Abstract
The burden of adverse pregnancy outcomes such as preterm birth and low birth weight is considerable across the world. Several risk factors for adverse pregnancy outcomes have been identified. One risk factor for adverse pregnancy outcomes receiving considerable attention in recent years is gestational exposure to endocrine-disrupting chemicals (EDCs). Humans are exposed to a multitude of environmental chemicals with known endocrine-disrupting properties, and evidence suggests exposure to these EDCs have the potential to disrupt the maternal-fetal environment culminating in adverse pregnancy and birth outcomes. This review addresses the impact of maternal and fetal exposure to environmental EDCs of natural and man-made chemicals in disrupting the maternal-fetal milieu in human leading to adverse pregnancy and birth outcomes—a risk factor for adult-onset noncommunicable diseases, the role lifestyle and environmental factors play in mitigating or amplifying the effects of EDCs, the underlying mechanisms and mediators involved, and the research directions on which to focus future investigations to help alleviate the adverse effects of EDC exposure.
Keywords: endocrine-disrupting chemicals, pregnancy, placenta, birth outcomes
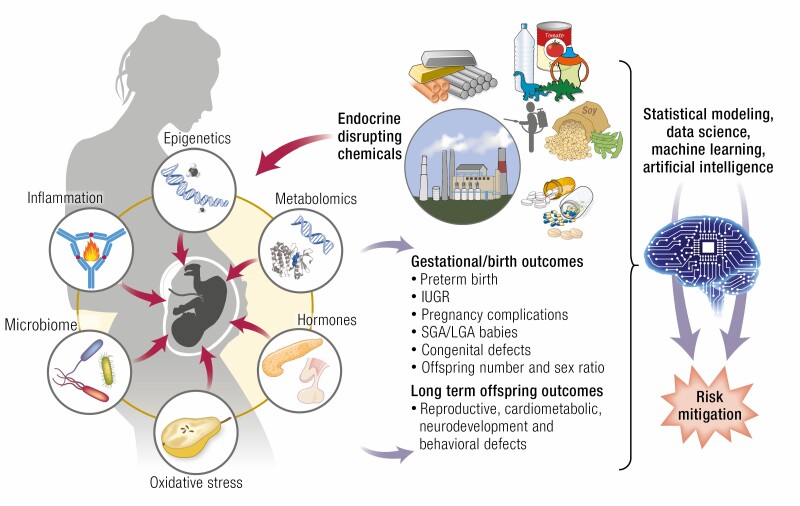
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
One of the major risk factor affecting adverse birth outcomes is gestational exposure to endocrine-disrupting chemicals (EDCs) with disruptions manifested at the level of maternal, fetal/neonatal and placental milieu.
Lifestyle factors such as diet and stress interact with EDCs to mitigate or amplify the effects of EDCs.
EDC action involves mediators such as inflammation, oxidative stress, hormonal and metabolomic changes, microbiome and epigenetic alterations.
Risk assessments would require consideration of EDC exposure burden in relation to site, type and matrix of EDC measurement, dose-response relationships, susceptibility windows, fetal/neonatal sex, lifestyle factors, placental contribution, and analytical approaches employed for appraising composite risk and target interventions for mitigating the adverse effects of EDC exposure.
The development of any organism into an adult depends on an intricate interaction of processes that regulate cell proliferation and differentiation along with integration of numerous factors that include genetics and environment. These complex processes need to be coordinated over various developmental windows that span prenatal and postnatal life. Organismal developmental plasticity, by which a single genotype can give rise to a range of different physiologic or morphologic states in response to prevailing environmental conditions, characterizes early periods of development (1). By adolescence, individuals lose this plasticity and mature into an adult phenotype of physiological capacity and morphology. Developmental plasticity during early life comprises successive or parallel windows of susceptibility of organ systems to environmental insults. Developmental adaptations made in the short term to early-life insults ensure immediate survival by selectively reducing functional capacity of some organs so as to channel resources to critical organs (2). Epidemiological and experimental data, however, indicate that such adaptive responses can prove to be maladaptive in the long term, compromising health (3). For example, babies born during the 1944 to 1945 Dutch famine, who faced malnutrition during intrauterine life, had increased risk of many chronic cardiovascular and metabolic diseases (4-6). The long-term health outcomes resulting from developmental impact were formulated by David Barker as the fetal origin of disease theory, which is now formalized as the developmental origin of health and disease (DOHaD) hypothesis (7). Since its formulation, epidemiological and animal experimentation studies have provided a large body of evidence confirming the validity of this hypothesis.
Developmental insults leading to maladaptive responses range from maternal disease, nutritional deficit/excess, stress states, and exposure to environmental factors that include lifestyle choices and chemical and climate exposure (8). Of these, the impact of early exposure to environmental chemicals capable of disrupting the endocrine system, referred to as endocrine-disrupting chemicals (EDCs), has gained prominence in recent years. This review focuses on (a) the early impacts of environmental EDCs in modulating the maternal-fetal environment, predominantly in humans (barring a few examples from experimental animal models relative to causality) resulting in adaptive changes in the fetus and adverse pregnancy outcomes; (b) the mechanisms and intermediaries involved; (c) the long-term health consequences in brief (an outcome extensively reviewed previously [8-10]); (d) the role that genetic makeup, lifestyle, and environmental factors play in modifying the effects of EDCs, and (e) research directions on which to focus future investigations that would aid in the development of intervention strategies to mitigate the adverse developmental effects of EDCs.
Endocrine-Disrupting Chemicals
Hormones are major factors in the maternal and fetal milieu that influence the developmental trajectory of the offspring in a dose-, time-, and organ-specific manner. Any agent that acts as an agonist or antagonist of endogenous hormones thus has the potential to influence developmental trajectory of the offspring. In this context, EDCs as defined by the US Environmental Protection Agency (EPA) are “exogenous agents that interfere with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction, and developmental process” (11). Through their action EDCs have the potential to disrupt normal endocrine functions and disturb the maternal and fetal endocrine milieus (12, 13). Although certain chemicals were known for their hormone-disrupting function by the mid-20th century, the term endocrine disrupter was first used in the 1991 Wingspread meeting (14). Initial research into EDCs focused mostly on chemicals with steroidal capacity and has now expanded to include chemicals that span a wide range of hormonal functions.
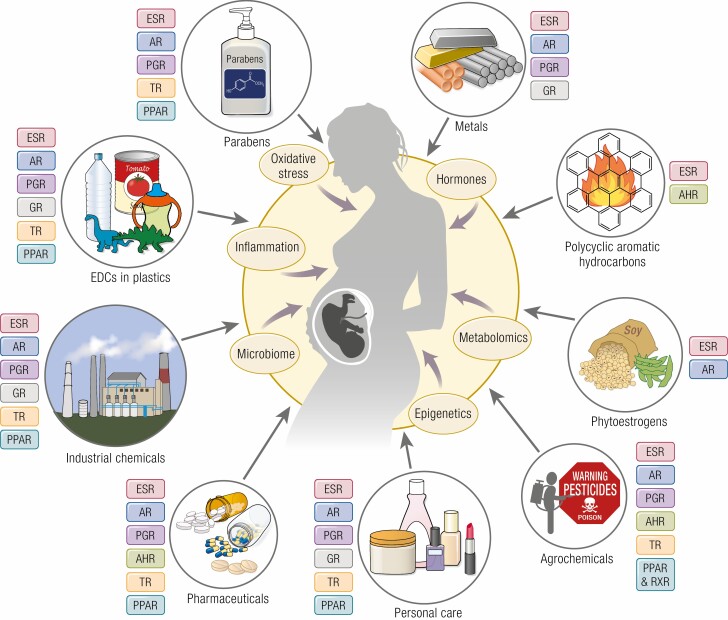
The Toxic Substances Control Act mandates the US EPA maintain a list of chemicals that are manufactured, processed, or imported into the United States that includes about 86 000 chemical substances. A 2012 estimate by the World Health Organization indicated about 800 chemicals, used in everyday life, to be EDCs (15). In 2020, the Endocrine Disruption Exchange database (https://endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list) listed 1482 chemicals with endocrine-disrupting potential highlighting the increase in number of chemicals recognized as EDCs. Broadly speaking, EDCs include both natural and anthropogenic chemicals that humans are exposed to in everyday life (Fig. 1) (13, 16). Naturally occurring compounds with endocrine-disrupting potential include metals and metalloids, parabens, polyaromatic hydrocarbons (PAHs), and phytoestrogens. Man-made synthetic chemicals are commonly used in agricultural practices (pesticides, insecticides, and fungicides), packaging (food-storage materials, plastics), industry (solvents, flame retardants, preservatives, emulsifiers, fracking chemicals), consumer products (household chemicals, cosmetics, flame retardants, building materials, children’s toys, electronics, cookware), and medical care (birth control pills, biocides, intravenous bags and tubing, disposable gloves, disinfectants). Although chemicals such as metals and PAHs exist naturally, increase in their exposure occur as a result of human activity such as mining for metals and burning fossil fuel.
Figure 1.
Schematic showing the different classes of endocrine-disrupting chemicals (EDCs) that pregnant women are commonly exposed to during everyday life, their mechanism of endocrine disruption and mediators involved in influencing maternal, placental, and fetal milieu. AhR, arylhydrocarbon receptor; AR, androgen receptor; ESR, estrogen receptor; GR, glucocorticoid receptor; PGR, progesterone receptor; PPAR, peroxisome proliferator activated receptor; TR, thyroid receptor.
Relevant to the focus of this review, EDCs are detectable in the maternal-placental-fetal unit during different stages of gestation and at delivery (Table 1). Several of these EDCs were detected in pregnant women as evidenced from the National Health and Nutrition Examination Survey (NHANES) in the United States and the Maternal-Infant Research on Environment Chemicals study in Canada (17, 18). About 40 to 50 chemicals have been measured in women during pregnancy (17, 19), highlighting the potential risk to maternal and fetal health from such exposures. Emerging epidemiological studies, both prospective and retrospective, have documented adverse effects of EDCs that range from poor gestational/early birth outcomes (13, 19, 20) to long-term adverse health effects in the offspring (13, 21-24). Exposure to EDCs also come at increased economic consequences although specific estimates relative to exposure during pregnancy are not available. Conservative estimates from 2016 based on the attributable fraction of a risk factor that could decrease the number of cases of disease or deaths by reducing the risk factor places disease costs of EDC exposures at about $340 billion in the United States and €163 billion in Europe (25, 26). The majority of the cost in the United States appears to be associated with polybrominated diphenyl ether (PBDE) exposure ($266 billion), whereas in the European Union organophosphate pesticides seem to be the largest contributors (€146 billion) (25, 26).
Table 1.
Common endocrine-disrupting chemicals with their known role in endocrine disruption and detection in humans
| EDC class | Example EDC | Endocrine disruption | EDC presence detected in | |||||
|---|---|---|---|---|---|---|---|---|
| Maternal urine | Maternal blood | Placenta | Umbilical cord blood | Amniotic fluid | Fetal tissue | |||
| Naturally occurring EDCs | ||||||||
| Metals | Cd Hg Ar Cr |
Pb, Cd, and Hg: placental hormone biosynthesis (27, 35) Ar: steroid receptor function (27) |
TM1
heavy metals: As, Cd, Cr, Pb, Tl, V (19, 36–38) As (39) Cd (39-41) TM2 As (38, 42-44) Cd (40) TM3/Term U (45-47) As (38, 39, 41, 48, 49) Cd (40) Ni (50) |
TM1 Heavy metals (37, 39) TM3/Term Heavy metals (34, 39, 51, 52) Hg (53) Cd (54) |
Term
Heavy metals (32-34, 52) Cr (35) Pb (55, 56) |
Term
Heavy metal (34, 39, 52, 57, 58) Hg (53, 59) |
Unspecified Heavy metals (60) Term Heavy metals (51) |
|
| Parabens | Methylparaben Propylparaben Butylparaben Ethylparaben | Estrogenic and antiandrogenic actions (66) progesterone receptor, thyroid receptor and PPAR (67) |
TM1
(19) TM3/Term (63, 68) |
TM1
(68) TM2 (69) |
TM3/Term (64, 68) |
Term
(68) |
Term
(68) |
|
| PAHs | Benzo(a) pyrene benzo(a) anthracene benzo(b) fluranthene indeno(1,2,3-cd) pyrene naphthalene | Aryl hydrocarbon receptor binding (75, 76); inhibit estrogen receptor action (80) |
Unspecified
(17) TM3/Term (77, 78) |
TM2
(79) TM3/Term (73) |
TM3/Term (72, 73, 81) |
TM3/Term
(73) |
TM3/Term (74) | TM2 (79) |
| Phytoestrogens | Lignans, isoflavones (daidzein, genistein, and glycitein) coumestans (coumestrol) | Estrogen receptor agonists (86) |
TM3/Term
(84) |
TM3/Term
(84, 85) |
Term
(83-85, 88) |
TM2
(89, 90) Term (85, 91) |
||
| EDCs in plastics | ||||||||
| Bisphenols | BPA, BPB, BPC, BPE, BPF, BPS, BPZ, BPAF, and BPAP |
Estrogenic activity (101); Anti-androgenic activity and modulation of glucocorticoid, PPAR and thyroid systems (102-104) |
TM1
(19, 40) TM3/Term (40) |
TM1 (20) (20, 69) TM2 (43, 69) TM3/Term (20, 50, 105, 106) |
Term
(94, 105) |
Unspecified
(107) TM2 (108) Term (20, 50, 105, 106) |
TM2
(98, 99) TM3 (98) |
Unspecified
(107) TM1-TM2 (100) TM2 (108) |
| Phthalates | DEHP MEHP DBP DMP |
Estrogenic, anti-estrogenic, anti-androgenic and metabolic actions (115-117) |
Unspecified
(17) TM1 (19, 40, 110) TM2 (112) TM3/Term (40, 110-112) |
TM2 (43) TM3/Term (112-114) |
Term
(112-114) |
Unspecified
(118) TM2 (119) |
||
| Industrial chemicals | ||||||||
| Organo-halogens | PCBs PBDEs TBBPA HBCDD PFAS (PFOS and PFOA) |
PCB and PBDE: disruption of estrogen, androgen or thyroid signaling (129) PFAS: estrogenic activity (123) Halogenated bisphenol: estrogenic and PPAR action (130) |
Unspecified
(17) |
TM1
PCB and PBDE (126) TM2 PCB and PBDE (43, 127) PFAS (43, 125) TM3/Term PFAS (113) PBDE and PFOS (50) |
TM2
PFAS (125) |
TM2
PCB and PBDE (127) TM3/Term PFAS (113) PBDE and PFOS (50) PCB and PBDE (126) |
TM1
PFAS (124, 125) TM2 PCB and PBDE (127) PFAS (124, 125) Term PFAS (125) |
TM1
PFAS (124, 125) TM2 PFAS (124, 125) PCB and PBDE (127) Term PFAS (125) |
| Agrochemicals | ||||||||
| Pesticides | OC, OP, carbamates | Organochlorines: interference of AHR action (136) organophosphates and carbamates: inactivation of acetylcholinesterase enzyme (141) |
TM1
DDE (143) TM2 DDE (143) |
TM3/Term
DDE (122, 159, 160) OP (50) |
TM3/Term DDE (122) DDT and HCH (81) |
TM3/Term
DDE (122, 159, 160) OP (50) |
||
| Herbicides | Atrazine, simazine, and propazine | Increases aromatase expression (146) |
TM1
(143) TM2 (143, 161, 162) TM3 (162) |
|||||
| Fungicide | Vinclozolin | Anti-androgenic effects (149, 150) |
TM3/Term
(148) |
|||||
| Antifouling agents | Tributyltin, Chlorothalonil, Dichlofluanid, Diuron | Agonistic ligand for RXR and PPAR γ (156) Placental steroidogenesis and chorionic gonadotropin secretion (157) |
TM3/Term
(155, 163) |
|||||
| Anti-bacterials | Triclosan triclocarban | Interferes with estrogen, androgen, and thyroid hormone action (174, 175) |
TM1
(19, 176, 177) TM3/Term (171) |
TM1
(69) TM2 (69) TM3/Term (172, 173) |
TM3/Term
(173) |
TM3/Term
(172) |
||
| Medical products | ||||||||
| Pharma-ceuticals | Diethylstilbestrol | Estrogen receptor agonist anti-androgenic action (180-184) |
Unspecified
(179) |
|||||
| Medical Supplies (Bisphenols and Phthalates) | see above sections for detection of bisphenols and phthalates |
Abbreviations: AHR, arylhydrocarbon receptor; EDC, endocrine-disrupting chemical; HBCDD, hexabromocyclododecane; HCH, hexachlorocyclohexane; MDA, malondialdehyde; MEHP, mono(2-ethylhexyl) phthalate; mid to late, second and third trimester; mRNA, messenger RNA; miRNA, microRNA; OC, organochlorines; OP, organophosphorus; PAHs, polyaromatic hydrocarbons; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFAS, perfluorinated alkylated substance; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PPAR, peroxisome proliferator activated receptor; RXR, retinoid X receptor; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TBBPA, tetrabromobisphenol A; TM, trimester; TNF, tumor necrosis factor; TSH, thyrotropin.
Naturally Occurring Endocrine-Disrupting Chemicals
This section focuses on naturally occurring products with proven endocrine-disrupting activity that humans are exposed to through diet or inhalation.
Metals
Certain metals called trace metals are indispensable for living organisms and are sourced through food—these metals when in excess can be detrimental. Other metals although found naturally can be toxic when released into the environment in large quantities by industrial activities and enter humans and animals along the food chain, leading to higher levels of exposure and serious health risks. Most studies assessing the endocrine-disrupting potential of metals have focused on cadmium (Cd), mercury (Hg), arsenic (As), and lead (Pb). In recent years’ other metals, especially chromium (Cr), manganese (Mn) and zinc (Zn) are also being acknowledged as EDCs (27). Inappropriate exposure to most metals is through food and water contamination that occurs mostly from pollution from industrial activity (28-30). Consumer exposures to these metals can also occur from their presence in tobacco smoke (Cd), paints and batteries (Cd, As, Hg, and Pb), and dental amalgam (Hg) (28-30). The 1999 to 2016 NHANES reported detection rates of 83 to 99% based on urinary levels of As, Pb, Hg and Cd both in pregnant and nonpregnant women of child bearing age. Importantly, the majority of these metal concentrations were found to be higher in pregnant compared to nonpregnant women, the result of usage of multivitamin and multimineral supplements or dietary changes (31). These metals have also been found in placenta, newborn cord blood, and amniotic fluids (see Table 1) (27, 32-34), indicative of placental transfer. A wide range of endocrine disruptions is attributed to metals such as disruptions of placental progesterone synthesis through inhibition of cytochrome P450 enzymes by Cd and Hg and steroidal hormone receptor action such as estrogen androgen and glucocorticoid receptors by As (27, 35).
Parabens
Parabens are aliphatic or aromatic alkyl esters of p-hydroxybenzoic acid, which are naturally present in some fruits and vegetables. They are of environmental concern because of their widespread use as preservatives in cosmetics, drugs, and foods (61). Although categorized as “generally recognized as safe” by the US Food and Drug Administration, emerging data suggest adverse effects of parabens, particularly during early developmental stages (61, 62). The most commonly used parabens are methylparaben, propylparaben, butylparaben, and ethylparaben. Parabens have been detected in maternal urine (19, 63). A significant correlation exists between maternal urinary paraben concentrations and their newborn infant levels, supporting transfer of parabens from mother to fetus (63). Parabens have also been detected in human placental tissue (64) and amniotic fluid (65) (see Table 1). Parabens have both estrogenic and antiandrogenic actions as the basis for their endocrine disruptions (66) but are also known to influence the progesterone receptor, thyroid receptor and peroxisome proliferator-activated receptor (PPAR) (67).
Polycyclic aromatic hydrocarbons
PAHs are naturally occurring organic compounds in coal, crude oil, and gasoline that result from incomplete combustion or pyrolysis of organic matter. These are mainly generated by combustion processes, such as wood burning, motor vehicle exhaust, cooking and agricultural waste burning (70). Cigarette smoking is another source of exposure to many PAHs (71). As part of the atmospheric pollutants they include compounds like benzo(a) pyrene, benzo(a) anthracene, benzo(b) fluoranthene, benzo(k) fluoranthene, chrysene, dibenzo(a, h) anthracene, indeno(1,2,3-cd) pyrene and naphthalene, also classified as carcinogens by the EPA. The 2003 to 2004 NHANES detected urinary PAHs in approximately 99% to 100% of US pregnant women (17). PAHs and PAH-DNA adducts have been detected in maternal blood, cord blood and placentae (72, 73), amniotic fluid (74) and fetal tissues (79) (see Table 1). The effects of PAHs in disrupting endocrine functions are mediated through their ability to bind aryl hydrocarbon receptor (AHR) (75, 76) but also have been shown to involve estrogen receptor action (80).
Phytoestrogens
Phytoestrogens, naturally occurring plant compounds, are known for their weak estrogenic effects (82). The presence of soy, a major source of phytoestrogen in many baby formulas, is of particular developmental concern. Phytoestrogens are categorized as lignan, isoflavones (daidzein, genistein, and glycitein), and coumestans (coumestrol). Phytoestrogens can be transferred from mother to fetus (83) and have been detected in maternal blood, maternal urine, cord blood, and amniotic fluid (84, 85) (see Table 1). The main endocrine-disrupting role of phytoestrogens involves their binding to estrogen receptors, with coumestrol having the highest affinity, followed by genistein (86). Phytoestrogens have also been found to have inhibitory effects on placental growth factor and chorionic gonadotropin biosynthesis (86, 87).
Anthropogenic Chemicals
This section specifically deals with man-made synthetic chemicals, the environmental burden of which has significantly increased with the industrial revolution and urbanization.
Endocrine-disrupting chemicals in plastics
Bisphenols are industrial chemicals used in the manufacture of plastics. Bisphenol A (BPA), which has been in use since 1960, is found in certain plastics and resins such as polycarbonate plastics (eg, water bottles) and epoxy resins used to coat the inside of metal products (food cans, bottle tops, and water supply lines) (92). It is also present in thermal paper used for cash receipts and some dental sealants and composites (92). More recently analogues of BPA such as BPB, BPC, BPE, BPF, BPS, BPZ, BPAF, and BPAP have been used as BPA replacements (93). Owing to their widespread usage, BPA and its analogues have been detected in 75% of food samples tested (91) and are regularly detected in human urine and blood (94, 95). According to the 2013 to 2014 US NHANES, 96%, 88%, and 66% of the women of reproductive age (15-44 years) had urinary concentrations of BPA, BPS, and BPF, respectively (96) with more than 90% of pregnant women in the United States having measurable urinary levels of BPA (17). In addition to maternal urine and maternal plasma (20), BPA levels have also been detected in placentae (97), neonatal cord blood (20), amniotic fluid (98, 99) and fetal tissues (100), supportive of placental transfer (see Table 1). BPA is well known for its estrogenic activity (101); however, it has also been shown to have anti-androgenic activity and to modulate glucocorticoids, PPAR, and thyroid systems (102-104). Emerging data indicate that the replacement BPA analogues also have comparable endocrine-disrupting potential (93).
Phthalates are another group of EDCs used in the plastics industry for their ability to enhance the flexibility of plastics. The high-molecular-weight forms of diesters of phthalic acid are used as plasticizers in polyvinyl chloride products used as building materials, medical devices, and in food processing or packaging (96). Among these, phthalate di(2-ethylhexyl)-phthalate (DEHP) is the most widely used. Phthalates have been found in approximately 100% of humans tested (109), and according to the 2003 to 2004 NHANES study, 90% of pregnant women showed detectable levels of phthalates (17). Phthalates have been found in maternal urine (19, 110-112), maternal and cord blood (113, 114) and amniotic fluids (see Table 1). The endocrine-disrupting capability of phthalates includes antiandrogenic, estrogenic, antiestrogenic, and actions of glucocorticoid, thyroid, and metabolic receptors (115-117).
Industrial chemicals
In addition to the bisphenols and phthalates used in the manufacture of plastics, resins, and emulsifiers, several other chemicals are used as solvents, flame retardants, as well as in the manufacturing of consumer and industrial products including textiles, plastics, wire insulation, and automobiles. Among these, organohalogens, including polychlorinated biphenyls (PCBs) and brominated flame retardants (brominated flame retardants [eg, PBDEs], tetrabromobisphenol A [TBBPA], hexabromocyclododecane [HBCDD]) and perfluorinated alkylated substances (PFASs), are the most widely used (120). They are known for their persistence in the environment, bioaccumulation in living organisms, long-range transport beyond the geographical regions of their use, and long-term health effects in wildlife and humans (121, 122). Although many of these compounds have been banned or severely restricted for use in developed countries—some for as long as 4 decades—these PCB and PBDE congeners are still detectable in the environment and in populations (123). Precursors or metabolic intermediates of PFASs, which have received increased attention recently, include perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA), and these have been detected in many food sources and drinking water (123). The 2003 to 2004 NHANES study found detectable levels of organohalogens from multiple classes in 99% to 100% of pregnant women (17). In addition, PFASs have been detected in maternal and neonatal cord blood (113, 122), placenta (122) and embryonic and fetal tissues (124, 125) (see Table 1). Similarly, PCB and PBDE have also been detected in maternal and cord blood, placenta and fetal tissues (126, 127) (see Table 1). The major effects of PCB and PBDE are associated with developmental neurotoxicity (128). Although the mode of disruption in endocrine systems is not known, interference with estrogen, androgen and thyroid signaling have been suggested (129). PFASs are known to cause developmental problems through disruption of endocrine and immune systems, and many PFASs are also known to have estrogen-like activities (123). The halogenated bisphenol in addition to acting through estrogen receptors has also been known to interact with PPAR (130).
Agrochemicals
Chemicals used in agriculture for crop protection, including pesticides, herbicides, fungicides and insecticides, comprise those belonging to organochlorines, organophosphorus, carbamates, pyrethroids, and neonicotinoids classes (131). Waterway transportation and harvesting of aquatic and marine food has also led to the introduction of another class of chemicals mainly used as anti-fouling agents, which include chemicals like tributyltin (TBT), chlorothalonil, dichlofluanid, diuron, and Zn pyrithione (132), that contaminate seafood. In addition, anabolic steroids, like the synthetic androgen, trenbolone, are used in the livestock industry to promote muscle growth and are routinely detected in various meats (133, 134).
Pesticides.
Organochlorine pesticides include biocidal chemicals such as DDT and its metabolites, hexachlorobenzene (HCB), lindane, and dieldrin (83). The use of DDT is now banned in many countries (135). The endocrine-disruptive functions for organochlorines include disruption of cytochrome enzyme expression through interference with AHR action (136) and alteration of thyroid hormone levels (137). Organophosphorus compounds, esters of phosphoric acids, such as chlorpyrifos and triazophos are among the most widely used insecticides. Organophosphorous compounds are inhibitors of the acetylcholinesterase enzyme involved in neuronal signal transmission (138). Metabolites are known to bind steroid and adrenergic hormone receptors and interfere with their signal transduction (139, 140). Other insecticides are chemicals within the carbamate ester functional group such as aldicarb, carbofuran, carbaryl, fenobucarb, oxamyl, and methomyl. Unlike organophosphorous compounds, these reversibly inactivate the acetylcholinesterase enzyme (141), and inhibit steroidogenesis as well as steroid hormone action (140).
Exposure to these chemicals can occur via household use of pesticide products, dietary exposure to pesticide residues, exposure to chemical drift from agricultural application, or agricultural use. Biomonitoring has found widespread exposures to agrochemicals in the human population. Despite being banned, DDT and its metabolites have been detected in maternal circulation (122) and placentae (142). In the CHAMACOS (Center for the Health Assessment of Mothers and Children of Salinas) cohort from the agricultural Salinas Valley in California, at least one of the pesticide metabolite of either carbamate, organochlorine, organophosphorus or pyrethroid was detected in 78% of the pregnant women (143) (Table 1).
Herbicides
Triazine herbicides that include atrazine, simazine, and propazine are used in large amounts to control weeds. Because of their widespread use in agriculture, it is of human exposure concern (144). Many earlier epidemiological studies focused on cancer risk; however, later on the developmental effects due to their endocrine-disruptive function began to be recognized (145). In the CHAMACOS cohort the herbicide triazine has been detected in at least 78% of pregnant women (143) (see Table 1). Endocrine-disrupting properties of atrazine have been linked to one or more of its metabolites and involves disruption of steroid synthesis through increased expression of aromatase (146).
Fungicides.
Dicarboximide, a fungicidal chemical like vinclozolin, is applied to various food crop, grass, and ornamental plants (143, 147). Studies relative to the presence of vinclozolin in maternal and fetal units are limited; one such study has shown the presence of vinclozolin in umbilical cord blood, indicating transplacental transfer (148). The effects of developmental vinclozolin exposures are well studied in animal models, in which it is demonstrated to have anti-androgenic effects (147, 149, 150), although data on its effects in human are limited (151).
Antifouling agents.
A well-known antifouling agent used in paints for the protection of ship hulls along with other derivatives (phenyltins and octyltins) is the potent biocide TBT, an organotin. Although the use of TBT has been banned since 2001 by the International Maritime Organization, its use continues unabated (152). Organotin compounds are also used as stabilizers in the manufacture of polyvinyl chloride plastics and polyurethane foams (152). They are commonly detected in human samples (153) and a few studies have also reported the presence of TBTs in human placentae (154, 155). Organotins are obesogenic endocrine disruptors as they are agonistic ligands for nuclear receptors retinoid X receptor (RXR) and PPAR γ (156). They have also been shown to affect placental steroidogenesis and chorionic gonadotropin secretion (157, 158).
Cosmetics and consumer products
Phthalates, parabens, and phenols, discussed earlier in the context of their use in plastics, are also used in a wide variety of consumer products such as perfumes, deodorants, soaps, shampoo, nail polish and cosmetics. The low-molecular-weight (LWW) phthalates such as dibutyl-phthalate (DBP) and dimethyl-phthalate (DMP) are commonly used, while parabens are used as preservatives in personal care products and cosmetics (164-166). The use of personal care products among pregnant women corresponded positively to the detection of phthalate metabolites in their urine, pointing to the risk of developmental exposure (167-169).
Triclosan and triclocarban are used as antimicrobials in products such as soaps, toothpastes, detergents, clothing, toys, carpets, plastics, and paints. As a result, these products have been detected in a wide variety of matrices worldwide. Triclosan and triclocarban persist in the environment and provide substrates for generating other toxic and carcinogenic compounds including dioxins, chloroform, and chlorinated anilines (170). They have been routinely detected in the urine of pregnant women (19). Among pregnant women from an urban population (Brooklyn, New York), the frequency of detection was 100% for triclosan and 87% for triclocarban (171). They were also detected in maternal blood, cord blood, and amniotic fluid, indicating transplacental transfer (172, 173) (see Table 1). The endocrine-disrupting role of triclosan is not well understood, but animal studies support a role in the disruption of adipocyte differentiation together with interference in estrogen, androgen, and thyroid hormone action (174, 175).
