Abstract
Periodontal diseases, such as gingivitis and periodontitis, are inflammatory diseases triggered by pathogenic bacteria that lead to damage of the soft tissue and bone supporting the teeth. Amongst the identified oral periodontopathogenic bacteria, Porphyromonas gingivalis is able to enhance oral dysbiosis, which is an imbalance in the beneficial commensal and periodontal pathogenic bacteria that induces chronic inflammation. Given the critical role of oral pathogenic bacteria like P. gingivalis in the pathogenesis of periodontitis, local and/or systemic antibacterial therapy has been suggested to treat this disease, especially in its severe or refractory forms. Nevertheless, the majority of the antibacterial agents currently used for the treatment of periodontal diseases are broad-spectrum, which harms beneficial bacterial species that are critical in health, inhibit the growth of pathogenic bacteria, contribute in protecting the periodontal tissues to damage and aid in its healing. Thus, the development of more effective and specific antibacterial agents is needed to control oral pathogens in a polymicrobial environment. The strategies for the development of novel antibacterial agents include natural product isolation as well as synthetic and semi-synthetic methodologies. This review presents an overview of the periodontal diseases gingivitis and periodontitis along with current antibacterial treatment options (i.e., classes of antibacterial agents and the mechanism(s) of resistance that hinder their usage) used in periodontal diseases that specifically target oral pathogens such as P. gingivalis. In addition, to help medicinal chemists gain a better understanding of potentially promising scaffolds, this review provides an in-depth coverage of the various families of small molecules that have been investigated as potential anti-P. gingivalis agents, including novel families of compounds, repositioned drugs, as well as natural products.
Local and/or systemic antibacterial therapy has been extensively studied and suggested to control periodontopathogens like P. gingivalis. However, more effective and specific antibacterial agents against oral pathobionts remain to be developed.
1. Introduction
1.1. Periodontal diseases: gingivitis and periodontitis
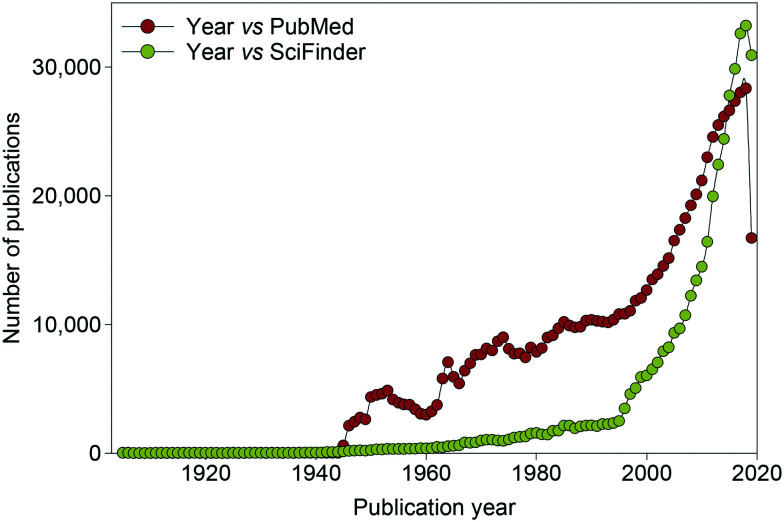
Periodontal diseases, also known as gum diseases, are inflammatory diseases initiated by oral pathogenic bacteria, which result in the destruction of the bone and tissue supporting the tooth (e.g., gingiva, alveolar bone, and periodontal ligament).1–3 Periodontal diseases comprise the early reversible stage of gum disease, gingivitis, which causes red and swollen gums that bleed when brushing or flossing teeth. When left untreated, this can lead to the severe form of gum disease, periodontitis, which can cause irreversible damage potentially leading to tooth loss. In the United States, 64.7 million adults over the age of 30 have periodontitis.4–6 There are treatment options for periodontitis such as removal/debridement of subgingival dental plaque, surgery to remove the infected tissue and antibiotics, but the lack of clear guidelines for choosing an antibiotic that selectively targets pathogenic bacteria can lead to the development of antibiotic resistance by some bacterial species (see sections 2.1–2.2). In general, there is an increasing interest in expanding the knowledge and arsenal of antibacterial agents, as demonstrated by the steady increase in publications related to antibacterial research in the last 80 years (Fig. 1).
Fig. 1. Graph displaying the number of publications related to antibacterial research published per year from 1905 to January 2020.
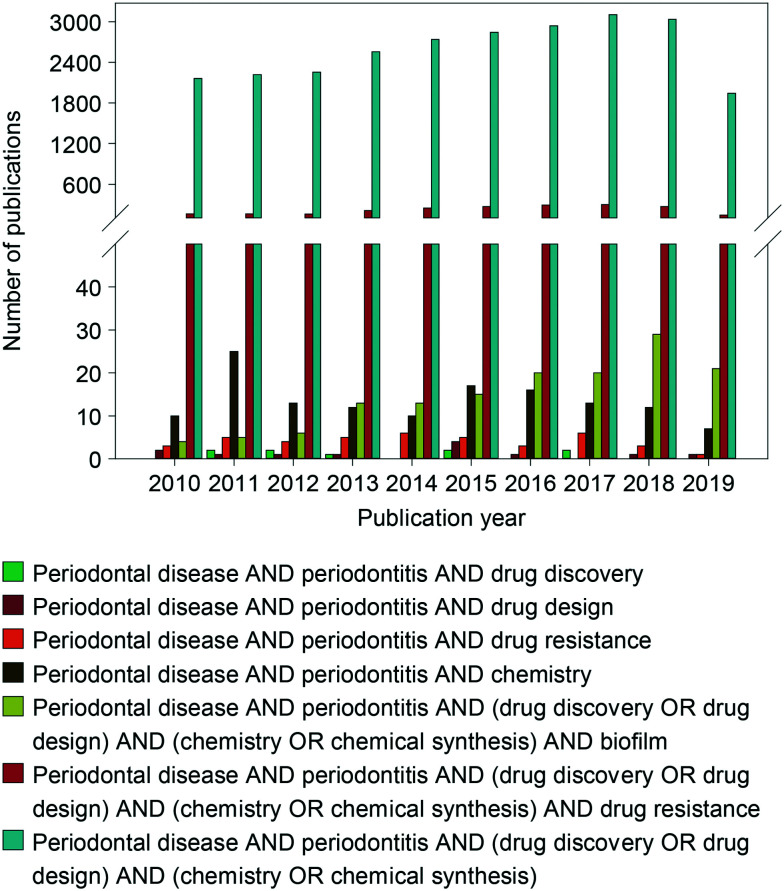
Additionally, there are some interesting reviews that discuss current antibacterial agents clinically used to treat periodontal diseases such as periodontitis along with a few novel non-approved molecules (Fig. 2). However, few of them discuss the drug discovery and development of novel antibacterial agents used to target specific periodontal pathogens along with where treatment is heading in the future. The goal of the current review is to fill that gap. In order to combat antibacterial resistance, the future of medicine should head towards the discovery of novel antibiotics, which target specific pathogenic bacteria causing the diseases, without significantly perturbing the presence of commensal bacteria critical for health and homeostasis. The periodontopathogenic Gram-negative anaerobic bacterium Porphyromonas gingivalis is a major keystone pathogen associated with periodontitis capable of causing tissue damage and invading the weakened epithelial cell layers leading to systemic diseases in the same way as other pathogenic bacteria, such as Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Prevotella intermedia, and Fusobacterium nucleatum.7,8 Of note, these bacterial species along with other oral bacteria have been associated with diabetes, Alzheimer's disease, cardiovascular diseases, polycystic ovary syndrome, and rheumatoid arthritis.9–15 Therefore, it is of interest for researchers to develop novel antibacterial agents that specifically target P. gingivalis. In order to design and synthesize an antibacterial agent that specifically targets P. gingivalis, it is critical to understand how this oral pathogen colonizes the oral cavity and interacts with other pathogenic bacteria in a biofilm (Fig. 3). In addition, having a deep knowledge of the breadth of antibacterial agents used to treat periodontal diseases currently on the market as well as their mechanism(s) of action and bacterial mode(s) of resistance is important. This review will summarize the copious amount of research articles related to the discovery and development of novel antibacterial agents used to target P. gingivalis as treatment options for chronic periodontitis. To provide a thorough overview of where we stand in our quest for the discovery and development of novel anti-P. gingivalis agents, in addition to emerging active potential antibacterial agents, this review will discuss scaffolds found to be inactive against P. gingivalis as well as discuss where future treatment options are heading (note: as most of the studies presented in this review covering the use of antibacterial agents for the treatment of periodontal diseases were published before the new classification of periodontal diseases, the terms chronic and aggressive periodontitis, now simply referred to as one all-encompassing term called “periodontitis”, will be used in this review).16
Fig. 2. Graph displaying the number of reviews published per year from 2010–2019. The various combination of terms displayed below the graph were searched in PubMed.
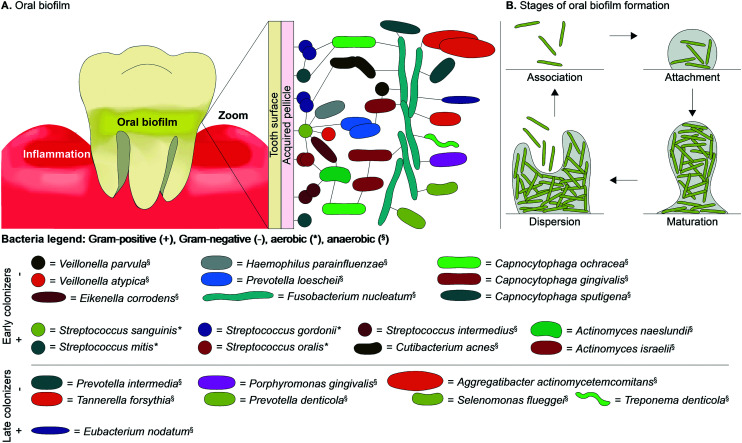
Fig. 3. A. An overview of the location of commensal and pathogenic bacterial species found in oral biofilms as early or late Gram-positive and Gram-negative colonizers along with the B. stages of oral biofilm formation.
1.2. Oral biofilms: formation of oral biofilms, oral bacterial species within oral biofilms, and their organization within oral biofilms
There are over 700 bacterial species found in the oral cavity (including cocci, spirilla, bacilli, spirochaetes, and vibrios), with only a small number of those bacterial species being pathogenic leading to inflammation and bone destruction (Table 1 and Fig. 3A).1,7,17 Periodontal diseases are caused by the shift of bacterial species in dental plaque from mostly Gram-positive bacteria, such as Actinomyces and Streptococcus spp., in healthy gingival to predominantly Gram-negative pathogenic species in periodontal diseases.2 Dental plaque, also known as an oral biofilm, consists of polymicrobial communities embedded on a tooth surface encased in an extracellular matrix (ECM).17 Oral biofilms form on the surface of the tooth due to their non-shedding nature that provides microbes, such as bacteria, with a favorable environment where they will not be disturbed and can mature. The volume of oral biofilms is composed of about 20% microorganisms and 80% ECM (Fig. 3B). The two main types of oral biofilms are supragingival, above the gingival margin line associated with dental caries, or subgingival biofilms, below the gingival margin line associated with periodontal diseases. This review will focus on pathogenic bacteria, such as P. gingivalis, that reside in subgingival plaque frequently found in patients with periodontal diseases.
Major early colonizers and late colonizers of oral biofilms associated with periodontal diseases.
| Early colonizers | |||||||
|---|---|---|---|---|---|---|---|
| Bacterial species (in alphabetical order of Gram-positive and Gram-negative) | Shape | Gram-positive/Gram-negative | Aerobic/anaerobic | Symbiotic/antagonistic interaction | Complex | Found in type of periodontal diseases | Ref. |
| Actinomyces israelii | Bacillus | + | b | Competitive interaction with P. gingivalis | Blue | Chronic periodontitis | 7, 26, 38 |
| Actinomyces naeslundii | Bacillus | + | b | ✗ S. sanguinis | Blue | Chronic periodontitis | 7, 17, 26 |
| ✗ S. gordonii | |||||||
| Competitive interaction with P. gingivalis | |||||||
| Streptococcus gordonii | Coccus | + | a | ✗ A. naeslundii | Yellow | Commensal | 17 |
| ✗ S. mutans | |||||||
| ✗ S. sanguinis | |||||||
| ✓ S. gordonii | |||||||
| Streptococcus intermedius | Coccus | + | b | N/A | Yellow | Chronic periodontitis | 39 |
| Streptococcus mitis | Coccus | + | a | Competitive interaction with P. gingivalis | Yellow | Commensal | 7 |
| Streptococcus mutans | Coccus | + | b | ✗ A. actinomycetemcomitans and competitive interaction with P. gingivalis | None | Dental caries | 7, 17 |
| ✗ S. gordonii | |||||||
| ✗ S. oralis | |||||||
| ✗ S. sanguinis | |||||||
| Streptococcus oralis | Coccus | + | a | ✗ S. mutans | Yellow | Commensal | 17 |
| Streptococcus sanguinis | Coccus | + | a | ✗ A. actinomycetemcomitans | Yellow | Commensal | 7, 17 |
| ✗ A. naeslundii | |||||||
| ✗ P. gingivalis | |||||||
| ✗ S. mutans | |||||||
| ✗ S. gordonii | |||||||
| Campylobacter concisus | Spirilla | − | b | N/A | Green | Periodontitis | 26, 40 |
| Campylobacter rectus | Bacillus | − | b | ✓ P. gingivalis | Orange | Chronic periodontitis | 1, 7, 26, 40 |
| Capnocytophaga gingivalis | Bacillus | − | b | N/A | Green | Chronic periodontitis | 26 |
| Capnocytophaga ochracea | Bacillus | − | b | N/A | Green | Periodontitis | 26, 41 |
| Capnocytophaga sputigena | Bacillus | − | b | N/A | Green | Periodontitis | 26, 42 |
| Eikenella corrodens | Bacillus | − | b | ✗ P. gingivalis | Green | Chronic periodontitis | 1, 26, 43 |
| Fusobacterium nucleatum | Bacillus | − | b | ✓ P. gingivalis | Orange | Chronic and aggressive periodontitis | 7, 17, 19, 23, 26, 27 |
| ✓ T. forsythia | |||||||
| Veillonella atypica | Coccus | − | b | ✓ S. gordonii | None | Early childhood caries and chronic periodontitis | 44–46 |
| Veillonella parvula | Coccus | − | b | ✓ F. nucleatum | Purple | Generally commensal, but was found in early childhood caries and chronic periodontitis | 26 |
Aerobic.
Anaerobic.
| Late colonizers | |||||||
|---|---|---|---|---|---|---|---|
| Bacterial species (in alphabetical order of Gram-positive and Gram-negative) | Shape | Gram-positive/Gram-negative | Aerobic/anaerobic | Symbiotic/antagonistic interaction | Complex | Periodontal diseases associated with | Ref. |
| Eubacterium nodatum | Bacillus | + | b | ✓ red complex species | Orange | Chronic periodontitis | 17, 23, 26, 27 |
| Filifactor alocis | Bacillus | + | b | ✓ P. gingivalis | None | Localized aggressive and refractory periodontitis | 47–49 |
| Aggregatibacter actinomycetemcomitans | Bacillus | − | b | ✗ S. mutans | None | Chronic, localized aggressive, and generalized aggressive periodontitis | 23 |
| ✗ S. sanguinis | |||||||
| Porphyromonas gingivalis | Bacillus | − | b | ✗ S. sanguinis | Red | Chronic, localized aggressive, and generalized aggressive periodontitis | 1, 7, 17, 23, 24, 26, 27, 31 |
| ✓ A. actinomycetemcomitans | |||||||
| ✓ C. rectus | |||||||
| ✓ F. nucleatum | |||||||
| ✓ T. denticola | |||||||
| Prevotella denticola | Bacillus | − | b | N/A | None | Chronic and aggressive periodontitis | 23 |
| Prevotella intermedia | Bacillus | − | b | ✓ red complex species | Orange | Chronic, localized aggressive, and generalized aggressive periodontitis | 1, 17, 23, 26, 27 |
| Prevotella nigrescens | Bacillus | − | b | ✓ red complex species | Orange | Chronic and aggressive periodontitis | 1, 17, 26, 27 |
| Selenomonas flueggei | Bacillus | − | b | N/A | None | Chronic periodontitis | 1 |
| Tannerella forsythia | Bacillus | − | b | ✓ F. nucleatum | Red | Chronic, localized aggressive, and generalized aggressive periodontitis | 1, 7, 23, 26 |
| Treponema denticola | Spiral | − | b | ✓ P. gingivalis | Red | Chronic and generalized aggressive periodontitis | 1, 7, 19, 23, 26, 31 |
Oral biofilm development begins with association of planktonic bacterial species near the surface of the tooth (Fig. 3B). The next stage of development, attachment, is initiated by the acquired pellicle that is made up of salivary glycoproteins (e.g., phosphate-rich proteins, proline-rich proteins, mucins, enzymes, etc.)18 adsorbed to the dental surface and acts as a receptor for early colonizers (Fig. 3A). Studies show that within as little as four hours, biofilm formation occurs. These biofilms mainly contain primary early colonizers such as Streptococcus spp. (e.g., S. gordonii, S. intermedius, S. mitis, S. oralis, and S. sanguinis). Streptococcus spp. attach to the acquired pellicle due to their ability to bind to specific receptors of the acquired pellicle and thrive in aerobic conditions. Oral bacteria, such as S. sanguinis, have a unique ability to colonize the tooth surface first due to fimbriae that mediate attachment to the tooth surface along with pili that bind to salivary components to initiate biofilm formation.19 Once attachment of the early colonizing Streptococcus spp. occurs with weak binding, they produce an ECM that contains proteins, nucleic acids, lipids, extracellular DNA, and polysaccharides. The ECM proteins protect the bacteria and allow them to strongly attach to the acquired pellicle due to their ability to mediate cell–cell and cell–surface adhesions to form a structured ECM (Fig. 3B).19,20 The components of the ECM are produced by bacteria themselves in addition to saliva and gingival crevicular fluid. The ECM contains water channels or open areas that carry nutrients and other agents to the bacteria and carries waste out of the biofilm.1,21 ECM proteins allow the biofilm to maintain its structure, protect the bacteria from external forces, and maintain its integrity. Polysaccharides produced by bacteria colonizing the biofilm enable the bacteria to adhere to surfaces and other bacterial species.20 The ECM also allows bacteria to resist antibiotics in various ways: (i) ECM proteins that prevent antibiotics from reaching bacteria within biofilms, and (ii) transfer of resistance genes (e.g., β-lactamase discussed in section 2.2) by bacteria that are in close proximity to one another.
Beneficial commensal oral bacteria along with pathogenic bacteria are seen in healthy and diseased sites of the oral cavity; the main difference being the proportion of beneficial and pathogenic bacteria.22 In periodontal health, the Gram-negative pathogenic bacteria are kept at low concentrations due to the balance of beneficial bacteria controlling the growth of pathogenic bacteria through antagonistic interactions. A primary example of a commensal Gram-positive aerobic species prevalent in periodontal health that counterbalances a pathogenic Gram-negative bacterium is S. sanguinis. Excess O2 is expelled from S. sanguinis in the form of hydrogen peroxide (H2O2), which acts as an antimicrobial agent by inhibiting glycolysis or protein synthesis. Increased concentration of H2O2 results in the growth inhibition of Actinomyces naeslundii, pathogenic/cariogenic bacteria such as Streptococcus mutans, and the Gram-negative anaerobic pathogenic species A. actinomycetemcomitans frequently found in the formerly called localized aggressive periodontitis.17,23 Antagonistic interactions by the excretion of H2O2 is additionally exemplified by S. gordonii inhibiting the growth of A. naeslundii and S. mutans. S. mutans is not depicted in Fig. 3A due to the presence of bacterial species S. oralis that grows rapidly and inhibits the growth of S. mutans and because it is mostly found in supragingival plaque. There are synergistic interactions where pathogenic bacterial species protect other bacteria from H2O2 by its consumption through protein oxidation.17 When cultured without A. actinomycetemcomitans, S. sanguinis inhibits the growth of the pathogenic bacteria P. gingivalis by secreting H2O2. However, P. gingivalis is able to grow in the presence of A. actinomycetemcomitans that reduces H2O2 due to production of the enzyme cytoplasmic catalase by expression of the katA gene.3
Periodontal diseases are initiated by the increase in proportion of pathogenic bacteria where the level cannot be maintained through antagonistic interactions. Once the disease starts, the level of pathogenic bacteria can increase due to the positive interactions they have with each other (i.e., synergism, mutualism, and commensalism), further enhancing progression of the disease. Synergism is seen with early colonizers of oral biofilms such as Streptococci and Actinomyces species that are generally non-pathogenic and consume oxygen (Fig. 3B).7 As the biofilm matures, the environment becomes favorable to more anaerobic pathogenic bacteria with an oxygen gradient and a flow of nutrients along with horizontal gene transfer including genes for antibiotic resistance (mechanisms of resistance discussed in section 2.2). The survival of pathogenic bacteria could be enhanced by synergistic interactions between P. gingivalis and Treponema denticola, which is a motile bacterial species that can create pores within the biofilm allowing nutrients to flow to P. gingivalis.24 Many of the pathogenic bacteria (e.g., the pairs of P. gingivalis and T. denticola as well as Tannerella forsythia and Fusobacterium nucleatum) grow better together than they do apart, which is a relationship known as mutualism. Additionally, the by-product of one bacterial species can increase the survival of the other bacterial species without affecting the first bacterial species in the case of P. gingivalis and Campylobacter rectus.
Despite a high and diverse number of oral bacterial species in the oral microbiome, there are a small number of bacterial species (mainly late colonizers) predominantly found to be associated with the various forms of periodontal diseases (Table 1). Additionally, there are a number of factors that can influence the type of bacterial species seen with high frequency in periodontitis, a major factor being age. In the formerly called localized aggressive periodontitis (now referred to as periodontitis), found in young children, there is a high level of A. actinomycetemcomitans. In the generalized form of aggressive periodontitis, found in individuals younger than 30 years of age, there is however a lower level of A. actinomycetemcomitans and an increased level of P. gingivalis along with other species including Campylobacter, Prevotella, Tannerella, and Treponema. In the formerly called chronic periodontitis (now referred to as periodontitis), the focus of this review, which develops over a long period of time and is often seen in adults 30 years and older, the predominant pathogenic bacterial species include Aggregatibacter, Campylobacter, Eikenella, Fusobacterium, Parvimonas, Porphyromonas, Prevotella, Selenomonas, Tannerella, and Treponema.1
The concentration of bacterial species in saliva during periodontal diseases are 108 to 109 bacteria per mL compared to a concentration of 103 during periodontal health.1,25 In the subgingival plaque, the major bacterial species found in patients with periodontitis seem to co-exist in six major complexes: yellow, blue, green, purple, orange, and red.26 Early colonizers such as Streptococcus spp. are Gram-positive commensal beneficial bacteria that make up the majority of the bacterial species found in supragingival and subgingival plaque of healthy periodontal patients and comprise the yellow complex.19 Additional early colonizers include the Gram-positive facultative anaerobic Actinomyces spp. that are a part of the blue complex along with the green complex, which contains Eikenella corrodens, Capnocytophaga ochracea, Capnocytophaga gingivalis, Capnocytophaga sputigena, and Campylobacter concisus, as well as the purple complex comprising Actinomyces odontolyticus and Veillonella parvula.26 Once the members of the yellow, blue, green, and purple complexes colonize the oral biofilm, the orange complex species (e.g., F. nucleatum, Eubacterium nodatum, P. intermedia, Prevotella nigrescens, C. rectus, Campylobacter showae, Peptostreptococcus micros, Campylobacter gracilis, and Streptococcus constellatus) colonize the biofilm.26 As the environment becomes favorable to more anaerobic pathogenic bacteria, the red complex comprising P. gingivalis, T. forsythia, and T. denticola can attach.
