians will have to assess the risk-benefit calculus for each patient prior to initiating enzalutamide as first-line therapy in combination with standard-of-care ADT.
ARCHES was a large, multicenter, double-blind phase 3 trial that randomly allocated 1150 men with mCSPC to ADT + enzalutamide (N = 574) or ADT + placebo (N = 576) (30). The ADT + enzalutamide arm achieved a superior primary endpoint of radiographic PFS (not reached vs 19.0 months; HR 0.39; 95% CI 0.30-0.50; P < 0.001) as well as key secondary endpoints such as median time to PSA progression (P < 0.001), objective response rate (P < 0.001), and median time to starting antineoplastic therapy (P < 0.001). Overall survival data was not mature at time of publication in 2019 but was planned for analysis upon occurrence of 342 deaths. Reduction in risk of radiographic progression or death was statistically significant within the prespecified subgroups, including those who did or did not receive prior docetaxel and those with high- vs low-volume metastatic burden. The toxicity profile was broadly similar in both arms with no new adverse events or seizures observed with enzalutamide treatment.
Apalutamide
Apalutamide is a selective, nonsteroidal, competitive AR inhibitor that was the result of a discovery effort using structure-activity relationship chemistry to find molecules with full AR antagonist activity (120). This small-molecule drug binds to the AR ligand-binding domain, inhibiting AR translocation into the nucleus, DNA binding, and downstream transcription of AR-related genes. Currently approved indications in the United States include mCSPC and nmCRPC. Initial clinical proof-of-concept phase 1 studies were done in mCRPC to define optimal dosing (121). A subsequent phase 2 trial was done in nmCRPC patients with PSA ≥8 ng/mL or PSA doubling time ≤10 months; results showed a sustained reduction in PSA, median MFS not reached and an acceptable toxicity profile (122). Success of this phase 2 trial informed the rationale and design of the phase 3 pivotal SPARTAN trial in nmCRPC patients (123). This randomized, double-blind trial enrolled 1207 men with nmCRPC and a PSA doubling time <10 months into an apalutamide treatment arm (N = 806) or placebo arm (N = 401). A preplanned primary analysis completed upon occurrence of 378 events showed a significantly longer (>2 years) median MFS of 40.5 months with apalutamide compared to 16.2 months with placebo (HR for death or metastasis 0.28; 95% CI 0.23-0.35; P < 0.001). This equated to a 70% lower risk of death or metastasis with apalutamide treatment as well as statistically significant improvement in all secondary endpoints, including PFS, time to metastasis, and time to symptomatic progression (P < 0.001). The majority of adverse events that were more common in the apalutamide arm were mild with no new toxicities identified compared to prior trials.
The SPARTAN trial was followed by publication of TITAN, a phase 3 trial that demonstrated efficacy of apalutamide when added to ADT for treatment of mCSPC (31). This was a multicenter, double-blind, placebo-controlled trial that allocated 1052 patients with confirmed metastasis and not receiving ADT at time of progression to ADT + apalutamide (N = 525) or ADT + placebo (N = 527). Most patients (62.7%) had high-volume disease, 16.4% had undergone prior prostatectomy or radiotherapy and 10.7% had previously received docetaxel. With a median follow-up of 22.7 months, PFS at 24 months was 68.2% in the ADT + apalutamide arm compared to 47.5% in the ADT + placebo arm (HR for death or progression 0.48; 95% CI 0.39-0.60; P < 0.001). Notably, OS at 24 months was more favorable in the combination treatment arm at 82.4% compared to 73.5% in the ADT + placebo arm (HR for death 0.67; 95% CI 0.51-0.89; P = 0.005). The benefit of reduced risk of progression or death was observed in various subgroups, including both low- and high-disease volume, prior docetaxel use and presence bone metastases at baseline. The investigators point out, however, that sample sizes were limited in the subgroup analyses.
Darolutamide
Darolutamide is a selective, nonsteroidal, competitive AR antagonist that also inhibits AR nuclear translocation and downstream transcription of target genes. Discovered as part of a broader drug screen, this compound potently binds to AR and has full antagonist activity against a rare AR mutation that may drive resistance to other AR antagonists (124). The drug is currently approved for treatment of nmCRPC in combination with ADT with an ongoing phase 3 trial in mCSPC patients (ARASENS; NCT02799602) (125). Early trials of darolutamide demonstrated clinical activity (reduced tumor volume and PSA levels) and acceptable toxicity pre- and post-chemotherapy in patients with mCRPC (126,127).
The pivotal phase 3 ARAMIS trial in patients with nmCRPC was the basis of the FDA’s Fast Track designation of the drug and its subsequent approval in July 2019 (128). This large, randomized, double-blind phase 3 trial recruited 1509 nmCRPC patients with a PSA doubling time <10 months and randomly assigned them to darolutamide treatment (N = 955) or placebo (N = 554) while being continued on ADT in both arms. Patients in the treatment arm had a median MFS of 40.4 months compared to 18.4 months in the placebo arm (HR for death or metastasis 0.41; 95% CI 0.34-0.50; P < 0.001). This conferred a 59% reduction in risk of death or metastasis for patients receiving darolutamide. Similarly, improved efficacy was observed for all secondary endpoints, including PFS (P < 0.001), OS (P = 0.045), time to pain progression (P < 0.001), time to cytotoxic chemotherapy (P < 0.001) and time to symptomatic skeletal event (P = 0.01). The drug was generally well tolerated with certain adverse events such as fatigue, falls, fractures, and seizures being less common in ARAMIS than in SPARTAN (apalutamide) and PROSPER (enzalutamide). The final analysis of outcomes in ARAMIS was presented at the American Society of Clinical Oncology Virtual Scientific Program in June 2020 and showed OS at 3 years at 83% in the treatment arm compared to 77% in the placebo arm with an associated reduction in death of 31% (HR 0.69; 95% CI 0.53-0.88; P = 0.003) (129). The improved efficacy observed for the secondary endpoints was sustained with darolutamide treatment over the extended follow-up period.
