Abstract
The first cases of coronavirus disease 2019 (COVID-19) were detected in Wuhan, China, in December 2019. Since this time a concerted global effort of research and observational data gathering has meant that a great deal has been learnt about the impact of COVID-19 in patients with lymphoid malignancies. Approximately one-third of patients with lymphoid malignancies who acquire COVID-19 and have it severely enough to require hospital assessment will die from this infection. Major risk factors for a poor outcome are age and co-morbidities, but when these are taken into account lymphoma patients have a slightly greater than 2-fold increased risk compared to the general population. Notably, despite early concerns regarding the particular vulnerability of lymphoma patients due to the immunosuppressive effects of therapy, active treatment, including B-cell depleting agents such as rituximab, do not appear to be associated with an increased risk of a poorer outcome. Indeed, some treatments such as ibrutinib may be beneficial due to their modulation of the potential fatal hyperinflammatory phase of infection. There are risks associated with hemopoietic stem cell transplantation, but the collective experience is that these can be minimized by preventive strategies and that the majority of transplant recipients with COVID-19 infection will survive. Many questions remain including those regarding the outcome of COVID-19 infection in the rarer lymphoid malignancies and the efficacy of COVID-19 vaccines in lymphoma patients. This review aims to discuss these issues and present a summary of the current knowledge of the impact of COVID-19 in lymphoid malignancies.
Keywords: COVID-19, Lymphoma, Leukemia, Chemoimmunotherapy, Hemopoietic stem cell transplantation, Vaccination
Core Tip: Patients with lymphoid malignancies who have coronavirus disease 2019 (COVID-19) severely enough to require hospital assessment have an approximately one-third chance of dying from the infection, representing a slightly greater than 2-fold increased risk compared to the general population. Despite initial concerns, treatment for lymphoma is not associated with increased risk for poor outcome. Current evidence for the efficacy of COVID-19 vaccines in patients with lymphoid malignancies is extremely limited, so it will be crucial to conduct studies to address this issue over the coming months.
INTRODUCTION
The first cases of coronavirus disease 2019 (COVID-19) were detected in Wuhan, China, in December 2019. The disease, caused by a novel RNA beta coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was initially reported as predominantly causing a pulmonary syndrome, typified by fevers in combination with breathlessness and cough[1]. However, it is now appreciated that COVID-19 can cause a wide range of symptoms of variable severity, including fatigue, myalgia, headache, anosmia, pharyngitis, coryza, nausea and diarrhoea[2]. Since initial detection of the virus, more than 130 million cases of COVID-19 have been confirmed worldwide, with more than 2.8 million deaths[3]. Initial reports from China have indicated that COVID-19 has an overall mortality rate of 1.4%. However, the prognosis varies widely between groups, with those people over the age of 60 years and those with underlying conditions, including hypertension, diabetes, cardiovascular disease, chronic respiratory disease and cancer, at a significantly higher risk for severe disease and death[4].
There has been a great deal of concern that patients with lymphoid malignancies such as lymphomas and lymphoid leukemias would be at particular risk from COVID-19. The initial reports from China showed that patients with cancer were over-represented among individuals who developed severe COVID-19 after contracting the virus[5]. Patients with lymphoid malignancies could be expected to be at increased risk of adverse outcomes from this viral infection, both due to being immuno-compromised as a consequence of the underlying cancer, and due to the myelosuppressive and lymphodepleting effects of therapy. A number of retrospective studies have reported outcomes of patients with lymphoid malignancies who became infected with SARS-CoV-2 during or shortly after treatment[6-21]. These were pooled into a large meta-analysis of 3377 patients with hematological malignancies who developed COVID-19 with a primary outcome of risk of death[22]. Among all blood cancers the overall risk of death was 34%, rising to 39% when combining data for hospitalized patients. Within this the pooled risk of death was also calculated by hematologic malignancy subtype with lymphomas including/excluding chronic lymphocytic leukemia (CLL) having a risk of death of 32%, with CLL specifically having a risk of 31%. This was comparable to myeloproliferative neoplasms (34%) and plasma cell dyscrasias (33%), but somewhat less than acute leukemias (41%) and acquired bone marrow failure syndromes (53%). Notably the primary risk factor for COVID-19 mortality was age with patients aged 60 years and older having a significantly higher risk of death than patients under 60 years. While these “headline” figures are rather high, one of the major limitations of these retrospective studies was that almost all of them focused on patients who were either assessed in hospital, or were actually hospitalized for their COVID-19. Invariably, these patients had more severe infections than those who remained at home, who were not necessarily detected and included in these studies, making these mortality statistics an over-estimation. Ascertaining the true mortality rates remains challenging and governments around the world continue to advise patients with mild COVID-19 symptoms to self-isolate at home. At the time of our own study the United Kingdom was focused on hospital-based testing for suspected COVID-19, representing a comparable group of patients to the meta-analysis[23]. This allowed an estimation of a crude case fatality rate of 14% suggesting that blood cancer patients have a 2-2.5 -fold greater risk of dying from COVID-19 than the general population. The largest single study to date also likely has the best estimate of true population mortality risk from COVID-19 for hematological cancer patients as they used population-based data from a countrywide Ministry of Health database[18]. This reported a risk of death 14%, which was twice that of a control population in their study (7%) and was comparable to the estimated risk of death of 13% in patients with all cancers[24]. A further study from Italy of 536 patients with hematologic malignancies and COVID-19 reported a mortality rate 37%, with a standardized mortality ratio for of 2.04 increased risk when compared with the impact of COVID-19 in the general Italian population[13]. Taken together, these studies have fairly consistently demonstrated that approximately one-third of patients with hematological malignancies who acquire COVID-19 and have it severely enough to require hospital assessment and/or admission will die from this infection. The major risk factors are age and co-morbidities, but when these are taken into account patients with blood cancers have a slightly greater than 2-fold increased risk compared to the general population.
IMPACT OF COVID-19 BY LYMPHOMA SUBTYPE
Many of the larger studies have pooled all patients with hematological cancers together. While this is useful, clearly there is very significant heterogeneity within this group of diseases, in respect of pathophysiology, clinical characteristics, and the type and intensity of treatment. Therefore, studies which have included patients with a single disease/disease group can give more “granularity” and aid physicians in informing their patents. At the time of writing, the lymphoid malignancy with the most data in this regard is CLL. Patients with this leukemia could be hypothesized to be particularly vulnerable to SARS-CoV-2 infection. This is due to the fact that CLL is frequently accompanied by an immunodeficiency which can be further aggravated by therapy, and also that it typically effects older adults (median age at diagnosis 70 years) who are higher risk due to their age[25,26]. A number of studies have now looked at the impact of COVID-19 in CLL patients specifically. Perhaps, due to the geography of the pandemic one of the first reports was from an Italian group who assessed 47 symptomatic CLL patients were found to be positive for COVID-19[27]. Of the 46 evaluable patients, 14 died, equating to a morality rate of 30.4%. The median age of these patients was 75 years, meaning that the mortality rate of this group was only a little higher than the mortality rate of 25.5% in 70-79-year-olds in the general Italian population at the same time. The European Research Initiative on CLL group reported outcomes of 190 CLL patients who presented in the first wave of the pandemic. 151 (79%) presented with severe COVID-19 (requiring oxygen and/or intensive care admission) which was associated with more advanced age (≥ 65 years) with a mortality rate of 36.4%[15]. Mato et al[12] reported data from a further international (predominantly United States) multi-center cohort of 198 patients. This again revealed a relatively high rate of severe disease and hospital admissions with an overall case fatality rate of 33%. This rose to 37% in those requiring admission, a remarkably similar figure to the other study. Across these two major studies the main risk factors were mainly those already known for COVID-19 itself: age and co-morbidities. Interestingly, hypogammaglobulinemia, a marker of the CLL-associated immunodeficiency, did not impact upon the outcome. It could be hypothesized that the immune defect associated with this defect could be a “double-edged” sword. On one hand, a weakened immune system may not be as capable of eliminating SARS-CoV-2, yet on the other, it might help to prevent a fatal immune and inflammatory over-reaction[28].