Orange and red complexes are closely associated together and facilitate the progression of periodontal diseases through synergistic interactions. The red complex bacteria are usually found with those in the orange complex and it is rare to see them without one another in periodontal pockets associated with periodontal diseases. When the concentration of orange complex species increases, the amount of red complex species also increases in the oral biofilm. F. nucleatum is a Gram-negative anaerobic bacterium that acts as a bridge between early and late colonizers of oral biofilms. Although there may be an increased presence of oxygen that would normally inhibit the growth of anaerobic bacteria, F. nucleatum provides a microenvironment with high concentrations of carbon dioxide (CO2) that facilitates the growth of P. gingivalis by providing a CO2 rich, or capnophilic, environment for P. gingivalis to thrive in.17,27P. gingivalis in culture can survive at O2 levels of 3% and 6%, but if the O2 levels are 10% or more, then P. gingivalis is unable to grow unless co-cultured with F. nucleatum that creates a CO2 rich environment that P. gingivalis can survive in even at O2 levels of 10% and 20%.27
When bacteria colonize a biofilm, changes in their gene expression occur to facilitate gene transfer, nutritional cooperation, and cell–cell signaling. As the bacterial cell density within the oral biofilm increases, bacteria communicate through quorum sensing systems where they release chemical signaling molecules called autoinducers. Once the level of autoinducers is above a certain threshold, this causes changes in gene expression of the bacteria within the biofilm (e.g., expression of virulence factors).1 Gram-negative bacteria in the orange and red complexes are considered pathogenic due to the virulence factors that destroy the surrounding tissue and aid in invasion of gingival tissue.25 In periodontal health, the ECM components (e.g., fibronectin, vitronectin, elastin, type I collagen, etc.) that make up the oral biofilm are essential for the maintenance and repair of gingival tissues and periodontal ligament cells damaged by pathogenic bacteria. In particular, the ECM glycoproteins fibronectin and vitronectin bind to integrins, which are transmembrane receptor proteins that promote cell–ECM adhesion. This causes cellular signal transduction that results in the promotion of healing gingival tissues and periodontal ligament cells. Fibronectin is essential for periodontal ligament cells in terms of rapid reproduction, known as proliferation, as well as the migration of cells in response to an increase or decrease in concentration of certain extracellular signals, known as chemotaxis. Vitronectin is involved in cell adhesion and spreading by protecting gingival connective tissue.28
After the initial attachment of Streptococcus spp. and Actinomyces spp., the development of the ECM allows these bacterial species to adhere to the acquired pellicle and act as substrates or binding sites for other early colonizers. Attachment of subsequent bacterial species is a highly organized process where specific interactions occur between each individual cell type. The bacteria within a biofilm not only have to be close together to form a mature biofilm, they also need to have specific interactions (e.g., exchange of metabolites and genetic material, physical interactions, and communication through signaling). Interactions between bacterial species include co-adhesion and co-aggregation. Co-adhesion includes interactions between bacterial cells attached to a surface recognizing planktonic cells of a different strain or species. Co-aggregation is a term used to describe the interactions of planktonic cells recognizing and binding to planktonic cells of a different strain or species.18,29,30 The bacterial species S. oralis and S. sanguinis contain receptors on their cell surface that bind to adhesion proteins on the surface of other early colonizers, such as Actinomyces spp. (e.g., A. naeslundii), Veillonella spp. (e.g., Veillonella atypica), Haemophilus spp. (e.g., Haemophilus parainfluenzae), Prevotella spp. (e.g., Prevotella loescheii), Eikenella spp. (e.g., E. corrodens), Fusobacterium spp. (e.g., F. nucleatum) and Capnocytophaga spp. (e.g., C. ochracea) (Fig. 3A).18,29,30 With the initial attachment of bacterial species to streptococci, more receptors and adhesion proteins are available for other bacterial species to interact with, which increases the diversity of the biofilm. These specific interactions have been shown to yield co-aggregation partners. A stepwise formation of the oral biofilm based on co-aggregation partners depicts the interactions of bacterial species leading to the formation of a mature biofilm in Fig. 3A. Each sequential partner attaches to the next bacterial species starting with Streptococcus spp. that attaches to A. naeslundii, which itself attaches to C. ochracea, followed by Actinomyces israelii, which then attaches to C. gingivalis. As the biofilm matures there is a decrease in abundance of Streptococcus spp. (e.g., S. oralis, S. gordonii, and S. sanguinis), Actinomyces spp. (e.g., A. naeslundii), Rothia dentocariosa, and Veillonella spp. (e.g., Veillonella dispar) as well as an increase in abundance of bacterial species associated with periodontal diseases such as Aggregatibacter, Prevotella, Porphyromonas, Treponema, Tannerella, and Fusobacterium spp.22 Additional bacterial species with increased abundance in diseased sites include Porphyromonas endodontalis, P. nigrescens, Treponema medium, and Parvimonas micra. F. nucleatum is an interesting early colonizer that interacts with early and late colonizers as a result of its cell wall containing adhesion proteins rather than receptors. This bacterial species can interact with many different species including early colonizers such as V. parvula in addition to late colonizers such as A. actinomycetemcomitans, C. sputigena, P. gingivalis, Prevotella denticola, P. intermedia, Selenomonas flueggei, and T. denticola.29 The co-aggregation partners P. gingivalis and T. denticola in the red complex help facilitate the growth of one another and facilitate the maturation of the oral biofilm by producing growth factors (i.e., succinic acid in T. denticola and isobutyric acid in P. gingivalis).1,31 Once the biofilm reaches the stage of maturation, depending on the available attachment site, nutrient level, and other factors, the bacteria can disperse to resume planktonic life or form another biofilm.
The pathogenic bacteria P. gingivalis is a black pigmented Gram-negative anaerobe found in both healthy and diseased periodontal pockets. The ability of P. gingivalis to be the etiological agent and keystone pathogen of periodontal diseases relies on the slight increase in abundance of P. gingivalis within the periodontal pocket along with its serotype (note: different strains of P. gingivalis have lower or higher levels of pathogenicity). Keystone pathogen is a term used to describe the ability of a low-abundance pathogenic bacterial species to cause a large amount of damage through organizing the commensal microbial community into a dysbiotic community that progresses an inflammatory disease, such as periodontal diseases.32,33P. gingivalis has been shown to communicate with subgingival oral bacteria within the biofilm deploying a pathogenic influence that elevates the pathogenicity of the biofilm community.24 Different strains of P. gingivalis express various virulence factors (e.g., fimbriae, collagenase, hemolysins, proteases, fatty acids, and endotoxins) that aid in its ability to trigger inflammation and the progression of periodontitis.34–36 Fimbriae on the surface of more virulent strains of P. gingivalis are used to adhere to and invade host cells.34,37 The fimbriae are required for specific cell–cell interactions, cell–surface interactions, and cell–ECM protein interactions.34 ECM proteins have shown specific affinities towards P. gingivalis fimbriae. The ability of P. gingivalis fimbriae to bind to ECM proteins such as fibronectin and vitronectin could hinder gingival tissue repair.28,37 As a late colonizer that requires an anaerobic environment within the oral biofilm, the location of P. gingivalis deep in the periodontal pocket allows P. gingivalis to be situated closer to sulcular epithelium rather than the tooth itself.30 Not only can the P. gingivalis fimbriae bind to ECM proteins, the fimbriae can also competitively inhibit the binding of ECM proteins to specific integrins (i.e., αvβ3 and α5β1) on the surface of gingival tissue. Healing of the gingival tissue could be delayed and P. gingivalis could invade the damaged tissue. For more information regarding common virulent strains of P. gingivalis associated with periodontal disease based on their subunits of fimbriae the reader is referred to a previous in-depth review.34
Overall, the development of an oral biofilm is not random, it is specific and follows a stepwise formation process (Fig. 3B). Planktonic bacteria begin the first stage called association, where they begin to come closer together. As the biofilm matures the number of different genera and species of oral bacteria increases to form a diverse community. When the environment becomes favorable (i.e., when formation of the acquired pellicle occurs) the bacteria attach to the surface of the tooth allowing for various bacterial species to attach to them to form a mature biofilm. When the environment becomes unfavorable (i.e., lack of proper nutrients) the bacteria can disperse and detach from the mature biofilm and resume planktonic life or form a new biofilm. Mature oral biofilms contain predominantly oral bacterial species, which are the cause of periodontal diseases, but can additionally be colonized by other microbial species (e.g., viruses, yeasts, and archaea).25
2. Current treatment options used to target Porphyromonas gingivalis
2.1. Classes of antibacterial agents used to treat periodontal diseases and their mechanisms of action
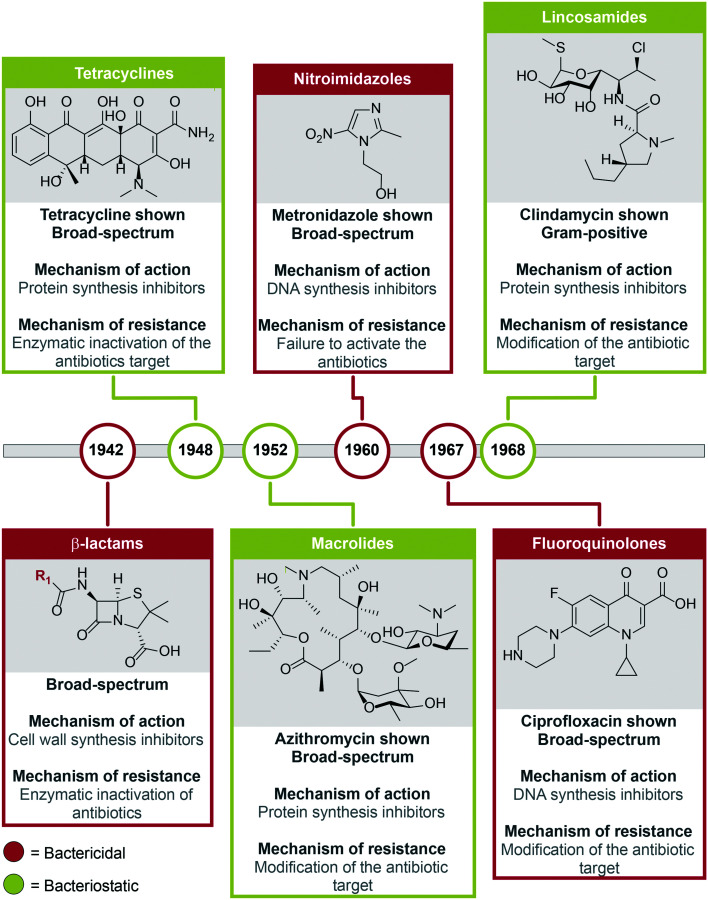
For the treatment of periodontal diseases, there are noninvasive (e.g., scaling, root planing alone or combined with antibiotics) and invasive (e.g., flap surgery, bone grafting, soft tissue grafts, guided tissue regeneration, and tissue-stimulating proteins) techniques.50–59 In the early stages of periodontal diseases, the noninvasive techniques can be used. However, if left untreated, the more aggressive and expensive surgical options must be utilized as the diseases progress.50 Gingivitis can be managed with good plaque control through brushing and flossing teeth along with antiseptic (e.g., chlorhexidine) oral mouth rinses. Chlorhexidine inhibits the growth of biofilm forming bacteria such as P. gingivalis, but it does not eradicate the biofilm.50,60 As periodontal diseases progress in severity and extension, other forms of treatment are needed to eradicate periodontopathogenic bacteria to stop the progression. For example, antibiotics are used as an adjunctive therapy to mechanical debridement such as scaling and root planing in severe and refractory forms of periodontitis (Table 2).50,60 Mechanical debridement is not always sufficient to remove/reduce subgingival pathogenic bacteria that becomes clinically inaccessible in deep periodontal pockets and is already likely invading soft gingival tissues. Thus, the use of antibiotics could contribute to lower the number or completely eradicate pathogenic bacteria, which as a consequence reduce and control pathologic inflammation-induced tissue destruction. Given the polymicrobial and chronic nature of periodontal diseases, the prolonged use of antibiotics brings disadvantages such as side effects, poor efficacy and poor specificity against oral pathogenic bacteria, as well as development of drug-resistant bacteria (Table 2).61–63 Several classes of antibiotics used for the treatment of periodontal diseases have as their main mechanisms of action to inhibit (i) cell wall, (ii) protein, and (iii) DNA synthesis (Fig. 4). Interestingly, folate synthesis inhibitors seem to be a group of antibacterial compounds that could be used in the future as a safer and pathogen-specific adjunctive therapy for periodontal disease (Fig. 5). In the following sections, the main examples for each of these antimicrobials used to control periodontal diseases and their effect in oral pathogens such as P. gingivalis will be presented.
Classes of antibacterial agents with the various oral diseases they treat, oral bacteria they target, and their disadvantages.
| Classes of antibiotics | Diseases targeted | Oral bacteria against which antibiotics are active | Associated problems | Ref. |
|---|---|---|---|---|
| β-Lactams | Localized and generalized aggressive periodontitis | A. actinomycetemcomitans | Due to bacterial resistance they should be used in combination with β-lactamase inhibitors | 88 |
| Tetracyclines | Localized aggressive periodontitis | A. actinomycetemcomitans, P. gingivalis, P. intermedia, E. corrodens, F. nucleatum, and P. micra | Adverse effect of metal chelation | 88 |
| Macrolides | Aggressive and chronic periodontitis | P. gingivalis | Not as effective as MTZ + AMX combination therapy | 60 |
| Nitroimidazoles | Localized aggressive periodontitis (when used in combination therapy with other antibiotics) | Active against most Gram-negative species, including P. gingivalis, F. nucleatum, and P. intermedia | Only active against planktonic species of P. gingivalis | 72, 88 |
| Fluoroquinolones | Chronic periodontitis | Active against all strains of A. actinomycetemcomitans | Active against Gram-negative bacteria, but little activity against anaerobic bacteria | 64 |
| Lincosamides | Chronic periodontitis | P. gingivalis and P. intermedia | A. actinomycetemcomitans displays resistance to lincosamides | 89 |
Fig. 4. Classes of antibacterial agents commonly used to treat periodontal diseases and their year of introduction.
Fig. 5. Representative structures of two sulfonamide antimicrobial agents.
2.1.1. Cell wall synthesis inhibitors
The most clinically relevant target for antibiotics is bacterial cell wall biosynthesis. In the clinic, the classes of cell wall synthesis inhibitors more commonly used are the penicillins (e.g., amoxicillin (AMX))50,60 and cephalosporins (e.g., cephalexin and cephradine),64 followed by the monobactams (e.g., aztreonam).65 The naturally occurring penicillin, cephalosporin, and monobactam classes of antibiotics are β-lactams, which contain a four-membered β-lactam ring (Fig. 4). The β-lactam antibiotics were introduced into the market in 1942. They have a broad-spectrum of activity and are used to treat localized and generalized aggressive periodontitis by inhibiting the last step in peptidoglycan synthesis. The monobactam aztreonam is used for root canal irrigation. However, aztreonam is not as commonly utilized as the other β-lactam antibiotics for the treatment of periodontal diseases, because Gram-positive bacteria have become resistant to it and it is not active against Gram-negative anaerobic bacteria.
2.1.2. Protein synthesis inhibitors
For the treatment of periodontal diseases, there are three main classes of antibiotics used to block bacterial protein synthesis. These classes comprise the bacteriostatic tetracyclines (marketed in 1948), macrolides (1952), and lincosamides (1968) (Fig. 4). The broad-spectrum natural product tetracycline (TET) along with its semi-synthetic derivatives minocycline (MIN) and doxycycline (DOX) contain four fused cyclic six-membered rings in their structures. The TET class of antibacterial agents displays activity against Gram-negative anaerobic bacteria such as A. actinomycetemcomitans, C. rectus, E. corrodens, and Capnocytophaga spp.66 Although TET is used for many common bacterial infections, it causes adverse effects such as staining of the teeth, nausea, and diarrhea.64 MIN is used for the treatment of localized aggressive periodontitis normally occurring in children. For the treatment of chronic periodontitis, DOX is used as an adjunctive therapy to scaling and root planing, as it has been found to be highly effective at inhibiting P. gingivalis and A. actinomycetemcomitans growth when isolated from periodontal pockets. The systemic use of DOX does not completely eradicate P. gingivalis from the oral cavity, as the amount of P. gingivalis found in the gingival crevicular fluid is not reduced.36 Gingival crevicular fluid is found in the gingival sulcus and it is an inflammatory exudate that comprises serum, inflammatory mediators, antibodies, proteins, various cells, and bacteria from adjacent plaque.67 Therefore, once a mature biofilm is re-established P. gingivalis can re-colonize the biofilm.
The broad-spectrum naturally occurring polyketide class of antibiotics, macrolides, inhibits protein synthesis by interfering with translation (Fig. 4). Macrolides comprise the natural products spiramycin and erythromycin (ERY).50,60,64 The poor bioavailability of ERY led to the development of its semi-synthetic derivatives clarithromycin (CLR) and azithromycin (AZM). Spiramycin is a systemic antibiotic used as an effective treatment option for periodontitis, but it is active against Gram-positive bacterial species, which could lead to oral dysbiosis. The less commonly used macrolide, CLR, is prescribed for patients allergic to penicillin and has been studied in a clinical trial for the treatment of chronic periodontitis.68 The clinical trial revealed that CLR can be used as an adjunctive therapy to scaling and root planing, as it reduced probing depth and enhanced clinical attachment level. Gram-negative anaerobic bacilli are the primary target of AZM used for the treatment of aggressive/severe forms of periodontitis. In addition to macrolides, lincosamides are natural products with the same mechanism of action, but lincosamides are only active against Gram-positive bacterial species. The semi-synthetic derivative of lincomycin, clindamycin, is a lincosamide used for patients allergic to penicillin to treat acute periodontal abscesses. Clindamycin is less commonly used as it can lead to an increased risk of intestinal Clostridium difficile infections.69
2.1.3. DNA synthesis inhibitors
Two classes of synthetic broad-spectrum antibacterial agents, the bactericidal fluoroquinolones (marketed in 1967) and nitroimidazoles (1960) have been shown to inhibit DNA synthesis (Fig. 4).50,60,64 For the treatment of aggressive periodontitis, the fluoroquinolone, ciprofloxacin (CIP), is active against all strains of A. actinomycetemcomitans and has been shown to help facilitate growth of beneficial commensal Streptococcus spp.70 CIP inhibits DNA replication, but because of an increase in CIP bacterial resistance, it is commonly used in combination with metronidazole (MTZ), which is a nitroimidazole used for the treatment of localized aggressive periodontitis that inhibits bacterial nucleic acid synthesis. When tested against planktonic P. gingivalis and F. nucleatum, MTZ inhibited growth with minimum inhibitory concentration (MIC) values of 0.125 μg mL−1 and 1 μg mL−1, respectively.71 However, MTZ is normally used in combination with either AMX, for advanced periodontitis, or CIP, for advanced chronic periodontitis due to its inactivity when tested alone against preformed biofilms of P. gingivalis.72
2.1.4. Folate synthesis inhibitors
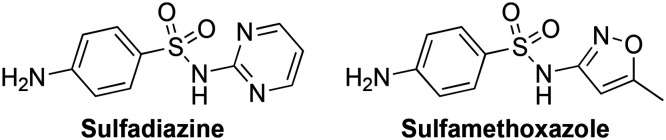
Although not currently used in the clinic for the treatment of periodontal diseases, folate synthesis inhibitors such as the sulfonamides (put on the market in 1965) show great potential as antibacterial agents against P. gingivalis (Fig. 5). Sulfonamides, also known as sulfa drugs, are synthetic antibacterial agents that contain a sulfur double bonded to two oxygen atoms and single bonded to one nitrogen, and one carbon. Sulfonamides inhibit tetrahydrofolate biosynthesis by targeting a key biosynthetic enzyme, dihydropteroate synthase. Common sulfonamides used in the clinic to treat bacterial infections include sulfadiazine and sulfamethoxazole (Fig. 5).73 For the treatment of periodontal diseases, the sulfonamide anti-asthma drug, zafirlukast (ZAF), which has been shown to be active against P. gingivalis, could be a promising molecule warranting further investigation (see section 3.1.4.).
2.2. Periodontal pathogens modes of resistance to various classes of antibacterial agents
There are four common modes of resistance to the antibacterial agents used to treat periodontal diseases: (i) enzymatic inactivation of antibacterial agents, (ii) limiting access of the antibacterial agent to its target(s), (iii) modification of the antibacterial agents' targets, and (iv) failure to activate the antibacterial agents. Understanding the modes of resistance provides a basis to discuss the future direction of rational development of novel antibacterial agents that can overcome these resistance mechanisms. As this review is primarily focused on the development of novel antibacterial agents active against P. gingivalis, this section will not go into all of the details, but instead will provide a brief overview of these antibacterial resistance mechanisms (for in-depth reviews, readers are directed to ref. 74–77).
2.2.1. Enzymatic inactivation of antibacterial agents
The inappropriate and excessive use of antibacterial agents over the years has been the major cause of bacterial resistance.78 Within five years of widespread penicillin use, the first case of bacterial resistance to this antibiotic was observed.79 The ability of Gram-negative oral bacterial species to produce β-lactamase, an enzyme that cleaves the β-lactam ring and makes the antibacterial agent inactive, has led to an increase in resistance to the penicillin, cephalosporin, and monobactam classes of antibiotics. Oral bacterial species with increased resistance to β-lactam antibacterial agents include the pathogenic Prevotella spp., Porphyromonas spp., and Fusobacterium spp.80 Bacterial resistance to the TET class of antibiotics occurs through oxidation of TET by TET destructase enzymes, which are class A flavin-monooxygenases.75
2.2.2. Limiting access of the antibacterial agent to its target(s)
Due to the lack of a lipopolysaccharide (LPS) outer membrane, it is less common for Gram-positive bacteria to limit access of antibacterial agents to their target compared to Gram-negative bacteria.74 Gram-negative bacteria are harder to kill because their cell walls contain an LPS outer membrane that acts as a barrier to prevent antibacterial agents from entering the cell and efflux pumps that prevent accumulation of high concentrations of the drug within the cell. Classes of antibacterial agents (e.g., tetracyclines, macrolides, and fluoroquinolones) with vastly different structures are exported from the cell by efflux pumps, which has led to multidrug-resistant bacteria.
