Abstract
The kexin-like proprotein convertases perform the initial proteolytic cleavages that ultimately generate a variety of different mature peptide and proteins, ranging from brain neuropeptides to endocrine peptide hormones, to structural proteins, among others. In this review, we present a general introduction to proprotein convertase structure and biochemistry, followed by a comprehensive discussion of each member of the kexin-like subfamily of proprotein convertases. We summarize current knowledge of human proprotein convertase insufficiency syndromes, including genome-wide analyses of convertase polymorphisms, and compare these to convertase null and mutant mouse models. These mouse models have illuminated our understanding of the roles specific convertases play in human disease and have led to the identification of convertase-specific substrates; for example, the identification of procorin as a specific PACE4 substrate in the heart. We also discuss the limitations of mouse null models in interpreting human disease, such as differential precursor cleavage due to species-specific sequence differences, and the challenges presented by functional redundancy among convertases in attempting to assign specific cleavages and/or physiological roles. However, in most cases, knockout mouse models have added substantively both to our knowledge of diseases caused by human proprotein convertase insufficiency and to our appreciation of their normal physiological roles, as clearly seen in the case of the furin, proprotein convertase 1/3, and proprotein convertase 5/6 mouse models. The creation of more sophisticated mouse models with tissue- or temporally-restricted expression of specific convertases will improve our understanding of human proprotein convertase insufficiency and potentially provide support for the emerging concept of therapeutic inhibition of convertases.
Keywords: proprotein convertase, precursor processing, knockout mouse models, PCSK
Graphical Abstract
ESSENTIAL POINTS
Kexin-like proprotein convertases constitute a widely distributed family of 7 enzymes that perform essential functions in the maturation of protein precursors
Human polymorphisms and mutations in the genes encoding these enzymes result in a spectrum of insufficiency disease phenotypes, from mild obesity to neonatal failure to thrive
Knockout mouse models have been created which in many, but not all cases recapitulate human insufficiency syndromes
In this review we compare the phenotypes of convertase mutant mouse models to those of human insufficiency syndromes
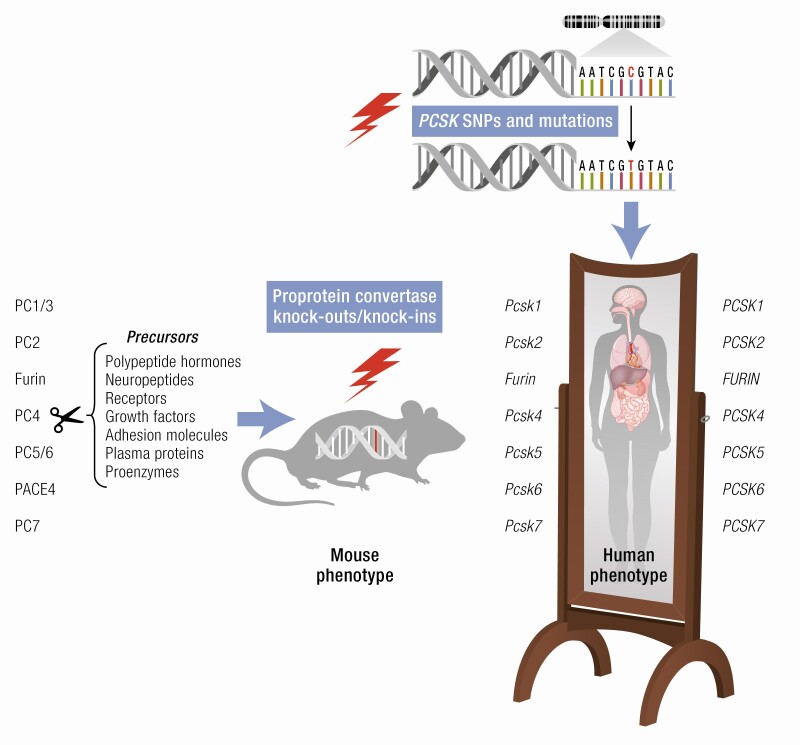
The proprotein convertases (PCs) are a family of serine proteinases that are typically involved in secretory protein maturation. As described below, these enzymes vary in tissue distribution and specificity, participate in a wide variety of substrate cleavage reactions, and have been implicated in many human diseases. Patients lacking one or both functional alleles, resulting in partial to complete enzyme deficiency, have been reported for most PCs. Furthermore, recent genome-wide association studies (GWASs) of single nucleotide polymorphisms (SNPs) and other mutations present in human populations have identified variants in both coding and noncoding regions of PC genes that are clearly linked to a substantial number of pathological conditions.
Mice have historically been used to model human disease because of their genetic and physiological similarity to humans. Additional advantages include their ease of gene manipulation, availability, and cost-effectiveness. Over the past 2 decades, many different null mouse strains, targeting all PC genes, have been generated. These mouse models have proved to be indispensable tools in dissecting the physiological functions of each PC; in the identification of specific substrates; and in the determination of convertase redundancy. A comprehensive review of existing mouse models of PC insufficiency has not been published recently (1), although several excellent reviews on individual PCs are available; for example, for PC1/3 (2, 3), furin (4, 5), and PC9 (6-8). The aim of this review is to summarize current knowledge regarding the various germline and conditional PC knockout (KO) mouse models, and to apply lessons learned from phenotypic assessments of null mice to human convertase insufficiency syndromes. We will specifically address the advantages and limitations of these mouse models and their relevance to human PC polymorphisms and mutations. We begin by providing a general introduction to convertase structure and biochemistry, and then discuss each convertase individually, focusing on unique features and more recent studies. We omit extensive discussion of PC9 as well as of SKI-1/S1P (PC8), neither of which possess substrate specificity similar to that of the first 7 family members. We conclude with a discussion of the state of the field, and suggestions for future work.
General Information: The Proprotein Convertases
The proprotein convertases constitute a large family of Ca2+-dependent serine endoproteases. In mammals, this family is comprised of 9 members, PC1/3, PC2, furin, PC4, PC5/6, PACE4, PC7, SKI-1/S1P, and PCSK9/NARC-1, respectively encoded by the genes PCSK1 to PCSK9. Certain members possess species-specific additional protein isoforms that differ in tissue expression profiles and subcellular localization and function and are produced by alternative splicing of their precursor RNAs. For example, humans, but not rodents, express 8 PACE4 isoforms (9, 10).
Based on structural similarity to bacterial and yeast proteases, the enzymes can be grouped as follows into 3 subfamilies. The specificity of each proprotein convertase varies by family member.
PCSK family members 1 to 7 belong to the kexin-like subfamily. These enzymes cleave proprotein substrates at the general consensus motif (K/R)-(X)n-(K/R)↓, where (K/R) indicates either a lysine or an arginine residue, and the arrow (↓) indicates the site of cleavage, n = 0, 2, 4, or 6, and X stands for any amino acid except Cys (11). A (K/R)-R motif at the P1-P2 positions is the minimal cleavage motif; an Arg (or rarely, Lys) at the P1 position is absolutely required, while an upstream basic amino acid residue at subsite P4 or P6 can occasionally compensate for the lack of a basic residue at the P2 position for proprotein convertases acting within the regulated secretory pathway, namely PC1/3 and PC2. PCs acting within the constitutive secretory pathway, such as furin, require at least 2 other basic residues at the P2, P4, or P6 subsites in addition to a P1 Arg (12).
PCSK8 belongs to the pyrolysin subfamily; these enzymes cleave after the hydrophobic residue in the consensus motif (R/K)-X (hydrophobic)-X↓, where X is any amino acid, preferentially Leu or Thr, but excluding Val, Pro, Glu, Asp, or Cys (13). PCSK8 is mainly involved in lipid homeostasis, mediated through cleavage of membrane-bound transcription factors such as sterol regulatory element binding proteins (reviewed in (14)).
PCSK9 belongs to the proteinase K subfamily and is atypical in that it does not cleave any substrates in trans, but acts only as its own substrate during intramolecular self-cleavage. Two different intramolecular cleavage sites have been reported: (Y/I)VV(V/L)(L/M)↓ (15) and LVFAQ↓ (16). PCSK9 specifically regulates serum low-density lipoprotein (LDL) levels by targeting hepatocyte LDL receptors for endosomal degradation (see (6-8) for extensive reviews).
Several computational approaches have been developed for accurate prediction of protease-specific substrates and their cleavage sites, with widespread availability of convertase-specific cleavage algorithms and databases (17, 18). While general rules regarding consensus sites are useful in cleavage prediction, experimental work in cells and tissues is required to assess whether a given cleavage actually occurs efficiently. For example, furin preferentially recognizes sites that contain the sequence motif Arg-Xaa-(Lys/Arg)-Arg ↓. However, in vitro and cell-based cleavage assays have shown that select precursors that do not fully fit consensus sequences can still be cleaved by furin, while certain substrates with conserved sequences may fail to be cleaved by furin in vitro (reviewed in (19)). Another complication is that substrates that are rapidly secreted by the constitutive pathway may not physically interact with PC1/3 or PC2, which are normally stored in regulated secretory granules.
This review will focus only on the proprotein convertase subfamily with specificity for basic residues: the kexin-like proprotein convertases.
Kexin-like Proprotein Convertases
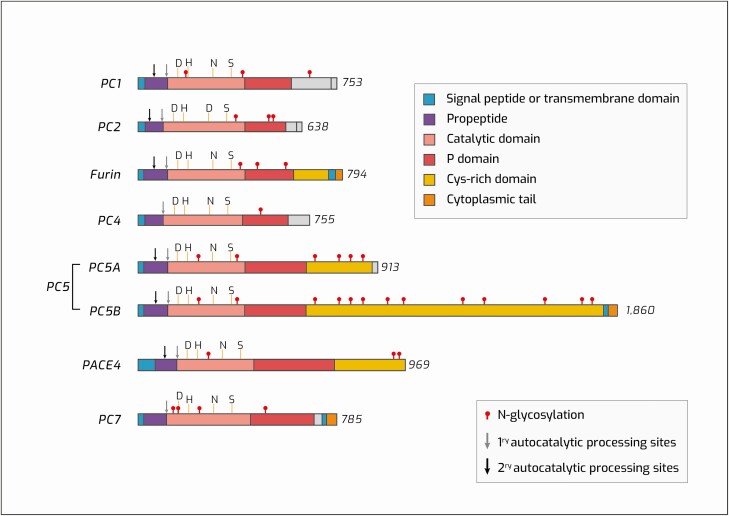
As shown in Fig. 1, PCs are synthesized in the endoplasmic reticulum (ER) as zymogens, consisting of a signal peptide, followed by a chaperone-active proprotein domain; a generally well-conserved catalytic domain; a “P” or “HomoB” domain; and variable carboxy-terminal domains that often contribute information for subcellular targeting. This includes a transmembrane domain for furin, PC5/6B, and PC7 (Fig. 1). Propeptide cleavage at the primary site, a Ca2+-dependent autocatalytic event, occurs within the ER for all PCs except PC2, and is generally required for further transit through the secretory pathway. However, full maturation of the zymogen to an active enzyme species occurs only after the dissociation of propeptide, often caused by secondary propeptide cleavage (see Fig. 1 for site illustration) and/or pH-mediated dissociation of prodomain peptides.
Figure 1.
Proprotein convertase structure. This figure depicts the structures of the various basic residue-specific proprotein convertases (human). The membrane-spanning signal peptides and transmembrane domains (when present) are shown in blue. The comparatively large propeptides (purple) are cleaved after basic residues, often at 2 sites, the primary (light arrow) and secondary (dark arrow) cleavage site; the primary site is always an intermolecular cleavage. The catalytic domain (pink) is the most conserved domain for each enzyme; shown here are the catalytic residues D (Asp), H (His) and S (Ser), as well as the oxyanion hole residue N (Asn) (D = Asp only in PC2). The P or homoB domain (red) is thought to confer stability to the catalytic domain and is required for expression of active enzyme. The carboxy-terminal segments following the P domain are quite variable between convertases; certain enzymes contain a Cys-rich domain (yellow) which can interact with surface glycoproteins. Cytoplasmic tails (orange) present in furin, PC5B (PC5/6B) and PC7 have been shown to confer subcellular targeting information. All enzymes are N-glycosylated to a variable extent (shown with red circles). Of note, other posttranslational modifications not depicted here, including phosphorylation and palmitoylation, may also be present. Adapted from Seidah NG, Nat Rev Drug Discov. 2012 (1).
As described below, PCs activate, and in some cases inactivate, a large number of precursor proteins, including precursors to peptide hormones, neuropeptides, and growth factors; many plasma membrane receptors; adhesion molecules; other enzymes; and various pathogen-derived proteins.
PC1/3 (gene name: PCSK1)
Unique features and distribution; substrates
PC1/3 was one of the earliest mammalian convertases to be discovered. Its dually numbered name derives from its codiscovery by both the Steiner (20) and the Seidah (21) groups. Its specific features, as well as human polymorphisms and mutations, have been described extensively in 2 comprehensive reviews (2, 3). A unique feature of PC1/3 is its autocatalytic carboxy-terminal processing within the regulated secretory pathway, which yields multiple active forms with molecular masses of 87, 74, and 66 kDa. These processed forms tend to self-oligomerize to form more stable dimers, oligomers, and aggregates; oligomerization may contribute to the control of PC1/3 activity within secretory granules (22).
PC1/3 binds to the neural- and endocrine-specific secretory protein proSAAS (23), which is thought to block PC1/3 from inactivating oligomerization events, although no direct evidence for this currently exists (22). ProSAAS, encoded by the gene PCSK1N, is a 225-residue polypeptide originally discovered via mass spectrometric screening of peptides extracted from the brains of Cpefat/Cpefat mice (23, 24). The C-terminal region of proSAAS contains the potent inhibitory hexapeptide Leu-Leu-Arg-Val-Lys-Arg, which inhibits PC1/3 at low nanomolar concentrations (25, 26).
PC1/3 is concentrated in a variety of neural and neuroendocrine tissues, including brain (and is especially rich in the arcuate and paraventricular nuclei of the hypothalamus), pituitary, adrenal, thyroid, small intestine (enteroendocrine L and K cells), and pancreatic α and β cells (reviewed in (2)). In addition, PC1/3 is also atypically expressed in immune cells (macrophages and lymphocytes) and organs (spleen, thymus, and lymphatic ganglia) (27).
PC1/3 often overlaps with PC2 in substrate preference and specificity, but each enzyme has a specific cleavage site preference and a specific tissue distribution that ultimately results in divergent tissue-specific products, often with differing functions. For example, proglucagon is cleaved by PC2 in islet α cells to mainly release glucagon, while in the absence of PC2, in intestinal L cells, this same precursor is exclusively cleaved by PC1/3 to release the glucagon-containing fragment oxyntomodulin and the glucagon-like peptides (GLP)-1 and GLP-2 (28). PC1/3 has a more restricted cleavage specificity than PC2; for example, PC1/3 is less efficient at cleaving Arg-Xaa, where Xaa is a (Pro, Gly) or aromatic (Tyr, Trp, Phe) residue at the P1′ position (29). PC1/3 thus typically generates larger peptide products and a smaller number of total cleaved products than PC2 (29, 30). As previously summarized in a comprehensive table (31) that includes many substrates identified through proteomics efforts conducted on tissues prepared from convertase-null mice (29), PC1/3 action has been implicated in the processing of over 25 different prohormones and neuropeptide precursors, including but not limited to: the 3 opioid peptide precursors, proopiomelanocortin (POMC), proenkephalin, and prodynorphin; proagouti-related peptide (proAGRP), progrowth-hormone releasing hormone (proGHRH), prothyrotropin-releasing hormone; proinsulin; proglucagon, provasopressin, proghrelin, proneuropeptide Y, islet amyloid polypeptide (amylin), prococaine/amphetamine-regulated transcript (proCART); procalcitonin, procholecystokinin (proCCK; mainly in intestinal I cells), proneurotensin, prosomatostatin, prothyrotropin-releasing hormone, and proVGF. Other recently identified PC1/3 substrates include proglucose-dependent insulinotropic polypeptide (proGIP) (32), propeptide YY (33), and probrain-derived neurotrophic factor (34) (proBDNF), although it should be noted that other data implicate furin (35) and/or PC7 (36) in BDNF synthesis.
In many cases, PC2 also participates in the further cleavage of these precursors in a tissue-specific manner (see below). It should be pointed out that the involvement of specific convertases has not yet been established for certain peptide hormone precursors, for example proneuropeptide FF, proneuropeptide Y and promotilin.
Human PCSK1 polymorphisms and mutations
The first documentation of a patient with a PC1/3 mutation was published in 1995 (37). This patient, with a history of severe early-onset obesity, reactive hypoglycemia, hypogonadotropic hypogonadism, and hypocortisolism, with biochemical evidence of impaired processing of adrenocorticotropin (ACTH) and insulin precursors, was diagnosed as a PCSK1 compound heterozygote only in adulthood (38). On one PCSK1 allele she carried a missense G593R mutation in the P domain that resulted in total ER retention of this gene product, while her other allele contained a splice site mutation in the catalytic domain, which led to the production of completely inactive, truncated PC1/3. In the 25 years since this initial case, an increasing number of additional infants and children with congenital enteropathy have been diagnosed with PCSK1 deficiency, totaling 26 cases to the present date; more than half of these were reported in a comprehensive study by Martín et al. (39). The majority of these rare PC1/3 pathogenic variants are located in the catalytic domain, while a small number are in the P domain; many of these PC1/3 mutant proteins are completely inactive as a consequence of their ER retention (eg, G593R, G209R), which renders them subject to ER-associated degradation. Other pathogenic variants, although secreted, are also enzymatically inactive due to catalytic dysfunction (eg, N309K and F548S) (40).
PC1/3 deficiency is a rare disorder inherited as an autosomal recessive trait with an age-dependent, complex endocrinological phenotype of varying penetrance. The predominant phenotype is severe malabsorptive diarrhea (96%) with profound failure to thrive, and metabolic acidosis requiring total parenteral nutrition (92%) within the first month of life; the intestinal architecture is however normal or only mildly distorted. Most importantly, the initial severe diarrhea subsequently improves over time, permitting enteral feeding by 18 months of age. Many PCSK1-deficient patients develop hyperphagia, resulting in significant weight gain despite persistent loose stool. The second most common symptom is polyuria-polydipsia (83% of patients), with central diabetes insipidus identified in 61% of cases. Elevated serum proinsulin has also been reported in many cases; a longer half-life of proinsulin than of insulin causes postprandial hypoglycemia in over half of the reported cases of PCSK1 insufficiency. Central adrenal insufficiency, hypothyroidism, and gonadotropin deficiency are additional endocrinopathies reported in these patients (reviewed in (2, 41)). PC1/3-deficient patients share many clinical features with Prader–Willi syndrome (PWS) patients. A study by Burnett et al. (42) suggests that PC1/3-mediated defects in prohormone processing may underlie the appetitive phenotype in PWS. They observed decreased PC1/3 mRNA and protein expression as well as of its transcription factor, NHLH2 (Nescient Helix-Loop-Helix 2), both in induced pluripotent stem cell–derived neurons from PWS patients and in a mouse model of human PWS. However, the role of PCSK1 deficiency in the neuroendocrine features of PWS was called into question by a recent study that reported neither a reduction in the expression of Pcsk1 nor of its transcription factor in a mouse model of PWS (43).
A large number of GWA and quantitative trait locus (QTL) studies carried out in European and several other populations have established that several common nonsynonymous polymorphisms as well as rare heterozygous mutations in PCSK1 are linked both to increased obesity risk and to disturbances in glucose homeostasis (reviewed in (2, 3)). Indeed, PCSK1 variants constitute the third most prevalent cause of monogenic obesity (reviewed in (3)). The most extensively studied nonsynonymous variants most reproducibly reported to be associated with obesity, in multiple populations, are rs6232, rs6234/rs6235, encoding the N221D and Q665E/S690T variations, respectively. Subgroup meta-analysis clearly shows association of these common SNPs with obesity; metabolic traits also vary in an age- and ethnicity-dependent fashion (reviewed in (2, 3)). Nonsynonymous PCSK1 exonic (rs6234) and intronic variants, including rs3811951 and rs156019, are also strongly associated with the risk of coronary artery disease in type 2 diabetes (T2D) (44), although it is not yet known whether these variants are gain or loss of function. Lastly, the known PC1/3 hypermorph S357G (rs1050622) confers PC2-like specificity to PC1/3 (45). This rare mutation was originally found in a lung carcinoid sample (46); whether this substitution contributes to tumor proliferation or function is not yet known, and needs to be investigated through PCSK1 sequencing of multiple bronchial carcinoid samples. Given that hypomorphs are associated with obesity, a potential association of this hypermorph variant with thinness would also be of interest, though difficult to study given the rare nature of this lung cancer type.
Given the apparent involvement of PC1/3 in hypothalamic melanocortin production (an anorexic peptide), pharmacologic agents that promote PC1/3 activity could theoretically result in enhanced α-MSH production. Proof of principle of such activators has been reported (47). The fact that a natural PC1/3 hypermorph exists (S357G (45)) also provides further confidence that it might be possible to design convertase activators once the crystal structure of this enzyme is known. Unfortunately, much still remains to be learned regarding both PC1/3 structure and enzymology. For example, the early finding by the Mains group that tethering the C-terminal tail activates the enzyme (48) remains to be explained on a molecular level.
Mouse models of PC1/3 insufficiency
The first PC1/3 KO mouse model was generated in 2002 by targeted deletion of the promoter region and the first exon (49). These PC1/3 KO mice have a high perinatal (40%) and postnatal (40%) mortality rate, and those that do survive display severe postnatal dwarfism, likely due to reduced proGHRH processing (49). In patients, only a few cases of linear growth deficiencies have been observed (39). These growth differences between the human and mouse models of PC1/3 insufficiency have been ascribed to species differences in the cleavage site of proGHRH; the presence of Arg vs Gln at the P2 residue renders human, but not mouse proGHRH susceptible to furin-mediated cleavage, thus facilitating its activation (49). However, the fact that certain PCSK1 patients exhibit growth deficiencies supports a role for this enzyme in proGHRH processing in humans as well as in mice (39).
