Abstract
Study Objectives
Sleep loss produces large individual differences in neurobehavioral responses, with marked vulnerability or resilience among individuals. Such differences are stable with repeated exposures to acute total sleep deprivation (TSD) or chronic sleep restriction (SR) within short (weeks) and long (years) intervals. Whether trait-like responses are observed to commonly experienced types of sleep loss and across various demographically defined groups remains unknown.
Methods
Eighty-three adults completed two baseline nights (10 h–12 h time-in-bed, TIB) followed by five 4 h TIB SR nights or 36 h TSD. Participants then received four 12-h TIB recovery nights followed by five SR nights or 36 h TSD, in counterbalanced order to the first sleep loss sequence. Neurobehavioral tests were completed every 2 h during wakefulness.
Results
Participants who displayed neurobehavioral vulnerability to TSD displayed vulnerability to SR, evidenced by substantial to near perfect intraclass correlation coefficients (ICCs; 78%–91% across measures). Sex, race, age, body mass index (BMI), season, and sleep loss order did not impact ICCs significantly. Individuals exhibited significant consistency of responses within, but not between, performance and self-reported domains.
Conclusions
Using the largest, most diverse sample to date, we demonstrate for the first time the remarkable stability of phenotypic neurobehavioral responses to commonly experienced sleep loss types, across demographic variables and different performance and self-reported measures. Since sex, race, age, BMI, and season did not affect ICCs, these variables are not useful for determining stability of responses to sleep loss, underscoring the criticality of biological predictors. Our findings inform mathematical models and are relevant for the general population and military and health professions.
Keywords: sleep loss, individual differences, trait-like, phenotype, sleepiness, alertness, fatigue, psychomotor vigilance test, cognitive, demographics
Statement of Significance.
In a large, diverse sample, we observed robust, stable individual differences in neurobehavioral responses to two commonly experienced types of sleep loss, acute total sleep deprivation (one night without sleep) and chronic sleep restriction (5 consecutive nights of 4 hours nightly sleep opportunity). Individuals who displayed neurobehavioral vulnerability to one type of sleep loss consistently displayed vulnerability to the other type of sleep loss. Sex, race, age, body mass index, and testing season did not significantly affect such stability, highlighting the need for biological predictors for vulnerability or resilience to sleep loss. These results provide important and novel information for predictive mathematical models and have direct relevance for the population at large and for individuals in military and health professions.
Introduction
The differences among healthy people in neurobehavioral decrements in response to sleep loss are large and stable over time [1–7]. Among healthy adults, approximately one third have profound performance deficits with even moderate sleep loss; one third have moderate deficits, and one third have few or no performance deficits, even when sleep loss is severe [8–10]. Thus, short-term trait-like, phenotypic susceptibility among individuals accounts for 50%–95% of the variance (depending on the measure) in the severity of neurobehavioral decrements due to sleep loss [8–10]. Re-exposure to acute total sleep deprivation (TSD) after 1–6 weeks reveals differential neurobehavioral vulnerability in various measures, such as objective alertness and performance tasks and self-reported mood and sleepiness tasks, which are sensitive to sleep loss [1, 3, 11, 12]. Long-term stability also has been observed in individuals who experienced months to years between exposures of TSD or chronic sleep restriction (SR), with intraclass correlation coefficients (ICCs) ranging from 0.72 to 0.95 [7]. Stability in higher-order cognitive tests also has been found when SR and circadian misalignment were combined [13].
One small prior study compared neurobehavioral deficits across 64 h of TSD and chronic, severe SR using ICCs [6]. The results were comparable to those found from repeated exposures of TSD [1, 3, 11, 12]. This result is likely due to the protocol [6] consisting of seven nights of 3 h time-in-bed (TIB) in the SR condition (although, in that study actual sleep duration during SR was not assessed), a SR amount so severe (in terms of both chronicity and sleep duration) that it is essentially equivalent to TSD [14, 15]. Critically, whether such reported stability extends to greater TIB durations for SR and/or to varying durations of TSD, or what possible influence the circadian system and/or sleep inertia play in such differential vulnerability remains unknown [6]. In the present study, we aimed to determine if responses to one acute night of TSD and five nights of 4 h TIB chronic SR were trait-like using more realistic and more commonly experienced durations of sleep loss shown to be neurobehaviorally equivalent by mathematical models [15].
Stable and trait-like interindividual differences have been observed in electroencephalogram power spectra responses to SR (5 h TIB) and 1-h naps [16]. These differences also have been observed in TSD, including polysomnographic sleep and slow-wave energy responses to sleep loss across 2–3 days [17–19], and in heart rate, heart rate variability, percentage of eyelid closure, blink rate, and electroencephalogram alpha power across 2.5–15 months [20].
A few prior studies have reported small sex differences in cognitive performance responses to sleep loss (reviewed in Ref. [21]). It remains unknown, however, whether there is a sex difference in the stability of trait-like neurobehavioral responses. Photoperiod affects neuroplasticity and mood and cognition [22], and affects neurobehavioral performance both separately [23] and in conjunction with the circadian system [22]. Thus, the season of testing is an environmental factor that may influence the repeatability of neurobehavioral responses to sleep loss but has not been systematically examined. Similarly, the stability of trait-like neurobehavioral responses for other key demographic factors have not been explicitly investigated, including body mass index (BMI), age, and race.
Some studies have found task-dependent variability in response to acute TSD, with differential susceptibility across cognitive domains as measured by different neurobehavioral tests [7, 11, 24–27]. Further research is needed to understand the relationships between performance outcomes on different cognitive tasks and between self-reported and objective measures of sleepiness and fatigue with respect to vulnerability and resistance to sleep loss comparing commonly experienced durations of deprivation for TSD and chronic SR.
In this study, we sought to address three gaps in prior research: (1) we determined whether trait-like neurobehavioral response deficits in the largest, most diverse sample to date are maintained between two common durations of sleep loss, acute TSD and chronic SR, separated by recovery within the same protocol; (2) we sought to explicitly test for the first time whether various demographically defined groups (sex, race, age, BMI) or environmentally defined groups (testing season) show comparable neurobehavioral stability for TSD and chronic SR exposures; (3) we determined the relationships among various cognitive performance measures and self-reported measures of sleepiness and fatigue across different, common types of sleep loss separated by a few days. We hypothesized an individual’s vulnerability or resistance to TSD and to chronic SR of 4 h per night for five consecutive nights in the same protocol would remain highly stable and that demographically and environmentally defined groups would show comparable stability. We also hypothesized objective performance measures would be related and self-reported measures would be related, but that measures would not be related across cognitive and self-reported domains for different types of sleep loss.
