Abstract
Phenyl rings are one of the most prevalent structural moieties in active pharmaceutical ingredients, even if they often contribute to poor physico-chemical properties. Herein, we propose the use of a bridged piperidine (BP) moiety as a phenyl bioisostere, which could also be seen as a superior phenyl alternative as it led to strongly improved drug like properties, in terms of solubility and lipophilicity. Additionally, this BP moiety compares favorably to the recently reported saturated phenyl bioisosteres. We applied this concept to our γ-secretase modulator (GSM) project for the potential treatment of Alzheimer's disease delivering clinical candidates.
We propose the use of a bridged piperidine moiety as a phenyl bioisostere, leading to strongly improved drug like properties. This concept was applied to the discovery of γ-secretase modulators for the potential treatment of Alzheimer's disease.
1. Introduction
The unmet need in Alzheimer's disease (AD) for effective disease modifying therapies (DMT) is huge since currently approved treatments are purely symptomatic and provide only modest and transient, short-lasting benefit. AD is an age-related chronic neurodegenerative disease that manifests in progressive cognitive decline followed by gradual changes in function, ultimately resulting in death.1 The pathological hallmarks of AD are brain deposits of amyloid (“plaques”)2,3 and tau protein (“tangles”),3,4 with amyloid deposits appearing first, decades before any measurable cognitive impairment. The main component of the amyloid plaques in AD brains is amyloid beta (Aβ), which consists of peptides of various amino acid lengths. These peptides are derived from the amyloid precursor protein (APP), which is cleaved by β-secretase and γ-secretase to yield Aβ.5,6 Aβ molecules can aggregate to form oligomers which can exist in various forms and finally lead to the amyloid pathology in AD.
Modulating – instead of inhibiting – γ-secretase represents a very compelling approach to reduce neurotoxic Aβ42, the major constituent of amyloid plaques, by shifting the cleavage to form smaller, more soluble Aβ peptides. First, because the imbalance between production and clearance of Aβ42 and related Aβ peptides is a very early, initiating factor in amyloid plaque formation. Secondly, because mutations causing familial AD occur either in the substrate APP or in the protease of the reaction that generates Aβ (i.e. presenilin, the catalytic site of γ-secretase). Early-onset familial AD forms are all characterized by increased production of Aβ42. Soluble Aβ42 oligomers from AD patients' brains can decrease synapse density, inhibit long-term potentiation, and enhance long-term synaptic depression also in rodent hippocampus; injecting them into healthy rats impairs their memory. From a safety aspect, GSMs are different from γ-secretase inhibitors (GSIs), which have been abandoned as potential AD therapies due to their serious safety liabilities. This is due to the fact that GSMs do not inhibit processing of Notch and other protein substrates of γ-secretase.7
2. Study design and rational
This collective evidence triggered our interest for preparing GSMs for the potential treatment of AD and explains why a number of GSMs, reaching various development stages, have been reported.8–10 A decade ago, we disclosed a novel series of GSM of general formula 1 (Fig. 1) containing a phenyl linker between the hetero-aryl moiety and the aminotriazole fragment.11 Shortly afterwards, we realized that an aromatic linker was not mandatory for potency and that it could therefore be advantageously replaced by a saturated bridged piperidine moiety, leading to compounds of the type 2.12 More recently, this has been used in the design of a novel series as exemplified by the compound 3 with interesting properties.13 We then incorporated this bridged-piperidine moiety in molecule (S)-4, which upon successful minitox and dose range finding toxicology studies, underwent entry into human enabling GLP-Tox study.14
Fig. 1. Chemical structures of compounds 1–4.
