Abstract
Cancer is a huge burden on the healthcare system and is foremost cause of mortality across the globe. Among various therapeutic strategies, chemotherapy plays an enormous role in overcoming the challenges of treating cancer, especially in late stage detection. However, limitations such as extreme side/adverse effects and drug resistance associated with available drugs have impelled the development of novel chemotherapeutic agents. In this regard, we have reviewed the development of β-carboline-based chemotherapeutic agents reported in last five years. The review mainly emphasizes on the molecular hybrids of β-carbolines with various pharmacophores, their synthetic strategies, and in vitro anticancer evaluation. In addition, the mechanisms of action, in silico studies, structural influence on the potency and selectivity among diverse cancer cell lines have been critically presented. The review updates readers on the diverse molecular hybrids prepared and the governing structural features of high potential molecules that can help in the future development of novel cytotoxic agents.
The present review elaborates development of β-carboline-based molecular hybrids in past 5 years, their synthesis and biological evaluation. Interestingly, these hybrids have exhibited excellent cytotoxic profile with minimal effect on normal cells.
1. Introduction
Cancer is now a leading cause of mortality and a huge burden to the human healthcare system world-wide. It may originate from genetic interferences or environmental causes or a combination of both. Among more than 100 different type of cancers, breast, lung, prostate, and colorectal cancer cases are dominant. Oral and laryngopharynx cancer are another notable cause of mortality, which might be attributed to the use of tobacco.1 In this regard, natural products with great diversity play dominant roles in human life and daily activities including the treatment of health problems.2a,b Among such natural products, β-carboline alkaloids such as harmine, vasicine, and harmaline are present in the seeds of Peganum harmala (Zygophyllaceae), which are basically tricyclic pyrido[3,4-b]indole ring systems.2c The different class of β-carbolines belong to completely aromatized, dihydro, or tetrahydro pyridine moiety with diverse biological significance as anti-inflammatory,2d antidepressant,2e antimalarial,2f antileshmeniasis,2g antidiabetic,2h anticancer,2f,i–k antioxidant,2l and antianxiety2m (Fig. 1).2
Fig. 1. Structural diversity of the β-carboline scaffold and various biologically-relevant molecules.
The β-carboline scaffold constitutes the chief motif of several pharmacological agents and commercially available drugs with different therapeutic applications, such as yohimbine and tadalafil (used in erectile dysfunction), vinpocetine (used for cerebrovascular disorder), abecarnil (used as antianxietic), vincamine (used as peripheral vasodilator), rescinnamine (used as ACE inhibitor), ajmaline (used as antiarrhythmiatic), reserpine (used as antihypertensive), and deserpidine (used as antipsychotic and antihypertensive) (Fig. 2).3 The anticancer/cytotoxic effect of β-carboline alkaloids is mainly attributed to their ability to target DNA,4a the modulation of p-53 signaling pathways,2j inhibition of topoisomerase I and II,4b histone deacetylase,4c telomerase,4d minor groove binding,4a kinesin spindle protein (KSP-ATPase),4e and other kinases.4f The photolytic cleavage of DNA has also been reported for various β-carboline derivatives.4g,h Apart from simple derivatives and metal complexes of natural β-carbolines, the molecular hybridization approach has been extensively explored with several pharmacophoric scaffolds to achieve more potent and promising molecules, especially with potential cytotoxic profile.5a,b Molecular hybridization not only helps to improve the potency/efficacy of molecules but also important to overcome drug resistance due to the involvement of more than one biological target and different mechanisms of action.5c–e
Fig. 2. Representative β-carboline containing molecules in clinical use.
Some representative reviews published previously revolve around certain aspects of β-carbolines obtained either from natural source or synthetic molecules.6 Zhang et al., in 2015,5b collated the anticancer activity of β-carbolines with their structure–activity relationship (SAR) and mechanism of actions, followed by a review on pharmacological and toxicological aspects of this scaffold by Khan et al., in 2017.6a Similarly, Kumar and co-workers updated the development of anticancer β-carboline derivatives and metal complexes reported during 2011–2016.6b Wang and co-authors reviewed the monomeric and dimeric units of β-carboline alkaloids with an emphasis on their occurrences, structural diversity, and associated biological responses.6c Sahoo et al., in 2019, focused on natural norharmane alkaloids regarding their synthesis and SAR in connection to their cytotoxic abilities.6d Recently, Manasa et al., provided an assessment regarding β-carboline natural alkaloids and their derivatives with anticancer potential along with the SAR.6e An interesting review published by Piechowska et al., emphasized the presence of β-carbolines in food items, which reduces the risk of neurodegenerative diseases.6f In the present review, we aim to provide an update on the recent developments (2015–2020) of β-carboline-based hybrid molecules with anticancer potential. The review describes the synthetic strategies, in vitro anticancer potential, and mechanisms of action of such hybrids.
2. Synthesis of functionalized β-carboline framework
The synthesis of β-carboline core structures bearing different functional groups has been summarized in Scheme 1. The presence of multiple functionalities and their straight-forward conversion to other functional groups has enabled the demanding coupling of β-carboline core with additional pharmacophores.7l-Tryptophan (1, an amino acid) is the most often used starting material, which was readily esterified with an alcohol in acidic medium, followed by Pictet–Spengler cyclization, which afforded tetrahydro-β-carbolines (3). Alternatively, the cyclization of tryptamine (6) also delivered tetrahydro-β-carbolines (7), which on aromatization yielded β-carboline 4.8a–c The tetrahydro-β-carbolines 3 were either subjected to decarboxylative aromatization to form simple β-carboline core (4)8d–f or aromatized using different oxidative reagents such as KMnO4 or S8 to obtain aromatic β-carboline esters (5).8g The reduction of esters (5) using LiBH4 or LiAlH4 furnished alcohol (8), which upon subsequent oxidation with DMP/MnO2 allowed the formation of β-carboline aldehyde (9).8f On the other hand, the azidation of alcohol (8) directly with DPPA or sulphonation with mesyl/tosyl to 10, followed by azidation with NaN3, produced β-carboline azides (11), which upon further reduction afforded β-carboline amine (12, Scheme 1).8h,i
Scheme 1. Synthesis of the β-carboline core with various functional groups (*reg = reagents).
As briefed, the primary functional groups present in β-carboline are acid/ester, alcohol, aldehyde, and amine, which can be coupled with different pharmacophoric moieties to produce molecules with potential biological profile. Apart from these functionalities, β-carboline ester (5) was converted to its corresponding carbohydrazides (13) by treatment with hydrazine hydrate, followed by diazotization to carbonyl azides (14), which further transformed to β-carboline amine (15) by means of Curtius rearrangement (Scheme 1).8j,k The literature discussed in the successive part describes the complete synthetic strategies using any of the reagents/conditions presented in Scheme 1 and should be considered for citations. However, only some of the synthetic works/reviews that have not been involved in biological studies are cited in this section.
3. β-Carboline hybrids as cytotoxic agents
3.1. β-Carboline-hydantoin hybrids
In continuation of our extensive efforts for finding new potent cytotoxic scaffolds based on different heterocycles,9a–i in 2014, we started working on derivatives/hybrids of β-carboline with the same integrity. These β-carboline hybrids may hold great promise as therapeutic agents for intervention in human cancers. HR22C16 (compound 16, Scheme 2), a small-molecular probe for studying the dynamics of cell division, was identified in 2003.10a Based on this potent Eg5 inhibitor, new tetrahydro-β-carboline-hydantoin hybrids were prepared and their in vitro anticancer potential was evaluated. The synthesis was accomplished by the reaction of tetrahydro-β-carboline acid (3) with isocyanates (compounds 17 and 18, Scheme 2). Among the synthesized library; compound 18a (with meta-hydroxyphenyl at C1 and para-chlorophenyl in hydantoin) was found to be significantly cytotoxic with IC50 values of 16.0, 6.5, and 6.08 μM against the A549, HeLa, and PC3 cell lines, respectively, which were close to the IC50 of etoposide (50.0, 4.71, and 14.4 μM). In addition, compound 18a was found to be 5-fold more potent against the PC3 cell line as compared to normal prostate epithelial cells (RWPE), which indicated superior selectivity towards the cancer cells. The morphological changes of the tested cells such as membrane blebbing, cell shrinkage, apoptotic body formation, and chromatin condensation with the destructive fragmentation of the nucleus indicated the induction of cell apoptosis.10b
Scheme 2. Synthesis of β-carboline-hydantoin hybrids with anticancer activity.
Inspired from natural marine product aplysinopsins (19), aromatic N9-substituted β-carboline-hydantoin hybrids were designed and synthesized to restrict the conformational flexibility (compounds 20, Scheme 3).10c The prepared library was evaluated against various human cancer cell lines and their potency was compared with that of 5-fluorouracil (5-FU). The hybrids produced satisfactory results during the in vitro assay, wherein compound 20a and 20b showed potent IC50 values of 0.37, 1.18, 2.96 and 1.70, 1.14, 3.09 μM on MCF-7, MDA-MB-231, and A549, respectively. Interestingly, compound 20a (N9-p-CN-Bn) was found to be 83-fold more potent for MCF-7 compared to 5-FU and displayed high selectivity towards cancer cells. Electron-rich benzyl substitution with N9-p-OMe-Bn also produced the highly potent molecule 20b. The molecules with strong electron-withdrawing groups such as p-NO2-Bn and p-CF3-Bn were poorly active, while the slight electro-negative substitutions, such as p-CN/F, were showed a potency similar to that of 20b. An attempt was also made to check the effect of substitution at various positions, where in contrast to the para-position, substitution at other positions decreased the potency (Fig. 3).10d
Scheme 3. Synthesis of β-carboline-1-one-hydantoin hybrids with restricted conformation.
Fig. 3. Structural features of β-carboline-hydantoin hybrids producing highly potent frameworks.
3.2. β-Carboline-chalcone/pyrazole hybrids
In the field of medicinal chemistry, chalcones have a unique impact on the broad spectrum of biological activity including anticancer potential.11a The hybrids of chalcones with β-carboline were designed and synthesized through the reaction of β-carboline aldehydes (9) with aryl/heteroaryl ketones in the presence of barium hydroxide (compounds 21, Scheme 4). Further, the hybrids were evaluated for their in vitro cytotoxicity against various cancer cell lines and tested for their safety profile on normal cells. Among the series (with Ar ≠ heterocycles), some of the compounds showed an effective cytotoxic profile against A549 cells in less than 10 μM inhibitory concentrations. However, the compounds showed a significant elevation in the thermal denaturation (ΔTm) of DNA in comparison to doxorubicin. Moreover, compound 21a (Ar = 2-furanyl) showed a potent IC50 of 1.86 μM for MCF-7 (doxorubicin IC50 = 2.18 μM); also, significant potency was observed for other cancer cell lines. In addition, these hybrids showed no significant toxicity towards normal human embryonic kidney cells (HEK-293). The biological activity of these hybrids was attributed to the ability to induce apoptosis, photo-cleavage of DNA, and inhibition of topoisomerase I.11b 4-Fluoro-3-methoxy-phenyl at the C1 of β-carboline with 4-methoxyphenylchalcone displayed the highest potency on A549. Similarly, the trimethoxyphenyl group at C1-β-carboline and chalcones produced significant activity with IC50 of 5.30 μM and 3.67 μM for A549 and B-16, respectively. The heteroaryl group (2-furanyl) on chalcone with p-CF3-Ph at C1-β-carboline resulted in a highly potent molecule. The in silico molecular modelling studies were in agreement with the experimental results and showed DNA intercalation as the binding mode. Barring the stacking of the β-carboline moiety within the DNA base pairs, alternative binding to the minor groove of DNA was also observed. Further, the best orientation from the DNA–ligand complex was subjected to molecular dynamics simulation. The study revealed the stability of the complex up to 5 ns without significant fluctuation from the initial orientation, which indicated strong DNA intercalation.11b
Scheme 4. Synthesis of β-carboline-chalcone/pyrazole hybrids.