Methods
Participants
Eighty-three healthy individuals, representative of the racial composition of Philadelphia County, PA, between the ages of 21–50 years were recruited in response to study advertisements. Participants reported habitual nightly sleep durations between 6.5 and 8.5 h, with habitual bedtimes between 2200h and 0000h and habitual awakenings between 0600h and 0930h; these were confirmed via wrist actigraphy. Chronotype was determined via the Composite Scale of Morningness and Eveningness, with extreme morning and evening types excluded [28]. Participants did not engage in habitual napping and did not present with sleep disturbances (i.e. no complaints of daytime sleepiness, insomnia, or other sleep–wake disturbances) [29]. They did not have any acute or chronic psychological and medical conditions, as determined by questionnaires, interviews, physical exams, clinical history, and urine and blood tests (including a fasting blood glucose test). They were not taking any regular medications (except oral contraceptives) and were nonsmokers with BMIs between 17.3 and 30.9 kg/m2. They did not participate in transmeridian travel or shift work or have irregular sleep–wake routines in the 60 days before the study. Participants were monitored at home with actigraphy, sleep–wake diaries, and time-stamped call-ins to determine bedtimes and waketimes during the 7–14 days before the laboratory phase and the 7 days following the laboratory phase. Sleep disorders were excluded on the first laboratory night by oximetry and polysomnography (PSG) measurements, if applicable—in our sample, no participants demonstrated a sleep disorder. Participants were not allowed to use tobacco during the 7 days before the study, as verified by blood and urine screenings. The protocol was approved by the University of Pennsylvania’s Institutional Review Board. All participants provided written informed consent in accordance with the Declaration of Helsinki. They received compensation for participation.
Procedures
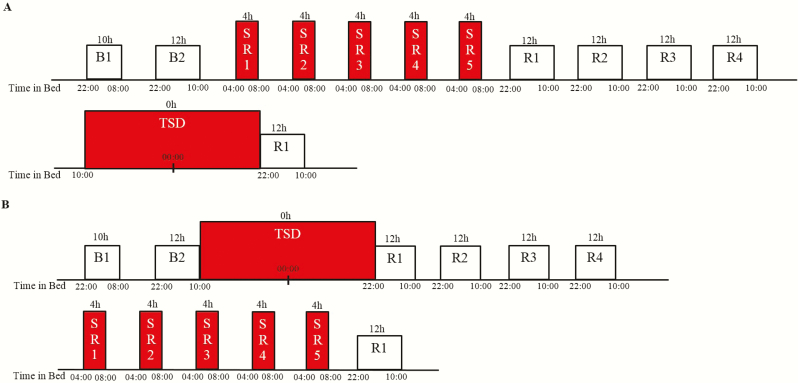
Participants engaged in a 13-day laboratory study in which they were studied continuously and received daily checks of vital signs and symptoms by nurses (with a physician on call). All participants experienced two types of sleep loss during the protocol, SR and TSD, with the order of sleep loss exposures counterbalanced across conditions. In addition, there were four nights of 12 h recovery sleep (2200 hours–1000 hours) between sleep loss conditions in order to ensure complete recuperation and return of neurobehavioral variables to baseline, and along with counterbalancing, to eliminate any possible lingering effects of one sleep loss condition on the other. Participants were randomized as a group (N = 4 per group) to one of the two conditions after two initial nights of baseline sleep of 10 h (2200 hours–0800 hours) and 12 h (2200 hours–1000 hours) TIB, respectively, and were blinded to condition assignment until after the second night of baseline sleep (Figure 1). Participants randomized to Condition A (N = 41) underwent five consecutive nights of sleep restricted to 4 h TIB per night (SR1-5, 0400 hours–0800 hours) followed by four consecutive nights of 12 h recovery sleep (2200 hours–1000 hours), one night of TSD (0 h TIB) during which they were kept awake for 36 h (1000 hours–2200 hours the following day), and then a final night of recovery sleep (2200 hours–1000 hours). Participants randomized to Condition B (N = 42) underwent one night of TSD (0 h TIB) during which they were kept awake for 36 h (1000 hours–2200 hours the following day), followed by four consecutive nights of 12 h recovery sleep (2200 hours–1000 hours), five consecutive nights of sleep restricted to 4 h TIB per night (SR1-5, 0400 hours–0800 hours) and then a final night of recovery sleep (2200 hours–1000 hours). . For both conditions, PSG was recorded during the SR1 and SR5 sleep opportunities as previously described [30].
Figure 1.
Experimental protocol. (A) Condition A: 2 nights of baseline sleep (B1 and B2) followed by 5 nights of sleep restriction (SR), followed by 4 nights of recovery sleep (R1–R4) and then one night of acute total sleep deprivation (TSD); (B) Condition B: 2 nights of baseline sleep (B1 and B2) followed by one night of acute TSD followed by 4 nights of recovery sleep (R1–R4) and then 5 nights of SR. The order of sleep loss was counterbalanced, with all participants receiving both types of sleep loss in a crossover design.
Participants were ambulatory and were permitted to perform sedentary activities such as watching television, reading, and playing video or board games between neurobehavioral test bouts (completed while seated at a computer); however, they were not allowed to exercise. Ambient temperature was maintained between 22°C and 24°C. Laboratory light levels remained constant at less than 50 lux during scheduled wakefulness and less than 1 lux during scheduled sleep periods. Participants were monitored continuously by trained staff throughout the study to ensure adherence.
Neurobehavioral measures
A precise computer-based neurobehavioral test battery was administered every 2 h during wakefulness and contained the following tasks: the 10-min Psychomotor Vigilance Test (PVT) [31, 32], the Digit Symbol Substitution Task (DSST) [33], the Forward and Backward Digit Span (DS) Task [33], the Karolinska Sleepiness Scale (KSS) [34], and the Profile of Mood States (POMS) [35]. The number of lapses (reaction times [RT] >500 ms) and errors (false starts with premature reactions without a stimulus or coincident false starts with RT <100 ms) and response speed were analyzed for the PVT.
In addition to these test bouts, a modified Maintenance of Wakefulness Test (MWT) [36–40]—a physiological measure of the ability to resist sleep—was administered at baseline, after five nights of SR and after TSD (a single trial was conducted between 1430h and 1600h) using a standard recording montage. Before each trial, the lights were dimmed to less than 10 lux and participants were instructed, “Keep your eyes open and try not to fall asleep.” Each trial was terminated at the first microsleep (10 s of theta activity) determined by the C3-A2 derivation or at 30 min if sleep onset did not occur. MWT scores represented either the time (minutes) to microsleep initiation or 30 min (if no microsleep occurred).