Phenyl rings are one of the most prevalent structural moieties in pharmaceutically active compounds with more than 500 drugs as well as agrochemicals containing this 6-membered aromatic structure.15 Due to their high aromaticity (Fsp3 = 0),16 they may contribute to undesirable biopharmaceutical properties, such as low permeability, solubility and non-specific binding17 – especially in cases in which the analogs contain additional aromatic moieties.18 Non-classical phenyl bioisosteres with high fraction of sp3 centers have recently gained a lot of attention, as a means to “escape from flatland” and improve physicochemical properties, as well as gain intellectual property rights.15,19–21 These types of sp3-rich phenyl bioisosteres is best utilized in scenarios in which the phenyl ring of the parent molecule does not engage in any productive π–π stacking interaction. An example of a non-classical phenyl bioisostere mimicking para-di-substituted phenyls is the bicyclo[1.1.1]pentane (BCP) unit, which was successfully applied to, e.g., the γ-secretase inhibitor avagacestat,22 lipoprotein-associated phospholipase A2 inhibitors,23 resveratrol24 and indoleamine-2,3-dioxygenase 1 inhibitors.25 Recent improvements in synthetic methods to access the BCP moiety, as well as its close analogues now allows ready integration of this bridgehead moiety.26–31 Relative to the BCP system, the C–C distance of the corresponding bicyclo[2.2.2]octane motif (BCO; BCO – 2.7 Å vs. BCP – 1.9 Å) (Fig. 2) is closer to that of a phenyl group (2.8 Å) and its use has been successfully exemplified in daclatasvir analogs,32 and an MDM2 inhibitor phase 1 candidate.33 The cubane C–C distance (2.6 Å) is similar to that of the BCO moiety and, therefore, cubanes have also been included in the list of non-traditional phenyl bioisosteres.34 Recently, cubanes have been explored with parent molecules such as the chemotherapeutic vorinostat, anesthetic benzocaine35 as well as in the optimization of antimalarial agents.36 Recently a new generation of benzene mimetics, resembling meta-disubstituted benzenes, was also reported.37
Fig. 2. Panel A. Views of phenyl, BP, BCP, BCO linkers connected to a C-atom. Panel B. Views of phenyl, BP, BCP, BCO linkers connected to a N-atom and an aromatic moiety. Panel C. Characteristics of the linkers. Distance (D) expressed in Å, angle in ° and van der Waals volume of the linker itself in ml mol−1.
In this research article, we aim at providing a comparison between our newly proposed bridged piperidine (BP) as phenyl bioisostere and its BCP, BCO and phenyl homologues (Fig. 2).
The panel A displays those four moieties simply bound to a C-atom on both sides, whereas the second panel B presents them link to an aromatic moiety on one side and a N-atom on the other, with representative data presented in panel C. In this case, the aromatic moiety influences the pyrimidality of the BP nitrogen atom. In this manuscript, we focus on a comparison of the N-link version of these groups. The N-linked bioisosteres are particularly important since they remove the structural alert of the corresponding aniline.
For this purpose, we planned to systematically use those four linkers within three chemically different GSM series (Fig. 3). We will compare their impact on the in vitro potency, with further emphasis on key physicochemical properties such as lipophilicity and solubility.
Fig. 3. Three series of study design compounds with different linkers.
3. Results and discussion
The resulting 16 compounds described Fig. 3 have been prepared and tested in vitro for potency, solubility and lipophilicity. The data are summarized in Tables 1–3 for the series 1–3, respectively.
Potency and physicochemical properties of the series 1 with the 4 linkers.
IC50 Aβ42 in nM in human H4 cells.
Log D determination by high-throughput shake-flask.
Solubility expressed in μg mL−1 and measured in Lysa assay.
Potency and physicochemical properties of the series 2 with the 4 linkers.
IC50 Aβ42 in nM in human H4 cells.
Log D determination by high-throughput shake-flask.
Solubility expressed in μg mL−1 and measured in Lysa assay.
Potency and physicochemical properties of the series 3-1 and 3-2 with the 4 linkers.
| Cpd series 3-2 | 17 | 18 | 19 | 20 |
|---|---|---|---|---|
| R = p-OCF3 | ||||
| IC50a | 227 | 46 | 597 | 221 |
| Log Db | >4 | 3.5 | 2.8 | 3.7 |
| Solubilityc | <0.1 | 106 | 3.2 | 0.4 |
IC50 Aβ42 in nM in human H4 cells.
Log D determination by high-throughput shake-flask.
Solubility expressed in μg mL−1 and measured in Lysa assay.
In this triazole series 1, whilst the compound 5, containing the phenyl as a linker showed poor-drug like properties with a solubility below 0.1 μg mL−1 and a very high lipophilicity (log D > 4), the use of the phenyl bioisosteres BP, BCP and BCO improves those parameters. Of special note, the BP derivative 6 afforded the strongest impact on solubility (104 μg mL−1) and on lipophilicity (log D = 3.6), whilst being also the most potent (42 nM) of the series. The pKa of the nitrogen atom of the BP motif adjacent to the oxadiazole has been experimentally determined to be 2.3. The BCP has a similar improved solubility as compared to BP, whereas the influence of BCO, as certainly expected, is more limited. Fig. 4, displays an excellent alignment between the phenyl derivative 5 and its corresponding BP analogue 6.