Further, these molecules were transformed into pyrazole hybrids as the bioisostere of chalcones through an easy reaction with hydrazine hydrate (compounds 22, Scheme 4). Nevertheless, similar potency and selectivity was observed as chalcones, in which the most potent compound 22a produced an IC50 of 2.39, 3.63, 1.94, 2.63, and 2.75 μM for A549, DU-145, MCF-7, HeLa, and ACHN, respectively. The high IC50 concentration of 95.37 μM for HEK-293 indicated the excellent selectivity of 22a towards the cancer cells rather than the normal cells.11c
R. Cao and group explored N2-alkylated salts of β-carbolines as cytotoxic agents.12a–c The results revealed the high potency of such N2-quaternized salts (IC50 ≤ 10 μM) compared to the respective neutral β-carbolines (IC50 = 10–100 μM).12c Chauhan et al., reported highly potent β-carboline grafted with chalcones at C1-β-carboline having an IC50 of 2.25 μM towards the MCF-7 cell line (compound 23 and 24, Scheme 5).12d Encouraged by these efforts, a new series of β-carboline-chalcone hybrids and their N2-alkylated salts were designed, synthesized, and tested for their anti-proliferative activity against human cancer cell lines (compounds 25 and 26, Scheme 5). The in vitro cytotoxic assay showed that the neutral hybrids (25) were less potent as compared to the N2-alkylated salts of these hybrids (26) in the tested cell lines, viz., BxPC-3, HeLa, C4–2, PC-3, HEK293T, MDA-MB-231, and NIH3T3. Interestingly, compound 26a showed good anti-proliferative activity in all the mentioned cell lines with IC50 values of 20.0, 22.1, 16.13, 22.02, 17.18, 15.95, and 55.23 μM, respectively. Compound 26a showed induced apoptotic cell death in MDA-MB-231 cells. The β-carboline-C1-chalcone hybrids with N2-quaternary ammonium salts showed better potency compared to the corresponding neutral hybrids; for example, the N2-benzyl/propargyl groups produced significant response. The other aromatic enaminones, tested with the N2-benzyl group, had marginal or no activity except for 3,4,5-trimethoxyphenyl chalcone. N2-Butyl substitution with 3,4-dimethoxyphenylenaminone was completely inactive (Fig. 4).12e
Scheme 5. Synthesis of β-carboline-chalcone hybrids and their quaternary salts.
Fig. 4. Structural features of quaternary salts of β-carboline-chalcone hybrids producing highly potent frameworks.
3.3. β-Carboline-cinnamide hybrids
Cinnamic acid and its derivatives have been well-known for their promising anticancer potential.13a,b Encouraged by the potential of this naturally occurring scaffold, hybrids of β-carboline-linked C3-trans-cinnamides were designed and synthesized by the amide coupling of β-carboline amine (12) and cinnamic acids using hexafluorophosphate benzotriazole tetramethyl uronium (HBTU) (compounds 27, Scheme 6). These hybrids were evaluated for their anticancer activity against different human cancer cell lines and were found to be highly potent with activity in the nanomolar range. In particular, compound 27a showed the highest potency with IC50 values 18.86, 13.84, 16.31, and 22.32 nM for A549, MCF-7, B16, and HeLa cell lines, respectively. In addition, the selectivity towards the cancer cells was indicated by the high IC50 of 37.16 nM for normal cells (NIHT3T). Notably, the biological effect of these hybrids was attributed to apoptosis induction, cleavage of pBR-322 DNA, and inhibition of topoisomerase I. Together, the DNA binding experiments as well as molecular modelling studies revealed DNA intercalation at the topoisomerase I binding site. Moreover, the cinnamide part at the C3 position extended towards the DNA minor groove and formed π–π stacking. In the series, hybrids with p-OMe-Ph at C1-β-carboline or 3,4,5-tri-OMe-Ph on cinnamide were considerably more active with IC50 < 24 nM compared to the other conjugates. In particular, p-OMe-Ph at C1-β-carboline with 3-OH/4-Cl/3,4,5-tri-OMe-Ph on cinnamide exhibited the highest potency (Fig. 5). However, the replacement of cinnamide with acrylamide diminished the activity.13c
Scheme 6. Synthesis of β-carboline-cinnamide hybrids.
Fig. 5. Structural features of the most potent β-carboline-cinnamide hybrid.

3.4. β-Carboline-hydroxamic acid hybrids
Vorinostat (28) (suberanilohydroxamic acid – SAHA) has been tested for the treatment of cutaneous T-cell lymphoma, where unsatisfactory clinical trials invigorated medicinal chemists to proselytize and develop molecules containing hydroxamic acid.14a In this regard, Zhang and co-workers designed, synthesized β-carboline-hydroxamic acid hybrids (Schemes 7–9), and revealed their cytotoxicity by targeting the HDAC and DNA via the regulation of the p53 signalling pathway. The hybrids have four major categories of linkers, namely, aliphatic amide/urea,14b aromatic amide,14c aromatic amine,14d and (iv) aliphatic cinnamic acid.14e
Scheme 7. Synthesis of β-carboline-hydroxamic acid hybrids with the aliphatic amide/urea linker.
Scheme 9. Synthesis of β-carboline-hydroxamic acid hybrids with the aromatic amine linker (32) and the aliphatic cinnamic acid linker (33).
The first class of molecules with aliphatic amide/urea linker (compounds 29 and 30, Scheme 7) were found to have significant in vitro anti-proliferative activity in human colorectal cancer cell lines and were found to have potent HDAC inhibition. Among the series of hybrids, compound 30a exhibited the most potent in vitro anti-proliferative effect with an IC50 of 0.83, 0.94, 1.63, and 1.16 μM for HCT116, SW620, LOVO, and SW480 cell lines, respectively (7-fold higher potent than SAHA). Compound 30a demonstrated an IC50 of 0.27 μM for HDAC inhibition and was found to be a strong cell apoptosis inducer. Moreover, the cleavage of PARP and caspase-3 along with increased histone H3/α-tubulin acetylation and the activation of the p53 signalling pathway against HCT116 cells was also credited for its biological responses.14b
The replacement of aliphatic amide/urea linker with aromatic amide linker led to a second series of hydroxamic acid hybrids (compounds 31, Scheme 8), which were tested for their anti-proliferative activity. Among the evaluated compounds, 31a exhibited highly potent anti-proliferative activity with IC50 of 1.79, 1.60, 2.46, and 4.25 μM for the cell lines HCT116, HepG2, SMMC-7721, and H1299, respectively. In addition, compound 31a was found to be a highly effective inhibitor of HDAC with an IC50 of 0.092 μM, which is 5-folds more efficient than SAHA (IC50 = 0.48 μM). Moreover, no significant toxicity was observed for normal human LO2 cells.14c
Scheme 8. Synthesis of β-carboline-hydroxamic acid hybrids with the aromatic amide linker.
The third class of hybrids was designed by replacing the amidic linker with aromatic amine linker (compounds 32, Scheme 9). These hybrids were synthesized and evaluated against different human cancer cells for their anti-proliferative activity and HDAC inhibition. Among the synthesized library, compound 32a showed the high potency with IC50 values of 0.78, 0.87, 0.53, 1.56, and 0.96 μM for the cell lines HCT116, SUMM-7721, HepG2, MCF-7, and Huh7, respectively. It also demonstrated a 10-fold increase in the anti-proliferative potency compared to that of vorinostat 28.14d It also showed potent HDAC inhibition with IC50 values of 2.8, 0.75, and 1.1 nM for HDAC1/C3/C6. These molecules inhibited HDAC by inhibiting histone H3 and α-tubulin acetylation. In addition, hybrid 32 showed anti-metastatic activity by reducing the protein level of MMP2 and MMP9 as well as by inhibiting the MAPK signalling pathway.14d
Recently, in 2019, hydroxyl cinnamic acid linker was introduced in place of aliphatic/aromatic amide/amine linker, which led to the fourth series of hybrids (compounds 33, Scheme 9). The hybrids were prepared and evaluated against human cancer cell lines, amongst which compound 33a produced potent activity with IC50 values of 2.9, 1.4, 1.0, and 1.1 μM for SMMC-7721, HepG2, Bel7402, and Huh7 cell lines, respectively. Compound 33a was also found to be very effective as an HDAC inhibitor with an IC50 value of 27 nM by the acetylation of histone H3 and α-tubulin. Perhaps these molecules induced apoptosis by regulating the expression of apoptotic proteins Bax, Bcl-2, and caspase-3. Interestingly, these compounds significantly inhibited the PI3K/Akt/mTOR signalling pathways, which are involved in cancers.14e
The replacement of the phenyl ring of vorinostat (28, Scheme 7) with β-carboline produced highly potent anti-proliferative scaffolds (29–33) via HDAC inhibition. The terminal hydroxamic acid group was found to be crucial for the chelation of zinc ion at the active pocket of HDAC.14f In addition, the linker between these two pharmacophores was found to have distinctive influence on the potency as well as the selectivity of the congeners. Among the linkers screened, the following order of potency was observed: aryl amine (2.8 nM) > cinnamic acid (28 nM) > aryl amide (0.092 μM) > urea amide (0.27 μM) (Fig. 6).14b–e
Fig. 6. Structural features of potent hydroxamic acid hybrids having different linker units.

3.5. β-Carboline-dithiocarbamate hybrids
Dithiocarbamates express important natural or synthetic class of chemopreventive agents such as brassinin15a (34) and sulforamate15b (35), which act via the inhibition of the proteasome enzyme(s).15c,d Moreover, the structure–activity relationship (SAR) indicated the significance of the dithiocarbamate motif towards the chemopreventive nature of brassinin.15a Enthused by potent activities of dithiocarbamates, Kamal et al., disclosed the synthesis and in vitro cytotoxicity evaluation of β-carboline-dithiocarbamate hybrids (compounds 36, Scheme 10). The synthesis was accomplished by treating the β-carboline amine (12) with carbon-disulphide and alkyl halide. The in vitro cell-based assays proved the potent cytotoxicity of the synthesized compounds on selected human cancer cell lines. However, the corresponding amine precursors (12, Scheme 10) were also evaluated but they exhibited poor potency, demonstrating the substantial role of dithiocarbamates towards the cytotoxic profile. Among the hybrids evaluated, compound 36a produced highest potency with IC50 of 0.79 and 1.47 μM for the DU-145 and HeLa cancer cells (found more potent than doxorubicin), respectively. Moreover, compound 36b also showed excellent potency with IC50 of 1.34 and 3.45 μM for the same cancer cell lines, respectively. Believably, these molecules had different binding modes with DNA from the usual β-carboline alkaloids. They displayed promising apoptosis induction and DNA topoisomerase II inhibition, unlike other β-carboline hybrids. The molecular docking analysis was also consistent with the experimental results. These molecules bound well to the ATP binding site of topoisomerase II and displayed good interactions at the binding pocket similar to the co-crystal.15e
Scheme 10. Synthesis of β-carboline-dithiocarbamate hybrids.
The structural composition distinctively varied the pharmacological potency and the incorporation of dithiocarbamates increased the potency of congeners. The electronic nature of β-carboline-C1-aryl had a larger influence, where p-F/CF3-Ph produced better potency probably due to the lipophilic nature of fluorine. Methyl-dithiocarbamates were found to be the most appropriate with remarkable cytotoxicity. Moreover, the N9-methyl substitution displayed increased activity (IC50 < 20 μM) compared to free-N9-H (IC50 < 60 μM) (Fig. 7).15e
Fig. 7. Structural features of potent β-carboline-dithiocarbamate hybrids.