Mechanisms of Resistance
Androgen receptor alterations
One area that has received much attention as a potential mechanism of resistance in CRPC is alterations in the AR and its expression. Such alterations are rare in treatment-naïve prostate cancer but highly common in CRPC, suggesting they are selected for during treatment. It has been reported that roughly half of CRPC tumors have AR gene amplification (130,131). As well, amplification of AR enhancers leading to increased AR expression has been reported to occur even more frequently (132,133). Overexpression of AR via other mechanisms is also known; these mechanisms, which may include deregulation of microRNAs that regulate AR (134), deregulation of co-regulators or transcription factors (135), and loss of suppressors (136), are subjects of ongoing research.
Mutations in the AR gene are also common in CRPC and may lead to its activation by alternate ligands. The T878A point mutation first identified in the LNCaP cell line 3 decades ago and also found in patient samples allows activation of the AR by various other steroid hormones as well as certain antiandrogens (137). More recent work has identified various mutant ARs that are common in circulating cell-free DNA (138-142) and has shown that AR antagonists, including bicalutamide, hydroxyflutamide, enzalutamide, and apalutamide can become AR agonists in the presence of certain mutations (139). In a recent study of circulating tumor DNA from nearly 900 advanced prostate cancer patients, the most common AR mutation was L702H (25% of patients) followed by T878A (14%) and H875Y (11%) (143). Both AR gain and AR mutations have been associated with worse outcomes on enzalutamide and abiraterone (144).
Androgen receptor splice variants also have seen enormous research interest. Numerous such variants have been identified, most of which lack the ligand-binding domain and are therefore thought to be constitutively active (145). The splice variant that has received the most interest is AR-V7 (146,147). Detection of AR-V7 in circulating tumor cells has been associated with resistance to enzalutamide and abiraterone (148). AR-V7 expression is very rarely observed in primary, castrate-sensitive prostate cancer but is frequently detected in CRPC, in up to 75% of cases (149). Additionally, in a prospective multicenter study, AR-V7 in circulating tumor cells was shown to correlate with faster progression on enzalutamide and abiraterone (150). It has also been reported that AR-V7 positive patients respond better to taxane chemotherapy than to androgen-targeted treatments, with the reverse outcome for AR-V7 negative patients (151). From a mechanistic standpoint, the relative contributions of AR variants vs full-length AR to treatment resistance are a subject of ongoing work.
3βHSD1 germline variant
With the understanding that extragonadal precursor steroids have roles in driving CRPC progression comes the possibility that alterations in enzymes crucial for conversion from extragonadal precursors to potent androgens could be important. In any pathway from the abundant, adrenally produced precursors DHEA and DHEA-S toward production of the most potent androgen DHT, a critical and rate-limiting step is catalyzed by the enzyme 3βHSD1. A role for a missense-encoding germline variation in 3βHSD1 in CRPC was identified in work by Chang et al (60). Notably, 3βHSD1 is also necessary for potent androgen synthesis from de novo pathways originating from cholesterol.
The alternative form of 3βHSD1 is created by a germline single nucleotide polymorphisms (rs1047303) in the gene HSD3B1 that encodes the enzyme, converting A to C at position 1245 and thus exchanging an asparagine for a threonine at amino acid position 367. The HSD3B1(1245C) allele has frequencies that vary widely across different ethnic groups and is most common among people of European, particularly southern European ancestry. Frequencies of the C allele are ~0.4 in Italy and Spain (152). By contrast, African and East Asian populations have C allele frequencies below 0.1, and populations in other regions of the world fall between these extremes (152).
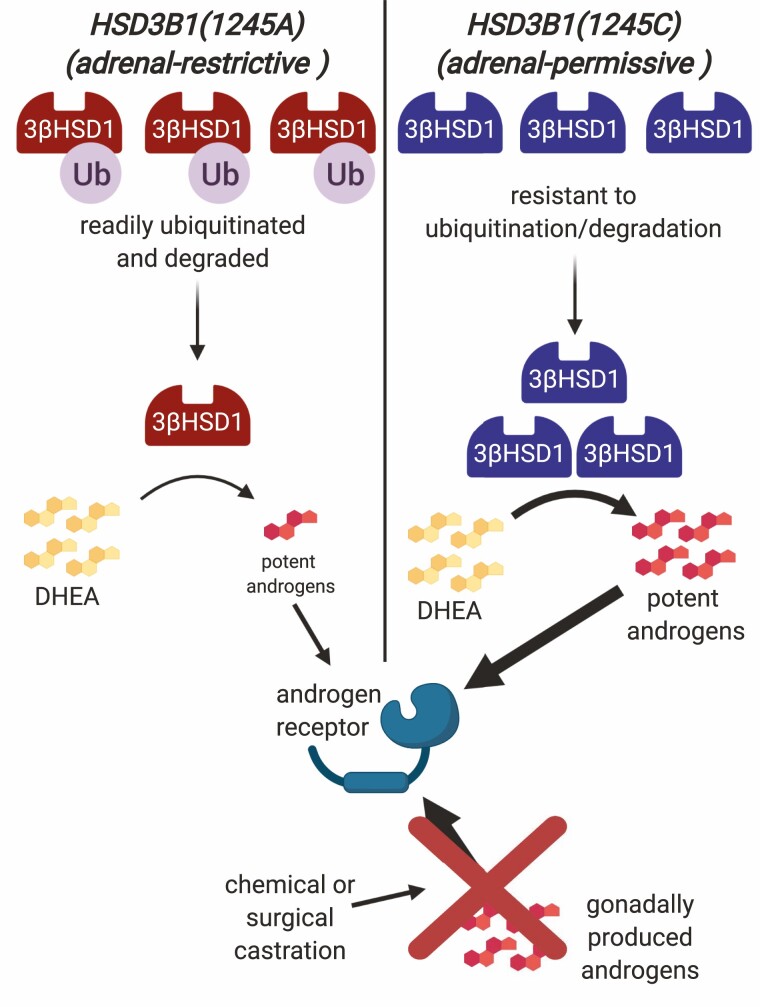
In their study published in 2013, Chang et al showed that prostate cancer cells with 3βHSD1(367T) encoded by HSD3B1(1245C) have far more rapid conversion of DHEA to Δ 4-androstenedione and thus greatly increased flux toward DHT compared to cells with 3βHSD1(367N) encoded by HSD3B1(1245A) (60). They further showed that the 2 forms of the enzyme have similar enzyme kinetics but that cells with 3βHSD1(367T) express much higher protein levels of 3βHSD1 than cells with 3βHSD1(367N). This difference in protein levels was explained by a differential tendency for ubiquitination and degradation. The 367T form of the enzyme was shown to have a protein half-life more than 10 times as long as that of the 367N form, and it was shown that 3βHSD1(367N) is readily polyubiquitinated whereas 3βHSD1(367T) is resistant to polyubiquitination (Fig. 2). It was further shown that autocrine mobility factor receptor, a membrane-anchored ubiquitin ligase acting through the endoplasmic reticulum-associated protein degradation pathway, preferentially binds to 3βHSD1(367N) compared to 3βHSD1(367T) and is necessary for ubiquitination and degradation of the enzyme. As a consequence, cells expressing the HSD3B1(1245C) allele and 367T form of the protein accumulate much higher levels of the 3βHSD1 enzyme and can produce much more of the potent androgen DHT from the pool of adrenally derived DHEA (60).