Fig. 4. Structural alignment between 5 (in grey) and 6 (in blue).
In the second evaluated pyrimidine series (2), we observed a similar effect as in the first series. Both, the BP and BCP linkers significantly improved the solubility whereas BCO stood below the measurable threshold. In terms of potency, the BP was again the most potent.
Finally in the third series, 3-1 and 3-2, all the three bioisosteres significantly improved the drug-like properties as compared to the original phenyl linker derivatives 13 and 17. The largest impacts are achieved with the BP and BCP with regards of the drastically increased aqueous solubility, particularly for the BP from <0.1 to >100 μg mL−1, and reduction of up to 1 log unit of the lipophilicity for the BCP derivative. Overall, the three tables present a consistent picture, that the BP derivatives offer superior profiles in comparison to the phenyl alternatives.
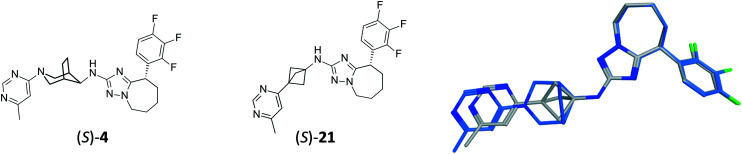
As a final match pair analysis, we replaced the bridged piperidine moiety of our clinical candidate (S)-4 with a BCP, leading to compound (S)-21, as a way to reduce its lipophilicity (Fig. 5). To prepare compound (S)-21, a novel route had to be developed for the introduction of the pyrimidine moiety on a BCP derivative, and is described Scheme 3.
Fig. 5. Chemical structure of (S)-4, its match pair BCP analog (S)-21 and their structural alignment.
Scheme 3. Reagents and conditions: (a) Fe(Pc), TBHP, Cs2CO3, di-tert-butyl azodicarboxylate, MeCN, −20 °C to RT, 52%; (b) 6 M HCl in MeOH, RT; (c) RANEY® Ni, H2, MeOH, RT, quantitative; (d) 1,1′-thiocarbonylbis(pyridin-2(1H)-one, iPr2NEt, CH2Cl2, RT; (e) 7 M NH3 in MeOH, RT; (f) MeI, EtOH, 75 °C, 71%; (g) 6-chloro-2-(2,3,4-trifluorophenyl)hexanoic acid, Et3N, T3P®, DMF, RT; h) N2H4·H2O, DMF, 60 °C, 25%; (i) LiCl, NaOtBu, DMF, 50 °C, 74%; (j) chiral HPLC separation; (k) tBuXPhos, Cs2CO3, Pd2(dba)3, 1,4-dioxane, 110 °C, failed.
The data are summarized Table 4. As expected, based on the alignment Fig. 5, the in vitro potency remains similar. The lipophilicity is reduced (log D from 3.8 to 3.4), whereas solubility remains comparable.
Potency and physicochemical properties of compound (S)-4 and (S)-21.
IC50 Aβ42 in nM in human H4 cells.
Log D determination by high-throughput shake-flask.
Solubility expressed in μg mL−1 and measured in Lysa assay.
4. Chemistry
4.1. Preparation of the amino-linker-oxadiazole derivatives
The preparation of the versatile amino-linker oxadiazole derivatives 22–25 is described Scheme 1. Derivative 22 bearing the phenyl linker, 4-(5-methyl-1,3,4-oxadiazol-2-yl)aniline is commercially available, the analogue 23 with the bridge piperidine moiety as a linker was prepared according to our reported procedure.13 For the BCP and BCO analogues, the synthesis started from methyl 3-aminobicyclo[1.1.1]pentane-1-carboxylate 26a and methyl 4-aminobicyclo[2.2.2]octane-1-carboxylate 26b respectively. Boc protection of the amino function followed by treatment with hydrazine converted the esters into the corresponding hydrazides 27a–27b in high yield. The intramolecular cyclisation was promoted with a propylphosphonic anhydride solution (T3P®) to yield the oxadiazoles 28a and 28b. Finally, a TFA-mediated deprotection afforded the derivatives 24 and 25.
