Abstract
In children who receive neurotoxic chemotherapy, peripheral neurotoxicity occurs frequently, necessitates dose reduction or treatment cessation, and affects function and long-term quality of life. No treatments exist for peripheral neurotoxicity and few assessment measures are specific to children. We did a systematic review to analyse the published literature concerning the evaluation of assessment measures for paediatric chemotherapy-induced peripheral neurotoxicity. We searched PubMed, CINAHL, PsycINFO, and Embase on Nov 7–8, 2018; of 1409 articles, seven met the inclusion criteria. A total of 335 children (excluding ten healthy controls) were enrolled in the seven studies and the sample sizes ranged from 17 to 86 individuals. 276 (82%) of the 335 children were actively undergoing chemotherapy treatment. Most studies did not comprehensively evaluate the psychometric properties of assessment measures for chemotherapy-induced peripheral neurotoxicity. By use of a narrative analysis that combined approaches from the Joanna Briggs Institute (Adelaide, SA, Australia) and the quality of diagnostic accuracy studies assessment method (known as QUADAS), only one study was deemed high quality. We identified two variants of the Total Neuropathy Score, two grading scales, two semi-objective tests, one patient-reported outcome, and several mobility measures. The National Cancer Institute Common Terminology Criteria for Adverse Events and the Balis grading scales showed lower sensitivity and specificity than the items of the Total Neuropathy Score. Although there is insufficient evidence to support the use of most approaches to assess chemotherapy-induced peripheral neurotoxicity in children, two variants of the Total Neuropathy Score, the pediatric-modified Total Neuropathy Score and the Total Neuropathy Score-pediatric vincristine, are promising but require further testing. Other approaches are less sensitive or less feasible. A patient-reported outcome measure for chemotherapy-induced peripheral neurotoxicity in children is needed.
Introduction
Considerable advances in survival from childhood cancer have led to a substantial and increasing population of childhood cancer survivors; therefore, considering the long-term consequences of cancer treatment in childhood is crucial. Chemotherapy-induced peripheral neurotoxicity (CIPN), a side-effect of cancer treatment affecting up to 78% of paediatric patients receiving neurotoxic chemotherapy,1 is associated with sensory, motor, and autonomic nervous system dysfunctions with long-lasting effects on function, school performance, and quality of life.1-6 Thus, understanding the clinical manifestations of CIPN and identifying the best methods to monitor the development and progression of toxic effects is important.
The manifestations of CIPN vary by chemotherapy type, treatment schedule, and cumulative dosage, and can include numbness, tingling, neuropathic pain, and muscle weakness and cramps, which generally affect the toes and fingers bilaterally first and then advance proximally. Signs of autonomic dysfunction, such as constipation, urinary retention, and orthostatic hypotension, are less common.1,3,7
Various neurotoxic drugs, which mainly cause moderate-to-severe sensory CIPN in 2–40% of paediatric patients,7 are used in the treatment of childhood cancers. Vinca alkaloids, bortezomib, and thalidomide are mainly used to treat haematological malignancies. Carboplatin, cisplatin, and, infrequently, oxaliplatin, paclitaxel, docetaxel, and ixabepilone, are used to treat solid tumours. Clinical characteristics of CIPN vary by drug class. For example, vinca alkaloids (eg, vincristine) cause moderate-to-severe sensory, motor, and autonomic neuropathy (motor and autonomic symptoms are more frequent for this class of drugs than for others) in up to 52% of patients.1,7 Bortezomib-related CIPN is distinguished by pronounced neuropathic pain due to damage to the small nerve fibres that transmit painful stimuli to the CNS.8 Thalidomide, which is commonly used to treat both haematological malignancies and solid tumours and causes primarily a sensory axonal polyneuropathy in adults, instead causes motor neuropathy in children.9,10
Some evidence suggests that CIPN might be more severe for children receiving concurrent azole antifungal treatment,11-13 with pre-existing peripheral nerve disease,14 or who have a genetic predisposition for CIPN than for paediatric patients without these factors.15-18 However, knowledge about the potential influence of risk factors on CIPN incidence, severity, and long-term outcomes is lacking. Furthermore, important differences exist between adult and paediatric CIPN. Unlike the clear evidence linking older age and increased cumulative doses of neurotoxic chemotherapy to worse CIPN outcomes in adults,19,20 the evidence in children is contradictory.5,21-25
No effective treatments for paediatric CIPN have been found in the few clinical trials that have been done;26-30 the only established strategies for the amelioration of CIPN are dose reduction or dose cessation. The paucity of psychometrically strong CIPN measures specific to children limits the rigour of studies that evaluate treatments for CIPN; previous reviews have highlighted challenges to assessing CIPN in children.1,3,31,32 For example, children might not have the language skills to describe numbness, tingling, and neuropathic pain. Short attention spans, trepidation about impending cancer treatment procedures, and co-occurring symptoms (eg, nausea and fatigue) can make it difficult for children to focus and cooperate during neurological examinations. Furthermore, children’s more basic level of reading comprehension limits the feasibility of the use of existing CIPN patient-reported outcomes.3,31,32 These challenges create particular difficulties in assessing children younger than 5 years of age.33 Other CIPN assessment methods, including nerve conduction studies and quantitative sensory testing, are not appropriate for routine use in children, as these assessments cause discomfort or require patient compliance. Available clinician-graded scales often lead to underestimation of CIPN and are insensitive to change over time.33-35
Thus, research is needed to identify the best methods for the assessment of paediatric CIPN. A reliable, valid, sensitive, responsive, clinically feasible, and age-appropriate gold standard measure, which facilitates both objective and subjective assessments, is needed. Knowledge of the current state of paediatric CIPN assessment will inform future standards of best practice and research priorities. The purpose of this Review is to analyse the published literature concerning the evaluation of assessment measures for paediatric CIPN that have been specifically tested for evidence of several psychometric properties (appendix p 1).
Methods
Search strategy and selection criteria
The reporting of this systematic Review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (known as PRISMA) criteria.36 On Nov 7–8, 2018, GAK-L and CK searched PubMed, CINAHL, PsycINFO, and Embase for articles published between the dates of database inception and Nov 8, 2018. Search terms included words and phrases describing the population, problem of interest (eg, CIPN, neurotoxicity, and neuropathic pain), measurement tools and surveys, and psychometric terminology. The full search strategy is included in the appendix (p 2).
