Abstract
Aims
Arterial stiffness is an underlying risk factor and a hallmark of cardiovascular diseases. The endothelial cell (EC) glycocalyx is a glycan rich surface layer that plays a key role in protecting against EC dysfunction and vascular disease. However, the mechanisms by which arterial stiffness promotes EC dysfunction and vascular disease are not fully understood, and whether the mechanism involves the protective endothelial glycocalyx is yet to be determined. We hypothesized that endothelial glycocalyx protects the endothelial cells lining the vascular wall from dysfunction and disease in response to arterial stiffness.
Methods and results
Cells cultured on polyacrylamide (PA) gels of substrate stiffness 10 kPa (mimicking the subendothelial stiffness of aged, unhealthy arteries) showed a significant inhibition of glycocalyx expression compared to cells cultured on softer PA gels (2.5 kPa, mimicking the subendothelial stiffness of young, healthy arteries). Specifically, gene and protein analyses revealed that a glycocalyx core protein Glypican 1 was inhibited in cells cultured on stiff PA gels. These cells had enhanced endothelial cell dysfunction as determined by enhanced cell inflammation (enhanced inflammatory gene expression, monocyte adhesion, and inhibited nitric oxide expression), proliferation, and EndMT. Removal of Glypican 1 using gene-specific silencing with siRNA or gene overexpression using a plasmid revealed that Glypican 1 is required to protect against stiffness-mediated endothelial cell dysfunction. Consistent with this, using a model of age-mediated stiffness, older mice exhibited a reduced expression of Glypican 1 and enhanced endothelial cell dysfunction compared to young mice. Glypican 1 gene deletion in knockout mice (GPC1−/−) exacerbated endothelial dysfunction in young mice, which normally had high endothelial expression, but not in old mice that normally expressed low levels. Endothelial cell dysfunction was exacerbated in young, but not aged, Glypican 1 knockout mice (GPC1−/−).
Conclusion
Arterial stiffness promotes EC dysfunction and vascular disease at least partly through the suppression of the glycocalyx protein Glypican 1. Glypican 1 contributes to the protection against endothelial cell dysfunction and vascular disease in endothelial cells.
Keywords: Endothelial cell dysfunction, Glycocalyx, Arterial stiffness, Vascular disease
1. Introduction
With increased age or as a result of hypertension arteries lose elasticity and thicken, giving rise to a stiffened arterial wall.1,2 Arterial stiffness is an underlying risk factor for age-related cardiovascular diseases such as atherosclerosis and stroke.3 Endothelial cell (EC) dysfunction, characterized by increased proliferation, permeability, and inflammation is an important mechanism driving these age-mediated vascular diseases, and arterial stiffness is a trigger for EC dysfunction to occur.4,5 ECs lining the vasculature are highly sensitive to mechanical forces (shear stress and stretch—circumferential stress) and respond to them by altering gene expression and downstream signalling pathways.6–8 Endothelial cells are covered by a multifunctional surface layer of glycans that is referred to as the endothelial glycocalyx (GCX). The common classes of glycans found in the GCX are glycoproteins and proteoglycans. Heparan sulfate proteoglycans (HSPG) are composed of core proteins, including Glypicans that are bound to the plasma membrane by GPI anchors and Syndecans that are transmembrane, with covalently bound glycosaminoglycan (GAG) chains. The GAG chains extend into the extracellular space and are in intimate contact with flowing blood. Heparan sulfate (HS, GAG associated with Glypicans and Syndecans), chondroitin sulfate (CS, GAG associated with Syndecans), and Hyaluronic acid (HA, non-sulfated GAG that binds to surface receptors, e.g. CD44) are the dominant GAGs on most cell surfaces. More details of GCX structure are described in recent review papers.9–11
The GCX plays an essential role in maintaining EC integrity and vascular homeostasis by preserving barrier function, suppressing inflammation and cell turnover, and mediating flow-induced nitric oxide release.9,12–16 The GCX is a mechanosensor that translates fluid shear forces into genetic and functional changes in ECs. The core protein Glypican 1 plays a role in nitric oxide release in response to atheroprotective, unidirectional shear stress, whereas Syndecan 4 and Syndecan 1 have been suggested to control cell alignment under these atheroprotective flow conditions.14,15,17 Intriguingly, atheroprone, low wall shear stress promotes loss of the GCX, leading to EC dysfunction and atherosclerosis.12,17–19 In addition to atherosclerosis, the GCX is also lost under other disease conditions such as hypertension, stroke, and diabetes.20–23 Interestingly, arterial stiffness is involved in the pathology of many of these diseases; it triggers EC dysfunction and is considered both a cause and/or consequence of the disease condition.1,5 It is not known, however, whether arterial stiffness affects GCX expression or whether stiffness-mediated EC dysfunction is controlled through the protective GCX. Here, we demonstrate that GCX core protein Glypican 1 is inhibited by enhanced stiffness, and that Glypican 1 promotes protection against EC dysfunction (inflammation and proliferation) in response to stiffness in vitro and in vivo.
2. Methods
Detailed methods are provided in the Supplementary material online.
2.1 Cell culture and polyacrylamide gels
Human umbilical vein endothelial cells (HUVEC) (Lonza) obtained from multiple pools of human donors and from at least five different batches were used for experiments at passages 4–6. HUVECs were cultured in 20% FBS (Atlanta Biologicals) EBM2 media (Lonza) and supplemented with EGM™-2 SingleQuots (Lonza). Rat fat pad endothelial cells (RFPEC) (a gift from Dr David C. Spray, Albert Einstein College of Medicine, Bronx, NY) were used at passages 23–34 and cultured as described previously.14 Cells were maintained at 37°C and 5% CO2. Polyacrylamide (PA) gels were synthesized at substrate stiffness levels at 2.5, 5, 10, and 100 kPa or on glass as a control to mimic the subendothelial layer stiffness of healthy and diseased arteries as described previously.24–27 Cells were seeded on fibronectin-coated PA gels and placed into 6-well plate cell culture dishes (Corning). Alternatively, Cells (600 000 cells/per well) were seeded directly onto fibronectin-coated glass coverslips for the glass control. Following the cells reaching confluency (48 h), they were processed for experiments.
2.2 Assessment of cell function
To assess the contribution of Glypican 1 to cell function changes mediated by stiffness, expression of Glypican 1 was inhibited by gene silencing using siRNA (hs.Ri.GPC1.13.2, IDT). A scrambled, non-targeting siRNA was used as a control (51-01-14-03, IDT). Cells were transfected with the siRNA molecules using Lipofectamine™ RNAiMAX (Life Technologies) following the manufacturer’s instructions. Alternatively, Glypican 1 was overexpressed using a Glypican 1 gene plasmid (RC208602, OriGene), as a control, a green fluorescent protein (GFP) plasmid was used (SC-108083, Santa Cruz). The cells were transfected using Lipofectamine™ 3000 (Life Technologies) following the manufacturer’s instructions. Following 24 h of transfection, cell function was assessed: for inflammation by a monocyte adhesion assay as described previously28 and by assessing the expression of inflammatory molecules by immunostaining and qPCR; for anti-inflammatory nitric oxide signalling by immunostaining and qPCR; for proliferation by using ki67 antibodies (∼30 cells/frame were counted) and for Endothelial–mesenchymal transition (EndMT) by measuring the expression of EndMT markers.29,30
2.3 Gene expression by q-PCR
Cells were lysed using RLT lysis buffer (RNEasy Minikit, Qiagen) and mRNA was isolated using the RNeasy® Minikit (Qiagen) following the manufacturer’s instructions. This was followed by reverse transcription using high-capacity cDNA reverse transcription kit (ThermoScientific) following the manufacturer’s instructions. The reaction took place using a thermal cycler (DNA Engine, Biorad) and thermal profile was determined following the manufacturer’s recommendations. Gene expression was assessed by qPCR on a ABI Prism 7000 Sequence detection system, using gene-specific primers (Supplementary material online, Table S1 lists human primer sequences). The data were analysed using the comparative Ct (2−ΔΔCT) method.
