Abstract
Iterative cycles of epithelial-mesenchymal transition (EMT) and mesenchymal to epithelial transition (MET) are responsible for epithelial plasticity necessary to achieve functional wound closure. Restoration of barrier function of the repaired skin is a hallmark of functional wound closure. Both EMT and MET are subject to control by glycemic status. New work in this issue supports the notion that hyperglycemia blunts epithelial plasticity.
Skin wound repair, regenerative and near-perfect under fetal conditions, remain intact with scarring in healthy adults but it stalls under diabetic conditions (Gnyawali et al., 2020). During morphogenesis, epithelial cells dismantle cell adhesion and tight junction structures in an effort to acquire a mesenchymal phenotype. This process, termed epithelial-mesenchymal transition (EMT), is prominent in fetal skin. It is noted after epidermal development and it gives rise to dermal α-smooth muscle actin expressing cells. In adults, these cells contribute to wound contraction and re-epithelialization resulting in wound closure with a characteristic scar phenotype (Kong et al., 2006). EMT is marked by the induction of prototypic epithelial markers coupled with the loss of apical–basal polarity and increased cell motility caused by cytoskeleton reorganization. Re-epithelialization of wounds relies on turning down intercellular adhesion followed by keratinocyte migration in the epidermis proximal to wound margins. Purse-string wound contraction caused by EMT-derived myofibroblasts, at the same time, prepares the underlying connective tissue bed. In this issue of the JID, Tan et al report that hyperglycemia restrains keratinocyte EMT. Specifically, their work shows that acetylcholine-induced EMT is at risk under conditions of hyperglycemia jeopardizing diabetic wound repair (Tan et al., 2020). This work draws attention to the significance of non-neuronal acetylcholine in the regulation of diabetic wound healing. Cholinergic pathways, nicotinic and muscarinic receptors are known to be present in keratinocytes. Impairment in these receptors can cause Grover disease, an eruption of intraepidermal acantholysis presenting as crusted reddened papules (Paslin, 2012). In vitro studies show that acetylcholine improves cell migration (Uberti et al., 2017) and, in vivo, cholinergic peptides augment skin wound closure(Chernyavsky et al., 2012).
Covering of wounds without discharge is an inadequate marker of wound closure since biofilm infected wounds may achieve such closure without restoring the barrier function of the repaired skin. Repair that results in barrier function deficient skin is faulty as it compromises the biomechanical properties of closed wounds in a way that favors wound recurrence. The concept of functional wound closure has thus emerged. Functional wound closure is achieved when covering of the wound defect is achieved without discharge and with evidence of restored barrier function at the site of closure (Roy et al., 2014, Roy et al., 2020). Of interest in this context, cholinergic pathways in keratinocytes induce the production of antimicrobial peptides and improve barrier function of skin (Curtis and Radek, 2012). In wound healing, EMT influences vascularization as well as re-epithelialization. Cutaneous EMT regulates wound angiogenesis and closure in a glycemic status-dependent manner (Singh et al., 2019). Stalled wound re-epithelialization and compromised angiogenesis are hallmarks of impaired diabetic wound healing. Work by our group recently identified zinc-finger E-box-binding 1 (ZEB1) as a significant mechanistic hub across epithelial and endothelial cells in wounds. In both epithelial as well as endothelial cell compartments of wound tissue, ZEB1 is responsive to the glycemic status of the injury microenvironment. In epithelial cells, hyperglycemia impaired the ZEB1-EMT pathway towards wound-epithelialization. In endothelial cells, ZEB1 was directly implicated in hyperglycemia-induced dysfunction (Singh et al., 2019). These findings establish a direct link of EMT with critical facets of wound healing including functional closure and wound-site vascularization.