They have been a few reports of the outcomes of COVID-19 more specifically in patients with lymphoma. A study by Lamure et al[29] investigated the outcomes of 89 patients, the majority of whom had recently treated (within the last year) B-cell non-Hodgkin lymphoma. With a median follow-up of 33 d from admission, 30-d overall survival was 71%, with age ≥ 70 years and relapsed/refractory lymphoma being risk factors for a poorer outcome in a multivariate analysis. They did not see any differences in outcomes of patients with B-cell vs T-cell lymphomas, but they only included 7 patients in the latter group. Recent bendamustine treatment was also identified as a potential risk factor. However, the numbers of patients were few and this characteristic was strongly associated with (and probably confounded by) relapsed/refractory lymphoma. Notably they concluded that survival of patients younger than 70 years without relapsed/refractory lymphoma was comparable to that of the general population[29]. A further Spanish study reported on 177 patients, 89% of who had non-Hodgkin lymphoma. The overall mortality rate was 34.5%, with age > 70 years, heart disease, chronic kidney disease, CURB-65 score ≥ 2 and active disease significantly increasing the risk of death in a multivariate analysis. Interestingly they did also note that the persistence of a positive polymerase chain reaction for SARS-CoV-2 after week 6 was significantly associated with mortality, suggesting that longer term viral suppression is an important component of recovery[30].
Not unexpectantly current published data is limited to small case series and case reports when it comes to the rarer forms of lymphoma. A Parisian study reported outcomes for 13 patients with primary central nervous system lymphoma. The mortality rate was 23% in this group, 11 (85%) of whom were undergoing chemotherapy at the time of infection. Two additional patients (15%) required mechanical ventilation, but two patients (15%) had no COVID-19 symptoms. A medical history of diabetes mellitus was more common in patients with severe disease. Chemotherapy was resumed after COVID-19 recovery in nine patients (69%) after a median delay of 16 d with no unusual chemotherapy complications nor incidents of SARS-CoV-2 reactivation[31]. Gonzaga et al[32] reported on the outcome of 2 patients with Sezary syndrome who acquired COVID-19. Unfortunately, both patients died, one attributable to COVID-19 and the other due to progressive disease. In contrast another patient who was receiving treatment for lymphoma type adult T-cell leukemia-lymphoma recovered after developing severe COVID-19 pneumonia with favipiravir therapy. Interestingly, there have also been a few reports of COVID-19 being beneficial to lymphoma patients, presumably due to an “immunostimulatory effect”. Challenor and Tucker[33] reported the case of a 61-year-old man who went into remission after SARS-CoV-2 infection without treatment. Sollini et al[34] also report a case of a patient with follicular lymphoma, who having achieved a partial remission after bendamustine-based therapy, went onto achieve a complete remission after asymptomatic COVID-19. In addition, Pasin et al[35] report an interesting case of a patient with natural killer (NK)/T-cell lymphoma who having been refractory to previous immuno-chemotherapy, subsequently developed a transient remission at the time if SARS-CoV-2 infection. As NK cells express angiotensin converting enzyme 2, the binding site for this virus, they hypothesize that a direct oncolytic effect of the virus combined with production of proinflammatory cytokines led to NK-cell apoptosis, something seen with other RNA viruses. Clearly, more data needs to be collected on these and other types of lymphoid malignancies, something that will almost certainly occur as the pandemic progresses.
INTERACTION OF COVID-19 AND TREATMENT OF LYMPHOMA
While a large part of this involves the management of bacterial infections, particularly in the context of concurrent neutropenia, infection with and re-activation of viruses are also a feature of the clinical course of many lymphoma patients on treatment. Prolonged symptoms from seasonal “flu” and “cold” viruses and reactivation of viruses such as hepatitis B and varicella zoster are common complications of treatment, particularly after depletion of the B-cell compartment with anti-CD20 monoclonal antibodies such as rituximab. Given that most effective lymphoma therapies are also lymphodepleting it could be expected that anti-lymphoma drugs would compromise the normal immune response to SARS-CoV-2 leading to prolonged and more severe infection. However, even in the early stages of the pandemic it was clear that this was not so straightforward. The infection typically begins with relatively mild symptoms, which if the infection is not controlled, then can become more severe at around day 10 associated with a cytokine-induced inflammatory storm as the “adaptive” immune response takes off. Therefore, it could also be hypothesized that the immunosuppressive effect of many lymphoma treatments could actually be beneficial at this stage by limiting this hyperinflammation, thereby avoiding severe pneumonitis and thrombotic sequelae. In light of this, a number of guidelines, consensus statements and recommendations regarding the management of lymphoma(s) were published at the start of the pandemic[36-43]. They invariably recommended a common-sense approach. Patients with aggressive lymphoma were to be treated as usual, while minimizing time in the hospital by use of measures including the wider use of granulocyte colony stimulating factor prophylaxis and subcutaneous administration of rituximab. In contrast, the advice for patients with more indolent lymphomas was to continue expectant management where possible and to use oral regimens where reasonable. In all cases virtual consultations were to be encouraged, particularly for patients in complete remission or for those in which no immediate change in therapy was expected. However, there was a clear concern that patients with lymphoid malignancies were going to be at particular risk from COVID-19 due to the combined immunosuppression from their underlying disease and its treatment.
Interestingly, multiple studies have consistently reported little or no negative impact of therapy on outcomes from COVID-19. The large meta-analysis of over 3000 patients with hematological cancers showed no association of poorer outcome with concurrent treatment, as have many smaller studies[17,22]. Similarly, in the two largest lymphoma-specific COVID-19 studies, there was no association of active treatment with poor outcome[29,30]. In particular there was no excess mortality identified with anti-CD20 treatment despite the anticipated risk of depleting the B-cell compartment and inhibiting humoral immunity. While, there have been several reports of prolonged viral shedding and/or pneumonia symptoms, and failure of SARS-CoV-2 antibody responses in patients treated with rituximab, this has not translated into a significant impact on survival in the larger studies[44-46]. It is possible that modulation of the “hyperinflammatory” phase of COVID-19 is playing a role; it is also possible that the relative sparing of T-cell responses may be enough to control the virus. As a consequence, most expert bodies are recommending continuing treat lymphoid malignancies as usual whilst highlighting the importance of a risk-benefit analysis in each individual patient scenario. While there does not appear to be any additional risk from treatment per se, COVID-19 does pose a significant risk to lymphoma patients in itself, particularly those who are older with multiple co-morbidities. Therefore, infection with SARS-CoV-2 needs to be avoided in lymphoma patients who should generally be regarded as clinically vulnerable and advised to “shield”. Visits to hospital (and hence potential exposure to the virus) should be reduced by choosing oral regimens over infusional ones where possible (e.g., ibrutinib or acalabrutinib for the treatment for CLL) and avoiding treatments with marginal benefit (e.g., maintenance rituximab for follicular lymphoma), particularly when COVID-19 infection rates in the general population are high.