Pharmaceuticals and medical supplies
Exposure to endocrine disruptors can also occur during medical treatment because of the use of chemicals in the manufacture of medical equipment, such as tubing, syringes, pills, and implants. Some common medical exposures include phthalates, bisphenols, and parabens. In neonatal intensive care units, BPA was detected in almost 60%, and parabens in more than 85%, of medical devices tested (178). Phthalates are also present in equipment used in neonatal intensive care units, including platelet transfusion, and hemodialysis and extracorporeal membrane oxygenation devices (164).
Other pollutants
In addition to the EDCs discussed earlier, natural and synthetic chemicals such as PAHs, ozone, nitrogen oxides, and particulate matter (PM) are also released as a result of industrial activity. Such activities along with urbanization have led to environmental pollution of the ground, water, and air through industrial and domestic sludge and effluents. Biosolids, which are processed human sewage sludge, have been shown to contain various EDCs and use of this in agriculture and livestock is another source of exposure (185). Air pollution is also considered to contribute to endocrine disruptions with estrogenicity, antiandrogenicity, and thyroidicity demonstrated both in indoor and outdoor air (186). A major contributor of air pollution is PM, which may contain dust, soil, acids, organic molecules, and some metals. It is categorized according to the size of the particles as PM10 (2.5-10 µm), PM2.5 (<2.5 µm), and PM0.1 (<0.1 µm). The compounds present in PM are also known to have profound effects on the functioning of the endocrine system (187). Nanoparticles, synthetic materials that are gaining increased use in several industrial, consumer, and medical applications, have the potential to influence the endocrine system (188). Another source of exposure is the use of cigarettes. Tobacco smoke contains a mix of 7000 chemicals including EDCs such as benzene, a PAH, and vinyl chloride, a plasticizer (189). Smoking is associated with disruption of metabolic pathways via interaction with PPAR, thyroid hormone receptors, farnesoid X receptor, liver X receptor, and RXRs (190). Except for the detection of PAHs in maternal, placental, and fetal samples (see Table 1), the assessment of gestational exposure to other PM and airborne pollutants have been based on geospatial environmental assessment, occupation, and response to questionnaire (191-194).
Effect of Endocrine-Disrupting Chemicals on Pregnancy Outcomes
Because hormones play an important role in not only the establishment and maintenance of pregnancy, but also fetal development, EDCs that act as hormone mimics or interfere with their action have the potential to affect these variables. The association of EDC exposure with pregnancy outcomes is manifested as a range of adverse effects depending on the EDC, time of detection during pregnancy, and the compartment in which the EDC is measured. Such adverse outcomes range from preterm birth, fetal intrauterine growth restriction (IUGR), changes in birth weight and size, small for gestational age (SGA) babies, increase in gestational length, macrosomia, large for gestational age (LGA) babies, and congenital anomalies. Although many of these outcomes are established risk factors for adult-onset diseases (195-198), longitudinal studies linking the impact of EDCs in the maternal-fetal milieu to adult consequences will understandably not be available in the foreseeable future. Several pregnancy cohorts addressing the impact of maternal EDC exposures on gestational and birth outcomes are now following their children. The health consequences of gestational EDC exposure in children are briefly discussed in later.
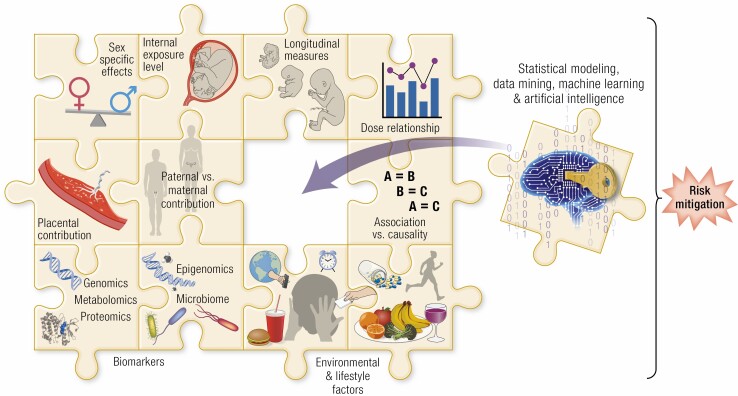
In spite of the wealth of associative information available, determining the true impact of maternal/fetal exposure to EDCs on the range of pregnancy and infant outcome is challenging for several reasons. These include 1) differences in the timing of EDC measurement relative to pregnancy progression; 2) exposure level of EDC considering the non-monotonic responses to their exposure; 3) the matrix in which EDC measurement is undertaken, which include: maternal urine, maternal plasma/serum, placenta, cord blood and amniotic fluid; 4) background contamination due to ubiquitous presence of many EDCs; 5) differences in methodological approaches used for measuring EDCs; 6) single time point assessment during pregnancy and predominantly at term; 7) lack of longitudinal assessment of exposure to EDCs considering the organ-specific differences in susceptibility windows during gestation; 8) impact of fetal sex in modulating effects of EDCs 9) soundness of statistical approaches employed; and 10) lack of attention to confounders such as ethnicity, age, diet, prepregnancy weight, weight gain, and lifestyle factors. Furthermore, humans are exposed to multiple EDCs in parallel that may have additive, synergistic, or antagonistic effects and addressing one EDC at a time will not reflect true life exposure burden. The following sections address the impact of EDCs on various pregnancy outcomes. In addressing this, the association of gestational and birth outcomes based on geospatial environmental surveys, occupation, and/or response to questionnaire that lack direct measures of maternal or fetal presence of the EDCs are not considered. It needs to be recognized that the impacts of EDCs on pregnancy outcomes are based on observed associations between EDC presence in maternal/fetal compartments and outcomes, and hence should be viewed as providing biological plausibility and not establishing causality.
Gestational Length
Gestational length in humans, from the onset of the last menstrual period, averages 280 days. Although in 70% of all human pregnancies delivery occurs within 10 days of the estimated due date (199), deliveries can occur within 259 days; these deliveries are designated as preterm birth (PTB) (200) or can occur later than 280 days, which may lead to higher risk of macrosomia (201). In addition to higher risk for hospitalization, such outcomes are risk factors for adult-onset diseases (202, 203). Although the gestational age can be influenced by many factors including sociodemographic, nutritional, medical, obstetric, and environmental factors, emerging evidence points to environmental EDCs as contributory factors (204, 205). The association of EDCs with gestational age at delivery has been found to vary depending on the EDC, ranging from PTB (eg Pb, nickel [Ni], parabens, DDE, PFOS), lack of effect on gestational age (eg, triclosan, Bis(1,3-dichloro-2-propyl) phosphate, diphenyl phosphate), and longer gestational age (eg, Cr, lignans, BPA, PCB) (Table 2). In addition to effects from the parent compound, effects also vary based on the type of EDC metabolites they produce. For instance, among phthalate metabolites, maternal urinary DBP and diisobutyl phthalate (DiBP) were associated with an increase in PTB (206, 207) as opposed to mono(2-ethyl-5-hydroxyhexyl) phthalate (MEOHP) with a decrease in PTB (208). These varying outcomes, which depend on the type and amount of EDC, site of measure, and timing of assessment, substantiate the difficulties in making generalized statements regarding the impact of EDCs on gestational length and emphasizes the need to consider all these variables when addressing the effects of EDCs.
Table 2.
Endocrine-disrupting chemicals and their associations with pregnancy and birth outcomes
| EDC | Gestational length | IUGR | Pregnancy complications | Birth weight | Congenital deformities | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early | Mid to late term | Early | Mid to late term | Early | Mid to late term | Early | Mid to late term | Early | Mid to late term | |
| Naturally occurring EDCs | ||||||||||
| Metals | Maternal urine: Se and Cu ↑ PTB (209) | Maternal urine: U (46); Ni (49); Pb (54, 200); Cd (53, 210) ↑ PTB Placental: Cr ↑ gestational length (212) |
Maternal urine: As, Ba, and Pb ↓ fetal femur length (19) | Maternal urine: Cd ↑ IUGR (51) Pb ≠ IUGR (55) Maternal hair Mn ↑ chest circumference (238) |
Maternal urine: Ni, As, Sb, Co, and V ↑ GDM (251) | Maternal term urine: Cr ↑ preeclampsia (45) Sb ↑ GDM (41) |
Maternal blood and urine: As and Pb (257) Maternal urine Cd ↑ LBW (39) |
Maternal urine: Cd ↑ LBW (48, 272) ≠ (57) Maternal blood: Cd ↑ LBW (211) Placental: Cd ↑ LBW and length (202) Pb ↑ LBW (43, 57); ↑ higher birth weight (259) ≠ birth weight (55) Hg ↑ LBW (52, 57) ≠ birth weight (274) As (267) and Hg (267) ↑ SGA |
Maternal blood: Pb ↑ and Se ↓ (301) Pb ↑ (302) congenital heart defects Maternal blood As, Pb, Cd and Cu ↑ and Zn and Se ↓ neural tube defects (303) |
|
| Parabens | Maternal urine: ethyl paraben ↑ PTB (213) | Maternal TM3 urine and cord blood: BuPB ↑ PTB (214) | Maternal urine: methyl, ethyl, propyl paraben (254); butyl and propylparaben (253) ↑ GDM | Maternal urine: ≠ birth weight and length (273) | Maternal urine: ↑ LBW (262); ↑ birth length (275) Maternal TM3 urine and cord blood: BuPB ↓ birth weight (214) Maternal term urine: EtPB and BuPB ↑ birth weight; Et PB ↑ birth weight in ♂ (276, 278) |
Maternal serum: propyl paraben ↑ cryptorchidism and reduced AGD (296) | Placental: propyl paraben ↑ cryptorchidism (304) | |||
| PAHs | Cord blood: (215) Placenta: (72, 216, 217) ↑ PTB |
Maternal blood and fetal tissue: PAHS ↑ abortions (by 14 wks) (79) | Placenta: ↑ LBW (72) Maternal urine: 1-OHP ↑ LBW, birth length and head circumferences (277, 279) 2-hydroxy fluorene ↑ LBW (280) |
Placenta: ↑ neural tube defects (81) | ||||||
| Phyto- estrogens | Maternal blood: genisten ↑ PTB (218) Maternal urine: lignans ↑ gestational length (219) |
Maternal blood: ≠ birth weight (83) | Maternal urine isoflavones: ≠ fetal AGD (293) | |||||||
| EDCs in plastics | ||||||||||
| Bisphenols | Maternal urine: BPA ↑ gestational length (20) Maternal term urine: BPS ↑ gestational length in ♀ (220) |
Maternal urine: BPA ≠ fetal measures (236) | TM2 maternal urine: BPA ↑ low birth length and head circumference (239) Term maternal urine: BPA ≠ fetal measures (236); ↑ birth length in ♂ (240) Maternal urine BPA: ↓ fetal weight and head circumference (241) |
Maternal blood: BPA fetal loss (237) Maternal urine: BPA ↑ preeclampsia (249) ≠ glucose levels (252) |
Maternal urine BPA ≠ preeclampsia (249) ↑ blood glucose (252) ↑ GDM (257, 259) | Maternal urine: BPA ↑ LBW (19, 20) | Maternal urine ↑ LBW (239, 243, 244) ≠ birth weight (236) Placental BPA ↓ birth weight (268) TM2 amniotic fluid BPA ↓ birth weight (99) |
Maternal serum: BPA ↑ cryptorchidism and reduced AGD (296) Maternal urine: BPA ↑ implantation failure (305) |
Placental: BPA ↑ cryptorchidism (304) | |
| Phthalates | Maternal urine: DEHP ↑ PTB (206) ↑ gestational length (208, 221) MEHP and MEOHP ↓ PTB (208) |
Maternal urine: DBP and DiBP Phthalate metabolites ↑ PTB (206, 207) | Maternal urine: MBzP and MnBP ↑ IUGR (in ♂); DEHP ≠ fetal measures (310) |
Maternal urine: DEHP ↑ IUGR (242) TM2 phthalates ↑ decrease head and chest circumference (239) |
Maternal urine: DEHP ↓ impaired glucose tolerance (246) MEP and MOCP ↑ blood glucose (250) | Maternal urine phthalate metabolites ≠ preeclampsia (249) MEP ↑ impaired glucose tolerance; DEHP ↓ glucose tolerance (256) | Maternal urine: ↑ birth weight (19) | Maternal urine: DEHP ↑ LBW (242) MBzP and MnBP ↑ reduced fetal growth parameters and ↑ birth weight (in ♂) (236) |
Maternal urine: MEP ↑ implantation failure (305) | Maternal urine: DEHP ↑ reduced AGD (293, 295) |
|
Average TM1-TM3 MEP ↑ impaired glucose tolerance (255, 256) MEP and DEHP ↑ preeclampsia (249) |
Phthalate (average TM1-TM3 urine) ↑ impaired glucose tolerance (255) Maternal TM2 and TM3 urine MBzP ↑ blood pressure (260) |
MEHP and MEHHP ↓ in ♂ and MMP and MEP↓ in ♀ birth weight (281) Maternal TM3 urine: MMP, MBP, MEHP, MEOHP, and MEHHP ↓ birth weight (282) Cord blood and meconium: DBP ↓ birth weight, DEHP ↓ birth length (283) |
||||||||
| Industrial chemicals | ||||||||||
| Organo-halogens | Maternal plasma PFAS (222) PFOS and PFNA ↑ PTB (223) | Maternal serum PCB ↑ gestational length (224, 225) | Cord blood: PBDE congeners ↑ FGR (235) | Maternal serum: PBDE ↑ GDM: (261) | PFAS ↓ birth weight (222, 284); PFAS and PFNA ↑ LBW (223) | Maternal serum: PCB (285); PCB, PFOA, HCB (286) ↑ SGA | ||||
| Agrochemicals | ||||||||||
| Pesticides | Maternal adipose tissue DDT and maternal serum DDE ↑ PTB (226, 227) | Maternal urine: dialkyl phosphates ↑ reduced fetal weight and length (237) | Maternal plasma: DDT/DDE ↑ Hypertensive disorders: (248) | Maternal blood: DDT (287) | Cord blood: β-HCH ↓ birth weight and ponderal index (in ♂) (289) | Maternal serum: DDE ↑ hypospadias, cryptorchidism (297, 298) Placental: DDT and HCH ↑ neural tube defects (81) |
||||
|
Maternal plasma DDE (218); cord HCH (228) ↑ PTB Maternal urine isopropyl-phenyl phenyl phosphate: ↑ PTB (in ♀) and ↓ PTB (in ♂); Bis(1,3-dichloro-2-propyl) phosphate and diphenyl phosphate ≠ gestational length (229) Maternal plasma DDT ↑ gestational length (230) |
Maternal urine: parathion and diazinon (288) ↑ LBW |
chlorpyrifos, diazinon, and propoxur ↓ birth weight and/or length (290) p,p′-DDE, total DDT, β-BHC; p,p′-DDT, p,p′-DDD, HCB and mirex ↓ birth weight (291) Maternal urine: isopropyl-phenyl phenyl phosphate ↑ LBW (229) Maternal plasma DDT ↑ birth weight and length (230) |
||||||||
| Herbicides | TM2 maternal urine: atrazine ↑ reduced head circumference (161) | |||||||||
| Fungicide | Cord blood: tributylin: ↑ LBW (148) | |||||||||
| Antifouling agents | ||||||||||
| Personal care products | ||||||||||
| Anti-bacterial | Maternal TM3 urine and cord blood: triclocarban ↑ PTB; triclosan ≠ gestational length (214) | Maternal blood triclosan: ↓ (250) ≠ (176) GDM |
Maternal and cord blood: triclosan ↑ Congenital malformations of circulatory system, eye, ear, face, neck, urinary system and musculoskeletal system (173) | |||||||
| Medical products | ||||||||||
| Pharmaceuticals | Diethylstilbestrol ↑ PTB (231, 232) | Diethylstilbestrol ↑ SGA (231) | Diethylstilbestrol: ↑ Cryptorchidism and hypoplasia of the penis (299) | |||||||
| Medical Supplies (Bisphenols and Phthalates) | see above sections for effects of bisphenols and phthalates |
Abbreviations: ↑, increase; ↓, decrease; ≠, no association; ∑, sum; ♂, male; ♀, female; AGD, anogenital distance; CRP, C-reactive protein; DBP, dibutyl-phthalate; DDE, diphenyl dichloro ethylene; DEHP, phthalate di(2-ethylhexyl)-phthalate; DMP, dimethyl-phthalate; E2, estradiol; E3, estriol; Early, first trimester; EDC, endocrine-disrupting chemical; FSH, follicle-stimulating hormone; GDM, gestational diabetes mellitus; IFNG, interferon γ; IGF1, insulin-like growth factor; IL, interleukin; IUGR, intrauterine growth restriction; LBW, low birthweight; MDA, malondialdehyde; mid to late, second and third trimester; mRNA, messenger RNA; miRNA, microRNA; PAHs, polyaromatic hydrocarbons; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFAS, perfluorinated alkylated substance; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PTB, preterm birth; SGA, small for gestational age; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TM, trimester; TNF, tumor necrosis factor; TSH, thyrotropin.
Intrauterine growth restriction
IUGR is defined as diminished in utero growth of the fetus documented by at least 2 intrauterine growth measurements (233). Normal fetal growth depends mainly on sufficient delivery of oxygen and nutrients via the placenta. Factors that can disrupt placental function, including EDCs (234), contribute to IUGR. As with gestational length, the impact of the EDCs on IUGR vary depending on the EDC studied, time of measurement, the maternal/fetal matrix in which it is measured, and fetal sex (see Table 2). For example, whereas placental Pb measure (55) was not associated with abnormal fetal measures, first-trimester maternal Pb was inversely associated with femur length (19). Maternal presence of Cd (51) and PBDE (235), phthalate metabolites MBzP and MnBP (226), and organophosphate pesticides (237) for the most part have been reported to be associated with reduced fetal weight (Table 2). In addition, a sex-specific association for first- and third-trimester urinary phthalate with IUGR became apparent only when stratified by fetal sex, with strong associations with risk for IUGR among male fetuses (236, 242). Although a standardized approach to assess IUGR across EDCs has not been employed, available evidence points to several EDCs negatively affecting estimated fetal weight and ultrasound assessed fetal measures (head, chest and abdominal circumference, femur and fetal length depending on the time of pregnancy, matrix of EDC measurement, and fetal sex) (see Table 2). Based on the DOHaD hypothesis, alterations in these fetal growth indicators have the potential to serve as surrogate markers for long-term health outcomes and hence are a valuable resource.
Maternal Complication of Pregnancy
The prevalence of medical problems in pregnancy is increasing (245) with early pregnancy disorders manifested as spontaneous abortions, and late pregnancy disorders as maternal hypertensive disease (preeclampsia [PE] and chronic hypertension) and gestational diabetes mellitus (GDM). While demographic and lifestyle factors contribute to these pregnancy complications, evidence is emerging that environmental chemicals may be additional contributors (246-248). For instance, women with higher levels of benzo[a]pyrene DNA adducts during the second trimester (77) and unconjugated maternal blood BPA at 4 to 5 weeks of gestation (249) have manifested higher odds of early pregnancy loss. On the other hand, for gestational hypertensive disorders, the impact varied from gestational hypertension with the presence of DDT/DDE (250) to PE with BPA, phthalates (251) and Cr (45) (see Table 2). Similarly, the association of EDC with GDM also varied from no associations (176) to an inverse association with the presence of triclosan (252) and strong associations with the detection of the metals Ni, As, Sb, Co, and V (253), BPA (254), parabens (255, 256) and phthalates DEHP and MBP (257). Furthermore, the association of BPA and DEHP with PE and GDM appeared to vary depending on when in pregnancy they were measured, with a strong association found between first-trimester levels, but not with mid and late-gestation levels (251) for PE. Similarly, in contrast to first-trimester BPA (254) and phthalate DEHP (258) showing no associations with GDM, second-trimester urinary BPA levels (254) and the average of first- and third-trimester levels of DEHP (257) were associated with increased blood glucose and GDM, respectively. Another study showed that the association of first trimester phthalate levels varied depending on the metabolite, with DEHP having no association, whereas MEP and MOCP were associated with increased maternal glucose levels (252). These studies emphasize the need for considering not only various EDCs but also their metabolites and timing of pregnancy in addressing risks.
Birth Weight and Size
Birth weight and size are important predictors not only of neonatal morbidity and mortality (252), but also of long-term health of the offspring, predicted by the DOHaD hypothesis (3). These variables can be influenced by several factors that include demographic, socioeconomic, nutritional and lifestyle factors (262), with recent evidence pointing to additional detrimental impact following gestational exposure to EDCs (43, 263-265). The impact of the EDCs on birth size and weight also varied depending on the EDC, time of pregnancy measurement, whether maternal or fetal measures were considered and fetal sex (see Table 2). For BPA, the association with birth weight varied from no association with first-trimester and third-trimester maternal urinary levels of BPA (236, 239, 266) or maternal term blood (267), to associations with low birth weight with increased levels of maternal first-trimester circulating (20), second-trimester amniotic fluid (99), and term placental (268) BPA levels (see Table 2). Some EDCs such as PFAS, PCBs, PBDEs, organochlorines, or organophosphates (43), and As (269) showed no impact or small effects on birth weight. For phthalates, as is the case with fetal weight, the association with birth weight appeared to depend on the metabolite studied: MEHHP and MEOHP metabolites were associated with decreases (242), whereas MBzP in males was associated with higher (236) birth weight. Similarly, metabolite and sex-dependent associations were also evident for PFOS, with one study finding higher PFOS in first-trimester maternal plasma associated with low birth weight in male infants (270), but another study finding associations for all maternal PFASs with low birth weight in female neonates (271). The association for Cd varied with the matrix analyzed, with maternal term urinary (48, 272) and placental (212) levels showing associations, whereas cord blood levels surprisingly showed no association with low birth weight (57). These studies continue to emphasize when and where during pregnancy EDCs are measured, the matrix they are measured in, the parent or metabolite assessed, and the sex of the newborn are important considerations in determining the impact of EDCS on birth weight.
Birth Anomalies
The evidence relative to the involvement of EDC in congenital defects is limited, with the majority of the evidence focusing on the susceptibility of male fetuses (292). Triclosan and triclocarban in maternal and cord blood at term were associated with congenital malformations of the circulatory system, eye, ear, face, neck, urinary system, and musculoskeletal system (173), although the sex specificity of these outcomes is not known. The maternal term (293) and cord blood (294) levels of DEHP were linked with reduced male anogenital distance, a marker for insufficient fetal androgenization, along with reduced penis size and incomplete testicular descent (295) (see Table 2). Similarly, maternal serum BPA and propyl parabens (296), and the organochlorine pesticides DDT and DDE (297, 298), were found to be associated with increased male cryptorchidism, hypoplasia of the penis, and reduced anogenital distance (see Table 2). These findings highlight the potential for EDCs to affect congenital male reproductive tract defects such as congenital hypospadias and cryptorchidism, and reduced anogenital distance, a known marker of masculinization (300). The impact of EDCs on female congenital anomalies are understudied and is an area for future investigation.
Offspring number and sex distribution
Sex ratio is measured as the ratio of male to female births and is considered to be about 1.06 (106 boys for every 100 girls born) (306). Although sex determination is genetic, sex ratio of birth outcomes appears to be influenced by numerous factors and may be dictated by seasonal changes, maternal age, interpregnancy intervals, family size, birth order, length of follicular phase and moment of conception, age of the fathers, in vitro fertilization, and maternal diabetes (307). Growing evidence, particularly in fish, amphibians, and reptiles, implicates anthropogenic disturbance, such as environmental chemical contamination, in overriding the genetic determination of gonads and altering the sex ratio (308). However, to what extent these observations can be expanded into higher species including mammals remains to be determined. Limited evidence available from analysis of birth records spanning many decades has pointed to significant decreases in the proportion of boys born in industrial countries, including the United States, Denmark, Finland, and the Netherlands (306, 309-311). In addition, some studies have linked changes in sex ratio to geographical and occupational exposure, for example, the use of pesticides in Russia (312). Although these observations raise the possibility for environmental chemicals to have the potential to affect sex ratios, direct evidence in humans is still lacking and would require decades of work involving larger cohorts.
Dose Effect of Endocrine-Disrupting Chemicals on Gestational and Birth Outcomes
Dose-response relationships are central to toxicological assessments of various hazards and risk assessment of environmental toxicants are typically based on linear dose-response effects. Some reports of associations with gestational and birth outcomes with EDCs appear to have linear dose-response relationships. For instance, early pregnancy loss was associated with presence of DDT in maternal blood in a dose-dependent manner, with relative odds increasing by 1.17 with every 10-ng/g increase in serum total DDT (313). In contrast, midpregnancy maternal urinary BPA levels were associated with decreasing anogenital distance, with higher quartiles showing strong associations (314). Likewise, dose-specific effects diminishing birth weight were evident with maternal first-trimester blood levels of BPA (20) and PFAS (271), urinary levels of BPS (19) and heavy metal V (315), maternal third-trimester urinary levels of As (37), methyl paraben (316), and 4-tert-octylphenol (317). In contrast, dose-dependent increases in maternal first-trimester urinary levels of the phthalate metabolite MCPP were associated with an increase in birth weight (19). Linear dose assessments, however, have usually been based on establishing quartiles without consideration of the entire dose range.
Hormonal responses in general are nonlinear and manifest nonmonotonic dose-response with U- or inverted U-shaped, or “biphasic,” dose-dependent effects and this kind of relationship is also evident with EDCs (318) both in animal models and epidemiological assessments in humans (319). For instance, an inverse U-shaped association was evident between maternal urinary BPA and birth weight, whereas the opposite, a U-shaped association, was present between phthalate MCPP and MECPP, and birth weight (275). Non-linear, but dose-dependent effects of PTB suggestive of a nonmonotonic relationship were also evident, with maternal circulating levels of PFAS (222, 223) and early pregnancy urinary levels of V (315). Such nonmonotonic dose effects are not confined just to birth outcomes. For example, reported inverse U-shaped relationships between maternal serum concentrations of PCBs with vitamin D3 (320), cubic spline association of maternal first-trimester circulating As levels with impaired glucose tolerance (321), and a U-shaped dose–response relationship between maternal third-trimester urinary MnBP and placental steroidogenic gene expression (322), are indicative of nonmonotonic relationships of EDC with maternal and fetal outcomes. These findings emphasize the dose-specific nature of EDC impact and caution against assuming linearity in dose response as often done in toxicological studies.
Effects of Endocrine-Disrupting Chemical Mixtures on Gestational and Birth Outcomes
Most of the studies discussed earlier have assessed the associations of gestational and birth outcomes with the maternal or fetal presence of an individual EDC. Considering that humans, in real-life scenarios, are routinely exposed to multiple chemicals of different classes with similar and differing signaling mechanisms, the cumulative effects of EDC exposure are of ultimate importance. Working via common or diverse receptor pathways, these environmental chemicals may lead to additive, synergistic, or antagonistic outcomes (see Fig. 1), with the overall effect dictated by the net effect of different chemicals in the mixture (323, 324). For example, combinations of 11 xenoestrogens were effective in producing an effect such as increased activity of yeast estrogen receptor α reporter, while each EDC tested at the same cumulative dose level as in the overall mixture failed to produce any effects (325). This emphasizes the clear need to assess the effects of EDC mixtures, although the current regulatory guidelines governing allowable concentrations of environmental EDCs have been developed on a chemical by chemical basis (326). Because of this, mixture risk assessment (MRA), defined as the determination of the cumulative risk to human health or the environment due to exposure to different chemicals via multiple routes, is gaining prominence (326). Initial studies of MRA of maternal exposures involved the same classes of chemicals (eg, phthalates) or chemicals with the same mode of action (eg, xenoestrogens). One study found that whereas analysis of individual phthalate metabolites showed a modest association with low birth weight for each metabolite, inclusion of all metabolites in the analysis increased the strength of the association (327), indicating the combined synergistic effects of phthalate metabolites. Such associations were also evident for other EDC classes in which early gestational presence of persistent organic pollutants in maternal plasma showed weak associations with birth anthropometry when assessed individually, but when analyzed as a class of EDCs such as organochlorine pesticide, PBDE, or PCB mixtures, manifested strong associations with different birth measurements (328, 329). Similarly, whereas individual PAH metabolites in maternal second-trimester urine showed no individual association with birth weight and birth measures, the sum of the PAH metabolites showed a significant interaction with cephalization index (head circumference to body weight ratio), a measure of brain growth (330). Measures of individual organochlorines showed both positive and negative associations depending on the fetal measure, whereas as a mixture they mainly manifested as negative associations (329). These reports are consistent with chemicals with similar action or classes having additive or synergistic effects on pregnancy and birth outcomes.
Assessing a single class of EDC for cumulative effects, however, is not effective in determining the MRA, because humans are exposed to different EDCs belonging to categories of metals, plastics, plasticizers, persistent organic pollutants, and agrochemicals, among others. About 40 to 50 different EDCs have been detected in maternal and fetal samples (17, 19), although these numbers reflect only a fraction of the exposome. As these chemicals are of divergent characteristics with similar as well as dissimilar modes of action (see Fig. 1), statistical modeling through cluster- and principal–component based analyses are being employed to gain an understanding of the impact of complex chemical mixtures (18, 331). The approach of statistical modeling in MRA is in its infancy and standardization of this approach is needed as various factors such as biomonitoring approaches, interaction between chemicals, geographical locations, socioeconomic status of the participants all influence the outcome (332). A very limited number of studies have assessed the maternal presence of EDCs as mixtures and their effects on gestational and birth outcomes. For instance, modeling of 43 EDCs present in the maternal milieu through cluster and principal component analysis (331) revealed an inverse association of the exposome with birth measures (277). Another study found clusters of chemicals containing phenols, phthalate metabolites, several metals, organophosphate and organochlorine pesticides, PCBs, and several PFAS were associated with low birth length (277). Likewise, a principal component containing oxychlordane, trans-nonachlor, benzophenone-3, triclosan, As, dialkylphosphate (DAP), and PCBs was associated with reduced birth length only (277). Similarly, whereas maternal first-trimester urinary EDCs individually showed an association with low levels of maternal and cord blood oxidative stress markers, these EDCs modeled as mixtures through principal component analysis showed both positive and negative associations in a sex-specific manner (333). Analysis of associations of EDC mixture with inflammatory cytokines also showed both positive and negative relationships that varied depending on the EDC mixture and cytokine (334). Emerging studies in humans exposed to complex chemical mixtures used in fracking industries or air pollution through geographical proximity or occupation, have also manifested poor gestational and birth outcomes (335-340). Animal studies substantiate causal relationships by demonstrating the association of exposure to fracking chemical mixture (341) or biosolids (185) with adverse developmental health and reproductive outcomes. These emerging data point to the differing impact of the maternal-fetal exposome as a whole on gestational and birth outcomes compared with individual EDC components.
