Abstract
In connection with our continuous efforts to generate new derivatives from lead compounds isolated from traditional medicinal plants, a series of aloe-emodin derivatives (6a–6e) were synthesized and assessed for their potential anticancer activity against a panel of cancer cell lines. The results showed that most of the derivatives are more active than the aloe-emodin and particularly, 6b and 6e manifested potent activity with IC50 values of 1.32 & 1.6 μM and 0.99 & 2.68 μM against MDA-MB-231 and MCF-7 cells, respectively. Moreover, 6b and 6e induce early and late apoptosis as well as arrest the cell cycle at the G2/M phase in MDA-MB-231 cells. In conclusion, the results confirmed that the aloe-emodin derivatives could be a potential drug candidate for better treatment of breast cancer.
In connection with our continuous efforts to generate new derivatives from lead compounds isolated from traditional medicinal plants, a series of aloe-emodin derivatives were synthesized and assessed for potential anticancer activity against a panel of cancer cell lines.
1. Introduction
Cancer is defined as uncontrolled cell growth and ability to spread over to vital organs. Today, cancer is one of the most deadly diseases with the highest rate of mortality after heart disease in the world. The global cancer burden is estimated about 13.2 million deaths each year and expected to have approximately 21.4 million new cases by 2030.1,2 Despite the continuous efforts to treat cancer over the past few decades, human mortality rates for cancer have not been significantly reduced. Although chemotherapy has become the mainstay and effective method for treating cancers, the non-selectivity between cancer cells and normal cells leads to adverse side effects, and resistance is one of the severe limitations to its therapeutic success.3,4 Therefore, developing safe and effective anticancer agents with novel mechanisms of action are indeed of great interest in the field of chemistry.
The past decade has witnessed plant based lead compounds for the development of new pharmaceuticals and the majority of these drugs have been evolved from traditional medicine.5 Traditional medicine of plant origin still holds an important position, as 80% of world population rely on plants for primary health care.6 More than half of the currently available drugs are either pure natural compounds or their derivatives, and in the case of cancer, this proportion surpassed 60%.7Rheum emodi wall ex Meissn is one of the most popular traditional medicinal herbs which finds an extensive usage in Ayurvedic and Unani systems of medicine.8 There has been great clinical interest in using rhubarb (the rhizomes of Rheum species) for the treatment of blood stagnation syndrome, also called ‘Oketsu syndrome’, diabetes, atherosclerosis, ischemia, and inflammation, and it has been used in Japanese and Chinese traditional medicines. Aloe-emodin (1), is one of the major anthraquinones from the rhizomes of Rheum emodi, which has been reported for a wide range of pharmacological activities including antibacterial, antiviral, anticancer, hepatoprotective, anti-proliferative and anti-inflammatory effects.9,10
In view of the fact that natural products (NPs) and their related analogues are attractive sources of new chemical entities for drug discovery and development,7 our group has been engaged in the design and synthesis of more potent and selective analogues, through the chemical modification of lead compounds isolated from traditional medicinal plant extracts.11–13 In line with this interest, the current study was designed to synthesise the novel analogues of aloe emodin (1) and assess their anticancer activity against a panel of cancer cell lines. On a careful review of specific structural similarities of some of the most well-known anticancer agents and aloe emodin (1), it was noticed that the anthraquinone moiety is common in most of the known standard drugs (Fig. 1).
Fig. 1. Representative anthraquinone drugs used in the anticancer drug discovery and development.
On the basis of these observations, we proposed to link the pyrazole moiety to aloe emodin (Fig. 2) for the development of new structurally diverse molecular scaffolds to combat cancer. These derivatives are expected to be more flexible to fit in the binding site of the target which leads to the potent anticancer activities. It is well known that heterocyclic moieties like pyrazoles provide an extra binding site to interact with a variety of enzymes and receptors in biological systems.14,15 Therefore, the strategic attachment of such moieties could enhance various drug-like properties such as polarity and solubility in the parent natural product lead. Recently, a few approaches have been developed to synthesize the aloe-emodin derivatives and also demonstrated the various biological activities including anti-inflammatory, xanthine-oxidase, and acetylcholinesterase inhibitory activities.16–18 Herein, we report the synthesis of pyrazole linked aloe-emodin derivatives and evaluation of their anticancer activities.
Fig. 2. A design strategy for pyrazole linked aloe-emodin derivatives.