In many respects similar to PCSK1-deficient humans, PC1/3 KO mice exhibit POMC processing defects, hyperproinsulinemia, and reduced production of intestinal GLP-1 and -2 (49). Despite undetectable ACTH levels, PC1/3 KO mice exhibit normal corticosterone levels, unlike the hypocortisolemia exhibited by PC1/3-deficient patients. PC1/3 KO mice also do not develop obesity and glucose intolerance, which are the most common features of PCSK1-deficient patients. Interestingly, heterozygote PC1/3 KO mice tend to be mildly obese and glucose intolerant. PC1/3 KO homozygotes also suffer from gastrointestinal dysfunction, manifested as chronic mild diarrhea associated with bulky moist stools that resembles the early enteropathy observed in PCSK1-deficient patients (49). PC1/3 KO mice also exhibit an innate immune defect, and Th1 pathway activation, with massive cytokine secretion indicative of a proinflammatory response, was observed when mice are challenged with lipopolysaccharides (27). However, no immune deficiencies have been reported in PC1/3-deficient patients, nor have any studies evaluated the effect of bystander infections on the PC1/3-deficient phenotype in humans. While this issue remains to be systematically investigated, these differences could possibly be explained by the many dissimilarities between the mouse and human immune systems (50).
A Pcsk1 mutant mouse bearing a missense mutation (N222D) near the calcium binding pocket in the catalytic domain was generated by N-ethyl-N-nitrosourea (ENU)-mediated mutagenesis (51). Binding of calcium to this site is required for enzymatic activity (52), thus explaining why variants in the vicinity of this binding pocket impact enzyme activity. The reduced enzymatic activity of the variant PC1/3 protein (as opposed to a complete absence of activity) may be sufficient to process proGHRH, but not proinsulin and POMC, as levels of the latter precursors were increased in this mutant mouse. Plasma ACTH and corticosterone levels were near normal in N222D homozygote mice, despite increased POMC levels. A similar increase in POMC, with near-normal ACTH despite negligible PC1/3 activity, has also been observed in patients with PC1/3 insufficiency (53). N222D mice exhibit glucose intolerance after a high-fat diet secondary to failure of β cell compensation with increasing metabolic demand. Most importantly, N222D mice develop adult-onset obesity, partially phenocopying PC1/3-deficient human obesity (51).
PC1/3-N222D is partially retained in the ER and causes dominant-negative deleterious effects on wild-type PC1/3 trafficking, targeting the complex for degradation (54, 55). Differential protein stability may help to explain why mice bearing the N222D mutation, but not PC1/3 null mice, develop obesity (54). Interestingly, N222D mutant mice exhibit increased secretion of the lipoprotein ApoA1; high-density lipoprotein (HDL) cholesterol concentrations, however, remain unchanged (56). There are no studies as yet reporting serum ApoA1 changes in PCSK1-deficient humans.
As discussed above, the common N221D polymorphism is highly associated with obesity risk in humans. To explore the biology underlying this risk, a CRISPR-edited mouse line expressing the PC1/3-N221D mutation was generated (57). Unlike the profound loss of enzymatic activity caused by the N222D mutation (54, 55), nonsynonymous mutation at the neighboring asparagine residue, N221D resulted in only a slight (but also significant) decrease in PC1/3 activity in vitro (57), confirming prior results (58, 59). These data highlight the very different contributions of the 2 adjoining asparagines near the calcium binding pocket of PC1/3 to total enzyme activity. Surprisingly, no change in body weight was observed on either a regular or a high-fat diet (57), although levels of hypothalamic anorexic peptides such as α-MSH were altered. In new work, we have observed that conditional deletion of Pcsk1 in POMC-expressing neurons also does not result in obesity, suggesting that differential POMC processing may not underlie either the mouse (or human) obesity phenotype (Shakya, Surbhi, Low, Verchere, White and Lindberg, manuscript submitted for publication). A definitive causal role for POMC processing in human PC1/3 deficiency syndromes remains to be established.
Lastly, a novel Pcsk1-deficient mouse model involving a pV96L missense mutation has been identified in an ENU mutagenesis screen for age-related diseases (60). This Pcsk1 96L/96L mutation alters the first nucleotide of the mouse exon 3, leading to mis-splicing and skipping of that exon, and a truncated protein. Like N222D, the Pcsk1 96L/96L mutation is a hypomorph and exhibits ER retention in Neuro2a cells (60). Similar to N222D mice, Pcsk1 pV96L homozygous mice exhibit obesity, hyperphagia, glucose intolerance, insulin resistance, and hyperproinsulinemia. Unlike N222D mice, pV96L variant mice additionally manifest transient diarrhea and shortened body length, better mimicking the human PCSK1 deficiency syndrome.
The above data clearly support the idea that with only few exceptions, Pcsk1-deficient mice act as valuable models to explain the complex human deficiency phenotype of congenital endocrinopathy and metabolic disease. The origin of the obesity phenotype is not yet clear; while initially this phenotype was thought to derive from aberrant hypothalamic POMC processing, the above N221D data, as well as new data using Pcsk1-floxed, POMC-Cre mice, do not support this contention. The various Pcsk1-deficient mouse models can be used to further explore the contribution of this enzyme to obesity phenotypes.
α-cell specific PCSK1 cKO mice
PC1/3 conditional KO mice specifically lacking PC1/3 in pancreatic α cells (made by crossing Pcsk1 floxed mice with Gcg-Cre driver mice) were generated to study whether α cell secretion of GLP-1 might exhibit a paracrine effect on β cell function, maintaining glucose homeostasis (61). Homozygotes exhibit an age-dependent decrease in GLP-1 islet content and secretion; insulin levels, systemic GLP-1 content, and intraperitoneal glucose tolerance, however, remain intact under normal feeding conditions. With increasing metabolic demand, induced following administration of a high fat diet and streptozocin treatment, these mice show impaired β cell function, manifested as reduced insulin secretion and glucose intolerance. Compared to mice, human pancreatic islets contain a higher content of GLP-1. These data support the idea that α cell-secreted GLP-1 plays an important role in β cell adaptation during metabolic stress, and suggest that therapeutic stabilization of α cell-derived GLP-1 content might be useful in the treatment of type 2 diabetes (61).
PC2 (gene name: PCSK2)
Unique features and distribution; substrates
PC2 was the second prohormone convertase to be discovered (reviewed in (62)). Along with furin and PC7, PC2 represents one of the earliest convertases found in evolution and has been identified in unicellular organisms such as rotifers (UniProt ID; A0A3M7QWU5). Specific features unique to this enzyme include the presence of an Asp in the oxyanion hole; and the ability of the precursor enzyme to escape the ER without undergoing propeptide cleavage. Thus far proPC2 is also unique in its absolute requirement for another protein, the neuroendocrine chaperone 7B2, for eventual maturation of the zymogen to an active enzyme species (63, 64), a process that occurs late in the regulated secretory pathway. Like PC1/3, PC2 is contained within dense core secretory granules in endocrine, neural, and neuroendocrine cells. PC2 is particularly rich in the neuro-intermediate lobe; hypothalamus; pancreas (especially α cells); and intestinal L cells (reviewed in (65)).
Due to the broader cleavage specificity of PC2, most precursors that are cleaved by PC1/3 will also serve as substrates for PC2; one notable exception is proghrelin (66). Peptidomics analyses of PC2 null mouse brain extracts have been extremely useful in defining PC2 substrates (67); these include, but are not limited to the following precursors (see (31) for specific literature references): proAGRP, chromogranins A and B, proCART, neuroendocrine secretory protein 55, proaugurin, proCCK (in neurons), POMC, procorticotropin-releasing hormone (proCRH), prodynorphin, proenkephalin, progalanin, progastrin, proglucagon, proGHRH, proinsulin, proamylin, promelanin-concentrating hormone, proneuropeptide Y, proneurotensin, proorphanin, proQRFamide, proSAAS, prosomatostatin, prospexin, protachykinins A and B, prothyrotropin-releasing hormone, provasopressin, secretogranin 2, and 7B2.
Human PCSK2 polymorphisms and mutations
No cases of PCSK2 deficiency have been reported in humans, suggesting that the presence of active PC2 is vital to human health (reviewed in (68)). However, several GWAS and related studies have described PCSK2 polymorphisms that are linked with T2D and/or with glucose homeostatic traits (69-74). Yoshida et al. reported that a highly polymorphic polymorphism in intron 2 is associated with T2D in Japanese subjects (69). A GWAS study of T2D implicated the following PCSK1 SNPs: rs2021785, rs1609659, rs4814597, and rs226902325 (70). A common variant, rs2021785, increases susceptibility to T2D in both African-Americans and Han Chinese (70, 71); this SNP was also associated with reduced glucose-stimulated insulin secretion and lower fasting glucagon levels in a Scandinavian population (72). To the contrary, other intronic PCSK2 variants were found to lower T2D risk. Specifically, the rs6044695 and rs2284912 SNPs were associated with a lower incidence of diabetes during a 5-year follow-up (73). Whether each of these variants is associated with gain or loss of PC2 function and/or expression is presently unknown, and should be investigated.
Unlike intronic variants that are present at minor allele frequencies of up to 8%, exonic variants of PCSK2 are extremely rare (minor allele frequency of less than 0.05%). One rare exonic variant (R430W/rs200711626) was found to be enriched in an older Amish population; interestingly, this R430W variant was almost twice as common in T2D individuals within this population, though this association failed to reach significance likely due to modest sample sizes (74). In vitro data show that the variant enzyme exhibits a broadened pH optimum, with increased enzyme activity between pH 5 and 6 as compared to the wild-type enzyme, implying gain of function; however, no increase in glucagon production was detected in proglucagon- and R430W human PC2-transfected GH4C1 cells (74); thus, this idea still requires further experimental support.
In addition to their clear association with glucose homeostasis traits, PCSK2 variants are also linked with neurodevelopmental and neurodegenerative disorders. The PCSK2 variant rs6080539 was significantly associated with amyotrophic lateral sclerosis (ALS) in a study carried out in US veterans (75). A comparative analysis of mRNA expression changes in the brains of Alzheimer’s disease (AD) patients and a mouse model of AD (3xTg-AD) showed that both PCSK1 and PCSK2 are significantly decreased in the AD hippocampus (76). Immunoreactivity for both PC1/3 and PC2 was decreased in select hypothalamic nuclei in brains obtained from Huntington’s disease patients (77). The PCSK2 SNP rs7274133 is significantly associated with autism in a recent genome-wide association analysis in a Han Chinese population (78). Though many of these PCSK2 GWAS studies require further validation in larger (as well as other) populations, they are intriguing and warrant further investigation into a possible mechanistic role of PC2 in various neurological disorders. Although PC2, unlike furin (4), is not known to contribute either directly or indirectly to β-amyloid peptide formation, PC2 is required for the production of many critical neuropeptides (see above) that could possibly contribute to the pathogenesis of neurological disorders, including AD (79) and autism spectrum disorders (80-82).
Mouse models of PC2 insufficiency
PC2 KO mice were first generated in 1997 by Steiner and colleagues (83) by insertion of the neomycin resistance gene into the third exon of the mouse PC2 gene. Although the majority of the mutant proPC2 is still synthesized, this insertion prevents the protein from undergoing autoactivation and post-ER trafficking. PC2 KO mice are surprisingly healthy with only a mild overall phenotype despite extensive disruption in neuropeptide and peptide hormone precursor processing (29, 84). PC2 KO mice appear normal at birth, followed by slight postnatal growth retardation. Since α and δ cells primarily express PC2 (but not PC1/3), processing of proglucagon to glucagon and prosomatostatin to mature somatostatin (SS-14) is severely impaired in PC2 KO mice. Thus, the dominant phenotype of this mouse is chronic hypoglycemia, due to the complete lack of glucagon; and marked hyperplasia of pancreatic α and δ cells, due to the lack of a negative metabolic feedback loop. Despite the lack of PC2, proinsulin processing is only partially blocked; about two-thirds of proinsulin is processed to insulin in the maturing secretory granules of β cells by coexpressed PC1/3. PC2 null mice also exhibit elevated des-31,32 proinsulin, an intermediate cleavage form generated by the action of PC1/3, and lack detectable generation of des-64,65 intermediates. This results in flattened glucose tolerance curves (83, 85-87).
Since PC2 absolutely requires the 7B2 chaperone protein for its enzymatic maturation and activity, 7B2 KO mice represent a second mouse model of PC2 insufficiency (63). 7B2 KO mice lack PC2 activity and exhibit a phenotype similar to that of PC2 KO mice when both nulls are placed upon the same background (88). However, when bred into a specific 129Sv substrain, 7B2 KO mice exhibit intermediate lobe ACTH hypersecretion, hypercorticosteronemia, loss of proglucagon processing, and early lethality due to impaired glucose regulation (89, 90); a similarly lethal phenotype is observed when the PC2 KO is bred onto this same 129Sv substrain background (88). These data indicate that genetic background strongly influences the phenotype of at least some convertase null mutants. Strain-specific contributions to endocrine phenotype represent an interesting area for further study since the genetic modifiers involved may pertain to humans.
Recent studies have highlighted differential species-specific PC1/3 and PC2 expression in β cells, with mouse, pig, and dog expressing abundant PC2, but undetectable PC2 levels in human and rodent β cells. In primary human islets, proglucagon, but not proinsulin processing was disrupted under PC2-inhibiting chase conditions, suggesting that PC2 does not contribute heavily to proinsulin processing in humans (91). Davalli et al. recently reported undetectable PC2 immunoreactivity in the β cells of mice bearing human islet transplants (92). Taken together, these human findings may support the idea that PC2 is quantitatively less important than PC1/3 in proinsulin processing (85, 91, 92). However, this work needs to be confirmed in other human studies.
Contrary to Pcsk1 deficiency, Pcsk2 deficiency protects mice from obesity. PC2 KO mice show reduced adiposity, a reduced refeeding response, resistance to high-fat diet–induced weight gain, reduced circulating triglycerides, and improved glucose tolerance (93). A more prominent effect is seen in females. In agreement with this, genetically obese mice express significantly higher levels of Pcsk2 mRNA in brain and stomach, suggesting an inverse correlation of Pcsk2 gene expression with body weight (93). Plasma levels of gastrointestinal motility-inhibiting and satiety peptides such as GLP-1, somatostatin, and pancreatic polypeptide Y are dramatically higher while the levels of gastrointestinal motility-stimulating peptides (eg, substance P) are lower in PC2 KO mice, possibly accounting for the finding of improved glucose tolerance and reduced obesity. These authors speculate that compensatory ectopic alternative processing of precursors by other convertases, particularly PC1/3, occurs in these mice. No compensatory increases in the levels of PC1/3 in α cells (86), and/or of other PCs such as PC1/3, furin, and PC5/6, have been reported in PC2 KO mice (94).
PC2 KO mice develop salt-induced hypertension, which has been ascribed to deficient PC2-dependent processing of POMC into the natriuretic peptide γ-MSH (95). PC2 KO mice also exhibit reduced nociception in models of stress-induced analgesia as well as altered morphine tolerance and dependence due to lowered levels of endogenous opioids derived from prodynorphin, proneurotensin, proenkephalin, pro-orphanin FQ/Nociceptin, and POMC (96, 97). Behavioral and electrophysiological analyses were recently carried out in these mice (98). PC2 KO mice display increased escape latency in the Morris water maze model, indicating cognitive impairment. Interestingly, in these mice, hippocampal long-term potentiation, which measures synaptic plasticity, is increased, and paired-pulse facilitation is decreased. Although as yet unvalidated by independent studies, these data indicate that PC2, most likely by regulating neuropeptide transmitter production, may be involved in regulating hippocampal transmission and plasticity, learning, and memory (98). Further behavioral and electrophysiological analyses of various brain regions might help to explain the genetic association of PC2 polymorphisms with neurodegenerative disease and autism spectrum disorders (75-78).
Collectively, the phenotype of the Pcsk2 null mouse suggests an important role for PC2 in blood sugar and body weight homeostasis, and this is in good agreement with the association of PCSK2 variants with diabetes in humans (69-72, 74).
FURIN (gene names: FURIN and PCSK3)
Unique features and distribution; substrates
In 1986, furin was the first mammalian family member to be discovered, as a homolog of the yeast enzyme kexin. Furin, the translational product of the fur gene, received its name due to its location (FES/FPS proto-oncogene Upstream Region) (99). As the prototype PC, furin is the most extensively studied and best characterized convertase, with over 2800 papers listed in PubMed at the time of writing. Furin is ubiquitously expressed; furin RNA is particularly enriched in liver, salivary gland and placenta (www.proteinatlas.org/ENSG00000140564-FURIN). Within cells, furin is located principally within the trans-Golgi network (TGN) of the constitutive secretory pathway, but cycles between the TGN and the cell surface, in part via the endosomal system. During this process, furin can be shed into the extracellular space as a soluble, enzymatically active species (reviewed in (4)).
Furin processes soluble and membrane-bound precursors for highly diverse categories of proteins. A useful database of furin substrates has been compiled (available online at http://www.nuolan.net/substrates.html), which includes experimentally verified furin substrates together with cleavage site and original references (100). Briefly, this list includes the precursors to peptide hormones (GHRH, parathyroid hormone, natriuretic peptides, thyrotropin-releasing hormone, and endothelin-1); secretory chaperones (7B2 and proSAAS); growth factors (transforming growth factor beta [TGF-β1/β2], insulin-like growth factors [IGF1/IGF2], vascular endothelial growth factors [VEGF-C, VEGF-D], platelet-derived growth factors [PDGF-А/PDGF-B], nerve growth factor, bone morphogenetic protein 4 [BMP-4]); tumor necrosis factor superfamily members (including TNF SF13 and chemokine ligand-10); a variety of matrix metalloproteinases (MMP2, MMP3, MMP11, MMP14, MMP16, MMP21, MMP28); members of a disintegrin and metalloproteinase (ADAM) protein family (ADAM9, ADAM15, ADAM19); ADAM domain containing family members with thrombospondin type motifs (ADAMTS1, ADAMTS4, ADAMTS9); many other enzyme precursors, including those to amyloid precursor protein cleaving enzymes (alpha secretases, beta secretase), N-acetylglucosamine-1-phosphodiester alpha-N-acetylglucosaminidase, PCSK9, superoxide dismutase-3, sulfatase; adhesion molecules (integrins, vitronectin); plasma proteins (albumin, coagulation factor VII/IX/X, von Willebrand factor, protein C, complement component); membrane receptors (for insulin, LDL, notch, sortilin, vitamin B12, hepatocyte growth factor, zona pellucida sperm receptor); structural proteins (collagen, fibrillin); membrane channels (sodium channel subunits); and angiogenesis inhibitors (semaphorins). Of note, many of these evaluations of the substrate specificity of furin have been accomplished through cellular coexpression and in vitro studies, and hence may not represent substrate cleavage under physiological conditions. However, recent tissue-specific KO data do provide support for many if not most of these substrate assignments (see below). In vivo studies showing cellular co-localization of substrate and furin in specific tissues can provide additional support in demonstrating physiological relevance. It should also be mentioned that other furin-like PCs, including PACE4, PC5/6, and PC7, are broadly distributed in tissues and cell lines and can redundantly cleave many of these substrates to a variable extent (reviewed in (65)).
Various floxed furin mouse lines have now been generated to better study the physiological roles of furin; these studies have confirmed that furin can both uniquely and/or redundantly cleave substrates in a tissue-specific manner. For example, in the mammary gland, furin inactivation impairs insulin receptor precursor (proIR) processing and decreases mammary tumor progression (101), while in the liver, furin-mediated proIR cleavage appears to be fully redundant with other furin-like PCs (102). Species differences in substrate cleavage sites have also been reported which result in differential substrate cleavage; for example, there is strong evidence that proGHRH is cleaved by furin in humans while being exclusively cleaved by PC1/3 in rodents (39).
Furin also plays a significant role in viral and bacterial pathogenicity by processing the envelope glycoproteins of viruses from many different families (Herpes-, Corona-, Flavi-, Toga-, Borna-, Bunya-, Filo-, Orthomyxo-, Paramyxo-, Pneumo- and Retroviridae), as well as in the activation of many bacterial protoxins, including anthrax toxin, diphtheria toxin, and Pseudomonas exotoxin (reviewed in (103, 104)). Additional information concerning the specific cleavage sites of various viral and bacterial glycoproteins can be found in the above-mentioned furin substrate database.
The wide distribution of furin means that the number of known furin substrates grows continuously; indeed, it is difficult to accurately capture all known furin substrates from the literature in any list. Miscellaneous substrates that are not currently listed in the above-cited furin database include the precursors to somatostatin (105); neurotrophin-3 and BDNF (35); renin receptor (106); subunit Ac45 of the V-ATPase pump (107); hepcidin (108); brain-specific angiogenesis inhibitor 2 (BAI2) (109); BMP-10 (110); N-cadherin (111); E-cadherin (112); human toll-like receptor-7 (113); apelin (114); kisspeptin (115); endothelial lipase (EL) and EL inhibitor (angiopoietin-like 3) (116); osteocalcin (OCN) (117); augurin (118); adrenomedullin (119); and dispatched (120).
Most furin-mediated precursor cleavage results in activation; however, certain proteins are inactivated by furin cleavage, for example, PCSK9 (121), fibroblast growth factor 23 (122), EL (116), and MMP-2 proforms (123).
Human FURIN polymorphisms and mutations
A variety of GWAS publications demonstrate association of FURIN variants with metabolic disorders, most notably with hypertension, but also with complex diseases such as atherosclerosis and schizophrenia. Li et al. first demonstrated a possible association between the common FURIN SNP rs2071410 and hypertension in the Xinjiang, Kazakh, and Uygur populations, the ethnic minority group with the highest prevalence of hypertension in China (124). A number of GWAS involving large populations which were conducted to identify genomic loci influencing blood pressure consistently find that FURIN gene variants are strongly linked to blood pressure measurements. FURIN variants mostly associated with individuals of European ancestry include: rs2521501, rs2071410, rs6227, and rs4702 (125-127). FURIN variants rs12917264, rs75493298, rs1573644, and rs74037507 exhibit significant intensity- and race-dependent associations with postexercise hypotension among adults with hypertension (128). In addition to hemodynamic traits, FURIN SNPs are also linked with other measures of metabolic syndromes. The rs17514846 SNP shows association with increased HDL cholesterol and decreased serum triglyceride concentrations, conferring protection against metabolic syndrome (129). The underlying molecular mechanisms for these associations remain to be investigated.
Indeed, whether these SNPs are gain or loss of function is not clear in most cases. Certain FURIN variants, for example rs2071410, are located within an intron (124, 126) and are thus predicted to negatively affect the splicing of furin mRNA transcripts, though this has not been experimentally demonstrated. The rs4702 SNP causes allele-dependent downregulation of furin expression, governed by the microRNA miR338 (127, 130). Sole examination of mRNA expression, however, will not yield a complete picture of the functionality of variant enzymes. Hou et al. showed that the rs4702 variant is indeed functional; rs4702 G allele–specific downregulation of furin expression by miR-338-3p results in both reduced enzyme maturation and reduced secretion of BDNF in human embryonic kidney 293T cells (130). The downstream effects of other furin variants remain to be elucidated.