There has been particular focus regarding the potential of ibrutinib as a potential immuno-modulator of COVID-19. Ibrutinib is used for the treatment of several B-cell disorders, including CLL, mantle cell lymphoma and Waldenstrom macroglobulinemia (WM)[47]. In addition to its inhibition of B-cell receptor signaling by Burton's tyrosine kinase (BTK) it is also known to inhibit interleukin-2 inducible T-cell kinase (ITK) modulating T-cell responses[48]. There were early reports of ibrutinib potentially having a beneficial effect in SARS-CoV-2 infection, protecting against pulmonary injury, both in the context of treatment for CLL and WM[49,50]. The effect has been hypothesized to be due not only to “off-target” inhibition of ITK, but also of inhibition of Src family kinases and attenuation of M1 macrophage polarization with the net effect of reducing viral entry and inflammatory cytokine responses in the lungs[51,52]. Whether or not the anti-platelet effect of ibrutinib could also help combat the pro-thrombotic events associated with severe COVID-19 has not been explored. Interestingly, a small clinical study has suggested that BTK inhibition could be the most important component of ibrutinib’s immunomodulatory activity. Roschewski et al[53] assessed the efficacy of 19 patients without hematological malignancies who were hospitalized with severe COVID-19 (11 on supplemental oxygen and 8 on mechanical ventilation), 18 of whom had increasing oxygen requirements at baseline. Acalabrutinib is a more selective inhibitor of BTK and should not have any effect on ITK and Src kinases. Analysis revealed a rapid normalization of inflammatory markers such as C-reactive protein and interleukin-6 with a temporal correlation with improved oxygenation. These results suggested that targeting excessive host inflammation with a BTK inhibitor is a therapeutic strategy in severe COVID-19 and has led to an ongoing international prospective randomized controlled clinical trial. A protective effect of BTK inhibition was also observed in the European study of outcomes of CLL patients with SAR-CoV-2 infection, with lower rates of hospitalization rate for severe COVID-19 for patients on ibrutinib vs those on other regimens or off treatment[15]. However, an effect was not seen in the Mato et al[12] report, although in many cases therapy was withheld once COVID-19 was diagnosed. Again, further work is required to investigate this, but it would seem reasonable to continue BTK inhibitors in patients who are diagnosed with COVID-19 on the basis of the available evidence. Certainly, discontinuation of effective anti-lymphoma therapy has its own risks, particularly in patients with more aggressive lymphoma subtypes, as exemplified by a report of patient who developed rapid progression of their mantle cell lymphoma after ibrutinib was discontinued for intercurrent COVID-19[54].
Further questions remain around the use of other immunomodulatory drugs for lymphoid malignancies in the context of COVID-19. Immune checkpoint blockade with drugs targeting programmed cell death 1 and other immuno-inhibitory molecules is widely used in the solid cancer field where they “release the brakes” of immune tolerance mechanisms leading to effective anti-tumor responses[55]. These agents are less commonly used in lymphoma where the main indications are in relapsed Hodgkin lymphoma and Richter syndrome. Again, the potential impact of immune checkpoint blockade in patients with COVID-19 could be hypothesized to be double-edged, with these agents potentially enhancing immunological control of the viral infection, yet also contributing to inflammation and aggravating the clinical course of COVID-19. Reports of these drugs in lymphoma are currently limited to a single case report. O’Kelly et al[56] report a case of a 22 year-old female with multiply relapsed Hodgkin lymphoma having pembrolizumab who developed severe COVID-19 requiring high levels of oxygen supplementation but not intubation, who subsequently recovered. A recently published study of 35 patients receiving immune checkpoint blockade in solid cancers concluded that COVID-19 related mortality in this population did not appear to be higher than previously published mortality rates for patients with cancer suggesting that this type of treatment does not increase the risk[57]. Another class of anti-lymphoma drugs that could be hypothesized to have an impact on the course of COVID-19 are the immunomodulatory imide drugs such as thalidomide and lenalidomide. While being used most commonly in the treatment of multiple myeloma, lenalidomide is well known to have activity in lymphomas including follicular lymphoma, mantle cell lymphoma and CLL[58,59]. At the time of writing the reports of the impact of these drugs on COVID-19 outcomes in myeloma patients remain equivocal; there are no reports of the outcome of COVID-19 with intercurrent use of these drugs in lymphoma. The potential mechanisms by which treatments for lymphoma may modulate COVID-19 infection is summarized in Figure 1.
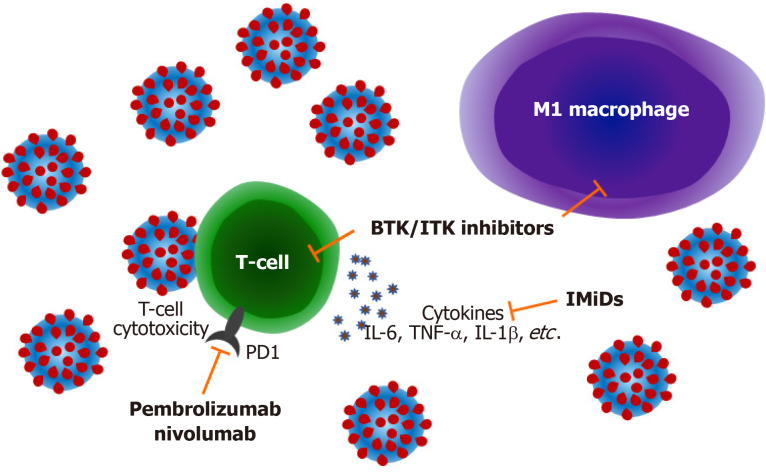
Figure 1.
Mechanisms by which lymphoma treatments may modulate coronavirus disease 2019 infection. Inhibition of Burton's tyrosine kinase and interleukin-2 inducible T-cell kinase modulates T-cell immune responses decreasing production of pro-inflammatory cytokines such as interleukin-6, tumour necrosis factor α and interleukin-1b and also attenuating M1 macrophage polarization reducing pulmonary inflammation. Immune checkpoint blockade with drugs targeting programmed cell death 1 may improve antiviral cytotoxic T-cell responses. Immunomodulatory imide drugs can also block cytokine responses and improve T-cell function. BTK: Burton's tyrosine kinase; ITK: Interleukin-2 inducible T-cell kinase; PD1: Programmed cell death 1; IMiDs: Immunomodulatory imide drugs; IL: Interleukin; TNF-α: Tumour necrosis factor α.