2.2.3. Modification of the antibacterial agents' targets
To interfere with the ability of antibacterial agents to bind to their target(s), bacteria have developed resistance by modifying these targets. This can occur in two ways: (i) spontaneous mutations in the target that impede binding and (ii) chemical modifications that still allow the target to function normally, while inhibiting binding of the antibacterial agent to the target. Spontaneous mutations in the enzyme dihydropteroate synthase leads to resistance to the sulfonamide class of antibacterial agents.81 In Gram-negative bacterial species, the most common mechanism of resistance to β-lactam antibacterial agents is through enzymatic inactivation by the production of β-lactamases. In Gram-positive bacteria, the most common mechanism of resistance consists of preventing the antibacterial agents from binding to their β-lactams target, the penicillin binding protein, by its mutation.77 Bacterial resistance to the TET class of antibacterial agents is by the tetracycline binding protein Tet(O), which is a ribosomal protection protein that promotes the release of TET from the ribosome.82 Resistance to TET through ribosomal protection has been observed in P. intermedia and P. nigrescens.83 Methylation of the adenine residues in 23S rRNA by RNA methyltransferases causes antibacterial resistance to macrolides and lincosamides. Resistance to macrolides due to 23S rRNA methylation has been observed in the pathogenic bacteria T. denticola and Prevotella spp., while resistance to lincosamides has been observed in Treponema pallidum.84–86
2.2.4. Failure to activate the antibacterial agents
In order for the nitroimidazole class of antibacterial agents to inhibit bacterial DNA synthesis, they must first be activated by flavodoxins, which are electron-transport proteins. Bacterial species develop resistance to nitroimidazoles by decreasing the expression of flavodoxins. MTZ resistance has been observed in the oral bacterial species A. actinomycetemcomitans, and in some Porphyromonas spp., Actinomyces spp., and Parvimonas spp.87
Although there are several periodontal pathogens that have been associated with periodontal diseases, such as A. actinomycetemcomitans, T. denticola, T. forsythia, F. nucleatum, P. intermedia, C. rectus, E. corrodens, P. micros, and Selenomas spp., this review focuses on the advantages and disadvantages of the antimicrobials used to treat periodontitis with particular emphasis on the pathogen P. gingivalis as a major contributor to the pathogenesis of periodontal diseases and its association with other systemic diseases. Many of the currently used antibiotics are broad-spectrum, which leads to poor efficacy and the development of drug-resistant bacteria. More pathogen targeted therapy should be used to treat periodontal diseases that would allow the control of pathogenic bacteria (e.g., P. gingivalis), without significantly affecting the normal oral microbiota critical for health.
3. Discovery and development of new compounds active against P. gingivalis
In the following sections (3.1–3.3), several identified compounds will be compared either to molecules they are derived from or to antibacterial agents that are already used to treat periodontal diseases in terms of activity against bacterial growth, activity as inhibitors of bacterial virulence factors, and/or improvement in properties (e.g., solubility, cytotoxicity, etc.).
In oral subgingival biofilms, increased P. gingivalis numbers favor the expression of many virulence factors (e.g., proteases, fimbriae, gingipains, LPS, collagenase, capsule, GroEL, etc.) by P. gingivalis, which contribute to the development of periodontal diseases by promoting biofilm formation, adhesion, anaerobic growth, invasion, tissue degradation, intracellular survival in gingival epithelial cells, etc. These virulence factors therefore offer a wide range of targets for the development of anti-P. gingivalis agents.
When developing a compound to combat P. gingivalis, most researchers will first look at inhibiting the growth of P. gingivalis by MIC or percent growth inhibition testing. If the compounds are inactive against P. gingivalis, most groups would not continue on to biofilm testing. For some of the seemingly inactive compounds in the following studies throughout the review, not performing biofilm studies would be a mistake as the compounds may have surprising activity. As described in section 1.2, P. gingivalis has the ability to express virulence factors, such as fimbriae, which aid in its ability to further progress periodontitis. Some compounds may be able to stop the progression of periodontal diseases by inhibiting the ability of P. gingivalis to express fimbriae, which would inhibit its ability to bind to the early colonizers in a mature biofilm. Researchers should make sure to always include cytotoxicity studies in their experiments as there may be an increased concentration needed to inhibit biofilm formation.
In order to control P. gingivalis by inhibiting its bacterial growth, biofilm growth, and the expression of virulence factors, researchers have utilized three main strategies: development of novel compounds through new scaffolds or with derivatives of FDA-approved drugs (section 3.1), repositioning of FDA-approved drugs (section 3.2), and isolation and derivatization of natural products (section 3.3).
3.1. Novel compounds
3.1.1. Triazole derivatives
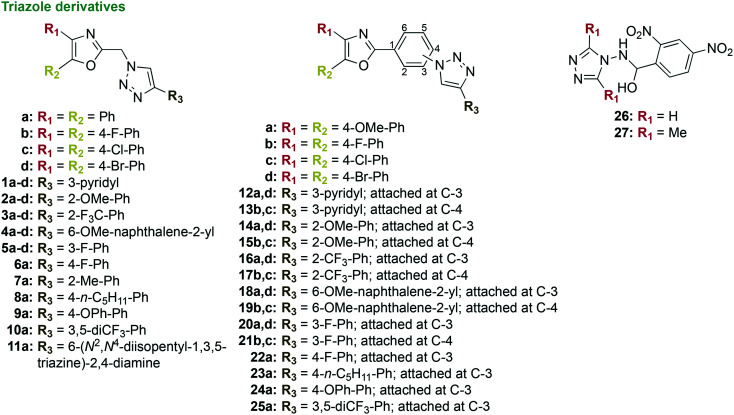
The azole class of drugs has traditionally been used in the clinic as antifungal agents. The azoles have also been investigated against oral bacterial species. In order for colonization and re-colonization of P. gingivalis within a biofilm to occur, it must first adhere to early colonizers such as Streptococcus spp. Preventing the colonization of an oral biofilm by periodontal pathogens, such as P. gingivalis, helps reduce the virulence of the bacterial species and slows the progression of periodontal diseases. In an investigation with expansive structure–activity relationship (SAR) studies, small molecules containing 1,2,3-triazoles were designed to be structurally similar to the natural peptide substrate that is recognized by the minor fimbrial antigen (Mfa) of P. gingivalis (Fig. 6).90 Binding of these small molecules to Mfa inhibits the protein–protein interaction that occurs between the Mfa of P. gingivalis and the antigen I/II polypeptide of S. gordonii. To inhibit the adherence of P. gingivalis to early colonizing oral bacterial species, 50 1,2,3-triazole derivatives were synthesized in three to five standard synthetic steps involving esterification, cyclization, and click reactions in overall yields of 33–100%. Out of the 50 derivatives, 26 contained 2-(azidomethyl)-4,5-diaryloxazoles (1–11), while 24 contained 2-(azidophenyl)-4,5-diaryloxazoles (12–25) linked by a substituted 1,2,3-triazole. None of the compounds in this study inhibited the growth of S. gordonii or P. gingivalis. For example, compound 11 displayed percent growth inhibition of 73% at the high concentration of 60 μM against P. gingivalis. Although these compounds did not inhibit the growth of P. gingivalis, four of them, 4c, 15b, 20a, and 20d displayed strong activity in terms of inhibition of P. gingivalis adherence to S. gordonii with IC50 values of 5.3, 7.7, 5.9, and 5.0 μM (2.80, 3.90, 3.06, and 3.08 μg mL−1), respectively. The rest of the compounds displayed moderate to no inhibition of activity. As there was no standard antibacterial agent used for comparison in these assays, it is difficult to determine the potential value of these compounds as inhibitors of P. gingivalis adherence for the treatment of chronic periodontitis.
Fig. 6. Three new scaffolds as representative examples of compounds that contain triazoles in their structures that were tested as potential antibacterial agents against oral bacterial species.
In a follow up study, five of the most potent inhibitors 4b, 4c, 14d, 15b, and 18a were further examined with a focus on inhibition of three-species biofilms, inhibition of P. gingivalis virulence, and low toxicity profile (Fig. 6).91 The dual-species biofilm of S. gordonii and P. gingivalis demonstrated the triazole derivatives' ability to inhibit P. gingivalis adherence to the early colonizing bacterial species in a two bacterial species biofilm model. As the oral cavity contains a more complex multi-species biofilm, S. gordonii and P. gingivalis normally adhere to the bridging oral bacterial species F. nucleatum. When tested against three-species biofilms comprising S. gordonii, F. nucleatum, and P. gingivalis, all triazole derivatives 4b, 4c, 14d, 15b, and 18a displayed the ability to inhibit the incorporation of P. gingivalis into the biofilm with IC50 values of 10–20 μM (5.27–9.89 μg mL−1). Additionally, 15b displayed an ability to disrupt preformed three-species biofilms with 61% inhibition at 20 μM (10.1 μg mL−1). Therefore, these compounds could act as inhibitors of P. gingivalis colonization in the oral cavity. Four out of the five compounds (excluding 4c) exhibited the ability to reduce P. gingivalis virulence in vivo using a mouse model of periodontitis. These compounds reduced alveolar bone resorption in P. gingivalis-infected mice. Triazole derivative 15b displayed good safety profiles in cytotoxicity experiments with telomerase immortalized human gingival keratinocytes (TIGK) and murine J774A.1 macrophage cell lines. Cytotoxicity assays comprised determination of the release of lactate dehydrogenase, quantification of adenosine triphosphate (ATP) levels, and apoptosis assays with TIGK and J774A.1 cells, as well as hemolysis assays with sheep red blood cells (RBCs). Although compound 15b displayed potent inhibition activity along with a good toxicity profile, there were no control antibacterial agents used and a conclusion about the triazole derivatives potential for clinical use as inhibitors remains to be determined.
In addition to the derivatization of 1,2,3-triazoles, 1,2,4-triazoles were also investigated for their ability to inhibit the growth of P. gingivalis (Fig. 6).92 A one-step synthesis consisting of nucleophilic addition of the nitrogen at the N4-position on the 1,2,4-triazole ring to the carbonyl carbon of 2,4-dinitrobenzaldehyde yielded two 1,2,4-triazole derivatives 26 and 27. These triazole derivatives were tested against three strains of P. gingivalis; one strain was fimbriated and more virulent, while the other two strains were not fimbriated and less virulent. When tested against the three strains of P. gingivalis, the MIC values were very similar, with the best MIC values being 0.062 mg mL−1 (62 μg mL−1, 221 μM) for the most active compound 26. Through colony forming unit (CFU) assays, 26 was found to inhibit 99.99% of P. gingivalis growth with a minimum bactericidal concentration (MBC) value of 0.25 mg mL−1 (250 μg mL−1, 892 μM). Zone of inhibition assays revealed that the two triazole derivatives displayed inferior antibacterial activity compared to the control antibiotics ERY, MTZ, and TET, with inhibition of P. gingivalis growth at ≥1 μg mL−1. No growth inhibition was observed with the control antibiotic gentamicin. Slightly higher toxicity of 26 was seen compared to 27 when tested in human cervical cancer (HeLa) cells. The lack of growth inhibition activity against P. gingivalis at higher concentrations of 26 reveals that the derivatization of 1,2,4-triazoles should not be further pursued for the treatment of P. gingivalis.
In brief, the azoles display little to no activity in terms of inhibiting growth of P. gingivalis. However, some should be further investigated in mature biofilm studies with other early colonizing Streptococcus spp., as the 1,2,3-triazoles showed the ability to inhibit P. gingivalis incorporation into biofilms.
3.1.2. Heterocyclic derivatives
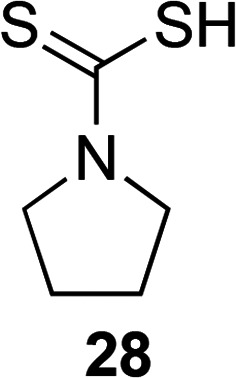
As the five-membered heterocyclic triazole ring has been used to develop novel antibacterial agents to combat oral bacterial species, the five-membered heterocyclic pyrrolidine ring has also been derivatized in an attempt to combat these pathogenic bacterial species. A preliminary study explored the use of a commercially available pyrrolidine derivative, pyrrolidine dithiocarbamate (PDTC, 28, Fig. 7), which is an antioxidant, as a potential treatment option to combat P. gingivalis.93 PDTC displayed low MIC values of 1 μM (0.147 μg mL−1) when tested against P. gingivalis compared to the control antioxidant curcumin that had an MIC value of ∼40 μM. Other control antioxidants such as parthenolide, quercetin, epigallocatechin gallate (EGCG), and resveratrol were studied, but they did not inhibit 100% of the growth of P. gingivalis at any of the concentrations tested. Reduced activity was seen when 28 was tested against three other bacterial species, A. actinomycetemcomitans, Staphylococcus aureus, and Escherichia coli (MIC = 30, 30, and 400 μM, respectively). Preliminary studies into the mechanism of action of 28 revealed that this known metal chelator with zinc ionophore activity requires zinc to enhance antibacterial activity. With its potent activity against P. gingivalis and promising preliminary mechanism of action data, further investigation into the safety profile, resistance, and anti-biofilm studies of the antioxidant 28 should be performed.
Fig. 7. A representative example of compounds that contain heterocycles in their structures that were tested as potential antibacterial agents and found active against P. gingivalis.
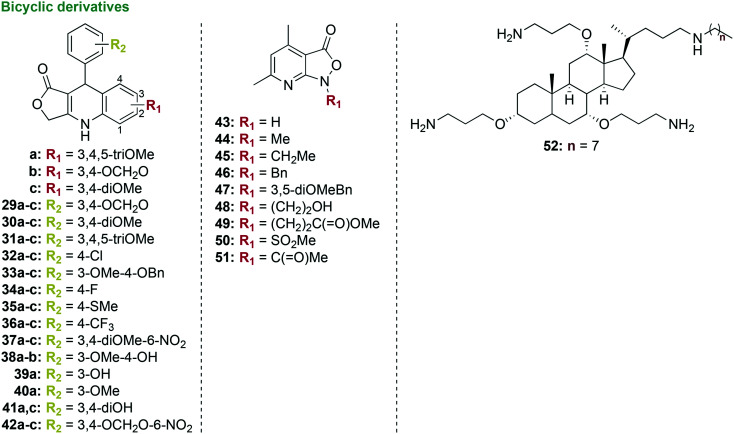
3.1.3. Bicyclic derivatives
Bicyclic compounds containing quinolines were also investigated for their antimicrobial activity (Fig. 8).94 A series of 39 lactone 1,4-dihydroquinoline derivatives were synthesized in a one-step microwave-assisted high-yielding reaction between tetronic acid, substituted anilines (e.g., methoxy and methylenedioxy), and substituted aromatic aldehydes (e.g., methoxy-, methylenedioxy-, halogen-, nitro-, benzyl-, trifluoromethyl-, hydroxy-, and methylthio-containing substituents). Three of the 29 derivatives, 36c, 37b, and 42b, displayed some activity against P. gingivalis with MIC values of 12.5–25 μg mL−1, which are worse than that of the control chlorhexidine (MIC = 0.922 μg mL−1). None of the compounds displayed activity when tested against other oral bacterial species, such as P. nigrescens, S. mitis, and S. sanguinis, as well as Mycobacterium species, such as M. tuberculosis, M. avium, and M. kansasii. With promising activity against P. gingivalis, further lead optimization with the three most active compounds could be performed using bioisosteric replacement of the substituents on the dihydroquinoline and benzylic rings, in addition to cytotoxicity and mechanism of action studies.
Fig. 8. Three new scaffolds as representative examples of compounds that contain bicyclic moieties in their structures that were tested as potential antibacterial agents against oral bacterial species.
Additionally, a series of 4,6-dimethylisoxazolo[3,4-b]pyridine-3(1H)-one (43, Fig. 8) derivatives were synthesized through alkylation, acetylation, and sulfonylation reactions to yield eight derivatives (43–51, Fig. 8) that were tested as antibacterial agents.95 The isoxazolone derivatives were tested against 68 strains of aerobic and anaerobic bacterial species, including one strain of P. gingivalis, 12 reference bacterial strains, and clinically isolated bacteria from the oral cavity, intestinal tract, and respiratory system. Derivatives 44–51 displayed no activity against P. gingivalis (MIC = 100 to >200 μg mL−1) when compared to the parent compound 43 (lowest MIC value = 50 μg mL−1), which itself was way less active than the control MTZ (MIC <0.4 μg mL−1). Thus, these isoxazolone derivatives appear not to be promising as antibacterial agents for the control of P. gingivalis.
A preliminary investigation led to the development of small molecule cationic steroid antimicrobial (CSA) derivatives active against P. gingivalis and S. mutans (Fig. 8). These CSA derivatives mimic cationic antibacterial peptides, but are more cost effective to synthetize, as they are small molecules synthesized in ten-steps including mesylation and reduction to yield a final compound in moderate yield.96,97 The most active compound 52 displayed good activity when tested against 23 strains of S. mutans and 24 strains of Porphyromonas spp. with MIC values of 1–8 μg mL−1 and 1–16 μg mL−1, respectively.98 As they display increased antibacterial activity, future cytotoxicity studies with human oral epithelial cells and biofilm studies with P. gingivalis and S. mutans should be pursued, particularly with compound 52.
Overall, bicyclic derivatives containing quinolines and cationic steroid scaffolds show potential as anti-P. gingivalis agents and should undergo cytotoxicity and anti-biofilm studies.
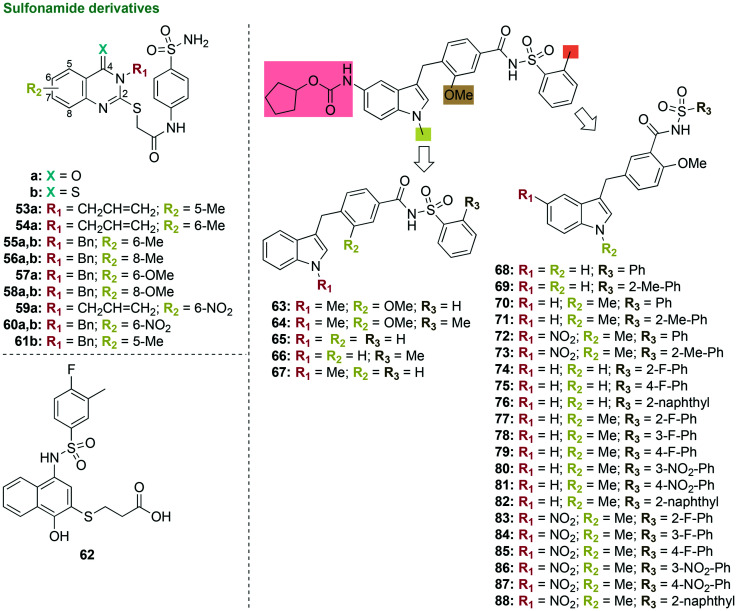
3.1.4. Sulfonamide derivatives
The sulfonamide antibiotics are commonly used to treat bacterial infections. Sulfonamide-based quinazoline derivatives, such as 53–61, were investigated as potential inhibitors of carbonic anhydrases (γ-CAs), which are crucial for the survival of bacterial species, including P. gingivalis (Fig. 9).99P. gingivalis γ-CAs are enzymes that catalyze the physiologically important reaction of CO2 hydration to bicarbonate and protons. Additionally, P. gingivalis γ-CAs are involved in biosynthetic reactions by supplying CO2 and bicarbonate, along with transportation of CO2 and bicarbonate, and regulation of pH. A three to four-step synthesis yielded 13 sulfonamide derivatives.99 Out of the 13 sulfonamide derivatives, compound 55a, displayed strong inhibitory activity with an inhibition constant (Ki) value of 3.5 nM (0.0035 μM, 0.0017 μg mL−1) that was superior to that of the control acetazolamide (Ki = 324 nM), a sulfonamide antibiotic FDA-approved in 1953 to prevent and reduce the symptoms of altitude sickness.100 The strong inhibitory activity of 55a against P. gingivalis γ-CAs suggested that MIC testing against P. gingivalis, cytotoxicity studies, as well as anti-biofilm studies should be performed to determine its potential as an anti-P. gingivalis agent.
Fig. 9. Four new scaffolds as representative examples of compounds that contain sulfonamide moieties in their structures that were tested as potential antibacterial agents and found active against P. gingivalis.
In an in silico study, a virtual screening of more than 9 million small molecules from the ZINC database of commercially available chemicals was used to explore the potential of these compounds as inhibitors of the enzyme meso-2,6-diaminopimelate dehydrogenase from P. gingivalis.101 The enzyme diaminopimelate dehydrogenase catalyzes a key step in lysine biosynthesis, which is essential for P. gingivalis survival as the pathway is involved in peptidoglycan biosynthesis. This study suggested a new target for the treatment of P. gingivalis. Unfortunately, when 11 compounds were tested in vitro against that target (62, Fig. 9), no inhibition activity was observed (lowest IC50 value = 157 ± 26 μM for compound 62). When tested against P. gingivalis and S. sanguinis, no activity was seen (lowest MIC value = 167 μM against P. gingivalis by compound 62). There was also no control antibiotic used for comparison. These compounds should not be further pursued as inhibitors or antibacterial agents due to their lack of activity.
A successful strategy employed by researchers to find new antibacterials is to reposition drugs that were FDA-approved for the treatment of a different disease. Screening of a drug repositioning library led to the identification of ZAF, which was FDA-approved in 1996 for the treatment of asthma, as an active drug against P. gingivalis.102 In a preliminary investigation into derivatization of the parent compound ZAF, 11 first generation ZAF derivatives were synthesized using a two- to four-step linear synthesis (63–73, Fig. 9).62 ZAF was modified by removal of the cyclopental carbamate group on the indole ring by replacement with either a hydrogen or nitro group, removal of the N-methyl group on the indole ring, change in substituents and substitution patterns on the benzoyl ring (e.g., indole, methoxy, and arylsulfonamide), and removal of the methyl group on the arylsulfonamide ring. When tested against P. gingivalis, five compounds, 67 and 70–73, displayed good activity with 90–100% growth inhibition at 10 μM, which was comparable to or superior to the activity of the controls TET at 2.25 μM and ZAF at 25 μM and 50 μM. The three most active compounds displayed selective activity against P. gingivalis with no activity seen when tested against other oral bacterial species such as A. naeslundii, A. actinomycetemcomitans, V. parvula, F. nucleatum, and S. sanguinis. Bactericidal activity was observed in CFU assays with compound 72 at 10 μM along with 60% inhibition of P. gingivalis growth at 1 μM. In cytotoxicity experiments, an increased safety profile was observed with compounds 71–73 compared to the parent ZAF when tested against immortalized human oral keratinocyte cells (OKF6) with 50–70% cell viability at 1 μM and 10 μM. The three most active compounds 71–73 containing 2-methoxy-5-indoyl substituents on the benzoyl ring displayed increased activity against P. gingivalis compared to the parent ZAF as well as the other derivatives synthesized with 3-methoxy-5-indoyl substituents on the benzoyl ring. As these compounds displayed increased activity against P. gingivalis when compared to ZAF, further investigation into structure optimization was pursued.