2.4 Mice
All animal experiments had local approval and all procedures conform to the NIH guidelines on the protection of animals used for scientific purposes. Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of The City College of New York. A pair of heterozygous mutants on the CD1 background (GPC1−/+) was a gift from Dr Arthur Lander’s lab.31 These mice were bred to produce homozygous mutants and wildtype controls. The mice were euthanized by CO2 asphyxiation and the vasculature was fixed by pressure perfusion as previously described.12 Briefly, a midline surgical incision was made from the abdominal wall to the thoracic wall and the heart was exposed. The inferior vena cava and right atrium were severed and 30 mL of PBS containing 1% BSA were pressure perfused to clear the blood. The vessels were then pressure perfused for 5 min with PBS containing 2% paraformaldehyde. The aorta was dissected and stored in PBS until immunostained. The descending aorta was used for immunostaining, imaging, and quantitation in all experiments. To assess the contribution of age-mediated stiffness on endothelial cell dysfunction, mice (GPC1−/− and GPC1+/+ wildtype controls) were either used at 29–32 weeks (older group) or at 6–8 weeks (young group). For all experiments, at least 5–10 animals were used.
2.5 Statistical analysis
Differences between samples were analysed using a Student’s t-test for comparisons between two groups or ANOVA for comparison among more than two groups (*P < 0.05, **P < 0.01, ***P < 0.001). The mean ± standard error of mean (S.E.M.) were plotted in all graphs.
3. Results
3.1 Substrate stiffness inhibited glycocalyx core protein Glypican 1 and its HS side chains
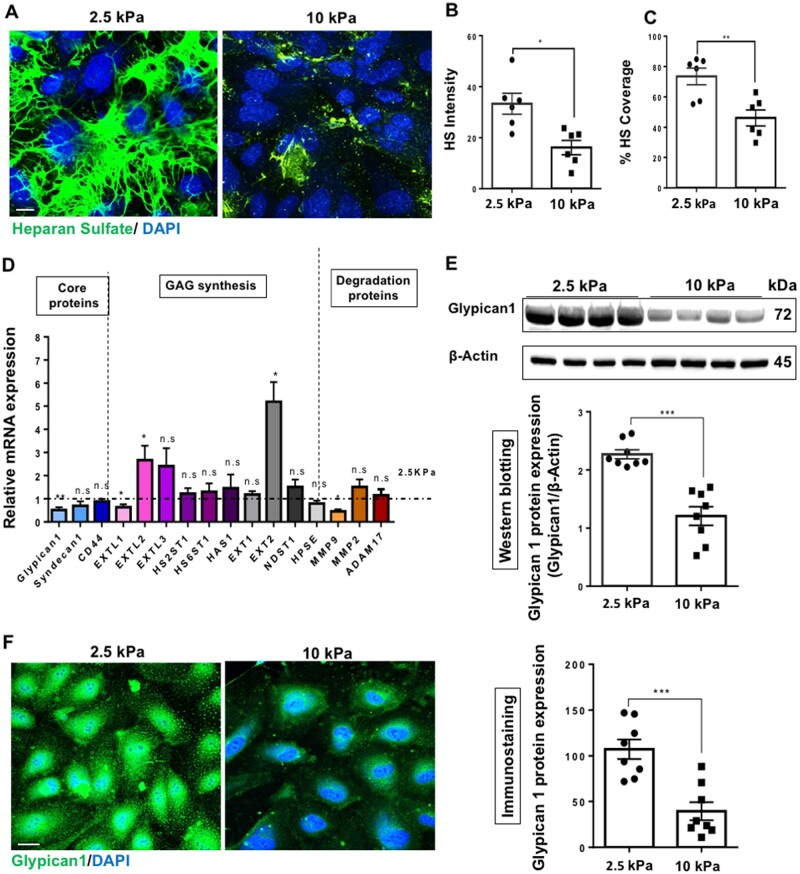
It has been reported using studies in human, porcine, bovine, and mice that a subendothelial stiffness of 2.5–5 kPa is reflective of healthy arteries, whereas subendothelial stiffness levels of ∼10 kPa and above is reflective of aged/diseased arteries.26,32–36 We assessed the effects of stiffness on GCX expression by culturing cells on polyacrylamide (PA) gels at a substrate stiffness of either 2.5 kPa (soft gels, mimicking the subendothelial layer stiffness of young/healthy arteries) or at 10 kPa (stiff gels, mimicking the subendothelial layer stiffness of aged/unhealthy arteries).24,26,35 Following 48 h of seeding on the PA gels, it was revealed that glycocalyx expression as assessed by immunostaining for the most abundant glycocalyx GAG side chain HS was markedly inhibited on stiff gels in HUVECs (Figure 1A). This reduced HS expression was revealed as fluorescent intensity (Figure 1B) and percentage coverage (Figure 1C). RFPECs also showed reduced HS expression on 10 kPa gels (Supplementary material online, Figure S1A–C). The expression of hyuluronic acid (HA), a non-sulfated GAG, was not reduced on 10 kPa gels relative to 2.5 kPa gels (Supplementary material online, Figure S2). Gene expression analysis for genes encoding GCX core proteins and GAG synthesis proteins revealed that Glypican 1 core protein gene and EXTL1 GAG synthesis protein-encoding gene were consistently downregulated in HUVECs (Figure 1D) and RFPECs (Supplementary material online, Figure S1D) cultured on 10 kPa compared to 2.5 kPa gels. To gain a further insight into the mechanism by which the GCX is inhibited on 10 kPa gels, the qPCR study included genes encoding enzymes that have been shown to degrade the GCX in other contexts.37 The degradation genes did not display a significant increase in expression in cells cultured on stiff gels, suggesting that the mechanism by which GCX expression is reduced on stiff gels does may not involve enzymatic degradation. Consistent with this, if Glypican 1 was cleaved it would be present in the cell culture media. Therefore, as an in-direct measure of Glypican 1 cleavage; enzyme-linked immunosorbent assay showed no significant difference in Glypican 1 levels in cell culture media collected from cells seeded on either soft (2.5 kPa) or stiff (10 kPa) gels following 4, 24, and 48 h of cell seeding (Supplementary material online, Figure S3). These data further suggest that enzyme degradation of Glypican 1 was not responsible for the reduction of Glypican 1 on stiff gels.
Figure 1.
Substrate stiffness inhibits the glycocalyx core protein Glypican 1 and its heparan sulfate side chains. Human umbilical vein EC (HUVECs) were cultured onto polyacrylamide gels of stiffness either 2.5 kPa (soft gel) or 10 kPa (stiff gel) until confluent (48 h). (A) Heparan sulfate (HS) (green) expression was assessed by immunostaining. Cell nuclei were identified using 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar= 10 μm. Heparan sulfate expression was quantified as (B) fluorescent intensity and (C) percentage coverage, N = 6. (D) The expression of GCX genes encoding core proteins, GAG synthesis, and degradation protein genes was assessed by qPCR from cells cultured on either 2.5 or 10 kPa gels. Gene expression in cells cultured on 10 kPa gels is normalized to expression on cells cultured on 2.5 kPa gels (dotted line). Hypoxanthine phospho-ribosyl transferase (HPRT) was used as a housekeeping gene, N = 9. Glypican 1 protein expression was assessed by (E) western blotting and quantified as relative protein expression to housekeeping protein ß-actin and (F) immunostaining (green). Cell nuclei were identified using DAPI (blue) N = 8. Expression was assessed by measuring fluorescent intensity and normalized as a percentage (expression in 10 kPa normalized to 2.5 kPa). Scale bar= 20 μm. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01, and ****P < 0.0001 using a Student’s t-test. N.S. indicates no statistically significant difference was found.
We next determined protein expression of Glypican 1 and EXTL1. Consistent with Glypican 1 mRNA expression, Glypican 1 protein levels as measured by western blotting (Figure 1E) and immunostaining (Figure 1F) were also downregulated in cells cultured on 10 kPa gels. However, this was not the case with EXTL1 protein (Supplementary material online, Figure S4), where there was no significant difference in EXTL1 protein expression in cells cultured on 10 or 2.5 kPa gels. Taken together, these data suggest that substrate stiffness inhibits GCX expression by inhibiting the expression of Glypican 1 and its associated HS GAG side chains.