Statistical analyses
To maximize the analysis of as many test bouts as possible, response to sleep loss was assessed using the average value of data collected every 2 h from 2200h/0000h to 2000h during TSD, as has been done in our previous studies [7], and using the average value of data collected every 2 h from 0800h/1000h to 2000h after the fifth night of SR. Sleep loss difference from baseline variables were not calculated, as has been done in our other studies [7] since participants had the same baseline for both TSD and SR exposures. A secondary analysis examined test bouts only between 0800h/1000h and 2000h during TSD and SR5 to remove possible circadian system influences during TSD and sleep inertia influences during SR5 on ICCs from the larger, more inclusive analysis. One-way analysis of variance (ANOVAs) compared pre-study chronotype, sleep and demographic measures, and SR1 and SR5 sleep duration between Conditions A and B. ICCs [41] and their 95% confidence intervals (CIs) (two-way mixed, absolute agreement, average measures; SPSS v25) assessed the interindividual differences and intraindividual stability of neurobehavioral responses (absolute values) to TSD and SR for the entire study population and for groups defined by order of sleep loss (Condition A [N = 41] vs. Condition B [N = 42]), testing season (spring/summer [N = 42] vs. fall/winter [N = 41]), sex (male [N = 47] vs. female [N = 36]), race (African American [N = 60] vs. Caucasian [N = 15]), age (median split, younger [N = 42] vs. older [N = 41]) and BMI (median split, underweight/normal weight [N = 42] vs. overweight/obese [N = 41] as defined using the World Health Organization classification). The following ranges characterize ICCs and reflect the stability of interindividual differences: 0.0–0.2 (slight); 0.2–0.4 (fair); 0.4–0.6 (moderate); 0.6–0.8 (substantial); and 0.8–1.0 (almost perfect) [41]. The overlap of CIs was used to assess whether systematic differences in stability existed between different groups or different test bout intervals [42–44]. Spearman’s rho assessed the relative rank of individuals’ averaged SR-TSD responses across neurobehavioral measures.
Results
Eighty-three healthy adults (mean ± SD, 34.7 ± 8.9 years; 36 females) (aged 21–50 years, 72.3% African American; 43.4% female) participated in the study, with N = 41 randomly assigned to Condition A (experienced five consecutive nights of SR first) and N = 42 randomly assigned to Condition B (experienced one night of TSD first). There were no significant differences between sleep loss order of conditions in age (p > 0.05), BMI (p > 0.05), the percentage of participants who were African American (p > 0.05) or female (p > 0.05), or in chronotype (p > 0.05), or in pre-study actigraphic sleep duration (p > 0.05), onset (p > 0.05), offset (p > 0.05), or midpoint (p > 0.05) (Table 1). During SR1, participants slept an average of 3:39 ± 15.54 min, with no significant differences in duration between the two conditions (p > 0.05). Similarly, during SR5, participants slept an average of 3:47 ± 8.76 min, with no significant differences between the two conditions (p > 0.05).
Table 1.
Participant characteristics (mean ± SD)
| N | Age (y) | Body mass index (kg/m2) | Female N (%) | African American N (%) | Chronotype* | Sleep duration (h)† | Sleep onset (time ± h)† | Sleep offset (time ± h)† | Sleep midpoint (time ± h)† | |
|---|---|---|---|---|---|---|---|---|---|---|
| All participants | 83 | 34.7 ± 8.9 | 24.7 ± 3.4 | 36 (43.4%) | 60 (72.3%) | 41.9 ± 5.6 | 8.0 ± 0.6 | 23:35 ± 0.9 | 7:35 ± 0.8 | 3:36 ± 0.8 |
| Condition A (SR first) | 41 | 33.9 ± 9.0 | 24.9 ± 3.3 | 18 (43.9%) | 31 (75.6%) | 41.5 ± 5.8 | 8.0 ± 0.5 | 23:33 ± 0.9 | 7:39 ± 0.8 | 3:38 ± 0.8 |
| Condition B (TSD first) | 42 | 35.5 ± 8.8 | 24.4 ± 3.4 | 18 (42.9%) | 29 (69.0%) | 42.3 ± 5.5 | 8.0 ± 0.6 | 23:37 ± 0.9 | 7:32 ± 0.8 | 3:35 ± 0.8 |
*Composite Scale of Morningness and Eveningness [28].
†Determined by wrist actigraphy (1 week prior to study entry).
Cognitive performance
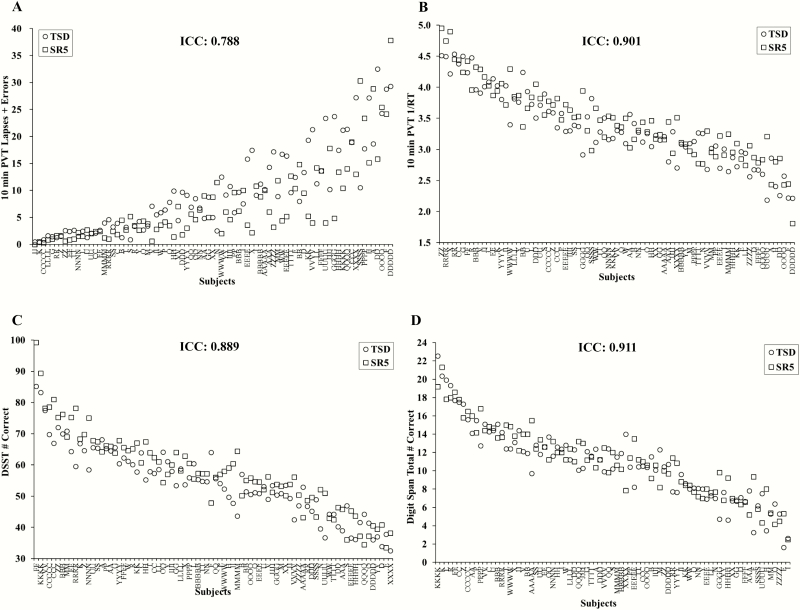
Cognitive performance was consistent across exposures to SR and TSD with a substantial ICC for 10-min PVT lapses and errors: 0.788 (95% CI, 0.662, 0.866), and almost perfect ICCs for all other measures: 10-min PVT response speed (1/RT): 0.901 (95% CI, 0.845, 0.936); DSST: 0.889 (95% CI, 0.768, 0.940); and DS: 0.911 (95% CI, 0.863, 0.942) (Figure 2). There were large phenotypic individual differences in cognitive responses across participants to TSD: average 10-min PVT lapses and errors ranged from 0.36 to 32.5; average 10-min PVT 1/RT ranged from 2.18 to 4.51 s; average DSST performance ranged from 32.5 to 85.2 correct responses; and average DS performance ranged from 1.64 to 22.55 correct responses. Similarly, there were large phenotypic individual differences in cognitive responses across participants to SR: average 10-min PVT lapses and errors ranged from 0.00 to 37.83; average 10-min PVT 1/RT ranged from 1.81 to 4.95 s; average DSST performance ranged from 33.5 to 99.2 correct responses; and average DS performance ranged from 2.5 to 21.33 correct responses.