Mechanisms Through Which Endocrine-Disrupting Chemicals Affect Pregnancy and Birth Outcomes
Maternal and fetal milieus are tightly controlled throughout pregnancy to maintain the energy demands of the growing fetus. The placenta serves as a conduit between the mother and fetus to maintain fetal homeostasis. EDCs affect pregnancy not only by acting directly as hormonal agonists/antagonists to influence endocrine functions, but also indirectly by disrupting maternal, placental, and fetal homeostasis.
Altered Maternal Milieu
The effects of EDCs on the maternal environment may be mediated by having an impact on the inflammatory and oxidative state, hormonal milieu, metabolomic profile, and microbiome.
Inflammation
The inflammatory state varies during the entire course of pregnancy, with 3 separate immunological states described: a proinflammatory state during early pregnancy with high levels of cytokines, an anti-inflammatory phase during midgestation and a return to a proinflammatory state during the late gestation and term (342). Labor/delivery occurring spontaneously is also characterized by a strong inflammatory state with an influx of immune cells along with increased levels of cytokines (342, 343). Disruption of this delicate balance can lead to pregnancy complications such as implantation failure, recurrent pregnancy loss, intrauterine growth restriction, PTB, PE, and GDM (344-347).
EDCs are known to modulate the immune system through their action on the immune regulatory network, cellular and humoral response, survival, maturation, and cytokine production by immune cells (348, 349). In line with this, several reports, including ours (334), have shown that EDCs such as BPA (350) and triclosan (351) increase proinflammatory cytokines, including interleukin 6 (IL-6) and PBDE and PFAS increase IL-6 and tumor necrosis factor α (TNF) in the maternal circulation (352). In addition, markers reflective of the inflammatory state are also associated with maternal exposure to EDCs. These include increases in 1) maternal circulating C-reactive protein (CRP), a marker of systemic inflammation with increased urinary BPA analogues BPB and BP3 across pregnancy (351) and 2) 8-iso-prostaglandin F2α, a product of enzymatic sources linked to inflammation, with multiple LMW and high-molecular-weight (HMW) phthalate metabolites in maternal third-trimester urine (353). Similarly, principal component analysis modeling of maternal first-trimester urinary EDC mixtures showed both positive and negative associations with inflammatory cytokines depending on the chemical mixture and the cytokine measured (334). For example, principal component grouping with higher weighting for metals and phthalates in maternal urine was associated with higher levels of IL8 and interferon γ (IFNG) in maternal first-trimester plasma. In contrast, principal component grouping with lower weighting for metals was associated with lower levels of IL1B levels in maternal term plasma (334). This direct and indirect evidence on the EDC influence on inflammatory state during pregnancy mostly comes from assessments carried out during late pregnancy or term for the majority of EDCs, with very limited information available from early pregnancy (Table 3).
Table 3.
Early and late gestational presence of endocrine-disrupting chemicals and their associations with maternal and fetal mediators
| EDC classes | Inflammation | Oxidative stress | Hormonal changes | Metabolomic changes | Microbiomic changes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early | Mid to late term | Early | Mid to late term | Early | Mid to late term | Early | Mid to late term | Early | Mid to late term | |
| Metals | Maternal: Se, Mo, Cd, Ni, Cu, Zn, Pb ↑ MCP3 and IL8 (334) | Maternal: Se, Mo, Ni ↑ maternal MCP3 (334) Maternal W and U ↓ cord MCP1 (334) Maternal Mo, Ni, Cd, Zn ↑ cord MCP1a and MCP3 (334) Maternal As ↑ IL1B, IL8, IFNG, TNF (355) |
Maternal Pb and Cu ↓ cord nitrotyrosine and chlor- tyrosine (333) | Maternal V, As, Pb ↓ free T3 (36) Maternal As, Se, Mn, Ni, Sb ↓ free and total T3 or T4 (381) Maternal Ce and Yb ↓ cord TSH (382) |
Maternal Cd ↑ urine L-cystine, L-tyrosine, and dityrosine; ↓histamine, and uric acid (256) ↑ uric acid (399) | Maternal toenail As level ↑ butyry- lqlycine and tartrate (400) Maternal TM2: higher Cu and lower Mo: ↑ glucose level (394) Maternal Ar ↑ TM2 cord 17- methylstearate, laurate (12:0) and 4- vinylphenol sulfate (400) |
Maternal Methyl Hg Gut microbiome composition changed (416) | Maternal Methyl Hg No effect on gut microbiome composition (416) | ||
| Parabens | Maternal urine: butyl paraben ↓ CRP (351) Ethyl paraben ↓ IL1B (356) |
Maternal urine: butyl, ethyl, methyl, propyl paraben ↑ 8-hydroxy- deoxyguanosine and 8- isoprostane (362) Maternal term urine: Methyl and ethyl paraben ↑ malondealdehyde (MDA) and 8- hydroxydeoxy- guanosine (63) |
Maternal butyl paraben ↓ E2 and free T4 (375); ↑ total T4 (376) Maternal methyl and propyl paraben ↓ mid-pregnancy E2 and ↑ late-pregnancy E2 (375) Maternal propyl paraben Maternal: ↓ free T4 (376) Maternal term urine: EtPB ↑ cord blood triiodothy- ronine levels (278) |
Maternal urine: Parabens affect purine metabolism, fatty acid β-oxidation, and other pathways: methyl paraben ↑ hypoxanthine and 7- methylxanthine; ethyl paraben ↑ benzoic acid; propyl paraben ↑ trimethylamine N-oxide, dihydrobiopterin, and 2,6-dimethy- lheptanoyl carnitine (401) | Maternal butyl paraben ↑ TM2 glucose Propyl paraben ↓ glucose (353) |
|||||
| PAHs | Placental PAH DNA adducts: ↑ TBARS and 7-ethoxy- coumarin O-deethylase, ↓ glutathione S-transferase activity (367) | Maternal urine: 1- hydroxypyrene ↑ MDA (366, 367); placental 1- hydroxypyrene: ↑8-OHdG (75) | Maternal Hydroxylated PAH ↓ E2 and T (280) | |||||||
| Phytoestrogens | Maternal equal ↑ E3 (84) Maternal matairesinol, enterodiol and entero- lactone ↑ urine hydroxylation products of estrogens (219) |
|||||||||
| Bisphenols | Maternal BPA ↑ IL6 (350) Maternal BP3 ↓ CRP (351) |
Maternal BPA ↑ 3-nitrotyrosine (364) | Maternal BPA ↑ cord 3-nitrotyrosine (364) Maternal BP3 ↑ 8-hydroxy- deoxyguanosine (8-OHdG) and 8-isoprostane (363) Maternal BPS ↑ 8-isoprostane (363) |
BPA: baseline cortisol↑ in female, baseline cortisol↓ in male (383) Maternal BPF: ↑ free T3 (384) |
Maternal BPA ↓ maternal TSH (369, 370); ↑ maternal free T4 (370) ↓ maternal T (374) ↑ cord E2 (294) Maternal BPA ↓ CRH (364) Maternal BPF ↑ free T4 (364) |
BPA ↑ maternal palmitic acid; oleic acid and total free fatty acids (364) | Maternal BPA ≠ glucose levels (249) ↑ urine - endocanna binoid, palmitoleamide and lysophosphatidy- lethanolamine 18:0 (402) Cord ↓leptin and ↑high-molecular-weight adiponectin (445) |
|||
| Phthalates | Maternal MnBP, MBzP, MCOMHP, meCPP, MEHP ↑ MCP3 and IL8 (353) | Maternal MnBP, mCINP, meCPP, MEHP, MEHHP, MEOHP ↑ MCP3 (353) Maternal MnBP/ MEHP ↑ cord MCP1a /MCP3 (353) Maternal MnBP, MIP, MNP ↑ 8-iso-prostaglandin F2α (351) |
Maternal MCPP and MCINP ↓ chlor-tyrosine (333) | Maternal MBzP and MCOMHP ↓ cord nitro-tyrosine (333) | Maternal DEHP slower rise in hCG (305) Maternal MiBP, MEHP, MEOHP ↑ E1 and E2 (378) Maternal MBzP ↑ E2 (378) Maternal MCNP and DEHP ↓ free T (378) Maternal urine: MnBP, MBzP, MCIP ↑ hCG (386) |
Maternal MEHP and DEHP ≠ cord E2, T, FSH, LH, T3, T4, and TSH (282) Maternal MEP: ↓ maternal total T4 (372); ↑ total and free T (377) Maternal DEHP: ↓ maternal total and free T (377) |
Maternal MEP ↑ blood glucose (396) Maternal DEHP ↓ triglycerides, palmitic, oleic, linoleic, and α-linolenic acids (397) Maternal MEP, MnBP, MiBP, DEHP ↑ nicotinamide mononucleotide, cysteine T2, cystine, and L-aspartic acid (398) MiBP, MnBP, and DEHP ↓ Cord leptin (445) |
|||
|
↓ cord cortisol, cortisone and glucocorticoid/adrenal androgen ratio, and ↑ cord DHEA and DHEA/androstenedione ratio (439) Maternal MBszP ↓ TSH (372) Maternal MEP, MnBP, MiBP, DEHP ↓ total T4 Cord: ↓ TSH and total T4 (372) ↓ androstenedione, T and DHEAS and ↑E2 and E3 (114) |
||||||||||
| Organohalogens | Maternal PBDE and PFAS ↑ IL6 and TNF (352) | Maternal serum HCB and PCB congeners ↓ total T3 and ↑ free T4 (387) PFOS: ↓ maternal TM3 cortisone (388) |
PFOS and PFNA ↓ newborn TSH (430) PFOA and PFOS Maternal hypothyroid (389) Cord: PFOS ↑ DHEA and cortisol; PFOA ↓ DHEA (441) PCB ↓ newborn T3 and T4 (436,437) ↓ T and ↑ E2 (443) PCDF ↑ maternal 4-hydroxyl E2; PCDD ↓4-hydroxyl E2/ 2-hydroxyl E2 ratio (390) PBDE ↑ cord TSH (436) ≠ maternal and cord E2 (444) |
PBDE ↑ maternal fasting glucose (395) PFAS ↓ maternal glucose (403) PFOS ↑ maternal glucose; PFOS metabolite inverse-U shaped association (404) |
||||||
| Agrochemicals | Maternal HCH, DDD, DDE ↑ IL6 and IL4 (357) Maternal plasma PBDEs ↑ IL6 and TNF; PFAS ↑ IL6; hydroxylated PBDE metabolites ↓ IL10 (352); ≠ CRP (354) |
DDE and HCB maternal (366) and cord (429) ↓ total T3 and T4; ↓ TSH (389) DAP ≠ maternal and cord TSH, T3 and T4 (374) Maternal pyrethroids ↑ TSH (373) Chlordanes, cis-HCB, heptachlor epoxide, Mirex, and toxaphenes Cord: ↓ T, cortisol, cortisone, prolactin, ↑ DHEA, FSH (♂) (442) DDT Cord: ↓ DHEA and ↑ cortisol (♀) (442) |
Maternal urine pesticides: ↑ glycine, threonine, lactate and glycerop- hosphoc- holine and ↓ citrate (405) | |||||||
| Antifouling agents | ||||||||||
| Personal care products | Maternal urine: Triclosan ↑ CRP, IL10, TNF; 2,5- dichlorophenol ↑ CRP (356) | Maternal triclosan ↑ 8-hydroxy- deoxyguanosine (363) | Maternal triclosan ≠ TSH, T3 or T4 (385) | Maternal triclosan ↑ E3 (376) Maternal triclocarban ↑ T3 and T3/T4 (375) |
||||||
| Pharmaceuticals | see sections above for effects of bisphenols and phthalates |
Abbreviations: ↑, increase; ↓, decrease; ≠, no association; ∑, sum; ♂, male; ♀, female; CRP, C-reactive protein; DDE, diphenyl dichloro ethylene; DEHP, phthalate di(2-ethylhexyl)-phthalate; E2, estradiol; E3, estriol; Early, first trimester; EDC, endocrine-disrupting chemical; FSH, follicle-stimulating hormone; IFNG, interferon γ; IGF1, insulin-like growth factor; IL, interleukin; MDA, malondialdehyde; mid to late, second and third trimester; mRNA, messenger RNA; miRNA, microRNA; PAHs, polyaromatic hydrocarbons; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFAS, perfluorinated alkylated substance; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TM, trimester; TNF, tumor necrosis factor; TSH, thyrotropin.
Oxidative stress
Metabolic activity leads to the production of reactive species (reactive oxygen/nitrogen species) that are neutralized by a cellular antioxidant defense mechanism, failure of which leads to oxidative stress. During pregnancy, physiological concentrations of reactive species participate in placental development (358). Increases in metabolic activity of the placenta and the growing fetus (359) increase production of reactive species (360) that are offset by corresponding increases in antioxidants (361). Failure of this protective mechanism is associated with adverse pregnancy and birth outcomes such as miscarriage, PE, fetal growth restriction and preterm labor (362).
The presence of EDCs in maternal and fetal milieu were associated with elevations in biomarkers of oxidative stress. For example, increases in oxidative stress biomarkers hydroxydeoxyguanosine (8-OHdG), a marker of DNA damage and 8-isoprostane, a biomarker for lipid peroxidation with maternal urinary dichlorophenols, benzophenone-3, triclosan, and parabens across pregnancy, 8-8-isoprostane with BPS (363), and nitrotyrosine- a marker of cell damage and inflammation with maternal urinary BPA (364) have been reported (see Table 3). In contrast, the presence of HMW phthalate metabolites at 13 weeks of gestation were associated with low levels of 8-isoprostane (365), maternal first-trimester urinary phthalate metabolites MCPP and MCINP with low levels of maternal first trimester chlortyrosine, a marker of myeloperoxidase-catalyzed oxidation, and MBzP and MCOMHP with nitrotyrosine (333). As opposed to these individual associations, analysis of maternal first-trimester urinary EDCs as mixtures has revealed associations varying with sex, including both positive and negative associations depending on the chemical composition of the EDC mixture and biomarker assessed (333). For example, principal component analysis with lower weighting for maternal first-trimester urinary metals and phthalates, was associated with higher levels of chlortyrosine in maternal first-trimester plasma. These studies point to the impact of EDCs on the pregnancy oxidative state. However, as with inflammation, outside of EDCs such as bisphenols and phthalates, information is limited on the effect of several EDC classes on oxidative stress in pregnancy.
Hormones and growth factors
Pregnancy is associated with profound physiological changes that are driven by dynamic changes in maternal, placental, and fetal hormones. Hormones control pregnancy at every stage, from the process of implantation (progesterone) and maintenance of pregnancy (chorionic gonadotropins and progesterone), to parturition (glucocorticoids and corticotropin-releasing hormone, as well as estradiol [E2] and oxytocin) (368). The physiological hormonal homeostasis during pregnancy is tightly regulated (369), and available evidence so far indicates that EDCs adversely affect pregnancy hormonal homeostasis (see Table 3). For instance, EDCs have been shown to disrupt secretion of anterior pituitary thyrotropin (TSH), which regulates the release of 3,5,3′-triiodothyronine (T3) and thyroxine (T4) secretion, which are important for fetal development, particularly the central nervous system (see Table 3). Studies have also indicated that maternal levels of TSH and thyroid hormones were influenced by third-trimester maternal EDCs in an EDC-specific manner with BPA (370, 371), MBzP (372), and organochlorine pesticides (366) decreasing, pyrethroids and triclocarban increasing (373), and organophosphate DAP metabolites (374) having no association with TSH levels. Similarly, whereas BPA (370) and BPF (375) were shown to increase free T4, phthalate metabolites (MEP, MnBP, MiBP, and DEHP metabolites) decreased maternal total T4 (372).
Disruptions in maternal steroidal hormones particularly sex steroids—key regulators of sexual differentiation and sexual dimorphism—were also found to be associated with EDC exposure (see Table 3). For instance, late pregnancy butyl paraben has been found to be associated with a decrease in maternal blood levels of E2 (376) as opposed to BPA being associated with decreased levels of testosterone (T) in maternal blood (375). Associations for some EDCs depended on the time of measurement during pregnancy or the EDC metabolite with methyl and propyl paraben linked with decreased and increased maternal serum E2 levels during mid- and late pregnancy, respectively (376). Similarly, urinary MEP levels were associated with increased levels of total and free T (377), in contrast to MCNP and DEHP levels being associated with decreased levels of free T (378).
Gestational EDC exposure was also associated with disruptions in maternal growth factors, particularly angiogenic factors, which have a bearing on placentation and placental function because the placenta is an organ with extensive vascular network promoting nutrient and waste exchange between the mother and fetus. The disruptive effects of EDCs that were manifested at the level of members of placental growth factors (PIGFs) and vascular endothelial growth factors (VEGFs) included (i) maternal urinary DEHP or BPA throughout pregnancy with decreased maternal circulating PlGF and soluble Flt1 (VEGF receptor) to PlGF ratio (379, 380); (ii) maternal third-trimester urinary PAH metabolites 2-naphthol, 2- and 3- hydroxyphenanthrene (PHE), and 4-PHE with decreased PIGF; (iii) third-trimester maternal circulating 9-PHE with increased maternal circulatory soluble Flt1 in (390); and (iv) maternal third trimester urinary metals both individually and as mixtures with changes in maternal angiogenic factors (45) (see Table 3). These findings suggest that EDCs have the potential to disrupt the maternal hormonal balance critical for proper maintenance of maternal, fetal, and placental function.
Metabolome
Considering that hormones help maintain general homeostasis, the impact of EDCs on the metabolic milieu may be mediated indirectly via disruption of the hormonal milieu. Alternatively, EDCs can directly affect the metabolic milieu by altering the enzymes involved in xenobiotic metabolism (391). The negative impact of EDCs on GDM and hypertensive disorders discussed earlier (Table 2) have resulted from disruption in glucose homeostasis (392) and renal function (393), respectively. That multiple EDCs affect glucose homeostasis (see Table 3) were supported by findings that Cu (394), butyl paraben, PBDE (395), and the phthalate metabolite MEP (396) are associated with higher second-trimester maternal blood glucose levels, key contributors to GDM. Likewise, EDCs also affect other aspects of metabolism such as lipid and amino acid metabolism. For example, first-trimester BPA was associated with higher levels of circulating maternal palmitic acid, oleic acid and total free fatty acids (364). In contrast, whereas second- and third-trimester urinary DEHP phthalate metabolites were associated with decreased levels of triglycerides, palmitic, oleic, linoleic, and α-linolenic acids (397), phthalate metabolites MEP, MnBP, MiBP, and DEHP were associated with higher levels of nicotinamide mononucleotide, cysteine, cystine, and L-aspartic acid (398). Therefore, the effects of EDCs in disrupting the balance of metabolites required for fetal growth, differentiation, and maintenance of metabolic and oxidative homeostasis may in part contribute to their negative effects on pregnancy outcomes.
Microbiome
Microbial organisms inhabit the skin, and gastrointestinal and urogenital tract (406) and therefore are part of the maternal compartment. With the identification of microbiome colonization in the placenta and fetal gut (407, 408), it is now clear that the fetal compartment also harbors a microbiome. The presence of microbiota aids in driving many host responses and thereby can influence the host phenotype in health and disease states (409). Growing evidence has also indicated that the microbiome can influence maternal, birth, and long-term child health outcomes (410, 411). The composition of microbiota has been shown to be influenced by host health and diet, with emerging evidence indicating EDCs also play a role (412, 413). The interaction between EDCs and host microbiota has been shown to be complex: While EDC exposure disrupts the microbiome or causes dysbiosis (eg due to antimicrobial actions of parabens or triclosan [403, 404]), the microbiome-produced enzymes and metabolites affect EDC biotransformation and its bioavailability (414).
Evidence that microbiota may be protective against EDCs comes from a study that showed consumption of probiotic yogurt by pregnant women reduces the increased mid-to-late pregnancy Pb and As (415). While this study suggests that the microbiota has the potential to modulate EDC bioavailability, limited data are also available suggesting that the presence of maternal EDCs induces changes in maternal microbiota. One study reported maternal blood levels of methyl Hg to be associated with changes in gut microbiota during the first trimester, but not the third trimester (416) (see Table 3). Most information relative to the impact of EDCs on microbiota come from animal models. For instance, perinatal treatment of mice with Pb (417) and BPA (418), as well as exposure to PM2.5 (419), resulted in gut dysbiosis both in dams and offspring. Considering that the mode of delivery between vaginal and cesarean delivery has a demonstrable effect on the infant microbiome (420, 421) and infant health outcomes (422, 423), the finding that maternal EDCs have the potential to alter the vaginal microbiome (424) suggests they can also affect the infant microbial transfer. This field is in its infancy, but limited available data from human and animal studies point to EDC exposure affecting maternal and fetal microbiota and thereby influencing pregnancy, birth, and child health outcomes in humans.
The Effect of Endocrine-Disrupting Chemicals on Fetal/Neonatal Milieu
As discussed earlier, environmental EDCs can affect not only the maternal milieu but can also cross the placenta to reach the fetus. Fetal compromise may therefore be mediated either through direct action of the EDCs on the fetus or indirectly by the effects of EDCs on the maternal milieu and/or placental function. In humans, the majority of assessment of changes in the fetal/neonatal milieu were accomplished by detecting changes in umbilical cord blood collected at birth. As such, the effects of EDCs on the fetal developmental trajectory and changes in the fetal hormonal milieu throughout gestation are not available.
Inflammatory and oxidative states
Inflammation and oxidative stress states in the fetus are strongly associated with PTB and low birth weight (425, 426). Considering that inflammation and oxidative stress go hand in hand with the developing embryo and fetus having low antioxidant capacity, excessive production of reactive oxygen species can lead to an adverse oxidative and inflammatory state that is detrimental to fetal growth (427). In spite of its importance, studies addressing the impact of EDCs on cord blood levels of inflammatory and oxidative stress markers have been limited to outcomes relative to maternal levels of EDCs (see Table 3). Available findings indicate: 1) a decreased inflammatory cytokine CCL2 association with W and U; 2) decreased IL33 and thymic stromal lymphopoietin (TSLP) with presence of Pb, PCB118, DDE, DEP, and DETP (428); 3) increased CCL2 and CCL7 with Ni, Cd, Zn, MnBP and MEHP (327); and 4) increased TNF with PBDE (429) in maternal urine or circulation (see Table 3). Airborne PM10 in term maternal blood was linked with contrasting changes in proinflammatory and anti-inflammatory cytokines with increased IL1B and decreased IL10 in cord blood (430). One study that addressed cord blood levels of EDC with outcomes found the pesticide chlordane (CHD) in cord blood was associated with lower levels of the proinflammatory IL-1B and permethrin with low levels of anti-inflammatory cytokine IL-10 (431).
Available evidence has also pointed to maternal EDC exposure–specific changes in oxidative stress biomarkers in the neonatal milieu (432). These include low cord blood levels of oxidative stress biomarkers nitrotyrosine and chlor-tyrosine with the presence of metals Pb and Cu, and nitro-tyrosine with the presence of phthalate metabolites MBzP and MCOMHP in maternal first-trimester urine (333). In contrast, maternal first-trimester plasma levels of BPA were associated with high levels of nitrotyrosine in cord blood (364). On the other hand, PAH and PM2.5 in maternal blood during late pregnancy showed no association with markers of oxidative DNA damage (8-oxo-7,8-dihydro-2′-deoxyguanosine) and lipid peroxidation (15-F2t-isoprostane) in newborn blood (433). These findings point to maternal EDCs having the potential to disrupt cord blood inflammatory and oxidative stress status.
Hormones
In addition to genetic factors, autocrine, paracrine, and endocrine networks of hormones and growth factors shape the development of the fetus by coordinating maternal-placental-fetal interactions and fetal maturation (434). Fetal endocrine function begins early in gestation and continues to term and is able to adapt to maternal changes such as hypoxemia and hypoglycemia (435). As such, exposure to EDCs that could disrupt the fetal endocrine milieu could also contribute to altered fetal growth with adverse gestational, birth, and long-term health outcomes. Considerable evidence exists relative to the impact of maternal EDCs on reducing newborn thyroid status (see Table 3). These include 1) decreased cord blood levels of TSH with presence of Ce and Yb (382); phthalate metabolites MEP, MnBP, MiBP, DEHP (372) and DDT (436), 2) decreased T3 and T4 with presence of PCB (437) and DDT (436); 3) decreased free and total T3 with presence of HCH; 4) reduced free and total T4 but increased TSH with presence of CHD in cord blood (436) and reduced total T4 with MEP, MnBP, MiBP, and DEHP metabolites (372) (Table 3). In contrast, no association of cord blood TSH and T3 or T4 were found with maternal blood levels of organophosphate DAP metabolites throughout pregnancy (374). Similar to the relationship with maternal DDE and HCB, cord blood levels of these chemicals were also associated with reduced levels of total T3 and T4 (435).
The association of cord blood EDCs has also extended to cord blood levels of steroidal hormones, with methyl and propyl paraben associated with reduced T levels (438), BPA with increased E2 levels (294), phthalate metabolites with reduced levels of cortisol and cortisone, and glucocorticoid/adrenal androgen ratio, and increased DHEA and DHEA/androstenedione ratio (439) and PCB with decreased maternal T and increases in maternal E2 levels (440). The effects of PFAS appear to be metabolite specific, with PFOS associated with increased maternal DHEA and cortisol, whereas PFOA is associated with decreased DHEA levels in cord blood (441). Importantly, EDC effects in newborns showed sex specificity, with agrochemicals CHD, HCB, heptachlor epoxide, Mirex, and toxaphenes associated with reduced cord blood levels of T, cortisol, cortisone, and prolactin, as well as with increased DHEA and follicle-stimulating hormone among boys (442), as opposed to DDT being associated with decreased cord blood DHEA and increased cortisol levels among girls (442). These studies indicated EDCs altered fetal hormonal milieu potentially in a sex-specific manner, a crucial aspect not investigated in many studies.
Metabolomics
In the growing fetus there is a great demand for the metabolites that serve as an energy source and building blocks to aid in rapid tissue differentiation and metabolism (445). Therefore, factors that regulates fetal metabolites themselves are under tight regulation. For example, fetal glucose metabolism has been found to be dependent not only on maternal glucose status but also on fetal glucose and insulin secretion (446). Similarly, the concentration of leptin in neonatal cord blood has been suggested to be an indicator of fetal adiposity (447). Evidence relative to the impact of EDCs on fetal metabolites is rather limiting. Limited evidence has indicated a potential impact of EDCs on cord blood levels of glucose and energy regulatory molecules (see Table 3). These include associations of 1) BPA and phthalate metabolites MiBP, MnBP, and DEHP in maternal first-trimester serum with reduced levels of leptin; 2) maternal first-trimester circulating BPA levels with increased HMW adiponectin levels (448); and 3) maternal first-trimester urinary DAP with increased insulin (449) in neonates. Cord blood levels of EDCs have also been shown to be related to decreased cord blood levels of metabolic factors such as insulin with increased PCB153 (449) as opposed to increased fatty acid metabolites 17-methylstearate, laurate (12:0), and 4-vinylphenol sulfate with inorganic As (400). Of importance, cord blood level of the persistent organic pollutant DDE showed a sexually dimorphic association with low adiponectin and insulin levels in cord blood of girls but not boys (449). Although the changes in fetal metabolome are apparent for some commonly studied EDCs, similar information for many other EDCs is lacking, opening a fertile area for future investigations.
Microbiome
Although the mechanisms are poorly understood, there is general agreement that microbial colonization of the fetal gut occurs in utero (450, 451). The presence of bacteria in amniotic fluid without premature rupture of membranes supports transmission of bacteria from the mother and bacterial colonization in utero (408, 452-454). Although EDC effects on the microbiome profile in the fetus are not known, the altered fetal microbiome, analyzed through detection in the meconium, has been found to be associated with gestational complications like GDM (455), and linked with adverse birth outcomes like PTB (454, 456) and long-term health of the offspring (457). Because maternal and fetal/neonatal presence of EDCs has been linked with such adverse gestational and fetal outcomes, part of the effects may involve an altered fetal microbiome.
Epigenetics
Epigenetic alterations involve heritable changes to the genome that are induced through modifications to DNA or histones and expression of noncoding RNAs (458). The DNA modification chiefly occurs through methylation, leading mostly to silencing of gene expression (459, 460). On the other hand, histones may be modified through methylation, acetylation, phosphorylation, sumoylation, and ubiquitination and have permissive or repressive effects on gene expression (461). Noncoding RNAs such as micro (miRNA), small RNA, long RNA (lncRNA), and circular RNA have a role in posttranscriptional regulation of gene expression (462, 463). These various epigenetic mechanisms affect gene function through which EDCs mediate short- and long-term health outcomes (13, 464-466). Evidence supports the association of EDCs in maternal and cord blood with epigenetic alterations in the fetal epigenome (467) (see Table 3). These include the association of 1) increased methylation of long interspersed nuclear elements (LINE) 1 and p16 gene with inorganic As (468); 2) lower Alu methylation with DDT/DDE (468), and MEP (469); 3) decreased TNF methylation with PBDE levels (429); 4) decreased methylation of LINE1, insulin-like growth factor 2 (IGF2) and PPARα with MCPP; and 5) decreased PPARα methylation with presence of MBzP and DEHP (470) in the maternal milieu. Importantly, some of the EDC impact has been found to be sex specific with maternal urinary As (471, 472) and Cd (473) showing increased methylation changes in boys as opposed to being decreased in girls. Similarly, presence of maternal first-trimester urinary BPA was associated with decreased methylation of IGF2, and PPARα in cord blood leukocytes only in female neonates (470). Some studies have associated fetal or newborn levels of EDCs with epigenetic outcomes. For instance, BPA in the human fetal liver showed increased methylation in CpG islands and decreased methylation in CpG shores, shelves, and repetitive regions in fetal liver when using an epigenome-wide approach (474). Total and methyl Hg in cord blood were also found to be associated with differential methylation of genes ANGPT2, PRPF18, FOXD2, and TCEANC2 (475). These studies provide evidence that sex-specific associations underlie maternal or fetal presence of EDC with changes in epigenetic biomarkers. Similar studies addressing the impact of EDCs on epigenetic alterations in key genes controlling maternal and fetal function are very much needed to help develop targeted interventions.
