Abstract
Background
Tildrakizumab is a high-affinity, humanized IgG1κ monoclonal antibody targeting the p19 subunit of IL-23, which is a key regulatory cytokine in psoriasis. Based on evidence from clinical trials, tildrakizumab is approved for the treatment of moderate-to-severe plaque psoriasis in patients eligible for systemic therapy.
Methods
We report our clinical experience with 26 patients followed up to 24 weeks.
Results
No adverse event was observed and no patient discontinued tildrakizumab. Whilst no patient had a Psoriasis Area and Severity Index (PASI) score of <5 at baseline, at week 4, 8 (32%) patients had a PASI score of <3 and 6 of these had a PASI score of 0. At week 12, 19 (79%) patients had a PASI score of <5 and, among these, 18 had a PASI score of <3 of whom 16 had a PASI score of 0. At week 24, 22 (96%) patients had a PASI score of <3 and 20 (87%) had a PASI score of 0. Quality of life was improved after 4 weeks and through the whole period of observation.
Conclusion
Our experience in the real-life setting confirms the efficacy and safety of tildrakizumab as demonstrated by clinical trials.
Keywords: plaque psoriasis, Psoriasis Area and Severity Index, real life, tildrakizumab
Background
Tildrakizumab is a high-affinity, humanized IgG1κ monoclonal antibody targeting the p19 subunit of IL-23, which is a key regulatory cytokine in psoriasis and stimulates the differentiation, proliferation and survival of T helper 17 cells.1,2 Two phase III trials demonstrated that tildrakizumab 100 mg and 200 mg were more effective in terms of Physician’s Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI) 75 at week 12 in comparison with placebo and etanercept and were well tolerated without an increased incidence of serious adverse events.3 The efficacy of tildrakizumab has been shown to be sustained at 28 weeks and at 52 weeks of treatment and more than half of week 28 partial responders (PASI 50–75) improved their PASI responses to a PASI score of ≥75 at week 52.3,4 The pooled analysis of data from the two phase III trials found that responders at week 28 maintained their response through treatment for 3 years. In the same analysis, improvement of PASI scores at week 148 was found in partial responders and non-responders to etanercept switched to tildrakizumab 200 mg.5 Based on evidence from clinical trials, tildrakizumab is approved for the treatment of moderate-to-severe plaque psoriasis in patients eligible for systemic therapy.6 Since approval, little has been published about the clinical use of this drug, suggesting that the reporting of experiences may be helpful to dermatologists to maximize the efficiency of this therapy.
Methods
In total, 26 patients with moderate-to-severe plaque psoriasis (body surface area involvement ≥10%, PGA score ≥3 and PASI score ≥12) at baseline and eligible for systemic therapy, who were referred to our clinic between March 2020 and September 2020, were administered 100 mg tildrakizumab at weeks 0 and 4 and then every 12 weeks by subcutaneous injection. This treatment was performed as recommended according to current clinical practice.6
Patients were assessed at baseline and at weeks 4, 12 and 24 by a clinical visit and recordings of PASI, PGA and Dermatology Life Quality Index (DLQI). At the moment of data collection, 23 patients had been followed up to week 24.
All patients signed informed consent to the use of clinical data for publication.
Statistical analysis
Data were summarized by descriptive analysis. Means, median and standard deviations (SDs) were calculated for continuous variables, whilst absolute values and frequency (percentage) were calculated for categorical variables. Comparison of mean values was performed by a t-test. The Wilcoxon-signed rank test was used to compare repeated measurements in each group and the Mann–Witney test was used to compare mean data between groups. All analyses were performed with IBM SPSS Statistics for Windows, Version 26.0.
