Abstract
Objective
Children with a solitary functioning kidney have an increased risk of developing renal injury that is hypothesized to be caused by glomerular hyperfiltration. In this study, we aimed to assess the early signs of renal injury and ambulatory blood pressure profiles in children with a solitary functioning kidney.
Materials and Methods
Data of children with normal office blood pressure measurement and a solitary functioning kidney were reviewed (serum creatinine and urine albumin and β2 microglobulin excretions), and 23 age-, weight-, and height-matched healthy children were considered as a control group. The size of the kidney was measured by renal ultrasound, and the presence of compensatory hypertrophy was calculated for all the subjects. Also, the subjects were additionally assessed for blood pressure (BP) pattern and the presence of hypertension by 24-hambulatory blood pressure monitoring.
Results
The solitary functioning kidney demonstrated compensatory hypertrophy in 36 out of the patients (86%) at a mean age of 14.0 (SD 3.0) years. Increased urine albumin and β2 microglobulinuria, which are signs of kidney damage, were found in 7 (17%) and 5(12%) patients. Compared with the controls, patients had significantly higher mean blood pressure standard deviation scores (p>0,001), and ambulatory blood pressure monitoring identified masked hypertension in 7 (17%) children and prehypertension in 6 (14%) patients. Therefore, renal injury, defined as the presence of hypertension and/or albuminuria and/or β2 microglobulinuria and/or hypertension, was present in 36% of all children with a solitary functioning kidney.
Conclusion
Children with a solitary functioning kidney need prolonged follow-up to detect early signs of renal injury and prevent end-organ damage later in life. Ambulatory blood pressure monitoring is an essential tool in the diagnosis and clinical management of solitary functioning kidney patients.
Keywords: Ambulatory blood pressure monitoring, children, glomerular hyperfiltration, hypertension, proteinuria, solitary functioning kidney
What is already known on this topic?
The decrease in the nephron number causes glomerular hyperfiltration in the remaining intact nephrons.
Glomerular hyperfiltration leads to chronic kidney damage manifested by proteinuria and hypertension in the long term.
Individuals with renal agenesis (solitary kidney) are at risk for chronic kidney damage at later ages.
What this study adds on this topic?
A significant proportion of children with a functional solitary kidney show early signs of kidney damage in the second decade of life.
Tubular proteinuria may also guide the follow-up of kidney damage in addition to microalbuminuria,
Hypertension investigation in these patients should be performed with 24-hour Ambulatory Blood Pressure Monitoring, as important additional findings can be obtained.
Introduction
Unilateral renal agenesis and multicystic dysplastic kidney are disorders that cause a congenital reduction in the nephron number. A decrease in functional nephron number causes changes in glomerular hemodynamics in remaining intact nephrons (1). In this process, which is defined as glomerular hyperfiltration, the intraglomerular pressure per nephron is increased with the increase of hydrostatic pressure. Thus, it is tried to minimize the functional consequences of structural loss with compensatory hypertrophy developing in a solitary functioning kidney (SFK). However, these maladaptive changes in intact nephrons result in a vicious cycle that starts with proteinuria and hypertension as a result of glomerular overload and progresses to glomerulosclerosis, resulting in a further increase in nephron loss (1, 2). Knowing these processes that lead to chronic kidney disease in situations that lead to nephron loss necessitates the investigation of the long-term results of glomerular hyperfiltration in children born with congenital SFK (2, 3). It is very important for the prognosis of patients to be diagnosed in the early period and to take the necessary precautions in patients with damage in the solitary functioning kidney. However, contradictory results, especially hypertension, are obtained in patients with SFK due to both patient choices and differences in the methods used (4–7).
In this study, early detection of structural and functional changes in the kidney in children with SFK utilizing glomerular filtration rate (GFR) as well as proteinuria and 24-hour ambulatory blood pressure monitoring (ABPM), thus contribute to the protection of patients from chronic kidney disease process by taking necessary precautions.