The Effect of Endocrine-Disrupting Chemicals on the Placenta
The placenta, a transient organ, serves as an interface between maternal and fetal vascular beds to aid in nutrient and waste exchange and support of in utero life. It acts as a buffer to protect the fetus from immunologic, endocrine and environmental insults and participates in cholesterol and steroid biosynthesis (476). The placenta is also a dynamic organ with the ability to adapt to maternal and fetal needs, and the large size and surface area of the placenta along with the presence of transporters and metabolizing proteins aid in this function (477). Disruptions in placental homeostasis could affect the fetal growth trajectory, leading to IUGR and SGA babies (478). Environmental exposure to EDCs has been shown to disrupt placental function (234, 479). Some EDCs could cross the placental barrier directly affecting the fetus while others could induce changes in the biosynthesis of placental hormones and function and thus indirectly affect the fetal developmental trajectory. Placental dysfunctions could also be facilitated via reduced nutrient availability, diminished vascular supply, and/or compromised hormonal, inflammatory, oxidant and metabolic states all of which could affect the maternal and fetal milieu. Although the placenta begins to form around 10 days into pregnancy and is important for maintenance of the pregnancy throughout, information on EDC impact on early pregnancy placental changes are not available in humans, with the majority of epidemiological studies being confined to placentae obtained at the time of delivery.
Placental structure and size
With the presence of syncytiotrophoblast, cytotrophoblast, and fetal endothelial cells, the human placenta acts as a physical barrier to separate fetal blood from the intravillous space that contains the maternal blood through which transfer of nutrients, gas, and other molecules are facilitated (480). The thickness of the placenta is one of the determinants of the barrier function that affects the permeability and bi-directional transfer of substances between the mother and fetus. At term, the placenta is discoid shaped, of 15 to 25cm in diameter, with an approximately 3-cm thickness and weighing about 500 to 600 g. The examination of the placenta soon after the delivery provides valuable information because altered placental morphology is associated with fetal and maternal abnormalities (481). Currently, placental morphology is assessed at delivery through gross examination and histological techniques, and there is a need for advanced imaging to facilitate early detection of placental abnormalities. Although studies addressing the association of changes in placental morphology with EDCs are limited, available evidence has indicated an association between increased placental width with maternal first-trimester urinary MBP as well as increased placental thickness with second trimester urinary MMP, MBP, MEOHP, and MEHHP, and MBP and third-trimester urinary MEH (482). High concentrations of placental Cd were also associated with smaller volumes of fetal capillaries and capillary surface-to-volume ratio, increased maternal blood space relative to placental volume, and a thickened trophoblast component of the villous membrane (483). One study indicated PBDE congeners in the umbilical cord blood at birth was associated with reduced placental length, breadth and surface area (484). These findings, along with reports that the presence of PBDE decreased cytotrophoblast differentiation marker integrin α-1 and increased interstitial/endovascular uterine invasion protein vascular endothelial-cadherin (485), suggest that EDC affects placental formation and function involving placental structural changes.
Placental efficiency
Optimal fetal growth depends on adequate provision of nutrients by the placenta. Any placental compromise leading to inadequate nutrient supply would result in the fetus failing to achieve its growth potential. A positive correlation between placental weight and birth weight suggesting that the size of the placenta dictates the size of the fetus is well established from studies in animals (486) and humans (487). The ratio between the birth weight and placental weight serves as an index of placental efficiency. A reduction in this ratio indicates the inability of the placenta to adapt its nutrient transfer capacity to compensate and overcome growth restriction (488, 489). Only a few studies have capitalized on this index with respect to EDC impact. One such study has found the late-gestational presence of phenols triclosan and benzophenone-3, and phthalates MCNP and MCOP to be associated with a reduced birth weight to placental weight ratio (490). Considering the value of this index in determining immediate and long-term health outcomes (202), future studies need to incorporate this easily obtainable measure in their investigations.
Mediators of placental function
Functional homeostasis of the placenta is essential for efficient fetal support. Disruptions in placental function may be mediated via changes in inflammatory cascade, oxidative stress, and hormonal support, with many of these changes involving epigenetic alterations. Although most of these data come from animal models, placental explants, and cell lines (491-494), limited studies provide evidence (Table 4) pointing to the EDC-associated changes in mediators of placental function in humans, and these are discussed below. The presence of EDC in the maternal compartment has been found to be associated with changes in placental inflammatory cytokines reflective of its state of inflammation. The association of maternal urinary phthalates with placental cytokine expression appear to be EDC metabolite and sex specific (see Table 4). For instance, maternal first-trimester urinary MBP was associated with increased IL-1B, IL-6, and CRP in placentae associated with male fetuses and increased IL-6, CRP, CCL2, IL-8, IL-10, and CD68 in those associated with female fetuses (495). In contrast, maternal first-trimester urinary MEOHP was associated with decreased CRP, CCL2, CD68 mRNA only in female placentae, whereas MBzP was linked to increased TNF, CCL2, and CD68 mRNA in male placentae collected at term (495). The association with metals depended on the time of the pregnancy, with maternal first-trimester urinary levels of As linked with decreased in IL-1B, TNF, IFNG (355) and Tl with increased CD68 and decreased IL-4 and CD206 (496). While second-trimester levels of As were associated with decreased CCL2, IL-6 and TNF (497) third-trimester Tl was associated with increased placental TNF, IL-6, and CD68 expression (496).
Table 4.
Early and late gestational presence of endocrine-disrupting chemicals and their associations with placental changes
| EDC Classes | Inflammatory and oxidative state | Hormonal and metabolomic changes | Epigenetic changes | |||
|---|---|---|---|---|---|---|
| Early | Mid to late /term | Early | Mid to late /term | Early | Mid to late /term | |
| Metals | Maternal TM1 As: ↑8-oxoguanine, IL1B, TNF, IFNG (355) Maternal TM1 Tl: ↑ CD68; ↓ IL4 AND CD206 (496) Maternal TM2 Co: ↓CCL2, IL6 and TNF (497) Placental Mn: CD68 colocalication with Mn (499) Placental Cd: ↑ Di- and nitrotyrosine; ↓antioxidants (34) Maternal TM3 As: ↑8-oxoguanine and IL1B (355) Maternal TM3 Tl: ↑ TNF, IL6 and CD68 (496) |
Placental Cd: ↓ placental progesterone production (500); ↓ leptin (502) Maternal TM3 As:↑leptin (355) |
Maternal urine Pb: ↓ methylation at 9 CpGs (513) | Placental Cd: 17 differentially methylated CpG sites with ↑ TNFAIP2, EXOC3L4, GAS7, SREBF1, ACOT7, and RORA (506) ↑ miR-1537 and ↓ let-7 family members (514) Placental Hg: ↓ let-7 family members (514) |
||
| Parabens | ∑parabens ↑miR-128 (512) | |||||
| PAHs | Placental benzopyrene: ↑ oxidant malondialdehyde and ↓ antioxidant glutathione (216) | Cord-blood PAHs: ↑IGF1 and IGFBP3 (501) | ||||
| Phytoestrogens | ||||||
| Bisphenols | TM2 aborted fetus BPA: ↑ miR-146a and 33 other miRNAs (511) Placenta BPA ↑ LINE1 methylation (515) Maternal ∑phenols: ↑miR-128; ↓miR-142, miR-15a-5p (512) |
|||||
| Phthalates | Maternal MBP: ↑mRNA expression of IL1B, IL6, and CRP (♂); ↑IL6, CRP, CCL2, IL8, IL10, and CD68 (♀) (495) Maternal MEOHP: ↓ CRP, CCL2, CD68 mRNA (♀) (495) Maternal MBzP: ↑TNF, CCL2 and CD68 mRNA (♂) (495) |
Maternal MEHHP, MEOHP, MECPP, ΣDEHP: ↓PlGF (379) | Maternal MnBP: U-shaped dose–response with steroidogenic genes (322) Maternal MEHHP, MEOHP, MECPP, ΣDEHP: ↓PlGF (379) |
Terminated pregnancy ∑phthalates: 2214 differentially methylated single CpG sites; 282 differentially methylated regions (518) | MEOHP or MEP: ↓ IGF2DMR0 methylation (♂ and ♀) (509) Maternal ∑LMP (MiBP, MnBP, MEP): ↑miR-185 (512) Maternal MECPP, MEHHP, MEHP: ↓IGF2DMR0 methylation (♀) (509) Maternal MCNP and MHiBP: ↑ placental lncRNAs (510) Maternal DEHP: ↓ LINE1 methylation (516) |
|
| Organohalogens | Placental PBDE: ↓ T4 (examined in males only) (163) Cord PBDE: ↑IGF1 and IGFBP3 (501) |
Placental PBDE: ↑ global DNA methylation (507) Placental PCB: ↑ H19 methylation (507) Maternal BDE ↑ DIO3 methylation (♀) (507) Maternal POP: 214 differentially methylated CpG sites (517) Maternal PBDE ↑ miR-188-5p ↓ let-7c; PCB ↑ miR-1537 (514) |
||||
| Agro- chemicals | Placental methoxychlor: ↓ free T3 (♂ examined) (163) | Maternal DDE: ↑ DIO3 methylation (♀) (508) Maternal DDT: ↑ MCT8 methylation (♂) (508) |
||||
| Antifouling agents | Placental tributyltin and ∑organotin: ↓ free T3 (♂ examined) (163) | |||||
| Personal care products | see above sections for effects of parabens, bisphenols and phthalates | |||||
| Pharmaceuticals | see above sections for effects of bisphenols and phthalates |
Abbreviations: ↑, increase; ↓, decrease; ≠, no association; ∑, sum; ♂, male; ♀, female; CRP, C-reactive protein; DDE, diphenyl dichloro ethylene; DEHP, phthalate di(2-ethylhexyl)-phthalate; Early, first trimester; EDC, endocrine-disrupting chemical; IFNG, interferon γ; IGF1, insulin-like growth factor; IL, interleukin; mid to late, second and third trimester; mRNA, messenger RNA; miRNA, microRNA; PAHs, polyaromatic hydrocarbons; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TM, trimester; TNF, tumor necrosis factor.
As is the case with inflammatory markers, EDCs were found to also be associated with changes in placental oxidative stress status. Causal links for such association come from many in vitro studies and animal models (498). Limited studies in human have indicated 1) first- and third-trimester maternal urinary levels of As are associated with increased oxidative marker 8-oxoguanine (355); 2) placental presence of Cr are associated with increased dityrosine and nitrotyrosine, with the increase higher in placentae involving male than female fetuses and a concurrent reduction in antioxidant proteins also showing sexual dimorphism with decreased activities of GPX and catalase in male placentae, and decreased GPX and increased SOD activities among female placentae (34); and 3) placental benzopyrene is associated with increased oxidant malondialdehyde and decreased antioxidant glutathione (216).
Because the placenta is also an endocrine organ, EDCs can also affect placental hormonal production (see Table 4). Evidence to date point to 1) reduced placental progesterone production with presence of Cd in the placenta (500); 2) a nonmonotonic U-shaped dose–response relationship with expression of steroidogenic genes aromatase, 17β-hydroxysteroid dehydrogenase, cholesterol side chain cleavage, and Cytochrome P450 1B1 in term placenta with presence of maternal third-trimester urinary MnBP (322); 3) increased placental leptin with third-trimester maternal urinary As (355); 4) increased placental IGF1 and IGFBP3 expression with cumulative cord blood levels of PAH and PBDEs (499); and 5) changes in placental thyroid hormones linked with placental persistent organic pollutants (163).
Epigenetic mechanisms are involved in the regulation of gene expression during development, and the placenta also undergoes dynamic epigenomic changes throughout pregnancy. Therefore, alterations in these placental epigenetic marks have been found to be associated with placental defects affecting the mother and fetus and long-term health of offspring (503, 504). Because of this, assessment of the placental epigenome is also important for understanding the developmental impact of EDCs (505). A limitation of placental studies in humans is that, although the placenta undergoes dynamic epigenetic changes throughout pregnancy, most observations have been limited to term, and early changes have been missed. Numerous reports have provided evidence in support of involvement of epigenetic alterations relative to EDC effects on the placenta (see Table 4). For instance, presence of Cd in term placenta was associated with about 17 differentially methylated CpG sites and increased expression of genes TNFAIP2, EXOC3L4, GAS7, SREBF1, ACOT7, and RORA (504). Similarly, the organohalogen PBDE present in placenta was associated with a global increase in DNA methylation in contrast to PCB being associated with increased methylation at the H19 locus (507). Maternal levels of EDCs have also shown to be associated with placental DNA methylation. Evidence supporting this premise includes a sex-specific association of maternal serum BDE and DDE at delivery with increased methylation of thyroid hormone-related gene deiodinase type 3 (DIO3) in females and the association of DDT with methylation of monocarboxylate transporter 8 in males (508). Other studies have reported that a sex-specific association of methylation of a regulatory region in IGF2 in the placenta depended on the phthalate metabolite, with maternal first-trimester urinary MECPP, MEHHP, MEHP linked with a decreased IGF2 methylation in females and MEOHP and MEP with a decrease in both sexes (509). EDCs have also affected the expression of noncoding RNAs in the placenta with maternal term urinary MCNP and MiBP showing an association with increased expression of lncRNAs (510). In one study, the presence of BPA in an aborted fetus at the second trimester was found to be associated with increased expression of 33 miRNAs in the placenta (511). Assessment of the cumulative effects of maternal first-trimester urinary EDCs showed that parabens in aggregate were associated with an increased placental miR-128, LMW phthalates (MiBP, MnBP, MEP) collectively with increased miR-185, and phenols altogether with increased miR-128 and decreased miR-142 and miR-15a-5p (512). These observations indicate that, as in the maternal and fetal compartment, altered placental inflammatory, oxidative, endocrine, and metabolic states and associated epigenetic modifications integrate to mediate EDC action and affect gestational and birth outcomes.
Long-term Consequences of Gestational Endocrine-Disrupting Chemical Exposure
The landmark studies with the Dutch Hunger Winter birth cohort have shown that the impact on birth weight of the offspring varies depending on the window of exposure to famine and undernutrition during pregnancy (519). The observations by Barker and colleagues that children with low birth are at high risk of developing coronary diseases led to the proposal of fetal origin of disease hypothesis (6). Later, the recognition that adverse exposures expand beyond just nutrient restriction and include environmental exposure to EDCs culminated in the DOHaD hypothesis (7). The impact of EDCs on the maternal and fetal milieu on birth outcomes, a risk factor for development of adult-onset noncommunicable diseases (5), is discussed earlier and is summarized in Table 2. Possible long-term consequences of maternal/fetal exposure to EDCs based mostly on animal studies have been extensively reviewed (16, 520-526). Observations from children born during past environmental disasters and emerging evidence from cohort-based studies that have measures of EDC during pregnancy and available outcomes in children have provided evidence that developmental EDC exposure can lead to adverse health consequences in children. Information from past disasters supportive of gestational programming by EDCs include exposure to i) Hg in Japan’s Minamata Bay that led to severe brain dysfunction in their children (527); ii) PCB in rice oil in Japan and Taiwan that resulted in children with poor cognitive development (528); and iii) dioxin from a chemical factory explosion in Seveso, Italy, that was associated with development of reproductive and cardiometabolic disorders and increased risk for cancer among children and decreased sperm count in their grandsons (529). Prospective birth cohort studies have started to link gestational presence of EDCs with neurobehavioral, immunologic, metabolic, and reproductive disruptions. Evidence linking maternal presence of EDCs with neurodevelopment include (1) a positive association of maternal urinary BPA concentrations at 16 weeks of gestation with externalizing (aggression and hyperactivity) behaviors in children at age 2 years (530), and anxious and depressed behavior at age 3 years (531) with this association strongest among girls; (2) an association of maternal third-trimester urinary HMW phthalates with sex-specific behavioral changes with better orientation and motor scores among boys and poorer orientation and quality of alertness among girls (532), (3) a linkage of LMW phthalates with more self-reported hyperactivity, attention problems extending to age 16 years in the CHAMCOS cohort (533); (4) an association of maternal PBDEs at 16 weeks of gestation with decreased PFASs, PFOA, and PFNA, with increased reading scores among 8-year-old children (534); and (5) an association of higher levels of gestational urinary DEHP and DBP metabolites in Korean mothers, with lower mental and physical developmental scores among infants (535).
Relative to offspring metabolic outcomes, first-trimester BPA levels were found to be associated with decreases in girls and increases in boys of body mass index (BMI) and adiposity measures at age 4 years (536). Association of PFAS was metabolite specific, with gestational presence of PFOA at 16 weeks of gestation being associated with an increase in BMI from age 4 weeks to 12 years, a risk factor for adult obesity and cardiometabolic disease, as opposed to PFOS and PFHxS exposure being associated with lower BMI during the first 12 years of child’s life (537). Importantly, the associations with PFAS appeared to be sex dependent, with prenatal exposure to PFASs linked with small increases in adiposity measurements in midchildhood among girls only (538). For phthalates, the associations with adiposity measures varied not only by phthalate metabolite and sex but also by timing of exposure. First-trimester presence of MIBP was associated with adiposity measures such as increase in skinfold thickness, BMI for age, and waist circumference among girls and diametrically opposite in boys in the presence of second-trimester MBzP (539).
At the reproductive level, gestational phthalates in a metabolite-specific manner were linked with changes in peripubertal serum T levels in girls (540). Specifically, MEP across pregnancy, MiBP in the second trimester and MBzP in the third trimester, were associated with higher T levels, while third-trimester DEHP was linked with higher DHEA-S in girls (540). Second-trimester levels of BPA were also linked with higher serum T in peripubertal girls. This same study also found MEHP across pregnancy to be associated with lower odds of having a Tanner stage greater than 1 for breast development while MEHP in the third trimester was associated with higher odds of having a Tanner Stage >1 for pubic hair development (540). Detailed information from a large diverse cohort on the impact of gestational EDC exposure on child health outcomes will be forthcoming through the National Institutes of Health (NIH) initiative Environmental Influences on Child Health Outcomes Program. This program with nearly 50 cohorts spread across the United States will relate environmental chemical exposure from more than 50 000 mother-child dyads (541) on various childhood outcomes.
The impact of gestational EDC exposure on long-term health outcomes extending into adulthood are not available at the present time. A causal link between EDC exposure and long-term outcome can only be surmised from findings in children born to pregnant women treated with diethylstilbestrol (DES), a synthetic estrogen widely used before 1971 to prevent miscarriage. Daughters of mothers treated with DES during pregnancy were associated with increased odds for infertility, spontaneous abortion, ectopic pregnancy, PE, still birth, preterm delivery, early menopause, cervical intraepithelial neoplasia, and breast cancer (542, 543). In sons born from DES-treated pregnancy, early effects on reproductive tract malformations such as cryptorchidism were well known; however, the long-term effects were inconclusive (544). Male and female offspring were also characterized by varying degrees of psychological effects including depression and anxiety behavior (545). In addition, transgenerational transmission of traits that have been well established in animal models (546-548) are beginning to emerge among grandchildren of women exposed to DES or dioxin during pregnancy. These include significantly elevated odds for attention-deficit/hyperactivity disorder among grandchildren of DES exposed pregnant women (542) and decreased sex ratio and elevated thyroidal hormones among grandchildren of Seveso women (529). These findings indicate that many of the traits induced by in utero exposure to EDCs not only have short-term and long-term effects on the offspring but have the potential to be passed on to subsequent generations.
Interaction of Endocrine-Disrupting Chemicals With Genetics, Diet, Stress, and Lifestyle
As discussed in earlier sections, pregnant women are exposed to a large number of EDCs that affect the maternal, placental, and fetal milieu either by acting directly as hormone agonists or antagonists or indirectly influencing inflammatory and oxidative status, hormonal, metabolomics, and microbial factors. In addition to the maternal, placental, and fetal milieu interacting with each other to dictate the effects of EDCs on pregnancy outcomes, other factors such as diet, stress, lifestyle, and their genetic makeup can modulate the impact of EDCs.
With diet being a major source of exposure to EDCs (549), many of the persistent organic pollutants because of their lipophilic nature can bioaccumulate (550), leading to biomagnification in animals including humans who are higher up in the food chain (550, 551). Results from a longitudinal pregnancy study in the United Kingdom demonstrated that pregnant mothers who ate meat have higher odds than vegetarian mothers for development of hypospadias, with the odds reduced even further when mothers only consumed organic food (552). Such findings suggested that consuming food exposed to chemical fertilizer and pesticides via nonorganic food were likely associated with higher incidences of hypospadias. Studies in animal models and prospective studies in humans have indicated that a well-balanced diet containing antioxidants, long-chain ω 3 polyunsaturated fatty acids, and flavonoids could provide protection from adverse effects of EDCs by reducing oxidative stress and inflammation (553-556) and odds of IUGR in animal models and humans (557), outcomes associated with EDC exposure (see Table 2). Similarly, diets rich in antioxidants and anti-inflammatory supplements have been shown to reduce maternal urinary Cd-associated increase in circulating inflammatory biomarkers (CRP, γ-glutamyltransferase [GGT], and alkaline phosphatase) (558). The protection from maternal BPA-associated implantation failure and reduction in live birth rate by soy dietary supplementation (559) as well as reduction in incidences of hypospadias among offspring of women with occupational exposure to phthalate containing hair spray products (560), and increased odds of live birth among mothers exposed to air pollution by folate supplementation (561) have provided additional support for diet-EDC interaction. Considering that part of the effects of endocrine disruptors involved epigenetic modulation, the classic demonstration that dietary supplementation of methyl donors such as folic acid antagonized the shift in coat color in the agouti mouse induced by BPA via DNA hypomethylation (562), provides an avenue for such modulatory effects of EDC.
As with diet, exposure to psychosocial stress is independently linked with adverse pregnancy and birth outcomes such as gestational hypertension, GDM, abortions, PTB, and low birth weight (563-565), outcomes also affected by exposure to EDCs. Because humans are exposed to psychosocial stress due to racial disparities and discrimination, poverty, and depression or anxiety in parallel, the interaction between these 2 variables is an area of concern (566, 567). The recognized impact of psychosocial stress and EDCs on glucocorticoids during pregnancy (see Table 3) has provided a common mediator for these 2 variables to interact at the level of the hypothalamus-pituitary-adrenal axis. For example, modification of EDC effects by maternal stress was demonstrated by a meta-analysis that showed exposure both to high levels of stress and EDCs (as a result of smoking and traffic pollution) were independently associated with reduced birth weight, but the combination of the 2 has significant negative outcome on birth weight (568). Another study demonstrated that the relationship between EDC and gestational length was stronger among women with more adverse life events (569). In this study, maternal urinary triclocarban and BPS across pregnancy were strongly linked with shorter gestational length as opposed to methyl- and propyl-parabens, which were strongly linked with increased gestational length in mothers with stress-inducing negative life events during pregnancy.
While lifestyle choices such as use of personal care products, smoking, eating fast food, canned food, and high-caloric diet vs organic and vegetarian diet can influence the amount of EDC exposure, other lifestyle choices like exercise vs sedentariness, smoking, and alcohol and/or drug usage can also dictate the outcomes of EDC action. A sedentary lifestyle during pregnancy was found to be associated with negative pregnancy outcomes both for mother and child, including gestational weight gain, hypertension, and altered birth weight (570), outcomes also associated independently with EDC exposure. The reported additive effect of a sedentary lifestyle with the urinary phthalate metabolite MEOHP on circulating levels of progesterone, follicle-stimulating hormone, and luteinizing hormone (571) suggest an interaction between sedentary lifestyle and EDC exposure during pregnancy. To what extent this is related to sedentary lifestyle contributing to excessive weight gain and obesity (570) needs to be ascertained. The relationship between maternal second-trimester circulating PBDE with second- and third-trimester inflammatory cytokine TNF was much stronger among obese women compared to nonobese women when compared with PBDE alone (352). This suggests disease status, diet, or lifestyle, modification, which are potential contributors to the obesity and consequent changes in the mother’s metabolic milieu may modulate EDC action. Likewise, the smoking status of mothers during pregnancy was shown to modify EDC association with birth outcomes, with an increase in odds of SGA associated with HCB and PFOS exposure found only in mothers who smoke (265).
A challenging public health problem in the United States is the racial/ethnic disparities in pregnancy outcome, with infant mortality and low birth weight rates shown to be higher among African American women compared with White women (572, 573) and associated economic burden (574). The disproportionate exposure of African American women to environmental EDCs (575-577) appear to be one contributing factor for this disparity in pregnancy outcomes. In addition, African American communities were found more likely to experience widespread poverty, have lower high school graduation rates, high unemployment rates, food insecurity, increased access to poor-quality foods such as canned and fast foods, and less access to physical activity and health care (578-581). As discussed earlier, considering these changes are associated with changes in diet and lifestyle and increase in psychosocial stress, they have the potential to interact with EDCs to modulate their effects.
The genotype or genetic make-up is a major contributor relative to susceptibility of an individual to EDC exposure. Genome mapping shows that the genome is 99.9% identical among unrelated individuals (582). The main source of the variation amongst individuals are polymorphisms of which the most common are single-nucleotide variations (SNVs, formerly single-nucleotide polymorphisms [SNPs), where a single nucleotide in the genome sequence is altered (583). These variations can decree the susceptibility of the individual for the EDC through an altered target receptor that can either reduce or enhance binding of the EDC or through changes in xenobiotic metabolizing enzymes that dictate bioavailability by neutralization, biotransformation, or potentiation (584). That genetic susceptibility can also be modified by environmental factors including diet and lifestyle factors has led to the concept of gene-environment interaction (GxE), which forms the basis of many developmental disorders (585) including alterations in pregnancy milieu. This GxE instills differential susceptibility depending on the genes that are involved in responsivity to environmental states. As such these genes can increase the risk for adverse pregnancy outcomes in a negative environment, or alleviate the risks in positive environments (586). In the Seveso Women’s Health Study, women exposed to dioxin found neither an SNV in AHR nor dioxin exposure was independently associated with birth weight, but considered together the association was significantly stronger with birth weight (587), thus supporting the GxE interaction in modifying the susceptibility to EDC exposures. These findings highlight the complexity associated with the risk assessment of EDC exposure and emphasize the need to consider genetics and lifestyle factors to understand the true impact of EDCs on pregnancy outcomes, an aspect not considered in most studies.
Conclusions and Future Directions
The evidence discussed thus far indicates that EDCs across all classes of chemicals have the potential to impinge on the pregnancy milieu leading to adverse pregnancy, birth, and long-term health outcomes. As discussed in detail in earlier sections, the actions of EDCs may be direct or facilitated via altered maternal and fetal placental homeostasis and function and involve alterations in inflammatory, oxidative, hormonal, metabolomic, microbiomic, and epigenomic status subject to modulation by genetic makeup and environmental and lifestyle factors. The limitations of this field and the directions future research (see Fig. 2) should take are discussed as follows.
Figure 2.
Schematic identifying the key gaps and challenges in integrating the knowledge to understand the complex processes and outcomes affected by endocrine-disrupting chemical exposure during pregnancy. These challenges may be potentially overcome through sophisticated statistical modelling involving data science, machine learning and artificial intelligence to ultimately develop effective risk mitigation strategies.
Need for Determining Internal Exposure Levels
For several EDCs, the biomonitoring assessment of exposure is based on measures of EDC metabolites in early morning or 24 hour voids of urine (588). Questions have been raised for certain EDCs on the validity of 24-hour urine measure because of the potential for day-to-day variability in exposure (589, 590). In view of differences in clearance rates and the potential for bioaccumulation, urinary measures do not provide actual internal levels and patterns of EDC exposures. This is important even for EDCs that clear quickly in view of rapid signaling mechanisms that these chemicals can operate through (589, 590). For instance, repetitive frequent exposures to an EDC such as BPA were effective in inducing effects at the cellular level, in spite of their rapid clearance (591). In light of this understanding, there is a clear need to develop techniques to accurately estimate internal exposure levels of EDCs.
Need for Longitudinal Measures of Endocrine-Disrupting Chemicals
Another limitation of studies addressing human exposure to EDCs during pregnancy are snapshot, single time-point assessments. Humans, however, are continuously exposed to EDCs that have the potential to bioaccumulate in tissues and be released back into circulation at a later time. Furthermore, the susceptible developmental windows differ throughout pregnancy depending on the parameter being assessed. For instance, assessment of ambient air pollution across pregnancy has narrowed down susceptible developmental windows for PTB associated with air pollution to be the first 2 trimesters of pregnancy (592). A few studies have determined BPA, phthalate, triclosan and metals at more than one-time point during pregnancy (see Table 2). One such study, measuring urinary levels of phthalate metabolites at 4 time points during pregnancy, showed differing exposure levels across gestation (206). Another aspect to consider is that duration of EDC exposure varies with the EDC half-life (593). When urine samples provide the main solution matrix for EDC assessments, EDCs with a longer half-life (eg, persistent organic pollutants, PFAS) have greater measurement reliability, whereas EDCs with a short half-life (eg, PAH, BPA), show greater variability among individuals and thus low reproducibility, even when urine collections are taken from the same individual (589, 590). Reliable techniques for repeated assessment of EDCs are therefore required spanning the entire pregnancy. When considering longitudinal assessment of EDC exposure, and when feasible, consideration needs to be given to preconception exposure to EDCs, as gamete exposure is also germane. In fact, exposure to EDCs during preconception has been found to be associated with poor pregnancy and birth outcome (594), suggesting EDC susceptible developmental windows begin well before pregnancy.
Need for Establishment of Endocrine-Disrupting Chemical Dose-Response Relationships
Traditionally, risk assessments of chemicals are based on establishing dose-response relationships to define maximal-effect level (Emax; a toxicant dose beyond which any increase will not increase the response), no observed adverse effect level (NOAEL), and/or the lowest observed adverse effect level. EDCs, like many hormones, however, follow nonmonotonic dose-response relationships (595). In today’s regulatory environment, NOAEL is considered a conservative default threshold below which an EDC is not expected to induce adverse effects (596). As discussed earlier for gestational and birth outcomes, inverted–U-shape profiles, with adverse effects at the intermediate exposure level and reduced or no effect observed at low- and high-exposure levels, and U-shaped profiles, with the adverse effects at lower and higher EDC exposure levels, both have been reported (318, 593). Standard approaches of dose testing to establish the NOAEL doses are therefore not valid for EDCs, and future studies should assess the nonmonotonicity of each dose relationship on adverse gestational outcomes.
Need for Assessment of Cumulative Endocrine-Disrupting Chemical Burden and Impact
As discussed earlier, most studies assess the impact of a single EDC or EDC class, but humans are exposed to multiple EDCs with differing chemical classifications. Considering the varying signaling mechanisms and potential for cross talk through common and divergent target receptors affected (see Fig. 1), there is a clear need to relate outcomes to the mixture of EDCs to which one is exposed. Although studies of this nature are emerging, many focus on a single class of chemicals, such as mixtures of phthalate metabolites. Because humans are exposed to multiple EDC classes in parallel, expansion of such studies to “real-life” EDC mixtures encompassing several classes is needed, combined with in vitro and in vivo models to establish true MRA. Such “bottom-up” approaches are necessary for EDC MRA, as current legislative efforts in regulating EDC address regulatory issues, one chemical at a time (597). In developing legislation, it is important to recognize that human EDC exposure varies geographically. For example, a Danish study comparing rural vs urban distribution of common EDCs in pairs of mothers and children found no difference in phthalate presence, but detected more BP3 and parabens among urban mothers and children (598), indicating a need for geographical considerations in the analysis of cumulative exposure burden. Alternate matrices such as hair, nails, meconium, and shed deciduous teeth (in children) should be capitalized on to gain an understanding of cumulative EDC burden.