Children and adolescents aged 0–18 years with CIPN comprised the target population; control groups were included if the groups exposed to neurotoxic agents could be independently analysed. We included articles if they discussed the development of an instrument to measure paediatric CIPN or if they evaluated the psychometric properties of such an instrument (appendix p 1). We excluded articles if they were not written in English; were animal studies or basic science research (in vitro studies); were conference abstracts or proceedings, case studies, expert opinions, correspondence, dissertations, or grey literature; included patients without a cancer diagnosis; described patients who were not receiving or had not received chemotherapy; and were not designed to assess the psychometric properties of a paediatric CIPN instrument. We reviewed the titles and abstracts of studies identified in the search against the inclusion and exclusion criteria. We assessed the full text of studies we identified and included those that met all criteria in the Review.
Data extraction and analysis
We assessed each article using a narrative analysis approach and the risk of bias using an established method37 that combines Joanna Briggs Institute (Adelaide, SA, Australia) recommendations for assessing associational, cross-sectional, and case-control studies,38-40 and the quality of diagnostic accuracy studies (QUADAS) assessment method.41 Two reviewers (EMLS and one other reviewer per paper [GC, PA, or SP]) rated the eight Joanna Briggs Institute characteristics (table 1), and three reviewers (EMLS, RS, and SP) rated the seven QUADAS characteristics, in which a score of 0 means the characteristic is not shown and a score of 1 means that the characteristic is shown (table 2). Summed QUADAS scores from the three independent reviewers were averaged. An average score of 0–3 reflected poor quality studies, 4–5 reflected moderate quality studies, and 6–7 reflected high quality studies. Empirical evidence supports satisfactory inter-rater reliability (r=0·78) of the QUADAS method to evaluate the quality of diagnostic accuracy studies.41
Table 1:
Critical appraisal of articles reviewed by use of the Joanna Briggs Institute recommendations
| CIPN measure tested |
Eligibility defined |
Sample and setting described |
Comparison groups defined |
Objective criteria for measuring condition |
Adequate training of study staff |
Attention to procedural fidelity |
Strategies to consider confounders |
Appropriate statistics and statistical power |
Total score |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Gilchrist et al (2009)42 | ped-mTNS | 1·0 | 1·0 | 0·0 | 1·0 | NA* | 0·0 | 0·5 | 0·0† | 3·5 |
| Gilchrist and Tanner (2013)35 | ped-mTNS | 1·0 | 1·0 | 1·0 | 1·0 | 1·0 | 1·0 | 0·5 | 0·0 | 6·5 |
| Gilchrist et al (2014)34 | ped-mTNS and CTCAE version 3.0 | 1·0 | 1·0 | 0·0 | 1·0 | 1·0 | 0·0 | 0·5 | 0·0 | 4·5 |
| Gilchrist and Tanner (2018)43 | ped-mTNS | 1·0 | 1·0 | 0·0 | 1·0 | 0·0 | 0·0 | 0·5 | 0·0 | 3·5 |
| Lieber et al (2018)44 | rPed-mTNS, nerve conduction studies, quantitative sensory testing | 1·0 | 1·0 | 1·0 | 0·0 | 0·0 | 0·0 | 0·5 | 0·0 | 3·5 |
| Smith et al (2013)33 | TNS-PV, CTCAE version 4.0, the Balis scale, and the FACES scale | 1·0 | 1·0 | 0·0 | 1·0 | 1·0 | 1·0 | 0·5 | 1·0 | 6·5 |
| Wright et al (2017)45 | Gait analysis | 1·0 | 1·0 | 1·0 | 1·0 | 0·0 | 0·0 | 0·5 | 0·0 | 4·5 |
A score of 0 means that the characteristic is not shown by the study. A score of 1 means that the characteristic is shown by the study. A score of 0·5 means that the characteristic is partially shown by the study. CIPN=chemotherapy-induced peripheral neurotoxicity. ped-mTNS=pediatric-modified Total Neuropathy Score. NA=not applicable. CTCAE=National Cancer Institute Common Terminology Criteria for Adverse Events. rPed-mTNS=reduced pediatric-modified Total Neuropathy Score. TNS-PV=Total Neuropathy Score-pediatric vincristine.
Scores obtained by a single reviewer and validated by a second reviewer. Training of study staff was not required.
This study by Gilchrist and colleagues was a pilot study.
Table 2:
Critical appraisal of articles reviewed by use of the QUADAS assessment method
| CIPN measure tested |
Participants had CIPN |
Random selection |
Reliability estimates ≥0·70 |
Evidence of construct validity |
Comparison to reference standard |
Procedural detail |
Instrument scoring procedures described |
Total QUADAS score |
|
|---|---|---|---|---|---|---|---|---|---|
| Gilchrist et al (2009)42 | ped-mTNS | 1·0 | 0·0 | 0·0* | 0·0 | 0·3 | 0·7 | 0·7 | 2·7 |
| Gilchrist and Tanner (2013)35 | ped-mTNS | 1·0 | 0·3 | 1·0 | 1·0 | 0·0 | 1·0 | 0·7 | 5·0 |
| Gilchrist et al (2014)34 | ped-mTNS and CTCAE version 3.0 | 1·0 | 0·3 | 0·0* | 0·0 | 1·0 | 0·7 | 0·7 | 3·7 |
| Gilchrist and Tanner (2018)43 | ped-mTNS | 1·0 | 0·3 | 0·0* | 0·7 | 0·0 | 0·7 | 0·3 | 3·0 |
| Lieber et al (2018)44 | rPed-mTNS, nerve conduction studies, and quantitative sensory testing | 0·7 | 0·3 | 0·0* | 0·0 | 1·0 | 0·3 | 0·7 | 3·0 |
| Smith et al (2013)33 | TNS-PV, CTCAE version 4.0, the Balis scale, and the FACES scale | 1·0 | 0·0 | 1·0 | 1·0 | 1·0 | 1·0 | 1·0 | 6·0 |
| Wright et al (2017)45 | Gait analysis | 1·0 | 0·3 | 0·0* | 0·7 | 0·7 | 0·3 | 0·3 | 3·3 |
A score of 0 means that the characteristic is not shown by the study. A score of 1 means that the characteristic is shown by the study. Scores reflect the mean of 3 scores from three independent reviewers. QUADAS=quality of diagnostic accuracy studies. CIPN=chemotherapy-induced peripheral neurotoxicity. ped-mTNS=pediatric-modified Total Neuropathy Score. CTCAE=National Cancer Institute Common Terminology Criteria for Adverse Events. rPed-mTNS=reduced pediatric modified Total Neuropathy Score. TNS-PV=Total Neuropathy Score-pediatric vincristine.
Scored 0 because reliability estimates were not done.