Furin appears to play a pivotal role in the pathogenesis of atherosclerosis. Furin is expressed in a lesion stage–specific manner in various cell types, for example in endothelial cells, vascular smooth muscle cells, and mononuclear inflammatory cells within the atherosclerotic plaques (131); indeed, furin is the only PC whose expression is increased in atherosclerotic plaques from different vascular regions (132). A large population-based cohort study identified the rs17514846 SNP, located within an intronic region, as a new risk locus for coronary artery disease. Expression of the risk-associated allele results in higher furin expression in monocytes and endothelial cells, with increased migratory and proliferative ability. Furthermore, the risk allele is associated with greater carotid intima media thickness, a surrogate measure of atherosclerosis (133). The FURIN rs2071410 variant, previously linked with hypertension risk, also shows strong association with the incidence and prognosis of transient ischemic attack (134). Furin is partially shed from the cell surface into the circulation as an active enzyme, and its levels have been measured using enzyme-linked immunosorbent assay in patients with metabolic, cardiac, and immune disorders. Plasma furin levels are significantly associated with the incidence of hypertension (135), diabetes (136); with diabetes-associated cardiovascular complications (137); with myocardial infarction-associated complications (138); with rheumatic arthritis (139); with Sjögren’s syndrome (140), systemic lupus erythematosus (141). Further studies are warranted to confirm these findings in independent populations before shed furin can be considered as an objective serum biomarker for these and other metabolic and autoimmune disorders.
Integrative analysis of genetic associations from schizophrenia GWAS and brain expression quantitative trait (eQTL) analyses have identified FURIN as an important schizophrenia risk gene. Fine mapping was used to identify a FURIN cis-eQTL as a potentially causal variant (rs4702, probability = .94) (142). In vitro studies showed that the high-risk allele downregulates FURIN mRNA levels, an effect associated with reduced furin-mediated intracellular maturation of proBDNF (35, 130). BDNF is a key neurotrophin associated with several psychiatric disorders, including schizophrenia (143). However, a direct connection of reduced proBDNF processing in schizophrenic brain has not been unequivocally confirmed in autopsy studies. Schrode et al. further validated this FURIN cis-eQTL using CRISPR-mediated allelic conversion in hiPSC-derived neural cells; these studies indicate that genotype-associated changes in FURIN expression are cell-type dependent (142).
Furin variants also appear to contribute to viral susceptibility. A common SNP (rs4932178) in the furin gene promoter was found to influence the clinical outcome of hepatitis B infection in a Han Chinese population. Individuals carrying the T allele exhibited persistent hepatitis B infection which was associated with significantly elevated furin transcription (144). This same SNP has also been studied in patients with colorectal cancer and arterial disease; however, the regulatory effect of this SNP on furin expression was not replicated in these studies (132, 145, 146). Most recently, the Covid-19 virus has been shown to have evolved a novel furin consensus cleavage site in the spike protein, which is postulated to contribute greatly to the increased infectivity of this new pathogen (147).
Mouse models of furin insufficiency
Furin, which is expressed at embryonic day (E) 7.5, is crucial for embryonic development (148). Complete inactivation of the furin gene impairs processing of multiple members of the TGF-β family of proteins, such as lefty and bone morphogenetic protein, both important for heart development. Hence furin KO mice die between days 10.5 and 11.5 from hemodynamic insufficiency associated with the failure of heart tube morphogenesis (148). This indicates a nonredundant function of furin during early embryogenesis. However, mice lacking a single furin allele are viable and appear relatively normal, suggesting that ~50% of furin dosage is sufficient to mediate its critical functions (148).
To bypass the early lethality of furin KO mice and to better study the physiological role of furin in specific tissues, a variety of floxed furin mouse lines have been engineered (103, 149). These 9 different mouse lines, described below, reveal the many important physiological roles that furin plays in different tissues.
Liver-specific furin cKO mice
Furin-floxed mice were crossed with Mx-Cre mice to generate liver-specific furin KO mice; the Mx-Cre mouse expresses Cre recombinase under the control of interferon-inducible Mx promoter. This was the first temporally-controlled conditional furin KO mouse generated (102). Although these mice exhibit partial deletion of furin in several other tissues, near-complete inactivation of the floxed furin allele is observed in liver. Unlike the general furin KO mouse, liver-specific inactivation of the furin gene results in only a mild phenotype, with near-normal processing of typical substrates such as the precursors to the insulin receptor, albumin, α5 integrin, lipoprotein receptor-related protein, vitronectin and α1-microglobulin/bikunin. This suggests the existence of considerable functional redundancy in PC processing activity in the liver (102, 150). A second hepatocyte-specific conditional furin cKO was generated by crossing furin-floxed mice with Alb-Cre mice, which express Cre exclusively in post-natal liver under the control of the albumin promoter (151). Similarly to the inducible Mx-Cre/LoxP discussed above, the Alb-Cre/LoxP furin cKO does not exhibit an overt phenotype. However, in these mice, and likely also in the prior mouse model, complete disappearance of inactive PCSK9 forms is seen in plasma, clearly indicating that hepatocyte-derived furin is the convertase responsible for the in vivo cleavage of PCSK9. Thus, by regulating PCSK9 levels, furin indirectly regulates cholesterol metabolism (151).
Furin overexpression is common feature of many types of human cancer, and furin expression correlates both with tumor aggressiveness and with metastasis (reviewed in (152)). Conditional furin KO mice were generated to gain mechanistic insight into cancer pathogenesis and progression, as well as to explore the therapeutic potential of furin inhibition. Furin was genetically ablated in the hepatocytes of ASV-B mice, a well-established human hepatocellular carcinoma model, by intercross with the above-mentioned Alb-Cre/LoxP furin cKO mice (153). Near-complete loss of furin expression (>95%) in the livers of these conditional mice increased hepatocyte proliferation rates and enhanced early-onset tumor development (153). These data agree with clinical data for hepatocellular carcinoma that show that patients with high hepatoma furin expression (vs adjacent normal tissues) exhibit improved postoperative disease-free survival rates. Furin-mediated repression of hepatoma growth is further supported by mouse xenograft models. Furin-overexpressing xenografts, compared to mock controls, showed increased tumor growth when injected with a synthetic furin inhibitor (154). Taken together, these data suggest that furin inhibition does not represent a viable therapeutic option for liver cancer (153), but may be beneficial for other cancers such as lung, head and neck, breast, pancreas, colon, and brain, in which furin overexpression is correlated with a more aggressive or metastasizing phenotype (reviewed in (152, 155)). Liver-specific furin inhibition associated with tumor progression (153) is intriguing and implies that the correlation of furin expression with tumor progression may depend on the specific type of tumor. Thus, depending upon tumor environments and/or specific molecular mechanisms underlying tumor formation, furin inhibition may decrease or increase tumor progression (reviewed in (152)).
Endothelial cell-specific furin cKO mice
The furin KO mouse exhibits profound yolk sac vasculature defects, indicative of endothelial cell-specific functions for furin (148). This idea was later confirmed by the generation of endothelial-specific furin KO (ecKO) mice under the control of the endothelial cell-specific Tie2 promoter (119). ecKO mice die shortly after birth due to ventricular septal defects, supporting a nonredundant role of endothelially expressed furin in cardiogenesis. Surprisingly, no apparent vessel abnormalities are observed in this mouse model. Primary cultures of endothelial cells from furin ecKO mice fail to grow, but growth can be efficiently rescued upon addition of purified soluble furin (148), suggesting the need for furin-specific cleavage of an as-yet unidentified growth factor.
Cardiomyocyte-specific furin cKO mice
The critical role of furin in early cardiac development is further strengthened by a recent conditional KO of furin in cardiac progenitor cells (Isl1-Cre/Furinfl/fl, Nkx2-5IRESCre/Furinfl/fl) and in differentiated cardiomyocytes (Mlc2-cre/Furinfl/fl) (156). Embryos with a deletion of furin in cardiac progenitor cells die around mid-gestation from cardiac morphological defects, while deletion of furin in differentiated cardiomyocytes results in viable mice with a less penetrant cardiac conduction abnormality. Both early cardiac progenitor differentiation defects and cardiac conduction defects phenocopy mice and humans bearing a mutation in the Nkx2-5 homeodomain transcription factor, suggesting a common mechanism. Further analysis has now shown that Nkx2-5 is in fact the upstream transcriptional repressor of furin (156).
T-cell specific furin cKO mice
Furin is highly expressed in T helper type 1 cells (157) and plays an indispensable role in maintaining peripheral immune tolerance (158). CD4-Cre-driven furin deletion in T cells results in overactivation of effector T cells, aberrant T-helper cell polarization, and functionally defective regulatory T cells (Tregs) that fail to produce mature forms of the anti-inflammatory cytokine TGF-β1 (158) (reviewed in (149)). This eventually leads to the loss of immunologic tolerance and the spontaneous development of autoimmunity in aging animals (158) (reviewed in (149)). Furthermore, furin directly regulates T cell activation by modifying T cell receptor–induced transactivation (159). In a mouse model of rheumatoid arthritis, the Type 1 T helper (Th1)/Th2 balance was found to be skewed, with reduced Treg number and function. Systemic furin administration restores local Th1/Th2 balance and prevents joint destruction (160). A similar decrease in the proportion of Treg and elevated expression of furin has been reported in samples of peripheral blood and synovial fluid leucocytes taken from rheumatoid arthritis patients (139, 161). siRNA-mediated furin knockdown in cultured fibroblast-like synoviocytes, derived from patients with rheumatoid arthritis, further enhances their aggressive phenotype (162). These data indicate that elevated furin activity, by increasing T cell–dependent peripheral tolerance, may prevent the development of autoimmune disease, including rheumatoid arthritis.
In addition to its involvement in peripheral immune tolerance, T cell–expressed furin also plays a critical role in cell-mediated immunity. CD4-Cre-furfl/fl mice infected with a prototypic Th1 pathogen, Toxoplasma gondii, succumb to death from overwhelming infection due to the failure of furin-deficient T cells to generate an appropriate protective Th1 response (163). Increasing clinical data suggest that elevated levels of dysfunctional T cells play a major role in cancer pathogenesis. In an oncogene-induced triple negative breast cancer mouse model, T cell–specific furin deletion leads to decreased tumor size and lung metastasis (164). To the contrary, T cell–specific furin cKO mice subjected to chemically induced skin carcinomas exhibit a marked increase in tumor progression (165). These data support the idea that T cell–specific KO of furin can either increase or decrease tumor progression, depending upon the specific tumor environment and/or the varying molecular mechanisms underlying tumor formation.
Myeloid cell–specific furin cKO mice
In addition to its role in T cell–mediated adaptive immunity, furin also plays an important role in maintenance of innate immunity by regulating proinflammatory M1/anti-inflammatory M2-type macrophage balance (166). Mice with a conditional deletion of furin in myeloid cells, chiefly in activated macrophages and granulocytes (ie, lysozyme M-positive cells; LysM-Cre driver) appear healthy but exhibit a reduced number of splenocytes, elevated levels of the proinflammatory cytokines, and reduced levels of anti-inflammatory cytokines. Homozygote LysM-Cre-floxed mice challenged with lipopolysaccharide also show increased mortality, indicating that furin-mediated regulation of macrophage polarization is involved in host response to infection (166).
Salivary gland-specific furin cKO mice
Overexpression of the proto-oncogene pleomorphic adenoma gene 1 (PLAG1) occurs in various tumors, including salivary gland pleomorphic adenomas (167). To study the potential involvement of furin in PLAG1-induced tumor development, mice with a conditional furin KO in salivary glands were generated (168) Conditional inactivation of furin results in a delay in PLAG1-induced tumor development in a gene dosage–dependent manner, with homozygotes developing tumors later than heterozygotes. How furin expression might regulate PLAG1-induced salivary gland tumorigenesis has not been explained in terms of a molecular pathway (168), but these data support the idea that furin inhibition could represent a valuable therapeutic option for PLAG1-induced tumors.
Pancreas-specific furin cKO mice
Unlike PC1/3 and PC2, convertases that process substrates stored in regulated secretory granules, furin is thought to be mainly involved in the proteolytic maturation of constitutive secretory pathway substrates. However, both pathways are present in tissues such as the pancreas and brain. In order to explore the contribution of furin to regulated pathway biology, Louagie et al. conditionally knocked out furin in pancreatic islets by crossing floxed mice with Pdx-Cre driver mice (107). Conditional inactivation of furin expression in pancreatic islets reduced insulin and glucagon secretion secondary to impaired intragranular acidification; furin processes the Ac45 subunit of the vacuolar-type H+-ATPase in pancreatic β cells and thus helps to maintain an acidic pH in secretory granules. Since an acidic pH is essential for prohormone convertase maturation, as well as for enzymatic activity, loss of this pH gradient impacts proinsulin and proglucagon processing (107). A similarly impaired V-ATPase complex was observed in a recent β cell-specific furin KO mouse (RIP-Cre, lacking the human growth hormone expression enhancer) (169). Additionally, β cell furin cKO mice exhibit a reduced β cell mass, increased numbers of immature secretory granules, impaired insulin receptor precursor cleavage, and age-dependent glucose intolerance. These data show that furin, by regulating secretory granule biogenesis, granule acidification and β cell proliferation, can help to maintain glucose homeostasis (169). In a recent epidemiological study, higher fasting plasma levels of furin were found to be associated with dysregulated metabolic and hemodynamic traits and an increased incidence of diabetes in a middle-aged population (136).
Oocyte-specific furin cKO mice
Furin is abundantly expressed during follicular oocyte maturation. To specifically delete furin in primary oocyte follicles and in primordial oocyte follicles, furin-floxed mice (169) were crossed with Zp3-Cre mice or Gdf9-Cre mice respectively (170). Both homozygous cKO mouse lines show developmental arrest of early secondary follicles and infertility that phenocopies oocyte development failure seen in ADAMTS-1 null mice. In support, only a precursor form of ADAMTS1 is detected in oocytes from these homozygous KO mice. These data imply that furin, by activating the ADAMTS-1 precursor, participates in normal follicular development and in the maintenance of fertility (170).
Osteoblast-specific furin cKO mice
A recent study showed that furin nonredundantly regulates bone endocrine function (117). Furin mRNA is highly expressed in primary osteoblasts and is robustly induced during osteoblast differentiation. In vitro studies indicated that furin is likely the primary convertase for intracellular cleavage of the osteocalcin (OCN) precursor within osteoblasts. Mice lacking furin specifically in osteoblasts (furinosb–/–; furinfl/flOCN-Cre) were generated to study pro-OCN processing in vivo (117). In agreement with the in vitro work, homozygous furinosb–/– mice accumulate pro-OCN and also show decreased circulating levels of undercarboxylated OCN. Interestingly, the lack of mature OCN does not overtly affect osteoclast differentiation and bone mineralization but negatively impacts body energy metabolism, including age-dependent glucose intolerance. The possible presence of other furin-cleaved osteoblast products that might regulate feeding requires further investigation (117).
Virally-mediated furin knockdown studies
Viral studies have also proven useful in assigning physiological roles for furin. For example, furin action is implicated in the development of epilepsy, since lentivirally-mediated knockdown of furin expression suppresses epileptic seizure activity and severity, while furin-overexpressing (transgenic) mice show increased susceptibility and heightened epileptic activity (171). In line with this idea, furin expression is increased in the temporal neocortex of epileptic patients and in the hippocampus and cortex of epileptic mice. Whole cell patch clamp recording showed that furin influences neuronal activity by regulating postsynaptic gamma-aminobutyric acid A receptor–mediated synaptic transmission. The specific furin substrates that might mediate the regulation of gamma-aminobutyric acid A receptor activity have not yet been identified (171).
Summary
In summary, the critical role of furin in a variety of physiological and many pathological states have made it an attractive target for drug development, particularly in certain forms of cancer and infectious disease (172, 173). The crystal structure of soluble human furin was elucidated over 17 years ago (174), which has facilitated the development of specific structure-based novel furin inhibitors (175) (reviewed in (173)); certain of these drugs have already entered clinical trials (172, 176).
PC4 (gene name: PCSK4)
Unique features and distribution; substrates
PC4 is the major PC present in the male reproductive system. In testes, this convertase is concentrated in germ cells, mostly in spermatocytes and round spermatids; it is also detected in mature sperm (177). In the female reproductive system, PC4 is expressed in macrophage-like cells of the ovary, as well as in unfertilized and fertilized oocytes (178, (reviewed in 179)). PC4 is also expressed in human placenta (180) and endometrium (181). Although PC4 is enriched mainly in reproductive tissues, PCSK4 transcripts have been detected in a variety of tissues including pituitary gland, kidney, pancreas, liver, adrenal gland, etc. (182), as well as in various brain regions (https://www.proteinatlas.org/ENSG00000115257-PCSK4/brain). The presence of the PC4 protein in these nonreproductive tissues has, however, not yet been reported. The structure of human PC4 is shown in Fig. 1; while a transmembrane domain is predicted, as of yet it is not clear whether the majority of PC4 actually contains this domain. One characteristic that PC4 shares with PC7 is that the prodomain of both enzymes lacks a basic secondary cleavage site; however, unlike PC7, the biosynthesis and trafficking of PC4 within the secretory pathway has yet to be fully elucidated (183).
While a variety of PC substrates are known to be expressed in gonadal cells, only a small number of precursors have been validated as authentic PC4-specific substrates (184). One exclusive natural PC4 substrate in reproductive tissues is the peptide precursor propituitary adenylate cyclase–activating polypeptide (proPACAP), as its bioactive products, PACAP38 and PACAP27, are undetectable in PC4 KO mouse testes and ovaries (185). PACAP, through a cAMP-mediated signaling pathway, regulates follicle development, oocyte maturation, and ovarian and testicular steroidogenesis, and has been implicated both in sperm capacitation and in acrosome reactions during fertilization. In addition to its local effects at the gonadal level, PACAP centrally regulates reproduction at the hypothalamo-hypophyseal level by regulating the synthesis and release of gonadotropin releasing hormone and gonadotropins (reviewed in (186)). In transfection studies, proPACAP processing in endocrine/neuroendocrine cells was shown to be able to occur via the action of PC1/3 and PC2 (187), enzymes normally expressed in the hypothalamus. ADAM2, a sperm plasma membrane protein involved in sperm–egg plasma membrane interaction, has been identified as a second natural substrate of sperm PC4 (188). In addition, the sperm fertilization molecule acrosin-binding protein (ACRBP)/sp32 is not proteolytically processed in PC4 KO mice, and clearly represents another natural substrate for PC4 (189). Lastly, PC4 has been shown to efficiently convert the growth factor precursor proIGF-II to mature IGF-II (1–67) in placenta, assisting the regulation of fetoplacental growth (180). Of note, proIGF-II can also be cleaved by other PCs, including furin, PACE4, PC5/6B, but less efficiently than by PC4 (190). Since only a small number of endogenous substrates for PC4 have been identified to date, further proteomics studies of the PC4 KO mouse model, with emphasis on substrate identification, are needed to obtain a full understanding of its roles in human physiology and disease.
Human PCSK4 polymorphisms and mutations
Despite a large degree of homology (>90%) between the human and murine PCSK4 genes and the high expression of PCSK4 in human sperm plasma membranes, human placenta, and endometrium (179), only limited numbers of GWAS publications describe PCSK4 polymorphisms associated with infertility or other disease phenotypes. Recently, semen analysis of patients with infertility has identified duplications in 19p13.3; the most functionally correlated genes identified in these chromosomal loci are within a network of 7 genes which include PCSK4 (191). All 7 genes are associated with biological processes regulating male fertility; whether duplication of a single candidate gene is sufficient to cause infertility is not yet known (191), and thus the involvement of PCSK4 remains to be definitively determined. Furthermore, the study was carried out in a small population and thus requires replication in larger cohort of infertile patients. PCSK4 mRNA can be detected in human ejaculate and has been proposed as a potential noninvasive biomarker to evaluate the quality of sperm in the diagnosis and treatment of male infertility (reviewed in (179)). Pregnant women carrying intrauterine growth–restricted fetuses exhibit higher serum proIGF-II levels (180); whether this is associated with PCSK4 SNPs has not yet been studied. PCSK4 has also been identified as a member of a 6-gene signature panel with prognostic value for patients with endometrial carcinoma (192).
Mouse models of PC4 insufficiency
A global PC4 KO mouse line was generated by insertion of a lacZ–neoR domain into the Pcsk4 locus, causing deletion of exons 3-6 and resulting in an enzymatically inactive, truncated protein (177). Consistent with the distribution of this convertase in humans, PC4 KO male mice are severely subfertile. In vitro fertilization using PC4-deficient sperm is also greatly impaired (177). Reduced proteolytic processing of sperm surface proteins, and morphological defects in the head/acrosomes, are thought to impact the motility and binding of sperm to egg zona pellucida, resulting in incompetent fertilization (discussed in (193)). Moreover, eggs fertilized by PC4-deficient spermatozoa fail to develop to the blastocyst stage (177). The specific role of PC4 in sperm maturation and fertilization has gained attention as a possible pharmacologic target, and the development of synthetic PC4 inhibitors and/or antibodies has been suggested as a potential strategy for nonhormonal contraception or immunocontraception (194, 195). More extensive studies of this mouse may reveal novel causes for unexplained infertility in men.
Somewhat surprisingly, PC4 KO female mice are only mildly subfertile, and the ovaries of female PC4 KO mice exhibit delayed folliculogenesis secondary to macrophage hyperactivation (177, 178). Macrophages are the most abundant immune cells in the ovary, and macrophage-derived molecules play many roles in ovarian events, including follicular growth, steroidogenesis and luteinization (178, 196). Investigations of placental development, embryo implantation and fetal growth have not yet been carried out in PC4 KO mice, and would contribute to a better understanding of the role of this convertase in reproductive biology.
PC5/6 (gene name: PCSK5)
Unique features and distribution; substrates
The convertase PC5/6 (also designated PC5 or PC6) exists as 2 alternatively-spliced isoforms: soluble PC5/6A (915 residues); and membrane-bound PC5/6B (1877 residues) (197, 198). As shown in Fig. 1, as compared to PC5/6A (PC5A), PC5/6B (PC5B) has a longer cysteine-rich domain (CRD) and has additional transmembrane domain sequence and a cytosolic tail. The PC5/6 A isoform is sorted to both the regulated and constitutive secretory pathways, while the C-terminally extended B isoform is localized to the Golgi (199). Stretches of basic residues found in the CRD of PC5/6A bind to cell surface heparan sulfate proteoglycans and to tissue inhibitors of metalloproteases, and this is where secondary propeptide cleavage and activation of PC5/6 is thought to take place (200). At the cell surface, PC5/6A cleaves heparan sulfate proteoglycan–bound proteins such as lefty, endothelial lipase, ADAMTS4, and PCSK9 (201). Like furin, membrane-bound PC5/6B cycles from the cell surface back to the TGN through endosomes, cleaving substrates at various sites (201, 202). PC5/6 exhibits a wide tissue distribution, with PC5/6A being the predominant isoform in most tissues except intestine and kidney, where PC5/6B transcripts predominate (203).