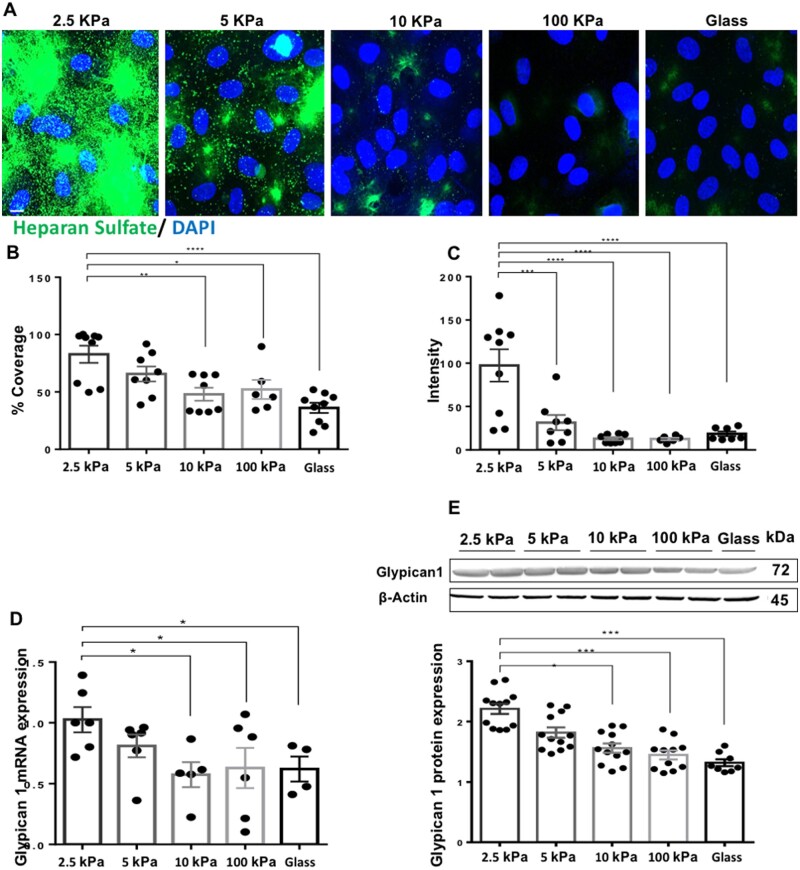
To establish a more direct link between increased stiffness and the downregulation of Glypican 1 and HS, cells were seeded on gels at substrate stiffness levels of 2.5, 5, 10, and 100 kPa, or on glass as a control. HS expression showed a stiffness-dependent decrease in expression (Figure 2A–C), with the highest expression seen in cells cultured on soft, 2.5 kPa gels and lowest on stiff, 10–100 kPa gels and on glass. Consistent with this, Glypican 1 mRNA (Figure 2D) and protein (Figure 2E) levels also showed a stiffness-dependent decrease in expression. These observations confirm that increased substrate stiffness inhibits Glypican 1 and its associated HS GAG side chains.
Figure 2.
Substrate stiffness inhibits Glypican 1 and its heparan sulfate side chains in a gradient-specific manner. Cells were cultured on polyacrylamide gels at a stiffness of 2.5, 5, 10, and 100 kPa or on glass as control. (A) Heparan sulfate expression (green) was assessed by immunostaining and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar= 10 μm. Heparan sulfate expression was quantified as (B) Percentage coverage, N = 6–9 or (C) fluorescent intensity, N = 6–9. (D) Glypican 1 expression was assessed by qPCR. HPRT was used as a housekeeping gene N = 4–6. (E) Glypican 1 protein expression was assessed by (E) western blotting and quantified as relative protein expression to housekeeping protein ß-Actin, N = 8–12. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 using an ANOVA test.
3.2 Substrate stiffness promotes EC inflammation, proliferation, and EndMT
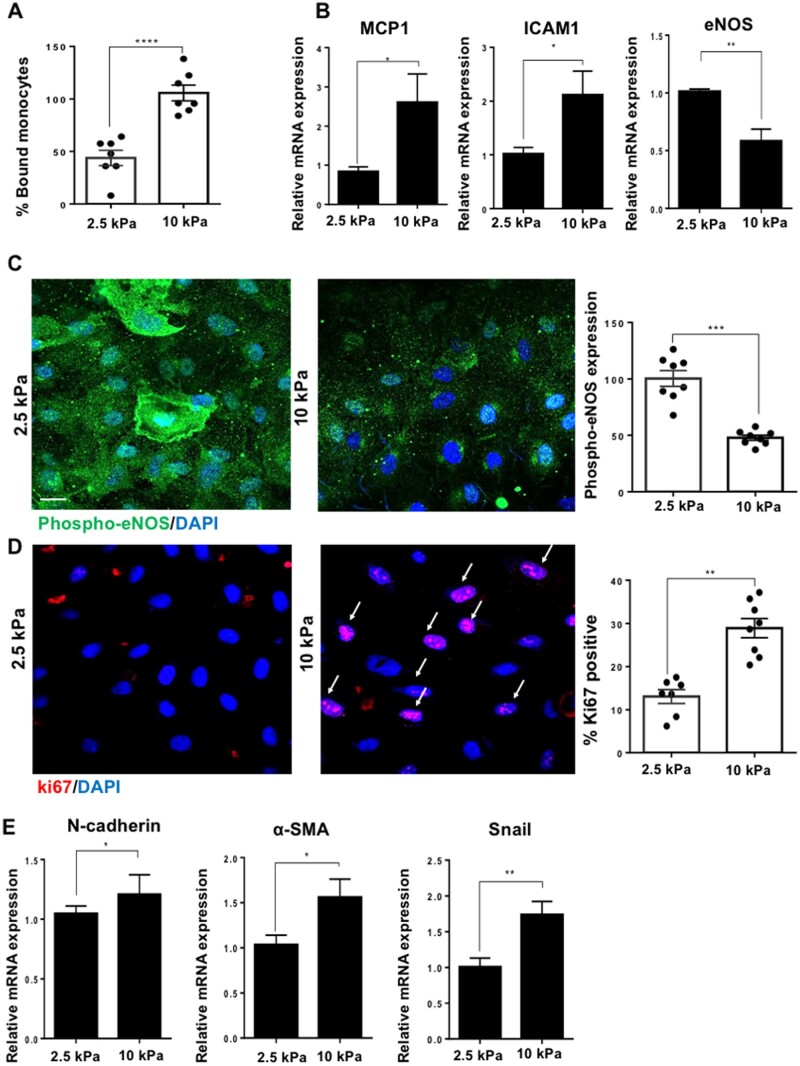
Stiffness has been shown to promote EC dysfunction (enhanced inflammation and proliferation) previously;24,26,38 we proceeded to test this in our system in preparation for the next sections on Glypican 1. We assessed inflammation using an in vitro monocyte adhesion assay which revealed that HUVEC grown on 10 kPa gels had enhanced adhesion of monocytes compared to cells cultured on 2.5 kPa gels (Figure 3A). Consistently, the expression of pro-inflammatory molecules monocyte chemoattractant protein 1 (MCP1) and intercellular adhesion molecule 1 (ICAM1) was increased in cells cultured on 10 kPa compared to 2.5 kPa gels (Figure 3B), whereas the mRNA expression of eNOS, an anti-inflammatory gene, showed the reverse trend. We then assessed the protein expression of phosphorylated endothelial nitric oxide synthase (peNOS) (Ser 1177), the active form of eNOS and a marker of active, anti-inflammatory nitric oxide signalling and found that phosphorylated eNOS expression was enhanced in cells cultured on 2.5 vs. 10 kPa gels (Figure 3C). The protein expression of total eNOS did not show a significant difference between cells grown on 2.5 or 10 kPa gels (Supplementary material online, Figure S5).
Figure 3.
Stiffness promotes EC dysfunction by increasing inflammation, proliferation, EndMT, and by inhibiting nitric oxide signalling. Cells were cultured on either 2.5 or 10 kPa gels until confluent (48 h). (A) Cell inflammation was assessed by a monocyte adhesion assay and data were quantified as percentage bound monocytes on the surface of ECs, N = 7. (B) Inflammatory marker gene expression was assessed by qPCR. HPRT was used as a housekeeping gene, N = 6. (C) To assess active nitric oxide signalling, we assessed the expression of phosphorylated-eNOS (phospho-eNOS, green) and nuclei were identified using DAPI (blue). The expression of phospho-eNOS was quantified as fluorescent intensity and was normalized as a percentage (expression in 10 kPa normalized to 2.5 kPa) N = 8. (D) Cell proliferation was assessed by ki67 (red) immunostaining and nuclei were stained with DAPI (blue). Arrows indicate ki67-positive nuclei. The data were quantified as percentage positive cells for ki67, N = 7–8. Scale bar= 20 μm. (E) Endothelial–mesenchymal transition (EndMT) gene marker expression was assessed by qPCR, N = 9. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01, and ***P < 0.001 using a Student’s t-test.