Materials and Methods
In a single-center, retrospective study, the medical records of 67 patients who were followed up with a diagnosis of ITB in the Division of Pediatric Nephrology at İstanbul Medeniyet University School of Medicine between January 2010 and January 2016 were examined. Among these patients, patients with cortical damage detected in their SFK by DMSA scintigraphy, patients whose clinical and biochemical data cannot be obtained for the last 6 months, and whose GFR is <90 mL/min/1.73 m2, those who have additional urological anomalies that may affect renal function, patients in whom SFK is a part of the syndromic disease, patients with SFK after nephrectomy performed for reasons such as a tumor, stones, trauma, infection in the postnatal period, patients who are hypertensive and using antihypertensive/antiproteinuric drugs, and patients who are not suitable for in-life blood pressure application were excluded. The patient group was formed with 42 cases meeting the criteria. The control group was formed with 23 healthy children who could voluntarily undergo ABPM with the results of biochemistry and ultrasonography (US) at their admission to other clinics, who were similar in age and gender, and who did not have a history of the nephrological disease. The weight and height measurements and body mass indexes of the patient and control groups were calculated and SDS calculations were made according to the charts prepared for Turkish children with the LMS method (8, 9). In the patient group, the diagnosis of renal agenesis and multicystic dysplasia was made by abdominal US examination and the diagnosis was confirmed by DMSA scintigraphy. Renal US examinations of the children in the patient and control groups were performed in the Aplio 500 Toshiba (Toshiba Medical Systems, Tokyo, Japan) branded device; with a 3.5 MHz 6C1 convex probe and 7.5 MHz 11L4 superficial probe. Kidney long axis was measured taking into account the extreme distance between the upper and lower poles in coronal sections passing through the hilus level while lying in a supine or slightly right or slightly left lateral position and was compared with the 95th percentile values previously determined according to age and gender (10). Renal size over the 95th percentile (>2SD) was defined as compensatory hypertrophy. The kidney sizes of the children in the patient and control groups were compared to their heights because of the known relationship between kidney size and height. Kidney-height index was obtained by proportioning the size obtained by taking the ITB size for the patients and the average of the anterior and posterior long axes of the right and left kidneys (right kidney+left kidney/2) for the control group; Kidney-Height Index=kidney anteroposterior long axis (mm)/child’s height (m).
Serum creatinine (mg/dL) measurements were recorded in all cases, urine microalbumin (mg/L), urine creatinine (mg/dL), urine β2 microglobulin (ng/mL) were recorded in the patient group. In the patient group, microalbumin and β2 microglobulin measurements obtained from the second urine in the morning were proportioned to urine creatinine values, the upper values of normal were accepted as 30 mg/gr creatinine and 40.7 mg/mol creatinine, respectively. Determination of creatinine in serum and urine was done by Jaffe’s reaction, urine microalbumin determination was done by the turbidimetric method, and Urine β2 microglobulin measurement was done by microparticle immunoassay method. Glomerular Filtration Rate (GFR) was calculated by Schwartz Formula (11).
24-h ABPM was performed for the assessment of blood pressure (BP) in all children in the patient and control groups. Mobil O Graph New Generation 24h ABPM Classic (I.E.M. GmbH; Stolberg, Germany) branded ABPM device was used in the study. After the information of the case was recorded, the device was set to measure automatically every 30 minutes during the day and every 60 minutes at night with the aid of a cuff attached to the child’s non-dominant arm for 24 hours. In the evaluation of in-life blood pressure monitoring measurements, the number of successful measurements above 80% during the measurement was considered acceptable. Height-specific SDS calculations were made with the LMS method of the obtained data (24-hour, mean for day and night, systolic, diastolic blood pressures) (12). Blood pressure load values were determined automatically by the device by proportioning the number of measurements remaining above the 95th percentile values previously recorded on the device according to the gender and height of the patient to the total number of measurements. Also, the rate of decrease in systolic and diastolic blood pressure during sleep was calculated by the device by proportioning the difference between the mean awake and asleep BP values to the awake BP values. Hypertension with ABPM was accepted as 24-hour mean BP above 95th percentile (>1.96 SDS). Mean BP values between the 90–95th percentile (1.64–1.96 SDS) were defined as prehypertension (high normal BP) (13).