PC5/6 processes and activates precursors of multiple cytokines and adhesion factors, such as the precursors to matrix metalloproteinases, N-cadherin, insulin-like growth factor, and others. A major PC5/6 substrate is growth differentiating factor 11, a secreted member of the TGF-β superfamily, a protein which plays a critical role in embryo development (particularly in anterior/posterior patterning, nephrogenesis, skeletal and anorectal development) (204). Inhibin and activin are glycoproteins structurally related to the TGF-β family whose processing by PC5/6 plays an important role in folliculogenesis (205). In the endometrium, PC5/6 cleaves the precursors of many important cell surface glycoproteins, such as dystroglycan, integrin-α; growth factors such as BMP-2; and structural proteins such as caldesmon and tropomyosin, thought to facilitate endometrial receptivity (206, 207).
PC5/6 inactivates endothelial and lipoprotein lipases, key enzymes in high density lipoprotein metabolism (208, 209). PC5/6-mediated cleavage of the precursors of integrin, matrix metalloproteinase, and tyrosine phosphatase receptor protein tyrosine phosphatase-µ results in trophic effects in vascular endothelial cells (210, 211). By processing the brain neural adhesion molecule L1, PC5/6 also assists in the processes of cell migration, adhesion, neurite outgrowth, myelination, and neuronal differentiation (212, 213). PC5/6 also processes and inactivates the adhesion protein N-cadherin (111, 198). Osteopontin, a small secreted protein that plays an important role in bone mineralization, has also recently been identified as a novel substrate for PC5/6 (214).
PC5/6A isoforms share certain substrate specificities with PC1/3 and PC2, and can also cleave hormone and neuropeptide precursors within the regulated secretory pathway; these include proglucagon (215), proGHRH (216), proneurotensin/neuromedin (217), proCCK (218), and proCART (219).
The wide variety of PC5/6 substrates identified to date, which undoubtedly do not represent a complete list, supports the important role of this enzyme in a range of cellular and physiological processes. The unusual cell biology of this enzyme, with isoform-specific activation at both the cell surface and within the constitutive and regulated secretory pathway, also lends this convertase a specific spatially restricted role that distinguishes it from furin and other constitutively expressed convertases.
Human PCSK5/6 polymorphisms and mutations
PCSK5 has been identified as a candidate gene in certain rare congenital diseases. Several research groups have independently reported nonsynonymous missense and frameshift variants of PCSK5 in patients with VACTERL, a rare congenital disease encompassing Vertebral defects, Anorectal malformations, Cardiac defects, Tracheoesophageal fistula, Renal anomalies, and Limb abnormalities (204, 220, 221). Pathogenic PCSK5 variants are however also carried by healthy parents and found in the general population, which implies a low penetrance (221).
In humans, PCSK5 is chiefly known as a regulator of HDL metabolism. Multiple PCSK5 variants that both positively and negatively modulate HDL-C levels have been reported; the most consistent and strongest association is with the intronic variants rs11144782 and rs11144766 (222). In addition to their negative association with HDL-C levels, these variants are also associated with increased levels of plasma triglycerides; very low–density lipoprotein cholesterol-C; and apolipoprotein B (222). The association between PCSK5 polymorphisms and HDL-C concentrations is further reinforced by the observation that allele-specific PCSK5 variants interact in a sex-specific manner with dietary polyunsaturated fatty acids to modulate HDL-C levels (223). PC5/6 is known to directly inactivate endothelial lipase and lipoprotein lipase, enzymes that play pivotal role in HDL metabolism (208, 209). PC5/6 was found to be enriched in atherosclerotic plaques, specifically in mononuclear cells such as macrophages/ foam cells, co-localized with its putative substrate integrin (131). However, contrary to the increased protein expression reported in this latter study, Turpeinen et al. reported significant downregulation of PCSK5 gene expression in carotid and aortic plaques (132). A second, more preliminary study reported a significant association of PCSK5 variants with lower extremity artery disease, though this study did not find a correlation with HDL-C values (224). These conflicting results require independent validation with larger numbers of patients. Mechanistic studies addressing how PCSK5 variants might regulate lipoproteins have not yet been carried out and should be the focus of future scientific studies that will help to explain its genetic association with both lipid levels and cardiovascular disease.
Mouse models of PC5/6 insufficiency
The first PC5/6 KO mice were generated by Essalmani et al. by removal of exon 4, common to both splice forms (225). Homozygous mice exhibited early embryonic lethality between E4.5 and E7.5, while heterozygotes were healthy and fertile (225). To bypass embryonic lethality, this group generated epiblast-specific conditional deletion of the first exon in Pcsk5 by crossing floxed mice with mice bearing a Meox2-Cre transgene. These homozygotes successfully passed through the implantation stage, but succumbed to death at birth (203). In another PC5/6 insufficiency model, a high-throughput ENU-induced chemical mutagenesis screen was used to identify a novel mutant mouse line bearing a PC5/6 C470R mutation (204). In vitro studies showed that this mutation, which perturbs the structure of the P domain, results in ER retention.
In agreement with human studies, homozygote PC5/6 cKO mice generated by breeding with Meox2-Cre-floxed pairs and Pcsk5 C470R mutant mice generated via chemical mutagenesis both exhibit major cardiac and noncardiac defects. Noncardiac malformations include anteroposterior axis defects with additional thoracic and lumbar vertebrae, and the lack of tails and kidneys; these defects nicely mirror human VACTERL traits. Cardiac malformations in these Pcsk5-deficient homozygotes include lesions such as dextrocardia, atrial and ventricular septal defects, and a common arterial trunk, all of which recapitulate human congenital heart disease (203, 204). Thus, these mouse lines represent good models for the VACTERL/caudal regression and Currarino syndromes with sacral and anorectal anomalies.
The spatiotemporal requirements of Pcsk5 expression in developing embryonic hearts were studied using conditional ablation of Pcsk5 from extraembryonic and lateral mesoderm using Mesp1-Cre driver mice (226). These studies reveal an essential and early role for PC5/6 in the cranio-cardiac mesoderm during heart development (226). In addition, PC5/6 also plays an important role in the cardiovascular system of adult mice. Endothelial cell–restricted PC5/6 KO mice (Pcsk5-floxed x Tie2-Cre) lack abnormal phenotypes at younger ages but begin developing cardiovascular hypotrophy and left-ventricular diastolic stiffness and dysfunction with advancing age (227). Reduced collagen deposition is observed in blood vessels, as well as in fibroblasts cocultured with endothelial cells derived from PC5/6 ecKO mice (227).
PC5/6 has been demonstrated in human atherosclerotic plaques (131), and PC5/6 expression is strongly upregulated in rat and pig vascular smooth muscle cells following vascular injury (228). Thus, the development of conditional PC5/6 KO mice may be useful in the identification of new targets for cardiovascular therapy. In addition, the role of PC5/6 in lipid metabolism and endothelial function should be further studied in these mice. Indeed, plasma lipid levels have not yet been measured in any Pcsk5-deficient mouse model; as discussed above, human PCSK5 variants are associated with altered levels of HDL and with other lipoprotein aberrations.
The intestine is highly enriched in PC5/6, specifically in the 6B isoform. In human cancers, PCSK5 is downregulated in many tumor types, including intestinal tumors (198). Consistent with the PCSK5 gene expression profile of colon tumors, a mouse model of colorectal cancer (ApcMin/+) exhibits downregulation of Pcsk5 and upregulation of furin expression. Mice bearing an enterocyte-specific conditional KO of Pcsk5 crossed with adenocarcinoma-susceptible ApcMin/+ mice show increased numbers of duodenal tumors and reduced survival rates compared with either parental line. These data imply that PC5/6 overexpression might play a protective role in human intestinal tumors (198), an idea that could be further profitably explored using this complex mouse model.
PACE4 (gene name: PCSK6)
Unique features and distribution; substrates
PACE4 (first termed Paired Basic Amino Acid Cleaving Enzyme 4) and alternatively named SPC4, was, as its name implies, the fourth mammalian PC to be discovered (229). The PCSK6 gene is the largest proprotein convertase gene, with 25 exons and uniquely large introns (10). PACE4 is broadly expressed in human tissues, including the heart, brain, placenta, lung, liver, uterine endometrium, and ovary, with particularly high expression in the corpus callosum, the spinal cord, and the liver (230, 231). As shown in Fig. 1, the PACE4 protein does not possess a transmembrane domain, but is tethered on the cell surface and/or in the extracellular matrix via its large cysteine-rich domain; these are also the loci where PACE4-mediated substrate processing largely takes place (reviewed in (150)). Multiple alternatively-spliced transcript variants of PCSK6, with distinct carboxy terminal structures, tissue distribution, intracellular localization and functions have been described. Indeed, 8 isoforms of human PACE4, including PACE4A-I, PACE4A-II, PACE4B, PACE4C, PACE4CS, PACE4D, PACE4E-I, and PACE4E-II have been reported; these include forms that are truncated, catalytically inactive, and/or retained in the ER (10). PACE4A, PACE4D and PACE4E mRNAs have been reported in various rat tissues (9); however, the presence of PACE4 isoforms has not been validated in independent studies, and the relative abundance of such forms in human tissues and cell lines requires further investigation.
In accordance with its cell surface/extracellular matrix localization, PACE4 accomplishes the processing of a number of membrane-bound cell surface precursors (reviewed in (65)). During mouse embryonic development, PACE4-mediated proteolytic cleavage of the TGF-β family of cytokines (for example BMP, nodal, and lefty) facilitates central nervous system patterning and the formation of the left–right axis (232). PACE4 also activates MMPs, including the membrane-type (MT) MMP precursors MT1- and MT2-MMP, major proteinases involved in cancer cell invasiveness (233, 234). In addition to these MMPs, PACE4 is also involved in the activation of MMPs involved in articular cartilage degradation, for example ADAMTS-4 and ADAMTS-5 (235). PACE4 also activates growth factor precursors, including insulin-like growth factor 2 (190) and progrowth differentiation factor-15 (236). The precursors of angiopoietin-like 3, an endothelial lipase inhibitor (116), and of insulin receptor isoform B (237) are cleaved at the cell surface by PACE4. PACE4 was identified as the long-sought physiological activator of corin, the major proatrial natriuretic peptide (ANP) cleaving enzyme (238, 239), which it appears to cleave in an interesting cosecretional reaction. PACE4 has also been suggested to be responsible for maintaining iron homeostasis through cleavage and shedding of the transferrin receptor-2 (240).
Human PCSK6 polymorphisms and mutations
Polymorphisms in the PCSK6 gene have been linked with various cardiovascular disease risk factors, including hypertension. Genetic association of PCSK6 variants with the regulation of diastolic blood pressure was first demonstrated in 2004 in a small study carried out in Chinese individuals; the PACE4 haplotype was significantly higher in individuals with high diastolic blood pressure than in controls (241). The novel PCSK6 variant (D282N) was later identified in another small study involving hypertensive patients (238). In vitro functional analyses show that the D282N substitution reduces PACE4-mediated corin activation in a dominant-negative fashion (238), implying oligomerization of PACE4 forms. PACE4 isoforms are upregulated at both the mRNA and protein levels in carotid plaques of patients with cardiovascular disease; the secreted isoform is the most significantly upregulated (242). Several independent cohort studies have further validated increased upregulation of PCSK6 expression in atherosclerotic lesions at both the mRNA and protein levels (243). A chromosomal locus containing the PCSK6 gene has also been linked to vessel wall instability and aortic dissection (244). PCSK6 has been identified as a potential candidate gene associated with atrial septal defects, one of the most common forms of human congenital heart disease. Whole genome linkage analyses indicate that the PCSK6 intronic variant rs4965381 is significantly associated with the septal defect phenotype (245).
PCSK6 variants are also associated with inflammatory joint conditions, including osteoarthritis and rheumatoid arthritis. The intronic rs900414 SNP is associated with protection against pain in knee osteoarthritis (246), while the rs4965833 polymorphism is associated with increased susceptibility to this disease (247). A third SNP, rs8029797, is highly associated with rheumatoid arthritis (248). Whether these variants are gain-or loss-of function is not clear, and would be interesting to learn; presumably, gain-of-function variants would result in increased osteoarthritis due to increased cleavage of MMP precursors.
PCSK6 variants have been implicated in the biological mechanisms that underlie the establishment of brain lateralization, cerebral asymmetry, and thus handedness. Handedness and cerebral asymmetry have been linked to neurodevelopmental disorders such as dyslexia (249). A GWAS for a quantitative measure of relative hand skill (the peg-board task) carried out in individuals with dyslexia revealed that 2 intronic PCSK6 SNPs, rs9806256 and rs11855415, are associated with handedness; rs11855415 represents the most highly associated variant (231). This allelic variant was not detected in the general population (231) nor in a healthy cohort not exhibiting dyslexia (250). Interestingly, a novel intronic variable number tandem repeat polymorphism in PCSK6 (rs10523972) was associated with handedness in the general population (250). Given the fact that very few proven dyslexia susceptibility genes are known to date (249), PCSK6 represents a promising candidate locus for further investigation.
PACE4 expression is clearly linked with tumor progression (reviewed in (251)). PACE4 is the PC most specifically overexpressed in prostate cancer, and has been validated as an important target in this disease (reviewed in (252)). PACE4 has been shown to play a critical role in the pathogenesis of prostate cancer through alternative splicing-mediated formation of a novel oncogenic C-terminally modified PACE4 isoform (PACE4-altCT). PACE4-altCT is retained intracellularly, where it cleaves progrowth differentiating factor 15 (glial-derived growth factor 15; also known as prostate differentiation factor), an abundantly expressed growth factor in prostate cancer (236). This mechanism remains to be investigated in other systems. A preliminary scan of various malignancies revealed that this oncogenic switch also occurs in other cancer types; the PACE4 mRNA splicing index varies across human tissues and different cancer types, with the highest index observed for tumors of lung, thyroid, adrenal, and testes (236). However, a subsequent study in malignant thyroid nodules showed contradictory results, with statistically lower expression of PACE4-altCT (253). Further research examining splicing switches in different tumor environments may help to explain the possible role of differential isoform expression in tumor progression. Unfortunately, the alternatively-spliced PACE4-altCT isoform is absent in mice, meaning that mice cannot be used as a model for mechanistic studies (236). From a clinical perspective, it is interesting to note that in vivo inhibitors of PACE4 activity, both silencing reagents and PACE4 inhibitors, have been found to have beneficial effects in a variety of cancer cell lines and xenograft cancer models (254).
Mouse models of PC6 insufficiency
PACE4 KO mice, generated by deleting an internal fragment, exhibit certain similarities to furin KO mice, but their phenotype is not as penetrant (reviewed in (184)). Homozygous PACE4 KO mice display complex craniofacial and cardiac malformations, altered left–right patterning, and visceral organ isomerism, while heterozygotes are normal and fertile. Twenty-five percent of homozygous KO embryos die prenatally, usually between E13.5 and E15.5, mainly as a consequence of cardiac malformation (septal defects) while the remaining homozygous KO mice are viable without any obvious cardiovascular abnormality (150, 232). The nonpenetrant phenotype of PACE4 KO mice implies some degree of functional redundancy by other convertases (reviewed in (184)). Though humans with complete PACE4 deficiency have not yet been reported, the presence of PCSK6 variants likely associated with PACE4 insufficiency has been linked with cardiac malformations, such as atrial septal defects and aortic dissection, in humans (244, 245).
PACE4 KO mice display improved pain thresholds in battery of algesiometric assays (246). Similar protection against painful stimuli is also observed in osteoarthritis patients bearing a specific PCSK6 variant (246). Since PACE4 is the main activator of the cartilage matrix–degrading proteases ADAMTS-4 and ADAMTS-5, and also regulates chondrogenic differentiation, PACE4 has been touted as a promising target for the development of osteoarthritis therapeutics (235, 255). However, this field has received little attention in the last decade, perhaps due to difficulty in identifying PACE4-specific small molecule inhibitors.
Similar to humans possessing PCSK6 variants, PACE4 KO mice exhibit impaired corin activation, undetectable proANP processing activity, and develop salt-sensitive hypertension (238). In addition to its likely ANP-related role in blood pressure regulation, PACE4 contributes to the progression of other cardiovascular diseases, such as atherosclerosis and myocardial infarction, by regulating vascular smooth muscle cell (SMC) and cardiac remodeling (243, 256). A study of primary SMCs and aortic explants isolated from Pcsk6 KO mice indicated reduced neointimal response and decreased SMC migration and proliferation (243). Gene expression profiling of carotid arteries from PACE4 KO mice indicates downregulation of all SMC contractile markers, as well as downregulation of many extracellular matrix molecules, especially proteoglycans (243). A positive association between Pcsk6 expression and SMC activation was corroborated using a rat carotid artery injury model (243), in agreement with the multiple independent human studies that show that PACE4 is one of the most significantly enriched molecules in carotid plaques and is associated with plaque instability (242, 243). In a mouse model of myocardial infarction, cardiomyocyte Pcsk6 mRNA and PACE4 immunoreactivity are both upregulated (256). Cardiomyocyte secretome analysis following hypoxia-induced cardiac ischemia in rats showed that PACE4 is the second most upregulated protein (256). Consistent with this idea, patients suffering from acute myocardial infarction show distinct serum PACE4 kinetics, with levels peaking on day 3 postinfarction and declining afterward. Interestingly, adeno-associated virus 9-mediated, cardiomyocyte-specific overexpression of Pcsk6 in mice shows that Pcsk6 expression potentiates adverse cardiac remodeling post-infarction by upregulating collagen production (256). PACE4 inhibitors may thus represent effective disease-modifying therapeutics in various cardiovascular diseases.
PC7 (gene name: PCSK7)
Unique features and distribution; substrates
PC7 is one of the most ancient, least conserved, and most distinct members of the subtilisin-like proprotein convertase family (257-259). PC7 is widely distributed in the body and is particularly enriched in tissues such as colon, duodenum, lymphoid-associated tissues, liver, central nervous system, pituitary, kidney, heart (257, 260), and the immune system (reviewed in (261)). Intracellularly, PC7 is mainly localized to the TGN and, like furin, shuttles between this compartment, the cell surface, and the endosome (262). The intracellular localization, trafficking, and zymogen activation of PC7 is however distinct from furin and other PCs. The cytosolic tail of PC7 contains a unique contiguous amino acid motif, Pro-Leu-Cys (PLC726) that is essential for its endosomal internalization from the cell surface (259). Furthermore, the PC7 cytosolic tail contains a cluster of 5 basic amino acids (His-Arg-Ser-Arg-Lys-Ala-Lys714) in the vicinity of 2 reversibly palmitoylated residues (Cys699 and Cys704) which are both essential for the TGN localization and endocytic trafficking of PC7. These trafficking motifs are distinct from those of furin and PC5/6B, which both require a cluster of acidic residues for TGN localization (262-264). Other work has shown that Leu is the only critical residue in the PLC726 motif; and that it, together with Glu-719 and Glu-721, facilitates substrate cleavage in addition to endosomal sorting (265). In addition to the conventional coat protein complex II (COPII) carrier-dependent ER-to-TGN route, PC7 has also been implicated in an unconventional and more rapid secretory pathway, COPII-independent trafficking, to reach the cell surface (260). The sorting mechanisms underlying this unconventional secretory pathway of PC7 still require further investigation.
Due to its wide expression, PC7 functions to process a variety of different protein precursors, often redundantly with other proprotein convertases (mainly furin). These precursors include proBMP4, proPDGF, proVEGF-C (266), proparathyroid hormone (267), proBDNF (36), proCCK (268); and the precursors to E-cadherin (112), ADAM10 (269), ANGPTL4 (270), sortilin, and apolipoprotein F (ApoF) (271).
Like furin, PC7 is expressed in T lymphocytes, the main target of HIV infection, and redundantly with furin cleaves viral glycoprotein gp160 into gp120/gp41; however, further cleavage of gp120 to gp77/gp53 is accomplished only by furin (272). PC7 also cleaves the Ebola virus and respiratory syncytial viral glycoproteins more effectively than furin (273). By virtue of its unique endosomal sorting and trafficking motifs, PC7 can nonredundantly cleave specific substrates, for example, human and rat transferrin receptor-1 (TfR1) (265, 274) and proEGF (275). PC7 uniquely cleaves human and rat TfR1 into a soluble shed form within endosomes (274). C-terminally truncated enzymes lacking the trafficking motif described above result in the formation of soluble PC7, associated with the loss of hTfR1 shedding activity (265). PC7 also participates in MHC I-mediated antigen presentation by rescuing an unstable MHC I peptide complex in post-ER compartments (276). This list of substrates cleaved by PC7 is not exhaustive, and other substrates are likely to be discovered in future, especially given that proteomics studies have apparently not yet been performed using PC7 KO tissues.
Human PCSK7 polymorphisms and mutations
Many different GWAS reports link PCSK7 variants with hepatic, metabolic and cardiovascular disease. PCSK7 variants influence circulating lipids and contribute to dyslipidemia by altering hepatic lipid metabolism (277). Both rare and common variants of PCSK7 have been extensively associated with circulating lipid profiles. Peloso et al. reported that a rare loss-of-function PCSK7 variant (rs142953140) is associated with increased circulating high density lipoproteins (HDL) and decreased triglycerides (278); another rare gain-of-function variant, rs11542139, is associated with reduced circulating lipid levels (277). In addition to rare variants, the common gain-of-function variants rs508487, rs236911, and rs236918, have been associated with elevated triglyceride levels (277, 279). A noncoding variant, rs236918, has previously been shown to positively correlate with higher circulating levels of soluble human transferrin receptor-1 (TfR1) (280) and with chronic liver damage in hereditary iron overload (281); a recent study found that this same variant is an important risk factor for nonalcoholic fatty liver disease (277).
A different PCSK7 SNP, rs2277287, is associated with lipid parameters in a population- and sex-specific manner (282). The rs508487 SNP is also in linkage disequilibrium with a promoter variant of the liver-derived apolipoprotein AV (ApoAV), an indirect activator of lipoprotein lipase (283) and is correlated with the levels of atherogenic small dense LDL particles (284). Additionally, data from a human obesity transcriptome project indicate very high expression of hepatocyte PCSK7 in individuals with severe obesity; this expression is correlated with the expression of genes involved in hepatic de novo lipogenesis, lipid oxidation, and hepatic fibrogenesis, suggesting a common pathway (277). These data support the idea that linkage of PCSK7 variants with the pathogenesis of hepatic and metabolic diseases is mediated through its regulation of lipid homeostasis.