Scheme 1. Reagents and conditions: (a) i. Boc2O, iPr2NEt, THF, RT, overnight; ii. Hydrazine hydrate (80% in water), MeOH, 80 °C. (b) T3P® (50% in EtOAc), AcOH, Et3N, EtOAc, microwave 150 °C, 0.5 h. (c) TFA, CH2Cl2, RT, 1 h.
4.2. Preparation of final derivatives 5–20
The 4 amino-linker-oxadiazole derivatives 22–25 were coupled with the building blocks 29–31 (preparation described in ESI†) in order to provide in a single step the set of compounds 5–20 used to assess and compare their potency and physicochemical properties (Scheme 2). All couplings were performed using a palladium catalyzed Buchwald–Hartwig process with the exception of the coupling of the building block 30 with the amino linker-oxadiazole 23–25 which was performed as nucleophilic aromatic substitutions (SNAr).
Scheme 2. Conditions: (a) Buchwald–Hartwig coupling (b) SNAr.
4.3. Preparation of compound (S)-21
The synthesis of the key pyrimidyl-[1.1.1]-bicylopentylamine scaffold 35 relied on a one-pot radical multicomponent iron(ii) phthalocyanine catalysed carboamination of [1.1.1]-propellane,38 to prepare hydrazide 34. Deprotection under acidic conditions and hydrogenation readily afforded amine 35. Unfortunately, a convergent approach featuring Buchwald–Hartwig coupling of bromide 38 with amine 35 failed, with protodebromination of 38 and fragmentation of the [1.1.1]-bicyclopentane core,39 being the only products. Hence, a more linear elaboration to the final product was used; conversion of amine 35 into N-methylthiourea 36, followed by acylation and cyclisation afforded the triazole 37, which subsequently underwent a regioselective base-induced cyclisation to afford racemic 21. A preparative chiral HPLC separation delivered the desired [1.1.1]-bicyclopentyl analog (S)-21.
5. Conclusions
The bridged piperidine (BP) moiety was successfully used as a phenyl bioisostere, displaying a systematic enhancement of the drug-like properties in terms of solubility and lipophilicity. Within this specific environment, an heterocycle on one side, and a N-link moiety on the other, this BP moiety compares favourably to the recently reported saturated phenyl bioisosteres BCP and BCO, thus being successfully employed within our GSM project. We believe that this alternative to the phenyl moiety will certainly find its space within the medicinal chemists' arsenal for the preparation of novel drugs.
Abbreviations used
- Aβ
Amyloid beta
- AD
Alzheimer's disease
- APP
Amyloid precursor protein
- DMF
N,N-Dimethylformamide
- BCO
Bicyclo[2.2.2]octane
- BCP
Bicyclo[1.1.1]pentane
- BP
Bridged piperidine
- GS
γ-Secretase
- GSM
γ-Secretase modulator
Calculations and alignments
Density functional calculations were performed using B3LYP/6-311G**. The Jaguar package (release 2020-2, Schrödinger, LLC, New York, NY) was used for all calculations. Initial conformations were assigned using Amber10:EHT forcefield minimization in MOE (release 2020.09, Chemical Computing Group, Montreal). Overlays of minimized structures were performed using rigid body alignment in MOE.
Conflicts of interest
There is no conflict of interest to declare.
Supplementary Material
Acknowledgments
We thank Thierry Meyer and Beat Frei for the synthesis of some of the compounds described. The research described in the manuscript was funded by F. Hoffmann-La Roche AG.