Need to Establish Sex-Specific Measures and Outcomes
It is becoming increasingly apparent that EDC effects differ between the sexes (599). The finding that efficiency of the placental transfer of metals Ti and Ag were significantly higher among male than female human fetuses (600) indicates fetal sex-related differences in placental transfer of EDC may also contribute to sex-specific differences in detrimental gestational and birth outcomes. In addition, the placenta, which is mostly of fetal origin, also manifests sexually dimorphic traits (601). Only a few human studies, however, have considered fetal sex when examining the placenta as a determining factor limiting the impact of EDCs (493). These studies have shown a sex-specific impact of EDCs on placental epigenetics (602) or maternal/neonatal thyroid hormone levels (603). Because of the differences in how males and females respond to stressors, including chemicals, since 2014 the NIH has implemented policies to encourage the understanding of sex-specific actions and effects on health outcomes (604), an aspect that should be incorporated into measures of EDCs and their effects.
Need to Consider Genetic, Environmental and Life Style Factors in Modulating Susceptibility to Endocrine-Disrupting Chemical Exposure
Considering the difference in genetic makeup and the burden of exposures to chemical and non-chemical stressors between people from different racial/ethnic background and their influence on susceptibility to EDCs, population-based studies should account for such differences when assessing EDC impact. In support of this premise, homozygosity in a specific estrogen receptor α haplotype with SNV among cryptorchid individuals showed heightened susceptibility to estrogenic EDCs (605). As discussed earlier, factors that can modulate susceptibility to EDCs include diet, physical activity, smoking, and medications. For instance, lack of exercise and/or a high-caloric diet intake can promote obesity leading to sequestration of lipophilic EDCs in fat depots resulting in increased exposure and susceptibility to EDCs (606). Considering many of these factors pose risks themselves, relative to the outcomes of EDC impact, epidemiological studies need to account for such variables and their potential interactions with EDCs. With more women having children later in their life (607), and one study reporting increased exposure levels of EDCs in older women (608), another fruitful area of research involves maternal age and its influence on the impact of EDCs.
Need to Develop Approaches to Understand the Placental Impact of Endocrine-Disrupting Chemicals
A major limitation relates to the lack of knowledge relative to early exposure level and effect of EDCs on the placenta. Development of the placenta occurs before that of the fetus to meet the demands of the growing fetus, and its vasculature is pliable to adapt to environmental conditions and to changes in maternal blood supply; because of this, the placenta undergoes considerable remodeling throughout pregnancy (609). The effect of EDCs on these variables cannot be assessed at delivery alone. Where possible, birthweight to placental weight ratio should be capitalized on as a surrogate to gain an understanding of EDC impact on placental efficiency/compromise. Considering that it is not safe or ethical to collect placental or fetal tissue samples during early and mid-pregnancy, only a few studies have shown EDC effects on placentae and fetal tissue using aborted or early-terminated pregnancies (610). Advancements in noninvasive approaches such as Doppler imaging and fetal ultrasound may aid in understanding adverse impacts of EDCs during pregnancy. Ongoing efforts to model the maternal-fetal interface with advancements in culture conditions and development of the trophoblast organoid and placenta-on-a-chip model (611) may also pave the way for more rapid assessments of EDC effects, and gain mechanistic insights.
Need for Consideration of Paternal Contribution
Much of this review has focused on the maternal exposure to EDCs and their impact on gestational and birth outcomes. Although half of each fetus’ nuclear chromosomal complement comes from the father, paternal contributions toward birth outcomes are rarely considered. Limited studies have looked at associations in which either paternal occupational exposure to (612) or urinary measures of (613, 614), EDCs such as flame retardants, metals, BPA, and phthalates, are associated with poor gestational outcomes such as implantation failure and hypospadias. These findings highlight the need to consider paternal exposure in assessing the impact of EDCs before birth.
Need to Establish Early Biomarkers
Another approach to overcome the limitations of early detection is to identify biomarkers of maternal, placental, and fetal dysfunction induced by EDCs especially those with short half-lives (593). Advanced “-omics” technologies, including genomic, epigenomic, transcriptomic, proteomic, metabolomics, microbiomics and metagenomics, can be used to identify novel unbiased markers (615) to predict maternal, placental, and fetal changes attributable to EDC effects and aid in early detection, thus providing opportunities for early intervention to eliminate detrimental effects of EDCs. Such approaches are being used for clinical conditions, including placental changes associated with PE (616, 617).
Need for Assessment of Endocrine-Disrupting Chemical Measures Following Safe Interventions
Lifestyle factors, including dietary choices, not only dictate exposure, but can also interact with EDCs to affect their outcome as discussed earlier. Because pregnancy is a major susceptibility window for developmental effects of the EDC, reproductive health professionals have started counseling on lifestyle changes (618). Changes in use of personal care products, diet, and physical activity are reported during pregnancy (619, 620) that may affect EDC exposure and action. Assessment of EDC measures and outcomes are therefore needed before and following counseling during the preconception and prenatal periods to encourage lifestyle changes that reduce EDC exposure, such as eating a well-balanced diet, avoiding processed food, reducing the use of cosmetics, and avoiding the use of pesticides (621).
Need for Linking Association with Causality
In human, risk assessments are made by studying associations between exposure domain or the presence of EDC in the maternal or fetal milieu with the outcome being studied. These studies merely point to potential relationships and do not address cause and effect (622), which can only be surmised through randomized trials. Some examples of such studies are the release of BPA from resin-based dental restorations and its effects on executive functioning in children in New England Children’s Amalgam Trial (623) and a randomized double-blind, placebo-controlled study on long-term treatment with phytoestrogens on endometrial hyperplasia (624). For ethical and safety reasons, studies involving the administration of EDCs during human pregnancy outcomes are not possible. Therefore, observational studies in humans need to be paired with experimental studies involving animal models that manifest similar developmental trajectories as humans to establish causal relationships (625). Studies with animal models, which allow the direct administration of EDCs in a controlled manner, can help establish cause and effect across subsequent generations. These studies needs to be carried out in translationally relevant animal models, because mechanistic studies in animal models that are developmentally dissimilar to humans may not aid in translation to humans. Use of humanized rodent models may help to a certain degree to overcome this challenge (626). Another aspect to consider is that these studies should model human internal exposure levels of EDCs derived from epidemiological studies. For instance, administration of a mixture of epidemiologically determined concentrations of various phthalate metabolites present in pregnant women in Illinois to pregnant mice showed sex-specific adverse effects such as reduction in anogenital distance in females, but not males, and long-term effects on reproductive function in both sexes (627, 628).
Need for Implementing Better Statistical Modeling Approaches
The assessment of EDC effects during pregnancy presents many challenges, including dissimilarity in the chemical structure and distribution, latency of effects, as well as confounders provided by differences in age, BMI, ethnicity, diet, geographical location, and occupation. A large body of data is being gathered through observational epidemiologic studies that incorporate medical records, EDC measures at different gestational time points, “-omic” measures (transcriptome, metabolome, epigenome, inflammasome, etc.), outcome assessments in children at different ages that are highly variable, complex, and poorly structured. These add to the levels of complexity that are inadequately addressed by traditional toxicity tests to predict population‐level effects (629). Additionally, obtaining insights through knowledge gained via complex, high-dimensional and heterogeneous data sets poses a major challenge in terms of understanding the true impact of EDCs. Mathematical modeling tools are therefore required to provide insight into relationships between EDC exposure and adverse outcomes. Tools such as mediation analysis are available to help determine the contribution of an intermediate that links the exposure-outcome association (630), and these techniques have undergone considerable improvement over recent decades to handle multiple mediators and interactions (631). With advancements in data science, computing, and machine learning, the application of Artificial Intelligence (AI) is another means through which machine learning algorithms can be applied to analyze large volumes of complex data (big data) like EDC mixtures and confounding variables to identify biomarkers and link to disease outcomes (632). By deciphering patterns, making predictions, pointing to new effective paradigms to obtain end-to-end learning models and developing a holistic interpretable blueprint to facilitate applicability, AI can help in risk assessment. As these determinations from AI are believed often to exceed the accuracy and efficiency of people (633) the mounting challenges due to the complicated nature of the data and insufficient domain knowledge can be overcome. The use of these sophisticated data science and AI approaches accounting for all confounding variables arising from multiple chemicals, periods of exposure, dose, sex and demographic diversity in determining EDC pregnancy outcomes is the way of the future.
In conclusion, evidence to date points to EDCs directly and indirectly affecting the maternal, placental, and fetal milieu and engaging many intermediaries that can be targeted for intervention and exposure mitigation. However, establishment of cause and effect relationships relative to pregnancy perturbations and outcomes stemming from EDC burden would require careful consideration and integration of several aspects identified earlier. These data should inform regulatory assessments of chemicals that are introduced into the environment. Currently, the EPA’s Endocrine Disruptor Screening Program screens chemicals for potential effects on estrogen, androgen, and thyroid hormone systems, although a multitude of evidence points to the disruption of metabolic pathways (634). Therefore, legislative efforts requiring testing a broader range endocrine disruption is needed to prevent introduction of new chemicals and regulate the use of existing chemicals. Therefore, a concerted effort by clinicians, epidemiologists, basic scientists, and statisticians would be required to meet these needs and aid in the development of informed legislation for mitigation of exposure to existing and newer EDCs, reduce the burden of short-lived EDCs and develop interventions to overcome the effects of persistent EDCs. These and the continued education of healthcare providers and the general public will aid in preventing adverse outcomes and promoting a healthy mother and child both in the short- and long-term.
Acknowledgments
The authors would like to acknowledge the critical reading and suggestions made by Dr David Abbot, University of Wisconsin-Madison, and Dr Jaclyn Goodrich, School of Public Health, University of Michigan, to improve the readability and flow of the manuscript.
Financial Support: This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS; grant Nos. R01 ES 030374 and UG3OD02325/UH3OD023), the NIEHS/US Environmental Protection Agency (EPA) Children’s Environmental Health and Disease Prevention Center (grant No. P01 ES022844/RD 83543601), and Michigan Lifestage Environmental Exposures and Disease (M-LEEAD) NIEHS Core Center (grant No. P30 ES017885). M.P. is supported by a Ruth L. Kirschstein Institutional Training Grant (No. T32 ES007062). The contents of this work are solely the responsibility of the grantees and do not necessarily represent the official views of the NIH or the EPA. Further, the EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Glossary
Abbreviations
- AHR
arylhydrocarbon receptor
- AI
artificial intelligence
- AR
androgen receptor
- As
arsenic
- BMI
body mass index
- BPA
bisphenol A
- BPB
bisphenol B
- BPS
bisphenol S
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
- Cd
cadmium
- Ce
cesium
- CHD
chlordane
- Cr
chromium
- CRP
C-reactive protein
- DAP
dialkylphosphate
- DBP
dibutyl-phthalate
- DDE
diphenyl dichloro ethylene
- DDT
dichlorodiphenyltrichloroethane
- DEHP
phthalate di(2-ethylhexyl)-phthalate
- DES
diethylstilbestrol
- DMP
dimethyl-phthalate
- DOHaD
developmental origin of health and disease
- E2
estradiol
- EDCs
endocrine-disrupting chemicals
- EPA
US Environmental Protection Agency
- ESR
estrogen receptor
- GDM
gestational diabetes mellitus
- GR
glucocorticoid receptor
- GxE
gene-environment interaction
- HCB
hexachlorobenzene
- Hg
mercury
- HMW
high molecular-weight
- IFNG
interferon γ
- IGF1
insulin-like growth factor
- IL
interleukin
- IUGR
intrauterine growth restriction
- LGA
large for gestational age
- LINE
long interspersed nuclear elements
- LMW
low-molecular-weight
- lncRNA
long noncoding RNA
- MBzP
mono-benzyl phthalate
- MCINP
mono(carboxy-isononyl) phthalate
- MCPP
mono- (3-carboxypropyl) phthalate
- MCOMHP
mono (6-COOH-2-methylheptyl) phthalate
- miRNA
microRNA
- Mn
manganese
- MRA
mixture risk assessment
- mRNA
messenger RNA
- MEHHP
Mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
mono-(2-ethyl-5-oxohexyl) phthalate
- Ni
nickel
- NIH
National Institutes of Health
- NHANES
National Health and Nutrition Examination Survey
- NOAEL
no observed adverse effect level
- PAHs
polyaromatic hydrocarbons
- Pb
lead
- PBDE
polybrominated diphenyl ether
- PCB
polychlorinated biphenyl
- PE
preeclampsia
- PFAS
perfluorinated alkylated substance
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonic acid
- PGR
progesterone receptor
- PM
particulate matter
- PPAR
peroxisome proliferator-activated receptor
- PTB
preterm birth
- RXR
retinoid X receptor
- SGA
small for gestational age
- T
testosterone
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TBT
tributyltin
- TM
trimester
- TNF
tumor necrosis factor
- TSH
thyrotropin
- V
vanadium
- VEGF
vascular endothelial growth factor
- Zn
zinc
Additional Information
Disclosures: The authors have nothing to disclose.
References
- 1. West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci U S A. 2005;102(Suppl 1):6543-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20. [DOI] [PubMed] [Google Scholar]
- 3. Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. 2004;9(5):419-425. [DOI] [PubMed] [Google Scholar]
- 4. Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349-353. [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S-595S. [DOI] [PubMed] [Google Scholar]
- 6. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412-417. [DOI] [PubMed] [Google Scholar]
- 7. Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15(4):183-187. [DOI] [PubMed] [Google Scholar]
- 8. Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental Programming, a Pathway to Disease. Endocrinology. 2016;157(4): 1328-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75(12):951-970. [DOI] [PubMed] [Google Scholar]
- 10. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94(4):1027-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. United States Environmental Protection Agency. Endocrine Disruption. 2015. Updated June 24, 2019. Accessed December 24, 2015.http://www.epa.gov/endocrine-disruption. [Google Scholar]
- 12. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015; 36(6):E1-E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hotchkiss AK, Rider CV, Blystone CR, et al. Fifteen years after “Wingspread”–environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105(2):235-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergman Å, Heindel JJ, Jobling S, Kidd K, Zoeller TR, World Health Organization . State of the Science of Endocrine Disrupting Chemicals 2012. World Health Organization,Geneva, Switzerland; 2013. [Google Scholar]
- 16. Sargis RM, Simmons RA. Environmental neglect: endocrine disruptors as underappreciated but potentially modifiable diabetes risk factors. Diabetologia. 2019;62(10):1811-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee WC, Fisher M, Davis K, Arbuckle TE, Sinha SK. Identification of chemical mixtures to which Canadian pregnant women are exposed: the MIREC Study. Environ Int. 2017;99:321-330. [DOI] [PubMed] [Google Scholar]
- 19. Goodrich JM, Ingle ME, Domino SE, et al. First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother-Infant Pairs study. J Dev Orig Health Dis. 2019;10(4):447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure. J Clin Endocrinol Metab. 2015;100(11):E1394-E1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woodruff TJ. Bridging epidemiology and model organisms to increase understanding of endocrine disrupting chemicals and human health effects. J Steroid Biochem Mol Biol. 2011;127(1-2):108-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chevalier N, Fénichel P. Endocrine disruptors: new players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015;41(2):107-115. [DOI] [PubMed] [Google Scholar]
- 23. Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1-24. [DOI] [PubMed] [Google Scholar]
- 24. Guo YL, Lambert GH, Hsu CC, Hsu MM. Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int Arch Occup Environ Health. 2004;77(3):153-158. [DOI] [PubMed] [Google Scholar]
- 25. Attina TM, Hauser R, Sathyanarayana S, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016;4(12):996-1003. [DOI] [PubMed] [Google Scholar]
- 26. Trasande L, Zoeller RT, Hass U, et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016;4(4):565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12(3):206-223. [DOI] [PubMed] [Google Scholar]
- 28. Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012;5(2):47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity—a review. Hum Exp Toxicol. 2007;26(10):823-832. [DOI] [PubMed] [Google Scholar]
- 30. Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf. 2003;56(1):174-179. [DOI] [PubMed] [Google Scholar]
- 31. Watson CV, Lewin M, Ragin-Wilson A, et al. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999-2016. Environ Res. 2020;183: 109208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zadorozhnaja TD, Little RE, Miller RK, et al. Concentrations of arsenic, cadmium, copper, lead, mercury, and zinc in human placentas from two cities in Ukraine. J Toxicol Environ Health A. 2000;61(4):255-263. [DOI] [PubMed] [Google Scholar]
- 33. Iwai-Shimada M, Kameo S, Nakai K, et al. Exposure profile of mercury, lead, cadmium, arsenic, antimony, copper, selenium and zinc in maternal blood, cord blood and placenta: the Tohoku Study of Child Development in Japan. Environ Health Prev Med. 2019;24(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banu SK, Stanley JA, Taylor RJ, et al. Sexually Dimorphic Impact of Chromium Accumulation on Human Placental Oxidative Stress and Apoptosis. Toxicol Sci. 2018;161(2):375-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood). 2004;229(5):383-392. [DOI] [PubMed] [Google Scholar]
- 36. Sun X, Liu W, Zhang B, et al. Maternal Heavy Metal Exposure, Thyroid Hormones, and Birth Outcomes: A Prospective Cohort Study. J Clin Endocrinol Metab. 2019;104(11):5043-5052. [DOI] [PubMed] [Google Scholar]
- 37. Liu H, Lu S, Zhang B, et al. Maternal arsenic exposure and birth outcomes: A birth cohort study in Wuhan, China. Environ Pollut. 2018;236:817-823. [DOI] [PubMed] [Google Scholar]
- 38. Bocca B, Ruggieri F, Pino A, et al. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. Concentrations in maternal blood, urine and cord blood. Environ Res. 2019;177:108599. [DOI] [PubMed] [Google Scholar]
- 39. Cheng L, Zhang B, Zheng T, et al. Critical Windows of Prenatal Exposure to Cadmium and Size at Birth. Int J Environ Res Public Health 2017;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robinson O, Basagaña X, Agier L, et al. The Pregnancy Exposome: Multiple Environmental Exposures in the INMA-Sabadell Birth Cohort. Environ Sci Technol. 2015;49(17):10632-10641. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Q, Li X, Liu X, et al. Association between maternal antimony exposure and risk of gestational diabetes mellitus: A birth cohort study. Chemosphere. 2020;246:125732. [DOI] [PubMed] [Google Scholar]
- 42. Romano ME, Enquobahrie DA, Simpson C, Checkoway H, Williams MA. Maternal body burden of cadmium and offspring size at birth. Environ Res. 2016;147:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woods MM, Lanphear BP, Braun JM, McCandless LC. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: a Bayesian analysis of the HOME Study. Environ Health. 2017;16(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moynihan M, Telléz-Rojo MM, Colacino J, et al. Prenatal Cadmium Exposure Is Negatively Associated With Adiposity in Girls Not Boys During Adolescence. Front Public Health. 2019;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bommarito PA, Kim SS, Meeker JD, et al. Urinary trace metals, maternal circulating angiogenic biomarkers, and preeclampsia: a single-contaminant and mixture-based approach. Environ Health. 2019;18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Liu W, Bao S, et al. Association of adverse birth outcomes with prenatal uranium exposure: a population-based cohort study. Environ Int. 2020;135:105391. [DOI] [PubMed] [Google Scholar]
- 47. Lewis RC, Meeker JD, Basu N, et al. Urinary metal concentrations among mothers and children in a Mexico City birth cohort study. Int J Hyg Environ Health. 2018;221(4):609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C. prenatal heavy metal exposure and adverse birth outcomes in Myanmar: a birth-cohort study. Int J Environ Res Public Health 2017;14:1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen X, Li Y, Zhang B, et al. Maternal exposure to nickel in relation to preterm delivery. Chemosphere. 2018;193:1157-1163. [DOI] [PubMed] [Google Scholar]
- 50. Maekawa R, Ito R, Iwasaki Y, et al. Evidence of exposure to chemicals and heavy metals during pregnancy in Japanese women. Reprod Med Biol. 2017;16(4):337-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sabra S, Malmqvist E, Saborit A, Gratacós E, Gomez Roig MD. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS One. 2017;12(10):e0185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trdin A, Snoj Tratnik J, Mazej D, et al. Mercury speciation in prenatal exposure in Slovenian and Croatian population—PHIME study. Environ Res. 2019;177:108627. [DOI] [PubMed] [Google Scholar]
- 53. Wang H, Liu L, Hu YF, et al. Association of maternal serum cadmium level during pregnancy with risk of preterm birth in a Chinese population. Environ Pollut. 2016;216:851-857. [DOI] [PubMed] [Google Scholar]
- 54. Ahamed M, Mehrotra PK, Kumar P, Siddiqui MK. Placental lead-induced oxidative stress and preterm delivery. Environ Toxicol Pharmacol. 2009;27(1):70-74. [DOI] [PubMed] [Google Scholar]
- 55. Falcón M, Viñas P, Luna A. Placental lead and outcome of pregnancy. Toxicology. 2003;185(1-2):59-66. [DOI] [PubMed] [Google Scholar]
- 56. Ludvigsson J, Andersson-White P, Guerrero-Bosagna C. Toxic metals in cord blood and later development of Type 1 diabetes. Pediatr Dimens 2019;4(2):10.15761/PD.1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang M, Xu C, Lin N, et al. Lead, mercury, and cadmium in umbilical cord serum and birth outcomes in Chinese fish consumers. Chemosphere. 2016;148:270-275. [DOI] [PubMed] [Google Scholar]
- 58. Murcia M, Ballester F, Enning AM, et al. Prenatal mercury exposure and birth outcomes. Environ Res. 2016;151:11-20. [DOI] [PubMed] [Google Scholar]
- 59. Long M, Ghisari M, Kjeldsen L, et al. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol Autism. 2019;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ovayolu A, Ovayolu G, Karaman E, Yuce T, Ozek MA, Turksoy VA. Amniotic fluid levels of selected trace elements and heavy metals in pregnancies complicated with neural tube defects. Congenit Anom (Kyoto). 2020;60(5):136-141. [DOI] [PubMed] [Google Scholar]
- 61. Błędzka D, Gromadzińska J, Wąsowicz W. Parabens. From environmental studies to human health. Environ Int. 2014;67:27-42. [DOI] [PubMed] [Google Scholar]
- 62. Pycke BF, Geer LA, Dalloul M, Abulafia O, Halden RU. Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ Int. 2015;84:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kang S, Kim S, Park J, et al. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci Total Environ. 2013;461-462:214-221. [DOI] [PubMed] [Google Scholar]
- 64. Jiménez-Díaz I, Vela-Soria F, Zafra-Gómez A, et al. A new liquid chromatography-tandem mass spectrometry method for determination of parabens in human placental tissue samples. Talanta. 2011;84(3):702-709. [DOI] [PubMed] [Google Scholar]
- 65. Frederiksen H, Taxvig C, Hass U, Vinggaard AM, Nellemann C. Higher levels of ethyl paraben and butyl paraben in rat amniotic fluid than in maternal plasma after subcutaneous administration. Toxicol Sci. 2008;106(2):376-383. [DOI] [PubMed] [Google Scholar]
- 66. Boberg J, Taxvig C, Christiansen S, Hass U. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 2010;30(2):301-312. [DOI] [PubMed] [Google Scholar]
- 67. Nowak K, Ratajczak-Wrona W, Górska M, Jabłońska E. Parabens and their effects on the endocrine system. Mol Cell Endocrinol. 2018;474:238-251. [DOI] [PubMed] [Google Scholar]
- 68. Song S, He Y, Zhang T, et al. Profiles of parabens and their metabolites in paired maternal-fetal serum, urine and amniotic fluid and their implications for placental transfer. Ecotoxicol Environ Saf. 2020;191:110235. [DOI] [PubMed] [Google Scholar]
- 69. Berger K, Gunier RB, Chevrier J, et al. Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ Res. 2018;165:379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mishra N, Ayoko GA, Morawska L. Atmospheric polycyclic aromatic hydrocarbons in the urban environment: occurrence, toxicity and source apportionment. Environ Pollut. 2016;208(Pt A):110-117. [DOI] [PubMed] [Google Scholar]
- 71. Vu AT, Taylor KM, Holman MR, Ding YS, Hearn B, Watson CH. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem Res Toxicol. 2015;28(8):1616-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suter MA, Aagaard KM, Coarfa C, et al. Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB). Biochem Biophys Res Commun. 2019;516(2):344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dong X, Wang Q, Peng J, Wu M, Pan B, Xing B. Transfer of polycyclic aromatic hydrocarbons from mother to fetus in relation to pregnancy complications. Sci Total Environ. 2018;636:61-68. [DOI] [PubMed] [Google Scholar]
- 74. Machado Jde B, Chatkin JM, Zimmer AR, Goulart AP, Thiesen FV. Cotinine and polycyclic aromatic hydrocarbons levels in the amniotic fluid and fetal cord at birth and in the urine from pregnant smokers. PLoS One. 2014;9(12):e116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Al-Saleh I, Alsabbahen A, Shinwari N, et al. Polycyclic aromatic hydrocarbons (PAHs) as determinants of various anthropometric measures of birth outcome. Sci Total Environ. 2013;444:565-578. [DOI] [PubMed] [Google Scholar]
- 76. Drwal E, Rak A, Gregoraszczuk EL. Review: polycyclic aromatic hydrocarbons (PAHs)—action on placental function and health risks in future life of newborns. Toxicology. 2019;411:133-142. [DOI] [PubMed] [Google Scholar]
- 77. Ferguson KK, McElrath TF, Pace GG, et al. Urinary polycyclic aromatic hydrocarbon metabolite associations with biomarkers of inflammation, angiogenesis, and oxidative stress in pregnant women. Environ Sci Technol. 2017;51(8):4652-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wierzba W, Radowicki S, Bojar I, Pinkas J. Effects of environmental pollution with aromatic hydrocarbons on endocrine and metabolic functions of the human placenta. Ann Agric Environ Med. 2018;25(1):157-161. [DOI] [PubMed] [Google Scholar]
- 79. Wu J, Hou H, Ritz B, Chen Y. Exposure to polycyclic aromatic hydrocarbons and missed abortion in early pregnancy in a Chinese population. Sci Total Environ. 2010;408(11):2312-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee S, Hong S, Liu X, et al. Endocrine disrupting potential of PAHs and their alkylated analogues associated with oil spills. Environ Sci Process Impacts. 2017;19(9):1117-1125. [DOI] [PubMed] [Google Scholar]
- 81. Ren A, Qiu X, Jin L, et al. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc Natl Acad Sci U S A. 2011;108(31):12770-12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tucak M, Horvat D, Cupic T, et al. Forage legumes as sources of bioactive phytoestrogens for use in pharmaceutics: a review. Curr Pharm Biotechnol. 2018;19(7):537-544. [DOI] [PubMed] [Google Scholar]
- 83. Todaka E, Sakurai K, Fukata H, et al. Fetal exposure to phytoestrogens—the difference in phytoestrogen status between mother and fetus. Environ Res. 2005;99(2):195-203. [DOI] [PubMed] [Google Scholar]
- 84. Nagata C, Iwasa S, Shiraki M, et al. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan). Cancer Causes Control. 2006;17(9):1107-1113. [DOI] [PubMed] [Google Scholar]
- 85. Adlercreutz H, Yamada T, Wähälä K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180(3 Pt 1):737-743. [DOI] [PubMed] [Google Scholar]
- 86. Tang ZR, Xu XL, Deng SL, Lian ZX, Yu K. Oestrogenic endocrine disruptors in the placenta and the fetus. Int J Mol Sci 2020; 21(4): pii: E1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jeschke U, Briese V, Richter DU, et al. Effects of phytoestrogens genistein and daidzein on production of human chorionic gonadotropin in term trophoblast cells in vitro. Gynecol Endocrinol. 2005;21(3):180-184. [DOI] [PubMed] [Google Scholar]
- 88. Mustafa AM, Malintan NT, Seelan S, et al. Phytoestrogens levels determination in the cord blood from Malaysia rural and urban populations. Toxicol Appl Pharmacol. 2007;222(1):25-32. [DOI] [PubMed] [Google Scholar]
- 89. Foster WG, Chan S, Platt L, Hughes CL Jr. Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol Lett. 2002;129(3):199-205. [DOI] [PubMed] [Google Scholar]
- 90. Jarrell J, Foster WG, Kinniburgh DW. Phytoestrogens in human pregnancy. Obstet Gynecol Int. 2012;2012:850313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Engel SM, Levy B, Liu Z, Kaplan D, Wolff MS. Xenobiotic phenols in early pregnancy amniotic fluid. Reprod Toxicol. 2006;21(1):110-112. [DOI] [PubMed] [Google Scholar]
- 92. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132-155. [DOI] [PubMed] [Google Scholar]
- 93. Gramec Skledar D, Peterlin Mašič L. Bisphenol A and its analogs: do their metabolites have endocrine activity? Environ Toxicol Pharmacol. 2016;47:182-199. [DOI] [PubMed] [Google Scholar]
- 94. Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655-4662. [DOI] [PubMed] [Google Scholar]
- 95. Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol. 2015;49(19):11834-11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pacyga DC, Sathyanarayana S, Strakovsky RS. Dietary predictors of phthalate and bisphenol exposures in pregnant women. Adv Nutr. 2019;10(5):803-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Overmeire I, Vrijens K, Nawrot T, Van Nieuwenhuyse A, Van Loco J, Reyns T. Simultaneous determination of parabens, bisphenols and alkylphenols in human placenta by ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1121:96-102. [DOI] [PubMed] [Google Scholar]
- 98. Edlow AG, Chen M, Smith NA, Lu C, McElrath TF. Fetal bisphenol A exposure: concentration of conjugated and unconjugated bisphenol A in amniotic fluid in the second and third trimesters. Reprod Toxicol. 2012;34(1):1-7. [DOI] [PubMed] [Google Scholar]
- 99. Pinney SE, Mesaros CA, Snyder NW, et al. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod Toxicol. 2017;67:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans. J Biochem Mol Toxicol. 2013;27(2):116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci. 2000;54(1):154-167. [DOI] [PubMed] [Google Scholar]
- 102. Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75(1):40-46. [DOI] [PubMed] [Google Scholar]
- 103. Roelofs MJ, van den Berg M, Bovee TF, Piersma AH, van Duursen MB. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology. 2015;329:10-20. [DOI] [PubMed] [Google Scholar]