The families of the patient and control groups in our study were informed about the study and their consent was obtained. The study was approved by the İstanbul Medeniyet University Göztepe Training and Research Hospital Clinical Research Ethics Committee on December 1, 2015 with protocol number 2015/0158, following the Declaration of Helsinki.
Statistical Analysis
Statistical Package for Social Sciences program (SPSS) for Windows version 15.0 (SPSS Inc.; Chicago, IL, USA) software program was used to evaluate the research data. Whether the continuous variables were distributed close to normal was evaluated with the Shapiro-Wilk normality test. Normally distributed variables were defined as mean ± standard deviation (SD), categorical data were defined by number and percentage (%). Student’s t-test was used in independent groups for measurements with a normal distribution. Mann-Whitney U test was used for intergroup examinations that did not conform to the normal distribution in the examinations. Median values were taken into account. The Chi-square test was used to evaluate categorical variables. A P-value of less than 0.05 was considered statistically significant.
Results
When 42 patients with SFK included in the study were examined, it was found that 22 had congenital unilateral renal agenesis and 20 had multicystic dysplasia. 30 of the patients were male and the male: female ratio was 2.5. Twenty-four (57%) patients had left kidney failure. The age of the patients was 14.0±3.0 years and the control group was 13.0±3.1 years. There was no significant difference between the patient and control groups in terms of age, gender, and anthropometric measurements (Table 1).
Table 1.
Comparison of demographic and anthropometric measurements of patient and control groups
| Control (n=23) | Patient (n=42) | P | |
|---|---|---|---|
| Age, yeara | 13.0±3,1 | 14.0±3,0 | 0.24 |
| Gender (M/f)b | 16/7 | 30/12 | 0.88 |
| Weight-SDSc | −0.19±1.13 | −0.01±1.36 | 0.43 |
| Height-SDSc | 0.14±1.19 | −0.26±1.08 | 0.18 |
| BMI-SDSc | −0.31±1.13 | 0.10±1.40 | 0.15 |
t-test,
Chi-Square test,
Mann-Whitney U test
Serum creatinine values were detected as 0.68±0.11 mg/dL in the patient group and 0.58±0.06 mg/dl in the control group; GFR values were found to be 140.9±22.6 mL/min/1.73 m2 in the patient group and 154.0±20.4 mL/min/1.73 m2 in the control group. A statistically significant difference was found between the patient and control groups in terms of serum creatinine and GFR means (P<0.001 and P=0.023, respectively). According to urinary US measurements, the mean of kidney long axis was 119.2±15.1 mm in the patient group and 101.6±13.7 mm in the control group. A statistically significant difference was found between the two groups in terms of kidney sizes (P<0.001). Kidney size was found to be above the 95th percentile (>2 SD) values in 36 (86%) of the patients, and this result was considered as compensatory hypertrophy. The kidney-height index was 77±10 mm/m in the patient group and 66±10 mm/m in the control group, and there was a significant difference between the two groups (P<0.001) (Table 2).
Table 2.
Comparison of kidney functions and kidney size and index of patient and control groups
| Control (n=23) | Patient (n=49) | P a | |
|---|---|---|---|
| Serum creatinine, mg/dL | 0.58±0.06 | 0.68±0.11 | <0.001 |
| GFR, mL/dk/1.73 m2 | 154.0±20.4 | 140.9±22.5 | 0.023 |
| Kidney Long Axis, mm | 101.6±13,7 | 119.2±15.1 | <0.001 |
| Kidney-Height Index, mm/m | 66±10 | 77±10 | <0.001 |
t-test
When the microalbumin/creatinine and β2 microglobulin/creatinine ratios were evaluated in the second-morning urine in the patient group, it was found that microalbumin levels were higher than the limit value in 7 (17%) patients and β2 microglobulin levels were higher than the limit value in 5 (12%) patients.