Cells grown on 10 kPa gels had a higher percentage of ki67-positive cells compared to those grown on 2.5 kPa gels, indicating that there is enhanced cell proliferation on stiff gels (Figure 3D). Consistently, RFPECs cultured on 10 kPa gels had enhanced inflammation, proliferation, and reduced nitric oxide signalling compared to cells cultured on 2.5 kPa gels (Supplementary material online, Figure S6). Finally, since stiffness has been shown to induce endothelial–mesenchymal transition,39 a process that is known to trigger proliferation and EC dysfunction we screened for EndMT marker genes N-cadherin, α-smooth muscle cell actin (α-sma) and Snail (Figure 3E). All EndMT markers showed increased expression in cells cultured on 10 vs. 2.5 kPa gels. Endothelial cell marker Cluster of Differentiation 31 (CD31) did not show a difference in expression between cells cultured on stiff or soft gels (Supplementary material online, Figure S7), indicating that stiffness triggers partial EndMT rather than a complete EndMT. MAPK signalling through p38 has been previously linked to arterial stiffness and EC dysfunction (inflammation, proliferation, and EndMT), we therefore tested the hypothesis that stiffness promotes p38 phosphorylation. We found p38 phosphorylation was enhanced in cells cultured on stiff gels compared to those cultured on soft gels (Supplementary material online, Figure S8).
3.3 Stiffness promotes EC inflammation, proliferation, and EndMT in association with the suppression of Glypican 1
Since stiffness enhanced EC inflammation, proliferation, and EndMT, and inhibited the expression of the GCX core protein Glypican 1, we tested the hypothesis that stiffness promotes EC dysfunction, at least in part, through suppressing Glypican 1.
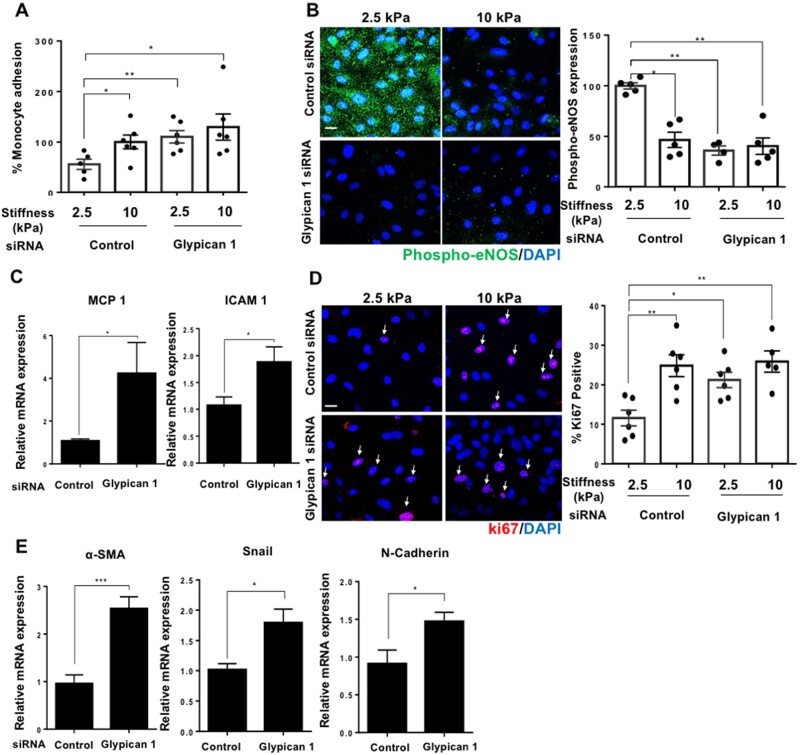
We tested the potential role of Glypican 1 in stiffness-mediated EC dysfunction using siRNA. Glypican 1 siRNA treatment inhibited Glypican 1 mRNA and protein levels (Supplementary material online, Figure S9). The silencing of Glypican 1 was associated with enhanced monocyte adhesion (Figure 4A), suppressed phospho-eNOS expression (Figure 4B), and increased the expression of inflammatory molecules MCP1 and ICAM1 (Figure 4C) in cells grown on 2.5 kPa gels, whereas Glypican 1 silencing had no significant effect on cells grown on 10 kPa gels (These cells already have a lower level of Glypican 1). These observations indicate that Glypican 1 silencing is associated with reversal of the anti-inflammatory phenotype seen in cells cultured on soft, 2.5 kPa gels, and that Glypican 1 may play a protective, anti-inflammatory role. We next assessed the effect of Glypican 1 silencing on proliferation, which was increased in cells grown on 2.5 kPa gels but had no effect on cells grown on 10 kPa gels (Figure 4D). The expression of EndMT marker genes, N-cadherin, α-sma, and Snail (Figure 4E) was also enhanced in cells cultured on 2.5 kPa gels following Glypican 1 silencing. Additionally, Glypican 1 silencing enhanced p38 phosphorylation (Supplementary material online, Figure S10). Collectively, these observations reveal that Glypican 1 abundance is associated with inhibited inflammation, proliferation, EndMT, and enhanced phospho-eNOS expression in cells grown on soft gels, and that the silencing of Glypican 1 reverses these protective effects resulting in a dysfunctional phenotype resembling cells grown on stiff, 10 kPa gels.
Figure 4.
Glypican 1 silencing enhances EC dysfunction in cells cultured on soft 2.5 kPa gels, while having no effect on cells cultured on stiff 10 kPa gels. Cells cultured on 2.5 or 10 kPa polyacrylamide gels were treated with an siRNA to silence Glypican 1 expression; alternatively, cells were treated with a control, non-targeting scrambled siRNA, followed by assessment of cell function. (A) Inflammation was assessed by a monocyte adhesion assay and data were quantified as percentage bound monocytes on the surface of ECs, N = 5–6. (B) Expression of phospho-eNOS (green) was assessed by immunostaining, nuclei were stained with DAPI (blue) N = 4–5. Scale bar = 20 μm. (C) Inflammatory marker expression was assessed by qPCR, HPRT was used as a housekeeping gene N = 6. (D) Cell proliferation was assessed by ki67 (red) immunostaining and nuclei were stained with DAPI (blue). The data were quantified as percentage of cells positive for ki67, N = 5–6. (E) EndMT gene marker expression was assessed by qPCR, N = 7. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01, and ***P < 0.001 using Student’s t-tests and ANOVA tests.
3.4 Age-mediated stiffness inhibited Glypican 1 and induced EC inflammation and proliferation in vivo
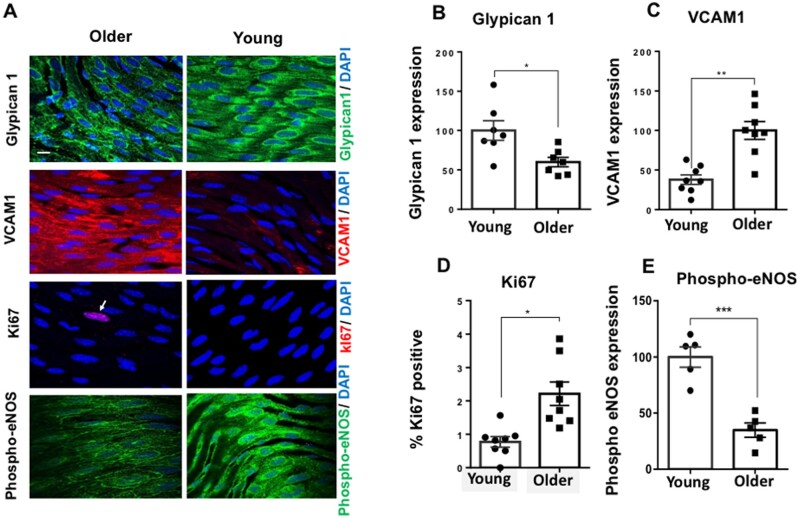
To test for the association of Glypican 1 in stiffness-mediated EC dysfunction in an in vivo setting, we utilized age-mediated stiffness as a model. It has been shown previously that young healthy arteries in murine and bovine exhibit a subendothelial layer stiffness of ∼2.5 kPa.35,40,41 With increased age or in disease conditions this subendothelial layer stiffness is increased: the arteries of 16-week-old mice have a reported stiffness of 4.8 kPa whereas older (28–32 weeks old) mice arteries exhibit a subendothelial layer stiffness that is >5 kPa but lower than that seen in old mice (72–88 weeks old; 17 kPa) giving them an approximate subendothelial layer stiffness of 7–15 kPa.24,26,40,41 Subendothelial stiffness, however, has not been measured in the Glypican 1 knockout mouse. To maintain consistency with our in vitro work, we assessed the expression of Glypican 1 by en face immunostaining in the descending aortae of young (6–8 weeks old) and older (28–32 weeks old) wildtype (WT) mice (Figure 5A). Glypican 1 levels were suppressed in older arteries compared to the young arteries (Figure 5B). We next assessed inflammation and proliferation by measuring VCAM1 as a marker of inflammation (Figure 5C) and ki67 for proliferation (Figure 5D) which both showed enhanced expression in EC in older arteries compared to younger arteries. Phospho-eNOS expression displayed the reverse trend; it was suppressed in EC in older vs. younger arteries (Figure 5E). These in vivo data for young and old mice show the same trends as our in vitro data for cells cultured on 2.5 and 10 kPa gels. In vivo, age-mediated stiffness inhibited Glypican 1 and induced EC dysfunction by enhancing inflammation, proliferation, and suppressing phospho-eNOS expression.