Results
Characteristics of included studies
We identified 1409 studies in the initial search. 1355 were excluded after a title and abstract screen and the full text of 54 studies was assessed (figure). Seven studies met the full inclusion and exclusion criteria and were included in the Review (figure; appendix pp 3-8). Five of the seven studies used prospective cross-sectional or case-control designs; the other two studies were longitudinal in design. Participants in the studies had a broad range of cancer types, such as leukaemia, lymphoma, and solid tumours. A total of 335 children (excluding ten healthy controls) were enrolled in the seven studies and the sample sizes ranged from 17 to 86 individuals. 276 (82%) of the 335 children were actively undergoing chemotherapy treatment. Two studies42,45 included a mixed study population: patients who had previously received neurotoxic chemotherapy and patients who were receiving neurotoxic chemotherapy at the time of the study. One study44 included only paediatric survivors who had completed neurotoxic chemotherapy and four studies33-35,43 included children who were undergoing treatment at the time of the study. Several tools and measures were analysed in these studies (panel 1).
Figure:
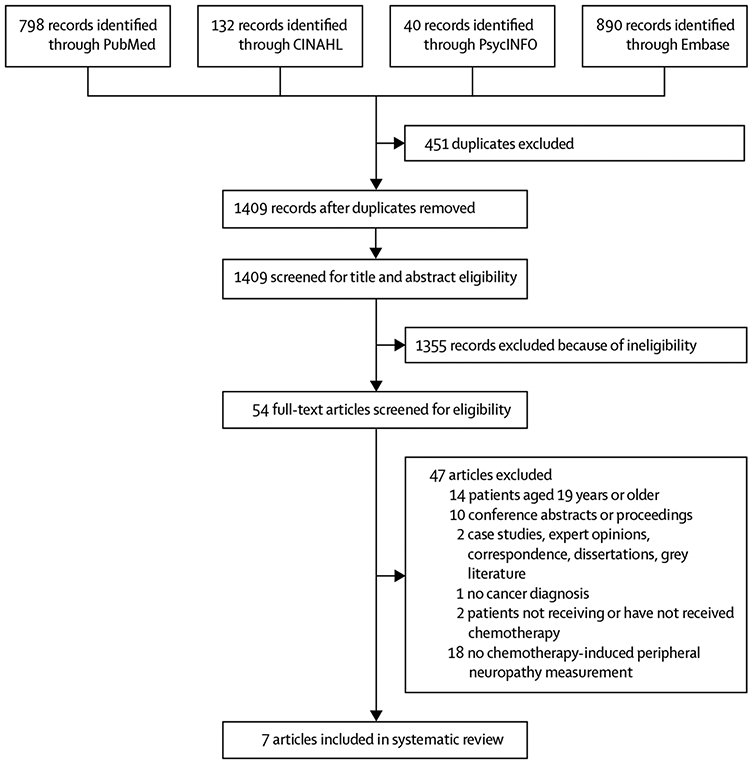
Flowchart of article selection process
Panel 1: Tools and measures analysed in the selected studies.
Two paediatric variants of the Total Neuropathy Score: pediatric-modified Total Neuropathy Score34,35,42,43 and the Total Neuropathy Score-pediatric vincristine33
Two grading scales: the National Cancer Institute Common Terminology Criteria for Adverse Events (known as NCI CTCAE)33,34 and the Balis grading scale33
Two objective assessments: nerve conduction and quantitative sensory testing44
The FACES pain scale33
Several mobility measures (eg, gate deviation index and passive ankle dorsiflexion range of motion)45
Paediatric variants of the Total Neuropathy Score
The pediatric-modified Total Neuropathy Score (ped-mTNS) is a composite measure of eight neuropathy signs and symptoms. Three types of signs and symptoms are assessed via a scripted interview: sensory (ie, numbness, tingling, and pain), motor (ie, difficulty buttoning, zipping, walking, and managing stairs), and autonomic (ie, dizziness, and hot or cold hands or feet).42 Using semi-objective examination techniques, a trained clinician assesses light touch, pin sensation, vibration perception, strength, and deep tendon reflexes. Each item is scored from 0 to 4 and the eight item scores are summed (total score range=0–32). A higher score reflects more severe symptoms, or more proximal extension of neurological deficits, and scores more than or equal to 5 indicate the presence of CIPN when compared with normal controls.35
Four studies by Gilchrist and colleagues34,35,42,43 assessed the psychometric properties of the ped-mTNS. All four studies provided data describing validity, and two studies35,42 provided data describing reliability. At a single institution,35 41 children and adolescents (aged 5–18 years) with cancer and 41 gender-matched and age-matched control individuals participated in a cross-sectional, descriptive study to examine the reliability (internal consistency reliability, intrarater reliability, and inter-rater reliability) of ped-mTNS. Internal consistency reliability was shown by a high Cronbach’s alpha coefficient (α=0·76). Results from inter-item correlational analysis also provide evidence of internal consistency reliability on the basis of moderate-to-strong individual item score correlations. Another study42 did not find adequate correlations for internal consistency among pin and vibration sensibility and sensory symptoms, although motor symptoms, vibration sensibility, and tendon reflexes were moderately correlated. Excellent intrarater (intraclass correlation coefficient 0·99, 95% CI 0·96–0·99) and inter-rater (0·98, 0·95–0·99) reliability data were reported in one study.35 Gilchrist’s four papers provide data regarding the construct validity (ie, contrasting group validity, or convergent validity, or both) of ped-mTNS.34,35,42,43 Gilchrist’s earliest study42 compared two different approaches for assessing vibration sensitivity: a tuning fork and a biothesiometer. Convergent validity of the two approaches was shown by moderately strong correlations of vibration sensibility at the finger (r=−0·72) and toe plantar surfaces (r=−0·63). Another study35 reported that children receiving neurotoxic chemotherapy had significantly worse ped-mTNS total scores than gender-matched and age-matched controls (8·7 [SD 4·2] vs 1·4 [0·9]; p<0·001). Although autonomic symptoms and pin sensitivity did not differ between patients and healthy controls, the authors opted to retain these items for clinical considerations. Two of the four studies35,43 provide evidence of convergent validity on the basis of statistically significant negative associations among mean ped-mTNS and Bruininks-Oseretsky Test of Motor Proficiency-2 balance item scores. One study35 found no correlation between ped-mTNS total scores and cumulative vincristine dosage. However, comparisons of ped-mTNS with cumulative vincristine dosage might not be valid for paediatric psychometric studies because of the variation in how children metabolise vincristine and the subsequent differences in systemic drug exposure and blood concentration (area under the curve).18
Three of four studies34,35,42 assessed the sensitivity of the ped-mTNS: the degree by which individual items or total scores reflect the entire scoring ranges. All three studies illustrate a floor effect, which is an absence of scores at the top of the individual item (0–4) and total score (0–32) ranges. When compared with the biothesiometer, results from one study42 suggest that the tuning fork has better sensitivity to detect CIPN because the fork identified four more patients with diminished vibration sensibility than the biothesiometer. Although the sensitivity of the tuning fork was excellent (1·0), specificity was only moderate (0·6). Another study34 provided strong evidence that the ped-mTNS is more sensitive than a combined sensory and motor Common Terminology Criteria for Adverse Events (CTCAE) score. Only one study42 assessed the feasibility of the ped-mTNS for use in children aged 5–18 years, finding that trained evaluators obtained results for all eight ped-mTNS-based assessments from these children in less than 10 min.