Electronic supplementary information (ESI) available: Full protocols for the following in vitro assays: cellular Aβ secretion assay, lipophilicity (log D) and solubility (Lysa). Synthetic routes and procedures, characterisation of the compounds described (1H NMR and MS). 1H NMR spectra of newly prepared compounds 7, 8, 11, 12, 15, 16, 19, 20, 21, 24 and 25. See DOI: 10.1039/d1md00043h
References
- Alzheimer's Association 2019 Alzheimer's disease facts and figures. Alzheimer's Dementia. 2019;15:367–429. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Hardy J. Allsop D. Amyloid deposition as the central event in the etiology of Alzheimer's disease. Trends Pharmacol. Sci. 1991;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Lee V. M. Y. Balin B. J. Otvos, , Jr L. Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Coronel R. Palmer C. Bernabeu-Zornoza A. Monteagudo M. Rosca A. Zambrano A. Liste I. Physiological effects of amyloid precursor protein and its derivatives on neural stem cell biology and signaling pathways involved. Neural Regener. Res. 2019;14(10):1661–1671. doi: 10.4103/1673-5374.257511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp W. G. Sarkar I. N. Origins of amyloid-β. BMC Genomics. 2013;14:290. doi: 10.1186/1471-2164-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde T. E. Koo E. H. Felsenstein K. M. Osborne B. A. Miele L. γ-Secretase inhibitors and modulators. Biochim. Biophys. Acta, Biomembr. 2013;1828(12):2898–2907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursavich M. G. Harrison B. A. Blain J.-F. Gamma Secretase Modulators: New Alzheimer's Drugs on the Horizon? J. Med. Chem. 2016;59(16):7389–7409. doi: 10.1021/acs.jmedchem.5b01960. [DOI] [PubMed] [Google Scholar]
- Boy K. M. Guernon J. M. Zuev D. S. Xu L. Zhang Y. Shi J. Marcin L. R. Higgins M. A. Wu Y.-J. Krishnananthan S. Li J. Trehan A. Smith D. Toyn J. H. Meredith J. E. Burton C. R. Kimura S. R. Zvyaga T. Zhuo X. Lentz K. A. Grace J. E. Denton R. Morrison J. S. Mathur A. Albright C. F. Ahlijanian M. K. Olson R. E. Thompson L. A. Macor J. E. Identification and Preclinical Evaluation of the Bicyclic Pyrimidine γ-Secretase Modulator BMS-932481. ACS Med. Chem. Lett. 2019;10(3):312–317. doi: 10.1021/acsmedchemlett.8b00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. E. Carrieri C. Dela Cruz F. Fullerton T. Hajos-Korcsok E. He P. Kantaridis C. Leurent C. Liu R. Mancuso J. Mendes da Costa L. Qiu R. Pharmacokinetic and Pharmacodynamic Effects of a γ-Secretase Modulator, PF-06648671, on CSF Amyloid-β Peptides in Randomized Phase I Studies. Clin. Pharmacol. Ther. 2020;107(1):211–220. doi: 10.1002/cpt.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K., Goetschi E., Green L., Jolidon S., Knust H., Limberg A., Luebbers T. and Thomas A., Preparation of phenyltriazolopyridinylimidazolylphenylamine derivatives and analogs for use as gamma secretase modulators, EP511842011092272, 20110128, 2011
- Baumann K., Green L., Limberg A., Luebbers T. and Thomas A., Preparation of bridged piperidine derivatives for use as amyloid beta modulators, EP533012012116965, 20120228, 2012
- Rodriguez Sarmiento R. M. Bissantz C. Bylund J. Limberg A. Neidhart W. Jakob-Roetne R. Wang L. Baumann K. Stepwise Design of γ-Secretase Modulators with an Advanced Profile by Judicious Coordinated Structural Replacements and an Unconventional Phenyl Ring Bioisostere. J. Med. Chem. 2020;63(15):8534–8553. doi: 10.1021/acs.jmedchem.0c00909. [DOI] [PubMed] [Google Scholar]