In GWAS studies, PCSK7 variants have been shown to interact with weight loss diets and insulin response. Individuals possessing the rs236918 SNP exhibit a greater decrease in fasting insulin and improvements in insulin sensitivity following a 6-month diet rich in carbohydrates (285). Whole-genome sequencing recently provided evidence for PCSK7 copy number variation as a potential novel pathogenesis gene for a rare congenital heart anomaly known as total anomalous pulmonary venous connection, a disease in which pulmonary veins fail to make their normal connection to the left atrium (286). Further replication of this finding in additional patients are needed to establish copy number variants of PCSK7 as causative in this congenital disease.
Mouse models of PC7 insufficiency
Though PC7 is widely expressed within the body, appearing early in embryogenesis, surprisingly, PC7 KO mice are viable and exhibit only subtle phenotypes (213, 287). However, PC7 expression is indispensable in lower vertebrates such as Xenopus and zebrafish, and, in these organisms, its loss results in severe defects in neural and eye development, leading to complete lethality (266, 288). These data imply that the level of redundancy and/or the critical nature of PC7-mediated substrate processing differs greatly between mammalian and nonmammalian species (266).
A battery of behavioral tests show that PC7 KO mice exhibit anxiolytic and novelty-seeking behavior as well as impaired cognitive performance resulting from partial (~40%) loss of processing of proBDNF into BDNF. Reduced BDNF levels occur secondary to increased degradation of proBDNF, ascribed to reduced processing of prosortilin in PC7 KO mice; sortilin is an intracellular chaperone that binds to the prodomain of BDNF and regulates its sorting to the secretory pathway (36, 289). Furthermore, PC7 KO mice show a region-specific reduction in brain cholecystokinin content (268). Compared with PC1/3 (290) and PC2 KO mice (291), PC7 KO mice exhibit a greater CCK reduction in certain brain areas (268). Other neuropeptides do not seem to have been examined in these PC7 KO brains to date.
PC7 is expressed in immune cells (reviewed in (261)), and in an in vitro study overexpression of PC7 was able to rescue an unstable MHC I peptide complex (276). However, PC7 KO mice show no significant differences in either survival rate, blood pressure or plasma cytokine profiles when challenged with lipopolysaccharide-induced endotoxemic conditions (27). As high levels of Pcsk7 mRNA are found in CD8+, CD4+ and NK cells, as well as in bone marrow (reviewed in (261)), the development of conditional nulls may be required to investigate the physiological role of PC7 in the innate and adaptive immune responses.
In agreement with clinical data showing involvement of PC7 in lipid metabolism, PC7 KO mice exhibit dyslipidemia in a diet-dependent manner. PC7 KO mice placed on a high fat diet, but not a normal chow diet, secrete larger quantities of ApoAV from liver to plasma, resulting in increased adipose lipoprotein lipase activity with adipocyte triglyceride accumulation and adipocyte hypertrophy. Interestingly, a recent in vitro study has provided evidence to support the idea that PC7 regulates ApoAV levels in a nonenzymatic fashion by facilitating its lysosomal degradation (283). Taken together, the many associations of PC7 variants with lipid metabolism strengthen the importance of collecting genetic information regarding this convertase prior to dietary intervention in the control of metabolic diseases (285).
Summary and Conclusions
Proprotein convertases participate in a huge range of physiological functions due to their generation of a large variety of mature molecules, which include polypeptide hormones, neuropeptides, receptors, growth factors, adhesion molecules, membrane-bound transcription factors, plasma proteins, viral and bacterial glycoproteins. Thus, it is not unexpected that polymorphisms and mutations in the genes encoding these enzymes results in systemic dysregulation linked to many different disease conditions (reviewed in (150, 292)). Genetic KOs in mice represent a powerful tool to understand the physiological functions of specific genes and to establish causal relationships between genetic variation and phenotype (293, 294). Over the past 3 decades, KO studies in mice have provided clear links between proprotein convertase function and human disease. However, the study of each convertase is far from complete, since both novel substrates and additional convertase functions are continuously being revealed. In addition, novel human variants for each convertase are also constantly being described, with ramifications for clinical care. For example, the “blue diaper” syndrome of PCSK1 insufficiency is still receiving increasing attention as novel inactivating mutations are identified in infants (60, 295). This has now led to a prescribed program of care for these children (296). Thus, the study of proprotein convertases represents an evolving field, both in the clinical and in the research arenas.
What are the major challenges in modeling human proprotein convertase insufficiency in mice?
Several important challenges remain to be overcome in modeling human proprotein convertase insufficiency in mice. Not the least of these is the fact that certain proprotein convertases exhibit a high degree of functional redundancy. On the one hand, functional redundancy of PCs ensures substrate cleavage and thus helps to maintain homeostasis (297) (reviewed in (65)). On the other hand, redundant and/or excessive proteolytic activity can potentially be deleterious in disease pathogenesis, for example in cancer (155, 298). Redundancy is however not all-or-none; PCs can cleave specific substrates to different extents. For example, EL and lipoprotein lipase are cleaved by different constitutively-expressed PCs with different efficiencies in vitro; the order of cleavage efficiency is PACE4 > PC5/6A > furin for EL, whereas for lipoprotein lipase, the order of cleavage is furin > PACE4 > PC5/6A (208). Since in vivo, different PCs are coexpressed to variable extents with a specific substrate, the physiological contribution of a given PC to a specific substrate cleavage event is at times difficult to judge. Thus, while in vitro and cell-based enzymatic assays can rule out the participation of given enzyme, and mouse KO studies are highly informative when a specific convertase accomplishes the majority of cleavage, in cases where robust functional redundancy occurs, substantial in vivo work using proteomics and different null mouse models is required to definitively assign the participation of a given convertase to a specific cleavage event. Much of this proteomics work remains to be done.
A related challenge lies in the fact that certain proprotein convertases exhibit both unique and/or redundant expression and functionality depending upon tissue and developmental stage (65, 150). For example, PC5/6 expression is indispensable during embryonic development, while the endothelial cell-restricted PC5/6 KO mouse lacks any overt phenotype until adulthood (225, 227). Similarly, the deletion of furin in cardiac progenitor cells results in embryonic lethality, while its deletion in these same cells only after cardiomyocyte differentiation results in viable mice with a less penetrant heart defect (156). This suggests that a certain degree of functional redundancy, or change in expression levels, may be able to compensate for the loss of a given convertase in adult, but not in developing mice. Since other constitutively-expressed proprotein convertase nulls also exhibit embryonic lethality (184), the extent of redundancy within these other convertases can be revealed using conditional compound KOs. Traditional Cre-flox conditional KOs are however labor-intensive to construct; and cross-breeding of different lines requires many months of breeding to acquire multiply-floxed mice. With the advent of simpler and faster novel genome editing technique such as loxP-stop-loxP–controlled CRISPR/Cas9, it is now feasible to delete multiple genes simultaneously (299). The application of novel cutting-edge genome editing techniques will speed the production of conditionally-deleted proprotein convertases, which will supply additional information regarding physiological situations in which functional redundancy does or does not exist (300, 301).
Complicating matters further, certain constitutively-expressed proprotein convertases can act on the same substrate in opposite directions, as exemplified by the activation of the adhesion molecule N-cadherin by furin, but its inactivation by PC5/6 (reviewed in (65)). Conversely, certain convertases complement each other by acting sequentially on a given substrate; for example, the sequential maturation of POMC by PC1/3 and PC2 (reviewed in (65)). The degree of redundancy and complementarity between PC family members at the tissue or organismal level could be theoretically be addressed by generating compound KO models by simple mating of individual lines of KO mice. However, to our knowledge, only one double KO (PC1/3 and PC2) has been generated so far; while both prohormone convertase null mouse lines are viable, double KO of both convertases results in embryonic lethality (D.F. Steiner, personal communication). A better approach would involve tissue-specific KOs. It will be interesting in future studies to determine whether select tissue-specific KOs of PC1/3 and PC2 can indeed be mated successfully; this could reveal the tissue origin of the lethal phenotype. Similarly, cross-breeding of tissue-specific KOs of other mouse lines (such as the cross of ASV-B mice with Alb-Cre/LoxP furin cKO mice; see "Furin" section above) will permit us to identify specific substrate: enzyme pairings involved in human disease.
What are the challenges in studying human proprotein convertase insufficiency?
While germline KO mice have been generated for all proprotein convertases, due to their rarity, SNPs and mutations resulting in human proprotein convertase insufficiency have been reported only for select convertases, namely PC1/3, PC5/6, and PC7. Surprisingly, despite the fact that Pcsk2 null mice are fairly healthy, nonsynonymous PCSK2 gene variants are rare, and no variants definitively associated with insufficiency have been identified. Thus, the physiological consequences of human PCSK2 insufficiency cannot easily be studied. However, the analysis of Pcsk2 expression in obese mice supports the idea of an inverse correlation that is consistent with the improved glucose tolerance and reduced obesity seen in PC2 null mice (83, 93). Likely due to the nonreplaceable role for furin in embryogenesis, and its wide array of physiological functions, complete furin deficiency has also not been reported in humans. Gene and protein expression analyses have however shown furin dysregulation in various pathological conditions, most importantly in malignancy and metabolic syndromes (5, 103). Furin is also an important target in microbial disease, most recently exemplified by its proposed role in the activation of the coronavirus spike protein responsible for the 2020 pandemic, in which furin action seems to play a unique role (302, 303). Thus the study of furin redundancy and specificity has important clinical ramifications. Human genetic association studies have identified many novel SNPs in convertase loci which are associated with various disease traits. The main challenges of GWA studies are 3-fold, confirming significant, causal, and direct (vs indirect) associations between SNPs and disease states (304). Only a limited number of significant associations have been well replicated, and only a small number of meta-analyses have been conducted for convertase SNPs (eg, (126, 305, 306)). Given that convertases are essential for the production of so many important molecules involved in normal physiological functions, total loss of convertase function is likely rare in humans (though total functional loss has been confirmed for at least one convertase: PC1/3). Furthermore, diseases linked to convertase mutations often represent recessive traits, which renders both the identification and the causal linkage of variants to disease difficult (307). One way to successfully address this is to study closed human populations such as the Amish, a well-defined community with a relatively homogenous lifestyle. Certain variants that are rare in most human populations have become common in the Amish, providing unique opportunities for genetic studies (74, 308). Supporting this idea, a rare missense exonic PCSK2 variant (R430W) was identified in an Amish population at a minor allele frequency of 330 times its frequency in the general population. The increased prevalence of this allele in Amish individuals with diabetes supports the idea that this gain-of-function mutation enhances susceptibility to diabetes (74), in line with the mouse data discussed above indicating that reduced Pcsk2 expression is correlated with protection from obesity and diabetes. However, functional experiments in humans—for example, fasting measurements of the PC2-specific product glucagon in subjects bearing the R430W allele—still need to be performed to support this idea.
Common variants of PCs are also increasingly being identified by GWAS and linked to complex disease traits; however, only a very small number of these convertase variants (both rare and common) have been thoroughly investigated in functional studies using cells (40, 45, 54, 72, 74, 130, 144) or mouse models (51, 57, 60); this area clearly requires additional work to infer the direction of association and mechanism. It is likely that many common convertase variants have small effect sizes, causing only subtle genetic changes and resulting in either no phenotype or only a partial gain and/or loss of function. Further studies using genetically closed populations as well as additional association studies in different populations will be informative in providing support for specific associations of convertase mutations with abnormalities detected in mouse models.
Do proprotein convertases represent legitimate drug targets?
The question is often raised as to whether, given their involvement in so many physiological processes, proprotein convertases could ever represent legitimate drug targets. This problem is especially acute for furin, which is both widely distributed and required for the constitutive synthesis of many important cellular constituents, for example, surface receptors (309). Despite this concern, the available evidence to date suggests that short-term inhibition of furin is not nearly as toxic as once assumed. This evidence includes both tissue-specific furin KO animal models (see "Furin" section above), as well as past feasibility studies using furin inhibitors in a variety of pathological conditions: topical use of furin inhibitors in combating bacterial eye infection (310, 311); systemic exposure to bacterial toxins (312); and even cancer (reviewed in (103)), which would presumably employ longer-term inhibitor exposure. As discussed above, several recent studies suggest that furin inhibitors may be of use in combating coronavirus infection (302, 303). In some of these cases, overlapping inhibition of related convertases has even been proposed to be beneficial (103). While as yet only few preclinical studies have been performed, it seems likely that furin inhibitors will one day represent an important addition to the current arsenal of protease inhibitors employed as drugs.
More speculatively, other proprotein convertases may also in future develop into appropriate drug targets. While genetic studies indicate that inhibition of PC1/3 is likely deleterious, it may be possible to develop allosteric stimulators of this enzyme (see (47)). By contrast, pancreatic inhibition of PC2 (47), which would preferentially block glucagon rather than insulin production, could potentially be of use in the management of diabetes, given that glucagon receptor antagonists are already in the clinic. Lastly, considerable effort has been devoted by the Day laboratory into optimizing inhibitors for PACE4 (313), which have been proposed for disease applications as varied as prostate cancer to osteoarthritis.
Can mouse models accurately model human proprotein convertase insufficiency?
Table 1 summarizes the information on the mouse models of proprotein convertase insufficiency available to date. No single proprotein convertase KO mouse model generated thus far to model human PC deficiency is without limitations. For example, similarities and differences between human and mouse deficiency phenotypes are noticeably visible in germline Pcsk1 null mice. These mice show robust growth retardation but do not develop obesity, a major feature observed in PCSK1-deficient humans, but do mimic other features of endocrinopathy to a variable extent (39, 49). It is interesting to note that the Pcsk1-deficient mouse bearing the N222D mutation does develop maturity-onset obesity, more accurately phenocopying human PCSK1 deficiency (51). A new Pcsk1-deficient mouse model bearing the pV96L variant manifests transient diarrhea and short body length, both common features of human PCSK1 deficiency syndromes (60), while the novel G209R Pcsk1 mutant mouse, which mimics a human mutation (39, 314), exhibits neonatal lethality due to absorption defects, similar to infants (Shakya, Surbhi, Low, and Lindberg, unpublished results). Collectively, these Pcsk1 data illustrate the range of phenotypes caused by variably inactivating convertase mutations and suggest that generating multiple mouse models exhibiting variable extents of insufficiency may assist our understanding of human convertase insufficiency syndromes.
Table 1.
Phenotypes of proprotein convertase global and conditional knockout micea
| Gene | Model | References | Phenotype |
|---|---|---|---|
| Pcsk1 | Global PC1/3 KO | (27, 49) | Homozygotes: 60% viable, dwarf, hyperproinsulinemia, chronic mild diarrhea, ACTH deficiency but normal corticosterone level, innate immune defect; heterozygotes: not growth retarded but mildly obese and glucose intolerant |
| N222D substitution knock-in | (51) | Homozygotes: maturity onset obesity, hyperphagia, islet hypertrophy, hyperproinsulinemia, near normal plasma ACTH and corticosterone, develop glucose intolerance on high fat diet, hypogonadism with reduced fertility; heterozygotes: intermediate phenotypes | |
| N221D substitution knock-in | (57) | Both homozygotes and heterozygotes: neither obese, nor glucose intolerant; of normal size | |
| V96L substitution knock-in | (60) | Homozygotes: Obese, hyperphagia, glucose intolerant, insulin resistant, hyperproinsulinemia, transient diarrhea, mild growth retardation | |
| α-Cell-specific PC1/3 cKO; Gcg-Cre | (61) | Age dependent decrease in local active GLP-1 islet content and secretion, reduced insulin secretion and glucose intolerance with increasing metabolic stress | |
| Pcsk2 | Global PC2 KO | (83) | Postnatal-growth retardation, α and δ hyperplasia, complete lack of glucagon, chronic hypoglycemia |
| (93) | Improved glucose tolerance, reduced adiposity, hypoleptinemia, resistance to high fat diet-induced weight gain | ||
| (96) | Reduced nociception | ||
| (97) | Altered morphine tolerance and dependence | ||
| (95) | Salt-induced hypertension | ||
| (88) | On a specific 129Sv substrain background, see Cushing’s syndrome; lethal between 5 and 8 weeks | ||
| Furin | Global Furin KO | (148) | Embryonic lethal between E10.5 and 11.5; no axial rotation, no heart looping, ventral closure and cardiac defects, and multiple tissue abnormalities |
| Liver-specific furin cKO Mx-Cre | (102) | Only mild phenotype, near-normal processing of typical substrates such as the precursors to the insulin receptor, albumin, α5 integrin, lipoprotein receptor-related protein, vitronectin, and α1-microglobulin/bikunin | |
| Hepatocyte-specific furin cKO; Albumin-Cre | (151) | No overt phenotype, reduced circulating level of inactivated PCSK9 forms, increased lysosomal degradation of low-density lipoprotein receptor | |
| Hepatocyte-specific furin cKO in ASV-B mice ASVB+/− Alb-Cre | (153) | Early tumor onset, increased hepatocyte proliferation and increased tumor mass | |
| Endothelial cell-specific furin cKO; Tie2-Cre | (119) | Neonatal lethality with cardiac and vascular defects | |
| Cardiac progenitor cell- specific furin cKOs; Isl1cre; and Nkx2-5IRES-Cre | (156) | Embryonic lethality; spectrum of cardiac abnormalities | |
| Differentiated cardiomyocyte-specific furin cKO; Mlc2-Cre | (156) | Viable mice with a less penetrant heart defect, especially of atrioventricular conduction abnormalities | |
| T cell-specific furin cKO CD4-Cre | (158, 163) | Overactivation of effector T cells, defective regulatory T cells, inflammatory bowel disease, autoimmunity in aging animals, increased susceptibility to pathogenic infection | |
| T cell-specific furin cKO in breast cancer model; CD4-Cre furin floxed x MMTVPyMT | (164) | Decreased tumor size and reduction in lung metastasis | |
| Myeloid cell specific furin cKO; LysMCre | (166) | Reduced number of splenocytes; elevated proinflammatory M1 and reduced anti-inflammatory M2-type macrophage and cytokines | |
| Salivary gland-specific furin cKO; PLAG1+MMTV-Cre | (168) | Both homozygotes and heterozygotes: delayed salivary gland tumor development | |
| Pancreas-specific furin cKO; Pdx1-Cre | (107) | Impaired dense-core secretory granule acidification, mild glucose intolerance, decreased insulin secretion | |
| β cell-specific furin cKO; RIP-Cre | (169) | Impaired dense-core secretory granule acidification, reduced β cell mass, impaired insulin receptor cleavage, worsening glucose intolerance with advancing age | |
| Oocyte follicle-specific furin cKO; Zp3-Cre and Gdf9-Cre | (170) | Both primary follicle cKO and primordial follicle-specific furin cKO show developmental arrest of early secondary follicles and infertility | |
| Osteoblast-specific furin cKO mice; OCN-Cre | (117) | Age-dependent glucose intolerance under fed condition; reduction in energy expenditure and increase in food intake | |
| Pcsk4 | Global KO | (177, 193) | Males severely subfertile, morphological defect in the head/acrosomes; females mildly subfertile, delayed folliculogenesis |
| Pcsk5 | Global KO | (225) | Homozygotes: embryonic lethal; noncardiac abnormalities include anteroposterior axis defects with additional thoracic and lumbar vertebrae, lack of tails and kidneys phenocopying human VACTERL traits; dextrocardia, atrial and ventricular septal defects, common arterial trunk. Heterozygotes: healthy and fertile |
| Epiblast-specific Pcsk5 cKO; Meox2-Cre | (203) | Embryo passes through the implantation stage but dies at birth; cardiac and noncardiac defect similar to Global KO | |
| C470R substitution knock-in (Vcc) | (204) | Cardiac and noncardiac defect similar to Global KO | |
| Cranio-cardiac mesoderm specific cKO; Mesp1-Cre | (226) | Embryonic lethal; septal defects | |
| Endothelial cell-specific Pcsk5 cKO; Tie2-cre | (227) | Cardiovascular dysfunction with advancing age | |
| Enterocyte-specific Pcsk5 cKO in ApcMin mice (Vil-Cre) | (198) | Enhance intestinal tumorigenesis in duodenum, reduction in overall survival time | |
| Pcsk6 | Global KO | (232) | Homozygotes: 25% are embryonic lethal with septal defects, complex craniofacial, cardiac malformations, altered left-right patterning, visceral organ isomerism; 75% without overt cardiac abnormalities; improved pain thresholds; impaired corin activation; Heterozygotes: healthy and fertile |
| Pcsk7 | Global KO | (36, 287) | Lack any overt phenotype; demonstrate anxiolytic and novelty-seeking behavior |
Abbreviation: cKO, conditional knockout.
a Homozygote phenotypes displayed unless otherwise stated.
Differences in phenotypes between the mouse and human insufficiency states can potentially be explained by species-specific disparities in a variety of parameters, including (1) species-specific differential proprotein convertase expression in various tissues; for example, PC2 is expressed abundantly in mouse β cells but may be expressed to a much lower extent in human β cells (91); (2) species-specific differences in substrate cleavage sites, as observed with proGHRH (49) and prolactase-phlorizin hydrolase (315); and (3) differential convertase isoform expression, as observed with PACE4 (9, 10). Finally, genetic background strongly influences the phenotype of certain convertase KOs, for example the lethal strain dependence of the PC2 KO mouse (88), and the genetic basis for this profound strain difference—which may also pertain to human disease—is not yet known. Species-specific differences can be partly overcome through use of humanized mouse models (316). There is growing interest on the development of ever-more sophisticated mouse models, often carrying multiple mutant alleles on specific genetically humanized backgrounds in order to more faithfully mimic human disease.
Having pointed out these limitations, it is clear that mouse convertase insufficiency models have indeed contributed greatly to our understanding of proprotein convertase biology and function. The future use of knock-in mice with specific point mutations that mimic human mutations will provide additional information to elucidate specific roles in disease mechanisms. For example, the PC5/6 KO mouse (204) clearly adds to our knowledge of the role of growth factor processing during development; but PC5/6 knock-in mice modeling specific human mutations have yet to be made. Similarly, the Pcsk1 G209R mouse, which mimics a known human mutation (39), will serve as an important model system to study the unexplained gastrointestinal nutrient absorption defect that results in considerable infant mortality.
In summary, variations and mutations within proprotein convertase genes are clearly involved in the pathogenesis of a variety of human diseases. While many of the physiological mechanisms behind these observations are not yet understood, further studies of mutant mice—likely using known human point mutations, conditional mouse models, and variably inactivating mutations—will enable a more complete understanding of convertase function in human disease and will further define specific convertase roles in precursor cleavage.