Results
Baseline characteristics
Baseline characteristics are reported in Table 1. Only 5/26 (19%) patients had concomitant psoriatic arthritis, and hypertension was the most frequent comorbidity (12/26, 46%). All patients had received previous systemic therapy and 21/26 (81%) had never been treated with biologic drugs. Two patients had received adalimumab, one had received etanercept, one had received ixekizumab and one had received ustekinumab. At baseline, mean PASI was 12.5±6.5, mean PGA was 2.6±1 and mean DLQI was 15.9±4.
Table 1.
Baseline characteristics of 26 patients treated with tildrakizumab.
| Characteristics | n (%)/mean±SD |
|---|---|
| Men | 14 (53.8) |
| Age (years) | 56±16 |
| Bodyweight (kg) | 75±16 |
| BMI (kg/m2) | 26.4±5.9 |
| Psoriasis duration (years) | 11.9±8.3 |
| Psoriatic arthritis | 5 (19.2) |
| Obesity | 4 (15.4) |
| Diabetes | 2 (7.7) |
| Hypercholesterolaemia | 5 (19.2) |
| Hypertension | 12 (46.2) |
| Previous systemic therapy | 26 (100) |
| Previous biologic therapy | 5 (19.2) |
| Psoriasis Area and Severity Index (PASI) | 12.5±6.5 |
| Physician’s Global Assessment (PGA) | 2.6±1 |
| Dermatology Life Quality Index (DLQI) | 15.9±4 |
One patient was lost to follow-up before 4 weeks of treatment and was excluded from the analysis of results.
Tolerability
No adverse event was reported during treatment with tildrakizumab, and no patients discontinued the treatment.
Treatment efficacy
The mean PASI score was reduced to 6.1±5.2 after 4 weeks of treatment (n=25), to 1.8±2.9 after 12 weeks (n=24), and to 0.6±2.1 after 24 weeks (n=23). All these changes from baseline were significant (p<0.001).
Whilst no patient had a PASI score of <5 at baseline, at week 4, 8 (32%) patients had a PASI score of <3 and 6 of these had a score of PASI 0. At week 12, 19 (79%) patients had a PASI score of <5 and, among these, 18 had a PASI score of <3 and, of these, 16 had a PASI score of 0. At week 24, 22 (96%) patients had a PASI score of <3 and 20 (87%) had a PASI score of 0.
PASI 90 was reached by 6/25 (24%) patients at 4 weeks, by 17/24 (71%) patients after 12 weeks and by 21/23 (91%) patients after 24 weeks. PASI 100 was reached by 6/25 (24%) patients at 4 weeks, by 16/24 (67%) patients after 12 weeks and by 20/23 (87%) patients after 24 weeks (Figure 1). In addition, mean PGA and DLQI scores were significantly reduced at each time point in comparison with baseline values (p<0.001 for both scores; Table 2).
Figure 1.
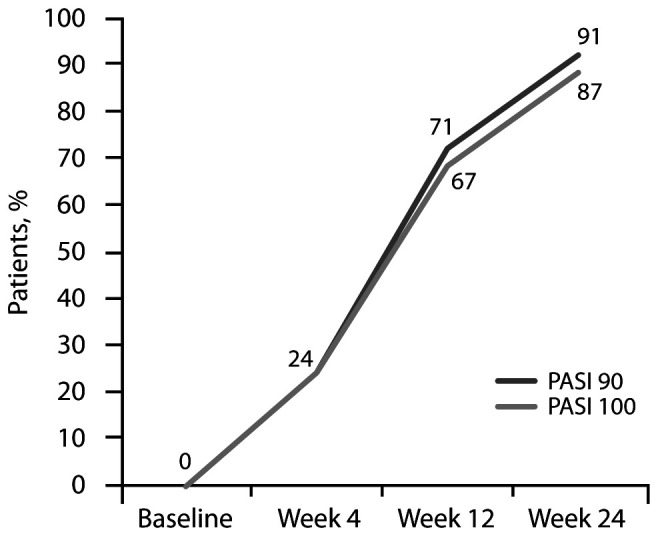
Proportion of patients with PASI90 and PASI100 by visit.