Figure 2.
Adrenal-permissive HSD3B1(1245C) allele as a mechanism of treatment resistance. Prostate tumor growth is driven by activation of the AR by potent androgens. Castration cuts off the supply of gonadally produced androgens. Adrenally produced DHEA can be converted in prostate cells to potent androgens to restore AR activation and tumor growth. A key step in the conversion of DHEA is mediated by enzyme 3βHSD1, which depending on HSD3B1 genotype occurs in either a form that is readily ubiquitinated and degraded (adrenal-restrictive) or that is resistant to ubiquitination and degradation (adrenal-permissive) and therefore accumulates at higher levels in cells. With the adrenal-permissive form, DHEA is much more rapidly converted to downstream potent androgens leading to stronger AR activation and faster tumor growth. Created with Biorender.com.
Because they either permit or restrict, respectively, the production of large quantities of potent androgens from adrenal precursors, the 2 versions of HSD3B1 have been termed adrenal-permissive (1245C) and adrenal-restrictive (1245A) alleles (153-155). The findings of Chang et al suggested a role for the adrenal-permissive allele in prostate cancer progression and the next step was to determine the clinical significance of its hypothesized role. That the adrenal-permissive allele plays a role in prostate cancer progression has now been validated in multiple cohorts. Hearn et al, in a multicohort study published in 2016, were the first to show that inheritance of the adrenal-permissive allele is associated with worse PFS and OS in prostate cancer patients undergoing ADT (156). These findings were later independently validated and expanded upon in several other studies (157-161). Consequently, HSD3B1(1245C) is a predictive biomarker predicting poorer outcomes for prostate cancer patients undergoing ADT. By contrast, in a cohort of patients undergoing treatment with the nonsteroidal CYP17A1 inhibitor ketoconazole, adrenal-permissive genotype was associated with longer time to treatment failure, suggesting a greater dependence on adrenal androgens in CRPC tumors with the HSD3B1(1245C) allele (162).
Two very recent studies have examined the relationship between HSD3B1 genotype and time to progression on next generation anti-androgen treatments abiraterone and enzalutamide (163,164). In both studies, patients homozygous for the adrenal-permissive 1245C allele had worse outcomes on either abiraterone or enzalutamide, highlighting the need for novel treatments targeting advanced prostate tumors harboring this variant (165).
Glucocorticoid-related mechanisms
The glucocorticoid receptor (GR) is another nuclear receptor that plays a critical role in regulating various aspects of human physiology, including metabolism, immune response, and response to stress (166). GR activation induces the expression of a set of genes that, although not identical, overlaps the set of genes induced by AR activation, and GR has emerged as another potential mechanism of resistance in CRPC (167).