24-hour, day and night blood pressure measurement values and SDS values of the patient and control groups according to the ABPM measurement results are shown in Table 3. Twenty-four-hour mean arterial pressure (MAP) values were 88.0±6.0 mm Hg in the patient group and 81.0±4.4 mmHg in the control group, and a significant difference was found between the patient and control groups (P<0.001). The 24-hour MAP-SDS values of the patients were also significantly higher than the control group (1.06±1.00 versus −0.22±0.65; P<0.001). According to 24-hour MAP-SDS measurements, 7 (17%) patients’ blood pressure was above 1.96 SDS (>95th percentile) and these patients were classified as hypertensive. Six patients (14%) had MAP-SDS values in the range 1.64–1.96 (90–95th percentile) and these patients were considered prehypertensive. Among the other criteria of in-life BP follow-up, the number of patients exceeding the limit of 25% of BP burden was 14 patients during the daytime, 20 patients at nighttime for systolic BP, and 11 patients at daytime and 8 patients at nighttime for diastolic BP. Daytime systolic and diastolic BP loads were significantly higher in the patient group (21.4±20.6 and 17.2±16.8, respectively) compared to the control group (4.2±3.8 and 4.2±4.5, respectively) (p <0.001 for both). Nighttime systolic BP burden was significantly higher in patients (29.6±27.9) compared to the control (12.5±15.4) group (P=0.041), while no significant difference was found for nighttime diastolic BP. There was also no significant difference in terms of systolic (8.4±5.2 vs. 8.5±5.3; p=0.68) and diastolic (13.0±6.3 vs. 14.6±6.6; p=0.29) BP reductions.
Table 3.
Comparison of 24-hour ambulatory blood pressure measures of the patient and control groups
| Parameter | Value, mm Hg | P a | SDS | P a | ||
|---|---|---|---|---|---|---|
| Control n:23 | Patient n:42 | Control n:23 | Patient n:42 | |||
| 24-hourMAP | 81.0±4.4 | 88.0±6.0 | <0,001 | −0.22±0.65 | 1.06±1.00 | <0.001 |
| 24-hour SPB | 110.5±6.0 | 113.8±8.0 | 0,082 | −0.53±0.68 | 0.16±0.99 | 0.006 |
| 24-hour DBP | 65.1±4.0 | 67.4±6.1 | 0,127 | −0.42±0.74 | 0.03±1.08 | 0.101 |
| Daytime SBP | 114.6±6.7 | 116.6±8.1 | 0,340 | −0.58±0.73 | −0.09±0.98 | 0.030 |
| Daytime DBP | 69.0±4.6 | 70.1±6.3 | 0,417 | −0.58±0.75 | −0.30±1.04 | 0.313 |
| Nighttime SBP | 103.9±5.3 | 107.0±7.9 | 0,060 | 0.13±0.61 | 0,64±0,85 | 0.015 |
| Nighttime DBP | 58.3±3.1 | 60.7±5.4 | 0,069 | 0.39±0.55 | 0.81±0.90 | 0.032 |
Mann-Whitney U test;
MAP: mean arterial pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure
When the renal injury markers of our patients were evaluated together, it was seen that microalbuminuria in one of or β2 microglobulinuria accompanied in one of 7 hypertensive patients, and microalbuminuria and β2 microglobulinuria were found together in 2 patients. Thus, when the presence of at least one marker was considered sufficient, it was found that 36% of our patients had a renal injury (Figure 1).
Figure 1.
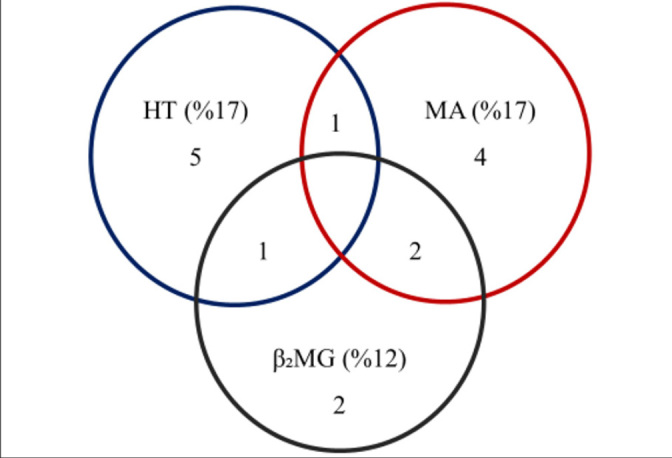
Distribution of renal injury markers in our patients
HT, hypertension; MA, microalbuminuria; β2MG, β2 microglobulin
Discussion
We determined the rate of subclinical renal injury as 36% in our patients according to proteinuria markers and hypertension criteria. In the KIMONO study, findings of renal injury were detected in 32% of children around the age of 10, and it was shown that renal injury rates increased rapidly after the age of 15 in patients with mild anomalies in SFK (14). Moreover, it has been shown that in one-third of the patients in whom renal injury findings become evident around the age of 15; there are already significant signs of renal injury before the age of 5 (15). The proportions in large series are similar to our findings. These findings emphasize the need for close monitoring of cases with especially mild renal damage signs starting from an early age. In such patients, both kidney-protective treatments should be initiated and they should be carefully protected from nephrotoxic drugs and agents.