Figure 2.
Individual differences and substantial phenotypic stability of cognitive measures to total sleep deprivation (TSD) and sleep restriction (SR) exposures. Neurobehavioral vulnerability to TSD and SR exposures showed trait-like stability across performance measures, as evident by substantial intraclass correlation coefficients (ICCs): (A) 10-min Psychomotor Vigilance Test (PVT) lapses and errors, ICC = 0.788; and almost perfect ICCs: (B) 10-min PVT 1/reaction time (RT), ICC = 0.901; (C) Digit Symbol Substitution Task (DSST) number correct, ICC = 0.889; and (D) Digit Span (DS) total number correct, ICC = 0.911. In all graphs, participants (denoted individually with letters) are ordered left to right from least to greatest response as determined by the average of the TSD (circle) and SR5 (square) scores. See text for ICC ranges.
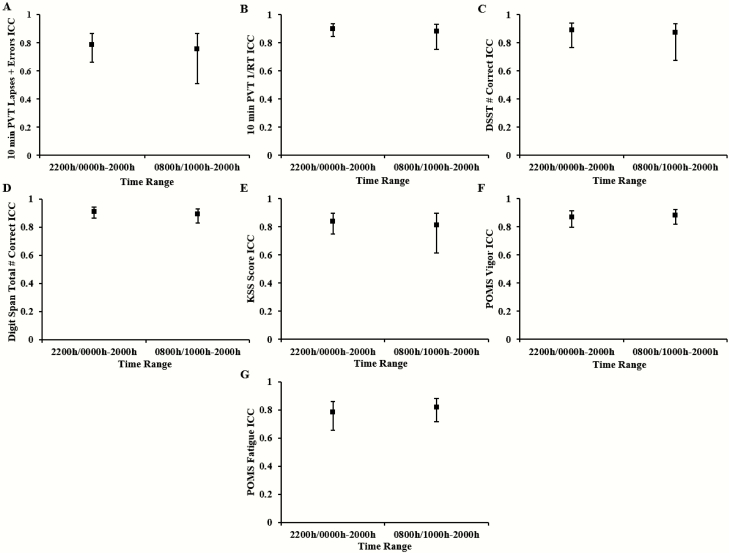
The ICCs from the secondary analysis comparing test bouts from 0800 hours/1000 hours to 2000 hours of TSD versus the same time range from SR5 remained substantial for the 10-min PVT lapses and errors: 0.756, and almost perfect for all other objective measures: 10-min PVT 1/RT: 0.88; DSST: 0.872; DS: 0.891 (Figure 3A–D). As shown in Figure 3, the CIs for each measure overlapped with the CIs for each measure from the analysis using all available TSD test bouts, indicating that there was no systematic difference in ICCs for test bout time range examined.
Figure 3.
Comparison of intraclass correlation coefficients (ICCs) of neurobehavioral measures by test bout range. ICCs and corresponding 95% confidence intervals (CIs) for test bouts from 2200 hours/0000 hours to 2000 hours of total sleep deprivation (TSD) and from 0800 hours/1000 hours to 2000 hours of the fifth night of sleep restriction (SR5) versus test bouts from 0800 hours/1000 hours to 2000 hours of TSD and from 0800 hours/1000 hours to 2000 hours of SR5 for (A) 10-min Psychomotor Vigilance Test (PVT) lapses and errors, (B) 10-min PVT 1/reaction time (RT), (C) Digit Symbol Substitution Task (DSST) number correct, (D) Digit Span (DS) total number correct, (E) Karolinska Sleepiness Scale (KSS) score, (F) Profile of Mood States (POMS) Vigor; and (G) POMS Fatigue. Overlapping confidence intervals (CIs) for each measure indicate that there was no systematic difference in ICCs for test bout time range used to measure stability. A test bout comparison for the Maintenance of Wakefulness Test (MWT) was not applicable because the MWT only was performed once during each sleep loss condition.
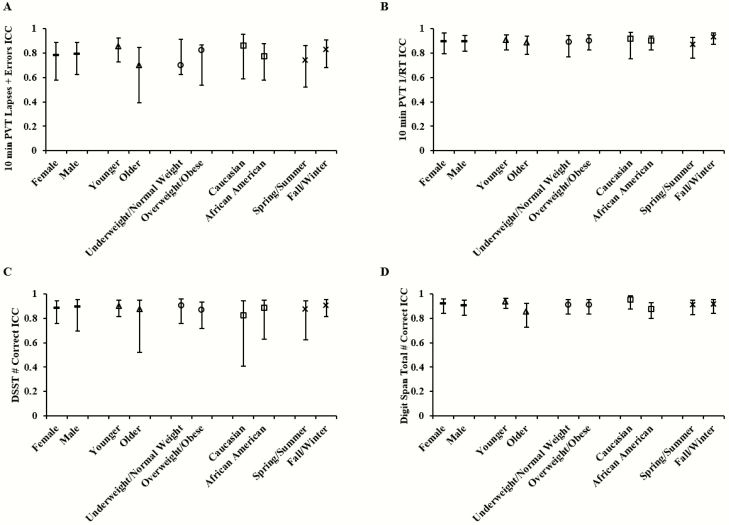
Sleep loss order and testing season did not appreciably affect the ICCs for any cognitive performance measure: ICC differences for sleep loss order condition ranged from 0.02 to 0.082 and for season of testing ranged from 0.007 to 0.088. Similarly, ICCs were not appreciably affected by demographic factors including by sex (group difference ranging from 0.002 to 0.015), age (group differences ranging from 0.018 to 0.156), race (group difference ranging from 0.019 to 0.084), or BMI (group difference ranging from 0 to 0.071). The 95% CIs of ICCs in each demographic and environmental group overlapped for all cognitive measures (Figure 4). A secondary analysis with test bouts from only between 0800h/1000h and 2000h was not performed for each demographic group because there were no differences in ICCs when comparing selected versus all test bouts.
Figure 4.