A discussion of the general principles of managing severe COVID-19 in lymphoid malignancies is beyond the scope of this review. However, one aspect that might be expected to be specifically relevant to these cancers is the use of convalescent plasma to treat COVID-19, given the hypogammaglobulinemia that frequently observed, particularly in CLL. As intravenous immunoglobulin replacement is indicated to prevent infections in these patients, it is reasonable to hypothesize that plasma containing anti-SARS-CoV-2 antibodies might be of particular benefit in these patient groups. Several studies have now looked at the efficacy of convalescent plasma in the general population. Initial randomized trials of convalescent plasma in patients with COVID-19 focused on hospitalized patients who were already moderately to severely ill, with these trials providing little evidence of clinical efficacy[60,61]. Subsequent observational studies have been more positive but generally the clinical benefits have been modest[62]. However, a recent randomized study has suggested that this “passive immunotherapy” can be effective if the right plasma is used for the right patients, with early administration of high-titer convalescent plasma against SARS-CoV-2 to mildly affected older adults reducing the progression of COVID-19[63]. While there have been no randomised studies investigating the use of convalescent plasma in patients with lymphoid malignancies, there have been several case reports and observational case series reporting efficacy in this patient group[64-70]. As a consequence, it seems reasonable to use convalescent plasma for high risk individuals in this patient group as long as the plasma contains high titers of SARS-CoV-2 antibodies and is given early enough in the patient’s course of infection.
IMPACT OF COVID-19 ON HEMOPOIETIC STEM CELL TRANSPLANTATION OF LYMPHOMA
High-dose chemotherapy with autologous hemopoietic stem cell transplantation (HSCT) represents a standard of care for many lymphoid malignancies, with allogeneic HSCT being potentially curative for other particular indications. Both types of transplantation are scenarios where COVID-19 infection could be expected to lead to particularly severe consequences, given the state of immune suppression that they induce. As a consequence, transplant organizations such as the European Society for Blood and Marrow Transplantation (EBMT) have been regularly issuing and updating recommendations regarding all aspects of transplantation during the pandemic[71]. The EBMT has been collecting data regarding the impact of COVID-19 on HSCT recipients and also those undergoing treatment with chimeric antigen receptor (CAR) T cells. While the 6-wk mortality in this patient group in the 1st wave was approximately 25%, preliminary data from the 2nd wave (August to December 2020) suggests a mortality rate slightly below 20%. This figure is not too dissimilar to that published by the group at the Memorial Sloan Kettering Cancer Center who observed that 22% of patients who had received cellular therapy (Allogeneic, 35; Auto, 37; CAR T, 5) had died after 30 d[72]. Notably the largest study published to-date did not observe any differences in 30-d overall survival when comparing recipients of allogeneic vs autologous HSCT[73]. Despite the theoretical risks associated with the procedure itself, the very nature of determining an individual’s eligibility for transplant typically excludes those at higher risk from COVID-19, which probably explains why these figures are lower than the fatality rates seen for patients with hematological malignancies outside the transplant setting. Many of the recommendations focus on avoiding SARS-CoV-2 infection by limiting risk of exposure to infected individuals as much as possible and strictly adherence to prevention practices such as hand hygiene and social distancing—something that applies to the donor as well as the recipient in allogeneic transplants[74]. The challenging question is what to do in patients that develop COVID-19 during preparation for transplantation? This includes those that acquire COVID-19 immediately before transplantation and those that develop and recover but have a persistently positive polymerase chain reaction test. Generally, the decision to proceed has to be assessed on a case-by-case basis weighing in the risks from COVID-19 infection vs the risks from delaying the transplant. The grade of lymphoid malignant (indolent vs aggressive) and availability of alternative salvage therapy will clearly play into these decisions. In addition to ongoing data collection by the bone marrow transplant registries there are now several published case reports and case series of patients successfully completing a bone marrow transplant despite intercurrent SARS-CoV-2 infection, including one report where all 11 patients survived without oxygen supplementation or mechanical ventilation[72,73,75-78]. Despite this, risks for lymphoma patients remain, with one study reporting a higher risk of mortality in autologous HSCT recipients when the indication was for lymphoma compared to myeloma—likely reflecting the increased intensity of the multi-agent high-dose chemotherapy used in lymphoma autograft conditioning[73]. Other potential factors identified as being predictive of poorer outcomes in HSCT include older age, being on steroids at the time of diagnosis of COVID-19, and COVID-19 infection within 1 year of HSCT[16].
IMPACT OF LYMPHOMA ON VACCINATION FOR COVID-19
The enormous societal and economic impact of the pandemic made it a global emergency to develop effective vaccines. In a testament to human ingenuity the first SAR-CoV-2 vaccine trails were being reported less than a year after the virus was initially identified[79-81]. A number of vaccines are in production with efficacy against laboratory-confirmed infection typically greater than 90%. Not surprisingly, the trials have excluded patients on treatment with immunosuppressive therapy or those diagnosis with an immunocompromising condition, which includes all patients with lymphoid malignancies. Therefore, at the time of writing there is no data on the efficacy of any of the leading SARS-CoV-2 vaccines in patients with lymphoid malignancies. As discussed above patients with these cancers could be expected to fail to mount an immune response to these vaccines. This is due both to the immune defects associated with the diseases themselves and also due to the impact of treatments. While little is known about the efficacy of COVID-19 vaccines in lymphoma patients, plenty of studies have demonstrated reduced rates of sero-conversion in patients vaccinated for other viruses in the past. Furthermore, one-third of CLL patients who had COVID-19 failed to mount a persistent antibody response in one study[69]. Therefore, it will be vital to design studies to assess their efficacy in patients with lymphoid malignancies, as even if current vaccines achieve the ideal of “herd immunity”, the presence of SARS-CoV-2 mutant strains will likely mean that lymphoma patients still require direct protection[82]. A further consideration is perhaps the opposite problem. As vaccines are widely rolled-out some patients with lymphoid malignancies will receive one or more doses during therapy. We have seen several cases at our centre when vaccination results in an increase in glycolytic lymphadenopathy as part of the normal immune response, something that can mimic lymphoma progression on fludeoxyglucose positron emission tomography/computed tomography[83].
CONCLUSION
The COVID-19 pandemic has been a challenge for all sections of society across the world. Despite this, a great deal has been learnt about this virus in a very short space of time, including its impact in patients with hematological malignancies. Multiple studies have consistently demonstrated that approximately one-third of patients with blood cancers who acquire COVID-19 and have it severely enough to require hospital assessment will die, representing a slightly greater than 2-fold increased risk compared to the general population. Perhaps surprisingly, several studies have shown little or no negative impact of concurrent or recent anti-cancer therapy on outcomes from COVID-19, with reports of agents such as the BTK inhibitors actually having a protective effect. This is important as it means that treatment should be initiated and continued as required, rather than being delayed due to concerns regarding the risks from COVID-19. Instead, the focus needs to be stopping lymphoma patients from acquiring SARS-CoV-2 in the first place, by advising them to shield and taking steps to reduce hospital visits. However, a great deal still remains unknown about the impact of this infection in patients with lymphoid malignancies. Particular questions remain around the outcomes of COVID-19 in rarer lymphomas, and about the interaction between lymphoma-associated and treatment-induced immunosuppression and vaccine responses. While it can be anticipated that these gaps in our knowledge will start to become filled over the coming months, the presence of novel SARS-CoV-2 mutants will almost certainly mean that many years of work lie ahead.