Failure to show expected kidney growth suggests that there is not enough spare capacity in the assumed healthy SFK and is considered an independent risk factor for renal injury (15). Compensatory hypertrophy has been reported in 59–83% of cases with SFK in different series (6, 15–17). We found relatively high rates of compensatory hypertrophy in our patient group. This difference may be due to the difference in single kidney etiology in the studies. The presence of mild dysplasia and unpredictable cell capacity in the healthy kidney are important determinants for the development of hypertrophy in patients with congenital SFK. Abou Jaoudé et al. (18) found that renal functions were better preserved in patients with congenital SFK than in patients with a single kidney, and they attributed this result to the potential of congenital SFK to demonstrate better functional adaptation. A rapid decrease in GFR is observed in patients with Wilms tumor after nephrectomy and compensatory hypertrophy rates in these patients are less than patients with congenital SFK. While there is only hypertrophy due to low mitotic activity in SFK that forms with vested reasons, it contributes to hyperplasia and compensation in cellular proliferation as well as hypertrophy in congenital SFK (18). Indeed, the warning of compensatory hypertrophy in fetal life begins very early in patients with congenital agenesis (3). Thus, the high rate of development of compensatory hypertrophy in our patients may also be due to the congenital SFK in all of our patients.
When the long-term clinical course of adult patients was examined, it was found that dialysis may be required in some patients with the risk of severe renal failure, as well as proteinuria and hypertension (19, 20). It is a well-known condition that GFR can still be detected normally in the early stages of chronic kidney disease (21). Microalbuminuria, first shown in patients with diabetes, is interpreted as the earliest marker of glomerular hyperfiltration damage in patients with SFK (22). The rates of microalbuminuria, which were found to be 12–18% in different series of random urine samples, were consistent with our series (5, 14, 18, 23). These rates show that microalbuminuria screening should be started from a young age in the case of congenital renal agenesis.
On the other hand, considering that hyperfiltration injury causes early damage to the proximal tubules, tubular proteinuria, as well as microalbuminuria, may be a good marker for early renal injury. In our patient group, 5 children (12%) had higher β2 microglobulin values. Moreover, microalbumin levels in urine were normal in 3 of these patients. Stefanowicz et al. (21) found high levels of β2 microglobulin in only 3 patients in a series who received chemotherapy and nephrectomy for Wilms’ tumor. When comparing the same group with patients with unilateral renal agenesis, they showed that urine β2 microglobulin levels were similar to those of Wilms tumor patients who had undergone chemotherapy (23). Thus, the monitoring of tubular proteinuria, independent of the primary etiology, together with microalbumin in urine, which represents the onset of hyperfiltration damage in the early period, may also contribute to the evaluation of the development of renal injury.