3.6. β-Carboline-imidazole/benzimidazole hybrids
Benzimidazoles were explored as potent cytotoxic agents by various mechanisms of actions such as the inhibition of receptor tyrosine kinase, topoisomerase II, and protein kinase CK2.16 The bis-benzimidazole derivative 37 was found to inhibit DNA topoisomerase I and helicase, and has undergone phase I clinical evaluation. The exhilarating potential of imidazole/benzimidazole led to the development of several hybrids of such scaffolds with β-carboline having good anti-proliferative activity.17,18d,e A series of β-carboline-benzimidazole conjugates were designed, synthesized, and evaluated as anti-proliferative agents. The synthesis of these conjugates was accomplished by coupling the β-carboline aldehyde (9) with ortho-phenylenediamines (OPDs) using lanthanum nitrate as the catalyst. The bioisosteric replacement of benzimidazole with pyridoimidazole produced the bioisosteres 38b–38d (compounds 38, Scheme 11). The in vitro anti-proliferative evaluation of conjugates 38 against human cancer cell lines produced potent biological responses. In particular, compound 38a showed potent cytotoxic activity with IC50 values of 1.8, 2.4, and 2.0 μM for HeLa, DU145, and A549 cell lines, respectively. The growth inhibition for hybrids 38 was also observed in the concentration range from 0.3 to 63 μM. Indeed, compound 38a exhibited remarkable anticancer activity against leukemia cancer cells with GI50 of 0.3 and 0.8 μM for RPMI-8226 and CCRF-CEM, respectively. The hybrids dominantly cleaved the pBR322 plasmid DNA by the irradiation of UV light and also showed the inhibition of topoisomerase I. The congeners also showed G-quadruplex DNA stabilization, inhibition of telomerase, and induced apoptosis towards the anticancer activity.4d The binding mode and interactions with DNA/topoisomerase I were examined by biophysical assays and molecular docking analysis. The results were consistent with DNA intercalation and minor groove binding, where the molecules were properly fitted in the active site of topoisomerase I.17 The best docked orientation displayed electrostatic interactions with Arg364, Lys374 (H-bonding), C112, A113, C10, G11 (planar π–π stacking) along with hydrophobic interactions involving Asp533, Ile535, Asn722, Arg364, Glu365, and Lys425 amino acids present close to the binding site.
Scheme 11. Synthesis of β-carboline-benzimidazole hybrids.
Among the hybrids 38, the substitution of p-OMe-Ph at C1 along with 6-F/OMe-benzimidazole at C3 of β-carboline produced the highest potency as the cytotoxic agent. In addition, other benzimidazole activating groups such as methyl substitution produced moderate cytotoxicity, while electron-withdrawing group (6-CF3) on C3-benzimidazole demolished the activity. Perhaps, the replacement of benzimidazoles with pyridoimidazole (compound 38b–38d, Scheme 11) drastically hindered the biological potency of the congeners.17
N-Heterocyclic carbenes, their precursors, and metal complexes have played crucial role towards the development of anticancer agents.18a–c In this strategy, β-carboline-based N-heterocyclic hybrids having the ability to generate carbene under biological conditions were synthesized and evaluated for their anti-proliferative/metastatic activities against breast and lung cancer cells (compounds 39, Scheme 12). Among the hybrids, some of the molecules produced high potency with IC50 of <10 μM; for example, 39a showed an IC50 of 4.48 μM against H1299 and 39b has an IC50 of 4.49 μM for MDA-MB-231. These hybrids exhibited anti-invasive effects against highly metastatic human breast cancer cells MDA-MB-231 via the anomaly of the MAP-kinase signalling pathway and the inhibition of matrix metalloproteinases.18d
Scheme 12. Synthesis of β-carboline-N-heterocyclic hybrids with the ability to generate carbene.
The imidazolium salts have an remarkable array of biological activities including the anticancer potential. Likely, an imidazolium salt (compound 40, Scheme 13) has produced the potent cytotoxicity.18e,f In this context, the hybrids of β-carboline-imidazolium salts were designed, synthesized, and evaluated against different cancer cell lines (compounds 41, Scheme 13). The in vitro cytotoxicity evaluation revealed the good potency of these congeners as anticancer agents. In particular, compound 41a was proved to be a highly cytotoxic molecule with IC50 of 3.24, 15.03, 8.78, 8.05, and 11.01 μM for HL-60, SMMC-7721, A549, MCF-7, and SW480 cancer cell lines, respectively. The biological responses of these hybrids were credited to apoptosis induction via the arrest of the G1 phase of the cell cycle. However, the neutral imidazole hybrids were poorly active compared to their corresponding imidazolium salts (Fig. 8).18g
Scheme 13. Synthesis of hybrids of β-carboline-imidazolium salts.
Fig. 8. Structure–activity relationship (SAR) of the hybrids of the β-carboline-imidazolium salt.
3.7. β-Carboline-pyridine hybrids
Pyridine, being an excellent bioisostere of phenyl as well as pyrimidine, has a considerable influence on its biological activities. A terpyridine (42, Scheme 14) and its metal complexes exhibited astonishing cytotoxic property via DNA intercalation.19a,b In the same fashion, β-carboline-pyridine hybrids were designed, synthesized, and evaluated for their biological activity. The synthesis involved the reaction of β-carboline aldehydes (9) with aryl/heteroaryl ketones in presence of barium hydroxide, which furnished the corresponding chalcone derivatives. These chalcones were subjected to Khronke reaction in the presence of ammonium acetate to obtain the designed β-carboline-pyridine hybrids (compounds 43, Scheme 14). The hybrids were evaluated for their DNA binding ability by assessing the thermal denaturation (ΔTm) of DNA from calf-thymus. Interestingly, among the hybrids, compound 43a showed strong DNA binding ability with highest melting stabilization, as indicated by ΔTm (6.3 °C at 0 h and 6.5 °C after 18 h of incubation). However, the reference standard doxorubicin was found to have 3-folds depressed ΔTm (2.4 °C at 0 h and 2.6 °C after 18 h of incubation), which indicated weaker DNA binding. However, most of the hybrids produced good DNA binding affinity, in particular, m-Cl-Ph at C1-β-carboline and 2,6-(bis-furan-2-yl)pyridyl at C3 exhibited the highest affinity towards DNA interactions. Molecular docking analysis revealed DNA intercalation as the binding mode of these ligands stabilized by planar π–π stacking and H-bonding of heteroatoms.19c
Scheme 14. Synthesis of β-carboline-pyridine hybrids.
3.8. β-Carboline-salicylic acid hybrids
As salicylic acid and its precursors are associated with the prevention and treatment of cancers,20a,b its hybrids with β-carboline were prepared and evaluated for their anticancer potential and apoptosis induction (compounds 44, Scheme 15). The in vitro cytotoxicity study of these hybrids along with 5-FU and harmine was performed against various human cancer cell lines. Interestingly, most of the designed molecules were found to be more potent than harmine as well as 5-FU. Among all, compound 44a was the most potent molecule with IC50 of 6.97, 7.12, 8.25, 7.89, and 13.1 μM for SMMC-7721, HepG2, HCT116, Ej, and H460, respectively. It showed excellent selectivity towards liver cancer cells (SMMC-7721) along with decreased mitochondrial membrane potential, which intervened with apoptotic proteins such as Bcl-2 and Bax in a dose-dependent manner. The influence of the structural variations of these hybrids was clearly reflected by the potency, where C1-methyl (44, R2 = Me) produced the most potent molecule, while the bulky substitution (4-OMe-Ph) decreased the activity. The length of the alkyl chain between the two pharmacophores was crucial; the activity increased until 5-carbon chain elongation, while further lengthening the linker decreased the potency. The replacement of salicylamide either with an acetamide or benzamide decreased the antitumor activity and directed the significance of the salicylic acid pharmacophore (Fig. 9).20c
Scheme 15. Synthesis of β-carboline-salicylic acid hybrids.
Fig. 9. Structural features of β-carboline-salicylic acid hybrids influencing the biological potency.

3.9. β-Carboline-thiazolidinedione hybrids
Thiazolidinedione and its derivatives have great impact on the development of anticancer agents, such as compounds 45a (IC50 = 1.3 and 3.1 μM for HL-60 and L1210, respectively) and 45b (IC50 = 0.13 and 0.05 μM for A549 and MDB-MB-231, respectively) have shown potent activity.21a–c In continuation, β-carboline-thiazolidinedione hybrids were designed, synthesized, and evaluated for their antitumor activity. The synthesis was accomplished using β-carboline-carbohydrazide (13) with isothiocyanates or alternatively treated with aldehydes, followed by reaction with 2-marcapto-acetic acid (compounds 46 and 47, Scheme 16). The hybrids were examined for in vitro cytotoxicity, among which compound 46a especially showed potent cytotoxic activity, having an IC50 of 1.59, 2.36, 0.19, 1.53, 3.91, 0.83, and 2.28 μM against U251, MCF-7, NCI/ADR-RES, 786-0, NCI-H460, OVCAR-3, and HT-29, respectively. The hybrids were credited for arresting the sub-G1 phase and the loss of cell membrane integrity.21d
Scheme 16. Synthesis of β-carboline-thiazolidinedione hybrids.
The thiazolidinedione hybrid with quinoline produced potent anti-tumor agents and among them, two are currently under clinical investigation (compound 48a–b, Scheme 17).22a The highly potent derivatives 48a–b encouraged the design of β-carboline-thiazolidinedione hybrids in identical fashion, barring the replacement of quinoline/quinoxaline.22b The synthesis was achieved by the simple coupling of β-carboline aldehydes (9) with active methylene of thiazolidinedione in basic ethanol medium (compounds 49, Scheme 17). The in vitro cytotoxicity of hybrids 49 was examined in different human cancer cell lines and exhibited considerable activity. In particular, compound 49a displayed the best cytotoxicity with an IC50 of 1.34, 6.66, 1.40, 1.80, 0.97, 4.51, 5.08, and 3.33 μM against PC-3, A549, MG-63, HCT-15, MDA-MB-231, A431, PANC-1, and L132, respectively. The high potency of 49a may also be credited to the presence of morpholine, which has its own remarkable potential as a cytotoxic agent.22c The hybrids were found to have the ability to induce apoptosis by membrane blebbing, chromatin condensation, and apoptotic body formation. The induction of apoptosis was evident from cell–cell adhesion, cell wall deformation, shrinkage of cells, and reduction in the number of viable cells. The different staining techniques such as AO/EB and DAPI along with the depolarization of mitochondrial membrane potential were also in support of the induction of apoptosis. The generation of reactive oxygen species (ROS), cell cycle arrest at the G1-phase, and the intercalation of CT-DNA were also been observed. The biophysical DNA binding affinity studies (relative viscosity, circular dichroism, and UV spectral study) along with molecular modelling analysis revealed the DNA intercalative property of these ligands. The best docked orientation of the ligand indicated the intercalation of planar β-carboline supported by strong π–π interactions. Morpholine substitution over thiazolidinedione oriented into the active site and the ligand was stabilized by different electrostatic and hydrophobic interactions. Further, MD simulation for 5 ns indicated the stability of the ligand–DNA complex without significant deviation from the initial orientations. The structural variations were found to have a distinct role in governing the potency and selectivity of these hybrids. The electron-withdrawing group on C1-aryl, such as 4-Cl-Ph, showed higher potency compared to electron-rich aromatic substitution such as 4-OMe-Ph and, which followed the order p-Cl-Ph > Ph > p-OMe-Ph. Among the thiazolidine N-substitutions, the potency followed the orders of morpholine > benzyl > phenacyl > free-NH.22b
Scheme 17. Synthesis of β-carboline-thiazolidine-2,4-dione hybrids.
3.10. β-Carboline-podophyllotoxin hybrids
Podophyllotoxins are a well-known class of scaffolds exerting their anticancer activity via different mechanisms of action, such as inhibition of microtubule, topoisomerase, and DNA intercalation.23a,b The β-carboline-podophyllotoxin hybrids were synthesized by amide coupling between β-carboline acids (5) and the corresponding amine (compounds 50, Scheme 18). The synthesized hybrids were evaluated for their in vitro cytotoxic potential against a panel of human cancer cell lines. Among the hybrids examined, compound 50a produced potent cytotoxic effects with IC50 of 1.87, 1.07, 2.64, 2.68, and 2.92 μM for A549, DU-145, MDA MB-231, HT-29, and HeLa, respectively. Notably, this compound has an IC50 of 103.28 μM for HEK-293 cells (human embryonic kidney cells), which directly imitates the high selectivity towards cancer cells rather than the normal cells. Hybrid 50a showed high efficacy towards DU-145 cancer cells, followed by potency towards A549. These hybrids led to cancer cell apoptosis by the inhibition of the cell cycle at the S and G2/M phases and act as catalytic inhibitors of topoisomerase IIα. Moreover, the biophysical DNA binding studies such as UV and fluorescence spectroscopy along with circular dichroism demonstrated the non-intercalative interactions of ligands such as groove or surface binding, causing the unwinding of CT-DNA. The in silico molecular modelling results were in correlation with DNA topoisomerase IIα inhibition assay, where the compounds occupied the ATP-binding site and formed strong interactions with the surrounding residues. The entry of ATP into the binding site was hindered due to the interaction of ligands with the residues below the ATP binding site and magnesium ions. Molecular dynamics revealed the ability of the amide functionality to orient the molecules perfectly in a curved shape in the minor groove and reflected the significance of the amide bond between two pharmacophores.23c
Scheme 18. Synthesis of β-carboline-podophyllotoxin hybrids.