- Ratni H. Alker A. Bartels B. Bissantz C. Chen W. Gerlach I. Limberg A. Lu M. Neidhart W. Pichereau S. Reutlinger M. Rodriguez-Sarmiento R.-M. Jakob-Roetne R. Schmitt G. Zhang E. Baumann K. Discovery of RO7185876, a Highly Potent γ-Secretase Modulator (GSM) as a Potential Treatment for Alzheimer's Disease. ACS Med. Chem. Lett. 2020;11(6):1257–1268. doi: 10.1021/acsmedchemlett.0c00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko A. Garbuz P. Shishkina S. V. Voloshchuk N. M. Mykhailiuk P. K. Saturated Bioisosteres of ortho-Substituted Benzenes. Angew. Chem., Int. Ed. 2020;59(46):20515–20521. doi: 10.1002/anie.202004183. [DOI] [PubMed] [Google Scholar]
- Lovering F. Bikker J. Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009;52(21):6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Auberson Y. P. Brocklehurst C. Furegati M. Fessard T. C. Koch G. Decker A. La Vecchia L. Briard E. Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem. 2017;12(8):590–598. doi: 10.1002/cmdc.201700082. [DOI] [PubMed] [Google Scholar]
- Hill A. P. Young R. J. Getting physical in drug discovery: a contemporary perspective on solubility and hydrophobicity. Drug Discovery Today. 2010;15(15/16):648–655. doi: 10.1016/j.drudis.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Bauer M. R. Di Fruscia P. Lucas S. C. C. Michaelides I. N. Nelson J. E. Storer R. I. Whitehurst B. C. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 2021 doi: 10.1039/D0MD00370K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykhailiuk P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 2019;17(11):2839–2849. doi: 10.1039/c8ob02812e. [DOI] [PubMed] [Google Scholar]
- Fessard T. C. Goncharenko K. Lefebvre Q. Salome C. Pushing the frontiers of accessible chemical space to unleash design creativity and accelerate drug discovery. Chimia. 2020;74(10):803–807. doi: 10.2533/chimia.2020.803. [DOI] [PubMed] [Google Scholar]
- Stepan A. F. Subramanyam C. Efremov I. V. Dutra J. K. O'Sullivan T. J. DiRico K. J. McDonald W. S. Won A. Dorff P. H. Nolan C. E. Becker S. L. Pustilnik L. R. Riddell D. R. Kauffman G. W. Kormos B. L. Zhang L. Lu Y. Capetta S. H. Green M. E. Karki K. Sibley E. Atchison K. P. Hallgren A. J. Oborski C. E. Robshaw A. E. Sneed B. O'Donnell C. J. Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem. 2012;55(7):3414–3424. doi: 10.1021/jm300094u. [DOI] [PubMed] [Google Scholar]
- Measom N. D. Down K. D. Hirst D. J. Jamieson C. Manas E. S. Patel V. K. Somers D. O. Investigation of a Bicyclo[1.1.1]pentane as a Phenyl Replacement within an LpPLA2 Inhibitor. ACS Med. Chem. Lett. 2017;8(1):43–48. doi: 10.1021/acsmedchemlett.6b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Y. L. Cui Y. T. Pendharkar V. Adsool V. A. Toward Resolving the Resveratrol Conundrum: Synthesis and in Vivo Pharmacokinetic Evaluation of BCP-Resveratrol. ACS Med. Chem. Lett. 2017;8(5):516–520. doi: 10.1021/acsmedchemlett.7b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Q. Zhang H. Guo L. Cheng M. Doty A. C. Ferguson H. Fradera X. Lesburg C. A. McGowan M. A. Miller J. R. Geda P. Song X. Otte K. Sciammetta N. Solban N. Yu W. Sloman D. L. Zhou H. Lammens A. Neumann L. Bennett D. J. Pasternak A. Han Y. Discovery of Potent and Orally Available Bicyclo[1.1.1]pentane-Derived Indoleamine-2,3-dioxygenase 1 (IDO1) Inhibitors. ACS Med. Chem. Lett. 2020;11(8):1548–1554. doi: 10.1021/acsmedchemlett.0c00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. Nhat Pham L. Selected Topics in the Syntheses of Bicyclo[1.1.1]pentane (BCP) Analogues. Asian. J. Org. Chem. 2020;9(1):8–22. [Google Scholar]
- Nugent J. Arroniz C. Shire B. R. Sterling A. J. Pickford H. D. Wong M. L. J. Mansfield S. J. Caputo D. F. J. Owen B. Mousseau J. J. Duarte F. Anderson E. A. A General Route to Bicyclo[1.1.1]pentanes through Photoredox Catalysis. ACS Catal. 2019;9(10):9568–9574. [Google Scholar]