Acknowledgments
We thank M. Weerakoddy for the preparation of Fig. 1.
Financial Support: This work was supported by National Institutes of Health, National Institute of Drug Abuse grant R01DA042351 to I.L.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- AD
Alzheimer’s disease
- ADAM
disintegrin and metalloproteinase
- ANP
atrial natriuretic peptide
- BMP
bone morphogenetic protein
- EL
endothelial lipase
- ER
endoplasmic reticulum
- ENU
N-ethyl-N-nitrosourea
- GHRH
growth hormone–releasing hormone
- GLP
glucagon-like peptide
- GWAS
genome-wide association study
- HDL
high-density lipoprotein
- IGF
insulin-like growth factor
- KO
knockout
- LDL
low-density lipoprotein
- MMP
matrix metalloproteinase
- MT
membrane type
- OCN
osteocalcin
- PC
proprotein convertase
- POMC
proopiomelanocortin
- proCART
prococaine/amphetamine-regulated transcript
- proCCK
procholecystokinin
- PDGF
platelet-derived growth factor
- PWS
Prader–Willi syndrome
- QTL
quantitative trait locus
- SNP
single nucleotide polymorphism
- T2D
type 2 diabetes
- TGF
transforming growth factor
- TGN
trans-Golgi network
- TNF
tumor necrosis factor
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
Additional Information
Disclosures: The authors confirm that they have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11(5):367-383. [DOI] [PubMed] [Google Scholar]
- 2. Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JW. PCSK1 mutations and human endocrinopathies: from obesity to gastrointestinal disorders. Endocr Rev. 2016;37(4):347-371. [DOI] [PubMed] [Google Scholar]
- 3. Ramos-Molina B, Martin MG, Lindberg I. PCSK1 variants and human obesity. Prog Mol Biol Transl Sci. 2016;140:47-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3(10):753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren K, Jiang T, Zheng XL, Zhao GJ. Proprotein convertase furin/PCSK3 and atherosclerosis: new insights and potential therapeutic targets. Atherosclerosis. 2017;262:163-170. [DOI] [PubMed] [Google Scholar]
- 6. Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53(12):2515-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res. 2018;122(10):1420-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malo J, Parajuli A, Walker SW. PCSK9: from molecular biology to clinical applications. Ann Clin Biochem. 2020;57(1):7-25. [DOI] [PubMed] [Google Scholar]
- 9. Tsuji A, Mori K, Hine C, Tamai Y, Nagamune H, Matsuda Y. The tissue distribution of mRNAs for the PACE4 isoforms, kexin-like processing protease: PACE4C and PACE4D mRNAs are major transcripts among PACE4 isoforms. Biochem Biophys Res Commun. 1994;202(3):1215-1221. [DOI] [PubMed] [Google Scholar]
- 10. Tsuji A, Hine C, Tamai Y, et al. Genomic organization and alternative splicing of human PACE4 (SPC4), kexin-like processing endoprotease. J Biochem. 1997;122(2):438-452. [DOI] [PubMed] [Google Scholar]
- 11. Seidah NG, Chrétien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848(1-2):45-62. [DOI] [PubMed] [Google Scholar]
- 12. Rockwell NC, Krysan DJ, Komiyama T, Fuller RS. Precursor processing by kex2/furin proteases. Chem Rev. 2002;102(12):4525-4548. [DOI] [PubMed] [Google Scholar]
- 13. Elagoz A, Benjannet S, Mammarbassi A, Wickham L, Seidah NG. Biosynthesis and cellular trafficking of the convertase SKI-1/S1P: ectodomain shedding requires SKI-1 activity. J Biol Chem. 2002;277(13):11265-11275. [DOI] [PubMed] [Google Scholar]
- 14. Pullikotil P, Benjannet S, Mayne J, Seidah NG. The proprotein convertase SKI-1/S1P: alternate translation and subcellular localization. J Biol Chem. 2007;282(37):27402-27413. [DOI] [PubMed] [Google Scholar]
- 15. Seidah NG, Benjannet S, Wickham L, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A. 2003;100(3):928-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roubtsova A, Chamberland A, Marcinkiewicz J, et al. PCSK9 deficiency unmasks a sex- and tissue-specific subcellular distribution of the LDL and VLDL receptors in mice. J Lipid Res. 2015;56(11):2133-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li F, Wang Y, Li C, et al. Twenty years of bioinformatics research for protease-specific substrate and cleavage site prediction: a comprehensive revisit and benchmarking of existing methods. Brief Bioinform. 2019;20(6):2150-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Southey BR, Amare A, Zimmerman TA, Rodriguez-Zas SL, Sweedler JV. NeuroPred: a tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucleic Acids Res. 2006;34(Web Server issue):W267-W272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327Pt (3):625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smeekens SP, Avruch AS, LaMendola J, Chan SJ, Steiner DF. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991;88(2):340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seidah NG, Gaspar L, Mion P, Marcinkiewicz M, Mbikay M, Chrétien M. cDNA sequence of 2 distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990;9(10):789. [DOI] [PubMed] [Google Scholar]
- 22. Hoshino A, Kowalska D, Jean F, Lazure C, Lindberg I. Modulation of PC1/3 activity by self-interaction and substrate binding. Endocrinology. 2011;152(4):1402-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fricker LD, McKinzie AA, Sun J, et al. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci. 2000;20(2):639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. Identification of peptides from brain and pituitary of Cpe(fat)/Cpe(fat) mice. Proc Natl Acad Sci U S A. 2001;98(17):9971-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, Fricker LD. The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J Biol Chem. 2000;275(31):23596-23601. [DOI] [PubMed] [Google Scholar]
- 26. Cameron A, Fortenberry Y, Lindberg I. The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1. FEBS Lett. 2000;473(2):135-138. [DOI] [PubMed] [Google Scholar]
- 27. Refaie S, Gagnon S, Gagnon H, et al. Disruption of proprotein convertase 1/3 (PC1/3) expression in mice causes innate immune defects and uncontrolled cytokine secretion. J Biol Chem. 2012;287(18):14703-14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rouillé Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J Biol Chem. 1995;270(44):26488-26496. [DOI] [PubMed] [Google Scholar]
- 29. Wardman JH, Zhang X, Gagnon S, et al. Analysis of peptides in prohormone convertase 1/3 null mouse brain using quantitative peptidomics. J Neurochem. 2010;114(1):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cameron A, Apletalina E, Lindberg I. 11 The enzymology of PC1 and PC2. In: Dalbey RE, ed. The Enzymes. San Diego: Academic Press; 2002:291-331. [Google Scholar]
- 31. Hoshino A, Lindberg I. Peptide Biosynthesis: Prohormone Convertases 1/3 and 2. Fricker LD, Devi L, eds. Morgan & Claypool Life Sciences Publishers; 2012. [Google Scholar]
- 32. Ugleholdt R, Poulsen ML, Holst PJ, et al. Prohormone convertase 1/3 is essential for processing of the glucose-dependent insulinotropic polypeptide precursor. J Biol Chem. 2006;281(16):11050-11057. [DOI] [PubMed] [Google Scholar]
- 33. Jorsal T, Rhee NA, Pedersen J, et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61(2):284-294. [DOI] [PubMed] [Google Scholar]
- 34. Zhang XY, Liu F, Chen Y, Guo WC, Zhang ZH. Proprotein convertase 1/3-mediated down-regulation of brain-derived neurotrophic factor in cortical neurons induced by oxygen-glucose deprivation. Neural Regen Res. 2020;15(6):1066-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379(3):247-250. [DOI] [PubMed] [Google Scholar]
- 36. Wetsel WC, Rodriguiz RM, Guillemot J, et al. Disruption of the expression of the proprotein convertase PC7 reduces BDNF production and affects learning and memory in mice. Proc Natl Acad Sci U S A. 2013;110(43):17362-17367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Rahilly S, Gray H, Humphreys PJ, et al. Brief report: impaired processing of prohormones associated with abnormalities of glucose homeostasis and adrenal function. N Engl J Med. 1995;333(21):1386-1390. [DOI] [PubMed] [Google Scholar]
- 38. Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303-306. [DOI] [PubMed] [Google Scholar]
- 39. Martín MG, Lindberg I, Solorzano-Vargas RS, et al. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology. 2013;145(1):138-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blanco EH, Ramos-Molina B, Lindberg I. Revisiting PC1/3 mutants: dominant-negative effect of endoplasmic reticulum-retained mutants. Endocrinology. 2015;156(10):3625-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pépin L, Colin E, Tessarech M, et al. A new case of PCSK1 pathogenic variant with congenital proprotein convertase 1/3 deficiency and literature review. J Clin Endocrinol Metab. 2019;104(4):985-993. [DOI] [PubMed] [Google Scholar]
- 42. Burnett LC, LeDuc CA, Sulsona CR, et al. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader-Willi syndrome. J Clin Invest. 2017;127(1):293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Polex-Wolf J, Lam BY, Larder R, et al. Hypothalamic loss of Snord116 recapitulates the hyperphagia of Prader-Willi syndrome. J Clin Invest. 2018;128(3):960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei X, Ma X, Lu R, et al. Genetic variants in PCSK1 gene are associated with the risk of coronary artery disease in type 2 diabetes in a Chinese Han population: a case control study. PLoS One. 2014;9(1):e87168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blanco EH, Peinado JR, Martín MG, Lindberg I. Biochemical and cell biological properties of the human prohormone convertase 1/3 Ser357Gly mutation: a PC1/3 hypermorph. Endocrinology. 2014;155(9):3434-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Creemers JW, Roebroek AJ, Van de Ven WJ. Expression in human lung tumor cells of the proprotein processing enzyme PC1/PC3. Cloning and primary sequence of a 5 kb cDNA. FEBS Lett. 1992;300(1):82-88. [DOI] [PubMed] [Google Scholar]
- 47. Vivoli M, Caulfield TR, Martínez-Mayorga K, Johnson AT, Jiao GS, Lindberg I. Inhibition of prohormone convertases PC1/3 and PC2 by 2,5-dideoxystreptamine derivatives. Mol Pharmacol. 2012;81(3):440-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bruzzaniti A, Mains RE. Enzymatic activity of soluble and membrane tethered peptide pro-hormone convertase 1. Peptides. 2002;23(5):863-875. [DOI] [PubMed] [Google Scholar]
- 49. Zhu X, Zhou A, Dey A, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A. 2002;99(16):10293-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731-2738. [DOI] [PubMed] [Google Scholar]
- 51. Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15(11):1884-1893. [DOI] [PubMed] [Google Scholar]
- 52. Than ME, Henrich S, Bourenkov GP, Bartunik HD, Huber R, Bode W. The endoproteinase furin contains 2 essential Ca2+ ions stabilizing its N-terminus and the unique S1 specificity pocket. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 5):505-512. [DOI] [PubMed] [Google Scholar]
- 53. Jackson RS, Creemers JW, Farooqi IS, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112(10):1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prabhu Y, Blanco EH, Liu M, et al. Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function. Endocrinology. 2014;155(7):2391-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stijnen P, Brouwers B, Dirkx E, et al. Endoplasmic reticulum-associated degradation of the mouse PC1/3-N222D hypomorph and human PCSK1 mutations contributes to obesity. Int J Obes (Lond). 2016;40(6):973-981. [DOI] [PubMed] [Google Scholar]
- 56. Choi S, Korstanje R. Proprotein convertases in high-density lipoprotein metabolism. Biomark Res. 2013;1(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jarvela TS, Shakya M, Bachor T, White A, Low MJ, Lindberg I. Reduced stability and pH-dependent activity of a common obesity-linked PCSK1 polymorphism, N221D. Endocrinology. 2019;160(11):2630-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benzinou M, Creemers JW, Choquet H, et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008;40(8):943-945. [DOI] [PubMed] [Google Scholar]
- 59. Creemers JW, Choquet H, Stijnen P, et al. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes. 2012;61(2):383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muhsin NIA, Bentley L, Bai Y, Goldsworthy M, Cox RD. A novel mutation in the mouse Pcsk1 gene showing obesity and diabetes. Mamm Genome. 2020;31(1-2):17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Traub S, Meier DT, Schulze F, et al. Pancreatic α cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Rep. 2017;18(13):3192-3203. [DOI] [PubMed] [Google Scholar]
- 62. Vivoli Vega M, Lindberg I. Prohormone convertase 2. In: Handbook of Biologically Active Peptides. Amsterdam: Academic Press; 2013:1797-1802. [Google Scholar]
- 63. Zhu X, Lindberg I. 7B2 facilitates the maturation of proPC2 in neuroendocrine cells and is required for the expression of enzymatic activity. J Cell Biol. 1995;129(6):1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee SN, Lindberg I. 7B2 prevents unfolding and aggregation of prohormone convertase 2. Endocrinology. 2008;149(8):4116-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seidah NG, Sadr MS, Chrétien M, Mbikay M. The multifaceted proprotein convertases: their unique, redundant, complementary, and opposite functions. J Biol Chem. 2013;288(30):21473-21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ozawa A, Cai Y, Lindberg I. Production of bioactive peptides in an in vitro system. Anal Biochem. 2007;366(2):182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang X, Pan H, Peng B, Steiner DF, Pintar JE, Fricker LD. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J Neurochem. 2010;112(5):1168-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen YC, Taylor AJ, Verchere CB. Islet prohormone processing in health and disease. Diabetes Obes Metab. 2018;20(Suppl (2):64-76. [DOI] [PubMed] [Google Scholar]
- 69. Yoshida H, Ohagi S, Sanke T, Furuta H, Furuta M, Nanjo K. Association of the prohormone convertase 2 gene (PCSK2) on chromosome 20 with NIDDM in Japanese subjects. Diabetes. 1995;44(4):389-393. [DOI] [PubMed] [Google Scholar]
- 70. Leak TS, Keene KL, Langefeld CD, et al. Association of the proprotein convertase subtilisin/kexin-type 2 (PCSK2) gene with type 2 diabetes in an African American population. Mol Genet Metab. 2007;92(1-2):145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zheng X, Ren W, Zhang S, et al. Association of type 2 diabetes susceptibility genes (TCF7L2, SLC30A8, PCSK1 and PCSK2) and proinsulin conversion in a Chinese population. Mol Biol Rep. 2012;39(1):17-23. [DOI] [PubMed] [Google Scholar]
- 72. Jonsson A, Isomaa B, Tuomi T, Eriksson JG, Groop L, Lyssenko V. Effect of a common variant of the PCSK2 gene on reduced insulin secretion. Diabetologia. 2012;55(12):3245-3251. [DOI] [PubMed] [Google Scholar]
- 73. Chang TJ, Chiu YF, Sheu WH, et al. Genetic polymorphisms of PCSK2 are associated with glucose homeostasis and progression to type 2 diabetes in a Chinese population. Sci Rep. 2015;5:14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Winters A, Ramos-Molina B, Jarvela TS, Yerges-Armstrong L, Pollin TI, Lindberg I. Functional analysis of PCSK2 coding variants: a founder effect in the Old Order Amish population. Diabetes Res Clin Pract. 2017;131:82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kwee LC, Liu Y, Haynes C, et al. A high-density genome-wide association screen of sporadic ALS in US veterans. PLoS One. 2012;7(3):e32768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hokama M, Oka S, Leon J, et al. Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cereb Cortex. 2014;24(9):2476-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van Wamelen DJ, Aziz NA, Zhao J, et al. Decreased hypothalamic prohormone convertase expression in Huntington disease patients. J Neuropathol Exp Neurol. 2013;72(12):1126-1134. [DOI] [PubMed] [Google Scholar]
- 78. Xia L, Ou J, Li K, et al. Genome-wide association analysis of autism identified multiple loci that have been reported as strong signals for neuropsychiatric disorders. Autism Res. 2020;13(3):382-396. [DOI] [PubMed] [Google Scholar]
- 79. Winsky-Sommerer R, Grouselle D, Rougeot C, et al. The proprotein convertase PC2 is involved in the maturation of prosomatostatin to somatostatin-14 but not in the somatostatin deficit in Alzheimer’s disease. Neuroscience. 2003;122(2):437-447. [DOI] [PubMed] [Google Scholar]
- 80. Zhang R, Xu XJ, Zhang HF, Han SP, Han JS. The role of the oxytocin/arginine vasopressin system in animal models of autism spectrum disorder. Adv Anat Embryol Cell Biol. 2017;224:135-158. [DOI] [PubMed] [Google Scholar]
- 81. Chen XY, Du YF, Chen L. neuropeptides exert neuroprotective effects in Alzheimer’s disease. Front Mol Neurosci. 2018;11:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Petrella C, Di Certo MG, Barbato C, et al. Neuropeptides in Alzheimer’s disease: an update. Curr Alzheimer Res. 2019;16(6):544-558. [DOI] [PubMed] [Google Scholar]
- 83. Furuta M, Yano H, Zhou A, et al. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A. 1997;94(13):6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J Neurochem. 2003;86(3):556-563. [DOI] [PubMed] [Google Scholar]
- 85. Furuta M, Carroll R, Martin S, et al. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. J Biol Chem. 1998;273(6):3431-3437. [DOI] [PubMed] [Google Scholar]
- 86. Furuta M, Zhou A, Webb G, et al. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J Biol Chem. 2001;276(29):27197-27202. [DOI] [PubMed] [Google Scholar]
- 87. Vincent M, Guz Y, Rozenberg M, et al. Abrogation of protein convertase 2 activity results in delayed islet cell differentiation and maturation, increased alpha-cell proliferation, and islet neogenesis. Endocrinology. 2003;144(9):4061-4069. [DOI] [PubMed] [Google Scholar]
- 88. Peinado JR, Laurent V, Lee SN, et al. Strain-dependent influences on the hypothalamo-pituitary-adrenal axis profoundly affect the 7B2 and PC2 null phenotypes. Endocrinology. 2005;146(8):3438-3444. [DOI] [PubMed] [Google Scholar]
- 89. Sarac MS, Zieske AW, Lindberg I. The lethal form of Cushing’s in 7B2 null mice is caused by multiple metabolic and hormonal abnormalities. Endocrinology. 2002;143(6):2324-2332. [DOI] [PubMed] [Google Scholar]
- 90. Westphal CH, Muller L, Zhou A, et al. The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell. 1999;96(5):689-700. [DOI] [PubMed] [Google Scholar]
- 91. Ramzy A, Asadi A, Kieffer TJ. Revisiting proinsulin processing: evidence that human β-cells process proinsulin with prohormone convertase (PC) 1/3 but Not PC2. Diabetes. 2020;69(7):1451-1462. [DOI] [PubMed] [Google Scholar]
- 92. Davalli AM, Perego L, Bertuzzi F, et al. Disproportionate hyperproinsulinemia, beta-cell restricted prohormone convertase 2 deficiency, and cell cycle inhibitors expression by human islets transplanted into athymic nude mice: insights into nonimmune-mediated mechanisms of delayed islet graft failure. Cell Transplant. 2008;17(12):1323-1336. [DOI] [PubMed] [Google Scholar]
- 93. Anini Y, Mayne J, Gagnon J, et al. Genetic deficiency for proprotein convertase subtilisin/kexin type 2 in mice is associated with decreased adiposity and protection from dietary fat-induced body weight gain. Int J Obes (Lond). 2010;34(11):1599-1607. [DOI] [PubMed] [Google Scholar]
- 94. Berman Y, Mzhavia N, Polonskaia A, et al. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J Neurochem. 2000;75(4):1763-1770. [DOI] [PubMed] [Google Scholar]
- 95. Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111(8):1251-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Croissandeau G, Wahnon F, Yashpal K, et al. Increased stress-induced analgesia in mice lacking the proneuropeptide convertase PC2. Neurosci Lett. 2006;406(1-2):71-75. [DOI] [PubMed] [Google Scholar]
- 97. Lutfy K, Parikh D, Lee DL, et al. Prohormone convertase 2 (PC2) null mice have increased mu opioid receptor levels accompanied by altered morphine-induced antinociception, tolerance and dependence. Neuroscience. 2016;329:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chang SY, DeVera C, Yang Z, et al. Hippocampal changes in mice lacking an active prohormone convertase 2. Hippocampus. 2020;30(7):715-723. [DOI] [PubMed] [Google Scholar]
- 99. Roebroek AJ, Schalken JA, Bussemakers MJ, et al. Characterization of human c-fes/fps reveals a new transcription unit (fur) in the immediately upstream region of the proto-oncogene. Mol Biol Rep. 1986;11(2):117-125. [DOI] [PubMed] [Google Scholar]
- 100. Tian S, Huang Q, Fang Y, Wu J. FurinDB: a database of 20-residue furin cleavage site motifs, substrates and their associated drugs. Int J Mol Sci. 2011;12(2):1060-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. He Z, Khatib AM, Creemers JWM. Loss of proprotein convertase furin in mammary gland impairs proIGF1R and proIR processing and suppresses tumorigenesis in triple negative breast cancer. Cancers (Basel). 2020;12(9):2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roebroek AJ, Taylor NA, Louagie E, et al. Limited redundancy of the proprotein convertase furin in mouse liver. J Biol Chem. 2004;279(51):53442-53450. [DOI] [PubMed] [Google Scholar]
- 103. Braun E, Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunology. 2019;8(8):e1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Klenk HD, Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2(2):39-43. [DOI] [PubMed] [Google Scholar]
- 105. Galanopoulou AS, Seidah NG, Patel YC. Direct role of furin in mammalian prosomatostatin processing. Biochem J. 1995;309(Pt 1):33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53(6):1077-1082. [DOI] [PubMed] [Google Scholar]
- 107. Louagie E, Taylor NA, Flamez D, et al. Role of furin in granular acidification in the endocrine pancreas: identification of the V-ATPase subunit Ac45 as a candidate substrate. Proc Natl Acad Sci U S A. 2008;105(34):12319-12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gagliardo B, Kubat N, Faye A, et al. Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process. J Hepatol. 2009;50(2):394-401. [DOI] [PubMed] [Google Scholar]
- 109. Okajima D, Kudo G, Yokota H. Brain-specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. J Recept Signal Transduct Res. 2010;30(3):143-153. [DOI] [PubMed] [Google Scholar]