- 104. Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1-2):27-34. [DOI] [PubMed] [Google Scholar]
- 105. Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703-A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Unal ER, Lynn T, Neidich J, et al. Racial disparity in maternal and fetal-cord bisphenol A concentrations. J Perinatol. 2012;32(11):844-850. [DOI] [PubMed] [Google Scholar]
- 107. Cao XL, Zhang J, Goodyer CG, Hayward S, Cooke GM, Curran IH. Bisphenol A in human placental and fetal liver tissues collected from Greater Montreal area (Quebec) during 1998–2008. Chemosphere. 2012;89(5):505-511. [DOI] [PubMed] [Google Scholar]
- 108. Zhang J, Cooke GM, Curran IH, Goodyer CG, Cao XL. GC-MS analysis of bisphenol A in human placental and fetal liver samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(2):209-214. [DOI] [PubMed] [Google Scholar]
- 109. James-Todd T, Stahlhut R, Meeker JD, et al. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Environ Health Perspect. 2012;120(9):1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: a preliminary analysis. Reprod Toxicol. 2016;65:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sol CM, Santos S, Asimakopoulos AG, et al. Associations of maternal phthalate and bisphenol urine concentrations during pregnancy with childhood blood pressure in a population-based prospective cohort study. Environ Int. 2020;138:105677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Johns LE, Ferguson KK, Soldin OP, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Caserta D, Pegoraro S, Mallozzi M, et al. Maternal exposure to endocrine disruptors and placental transmission: a pilot study. Gynecol Endocrinol. 2018;34(11):1001-1004. [DOI] [PubMed] [Google Scholar]
- 114. Kolatorova L, Vitku J, Vavrous A, et al. Phthalate metabolites in maternal and cord plasma and their relations to other selected endocrine disruptors and steroids. Physiol Res. 2018;67(Suppl 3):S473-S487. [DOI] [PubMed] [Google Scholar]
- 115. Harris CA, Sumpter JP. The endocrine disrupting potential of phthalates. In: Metzler M, ed. Endocrine Disruptors–Part I. Springer, Heidelberg, Germany; 2001:169-201. [Google Scholar]
- 116. Chen X, Xu S, Tan T, et al. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int J Environ Res Public Health. 2014;11(3):3156-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Desvergne B, Feige JN, Casals-Casas C. PPAR-mediated activity of phthalates: a link to the obesity epidemic? Mol Cell Endocrinol. 2009;304(1-2):43-48. [DOI] [PubMed] [Google Scholar]
- 118. Silva MJ, Reidy JA, Herbert AR, Preau JL Jr, Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol. 2004;72(6):1226-1231. [DOI] [PubMed] [Google Scholar]
- 119. Jensen MS, Nørgaard-Pedersen B, Toft G, et al. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect. 2012;120(6):897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Beszterda M, Frański R. Endocrine disruptor compounds in environment: as a danger for children health. Pediatr Endocrinol Diabetes Metab. 2018;24(2):88-95. [DOI] [PubMed] [Google Scholar]
- 121. Kodavanti PRS, Loganathan BG. Chapter 28: Polychlorinated biphenyls, polybrominated biphenyls, and brominated flame retardants. In: Gupta RC, ed. Biomarkers in Toxicology. 2nd ed. Academic Press, London, United Kingdom; 2019:501-518. [Google Scholar]
- 122. Müller MHB, Polder A, Brynildsrud OB, et al. Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ Res. 2019;170:433-442. [DOI] [PubMed] [Google Scholar]
- 123. Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mamsen LS, Björvang RD, Mucs D, et al. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int. 2019;124:482-492. [DOI] [PubMed] [Google Scholar]
- 125. Mamsen LS, Jönsson BAG, Lindh CH, et al. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ. 2017;596-597:97-105. [DOI] [PubMed] [Google Scholar]
- 126. Polevoy C, Arbuckle TE, Oulhote Y, et al. Prenatal exposure to legacy contaminants and visual acuity in Canadian infants: a maternal-infant research on environmental chemicals study (MIREC-ID). Environ Health. 2020;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zota AR, Mitro SD, Robinson JF, et al. Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDE metabolites (OH-PBDEs) in maternal and fetal tissues, and associations with fetal cytochrome P450 gene expression. Environ Int. 2018;112:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lam J, Lanphear BP, Bellinger D, et al. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect. 2017;125(8):086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Winneke G, Walkowiak J, Lilienthal H. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology. 2002;181-182:161-165. [DOI] [PubMed] [Google Scholar]
- 130. Riu A, le Maire A, Grimaldi M, et al. Characterization of novel ligands of ERα, Erβ, and PPARγ: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci. 2011;122(2):372-382. [DOI] [PubMed] [Google Scholar]
- 131. Sparks TC. Insecticide discovery: an evaluation and analysis. Pestic Biochem Physiol. 2013;107(1):8-17. [DOI] [PubMed] [Google Scholar]
- 132. Amara I, Miled W, Slama RB, Ladhari N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ Toxicol Pharmacol. 2018;57:115-130. [DOI] [PubMed] [Google Scholar]
- 133. Jannat B, Oveisi M, Sadeghi N, Hajimahmoodi M. Human health and trenbolone residue in bovine meat. J Environ Health Sci Eng. 2007;4:203-206. [Google Scholar]
- 134. Delatour T, Racault L, Bessaire T, Desmarchelier A. Screening of veterinary drug residues in food by LC-MS/MS. Background and challenges. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35(4):632-645. [DOI] [PubMed] [Google Scholar]
- 135. Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110(2):125-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bandiera SM, Torok SM, Letcher RJ, Norstrom RJ. Immunoquantitation of cytochromes P450 1A and P450 2B and comparison with chlorinated hydrocarbon levels in archived polar bear liver samples. Chemosphere. 1997;34(5-7):1469-1479. [DOI] [PubMed] [Google Scholar]
- 137. Li C, Cheng Y, Tang Q, et al. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in newborns in Yancheng, China. Environ Res. 2014;129:47-51. [DOI] [PubMed] [Google Scholar]
- 138. Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yang FW, Li YX, Ren FZ, Luo J, Pang GF. Assessment of the endocrine-disrupting effects of organophosphorus pesticide triazophos and its metabolites on endocrine hormones biosynthesis, transport and receptor binding in silico. Food Chem Toxicol. 2019;133:110759. [DOI] [PubMed] [Google Scholar]
- 140. Mnif W, Hassine AI, Bouaziz A, Bartegi A, Thomas O, Roig B. Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health. 2011;8(6):2265-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect. 1990;87:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lopez-Espinosa MJ, Granada A, Carreno J, Salvatierra M, Olea-Serrano F, Olea N. Organochlorine pesticides in placentas from Southern Spain and some related factors. Placenta. 2007;28(7):631-638. [DOI] [PubMed] [Google Scholar]
- 143. Castorina R, Bradman A, Fenster L, et al. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ Health Perspect. 2010;118(6):856-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gammon DW, Aldous CN, Carr WC Jr, Sanborn JR, Pfeifer KF. A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci. 2005;61(4):331-355. [DOI] [PubMed] [Google Scholar]
- 145. Goodman M, Mandel JS, DeSesso JM, Scialli AR. Atrazine and pregnancy outcomes: a systematic review of epidemiologic evidence. Birth Defects Res B Dev Reprod Toxicol. 2014;101(3):215-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect. 2001;109(10):1027-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hotchkiss AK, Ostby JS, Vandenbergh JG, Gray LE Jr. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav. 2003;79(2):151-156. [DOI] [PubMed] [Google Scholar]
- 148. Wickerham EL, Lozoff B, Shao J, Kaciroti N, Xia Y, Meeker JD. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ Int. 2012;47:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gray LE, Ostby J, Furr J, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248-264. [DOI] [PubMed] [Google Scholar]
- 150. Taxvig C, Hadrup N, Boberg J, et al. In vitro-in vivo correlations for endocrine activity of a mixture of currently used pesticides. Toxicol Appl Pharmacol. 2013;272(3):757-766. [DOI] [PubMed] [Google Scholar]
- 151. Bonde JP, Flachs EM, Rimborg S, et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: a systematic review and meta-analysis. Hum Reprod Update. 2016;23(1):104-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sousa ACA, Pastorinho MR, Takahashi S, Tanabe S. History on organotin compounds, from snails to humans. Environ Chem Lett. 2014;12:117-137. [Google Scholar]
- 153. Gadogbe M, Bao W, Wels BR, et al. Levels of tin and organotin compounds in human urine samples from Iowa, United States. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2019;54(9):884-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rantakokko P, Main KM, Wohlfart-Veje C, et al. Association of placenta organotin concentrations with congenital cryptorchidism and reproductive hormone levels in 280 newborn boys from Denmark and Finland. Hum Reprod. 2013;28(6):1647-1660. [DOI] [PubMed] [Google Scholar]
- 155. Rantakokko P, Main KM, Wohlfart-Veje C, et al. Association of placenta organotin concentrations with growth and ponderal index in 110 newborn boys from Finland during the first 18 months of life: a cohort study. Environ Health. 2014;13(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Grün F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl):S50-S55. [DOI] [PubMed] [Google Scholar]
- 157. Nakanishi T, Hiromori Y, Yokoyama H, et al. Organotin compounds enhance 17beta-hydroxysteroid dehydrogenase type I activity in human choriocarcinoma JAr cells: potential promotion of 17beta-estradiol biosynthesis in human placenta. Biochem Pharmacol. 2006;71(9):1349-1357. [DOI] [PubMed] [Google Scholar]
- 158. Nakanishi T, Kohroki J, Suzuki S, et al. Trialkyltin compounds enhance human CG secretion and aromatase activity in human placental choriocarcinoma cells. J Clin Endocrinol Metab. 2002;87(6):2830-2837. [DOI] [PubMed] [Google Scholar]
- 159. Barr DB, Ananth CV, Yan X, et al. Pesticide concentrations in maternal and umbilical cord sera and their relation to birth outcomes in a population of pregnant women and newborns in New Jersey. Sci Total Environ. 2010;408(4):790-795. [DOI] [PubMed] [Google Scholar]
- 160. Whyatt RM, Barr DB, Camann DE, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5):749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Chevrier C, Limon G, Monfort C, et al. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ Health Perspect. 2011;119(7):1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lewis RC, Cantonwine DE, Anzalota Del Toro LV, et al. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ Health. 2014;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Li ZM, Hernandez-Moreno D, Main KM, et al. Association of in utero persistent organic pollutant exposure with placental thyroid hormones. Endocrinology. 2018;159(10):3473-3481. [DOI] [PubMed] [Google Scholar]
- 164. Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134-9; discussion 181. [DOI] [PubMed] [Google Scholar]
- 165. García Ibarra V, Rodríguez Bernaldo de Quirós A, Paseiro Losada P, Sendón R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal Bioanal Chem. 2018;410(16):3789-3803. [DOI] [PubMed] [Google Scholar]
- 166. Hubinger JC, Havery DC. Analysis of consumer cosmetic products for phthalate esters. J Cosmet Sci. 2006;57(2):127-137. [PubMed] [Google Scholar]
- 167. Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Nakiwala D, Vernet C, Lyon-Caen S, et al. ; SEPAGES study group . Use of personal care products during pregnancy in relation to urinary concentrations of select phenols: a longitudinal analysis from the SEPAGES feasibility study. Int J Hyg Environ Health. 2020;227:113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhao H, Huo W, Li J, et al. Exposure to benzophenones, parabens and triclosan among pregnant women in different trimesters. Sci Total Environ. 2017;607-608:578-585. [DOI] [PubMed] [Google Scholar]
- 170. Halden RU, Lindeman AE, Aiello AE, et al. The Florence statement on triclosan and triclocarban. Environ Health Perspect. 2017;125(6):064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Pycke BF, Geer LA, Dalloul M, Abulafia O, Jenck AM, Halden RU. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol. 2014;48(15):8831-8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Shekhar S, Sood S, Showkat S, et al. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen Comp Endocrinol. 2017;241:100-107. [DOI] [PubMed] [Google Scholar]
- 173. Wei L, Qiao P, Shi Y, et al. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin Chim Acta. 2017;466:133-137. [DOI] [PubMed] [Google Scholar]
- 174. González-Casanova JE, Pertuz-Cruz SL, Caicedo-Ortega NH, Rojas-Gomez DM. Adipogenesis regulation and endocrine disruptors: emerging insights in obesity. Biomed Res Int. 2020;2020:7453786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rochester JR, Bolden AL, Pelch KE, Kwiatkowski CF. Potential developmental and reproductive impacts of triclocarban: a scoping review. J Toxicol. 2017;2017:9679738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Shapiro GD, Arbuckle TE, Ashley-Martin J, et al. Associations between maternal triclosan concentrations in early pregnancy and gestational diabetes mellitus, impaired glucose tolerance, gestational weight gain and fetal markers of metabolic function. Environ Res. 2018;161:554-561. [DOI] [PubMed] [Google Scholar]
- 177. Wang C, Chen L, Zhao S, et al. Impacts of prenatal triclosan exposure on fetal reproductive hormones and its potential mechanism. Environ Int. 2018;111:279-286. [DOI] [PubMed] [Google Scholar]
- 178. Iribarne-Durán LM, Artacho-Cordón F, Peña-Caballero M, et al. Presence of bisphenol A and parabens in a neonatal intensive care unit: an exploratory study of potential sources of exposure. Environ Health Perspect. 2019;127(11):117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. DAVIS ME, FUGO NW. Does administration of diethylstilbestrol to pregnant women result in increased output of urinary pregnanediol? Proc Soc Exp Biol Med. 1948;69(3):436-438. [DOI] [PubMed] [Google Scholar]
- 180. Korach KS, Metzler M, McLachlan JA. Estrogenic activity in vivo and in vitro of some diethylstilbestrol metabolites and analogs. Proc Natl Acad Sci U S A. 1978;75(1):468-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Herbst AL, Ulfelder H, Poskanzer DC, Longo LD. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. 1971. Am J Obstet Gynecol. 1999;181(6):1574-1575. [DOI] [PubMed] [Google Scholar]
- 182. Veurink M, Koster M, Berg LT. The history of DES, lessons to be learned. Pharm World Sci. 2005;27(3):139-143. [DOI] [PubMed] [Google Scholar]
- 183. Titus-Ernstoff L, Hatch EE, Hoover RN, et al. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer. 2001;84(1):126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med. 1995;332(21):1411-1416. [DOI] [PubMed] [Google Scholar]
- 185. Evans NP, Bellingham M, Sharpe RM, et al. Reproduction Symposium: does grazing on biosolids-treated pasture pose a pathophysiological risk associated with increased exposure to endocrine disrupting compounds? J Anim Sci. 2014;92(8):3185-3198. [DOI] [PubMed] [Google Scholar]
- 186. Oziol L, Alliot F, Botton J, et al. First characterization of the endocrine-disrupting potential of indoor gaseous and particulate contamination: comparison with urban outdoor air (France). Environ Sci Pollut Res Int. 2017;24(3):3142-3152. [DOI] [PubMed] [Google Scholar]
- 187. Darbre PD. Overview of air pollution and endocrine disorders. Int J Gen Med. 2018;11:191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Iavicoli I, Fontana L, Leso V, Bergamaschi A. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci. 2013;14(8):16732-16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Csaba G. Smoking, as epigenetically active endocrine disruptor: perinatal impact. Med Clin Arch. 2019;3. doi: 10.15761/MCA.1000158. [DOI] [Google Scholar]
- 190. Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bai W, Li Y, Niu Y, et al. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ Res. 2020;185:109471. [DOI] [PubMed] [Google Scholar]
- 192. Currie J, Ray SH, Neidell M. Quasi-experimental studies suggest that lowering air pollution levels benefits infants’ and children’s health. Health Aff (Millwood). 2011;30(12):2391-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Mukherjee A, Agrawal M. A global perspective of fine particulate matter pollution and its health effects. Rev Environ Contam Toxicol. 2018;244:5-51. [DOI] [PubMed] [Google Scholar]
- 194. Grippo A, Zhang J, Chu L, et al. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health. 2018;33(3):247-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Brown HL, Smith GN. Pregnancy complications, cardiovascular risk factors, and future heart disease. Obstet Gynecol Clin North Am. 2020;47(3):487-495. [DOI] [PubMed] [Google Scholar]
- 197. Guarner-Lans V, Ramirez-Higuera A, Rubio-Ruiz ME, Castrejon-Tellez V, Soto ME, Perez-Torres I. Early programming of adult systemic essential hypertension. Int J Mol Sci. 2020;21(4):1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Eriksson J. Developmental pathways and programming of diabetes: epidemiological aspects. J Endocrinol. 2019. doi: 10.1530/JOE-18-0680 [DOI] [PubMed] [Google Scholar]
- 199. Mongelli M, Wilcox M, Gardosi J. Estimating the date of confinement: ultrasonographic biometry versus certain menstrual dates. Am J Obstet Gynecol. 1996;174(1 Pt 1):278-281. [DOI] [PubMed] [Google Scholar]
- 200. Suff N, Story L, Shennan A. The prediction of preterm delivery: what is new? Semin Fetal Neonatal Med. 2019;24(1):27-32. [DOI] [PubMed] [Google Scholar]
- 201. Turkmen S, Johansson S, Dahmoun M. Foetal macrosomia and foetal-maternal outcomes at birth. J Pregnancy. 2018;2018:4790136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62-67. [DOI] [PubMed] [Google Scholar]
- 203. Stoelhorst GM, Rijken M, Martens SE, et al. ; Leiden Follow-Up Project on Prematurity . Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the Project on Preterm and Small for Gestational Age Infants 1983 and the Leiden Follow-Up Project on Prematurity 1996-1997. Pediatrics. 2005;115(2):396-405. [DOI] [PubMed] [Google Scholar]
- 204. Samuel TM, Sakwinska O, Makinen K, Burdge GC, Godfrey KM, Silva-Zolezzi I. Preterm Birth: a narrative review of the current evidence on nutritional and bioactive solutions for risk reduction. Nutrients. 2019;11(8):1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ferguson KK, Chin HB. Environmental chemicals and preterm birth: biological mechanisms and the state of the science. Curr Epidemiol Rep. 2017;4(1):56-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ferguson KK, Rosen EM, Rosario Z, et al. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int. 2019;132:105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Adibi JJ, Hauser R, Williams PL, et al. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kim SS, Meeker JD, Carroll R, et al. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int. 2018;121(Pt 1):582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Everett ED, Reller LB, Droegemueller W, Greer BE. Absence of bacteremia after insertion or removal of intrauterine devices. Obstet Gynecol. 1976;47(2):207-209. [PubMed] [Google Scholar]
- 211. Barn P, Gombojav E, Ochir C, et al. Coal smoke, gestational cadmium exposure, and fetal growth. Environ Res. 2019;179(Pt B):108830. [DOI] [PubMed] [Google Scholar]
- 212. Freire C, Amaya E, Gil F, et al. ; INMA Project . Placental metal concentrations and birth outcomes: the Environment and Childhood (INMA) project. Int J Hyg Environ Health. 2019;222(3):468-478. [DOI] [PubMed] [Google Scholar]
- 213. Aung MT, Ferguson KK, Cantonwine DE, McElrath TF, Meeker JD. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ Res. 2019;169:131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Geer LA, Pycke BFG, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater. 2017;323(Pt A):177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Guo Y, Huo X, Wu K, Liu J, Zhang Y, Xu X. Carcinogenic polycyclic aromatic hydrocarbons in umbilical cord blood of human neonates from Guiyu, China. Sci Total Environ. 2012;427-428:35-40. [DOI] [PubMed] [Google Scholar]
- 216. Agarwal P, Singh L, Anand M, Taneja A. Association between placental polycyclic aromatic hydrocarbons (PAHS), oxidative stress, and preterm delivery: a case-control study. Arch Environ Contam Toxicol. 2018;74(2):218-227. [DOI] [PubMed] [Google Scholar]
- 217. Singh VK, Singh J, Anand M, et al. Comparison of polycyclic aromatic hydrocarbon levels in placental tissues of Indian women with full- and preterm deliveries. Int J Hyg Environ Health. 2008;211(5-6):639-647. [DOI] [PubMed] [Google Scholar]
- 218. Wood SL, Jarrell JJ, Swaby C, Chan S. Endocrine disruptors and spontaneous premature labor: a case control study. Environ Health. 2007;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Tang R, Chen M, Zhou K, et al. Prenatal lignan exposures, pregnancy urine estrogen profiles and birth outcomes. Environ Pollut. 2015;205:261-268. [DOI] [PubMed] [Google Scholar]
- 220. Wan Y, Huo W, Xu S, et al. Relationship between maternal exposure to bisphenol S and pregnancy duration. Environ Pollut. 2018;238:717-724. [DOI] [PubMed] [Google Scholar]
- 221. Chin HB, Jukic AM, Wilcox AJ, et al. Association of urinary concentrations of early pregnancy phthalate metabolites and bisphenol A with length of gestation. Environ Health. 2019;18(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health. 2018;15(9):1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sagiv SK, Rifas-Shiman SL, Fleisch AF, et al. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol. 2018;187(4):793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Lackmann GM, Angerer J, Salzberger U, Töllner U. Influence of maternal age and duration of pregnancy on serum concentrations of polychlorinated biphenyls and hexachlorobenzene in full-term neonates. Biol Neonate. 1999;76(4):214-219. [DOI] [PubMed] [Google Scholar]
- 225. Lackmann GM, Göen T, Tölliner U, Schaller KH, Angerer J. PCBs and HCB in serum of full-term German neonates. Lancet. 1996;348(9033):1035. [DOI] [PubMed] [Google Scholar]
- 226. Bergonzi R, De Palma G, Specchia C, et al. Persistent organochlorine compounds in fetal and maternal tissues: evaluation of their potential influence on several indicators of fetal growth and health. Sci Total Environ. 2011;409(15):2888-2893. [DOI] [PubMed] [Google Scholar]
- 227. Tyagi V, Garg N, Mustafa MD, Banerjee BD, Guleria K. Organochlorine pesticide levels in maternal blood and placental tissue with reference to preterm birth: a recent trend in North Indian population. Environ Monit Assess. 2015;187(7):471. [DOI] [PubMed] [Google Scholar]
- 228. Fang J, Liu H, Zhao H, Zhou Y, Xu S, Cai Z. Association of in utero hexachlorocyclohexane exposure with gestational age. Ecotoxicol Environ Saf. 2019;174:263-269. [DOI] [PubMed] [Google Scholar]
- 229. Hoffman K, Stapleton HM, Lorenzo A, et al. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ Int. 2018;116:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Bravo N, Grimalt JO, Chashchin M, Chashchin VP, Odland JØ. Drivers of maternal accumulation of organohalogen pollutants in Arctic areas (Chukotka, Russia) and 4,4′-DDT effects on the newborns. Environ Int. 2019;124:541-552. [DOI] [PubMed] [Google Scholar]
- 231. Hatch EE, Troisi R, Wise LA, et al. Preterm birth, fetal growth, and age at menarche among women exposed prenatally to diethylstilbestrol (DES). Reprod Toxicol. 2011;31(2):151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Papiernik E, Pons JC, Hessabi M. Obstetrical outcome in 454 women exposed to diethylstilbesterol during their fetal life: a case-control analysis [article in French]. J Gynecol Obstet Biol Reprod (Paris). 2005;34(1 Pt 1):33-40. [DOI] [PubMed] [Google Scholar]
- 233. Darendeliler F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract Res Clin Endocrinol Metab. 2019;33(3):101260. [DOI] [PubMed] [Google Scholar]
- 234. Gingrich J, Ticiani E, Veiga-Lopez A. Placenta disrupted: endocrine disrupting chemicals and pregnancy. Trends Endocrinol Metab. 2020;31(7):508-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zhao Y, Song Q, Ge W, et al. Associations between in utero exposure to polybrominated diphenyl ethers, pathophysiological state of fetal growth and placental DNA methylation changes. Environ Int. 2019;133(Pt B):105255. [DOI] [PubMed] [Google Scholar]
- 236. Casas M, Valvi D, Ballesteros-Gomez A, et al. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell cohort. Environ Health Perspect. 2016;124(4):521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ferguson KK, van den Dries MA, Gaillard R, et al. Organophosphate pesticide exposure in pregnancy in association with ultrasound and delivery measures of fetal growth. Environ Health Perspect. 2019;127(8):87005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Mora AM, van Wendel de Joode B, Mergler D, et al. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Environ Res. 2015;136:47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Huang YF, Pan WC, Tsai YA, et al. Concurrent exposures to nonylphenol, bisphenol A, phthalates, and organophosphate pesticides on birth outcomes: A cohort study in Taipei, Taiwan. Sci Total Environ. 2017;607-608:1126-1135. [DOI] [PubMed] [Google Scholar]
- 240. Ding G, Wang C, Vinturache A, et al. Prenatal low-level phenol exposures and birth outcomes in China. Sci Total Environ. 2017;607-608:1400-1407. [DOI] [PubMed] [Google Scholar]
- 241. Snijder CA, Heederik D, Pierik FH, et al. Fetal growth and prenatal exposure to bisphenol A: the Generation R study. Environ Health Perspect. 2013;121(3):393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhao Y, Chen L, Li LX, et al. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr Res. 2014;76(4):401-408. [DOI] [PubMed] [Google Scholar]
- 243. Lee BE, Park H, Hong YC, et al. Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children’s Environmental Health) study. Int J Hyg Environ Health. 2014;217(2-3):328-334. [DOI] [PubMed] [Google Scholar]
- 244. Huo W, Xia W, Wan Y, et al. Maternal urinary bisphenol A levels and infant low birth weight: a nested case-control study of the Health Baby Cohort in China. Environ Int. 2015;85:96-103. [DOI] [PubMed] [Google Scholar]
- 245. Narayan B, Nelson-Piercy C. Medical problems in pregnancy. Clin Med (Lond). 2016;16(Suppl 6):s110-s116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho SM. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am J Perinatol. 2016;33(13):1313-1318. [DOI] [PubMed] [Google Scholar]
- 247. Kahn LG, Trasande L. Environmental toxicant exposure and hypertensive disorders of pregnancy: recent findings. Curr Hypertens Rep. 2018;20(10):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Krieg SA, Shahine LK, Lathi RB. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertil Steril. 2016;106(4):941-947. [DOI] [PubMed] [Google Scholar]
- 249. Lathi RB, Liebert CA, Brookfield KF, et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril. 2014;102(1):123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Murray J, Eskenazi B, Bornman R, et al. Exposure to DDT and hypertensive disorders of pregnancy among South African women from an indoor residual spraying region: The VHEMBE study. Environ Res. 2018;162:49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ Health Perspect. 2016;124(10):1651-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Fisher BG, Frederiksen H, Andersson AM, et al. Serum phthalate and triclosan levels have opposing associations with risk factors for gestational diabetes mellitus. Front Endocrinol (Lausanne). 2018;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wang X, Gao D, Zhang G, et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: a prospective cohort study. Environ Int. 2020;135:105370. [DOI] [PubMed] [Google Scholar]
- 254. Chiu YH, Mínguez-Alarcón L, Ford JB, et al. ; for EARTH Study Team . Trimester-specific urinary bisphenol A concentrations and blood glucose levels among pregnant women from a fertility clinic. J Clin Endocrinol Metab. 2017;102(4):1350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Bellavia A, Chiu YH, Brown FM, et al. ; EARTH Study Team . Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environ Res. 2019;168:389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Liu W, Zhou Y, Li J, et al. Parabens exposure in early pregnancy and gestational diabetes mellitus. Environ Int. 2019;126:468-475. [DOI] [PubMed] [Google Scholar]
- 257. Shaffer RM, Ferguson KK, Sheppard L, et al. ; TIDES Study team . Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ Int. 2019;123:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. James-Todd TM, Meeker JD, Huang T, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int. 2016;96:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Bellavia A, Cantonwine DE, Meeker JD, et al. Pregnancy urinary bisphenol—a concentrations and glucose levels across BMI categories. Environ Int. 2018;113:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The HOME Study. Environ Health. 2015;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Smarr MM, Grantz KL, Zhang C, et al. Persistent organic pollutants and pregnancy complications. Sci Total Environ. 2016;551-552:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Weng YH, Yang CY, Chiu YW. Risk assessment of adverse birth outcomes in relation to maternal age. PLoS One. 2014;9(12):e114843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Birks L, Casas M, Garcia AM, et al. ; Regina Gražulevičienė . Occupational exposure to endocrine-disrupting chemicals and birth weight and length of gestation: a European meta-analysis. Environ Health Perspect. 2016;124(11):1785-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. DiVall SA. The influence of endocrine disruptors on growth and development of children. Curr Opin Endocrinol Diabetes Obes. 2013;20(1):50-55. [DOI] [PubMed] [Google Scholar]
- 265. Govarts E, Iszatt N, Trnovec T, et al. Prenatal exposure to endocrine disrupting chemicals and risk of being born small for gestational age: pooled analysis of seven European birth cohorts. Environ Int. 2018;115:267-278. [DOI] [PubMed] [Google Scholar]
- 266. Ferguson KK, Meeker JD, Cantonwine DE, Chen YH, Mukherjee B, McElrath TF. Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environ Int. 2016;94:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Padmanabhan V, Siefert K, Ransom S, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28(4):258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Troisi J, Mikelson C, Richards S, et al. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta. 2014;35(11):947-952. [DOI] [PubMed] [Google Scholar]
- 269. Thomas S, Arbuckle TE, Fisher M, Fraser WD, Ettinger A, King W. Metals exposure and risk of small-for-gestational age birth in a Canadian birth cohort: The MIREC study. Environ Res. 2015;140:430-439. [DOI] [PubMed] [Google Scholar]
- 270. Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int. 2017;108:278-284. [DOI] [PubMed] [Google Scholar]
- 271. Wikström S, Lin PI, Lindh CH, Shu H, Bornehag CG. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr Res. 2020;87(6):1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Huang K, Li H, Zhang B, et al. Prenatal cadmium exposure and preterm low birth weight in China. J Expo Sci Environ Epidemiol. 2017;27(5):491-496. [DOI] [PubMed] [Google Scholar]
- 273. Agier L, Basagaña X, Hernandez-Ferrer C, et al. Association between the pregnancy exposome and fetal growth. Int J Epidemiol. 2020;49(2):572-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Ding G, Cui C, Chen L, et al. Prenatal low-level mercury exposure and neonatal anthropometry in rural northern China. Chemosphere. 2013;92(9):1085-1089. [DOI] [PubMed] [Google Scholar]
- 275. Philippat C, Mortamais M, Chevrier C, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120(3):464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R; EDEN Study Group . Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25(5):625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Kalloo G, Wellenius GA, McCandless L, et al. Exposures to chemical mixtures during pregnancy and neonatal outcomes: the HOME study. Environ Int. 2020;134:105219. [DOI] [PubMed] [Google Scholar]
- 278. Li W, Guo J, Wu C, et al. Effects of prenatal exposure to five parabens on neonatal thyroid function and birth weight: evidence from SMBCS study. Environ Res. 2020;188:109710. [DOI] [PubMed] [Google Scholar]
- 279. Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int. 2010;36(7):699-704. [DOI] [PubMed] [Google Scholar]
- 280. Huang X, Xu X, Dai Y, Cheng Z, Zheng X, Huo X. Association of prenatal exposure to PAHs with anti-Müllerian hormone (AMH) levels and birth outcomes of newborns. Sci Total Environ. 2020;723:138009. [DOI] [PubMed] [Google Scholar]
- 281. Zhang YW, Gao H, Mao LJ, et al. Effects of the phthalate exposure during three gestation periods on birth weight and their gender differences: a birth cohort study in China. Sci Total Environ. 2018;613-614:1573-1578. [DOI] [PubMed] [Google Scholar]