One study33 assessed another paediatric variant of the Total Neuropathy Score for use in children receiving vincristine: the Total Neuropathy Score-pediatric vincristine (TNS-PV), which includes items quantifying vibration sensibility, muscle strength, deep tendon reflexes, subjective autonomic symptoms, and distal to proximal extension of subjective sensory and motor symptoms. The TNS-PV differs from the ped-mTNS variant in four ways: (1) for assessing small nerve fibre function, temperature sensibility testing replaces pin testing, which is particularly uncomfortable for younger children; (2) monofilament tests of light touch are not included; (3) because constipation and laryngeal nerve paralysis (causing hoarseness) are unique manifestations of vincristine-induced CIPN, two additional items quantify these symptoms; and (4) there is no established cutoff point to differentiate normal from abnormal scores. In one study33 at four sites, investigators recruited 65 children (aged 1–18 years) with acute lymphoblastic leukaemia who were about to begin vincristine treatment; TNS-PV assessments were done at baseline and at each vincristine treatment over 15 weeks, resulting in 806 assessments. Study findings provide moderately strong evidence of the internal consistency and inter-rater reliability, construct validity, sensitivity, responsiveness, and feasibility of TNS-PV for use in children aged six years and older. The hoarseness and constipation items were not highly correlated with other TNS-PV items, suggesting that measurements of hoarseness and constipation might be capturing symptoms of medical problems unrelated to neurotoxicity. The average of just the vibration and reflexes scores (providing a two-item V-Rex score) showed construct validity and superior responsiveness when compared with the full TNS-PV. In contrast to the absence of correlation between the ped-mTNS and CTCAE,34 the TNS-PV scores were moderately to strongly correlated with both the CTCAE and Balis grading scale scores.
Grading scales
Two studies33,34 assessed the National Cancer Institute’s CTCAE grading scale; one study33 also assessed the Balis grading scale. Gilchrist and colleagues34 assessed the convergent validity of CTCAE version 3.0 via analysis of correlations with ped-mTNS scores.34 60 patients, aged 5–18 years, completed the ped-mTNS assessments and, within 24 h, a trained rater derived CTCAE scores from patient medical records. No correlations between ped-mTNS and combined motor and sensory CTCAE scores were found. The only ped-mTNS item that moderately correlated with CTCAE motor scores was strength testing (r=0·43). Smith and colleagues33 evaluated the convergent validity of CTCAE version 4.0 and Balis grading scales via comparison with the TNS-PV and vincristine cumulative dosage and the area under the curve. Study findings were mixed; although the CTCAE and Balis scale scores were moderately correlated with TNS-PV scores, only the CTCAE sensory and Balis motor scales correlated with vincristine cumulative dose. No statistically significant correlations with the area under the curve were found.
Sensitivity and specificity of the CTCAE were assessed in two studies.33,34 The CTCAE version 3.0 had lower sensitivity and specificity than did ped-mTNS’s light touch and manual strength testing.34 Specifically, 16 (84%) of 19 patients who received a combined sensory and motor CTCAE score of 0 showed evidence of CIPN on the basis of their ped-mTNS scores (ie, score ≥5). The sensory CTCAE did not detect sensory neurotoxicity in 24 (40%) of 60 patients and did not detect motor neurotoxicity in 9 (15%) of 60 of patients. Because of these findings, the authors suggested that the sensitivity of the ped-mTNS to detect subtle CIPN is superior to that of the CTCAE.34 Smith and colleagues33 also reported that only the Balis motor grading scale showed evidence of sensitivity by capturing scores encompassing the entire 0–4 range. Empirical evidence of grading scale responsiveness is scant; results from only one study33 suggest that the CTCAE sensory scale is responsive to change in neuropathy signs and symptoms over time.
Objective assessments
One study published by Lieber and colleagues44 evaluated objective approaches for the assessment of CIPN in children. This dual site, cross-sectional, observational study compared quantitative sensory testing to nerve conduction studies and CIPN pain assessed by a questionnaire in 46 patients with acute lymphoblastic leukaemia (aged 6–18 years) at a mean of 3·2 (SD 2·5) years after treatment with more than 12 mg/m2 of vincristine. Assessment was based on a five-item ped-mTNS, nerve conduction studies (only nerve conduction velocity in the median and sural sensory nerves), and quantitative sensory testing.46,47 The researchers excluded light touch, pinprick, and vibration sensation items from the eight-item ped-mTNS because these CIPN characteristics were assessed by use of quantitative sensory testing. Sensitivity and specificity of the variables of quantitative sensory testing and ped-mTNS were assessed via comparison to sensory nerve conduction studies. The researchers interpreted scores on the basis of published reference standards for nerve conduction velocity and quantitative sensory testing in an age-matched and gender-matched healthy control cohort.
Mean scores for several variables of quantitative sensory testing from the patients with leukaemia were worse than published mean reference scores from healthy controls (p range for the difference 0·019 to <0·0001), showing construct and contrasting group validity. The quantitative sensory testing variables of vibration and light touch detected 86% and 57% of patients with slowed nerve conduction velocity, showing the sensitivity of quantitative sensory testing to detect true abnormalities, although the reduced ped-mTNS did not detect decreased nerve conduction velocity.
FACES pain scale
Only one patient-reported outcome measure for assessing painful neurotoxicity has been tested: the FACES Scale.33 In Smith and colleagues’ study,33 FACES scores were collected repeatedly over 15 weeks in 65 children with acute lymphoblastic leukaemia who were receiving vincristine. Scores reflected the entire 0–5 range, but the mean score was low (0·19, SD 0·70). FACES scores moderately correlated with the TNS-PV neuropathic pain distal to proximal extension item, but not with cumulative vincristine dose or the area under the curve. Furthermore, the FACES scale was not responsive to change over time, perhaps because of the low incidence of painful neurotoxicity (948 [91·4%] of 1037 pain assessments done by patients reported no pain over the 15 weeks). FACES scores were obtainable in nearly all children, regardless of age.