Hypertension is the main marker of early glomerular hyperfiltration-induced renal damage in patients with a solitary functioning kidney (15). In-life BP monitoring is now considered the gold standard in the diagnosis and management of hypertension in children (24). Quite different results were obtained in previous studies with ABPM in patients with a solitary functioning kidney. Although some researchers did not find a difference in blood pressure values compared to the control group (5, 6), in other studies, BP values determined by ABPM were found to be significantly higher in children with congenital SFK (7, 16). Dursun et al. (17) could not show a difference in BP values compared to the control group but found an inverse relationship between 24-hour MAP-SDS and healthy kidney size. We found significant differences in ABPM criteria in our patient group when compared with healthy children. Moreover, during the outpatient clinic follow-ups, all of our patients’ blood pressures were evaluated as normal by office measurements. Thus, when the results obtained with ABPM were evaluated, we found masked hypertension in 7 patients. In the KIMONO study, one out of every five children with SFK was found to be hypertensive with ABPM (15). Westland et al. (16) found that blood pressure was above the 90th percentile in one of every 3 patients with SFK together with prehypertensive patients. In our study, 17% of our patients were found to be hypertensive and 14% prehypertensive, according to the 24-hour mean arterial pressure. Thus, more than 30% of our patients had blood pressure above the 90th percentile. While antihypertensive therapy was initiated in patients diagnosed with hypertension, prehypertensive patients were followed up by recommending lifestyle changes. The early detection of masked hypertension, which poses a risk for target organ damage, emphasizes both the superiority of ABPM over office measurements and the importance of performing blood pressure monitoring with ABPM in the group with SFK. This study contains several limitations despite the significant results found. On the other hand, analyzes that do not reach a significant level in our blood pressure data may also be due to the small sample size. Evaluating prospective and simultaneously repeated data with a higher number of cases could provide us with more reliable results. Also, the decision of high β2 microglobulin level in urine with a single measurement can be criticized. Indeed, recent infections or nephrotoxic agents such as analgesics may have temporarily affected tubular functions. However, the fact that other markers were found to be high in 3 of 5 patients supports the view that tubular functions may be affected in patients with SFK.
As a result, this study showed that children with SFK are at risk for renal injury due to glomerular hyperfiltration. Even patients with compensated functional kidneys may show signs of renal damage in their early lives. Therefore, patients with SFK should be followed closely in terms of proteinuria, and hypertension with ABPM, as well as GFR measurements.
Footnotes
Ethical Committee Approval: Ethical committee aproval was received from the Clinical Research Ethics Committee of İstanbul Medeniyet University Göztepe Training and Research Hospital (Date: December 1, 2015 No: 2015/0158) .
Informed Consent: Written informed consent forms were obtained from the parents and/or relatives of all the patients in the study.
Peer-review: Externally peer-reviewed.
Conflict of interest: The authors have no conflicts of interest to declare.
Author Contributions: Concept - H.G.B., C.C., Design - H.G.B., C.C.; Supervision - P.T., C.C.; Funding - H.G.B., C.C.; Materials - P.T., C.C.; Data Collection and/or Processing - P.T., C.C., H.G.B.; Analysis and/or Interpretation - H.G.B., C.C.; Literature Review - H.G.B., C.C.; Writing - H.G.B., C.C.; Critical Review - C.C., P.T.; Other - P.T., C.C.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: A potentially adverse response to renal ablation. J Am Soc Nephrol. 2001;12:1315–25. [Google Scholar]
- 2.Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: Definitions, mechanisms, and clinical implications. Nat Rew Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 3.Schreuder M. Safety in glomerular numbers. Pediatr Nephrol. 2012;27:1881–87. doi: 10.1007/s00467-012-2169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz A, Christensen CK, Christensen T, Sølling K. No microalbuminuria or other adverse effects of long-standing hyperfiltration in humans with one kidney. Am J Kidney Dis. 1989;13:131–36. doi: 10.1016/S0272-6386(89)80131-3. [DOI] [PubMed] [Google Scholar]