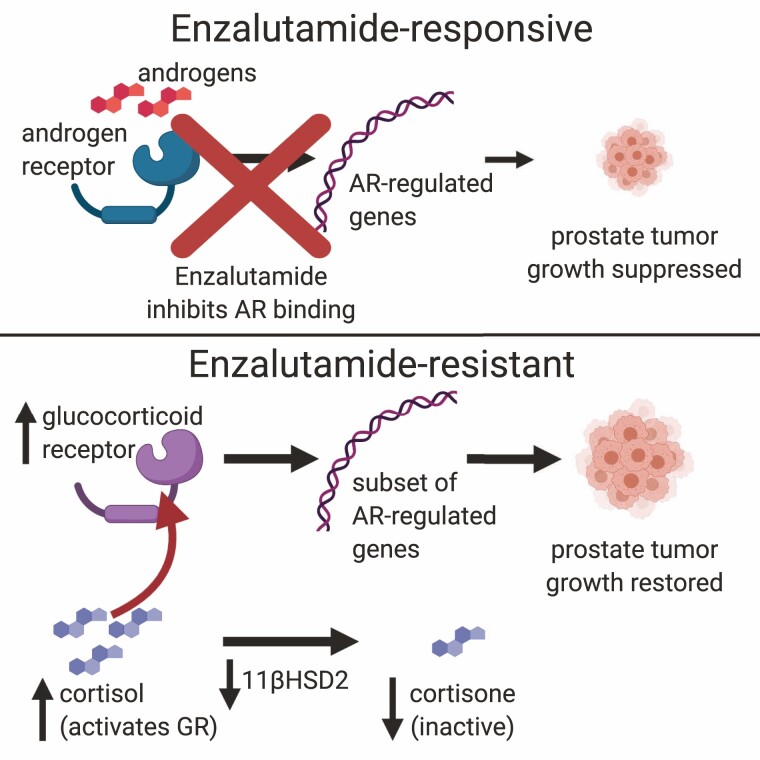
In a 2013 study of xenograft tumors in mice treated with enzalutamide or other antiandrogens, NR3C1, the gene encoding GR, was found to be one of the most highly upregulated genes in tumors that developed resistance. Knockdown of GR reversed enzalutamide resistance specifically in GR-expressing resistant tumors. The study further showed an association between increased GR expression and resistance to enzalutamide in patient bone metastases (168); other work around the same time also provided evidence for links between GR expression and enzalutamide resistance (169). More recent work has followed on this path by implicating alterations to glucocorticoid metabolism in GR-mediated enzalutamide resistance. GR is stimulated by cortisol and this stimulation is regulated by 11β-hydroxysteroid dehydrogenase-2 (11βHSD2), which converts cortisol to the inactive metabolite cortisone, thereby limiting GR stimulation. It was shown in xenograft models that exposure to enzalutamide decreased conversion of cortisol to cortisone in tumors. The underlying mechanism was shown to involve loss of 11βHSD2 protein mediated by the same endoplasmic reticulum-associated protein degradation pathway responsible for degradation of 3βHSD1. Overexpression of 11βHSD2 reversed the effects of enzalutamide resistance but had no effect on growth of untreated tumors (170). These results suggest the possibility that GR is another steroid metabolic pathway in which alterations could play important roles in mediating treatment resistance in CRPC (Fig. 3).
Figure 3.
Alterations in glucocorticoid receptor pathway as a mechanism of enzalutamide resistance. Enzalutamide inhibits binding of androgens to the AR, suppressing transcription of AR-regulated genes and therefore suppressing prostate tumor growth (top). The GR regulates an overlapping but not identical set of genes as AR, therefore activating a subset of the AR-regulated genes. Increased GR expression, along with downregulation of enzyme 11β-hydroxysteroid dehydrogenase-2 that converts GR agonist cortisol to the inactive metabolite cortisone, can lead to increased transcription of this subset of AR-regulated genes and restoration of tumor growth (bottom). Created with Biorender.com.
Future Directions
Tremendous progress has been made in elucidating the tumor biology of prostate cancer starting with the pioneering experiments of Huggins and Hodges in the 1940s. These insights led to drug discovery efforts that ultimately expanded the therapeutic armamentarium and dramatically improved survival, especially in men with metastatic disease. Despite this success, many unmet needs remain and will inform basic and translational research priorities in the coming decades. While the AR has been extensively exploited as a drug target, there is a need to characterize other novel targets which may improve survival benefit from existing therapies such as second-generation AR antagonists and abiraterone. The pipeline for advanced prostate cancer is robust with a diversity of targets and modalities, including steroidogenic enzymes (eg, 3βHSD1, CYP11A1, 11βHSD, etc), AR-related regulators and co-factors (eg, HSP90, GATA-2, etc), immunotherapy (eg, checkpoint inhibitors, vaccines, etc), radiopharmaceuticals (eg, Radium-223), and signaling pathways (eg, PI3K-AKT-mechanistic target of rapamycin).
In particular, the role of immunotherapy in prostate cancer remains to be defined with clinical trials to date demonstrating modest efficacy (171). Sipuleucel-T, a cancer vaccine that stimulates ex vivo antigen presenting cells, is the only approved immunotherapy agent in prostate cancer and has a demonstrated OS benefit. Efficacy of approved immunotherapy agents such as checkpoint inhibitors has been limited in prostate cancer for several reasons including low immunogenicity with low intratumoral T cell infiltration (ie, a “cold tumor”) (172), an immunosuppressive tumor microenvironment driven in particular by T regulatory cells and myeloid-derived suppressor cells (173,174) and immune evasion through various mechanisms (171). There are numerous ongoing clinical trials testing novel therapeutic approaches and combinations in metastatic prostate cancer with the aim of overcoming these limitations (175).The recent approval of PARP inhibitors (olaparib and rucaparib) for treatment of men with homologous recombination repair mutations marked a landmark event that demonstrated a “precision” approach to patient selection and treatment. The future treatment paradigm will be shaped by which emerging clinical states ultimately drive clinical decision-making. We are evolving beyond mCRPC with emerging clinical states such as mCSPC and nmCRPC providing greater flexibility to segment patients on basis of metastatic and castration status. Several therapeutic options such as second-generation nonsteroidal AR antagonists and abiraterone have been approved in these clinical states and allow us to “tailor” treatment based on patient status. Ultimately, there is reason to be optimistic in the field of prostate cancer as we translate growing knowledge of tumor biology into novel therapies.
Acknowledgments
Financial Support: This work was supported by grants from the National Cancer Institute (R01CA172382, R01CA190289, and R01CA236780) and the Prostate Cancer Foundation.
Additional Information
Disclosure Summary: Kunal Desai declares that he has no conflicts of interest that might be relevant to the contents of this manuscript. Jeffrey M. McManus declares that he has no conflicts of interest that might be relevant to the contents of this manuscript. Nima Sharifi has been a paid consultant for Pfizer and Celgene. Cleveland Clinic has patent applications on HSD3B1.
Data Availability
All data generated for this article are included in this published article.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2. SEER*Explorer: an interactive website for SEER cancer statistics. Surveillance Research Program. https://seer.cancer.gov. Accessed February 14, 2021.