Footnotes
Conflict-of-interest statement: The author has no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: March 9, 2021
First decision: April 6, 2021
Article in press: April 26, 2021
Specialty type: Virology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alberca RW, Cure E, de Melo FF S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. [cited 8 April 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aries JA, Davies JK, Auer RL, Hallam SL, Montoto S, Smith M, Sevillano B, Foggo V, Wrench B, Zegocki K, Agrawal S, Le Dieu R, Truelove E, Erblich T, Araf S, Okosun J, Oakervee H, Cavenagh JD, Gribben JG, Riches JC. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol. 2020;190:e64–e67. doi: 10.1111/bjh.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox TA, Troy-Barnes E, Kirkwood AA, Chan WY, Day JW, Chavda SJ, Kumar EA, David K, Tomkins O, Sanchez E, Scully M, Khwaja A, Lambert J, Singer M, Roddie C, Morris EC, Yong KL, Thomson KJ, Ardeshna KM. Clinical outcomes and risk factors for severe COVID-19 in patients with haematological disorders receiving chemo- or immunotherapy. Br J Haematol. 2020;191:194–206. doi: 10.1111/bjh.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnett C, Foldes D, Bailey C, Nesr G, Hui T, Hinton R, Gurung K, Phillips J, Saleem Z, Koshy R, Aduwa E, Colis M, Bimolah R, Katsomitrou V, Arami S, Kagdi H. Outcome of hospitalized patients with hematological malignancies and COVID-19 infection in a large urban healthcare trust in the United Kingdom. Leuk Lymphoma. 2021;62:469–472. doi: 10.1080/10428194.2020.1838506. [DOI] [PubMed] [Google Scholar]
- 9.Infante MS, González-Gascón Y Marín I, Muñoz-Novas C, Churruca J, Foncillas MÁ, Landete E, Marín K, Ryan P, Hernández-Rivas JÁ. COVID-19 in patients with hematological malignancies: A retrospective case series. Int J Lab Hematol. 2020;42:e256–e259. doi: 10.1111/ijlh.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lattenist R, Yildiz H, De Greef J, Bailly S, Yombi JC. COVID-19 in Adult Patients with Hematological Disease: Analysis of Clinical Characteristics and Outcomes. Indian J Hematol Blood Transfus. 2020:1–5. doi: 10.1007/s12288-020-01318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Moro F, Marquet J, Piris M, Michael BM, Sáez AJ, Corona M, Jiménez C, Astibia B, García I, Rodríguez E, García-Hoz C, Fortún-Abete J, Herrera P, López-Jiménez J. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190:e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, Patel K, Osterborg A, Wojenski D, Kamdar M, Huntington SF, Davids MS, Brown JR, Antic D, Jacobs R, Ahn IE, Pu J, Isaac KM, Barr PM, Ujjani CS, Geyer MB, Berman E, Zelenetz AD, Malakhov N, Furman RR, Koropsak M, Bailey N, Hanson L, Perini GF, Ma S, Ryan CE, Wiestner A, Portell CA, Shadman M, Chong EA, Brander DM, Sundaram S, Seddon AN, Seymour E, Patel M, Martinez-Calle N, Munir T, Walewska R, Broom A, Walter H, El-Sharkawi D, Parry H, Wilson MR, Patten PEM, Hernández-Rivas JÁ, Miras F, Fernández Escalada N, Ghione P, Nabhan C, Lebowitz S, Bhavsar E, López-Jiménez J, Naya D, Garcia-Marco JA, Skånland SS, Cordoba R, Eyre TA. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E, Krampera M, Cairoli R, Della Porta MG, Fracchiolla N, Ladetto M, Gambacorti Passerini C, Salvini M, Marchetti M, Lemoli R, Molteni A, Busca A, Cuneo A, Romano A, Giuliani N, Galimberti S, Corso A, Morotti A, Falini B, Billio A, Gherlinzoni F, Visani G, Tisi MC, Tafuri A, Tosi P, Lanza F, Massaia M, Turrini M, Ferrara F, Gurrieri C, Vallisa D, Martelli M, Derenzini E, Guarini A, Conconi A, Cuccaro A, Cudillo L, Russo D, Ciambelli F, Scattolin AM, Luppi M, Selleri C, Ortu La Barbera E, Ferrandina C, Di Renzo N, Olivieri A, Bocchia M, Gentile M, Marchesi F, Musto P, Federici AB, Candoni A, Venditti A, Fava C, Pinto A, Galieni P, Rigacci L, Armiento D, Pane F, Oberti M, Zappasodi P, Visco C, Franchi M, Grossi PA, Bertù L, Corrao G, Pagano L, Corradini P ITA-HEMA-COV Investigators. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, Gil Manso R, Colmenares R, Gil Alos D, Paciello ML, Zafra D, Garcia-Sanchez C, Villegas C, Cuellar C, Carreño-Tarragona G, Zamanillo I, Poza M, Iñiguez R, Gutierrez X, Alonso R, Rodríguez A, Folgueira MD, Delgado R, Ferrari JM, Lizasoain M, Aguado JM, Ayala R, Martinez-Lopez J, Calbacho M. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 2020;105:597–607. doi: 10.1111/ejh.13493. [DOI] [PubMed] [Google Scholar]
- 15.Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, Garcia-Marco JA, Hernández-Rivas JÁ, Mirás F, Baile M, Marquet J, Niemann CU, Reda G, Munir T, Gimeno E, Marchetti M, Quaglia FM, Varettoni M, Delgado J, Iyengar S, Janssens A, Marasca R, Ferrari A, Cuéllar-García C, Itchaki G, Špaček M, De Paoli L, Laurenti L, Levin MD, Lista E, Mauro FR, Šimkovič M, Van Der Spek E, Vandenberghe E, Trentin L, Wasik-Szczepanek E, Ruchlemer R, Bron D, De Paolis MR, Del Poeta G, Farina L, Foglietta M, Gentile M, Herishanu Y, Herold T, Jaksic O, Kater AP, Kersting S, Malerba L, Orsucci L, Popov VM, Sportoletti P, Yassin M, Pocali B, Barna G, Chiarenza A, Dos Santos G, Nikitin E, Andres M, Dimou M, Doubek M, Enrico A, Hakobyan Y, Kalashnikova O, Ortiz Pareja M, Papaioannou M, Rossi D, Shah N, Shrestha A, Stanca O, Stavroyianni N, Strugov V, Tam C, Zdrenghea M, Coscia M, Stamatopoulos K, Rossi G, Rambaldi A, Montserrat E, Foà R, Cuneo A, Ghia P. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varma A, Kosuri S, Ustun C, Ibrahim U, Moreira J, Bishop MR, Nathan S, Mehta J, Moncayo D, Heng J, Osman K, Adekola KUA. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34:2809–2812. doi: 10.1038/s41375-020-01019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood WA, Neuberg DS, Thompson JC, Tallman MS, Sekeres MA, Sehn LH, Anderson KC, Goldberg AD, Pennell NA, Niemeyer CM, Tucker E, Hewitt K, Plovnick RM, Hicks LK. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv. 2020;4:5966–5975. doi: 10.1182/bloodadvances.2020003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yigenoglu TN, Ata N, Altuntas F, Bascı S, Dal MS, Korkmaz S, Namdaroglu S, Basturk A, Hacıbekiroglu T, Dogu MH, Berber İ, Dal K, Erkurt MA, Turgut B, Ulgu MM, Celik O, Imrat E, Birinci S. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2021;93:1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenthøj A, Jakobsen LH, Sengeløv H, Ahmad SA, Qvist K, Rewes A, Poulsen CB, Overgaard UM, Mølle I, Severinsen MT, Strandholdt CN, Maibom J, Kodahl AR, Ryg J, Ravn P, Johansen IS, Helsø SN, Jensen-Fangel S, Kisielewicz J, Wiese L, Helleberg M, Kirk O, Clausen MR, Frederiksen H. SARS-CoV-2 infection among patients with haematological disorders: Severity and one-month outcome in 66 Danish patients in a nationwide cohort study. Eur J Haematol. 2021;106:72–81. doi: 10.1111/ejh.13519. [DOI] [PubMed] [Google Scholar]