Wound closure is only complete when defects caused by injury are covered by skin with restored barrier function. Thus, measurement of barrier function restoration is an important element characterizing wound closure (Ghatak et al., 2015, Li J. et al., 2018). Inherent reversible plasticity of skin cells is manifest during wound repair (Fig. 1). One aspect of this process is EMT↔MET (mesenchymal to epithelial transition) (Lamouille et al., 2014, Nieto et al., 2016). The spatiotemporal process that advances re-epithelialization in a way that restores epidermal barrier function requires partial EMT (Haensel and Dai, 2018). Extracellular cues trigger transcriptional, translational and post-translational regulation of transcription factors (TF) such as SNAIL, ZEB and basic helix-loop-helix TF to cause EMT. Transforming growth factor-β (TGFβ) family signaling pathways play a central role in relaying those cues. Of the many signaling pathways that enable EMT, the significance of non-neuronal cholinergic pathways in driving EMT in the skin remains poorly understood (Lamouille et al., 2014). Tan et al (Tan et al., 2020) report that under conditions of hyperglycemia, keratinocytes were resistant to acetyl choline induced EMT. Acetyl choline (ACh) dependent EMT was dependent on TGF-β1 signaling. In skin, activation of the parasympathetic nervous system by stimuli including stress causes release of ACh from nerve fibers. Among other outcomes, this causes sweating. In those suffering from wounds, stress is commonly experienced (Sen and Roy, 2019). Thus, it is plausible that ACh is present in elevated levels at sites of cutaneous wounds. Both ACh synthesizing choline acetyltransferase as well as the ACh degrading enzyme acetylcholinesterase are abundant in skin. Based on the work by Tan et al (Tan et al., 2020) it may be that ACh represents a physiological mechanism to augment EMT in wounds.
Figure 1:
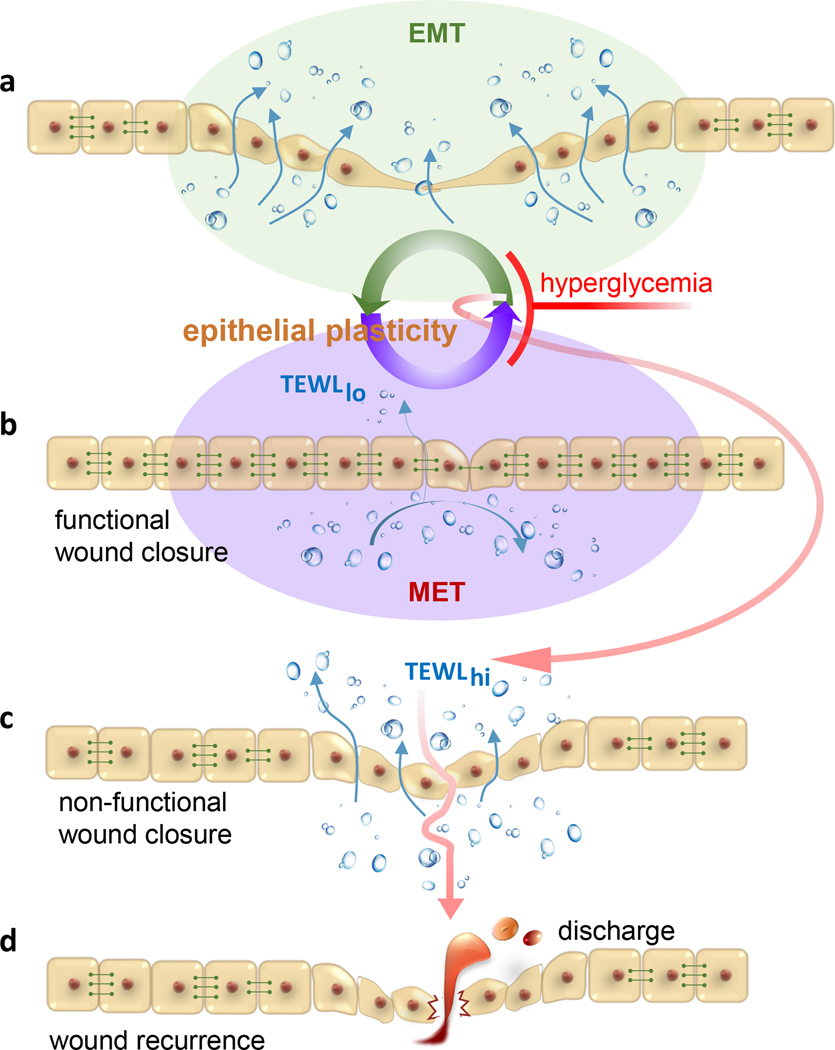
Hyperglycemia restrains cutaneous epithelial plasticity necessary for functional wound closure. A hypothetical paradigm depicts a-b wherein EMT↔MET is central for re-epithelialization and restoration of barrier function of the repaired skin. During EMT, epithelial cells dismantle cell adhesion and tight junction structures in an effort to acquire a mesenchymal phenotype favoring re-epithelialization. MET helps reconstitute apical junctional complexes (AJC) restoring barrier function of the repaired skin. Hyperglycemia is a barrier to such plasticity and as such hinders re-epithelialization, restoration of barrier function or both (c). The result is wound chronicity or non-functional wound closure. Closure of wound without restoration of skin barrier function predisposes the closed wound to recidivism (d) as evident in preclinical porcine studies. The incidence of wound recurrence is high in diabetic patients. TEWL, transepidermal water loss is a measure of skin barrier function. TEWLhi, deficient skin barrier function; TEWLlo, restored skin barrier function indicative of functional wound closure. Horizontal rivets between cells represent functional AJC. These are low or absent in mesenchymal cells compromising barrier function.