Figure 5.
Age-mediated stiffness inhibits Glypican 1 and induces EC dysfunction. To investigate the effects of age-mediated stiffness, descending aortae were collected from either young mice (6–8 weeks) or older mice (28–32 weeks) followed by en face immunostaining (A), for: Glypican 1 (green, top panel), VCAM1 (red), Ki67 (red), and phosho-eNOS. Nuclei were identified using DAPI. Arrows indicate ki67-positive nuclei. Expression of Glypican 1 (B), VCAM1 (C), and (E) Phospho-eNOS was quantified as fluorescent intensity and was normalized as a percentage (expression normalized to either young or older mice). For ki67 (D), the data were quantified as percentage positive cells. Arrows indicate ki67-positive nuclei. Scale bar = 10 μm. The data were pooled from five to eight animals. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01 using Student’s t-tests.
3.5 Age-mediated EC dysfunction is associated with suppression of Glypican 1
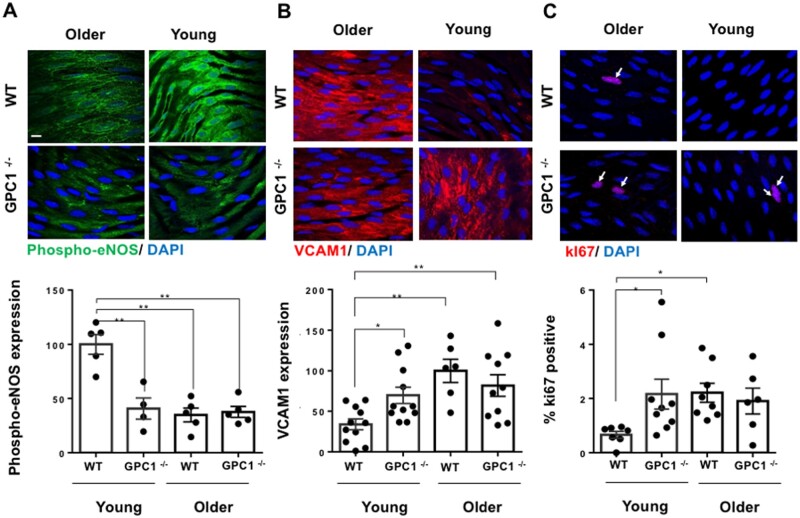
We next assessed the association of Glypican 1 with age-mediated stiffness using Glypican 1 knockout (KO) (GPC1−/−) and WT (GPC1+/+) young (6–8 weeks old), and older (28–32 weeks) mice. Young GPC1−/− mice showed a marked reduction in phospho-eNOS expression compared to Young GPC1+/+ mice, whereas in the older murine group, there was no significant difference in Phospho-eNOS expression between GPC1−/− and GPC1+/+ wildtype mice (Figure 6A). Conversely, GPC1−/− arteries exhibited an increase in EC inflammation as indicated by VCAM1 expression (Figure 6B) and enhanced proliferation as shown by ki67 positive cells (Figure 6C) in young GPC1−/− mice compared to GPC1+/+ wildtype mice; no significant differences were seen between GPC1−/− and GPC1+/+ older mice. These observations indicate that the loss of Glypican 1 is associated with an increase in EC dysfunction (inflammation and proliferation) in young arteries that is similar to the increase in EC dysfunction seen in older mouse arteries.
Figure 6.
Stiffness promotes EC dysfunction through suppression of Glypican 1 in mouse arteries. Descending aortae were collected from either young mice (6–8 weeks) or older mice (28–32 weeks) that were either Glypican 1 knockouts (GPC1−/−) or Glypican 1 wildtypes (GPC1+/+); this was followed by en face immunostaining for: (A) Phospho-eNOS (green), (B) VCAM1 (red), and (C) ki67. Expression was quantified by fluorescent intensity and normalized as a percentage for phospo-eNOS and VCAM1 (expression was normalized to either GPC+/+ young or older mice). For ki67, the data were quantified as percentage positive cells. Arrows indicate ki67-positive nuclei. The data were pooled from 4 to 11 animals. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01 using an ANOVA test.
3.6 Glypican 1 contributes to protecting endothelial cells from stiffness-mediated dysfunction
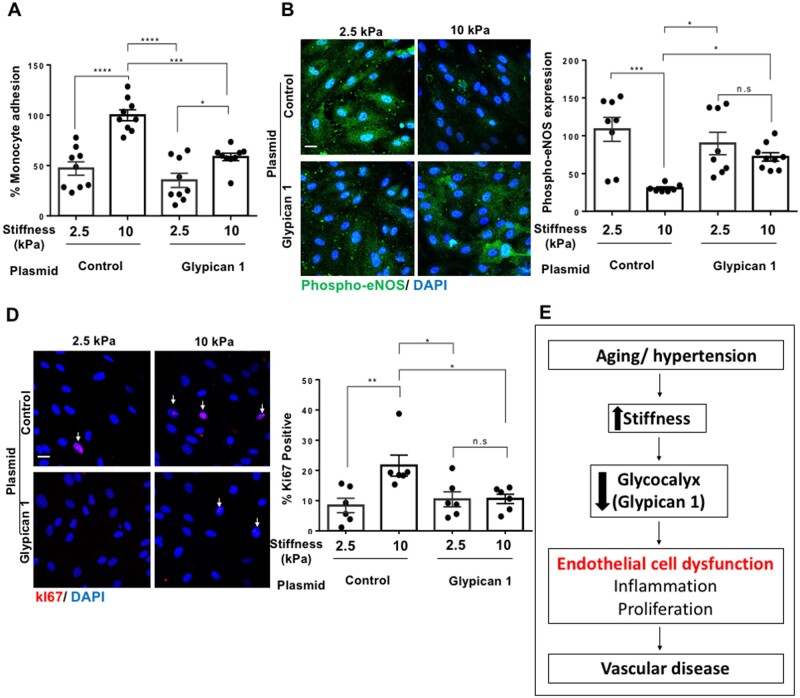
Stiffness promoted proliferation, inflammation, and suppressed protective nitric oxide signalling in endothelial cells in culture (2.5 vs. 10 kPa gels) and in mouse arteries (young vs. older arteries) in association with suppressed Glypican 1. To directly test the contribution of Glypican 1 in protecting endothelial cells from stiffness-mediated dysfunction, Glypican 1 was overexpressed in cells cultured on either 2.5 kPa (soft) or 10 kPa (stiff) polyacrylamide gels. As a control, a GFP expressing plasmid was used (Supplementary material online, Figure S11). Following 24 h of plasmid transfection, Glypican 1 protein levels were determined by western blotting, which revealed that Glypican 1 plasmid treatment enhanced Glypican 1 protein levels in ECs compared to cells treated with the control, GFP plasmid (Supplementary material online, Figure S12). We next assessed cell function. Glypican 1 overexpression suppressed monocyte adhesion (Figure 7A), enhanced protective Phospho-eNOS expression (Figure 7B), and reduced the percentage of ki67 positive cells (Figure 7C) in cells cultured on 10 kPa gels, while having no significant effect on cells cultured on 2.5 kPa gels (These cells already have a higher level of Glypican 1). These results reveal that Glypican 1 partially reversed stiffness-mediated inflammation and proliferation, making cells cultured on 10 kPa gels behave more like those cultured on soft, 2.5 kPa gels. The data suggest that age-mediated stiffness promotes EC dysfunction at least partly by suppressing GCX core protein Glypican 1 expression, leading to an increase in inflammation and proliferation which in turn influences vascular dysfunction and disease (Figure 7D).