- 20.Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, Ikhlef S, Banet A, Lapusan S, Sestilli S, Corre E, Paviglianiti A, Adaeva R, M 'Hammedi-Bouzina F, Labopin M, Legrand O, Dulery R, Mohty M. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Chen W, Li W, Zhao M, Wei Q, Zhang X, Mei H, Wang Y, Hu Y. Clinical characteristics, therapeutic management, and prognostic factors of adult COVID-19 inpatients with hematological malignancies. Leuk Lymphoma. 2020;61:3440–3450. doi: 10.1080/10428194.2020.1808204. [DOI] [PubMed] [Google Scholar]
- 22.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martín-Moro F, Razanamahery J, Riches JC, Zwicker J, Patell R, Vekemans MC, Scarfò L, Chatzikonstantinou T, Yildiz H, Lattenist R, Mantzaris I, Wood WA, Hicks LK. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Official UK Government. Data and insights on coronavirus (COVID-19). [cited 8 April 2021]. In: The Official UK Government Website [Internet]. Available from: https://coronavirus.data.gov.uk/
- 24.Giannakoulis VG, Papoutsi E, Siempos II. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute. Cancer Stat Facts: Leukemia — Chronic Lymphocytic Leukemia (CLL). [cited 8 April 2021]. In: National Cancer Institute [Internet]. Available from: https://seer.cancer.gov/statfacts/html/clyl.html .
- 26.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, Ramsay AG, Gribben JG. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuneo A, Scarfò L, Reda G, Varettoni M, Quaglia FM, Marchetti M, De Paoli L, Re F, Pietrasanta D, Rigolin GM, Orsucci L, Ibatici A, Gattei V, Mauro FR, Trentin L, Laurenti L, Marasca R, Foà R. Chronic lymphocytic leukemia management in Italy during the COVID-19 pandemic: a Campus CLL report. Blood. 2020;136:763–766. doi: 10.1182/blood.2020006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montserrat E. When CLL meets COVID-19. Blood. 2020;136:1115–1116. doi: 10.1182/blood.2020008092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamure S, Duléry R, Di Blasi R, Chauchet A, Laureana C, Deau-Fischer B, Drenou B, Soussain C, Rossi C, Noël N, Choquet S, Bologna S, Joly B, Kohn M, Malak S, Fouquet G, Daguindau E, Bernard S, Thiéblemont C, Cartron G, Lacombe K, Besson C. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: A retrospective multicentric cohort study. EClinicalMedicine. 2020;27:100549. doi: 10.1016/j.eclinm.2020.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JÁ, Navarro B, Núñez L, Alaez C, Córdoba R, Peñalver FJ, Cannata J, Estival P, Quiroz-Cervantes K, Riaza Grau R, Velasco A, Martos R, Domingo-González A, Benito-Parra L, Gómez-Sanz E, López-Jiménez J, Matilla A, Herraez MR, Penalva MJ, García-Suárez J, Díez-Martín JL, Bastos-Oreiro M. Risk Factors and Mortality of COVID-19 in Patients With Lymphoma: A Multicenter Study. Hemasphere. 2021;5:e538. doi: 10.1097/HS9.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurenge A, Ursu R, Houillier C, Abdi B, Tebano G, Quemeneur C, Choquet S, Di Blasi R, Lozano F, Morales A, Durán-Peña A, Sirven-Villaros L, Mathon B, Mokhtari K, Bielle F, Martin-Duverneuil N, Delattre JY, Marcelin AG, Pourcher V, Alentorn A, Idbaih A, Carpentier AF, Leblond V, Hoang-Xuan K, Touat M. SARS-CoV-2 infection in patients with primary central nervous system lymphoma. J Neurol. 2021 doi: 10.1007/s00415-020-10311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzaga Y, Santos MBF, Silva MM, Nucci M. COVID-19 infection in patients with Sézary syndrome: Report of two cases. Dermatol Ther. 2020;33:e14042. doi: 10.1111/dth.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192:415. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 34.Sollini M, Gelardi F, Carlo-Stella C, Chiti A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the "flare phenomenon" to the "abscopal effect". Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasin F, Mascalchi Calveri M, Calabrese A, Pizzarelli G, Bongiovanni I, Andreoli M, Cattaneo C, Rignanese G. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed. 2020;91:ahead of print. doi: 10.23750/abm.v91i3.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Cruz-Benito B, Lázaro-Del Campo P, Ramírez-López A, de Soto-Álvarez T, Sánchez-Vadillo I, García-Pérez E, Dos Santos-Ortas A, Humala-Barbier K, López-de la Guía A, Casado-Abad G, Jiménez-Yuste V, Canales-Albendea M. Managing the front-line treatment for diffuse large B cell lymphoma and high-grade B cell lymphoma during the COVID-19 outbreak. Br J Haematol. 2020;191:386–389. doi: 10.1111/bjh.17066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Ciaccio P, McCaughan G, Trotman J, Ho PJ, Cheah CY, Gangatharan S, Wight J, Ku M, Quach H, Gasiorowski R, Polizzotto MN, Prince HM, Mulligan S, Tam CS, Gregory G, Hapgood G, Spencer A, Dickinson M, Latimer M, Johnston A, Armytage T, Lee C, Cochrane T, Berkhahn L, Weinkove R, Doocey R, Harrison SJ, Webber N, Lee HP, Chapman S, Campbell BA, Gibbs SDJ, Hamad N. Australian and New Zealand consensus statement on the management of lymphoma, chronic lymphocytic leukaemia and myeloma during the COVID-19 pandemic. Intern Med J. 2020;50:667–679. doi: 10.1111/imj.14859. [DOI] [PubMed] [Google Scholar]
- 38.Hus I, Salomon-Perzyński A, Tomasiewicz K, Robak T. The management of hematologic malignancies during the COVID-19 pandemic. Expert Opin Pharmacother. 2021;22:565–582. doi: 10.1080/14656566.2020.1849143. [DOI] [PubMed] [Google Scholar]
- 39.Lang N, Kuruvilla J. Evolving management strategies for lymphomas during the COVID-19 pandemic. Leuk Lymphoma. 2020:1–19. doi: 10.1080/10428194.2020.1861277. [DOI] [PubMed] [Google Scholar]