In the following lead optimization study, the three most active first generation ZAF derivatives 71–73 (Fig. 9) were used as starting points for the generation, in three to four linear steps, of 15 second generation ZAF derivatives active against P. gingivalis (74–88, Fig. 9).103 The 15 second generation ZAF derivatives were modified on the indole ring at R1 and R2 positions (e.g., nitro and methyl groups), arrangement of the indole, methoxy, and arylsulfonamide substituents on the benzoyl ring, and substitution at the R3 position of the arylsulfonamide (e.g., fluoro and nitro phenyl, and naphthyl groups). When tested against P. gingivalis, six out of the 15 compounds, 78–80, 82, 84, and 87, displayed similar or superior percent growth inhibition values against P. gingivalis at 1 μM, 10 μM, and 100 μM compared to ZAF at 25 μM and 50 μM, its first generation derivatives, and TET at 2.81 μM. Compounds 78–80, 82, 84, and 87 displayed selective inhibition of P. gingivalis with little to no activity against other oral bacterial species such as A. actinomycetemcomitans, A. naeslundii, V. parvula, and S. sanguinis. When tested against OKF6 cells, compounds 78–80, 82, 84, and 87 displayed decreased cytotoxicity compared to the parent ZAF. In CFU assays, compounds 78–80, 82, 84, and 87 displayed bactericidal activity at 100 μM and two of the most active compounds, 84 and 87, displayed bactericidal activity at concentrations as low as 1 μM. Due to increased activity against P. gingivalis, increased safety profile in human oral epithelial cells, and selectivity, these compounds would need to be further optimized and tested in oral biofilms.
In general, the use of sulfonamide-containing scaffolds as potent inhibitors of enzymes crucial to P. gingivalis survival as well as the targeted inhibition of P. gingivalis growth show great promise for the development of anti-P. gingivalis agents as treatment of chronic periodontitis. Future experiments determining mechanism(s) of action, lead structure optimization, and anti-biofilm studies should be performed.
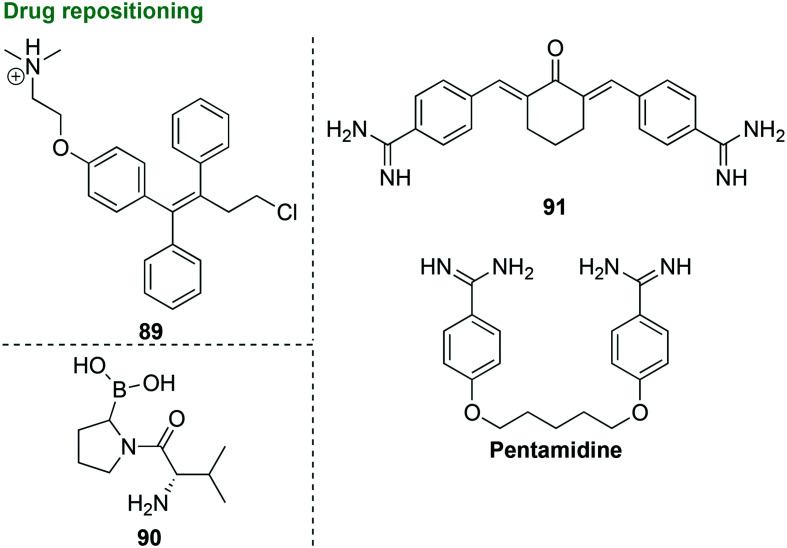
3.2. Drug repositioning
As demonstrated with ZAF in the previous section, repositioning of FDA-approved drugs that are not used for the treatment of periodontal diseases as new antibacterial agents active against P. gingivalis is a strategy used due to the high cost of novel drug development. Screening of a drug repositioning library led to the discovery of two FDA-approved drugs, ZAF (discussed above) and toremifene (89, Fig. 10) active against P. gingivalis.104 Toremifene (89) was FDA-approved in 1997 for the treatment of breast cancer as a 60 mg oral tablet. In a preliminary study, toremifene (89) displayed good antibacterial activity against P. gingivalis and S. mutans with MIC and minimum biofilm-inhibitory concentration (MBIC) values of 12.5 μM and 25 μM (5.1 μg mL−1 and 10.1 μg mL−1), respectively. Mechanism of action studies focused on DNA, RNA, and protein synthesis were performed in P. gingivalis and S. mutans and showed that toremifene (89) did not inhibit the synthesis of DNA, RNA, or protein. Instead toremifene (89) caused increased membrane permeabilization along with binding to LPS produced by P. gingivalis. The ability of toremifene (89) to bind to LPSs is important because the progression of periodontal diseases is promoted by virulence factors produced by P. gingivalis, such as LPSs, that contribute to the development of periodontal diseases. When tested in human oral gingival epithelial cells, toremifene (89) was toxic at 25 μM, while no hemolytic activity was observed when tested against horse RBCs at concentrations up to 100 μM. Toremifene (89) is currently used in the clinic at a much higher dose than the MIC values against P. gingivalis presented and is an ideal candidate as an antibacterial agent for use as a mouthwash due to its toxicity of oral epithelial cells.
Fig. 10. Four FDA-approved scaffolds and their derivatives as representative examples of compounds that could be used as potential antibacterial agents.
With the preliminary success of five-membered heterocyclic triazole derivatives (discussed in section 3.1.1) leading to strong inhibitors of P. gingivalis adherence to other oral bacterial species within biofilms, it is no surprise that other heterocyclic-based inhibitors have been investigated as potential therapeutic options. Proteases produced by P. gingivalis are important virulence factors that contribute to tissue destruction, adhesion, and degradation of the ECM. Inhibition of the P. gingivalis protease dipeptidyl peptidase 4 (DPP4), which is involved in collagen degradation through interactions with ECM proteins fibronectin and collagen, was investigated in a preliminary study that led to the screening of 450 potential inhibitors (90, Fig. 10).105 This preliminary investigation looked into the similarity in structure and function of the human DPP4 and P. gingivalis DPP4. Some of the library compounds were known inhibitors of the human DPP4 that are currently used as antidiabetic drugs and others were DPP4 inhibitors that have previously been developed and characterized as a part of a collection of mammalian DPP family inhibitors. The human and P. gingivalis DPP4 were found to have similar overall structures along with similar substrate specificity. Although inhibitors were active against the human DPP4, little to no activity was seen when tested against P. gingivalis DPP4. Out of the 450 inhibitors screened, 81 inhibitors displayed more than 50% growth inhibition at 100 μM and 33 displayed more than 50% growth inhibition of P. gingivalis at 10 μM. Out of the 81 compounds that exhibited 50% growth inhibition of P. gingivalis at 100 μM, only 16 displayed good IC50 values of 0.079–8 μM. With none of the compounds completely inhibiting the growth of P. gingivalis along with a low affinity towards P. gingivalis DPP4, these compounds show very limited promise as potential antibacterial agents.
Novel compounds tested as potential antibacterial agents active against P. gingivalis should display properties of inhibiting the growth of P. gingivalis and/or inhibiting the virulence factors produced by P. gingivalis that play key roles in the progression of periodontal diseases. The most important and widely studied virulence factors produced by P. gingivalis that contribute to its pathogenicity include gingipains, fimbriae, and LPSs.106 In a preliminary investigation, previously synthesized symmetrical aromatic compounds (synthesized by J. Stürzebecher from the Institute of Vascular Biology and Medicine, University Hospital of Jena) that contained benzamidines in their structures were found to be potent inhibitors of arginine-specific cysteine proteinases, such as gingipains (Arg-gingipains, Rgp) (91, Fig. 10). When tested against Rgp, benzamidine displayed no activity with a Ki of 277 μM, while its derivatives 91 and pentamidine (as a control) displayed better activity with Ki values of 0.51 and 40.3 μM, respectively.107 The benzamidine derivative pentamidine was FDA approved in 1989 for the treatment of Pneumocystis jiroveci pneumonia and has previously been shown to inhibit the growth of P. gingivalis with an MIC value of 1 ng mL−1 (0.001 μg mL−1).108 In a follow up study,10691 displayed poor activity against P. gingivalis (20% growth inhibition) at the tested concentration of 20 μM (7.17 μg mL−1) in CFU assays, benzamidine displayed no activity at 20 μM (2.40 μg mL−1), while pentamidine displayed the best activity with 90% growth inhibition at 20 μM (6.81 μg mL−1). No known antibacterial agent was used as a control in these experiments. The benzamidine derivatives were also tested as inhibitors of the protein GroEL, a virulence factor produced by P. gingivalis belonging to the heat shock protein 60 family. GroEL contributes to the progression of periodontal diseases and increases inflammation due to its role in stimulating the production of inflammatory cytokines. The virulence of P. gingivalis was reduced by 91 and pentamidine through binding of GroEL and inhibition of the ability of P. gingivalis to kill fertile white leghorn eggs. Based on these promising results, 91 should be further derivatized to increase its activity against P. gingivalis growth inhibition and pentamidine could be further developed as a potent inhibitor of P. gingivalis virulence. To conclude, the repositioning and/or derivatization of FDA-approved drugs, such as ZAF, toremifene, benzamidine, and pentamidine, could lead to novel therapeutics for P. gingivalis infections.
3.3. Natural products
An alternative to synthetizing novel compounds and drug repositioning is utilizing Nature's treasure trove of naturally produced antibacterial agents. Natural products are a source of many drugs and drug leads for different ailments. Therefore, it is no surprise that natural products have been explored for P. gingivalis treatment. Although many natural products have been tested, researchers are still in the early stages of developing/optimizing natural products for the treatment of P. gingivalis. The following sections will cover: (i) natural products from various organisms and their derivatives, (ii) plant essential oils and their derivatives, and (iii) inactive natural products.
3.3.1. Natural products and their derivatives
Similar to the drug repositioning and derivatization of ZAF discussed in section 3.1.4, scientists have also attempted to isolate natural products and test their crude extracts and purified compounds against P. gingivalis as well as other oral bacterial species. Modification of natural products occurs through a wide variety of methods such as isolation, derivatization, semi-synthesis, synthesis, and biotransformation.
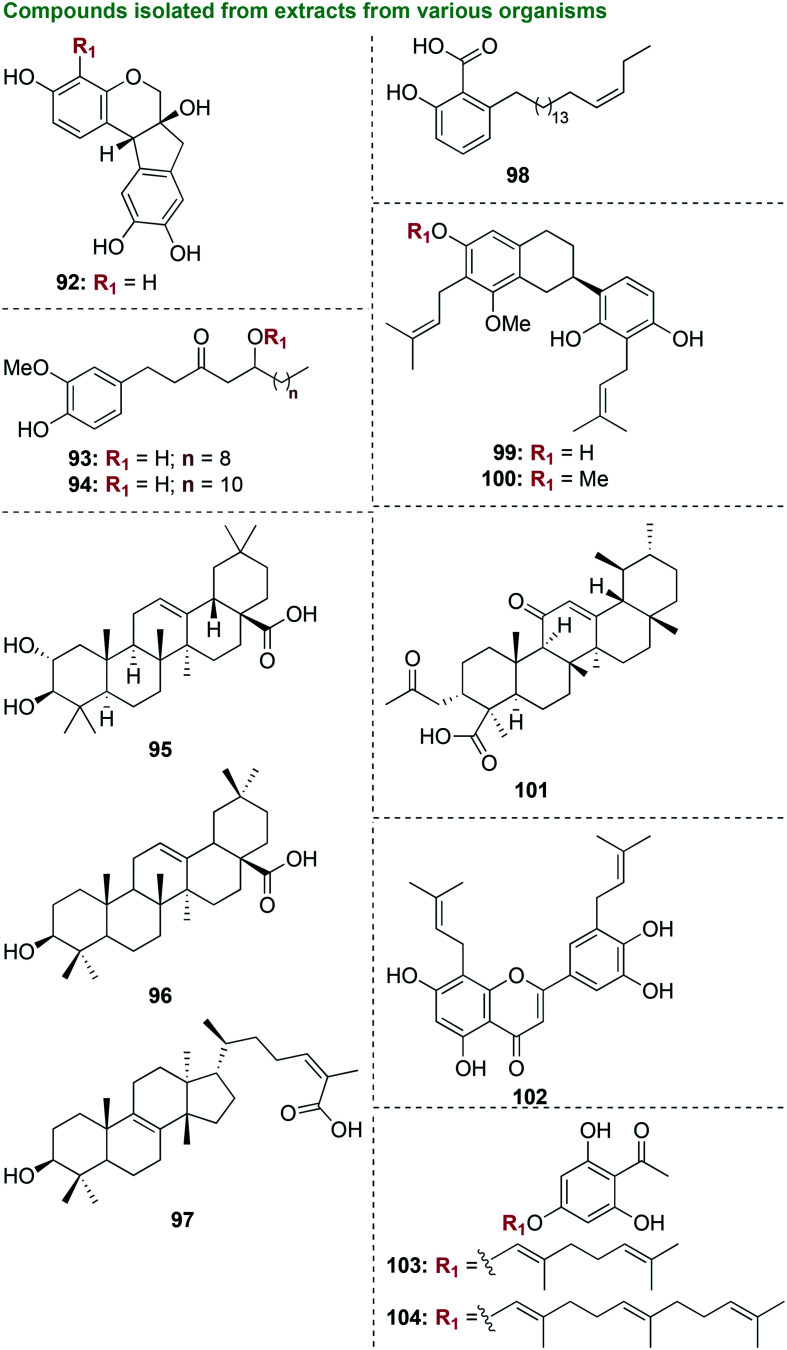
3.3.1.1. Compounds isolated from extracts from various organisms
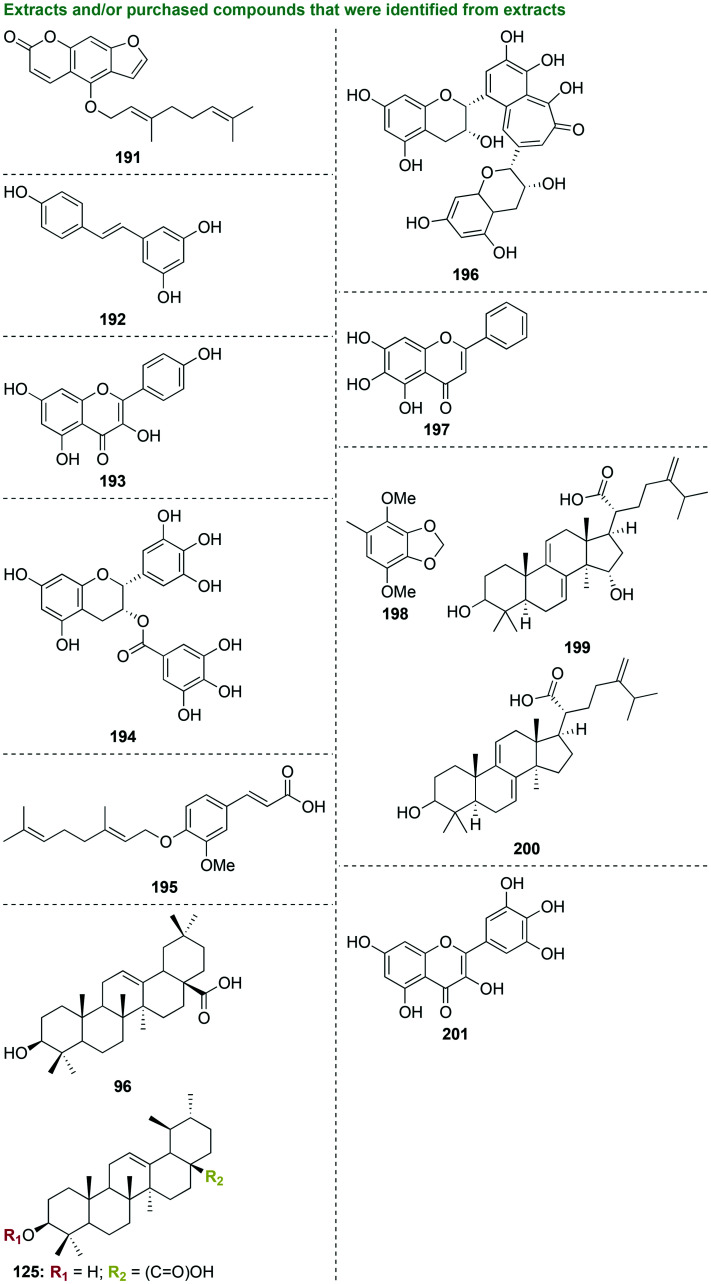
In an early stage investigation, a total of eight compounds were isolated from the methanolic extract of the bark of Haematoxylon brasiletto Karst.109 Starting with 1 kg of bark, 100 g was extracted by maceration with methanol along with 35.7 g of crude ethyl acetate (EtOAc)-soluble extract, which displayed the most antibacterial activity. Eight pure known compounds were isolated from EtOAc, all of which were phenolic compounds (92, Fig. 11). When tested against three strains of S. aureus, three strains of E. coli, one strain of Enterococcus faecium, Bacillus subtilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, S. mutans, P. gingivalis, and Candida albicans, the methanolic extract along with all of the pure compounds were inactive, with the exception of brazilin (92) against P. gingivalis (MIC value of 8.7 μg mL−1 that was worse than that of the control chlorhexidine gluconate (MIC = 0.6 μg mL−1)). With its selective activity of against P. gingivalis, brazilin (92) would be a good candidate for future investigations focused on mechanism of action studies to aid structure optimization, along with cytotoxicity and combination studies with known antibiotics to determine the potential of next generation phenolic compounds as anti-P. gingivalis agents.
Fig. 11. Ten scaffolds as representative examples of compounds that were isolated from extracts from various organisms that were tested as potential antibacterial agents against oral bacterial species.
In another preliminary study also focused on phenolic molecules, five polyphenolic ketones known as gingerols were isolated and purified by silica-gel column chromatography and preparative high-performance liquid chromatography (HPLC) from ginger, Zingiber officinale Roscoe from the family Zingiberaceae (93 and 94, Fig. 11).110 Dry rhizomes of Z. officinale (100 g) were purchased and 250 mg of active fractions were isolated. Ethanol and n-hexane extracts as well as isolated pure compounds were tested against P. gingivalis, P. endodontalis, and P. intermedia, and were found to be inactive with the exception of [10]-gingerol (93) and [12]-gingerol (94). The most active compound, [10]-gingerol (93), displayed moderate antibacterial activity against P. gingivalis, P. endodontalis, and P. intermedia with MIC/MBC values of 6/6 μg mL−1, 8/4 μg mL−1, and 14/14 μg mL−1, respectively. Future studies to determine mechanism of action as well as anti-biofilm experiments with P. gingivalis should be pursued with [10]-gingerol (93).
In a different preliminary study in which no pure small molecules were isolated, five extracts from the Mediterranean plants of Olea europaea (olive leaf extract and table olives extract), mastic gum, and Inula viscosa were tested for their antimicrobial activity.111 One of the main problems of using an extract as a treatment option is the many active and inactive compounds within the extract could have synergistic or antagonistic activity with one another. Therefore, the true potency of any one compound within the extract is not known until it is isolated and individually tested. The extracts were tested against a wide range of oral bacterial species comprising S. mutans, Streptococcus sobrinus, S. oralis, Enterococcus faecalis, P. gingivalis, P. intermedia, F. nucleatum, and P. micra. To determine the extracts specificity toward oral bacterial species they were tested against one strain of S. aureus and E. coli, normally found on the skin and intestinal flora, and one fungal strain of C. albicans, which can cause oral thrush, also known as oral candidiasis. All extracts were found to be inactive against all tested bacterial and fungal strains, with the exception of moderate activity seen with the mastic gum extract with an MIC value of 20 μg mL−1 against P. gingivalis. However, the MBC values with the mastic gum extract were high (MBC = 70 μg mL−1). The active natural products in the mastic gum extract should be isolated and tested alone with P. gingivalis to determine the most active compounds that could be further investigated.
In addition to the extracts described above, pure isolated compounds from the Mediterranean plant extracts of Olea europaea (olive) and Pistacia lentiscus (mastic gum) were tested for their antimicrobial activity (95–97, Fig. 11).112 Five known natural products were isolated from O. europaea (oleuropein, hydroxytyrosol, oleocanthal, oleacein, and maslinic acid (95)) and three were isolated from P. lentiscus (oleanonic aldehyde, 24Z-isomasticadienolic acid (97), and oleanolic acid (96)). The two pentacyclic triterpenoids maslinic acid (95) and oleanolic acid (96) along with the tetracyclic triterpenoid 24Z-isomasticadienolic acid (97) displayed good to moderate antimicrobial activity when tested against P. gingivalis with MIC/MBC values of 4.9/9.8 μg mL−1, 9.8/9.8 μg mL−1, and 2.4/9.8 μg mL−1, respectively. Additionally, maslinic acid (95) and 24Z-isomasticadienolic acid (97) displayed good activity against P. micra (MIC/MBC = 9.8/9.8 μg mL−1 and 2.4/9.8 μg mL−1, respectively). Unfortunately, all compounds were found to be inactive against the other eight bacterial species tested comprising S. mutans, S. sobrinus, S. oralis, E. faecalis, E. coli, S. aureus, P. intermedia, F. nucleatum, and one C. albicans fungal strain. It could be beneficial to perform anti-biofilm and cytotoxicity studies with the three most active compounds.
In another preliminary investigation now focused on plants from Mexico, one novel compound along with six known compounds were extracted in a methanol soluble extract and isolated from the tree bark of the Mexican plant Amphipterygium adstringens Standl (98, Fig. 11).113 The compounds consisted of four anacardic acids and three triterpenes. When tested against P. gingivalis and S. mutans, the methanol extract was inactive with MIC values of 82 μg mL−1 and 69 μg mL−1, respectively. The only compound to display good to moderate activity was 6-[16′Z-nonadecenyl]-salicylic acid (98), which displayed respective MIC values of 12 μg mL−1 and 7 μg mL−1 against P. gingivalis and S. mutans (worse than those of the control chlorhexidine gluconate (MIC = 1.4 μg mL−1 and 3.2 μg mL−1, respectively)). Lead optimization studies of 6-[16′Z-nonadecenyl]-salicylic acid (98) could focus on treatment of dental caries as this compound displayed increased activity with S. mutans.