- 3. Holm M, Doveson S, Lindqvist O, Wennman-Larsen A, Fransson P. Quality of life in men with metastatic prostate cancer in their final years before death - a retrospective analysis of prospective data. BMC Palliat Care. 2018;17(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Resnick MJ, Penson DF. Quality of life with advanced metastatic prostate cancer. Urol Clin North Am. 2012;39(4):505-515. [DOI] [PubMed] [Google Scholar]
- 5. Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000;55(3):323-327. [DOI] [PubMed] [Google Scholar]
- 7. Zhu Z, Chung YM, Sergeeva O, et al. Loss of dihydrotestosterone-inactivation activity promotes prostate cancer castration resistance detectable by functional imaging. J Biol Chem. 2018;293(46):17829-17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Auchus RJ, Sharifi N. Sex hormones and prostate cancer. Annu Rev Med. 2020;71:33-45. [DOI] [PubMed] [Google Scholar]
- 9. Scher HI, Morris MJ, Stadler WM, et al. ; Prostate Cancer Clinical Trials Working Group 3 . Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scher HI, Solo K, Valant J, Todd MB, Mehra M. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One. 2015;10(10):e0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crawford ED, Stone NN, Yu EY, et al. ; Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence (RADAR) Group . Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83(3):664-669. [DOI] [PubMed] [Google Scholar]
- 12. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. [DOI] [PubMed] [Google Scholar]
- 13. Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 6⁸Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668-674. [DOI] [PubMed] [Google Scholar]
- 14. Mosavi F, Johansson S, Sandberg DT, Turesson I, Sörensen J, Ahlström H. Whole-body diffusion-weighted MRI compared with (18)F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR Am J Roentgenol. 2012;199(5):1114-1120. [DOI] [PubMed] [Google Scholar]
- 15. Crawford ED, Koo PJ, Shore N, et al. ; RADAR III Group . A clinician’s guide to next generation imaging in patients with advanced prostate cancer (RADAR III). J Urol. 2019;201(4):682-692. [DOI] [PubMed] [Google Scholar]
- 16. Eiber M, Fendler WP, Rowe SP, et al. Prostate-specific membrane antigen ligands for imaging and therapy. J Nucl Med. 2017;58(Suppl 2):67s-76s. [DOI] [PubMed] [Google Scholar]
- 17. Beheshti M, Rezaee A, Geinitz H, Loidl W, Pirich C, Langsteger W. Evaluation of prostate cancer bone metastases with 18F-NaF and 18F-fluorocholine PET/CT. J Nucl Med. 2016;57(Suppl 3):55S-60S. [DOI] [PubMed] [Google Scholar]
- 18. Smith MR, Saad F, Chowdhury S, et al. ; SPARTAN Investigators . Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418. [DOI] [PubMed] [Google Scholar]
- 19. Sternberg CN, Fizazi K, Saad F, et al. ; PROSPER Investigators . Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. [DOI] [PubMed] [Google Scholar]
- 20. Small EJ, Saad F, Chowdhury S, et al. Final survival results from SPARTAN, a phase III study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(15_suppl):5516. [Google Scholar]
- 21. Xie W, Regan MM, Buyse M, et al. ; ICECaP Working Group . Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35(27):3097-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith MR, Mehra M, Nair S, Lawson J, Small EJ. Relationship between metastasis-free survival and overall survival in patients with nonmetastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18(2):e180-e189. [DOI] [PubMed] [Google Scholar]
- 23. Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013). Prostate Cancer Prostatic Dis. 2016;19(4):395-397. [DOI] [PubMed] [Google Scholar]
- 24. Dalela D, Sun M, Diaz M, et al. Contemporary trends in the incidence of metastatic prostate cancer among US men: results from nationwide analyses. Eur Urol Focus. 2019;5(1):77-80. [DOI] [PubMed] [Google Scholar]
- 25. James ND, de Bono JS, Spears MR, et al. ; STAMPEDE Investigators . Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158. [DOI] [PubMed] [Google Scholar]
- 27. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fizazi K, Tran N, Fein L, et al. ; LATITUDE Investigators . Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360. [DOI] [PubMed] [Google Scholar]
- 29. Davis ID, Martin AJ, Stockler MR, et al. ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chi KN, Agarwal N, Bjartell A, et al. ; TITAN Investigators . Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. [DOI] [PubMed] [Google Scholar]
- 32. Hahn AW, Higano CS, Taplin ME, Ryan CJ, Agarwal N. Metastatic castration-sensitive prostate cancer: optimizing patient selection and treatment. Am Soc Clin Oncol Educ Book. 2018;38:363-371. [DOI] [PubMed] [Google Scholar]
- 33. Kinsey EN, Zhang T, Armstrong AJ. Metastatic hormone-sensitive prostate cancer: a review of the current treatment landscape. Cancer J. 2020;26(1):64-75. [DOI] [PubMed] [Google Scholar]
- 34. Fink G. 60 years of neuroendocrinology: memoir: Harris’ neuroendocrine revolution: of portal vessels and self-priming. J Endocrinol. 2015;226(2):T13-T24. [DOI] [PubMed] [Google Scholar]
- 35. Harris G. Neural Control of the Pituitary Gland. London, UK: Edward Arnold; 1955. [Google Scholar]
- 36. Plant TM. 60 years of neuroendocrinology: the hypothalamo-pituitary-gonadal axis. J Endocrinol. 2015;226(2):T41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem Biophys Res Commun. 1971;44(1):205-210. [DOI] [PubMed] [Google Scholar]
- 38. Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43(6):1334-1339. [DOI] [PubMed] [Google Scholar]
- 39. Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism. 2018;86:3-17. [DOI] [PubMed] [Google Scholar]
- 40. Clavijo RI, Hsiao W. Update on male reproductive endocrinology. Transl Androl Urol. 2018;7(Suppl 3):S367-S372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsumoto T, Sakari M, Okada M, et al. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201-224. [DOI] [PubMed] [Google Scholar]
- 42. Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243(8):2012-2021. [PubMed] [Google Scholar]
- 43. Dai C, Chung YM, Kovac E, et al. Direct metabolic interrogation of dihydrotestosterone biosynthesis from adrenal precursors in primary prostatectomy tissues. Clin Cancer Res. 2017;23(20):6351-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1(4):293-297. [DOI] [PubMed] [Google Scholar]