3.11. β-Carboline-combretastatin hybrids
Combretastatin derivatives are well-known cytotoxic agents acting via varied mechanisms such as apoptosis induction, DNA intercalation, and topoisomerase II inhibition. The potent anti-proliferative activity of combretastatin A-4 (CA-4) was marked as the lead candidate in the discovery of anticancer agents. The flexible double bond configuration has limited its clinical use since only the cis-conformation is active.24a,b In effort to develop the rigid cis-isomer, the hybrids of β-carboline-combretastatin were designed, synthesized and further tested for the in vitro cytotoxic activity against human cancer cell lines. The synthesis of these hybrids involved amide coupling between the β-carboline amine (12) and CA-4 acids using EDC-HOBt as the coupling agent (compounds 51, Scheme 19). The hybrids were examined for their in vitro cytotoxicity, which exhibited considerable cytotoxicity with <2 μM IC50 for many of the analogues. For instance, compound 51a showed the highest potency with an IC50 of 1.01 and 1.51 μM against the A549 and DU-145 cell lines, respectively.24c However, another compound 51b from the series also produced potent activity with an IC50 of 1.17 and 1.37 μM against the A549 and DU-145 cell lines, respectively.
Scheme 19. Synthesis of β-carboline-combretastatin hybrids.
The cytotoxic effect of these hybrids was attributed to cell cycle arrest at the G2/M phase, apoptosis induction, DNA intercalation, and topoisomerase II inhibition. Molecular modelling analysis unfolded the understating of interactions of these hybrids and the results were consistent with DNA topoisomerase II inhibition assay. The compounds were occupied in the ATP binding pocket of topoisomerase II and showed strong electrostatic interactions. These ligands also displayed DNA intercalation due to the β-carboline ring, whereas the combretastatin moiety was extended out to the DNA minor groove. The prevailing role of structural variations of hybrids 51 has been well established and observed for their biological response. The 3,4,5-tri-OMe-Ph motif was found to be crucial for its efficacy. The other aromatic ring of combretastatin with Ar = 4-CF3/Me-Ph substitutions showed the highest potency. Moreover, the substitutions such as R2 = 4-OMe-Ph or 3,4,5-tri-OMe-Ph at C1-β-carboline also produced excellent cytotoxicity.24c
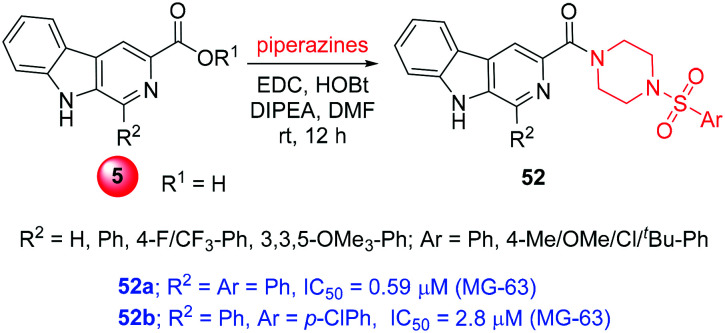
3.12. β-Carboline-sulfonyl piperazine hybrids
A new hybrid class of β-carboline-linked sulfonyl piperazine derivatives were prepared by amide coupling between β-carboline acids (5) and 4-(arylsulfonyl)piperazine using EDC-HOBt (compounds 52, Scheme 20). The anticancer activity of these congeners was examined with the help of in vitro cytotoxic studies. Among the series, most of the compounds showed good potency; in particular, compounds 52a and 52b exhibited highly potent activity with the respective IC50 values of 0.59 and 2.80 μM towards the MG-63 cancer cells. Fortunately, these hybrids showed least toxicity towards normal monkey kidney cells (Vero), having an IC50 of 8.41 μM and 9.95 μM for 52a and 52b, respectively. These compounds manifested the ability to inhibit topoisomerase II along with the binding to DNA, thus inhibiting the cell cycle. The experimental results of topoisomerase II inhibition and DNA binding were also supported by the in silico molecular modelling studies. Similar to other β-carboline-based topoisomerase II inhibitors, these molecules suitably docked in the ATP binding site of the enzyme topoisomerase IIα and produced consistent electrostatic interactions. Different H-bond interactions were observed for N9-H with Asp99 and –CO with Arg98 at the distance of 2.79 Å and 2.99 Å, respectively. Also, the π–cation interaction of sulfonyl phenyl was observed with Arg98 and Lys157 along with multiple hydrophobic interactions. Similarly, the DNA ligand docking simulation displayed strong N9-H bond interaction with DT8 (DNA base pair) along with β-carboline π–π stacking interactions. The C1-aryl substitutions with phenyl or para-chlorophenyl on sulfonyl produced the most potent congeners. The C1-phenyl on β-carboline produced better activity rather than the substituted phenyls.25
Scheme 20. Synthesis of β-carboline-sulfonyl piperazine hybrids.
3.13. β-Carboline-coumarin hybrids
Coumarin is another well-distinguished pharmacophore with a wide range of biological activities along with cytotoxic/anti-cancer potential.26a,b In the pursuit to obtain highly efficacious cytotoxic agents, the hybrids of β-carboline-coumarins were designed. The tetrahydro-β-carboline hybrids were synthesized using Pictet–Spengler cyclization, followed by controlled oxidation to dihydro-β-carboline to aromatic β-carboline hybrids (compounds 53–55, Scheme 21). Among the hybrids evaluated for their cytotoxic potential towards 60 different cancer cell lines, most of the molecules produced considerable cell growth inhibition. In particular, compound 53a and 55a showed 37.07% and 59.01% average growth inhibition of the NCI 60 cancer cell lines, respectively, at a single concentration of 10 μM. Moreover, these compounds exhibited significant cytotoxicity in the HeLa cancer cells with the GI50 of 33.33 μM and 23.4 μM. In the series of aromatic β-carboline, 6-Cl or 6-OMe-coumarin produced maximum potency. Similarly, tetrahydro-β-carboline was found to be more active than that of the corresponding dihydro-β-carboline towards the tested cancer cell lines. The molecular docking analysis with tubulin (1SA0) and KSP (1Q0B) displayed good electrostatic interaction (H-bond) with Ala250, Asp251, Leu252 of tubulin, or Asn29 of KSP.26c
Scheme 21. β-Carboline-coumarin hybrids and their anticancer activity.
3.14. β-Carboline-triazole hybrids
Triazole(s) are well-known pharmacophore with a broad range of biological activities. The triazole derivatives have been considerably explored as cytotoxic agents and have met with encouraging results.27a,b In the effort to achieve better potency and selectivity, β-carboline-triazole hybrids were prepared and evaluated for their anti-proliferative activity.9c For instance, C3-tethered β-carboline-triazole congeners were synthesized via the click coupling of β-carboline-azides (11) with different alkynes in the presence of copper catalyst (compounds 56, Scheme 22). These hybrids were tested for their in vitro cytotoxicity against different human cancer cell lines, where most of the hybrids exhibited good cytotoxic profile. The representative compound 56a produced the best potency with an IC50 of 3.67 and 5.44 μM, respectively, for HT-29 and HGC-27 cancer cells compared to harmine (IC50 = 4.28 and 10.56 μM). The structure–activity relationship revealed that the 3,4,5-tri-OMe-Ph at C1-β-carboline and (5-methylphthalimide)methyl on C4-triazole produced the best potency. A similar activity range was observed for 3,4-di-OMe-Ph at C1-β-carboline with naphthalimide on C4-triazole. The biophysical DNA binding assays (UV, CD, fluorescence spectral analysis, and DNA viscosity measurement) along with molecular modelling studies substantiated these hybrids as DNA minor groove binding agents.27c
Scheme 22. Synthesis of C3-thetered β-carboline-triazole hybrids.
Another series of β-carboline-triazole hybrids have been prepared by connecting the C1-β-carboline and C4-triazoles with an aryl-alkyl-ether linker (compounds 57, Scheme 23). The in vitro cytotoxicity results revealed considerable potency of compound 57a with an IC50 of 32.0 and 46.0 μM for HepG2 and HeLa cancer cell lines, respectively. The in vitro testing results were found to be the least potent for unaromatized β-carboline, while 3,4-di-Cl-Ph in triazole displayed the best activity among the hybrids. However, these molecules have also displayed significant antibacterial property against E. coli, S. aureus, B. cereus, and vancomycin-resistant strains E. faecium and E. faecalis.27d
Scheme 23. Synthesis of β-carboline-triazole hybrids with aryl-alkyl-ether linker.
The hybrids of tetrahydro-β-carboline-triazole grafted with chalcones were designed, synthesized, and evaluated for their anticancer potential against breast cancer cell lines (compounds 58, Scheme 24). All the compounds from the series were examined against the MCF-7 and MDA-MB-231 cancer cell lines and resulted in interesting activity. For instance, compound 58a was 5-fold more potent than tamoxifen with an IC50 of 10.33 μM on the MCF-7 cell line. Compound 58b showed 3-fold higher potency than tamoxifen against the MDA-MB-231 cell line with an IC50 of 21.99 μM. The analysis of the structural relationship with the activity demonstrated that the nature of aryl chalcone and the length of alkyl-aryl-ether played a critical role towards the cytotoxic potency of the hybrids. Thus, trimethoxyphenyl-chalcone and long 5C-alkyl chain afforded high selectivity and potency towards the MDA-MB-231 cells, while para-fluorophenyl-chalcone and shorter 3C-alkyl chain were dominant towards the MCF-7 cells. The hybrids with ferrocenyl-chalcone were found to be less potent rather than other aryl–chalcone hybrids.27e
Scheme 24. Synthesis of tetrahydro-β-carboline-triazole hybrids grafted with chalcone.
The polycyclic hybrids of tetrahydro-β-carboline-triazoles were synthesized and evaluated for their in vitro anticancer activity on MCF-7 and B16F10 (compounds 59 and 60, Scheme 25). Compound 59 showed potent cytotoxic activity with an IC50 of 6.45 μM against the MCF-7 cells and compound 60 also exhibited significant cytotoxicity with an IC50 of 4.01 μM against the B16F10 cells. The molecules demonstrated the ability to bind the DNA minor groove, induce apoptosis, arrest cell cycle, inhibit cell migration, and colony formation. Molecular docking with DNA indicated non-covalent binding of the ligands into the DNA minor groove, which lengthen the minor groove and distorted the helical axis by about 4 Å. The β-carboline N9-H, methoxy, and triazole participated in electrostatic H-bonding. In addition, π–π stacking was also observed with triazole moiety. In case of molecule 60, the trans-diastereomer was more efficient in adopting the crescent form to bind along the minor groove curvature. Further, molecular dynamics revealed the stability of the best-docked orientation for 4 ns without inducing large-scale conformational changes in the DNA double helix.27f
Scheme 25. Polycyclic tetrahydro-β-carboline-triazole hybrids.