Comparison of intraclass correlation coefficients (ICCs) of cognitive measures by demographic or environmental category. ICCs and corresponding 95% confidence intervals (CIs) for sex (female vs. male, both ICCs denoted with dashed points), age (younger vs. older, both ICCs denoted with triangle points), BMI (underweight/normal weight vs. overweight/obese, both ICCs denoted with circle points), race (Caucasian vs. African American, both ICCs denoted with square points), and season of testing (spring/summer vs. fall/winter, both ICCs denoted with x-marked points) for (A) 10-min Psychomotor Vigilance Test (PVT) lapses and errors, (B) 10-min PVT 1/reaction time (RT), (C) Digit Symbol Substitution Task (DSST) number correct; and (D) Digit Span (DS) total number correct. Overlapping CIs within each demographic or environmental subgroup indicate there was no systematic difference in ICCs between groups.
Self-reported sleepiness, vigor, and fatigue
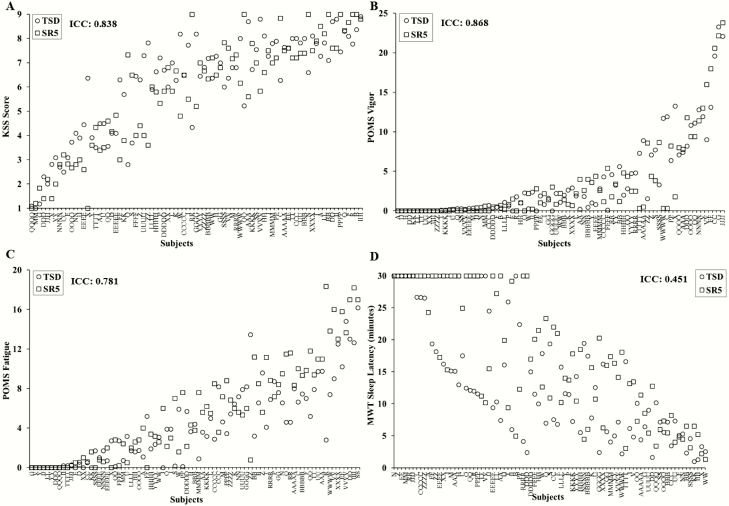
Self-reported ratings of sleepiness, fatigue and vigor were stable across exposures to SR and TSD, with almost perfect ICCs for KSS: 0.838 (95% CI, 0.748, 0.896) and POMS Vigor (POMS-V): 0.868 (95% CI, 0.796, 0.915) and a substantial ICC for POMS Fatigue (POMS-F): 0.781 (95% CI, 0.655, 0.860) (Figure 5A–C). There were large phenotypic individual differences in self-reported responses across participants to TSD: average KSS ratings ranged from 1.00 to 8.91; average POMS-V ratings ranged from 0 to 23.27; and average POMS-F ratings ranged from 0.00 to 16.18. Similarly, there were large phenotypic individual differences in self-reported responses across participants to SR: average KSS ratings ranged from 1.00 to 9.00; average POMS-V ratings ranged from 0.00 to 23.8; and average POMS-F ratings ranged from 0.00 to 18.33.
Figure 5.
Individual differences and substantial phenotypic stability of self-reported measures and physiological alertness to total sleep deprivation (TSD) and sleep restriction (SR) exposures. Neurobehavioral vulnerability to TSD and SR exposures showed trait-like stability across self-reported measures, as evident by almost perfect intraclass correlation coefficients (ICCs) for (A) Karolinska Sleepiness Scale (KSS) score, ICC = 0.838; and (B) Profile of Mood States (POMS) Vigor, ICC = 0.868, and a substantial ICC for (C) POMS Fatigue, ICC = 0.781. A physiological alertness measure also showed trait-like stability to SR and TSD exposures, as evident by a moderate ICC for the (D) Maintenance of Wakefulness Test (MWT), ICC=0.451. In all graphs, participants (denoted individually with letters) are ordered left to right from least to greatest response as determined by the average of the TSD (circle) and SR5 (square) ratings. See text for ICC ranges.
The secondary analysis computed ICCs for test bouts from 0800 hours/1000 hours to 2000 hours in TSD and SR5 for each self-reported measure. The ICCs remained almost perfect for the KSS: 0.812 and for POMS-V: 0.884, while POMS-F became almost perfect at 0.816 (ICC increase of 0.035) (Figure 3E–G). As shown in Figure 3, the CIs for each measure overlapped with the CIs for each measure from the analysis using all available TSD test bouts, indicating that there was no systematic difference in ICCs for test bout time range examined.
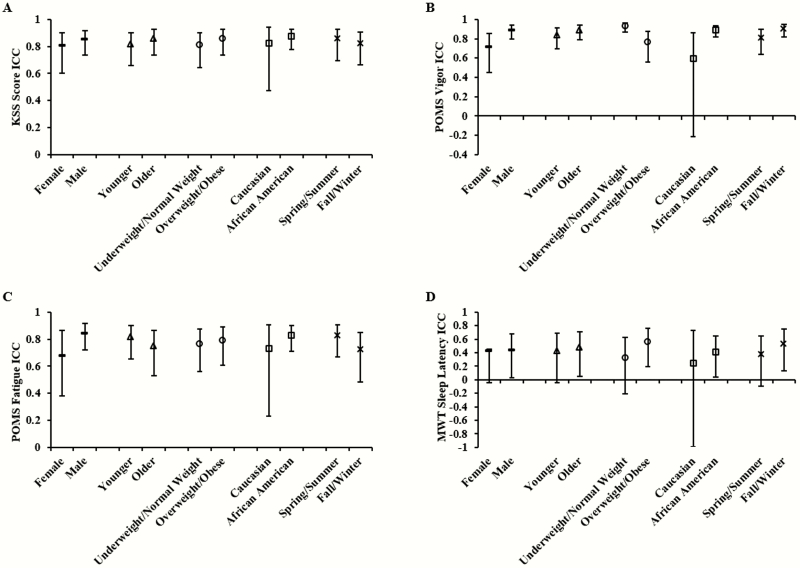
Sleep loss order and testing season did not appreciably affect the ICCs for any self-reported performance measure: ICC differences for sleep loss order condition ranged from 0.013 to 0.088 and for season of testing ranged from 0.038 to 0.102. Similarly, ICCs were not appreciably affected by demographic factors including by sex (group differences ranging from 0.048 to 0.165), age (group difference ranging from 0.045 to 0.066), race (group difference ranging from 0.052 to 0.296), or BMI (group difference ranging from 0.026 to 0.163). The 95% CIs of ICCs within each demographic and environmental group overlapped for all self-reported measures (Figure 6A–C). A secondary analysis with test bouts from only between 0800 hours/1000 hours and 2000 hours was not performed for each demographic group because there were no differences in ICCs when comparing selected versus all test bouts.
Figure 6.