- 45. Mousses S, Wagner U, Chen Y, et al. Failure of hormone therapy in prostate cancer involves systematic restoration of androgen responsive genes and activation of rapamycin sensitive signaling. Oncogene. 2001;20(46):6718-6723. [DOI] [PubMed] [Google Scholar]
- 46. Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644-648. [DOI] [PubMed] [Google Scholar]
- 48. Cai C, Wang H, Xu Y, Chen S, Balk SP. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res. 2009;69(15):6027-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taris M, Irani J, Blanchet P, Multigner L, Cathelineau X, Fromont G. ERG expression in prostate cancer: the prognostic paradox. Prostate. 2014;74(15):1481-1487. [DOI] [PubMed] [Google Scholar]
- 50. Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311(11):1143-1149. [DOI] [PubMed] [Google Scholar]
- 51. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180-1192. [DOI] [PubMed] [Google Scholar]
- 52. Jack G, Jerry A, Debra L, Suzanne G, Woods S, Daniel DLV. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab. 1978;46(3):440-444. [DOI] [PubMed] [Google Scholar]
- 53. Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653-4657. [DOI] [PubMed] [Google Scholar]
- 54. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815-2825. [DOI] [PubMed] [Google Scholar]
- 56. Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407-6415. [DOI] [PubMed] [Google Scholar]
- 57. Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39(4): 327-348. [DOI] [PubMed] [Google Scholar]
- 58. Chang K-H, Sharifi N. Prostate cancer--from steroid transformations to clinical translation. Nat Rev Urol. 2012;9:721. [DOI] [PubMed] [Google Scholar]
- 59. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7(9):a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang KH, Li R, Kuri B, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108(33):13728-13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marchais-Oberwinkler S, Henn C, Möller G, et al. 17β-Hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. J Steroid Biochem Mol Biol. 2011;125(1-2):66-82. [DOI] [PubMed] [Google Scholar]
- 63. Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43(2):209-223. [Google Scholar]
- 64. Kent JR, Bischoff AJ, Arduino LJ, et al. Estrogen dosage and suppression of testosterone levels in patients with prostatic carcinoma. J Urol. 1973;109(5):858-860. [DOI] [PubMed] [Google Scholar]
- 65. Prout GR, Kliman B, Daly JJ, MacLaughlin RA, Griffin PP, Young HH. Endocrine changes after diethylstilbestrol therapy: effects on prostatic neoplasm and pituitary-gonadal axis. Urology. 1976;7(2):148-155. [DOI] [PubMed] [Google Scholar]
- 66. Malkowicz SB. The role of diethylstilbestrol in the treatment of prostate cancer. Urology. 2001;58(2 Suppl 1):108-113. [DOI] [PubMed] [Google Scholar]
- 67. Blackard CE. The Veterans’ Administration Cooperative Urological Research Group studies of carcinoma of the prostate: a review. Cancer Chemother Rep. 1975;59(1):225-227. [PubMed] [Google Scholar]
- 68. Schröder FH, Collette L, de Reijke TM, Whelan P. Prostate cancer treated by anti-androgens: is sexual function preserved? EORTC Genitourinary Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 2000;82(2):283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bessone F, Lucena MI, Roma MG, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver Int. 2016;36(2):302-310. [DOI] [PubMed] [Google Scholar]
- 70. Friedman G, Lamoureux E, Sherker AH. Fatal fulminant hepatic failure due to cyproterone acetate. Dig Dis Sci. 1999;44(7):1362-1363. [DOI] [PubMed] [Google Scholar]
- 71. Waxman J, Man A, Hendry WF, et al. Importance of early tumour exacerbation in patients treated with long acting analogues of gonadotrophin releasing hormone for advanced prostatic cancer. Br Med J (Clin Res Ed). 1985;291(6506):1387-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol. 2001;3(Suppl 3):S10-S14. [PMC free article] [PubMed] [Google Scholar]
- 73. Capper CP, Rae JM, Auchus RJ. The metabolism, analysis, and targeting of steroid hormones in breast and prostate cancer. Horm Cancer. 2016;7(3):149-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153-163. [DOI] [PubMed] [Google Scholar]
- 75. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098-2106. [DOI] [PubMed] [Google Scholar]
- 76.Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355(9214):1491-1498. [PubMed] [Google Scholar]
- 77. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215-224. [DOI] [PubMed] [Google Scholar]
- 78. Andriole GL, Bostwick DG, Brawley OW, et al. ; REDUCE Study Group . Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192-1202. [DOI] [PubMed] [Google Scholar]
- 79. Chau CH, Figg WD. Revisiting 5α-reductase inhibitors and the risk of prostate cancer. Nat Rev Urol. 2018;15(7):400-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630-642. [DOI] [PubMed] [Google Scholar]
- 81. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(5):479-505. [DOI] [PubMed] [Google Scholar]
- 82. Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324(2):93-103. [DOI] [PubMed] [Google Scholar]
- 83. Limonta P, Montagnani Marelli M, Moretti RM. LHRH analogues as anticancer agents: pituitary and extrapituitary sites of action. Expert Opin Investig Drugs. 2001;10(4):709-720. [DOI] [PubMed] [Google Scholar]
- 84. Sun M, Choueiri TK, Hamnvik OP, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy: effects of androgen-deprivation therapy. JAMA Oncol. 2016;2(4):500-507. [DOI] [PubMed] [Google Scholar]
- 85. Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448-4456. [DOI] [PubMed] [Google Scholar]
- 86. Tan O, Bukulmez O. Biochemistry, molecular biology and cell biology of gonadotropin-releasing hormone antagonists. Curr Opin Obstet Gynecol. 2011;23(4):238-244. [DOI] [PubMed] [Google Scholar]
- 87. Debruyne F, Bhat G, Garnick MB. Abarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancer. Future Oncol. 2006;2(6):677-696. [DOI] [PubMed] [Google Scholar]
- 88. Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531-1538. [DOI] [PubMed] [Google Scholar]
- 89. Tombal B, Miller K, Boccon-Gibod L, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol. 2010;57(5):836-842. [DOI] [PubMed] [Google Scholar]
- 90. Shore ND, Saad F, Cookson MS, et al. ; HERO Study Investigators . Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187-2196. [DOI] [PubMed] [Google Scholar]