In another study, 20 known isoflavonoids and coumarins were isolated along with one new pterocarpan from Chinese licorice Glycyrrhiza uralensis and were tested as potential antibacterial agents (99 and 100, Fig. 11).114 Only seven of the compounds could be tested as the remaining compounds could not be isolated in sufficient amount for the assays. The licorice extract was shown to be inactive against all bacterial strains tested, while the two major components isolated, licoricidin (99) and licorisoflavan A (100), displayed the best activity against multiple bacterial strains.115 When tested against P. gingivalis, licoricidin (99) and licorisoflavan A (100) displayed moderate activity with 80% growth inhibition at 5 μg mL−1, worse than that of the control penicillin G (90% growth inhibition at 0.025 μg mL−1). Additionally, 90% growth inhibition at 5 μg mL−1 of licoricidin (99) and 2.5 μg mL−1 of licorisoflavan A (100) was exhibited when tested against P. intermedia. All compounds displayed little to no activity against F. nucleatum, S. mutans, and S. sobrinus. The good antibacterial activity displayed by licoricidin (99) and licorisoflavan A (100) warrants further investigation into their mechanism(s) of action and safety profiles.
Four boswellic acids that are composed of triterpenic acids were isolated from the oleo gum resin of the plant Boswellia serrata.116 These compounds were previously extracted, isolated, and quantified from 1 kg of B. serrata and yielded an extract of 490 g that was further purified to isolate the acidic mixture of boswellic acids as 280 g.117 When tested against P. gingivalis, S. mutans, E. faecalis, E. faecium, Actinomyces viscosus, S. sanguinis, F. nucleatum, and P. intermedia, the most active pentacyclic terpenoid acetyl-11-keto-β-boswellic acid (AKBA, 101) displayed good activity with MIC values of 2–4 μg mL−1, with the exception of F. nucleatum (MIC >128 μg mL−1). These values were similar to the control triclosan that is commonly found in body washes, toothpastes, and soaps as an antibacterial agent with MIC values of 1–4 μg mL−1 against all bacterial strains tested. Time-kill assays with AKBA (101) in S. mutans revealed bacteriostatic activity at 32 μg mL−1. At 8× MIC, AKBA (101) suppressed the emergence of mutants in S. mutans and A. viscosus. Additionally, AKBA (101) displayed good postantibiotic effect at 2× MIC at 5.7 h. In biofilm studies with AKBA (101), preformed biofilms of S. mutans and A. viscosus were eradicated and biofilm growth was inhibited with a MBIC50 value of 16 μg mL−1. With low MIC values, biofilm studies with P. gingivalis and cytotoxicity studies should be performed.
An investigation into inhibitors of gingipain virulence factors secreted by P. gingivalis such as arginine-specific (Rgp) and lysine-specific (Lys-gingipains, Kgp) cysteine proteinases led to the isolation of 17 prenylated flavonoids from the plant species Epimedium (102, Fig. 11).118 The compounds consisted of known and novel flavonoids isolated from 3 kg of Epimedium and extracted to yield a residue of 485 g. Four compounds completely inhibited P. gingivalis growth at 12.5 μM. Two of these compounds slightly inhibited P. gingivalis biofilm formation at 1.25 μM. Of these two compounds, epimedokoreanin B (102) displayed the most potent inhibition activity with Ki values of 1.67 ± 0.07 μM and 2.71 ± 0.22 μM for Rgp and Kgp, respectively. Mechanism of action studies revealed that epimedokoreanin B (102) is a non-competitive inhibitor of cysteine proteases, gingipains Rgp and Kgp. An important structural feature of the potent inhibitors is a hydrophobic prenyl functional group on the aromatic rings of the flavonoids, as the compounds with prenyl functional groups (e.g., epimedokoreanin B (102)) displayed better inhibition activity compared to known unprenylated flavonoids such as quercetin and luteolin. With its known mechanism of action, potent activity as an inhibitor, as well as moderate activity against P. gingivalis, epimedokoreanin B (102) is an ideal starting point for SAR studies to further optimize its activity.
Common prenyl functional groups attached to aromatic rings via a C–C or a C–O linkage for natural products include geranyl (C10) and farnesyl (C15) chains. In a preliminary investigation, the synthesis of two known phytochemical oxyprenylated secondary metabolites, 2′,6′-dihydroxy-4′-geranyloxyacetophenone (103) and 2′,6′-dihydroxy-4′-farnesyloxy-acetophenone (104), was performed in a single step using the prenylation of 2,4,6-trihydroxyacetophenone at the 4′-position to obtain 103 and 104 with geranyl or farnesyl chains in 55% and 62% yields (Fig. 11).119 These compounds were synthesized as they are normally produced/isolated in low amounts from the fruits and aerial parts of plants belonging to the Rutaceae family. When tested against S. sobrinus, S. mutans, A. actinomycetemcomitans, P. intermedia, and C. albicans, both compounds displayed little to no activity with MIC values of 25 to >100 μg mL−1, much worse than those of the controls penicillin G (MIC = 0.049–3.125 μg mL−1) and nystatin (MIC = 0.78–6.25 μg mL−1). When tested against P. gingivalis, both compounds displayed moderate activity with MIC of 12.5 μg mL−1, much worse than that of the control penicillin G (MIC = 0.098 μg mL−1). With an enticing one-step synthesis, compounds 103 and 104 are highly amenable to SAR studies to increase their selective inhibition of P. gingivalis.
For the treatment of periodontal diseases, isolated extracts would not be a viable option as the majority of extracts tested were inactive against oral bacterial species, while isolated compounds displayed activity. Amongst the compounds isolated from extracts, the triterpenic acids were the best followed by the triterpenoid scaffolds, then the phenolic compounds, anacardic acids, prenylated flavonoids, isoflavonoids, coumarins, and pterocarpans. Out of the four triterpenic acids isolated, one pentacyclic terpenoid, AKBA (101), displayed the best activity and should be further investigated as an antibacterial agent for the treatment of dental caries and periodontitis.
3.3.1.2. Purchased compounds that were identified from extracts
In a comprehensive study, the two known major components (105 and 106, Fig. 12) of Psoraleae, a traditional Chinese medicine, were investigated for their antibacterial and anti-inflammatory properties against P. gingivalis.120 These commercially available coumarin-based compounds vary in the position of the fused furan ring. When tested against planktonic P. gingivalis, psoralen (105) and angelicin (106) displayed good MIC values of 6.25 μg mL−1 and 3.125 μg mL−1, respectively. The cell viability and thickness of P. gingivalis biofilms was also decreased at these concentrations with each compound. The compounds displayed poor MBC values at 50 μg mL−1 against planktonic P. gingivalis. The low MIC values against planktonic P. gingivalis prompted the exploration into their effects on preformed biofilm growth as well as inhibition of biofilm formation of P. gingivalis. Moderate inhibition of P. gingivalis biofilm formation (MBIC50 values of 15.8 μg mL−1 and 7.5 μg mL−1 for psoralen (105) and angelicin (106), respectively) was observed. Preformed biofilms of P. gingivalis were eliminated by psoralen (105) and angelicin (106) with respective minimum biofilm reduction concentration (MBRC50) values of 24.5 μg mL−1 and 23.7 μg mL−1. The viability of P. gingivalis cells in preformed biofilms with the bacterial cells attached to a surface, also known as sessile cells, was reduced with sessile MIC50 (SMIC50) values of 5.8 μg mL−1 and 6.5 μg mL−1. When psoralen (105) and angelicin (106) were tested in human periodontal ligament cells, an osteogenesis-potentiating effect was observed in addition to no cytotoxic effect in this cell line or the monocyte-like THP-1 cells. With angelicin (106), alveolar bone loss and inflammation induced by LPS was reduced in in vivo experiments using wild-type male C57BL/6 mice. With the ability to disrupt preformed biofilms and inhibit biofilm formation of P. gingivalis along with anti-inflammatory properties, angelicin (106) should be further developed as an antibacterial agent.
Fig. 12. Five scaffolds as representative examples of purchased compounds that were identified from extracts and tested as potential antibacterial agents against oral bacterial species.
A cranberry polyphenol, A-type cranberry proanthocyanidins (107), isolated from the cranberry fruit Vaccinium macrocarpon, and a commercially available chalcone, licochalcone A (108), a flavonoid originally isolated in the licorice Glycyrrhiza inflata, were tested as antibacterial agents (Fig. 12).121 Licochalcone A (108) displayed good activity against P. gingivalis while compound 107 was inactive. When tested against planktonic P. gingivalis cells and preformed biofilms, licochalcone A (108) displayed good MIC values of 10 μg mL−1. Both compounds displayed synergy in combination with one another against planktonic and preformed biofilms of P. gingivalis. In combinations, these compounds also inhibited the adherence of P. gingivalis to the immortalized human oral epithelial cell line GMSM-K. When tested against tissue destructive enzymes of P. gingivalis, collagenase and human recombinant matrix metallopeptidase 9 (MMP-9), licochalcone A (108) displayed slight inhibition of activity against collagenase with 35% inhibition at 5 μg mL−1, but was not really active against MMP-9 (15% inhibition of MMP-9 at 5 μg mL−1). In a macrophage model, the compounds displayed synergistic interactions in combination with one another to reduce the LPS-induced secretion of IL-1β, TNF-α, and IL-8, which are pro-inflammatory mediators. For future investigations, combination studies with licochalcone A (108) and known antibiotics could potentially provide novel treatment options.
With the promising anti-P. gingivalis activity seen with oxyprenylated compounds 103 and 104 and the coumarin-based compounds 105 and 106 discussed above, compounds containing both groups would seem to be an interesting approach to target P. gingivalis. In an early phase investigation, two known coumarin-based compounds were tested against P. gingivalis (109 and 110, Fig. 12).122 One of these was commercially available with a geranyloxy group at the C-7 position, auraptene (109), and the other was synthesized through condensation, alkylation, and methylation reactions123 to yield lacinartin (110) with an isopentenyloxy moiety at the C-7 position. Unfortunately, these compounds were found to be inactive, with auraptene (109) inhibiting P. gingivalis growth by 42% at 100 μg mL−1 and lacinartin (110) inhibiting about 90% of P. gingivalis growth at 50 μg mL−1. Lacinartin (110) displayed poor activity through inhibition of P. gingivalis biofilm formation by 75% at 50 μg mL−1 and 100 μg mL−1. Although these compounds needed to be given at high concentrations to inhibit P. gingivalis growth, they displayed promise as potential anti-inflammatory agents. Both compounds inhibited the adherence of P. gingivalis to the immortalized human oral epithelial cell line GMSM-K. Additionally, they inhibited MMP-9 activity and decreased the secretion of cytokines TNF-α and IL-8. SAR studies will be needed to improve P. gingivalis growth inhibition for these compounds to be used as anti-inflammatory agents for the treatment of periodontal diseases.
All in all, coumarin-based and polyphenolic scaffolds yielded compounds with good activity against P. gingivalis, increased ability to inhibit P. gingivalis biofilm formation, decreased virulence of P. gingivalis, and/or displayed anti-inflammatory properties. These scaffolds should be tested in multi-species biofilms to show their true potential as anti-P. gingivalis agents.
3.3.1.3. Natural product derivatives
Nature provides an extensive selection of natural products that can be used as starting points for investigation into their activity against P. gingivalis. The natural products presented in this subsection include autoinducers secreted by bacteria, secondary metabolites from marine sponges, and compounds originating from fungi and plants (fruits, roots, aerial parts, and seeds) from California, Brazil, India, and Sri Lanka. These starting materials provide different structural scaffolds for derivatization through synthetic, semi-synthetic, and biotransformation pathways.
Although there are a wide variety of natural products, the same natural products can be isolated from various plants from different regions.112 Oleanolic acid (96, Fig. 11 and 13) was isolated in a previous study from Mediterranean plant extracts of P. lentiscus. In the current study, oleanolic acid (96) and nine other known compounds were isolated from the California fruits of the Thompson seedless raisins (Vitis vinifera).124 A total of 5 kg of raisins were used to yield 68.4 g of an hexane-soluble extract along with 86.2 g of an EtOAc-soluble extract. From the hexane-soluble extract, 340 mg of the triterpenoid oleanolic acid (96) was isolated. Oleanolic acid (96) was semi-synthetically derivatized in previous studies125–128 through one chemical step consisting of acylation or esterification reactions to yield six derivatives (111–116, Fig. 13). None of the known compounds, including oleanolic acid (96), displayed activity against P. gingivalis and S. mutans. Of the six semi-synthetic derivatives, the oleanolic acid sodium salt 116 displayed the best activity with MIC values of 3.9 μg mL−1 and 7.8 μg mL−1 against P. gingivalis and S. mutans, respectively (worse than the control chlorhexidine gluconate with MIC values of 0.3 μg mL−1 and 1.2 μg mL−1). Future investigations focused on structure optimization of compound 116 should include derivatives with more polar functionality to enhance anti-P. gingivalis activity.
Fig. 13. Seven scaffolds as representative examples of natural products and their derivatives that were tested as potential antibacterial agents against oral bacterial species.
A study focused on the treatment of oral bacterial species for root canal infections led to the isolation of six pimarane-type diterpenes from the Brazilian plant species Viguiera arenaria Baker as well as two semi-synthetic derivatives (117–124, Fig. 13).129 The six natural products, 117–121 and 124, were isolated from the roots of V. arenaria (8 g) to yield 2 g from n-hexane and 1 g of dichloromethane fractions.130 Two derivatives, 122 131 and 123,132 were semi-synthetically made in one step from the respective starting materials ent-8(14),15-pimaradien-3β-ol (119) and ent-pimara-8(14),15-dien-19-oic acid (118). All compounds (with the exception of 117) displayed excellent to moderate activity against at least one of the 14 oral bacterial species tested, including clinical isolates. Compounds 118–124 displayed good activity when tested against a clinical isolate of Prevotella buccae with a range of MIC values from 1.5–4.0 μg mL−1 (slightly worse than that of the control chlorhexidine (MIC = 0.92 μg mL−1)). When tested against a broad panel of oral bacterial species comprising two strains of P. gingivalis and one strain of P. nigrescens, P. intermedia, Bacteroides fragilis, A. naeslundii, P. micros, and A. actinomycetemcomitans, compounds 118, 119, and 123 displayed excellent to moderate activity with a range of MIC values from 0.5–10 μg mL−1 (similar to those of chlorhexidine (MIC = 0.92–7.38 μg mL−1)). None of the compounds displayed activity against F. nucleatum, E. faecalis, and clinical isolates of A. naeslundii, A. viscosus, and E. faecalis. The three most active compounds, 118, 119, and 123, displayed MBC values that were 1–8× their MIC values. Due to the broad spectrum of activity against Gram-negative bacteria, the most active compounds, 118, 119, and 123, should undergo further structure optimization, mechanism of action, and anti-P. gingivalis biofilm studies.
In another study, extracts, known isolated compounds (including oleanolic acid (96)), and semi-synthetic derivatives of ursolic acid (125) were tested for antibacterial activity (125–131,133Fig. 13). The extracts and pure known compounds were isolated from the aerial parts of the Brazilian plant Tibouchina candolleana. 1 kg of T. candolleana yielded crude extracts of n-hexane (10.0 g), dichloromethane (15.5 g), and ethanol (44.3 g). The four known isolated compounds comprised the triterpenes ursolic acid (125) and oleanolic acid (96), and the flavonoids luteolin and genistein. Four semi-synthetic derivatives (126–129) of ursolic acid (125) were prepared in one synthetic step consisting of acetylation or esterification. When tested against B. fragilis, A. naeslundii, P. gingivalis, P. nigrescens, F. nucleatum, Bacteroides thetaiotaomicron, and Peptostreptococcus anaerobius, all extracts, isolated compounds, and ursolic acid derivatives were inactive with the exception of ursolic acid (125) that displayed moderate activity against P. gingivalis and A. naeslundii with MIC values of 20 μg mL−1 (worse than those of the control chlorhexidine (MIC = 3.7 and 7.4 μg mL−1)). Due to their inactivity, these compounds should not be further investigated for anti-P. gingivalis activity.
In addition to semi-synthesis, biotransformation of natural products is a method used for structure modification. The fungus Mucor rouxii was used for derivatization of the pentacyclic triterpene oleanolic acid (96) through biotransformation (132–136, Fig. 13).134 Oleanolic acid (96) was purchased and five derivatives were made through biotransformation using a two-step protocol. Out of the five derivatives prepared, three were novel (132, 135, and 136), one was known, 21β-hydroxy-3-oxo-olean-12-en-28-oic acid (133), and the last one was the sodium salt derivative of 133 (134). When tested against P. gingivalis, compound 134 displayed good activity with MIC and MBC values both of 6.6 μM (3.25 μg mL−1), although inferior to those of the control chlorhexidine dihydrochloride (MIC and MBC = 1.5 μM). Additionally, the parent compound oleanolic acid (96) displayed excellent activity against A. naeslundii with MIC and MBC values of 2.1 μM (0.96 μg mL−1), which were better than those of chlorhexidine dihydrochloride (MIC and MBC = 3.18 μM). All compounds displayed little to no activity when tested against A. actinomycetemcomitans, E. faecalis, and P. nigrescens. SAR studies focused on derivatives with more polar functionality similar to compound 134 could be developed to improve activity against P. gingivalis.
As isolated natural products generally do not display as potent activity as semi-synthetic derivatives, (S)-bakuchiol (137, Fig. 14), the major constituent isolated from the seeds of Psoralea corylifolia Leguminosae, was derivatized in one to two steps using methylation, acetylation, epoxidation, and/or C–C bond formation via Heck and oxidative Heck coupling to yield 16 compounds (138–153, Fig. 14) with excellent activity against P. gingivalis.135 The meroterpene, (S)-bakuchiol (137), offered an ideal structure for SAR studies with three major sites of modification: (i) the hydroxyl group at the meta or para position of the aryl ring was modified (e.g., acetyl, methyl ether, nitro, and amino groups), (ii) the isopropylidene group was modified at C-8 with an aldehyde or C-6 to C-7 with an epoxide or to a single bond, and (iii) the ethylene group was modified from a double bond to a single bond or addition of an aryl group. All compounds were inactive when tested against F. nucleatum, with the exception of compound 146, which displayed good activity with an MIC value of 8 μg mL−1, worse than that of the control triclosan that is added in consumer products as an antibacterial agent, but that is not FDA approved (MIC = 2 μg mL−1). Among the 16 semi-synthetic derivatives, nine displayed excellent to good activity when tested against S. mutans, A. viscosus, S. sanguinis, P. intermedia, and P. gingivalis with MIC and MBC values in the range of 0.25–8 μg mL−1, similar to those of the control triclosan (MIC and MBC = 1–8 μg mL−1). In single-species biofilm studies with S. mutans along with multi-species biofilms (containing S. mutans, A. viscosus, F. nucleatum, and P. gingivalis), the three most active compounds, 146–148, displayed good MBIC50 values of 1–2 μg mL−1 against single-species biofilms and 4–16 μg mL−1 against multi-species biofilms. Mechanism of action studies revealed that compounds 146–148 permeabilized the membrane of S. mutans. Overall, modification of the ethylene group on the parent compound, (S)-bakuchiol (137), with the addition of an aryl group containing para-hydroxy or para-methoxy groups was a successful strategy for developing promising anti-P. gingivalis agents.
Fig. 14. Ten scaffolds as representative examples of natural products and their derivatives that were tested as potential antibacterial agents against oral bacterial species.
In a comprehensive study, curcumin (154; normally found as the major component in rhizomes of the plant Curcuma longa from the family Zingiberaceae) was purchased and semi-synthetically derivatized to yield quantum curcumin (155), which was tested against oral bacterial species (Fig. 14).136 Quantum curcumin (155) was synthesized in a two-step top-down method to yield a compound with a C C double bond at the C-4 position along with a hydroxyl group at the C-5 position rather than the carbonyl group seen in curcumin (154). When tested against P. gingivalis, A. viscosus, and S. mutans, quantum curcumin (155) displayed excellent activity with MIC values of 1.114 μM (0.42 μg mL−1), 2.228 μM (0.82 μg mL−1), and 8.913 μM (3.28 μg mL−1), respectively. These MIC values were much better than those of the control MTZ with respective MIC values of 1.46 mM, 2.921 mM, and 11.68 mM. In anti-biofilm studies, quantum curcumin (155) also displayed low MBIC50 and MBIC90 values in the range of 0.557 to >17.826 μM. A total of 11 clinical isolates of P. gingivalis were treated with quantum curcumin (155) and excellent MIC values were observed in the range of 0.557–8.913 μM. Mechanism of action studies revealed that quantum curcumin (155) inhibits Arg- and Lys-specific proteinases produced by P. gingivalis at 17.826 μM with 99% and 89% inhibition, respectively. Inhibition of gingipains is important as these are virulence factors that contribute to the progression of periodontal diseases. In cytotoxicity studies, quantum curcumin (155) was found to be non-toxic as no hemolysis against human RBCs was observed at concentrations up to 100 μM and no cell leakage was observed in cell viability assays using Vero (kidney) cells. With their excellent activity against P. gingivalis, mechanism of action revealed, and low toxicity, these highly promising compounds should be further studied in animal models.
Small signaling molecules called autoinducers mediate the cell-to-cell communication known as quorum sensing (discussed in section 1.2). The macroalga Delisea pulchra produces brominated furanones such as 3-(dibromomethylene)isobenzofuran-1(3H)-one (156a), which was derivatized in one to five steps using a Ramirez olefination reaction137 to yield 14 compounds that were tested for antibacterial activity. These compounds were also tested for inhibition of F. nucleatum, P. gingivalis, and T. forsythia biofilm formation by inhibiting autoinducer-2 (AI-2), which is secreted by F. nucleatum (156–168, Fig. 14).138 Replacement of the fused aromatic ring of the bicyclic structure with fused cyclohexyl or cyclopentyl groups along with substitution of the fused benzene ring with methyl and alkoxy groups with varying chain lengths afforded the three most active compounds, 167 (fused cyclopentyl group in place of the fused aromatic ring), 157a (methyl group at the 5-position), and 162a (methyl group at the 7-position). Unfortunately, none of the compounds inhibited the growth of planktonic F. nucleatum, P. gingivalis, or T. forsythia. However, compounds 157a, 162a, and 167 inhibited a little over 40% of F. nucleatum biofilm formation at 2 μM. Once pathogenic oral bacterial species colonize the biofilm, they secrete small signaling molecules that enable them to communicate with other bacterial species to increase biofilm production. By inhibiting these signaling molecules the bacteria can no longer communicate with one another and the biofilm formation is reduced. The mechanism of action of 157a, 162a, and 167 was determined to be inhibition of AI-2 activity, as the compounds inhibited ∼20–50% of activity at 2 μM (0.59–0.64 μg mL−1). The compounds also inhibited biofilm formation induced by F. nucleatum AI-2 in biofilms of F. nucleatum, P. gingivalis, and T. forsythia at 2 μM. In cytotoxicity studies, compounds 157a, 162a, and 167 displayed no cytotoxicity in human monocytic cell line THP-1, human gingival fibroblasts, and human oral keratinocyte cell line HOK-16B at 2 μM. Additionally, these three compounds did not induce an inflammatory response in THP-1 cells or human gingival fibroblasts. Compounds 157a, 162a, and 167 should be further investigated for their potential ability to control biofilm formation in animal models.