Figure 7.
Glypican 1 protects endothelial cells from stiffness-mediated dysfunction and vascular disease. Cells cultured on 2.5 or 10 kPa polyacrylamide gels were treated with a gene plasmid to overexpress Glypican 1; alternatively, cells were treated with a control, non-targeting GFP plasmid, followed by assessment of cell function. (A) Inflammation was assessed by a monocyte adhesion assay and data were quantified as percentage bound monocytes on the surface of ECs, N = 9. (B) Expression of phospho-eNOS (green) was assessed by immunostaining, nuclei were stained with DAPI (blue) N = 7–9. (C) Cell proliferation was assessed by ki67 (red) immunostaining and nuclei were stained with DAPI (blue). The data were quantified as percentage of cells positive for ki67, N = 6. Scale bar = 20 μm. Mean values ± S.E.M. are shown. *P < 0.05, **P < 0.01 and ***P < 0.001 using Student’s t-tests and ANOVA tests. (D) Summary schematic showing that age-mediated stiffness promotes EC dysfunction and vascular disease through the suppression of the glycocalyx core protein Glypican 1.
4. Discussion
We have shown, using in vitro (using HUVECs and RFPECs) and in vivo studies, that stiffness suppresses Glypican 1, a HS proteoglycan core protein in the endothelial GCX. Loss of Glypican 1 is associated with increases in inflammation, proliferation, and EndMT. Overexpression of Glypican 1 reverses the effects of stiffness. Therefore, our observations suggest that arterial stiffness promotes EC dysfunction and vascular disease, at least in part, through the suppression of Glypican 1. We also showed that substrate stiffness inhibited Glypican 1 along with glycocalyx HS expression on the surface of ECs .We have previously studied the interaction between HS and Glypican 1 and have shown that they both associate with one another on the surface of ECs.42 Interestingly, we detected an increase in HS synthesis genes EXT2 and EXTL2 in HUVECs cultured on a stiff matrix, which could suggest that a compensatory mechanism is in play. This was not detected however in RFPECs (Supplementary material online, Figure S1D) which may suggest that this proposed compensatory mechanism is cell-type specific.
The GCX has been shown previously to be altered by the shear stress of flowing blood; atheroprone shear stress suppresses GCX expression in ECs and is associated with the development of atherosclerosis.19 GCX expression, including Glypican 1, is inhibited in hypertension.20 Interestingly, atherosclerosis and hypertension are diseases that are characterized as age-mediated and arterial stiffness has been shown to play a vital role in the progression and development of these diseases.1,3 Several studies have demonstrated that atheroprone, low wall shear stress induces arterial stiffness,41,43 thus, it may be that the suppression of the GCX in EC exposed to low wall shear stress is due to enhanced stiffness or a combination of enhanced stiffness and low shear stress. Our in vitro studies on cells cultured on 2.5 and 10 kPa gels were carried out in the absence of shear stress to determine the independent effect of subendothelial stiffness on GCX expression and EC dysfunction. However, our in vivo studies carried out on ECs from either older or young mouse arteries naturally incorporated the effects of shear stress. The results from both of these studies showed consistently that subendothelial stiffness promoted EC dysfunction in association with the suppression of Glypican 1. In further support of these observations, we note that Kohn et al.44 studied the cooperative effects of shear stress and substrate stiffness on NO production by ECs in culture and observed that NO production was higher after exposure to 3 h steady shear stress (12 dyn/cm2) than under static conditions for cells grown on either 2.5 kPa gels or glass. But, under shear conditions, NO production was significantly lower on glass than on 2.5 kPa gels. This is consistent with our previous observations that the glycocalyx (Glypican 1) is the mechanosenor for shear-induced NO production14–16 and our current observations that the glycocalyx is reduced on stiff gels and arteries from older mice.
Arterial stiffness has not been measured in the GPC1−/− mouse, and our underlying assumption has been that young GPC1−/− mice do not have altered stiffness that would inherently induce endothelial dysfunction. While we have not tested this directly, we have shown in vitro that cells on soft gels behave like cells on stiff gels when Glypican 1 is knocked down, and that cells on stiff gels behave like cells on soft gels when Glypican 1 is over expressed. Previous studies have shown that enhanced endothelial cell stiffness in obesity decreases endothelial generation of nitric oxide.45 However, acute pharmacological reduction of nitric oxide in rabbit arteries does not alter vessel stiffness.46 This implies that even if Glypican 1 gene deletion reduces nitric oxide, that would not lead to increased stiffness.
It is well established that arterial stiffness increases with age.47–49Age-mediated stiffness has been shown to indirectly impact GCX expression; studies have demonstrated that the expression of GCX was reduced, as measured by GCX thickness, in old vs. young mice and human subjects.50,51 Our data show that stiffness directly inhibits GCX expression, specifically by suppressing the GCX core protein Glypican 1. The mechanism by which Glypican 1 is inhibited in response to substrate stiffness is yet to be completely determined. Glypican 1’s suppression by substrate stiffness could be through a transcriptional mechanism involving miRNAs. Glypican 1 has been shown to be a target for miRNA-149 in human melanoma and endothelial cells.52,53 Therefore, it could be that subendothelial stiffness promotes the expression of miRNA-149, which in turn targets Glypican 1 mRNA, leading to reduced Glypican 1 protein levels.
Another plausible mechanism could involve signal transduction from mechanosensitive integrins,54 which in turn alter signalling pathways in response to stiffness, leading to reduced Glypican 1 levels. Along with integrins, mechanosensitive ion channels such as Piezo may be acting upstream of Glypican 1 in promoting the mechanosensitive signal transduction pathway in response to subendothelial stiffness.55 Glypican 1 is located in lipid rafts and caveolae,37,56 and it has been shown that both pro-atherogenic flow18 and substrate stiffness57 result in caveolae instability and removal from the cell surface. Therefore, this suggests that caveolae removal in response to stiffness is a possible mechanism by which Glypican 1 is lost. However, our ELISA study aimed to assess whether Glypican 1 was shed via enzymatic degradation into the culture media, and the results of this study revealed that the levels of Glypican 1 were not affected by substrate stiffness. Thus, the data suggest that a shedding mechanism is unlikely.
Our observations that stiffness promotes EC dysfunction are in line with previous data that showed stiffness of 10 kPa or higher triggered enhanced inflammation, permeability, proliferation, and EndMT in cultured endothelial cells (both of arterial, i.e. BAEC and venous origins, i.e. HUVECs).26,39,58,59 Consistent with our observation, previous studies have shown that stiffness triggers cell dysfunction through Nf-kB and MAPK pro-inflammatory signalling pathways.60–62 These signalling pathways trigger inflammation by inducing the expression of MCP1 and ICAM1 and suppression of anti-inflammatory eNOS signalling.63–67
Our data showed that substrate stiffness triggered an increase in EndMT marker expression a-SMA and N-cadherin, while having no effect on CD31 expression. These observations are in line with studies that have shown that mechanical forces such as low shear stress inducing a partial EndMT in ECs in vitro and in vivo.29,30,68 A Partial transition has been linked with the induction of EC dysfunction and disease.29,30,68,69
We showed that stiffness triggers EC dysfunction through p38-mediated MAPK signalling. Increased stiffness promoted p38 phosphorylation, which was enhanced upon Glypican 1 silencing in cells cultured on soft gels. These data suggest that Glypican 1 may be protecting ECs from stiffness-mediated dysfunction by inhibiting p38 phosphorylation. This is consistent with previous work has linked MAPK, in addition to Rho GTPase, NF-kB, wnt/β-catenin, and YAP/TAZ signalling in response to stiffness resulting in inflammation, proliferation, and EndMT in ECs and other cell types.58,60–62,70–72 In addition, Glypican 1 has been shown to influence MAPK signalling in cancer cells.73 Future studies will aim to further address the mechanism(s) by which stiffness inhibits Glypican 1 and the molecular mechanism by which Glypican 1 inhibits EC dysfunction.