3.15. β-Carboline-indole hybrids
Indole is a privileged pharmacophore as it has been remarkably explored with pronounced biological potential.28a–c The indole-containing marketed drug, sunitinib, was used for the treatment of renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor by the inhibition of multi-target receptor tyrosine kinase (RTK).28d,e In the pursuit of highly potent cytotoxic agents, the hybrids of β-carboline-indole were prepared by the Knoevenagel condensation of β-carboline aldehyde 9 with 2-oxindole and their in vitro cytotoxicity was examined (compounds 61, Scheme 26). The E-configuration of the newly formed double bond was characterized with the help of NOE experiments (1H gDQFCOSY NMR). The primary MTT assay of the hybrids displayed promising cytotoxic activity against a panel of human cancer cell lines, such as HCT-15, HCT-116, A549, NCI-H460, and MCF-7. Among the congeners evaluated, hybrid 61a exhibited distinguished potency towards the entire tested cancer cell lines with IC50 of 1.43, 4.18, 2.50, 6.71, and 3.36 μM, respectively. Notably, it displayed high selectivity towards the cancer cells and least toxicity towards the normal cells (IC50 = 49.79 μM for HFL-1). Moreover, a critical relationship was observed with the structural modifications; the C1 substitutions with phenyls produced potent activity, while the heteroaryl rings decreased the activity. The presence of electron-donating groups over C1 phenyl enhanced the activity. In addition, free-NH or benzyl substitution of oxindole had a decisive impact on the activity and C5-halo-substitution also exhibited excellent cytotoxic activity. On treatment, the morphological changes in the tested cells, AO/EB staining, DAPI nucleic acid staining, and the reduction of mitochondrial membrane potential suggested the induction of apoptosis. The cell cycle analysis showed the elevation of the G0/G1 phase cell population from 68.7% to 80.61% in the 1–2.5 μM concentration range. Further, the molecular docking analysis with DNA demonstrated the intercalation of planar β-carboline towards the GC base pairs, assisted by the right alignment of the C1-substituents. In addition, the oxindole moiety was also oriented perfectly between the DNA base pairs to have strong π-π interactions. Molecular dynamics revealed the stability of the best-docked orientation up to 20 ns without significant deviation from the initial orientation. The biophysical assays, molecular docking, and simulation studies were consistent with the DNA intercalation of these ligands.28f
Scheme 26. Synthesis of β-carboline-indole hybrids.
Further, a class of β-carboline-bis-indole hybrids was prepared and evaluated for their in vitro anti-proliferative activity against various cancer cell lines (compounds 62, Scheme 27). Among the hybrids examined, most of the compounds exhibited considerable anti-proliferative activity. For example, compounds 62a and 62b were proved to be highly active with the respective IC50 of 1.80 and 1.86 μM for the DU-145 cell line. Moreover, these compounds were recognized as apoptosis inducers, inhibitors of the cell cycle (G2/M phase), and topoisomerase I, and cleaved the pBR322 plasmid under UV light. However, different substituents have a distinguishable impact on the biological activity. In particular, the presence of electron-deficient C1-aryl has higher potency compared to electron-rich C1-aromatic groups. C2-methylated indole has reduced activity than C2-unsubstituted molecules, while substitution at C5 followed the activity pattern of H > Br > NO2 > CN > OMe.28g
Scheme 27. Synthesis of β-carboline-bis-indole hybrids.
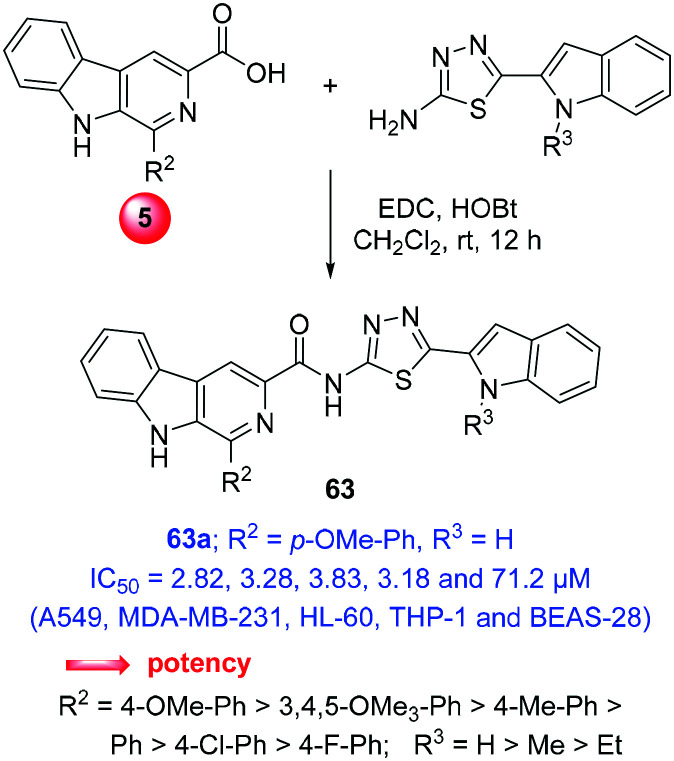
Recently, a series of β-carboline-indole hybrids adjoined with thiadiazole-carboxamide was prepared by amide coupling between β-carboline acid (5) and 2-amino-5-(indol-2-yl)thiadiazole with the help of EDC-HOBt (compounds 63, Scheme 28). Preceding this, 2-amino-5-(indol-2-yl)thiadiazole was prepared from indole-2-carboxylic acid in a sequence of four-steps, viz., esterification, N-alkylation, ester hydrolysis, and reaction with thiosemicarbazide in phosphorous-oxychloride. Further, these hybrids exhibited DNA intercalative topoisomerase IIα inhibition and thus anti-proliferative activity. The in vitro cytotoxicity and apoptosis assays revealed promising results with nuclear and morphological alterations, and mitochondrial membrane depolarization against a panel of human cancer cell lines. Particularly, compound 63a exhibited most potent IC50 of 2.82, 3.28, 4.09, 4.78, 3.83, 3.18 μM for A549, MDA-MB-231, BT-474, HCT-116, HL-60 and THP-1, respectively.28h
Scheme 28. Synthesis of β-carboline-indole hybrids adjoined with thiadiazole.
Notably, the safety/selectivity index of 63a against normal human lung epithelial cells (BEAS-28) was found in the range of 14.9–25.26 with an IC50 of 71.2 μM. The enzyme inhibition assay demonstrated the induction of nicks and linearized the kDNA, indicating the catalytic inhibition of topoisomerase IIα. Further, the relative viscosity experiment and absorbance spectroscopy revealed the DNA intercalation of these ligands. The induction of cell apoptosis was inferred from different nuclear/non-nuclear staining such as AO or DAPI along with the changes in mitochondrial membrane potential and the arrest of cell cycle in the G0/G1 phase. In addition, molecular modelling studies affirmed the intercalative binding of these molecules in the active pocket of topoisomerase IIα. The critical analysis of structural features and biological response revealed that the electron-rich β-carboline-C1-aryl with unsubstituted indole-NH produced the best potency.28h
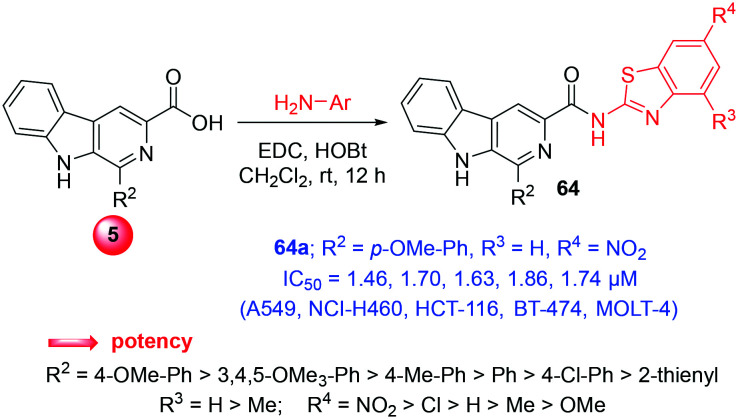
3.16. β-Carboline-benzothiazole hybrids
Benzothiazole (BT) is an important pharmacophore, which shows a broad range of therapeutic profile including anti-proliferative action.29a–c In the quest to obtain promising anticancer agents, β-carboline-benzothiazole hybrids were synthesized via the amide coupling of β-carboline acid (5) and various substituted 2-aminobenzothiazoles (compounds 64, Scheme 29). The in vitro cytotoxic ability of the hybrids was established on adherent (A549, NCI-H460, HCT-116, BT-474) and suspension (MOLT-4, THP-1, HL-60) human cancer cell lines. Among the hybrids, compound 64a displayed potent cytotoxicity with an IC50 of 1.46, 1.70, 1.63, 1.86, 1.74, 2.07, and 9.98 μM against the cancer cell lines. In addition, hybrid 64b also exhibited similar potency with IC50 of 1.81, 2.01, 1.97, 2.06, 1.93, 4.51, and 11.05 μM for the same cell lines. Notably, these congeners were found to be 13-folds more potent than harmine and were found to have comparable IC50 to doxorubicin as well. In addition, biophysical assays and molecular modelling studies unveiled these hybrids as intercalative topoisomerase II inhibitors and DNA binding agents with the ability to induce apoptosis.29d The structural influence on the biological response was distinguished as C1-β-carboline aryls, which were more active when compared to the heteroaryl groups. Moreover, unsubstituted C4-BT (R3 = H) or C6-BT substitutions (R4) with electron-withdrawing groups were also more potent.29d
Scheme 29. Synthesis of β-carboline-benzothiazole hybrids.
4. Conclusions
In summary, the current review demonstrates the design of molecular hybrids, their synthesis, and evaluation of in vitro anti-cancer potential in the last 5 years (2015–2020). The cytotoxic potential of the β-carboline motif grafted with various pharmacophores such as indole, imidazole, benzimidazole, benzothiazole, coumarin, pyridine, and hydantoin has been unfolded. Interestingly, hybrids with lead molecules such as combretastatin and podophyllotoxin that appeared in the literature have also been discussed. Moreover, the well-known chemical motifs such as dithiocarbamate, hydroxamic acid, salicylic acid, chalcone, cinnamide, pyrazole, thiazolidinedione, piperazine, and triazole were also very well explored as anticancer agents by employing β-carboline as the core. Interestingly, these hybrids not only produced excellent cytotoxic effects against most of the human cancer cell lines but were also adequately selective towards the cancer cells and exhibited excellent in vitro response in sub-micromolar/nanomolar concentrations. For instance, the β-carboline-chalcone hybrid 12a has shown highly potent cytotoxicity with an IC50 of 13.84 nM against the breast cancer cell line MCF-7. However, these agents can be further examined in vivo, which may assist in the identification of suitable lead molecules for the future development of novel anticancer agents with high efficacy and safety.
Note: Cell lines present in this review
- Cell lines name
Description
- A549/H1299/NCI-H460
Lung adenocarcinoma cells
- HeLa
Cervical carcinoma cells
- PC-3/DU-145
Prostate cancer cells
- MCF-7/MDA-MB-231/BT-474
Breast cancer cells
- ACHN/786-0
Renal cancer cells
- BxPC-3/PANC-1
Pancreatic cancer cells
- C4–2
Castration-resistant prostate cancer cells
- B-16
Mouse melanoma cancer cells
- HCT116/HCT15/HT-29
Colon cancer cells
- SW-620/SW-480/LOVO
Colon cancer cells
- HepG2/Huh7/Bel7402
Hepatocellular carcinoma cells
- SMMC-7721/SUMM-7721
Hepatocellular carcinoma cells
- RPMI-8226/CCRF-CEM/HL-60
Leukemia cancer cells
- Ej
Bladder carcinoma cells
- U251
Glioma cancer cells
- NCI-ADR-RES/OVCAR-3
Ovarian cancer cells
- MG-63
Osteosarcoma cells
- A-431/B16F10
Skin cancer cells
- L-132
Human pulmonary epithelial cells
- HGC-27
Human gastric carcinoma cells
- THP-1
Acute monocytic leukemia cells
- MOLT-4
Acute lymphoblastic leukemia cells
- RWPE
Normal prostate epithelial cells
- HEK-293/HEK293T
Normal human embryonic kidney cells
- NIH3T3
Normal murine fibroblast cells
- HFL-1
Normal lung fibroblasts cells
- BEAS-28
Normal human lung epithelial cells
- Vero
Normal monkey kidney cells
Conflicts of interest
There is no conflict of interest to declare.
Acknowledgments
Authors are thankful to Department of Pharmaceuticals (DoP), Ministry of Chemicals & Fertilizers, Govt. of India, New Delhi, for the award of NIPER fellowship; NIPER-H Research Communication No.: NIPER-H/2020/135.
Biographies
Biography
Jay Prakash Soni.