- Caputo D. F. J. Arroniz C. Durr A. B. Mousseau J. J. Stepan A. F. Mansfield S. J. Anderson E. A. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 2018;9(23):5295–5300. doi: 10.1039/c8sc01355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J. Shire B. R. Caputo D. F. J. Pickford H. D. Nightingale F. Houlsby I. T. T. Mousseau J. J. Anderson E. A. Synthesis of All-Carbon Disubstituted Bicyclo[1.1.1]pentanes by Iron-Catalyzed Kumada Cross-Coupling. Angew. Chem., Int. Ed. 2020;59(29):11866–11870. doi: 10.1002/anie.202004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychek R. M. Hutskalova V. Bas Y. P. Zaporozhets O. A. Zozulya S. Levterov V. V. Mykhailiuk P. K. Difluoro-Substituted Bicyclo[1.1.1]pentanes for Medicinal Chemistry: Design, Synthesis, and Characterization. J. Org. Chem. 2019;84(23):15106–15117. doi: 10.1021/acs.joc.9b01947. [DOI] [PubMed] [Google Scholar]
- Ma X. Sloman D. L. Han Y. Bennett D. J. A Selective Synthesis of 2,2-Difluorobicyclo[1.1.1]pentane Analogues: “BCP-F2”. Org. Lett. 2019;21(18):7199–7203. doi: 10.1021/acs.orglett.9b02026. [DOI] [PubMed] [Google Scholar]
- Zhong M. Peng E. Huang N. Huang Q. Huq A. Lau M. Colonno R. Li L. Discovery of functionalized bisimidazoles bearing cyclic aliphatic-phenyl motifs as HCV NS5A inhibitors. Bioorg. Med. Chem. Lett. 2014;24(24):5731–5737. doi: 10.1016/j.bmcl.2014.10.057. [DOI] [PubMed] [Google Scholar]
- Aguilar A. Lu J. Liu L. Du D. Bernard D. McEachern D. Przybranowski S. Li X. Luo R. Wen B. Sun D. Wang H. Wen J. Wang G. Zhai Y. Guo M. Yang D. Wang S. Discovery of 4-((3'R,4'S,5'R)-6′′-Chloro-4'-(3-chloro-2-fluorophenyl)-1'-ethyl-2′′-oxodispiro[cyclohexane-1,2'-pyrrolidine-3',3′′-indoline]-5'-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017;60(7):2819–2839. doi: 10.1021/acs.jmedchem.6b01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekie T. A. Williams C. M. Rendina L. M. Kassiou M. Cubanes in Medicinal Chemistry. J. Med. Chem. 2019;62(3):1078–1095. doi: 10.1021/acs.jmedchem.8b00888. [DOI] [PubMed] [Google Scholar]
- Chalmers B. A. Xing H. Houston S. Clark C. Ghassabian S. Kuo A. Cao B. Reitsma A. Murray C.-E. P. Stok J. E. Boyle G. M. Pierce C. J. Littler S. W. Winkler D. A. Bernhardt P. V. Pasay C. De Voss J. J. McCarthy J. Parsons P. G. Walter G. H. Smith M. T. Cooper H. M. Nilsson S. K. Tsanaktsidis J. Savage G. P. Williams C. M. Validating Eaton's Hypothesis: Cubane as a Benzene Bioisostere. Angew. Chem., Int. Ed. 2016;55(11):3580–3585. doi: 10.1002/anie.201510675. [DOI] [PubMed] [Google Scholar]
- Tse E. G. Houston S. D. Williams C. M. Savage G. P. Rendina L. M. Hallyburton I. Anderson M. Sharma R. Walker G. S. Obach R. S. Todd M. H. Nonclassical Phenyl Bioisosteres as Effective Replacements in a Series of Novel Open-Source Antimalarials. J. Med. Chem. 2020;63(20):11585–11601. doi: 10.1021/acs.jmedchem.0c00746. [DOI] [PubMed] [Google Scholar]
- Levterov V. V. Panasyuk Y. Pivnytska V. O. Mykhailiuk P. K. Water-Soluble Non-Classical Benzene Mimetics. Angew. Chem., Int. Ed. 2020;59(18):7161–7167. doi: 10.1002/anie.202000548. [DOI] [PubMed] [Google Scholar]
- Kanazawa J. Maeda K. Uchiyama M. Radical Multicomponent Carboamination of [1.1.1]Propellane. J. Am. Chem. Soc. 2017;139(49):17791–17794. doi: 10.1021/jacs.7b11865. [DOI] [PubMed] [Google Scholar]
- Locke G. M. Bernhard S. S. R. Senge M. O. Nonconjugated Hydrocarbons as Rigid-Linear Motifs: Isosteres for Material Sciences and Bioorganic and Medicinal Chemistry. Chem. – Eur. J. 2019;25(18):4590–4647. doi: 10.1002/chem.201804225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.