Our in vivo data are in line with extensive work showing the effect of age-mediated stiffness on EC dysfunction and vascular disease.4,5,74 We provide a previously unknown molecular mechanism by which stiffness promotes EC dysfunction—through its suppression of Glypican 1 which plays a protective role in ECs. The function of Glypican 1 in ECs has not been studied extensively. Previous work from our laboratory showed that in response to atheroprotective high unidirectional shear stress Glypican 1-mediated shear-induced nitric oxide production.14,15,75 This suggests that Glypican 1’s role in protecting EC from inflammation and dysfunction may in part be conserved in ECs in response to multiple mechanical stimuli (shear and substrate stiffness). Other functions of Glypican 1 have been studied extensively in cancer cells, where Glypican 1 controls cell cycle progression, survival, and cell migration.76–78 We showed using complimentary gene silencing and gene overexpression approaches that Glypican 1 plays a protective role against stiffness-mediated endothelial cell dysfunction. The use of a Glypican 1-expressing plasmid increased Glypican 1 levels in ECs compared to those treated with a control, GFP plasmid. This increase in Glypican 1 levels was sufficient in inhibiting stiffness-mediated EC dysfunction. The therapeutic potential of Glypican 1 in targeting arterial stiffness-mediated vascular diseases such as hypertension and atherosclerosis can be tested in future studies using EC-specific promoter-driven adeno-associated virus (AAV)-mediated Glypican 1 gene overexpression in endothelial cells in mice.
Taken together, our work reveals that stiffness inhibits the mechanosensitive GCX by suppressing Glypican 1, and that Glypican 1 plays a protective role in inhibiting EC dysfunction. Glypican 1 may be an attractive therapeutic candidate to reverse the effects of stiffness on EC dysfunction thereby potentially attenuating vascular disease development.
Data availability
The data underlying this article are available in the article and in its Supplementary material online.
Translational perspective
This work reveals for the first time the effect of arterial stiffness on the glycocalyx protein Glypican 1 in endothelial cell function. Stiffness promotes endothelial cell dysfunction and subsequently vascular disease at least partly through the suppression of Glypican 1. Thus, Glypican 1 could be a novel therapeutic candidate for the treatment/reversal of arterial stiffness-mediated vascular disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
The authors wish to thank Dr Arthur Lander’s lab, the gift of the GPC 1 knockout animals. We also wish to thank Dr Ye Zeng for his valuable consultation regarding the interaction between HS and Glypican 1.
Conflict of interest: none declared.
Funding
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number F32HL145913, and by NIH Grants 5F32HL145913-02, RO1 HL094889, and RO1 CA204949.
References
- 1. Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension 2014;64:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wagenseil JE, Mecham RP.. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 2012;5:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ.. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duprez Daniel A. Arterial stiffness and endothelial function. Hypertension 2010;55:612–613. [DOI] [PubMed] [Google Scholar]
- 5. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Iwamoto A, Kajikawa M, Matsumoto T, Oda N, Kishimoto S, Matsui S, Hashimoto H, Aibara Y, Yusoff Hidaka MF, Kihara T, Chayama Y, Noma K, Nakashima K, Goto A, Tomiyama C, Takase H, Kohro B, Suzuki T, Ishizu T, Ueda T, Yamazaki S, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A, Higashi Y. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: FMD‐J (Flow‐Mediated Dilation Japan) Study A. J Am Heart Assoc 2018;7:e008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 2009;6:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA.. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 2016;126:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chistiakov DA, Orekhov AN, Bobryshev YV.. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol 2017;219:382–408. [DOI] [PubMed] [Google Scholar]
- 9. Tarbell JM, Cancel LM.. The glycocalyx and its significance in human medicine. J Intern Med 2016;280:97–113. [DOI] [PubMed] [Google Scholar]
- 10. Weinbaum S, Tarbell JM, Damiano ER.. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007;9:121–167. [DOI] [PubMed] [Google Scholar]
- 11. Tarbell JM, Simon SI, Curry F-R.. Mechanosensing at the vascular interface. Annu Rev Biomed Eng 2014;16:505–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM.. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 2016;252:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russell-Puleri S, Dela Paz NG, Adams D, Chattopadhyay M, Cancel L, Ebong E, Orr AW, Frangos JA, Tarbell JM.. Fluid shear stress induces upregulation of COX-2 and PGI2 release in endothelial cells via a pathway involving PECAM-1, PI3K, FAK, and p38. Am J Physiol Heart Circ Physiol 2017;312:H485–H500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartosch AMW, Mathews R, Tarbell JM.. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys J 2017;113:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ebong EE, Spray DC, Tarbell JM.. The endothelial glycocalyx in vitro: its structure and the role of heparan sulfate and Glypican-1 in eNOS activation by flow. FASEB J 2010;24:784.8. [Google Scholar]
- 16. Mitra R, O’Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE.. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep 2017;19:63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, Schwartz MA.. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A 2014;111:17308–17313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harding IC, Mitra R, Mensah SA, Herman IM, Ebong EE.. Pro-atherosclerotic disturbed flow disrupts caveolin-1 expression, localization, and function via glycocalyx degradation. J Transl Med 2018;16:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bar A, Targosz‐Korecka M, Suraj J, Proniewski B, Jasztal A, Marczyk B, Sternak M, Przybyło M, Kurpińska A, Walczak M, Kostogrys RB, Szymonski M, Chlopicki S.. Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/low‐density lipoprotein receptor‐deficient mice. J Am Heart Assoc 2019;8:e011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson JW, Ferdaus MZ, McCormick JA, Minnier J, Kaul S, Ellison DH, Barnes AP.. Endothelial transcriptomics reveals activation of fibrosis-related pathways in hypertension. Physiol Genomics 2018;50:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fumiaki Kumase YM, Mohri S, Takasu I, Ohtsuka A, Ohtsuki H.. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama 2010;64:277–283. [DOI] [PubMed] [Google Scholar]
- 22. Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang C-L, Kanenishi K.. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol 2004;107:532–538. [DOI] [PubMed] [Google Scholar]
- 23. Martens RJH, Vink H, van Oostenbrugge RJ, Staals J.. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis 2013;35:451–454. [DOI] [PubMed] [Google Scholar]
- 24. Lampi MC, Faber CJ, Huynh J, Bordeleau F, Zanotelli MR, Reinhart-King CA.. Simvastatin ameliorates matrix stiffness-mediated endothelial monolayer disruption. PLoS One 2016;11:e0147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reinhart-King CA, Dembo M, Hammer DA.. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir 2003;19:1573–1579. [Google Scholar]
- 26. Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA.. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 2011;3:112ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bangasser BL, Shamsan GA, Chan CE, Opoku KN, Tüzel E, Schlichtmann BW, Kasim JA, Fuller BJ, McCullough BR, Rosenfeld SS, Odde DJ.. Shifting the optimal stiffness for cell migration. Nat Commun 2017;8:15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Son DJ, Kumar S, Takabe W, Woo Kim C, Ni C-W, Alberts-Grill N, Jang I-H, Kim S, Kim W, Won Kang S, Baker AH, Woong Seo J, Ferrara KW, Jo H.. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun 2013;4:3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahmoud MM, Kim HR, Xing R, Hsiao S, Mammoto A, Chen J, Serbanovic-Canic J, Feng S, Bowden NP, Maguire R, Ariaans M, Francis SE, Weinberg PD, van der Heiden K, Jones EA, Chico TJA, Ridger V, Evans PC.. TWIST1 integrates endothelial responses to flow in vascular dysfunction and atherosclerosis. Circ Res 2016;119:450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahmoud MM, Serbanovic-Canic J, Feng S, Souilhol C, Xing R, Hsiao S, Mammoto A, Chen J, Ariaans M, Francis SE, Van der Heiden K, Ridger V, Evans PC.. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci Rep 2017;7:3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jen Y-H, Musacchio M, Lander AD.. Glypican-1 controls brain size through regulation of fibroblast growth factor signaling in early neurogenesis. Neural Dev 2009;4:33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Damughatla AR, Raterman B, Sharkey-Toppen T, Jin N, Simonetti OP, White RD, Kolipaka A.. Quantification of aortic stiffness using MR elastography and its comparison to MRI-based pulse wave velocity. J Magn Reson Imaging 2015;41:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stroka KM, Aranda-Espinoza H.. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 2011;118:1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Engler AJ, Richert L, Wong JY, Picart C, Discher DE.. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surf Sci 2004;570:142–154. [Google Scholar]