2. Results and discussion
2.1. Chemistry
The natural product aloe emodin, a 1,8-dihydroxyanthraquinone, was isolated (on the gram scale) from the chloroform extract of rhizomes of Rheum emodi. It is well known that free hydroxyl groups at C-1 & C-8 of anthraquinones are essential for cytotoxic activity (Fig. 1).19 Keeping the view of free hydroxyl group importance, the present study focused on the CH2OH group at a C-3 position for the attachment of the pyrazole moiety on emodin. As shown in Scheme 1, aloe emodin (1) was subjected to oxidation reaction using pyridinium chlorochromate (PCC) in the presence of DCM to afford the corresponding aldehyde.18 Subsequently, aldehyde (2) reacted with phenyl hydrazines (3a–k) in methanol/ethanol at room temperature to provide their corresponding phenyl hydrazones/Schiff base compounds (4a–k) in good yields. The 1,3-dipolar cycloaddition of an ylide to an alkene involving the [3 + 2] principle is a flexible route to attain five membered heterocycles. Thus, [3 + 2] cycloaddition reaction of hydrazones (4a–k) with diethyl but-2-ynedioate under heating conditions at 130 °C afforded the corresponding pyrazole linked aloe-emodin derivatives (5a–5k) in moderate to good yields. In a similar way, cycloaddition of Schiff base compounds with dimethyl but-2-ynedioate at 130 °C afforded the corresponding pyrazole linked aloe emodin derivatives (6a–6e) in good yields. All the reactions preceded in a smooth manner and the structures of all the synthesized compounds were characterized using 1H NMR and 13C NMR, IR, and mass spectral analysis. The 1H NMR spectrum of key intermediate 4a showed a peak at δH 8.11 due to the H-15 proton while 13C NMR showed the absence of the carbonyl signal indicating the formation of the Schiff base (Fig. S8 and S9 in the ESI‡ data).
Scheme 1. Synthesis of compounds 5a–k & 6a–e.
2.2. Biological activity
2.2.1. Cytotoxicity studies
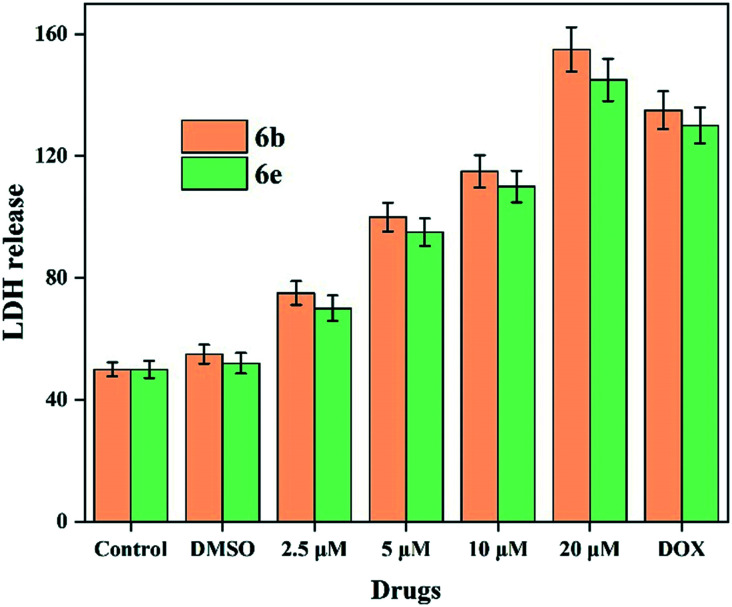
All the compounds were tested for their cytotoxicity against various cancerous (MCF-7, MDA-MB-231 and HepG2) and non-cancerous (HEK 293) cell lines using the MTT assay.20 As shown in Table 1, compounds 6b and 6e showed significant cytotoxic activity against the MDA-MB-231 cell line with IC50 values of 1.32 & 1.66 μM, 0.99 & 2.68 μM against MCF-7, 5.62 & 3.99 μM against HepG2, 9.57 & 6.06 μM against B16F10 and 30.11 & 21.22 μM against HEK 293 cells, respectively. Comparing with other tested compounds, 6b and 6e were selected for further analysis due to their specific activity against cancer cells and less toxicity against normal cells. Moreover, the anticancer activities of the selected compounds were more or less equal to the standard anticancer drug i.e. doxorubicin. Further, the effects of these compounds on the membrane integrity of the MDA-MB-231 cells were analysed using the LDH leakage assay. The results showed that compounds 6b & 6e significantly increase the LDH leakage which is confirmed by measuring the amount of formazan products (Fig. 3). The results showed that the increase in concentration of compounds 6b & 6e inhibits the growth of breast cancer cells by increasing the LDH release and the results were compared with the standard drug. Together, these results proved that the selected compounds 6b & 6e are highly sensitive to breast cancer cells and could be potential anticancer agents. Hence, compounds 6b and 6e were selected to study the effect on the molecular mechanism of their activity against MDA-MB-231 cells.