Comparison of intraclass correlation coefficients (ICCs) of self-reported and physiological alertness measures by demographic or environmental category. ICCs and corresponding 95% confidence intervals (CIs) for sex (female vs. male, both ICCs denoted with dashed points), age (younger vs. older, both ICCs denoted with triangle points), BMI (underweight/normal weight vs. overweight/obese, both ICCs denoted with circle points), race (Caucasian vs. African American, both ICCs denoted with square points), and season of testing (spring/summer vs. fall/winter, both ICCs denoted with x-marked points) for A) Karolinska Sleepiness Scale (KSS) score, (B) Profile of Mood States (POMS) Vigor, (C) POMS Fatigue; and (D) Maintenance of Wakefulness Test (MWT). Overlapping CIs within each demographic or environmental subgroup indicates there was no systematic difference in ICCs between groups.
Physiologic alertness
Physiologic alertness was measured using the MWT. MWT latency was moderately consistent across SR and TSD exposures: ICC = 0.451 (95% CI, 0.163, 0.641) (Figure 5D). There were large phenotypic individual differences in MWT responses across participants to TSD: average latency ranged from 1.00 to 30 min (30 min indicates that no microsleeps occurred for the duration of the test). Similarly, there were large phenotypic individual differences in MWT responses across participants to SR: average latency ranged from 1.33 to 30 min (30 min indicates that no microsleeps occurred for the duration of the test).
Sleep loss order and testing season did not appreciably affect the ICCs for physiological alertness, whereby the ICC difference for sleep loss order condition was 0.232 and for season of testing it was 0.161. Similarly, ICC group differences were not appreciably affected by demographic factors including by sex (0.012), age (0.045), race (0.166), or BMI (0.238). The 95% CI of ICCs overlapped in each demographic category, indicating no differences in stability within each group (Figure 6D).
Neurobehavioral measures: relative rank relationships
Cognitive performance and self-reported ratings showed consistency across the different neurobehavioral responses to SR and TSD (Table 2). During SR and TSD, PVT lapses and errors were positively correlated with PVT 1/RT (ρ = 0.70, p < 0.001), DSST (ρ = 0.37, p < 0.001) and DS (ρ = 0.44, p < 0.001). PVT 1/RT was also positively correlated with DSST (ρ = 0.41, p < 0.001) and DS (ρ = 0.56, p < 0.001) and inversely correlated with POMS-V (ρ = −0.33, p < 0.05). DSST was positively correlated with DS (ρ = 0.50, p < 0.001) and KSS was positively correlated with POMS-F (ρ = 0.66, p < 0.001) and inversely correlated with POMS-V (ρ = −0.43, p < 0.001). There were no significant correlations between PVT lapses and errors and KSS, POMS-V, or POMS-F, or between PVT 1/RT and KSS or POMS-F (all p > 0.05). DSST was not significantly correlated with KSS, POMS-V, or POMS-F; DS was not significantly correlated with KSS or POMS-F; and POMS-V was not significantly correlated with POMS-F (all p > 0.05). Relative rank analyses were not performed with test bouts from only 0800 hours/1000 hours to 2000 hours for SR and TSD because there were no differences in ICCs when comparing selected versus all test bouts.
Table 2.
Spearman’s rank correlation coefficients for neurobehavioral measures for TSD and SR5 exposures
| PVT lapses and errors | PVT 1/RT | DSST | DS | KSS | POMS-V | POMS-F | |
|---|---|---|---|---|---|---|---|
| PVT lapses and errors | 0.70** | 0.37** | 0.44** | 0.11 | −0.19 | −0.06 | |
| PVT 1/RT | 0.70** | 0.41** | 0.56** | 0.04 | −0.33** | −0.05 | |
| DSST | 0.37** | 0.41** | 0.50** | −0.18 | −0.05 | −0.17 | |
| DS | 0.44** | 0.56** | 0.50** | −0.07 | −0.24* | −0.19 | |
| KSS | 0.11 | 0.04 | -0.18 | -0.07 | −0.43** | 0.66** | |
| POMS-V | −0.19 | −0.33** | -0.05 | -0.24* | −0.43** | −0.19 | |
| POMS-F | −0.06 | −0.05 | -0.17 | -0.19 | 0.66** | −0.19 |
**p < 0.01, *p < 0.05.
Discussion
This study provides evidence for phenotypic stability of individual neurobehavioral responses across two commonly experienced forms of sleep loss, acute TSD and chronic SR. Trait-like stability was observed in performance and self-reported measures with substantial to almost perfect ICCs between 78% and 91%, indicating that participants who displayed vulnerability to TSD also displayed vulnerability to SR. ICCs for various demographic groups also showed marked stability, with no significant differences between subgroups. Neurobehavioral outcomes showed consistency across objective measures, and consistency across self-reported measures, but generally not between objective and self-reported domains.
Our findings replicate studies comparing TSD-TSD exposures across short time intervals [1, 3, 11, 12], and TSD-TSD [7, 20] and SR-SR [7] exposures across long time intervals, with respect to range of performance responses and robust ICCs. Our results also are similar to a study comparing two nights of TSD and chronic, severe SR [6]. ICCs from the current study are within the ranges for polysomnographic sleep and slow-wave energy responses to TSD–TSD exposures [18, 19], and within the ranges for heart rate, heart rate variability, percentage of eyelid closure, blink rate, and electroencephalogram alpha power responses to TSD–TSD exposures [20]. In addition, the ICCs reported in this study are generally more stable than the ranges found for energy balance responses to TSD–SR exposures and to long duration SR–SR exposures [45, 46].
The MWT was the least stable measurement for the entire study population, and also when divided by groups defined based on sex, age, race, BMI, season of testing, and sleep loss order. It was the only measure with a moderate ICC, compared to all other measures that showed substantial or almost perfect ICCs. The lower MWT ICCs are similar to those reported by Rupp et al. [6]. The MWT required participants to sit still and stay quiet during testing; this behavioral test component may have enhanced the differences within individuals and decreased the variability between individuals. Additionally, the lack of variability in MWT responses, with many individuals staying awake for the full 30 min, likely contributed to the lower ICC.
The secondary analysis comparing the ICCs and CIs of 0800 hours/1000 hours to 2000 hours TSD versus 0800 hours/1000 hours to 2000 hours SR5 test bouts was performed to assess the stability of responses after removing possible circadian system influences on TSD and possible sleep inertia influences on SR5. The comparison showed no significant differences, with CIs overlapping for all variables. Although there were small increases in the range of CIs for some measures, these were not substantial. Understanding how the circadian system and/or sleep inertia influences neurobehavioral performance stability during sleep loss requires further systematic investigation.