Mobility measures
One cross-sectional, case-control study45 evaluated assessments of CIPN-associated mobility by use of data from instrumented three-dimensional motion analysis and simultaneous surface electromyography of the gastrocnemius and tibialis anterior muscles. Gait characteristics of 17 children aged 5 years and older who had been treated with vincristine for acute lymphoblastic leukaemia, and showed signs of CIPN according to the CTCAE, were compared with assessments from ten healthy controls. Although the researchers did not intend to assess the psychometric properties of these methods, data comparisons with healthy controls allowed assessment of contrasting group construct validity. The individuals did a minimum of six barefoot walking trials along a walkway of 8 m. A three-dimensional motion analysis camera and software system recorded each trial, and surface electromyography data were collected simultaneously. Several additional methods, such as goniometer assessments of passive ankle dorsiflexion range of motion, physical strength examinations, and the unipedal hopping test, quantified gait and movements. When compared with healthy controls, children with CIPN had a spectrum of electromyography, kinematic, kinetic, and temporal spatial deviation throughout the gait cycle (eg, shorter step length, less dorsiflexion and plantar flexion, decreased hip extension and peak knee flexion, atypical timing of electromyography activity, and excessive gastrocnemius and tibialis coactivation). Contrasting group validity was supported; several tests showed statistically significant differences between the two groups.
Quality assessment
Based on the Joanna Briggs Institute (table 1) and QUADAS (table 2) quality assessments of the seven papers included in this Review, insufficient evidence supports the use of most approaches to assess paediatric CIPN. The strongest evidence is reported in two papers33,35 with the highest Joanna Briggs Institute and QUADAS scores; the researchers used rigorous research methods and tested numerous psychometric properties of two variants of the Total Neuropathy Score. However, the five studies33-35,42,43 that analysed variants of the Total Neuropathy Score, four of which are of low-to-moderate quality, have several limitations, suggesting that further psychometric testing is warranted before clinicians and researchers consider the ped-mTNS or the TNS-PV as a gold standard measure.
Discussion
Robust assessment tools designed specifically to detect treatment toxicities in children are a necessary step to improve identification of CIPN in routine clinical practice and in intervention trials. Current evidence suggests that the ped-mTNS and the TNS-PV are the most promising assessment tools; however, these scores are not yet gold standard measures of CIPN. Grading scales (eg, the CTCAE) do not have high sensitivity. Other tools and approaches described in this Review (ie, the FACES scale, quantitative sensory testing, and mobility measures) have not been adequately tested and might not be feasible for routine use, particularly in young children (ie, aged ≤5 years). Future research is needed to expand the scope of testing for the variants of the Total Neuropathy Score, with attention to further shortening the length of, or reducing the number of items in, the measures. In addition, a simple patient-reported outcome measure for CIPN is sorely needed.
The generalisability of the overall findings from the five studies33-35,42,43 that analysed variants of the Total Neuropathy Score is limited by the small sample sizes, which ranged from 20 to 86 participants, and the fact that four of five studies34,35,42,43 were done at a single institution and all five predominantly only assessed vincristine CIPN. Four of the five did not have control group comparisons.33,34,42,43 Intrarater (test-retest) and inter-rater reliability results33,35 should be interpreted carefully because of the short time interval (≤1 h) between the two tests: patients’ recall of initial answers and scores probably informed answers given during the second assessment, resulting in high intraclass correlation coefficients. In one study,35 independent t tests (instead of the preferred paired t tests) were used to evaluate differences between matched cases and controls, which might have compromised statistical validity.48 The cross-sectional designs used in four of the five studies34,35,42,43 limit the evaluation of whether the paediatric variants of the Total Neuropathy Score are responsive to change in neuropathy over time and thus are good tools for use in intervention studies. Not controlling for additional confounding variables also could have threatened the internal validity of these studies. The researchers did not take into account the potential influence of obesity,25 steroid-induced myopathy, and genetic determinants of risk for vincristine neurotoxicity.18,49 Moreover, obtaining accurate reports of pain, numbness, tingling, and pin and vibration sensations from children is often challenging because of competing distractions (eg, other distressing symptoms, noise, and sibling activity), cognitive limitations related to age and developmental stage, and the children’s anxiety about impending scheduled events (eg, painful procedures).
Despite the limitations and some mixed findings of the identified studies, moderately strong evidence supports the reliability and construct validity of the ped-mTNS. However, floor effects limit the sensitivity of this tool and item rescaling might improve performance. Very little evidence supports the responsiveness of the ped-mTNS to changes in neurotoxicity. One study50 that did not meet the eligibility criteria for inclusion in this Review provides data that could be used to support the ped-mTNS’s responsiveness to change. However, this study was not specifically designed to test psychometric properties, and, consequently, the researchers did not do analytical tests (ie, Cohen’s d), or use empirical evidence to support conclusions about responsiveness based on an a priori hypothesis. Furthermore, little evidence indicates that the measure is feasible for use outside research intensive environments. As for the TNS-PV, only one study33 evaluated numerous psychometric properties and provided moderately strong evidence supporting the use of this measure. No evidence supports the appropriateness of these variants of the Total Neuropathy Score for quantifying neurotoxicity caused by drugs other than vincristine. Before the ped-mTNS and the TNS-PV can be considered gold standard paediatric measures of CIPN, stronger psychometric evidence from longitudinal, case-control studies that use larger and more diverse populations is needed.
Given that five studies33-35,42,43 tested two similar Total Neuropathy Score variants for use in children, it is tempting to recommend, on the basis of the collective psychometric evidence, that either variant could be used. However, because each variant uses different items and provides a slightly different summary of neurological deficits, these variants are not directly comparable. For example, when compared with the ped-mTNS, the TNS-PV does not quantify light touch sensation, assesses sensibility to temperature but not to pinprick, and has additional items that evaluate constipation and hoarseness. Moreover, the objective assessment techniques used in the ped-mTNS and the TNS-PV were different (eg, biothesiometer vs tuning fork for vibration sensation and different testing locations and procedures for light touch, pin, strength, and tendon reflexes). Additional evidence from larger and adequately powered, multisite studies of children with various cancers and types of neurotoxicity is needed before these measures can be considered gold standards. Furthermore, shorter variants of the Total Neuropathy Score that require less time and training to administer than the full variant (eg, the V-Rex) will make objective CIPN assessments more feasible.