Mr. Jay Prakash Soni was born in 1993, in Madhya Pradesh, India. He completed his graduation in Pharmacy from VNS Institution of Pharmacy, Bhopal in 2015 and received his M.S. (Pharm) degree from the National Institute of Pharmaceutical Education and Research (NIPER) S.A.S. Nagar, Punjab, India. Later, he joined Dr. N. Shankaraiah's research group in July 2017 as a PhD scholar at NIPER-Hyderabad. His research interest involves the design and synthesis of NCEs with potential cytotoxic profile and molecular modelling studies.
Biography
Yogesh Yeole.

Mr. Yogesh Bapusaheb Yeole was born in Maharashtra, India. He graduated with a B. Pharma degree from Dr. D. Y. Patil Institute of Pharmaceutical Science and Research, Pune, India. Currently, he is pursuing his M.S. (Pharm.) degree at the National Institute of Pharmaceutical Education and Research, Hyderabad, India; and working in Dr. N. Shankaraiah's group.
Biography
Nagula Shankaraiah.

Dr. Nagula Shankaraiah was born in 1976 in India. He obtained his B.Sc. and M.Sc. degree in Chemistry from Kakatiya University, Warangal. He completed his Ph.D. under the guidance of Dr. Ahmed Kamal at CSIR-IICT, Hyderabad. Later, he worked with Prof. Leonardo S. Santos at the University of Talca as a post-doctoral fellow and also worked in UINICAMP, Brazil. Currently, he is an Associate Professor at the Department of Medicinal Chemistry at NIPER-Hyderabad. His prime research interest focuses on the design and synthesis of cytotoxic NCEs for cancer chemotherapy and the development of sustainable methodologies involving click reactions, C–H activation, and one-pot domino reactions.
References
- (a) Siegel R. L. Miller K. D. Jemal A. Ca-Cancer J. Clin. 2019;69:7. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]; (b) Siegel R. L. Miller K. D. Jemal A. Ca-Cancer J. Clin. 2020;70:7. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]; (c) Mathur P. Sathish Kumar K. Chaturvedi M. Das P. Sudarshan K. L. Santhappan S. Nallasamy V. John A. Narasimhan S. Roselind F. S. JCO Glob. Oncol. 2020;6:1063. doi: 10.1200/GO.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Bernardini S. Tiezzi A. Laghezza Masci V. Ovidi E. Nat. Prod. Res. 2018;32:1926. doi: 10.1080/14786419.2017.1356838. [DOI] [PubMed] [Google Scholar]; (b) Singh D. Devi N. Kumar V. Malakar C. C. Mehra S. Rattan S. Rawal R. K. Singh V. Org. Biomol. Chem. 2016;14:8154. doi: 10.1039/c6ob01216g. [DOI] [PubMed] [Google Scholar]; (c) Abramovitch R. A. Spenser I. D. Adv. Heterocycl. Chem. 1964;3:79. doi: 10.1016/s0065-2725(08)60542-5. [DOI] [PubMed] [Google Scholar]; (d) Jiao W. H. Gao H. Zhao F. Lin H. W. Pan Y. M. Zhou G. X. Yao X. S. Chem. Pharm. Bull. 2011;59:359. doi: 10.1248/cpb.59.359. [DOI] [PubMed] [Google Scholar]; (e) Ferraz C. A. A. de Oliveira Júnior R. G. Picot L. da Silva Almeida J. R. G. Nunes X. P. Fitoterapia. 2019;137:104196. doi: 10.1016/j.fitote.2019.104196. [DOI] [PubMed] [Google Scholar]; (f) Van Baelen G. Hostyn S. Dhooghe L. Tapolcsanyi P. Matyus P. Lemiere G. Dommisse R. Kaiser M. Brun R. Cos P. Maes L. Hajos G. Riedl Z. Nagy I. Maes B. U. W. Pieters L. Bioorg. Med. Chem. 2009;17:7209. doi: 10.1016/j.bmc.2009.08.057. [DOI] [PubMed] [Google Scholar]; (g) Costa E. V. Pinheiro M. L. B. Xavier C. M. Silva J. R. A. Amaral A. C. F. Souza A. D. L. Barison A. Campos F. R. Ferreira A. G. Machado G. M. C. Leon L. L. P. J. Nat. Prod. 2006;69:292. doi: 10.1021/np050422s. [DOI] [PubMed] [Google Scholar]; (h) Cooper E. J. Hudson A. L. Parker C. A. Morgan N. G. Eur. J. Pharmacol. 2003;482:189. doi: 10.1016/j.ejphar.2003.09.039. [DOI] [PubMed] [Google Scholar]; (i) Frederick R. Bruyere C. Vancraeynest C. Reniers J. Meinguet C. Pochet L. Backlund A. Masereel B. Kiss R. Wouters J. J. Med. Chem. 2012;55:6489. doi: 10.1021/jm300542e. [DOI] [PubMed] [Google Scholar]; (j) Chen Y. Qin M. Y. Wang L. Chao H. Ji L. N. Xu A. L. Biochimie. 2013;95:2050. doi: 10.1016/j.biochi.2013.07.016. [DOI] [PubMed] [Google Scholar]; (k) Akabli T. Lamchouri F. Senhaji S. Toufik H. Struct. Chem. 2019;30:1495. [Google Scholar]; (l) Herraiz T. Galisteo J. Free Radical Res. 2002;36:923. doi: 10.1080/1071576021000005762. [DOI] [PubMed] [Google Scholar]; (m) Aricioglu F. Altunbas H. Ann. N. Y. Acad. Sci. 2003;1009:196. doi: 10.1196/annals.1304.024. [DOI] [PubMed] [Google Scholar]; (n) Khan H. Patel S. Kamal M. A. Curr. Drug Metab. 2017;18:853. doi: 10.2174/1389200218666170607100947. [DOI] [PubMed] [Google Scholar]
- (a) Banoth K. K. Faheem Chandrasekhar K. V. G. Adinarayana N. Murugesan S. Heliyon. 2020;6:e04916. doi: 10.1016/j.heliyon.2020.e04916. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samala A. Murthy S. M. Gottumukkala K. M. Res. J. Pharm. Technol. 2018;11:3547. [Google Scholar]
- (a) Nekkanti S. Tokala R. Shankaraiah N. Curr. Med. Chem. 2017;24:2887. doi: 10.2174/0929867324666170523102730. [DOI] [PubMed] [Google Scholar]; (b) Deveau A. M. Labroli M. A. Dieckhaus C. M. Barthen M. T. Smith K. S. MacDonald T. L. Bioorg. Med. Chem. Lett. 2001;11:1251. doi: 10.1016/s0960-894x(01)00136-6. [DOI] [PubMed] [Google Scholar]; (c) Yang F. Zhao N. Ge D. Chen Y. RSC Adv. 2019;9:19571. doi: 10.1039/c9ra02985k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yadav K. Meka P. N. R. Sadhu S. Guggilapu S. D. Kovvuri J. Kamal A. Srinivas R. Devayani P. Babu B. N. Nagesh N. Biochemistry. 2017;56:4392. doi: 10.1021/acs.biochem.7b00008. [DOI] [PubMed] [Google Scholar]; (e) Takeuchi T. Oishi S. Watanabe T. Ohno H. Sawada J. Matsuno K. Asai A. Asada N. Kitaura K. Fujii N. J. Med. Chem. 2011;54:4839. doi: 10.1021/jm200448n. [DOI] [PubMed] [Google Scholar]; (f) Barsanti P. A. Wang W. Ni Z. J. Duhl D. Brammeier N. Martin E. Bussiere D. Walter A. O. Bioorg. Med. Chem. Lett. 2010;20:157. doi: 10.1016/j.bmcl.2009.11.012. [DOI] [PubMed] [Google Scholar]; (g) Toshima K. Okuno Y. Nakajima Y. Matsumura S. Bioorg. Med. Chem. Lett. 2002;12:671. doi: 10.1016/s0960-894x(01)00828-9. [DOI] [PubMed] [Google Scholar]; (h) Guan H. Liu X. Peng W. Cao R. Ma Y. Chen H. Xu A. Biochem. Biophys. Res. Commun. 2006;342:894. doi: 10.1016/j.bbrc.2006.02.035. [DOI] [PubMed] [Google Scholar]
- (a) Gopu S. Ravi Kumar V. Laxma Reddy K. Venkat Reddy P. Sirasani S. Nucleosides, Nucleotides Nucleic Acids. 2019;38:349. doi: 10.1080/15257770.2018.1549329. [DOI] [PubMed] [Google Scholar]; (b) Zhang M. Sun D. Anti-Cancer Agents Med. Chem. 2015;15:537. doi: 10.2174/1871520614666141128121812. [DOI] [PubMed] [Google Scholar]; (c) Viegas Jr C. Barreiro E. J. Fraga C. A. M. Curr. Med. Chem. 2007;14:1829. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]; (d) Fraga C. A. M. Expert Opin. Drug Discovery. 2009;4:605. doi: 10.1517/17460440902956636. [DOI] [PubMed] [Google Scholar]; (e) Ivasiv V. Albertini C. Goncalves A. E. Rossi M. Bolognesi M. L. Curr. Top. Med. Chem. 2019;19:1694. doi: 10.2174/1568026619666190619115735. [DOI] [PubMed] [Google Scholar]
- (a) Khan H. Patel S. Kamal M. A. Curr. Drug Metab. 2017;18:853. doi: 10.2174/1389200218666170607100947. [DOI] [PubMed] [Google Scholar]; (b) Kumar S. Singh A. Kumar K. Kumar V. Eur. J. Med. Chem. 2017;142:48. doi: 10.1016/j.ejmech.2017.05.059. [DOI] [PubMed] [Google Scholar]; (c) Dai J. Dan W. Schneider U. Wang J. Eur. J. Med. Chem. 2018;157:622. doi: 10.1016/j.ejmech.2018.08.027. [DOI] [PubMed] [Google Scholar]; (d) Sahoo C. R. Paidesetty S. K. Padhy R. N. Eur. J. Med. Chem. 2019;162:752. doi: 10.1016/j.ejmech.2018.11.024. [DOI] [PubMed] [Google Scholar]; (e) Manasa K. L. Yadav S. S. Srikanth D. Nagesh N. Alvala M. IOSR J. Pharm. Biol. Sci. 2020;15:1. [Google Scholar]; (f) Piechowska P. Zawirska-Wojtasiak R. Mildner-Szkudlarz S. Nutrients. 2019;11:814. doi: 10.3390/nu11040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Milen M. Abranyi-Balogh P. Chem. Heterocycl. Compd. 2016;52:996. [Google Scholar]; (b) Galvis C. E. P. Kouznetsov V. V. Synthesis. 2017;49:4535. [Google Scholar]; (c) Devi N. Kumar S. Pandey S. K. Singh V. Asian J. Org. Chem. 2018;7:6. [Google Scholar]; (d) Maity P. Adhikari D. Jana A. K. Tetrahedron. 2019;75:965. [Google Scholar]; (e) Love B. E. Org. Prep. Proced. Int. 1996;28:1. [Google Scholar]