We explicitly examined for the first time the stability of neurobehavioral responses by various demographic and environmental factors. There were no significant differences between ICCs for males and females in any measure. Individual vulnerability to sleep loss in both sexes was stable across TSD and chronic SR, with ICCs ranging from 0.679 to 0.919 in objective and self-reported measures (excluding the MWT), with CIs of all measures overlapping. Our findings contrast with past studies analyzing sex and interindividual differences in energy balance after repeated SR exposures [45] or TSD–SR exposures [46]. In those studies, males were more consistent in weight [45] and caloric intake [45, 46] changes after sleep loss, showing higher ICCs, and females were less consistent, showing lower ICCs [45, 46]. These inconsistencies may be due to sex differences in attitudes of food and behavioral dependence associated with energy balance in males and females [47]; neither of these factors are likely to influence performance, which may explain the higher ICCs for neurobehavioral variables.
In addition to sex, other demographic and environmental factors also showed no considerable differences between subgroups. While season has been reported to influence brain responses to sustained attention and working memory tasks using cross-sectional data [48], season of testing did not affect ICC stability to sleep loss across various measures. BMI classifications also did not affect the stability of neurobehavioral responses—similar findings have been reported for energy balance responses [45]. Stability of subgroups divided by age or race have not been investigated previously and did not show systematic differences within demographic groups. Yet, the 95% CIs for POMS Vigor and Fatigue and for the MWT in the Caucasian subgroup were large compared to the CIs of the African American subgroup. This may be partially attributed to the small sample size used for Caucasians (N = 15), in comparison to African Americans (N = 60), yielding a larger CI. Nevertheless, the 95% CIs overlapped for all measures and for all groups. Therefore, demographic and environmental factors cannot be reliably used for predicting whether individuals are vulnerable/resistant to sleep loss.
In addition to demographic factors reported here, individual differences have not been accounted for by sleep need or circadian chronotype; moreover, psychometric scales have not reliably identified neurobehaviorally vulnerable individuals [2, 11]. A study with monozygotic and dizygotic twin pairs found substantial differences in PVT responses to TSD, whereby 56.2% of the total variance in monozygotic twins was due to variance between pairs compared with only 14.5% in dizygotic twins, indicating the response to acute TSD is a highly stable, genetically determined trait [49]. Moreover, candidate gene, “omics” and other biomarker studies highlight a significant role for biological factors underlying individual differences to sleep loss [50–53]. However, additional studies are needed to further explore the biological underpinnings of such stable phenotypic responses.
For TSD–SR exposures, PVT lapses and errors and 1/RT measures were highly correlated with each other and with the DSST and/or DS; similarly, KSS scores were highly correlated with POMS-F and POMS-V scores. Overall, cognitive performance tests were not correlated strongly with the KSS or either POMS measure. Our findings are consistent with prior studies that found objective performance assessments are not congruent with self-reported ratings [2, 7, 11, 12, 24, 25], indicating participants’ ranking in terms of resistance or vulnerability varies depending on the task or measure. Our findings indicate self-reported ratings are insufficient to determine who will be vulnerable to sleep disturbance, and thus have important implications for the development of tests to determine impairment.
There are a few limitations to the current experiment. All participants were healthy, thus making it difficult to generalize our findings to individuals with sleep or mood disorders, or with other medical conditions. Similarly, participants were all between the ages of 21 and 50. Adolescents and older individuals may show less stability in their neurobehavioral responses to sleep loss across time, particularly since these life span periods are characterized by numerous marked neurobehavioral changes [54, 55]. In addition, although there was minimal variability in sleep timing due to our selection criteria, the timing of sleep loss was not adapted to participants’ habitual sleep schedules.
Although these data were collected in a laboratory setting, our results have implications for the military, health care workers, truck drivers, and workers in other applied settings where sleep loss is common, and in which individual differences in vulnerability to the neurobehavioral deficits caused by sleep deprivation could have hazardous consequences [56–58]. Importantly, our results also help inform, refine, and expand various existing mathematical models designed to predict neurobehavioral performance resulting from different types of sleep loss [15, 59–61]. We show, for the first time, robust differential vulnerability and phenotypic stability of neurobehavioral responses to two commonly experienced types of sleep loss, with nominal influences of various demographic and environmental factors, heralding the use of biomarkers and countermeasures for prediction and mitigation of this critical vulnerability.
Funding
This work was primarily supported by the Department of the Navy, Office of Naval Research (Award No. N00014-11-1-0361) to NG. Other support provided by National Aeronautics and Space Administration (NASA) (NNX14AN49G to NG) and Clinical and Translational Research Center grant UL1TR000003. None of the sponsors had any role in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of interest statement: None declared.
Acknowledgments
We thank the faculty and staff of the Unit of Experimental Psychiatry for their contributions to this study. NG designed the overall study, conducted statistical analyses of the data, and provided financial support. EY and NG prepared the manuscript and both authors reviewed and approved the final manuscript.