This Review includes one high-quality study of the Balis grading scale and the CTCAE,33 and one low-quality study34 of solely the CTCAE. Unfortunately, these studies provide insufficient and conflicting evidence of the psychometric properties of these grading scales. Of note, the internal validity evaluated in the low-quality study34 was compromised by the absence of prospective data collection. Furthermore, the single-site study design limited external validity.34 Results from these two studies cannot be extrapolated to CIPN caused by drugs other than vincristine. Although the researchers specifically tested the psychometric properties of these grading scales, they also considered the CTCAE as the gold standard tool to which variants of the Total Neuropathy Score were compared.33,34 Given the known limitations of the CTCAE (based on studies of CIPN in adults),37,51,52 these comparisons should be interpreted cautiously.
Both grading scale studies provide evidence of poor sensitivity, but findings concerning construct validity differ. Evidence from the low-quality study34 suggests that the CTCAE has poor construct validity when compared with the ped-mTNS. The high-quality study33 provides evidence that the CTCAE and Balis scale have construct validity when compared with the TNS-PV and that there is no correlation of the grading scales with vincristine dosage or area under the curve. Based on these findings and those from previously published reports describing grading scale limitations,37,51,52 the CTCAE and Balis scales might be most useful for obtaining crude CIPN measures within busy clinical settings, but should not be used when more sensitive measures of subtle changes in symptom progression are needed (eg, in intervention trials).
Study quality was low for two of three papers that reported on the strength of quantitative sensory testing,44 the FACES pain scale,33 and several mobility assessments.45 A major limitation of the study by Lieber and colleagues44 is the study’s reliance on the testing of nerve conduction velocity, rather than nerve conduction amplitude. Vincristine-induced CIPN, which mainly manifests as an axonopathy,53,54 is characterised by greater reductions in nerve amplitude than nerve velocity, which remains relatively well preserved. Also, although motor CIPN is common in paediatric patients,53 only sensory nerves were tested in Lieber and colleagues’ study. For these reasons, any conclusions regarding the sensitivity and specificity of quantitative sensory testing are probably inaccurate because the reference assessment (nerve conduction velocity solely measured in sensory nerves) is not a sufficiently valid indicator of vincristine-induced CIPN. Another considerable limitation of this study was the use of an unvalidated reduced ped-mTNS, which omits all objective sensory testing. Furthermore, the long battery of quantitative sensory testing requires patient attention and cooperation, limiting the feasibility for use of these tests in children. The internal and external validity of this study is lessened by an insufficient sample size.
Quantitative sensory testing and comprehensive mobility assessments might be impractical for use in the paediatric population. Tests requiring specialised equipment or assessment skills, like those described by Lieber and colleagues44 and Wright and colleagues,45 make routine assessments less feasible. Furthermore, these tests can be uncomfortable for patients. If additional appointments with a specialised provider are required, these tests will cause children to take additional time away from school and will increase the cost of care. Given the complexity inherent in the three-dimensional motion analysis and other mobility assessments, children who are anxious or feeling unwell because of various cancer-related and treatment-related side-effects might not be able to focus on or tolerate the tests.
The one high-quality study33 of the FACES scale did not have a strict control for pain not related to CIPN and has little generalisability beyond vincristine neurotoxicity. Given the numerous limitations of the low-quality study by Wright and colleagues,45 the use of three-dimensional motion analysis and surface electromyography cannot be recommended as reliable and valid measures of gait abnormalities associated with CIPN. Other limitations of the study by Wright and colleagues45 include the cross-sectional design, use of an insensitive CIPN screening measure (CTCAE) and poor methodological and statistical methods (eg, small sample size, no power analysis, and no details regarding assessor training and fidelity procedures), and the absence of a control for confounding variables. Moreover, routinely obtaining an extensive battery of mobility measures in young children might not be feasible.
Unfortunately, a paediatric patient-reported outcome measure for CIPN is not yet available.55 A major barrier to developing patient-reported outcome measures is that symptoms of numbness, tingling, and neuropathic pain are difficult for children to describe, and parental proxy assessments might be invalid. Variants of the Total Neuropathy Score provide important objective assessments of early preclinical signs in trials testing interventions for the prevention of CIPN, but these tools do not adequately quantify the patients’ symptoms. Also, testing the psychometric properties of a patient-reported outcome measure might be problematic. For example, obtaining valid intrarater reliability data can be difficult because patients’ symptoms are not static. Evidence suggests that CIPN severity might fluctuate throughout treatment cycles, while ultimately becoming increasingly severe over time.5,56 Furthermore, the severity of CIPN can vary from hour to hour or day to day. Obtaining multiple assessments over several days is the best approach to obtain valid data, albeit challenging to put into operation.
For children who can read and verbally describe subjective symptoms, the ideal patient-reported outcome measure would quantify subjective numbness, tingling, and pain by use of simple words and fun assessments that are age appropriate and based on tasks. However, patient-reported outcome measures are not feasible for use with children who are too young to read or provide descriptions of their signs and symptoms. For this population, one study33 provides evidence supporting the use of the V-Rex, which is an abbreviated version of the Total Neuropathy Score for quantifying objective indicators (ie, vibration sensibility and tendon reflexes). Moreover, the ideal patient-reported outcome measure would also be easy and inexpensive to administer, could be used alongside an abbreviated Total Neuropathy Score when a more comprehensive assessment is needed, and would not involve uncomfortable or complex testing procedures, nor require specially trained assessors or equipment. Such an easily administered tool could facilitate routine and rapid assessments by oncology nurses during chemotherapy infusions.
Conclusion
Very few high-quality studies have comprehensively tested the psychometric properties of tools for assessing CIPN in children receiving neurotoxic chemotherapy. Given the negative influence of CIPN on general health, normal development, and quality of life for a growing number of paediatric cancer survivors, the identification of effective CIPN measures is sorely needed. Without strong measures, future CIPN intervention studies that can identify ways to cure or mitigate CIPN will not be possible. To advance the science of paediatric assessments quickly and rigorously, we suggest a large international collaboration to assist with putting our recommendations into operation. These recommendations (panel 2) involve doing large scale validation and feasibility studies of existing and newly developed tools for CIPN assessment for use within heterogeneous, paediatric populations.
Panel 2: Future directions for the assessment of chemotherapy-induced peripheral neurotoxicity (CIPN) in children.