- (a) Pulka K. Curr. Opin. Drug Discovery Dev. 2010;13:669. [PubMed] [Google Scholar]; (b) Saha B. Sharma S. Sawant D. Kundu B. Tetrahedron Lett. 2007;48:1379. [Google Scholar]; (c) Gupta L. Srivastava K. Singh S. Puri S. K. Chauhan P. M. S. Bioorg. Med. Chem. Lett. 2008;18:3306. doi: 10.1016/j.bmcl.2008.04.030. [DOI] [PubMed] [Google Scholar]; (d) Kamal A. Sathish M. Prasanthi A. V. G. Chetna J. Tangella Y. Srinivasulu V. Shankaraiah N. Alarifi A. RSC Adv. 2015;5:90121. [Google Scholar]; (e) Shi B. Cao R. Fan W. Guo L. Ma Q. Chen X. Zhang G. Qiu L. Song H. Eur. J. Med. Chem. 2013;60:10. doi: 10.1016/j.ejmech.2012.11.033. [DOI] [PubMed] [Google Scholar]; (f) Wu Q. Cao R. Feng M. Guan X. Ma C. Liu J. Song H. Peng W. Eur. J. Med. Chem. 2009;44:533. doi: 10.1016/j.ejmech.2008.03.030. [DOI] [PubMed] [Google Scholar]; (g) Cao R. Peng W. Chen H. Hou X. Guan H. Chen Q. Ma Y. Xu A. Eur. J. Med. Chem. 2005;40:249. doi: 10.1016/j.ejmech.2005.04.008. [DOI] [PubMed] [Google Scholar]; (h) Gaikwad S. Kamble D. Lokhande P. Tetrahedron Lett. 2018;59:2387. [Google Scholar]; (i) Zheng B. Trieu T. H. Meng T. Z. Lu X. Dong J. Zhang Q. Shi X. X. RSC Adv. 2018;8:6834. doi: 10.1039/c7ra13434g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhao M. Bi L. Wang W. Wang C. Baudy-Floc'h M. Ju J. Peng S. Bioorg. Med. Chem. 2006;14:6998. doi: 10.1016/j.bmc.2006.06.021. [DOI] [PubMed] [Google Scholar]; (k) Cao R. Chen H. Peng W. Ma Y. Hou X. Guan H. Liu X. Xu A. Eur. J. Med. Chem. 2005;40:991. doi: 10.1016/j.ejmech.2005.04.008. [DOI] [PubMed] [Google Scholar]
- (a) Kamal A. Srinivasulu V. Sathish M. Tangella Y. Nayak V. L. Rao M. P. N. Shankaraiah N. Nagesh N. Asian J. Org. Chem. 2014;3:68. [Google Scholar]; (b) Kamal A. Reddy T. S. Vishnuvardhan M. V. P. S. Nimbarte V. D. Rao A. V. S. Srinivasulu V. Shankaraiah N. Bioorg. Med. Chem. 2015;23:4608. doi: 10.1016/j.bmc.2015.05.060. [DOI] [PubMed] [Google Scholar]; (c) Nekkanti S. Veeramani K. Kumari S. S. Tokala R. Shankaraiah N. RSC Adv. 2016;6:103556. [Google Scholar]; (d) Sharma P. Reddy T. S. Praveen N. Ram K. Bhargava S. K. Shankaraiah N. Eur. J. Med. Chem. 2017;138:234. doi: 10.1016/j.ejmech.2017.06.035. [DOI] [PubMed] [Google Scholar]; (e) Yadav U. Sakla A. P. Tokala R. Nyalam S. T. Khurana A. Digwal C. S. Talla V. Godugu C. Shankaraiah N. Kamal A. ChemistrySelect. 2020;5:4356. [Google Scholar]; (f) Sana S. Tokala R. Bajaj D. M. Nagesh N. Bokara K. K. Kiranmai G. Lakshmi U. J. Vadlamani S. Talla V. Shankaraiah N. Bioorg. Chem. 2019;93:103317. doi: 10.1016/j.bioorg.2019.103317. [DOI] [PubMed] [Google Scholar]; (g) Mani G. S. Anchi P. Sunkari S. Donthiboina K. Godugu C. Shankaraiah N. Kamal A. Bioorg. Med. Chem. Lett. 2020;30:127432. doi: 10.1016/j.bmcl.2020.127432. [DOI] [PubMed] [Google Scholar]; (h) Tokala R. Bora D. Sana S. Nachtigall F. M. Santosh L. S. Shankaraiah N. J. Org. Chem. 2019;84:5504. doi: 10.1021/acs.joc.9b00454. [DOI] [PubMed] [Google Scholar]; (i) Bora D. Tokala R. John S. E. Prasanth B. Shankaraiah N. Org. Biomol. Chem. 2020;18:2307. doi: 10.1039/d0ob00250j. [DOI] [PubMed] [Google Scholar]
- (a) Hotha S. Yarrow J. C. Yang J. G. Garrett S. Renduchintala K. V. Mayer T. U. Kapoor T. M. Angew. Chem., Int. Ed. 2003;42:2379. doi: 10.1002/anie.200351173. [DOI] [PubMed] [Google Scholar]; (b) Shankaraiah N. Nekkanti S. Chudasama K. J. Ram K. Sharma P. Kumar M. Naidu V. G. M. Srinivasulu V. Srinivasulu G. Kamal A. Bioorg. Med. Chem. Lett. 2014;24:5413. doi: 10.1016/j.bmcl.2014.10.038. [DOI] [PubMed] [Google Scholar]; (c) Bialonska D. Zjawiony J. K. Mar. Drugs. 2009;7:166. doi: 10.3390/md7020166. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yao C. H. Hsieh T. C. Song J. S. Lee J. C. Future Med. Chem. 2020;12:3. doi: 10.4155/fmc-2019-0276. [DOI] [PubMed] [Google Scholar]
- (a) Mahapatra D. K. Asati V. Bharti S. K. Expert Opin. Ther. Pat. 2019;29:385. doi: 10.1080/13543776.2019.1613374. [DOI] [PubMed] [Google Scholar]; (b) Shankaraiah N. Siraj K. P. Nekkanti S. Srinivasulu V. Sharma P. Ram K. Sathish M. Vishnuvardhan M. V. P. S. Ramakrishna S. Jadala C. Nagesh N. Kamal A. Bioorg. Chem. 2015;59:130. doi: 10.1016/j.bioorg.2015.02.007. [DOI] [PubMed] [Google Scholar]; (c) Kamal A. Srinivasulu V. Nayak V. L. Sathish M. Shankaraiah N. Bagul C. Reddy N. V. S. Rangaraj N. Nagesh N. ChemMedChem. 2014;9:2084. doi: 10.1002/cmdc.201300406. [DOI] [PubMed] [Google Scholar]
- (a) Cao R. Yi W. Wu Q. Guan X. Feng M. Ma C. Chen Z. Song H. Peng W. Bioorg. Med. Chem. Lett. 2008;18:6558. doi: 10.1016/j.bmcl.2008.10.043. [DOI] [PubMed] [Google Scholar]; (b) Zhang G. Cao R. Guo L. Ma Q. Fan W. Chen X. Li J. Shao G. Qiu L. Ren Z. Eur. J. Med. Chem. 2013;65:21. doi: 10.1016/j.ejmech.2013.04.031. [DOI] [PubMed] [Google Scholar]; (c) Cao R. Fan W. Guo L. Ma Q. Zhang G. Li J. Chen X. Ren Z. Qiu L. Eur. J. Med. Chem. 2013;60:135. doi: 10.1016/j.ejmech.2012.11.045. [DOI] [PubMed] [Google Scholar]; (d) Chauhan S. S. Singh A. K. Meena S. Lohani M. Singh A. Arya R. K. Cheruvu S. H. Sarkar J. Gayen J. R. Datta D. Chauhan P. M. S. Bioorg. Med. Chem. Lett. 2014;24:2820. doi: 10.1016/j.bmcl.2014.04.109. [DOI] [PubMed] [Google Scholar]; (e) Reddy P. O. V. Hridhay M. Nikhil K. Khan S. Jha P. N. Shah K. Kumar D. Bioorg. Med. Chem. Lett. 2018;28:1278. doi: 10.1016/j.bmcl.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) De P. Baltas M. Bedos-belval F. Curr. Med. Chem. 2011;18:1672. doi: 10.2174/092986711795471347. [DOI] [PubMed] [Google Scholar]; (b) Su P. Shi Y. Wang J. Shen X. Jhang J. Anti-Cancer Agents Med. Chem. 2015;15:980. doi: 10.2174/1871520615666150130111120. [DOI] [PubMed] [Google Scholar]; (c) Sathish M. Dushantrao S. C. Nekkanti S. Tokala R. Thatikonda S. Tangella Y. Srinivas G. Cherukommu S. Krishna N. H. Shankaraiah N. Nagesh N. Kamal A. Bioorg. Med. Chem. 2018;26:4916. doi: 10.1016/j.bmc.2018.08.031. [DOI] [PubMed] [Google Scholar]
- (a) Bubna A. K. Indian J. Dermatol. 2015;60:419. doi: 10.4103/0019-5154.160511. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ling Y. Xu C. Luo L. Cao J. Feng J. Xue Y. Zhu Q. Ju C. Li F. Zhang Y. Zhang Y. Ling X. J. Med. Chem. 2015;58:9214. doi: 10.1021/acs.jmedchem.5b01052. [DOI] [PubMed] [Google Scholar]; (c) Ling Y. Feng J. Luo L. Guo J. Peng Y. Wang T. Ge X. Xu Q. Wang X. Dai H. Zhang Y. ChemMedChem. 2017;12:646. doi: 10.1002/cmdc.201700133. [DOI] [PubMed] [Google Scholar]; (d) Ling Y. Guo J. Yang Q. Zhu P. Miao J. Gao W. Peng Y. Yang J. Xu K. Xiong B. Liu G. Tao J. Luo L. Zhu Q. Zhang Y. Eur. J. Med. Chem. 2018;144:398. doi: 10.1016/j.ejmech.2017.12.061. [DOI] [PubMed] [Google Scholar]; (e) Ling Y. Li Y. Zhu R. Qian J. Liu J. Gao W. Meng C. Miao J. Xiong B. Qiu X. Ling C. Dai H. Zhang Y. J. Nat. Prod. 2019;82:1442. doi: 10.1021/acs.jnatprod.8b00843. [DOI] [PubMed] [Google Scholar]; (f) Zhang L. Zhang J. Jiang Q. Zhang L. Song W. J. Enzyme Inhib. Med. Chem. 2018;33:714. doi: 10.1080/14756366.2017.1417274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Gaspari P. Banerjee T. Malachowski W. P. Muller A. J. Prendergast G. C. Duhadaway J. Bennett S. Donovan A. M. J. Med. Chem. 2006;49:684. doi: 10.1021/jm0508888. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) You M. Moriarty M. Moon R. C. Pezzuto J. M. Cancer Res. 1997;57:272. [PubMed] [Google Scholar]; (c) Buac D. Schmitt S. Ventro G. Rani Kona F. Ping Dou Q. Mini-Rev. Med. Chem. 2012;12:1193. doi: 10.2174/138955712802762040. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shinde S. D. Sakla A. P. Shankaraiah N. Bioorg. Chem. 2020;105:104346. doi: 10.1016/j.bioorg.2020.104346. [DOI] [PubMed] [Google Scholar]; (e) Kamal A. Sathish M. Nayak V. L. Srinivasulu V. Kavitha B. Tangella Y. Thummuri D. Bagul C. Shankaraiah N. Nagesh N. Bioorg. Med. Chem. 2015;23:5511. doi: 10.1016/j.bmc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- (a) El Rashedy A. A. Aboul-Enein H. Y. Mini-Rev. Med. Chem. 2013;13:399. doi: 10.2174/138955713804999847. [DOI] [PubMed] [Google Scholar]; (b) Refaat H. M. Eur. J. Med. Chem. 2010;45:2949. doi: 10.1016/j.ejmech.2010.03.022. [DOI] [PubMed] [Google Scholar]; (c) Singla P. Luxami V. Paul K. Bioorg. Med. Chem. 2015;23:1691. doi: 10.1016/j.bmc.2015.03.012. [DOI] [PubMed] [Google Scholar]; (d) Samundeeswari S. Chougala B. Holiyachi M. Shastri L. Kulkarni M. Dodamani S. Jalalpur S. Joshi S. Dixit S. Sunagar V. Hunnur R. Eur. J. Med. Chem. 2017;128:123. doi: 10.1016/j.ejmech.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Kamal A. Rao M. P. N. Swapna P. Srinivasulu V. Bagul C. Shaik A. B. Mullagiri K. Kovvuri J. Reddy V. S. Vidyasagar K. Nagesh N. Org. Biomol. Chem. 2014;12:2370. doi: 10.1039/c3ob42236d. [DOI] [PubMed] [Google Scholar]