- 5.Seeman T, Patzer L, John U. Blood pressure, renal function, and proteinuria in children with unilateral renal agenesis. Kidney Blood Press Res. 2006;29:210–15. doi: 10.1159/000095735. [DOI] [PubMed] [Google Scholar]
- 6.Shirzai A, Yıldız N, Bıyıklı N, Üstünsoy S, Benzer M, Alpay H. Is microalbuminuria a risk factor for hypertension in children with a solitary kidney? Pediatr Nephrol. 2014;29:283–88. doi: 10.1007/s00467-013-2641-2. [DOI] [PubMed] [Google Scholar]
- 7.Mei-Zahav M, Korzets S, Cohen L. Ambulatory blood pressure monitoring in children with a solitary kidney a comparison between unilateral renal agenesis and uninephrectomy. Pediatr Nephrol. 2001;6:263–67. doi: 10.1097/00126097-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Neyzi O, Furman A, Bundak R, Gunoz H, Darendeliler F, Bas F. Growth references for Turkish children aged 6 to 18 years. Acta Paediatr. 2006;95:1635–41. doi: 10.1080/08035250600652013. [DOI] [PubMed] [Google Scholar]
- 9.Bundak R, Furman A, Gunoz H, Darendeliler F, Bas F, Neyzi O. Body mass index references for Turkish children. Acta Pediatr. 2006;95:194–98. doi: 10.1080/08035250500334738. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum DM, Korngold E, TeeIe RL. Sonographic assessment of renal length in normal children. Am J Roentgenol. 1983;142:467–69. doi: 10.2214/ajr.142.3.467. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106:522–26. doi: 10.1016/S0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 12.Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F Germany Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: Normalized reference values and the role of body dimensions. J Hypertens. 2002;20:1995–2007. doi: 10.1097/00004872-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: A multicenter trial including 1141 subjects. J Pediatr. 1997;130:78–84. doi: 10.1016/S0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 14.Westland R, Schreuder MF, Bökenkamp A, Spreeuwenberg MD, Van Wijk JA. Renal injury in children with a solitary functioning kidney-the KIMONO study. Nephrol Dial Transplant. 2011;26:1533–41. doi: 10.1093/ndt/gfq844. [DOI] [PubMed] [Google Scholar]
- 15.Westland R, Kurvers RA, Van Wijk JA, Schreuder MF. Risk factors for injury in children with a solitary functioning kidney. Pediatrics. 2013;131:478–85. doi: 10.1542/peds.2012-2088. [DOI] [PubMed] [Google Scholar]
- 16.Westland R, Schreuder M, Vermeulen A, et al. Ambulatory blood pressure monitoring is recommended in the clinical management of children with a solitary functioning kidney. Pediatr Nephrol. 2014;467:2853–60. doi: 10.1007/s00467-014-2853-0. [DOI] [PubMed] [Google Scholar]
- 17.Dursun HK, Bayazit A, Cengiz N, et al. Ambulatory blood pressure monitoring and renal functions in children with a solitary kidney. Pediatr Nephrol. 2007;22:559–64. doi: 10.1007/s00467-006-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abou Jaoudé P, Dubourg L, Bacchetta J, Berthiller J, Ranchin B, Cochat P. Congenital versus acquired solitary kidney: Is the difference relevant? Nephrol Dial Transplant. 2011;26:2188–94. doi: 10.1093/ndt/gfq659. [DOI] [PubMed] [Google Scholar]
- 19.Argueso LR, Ritchey ML, Boyle ET, Milliner DS, Bergstralh EJ, Kramer SA. Prognosis of patients with unilateral renal agenesis. Pediatr Nephrol. 1992;6:412–16. doi: 10.1007/BF00873996. [DOI] [PubMed] [Google Scholar]
- 20.Sanna-Cherchi S, Ravani P, Corbani V, et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76:528–33. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 21.Stefanowicz J, Kosiak M, Romanowicz G, Owczuk R, Adamkiewicz-Drożyńska E, Balcerska A. Glomerular filtration rate and prevalence of chronic kidney disease in Wilms’ tumour survivors. Pediatr Nephrol. 2011;26:759–66. doi: 10.1007/s00467-011-1759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palatini P. Glomerular hyperfiltration: A marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant. 2012;27:1708–14. doi: 10.1093/ndt/gfs037. [DOI] [PubMed] [Google Scholar]
- 23.Stefanowicz J, Owczuk R, Kałużyńska B, et al. Renal function and solitary kidney disease: Wilms tumour survivors versus patients with unilateral renal agenesis. Kidney Blood Pres Res. 2012;35:174–81. doi: 10.1159/000332083. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhuri A. Pediatric ambulatory blood pressure monitoring: Diagnosis of hypertension. Pediatr Nephrol. 2013;28:995–99. doi: 10.1007/s00467-013-2470-3. [DOI] [PubMed] [Google Scholar]