- 91. Suzuki H, Uemura H, Mizokami A, et al. Phase I trial of TAK-385 in hormone treatment-naïve Japanese patients with nonmetastatic prostate cancer. Cancer Med. 2019;8(13):5891-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. MacLean DB, Shi H, Faessel HM, Saad F. Medical castration using the investigational oral GnRH antagonist TAK-385 (Relugolix): phase 1 study in healthy males. J Clin Endocrinol Metab. 2015;100(12):4579-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dearnaley DP, Saltzstein DR, Sylvester JE, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78(2):184-192. [DOI] [PubMed] [Google Scholar]
- 94. Saad F, Bailen JL, Pieczonka CM, et al. Second interim analysis (IA2) results from a phase II trial of TAK-385, an oral GnRH antagonist, in prostate cancer patients (pts). J Clin Oncol. 2016;34(2_suppl):200. [Google Scholar]
- 95. Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565-573. [DOI] [PubMed] [Google Scholar]
- 96. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202(6): 1199-1208. [DOI] [PubMed] [Google Scholar]
- 97. Eichenberger T, Trachtenberg J, Toor P, Keating A. Ketoconazole: a possible direct cytotoxic effect on prostate carcinoma cells. J Urol. 1989;141(1):190-191. [DOI] [PubMed] [Google Scholar]
- 98. Pont A, Williams PL, Azhar S, et al. Ketoconazole blocks testosterone synthesis. Arch Intern Med. 1982;142(12):2137-2140. [PubMed] [Google Scholar]
- 99. Ketoconazole blocks adrenal steroid synthesis. Ann Intern Med. 1982;97(3):370-372. [DOI] [PubMed] [Google Scholar]
- 100. Patel V, Liaw B, Oh W. The role of ketoconazole in current prostate cancer care. Nat Rev Urol. 2018;15(10):643-651. [DOI] [PubMed] [Google Scholar]
- 101. Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756-1764. [DOI] [PubMed] [Google Scholar]
- 102. Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17(8):2506-2513. [DOI] [PubMed] [Google Scholar]
- 103. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513-1520. [DOI] [PubMed] [Google Scholar]
- 104. Tannock IF, de Wit R, Berry WR, et al. ; TAX 327 Investigators . Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512. [DOI] [PubMed] [Google Scholar]
- 105. de Bono JS, Logothetis CJ, Molina A, et al. ; COU-AA-301 Investigators . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. [DOI] [PubMed] [Google Scholar]
- 107. Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97(2):507-516. [DOI] [PubMed] [Google Scholar]
- 108. Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563-4571. [DOI] [PubMed] [Google Scholar]
- 109. Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28(9):1481-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li Z, Alyamani M, Li J, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Alyamani M, Li Z, Berk M, et al. Steroidogenic metabolism of galeterone reveals a diversity of biochemical activities. Cell Chem Biol. 2017;24(7):825-832.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Alyamani M, Emamekhoo H, Park S, et al. HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J Clin Invest. 2018;128(8):3333-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ryan CJ, Smith MR, de Bono JS, et al. ; COU-AA-302 Investigators . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2): 138-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ryan CJ, Smith MR, Fizazi K, et al. ; COU-AA-302 Investigators . Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152-160. [DOI] [PubMed] [Google Scholar]
- 115. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jung ME, Ouk S, Yoo D, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J Med Chem. 2010;53(7):2779-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Beer TM, Armstrong AJ, Rathkopf DE, et al. ; PREVAIL Investigators . Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Alyamani M, Li J, Patel M, et al. Deep androgen receptor suppression in prostate cancer exploits sexually dimorphic renal expression for systemic glucocorticoid exposure. Ann Oncol. 2020;31(3):369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31(28):3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Smith MR, Antonarakis ES, Ryan CJ, et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol. 2016;70(6):963-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Apalutamide and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(26):2541-2542. [DOI] [PubMed] [Google Scholar]
- 124. Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Smith MR, Saad F, Hussain M, et al. ARASENS: A phase 3 trial of darolutamide in combination with docetaxel for men with metastatic hormone-sensitive prostate cancer (mHSPC). J Clin Oncol. 2018;36(6_suppl):TPS383-TPS. [Google Scholar]
- 126. Fizazi K, Smith MR, Tombal B. Clinical development of darolutamide: a novel androgen receptor antagonist for the treatment of prostate cancer. Clin Genitourin Cancer. 2018;16(5):332-340. [DOI] [PubMed] [Google Scholar]
- 127. Crawford ED, Stanton W, Mandair D. Darolutamide: an evidenced-based review of its efficacy and safety in the treatment of prostate cancer. Cancer Manag Res. 2020;12:5667-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fizazi K, Shore N, Tammela TL, et al. ; ARAMIS Investigators . Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. [DOI] [PubMed] [Google Scholar]
- 129. Fizazi K, Shore ND, Tammela T, et al. Overall survival (OS) results of phase III ARAMIS study of darolutamide (DARO) added to androgen deprivation therapy (ADT) for nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(15_suppl):5514. [Google Scholar]
- 130. Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A. 2019;116(23):11428-11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen WS, Aggarwal R, Zhang L, et al. ; West Coast Prostate Cancer Dream Team . Genomic drivers of poor prognosis and enzalutamide resistance in metastatic castration-resistant prostate cancer. Eur Urol. 2019;76(5):562-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;174(3):758-769.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Östling P, Leivonen SK, Aakula A, et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71(5):1956-1967. [DOI] [PubMed] [Google Scholar]
- 135. Zhang L, Altuwaijri S, Deng F, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang LG, Johnson EM, Kinoshita Y, et al. Androgen receptor overexpression in prostate cancer linked to Pur alpha loss from a novel repressor complex. Cancer Res. 2008;68(8):2678-2688. [DOI] [PubMed] [Google Scholar]
- 137. Veldscholte J, Risstalpers C, Kuiper GGJM, et al. A mutation in the ligand-binding domain of the androgen receptor of human lncap cells affects steroid binding characteristics and response to anti-androgens. Biochem Bioph Res Co. 1990;173(2):534-540. [DOI] [PubMed] [Google Scholar]