- 40.Oertel M, Elsayad K, Engenhart-Cabillic R, Reinartz G, Baues C, Schmidberger H, Vordermark D, Marnitz S, Lukas P, Ruebe C, Engert A, Lenz G, Eich HT. Radiation treatment of hemato-oncological patients in times of the COVID-19 pandemic : Expert recommendations from the radiation oncology panels of the German Hodgkin Study Group and the German Lymphoma Alliance. Strahlenther Onkol. 2020;196:1096–1102. doi: 10.1007/s00066-020-01705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papadavid E, Scarisbrick J, Ortiz Romero P, Guaglino P, Vermeer M, Knobler R, Stadler R, Bagot M. Management of primary cutaneous lymphoma patients during COVID-19 pandemic: EORTC CLTF guidelines. J Eur Acad Dermatol Venereol. 2020;34:1633–1636. doi: 10.1111/jdv.16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perini GF, Fischer T, Gaiolla RD, Rocha TB, Bellesso M, Teixeira LLC, Delamain MT, Scheliga AAS, Ribeiro GN, Neto JV, Baiocchi OCCG, Abdo ANR, Arrais-Rodrigues C, Fogliatto LM, Bigni RS, Schaffel R, Biasoli I, Pereira J, Nabhan SK, Souza CA, Chiattone CS Associação Brasileira de Hematologia. Hemoterapia e Terapia Celular (ABHH) How to manage lymphoid malignancies during novel 2019 coronavirus (CoVid-19) outbreak: a Brazilian task force recommendation. Hematol Transfus Cell Ther. 2020;42:103–110. doi: 10.1016/j.htct.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talaulikar D, Advani RH, Branagan AR, Buske C, Dimopoulos MA, D'Sa S, Kersten MJ, Leblond V, Minnema MC, Owen RG, Palomba ML, Tedeschi A, Trotman J, Varettoni M, Vos JM, Treon SP, Kastritis E, Castillo JJ. Consensus Statement on the Management of Waldenström Macroglobulinemia Patients During the COVID-19 Pandemic. Hemasphere. 2020;4:e433. doi: 10.1097/HS9.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kos I, Balensiefer B, Roth S, Ahlgrimm M, Sester M, Schmidt T, Thurner L, Bewarder M, Bals R, Lammert F, Stilgenbauer S, Kaddu-Mulindwa D. Prolonged Course of COVID-19-Associated Pneumonia in a B-Cell Depleted Patient After Rituximab. Front Oncol. 2020;10:1578. doi: 10.3389/fonc.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajima Y, Ogai A, Furukawa K, Arai R, Anan R, Nakano Y, Kurihara Y, Shimizu H, Misaki T, Okabe N. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27:387–389. doi: 10.1016/j.jiac.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda H, Tsukune Y, Watanabe N, Sugimoto K, Uchimura A, Tateyama M, Miyashita Y, Ochi Y, Komatsu N. Persistent COVID-19 Pneumonia and Failure to Develop Anti-SARS-CoV-2 Antibodies During Rituximab Maintenance Therapy for Follicular Lymphoma. Clin Lymphoma Myeloma Leuk. 2020;20:774–776. doi: 10.1016/j.clml.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O'Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY, Larkin KM, Stefanovski MR, Chappell DL, Frissora FW, Smith LL, Smucker KA, Flynn JM, Jones JA, Andritsos LA, Maddocks K, Lehman AM, Furman R, Sharman J, Mishra A, Caligiuri MA, Satoskar AR, Buggy JJ, Muthusamy N, Johnson AJ, Byrd JC. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treon SP, Castillo JJ, Skarbnik AP, Soumerai JD, Ghobrial IM, Guerrera ML, Meid K, Yang G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thibaud S, Tremblay D, Bhalla S, Zimmerman B, Sigel K, Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol. 2020;190:e73–e76. doi: 10.1111/bjh.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin AY, Cuttica MJ, Ison MG, Gordon LI. Ibrutinib for chronic lymphocytic leukemia in the setting of respiratory failure from severe COVID-19 infection: Case report and literature review. EJHaem. 2020 doi: 10.1002/jha2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chong EA, Roeker LE, Shadman M, Davids MS, Schuster SJ, Mato AR. BTK Inhibitors in Cancer Patients with COVID-19: "The Winner Will be the One Who Controls That Chaos" (Napoleon Bonaparte) Clin Cancer Res. 2020;26:3514–3516. doi: 10.1158/1078-0432.CCR-20-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roschewski M, Lionakis MS, Sharman JP, Roswarski J, Goy A, Monticelli MA, Roshon M, Wrzesinski SH, Desai JV, Zarakas MA, Collen J, Rose K, Hamdy A, Izumi R, Wright GW, Chung KK, Baselga J, Staudt LM, Wilson WH. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hindilerden F, Yönal Hindilerden İ, Diz Küçükkaya R. Rapid progression after ibrutinib discontinuation in a patient with mantle cell lymphoma who has severe coronavirus disease 2019 infection. Balkan Med J. 2021;38:141–142. doi: 10.4274/balkanmedj.galenos.2020.2020.9.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 56.O'Kelly B, McGettrick P, Angelov D, Fay M, McGinty T, Cotter AG, Sheehan G, Lambert JS. Outcome of a patient with refractory Hodgkin lymphoma on pembrolizumab, infected with SARS-CoV-2. Br J Haematol. 2020;190:e1–e3. doi: 10.1111/bjh.16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogiers A, Pires da Silva I, Tentori C, Tondini CA, Grimes JM, Trager MH, Nahm S, Zubiri L, Manos M, Bowling P, Elkrief A, Papneja N, Vitale MG, Rose AAN, Borgers JSW, Roy S, Mangana J, Pimentel Muniz T, Cooksley T, Lupu J, Vaisman A, Saibil SD, Butler MO, Menzies AM, Carlino MS, Erdmann M, Berking C, Zimmer L, Schadendorf D, Pala L, Queirolo P, Posch C, Hauschild A, Dummer R, Haanen J, Blank CU, Robert C, Sullivan RJ, Ascierto PA, Miller WH Jr, Stephen Hodi F, Suijkerbuijk KPM, Reynolds KL, Rahma OE, Lorigan PC, Carvajal RD, Lo S, Mandala M, Long GV. Clinical impact of COVID-19 on patients with cancer treated with immune checkpoint inhibition. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, Kukreti V, Wei E, Leung-Hagesteijn C, Li ZH, Brandwein J, Pantoja M, Johnston J, Gibson S, Hernandez T, Spaner D, Trudel S. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, Zhang H, Offner F, Scheliga A, Nowakowski GS, Pinto A, Re F, Fogliatto LM, Scheinberg P, Flinn IW, Moreira C, Cabeçadas J, Liu D, Kalambakas S, Fustier P, Wu C, Gribben JG AUGMENT Trial Investigators. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2019;37:1188–1199. doi: 10.1200/JCO.19.