- 110. Susan-Resiga D, Essalmani R, Hamelin J, et al. Furin is the major processing enzyme of the cardiac-specific growth factor bone morphogenetic protein 10. J Biol Chem. 2011;286(26):22785-22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maret D, Sadr MS, Sadr ES, Colman DR, Del Maestro RF, Seidah NG. Opposite roles of furin and PC5A in N-cadherin processing. Neoplasia. 2012;14(10):880-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bessonnard S, Mesnard D, Constam DB. PC7 and the related proteases Furin and Pace4 regulate E-cadherin function during blastocyst formation. J Cell Biol. 2015;210(7):1185-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hipp MM, Shepherd D, Gileadi U, et al. Processing of human toll-like receptor 7 by furin-like proprotein convertases is required for its accumulation and activity in endosomes. Immunity. 2013;39(4):711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shin K, Pandey A, Liu XQ, Anini Y, Rainey JK. Preferential apelin-13 production by the proprotein convertase PCSK3 is implicated in obesity. FEBS Open Bio. 2013;3(1):328-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Harihar S, Pounds KM, Iwakuma T, Seidah NG, Welch DR. Furin is the major proprotein convertase required for KISS1-to-Kisspeptin processing. PLoS One. 2014;9(1):e84958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Essalmani R, Susan-Resiga D, Chamberland A, et al. Furin is the primary in vivo convertase of angiopoietin-like 3 and endothelial lipase in hepatocytes. J Biol Chem. 2013;288(37):26410-26418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Al Rifai O, Chow J, Lacombe J, et al. Proprotein convertase furin regulates osteocalcin and bone endocrine function. J Clin Invest. 2017;127(11):4104-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ozawa A, Lick AN, Lindberg I. Processing of proaugurin is required to suppress proliferation of tumor cell lines. Mol Endocrinol. 2011;25(5):776-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kim W, Essalmani R, Szumska D, et al. Loss of endothelial furin leads to cardiac malformation and early postnatal death. Mol Cell Biol. 2012;32(17):3382-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stewart DP, Marada S, Bodeen WJ, et al. Cleavage activates dispatched for Sonic Hedgehog ligand release. Elife. 2018;7:e31678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Benjannet S, Rhainds D, Hamelin J, Nassoury N, Seidah NG. The proprotein convertase (PC) PCSK9 is inactivated by furin and/or PC5/6A: functional consequences of natural mutations and post-translational modifications. J Biol Chem. 2006;281(41):30561-30572. [DOI] [PubMed] [Google Scholar]
- 122. Tagliabracci VS, Engel JL, Wiley SE, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A. 2014;111(15):5520-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cao J, Rehemtulla A, Pavlaki M, Kozarekar P, Chiarelli C. Furin directly cleaves proMMP-2 in the trans-Golgi network resulting in a nonfunctioning proteinase. J Biol Chem. 2005;280(12):10974-10980. [DOI] [PubMed] [Google Scholar]
- 124. Li N, Luo W, Juhong Z, et al. Associations between genetic variations in the FURIN gene and hypertension. BMC Med Genet. 2010;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ganesh SK, Tragante V, Guo W, et al. ; CARDIOGRAM, METASTROKE; LifeLines Cohort Study . Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum Mol Genet. 2013;22(8):1663-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Turpeinen H, Seppälä I, Lyytikäinen LP, et al. A genome-wide expression quantitative trait loci analysis of proprotein convertase subtilisin/kexin enzymes identifies a novel regulatory gene variant for FURIN expression and blood pressure. Hum Genet. 2015;134(6):627-636. [DOI] [PubMed] [Google Scholar]
- 128. Cilhoroz BT, Schifano ED, Panza GA, et al. FURIN variant associations with postexercise hypotension are intensity and race dependent. Physiol Rep. 2019;7(3):e13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ueyama C, Horibe H, Yamase Y, et al. Association of FURIN and ZPR1 polymorphisms with metabolic syndrome. Biomed Rep. 2015;3(5):641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hou Y, Liang W, Zhang J, et al. Schizophrenia-associated rs4702 G allele-specific downregulation of FURIN expression by miR-338-3p reduces BDNF production. Schizophr Res. 2018;199:176-180. [DOI] [PubMed] [Google Scholar]
- 131. Stawowy P, Kallisch H, Borges Pereira Stawowy N, et al. Immunohistochemical localization of subtilisin/kexin-like proprotein convertases in human atherosclerosis. Virchows Arch. 2005;446(4):351-359. [DOI] [PubMed] [Google Scholar]
- 132. Turpeinen H, Raitoharju E, Oksanen A, et al. Proprotein convertases in human atherosclerotic plaques: the overexpression of FURIN and its substrate cytokines BAFF and APRIL. Atherosclerosis. 2011;219(2):799-806. [DOI] [PubMed] [Google Scholar]
- 133. Yang X, Yang W, McVey DG, et al. FURIN expression in vascular endothelial cells is modulated by a coronary artery disease-associated genetic variant and influences monocyte transendothelial migration. J Am Heart Assoc. 2020;9(4):e014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sun QX, Zhou HM, Du QW. Association of Rs2071410 on furin with transient ischemic attack susceptibility and prognosis in a Chinese population. Med Sci Monit. 2016;22:3828-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. He Y, Ren L, Zhang Q, et al. Serum furin as a biomarker of high blood pressure: findings from a longitudinal study in Chinese adults. Hypertens Res. 2019;42(11):1808-1815. [DOI] [PubMed] [Google Scholar]
- 136. Fernandez C, Rysä J, Almgren P, et al. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med. 2018;284(4):377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fathy SA, Abdel Hamid FF, Zabut BM, Jamee AF, Ali MA, Abu Mustafa AM. Diagnostic utility of BNP, corin and furin as biomarkers for cardiovascular complications in type 2 diabetes mellitus patients. Biomarkers. 2015;20(6-7):460-469. [DOI] [PubMed] [Google Scholar]
- 138. Wang YK, Tang JN, Han L, et al. Elevated FURIN levels in predicting mortality and cardiovascular events in patients with acute myocardial infarction. Metabolism. 2020;111:154323. [DOI] [PubMed] [Google Scholar]
- 139. Valli A, Ranta N, Grönholm A, Silvennoinen O, Pesu M, Isomäki P. Increased expression of the proprotein convertase enzyme FURIN in rheumatoid arthritis. Scand J Rheumatol. 2019;48(3):173-177. [DOI] [PubMed] [Google Scholar]
- 140. Ranta N, Valli A, Grönholm A, et al. Proprotein convertase enzyme FURIN is upregulated in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2018;36(Suppl 112)(3):47-50. [PubMed] [Google Scholar]
- 141. Wu T, Ding H, Han J, et al. Antibody-array-based proteomic screening of serum markers in systemic lupus erythematosus: a discovery study. J Proteome Res. 2016;15(7):2102-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schrode N, Ho SM, Yamamuro K, et al. Synergistic effects of common schizophrenia risk variants. Nat Genet. 2019;51(10):1475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nieto R, Kukuljan M, Silva H. BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry. 2013;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lei RX, Shi H, Peng XM, Zhu YH, Cheng J, Chen GH. Influence of a single nucleotide polymorphism in the P1 promoter of the furin gene on transcription activity and hepatitis B virus infection. Hepatology. 2009;50(3):763-771. [DOI] [PubMed] [Google Scholar]
- 145. Declercq J, Jacobs B, Biesmans B, et al. Single nucleotide polymorphism (rs4932178) in the P1 promoter of FURIN is not prognostic to colon cancer. Biomed Res Int. 2015;2015(5):321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Försti A, Li X, Wagner K, et al. Polymorphisms in the transforming growth factor beta 1 pathway in relation to colorectal cancer progression. Genes Chromosomes Cancer. 2010;49(3):270-281. [DOI] [PubMed] [Google Scholar]
- 147. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Roebroek AJ, Umans L, Pauli IG, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125(24):4863-4876. [DOI] [PubMed] [Google Scholar]
- 149. Declercq J, Creemers J. Therapeutic potential of furin inhibition: an evaluation using a conditional furin knockout mouse model. Colloquium Series on Protein Activation and Cancer. 2012;1(4):1-30. [Google Scholar]
- 150. Creemers JW, Khatib AM. Knock-out mouse models of proprotein convertases: unique functions or redundancy? Front Biosci. 2008;13:4960-4971. [DOI] [PubMed] [Google Scholar]
- 151. Essalmani R, Susan-Resiga D, Chamberland A, et al. In vivo evidence that furin from hepatocytes inactivates PCSK9. J Biol Chem. 2011;286(6):4257-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Jaaks P, Bernasconi M. The proprotein convertase furin in tumour progression. Int J Cancer. 2017;141(4):654-663. [DOI] [PubMed] [Google Scholar]
- 153. Declercq J, Brouwers B, Pruniau VP, et al. Liver-specific inactivation of the proprotein convertase FURIN leads to increased hepatocellular carcinoma growth. Biomed Res Int. 2015;2015(6):148651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Huang YH, Lin KH, Liao CH, Lai MW, Tseng YH, Yeh CT. Furin overexpression suppresses tumor growth and predicts a better postoperative disease-free survival in hepatocellular carcinoma. PLoS One. 2012;7(7):e40738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Khatib AM, Siegfried G, Chrétien M, Metrakos P, Seidah NG. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am J Pathol. 2002;160(6):1921-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Dupays L, Towers N, Wood S, David A, Stuckey DJ, Mohun T. Furin, a transcriptional target of NKX2-5, has an essential role in heart development and function. PLoS One. 2019;14(3):e0212992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pesu M, Muul L, Kanno Y, O’Shea JJ. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma. Blood. 2006;108(3):983-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Pesu M, Watford WT, Wei L, et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455(7210):246-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ortutay Z, Oksanen A, Aittomäki S, Ortutay C, Pesu M. Proprotein convertase FURIN regulates T cell receptor-induced transactivation. J Leukoc Biol. 2015;98(1):73-83. [DOI] [PubMed] [Google Scholar]
- 160. Lin H, Ah Kioon MD, Lalou C, et al. Protective role of systemic furin in immune response-induced arthritis. Arthritis Rheum. 2012;64(9):2878-2886. [DOI] [PubMed] [Google Scholar]
- 161. Niu Q, Cai B, Huang ZC, Shi YY, Wang LL. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int. 2012;32(9):2731-2736. [DOI] [PubMed] [Google Scholar]
- 162. Wu C, Song Z, Liu H, et al. Inhibition of furin results in increased growth, invasiveness and cytokine production of synoviocytes from patients with rheumatoid arthritis. Joint Bone Spine. 2017;84(4):433-439. [DOI] [PubMed] [Google Scholar]
- 163. Oksanen A, Aittomäki S, Jankovic D, et al. Proprotein convertase FURIN constrains Th2 differentiation and is critical for host resistance against Toxoplasma gondii. J Immunol. 2014;193(11):5470-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. He Z, Khatib AM, Creemers JWM. Loss of the proprotein convertase Furin in T cells represses mammary tumorigenesis in oncogene-driven triple negative breast cancer. Cancer Lett. 2020;484:40-49. [DOI] [PubMed] [Google Scholar]
- 165. Vähätupa M, Aittomäki S, Martinez Cordova Z, et al. T-cell-expressed proprotein convertase FURIN inhibits DMBA/TPA-induced skin cancer development. Oncoimmunology. 2016;5(12):e1245266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cordova ZM, Grönholm A, Kytölä V, et al. Myeloid cell expressed proprotein convertase FURIN attenuates inflammation. Oncotarget. 2016;7(34):54392-54404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Declercq J, Van Dyck F, Braem CV, et al. Salivary gland tumors in transgenic mice with targeted PLAG1 proto-oncogene overexpression. Cancer Res. 2005;65(11):4544-4553. [DOI] [PubMed] [Google Scholar]
- 168. De Vos L, Declercq J, Rosas GG, et al. MMTV-cre-mediated fur inactivation concomitant with PLAG1 proto-oncogene activation delays salivary gland tumorigenesis in mice. Int J Oncol. 2008;32(5):1073-1083. [PubMed] [Google Scholar]
- 169. Brouwers B, Coppola I, Vints K, et al. Loss of Furin in β cells Induces an mTORC1-ATF4 Anabolic Pathway that Leads to β cell Dysfunction. Diabetes. 2020:db200474. doi: 10.2337/db20-0474. [DOI] [PubMed] [Google Scholar]
- 170. Meng TG, Hu MW, Ma XS, et al. Oocyte-specific deletion of furin leads to female infertility by causing early secondary follicle arrest in mice. Cell Death Dis. 2017;8(6):e2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yang Y, He M, Tian X, et al. Transgenic overexpression of furin increases epileptic susceptibility. Cell Death Dis. 2018;9(11):1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Senzer N, Barve M, Kuhn J, et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther. 2012;20(3):679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Couture F, Kwiatkowska A, Dory YL, Day R. Therapeutic uses of furin and its inhibitors: a patent review. Expert Opin Ther Pat. 2015;25(4):379-396. [DOI] [PubMed] [Google Scholar]
- 174. Henrich S, Cameron A, Bourenkov GP, et al. The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat Struct Biol. 2003;10(7):520-526. [DOI] [PubMed] [Google Scholar]
- 175. Dahms SO, Hardes K, Becker GL, Steinmetzer T, Brandstetter H, Than ME. X-ray structures of human furin in complex with competitive inhibitors. ACS Chem Biol. 2014;9(5):1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ghisoli M, Barve M, Mennel R, et al. Three-year follow up of GMCSF/bi-shRNA(furin) DNA-transfected autologous tumor immunotherapy (Vigil) in metastatic advanced Ewing’s sarcoma. Mol Ther. 2016;24(8):1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mbikay M, Tadros H, Ishida N, et al. Impaired fertility in mice deficient for the testicular germ-cell protease PC4. Proc Natl Acad Sci U S A. 1997;94(13):6842-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tadros H, Chrétien M, Mbikay M. The testicular germ-cell protease PC4 is also expressed in macrophage-like cells of the ovary. J Reprod Immunol. 2001;49(2):133-152. [DOI] [PubMed] [Google Scholar]
- 179. Gyamera-Acheampong C, Mbikay M. Proprotein convertase subtilisin/kexin type 4 in mammalian fertility: a review. Hum Reprod Update. 2009;15(2):237-247. [DOI] [PubMed] [Google Scholar]
- 180. Qiu Q, Basak A, Mbikay M, Tsang BK, Gruslin A. Role of pro-IGF-II processing by proprotein convertase 4 in human placental development. Proc Natl Acad Sci U S A. 2005;102(31):11047-11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Freyer C, Kilpatrick LM, Salamonsen LA, Nie G. Pro-protein convertases (PCs) other than PC6 are not tightly regulated for implantation in the human endometrium. Reproduction. 2007;133(6):1189-1197. [DOI] [PubMed] [Google Scholar]
- 182. Badola S, Spurling H, Robison K, et al. Correlation of serpin-protease expression by comparative analysis of real-time PCR profiling data. Genomics. 2006;88(2):173-184. [DOI] [PubMed] [Google Scholar]
- 183. Gyamera-Acheampong C, Sirois F, Denis NJ, et al. The precursor to the germ cell-specific PCSK4 proteinase is inefficiently activated in transfected somatic cells: evidence of interaction with the BiP chaperone. Mol Cell Biochem. 2011;348(1-2):43-52. [DOI] [PubMed] [Google Scholar]
- 184. Scamuffa N, Calvo F, Chrétien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20(12):1954-1963. [DOI] [PubMed] [Google Scholar]
- 185. Li M, Mbikay M, Arimura A. Pituitary adenylate cyclase-activating polypeptide precursor is processed solely by prohormone convertase 4 in the gonads. Endocrinology. 2000;141(10):3723-3730. [DOI] [PubMed] [Google Scholar]
- 186. Winters SJ, Moore JP Jr. PACAP: A regulator of mammalian reproductive function. Mol Cell Endocrinol. 2020;518:110912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Li M, Shuto Y, Somogyvári-Vigh A, Arimura A. Prohormone convertases 1 and 2 process ProPACAP and generate matured, bioactive PACAP38 and PACAP27 in transfected rat pituitary GH4C1 cells. Neuroendocrinology. 1999;69(3):217-226. [DOI] [PubMed] [Google Scholar]
- 188. Iamsaard S, Vanichviriyakit R, Hommalai G, et al. Enzymatic activity of sperm proprotein convertase is important for mammalian fertilization. J Cell Physiol. 2011;226(11):2817-2826. [DOI] [PubMed] [Google Scholar]
- 189. Tardif S, Guyonnet B, Cormier N, Cornwall GA. Alteration in the processing of the ACRBP/sp32 protein and sperm head/acrosome malformations in proprotein convertase 4 (PCSK4) null mice. Mol Hum Reprod. 2012;18(6):298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Duguay SJ, Jin Y, Stein J, Duguay AN, Gardner P, Steiner DF. Post-translational processing of the insulin-like growth factor-2 precursor. Analysis of O-glycosylation and endoproteolysis. J Biol Chem. 1998;273(29):18443-18451. [DOI] [PubMed] [Google Scholar]
- 191. Singh V, Bala R, Chakraborty A, Rajender S, Trivedi S, Singh K. Duplications in 19p13.3 are associated with male infertility. J Assist Reprod Genet. 2019;36(10):2171-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang Y, Ren F, Chen P, Liu S, Song Z, Ma X. Identification of a six-gene signature with prognostic value for patients with endometrial carcinoma. Cancer Med. 2018;7(11):5632-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gyamera-Acheampong C, Tantibhedhyangkul J, Weerachatyanukul W, et al. Sperm from mice genetically deficient for the PCSK4 proteinase exhibit accelerated capacitation, precocious acrosome reaction, reduced binding to egg zona pellucida, and impaired fertilizing ability. Biol Reprod. 2006;74(4):666-673. [DOI] [PubMed] [Google Scholar]
- 194. Basak A, Goswami M, Rajkumar A, et al. Enediynyl peptides and iso-coumarinyl methyl sulfones as inhibitors of proprotein convertases PCSK8/SKI-1/S1P and PCSK4/PC4: design, synthesis and biological evaluations. Bioorg Med Chem Lett. 2015;25(10):2225-2237. [DOI] [PubMed] [Google Scholar]
- 195. Dahril A, Keumala V, Mustafa A. Human spermatozoa anti-proprotein convertase subtilisin/kexin type 4 synthesis using New Zealand rabbit for novel immunocontraception in males. Investig Clin Urol. 2019;60(4):303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11(4):790-797. [DOI] [PubMed] [Google Scholar]
- 197. Nakagawa T, Murakami K, Nakayama K. Identification of an isoform with an extremely large Cys-rich region of PC6, a Kex2-like processing endoprotease. FEBS Lett. 1993;327(2):165-171. [DOI] [PubMed] [Google Scholar]
- 198. Sun X, Essalmani R, Seidah NG, Prat A. The proprotein convertase PC5/6 is protective against intestinal tumorigenesis: in vivo mouse model. Mol Cancer. 2009;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. De Bie I, Marcinkiewicz M, Malide D, et al. The isoforms of proprotein convertase PC5 are sorted to different subcellular compartments. J Cell Biol. 1996;135(5):1261-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Nour N, Mayer G, Mort JS, et al. The cysteine-rich domain of the secreted proprotein convertases PC5A and PACE4 functions as a cell surface anchor and interacts with tissue inhibitors of metalloproteinases. Mol Biol Cell. 2005;16(11):5215-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Mayer G, Hamelin J, Asselin MC, et al. The regulated cell surface zymogen activation of the proprotein convertase PC5A directs the processing of its secretory substrates. J Biol Chem. 2008;283(4):2373-2384. [DOI] [PubMed] [Google Scholar]
- 202. Seidah NG, Mayer G, Zaid A, et al. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40(6-7):1111-1125. [DOI] [PubMed] [Google Scholar]
- 203. Essalmani R, Zaid A, Marcinkiewicz J, et al. In vivo functions of the proprotein convertase PC5/6 during mouse development: Gdf11 is a likely substrate. Proc Natl Acad Sci U S A. 2008;105(15):5750-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Szumska D, Pieles G, Essalmani R, et al. VACTERL/caudal regression/Currarino syndrome-like malformations in mice with mutation in the proprotein convertase Pcsk5. Genes Dev. 2008;22(11):1465-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Antenos M, Lei L, Xu M, Malipatil A, Kiesewetter S, Woodruff TK. Role of PCSK5 expression in mouse ovarian follicle development: identification of the inhibin α- and β-subunits as candidate substrates. PLoS One. 2011;6(3):e17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Paule S, Aljofan M, Simon C, Rombauts LJ, Nie G. Cleavage of endometrial α-integrins into their functional forms is mediated by proprotein convertase 5/6. Hum Reprod. 2012;27(9):2766-2774. [DOI] [PubMed] [Google Scholar]
- 207. Heng S, Cervero A, Simon C, et al. Proprotein convertase 5/6 is critical for embryo implantation in women: regulating receptivity by cleaving EBP50, modulating ezrin binding, and membrane-cytoskeletal interactions. Endocrinology. 2011;152(12):5041-5052. [DOI] [PubMed] [Google Scholar]
- 208. Jin W, Fuki IV, Seidah NG, Benjannet S, Glick JM, Rader DJ. Proprotein convertases [corrected] are responsible for proteolysis and inactivation of endothelial lipase. J Biol Chem. 2005;280(44):36551-36559. [DOI] [PubMed] [Google Scholar]
- 209. Jin W, Wang X, Millar JS, et al. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 2007;6(2):129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Campan M, Yoshizumi M, Seidah NG, Lee ME, Bianchi C, Haber E. Increased proteolytic processing of protein tyrosine phosphatase mu in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family. Biochemistry. 1996;35(12):3797-3802. [DOI] [PubMed] [Google Scholar]
- 211. Fritzsche J, Stawowy P. Time for endothelial cell proprotein convertase PC5/6 in cardiovascular medicine? J Mol Med (Berl). 2011;89(11):1061-1063. [DOI] [PubMed] [Google Scholar]
- 212. Kalus I, Schnegelsberg B, Seidah NG, Kleene R, Schachner M. The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J Biol Chem. 2003;278(12):10381-10388. [DOI] [PubMed] [Google Scholar]
- 213. Seidah NG. What lies ahead for the proprotein convertases? Ann N Y Acad Sci. 2011;1220:149-161. [DOI] [PubMed] [Google Scholar]
- 214. Hoac B, Susan-Resiga D, Essalmani R, Marcinkiweicz E, Seidah NG, McKee MD. Osteopontin as a novel substrate for the proprotein convertase 5/6 (PCSK5) in bone. Bone. 2018;107:45-55. [DOI] [PubMed] [Google Scholar]
- 215. Dey A, Lipkind GM, Rouillé Y, et al. Significance of prohormone convertase 2, PC2, mediated initial cleavage at the proglucagon interdomain site, Lys70-Arg71, to generate glucagon. Endocrinology. 2005;146(2):713-727. [DOI] [PubMed] [Google Scholar]
- 216. Dey A, Norrbom C, Zhu X, et al. Furin and prohormone convertase 1/3 are major convertases in the processing of mouse pro-growth hormone-releasing hormone. Endocrinology. 2004;145(4):1961-1971. [DOI] [PubMed] [Google Scholar]