Because of the insufficient evidence supporting the use of most approaches for the assessment of paediatric CIPN, we provide the following recommendations for future research:
To gauge their suitability as gold standard assessment tools for paediatric CIPN for various age groups, examine the psychometric properties and feasibility of the Total Neuropathy Score variants–pediatric-modified Total Neuropathy Score and Total Neuropathy Score-pediatric vincristine–in large, adequately powered, multisite studies in diverse paediatric populations with varied cancer diagnoses and neurotoxic drug exposures
Examine the responsiveness of assessment tools over time via prospective studies during active treatment to enable the identification of the measures best suited to early identification of toxic effects
Develop a patient-reported outcome measure that is age appropriate for routine clinical use with children with basic reading and verbal skills
Use appropriate reference standards (such as Total Neuropathy Score variants or sensory and motor nerve conduction studies) for psychometric validation studies
Further test the short-form variants of the Total Neuropathy Score, such as the V-Rex, that require less time and training to administer than the full variant, and can be used to obtain objective assessments in very young children who cannot yet read or verbally describe their symptoms
Supplementary Material
Acknowledgments
GC is the recipient of the Associazione Italiana per la Ricerca sul Cancro IG 18631 grant. SP is supported by the Cancer Institute of New South Wales Program Grant (14/TPG/1–05), National Health and Medical Research Council of Australia Project Grant (#1080521), and Career Development Fellowship (#1148595). RS is supported by the American University of Beirut for doctoral studies in Nursing Science. GAK-L is funded by the American Cancer Society Denny Hoelzer Sentinel Technologies Doctoral Degree Scholarship in Cancer Nursing (DSCN-17–082–01 – SCN). CK is a T32 NR016914–01 trainee in the National Institutes of Health, National Institute for Nursing Research on Complexity: Innovations in Promoting Health and Safety. This study was neither fully, nor in part funded by the National Institutes of Health. No author is in receipt of a grant from the National Institutes of Health related to this study, and no author is employed by the National Institutes of Health.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Ellen M Lavoie Smith, School of Nursing, University of Michigan, Ann Arbor, MI, USA.
Clare Kuisell, School of Nursing, University of Michigan, Ann Arbor, MI, USA.
Grace A Kanzawa-Lee, School of Nursing, University of Michigan, Ann Arbor, MI, USA.
Celia M Bridges, School of Nursing, University of Michigan, Ann Arbor, MI, USA.
Paola Alberti, Experimental Neurology Unit, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Milan Center for Neuroscience, Milan, Italy.
Guido Cavaletti, Experimental Neurology Unit, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Milan Center for Neuroscience, Milan, Italy.
Rima Saad, Hariri School of Nursing, American University of Beirut, Beirut, Lebanon.
Susanna Park, Brain and Mind Centre, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia.
References
- 1.Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA. Pediatric chemotherapy induced peripheral neuropathy: a systematic review of current knowledge. Cancer Treat Rev 2016; 50: 118–28. [DOI] [PubMed] [Google Scholar]
- 2.Tay CG, Lee VWM, Ong LC, Goh KJ, Ariffin H, Fong CY. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 2017; 64: e26471. [DOI] [PubMed] [Google Scholar]
- 3.Mora E, Smith EML, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res 2016; 6: 2416–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St Jude Lifetime Cohort Study. Arch Phys Med Rehabil 2013; 94: 1451–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoie Smith EM, Li L, Chiang C, et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst 2015; 20: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng DJ, Krull KR, Chen Y, et al. Long-term psychological and educational outcomes for survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. Cancer 2018; 124: 3220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandula T, Farrar MA, Cohn RJ, et al. Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol 2018; 75: 980–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velasco R, Alberti P, Bruna J, Psimaras D, Argyriou AA. Bortezomib and other proteosome inhibitors-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst 2019; 24 (suppl 2): S52–62. [DOI] [PubMed] [Google Scholar]
- 9.Liew WKM, Pacak CA, Visyak N, Darras BT, Bousvaros A, Kang PB. Longitudinal patterns of thalidomide neuropathy in children and adolescents. J Pediatr 2016; 178: 227–32. [DOI] [PubMed] [Google Scholar]
- 10.Islam B, Lustberg M, Staff NP, Kolb N, Alberti P, Argyriou AA. Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst 2019; 24 (suppl 2): S63–73. [DOI] [PubMed] [Google Scholar]
- 11.Langholz B, Skolnik JM, Barrett JS, et al. Dactinomycin and vincristine toxicity in the treatment of childhood cancer: a retrospective study from the Children’s Oncology Group. Pediatr Blood Cancer 2011; 57: 252–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriyama B, Henning SA, Leung J, et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55: 290–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikanjam M, Sun A, Albers M, et al. Vincristine-associated neuropathy with antifungal usage: a Kaiser northern California experience. J Pediatr Hematol Oncol 2018; 40: e273–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibañez-Juliá MJ, Berzero G, Reyes-Botero G, et al. Antineoplastic agents exacerbating Charcot Marie Tooth disease: red flags to avoid permanent disability. Acta Oncol 2018; 57: 403–11. [DOI] [PubMed] [Google Scholar]
- 15.Ceppi F, Langlois-Pelletier C, Gagné V, et al. Polymorphisms of the vincristine pathway and response to treatment in children with childhood acute lymphoblastic leukemia. Pharmacogenomics 2014; 15: 1105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egbelakin A, Ferguson MJ, MacGill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2011; 56: 361–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abaji R, Ceppi F, Patel S, et al. Genetic risk factors for VIPN in childhood acute lymphoblastic leukemia patients identified using whole-exome sequencing. Pharmacogenomics 2018; 19: 1181–93. [DOI] [PubMed] [Google Scholar]
- 18.Skiles JL, Chiang C, Li CH, et al. CYP3A5 genotype and its impact on vincristine pharmacokinetics and development of neuropathy in Kenyan children with cancer. Pediatr Blood Cancer 2018; 65: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 2014; 155: 2461–70. [DOI] [PubMed] [Google Scholar]