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, Ren L, Wei Q, Mei H, Hu C, Tao C, Yang R, Wang J, Yu Y, Guo Y, Wu X, Xu Z, Zeng L, Xiong N, Chen L, Man N, Liu Y, Xu H, Deng E, Zhang X, Li C, Wang C, Su S, Zhang L, Wu Y, Liu Z. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy N, Giunta DH, Pérez LG, Sánchez MDL, Gamarnik AV, Ojeda DS, Santoro DM, Camino PJ, Antelo S, Rainero K, Vidiella GP, Miyazaki EA, Cornistein W, Trabadelo OA, Ross FM, Spotti M, Funtowicz G, Scordo WE, Losso MH, Ferniot I, Pardo PE, Rodriguez E, Rucci P, Pasquali J, Fuentes NA, Esperatti M, Speroni GA, Nannini EC, Matteaccio A, Michelangelo HG, Follmann D, Lane HC, Belloso WH PlasmAr Study Group. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, van Helmond N, Verdun NC, Marks P, van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS, Fairweather D, Wright RS, Casadevall A. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med. 2021;384:1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L, Althabe F, Bergel E, Polack FP Fundación INFANT–COVID-19 Group. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betrains A, Godinas L, Woei-A-Jin FJSH, Rosseels W, Van Herck Y, Lorent N, Dierickx D, Compernolle V, Meyfroidt G, Vanderbeke L, Vergote V, Lagrou K, Verhamme P, Wauters J, Vermeersch P, Devos T, Maes P, Vanderschueren S. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2021;192:1100–1105. doi: 10.1111/bjh.17266. [DOI] [PubMed] [Google Scholar]
- 65.Ferrari S, Caprioli C, Weber A, Rambaldi A, Lussana F. Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies. Leuk Lymphoma. 2021:1–9. doi: 10.1080/10428194.2021.1872070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeyaraman P, Agrawal N, Bhargava R, Bansal D, Ahmed R, Bhurani D, Bansal S, Rastogi N, Borah P, Naithani R Delhi Hematology Group. Convalescent plasma therapy for severe Covid-19 in patients with hematological malignancies. Transfus Apher Sci. 2021:103075. doi: 10.1016/j.transci.2021.103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malsy J, Veletzky L, Heide J, Hennigs A, Gil-Ibanez I, Stein A, Lütgehetmann M, Rosien U, Jasper D, Peine S, Hiller J, Haag F, Schmiedel S, Huber S, Jordan S, Addo MM, Schulze Zur Wiesch J. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ormazabal Vélez I, Induráin Bermejo J, Espinoza Pérez J, Imaz Aguayo L, Delgado Ruiz M, García-Erce JA. Two patients with rituximab associated low gammaglobulin levels and relapsed covid-19 infections treated with convalescent plasma. Transfus Apher Sci. 2021:103104. doi: 10.1016/j.transci.2021.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roeker LE, Knorr DA, Pessin MS, Ramanathan LV, Thompson MC, Leslie LA, Zelenetz AD, Mato AR. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34:3047–3049. doi: 10.1038/s41375-020-01030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wright Z, Bersabe A, Eden R, Bradley J, Cap A. Successful Use of COVID-19 Convalescent Plasma in a Patient Recently Treated for Follicular Lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21:66–68. doi: 10.1016/j.clml.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The European Society for Blood and Marrow Transplantation. COVID-19 and BMT. [cited 8 April 2021]. In: The European Society for Blood and Marrow Transplantation [Internet]. Available from: https://www.ebmt.org/covid-19-and-bmt .
- 72.Shah GL, DeWolf S, Lee YJ, Tamari R, Dahi PB, Lavery JA, Ruiz J, Devlin SM, Cho C, Peled JU, Politikos I, Scordo M, Babady NE, Jain T, Vardhana S, Daniyan A, Sauter CS, Barker JN, Giralt SA, Goss C, Maslak P, Hohl TM, Kamboj M, Ramanathan L, van den Brink MR, Papadopoulos E, Papanicolaou G, Perales MA. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest. 2020;130:6656–6667. doi: 10.1172/JCI141777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, Dandoy C, Gauthier J, Gowda L, Perales MA, Seropian S, Shaw BE, Tuschl EE, Zeidan AM, Riches ML, Shah GL. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greiner J, Götz M, Malner-Wagner W, Wendt C, Enders M, Durst C, Michel D, von Harsdorf S, Jung S. Characteristics and mechanisms to control a COVID-19 outbreak on a leukemia and stem cell transplantation unit. Cancer Med. 2021;10:237–246. doi: 10.1002/cam4.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haroon A, Alnassani M, Aljurf M, Ahmed SO, Shaheen M, Hanbli A, Chaudhari N, El Fakih R. COVID - 19 post Hematopoietic Cell Transplant, a Report of 11 Cases from a Single Center. Mediterr J Hematol Infect Dis. 2020;12:e2020070. doi: 10.4084/MJHID.2020.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lázaro Del Campo P, Ramírez López A, de la Cruz Benito B, de Paz Arias R, de Soto Álvarez T, Sánchez Vadillo I, Humala Barbier K, García Pérez E, Dos Santos Ortas A, López de la Guía A, Gasior Kabat M, Baltasar Tello P, Jiménez Yuste V, Canales Albendea M. Hematopoietic cell transplantation during COVID-19 pandemic: experience from a tertiary hospital in Madrid. Expert Rev Hematol. 2021;14:1–5. doi: 10.1080/17474086.2021.1858789. [DOI] [PubMed] [Google Scholar]
- 77.Marino D, Finotto S, Basso U, Galiano A, Bolshinsky M, Amato O, Marson P, Tison T, Colpo A, Zagonel V. To Transplant or Not to Transplant During the SARS-CoV-2 Pandemic? Oncologist. 2021;26:e336–e337. doi: 10.1002/onco.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Onaka T, Iwai F, Kato-Ogura A, Yonezawa A. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection after allogeneic stem cell transplantation. Clin Case Rep. 2020 doi: 10.1002/ccr3.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O'Dell S, Schmidt SD, Corbett KS, Swanson PA 2nd, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH mRNA-1273 Study Group. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O'Dell S, Schmidt SD, Swanson PA 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH mRNA-1273 Study Group. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shadman M, Ujjani C. Vaccinations in CLL: implications for COVID-19. Blood. 2021;137:144–146. doi: 10.1182/blood.2020009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu G, Lu Y. COVID-19 mRNA Vaccination-Induced Lymphadenopathy Mimics Lymphoma Progression on FDG PET/CT. Clin Nucl Med. 2021;46:353–354. doi: 10.1097/RLU.0000000000003597. [DOI] [PubMed] [Google Scholar]