Table 2.
Mean value ± SD of PGA and DLQI at baseline and at visits.
| n | Baseline | Visit value | p value |
|---|---|---|---|
| PGA | |||
|
| |||
| 25 | 2.6±1 | Week 4 | <0.001 |
| 1.6±1.2 | |||
|
| |||
| 24 | 2.6±1 | Week 12 | <0.001 |
| 0.5±0.8 | |||
|
| |||
| 23 | 2.6±1 | Week 24 | <0.001 |
| 0.3±0.9 | |||
|
| |||
| DLQI | |||
|
| |||
| 26 | 15.9±4 | Week 4 | <0.001 |
| 9.1±2 | |||
|
| |||
| 24 | 15.8±4.0 | Week 12 | <0.001 |
| 2.3±1.6 | |||
|
| |||
| 23 | 15.7±4.1 | Week 24 | <0.001 |
| 2.2±4.2 | |||
DLQI, Dermatology Life Quality Index; PGA, Physician’s Global Assessment.
No significant difference was found between patients who had not previously been treated with biologic drugs versus those who were not naive to biologic drugs and in patients who were overweight versus those of normal weight; indeed, we acknowledge that the low number of patients may account for the impossibility to detect any role of previous treatments or body weight.
Psoriatic arthritis
The five patients with psoriatic arthritis had been diagnosed by a rheumatologist several years before our observation for plaque psoriasis. None of them had rheumatologic symptoms when tildrakizumab was initiated and no patient reported joint pain during treatment with tildrakizumab. They were all assessed up to week 24.
The mean PASI score was 15±9.3 at baseline, 8.4±6.9 at week 4, 2.8±3.9 at week 12 and 0.6±0.9 at week 24. Indeed, a PASI score of <3 (no patient had this score at baseline) was found in 1 patient at week 4, in 3 patients at week 12 and in all 5 patients at week 24. A PASI score of 0 was present in 3 patients at week 12 and was maintained in these 3 patients at week 24.
The mean PGA was 2.8±1.3 at baseline, 2.2±1.1 after 4 weeks, 0.8±1.1 after 12 weeks and 0.4±0.5 after 24 weeks. The DLQI score was 17.2±5.4 at baseline, 11±2.2 after 4 weeks, 2.2±1.5 after 12 weeks and 2±2 after 24 weeks.
Conclusion
Tildrakizumab 100 mg was well tolerated and no adverse event was reported by our patients during the whole 24-week follow-up period. No adverse event was reported and no patient discontinued the treatment. The therapy was as effective as expected based on data from clinical trials (Figure 2). Although we cannot draw conclusions from a scant sample, we can observe that psoriasis improvement in our experience was relevant after only 4 weeks of treatment, suggesting that the time to response may be shorter than demonstrated by clinical trials. The improvement of plaque psoriasis progressed after 12 weeks of therapy up to 24 weeks and PASI 100 was obtained by a high proportion of patients at the end of this period. This observation is in agreement with evidence from the two randomized trials, where the maximal efficacy of tildrakizumab was reached between week 22 and week 28.3 Moreover, the overall efficacy was higher in our patients in comparison with results from trials.3 A significant benefit on quality of life was present after only 4 weeks of treatment and it was maintained up to the 24 weeks of follow-up. Tildrakizumab was as effective in overweight patients, who are often difficult to treat, as in non-overweight patients.7,8 Although the sample number is limited to draw conclusions, we observed that tildrakizumab efficacy and safety were similar in patients who had previously received systemic biologic treatment for psoriasis and in patients naïve to biologic drugs.
Figure 2.