In vitro anticancer activity (IC50) of aloe emodin and its derivatives (1, 2, 5a–5k, and 6a–6e) against cancer cell lines. Concentration that promotes 50% of cell growth inhibition after 48 h of treatment. IC50 was determined by the MTT assay. The independent experiment was run at least three times.
| Compound | MDA-MB-231a | MCF-7a | HEPG2b | B16F10c | HEK-293d |
|---|---|---|---|---|---|
| 1 | 12.24 ± 0.15 μM | 4.14 ± 0.37 μM | 6.13 ± 0.25 μM | 3.12 ± 1.25 μM | 1.00 ± 0.78 μM |
| 2 | 4.99 ± 0.26 μM | 2.68 ± 0.74 μM | 3.15 ± 0.43 μM | 2.90 ± 1.27 μM | 4.49 ± 1.42 μM |
| 5a | 21.70 ± 0.9 μM | 3.18 ± 0.15 μM | 12.35 ± 0.53 μM | 2.22 ± 1.49 μM | 4.56 ± 1.23 μM |
| 5b | 0.90 ± 0.40 μM | 1.04 ± 1.35 μM | 1.00 ± 2.16 μM | 1.85 ± 1.43 μM | 1.03 ± 1.69 μM |
| 5c | 7.56 ± 0.37 μM | 1.13 ± 2.46 μM | 1.35 ± 2.54 μM | 1.57 ± 0.25 μM | 2.47 ± 1.28 μM |
| 5d | 3.96 ± 1.15 μM | 3.58 ± 1.42 μM | 6.23 ± 0.87 μM | 3.35 ± 0.27 μM | 9.76 ± 0.57 μM |
| 5e | 4.46 ± 1.85 μM | 0.87 ± 1.02 μM | 3.35 ± 1.66 μM | 1.15 ± 0.43 μM | 9.32 ± 0.80 μM |
| 5f | 6.20 ± 1.96 μM | 3.96 ± 1.45 μM | 2.16 ± 0.55 μM | 3.90 ± 0.49 μM | 1.09 ± 0.60 μM |
| 5g | 0.99 ± 0.32 μM | 6.35 ± 1.56 μM | 1.15 ± 0.69 μM | 3.46 ± 1.26 μM | 1.12 ± 1.57 μM |
| 5h | 2.16 ± 0.65 μM | 8.98 ± 0.97 μM | 1.26 ± 0.95 μM | 8.15 ± 2.85 μM | 9.07 ± 1.48 μM |
| 5i | 1.85 ± 0.99 μM | 7.26 ± 0.75 μM | 2.6 ± 0.24 μM | 5.40 ± 0.36 μM | 1.93 ± 0.32 μM |
| 5j | 1.15 ± 0.16 μM | 4.62 ± 0.95 μM | 1.34 ± 1.55 μM | 3.46 ± 0.79 μM | 1.97 ± 2.12 μM |
| 5k | 4.70 ± 0.36 μM | 3.86 ± 1.96 μM | 5.68 ± 1.24 μM | 2.15 ± 0.37 μM | 10.13 ± 1.35 μM |
| 6a | 6.78 ± 1.36 μM | 9.96 ± 1.85 μM | 3.41 ± 1.75 μM | 5.61 ± 0.25 μM | 8.86 ± 0.18 μM |
| 6b | 1.32 ± 0.13 μM | 0.99 ± 0.36 μM | 5.62 ± 0.18 μM | 9.57 ± 0.99 μM | 30.11 ± 0.05 μM |
| 6c | 1.49 ± 1.16 μM | 4.66 ± 0.49 μM | 3.46 ± 0.32 μM | 3.34 ± 1.34 μM | 5.01 ± 1.88 μM |
| 6d | 3.68 ± 0.63 μM | 1.16 ± 0.13 μM | 2.29 ± 0.46 μM | 6.09 ± 0.27 μM | 6.84 ± 0.65 μM |
| 6e | 1.66 ± 0.25 μM | 2.68 ± 0.25 μM | 3.99 ± 0.70 μM | 6.06 ± 0.14 μM | 21.22 ± 0.05 μM |
| Doxorubicin | 2.56 ± 0.27 μM | 2.02 ± 0.24 μM | 2.35 ± 2.16 μM | 3.28 ± 1.65 μM | 1.65 ± 1.85 μM |
Human breast cancer cells.