- 217. Barbero P, Rovère C, De Bie I, Seidah N, Beaudet A, Kitabgi P. PC5-A-mediated processing of pro-neurotensin in early compartments of the regulated secretory pathway of PC5-transfected PC12 cells. J Biol Chem. 1998;273(39):25339-25346. [DOI] [PubMed] [Google Scholar]
- 218. Cain BM, Vishnuvardhan D, Beinfeld MC. Neuronal cell lines expressing PC5, but not PC1 or PC2, process Pro-CCK into glycine-extended CCK 12 and 22. Peptides. 2001;22(8):1271-1277. [DOI] [PubMed] [Google Scholar]
- 219. Stein J, Shah R, Steiner DF, Dey A. RNAi-mediated silencing of prohormone convertase (PC) 5/6 expression leads to impairment in processing of cocaine- and amphetamine-regulated transcript (CART) precursor. Biochem J. 2006;400(1):209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Winberg J, Gustavsson P, Papadogiannakis N, et al. Mutation screening and array comparative genomic hybridization using a 180K oligonucleotide array in VACTERL association. PLoS One. 2014;9(1):e85313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Nakamura Y, Kikugawa S, Seki S, et al. PCSK5 mutation in a patient with the VACTERL association. BMC Res Notes. 2015;8:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Iatan I, Dastani Z, Do R, et al. Genetic variation at the proprotein convertase subtilisin/kexin type 5 gene modulates high-density lipoprotein cholesterol levels. Circ Cardiovasc Genet. 2009;2(5):467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Jang HB, Hwang JY, Park JE, et al. Intake levels of dietary polyunsaturated fatty acids modify the association between the genetic variation in PCSK5 and HDL cholesterol. J Med Genet. 2014;51(12):782-788. [DOI] [PubMed] [Google Scholar]
- 224. Popa SAV, Gorduza EV, Costache II. Preliminary evaluation of proprotein convertase subtilisin/kexin type 5 mutations in lower extremity artery disease. Biomed Res. 2017;28(10):4676-4679. [Google Scholar]
- 225. Essalmani R, Hamelin J, Marcinkiewicz J, et al. Deletion of the gene encoding proprotein convertase 5/6 causes early embryonic lethality in the mouse. Mol Cell Biol. 2006;26(1):354-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Szumska D, Cioroch M, Keeling A, Prat A, Seidah NG, Bhattacharya S. Pcsk5 is required in the early cranio-cardiac mesoderm for heart development. BMC Dev Biol. 2017;17(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Marchesi C, Essalmani R, Lemarié CA, et al. Inactivation of endothelial proprotein convertase 5/6 decreases collagen deposition in the cardiovascular system: role of fibroblast autophagy. J Mol Med (Berl). 2011;89(11):1103-1111. [DOI] [PubMed] [Google Scholar]
- 228. Stawowy P, Fleck E. Proprotein convertases furin and PC5: targeting atherosclerosis and restenosis at multiple levels. J Mol Med (Berl). 2005;83(11):865-875. [DOI] [PubMed] [Google Scholar]
- 229. Kiefer MC, Tucker JE, Joh R, Landsberg KE, Saltman D, Barr PJ. Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol. 1991;10(10):757-769. [DOI] [PubMed] [Google Scholar]
- 230. Mujoomdar ML, Hogan LM, Parlow AF, Nachtigal MW. Pcsk6 mutant mice exhibit progressive loss of ovarian function, altered gene expression, and formation of ovarian pathology. Reproduction. 2011;141(3):343-355. [DOI] [PubMed] [Google Scholar]
- 231. Scerri TS, Brandler WM, Paracchini S, et al. PCSK6 is associated with handedness in individuals with dyslexia. Hum Mol Genet. 2011;20(3):608-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Constam DB, Robertson EJ. SPC4/PACE4 regulates a TGFbeta signaling network during axis formation. Genes Dev. 2000;14(9):1146-1155. [PMC free article] [PubMed] [Google Scholar]
- 233. Hubbard FC, Goodrow TL, Liu SC, et al. Expression of PACE4 in chemically induced carcinomas is associated with spindle cell tumor conversion and increased invasive ability. Cancer Res. 1997;57(23):5226-5231. [PubMed] [Google Scholar]
- 234. Bassi DE, Lopez De Cicco R, Cenna J, Litwin S, Cukierman E, Klein-Szanto AJ. PACE4 expression in mouse basal keratinocytes results in basement membrane disruption and acceleration of tumor progression. Cancer Res. 2005;65(16):7310-7319. [DOI] [PubMed] [Google Scholar]
- 235. Malfait AM, Arner EC, Song RH, et al. Proprotein convertase activation of aggrecanases in cartilage in situ. Arch Biochem Biophys. 2008;478(1):43-51. [DOI] [PubMed] [Google Scholar]
- 236. Couture F, Sabbagh R, Kwiatkowska A, et al. PACE4 undergoes an oncogenic alternative splicing switch in cancer. Cancer Res. 2017;77(24):6863-6879. [DOI] [PubMed] [Google Scholar]
- 237. Kara I, Poggi M, Bonardo B, et al. The paired basic amino acid-cleaving enzyme 4 (PACE4) is involved in the maturation of insulin receptor isoform B: an opportunity to reduce the specific insulin receptor-dependent effects of insulin-like growth factor 2 (IGF2). J Biol Chem. 2015;290(5):2812-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Chen S, Cao P, Dong N, et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med. 2015;21(9):1048-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Volpe M, Rubattu S. Novel insights into the mechanisms regulating pro-atrial natriuretic peptide cleavage in the heart and blood pressure regulation: proprotein convertase Subtilisin/Kexin 6 is the corin activating enzyme. Circ Res. 2016;118(2):196-198. [DOI] [PubMed] [Google Scholar]
- 240. Guillemot J, Seidah NG. PACE4 (PCSK6): another proprotein convertase link to iron homeostasis? Haematologica. 2015;100(9):e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Li JP, Wang XB, Chen CZ, et al. The association between paired basic amino acid cleaving enzyme 4 gene haplotype and diastolic blood pressure. Chin Med J (Engl). 2004;117(3):382-388. [PubMed] [Google Scholar]
- 242. Perisic L, Hedin E, Razuvaev A, et al. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33(10):2432-2443. [DOI] [PubMed] [Google Scholar]
- 243. Rykaczewska U, Suur BE, Röhl S, et al. ; IMPROVE study group . PCSK6 is a key protease in the control of smooth muscle cell function in vascular remodeling. Circ Res. 2020;126(5):571-585. [DOI] [PubMed] [Google Scholar]
- 244. Suh JH, Yoon JS, Kwon JB, Kim HW, Wang YP. Identification of genomic aberrations by array comparative genomic hybridization in patients with aortic dissections. Korean J Thorac Cardiovasc Surg. 2011;44(2):123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Flaquer A, Baumbach C, Piñero E, et al. Genome-wide linkage analysis of congenital heart defects using MOD score analysis identifies 2 novel loci. BMC Genet. 2013;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Malfait AM, Seymour AB, Gao F, et al. A role for PACE4 in osteoarthritis pain: evidence from human genetic association and null mutant phenotype. Ann Rheum Dis. 2012;71(6):1042-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. He J, Yang H, Xu Z, et al. A functional polymorphism in the paired basic amino acid-cleaving enzyme 4 gene confers osteoarthritis risk in a population of Eastern China. Genet Mol Biol. 2020;43(1):e20190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wang F, Wang L, Jiang H, Chang X, Pan J. Inhibition of PCSK6 may play a protective role in the development of rheumatoid arthritis. J Rheumatol. 2015;42(2):161-169. [DOI] [PubMed] [Google Scholar]
- 249. Brandler WM, Paracchini S. The genetic relationship between handedness and neurodevelopmental disorders. Trends Mol Med. 2014;20(2):83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Arning L, Ocklenburg S, Schulz S, et al. PCSK6 VNTR polymorphism is associated with degree of handedness but not direction of handedness. PLoS One. 2013;8(6):e67251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Bassi DE, Mahloogi H, Klein-Szanto AJ. The proprotein convertases furin and PACE4 play a significant role in tumor progression. Mol Carcinog. 2000;28(2):63-69. [PubMed] [Google Scholar]
- 252. D’Anjou F, Routhier S, Perreault JP, et al. Molecular validation of PACE4 as a target in prostate cancer. Transl Oncol. 2011;4(3):157-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Fradet L, Temmar R, Couture F, Belzile M, Fortier PH, Day R. Evaluation of PACE4 isoforms as biomarkers in thyroid cancer. J Otolaryngol Head Neck Surg. 2018;47(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Levesque C, Couture F, Kwiatkowska A, et al. PACE4 inhibitors and their peptidomimetic analogs block prostate cancer tumor progression through quiescence induction, increased apoptosis and impaired neovascularisation. Oncotarget. 2015;6(6):3680-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Yuasa K, Futamatsu G, Kawano T, et al. Subtilisin-like proprotein convertase paired basic amino acid-cleaving enzyme 4 is required for chondrogenic differentiation in ATDC5 cells. FEBS J. 2012;279(21):3997-4009. [DOI] [PubMed] [Google Scholar]
- 256. Kuhn TC, Knobel J, Burkert-Rettenmaier S, et al. Secretome analysis of cardiomyocytes identifies PCSK6 (Proprotein Convertase Subtilisin/Kexin Type 6) as a novel player in cardiac remodeling after myocardial infarction. Circulation. 2020;141(20):1628-1644. [DOI] [PubMed] [Google Scholar]
- 257. Seidah NG, Hamelin J, Mamarbachi M, et al. cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proc Natl Acad Sci U S A. 1996;93(8):3388-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Creemers JW, van de Loo JW, Plets E, Hendershot LM, Van De Ven WJ. Binding of BiP to the processing enzyme lymphoma proprotein convertase prevents aggregation, but slows down maturation. J Biol Chem. 2000;275(49):38842-38847. [DOI] [PubMed] [Google Scholar]
- 259. Declercq J, Meulemans S, Plets E, Creemers JW. Internalization of proprotein convertase PC7 from plasma membrane is mediated by a novel motif. J Biol Chem. 2012;287(12):9052-9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Rousselet E, Benjannet S, Hamelin J, Canuel M, Seidah NG. The proprotein convertase PC7: unique zymogen activation and trafficking pathways. J Biol Chem. 2011;286(4):2728-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Garten W. Characterization of proprotein convertases and their involvement in virus propagation. In: Böttcher-Friebertshäuser E, Garten W, Klenk HD, eds. Activation of Viruses by Host Proteases. Springer International Publishing; 2018:205-248. [Google Scholar]
- 262. Declercq J, Ramos-Molina B, Sannerud R, et al. Endosome to trans-Golgi network transport of Proprotein Convertase 7 is mediated by a cluster of basic amino acids and palmitoylated cysteines. Eur J Cell Biol. 2017;96(5):432-439. [DOI] [PubMed] [Google Scholar]
- 263. Simmen T, Nobile M, Bonifacino JS, Hunziker W. Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol Cell Biol. 1999;19(4):3136-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Xiang Y, Molloy SS, Thomas L, Thomas G. The PC6B cytoplasmic domain contains 2 acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol Biol Cell. 2000;11(4):1257-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Durand L, Duval S, Evagelidis A, et al. The motif EXEXXXL in the cytosolic tail of the secretory human proprotein convertase PC7 regulates its trafficking and cleavage activity. J Biol Chem. 2020;295(7):2068-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Turpeinen H, Oksanen A, Kivinen V, et al. Proprotein convertase subtilisin/kexin type 7 (PCSK7) is essential for the zebrafish development and bioavailability of transforming growth factor β1a (TGFβ1a). J Biol Chem. 2013;288(51):36610-36623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Canaff L, Bennett HP, Hou Y, Seidah NG, Hendy GN. Proparathyroid hormone processing by the proprotein convertase-7: comparison with furin and assessment of modulation of parathyroid convertase messenger ribonucleic acid levels by calcium and 1,25-dihydroxyvitamin D3. Endocrinology. 1999;140(8):3633-3642. [DOI] [PubMed] [Google Scholar]
- 268. Anyetei-Anum EN, Blum A, Seidah NG, Beinfeld MC. Prohormone convertase 7 is necessary for the normal processing of cholecystokinin in mouse brain. Biochem Biophys Res Commun. 2017;482(4):1190-1193. [DOI] [PubMed] [Google Scholar]
- 269. Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15(10):1837-1839. [DOI] [PubMed] [Google Scholar]
- 270. Lei X, Shi F, Basu D, et al. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem. 2011;286(18):15747-15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Guillemot J, Essalmani R, Hamelin J, Seidah NG. Is there a link between proprotein convertase PC7 activity and human lipid homeostasis? FEBS Open Bio. 2014;4:741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Decroly E, Vandenbranden M, Ruysschaert JM, et al. The convertases furin and PC1 can both cleave the human immunodeficiency virus (HIV)-1 envelope glycoprotein gp160 into gp120 (HIV-1 SU) and gp41 (HIV-I TM). J Biol Chem. 1994;269(16):12240-12247. [PubMed] [Google Scholar]
- 273. Basak A, Zhong M, Munzer JS, Chrétien M, Seidah NG. Implication of the proprotein convertases furin, PC5 and PC7 in the cleavage of surface glycoproteins of Hong Kong, Ebola and respiratory syncytial viruses: a comparative analysis with fluorogenic peptides. Biochem J. 2001;353(Pt 3):537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Guillemot J, Canuel M, Essalmani R, Prat A, Seidah NG. Implication of the proprotein convertases in iron homeostasis: proprotein convertase 7 sheds human transferrin receptor 1 and furin activates hepcidin. Hepatology. 2013;57(6):2514-2524. [DOI] [PubMed] [Google Scholar]
- 275. Rousselet E, Benjannet S, Marcinkiewicz E, Asselin MC, Lazure C, Seidah NG. Proprotein convertase PC7 enhances the activation of the EGF receptor pathway through processing of the EGF precursor. J Biol Chem. 2011;286(11):9185-9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Leonhardt RM, Fiegl D, Rufer E, Karger A, Bettin B, Knittler MR. Post-endoplasmic reticulum rescue of unstable MHC class I requires proprotein convertase PC7. J Immunol. 2010;184(6):2985-2998. [DOI] [PubMed] [Google Scholar]
- 277. Dongiovanni P, Meroni M, Baselli G, et al. PCSK7 gene variation bridges atherogenic dyslipidemia with hepatic inflammation in NAFLD patients. J Lipid Res. 2019;60(6):1144-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Peloso GM, Auer PL, Bis JC, et al. ; NHLBI GO Exome Sequencing Project . Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am J Hum Genet. 2014;94(2):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Kurano M, Tsukamoto K, Kamitsuji S, et al. Genome-wide association study of serum lipids confirms previously reported associations as well as new associations of common SNPs within PCSK7 gene with triglyceride. J Hum Genet. 2016;61(5):427-433. [DOI] [PubMed] [Google Scholar]
- 280. Oexle K, Ried JS, Hicks AA, et al. Novel association to the proprotein convertase PCSK7 gene locus revealed by analysing soluble transferrin receptor (sTfR) levels. Hum Mol Genet. 2011;20(5):1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Stickel F, Buch S, Zoller H, et al. Evaluation of genome-wide loci of iron metabolism in hereditary hemochromatosis identifies PCSK7 as a host risk factor of liver cirrhosis. Hum Mol Genet. 2014;23(14):3883-3890. [DOI] [PubMed] [Google Scholar]
- 282. Qing-Hui Zhang R-XY, Gao H, Bin Y, et al. Association of the PCSK7 rs2277287 polymorphismand serum lipid levels in the Jing and Han populations. Int J Clin Exp Med. 2017;10(3):4986-5000. [Google Scholar]
- 283. Ashraf Y, Duval S, Sachan V, et al. Proprotein convertase 7 (PCSK7) reduces apoA-V levels. FEBS J. 2020;287(16):3565-3578. [DOI] [PubMed] [Google Scholar]
- 284. Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Huang T, Huang J, Qi Q, et al. PCSK7 genotype modifies effect of a weight-loss diet on 2-year changes of insulin resistance: the POUNDS LOST trial. Diabetes Care. 2015;38(3):439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Shi X, Cheng L, Jiao X, et al. Rare copy number variants identify novel genes in sporadic total anomalous pulmonary vein connection. Front Genet. 2018;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Villeneuve P, Feliciangeli S, Croissandeau G, et al. Altered processing of the neurotensin/neuromedin N precursor in PC2 knock down mice: a biochemical and immunohistochemical study. J Neurochem. 2002;82(4):783-793. [DOI] [PubMed] [Google Scholar]
- 288. Senturker S, Thomas JT, Mateshaytis J, Moos M Jr. A homolog of Subtilisin-like Proprotein Convertase 7 is essential to anterior neural development in Xenopus. PLoS One. 2012;7(6):e39380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Evans SF, Irmady K, Ostrow K, et al. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J Biol Chem. 2011;286(34):29556-29567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Cain BM, Connolly K, Blum AC, et al. Genetic inactivation of prohormone convertase (PC1) causes a reduction in cholecystokinin (CCK) levels in the hippocampus, amygdala, pons and medulla in mouse brain that correlates with the degree of colocalization of PC1 and CCK mRNA in these structures in rat brain. J Neurochem. 2004;89(2):307-313. [DOI] [PubMed] [Google Scholar]
- 291. Beinfeld MC, Blum A, Vishnuvardhan D, Fanous S, Marchand JE. Cholecystokinin levels in prohormone convertase 2 knock-out mouse brain regions reveal a complex phenotype of region-specific alterations. J Biol Chem. 2005;280(46):38410-38415. [DOI] [PubMed] [Google Scholar]
- 292. Taylor NA, Van De Ven WJ, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17(10):1215-1227. [DOI] [PubMed] [Google Scholar]
- 293. Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474(7351):337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Susaki EA, Ukai H, Ueda HR. Next-generation mammalian genetics toward organism-level systems biology. NPJ Syst Biol Appl. 2017;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Distelmaier F, Herebian D, Atasever C, et al. Blue diaper syndrome and PCSK1 mutations. Pediatrics. 2018;141(Suppl 5):S501-S505. [DOI] [PubMed] [Google Scholar]
- 296. Thiagarajah JR, Martin MG. Approach to Chronic Diarrhea in Neonates and Young Infants (<6 months). Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. Accessed August 17, 2020. https://www.uptodate.com/contents/approach-to-chronic-diarrhea-in-neonates-and-young-infants-less-than6-months?source=mostViewed_widget [Google Scholar]
- 297. Zhang J. Genetic redundancies and their evolutionary maintenance. Adv Exp Med Biol. 2012;751:279-300. [DOI] [PubMed] [Google Scholar]
- 298. López-Otín C, Hunter T. The regulatory crosstalk between kinases and proteases in cancer. Nat Rev Cancer. 2010;10(4):278-292. [DOI] [PubMed] [Google Scholar]
- 299. Chen J, Du Y, He X, Huang X, Shi YS. A convenient Cas9-based conditional knockout strategy for simultaneously targeting multiple genes in mouse. Sci Rep. 2017;7(1):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Kriseman M, Monsivais D, Agno J, Masand RP, Creighton CJ, Matzuk MM. Uterine double-conditional inactivation of Smad2 and Smad3 in mice causes endometrial dysregulation, infertility, and uterine cancer. Proc Natl Acad Sci U S A. 2019;116(9):3873-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Mounsey RB, Martin HL, Nelson MC, Evans RM, Teismann P. The effect of neuronal conditional knock-out of peroxisome proliferator-activated receptors in the MPTP mouse model of Parkinson’s disease. Neuroscience. 2015;300:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Wu C, Zheng M, Yang Y, et al. Furin: a potential therapeutic target for COVID-19. Iscience. 2020;23(10):101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Visscher PM, Wray NR, Zhang Q, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. 2017;101(1):5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Stijnen P, Tuand K, Varga TV, Franks PW, Aertgeerts B, Creemers JW. The association of common variants in PCSK1 with obesity: a HuGE review and meta-analysis. Am J Epidemiol. 2014;180(11):1051-1065. [DOI] [PubMed] [Google Scholar]
- 306. Nead KT, Li A, Wehner MR, et al. ; BioBank Japan, AGEN-BMI, GIANT Consortium . Contribution of common non-synonymous variants in PCSK1 to body mass index variation and risk of obesity: a systematic review and meta-analysis with evidence from up to 331 175 individuals. Hum Mol Genet. 2015;24(12):3582-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Génin E, Sahbatou M, Gazal S, Babron MC, Perdry H, Leutenegger AL. Could inbred cases identified in GWAS data succeed in detecting rare recessive variants where affected sib-pairs have failed? Hum Hered. 2012;74(3-4):142-152. [DOI] [PubMed] [Google Scholar]
- 308. McKusick VA. Beyond the Clinic: Genetic Studies of the Amish and Little People, 1960-1980s. Accessed July 25, 2020, https://profiles.nlm.nih.gov/spotlight/jq/feature/additional-resources [Google Scholar]
- 309. Bass J, Turck C, Rouard M, Steiner DF. Furin-mediated processing in the early secretory pathway: sequential cleavage and degradation of misfolded insulin receptors. Proc Natl Acad Sci U S A. 2000;97(22):11905-11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Karicherla P, Hobden JA. Nona-D-arginine therapy for Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2009;50(1):256-262. [DOI] [PubMed] [Google Scholar]
- 311. Karicherla P, Hobden JA. Nona-D-arginine amide for prophylaxis and treatment of experimental Pseudomonas aeruginosa keratitis. Curr Eye Res. 2010;35(3):220-224. [DOI] [PubMed] [Google Scholar]
- 312. Sarac MS, Cameron A, Lindberg I. The furin inhibitor hexa-D-arginine blocks the activation of Pseudomonas aeruginosa exotoxin A in vivo. Infect Immun. 2002;70(12):7136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Dianati V, Kwiatkowska A, Couture F, Desjardins R, Dory YL, Day R. Increasing C-terminal hydrophobicity improves the cell permeability and antiproliferative activity of PACE4 inhibitors against prostate cancer cell lines. J Med Chem. 2018;61(18):8457-8467. [DOI] [PubMed] [Google Scholar]
- 314. Bandsma RH, Sokollik C, Chami R, et al. From diarrhea to obesity in prohormone convertase 1/3 deficiency: age-dependent clinical, pathologic, and enteroendocrine characteristics. J Clin Gastroenterol. 2013;47(10):834-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Keller P, Zecca L, Boukamel R, Zwicker E, Gloor S, Semenza G. Furin, PC1/3, and/or PC6A process rabbit, but not human, pro-lactase-phlorizin hydrolase to the 180-kDa intermediate. J Biol Chem. 1995;270(43):25722-25728. [DOI] [PubMed] [Google Scholar]
- 316. Walsh NC, Kenney LL, Jangalwe S, et al. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.