- 35. Peloquin J, Huynh J, Williams RM, Reinhart-King CA.. Indentation measurements of the subendothelial matrix in bovine carotid arteries. J Biomech 2011;44:815–821. [DOI] [PubMed] [Google Scholar]
- 36. Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK.. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 2009;19:1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeng Y, Adamson RH, Curry F-R, Tarbell JM.. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol 2014;306:H363–H372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J.. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 2012;60:1455–1469. [DOI] [PubMed] [Google Scholar]
- 39. Souilhol C, Harmsen MC, Evans PC, Krenning G.. Endothelial–mesenchymal transition in atherosclerosis. Cardiovasc Res 2018;114:565–577. [DOI] [PubMed] [Google Scholar]
- 40. Le Master E, Fancher IS, Lee J, Levitan I.. Comparative analysis of endothelial cell and sub-endothelial cell elastic moduli in young and aged mice: role of CD36. J Biomech 2018;76:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Master E, Huang R-T, Zhang C, Bogachkov Y, Coles C, Shentu T-P, Sheng Y, Fancher IS, Ng C, Christoforidis T, Subbaiah PV, Berdyshev E, Qain Z, Eddington DT, Lee J, Cho M, Fang Y, Minshall RD, Levitan I.. Proatherogenic flow increases endothelial stiffness via enhanced CD36-mediated uptake of oxidized low-density lipoproteins. Arterioscler Thromb Vasc Biol 2018;38:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng Y, Waters M, Andrews A, Honarmandi P, Ebong EE, Rizzo V, Tarbell JM.. Fluid shear stress induces the clustering of heparan sulfate via mobility of Glypican-1 in lipid rafts. Am J Physiol Heart Circ Physiol 2013;305:H811–H820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim CW, Pokutta-Paskaleva A, Kumar S, Timmins LH, Morris AD, Kang D-W, Dalal S, Chadid T, Kuo KM, Raykin J, Li H, Yanagisawa H, Gleason RL Jr, Jo H, Brewster LP.. Disturbed flow promotes arterial stiffening through thrombospondin-1. Circulation 2017;136:1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kohn Julie C, Zhou Dennis W, Bordeleau F, Zhou Allen L, Mason Brooke N, Mitchell Michael J, King Michael R, Reinhart-King Cynthia A.. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J 2015;108:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aroor AR, Jia G, Sowers JR.. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol 2018;314:R387–R398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiba T, Sakuma K, Komatsu T, Cao X, Aimoto M, Nagasawa Y, Shimizu K, Takahashi M, Hori Y, Shirai K, Takahara A.. Physiological role of nitric oxide for regulation of arterial stiffness in anesthetized rabbits. J Pharmacol Sci 2019;139:42–45. [DOI] [PubMed] [Google Scholar]
- 47. Lee H-Y, Oh B-H.. Aging and arterial stiffness. Circ J 2010;74:2257–2262. [DOI] [PubMed] [Google Scholar]
- 48. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension 2015;65:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kohn JC, Lampi MC, Reinhart-King CA.. Age-related vascular stiffening: causes and consequences. Front Genet 2015;6:112–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, Rondina MT, Donato AJ.. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol 2018;315:H531–H539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osuka A, Kusuki H, Yoneda K, Matsuura H, Matsumoto H, Ogura H, Ueyama M.. Glycocalyx shedding is enhanced by age and correlates with increased fluid requirement in patients with major burns. Shock 2018;50:60–65. [DOI] [PubMed] [Google Scholar]
- 52. Lai X, Wang M, McElyea SD, Sherman S, House M, Korc M.. A microRNA signature in circulating exosomes is superior to exosomal Glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett 2017;393:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jin L, Hu WL, Jiang CC, Wang JX, Han CC, Chu P, Zhang LJ, Thorne RF, Wilmott J, Scolyer RA, Hersey P, Zhang XD, Wu M.. MicroRNA-149*, a p53-responsive microRNA, functions as an oncogenic regulator in human melanoma. Proc Natl Acad Sci U S A 2011;108:15840–15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC.. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A 2003;100:7988–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, Petrassi HM, Schumacher AM, Montal M, Bandell M, Patapoutian A.. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015;4:e07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cheng F, Lindqvist J, Haigh CL, Brown DR, Mani K.. Copper-dependent co-internalization of the prion protein and Glypican-1. J Neurochem 2006;98:1445–1457. [DOI] [PubMed] [Google Scholar]
- 57. Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P.. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 2011;144:402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. LaValley DJ, Zanotelli MR, Bordeleau F, Wang W, Schwager SC, Reinhart-King CA.. Matrix stiffness enhances VEGFR-2 internalization, signaling, and proliferation in endothelial cells. Converg Sci Phys Oncol 2017;3:044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yeh Y-T, Hur SS, Chang J, Wang K-C, Chiu J-J, Li Y-S, Chien S.. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS One 2012;7:e46889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Q, Doerschuk CM.. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol 2001;166:6877–6884. [DOI] [PubMed] [Google Scholar]
- 61. Lampi MC, Reinhart-King CA.. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci Transl Med 2018;10:eaao0475. [DOI] [PubMed] [Google Scholar]
- 62. Noguchi S, Saito A, Nagase T.. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int J Mol Sci 2018;19:3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US.. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am J Physiol Cell Physiol 2005;289:C521–C530. [DOI] [PubMed] [Google Scholar]
- 64. Salerno JC, Ghosh DK, Razdan R, Helms KA, Brown CC, McMurry JL, Rye EA, Chrestensen CA.. Endothelial nitric oxide synthase is regulated by ERK phosphorylation at Ser602. Biosci Rep 2014;34:e00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chrestensen CA, McMurry JL, Salerno JC.. MAP kinases bind endothelial nitric oxide synthase. FEBS Open Bio 2012;2:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim J, Lee K-S, Kim J-H, Lee D-K, Park M, Choi S, Park W, Kim S, Choi YK, Hwang JY, Choe J, Won M-H, Jeoung D, Lee H, Ryoo S, Ha K-S, Kwon Y-G, Kim Y-M.. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: role of a miR-155/eNOS axis in preeclampsia. Free Radic Biol Med 2017;104:185–198. [DOI] [PubMed] [Google Scholar]
- 67. Kassan M, Choi S-K, Galán M, Bishop A, Umezawa K, Trebak M, Belmadani S, Matrougui K.. Enhanced NF-κB activity impairs vascular function through PARP-1–, SP-1–, and COX-2–dependent mechanisms in type 2 diabetes. Diabetes 2013;62:2078–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen P-Y, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M.. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 2015;125:4514–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lovisa S, Zeisberg M, Kalluri R.. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol Metab 2016;27:681–695. [DOI] [PubMed] [Google Scholar]
- 70. Provenzano PP, Keely PJ.. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci 2011;124:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhong A, Mirzaei Z, Simmons CA.. The roles of matrix stiffness and ß-catenin signaling in endothelial-to-mesenchymal transition of aortic valve endothelial cells. Cardiovasc Eng Technol 2018;9:158–167. [DOI] [PubMed] [Google Scholar]
- 72. Ishihara S, Yasuda M, Harada I, Mizutani T, Kawabata K, Haga H.. Substrate stiffness regulates temporary NF-κB activation via actomyosin contractions. Exp Cell Res 2013;319:2916–2927. [DOI] [PubMed] [Google Scholar]
- 73. Aikawa T, Whipple CA, Lopez ME, Gunn J, Young A, Lander AD, Korc M.. Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J Clin Invest 2008;118:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L.. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 2012;3:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeng Y, Zhang XF, Fu BM, Tarbell JM, The role of endothelial surface glycocalyx in mechanosensing and transduction. In Fu BM, Wright NT (eds). Molecular, Cellular, and Tissue Engineering of the Vascular System. Cham: Springer International Publishing; 2018. p1–27. [DOI] [PubMed] [Google Scholar]
- 76. Matsuda K, Maruyama H, Guo F, Kleeff J, Itakura J, Matsumoto Y, Lander AD, Korc M.. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res 2001;61:5562–5569. [PubMed] [Google Scholar]
- 77. Hara H, Takahashi T, Serada S, Fujimoto M, Ohkawara T, Nakatsuka R, Harada E, Nishigaki T, Takahashi Y, Nojima S, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Morii E, Mori M, Doki Y, Naka T.. Overexpression of Glypican-1 implicates poor prognosis and their chemoresistance in oesophageal squamous cell carcinoma. Br J Cancer 2016;115:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zaslavsky A, Adams M, Wissmueller S, Campbell D, Klingemann H, Walsh B, Palapattu GS.. Glypican-1 as a novel immunotherapeutic target in prostate cancer. J Clin Oncol 2018;36:174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary material online.
Translational perspective
This work reveals for the first time the effect of arterial stiffness on the glycocalyx protein Glypican 1 in endothelial cell function. Stiffness promotes endothelial cell dysfunction and subsequently vascular disease at least partly through the suppression of Glypican 1. Thus, Glypican 1 could be a novel therapeutic candidate for the treatment/reversal of arterial stiffness-mediated vascular disease.