- 282. Song Q, Li R, Zhao Y, et al. Evaluating effects of prenatal exposure to phthalates on neonatal birth weight: structural equation model approaches. Chemosphere. 2018;205:674-681. [DOI] [PubMed] [Google Scholar]
- 283. Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge RS. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J Pediatr. 2009;155(4):500-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Rokoff LB, Rifas-Shiman SL, Coull BA, et al. Cumulative exposure to environmental pollutants during early pregnancy and reduced fetal growth: the Project Viva cohort. Environ Health. 2018;17(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Longnecker MP, Klebanoff MA, Brock JW, Guo X. Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology. 2005;16(5):641-647. [DOI] [PubMed] [Google Scholar]
- 286. Lauritzen HB, Larose TL, Øien T, et al. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatr Res. 2017;81(1-1):33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Vafeiadi M, Vrijheid M, Fthenou E, et al. Persistent organic pollutants exposure during pregnancy, maternal gestational weight gain, and birth outcomes in the mother-child cohort in Crete, Greece (RHEA study). Environ Int. 2014;64:116-123. [DOI] [PubMed] [Google Scholar]
- 288. Jaacks LM, Diao N, Calafat AM, et al. Association of prenatal pesticide exposures with adverse pregnancy outcomes and stunting in rural Bangladesh. Environ Int. 2019;133(Pt B):105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Fang J, Liu H, Zhao H, Wong M, Xu S, Cai Z. Association of prenatal exposure to organochlorine pesticides and birth size. Sci Total Environ. 2019;654:678-683. [DOI] [PubMed] [Google Scholar]
- 290. Perera FP, Rauh V, Whyatt RM, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26(4):573-587. [DOI] [PubMed] [Google Scholar]
- 291. Guo H, Jin Y, Cheng Y, et al. Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere. 2014;110:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Main KM, Skakkebaek NE, Virtanen HE, Toppari J. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab. 2010;24(2):279-289. [DOI] [PubMed] [Google Scholar]
- 293. Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35(3):236-244. [DOI] [PubMed] [Google Scholar]
- 294. Sunman B, Yurdakök K, Kocer-Gumusel B, et al. Prenatal bisphenol a and phthalate exposure are risk factors for male reproductive system development and cord blood sex hormone levels. Reprod Toxicol. 2019;87:146-155. [DOI] [PubMed] [Google Scholar]
- 295. Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108(2):177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Fisher BG, Thankamony A, Mendiola J, et al. Maternal serum concentrations of bisphenol A and propyl paraben in early pregnancy are associated with male infant genital development. Hum Reprod. 2020;35(4):913-928. [DOI] [PubMed] [Google Scholar]
- 297. Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect. 2005;113(2):220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Longnecker MP, Klebanoff MA, Brock JW, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol. 2002;155(4):313-322. [DOI] [PubMed] [Google Scholar]
- 299. Tournaire M, Devouche E, Epelboin S, Cabau A, Dunbavand A, Levadou A. Birth defects in children of men exposed in utero to diethylstilbestrol (DES). Therapie. 2018;73(5):399-407. [DOI] [PubMed] [Google Scholar]
- 300. Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166(6):E1-E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Ou Y, Bloom MS, Nie Z, et al. Associations between toxic and essential trace elements in maternal blood and fetal congenital heart defects. Environ Int. 2017;106:127-134. [DOI] [PubMed] [Google Scholar]
- 302. Liu Z, Yu Y, Li X, et al. Maternal lead exposure and risk of congenital heart defects occurrence in offspring. Reprod Toxicol. 2015;51:1-6. [DOI] [PubMed] [Google Scholar]
- 303. Demir N, Başaranoğlu M, Huyut Z, et al. The relationship between mother and infant plasma trace element and heavy metal levels and the risk of neural tube defect in infants. J Matern Fetal Neonatal Med. 2019;32(9):1433-1440. [DOI] [PubMed] [Google Scholar]
- 304. Fernández MF, Arrebola JP, Jiménez-Díaz I, et al. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod Toxicol. 2016;59:89-95. [DOI] [PubMed] [Google Scholar]
- 305. Chin HB, Jukic AM, Wilcox AJ, et al. Association of urinary concentrations of phthalate metabolites and bisphenol A with early pregnancy endpoints. Environ Res. 2019;168:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries: a sentinel health indicator? JAMA. 1998;279(13):1018-1023. [DOI] [PubMed] [Google Scholar]
- 307. Rittler M, Castilla EE. Endocrine disruptors and congenital anomalies. Cad Saude Publica. 2002;18(2):421-428. [DOI] [PubMed] [Google Scholar]
- 308. Wedekind C. Demographic and genetic consequences of disturbed sex determination. Philos Trans R Soc Lond B Biol Sci. 2017;372(1729):20160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Vartiainen T, Kartovaara L, Tuomisto J. Environmental chemicals and changes in sex ratio: analysis over 250 years in finland. Environ Health Perspect. 1999;107(10):813-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. van der Pal-de Bruin KM, Verloove-Vanhorick SP, Roeleveld N. Change in male:female ratio among newborn babies in Netherlands. Lancet. 1997;349(9044):62. [DOI] [PubMed] [Google Scholar]
- 311. Møller H. Change in male:female ratio among newborn infants in Denmark. Lancet. 1996;348(9030):828-829. [DOI] [PubMed] [Google Scholar]
- 312. Ryan JJ, Amirova Z, Carrier G. Sex ratios of children of Russian pesticide producers exposed to dioxin. Environ Health Perspect. 2002;110(11):A699-A701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Venners SA, Korrick S, Xu X, et al. Preconception serum DDT and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol. 2005;162(8):709-716. [DOI] [PubMed] [Google Scholar]
- 314. Sun X, Li D, Liang H, et al. Maternal exposure to bisphenol A and anogenital distance throughout infancy: a longitudinal study from Shanghai, China. Environ Int. 2018;121(Pt 1):269-275. [DOI] [PubMed] [Google Scholar]
- 315. Hu J, Xia W, Pan X, et al. Association of adverse birth outcomes with prenatal exposure to vanadium: a population-based cohort study. Lancet Planet Health. 2017;1(6):e230-e241. [DOI] [PubMed] [Google Scholar]
- 316. Chang CH, Wang PW, Liang HW, et al. The sex-specific association between maternal paraben exposure and size at birth. Int J Hyg Environ Health. 2019;222(6):955-964. [DOI] [PubMed] [Google Scholar]
- 317. Lv S, Wu C, Lu D, et al. Birth outcome measures and prenatal exposure to 4-tert-octylphenol. Environ Pollut. 2016;212:65-70. [DOI] [PubMed] [Google Scholar]
- 318. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam Horm. 2014;94:129-165. [DOI] [PubMed] [Google Scholar]
- 320. Morales E, Gascon M, Martinez D, et al. ; INMA Project . Associations between blood persistent organic pollutants and 25-hydroxyvitamin D3 in pregnancy. Environ Int. 2013;57-58:34-41. [DOI] [PubMed] [Google Scholar]
- 321. Shapiro GD, Dodds L, Arbuckle TE, et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ Int. 2015;83:63-71. [DOI] [PubMed] [Google Scholar]
- 322. Adibi JJ, Whyatt RM, Hauser R, et al. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ Health Perspect. 2010;118(2):291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Ribeiro E, Ladeira C, Viegas S. EDCs mixtures: a stealthy hazard for human health? Toxics 2017;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect. 2007;115(Suppl 1):98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110(9):917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Evans RM, Martin OV, Faust M, Kortenkamp A. Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci Total Environ. 2016;543(Pt A):757-764. [DOI] [PubMed] [Google Scholar]
- 327. Chiu YH, Bellavia A, James-Todd T, et al. ; EARTH Study Team . Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: a comparison of three statistical approaches. Environ Int. 2018;113:231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Govarts E, Portengen L, Lambrechts N, et al. Early-life exposure to multiple persistent organic pollutants and metals and birth weight: Pooled analysis in four Flemish birth cohorts. Environ Int. 2020;145:106149. [DOI] [PubMed] [Google Scholar]
- 329. Ouidir M, Buck Louis GM, Kanner J, et al. Association of maternal exposure to persistent organic pollutants in early pregnancy with fetal growth. JAMA Pediatr. 2020;174(2):149-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Polanska K, Dettbarn G, Jurewicz J, et al. Effect of prenatal polycyclic aromatic hydrocarbons exposure on birth outcomes: the Polish Mother and Child Cohort study. Biomed Res Int. 2014;2014:408939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Kalloo G, Wellenius GA, McCandless L, et al. Profiles and predictors of environmental chemical mixture exposure among pregnant women: the Health Outcomes and Measures of the Environment study. Environ Sci Technol. 2018;52(17):10104-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 2016;124(1):A6-A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Puttabyatappa M, Banker M, Zeng L, et al. Maternal exposure to environmental disruptors and sexually dimorphic changes in maternal and neonatal oxidative stress. J Clin Endocrinol Metab. 2020;105(2):492-–505.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Kelley AS, Banker M, Goodrich JM, et al. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci Rep. 2019;9(1):5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Stacy SL, Brink LL, Larkin JC, et al. Perinatal outcomes and unconventional natural gas operations in Southwest Pennsylvania. PLoS One. 2015;10(6):e0126425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Casey JA, Savitz DA, Rasmussen SG, et al. Unconventional natural gas development and birth outcomes in Pennsylvania, USA. Epidemiology. 2016;27(2):163-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ Health Perspect. 2014;122(4):412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Lee PC, Roberts JM, Catov JM, Talbott EO, Ritz B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern Child Health J. 2013;17(3):545-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. PM2.5 exposure and birth outcomes: use of satellite- and monitor-based data. Epidemiology. 2014;25(1):58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. van den Hooven EH, Pierik FH, de Kluizenaar Y, et al. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environ Health Perspect. 2012;120(1):150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Kassotis CD, Bromfield JJ, Klemp KC, et al. Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology. 2016;157(9):3469-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121-128. [DOI] [PubMed] [Google Scholar]
- 343. Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154-167. [DOI] [PubMed] [Google Scholar]
- 344. Walker JJ. Inflammation and preeclampsia. Pregnancy Hypertens. 2011;1(1):43-47. [DOI] [PubMed] [Google Scholar]
- 345. Tenório MB, Ferreira RC, Moura FA, Bueno NB, de Oliveira ACM, Goulart MOF. Cross-talk between oxidative stress and inflammation in preeclampsia. Oxid Med Cell Longev. 2019;2019:8238727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Humberg A, Fortmann I, Siller B, et al. ; German Neonatal Network, German Center for Lung Research and Priming Immunity at the beginning of life (PRIMAL) Consortium . Preterm birth and sustained inflammation: consequences for the neonate. Semin Immunopathol. 2020;42(4):451-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Khambule L, George JA. The role of inflammation in the development of GDM and the use of markers of inflammation in GDM screening. Adv Exp Med Biol. 2019;1134:217-242. [DOI] [PubMed] [Google Scholar]
- 348. Chalubinski M, Kowalski ML. Endocrine disrupters—potential modulators of the immune system and allergic response. Allergy. 2006;61(11):1326-1335. [DOI] [PubMed] [Google Scholar]
- 349. Rychlik KA, Sillé FCM. Environmental exposures during pregnancy: Mechanistic effects on immunity. Birth Defects Res. 2019;111(4):178-196. [DOI] [PubMed] [Google Scholar]
- 350. Ferguson KK, Cantonwine DE, McElrath TF, Mukherjee B, Meeker JD. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod Toxicol. 2016;66:93-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health. 2015;218(2):212-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Zota AR, Geller RJ, Romano LE, et al. Association between persistent endocrine-disrupting chemicals (PBDEs, OH-PBDEs, PCBs, and PFASs) and biomarkers of inflammation and cellular aging during pregnancy and postpartum. Environ Int. 2018;115:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. van T Erve TJ, Rosen EM, Barrett ES, et al. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ Sci Technol. 2019;53(6):3258-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Matilla-Santander N, Valvi D, Lopez-Espinosa MJ, et al. Exposure to perfluoroalkyl substances and metabolic outcomes in pregnant women: evidence from the Spanish INMA birth cohorts. Environ Health Perspect 2017;125:p.117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Ahmed S, Mahabbat-e Khoda S, Rekha RS, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119(2):258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Aung MT, Ferguson KK, Cantonwine DE, et al. Associations between maternal plasma measurements of inflammatory markers and urinary levels of phenols and parabens during pregnancy: a repeated measures study. Sci Total Environ. 2019;650(Pt 1):1131-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Mustafa M, Garg N, Banerjee BD, et al. Inflammatory-mediated pathway in association with organochlorine pesticides levels in the etiology of idiopathic preterm birth. Reprod Toxicol. 2015;57:111-120. [DOI] [PubMed] [Google Scholar]
- 358. Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11(6):342-352. [DOI] [PubMed] [Google Scholar]
- 359. Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol. 1991;165(6 Pt 1):1701-1704. [DOI] [PubMed] [Google Scholar]
- 360. Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19(8):581-586. [DOI] [PubMed] [Google Scholar]
- 361. Wang YP, Walsh SW, Guo JD, Zhang JY. Maternal levels of prostacyclin, thromboxane, vitamin E, and lipid peroxides throughout normal pregnancy. Am J Obstet Gynecol. 1991;165(6 Pt 1):1690-1694. [DOI] [PubMed] [Google Scholar]
- 362. Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634-1650. [DOI] [PubMed] [Google Scholar]
- 363. Ferguson KK, Lan Z, Yu Y, Mukherjee B, McElrath TF, Meeker JD. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: assessment of effects independent of phthalates. Environ Int. 2019;131:104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Veiga-Lopez A, Pennathur S, Kannan K, et al. Impact of gestational bisphenol A on oxidative stress and free fatty acids: human association and interspecies animal testing studies. Endocrinology. 2015;156(3):911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Tran V, Tindula G, Huen K, et al. Prenatal phthalate exposure and 8-isoprostane among Mexican-American children with high prevalence of obesity. J Dev Orig Health Dis. 2017;8(2):196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Obolenskaya MY, Teplyuk NM, Divi RL, et al. Human placental glutathione S-transferase activity and polycyclic aromatic hydrocarbon DNA adducts as biomarkers for environmental oxidative stress in placentas from pregnant women living in radioactivity- and chemically-polluted regions. Toxicol Lett. 2010;196(2):80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Kim H, Hwang JY, Ha EH, et al. Fruit and vegetable intake influences the association between exposure to polycyclic aromatic hydrocarbons and a marker of oxidative stress in pregnant women. Eur J Clin Nutr. 2011;65(10):1118-1125. [DOI] [PubMed] [Google Scholar]
- 368. Tal R, Taylor HS, Burney RO, et al. , eds. Endocrinology of Pregnancy. In: Endotext. MDText.com Inc; 2000. [Google Scholar]
- 369. Ishida J, Matsuoka T, Saito-Fujita T, et al. Pregnancy-associated homeostasis and dysregulation: lessons from genetically modified animal models. J Biochem. 2011;150:5-14. [DOI] [PubMed] [Google Scholar]
- 370. Aung MT, Johns LE, Ferguson KK, Mukherjee B, McElrath TF, Meeker JD. Thyroid hormone parameters during pregnancy in relation to urinary bisphenol A concentrations: a repeated measures study. Environ Int. 2017;104:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Wang X, Tang N, Nakayama SF, et al. Maternal urinary bisphenol A concentration and thyroid hormone levels of Chinese mothers and newborns by maternal body mass index. Environ Sci Pollut Res Int. 2020;27(10):10939-10949. [DOI] [PubMed] [Google Scholar]
- 372. Huang HB, Kuo PL, Chang JW, Jaakkola JJK, Liao KW, Huang PC. Longitudinal assessment of prenatal phthalate exposure on serum and cord thyroid hormones homeostasis during pregnancy—Tainan birth cohort study (TBCS). Sci Total Environ. 2018;619-620:1058-1065. [DOI] [PubMed] [Google Scholar]
- 373. Chevrier J, Rauch S, Obida M, Crause M, Bornman R, Eskenazi B. Sex and poverty modify associations between maternal peripartum concentrations of DDT/E and pyrethroid metabolites and thyroid hormone levels in neonates participating in the VHEMBE study, South Africa. Environ Int. 2019;131:104958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Mulder TA, van den Dries MA, Korevaar TIM, Ferguson KK, Peeters RP, Tiemeier H. Organophosphate pesticides exposure in pregnant women and maternal and cord blood thyroid hormone concentrations. Environ Int. 2019;132:105124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Aker AM, Ferguson KK, Rosario ZY, et al. A repeated measures study of phenol, paraben and triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ Health. 2019;18(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Aker AM, Watkins DJ, Johns LE, et al. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 2016;151:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014;147(4):401-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Sathyanarayana S, Butts S, Wang C, et al. ; TIDES Team . Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab. 2017;102(6):1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36(6):699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Ferguson KK, McElrath TF, Pace GG, et al. Correction to “Urinary polycyclic aromatic hydrocarbon metabolite associations with biomarkers of inflammation, angiogenesis, and oxidative stress in pregnant women”. Environ Sci Technol. 2019;53(4):2269. [DOI] [PubMed] [Google Scholar]
- 381. Guo J, Lv N, Tang J, et al. Associations of blood metal exposure with thyroid hormones in Chinese pregnant women: a cross-sectional study. Environ Int. 2018;121(Pt 2):1185-1192. [DOI] [PubMed] [Google Scholar]
- 382. Liu Y, Wu M, Zhang L, et al. Prenatal exposure of rare earth elements cerium and ytterbium and neonatal thyroid stimulating hormone levels: findings from a birth cohort study. Environ Int. 2019;133(Pt B):105222. [DOI] [PubMed] [Google Scholar]
- 383. Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: a repeated measures study. Environ Int. 2018;113:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Giesbrecht GF, Ejaredar M, Liu J, et al. ; APrON Study Team . Prenatal bisphenol a exposure and dysregulation of infant hypothalamic-pituitary-adrenal axis function: findings from the APrON cohort study. Environ Health. 2017;16(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Derakhshan A, Shu H, Peeters RP, et al. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ Int. 2019;133(Pt A):105123. [DOI] [PubMed] [Google Scholar]
- 386. Adibi JJ, Lee MK, Naimi AI, et al. Human chorionic gonadotropin partially mediates phthalate association with male and female anogenital distance. J Clin Endocrinol Metab. 2015;100(9):E1216-E1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Alvarez-Pedrerol M, Guxens M, Ibarluzea J, et al. Organochlorine compounds, iodine intake, and thyroid hormone levels during pregnancy. Environ Sci Technol. 2009;43(20):7909-7915. [DOI] [PubMed] [Google Scholar]
- 388. Dreyer AF, Jensen RC, Glintborg D, et al. Perfluoroalkyl substance exposure early in pregnancy was negatively associated with late pregnancy cortisone levels. J Clin Endocrinol Metab. 2020;105(8):e2834–e2844. [DOI] [PubMed] [Google Scholar]
- 389. Dufour P, Pirard C, Seghaye MC, Charlier C. Association between organohalogenated pollutants in cord blood and thyroid function in newborns and mothers from Belgian population. Environ Pollut. 2018;238:389-396. [DOI] [PubMed] [Google Scholar]
- 390. Wang SL, Chang YC, Chao HR, et al. Body burdens of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls and their relations to estrogen metabolism in pregnant women. Environ Health Perspect. 2006;114(5):740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135-162. [DOI] [PubMed] [Google Scholar]
- 392. Angueira AR, Ludvik AE, Reddy TE, Wicksteed B, Lowe WL Jr, Layden BT. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64(2):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Mirza FG, Cleary KL. Pre-eclampsia and the kidney. Semin Perinatol. 2009;33(3):173-178. [DOI] [PubMed] [Google Scholar]
- 394. Zheng Y, Zhang C, Weisskopf M, et al. A prospective study of early pregnancy essential metal(loid)s and glucose levels late in the second trimester. J Clin Endocrinol Metab. 2019;104(10):4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Liu X, Zhang L, Li J, et al. A nested case-control study of the association between exposure to polybrominated diphenyl ethers and the risk of gestational diabetes mellitus. Environ Int. 2018;119:232-238. [DOI] [PubMed] [Google Scholar]
- 396. Noor N, Ferguson KK, Meeker JD, et al. Pregnancy phthalate metabolite concentrations and infant birth weight by gradations of maternal glucose tolerance. Int J Hyg Environ Health. 2019;222(3):395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Jia X, Harada Y, Tagawa M, et al. Prenatal maternal blood triglyceride and fatty acid levels in relation to exposure to di(2-ethylhexyl)phthalate: a cross-sectional study. Environ Health Prev Med. 2015;20(3):168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Zhou M, Ford B, Lee D, et al. Metabolomic markers of phthalate exposure in plasma and urine of pregnant women. Front Public Health. 2018;6:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Li H, Huang K, Jin S, et al. Environmental cadmium exposure induces alterations in the urinary metabolic profile of pregnant women. Int J Hyg Environ Health. 2019;222(3):556-562. [DOI] [PubMed] [Google Scholar]
- 400. Wei Y, Shi Q, Wang Z, et al. Maternal/fetal metabolomes appear to mediate the impact of arsenic exposure on birth weight: a pilot study. J Expo Sci Environ Epidemiol. 2017;27(3):313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Zhao H, Zheng Y, Zhu L, et al. Paraben exposure related to purine metabolism and other pathways revealed by mass spectrometry-based metabolomics. Environ Sci Technol. 2020;54(6):3447-3454. [DOI] [PubMed] [Google Scholar]
- 402. Zbucka-Kretowska M, Zbucki R, Parfieniuk E, et al. Evaluation of bisphenol A influence on endocannabinoid system in pregnant women. Chemosphere. 2018;203:387-392. [DOI] [PubMed] [Google Scholar]
- 403. Starling AP, Adgate JL, Hamman RF, et al. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the healthy start study. Environ Health Perspect. 2017;125(6):067016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Preston EV, Rifas-Shiman SL, Hivert MF, et al. Associations of per- and polyfluoroalkyl substances (PFAS) with glucose tolerance during pregnancy in Project Viva. J Clin Endocrinol Metab. 2020; 105(8):e2864-e2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Bonvallot N, Tremblay-Franco M, Chevrier C, et al. Metabolomics tools for describing complex pesticide exposure in pregnant women in Brittany (France). PLoS One. 2013;8(5):e64433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes. 2017;12(Suppl 1):3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ. 2017;356:j831. [DOI] [PubMed] [Google Scholar]
- 410. Dunlop AL, Mulle JG, Ferranti EP, Edwards S, Dunn AB, Corwin EJ. Maternal microbiome and pregnancy outcomes that impact infant health: a review. Adv Neonatal Care. 2015;15(6):377-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. Neuman H, Koren O. The pregnancy microbiome. Nestle Nutr Inst Workshop Ser. 2017;88:1-9. [DOI] [PubMed] [Google Scholar]
- 412. Gálvez-Ontiveros Y, Páez S, Monteagudo C, Rivas A. Endocrine disruptors in food: impact on gut microbiota and metabolic diseases. Nutrients. 2020;12(4):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Rosenfeld CS. Gut dysbiosis in animals due to environmental chemical exposures. Front Cell Infect Microbiol. 2017;7:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Velmurugan G, Ramprasath T, Gilles M, Swaminathan K, Ramasamy S. Gut Microbiota, endocrine-disrupting chemicals, and the diabetes epidemic. Trends Endocrinol Metab. 2017;28(8):612-625. [DOI] [PubMed] [Google Scholar]
- 415. Bisanz JE, Enos MK, Mwanga JR, et al. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio. 2014;5(5):e01580-e01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Rothenberg SE, Wagner CL, Hamidi B, Alekseyenko AV, Andrea Azcarate-Peril M. Longitudinal changes during pregnancy in gut microbiota and methylmercury biomarkers, and reversal of microbe-exposure correlations. Environ Res. 2019;172:700-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Wu J, Wen XW, Faulk C, et al. Perinatal lead exposure alters gut microbiota composition and results in sex-specific bodyweight increases in adult mice. Toxicol Sci. 2016;151(2):324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Javurek AB, Spollen WG, Johnson SA, et al. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes. 2016;7(6):471-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Liu W, Zhou Y, Yong Li, et al. Effects of PM2.5 exposure during gestation on maternal gut microbiota and pregnancy outcomes. Chemosphere. 2020;247:125879. [DOI] [PubMed] [Google Scholar]
- 420. Montoya-Williams D, Lemas DJ, Spiryda L, et al. The neonatal microbiome and its partial role in mediating the association between birth by cesarean section and adverse pediatric outcomes. Neonatology. 2018;114(2):103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. 2016;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. 2018;392(10155):1349-1357. [DOI] [PubMed] [Google Scholar]
- 423. Stinson LF, Payne MS, Keelan JA. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43(3):352-369. [DOI] [PubMed] [Google Scholar]
- 424. Heinemann C, Reid G. Vaginal microbial diversity among postmenopausal women with and without hormone replacement therapy. Can J Microbiol. 2005;51(9):777-781. [DOI] [PubMed] [Google Scholar]
- 425. Kemp MW. Preterm birth, intrauterine infection, and fetal inflammation. Front Immunol. 2014;5:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Stefanovic V, Andersson S, Vento M. Oxidative stress-related spontaneous preterm delivery challenges in causality determination, prevention and novel strategies in reduction of the sequelae. Free Radic Biol Med. 2019;142:52-60. [DOI] [PubMed] [Google Scholar]
- 427. Dennery PA. Oxidative stress in development: nature or nurture? Free Radic Biol Med. 2010;49(7):1147-1151. [DOI] [PubMed] [Google Scholar]
- 428. Ashley-Martin J, Levy AR, Arbuckle TE, Platt RW, Marshall JS, Dodds L. Maternal exposure to metals and persistent pollutants and cord blood immune system biomarkers. Environ Health. 2015;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Dao T, Hong X, Wang X, Tang WY. Aberrant 5′-CpG methylation of cord blood TNFα associated with maternal exposure to polybrominated diphenyl ethers. PLoS One. 2015;10(9):e0138815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Latzin P, Frey U, Armann J, et al. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS One. 2011;6(8):e23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Neta G, Goldman LR, Barr D, Apelberg BJ, Witter FR, Halden RU. Fetal exposure to chlordane and permethrin mixtures in relation to inflammatory cytokines and birth outcomes. Environ Sci Technol. 2011;45(4):1680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Al-Gubory KH. Multiple exposures to environmental pollutants and oxidative stress: Is there a sex specific risk of developmental complications for fetuses? Birth Defects Res C Embryo Today. 2016;108(4):351-364. [DOI] [PubMed] [Google Scholar]
- 433. Ambroz A, Vlkova V, Rossner P Jr, et al. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int J Hyg Environ Health. 2016;219(6):545-556. [DOI] [PubMed] [Google Scholar]
- 434. Badiu CMDP. Williams textbook of endocrinology. Acta Endocrinol (Buchar). 2019; 15:416. [Google Scholar]
- 435. Symonds ME, Budge H, Stephenson T, McMillen IC. Fetal endocrinology and development–manipulation and adaptation to long-term nutritional and environmental challenges. Reproduction. 2001;121(6):853-862. [DOI] [PubMed] [Google Scholar]
- 436. Kim S, Park J, Kim HJ, et al. Association between several persistent organic pollutants and thyroid hormone levels in cord blood serum and bloodspot of the newborn infants of Korea. PLoS One. 2015;10(5):e0125213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Berg V, Nøst TH, Pettersen RD, et al. Persistent organic pollutants and the association with maternal and infant thyroid homeostasis: a multipollutant assessment. Environ Health Perspect. 2017;125(1):127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Kolatorova L, Vitku J, Hampl R, et al. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ Res. 2018;163:115-122. [DOI] [PubMed] [Google Scholar]
- 439. Araki A, Mitsui T, Goudarzi H, et al. Prenatal di(2-ethylhexyl) phthalate exposure and disruption of adrenal androgens and glucocorticoids levels in cord blood: The Hokkaido Study. Sci Total Environ. 2017;581-582:297-304. [DOI] [PubMed] [Google Scholar]
- 440. Warembourg C, Debost-Legrand A, Bonvallot N, et al. Exposure of pregnant women to persistent organic pollutants and cord sex hormone levels. Hum Reprod. 2016;31(1):190-198. [DOI] [PubMed] [Google Scholar]
- 441. Goudarzi H, Araki A, Itoh S, et al. The association of prenatal exposure to perfluorinated chemicals with glucocorticoid and androgenic hormones in cord blood samples: the Hokkaido Study. Environ Health Perspect. 2017;125(1):111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Araki A, Miyashita C, Mitsui T, et al. Prenatal organochlorine pesticide exposure and the disruption of steroids and reproductive hormones in cord blood: the Hokkaido study. Environ Int. 2018;110:1-13. [DOI] [PubMed] [Google Scholar]
- 443. El Majidi N, Bouchard M, Carrier G. Systematic analysis of the relationship between standardized biological levels of polychlorinated biphenyls and thyroid function in pregnant women and newborns. Chemosphere. 2014;98:1-17. [DOI] [PubMed] [Google Scholar]
- 444. Wan Y, Choi K, Kim S, et al. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ Sci Technol. 2010;44(13):5233-5239. [DOI] [PubMed] [Google Scholar]
- 445. Jones CT, Rolph TP. Metabolism during fetal life: a functional assessment of metabolic development. Physiol Rev. 1985;65(2):357-430. [DOI] [PubMed] [Google Scholar]
- 446. Hay WW Jr. Recent observations on the regulation of fetal metabolism by glucose. J Physiol. 2006;572(Pt 1):17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123(2):682-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Minatoya M, Araki A, Miyashita C, et al. Association between prenatal bisphenol A and phthalate exposures and fetal metabolic related biomarkers: The Hokkaido study on Environment and Children’s Health. Environ Res. 2018;161:505-511. [DOI] [PubMed] [Google Scholar]
- 449. Debost-Legrand A, Warembourg C, Massart C, et al. Prenatal exposure to persistent organic pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ Res. 2016;146:207-217. [DOI] [PubMed] [Google Scholar]
- 450. Romano-Keeler J, Weitkamp JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77(1-2):189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Wilczyńska P, Skarżyńska E, Lisowska-Myjak B. Meconium microbiome as a new source of information about long-term health and disease: questions and answers. J Matern Fetal Neonatal Med. 2019;32(4):681-686. [DOI] [PubMed] [Google Scholar]
- 452. DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. Plos One. 2008;3(8):e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15(2):111-122. [DOI] [PubMed] [Google Scholar]
- 454. Ardissone AN, de la Cruz DM, Davis-Richardson AG, et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. Plos One. 2014;9(3):e90784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Hu J, Nomura Y, Bashir A, et al. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One. 2013;8(11):e78257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Neu J. The microbiome during pregnancy and early postnatal life. Semin Fetal Neonatal Med. 2016;21(6):373-379. [DOI] [PubMed] [Google Scholar]
- 457. Neu J. Developmental aspects of maternal-fetal, and infant gut microbiota and implications for long-term health. Matern Health Neonatol Perinatol. 2015;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Kalish JM, Jiang C, Bartolomei MS. Epigenetics and imprinting in human disease. Int J Dev Biol. 2014;58(2-4):291-298. [DOI] [PubMed] [Google Scholar]