Gram-negative bacteria produce autoinducers, such as the AI-2 produced by F. nucleatum as well as the N-acyl homoserine lactone (HSL) produced by P. gingivalis, that contribute to the progression of periodontitis through the production of virulence factors and biofilm formation. The previously synthesized derivative of N-acyl HSL,139 compound 169, was used in combination with cefuroxime, ofloxacin, and MIN to study the effect on P. gingivalis biofilm disruption (Fig. 14).140 Alone, compound 169 was not active against P. gingivalis even at a concentration of 100 μM (20.4 μg mL−1). However, when compound 169 was used in combination with the known antibiotics cefuroxime, MIN, and ofloxacin, there was a synergistic effect that allowed for a much better biofilm disruption, as established by an ATP bioluminescence assay. The best combination that reduced the viability P. gingivalis cells in biofilms was compound 169 with cefuroxime, followed by MIN, and then by ofloxacin.
Inhibition of P. gingivalis biofilm formation was also investigated in a study where three active compounds, 170–172, were selected from screening 506 derivatives of oroidin (Fig. 14).141 Oroidin, which belongs to a class of marine alkaloids, is a secondary metabolite from the marine sponge Agelas conifera. The most active compounds 170–172 contained 2-aminoimidazole in their structures. Compounds 170–172 reduced the levels of P. gingivalis without affecting S. gordonii. In multi-species biofilms comprising P. gingivalis and S. gordonii, the most active compound, 170, displayed excellent IC50 values of 3.41 ± 0.92 μM against P. gingivalis biofilms. The biovolume of P. gingivalis was reduced by 90% at 20 μM and 40% at 2.5 μM with the important aspect of not affecting the commensal beneficial species S. gordonii. As the compounds did not inhibit the growth of P. gingivalis, the mechanism of action was investigated to determine if the compounds affected community mediators involved in the formation of multi-species biofilms. Compounds 170–172 reduced the mRNA levels of fimA and mfa1, which reduce the fimbrial adhesins Mfa1 and FimA to inhibit the ability of P. gingivalis to attach to the early colonizer S. gordonii. As the most active compound 170 showed exciting data as an inhibitor of the initial adherence of P. gingivalis to S. gordonii, it should be further tested in mature biofilm models to determine if it can still be as effective in a more complex system.
In sum, derivatives of natural products were generally more active against P. gingivalis than the parent compound from which they were derived. Not all derivatives inhibited the growth of P. gingivalis. The scaffolds containing brominated furones, HSL, and alkaloids with 2-aminoimidazoles inhibited P. gingivalis biofilm formation. When tested against oral bacterial species including P. gingivalis, the most active compounds were meroterpene derivatives, followed by curcumin, pimarane-type diterpenes, pentacyclic triterpenes, triterpenes, and flavonoids. Out of the 16 semi-synthetic meroterpene derivatives, nine displayed the best activity and should be further investigated in cytotoxicity studies.
3.3.2. Essential oils and their derivatives
Essential oils are extracts that contain many natural products that alone may not inhibit the growth of P. gingivalis or other oral bacterial species, but together in a mixture they may display synergistic activity against the targeted pathogen. Essential oils are traditional herbal medicine that display antibacterial activity against oral bacterial species and could be used to isolate active compounds within the extracts to determine the biologically active compounds that could be used to treat periodontitis. Essential oils and their derivatives will be presented in the two following subsections: (i) extracts and/or isolated compounds from various plants and (ii) purchased extracts and/or individual compounds tested against oral bacterial species. Compounds shown in these sections are representative structures of some of the many compounds isolated and purchased.
3.3.2.1. Extracts and/or isolated compounds from various plants
Cinnamon bark essential oil (CBEO) originally extracted from the inner bark of trees from the Sri Lankan cinnamon, Cinnamomum zeylanicum, contains 51 components that could be tested against P. gingivalis.142 In a thorough study, the major known constituents cinnamaldehyde (173), eugenol (174), and β-caryophyllene (175), as well as a minor component, β-pinene (176), were tested for anti-P. gingivalis activity (Fig. 15). When tested against P. gingivalis, cinnamaldehyde (173) displayed excellent activity with an MIC value of 2.5 μM (0.33 μg mL−1), which was better than those of CBEO (MIC = 6.25 μg mL−1) and the control tinidazole, a synthetic nitroimidazole antiprotozoal agent (MIC = 7.8 μM). In mechanism of action studies, CBEO and cinnamaldehyde (173) caused membrane permeabilization of P. gingivalis cells and caused the loss of DNA, RNA, and protein from cells resulting in cell death. P. gingivalis biofilm formation was inhibited with CBEO at 4.17 μg mL−1 by 74.5% and with cinnamaldehyde (173) at 1.67 μg mL−1 by 67.3%. Only slight inhibition of preformed P. gingivalis biofilm growth was displayed with CBEO, and cinnamaldehyde (173) displayed no inhibition. The major component of CBEO, cinnamaldehyde (173), shows great promise with low MIC values, but it should be tested in combination with an antibiotic that can disrupt preformed biofilms of P. gingivalis as it is not a viable option to kill P. gingivalis in mature biofilms on its own.
Fig. 15. Eight scaffolds as representative examples of compounds isolated from various plants that were tested as potential antibacterial agents against oral bacterial species.
In a preliminary investigation, compounds were isolated from the oleoresin of the tropical rainforest tree Copaifera langsdorffii and tested against P. gingivalis (Fig. 15).143 A total of 12 g of oleoresin was used to isolate four known diterpenes, (−)-copalic acid (177), (−)-acetoxycopalic acid (178), (−)-hydroxycopalic acid (179), and (−)-agathic acid (180), as well as two purchased diterpenes, sclareol (181) and manool (182). When tested against A. naeslundii, P. gingivalis, and P. anaerobius, (−)-copalic acid (177) displayed the best activity with MIC values of 6.2 μg mL−1, 3.1 μg mL−1, and 3.1 μg mL−1, respectively, which were similar or worse than those of the control chlorhexidine dihydrochloride (MIC = 1.8, 0.9, and 7.4 μg mL−1, respectively). All compounds displayed little to no activity against Bacteroides fragilis, Bacteroides thetaiotaomicron, F. nucleatum, and P. nigrescens. In time-kill assays, 177 displayed bactericidal activity at 3.1 μg mL−1, which was 1× MBC against P. gingivalis. In cytotoxicity studies, 177 displayed no toxicity in a human fibroblast cell line up to 62.5 μM. With its low MIC values and good bactericidal activity, (−)-copalic acid (177) warrants further investigation in P. gingivalis biofilm studies.
Just as CBEO was extracted from tree bark, essential oils isolated from the roots of trees were tested against P. gingivalis. In a study that unfortunately yielded inactive compounds against P. gingivalis, Miswak essential oil was extracted from chewing sticks called Miswak from Salvadora persica, which was nicknamed “the toothbrush tree” as the chewing sticks can be used as a natural toothbrush.144 When used as a toothbrush, the chewing sticks releases the major component benzyl isothiocyanate (183) and minor components benzyl cyanide (184) and benzaldehyde (185, Fig. 15). When tested against Haemophilus influenzae, A. actinomycetemcomitans, and P. gingivalis, low activity was seen with a dose dependent response of Miswak essential oil and benzyl isothiocyanate (183) inhibiting 100% of bacterial growth with CFU at 1170 μg mL−1, worse than what was observed with the control chlorhexidine (100% inhibition at 500 μg mL−1). Benzyl cyanide (184) and benzaldehyde (185) were found to be inactive. Due to their inactivity, these compounds should not be further investigated for anti-P. gingivalis activity.
Overall, the isolated compounds comprising cinnamaldehyde and the diterpenes showed great promise as anti-P. gingivalis agents. These compounds should, respectively, be further investigated in combination studies with known antibacterial agents and anti-biofilm studies.
3.3.2.2. Purchased extracts and/or individual compounds
Natural product essential oils extracted from plants are commonly used today and they are readily available for purchase along with their major constituents. Essential oils are extracted from various parts of plants such as the leaves and peel of the Thai lime Citrus hystrix de Candolle. In a study, the leaf and peel oil of Citrus hystrix as well as the terpenes citronellal (186), linalool (187), and pinene (188) were purchased and tested for their antibacterial activity (Fig. 16).145 Unfortunately, the leaf oil, peel oil, and major components were found to be inactive when tested against P. gingivalis, S. sanguinis, and S. mutans. When tested against P. gingivalis biofilm formation, leaf oil displayed 99% inhibition at 4.25 mg mL−1 (4250 μg mL−1). Due to their inactivity against oral bacterial species, these compounds should not be further pursued.
Fig. 16. Seven scaffolds as representative examples of purchased compounds that were tested as potential antibacterial agents against oral bacterial species.
In another study, clove essential oil, originally extracted from the flower buds of Eugenia caryophyllata L., was purchased along with its major components, eugenol (174) and β-caryophyllene (175), and these were tested for antibacterial activity (Fig. 15 and 16).146 When tested against 11 oral bacterial species comprising S. mutans, S. sanguinis, S. sorbinus, Streptococcus ratti, Streptococcus criceti, Streptococcus anginosus, S. gordonii, A. actinomycetemcomitans, F. nucleatum, P. intermedia, and P. gingivalis, clove essential oil, eugenol (174), and β-caryophyllene (175) were all found to be inactive with the lowest MIC value observed being 100 μg mL−1. In combination studies using checkerboard and time-kill assays, there was a slight improvement with activity of clove oil and eugenol (174) in combination with ampicillin and gentamicin with ≥4-fold reduction. These compounds could potentially be used in combination with known antibiotics to improve their activity against P. gingivalis as they are inactive when tested alone.
The major components of essential oils can come from many different sources, as seen with eugenol (174) and β-caryophyllene (175) from CBEO and clove oil. In a study that yielded inactive compounds, the essential oils of Orthosiphon stamineus Benth and Ficus deltoidea Jack and their components eugenol (174), β-caryophyllene (175), α-humulene (189), and germacrene D (190) were tested alone and in combination with AMX (Fig. 16).147 When tested against E. faecalis, S. mutans, S. mitis, S. salivarius, A. actinomycetemcomitans, P. gingivalis, and F. nucleatum, the essential oils and their components were found to be inactive with the lowest MIC value observed being 630 μg mL−1. These values were much worse than that of the control AMX (MIC = 50 μg mL−1). In combination studies, O. stamineus and F. deltoidea essential oils displayed an additive effect with AMX with a 2-fold reduction in MIC values in all bacterial species tested. In general, due to their inactivity, the purchased extracts and compounds described in this section should not be further pursued.
3.3.3. Inactive natural products
Investigations into natural product scaffolds that are inactive as antibacterial agents are rarely published in the scientific literature. However, these studies provide valuable information as to which scaffolds should be avoided leading to more productive research by focusing on those that show promise. In the studies described in this section, there were many natural products tested that were inactive, therefore, the scaffolds below are representative scaffolds. This section provides 13 representative natural products that were synthetized, isolated, or purchased that were found to be inactive against oral bacterial species such as P. gingivalis (Fig. 17).
Fig. 17. Thirteen scaffolds as representative examples of compounds that were tested as potential antibacterial agents and found to be inactive against oral bacterial species.
The peel of the lemon citrus fruit contains four natural products that were isolated and tested against S. mutans, P. intermedia, and P. gingivalis. These compounds were inactive against the bacterial species with 5-geranyloxypsolaren (191, Fig. 17) that displayed the best MIC value of 0.15 mM (51 μg mL−1) against S. mutans and P. gingivalis.148 The effects of resveratrol (192, Fig. 17), which is a natural product originally found in grape skin used as a dietary supplement, was purchased and thoroughly evaluated in an investigation with planktonic P. gingivalis, inhibition of biofilm formation studies, cytotoxicity assays, and membrane permeabilization assays.149 When tested against P. gingivalis, resveratrol (192) displayed no activity with an MIC value of 250 μg mL−1 and an MBC value of 500 μg mL−1. The evaluation of the crude extract and four fractions (chloroform, hexane, ethanol, and butanol) isolated from the aerial parts (leaves and soft stem) of the herb Phytolacca americana displayed no activity against P. gingivalis, S. mutans, and E. coli.150 The crude extract of P. americana displayed 100% growth inhibition of P. gingivalis at 1800 μg mL−1. The major constituents of P. americana were purchased and found to be inactive with the exception of kaempferol (193, Fig. 17) that displayed 84% growth inhibition of P. gingivalis at 8 μg mL−1. The tea catechin EGCG (194, Fig. 17) was purchased and evaluated against planktonic P. gingivalis, preformed biofilms, and established biofilms of P. gingivalis.151 EGCG (194) was found to be inactive with an MIC value of 500 μg mL−1 against planktonic P. gingivalis. Similar results were displayed in a separate study where green tea extracts and EGCG (194) were tested against three strains of P. gingivalis and were found to be inactive.152
The synthesis and evaluation of 10 known alcohol derivatives and 10 known ester derivatives of 3-(4′-geranyloxy-3′-methoxyphenyl)-2-trans propenoic acid (195, Fig. 17), which was originally isolated from the bark of Acronychia baueri Schott, against biofilm formation of P. gingivalis and S. mutans produced inactive compounds.153 The parent compound 195 displayed 80% inhibition of P. gingivalis biofilm formation at 31.3 μg mL−1 (78.1 μM). In another study, the pentacyclic triterpenoids oleanolic acid (96) and ursolic acid (125) were tested against a wide variety of bacterial strains (Fig. 17).154 Although oleanolic acid (96) was inactive against P. gingivalis with an MIC value of 625 μg mL−1, both oleanolic acid (96) and ursolic acid (125) displayed excellent activity against M. tuberculosis (MIC = 2.5 μg mL−1). A black tea extract and two major components, including theaflavin (196, Fig. 17), were found to be completely inactive against P. gingivalis, P. intermedia, F. nucleatum, and A. actinomycetemcomitans (MIC values in the range of 125–2000 μg mL−1).155 A separate in-depth study focused on the inactive theaflavin (196) investigated its activity against planktonic P. gingivalis, biofilm formation, biofilm reduction, biofilm viability, cytotoxicity in human gingival fibroblasts, and inhibition of virulence factors produced by P. gingivalis such as gingipains and collagenases.156 It is of no surprise that theaflavin (196) in this later study displayed no antibacterial activity with an MIC value of 125 μg mL−1 against planktonic P. gingivalis. Originally isolated from the root of Scutellaria baicalensis Georgi, the flavonoid baicalein (197, Fig. 17) was tested against 15 oral bacterial species, including P. gingivalis, and was found to be in active with MIC values in the range of 80–320 μg mL−1.157 Isolated from the mushroom Antrodia camphorate, the ethanol, EtOAc, chloroform, and water extracts as well as its three major components, 4,7-dimethoxy-5-methyl-1,3-benzodioxole (198), dehydrosulphurenic acid (199), and dehydroeburicoic acid (200), were tested for antibacterial activity (Fig. 17).158 When tested against P. gingivalis and S. mutans, compounds 198–200 were found to be inactive while the chloroform, EtOAc, and 95% ethanol extracts displayed good antibacterial activity with MIC values in the range of 4–16 μg mL−1. In another study that evaluated the polyphenol myricetin (201, Fig. 17) against four P. gingivalis strains, it was found that myricetin (201) was inactive with MIC values in the range of 62.5–125 μg mL−1.159
3.4. Overall summary of all molecules discussed in this review
A table (Table 3) summarizing all the data that were discussed in this review is presented to provide readers with a quick reference summary that can be used to identify promising compounds they might be interested in pursuing or learning more about.
Summary of activity of representative compounds presented in this review that were tested against P. gingivalis.
| Section 3.1. Novel compounds | ||||||
|---|---|---|---|---|---|---|
| Family | Cpd # | Inhibition of P. gingivalis growtha | Inhibition of P. gingivalis virulence factorsb | Anti-biofilm activityb | Cytotoxicityb | Appropriate controlsb |
| 3.1.1. Triazoles | 1–25 c | ✗ | YES: binds Mfa of P. gingivalis | Disrupt preformed biofilms and inhibited P. gingivalis incorporation into S. gordonii and F. nucleatum biofilms | NT: TIGK, J774A.1, and sheep RBCs | NC |
| 26–27 e | ✗ | NO: not active against fimbriated strain | N/A | T: HeLa | YES (ERY, MTZ, TET, and gentamicin) | |
| 3.1.2. Heterocyclic | 28 c | ✓ | N/A | N/A | N/A | NO (curcumin, parthenolide, quercetin, EGCG, and resveratrol) |
| 3.1.3. Bicyclic | 29–42 d | ✓ | N/A | N/A | N/A | YES (chlorhexidine) |
| 43–51 e | ✗ | N/A | N/A | N/A | YES (MTZ) | |
| 52 c | ✓ | YES: binds LPSs from P. gingivalis | N/A | N/A | NC | |
| 3.1.4. Sulfonamides | 53–61 c | N/A | YES: inhibits P. gingivalis γ-CAs | N/A | N/A | NO (acetazolamide) |
| 62 e | ✗ | NO: not active against meso-2,6-diaminopimelate dehydrogenase from P. gingivalis | N/A | N/A | NC | |
| 63–73 c | ✓ | N/A | N/A | NT: OKF6 | YES (TET and ZAF) | |
| 74–88 c | ✓ | N/A | N/A | NT: OKF6 | YES (TET and ZAF) | |
| Section 3.2. Drug repositioning | ||||||
|---|---|---|---|---|---|---|
| Family | Cpd # | Inhibition of P. gingivalis growtha | Inhibition of P. gingivalis virulence factorsb | Anti-biofilm activityb | Cytotoxicityb | Appropriate controlsb |
| 89 c | ✓ | YES: binds LPSs from P. gingivalis | Inhibited P. gingivalis and S. mutans biofilms | T: human oral gingival epithelial cells | YES (TET, chlorhexidine, rifampin, triclosan, ciprofloxacin, and melittin) | |
| NT: horse RBCs | ||||||
| 90 e | ✗ | NO: low affinity towards P. gingivalis DPP4 | N/A | N/A | NC | |
| 91 d | ✗ | YES: binds GroEL from P. gingivalis | N/A | N/A | NO (pentamidine and benzamidine) | |
| Section 3.3. Natural products | ||||||
|---|---|---|---|---|---|---|
| Family | Cpd # | Inhibition of P. gingivalis growtha | Inhibition of P. gingivalis virulence factorsb | Anti-biofilm activityb | Cytotoxicityb | Appropriate controlsb |
| 3.3.1. Natural products and their derivatives | 92 c | ✓ | N/A | N/A | N/A | YES (chlorhexidine gluconate) |
| 93–94 c | ✓ | N/A | N/A | N/A | NC | |
| 95–97 c | ✓ | N/A | N/A | N/A | NC | |
| 98 d | ✓ | N/A | N/A | N/A | YES (chlorhexidine gluconate) | |
| 99–100 c | ✓ | N/A | N/A | N/A | YES (penicillin G) | |
| 101 c | ✓ | N/A | Inhibited biofilm growth and disrupted preformed biofilms of S. mutans and A. viscosus | N/A | NO (triclosan) | |
| 102 c | ✓ | YES: inhibited gingipains Rgp and Kgp from P. gingivalis | Slight inhibition of P. gingivalis biofilm formation | N/A | NC | |
| 103–104 d | ✓ | N/A | N/A | N/A | YES (penicillin G) | |
| 105–106 c | ✓ | YES: reduced alveolar bone loss and inflammation induced by LPSs from P. gingivalis | Inhibited biofilm growth and disrupted preformed biofilms of P. gingivalis | NT: human periodontal ligament cells or monocyte-like THP-1 cells | NC | |
| 107–108 c | ✓ | YES: inhibited adherence of P. gingivalis to GMSM-K cells, inhibited activity against collagenase, and reduced LPS-induced secretion of IL-1β, TNF-α, and IL-8 | Disrupted preformed biofilms of P. gingivalis | N/A | NC | |
| NO: not as active against MMP-9 | ||||||
| 109–110 d | ✗ | YES: inhibited adherence of P. gingivalis to GMSM-K cells, inhibited MMP-9 activity, and reduced secretion of TNF-α and IL-8 | No inhibition of P. gingivalis biofilm formation | N/A | NC | |
| NO: no inhibition of P. gingivalis collagenase activity | ||||||
| 111–116 c | ✓ | N/A | N/A | N/A | YES (chlorhexidine gluconate) | |
| 117–124 c | ✓ | N/A | N/A | N/A | YES (chlorhexidine) | |
| 125–131 d | ✓ | N/A | N/A | N/A | YES (chlorhexidine) | |
| 132–136 c | ✓ | N/A | N/A | N/A | YES (chlorhexidine dihydrochloride) | |
| 137–153 c | ✓ | N/A | Inhibited growth of single-species biofilms of S. mutans along with multi-species biofilms (containing S. mutans, A. viscosus, F. nucleatum, and P. gingivalis) | N/A | NO (triclosan) | |
| 154–155 c | ✓ | YES: inhibited P. gingivalis gingipains | Inhibited biofilm formation of P. gingivalis and A. viscosus | NT: human RBCs and Vero cells | YES (MTZ) | |
| 156–168 c | ✗ | YES: inhibited AI-2 activity | Inhibited biofilm formation of F. nucleatum | NT: THP-1, human gingival fibroblasts, and HOK-16B | NO ((Z)-4-bromo-5-(bromomethylene)furan-2(5H)-one) | |
| 169 d | ✗ | N/A | Disrupted P. gingivalis biofilms in combination with other known antibiotics | N/A | YES (cefuroxime, ofloxacin, and MIN) | |
| 170–172 c | ✗ | YES: reduced mRNA levels of fimA and mfa1 | Inhibited the ability of P. gingivalis to attach to S. gordonii | N/A | NC | |
| 3.3.2. Essential oils and their derivatives | 173–176 c | ✓ | N/A | Inhibited biofilm formation of P. gingivalis and slightly inhibited preformed biofilm growth | N/A | NO (CBEO and tinidazole) |
| 177–181 c | ✓ | N/A | N/A | NT: human fibroblast cells | YES (chlorhexidine dihydrochloride) | |
| 183–185 e | ✗ | N/A | N/A | T: gingival fibroblasts | YES (chlorhexidine) | |
| NT: oral keratinocytes | ||||||
| 186–188 e | ✗ | N/A | Not active against P. gingivalis biofilms | N/A | YES (chlorhexidine) | |
| 189–190 e | ✗ | N/A | Not active against P. gingivalis biofilms | N/A | YES (AMX) | |
✓ indicates active below 49 μg mL−1 for the best representative compounds in the series as determined by either MIC, CFUs, or % growth inhibition. ✗ indicates not active (>50 μg mL−1) for the best representative compounds in the series as determined by either MIC, CFUs, or % growth inhibition.