Human liver cancer cells.
Human skin melanoma cancer cells.
Normal cells.
Fig. 3. LDH leakage analysis was performed on 6b & 6e in MDA-MB-231 cells. The assay was performed in triplicate and the mean values were presented and the error bar is meant to be SD.
2.2.2. Apoptosis studies
Annexin V/propidium iodide (PI) apoptosis induction assay was performed to differentiate between early apoptotic, late apoptotic, and necrotic cells.21 In this study, MDA-MB-231 cells were treated with the selected compounds 6b & 6e at 0, 5, 10 and 20 μM for 24 h and assessed for apoptosis. The results clearly showed the presence of early apoptotic cells (lower right quadrant) and late apoptotic cells (upper right quadrant) in 6b and 6e treated cells. Moreover, compounds 6b and 6e also increased the number of cells at both early and late apoptotic phases compared with the control group (Fig. 4). After the treatment, the percentage of early and late apoptotic cells was increased in the cells treated with different concentrations (5 and 10 μM) of compounds 6b & 6e compared to control cells. Together, the data suggest that there is an increase in the induction of apoptosis by both compounds 6b & 6e in a concentration dependent manner.
Fig. 4. Apoptosis studies of 6b & 6e at different concentrations in MDA-MB-231 cells by the Annexin V/PI assay using flow cytometry.
2.2.3. Cell cycle progression
The effect of compounds 6b & 6e on the cell cycle progression was also measured as it can also be evidence for anticancer activity.22 MDA-MB-231 cells were treated with 5, 10 and 20 μM each of compounds 6b & 6e for 24 h and the cells were permeabilized, stained with PI and subjected to flow cytometric analysis and MODFit software to find out the percentage of dividing cells in G0/G1, S, and/G2M phases. As shown in Fig. 5, 6b & 6e significantly affect the cell cycle process at the G0/G1 phase and arrest with extensive apoptosis induction in MDA-MB-231 cells. The sub G0 phase of the control cells was observed at 1.33% and the cells treated with different concentrations of 6b such as 5, 10 and 20 μM were observed at 3.22%, 8.20%, and 25.80%, respectively. In addition, compound 6e treatment also increased the number of cells at the sub G0 phase from 1.33% to 5.66%, 10.87% and 61.45% in 5, 10 and 20 μM, respectively. The increase of cells at the sub G0 phase in 6b & 6e treated MDA-MB 231 cells is indicating the substantial cell cycle inhibitory activity of 6b & 6e.
Fig. 5. Effects of compounds 6b and 6e on cell cycle progression of MDA-MB-231 cell lines by using flow cytometry.
2.2.4. Caspase-2, 3, 6, 8, and 9 activation assays
Further the effect of compounds 6b & 6e on the caspase activity in breast cancer cells was quantitatively measured using the caspase enzyme assay.23 As showed in Fig. 6, compounds 6b & 6e significantly increased the activity of the initiator caspases such as caspase-2, -8 and -9 and executioner caspases such as caspase-3 and caspase-6. Moreover, the results also indicated that both compounds increased the caspase activity with the increase of concentration which revealed that compounds 6b & 6e could be potential anticancer agents against breast cancer.
Fig. 6. Measurement of caspase-2, 3, 6, 8, and 9 activities by colorimetric assay. Dose-dependent induction of caspases in MDA-MB-235 cell lines. The assay was performed in triplicate and the mean values were presented and the error bar is meant to be SD.
3. Conclusion
In the present study, the pyrazole linked aloe-emodin derivatives were synthesized and assessed for their anticancer activities against a panel of cancer cell lines. Among the various derivatives, compounds 6b and 6e showed a significant inhibitory activity against MDA-MB-231 breast cancer cells in concentration dependent manner. The dual staining apoptosis assay showed that compounds 6b and 6e induce the early and late apoptosis in MDA-MB-231 cells and it was further confirmed with flow cytometry analysis, and the results showed that these compounds arrest the cell cycle at the G2/M phase of MDA-MB-231 cells. The caspase enzymatic activity assay showed that compounds 6b and 6e increased the activity of initiator caspases 2/8/9 and executioner caspases 3/6 in breast cancer cells. Together, the aloe-emodin pyrazole derivatives could be excellent anticancer agents for breast cancer treatment.