- 459. Chen ZX, Riggs AD. Maintenance and regulation of DNA methylation patterns in mammals. Biochem Cell Biol. 2005;83(4):438-448. [DOI] [PubMed] [Google Scholar]
- 460. Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14(3):204-220. [DOI] [PubMed] [Google Scholar]
- 461. Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074-1080. [DOI] [PubMed] [Google Scholar]
- 462. Carninci P, Hayashizaki Y. Noncoding RNA transcription beyond annotated genes. Curr Opin Genet Dev. 2007;17(2):139-144. [DOI] [PubMed] [Google Scholar]
- 463. Collins LJ, Schönfeld B, Chen XS. Chapter 4: the epigenetics of non-coding RNA. In: Tollefsbol T, ed. Handbook of Epigenetics. Academic Press; 2011:49-61. [Google Scholar]
- 464. Conradt E, Adkins DE, Crowell SE, Raby KL, Diamond LM, Ellis B. Incorporating epigenetic mechanisms to advance fetal programming theories. Dev Psychopathol. 2018;30(3):807-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Ho SM, Cheong A, Adgent MA, et al. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol. 2017;68:85-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci. 2018;19:3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Bommarito PA, Martin E, Fry RC. Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome. Epigenomics. 2017;9(3):333-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Kile ML, Baccarelli A, Hoffman E, et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ Health Perspect. 2012;120(7):1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Huen K, Calafat AM, Bradman A, Yousefi P, Eskenazi B, Holland N. Maternal phthalate exposure during pregnancy is associated with DNA methylation of LINE-1 and Alu repetitive elements in Mexican-American children. Environ Res. 2016;148:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Montrose L, Padmanabhan V, Goodrich JM, et al. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics. 2018;13(3):301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Broberg K, Ahmed S, Engström K, et al. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J Dev Orig Health Dis. 2014;5(4):288-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472. Pilsner JR, Hall MN, Liu X, et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS One. 2012;7(5):e37147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Kippler M, Engström K, Mlakar SJ, et al. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics. 2013;8(5):494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Faulk C, Kim JH, Jones TR, et al. Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver. Environ Epigenet. 2015;1(1):dvv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Bakulski KM, Lee H, Feinberg JI, et al. Prenatal mercury concentration is associated with changes in DNA methylation at TCEANC2 in newborns. Int J Epidemiol. 2015;44(4):1249-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Maltepe E, Fisher SJ. Placenta: the forgotten organ. Annu Rev Cell Dev Biol. 2015;31:523-552. [DOI] [PubMed] [Google Scholar]
- 477. Sibley CP. Understanding placental nutrient transfer—why bother? New biomarkers of fetal growth. J Physiol. 2009;587(Pt 14):3431-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Nugent BM, Bale TL. The omniscient placenta: metabolic and epigenetic regulation of fetal programming. Front Neuroendocrinol. 2015;39:28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Gingrich J. The Effects of Endocrine Disrupting Chemicals on Placental Development and Function. Michigan State University East Lansing, Michigan; 2020. [Google Scholar]
- 480. Myren M, Mose T, Mathiesen L, Knudsen LE. The human placenta—an alternative for studying foetal exposure. Toxicol In Vitro. 2007;21(7):1332-1340. [DOI] [PubMed] [Google Scholar]
- 481. Vrooman LA, Xin F, Bartolomei MS. Morphologic and molecular changes in the placenta: what we can learn from environmental exposures. Fertil Steril. 2016;106(4):930-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Zhu YD, Gao H, Huang K, et al. Prenatal phthalate exposure and placental size and shape at birth: a birth cohort study. Environ Res. 2018;160:239-246. [DOI] [PubMed] [Google Scholar]
- 483. Bush PG, Mayhew TM, Abramovich DR, Aggett PJ, Burke MD, Page KR. A quantitative study on the effects of maternal smoking on placental morphology and cadmium concentration. Placenta. 2000;21(2-3):247-256. [DOI] [PubMed] [Google Scholar]
- 484. Zhao Y, Song Q, Cao Z, et al. Umbilical cord blood PBDEs concentrations in relation to placental size at birth. Chemosphere. 2018;201:20-24. [DOI] [PubMed] [Google Scholar]
- 485. Varshavsky JR, Robinson JF, Zhou Y, et al. Association of polybrominated diphenyl ether (PBDE) levels with biomarkers of placental development and disease during mid-gestation. Environ Health. 2020;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Harding JE, Jones CT, Robinson JS. Studies on experimental growth retardation in sheep. The effects of a small placenta in restricting transport to and growth of the fetus. J Dev Physiol. 1985;7(6):427-442. [PubMed] [Google Scholar]
- 487. Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58(6):894-900. [DOI] [PubMed] [Google Scholar]
- 488. Hayward CE, Lean S, Sibley CP, et al. Placental adaptation: what can we learn from birthweight:placental weight ratio? Front Physiol. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Myatt L, Thornburg KL. Effects of prenatal nutrition and the role of the placenta in health and disease. Methods Mol Biol. 2018;1735:19-46. [DOI] [PubMed] [Google Scholar]
- 490. Philippat C, Heude B, Botton J, Alfaidy N, Calafat AM, Slama R; EDEN Mother–Child Cohort Study Group . Prenatal exposure to select phthalates and phenols and associations with fetal and placental weight among male births in the EDEN Cohort (France). Environ Health Perspect. 2019;127(1):17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Chavatte-Palmer P, Guillomot M. Comparative implantation and placentation. Gynecol Obstet Invest. 2007;64(3):166-174. [DOI] [PubMed] [Google Scholar]
- 492. Loch-Caruso R. A mechanistic-based approach for assessing chemical hazards to parturition. J Womens Health. 1999;8(2):235-248. [DOI] [PubMed] [Google Scholar]
- 493. Strakovsky RS, Schantz SL. Using experimental models to assess effects of bisphenol A (BPA) and phthalates on the placenta: challenges and perspectives. Toxicol Sci. 2018;166(2):250-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Yang C, Song G, Lim W. A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere. 2019;231:326-336. [DOI] [PubMed] [Google Scholar]
- 495. Wang JQ, Hu YB, Gao H, et al. Sex-specific difference in placental inflammatory transcriptional biomarkers of maternal phthalate exposure: a prospective cohort study. J Expo Sci Environ Epidemiol. 2020;30(5):835-844. [DOI] [PubMed] [Google Scholar]
- 496. Zhu YD, Liang CM, Hu YB, et al. Repeated measures of prenatal thallium exposure and placental inflammatory cytokine mRNA expression: The Ma’anshan birth cohort (MABC) study. Chemosphere. 2020;246:125721. [DOI] [PubMed] [Google Scholar]
- 497. Liang C, Wang J, Xia X, et al. Serum cobalt status during pregnancy and the risks of pregnancy-induced hypertension syndrome: a prospective birth cohort study. J Trace Elem Med Biol. 2018;46:39-45. [DOI] [PubMed] [Google Scholar]
- 498. Al-Gubory KH. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod Biomed Online. 2014;29(1):17-31. [DOI] [PubMed] [Google Scholar]
- 499. Niedzwiecki MM, Austin C, Remark R, et al. A multimodal imaging workflow to visualize metal mixtures in the human placenta and explore colocalization with biological response markers. Metallomics. 2016;8(4):444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Piasek M, Blanusa M, Kostial K, Laskey JW. Placental cadmium and progesterone concentrations in cigarette smokers. Reprod Toxicol. 2001;15(6):673-681. [DOI] [PubMed] [Google Scholar]
- 501. Xu X, Yekeen TA, Xiao Q, Wang Y, Lu F, Huo X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ Pollut. 2013;182:63-69. [DOI] [PubMed] [Google Scholar]
- 502. Stasenko S, Bradford EM, Piasek M, et al. Metals in human placenta: focus on the effects of cadmium on steroid hormones and leptin. J Appl Toxicol. 2010;30(3):242-253. [DOI] [PubMed] [Google Scholar]
- 503. Deshpande SS, Balasinor NH. Placental defects: an epigenetic perspective. Reprod Sci. 2018;25(8):1143-1160. [DOI] [PubMed] [Google Scholar]
- 504. Apicella C, Ruano CSM, Mehats C, Miralles F, Vaiman D. The role of epigenetics in placental development and the etiology of preeclampsia. Int J Mol Sci. 2019;20(11):2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Barouki R, Melén E, Herceg Z, et al. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ Int. 2018;114:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Everson TM, Punshon T, Jackson BP, et al. Cadmium-associated differential methylation throughout the placental genome: epigenome-wide association study of two U.S. birth cohorts. Environ Health Perspect. 2018;126(1):017010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Kappil MA, Li Q, Li A, et al. In utero exposures to environmental organic pollutants disrupt epigenetic marks linked to fetoplacental development. Environ Epigenet. 2016;2(1):dvv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Kim S, Cho YH, Won S, et al. Maternal exposures to persistent organic pollutants are associated with DNA methylation of thyroid hormone-related genes in placenta differently by infant sex. Environ Int. 2019;130:104956. [DOI] [PubMed] [Google Scholar]
- 509. LaRocca J, Binder AM, McElrath TF, Michels KB. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res. 2014;133:396-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Machtinger R, Zhong J, Mansur A, et al. Placental lncRNA expression is associated with prenatal phthalate exposure. Toxicol Sci. 2018;163(1):116-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. De Felice B, Manfellotto F, Palumbo A, et al. Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med Genomics. 2015;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. LaRocca J, Binder AM, McElrath TF, Michels KB. First-trimester urine concentrations of phthalate metabolites and phenols and placenta miRNA expression in a cohort of U.S. women. Environ Health Perspect. 2016;124(3):380-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Engström K, Rydbeck F, Kippler M, et al. Prenatal lead exposure is associated with decreased cord blood DNA methylation of the glycoprotein VI gene involved in platelet activation and thrombus formation. Environ Epigenet. 2015;1(1):dvv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Li Q, Kappil MA, Li A, et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics. 2015;10(9):793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere. 2015;124:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Zhao Y, Shi HJ, Xie CM, Chen J, Laue H, Zhang YH. Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ Mol Mutagen. 2015;56(3):286-292. [DOI] [PubMed] [Google Scholar]
- 517. Ouidir M, Mendola P, Buck Louis GM, Kannan K, Zhang C, Tekola-Ayele F. Concentrations of persistent organic pollutants in maternal plasma and epigenome-wide placental DNA methylation. Clin Epigenetics. 2020;12(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Grindler NM, Vanderlinden L, Karthikraj R, et al. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci Rep. 2018;8(1):6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci U S A. 2010;107(39):16757-16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Ghassabian A, Trasande L. Disruption in thyroid signaling pathway: a mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Front Endocrinol (Lausanne). 2018;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Heindel JJ. The developmental basis of disease: update on environmental exposures and animal models. Basic Clin Pharmacol Toxicol. 2019;125 Suppl 3:5-13. [DOI] [PubMed] [Google Scholar]
- 522. Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol. 2009;304(1-2):90-96. [DOI] [PubMed] [Google Scholar]
- 523. Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res C Embryo Today. 2011;93(1):34-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Newbold RR. Developmental exposure to endocrine-disrupting chemicals programs for reproductive tract alterations and obesity later in life. Am J Clin Nutr. 2011;94(6 Suppl):1939S-1942S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl. 2010;33(2):394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Sargis RM, Heindel JJ, Padmanabhan V. Interventions to address environmental metabolism-disrupting chemicals: changing the narrative to empower action to restore metabolic health. Front Endocrinol (Lausanne). 2019;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Yorifuji T, Kato T, Kado Y, et al. Intrauterine exposure to methylmercury and neurocognitive functions: Minamata disease. Arch Environ Occup Health. 2015;70(5):297-302. [DOI] [PubMed] [Google Scholar]
- 528. Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—what we have learned from Yusho disease. Environ Res. 2001;86(1):2-11. [DOI] [PubMed] [Google Scholar]
- 529. Eskenazi B, Warner M, Brambilla P, Signorini S, Ames J, Mocarelli P. The Seveso accident: a look at 40 years of health research and beyond. Environ Int. 2018;121(Pt 1):71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Braun JM, Yolton K, Dietrich KN, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Braun JM, Kalkbrenner AE, Calafat AM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Engel SM, Zhu C, Berkowitz GS, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Hyland C, Mora AM, Kogut K, et al. Prenatal exposure to phthalates and neurodevelopment in the CHAMACOS cohort. Environ Health Perspect. 2019;127(10):107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Vuong AM, Xie C, Jandarov R, et al. Prenatal exposure to a mixture of persistent organic pollutants (POPs) and child reading skills at school age. Int J Hyg Environ Health. 2020;228:113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Kim Y, Ha EH, Kim EJ, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119(10):1495-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Vafeiadi M, Roumeliotaki T, Myridakis A, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379-387. [DOI] [PubMed] [Google Scholar]
- 537. Braun JM, Eliot M, Papandonatos GD, et al. Gestational perfluoroalkyl substance exposure and body mass index trajectories over the first 12 years of life. Int J Obes (Lond). 2021;45(1):25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Mora AM, Oken E, Rifas-Shiman SL, et al. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect. 2017;125(3):467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Bowman A, Peterson KE, Dolinoy DC, et al. Phthalate exposures, DNA methylation and adiposity in Mexican children through adolescence. Front Public Health. 2019;7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Watkins DJ, Sánchez BN, Téllez-Rojo MM, et al. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ Res. 2017;159:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Buckley JP, Barrett ES, Beamer PI, et al. ; program collaborators for ECHO . Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) program. J Expo Sci Environ Epidemiol. 2020;30(3):397-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Kioumourtzoglou MA, Coull BA, O’Reilly ÉJ, Ascherio A, Weisskopf MG. Association of exposure to diethylstilbestrol during pregnancy with multigenerational neurodevelopmental deficits. JAMA Pediatr. 2018;172(7):670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today. 2013;99(2):134-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122(10):778-788. [DOI] [PubMed] [Google Scholar]
- 545. Vessey MP, Fairweather DV, Norman-Smith B, Buckley J. A randomized double-blind controlled trial of the value of stilboestrol therapy in pregnancy: long-term follow-up of mothers and their offspring. Br J Obstet Gynaecol. 1983;90(11):1007-1017. [DOI] [PubMed] [Google Scholar]
- 546. Nilsson EE, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of reproductive disease. Biol Reprod. 2015;93(6):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547. Walker DM, Gore AC. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol. 2011;7(4):197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549. Rashid H, Alqahtani SS, Alshahrani S. Diet: a source of endocrine disruptors. Endocr Metab Immune Disord Drug Targets. 2020;20(5):633-645. [DOI] [PubMed] [Google Scholar]
- 550. Connolly L. Endocrine-disrupting chemicals: origins, fates and transmission into the food chain. In: Shaw I, ed. Endocrine-Disrupting Chemicals in Food. 1st ed. Woodhead Publishing, Oxford, United Kingdom; 2009:103-125. [Google Scholar]
- 551. Schecter A, Päpke O, Tung KC, Staskal D, Birnbaum L. Polybrominated diphenyl ethers contamination of United States food. Environ Sci Technol. 2004;38(20):5306-5311. [DOI] [PubMed] [Google Scholar]
- 552. North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. BJU Int. 2000;85(1):107-113. [DOI] [PubMed] [Google Scholar]
- 553. Baldi F, Mantovani A. A new database for food safety: EDID (Endocrine disrupting chemicals—Diet Interaction Database). Ann Ist Super Sanita. 2008;44(1):57-63. [DOI] [PubMed] [Google Scholar]
- 554. Hofe CR, Feng L, Zephyr D, Stromberg AJ, Hennig B, Gaetke LM. Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicts reduced probability of polychlorinated biphenyl-associated risk for type 2 diabetes: National Health and Nutrition Examination Survey 2003-2004. Nutr Res. 2014;34(4):285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555. Majkova Z, Oesterling E, Toborek M, Hennig B. Impact of nutrition on PCB toxicity. Environ Toxicol Pharmacol. 2008;25(2):192-196. [DOI] [PubMed] [Google Scholar]
- 556. Watkins BA, Hannon K, Ferruzzi M, Li Y. Dietary PUFA and flavonoids as deterrents for environmental pollutants. J Nutr Biochem. 2007;18(3):196-205. [DOI] [PubMed] [Google Scholar]
- 557. Gonzalez-Bulnes A, Astiz S, Isabel B, Vazquez-Gomez M, Garcia-Contreras C. Chapter 19: possible benefits and risks of polyphenols supplementation during pregnancy. In: Watson RR, Preedy VR, Zibadi S, eds. Polyphenols: Mechanisms of Action in Human Health and Disease. 2nd ed. Academic Press, London, United Kingdom; 2018:249-260. [Google Scholar]
- 558. Colacino JA, Arthur AE, Ferguson KK, Rozek LS. Dietary antioxidant and anti-inflammatory intake modifies the effect of cadmium exposure on markers of systemic inflammation and oxidative stress. Environ Res. 2014;131:6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559. Chavarro JE, Mínguez-Alarcón L, Chiu YH, et al. ; EARTH Study Team . Soy intake modifies the relation between urinary bisphenol A concentrations and pregnancy outcomes among women undergoing assisted reproduction. J Clin Endocrinol Metab. 2016;101(3):1082-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Ormond G, Nieuwenhuijsen MJ, Nelson P, et al. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect. 2009;117(2):303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Gaskins AJ, Mínguez-Alarcón L, Fong KC, et al. Supplemental folate and the relationship between traffic-related air pollution and livebirth among women undergoing assisted reproduction. Am J Epidemiol. 2019;188(9):1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056-13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563. Frazier T, Hogue CJR, Bonney EA, Yount KM, Pearce BD. Weathering the storm; a review of pre-pregnancy stress and risk of spontaneous abortion. Psychoneuroendocrinology. 2018;92:142-154. [DOI] [PubMed] [Google Scholar]
- 564. Mutambudzi M, Meyer JD, Reisine S, Warren N. A review of recent literature on materialist and psychosocial models for racial and ethnic disparities in birth outcomes in the US, 2000-2014. Ethn Health. 2017;22(3):311-332. [DOI] [PubMed] [Google Scholar]
- 565. Shapiro GD, Fraser WD, Frasch MG, Séguin JR. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med. 2013;41(6):631-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566. Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Aff (Millwood). 2011;30(5):879-887. [DOI] [PubMed] [Google Scholar]
- 567. Solomon GM, Morello-Frosch R, Zeise L, Faust JB. Cumulative environmental impacts: science and policy to protect communities. Annu Rev Public Health. 2016;37:83-96. [DOI] [PubMed] [Google Scholar]
- 568. Vesterinen HM, Morello-Frosch R, Sen S, Zeise L, Woodruff TJ. Cumulative effects of prenatal-exposure to exogenous chemicals and psychosocial stress on fetal growth: systematic-review of the human and animal evidence. Plos One. 2017;12(7):e0176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569. Aker A, McConnell RER, Loch-Caruso R, et al. Interactions between chemicals and non-chemical stressors: the modifying effect of life events on the association between triclocarban, phenols and parabens with gestational length in a Puerto Rican cohort. Sci Total Environ. 2020;708:134719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. 2017;14(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Zhang J, Yin W, Li P, et al. Interaction between diet- and exercise-lifestyle and phthalates exposure on sex hormone levels. J Hazard Mater. 2019;369:290-298. [DOI] [PubMed] [Google Scholar]
- 572. Centers for Disease Control and Prevention (CDC). Infant mortality and low birth weight among black and white infants—United States, 1980-2000. MMWR Morb Mortal Wkly Rep 2002;51:589-592. [PubMed] [Google Scholar]
- 573. Hessol NA, Fuentes-Afflick E. Ethnic differences in neonatal and postneonatal mortality. Pediatrics. 2005;115(1):e44-e51. [DOI] [PubMed] [Google Scholar]
- 574. Attina TM, Malits J, Naidu M, Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: an exploratory analysis. J Clin Epidemiol. 2019;108:34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576. Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30(1):2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Zota AR, Adamkiewicz G, Morello-Frosch RA. Are PBDEs an environmental equity concern? Exposure disparities by socioeconomic status. Environ Sci Technol. 2010;44(15):5691-5692. [DOI] [PubMed] [Google Scholar]
- 578. Boles RE, Johnson SL, Burdell A, Davies PL, Gavin WJ, Bellows LL. Home food availability and child intake among rural families identified to be at-risk for health disparities. Appetite. 2019;134:135-141. [DOI] [PubMed] [Google Scholar]
- 579. Cervi MM, Agurs-Collins T, Dwyer LA, Thai CL, Moser RP, Nebeling LC. susceptibility to food advertisements and sugar-sweetened beverage intake in non-Hispanic black and non-Hispanic white adolescents. J Community Health. 2017;42(4):748-756. [DOI] [PubMed] [Google Scholar]
- 580. Payne-Sturges D, Gee GC. National environmental health measures for minority and low-income populations: tracking social disparities in environmental health. Environ Res. 2006;102(2):154-171. [DOI] [PubMed] [Google Scholar]
- 581. Romieu I, Dossus L, Barquera S, et al. ; IARC working group on Energy Balance and Obesity . Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582. Genc MR, Schantz-Dunn J. The role of gene-environment interaction in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):491-504. [DOI] [PubMed] [Google Scholar]
- 583. Butler JM. Chapter 12: Single nucleotide polymorphisms and applications. In: Butler JM, ed. Advanced Topics in Forensic DNA Typing: Methodology. Academic Press; 2012:347-369. [Google Scholar]
- 584. Aguilar M, Alfaro S, Aguilar R. Radioguided localisation of non-palpable lesions of the breast in Costa Rica: review of results of our first 800 patients in private practice. Ecancermedicalscience. 2017;11:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585. Esposito G, Azhari A, Borelli JL. Gene × environment interaction in developmental disorders: where do we stand and what’s next? Front Psychol. 2018;9:2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586. Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885-908. [DOI] [PubMed] [Google Scholar]
- 587. Ames J, Warner M, Mocarelli P, et al. AHR gene-dioxin interactions and birthweight in the Seveso Second Generation Health Study. Int J Epidemiol. 2018;47(6):1992-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Scher DP, Alexander BH, Adgate JL, et al. Agreement of pesticide biomarkers between morning void and 24-h urine samples from farmers and their children. J Expo Sci Environ Epidemiol. 2007;17(4):350-357. [DOI] [PubMed] [Google Scholar]
- 589. Li Z, Romanoff LC, Lewin MD, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol. 2010;20(6):526-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590. Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119(7):983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591. Upmeier A, Degen GH, Diel P, Michna H, Bolt HM. Toxicokinetics of bisphenol A in female DA/Han rats after a single i.v. and oral administration. Arch Toxicol. 2000;74(8):431-436. [DOI] [PubMed] [Google Scholar]
- 592. Warren J, Fuentes M, Herring A, Langlois P. Spatial-temporal modeling of the association between air pollution exposure and preterm birth: identifying critical windows of exposure. Biometrics. 2012;68(4):1157-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593. Lee DH, Jacobs DR Jr. Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord. 2015;16(4):289-297. [DOI] [PubMed] [Google Scholar]
- 594. Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112(4):613-621. [DOI] [PubMed] [Google Scholar]
- 595. Beausoleil C, Ormsby JN, Gies A, et al. Low dose effects and non-monotonic dose responses for endocrine active chemicals: science to practice workshop: workshop summary. Chemosphere. 2013;93(6):847-856. [DOI] [PubMed] [Google Scholar]
- 596. Slob W, Pieters MN. A probabilistic approach for deriving acceptable human intake limits and human health risks from toxicological studies: general framework. Risk Anal. 1998;18(6):787-798. [DOI] [PubMed] [Google Scholar]
- 597. Even Chorev N, Testa G. Acting on uncertainty: real-life mixtures of endocrine disrupting chemicals. BioSocieties. Published online April 28, 2020. doi:10.1057/s41292-020-00192-7 [Google Scholar]
- 598. Frederiksen H, Nielsen JK, Mørck TA, et al. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Environ Health. 2013;216(6):772-783. [DOI] [PubMed] [Google Scholar]
- 599. Barrett JR. Reconciling sex-related bias: an alternative method for data analysis. Environ Health Perspect. 2017;125(10):104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600. Li X, Li A, Zhang W, et al. A pilot study of mothers and infants reveals fetal sex differences in the placental transfer efficiency of heavy metals. Ecotoxicol Environ Saf. 2019;186:109755. [DOI] [PubMed] [Google Scholar]
- 601. Rosenfeld CS. Sex-specific placental responses in fetal development. Endocrinology. 2015;156(10):3422-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602. Vilahur N, Bustamante M, Morales E, et al. Prenatal exposure to mixtures of xenoestrogens and genome-wide DNA methylation in human placenta. Epigenomics. 2016;8(1):43-54. [DOI] [PubMed] [Google Scholar]
- 603. Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health. 2016;15(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605. Yoshida R, Fukami M, Sasagawa I, Hasegawa T, Kamatani N, Ogata T. Association of cryptorchidism with a specific haplotype of the estrogen receptor alpha gene: implication for the susceptibility to estrogenic environmental endocrine disruptors. J Clin Endocrinol Metab. 2005;90(8):4716-4721. [DOI] [PubMed] [Google Scholar]
- 606. Lim JS, Son HK, Park SK, Jacobs DR Jr, Lee DH. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int J Obes (Lond). 2011;35(5):744-747. [DOI] [PubMed] [Google Scholar]
- 607. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017; 66:1. [PubMed] [Google Scholar]
- 608. de Angelis P, Miller RK, Darrah TH, et al. Elemental content of the placenta: A comparison between two high-risk obstetrical populations, adult women carrying multiples and adolescents carrying singletons. Environ Res. 2017;158:553-565. [DOI] [PubMed] [Google Scholar]
- 609. Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction. 2010;140(6):803-813. [DOI] [PubMed] [Google Scholar]
- 610. Zhao Y, Ruan X, Li Y, Yan M, Qin Z. Polybrominated diphenyl ethers (PBDEs) in aborted human fetuses and placental transfer during the first trimester of pregnancy. Environ Sci Technol. 2013;47(11):5939-5946. [DOI] [PubMed] [Google Scholar]
- 611. Horii M, Touma O, Bui T, Parast MM. Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction. 2020;160(1):R1-R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612. Morales-Suárez-Varela MM, Toft GV, Jensen MS, et al. Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: a study in the Danish National Birth Cohort study. Environ Health. 2011;10(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. Carignan CC, Mínguez-Alarcón L, Williams PL, et al. ; EARTH Study Team . Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environ Int. 2018;111:232-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614. Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GM. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ Health. 2015;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Ning M, Lo EH. Opportunities and challenges in omics. Transl Stroke Res. 2010;1(4):233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616. Ackerman WE IV, Adamson L, Carter AM, et al. IFPA Meeting 2013 Workshop Report II: use of “omics” in understanding placental development, bioinformatics tools for gene expression analysis, planning and coordination of a placenta research network, placental imaging, evolutionary approaches to understanding pre-eclampsia. Placenta. 2014;35(Suppl):S10-S14. [DOI] [PubMed] [Google Scholar]
- 617. Kedia K, Smith SF, Wright AH, et al. Global “omics” evaluation of human placental responses to preeclamptic conditions. Am J Obstet Gynecol. 2016;215(2):238.e1-238.e20. [DOI] [PubMed] [Google Scholar]
- 618. Rouillon S, Deshayes-Morgand C, Enjalbert L, et al. Endocrine disruptors and pregnancy: knowledge, attitudes and prevention behaviors of french women. Int J Environ Res Public Health. 2017;14(9):1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619. Ferrari RM, Siega-Riz AM, Evenson KR, Moos MK, Carrier KS. A qualitative study of women’s perceptions of provider advice about diet and physical activity during pregnancy. Patient Educ Couns. 2013;91(3):372-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620. Lang C, Fisher M, Neisa A, et al. Personal care product use in pregnancy and the postpartum period: implications for exposure assessment. Int J Environ Res Public Health. 2016;13(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621. Sathyanarayana S, Focareta J, Dailey T, Buchanan S. Environmental exposures: how to counsel preconception and prenatal patients in the clinical setting. Am J Obstet Gynecol. 2012;207(6):463-470. [DOI] [PubMed] [Google Scholar]
- 622. Altman N, Krzywinski M. Association, correlation and causation. Nat Methods. 2015;12(10):899-900. [DOI] [PubMed] [Google Scholar]
- 623. Maserejian NN, Trachtenberg FL, Hauser R, McKinlay S, Shrader P, Bellinger DC. Dental composite restorations and neuropsychological development in children: treatment level analysis from a randomized clinical trial. Neurotoxicology. 2012;33(5):1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624. Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2004; 82:145-148, quiz 265 [DOI] [PubMed] [Google Scholar]
- 625. Hernandez LM, Blazer DG, eds. Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate. National Academies Press,Washington (DC); 2006. [PubMed] [Google Scholar]
- 626. Denayer T, Stöhr T, Van Roy M. Animal models in translational medicine: Validation and prediction. New Horizons in Translational Medicine. 2014;2(1):5-11. [Google Scholar]
- 627. Barakat R, Seymore T, Lin PP, Park CJ, Ko CJ. Prenatal exposure to an environmentally relevant phthalate mixture disrupts testicular steroidogenesis in adult male mice. Environ Res. 2019;172:194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628. Zhou C, Gao L, Flaws JA. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol. 2017;318:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629. McTavish K, Stech H, Stay F. A modeling framework for exploring the population-level effects of endocrine disruptors. Environmental Toxicology and Chemistry. 1998; 17:58-67. [Google Scholar]
- 630. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. [DOI] [PubMed] [Google Scholar]
- 631. Bellavia A, James-Todd T, Williams PL. Approaches for incorporating environmental mixtures as mediators in mediation analysis. Environ Int. 2019;123:368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632. National Academies of Sciences, Engineering, and Medicine. Leveraging Artificial Intelligence and Machine Learning to Advance Environmental Health Research and Decisions: Proceedings of a Workshop—in Brief. National Academies Press; 2019. [Google Scholar]
- 633. Wittwehr C, Blomstedt P, Gosling JP, et al. Artificial Intelligence for chemical risk assessment. Comput Toxicol. 2020;13:100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634. Kassotis CD, Vandenberg LN, Demeneix BA, Porta M, Slama R, Trasande L. Endocrine-disrupting chemicals: economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020;8(8):719-730. [DOI] [PMC free article] [PubMed] [Google Scholar]