Where work was conducted: Division of Sleep and Chronobiology, Department of Psychiatry, University of Pennsylvania Perelman School of Medicine, 1017 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104
References
- 1. Dijkman M, et al. Effects of reduced stimulation on neurobehavioral alertness depend on circadian phase during human sleep deprivation. Sleep Res. 1997;26:265. [Google Scholar]
- 2. Van Dongen HP, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 3. Van Dongen HP, et al. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med. 2004;75(3 Suppl):A147–A154. [PubMed] [Google Scholar]
- 4. Van Dongen HP, et al. Individual differences in vulnerability to sleep loss in the work environment. Ind Health. 2009;47(5):518–526. [DOI] [PubMed] [Google Scholar]
- 5. Chua EC, et al. Sustained attention performance during sleep deprivation associates with instability in behavior and physiologic measures at baseline. Sleep. 2014;37(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rupp TL, et al. Trait-like vulnerability to total and partial sleep loss. Sleep. 2012;35(8):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dennis LE, et al. Healthy adults display long-term trait-like neurobehavioral resilience and vulnerability to sleep loss. Sci Rep. 2017;7(1):14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goel N, et al. Predicting risk in space: genetic markers for differential vulnerability to sleep restriction. Acta Astronaut. 2012;77:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goel N, et al. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goel N, et al. Phenotyping of neurobehavioral vulnerability to circadian phase during sleep loss. Methods Enzymol. 2015;552:285–308. [DOI] [PubMed] [Google Scholar]
- 11. Van Dongen HP, et al. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–433. [PubMed] [Google Scholar]
- 12. Leproult R, et al. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R280–R290. [DOI] [PubMed] [Google Scholar]
- 13. Sprecher KE, et al. Trait-like vulnerability of higher-order cognition and ability to maintain wakefulness during combined sleep restriction and circadian misalignment. Sleep. 2019;42(8). doi:10.1093/sleep/zsz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Dongen HP. Connecting the dots: from trait vulnerability during total sleep deprivation to individual differences in cumulative impairment during sustained sleep restriction. Sleep. 2012;35(8):1031–1033. [PMC free article] [PubMed] [Google Scholar]
- 15. McCauley P, et al. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256(2):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ong JL, et al. Trait-like characteristics of sleep EEG power spectra in adolescents across sleep opportunity manipulations. J Sleep Res. 2019;28(5):e12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rusterholz T, et al. Interindividual differences in the dynamics of the homeostatic process are trait-like and distinct for sleep versus wakefulness. J Sleep Res. 2017;26(2):171–178. [DOI] [PubMed] [Google Scholar]
- 18. Tarokh L, et al. The spectrum of the non-rapid eye movement sleep electroencephalogram following total sleep deprivation is trait-like. J Sleep Res. 2015;24(4):360–363. [DOI] [PubMed] [Google Scholar]
- 19. Tucker AM, et al. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16(2):170–180. [DOI] [PubMed] [Google Scholar]
- 20. Chua EC, et al. Individual differences in physiologic measures are stable across repeated exposures to total sleep deprivation. Physiol Rep. 2014;2(9):e12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajali V, et al. Sex differences in sleep and sleep loss-induced cognitive deficits: the influence of gonadal hormones. Horm Behav. 2019;108:50–61. [DOI] [PubMed] [Google Scholar]
- 22. Porcu A, et al. Photoperiod-induced neuroplasticity in the circadian system. Neural Plast. 2018;2018:5147585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LeGates TA, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Dongen HP, et al. Individual differences in cognitive vulnerability to fatigue in the laboratory and in the workplace. Prog Brain Res. 2011;190:145–153. [DOI] [PubMed] [Google Scholar]
- 25. Frey DJ, et al. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13(4):305–315. [DOI] [PubMed] [Google Scholar]
- 26. Louca M, et al. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep. 2014;37(11):1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tucker AM, et al. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith CS, et al. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. [DOI] [PubMed] [Google Scholar]
- 29. Douglass AB, et al. The sleep disorders questionnaire. I: creation and multivariate structure of SDQ. Sleep. 1994;17(2):160–167. [DOI] [PubMed] [Google Scholar]
- 30. Spaeth AM, et al. Objective measurements of energy balance are associated with sleep architecture in healthy adults. Sleep. 2017;40(1). doi:10.1093/sleep/zsw018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim J, et al. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. [DOI] [PubMed] [Google Scholar]
- 32. Basner M, et al. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hartman DE. Wechsler adult intelligence scale IV (WAIS IV): return of the gold standard. Appl Neuropsychol. 2009;16(1):85–87. [DOI] [PubMed] [Google Scholar]
- 34. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. [DOI] [PubMed] [Google Scholar]
- 35. Bourgeois A, et al. Full-scale and short-form of the Profile of Mood States: a factor analytic comparison. J Sport Behav. 2010;33(4):355–376. [Google Scholar]
- 36. Banks S, et al. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33(8):1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goel N, et al. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4(6):e5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goel N, et al. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;75(17):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goel N, et al. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One. 2011;6(12):e29283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goel N, et al. Cognitive workload and sleep restriction interact to influence sleep homeostatic responses. Sleep. 2014;37(11):1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Landis JR, et al. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 42. Lu L, et al. Reliability analysis: calculate and compare intra-class correlation coefficients (ICC) in SAS. Paper presented at: SAS Conference; November 2007; Baltimore, MD.
- 43. Stolarova M, et al. How to assess and compare inter-rater reliability, agreement and correlation of ratings: an exemplary analysis of mother-father and parent-teacher expressive vocabulary rating pairs. Front Psychol. 2014;5:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koo TK, et al. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spaeth AM, et al. Phenotypic vulnerability of energy balance responses to sleep loss in healthy adults. Sci Rep. 2015;5:14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dennis LE, et al. Phenotypic stability of energy balance responses to experimental total sleep deprivation and sleep restriction in healthy adults. Nutrients. 2016;8(12). doi: 10.3390/nu8120823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spaeth AM, et al. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100(2):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer C, et al. Seasonality in human cognitive brain responses. Proc Natl Acad Sci U S A. 2016;113(11):3066–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuna ST, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012;35(9):1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goel N. Genetics of sleep timing, duration and homeostasis in humans. Sleep Med Clin. 2011;6(2):171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goel N. “Omics” approaches for sleep and circadian rhythm research: biomarkers for identifying differential vulnerability to sleep loss. Curr Sleep Med Rep. 2015;1(1):38–46. [Google Scholar]
- 52. Moreno-Villanueva M, et al. The degree of radiation-induced DNA strand breaks is altered by acute sleep deprivation and psychological stress and is associated with cognitive performance in humans. Sleep. 2018;41(7). doi:10.1093/sleep/zsy067 [DOI] [PubMed] [Google Scholar]
- 53. Goel N. Neurobehavioral effects and biomarkers of sleep loss in healthy adults. Curr Neurol Neurosci Rep. 2017;17(11):89. [DOI] [PubMed] [Google Scholar]
- 54. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. [DOI] [PubMed] [Google Scholar]
- 55. Kramer JH, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21(4):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Dongen HP, et al. Investigating systematic individual differences in sleep-deprived performance on a high-fidelity flight simulator. Behav Res Methods. 2006;38(2):333–343. [DOI] [PubMed] [Google Scholar]
- 57. Caldwell JA, et al. The effects of 37 hours without sleep on the performance of F-117 pilots. Mil Psychol. 2004;16(3):163–181. [Google Scholar]
- 58. Howard ME, et al. Deterioration in driving performance during sleep deprivation is similar in professional and nonprofessional drivers. Traffic Inj Prev. 2014;15(2):132–137. [DOI] [PubMed] [Google Scholar]
- 59. Rajdev P, et al. A unified mathematical model to quantify performance impairment for both chronic sleep restriction and total sleep deprivation. J Theor Biol. 2013;331:66–77. [DOI] [PubMed] [Google Scholar]
- 60. Ramakrishnan S, et al. Can a mathematical model predict an individual’s trait-like response to both total and partial sleep loss? J Sleep Res. 2015;24(3):262–269. [DOI] [PubMed] [Google Scholar]
- 61. McCauley P, et al. Dynamic circadian modulation in a biomathematical model for the effects of sleep and sleep loss on waking neurobehavioral performance. Sleep. 2013;36(12):1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]