- (a) Babaji S. Narayan P. Thenmozhi K. Rambhau P. Eur. J. Med. Chem. 2014;81:408. doi: 10.1016/j.ejmech.2014.05.036. [DOI] [PubMed] [Google Scholar]; (b) Porchia M. Pellei M. Marinelli M. Tisato F. Del F. Santini C. Eur. J. Med. Chem. 2018;146:709. doi: 10.1016/j.ejmech.2018.01.065. [DOI] [PubMed] [Google Scholar]; (c) Lam N. Y. S. Truong D. Burmeister H. Babak M. V. Holtkamp H. U. Movassaghi S. Ayine-tora D. M. Zafar A. Kubanik M. Oehninger L. Sohnel T. Reynisson J. Jamieson S. M. F. Gaiddon C. Ott I. Hartinger C. G. Inorg. Chem. 2018;57:14427. doi: 10.1021/acs.inorgchem.8b02634. [DOI] [PubMed] [Google Scholar]; (d) Dighe S. U. Khan S. Soni I. Jain P. Shukla S. Yadav R. Sen P. Meeran S. M. Batra S. J. Med. Chem. 2015;58:3485. doi: 10.1021/acs.jmedchem.5b00016. [DOI] [PubMed] [Google Scholar]; (e) Liu J. Wang M. Zhou Y. Yan J. Yang L. Li Y. Zhang H. Yang X. RSC Adv. 2015;5:63936. [Google Scholar]; (f) Liu L. X. Wang X. Q. Zhou B. Yang L. J. Li Y. Zhang H. B. Yang X. D. Sci. Rep. 2015;5:13101. doi: 10.1038/srep13101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhou B. Liu Z. Deng G. Chen W. Li M. Yang L. Li Y. Yang X. Zhang H. Org. Biomol. Chem. 2016;14:9423. doi: 10.1039/c6ob01495j. [DOI] [PubMed] [Google Scholar]
- (a) Altaf A. A. Shahzad A. Gul Z. Rasool N. Badshah A. Lal B. Khan E. J. Drug Des. Med. Chem. 2015;1:1. [Google Scholar]; (b) Zheng S. Zhong Q. Mottamal M. Zhang Q. Zhang C. Lemelle E. McFerrin H. Wang G. J. Med. Chem. 2014;57:3369. doi: 10.1021/jm500002k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shankaraiah N. Sharma P. Pedapati S. Nekkanti S. Srinivasulu V. Kumar N. P. Kamal A. Lett. Drug Des. Discovery. 2016;13:335. [Google Scholar]
- (a) Yu H. G. Huang J. A. Yang Y. N. Huang H. Luo H. S. Yu J. P. Meier J. J. Schrader H. Bastian A. Schmidt W. E. Schmitz F. Eur. J. Clin. Invest. 2002;32:838. doi: 10.1046/j.1365-2362.2002.01080.x. [DOI] [PubMed] [Google Scholar]; (b) Zhu Y. Fu J. Shurlknight K. L. Soroka D. N. Hu Y. Chen X. Sang S. J. Med. Chem. 2015;58:6494. doi: 10.1021/acs.jmedchem.5b00536. [DOI] [PubMed] [Google Scholar]; (c) Xu Q. B. Chen X. F. Feng J. Miao J. F. Liu J. Liu F. T. Niu B. X. Cai J. Y. Huang C. Zhang Y. Ling Y. Sci. Rep. 2016;6:36238. doi: 10.1038/srep36238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Kumar A. Vaidya A. Ravichandran V. Kumar S. Kishore R. Bioorg. Med. Chem. 2012;20:3378. doi: 10.1016/j.bmc.2012.03.069. [DOI] [PubMed] [Google Scholar]; (b) Wu J. Yu L. Yang F. Li J. Wang P. Zhou W. Qin L. Li Y. Luo J. Yi Z. Liu M. Chen Y. Eur. J. Med. Chem. 2014;80:340. doi: 10.1016/j.ejmech.2014.04.068. [DOI] [PubMed] [Google Scholar]; (c) Paulikova H. Vantova Z. Hunakova L. Cizekova L. Carna M. Kozurkova M. Sabolova D. Kristian P. Hamuľakova S. Imrich J. Bioorg. Med. Chem. 2012;20:7139. doi: 10.1016/j.bmc.2012.09.068. [DOI] [PubMed] [Google Scholar]; (d) Barbosa V. A. Barea P. Mazia R. S. Ueda-Nakamura T. da Costa W. F. Foglio M. A. Ruiz A. L. T. Goes. de Carvalho J. E. Vendramini-Costa D. B. Nakamura C. V. Sarragiotto M. H. Eur. J. Med. Chem. 2016;124:1093. doi: 10.1016/j.ejmech.2016.10.018. [DOI] [PubMed] [Google Scholar]
- (a) Knight S. D. Adams N. D. Burgess J. L. Chaudhari A. M. Darcy M. G. Donatelli C. A. Luengo J. I. Newlander K. A. Parrish C. A. Ridgers L. H. Sarpong M. A. Schmidt S. J. Van Aller G. S. Carson J. D. Diamond M. A. Elkins P. A. Gardiner C. M. Garver E. Gilbert S. A. Gontarek R. R. Jackson J. R. Kershner K. L. Luo L. Raha K. Sherk C. S. Sung C. M. Sutton D. Tummino P. J. Wegrzyn R. J. Auger K. R. Dhanak D. ACS Med. Chem. Lett. 2010;1:39. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tokala R. Thatikonda S. Sana S. Regur P. Godugu C. Shankaraiah N. New J. Chem. 2018;42:16226. doi: 10.1002/cmdc.201800402. [DOI] [PubMed] [Google Scholar]; (c) Kourounakis A. P. Xanthopoulos D. Tzara A. Med. Res. Rev. 2020;40:709. doi: 10.1002/med.21634. [DOI] [PubMed] [Google Scholar]
- (a) You Y. Curr. Pharm. Des. 2005;11:1695. doi: 10.2174/1381612053764724. [DOI] [PubMed] [Google Scholar]; (b) Cortese F. Bhattacharyya B. Wolff J. J. Biol. Chem. 1977;252:1134. [PubMed] [Google Scholar]; (c) Sathish M. Kavitha B. Nayak V. L. Tangella Y. Ajitha A. Nekkanti S. Alarifi A. Shankaraiah N. Nagesh N. Kamal A. Eur. J. Med. Chem. 2018;144:557. doi: 10.1016/j.ejmech.2017.12.055. [DOI] [PubMed] [Google Scholar]
- (a) Chaudhary A. Pandeya S. N. Kumar P. Sharma P. P. Gupta S. Soni N. Verma K. K. Bhardwaj G. Mini-Rev. Med. Chem. 2007;7:1186. doi: 10.2174/138955707782795647. [DOI] [PubMed] [Google Scholar]; (b) Nam N. Curr. Med. Chem. 2003;10:1697. doi: 10.2174/0929867033457151. [DOI] [PubMed] [Google Scholar]; (c) Jadala C. Sathish M. Reddy T. S. Reddy V. G. Tokala R. Bhargava S. K. Shankaraiah N. Nagesh N. Kamal A. Bioorg. Med. Chem. 2019;27:3285. doi: 10.1016/j.bmc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- Lakshmi Manasa K. Thatikonda S. Sigalapalli D. K. Sagar A. Kiranmai G. Kalle A. M. Alvala M. Godugu C. Nagesh N. Nagendra Babu B. Bioorg. Chem. 2020;101:103983. doi: 10.1016/j.bioorg.2020.103983. [DOI] [PubMed] [Google Scholar]
- (a) Thakur A. Singla R. Jaitak V. Eur. J. Med. Chem. 2015;101:476. doi: 10.1016/j.ejmech.2015.07.010. [DOI] [PubMed] [Google Scholar]; (b) Zhang L. Xu Z. Eur. J. Med. Chem. 2019;181:111587. doi: 10.1016/j.ejmech.2019.111587. [DOI] [PubMed] [Google Scholar]; (c) Samundeeswari S. Kulkarni M. V. Joshi S. D. Dixit S. R. Jayakumar S. Ezhilarasi R. M. ChemistrySelect. 2016;1:5019. [Google Scholar]
- (a) Kamal A. Prabhakar S. Janaki Ramaiah M. Venkat Reddy P. Ratna Reddy C. Mallareddy A. Shankaraiah N. Reddy T. L. N. Pushpavalli S. N. C. V. L. Pal-Bhadra M. Eur. J. Med. Chem. 2011;46:3820. doi: 10.1016/j.ejmech.2011.05.050. [DOI] [PubMed] [Google Scholar]; (b) Shankaraiah N. Sakla A. P. Laxmikeshav K. Tokala R. Chem. Rec. 2020;20:253. doi: 10.1002/tcr.201900027. [DOI] [PubMed] [Google Scholar]; (c) Shankaraiah N. Jadala C. Nekkanti S. Senwar K. R. Nagesh N. Shrivastava S. Naidu V. G. M. Sathish M. Kamal A. Bioorg. Chem. 2016;64:42. doi: 10.1016/j.bioorg.2015.11.005. [DOI] [PubMed] [Google Scholar]; (d) Salehi P. Babanezhad-Harikandei K. Bararjanian M. Al-Harrasi A. Esmaeili M. A. Aliahmadi A. Med. Chem. Res. 2016;25:1895. [Google Scholar]; (e) Sharma B. Gu L. Pillay R. P. Cele N. Awolade P. Singh P. Kaur M. Kumar V. New J. Chem. 2020;44:11137. [Google Scholar]; (f) Nekkanti S. Pooladanda V. Veldandi M. Tokala R. Godugu C. Shankaraiah N. ChemistrySelect. 2017;2:7210. [Google Scholar]
- (a) Wan Y. Li Y. Yan C. Yan M. Tang Z. Eur. J. Med. Chem. 2019;183:111691. doi: 10.1016/j.ejmech.2019.111691. [DOI] [PubMed] [Google Scholar]; (b) Dadashpour S. Emami S. Eur. J. Med. Chem. 2018;150:9. doi: 10.1016/j.ejmech.2018.02.065. [DOI] [PubMed] [Google Scholar]; (c) Singh A. K. Raj V. Saha S. Eur. J. Med. Chem. 2017;142:244. doi: 10.1016/j.ejmech.2017.07.042. [DOI] [PubMed] [Google Scholar]; (d) Demetri G. D. van Oosterom A. T. Garrett C. R. Blackstein M. E. Shah M. H. Verweij J. McArthur G. Judson I. R. Heinrich M. C. Morgan J. A. Desai J. Fletcher C. D. George S. Bello C. L. Huang X. Baum C. M. Casali P. G. Lancet. 2006;368:1329. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]; (e) Motzer R. J. Hutson T. E. Tomczak P. Michaelson M. D. Bukowski R. M. Oudard S. Negrier S. Szczylik C. Pili R. Bjarnason G. A. Garcia-del-Muro X. Sosman J. A. Solska E. Wilding G. Thompson J. A. Kim S. T. Chen I. Huang X. Figlin R. A. J. Clin. Oncol. 2009;27:3584. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tokala R. Thatikonda S. Vanteddu U. S. Sana S. Godugu C. Shankaraiah N. ChemMedChem. 2018;13:1909. doi: 10.1002/cmdc.201800402. [DOI] [PubMed] [Google Scholar]; (g) Kovvuri J. Nagaraju B. Nayak V. L. Akunuri R. Rao M. P. N. Ajitha A. Nagesh N. Kamal A. Eur. J. Med. Chem. 2018;143:1563. doi: 10.1016/j.ejmech.2017.10.054. [DOI] [PubMed] [Google Scholar]; (h) Tokala R. Sana S. Lakshmi U. J. Sankarana P. Sigalapalli D. K. Gadewal N. Kode J. Shankaraiah N. Bioorg. Chem. 2020;105:104357. doi: 10.1016/j.bioorg.2020.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Singh M. Singh S. K. Anti-Cancer Agents Med. Chem. 2014;14:127. doi: 10.2174/18715206113139990312. [DOI] [PubMed] [Google Scholar]; (b) Kamal A. Syed M. A. H. Mohammed S. M. Expert Opin. Ther. Pat. 2015;25:335. doi: 10.1517/13543776.2014.999764. [DOI] [PubMed] [Google Scholar]; (c) Sharma P. C. Sharma D. Sharma A. Bansal K. K. Rajak H. Sharma S. Thakur V. K. Appl. Mater. Today. 2020;20:100783. [Google Scholar]; (d) Tokala R. Mahajan S. Kiranmai G. Sigalapalli D. K. Sana S. John S. E. Nagesh N. Shankaraiah N. Bioorg. Chem. 2021;106:10448. doi: 10.1016/j.bioorg.2020.104481. [DOI] [PubMed] [Google Scholar]