N/A indicates not available for the best representative compounds in the series. NT indicates not toxic for the best representative compounds in the series. T indicates toxic for the best representative compounds in the series. NC indicates no control used for comparison. YES indicates that an appropriate antibacterial/antiseptic agent FDA-approved for periodontal therapy was used for comparison. NO indicates that either an antibacterial agent approved for indications other than periodontal therapy or not approved by the FDA for any indications was used for comparison.
Indicates compounds that displayed good anti-P. gingivalis activity as identified by growth inhibition, virulence factors inhibition, and/or anti-biofilm activity.
Indicates compounds that displayed moderate anti-P. gingivalis activity as identified by growth inhibition, virulence factors inhibition, and/or anti-biofilm activity.
Indicates compounds that displayed poor anti-P. gingivalis activity as identified by growth inhibition, virulence factors inhibition, and/or anti-biofilm activity.
Overall conclusion and perspective
Periodontal diseases can be treated with noninvasive techniques using antibacterial agents as adjunctive therapy to mechanical debridement. The discovery and use of penicillin first introduced into the market in 1942, revolutionized modern medicine and drug development. Nevertheless, it is imperative for researchers to develop novel antibacterial agents as many of the ones currently used for the treatment of periodontal diseases are broad-spectrum and bacterial species are continuously developing increased resistance to them.50 We encourage our colleagues to pursue novel targeted periodontal therapy as broad-spectrum antibiotics can lead to the disruption of the oral bacterial homeostasis leading to dysbiosis and chronic inflammatory disease.160 Targeted antibacterial therapy will be able to prevent disruption of the normal abundance of commensal bacterial communities, while eradicating specifically periodontopathogens such as P. gingivalis.
As targeting specific oral pathogenic bacterial species within biofilm structures is more difficult, the development of novel compounds active against P. gingivalis are in early stages of investigation. The early stages of development for these compounds generally focus on the inhibition of bacterial growth with MIC testing, percent growth inhibition assays, and CFU assays against planktonic oral bacterial species. Several of the small molecules either novel or known discussed in this review show great promise as anti-P. gingivalis agents. However, it is crucial to develop future studies using not only single- but multi-species biofilms, testing for drug resistance in planktonic bacteria and biofilms, cytotoxicity in oral epithelial cells, and testing new compounds in animal models of periodontal diseases. Researchers should also investigate the mechanism of action of these compounds as they may not inhibit growth of the bacteria, but they may be very active as inhibitors of bacterial virulence factors. This could be used as novel therapy as it could stop the progression of periodontal diseases if the virulence of pathogenic bacteria could be blocked. As many of these investigations are in their preliminary stages, it is difficult to conclusively say what type(s) of scaffolds should be used in the clinic in place of the currently used antibacterial agents for the treatment of periodontal diseases. Nevertheless, we hope this review will help guide researchers in a logical direction for in-depth studies based on the summary of the types of scaffolds that have so far demonstrated great targeted activity against P. gingivalis. Of note, many of the investigations are limited due to the use of inappropriate controls. At least one antibacterial agent currently used in the clinic for periodontal therapy should always be used as a control in the experiments to determine the novel compounds true potential as an antibacterial agent. Importantly, the use of future anti-P. gingivalis agents should consider their potential for topical use in addition to their systemic use to ensure that there is a high concentration of the treatment at the site of the infection.
Abbreviations
- AI-2
Autoinducer-2
- AKBA
Acetyl-11-keto-β-boswellic acid
- AMX
Amoxicillin
- ATP
Adenosine triphosphate
- AZM
azithromycin
- γ-CA
Carbonic anhydrases
- CBEO
Cinnamon bark essential oil
- CFU
Colony forming unit
- CIP
Ciprofloxacin
- CLR
Clarithromycin
- CO2
Carbon dioxide
- CSA
Cationic steroid antimicrobial
- DOX
Doxycycline
- DPP4
Dipeptidyl peptidase 4
- ECM
Extracellular matrix
- EGCG
Epigallocatechin gallate
- ERY
Erythromycin
- EtOAc
Ethyl acetate
- GMSM-K
Immortalized human oral epithelial cell line
- H2O2
Hydrogen peroxide
- HeLa
Human cervical cancer
- HOK-16B
Human oral keratinocyte cells line
- HPLC
High-performance liquid chromatography
- HSL
Homoserine lactone
- IL
Interleukin
- J774A.1
Murine macrophage
- Kgp
Lys-gingipains
- Ki
Inhibition constant
- LPS
Lipopolysaccharide
- MBC
Minimum bactericidal concentration
- MBIC
Minimum biofilm-inhibitory concentration
- MBRC
Minimum biofilm reduction concentration
- Mfa
Minor fimbrial antigen
- MIC
Minimum inhibitory concentration
- MIN
Minocycline
- MMP-9
Matrix metallopeptidase 9
- MTZ
Metronidazole
- OKF6
Immortalized human oral keratinocyte
- PDTC
Pyrrolidine dithiocarbamate
- RBC
Red blood cell
- Rgp
Arg-gingipains
- SAR
Structure–activity relationship
- SMIC
Sessile minimum inhibitory concentration
- TET
Tetracycline
- THP-1
Human monocytic cell line
- TIGK
Telomerase immortalized human gingival keratinocytes
- ZAF
Zafirlukast
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This work was supported by a National Institutes of Health (NIH) F31 fellowship DEO29661 (to K. C. H.).
References
- Harvey J. D. Periodontal Microbiology. Dent. Clin. North Am. 2017;61:253–269. doi: 10.1016/j.cden.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Loe H. Theilade E. Jensen S. B. Experimental gingivitis in man. J. Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Zhu B. Macleod L. C. Newsome E. Liu J. Xu P. Aggregatibacter actinomycetemcomitans mediates protection of Porphyromonas gingivalis from Streptococcus sanguinis hydrogen peroxide production in multi-species biofilms. Sci. Rep. 2019;9:4944. doi: 10.1038/s41598-019-41467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke P. I. Dye B. A. Wei L. Thornton-Evans G. O. Genco R. J. CDC Periodontal Disease Surveillance workgroup Beck J. Page G. D. R. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Andrian E. Grenier D. Rouabhia M. In vitro models of tissue penetration and destruction by Porphyromonas gingivalis. Infect. Immun. 2004;72:4689–4698. doi: 10.1128/IAI.72.8.4689-4698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front. Biosci. 2007;12:3965–3974. doi: 10.2741/2363. [DOI] [PubMed] [Google Scholar]
- Popova C. Dosseva-Panova V. Panov V. Microbiology of periodontal diseases. A review. Biotechnol. Biotechnol. Equip. 2013;27:3754–3759. doi: 10.5504/BBEQ.2013.0027. [DOI] [Google Scholar]
- Geng F. Liu J. Guo Y. Li C. Wang H. Wang H. Zhao H. Pan Y. Persistent exposure to Porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front. Cell. Infect. Microbiol. 2017;7:57. doi: 10.3389/fcimb.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. J. France J. Crean S. Singhrao S. K. The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to Alzheimer's disease, diabetes and cardiovascular diseases. Front. Aging Neurosci. 2017;9:408. doi: 10.3389/fnagi.2017.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcali A. Bostanci N. Ozcaka O. Ozturk-Ceyhan B. Gumus P. Buduneli N. Belibasakis G. N. Association between polycystic ovary syndrome, oral microbiota and systemic antibody responses. PLoS One. 2014;9:e108074. doi: 10.1371/journal.pone.0108074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka M. Kazar J. Pijak M. R. Gasparovic J. Wsolova L. Mongiellova V. The importance of the presence of Aggregatibacter actinomycetemcomitans in sulcus gingivalis of patients with cardiovascular diseases. Med. Sci. Monit. 2011;17:CR646–CR649. doi: 10.12659/MSM.882050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy S. S. Lynch C. Ermini F. Benedyk M. Marczyk A. Konradi A. Nguyen M. Haditsch U. Raha D. Griffin C. Holsinger L. J. Arastu-Kapur S. Kaba S. Lee A. Ryder M. I. Potempa B. Mydel P. Hellvard A. Adamowicz K. Hasturk H. Walker G. D. Reynolds E. C. Faull R. L. M. Curtis M. A. Dragunow M. Potempa J. Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019;5:eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli S. S. Rivera-Kweh M. F. Velsko I. M. Chen H. Zheng D. Bhattacharyya I. Gangula P. R. Lucas A. R. Kesavalu L. Chronic oral infection with major periodontal bacteria Tannerella forsythia modulates systemic atherosclerosis risk factors and inflammatory markers. Pathog. Dis. 2015;73:ftv009. doi: 10.1093/femspd/ftv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvikar S. L. Collier D. S. Fisher M. C. Unizony S. Cohen G. L. McHugh G. Kawai T. Strle K. Steere A. C. Clinical correlations with Porphyromonas gingivalis antibody responses in patients with early rheumatoid arthritis. Arthritis Res. Ther. 2013;15:R109. doi: 10.1186/ar4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J. Mydel P. Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:606–620. doi: 10.1038/nrrheum.2017.132. [DOI] [PubMed] [Google Scholar]
- Caton J. G. Armitage G. Berglundh T. Chapple I. L. C. Jepsen S. Kornman K. S. Mealey B. L. Papapanou P. N. Sanz M. Tonetti M. S. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018;45(Suppl 20):S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- Huang R. Li M. Gregory R. L. Bacterial interactions in dental biofilm. Virulence. 2011;2:435–444. doi: 10.4161/viru.2.5.16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz P. I. Kolenbrander P. E. Subgingival biofilm communities in health and disease. Revista Clínica De Periodoncia, Implantología Y Rehabilitación Oral. 2009;2:187–192. doi: 10.1016/S0718-5391(09)70033-3. [DOI] [Google Scholar]
- Zhu B. Macleod L. C. Kitten T. Xu P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018;13:915–932. doi: 10.2217/fmb-2018-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valen H. Scheie A. A. Biofilms and their properties. Eur. J. Oral Sci. 2018;126(Suppl 1):13–18. doi: 10.1111/eos.12425. [DOI] [PubMed] [Google Scholar]
- Saini R. Saini S. Sharma S. Biofilm: A dental microbial infection. J. Nat. Sci., Biol. Med. 2011;2:71–75. doi: 10.4103/0976-9668.82317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X. Rodriguez R. Trinh M. Gunsolley J. Xu P. Oral microbiome of deep and shallow dental pockets in chronic periodontitis. PLoS One. 2013;8:e65520. doi: 10.1371/journal.pone.0065520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könönen E. Gürsoy M. Subgingival distribution of microorganisms. Curr Oral Health Rep. 2014;1:262–271. doi: 10.1007/s40496-014-0033-8. [DOI] [Google Scholar]
- Zhu Y. Dashper S. G. Chen Y. Y. Crawford S. Slakeski N. Reynolds E. C. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One. 2013;8:e71727. doi: 10.1371/journal.pone.0071727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen T. Fiehn N. E. Dental biofilm infections - An update. APMIS. 2017;125:376–384. doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Haffajee A. D. Cugini M. A. Smith C. Kent Jr. R. L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Diaz P. I. Zilm P. S. Rogers A. H. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology. 2002;148:467–472. doi: 10.1099/00221287-148-2-467. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Amano A. Nakagawa I. Hamada S. Specific interactions between Porphyromonas gingivalis fimbriae and human extracellular matrix proteins. FEMS Microbiol. Lett. 1999;175:267–272. doi: 10.1111/j.1574-6968.1999.tb13630.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E. Palmer Jr. R. J. Rickard A. H. Jakubovics N. S. Chalmers N. I. Diaz P. I. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Mark Welch J. L. Rossetti B. J. Rieken C. W. Dewhirst F. E. Borisy G. G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E791–E800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H. M. Slakeski N. Butler C. A. Veith P. D. Chen Y. Y. Liu S. W. Hoffmann B. Dashper S. G. Reynolds E. C. The role of Treponema denticola motility in synergistic biofilm formation with Porphyromonas gingivalis. Front. Cell. Infect. Microbiol. 2019;9:432. doi: 10.3389/fcimb.2019.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Liang S. Payne M. A. Hashim A. Jotwani R. Eskan M. A. McIntosh M. L. Alsam A. Kirkwood K. L. Lambris J. D. Darveau R. P. Curtis M. A. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Darveau R. P. Curtis M. A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano A. Molecular interaction of Porphyromonas gingivalis with host cells: Implication for the microbial pathogenesis of periodontal disease. J. Periodontol. 2003;74:90–96. doi: 10.1902/jop.2003.74.1.90. [DOI] [PubMed] [Google Scholar]
- Liu H. Sun J. Dong Y. Lu H. Zhou H. Hansen B. F. Song X. Periodontal health and relative quantity of subgingival Porphyromonas gingivalis during orthodontic treatment. Angle Orthod. 2011;81:609–615. doi: 10.2319/082310-352.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez G. A. Acquier A. B. De Couto A. Busch L. Mendez C. F. Association between Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis in subgingival plaque and clinical parameters, in Argentine patients with aggressive periodontitis. Microb. Pathog. 2015;82:31–36. doi: 10.1016/j.micpath.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Nakagawa I. Amano A. Inaba H. Kawai S. Hamada S. Inhibitory effects of Porphyromonas gingivalis fimbriae on interactions between extracellular matrix proteins and cellular integrins. Microbes Infect. 2005;7:157–163. doi: 10.1016/j.micinf.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Vielkind P. Jentsch H. Eschrich K. Rodloff A. C. Stingu C. S. Prevalence of Actinomyces spp. in patients with chronic periodontitis. Int. J. Med. Microbiol. 2015;305:682–688. doi: 10.1016/j.ijmm.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Rams T. E. Feik D. Mortensen J. E. Degener J. E. van Winkelhoff A. J. Antibiotic susceptibility of periodontal Streptococcus constellatus and Streptococcus intermedius clinical isolates. J. Periodontol. 2014;85:1792–1798. doi: 10.1902/jop.2014.130291. [DOI] [PubMed] [Google Scholar]
- Liu F. Ma R. Wang Y. Zhang L. The clinical importance of Campylobacter concisus and other human hosted Campylobacter species. Front. Cell. Infect. Microbiol. 2018;8:243. doi: 10.3389/fcimb.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciantar M. Gilthorpe M. S. Hurel S. J. Newman H. N. Wilson M. Spratt D. A. Capnocytophaga spp. in periodontitis patients manifesting diabetes mellitus. J. Periodontol. 2005;76:194–203. doi: 10.1902/jop.2005.76.2.194. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Takamatsu N. Tominaga Y. Umeda M. Subgingival distribution of periodontopathic bacteria in adult periodontitis and their susceptibility to minocycline-HCl. J. Periodontol. 1998;69:92–99. doi: 10.1902/jop.1998.69.1.92. [DOI] [PubMed] [Google Scholar]
- Apolonio A. C. Carvalho M. A. Ribas R. N. Sousa-Gaia L. G. Santos K. V. Lana M. A. Nicoli J. R. Farias L. M. Production of antagonistic substance by Eikenella corrodens isolated from the oral cavity of human beings with and without periodontal disease. J. Appl. Microbiol. 2007;103:245–251. doi: 10.1111/j.1365-2672.2006.03211.x. [DOI] [PubMed] [Google Scholar]
- Han M. Liu G. Chen Y. Wang D. Zhang Y. Comparative genomics uncovers the genetic diversity and characters of Veillonella atypica and provides insights into its potential applications. Front. Microbiol. 2020;11:1219. doi: 10.3389/fmicb.2020.01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima I. Theodorea C. F. Thaweboon B. Thaweboon S. Nakazawa F. Identification of Veillonella species in the tongue biofilm by using a novel one-step polymerase chain reaction method. PLoS One. 2016;11:e0157516. doi: 10.1371/journal.pone.0157516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima I. Nakazawa F. The interaction between Streptococcus spp. and Veillonella tobetsuensis in the early stages of oral biofilm formation. J. Bacteriol. 2015;197:2104–2111. doi: 10.1128/JB.02512-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruni W. Chioma O. Fletcher H. M. Filifactor alocis: The newly discovered kid on the block with special talents. J. Dent. Res. 2014;93:725–732. doi: 10.1177/0022034514538283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruni A. W. Dou Y. Mishra A. Fletcher H. M. The biofilm community-rebels with a cause. Curr. Oral Health Rep. 2015;2:48–56. doi: 10.1007/s40496-014-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruni A. W. Roy F. Fletcher H. M. Filifactor alocis has virulence attributes that can enhance its persistence under oxidative stress conditions and mediate invasion of epithelial cells by Porphyromonas gingivalis. Infect. Immun. 2011;79:3872–3886. doi: 10.1128/IAI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen K. Jepsen S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 2016;71:82–112. doi: 10.1111/prd.12121. [DOI] [PubMed] [Google Scholar]
- Cobb C. M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996;1:443–490. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- Cobb C. M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002;29(Suppl 2):6–16. [PubMed] [Google Scholar]
- Harks I. Koch R. Eickholz P. Hoffmann T. Kim T. S. Kocher T. Meyle J. Kaner D. Schlagenhauf U. Doering S. Holtfreter B. Gravemeier M. Harmsen D. Ehmke B. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J. Clin. Periodontol. 2015;42:832–842. doi: 10.1111/jcpe.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S. Deschner J. Braun A. Schwarz F. Eberhard J. Calculus removal and the prevention of its formation. Periodontol 2000. 2011;55:167–188. doi: 10.1111/j.1600-0757.2010.00382.x. [DOI] [PubMed] [Google Scholar]
- Tomasi C. Wennstrom J. L. Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets: Outcome at furcation sites. J. Periodontol. 2011;82:210–218. doi: 10.1902/jop.2010.100308. [DOI] [PubMed] [Google Scholar]
- Tonetti M. S. Lang N. P. Cortellini P. Suvan J. E. Eickholz P. Fourmousis I. Topoll H. Vangsted T. Wallkamm B. Effects of a single topical doxycycline administration adjunctive to mechanical debridement in patients with persistent/recurrent periodontitis but acceptable oral hygiene during supportive periodontal therapy. J. Clin. Periodontol. 2012;39:475–482. doi: 10.1111/j.1600-051X.2012.01864.x. [DOI] [PubMed] [Google Scholar]
- Zandbergen D. Slot D. E. Cobb C. M. Van der Weijden F. A. The clinical effect of scaling and root planing and the concomitant administration of systemic amoxicillin and metronidazole: A systematic review. J. Periodontol. 2013;84:332–351. doi: 10.1902/jop.2012.120040. [DOI] [PubMed] [Google Scholar]
- Feres M. Soares G. M. Mendes J. A. Silva M. P. Faveri M. Teles R. Socransky S. S. Figueiredo L. C. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: A 1-year double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2012;39:1149–1158. doi: 10.1111/jcpe.12004. [DOI] [PubMed] [Google Scholar]
- Guerrero A. Griffiths G. S. Nibali L. Suvan J. Moles D. R. Laurell L. Tonetti M. S. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2005;32:1096–1107. doi: 10.1111/j.1600-051X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- Feres M. Figueiredo L. C. Soares G. M. Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67:131–186. doi: 10.1111/prd.12075. [DOI] [PubMed] [Google Scholar]
- Sum J. O'Rourke V. J. Factors affecting periodontal disease referral and the adherence to guidelines among general dentists. Aust. Dent. J. 2018;63:394–401. doi: 10.1111/adj.12641. [DOI] [PubMed] [Google Scholar]
- Thamban Chandrika N. Fosso M. Y. Alimova Y. May A. Gonzalez O. A. Garneau-Tsodikova S. Novel zafirlukast derivatives exhibit selective antibacterial activity against Porphyromonas gingivalis. MedChemComm. 2019;10:926–933. doi: 10.1039/C9MD00074G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi Jr. P. A., Rudy R. J., Jeong Y. N. and Coleman D., Non-surgical control of periodontal diseases: A comprehensive handbook, Srpinger, 1st edn, 2016, pp. 163–173 [Google Scholar]