Conflicts of interest
There is no conflict of interest to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge keen interest shown by Dr. S. Chandrasekhar, Director, CSIR-IICT, Hyderabad. GDK thanks DST for the financial support through the INSPIRE fellowship programme [IF150824]. IICT Communication No. IICT/Pubs./2020/174.
This work has been dedicated to our beloved colleague, late Dr. Surendar Reddy Bathula, Principal Scientist, CSIR-Indian Institute of Chemical Technology, Hyderabad – 500 007, India.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0md00315h
References
- Siegel R. L. Miller K. D. Jemal A. Ca-Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Thun M. J. DeLancey J. O. Center M. M. Jemal A. Ward E. M. Carcinogenesis. 2009;31:100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrama D. Reisfeld R. A. Becker J. C. Nat. Rev. Drug Discovery. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- Conklin K. A. Nutr. Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- Newman D. J. Cragg G. M. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Cragg G. M. Grothaus P. G. Newman D. J. Chem. Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- Newman D. J. Cragg G. M. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Pandith S. A. Hussain A. Bhat W. W. Dhar N. Qazi A. K. Rana S. Razdan S. Wani T. A. Shah M. A. Bedi Y. S. Hamid A. Lattoo S. K. S. Afr. J. Bot. 2014;95:1–8. [Google Scholar]
- Zargar B. A. Masoodi M. H. Ahmed B. Ganie S. A. Food Chem. 2011;128:585–589. [Google Scholar]
- Suresh Babu K. Srinivas P. V. Praveen B. Kishore K. H. Murty U. S. Rao J. M. Phytochemistry. 2003;62:203–207. doi: 10.1016/s0031-9422(02)00571-x. [DOI] [PubMed] [Google Scholar]
- Amujuri D. Siva B. Poornima B. Sirisha K. Sarma A. V. S. Lakshma Nayak V. Tiwari A. K. Purushotham U. Suresh Babu K. Eur. J. Med. Chem. 2018;149:182–192. doi: 10.1016/j.ejmech.2018.02.066. [DOI] [PubMed] [Google Scholar]
- Pavan Kumar P. Siva B. Venkateswara Rao B. Dileep Kumar G. Lakshma Nayak V. Nishant Jain S. Tiwari A. K. Purushotham U. Venkata Rao C. Suresh Babu K. Bioorg. Chem. 2019;91:103161. doi: 10.1016/j.bioorg.2019.103161. [DOI] [PubMed] [Google Scholar]
- Solipeta D. R. Bandi S. Vemulapalli S. P. B. Pallavi P. M. C. Vemireddy S. Balasubramania S. Mahabalarao S. K. H. Katragadda S. B. J. Nat. Prod. 2019;82:2731–2743. doi: 10.1021/acs.jnatprod.9b00346. [DOI] [PubMed] [Google Scholar]
- Faisal M. Saeed A. Hussain S. Dar P. Larik F. A. J. Chem. Sci. 2019;131:70. [Google Scholar]
- Ansari A. Ali A. Asif M. Shamsuzzaman S. New J. Chem. 2017;41:16–41. [Google Scholar]
- Liu J. Wu F. Chen C. Bioorg. Med. Chem. Lett. 2015;25:5142–5146. doi: 10.1016/j.bmcl.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Shi D.-H. Huang W. Li C. Wang L.-T. Wang S.-F. Bioorg. Med. Chem. 2013;21:1064–1073. doi: 10.1016/j.bmc.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Shi D.-H. Huang W. Li C. Liu Y.-W. Wang S.-F. Eur. J. Med. Chem. 2014;75:289–296. doi: 10.1016/j.ejmech.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Li Y. Jiang J.-G. Food Funct. 2018;9:6063–6080. doi: 10.1039/c8fo01569d. [DOI] [PubMed] [Google Scholar]
- Berényi Á. Minorics R. Iványi Z. Ocsovszki I. Ducza E. Thole H. Messinger J. Wölfling J. Mótyán G. Mernyák E. Frank É. Schneider G. Zupkó I. Steroids. 2013;78:69–78. doi: 10.1016/j.steroids.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Deng S. Yang Y. Han Y. Li X. Wang X. Li X. Zhang Z. Wang Y. PLoS One. 2012;7:e30714. doi: 10.1371/journal.pone.0030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. Schipany K. Hengstschläger M. Nat. Protoc. 2013;8:602–626. doi: 10.1038/nprot.2013.011. [DOI] [PubMed] [Google Scholar]
- Abu Bakar M. F. Mohamad M. Rahmat A. Burr S. A. Fry J. R. Food Chem. Toxicol. 2010;48:1688–1697. doi: 10.1016/j.fct.2010.03.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.