- 138. Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010;56(9):1492-1495. [DOI] [PubMed] [Google Scholar]
- 139. Lallous N, Volik SV, Awrey S, et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. 2016;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7(312):312re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21(10):2315-2324. [DOI] [PubMed] [Google Scholar]
- 142. Wyatt AW, Azad AA, Volik SV, et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016;2(12):1598-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ledet EM, Lilly MB, Sonpavde G, et al. Comprehensive analysis of AR alterations in circulating tumor DNA from patients with advanced prostate cancer. Oncologist. 2020;25(4):327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Conteduca V, Wetterskog D, Sharabiani MTA, et al. ; PREMIERE Collaborators; Spanish Oncology Genitourinary Group . Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Ann Oncol. 2017;28(7):1508-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wadosky KM, Koochekpour S. Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget. 2017;8(11):18550-18576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel Androgen Receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469-5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sharp A, Coleman I, Yuan W, et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129(1):192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37(13):1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Scher HI, Graf RP, Schreiber NA, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018;4(9):1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Thomas JL, Mack VL, Glow JA, Moshkelani D, Terrell JR, Bucholtz KM. Structure/function of the inhibition of human 3beta-hydroxysteroid dehydrogenase type 1 and type 2 by trilostane. J Steroid Biochem Mol Biol. 2008;111(1-2):66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sabharwal N, Sharifi N. HSD3B1 genotypes conferring adrenal-restrictive and adrenal-permissive phenotypes in prostate cancer and beyond. Endocrinology. 2019;160(9):2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Naelitz BD, Sharifi N. Through the looking-glass: reevaluating DHEA metabolism through HSD3B1 genetics. Trends Endocrinol Metab. 2020;31(9):680-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Thomas L, Sharifi N. Germline HSD3B1 genetics and prostate cancer outcomes. Urology. 2020;145:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hearn JWD, AbuAli G, Reichard CA, et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17(10):1435-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Agarwal N, Hahn AW, Gill DM, Farnham JM, Poole AI, Cannon-Albright L. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 2017;3(6):856-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Shiota M, Narita S, Akamatsu S, et al. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw Open. 2019;2(2):e190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Garcia Gil S, Ramos Rodriguez R, Plata Bello A, et al. Relationship between mutations in the HSD3B1 gene and response time to androgen deprivation therapy in the treatment of prostate cancer. Paper presented at: European Society of Oncology Pharmacy; October 25-27, 2018; Nantes, France. [Google Scholar]
- 160. Hearn JWD, Sweeney CJ, Almassi N, et al. HSD3B1 genotype and clinical outcomes in metastatic castration-sensitive prostate cancer. JAMA Oncol. 2020;6(4):e196496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hearn JWD, Xie W, Nakabayashi M, et al. Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 2018;4(4):558-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Almassi N, Reichard C, Li J, et al. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 2018;4(4):554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lu C, Terbuch A, Dolling D, et al. Treatment with abiraterone and enzalutamide does not overcome poor outcome from metastatic castration-resistant prostate cancer in men with the germline homozygous HSD3B1 c.1245C genotype. Ann Oncol. 2020;31(9):1178-1185. [DOI] [PubMed] [Google Scholar]
- 164. Khalaf DJ, Aragón IM, Annala M, et al. HSD3B1 (1245A>C) germline variant and clinical outcomes in metastatic castration-resistant prostate cancer patients treated with abiraterone and enzalutamide: results from two prospective studies. Ann Oncol. 2020;31(9):1186-1197. [DOI] [PubMed] [Google Scholar]
- 165. Sharifi N. Homozygous HSD3B1(1245C) inheritance and poor outcomes in metastatic castration-resistant prostate cancer with abiraterone or enzalutamide: what does it mean? Ann Oncol. 2020;31(9):1103-1105. [DOI] [PubMed] [Google Scholar]
- 166. Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34(9):518-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sharifi N. Steroid receptors aplenty in prostate cancer. N Engl J Med. 2014;370(10):970-971. [DOI] [PubMed] [Google Scholar]
- 168. Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Isikbay M, Otto K, Kregel S, et al. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5(2):72-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Li J, Alyamani M, Zhang A, et al. Aberrant corticosteroid metabolism in tumor cells enables GR takeover in enzalutamide resistant prostate cancer. eLife. 2017;6. doi: 10.7554/eLife.20183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cha HR, Lee JH, Ponnazhagan S. Revisiting immunotherapy: a focus on prostate cancer. Cancer Res. 2020;80(8):1615-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: facts and hopes. Clin Cancer Res. 2017;23(22):6764-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhao E, Wang L, Dai J, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1(2):152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Davidsson S, Andren O, Ohlson AL, et al. FOXP3+ regulatory T cells in normal prostate tissue, postatrophic hyperplasia, prostatic intraepithelial neoplasia, and tumor histological lesions in men with and without prostate cancer. Prostate. 2018;78(1):40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Almeida DVPd, Fong L, Rettig MB, Autio KA. Immune checkpoint blockade for prostate cancer: niche role or next breakthrough? Am Soc Clin Oncol Educ Book. 2020;(40):e89-e106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated for this article are included in this published article.