- Kapoor A. Malhotra R. Grover V. Grover D. Systemic antibiotic therapy in periodontics. Dent. Res. J. 2012;9:505–515. doi: 10.4103/1735-3327.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S. Saha S. G. Rajkumar B. Dhole T. K. Comparative antimicrobial efficacy of selected root canal irrigants on commonly isolated microorganisms in endodontic infection. Eur. J. Dent. 2017;11:12–16. doi: 10.4103/ejd.ejd_141_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil V. Mali R. Mali A. Systemic anti-microbial agents used in periodontal therapy. J. Indian Soc. Periodontol. 2013;17:162–168. doi: 10.4103/0972-124X.113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z. Mali M. Naseem M. Najeeb S. Zafar M. S. Human gingival crevicular fluids (GCF) proteomics: An overview. Dent. J. 2017;5:12. doi: 10.3390/dj5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep A. R. Kathariya R. Clarithromycin, as an adjunct to non surgical periodontal therapy for chronic periodontitis: A double blinded, placebo controlled, randomized clinical trial. Arch. Oral Biol. 2011;56:1112–1119. doi: 10.1016/j.archoralbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Duffy C. R. Huang Y. Andrikopoulou M. Stern-Ascher C. N. Wright J. D. Goffman D. D'Alton M. E. Friedman A. M. Clindamycin, gentamicin, and risk of Clostridium difficile infection and acute kidney injury during delivery hospitalizations. Obstet. Gynecol. 2020;135:59–67. doi: 10.1097/AOG.0000000000003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakasam A. Elavarasu S. S. Natarajan R. K. Antibiotics in the management of aggressive periodontitis. J. Pharm. BioAllied Sci. 2012;4:S252–S255. doi: 10.4103/0975-7406.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares L. J. de Avila E. D. Klein M. I. Panariello B. H. D. Spolidorio D. M. P. Pavarina A. C. Antimicrobial photodynamic therapy alone or in combination with antibiotic local administration against biofilms of Fusobacterium nucleatum and Porphyromonas gingivalis. J. Photochem. Photobiol., B. 2018;188:135–145. doi: 10.1016/j.jphotobiol.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Wright T. L. Ellen R. P. Lacroix J. M. Sinnadurai S. Mittelman M. W. Effects of metronidazole on Porphyromonas gingivalis biofilms. J. Periodontal Res. 1997;32:473–477. doi: 10.1111/j.1600-0765.1997.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Griffith E. C. Wallace M. J. Wu Y. Kumar G. Gajewski S. Jackson P. Phelps G. A. Zheng Z. Rock C. O. Lee R. E. White S. W. The structural and functional basis for recurring sulfa drug resistance mutations in Staphylococcus aureus dihydropteroate synthase. Front. Microbiol. 2018;9:1369. doi: 10.3389/fmicb.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reygaert W. C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley J. L. Wencewicz T. A. Tetracycline-inactivating enzymes. Front. Microbiol. 2018;9:1058. doi: 10.3389/fmicb.2018.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munita J. M. Arias C. A. Mechanisms of antibiotic resistance. Microbiol. Spectrum. 2016;2 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor G. Saigal S. Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol., Clin. Pharmacol. 2017;33:300–305. doi: 10.4103/joacp.JOACP_349_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway K. A. Rosella L. Henry D. The impact of WHO essential medicines policies on inappropriate use of antibiotics. PLoS One. 2016;11:e0152020. doi: 10.1371/journal.pone.0152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. Staphylococcal infection due to penicillin-resistant strains. Br. Med. J. 1947;2:863–865. doi: 10.1136/bmj.2.4534.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Wexler H. M. Goldstein E. J. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013;26:526–546. doi: 10.1128/CMR.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skold O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Updates. 2000;3:155–160. doi: 10.1054/drup.2000.0146. [DOI] [PubMed] [Google Scholar]
- Connell S. R. Trieber C. A. Dinos G. P. Einfeldt E. Taylor D. E. Nierhaus K. H. Mechanism of Tet(O)-mediated tetracycline resistance. EMBO J. 2003;22:945–953. doi: 10.1093/emboj/cdg093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres M. T. Chung W. O. Roberts M. C. Fierro J. F. Antimicrobial susceptibilities of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens spp. isolated in Spain. Antimicrob. Agents Chemother. 1998;42:3022–3023. doi: 10.1128/AAC.42.11.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y. Ning Y. Fenno J. C. 23S rRNA point mutation associated with erythromycin resistance in Treponema denticola. FEMS Microbiol. Lett. 2002;207:39–42. doi: 10.1111/j.1574-6968.2002.tb11025.x. [DOI] [PubMed] [Google Scholar]
- Arredondo A. Blanc V. Mor C. Nart J. Leon R. Azithromycin and erythromycin susceptibility and macrolide resistance genes in Prevotella from patients with periodontal disease. Oral Dis. 2019;25:860–867. doi: 10.1111/odi.13043. [DOI] [PubMed] [Google Scholar]
- Stamm L. V. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob. Agents Chemother. 2010;54:583–589. doi: 10.1128/AAC.01095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz A. N. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Clin. Infect. Dis. 2014;59:698–705. doi: 10.1093/cid/ciu395. [DOI] [PubMed] [Google Scholar]
- Soares G. M. Figueiredo L. C. Faveri M. Cortelli S. C. Duarte P. M. Feres M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral Sci. 2012;20:295–309. doi: 10.1590/S1678-77572012000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick S. Pfister W. Fiedler D. Straube E. Clindamycin promotes phagocytosis and intracellular killing of periodontopathogenic bacteria by crevicular granulocytes: An in vitro study. J. Antimicrob. Chemother. 2000;46:583–588. doi: 10.1093/jac/46.4.583. [DOI] [PubMed] [Google Scholar]
- Patil P. C. Tan J. Demuth D. R. Luzzio F. A. 1,2,3-Triazole-based inhibitors of Porphyromonas gingivalis adherence to oral streptococci and biofilm formation. Bioorg. Med. Chem. 2016;24:5410–5417. doi: 10.1016/j.bmc.2016.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. Patil P. C. Luzzio F. A. Demuth D. R. In vitro and in vivo activity of peptidomimetic compounds that target the periodontal pathogen Porphyromonas gingivalis. Antimicrob. Agents Chemother. 2018;62:e00400–e00418. doi: 10.1128/AAC.00400-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olczak T. Smiga M. Kwiecien A. Bielecki M. Wrobel R. Olczak M. Ciunik Z. Antimicrobial activity of stable hemiaminals against Porphyromonas gingivalis. Anaerobe. 2017;44:27–33. doi: 10.1016/j.anaerobe.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Kang M. S. Choi E. K. Choi D. H. Ryu S. Y. Lee H. H. Kang H. C. Koh J. T. Kim O. S. Hwang Y. C. Yoon S. J. Kim S. M. Yang K. H. Kang I. C. Antibacterial activity of pyrrolidine dithiocarbamate. FEMS Microbiol. Lett. 2008;280:250–254. doi: 10.1111/j.1574-6968.2008.01069.x. [DOI] [PubMed] [Google Scholar]
- Laurentiz R. S. Gomes W. P. Pissurno A. P. R. Santos F. A. Santos V. C. O. Martins C. H. G. Synthesis and antibacterial activity of new lactone 1,4-dihydroquinoline derivatives. Med. Chem. Res. 2018;27:1074–1084. doi: 10.1007/s00044-017-2129-x. [DOI] [Google Scholar]
- Sączewski J. Kędzia A. Jalińska A. New derivatives of 4,6-dimethylisoxazolo[3,4-b]pyridin-3(1H)-one: Synthesis, tautomerism, electronic structure and antibacterial activity. Heterocycl. Commun. 2014;20:215–223. [Google Scholar]
- Li C. Peters A. S. Meredith E. L. Allman G. W. Savage P. B. Design and synthesis of potent sensitizers of Gram-negative bacteria based on a cholic acid scaffolding. J. Am. Chem. Soc. 1998;120:2961–2962. doi: 10.1021/ja973881r. [DOI] [Google Scholar]
- Li C. Budge L. P. Driscoll C. D. Willardson B. M. Allman G. W. Savage P. B. Incremental Conversion of outer-membrane permeabilizers into potent antibiotics for Gram-negative bacteria. J. Am. Chem. Soc. 1999;121:931–940. doi: 10.1021/ja982938m. [DOI] [Google Scholar]
- Isogai E. Isogai H. Takahashi K. Okumura K. Savage P. B. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral Microbiol. Immunol. 2009;24:170–172. doi: 10.1111/j.1399-302X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- Alafeefy A. M. Ceruso M. Al-Tamimi A. M. Del Prete S. Capasso C. Supuran C. T. Quinazoline-sulfonamides with potent inhibitory activity against the alpha-carbonic anhydrase from Vibrio cholerae. Bioorg. Med. Chem. 2014;22:5133–5140. doi: 10.1016/j.bmc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Alafeefy A. M. Ceruso M. Al-Tamimi A. M. Del Prete S. Supuran C. T. Capasso C. Inhibition studies of quinazoline-sulfonamide derivatives against the gamma-CA (PgiCA) from the pathogenic bacterium, Porphyromonas gingivalis. J. Enzyme Inhib. Med. Chem. 2015;30:592–596. doi: 10.3109/14756366.2014.957202. [DOI] [PubMed] [Google Scholar]
- Stone V. N. Parikh H. I. El-rami F. Ge X. Chen W. Zhang Y. Kellogg G. E. Xu P. Identification of small-molecule inhibitors against meso-2,6-diaminopimelate dehydrogenase from Porphyromonas gingivalis. PLoS One. 2015;10:e0141126. doi: 10.1371/journal.pone.0141126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits E. Van der Massen I. Vandamme K. De Cremer K. De Brucker K. Thevissen K. Cammue B. P. A. Beullens S. Fauvart M. Verstraeten N. Michiels J. Roberts M. In vitro activity of the antiasthmatic drug zafirlukast against the oral pathogens Porphyromonas gingivalis and Streptococcus mutans. FEMS Microbiol. Lett. 2017;364:Fnx005. doi: 10.1093/femsle/fnx005. [DOI] [PubMed] [Google Scholar]
- Howard K. C. Gonzalez O. A. Garneau-Tsodikova S. Second generation of zafirlukast derivatives with improved activity against the oral pathogen Porphyromonas gingivalis. ACS Med. Chem. Lett. 2020;11:1905–1912. doi: 10.1021/acsmedchemlett.9b00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits E. Defraine V. Vandamme K. De Cremer K. De Brucker K. Thevissen K. Cammue B. P. Beullens S. Fauvart M. Verstraeten N. Michiels J. Repurposing toremifene for treatment of oral bacterial infections. Antimicrob. Agents Chemother. 2017;61:e01846–01916. doi: 10.1128/AAC.01846-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea D. Van Elzen R. De Winter H. Van Goethem S. Landuyt B. Luyten W. Schoofs L. Van Der Veken P. Augustyns K. De Meester I. Fulop V. Lambeir A. M. Crystal structure of Porphyromonas gingivalis dipeptidyl peptidase 4 and structure-activity relationships based on inhibitor profiling. Eur. J. Med. Chem. 2017;139:482–491. doi: 10.1016/j.ejmech.2017.08.024. [DOI] [PubMed] [Google Scholar]
- Frohlich E. Kantyka T. Plaza K. Schmidt K. H. Pfister W. Potempa J. Eick S. Benzamidine derivatives inhibit the virulence of Porphyromonas gingivalis. Mol. Oral Microbiol. 2013;28:192–203. doi: 10.1111/omi.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick S. Pfister W. Sturzebecher U. Jarema S. Sturzebecher J. Inhibitors of benzamidine type influence the virulence properties of Porphyromonas gingivalis strains. Acta Biochim. Pol. 2003;50:725–734. doi: 10.18388/abp.2003_3663. [DOI] [PubMed] [Google Scholar]
- Li Y. Miao Y. S. Fu Y. Li X. T. Yu S. J. Attenuation of Porphyromonas gingivalis oral infection by alpha-amylase and pentamidine. Mol. Med. Rep. 2015;12:2155–2160. doi: 10.3892/mmr.2015.3584. [DOI] [PubMed] [Google Scholar]
- Rivero-Cruz J. F. Antimicrobial compounds isolated from Haematoxylon brasiletto. J. Ethnopharmacol. 2008;119:99–103. doi: 10.1016/j.jep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Park M. Bae J. Lee D. S. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- Karygianni L. Cecere M. Skaltsounis A. L. Argyropoulou A. Hellwig E. Aligiannis N. Wittmer A. Al-Ahmad A. High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. BioMed Res. Int. 2014;2014:839019. doi: 10.1155/2014/839019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karygianni L. Cecere M. Argyropoulou A. Hellwig E. Skaltsounis A. L. Wittmer A. Tchorz J. P. Al-Ahmad A. Compounds from Olea europaea and Pistacia lentiscus inhibit oral microbial growth. BMC Complementary Altern. Med. 2019;19:51. doi: 10.1186/s12906-019-2461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Cruz B. E. Esturau N. Sanchez-Nieto S. Romero I. Castillo-Juarez I. Rivero-Cruz J. F. Isolation of the new anacardic acid 6-[16'Z-nonadecenyl]-salicylic acid and evaluation of its antimicrobial activity against Streptococcus mutans and Porphyromonas gingivalis. Nat. Prod. Res. 2011;25:1282–1287. doi: 10.1080/14786419.2010.534996. [DOI] [PubMed] [Google Scholar]
- Gafner S. Bergeron C. Villinski J. R. Godejohann M. Kessler P. Cardellina J. H. Ferreira D. Feghali K. Grenier D. Isoflavonoids and coumarins from Glycyrrhiza uralensis: Antibacterial activity against oral pathogens and conversion of isoflavans into isoflavan-quinones during purification. J. Nat. Prod. 2011;74:2514–2519. doi: 10.1021/np2004775. [DOI] [PubMed] [Google Scholar]
- Tanabe S. Desjardins J. Bergeron C. Gafner S. Villinski J. R. Grenier D. Reduction of bacterial volatile sulfur compound production by licoricidin and licorisoflavan A from licorice. J. Breath Res. 2012;6:016006. doi: 10.1088/1752-7155/6/1/016006. [DOI] [PubMed] [Google Scholar]
- Raja A. F. Ali F. Khan I. A. Shawl A. S. Arora D. S. Acetyl-11-keto-beta-boswellic acid (AKBA); Targeting oral cavity pathogens. BMC Res. Notes. 2011;4:406. doi: 10.1186/1756-0500-4-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. Khajuria A. Taneja S. C. Khajuria R. K. Singh J. Qazi G. N. Boswellic acids and glucosamine show synergistic effect in preclinical anti-inflammatory study in rats. Bioorg. Med. Chem. Lett. 2007;17:3706–3711. doi: 10.1016/j.bmcl.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Kariu T. Nakao R. Ikeda T. Nakashima K. Potempa J. Imamura T. Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. J. Periodontal Res. 2017;52:89–96. doi: 10.1111/jre.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifait L. Marquis A. Genovese S. Epifano F. Grenier D. Synthesis and antimicrobial activity of geranyloxy- and farnesyloxy-acetophenone derivatives against oral pathogens. Fitoterapia. 2012;83:996–999. doi: 10.1016/j.fitote.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Li X. Yu C. Hu Y. Xia X. Liao Y. Zhang J. Chen H. Lu W. Zhou W. Song Z. New application of psoralen and angelicin on periodontitis with anti-bacterial, anti-inflammatory, and osteogenesis effects. Front. Cell. Infect. Microbiol. 2018;8:178. doi: 10.3389/fcimb.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J. Appl. Microbiol. 2012;113:438–447. doi: 10.1111/j.1365-2672.2012.05329.x. [DOI] [PubMed] [Google Scholar]
- Marquis A. Genovese S. Epifano F. Grenier D. The plant coumarins auraptene and lacinartin as potential multifunctional therapeutic agents for treating periodontal disease. BMC Complementary Altern. Med. 2012;12:80. doi: 10.1186/1472-6882-12-S1-P80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese S. Epifano F. Curini M. Dudra-Jastrzebska M. Luszczki J. J. Prenyloxyphenylpropanoids as a novel class of anticonvulsive agents. Bioorg. Med. Chem. Lett. 2009;19:5419–5422. doi: 10.1016/j.bmcl.2009.07.110. [DOI] [PubMed] [Google Scholar]
- Rivero-Cruz J. F. Zhu M. Kinghorn A. D. Wu C. D. Antimicrobial constituents of Thompson seedless raisins (Vitis vinifera) against selected oral pathogens. Phytochem. Lett. 2008;1:151–154. doi: 10.1016/j.phytol.2008.07.007. [DOI] [Google Scholar]
- Zhu Y. M. Shen J. K. Wang H. K. Cosentino L. M. Lee K. H. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg. Med. Chem. Lett. 2001;11:3115–3118. doi: 10.1016/S0960-894X(01)00647-3. [DOI] [PubMed] [Google Scholar]
- Hichri F. Jannet H. B. Cheriaa J. Jegham S. Mighri Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chim. 2003;6:473–483. doi: 10.1016/S1631-0748(03)00066-3. [DOI] [Google Scholar]
- Kashiwada Y. Nagao T. Hashimoto A. Ikeshiro Y. Okabe H. Cosentino L. M. Lee K. H. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J. Nat. Prod. 2000;63:1619–1622. doi: 10.1021/np990633v. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y. Hashimoto F. Cosentino L. M. Chen C. H. Garrett P. E. Lee K. H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J. Med. Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- Carvalho T. C. Simao M. R. Ambrosio S. R. Furtado N. A. Veneziani R. C. Heleno V. C. Da Costa F. B. Gomes B. P. Souza M. G. Borges dos Reis E. Martins C. H. Antimicrobial activity of diterpenes from Viguiera arenaria against endodontic bacteria. Molecules. 2011;16:543–551. doi: 10.3390/molecules160100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio S. R. Schorr K. Da Costa F. B. Terpenoids of Viguiera arenaria (Asteraceae) Biochem. Syst. Ecol. 2004;32:221–224. doi: 10.1016/S0305-1978(03)00139-X. [DOI] [Google Scholar]
- da Costa F. B. Albuquerque S. Vichnewski W. Diterpenes and synthetic derivatives from Viguiera aspillioides with trypanomicidal activity. Planta Med. 1996;62:557–559. doi: 10.1055/s-2006-957971. [DOI] [PubMed] [Google Scholar]
- Dalo N. L. Sosa-Sequera M. C. Usubillaga A. On the anticonvulsant activity of kaurenic acid. Invest. Clin. 2007;48:349–358. [PubMed] [Google Scholar]
- Dos Santos F. M. de Souza M. G. Crotti A. E. Martins C. H. Ambrosio S. R. Veneziani R. C. Silva M. L. E. Cunha W. R. Evaluation of antimicrobial activity of extracts of Tibouchina candolleana (melastomataceae), isolated compounds and semi-synthetic derivatives against endodontic bacteria. Braz. J. Microbiol. 2012;43:793–799. doi: 10.1590/S1517-83822012000200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel C. S. de Souza A. C. de Carvalho T. C. de Sousa J. P. Ambrosio S. R. Martins C. H. Cunha W. R. Galan R. H. Furtado N. A. Biotransformation using Mucor rouxii for the production of oleanolic acid derivatives and their antimicrobial activity against oral pathogens. J. Ind. Microbiol. Biotechnol. 2011;38:1493–1498. doi: 10.1007/s10295-010-0935-y. [DOI] [PubMed] [Google Scholar]
- Reddy M. V. Thota N. Sangwan P. L. Malhotra P. Ali F. Khan I. A. Chimni S. S. Koul S. Novel bisstyryl derivatives of bakuchiol: Targeting oral cavity pathogens. Eur. J. Med. Chem. 2010;45:3125–3134. doi: 10.1016/j.ejmech.2010.03.049. [DOI] [PubMed] [Google Scholar]
- Singh A. K. Yadav S. Sharma K. Fridaus Z. Aditi P. Neogi K. Bansal M. Gupta M. K. Shanker A. Singh R. K. Prakash P. Quantum curcumin mediated inhibition of gingipains and mixed-biofilm of Porphyromonas gingivalis causing chronic periodontitis. RSC Adv. 2018;8:40426–40445. doi: 10.1039/C8RA08435A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. G. Aureggi V. Bryan C. S. Lautens M. Intramolecular cross-coupling of gem-dibromoolefins: A mild approach to 2-bromo benzofused heterocycles. Chem. Commun. 2009:5236–5238. doi: 10.1039/B912093A. [DOI] [PubMed] [Google Scholar]
- Park J. S. Ryu E. J. Li L. Choi B. K. Kim B. M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017;137:76–87. doi: 10.1016/j.ejmech.2017.05.037. [DOI] [PubMed] [Google Scholar]
- Smith K. M. Bu Y. Suga H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 2003;10:81–89. doi: 10.1016/S1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Asahi Y. Noiri Y. Igarashi J. Suga H. Azakami H. Ebisu S. Synergistic effects of antibiotics and an N-acyl homoserine lactone analog on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2012;112:404–411. doi: 10.1111/j.1365-2672.2011.05194.x. [DOI] [PubMed] [Google Scholar]
- Wright C. J. Wu H. Melander R. J. Melander C. Lamont R. J. Disruption of heterotypic community development by Porphyromonas gingivalis with small molecule inhibitors. Mol. Oral Microbiol. 2014;29:185–193. doi: 10.1111/omi.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Zhang Y. Shi Y. Q. Pan X. H. Lu Y. H. Cao P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018;116:26–32. doi: 10.1016/j.micpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Souza A. B. de Souza M. G. Moreira M. A. Moreira M. R. Furtado N. A. Martins C. H. Bastos J. K. dos Santos R. A. Heleno V. C. Ambrosio S. R. Veneziani R. C. Antimicrobial evaluation of diterpenes from Copaifera langsdorffii oleoresin against periodontal anaerobic bacteria. Molecules. 2011;16:9611–9619. doi: 10.3390/molecules16119611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albabtain R. Azeem M. Wondimu Z. Lindberg T. Borg-Karlson A. K. Gustafsson A. Investigations of a possible chemical effect of Salvadora persica chewing sticks. J. Evidence-Based Complementary Altern. Med. 2017;2017:2576548. doi: 10.1155/2017/2576548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsariya K. Phanthong P. Bunyapraphatsara N. Srisukh V. Chomnawang M. T. Synergistic interaction and mode of action of Citrus hystrix essential oil against bacteria causing periodontal diseases. Pharm. Biol. 2014;52:273–280. doi: 10.3109/13880209.2013.833948. [DOI] [PubMed] [Google Scholar]
- Moon S. E. Kim H. Y. Cha J. D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 2011;56:907–916. doi: 10.1016/j.archoralbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Azizan N. Mohd Said S. Zainal Abidin Z. Jantan I. Composition and antibacterial activity of the essential oils of Orthosiphon stamineus Benth and Ficus deltoidea Jack against pathogenic oral bacteria. Molecules. 2017;22:2135. doi: 10.3390/molecules22122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y. Hiramitsu M. Isolation and extraction of antimicrobial substances against oral bacteria from lemon peel. J. Food Sci. Technol. 2011;48:635–639. doi: 10.1007/s13197-011-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Lagha A. Andrian E. Grenier D. Resveratrol attenuates the pathogenic and inflammatory properties of Porphyromonas gingivalis. Mol. Oral Microbiol. 2019;34:118–130. doi: 10.1111/omi.12260. [DOI] [PubMed] [Google Scholar]
- Patra J. K. Kim E. S. Oh K. Kim H. J. Kim Y. Baek K. H. Antibacterial effect of crude extract and metabolites of Phytolacca americana on pathogens responsible for periodontal inflammatory diseases and dental caries. BMC Complementary Altern. Med. 2014;14:343. doi: 10.1186/1472-6882-14-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi Y. Noiri Y. Miura J. Maezono H. Yamaguchi M. Yamamoto R. Azakami H. Hayashi M. Ebisu S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J. Appl. Microbiol. 2014;116:1164–1171. doi: 10.1111/jam.12458. [DOI] [PubMed] [Google Scholar]
- Fournier-Larente J. Morin M. P. Grenier D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch. Oral Biol. 2016;65:35–43. doi: 10.1016/j.archoralbio.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Bodet C. Epifano F. Genovese S. Curini M. Grenier D. Effects of 3-(4′-geranyloxy-3′-methoxyphenyl)-2-trans propenoic acid and its ester derivatives on biofilm formation by two oral pathogens, Porphyromonas gingivalis and Streptococcus mutans. Eur. J. Med. Chem. 2008;43:1612–1620. doi: 10.1016/j.ejmech.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Wolska K. I. Grudniak A. M. Fiecek B. Kraczkiewicz-Dowjat A. Kurek A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010;5:543–553. [Google Scholar]
- Lombardo Bedran T. B. Morin M. P. Palomari Spolidorio D. Grenier D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and beta-defensin secretion in oral epithelial cells. PLoS One. 2015;10:e0143158. doi: 10.1371/journal.pone.0143158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L. Qi X. Huang S. Chen S. Wu Y. Zhao L. Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch. Oral Biol. 2015;60:12–22. doi: 10.1016/j.archoralbio.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Jang E. J. Cha S. M. Choi S. M. Cha J. D. Combination effects of baicalein with antibiotics against oral pathogens. Arch. Oral Biol. 2014;59:1233–1241. doi: 10.1016/j.archoralbio.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Lien H. M. Tseng C. J. Huang C. L. Lin Y. T. Chen C. C. Lai Y. Y. Antimicrobial activity of Antrodia camphorata extracts against oral bacteria. PLoS One. 2014;9:e105286. doi: 10.1371/journal.pone.0105286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D. Chen H. Ben Lagha A. Fournier-Larente J. Morin M. P. Dual action of myricetin on Porphyromonas gingivalis and the inflammatory response of host cells: A promising therapeutic molecule for periodontal diseases. PLoS One. 2015;10:e0131758. doi: 10.1371/journal.pone.0131758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]