- 20.Kerckhove N, Collin A, Condé S, Chaleteix C, Pezet D, Balayssac D. Long-term effects, pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: a comprehensive literature review. Front Pharmacol 2017; 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr 2010; 77: 97–100. [DOI] [PubMed] [Google Scholar]
- 22.van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: a systematic review. Crit Rev Oncol Hematol 2017; 114: 114–30. [DOI] [PubMed] [Google Scholar]
- 23.Bixby C, Cao L, Weiss A, Koh WY, Li E. Chemotherapy-induced peripheral neuropathy in pediatric patients: risk factors identified by retrospective review. Pediatr Blood Cancer 2017; 64: S67. [Google Scholar]
- 24.Li T, Luo L, Ren J, et al. Superior outcomes in relapsed/refractory childhood B-ALL than adult patients with CD19 CAR-T cell therapy: a single-center pilot prospective study of 23 patients. Blood 2019; 134 (suppl 1): 1353 (abstr). [Google Scholar]
- 25.Sajdyk TJ, Boyle FA, Foran KS, et al. Obesity as a potential risk factor for vincristine-induced peripheral neuropathy. J Pediatr Hematol Oncol 2019; published online October 18. DOI: 10.1097/MPH.0000000000001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradfield SM, Sandler E, Geller T, Tamura RN, Krischer JP. Glutamic acid not beneficial for the prevention of vincristine neurotoxicity in children with cancer. Pediatr Blood Cancer 2015; 62: 1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mokhtar GM, Shaaban SY, Elbarbary NS, Fayed WA. A trial to assess the efficacy of glutamic acid in prevention of vincristine-induced neurotoxicity in pediatric malignancies: a pilot study. J Pediatr Hematol Oncol 2010; 32: 594–600. [DOI] [PubMed] [Google Scholar]
- 28.Anghelescu DL, Faughnan LG, Jeha S, et al. Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2011; 57: 1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akbayram S, Akgun C, Doğan M, Sayin R, Caksen H, Oner AF. Use of pyridoxine and pyridostigmine in children with vincristine-induced neuropathy. Indian J Pediatr 2010; 77: 681–83. [DOI] [PubMed] [Google Scholar]
- 30.Tomasello C, Pinto RM, Mennini C, Conicella E, Stoppa F, Raucci U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: a preliminary study. Pediatr Blood Cancer 2018; 65: e27064. [DOI] [PubMed] [Google Scholar]
- 31.Smolik S, Arland L, Hensley MA, et al. Assessment tools for peripheral neuropathy in pediatric oncology: a systematic review from the Children’s Oncology Group. J Pediatr Oncol Nurs 2018; 35: 267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohrmann C, Armer J, Hayashi RJ. Challenges evaluating chemotherapy-induced peripheral neuropathy in childhood cancer survivors: which instrument should nurses use? J Pediatr Oncol Nurs 2017; 34: 106–14. [DOI] [PubMed] [Google Scholar]
- 33.Lavoie Smith EM, Li L, Hutchinson RJ, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs 2013; 36: E49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilchrist LS, Marais L, Tanner L. Comparison of two chemotherapy-induced peripheral neuropathy measurement approaches in children. Support Care Cancer 2014; 22: 359–66. [DOI] [PubMed] [Google Scholar]
- 35.Gilchrist LS, Tanner L. The pediatric-modified total neuropathy score: a reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer 2013; 21: 847–56. [DOI] [PubMed] [Google Scholar]
- 36.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffith KA, Merkies IS, Hill EE, Cornblath DR. Measures of chemotherapy-induced peripheral neuropathy: a systematic review of psychometric properties. J Peripher Nerv Syst 2010; 15: 314–25. [DOI] [PubMed] [Google Scholar]
- 38.Moola S, Munn Z, Sears K, et al. Conducting systematic reviews of association (etiology): the Joanna Briggs Institute’s approach. Int J Evid-Based Healthc 2015; 13: 163–69. [DOI] [PubMed] [Google Scholar]
- 39.Robertson-Malt S. Presenting and interpreting findings. Am J Nurs 2014; 114: 49–54. [DOI] [PubMed] [Google Scholar]
- 40.Joanna Briggs Institute. Critical Appraisal Tools. 2017. https://joannabriggs.org/critical_appraisal_tools (accessed May 7, 2019).
- 41.Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilchrist LS, Tanner L, Hooke MC. Measuring chemotherapy-induced peripheral neuropathy in children: development of the ped-mTNS and pilot study results. Rehabil Oncol 2009; 27: 7–15. [Google Scholar]
- 43.Gilchrist LS, Tanner LR. Short-term recovery of balance control: association with chemotherapy-induced peripheral neuropathy in pediatric oncology. Pediatr Phys Ther 2018; 30: 119–24. [DOI] [PubMed] [Google Scholar]
- 44.Lieber S, Blankenburg M, Apel K, Hirschfeld G, Hernáiz Driever P, Reindl T. Small-fiber neuropathy and pain sensitization in survivors of pediatric acute lymphoblastic leukemia. Eur J Paediatr Neurol 2018; 22: 457–69. [DOI] [PubMed] [Google Scholar]
- 45.Wright MJ, Twose DM, Gorter JW. Gait characteristics of children and youth with chemotherapy induced peripheral neuropathy following treatment for acute lymphoblastic leukemia. Gait Posture 2017; 58: 139–45. [DOI] [PubMed] [Google Scholar]
- 46.Maier C, Baron R, Tölle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010; 150: 439–50. [DOI] [PubMed] [Google Scholar]
- 47.Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006; 10: 77–88. [DOI] [PubMed] [Google Scholar]
- 48.Niven DJ, Berthiaume LR, Fick GH, Laupland KB. Matched case-control studies: a review of reported statistical methodology. Clin Epidemiol 2012; 4: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Sajdyk T, Smith EML, et al. Genetic variants associated with vincristine-induced peripheral neuropathy in two populations of children with acute lymphoblastic leukemia. Clin Pharmacol Ther 2019; 105: 1421–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilchrist LS, Tanner LR, Ness KK. Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer 2017; 64: 180–87. [DOI] [PubMed] [Google Scholar]
- 51.Frigeni B, Piatti M, Lanzani F, et al. Chemotherapy-induced peripheral neurotoxicity can be misdiagnosed by the National Cancer Institute Common Toxicity scale. J Peripher Nerv Syst 2011; 16: 228–36. [DOI] [PubMed] [Google Scholar]
- 52.Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol 1998; 9: 739–44. [DOI] [PubMed] [Google Scholar]
- 53.Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol 2014; 29: 932–37. [DOI] [PubMed] [Google Scholar]
- 54.Reinders-Messelink HA, Van Weerden TW, Fock JM, et al. Mild axonal neuropathy of children during treatment for acute lymphoblastic leukaemia. Eur J Paediatr Neurol 2000; 4: 225–33. [DOI] [PubMed] [Google Scholar]
- 55.Johnston DL, Sung L, Stark D, Frazier AL, Rosenberg AR. A systematic review of patient-reported outcome measures of neuropathy in children, adolescents and young adults. Support Care Cancer 2016; 24: 3723–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pachman DR, Qin R, Seisler D, et al. Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 2016; 24: 5059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