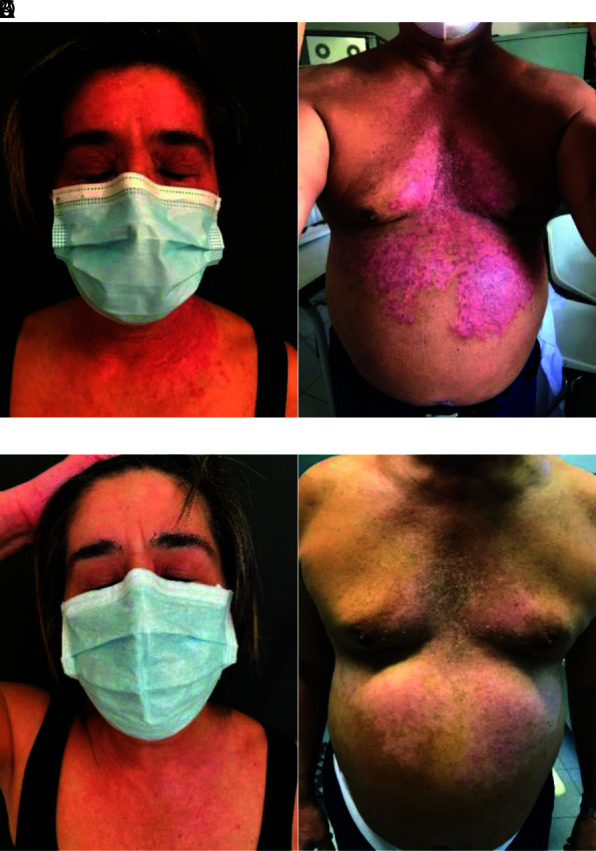
Exemplary patients before (A and B) and after (C and D) treatment with tildrakizumab for 4 weeks.
In conclusion, our experience confirms the efficacy and safety of tildrakizumab when used in real-life practice. Tildrakizumab represents an efficient strategy for subjects with moderate-to-severe disease, who need long-term persistent efficacy and safety of treatment.
Acknowledgements
Editorial assistance was provided by Laura Brogelli, PhD, and Aashni Shah (Polistudium, Italy), and this activity was funded by Almirall.
Footnotes
Contributions: All authors contributed to patient care, data collection, manuscript revision and approval. All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: MB has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, UCB Pharma. AP has served as consultant/investigator for AbbVie, Eli Lilly, Janssen-Cilag, Novartis and UCB Pharma. RC and EC have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/04/dic.2021-2-6-COI.pdf
Funding declaration: Almirall funded editorial assistance to manuscript preparation.
Correct attribution: Copyright © 2021 Burlando M, Castelli R, Cozzani E, Parodi A. https://doi.org/10.7573/dic.2021-2-6. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Kopp T, Riedl E, Bangert C, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222–226. doi: 10.1038/nature14175. [DOI] [PubMed] [Google Scholar]
- 2.Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930–939. doi: 10.1111/bjd.13932. [DOI] [PubMed] [Google Scholar]
- 3.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. doi: 10.1016/S0140-6736(17)31279-5. [DOI] [PubMed] [Google Scholar]
- 4.Elewski B, Menter A, Crowley J, et al. Sustained and continuously improved efficacy of tildrakizumab in patients with moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2020;31(8):763–768. doi: 10.1080/09546634.2019.1640348. [DOI] [PubMed] [Google Scholar]
- 5.Reich K, Warren RB, Iversen L, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol. 2020;182(3):605–617. doi: 10.1111/bjd.18232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilumetri product information. [Accessed May 4, 2021]. https://www.ema.europa.eu/en/documents/product-information/ilumetri-epar-product-information_it.pdf.
- 7.Giles JT, Ogdie A, Gomez Reino JJ, et al. Impact of baseline body mass index on the efficacy and safety of tofacitinib in patients with psoriatic arthritis. RMD Open. 2021;7(1):e001486. doi: 10.1136/rmdopen-2020-001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials. J Dermatolog Treat. 2020 doi: 10.1080/09546634.2020.1832187. [DOI] [PubMed] [Google Scholar]