Central cholinergic pathways have profound influence as on glycemic regulation (Healy et al., 2010). However, information on how hyperglycemia may modify cholinergic responses in peripheral organs is scanty. T2D blunts purinergic cutaneous vasodilatation but not muscarinic and nicotinic vascular responses or sweating (Fujii et al., 2018). Kevin Tracey’s cholinergic anti-inflammatory pathway provides important context to the work reported by Tan et al (Tracey, 2009). In an effort to understand the anti-inflammatory effects of a p38 MAPK inhibitor, these investigators identified an inflammatory reflex. In brief, it was proposed that the vagus nerve can sense peripheral inflammation and, in response, dispatch action potentials aimed at inhibiting pro-inflammatory cytokine production by the spleen. As part of molecular mechanisms that drive the cholinergic anti-inflammatory pathway, neurotransmitter ACh acts upon the α7 nicotinic ACh receptor (α7nAChR) subunit expressed on cytokine producing cells such as monocytes, macrophages and lymphocytes (Huston and Tracey, 2011). Neuroendocrine α7nAChR is also functionally active in skin cells such as epidermal keratinocytes, sebocytes and dermal fibroblasts. Both successful mounting, and timely resolution, of inflammation are necessary for wound healing (Khanna et al., 2010). Diabetic wound repair is complicated by persistent inflammation. Among several other factors (Das et al., 2018, Das et al., 2014, Das et al., 2016, Das et al., 2015), α7nAChR function is likely to play a considerable role in this regard. Selective agonists of α7nAChR accelerated the repair of diabetic wounds (Li J. Y. et al., 2018). nAChRs also accelerate diabetic wound angiogenesis (Jacobi et al., 2002). In diabetes, α7nAChR expression and function are blunted. Receptor for advanced glycation end (RAGE) products inactivate α7nAChR (Chandna et al., 2015). In the context of wound closure, it is important to note that α7nAChR is directly implicated in driving EMT (Zhang et al., 2016, Zhao et al., 2015). Whether diabetes-dependent impairment of α7nAChR function in skin cells impairs EMT during wound closure remains an open question.
Once re-epithelialization is achieved, epithelial cells must give up their migratory behavior, reconstitute apico-basal polarization and re-establish junctional complexes. Cells must reverse EMT and this can be achieved by MET (Thiery et al., 2009). Restoring barrier function of repaired skin requires reconstitution of apical junctional complexes encompassing tight junctions and adherens junctions. While specifics of EMT↔ MET mechanisms during wound healing have not been worked out yet, it may reasonably hypothesized to be an iterative process. Importantly, the same inducer can potentiate EMT and MET simultaneously in two different cell compartments. If clues from the formation of complex three-dimensional structures of internal organs are of any value, several rounds of EMT and MET are necessary for the final differentiation of specialized cell types (Thiery et al., 2009). Iterative EMT↔ MET may be viewed as stepwise cycles of epithelial plasticity necessary to achieve functional wound closure. Of extraordinary significance in the context of diabetic wounds is the evidence that tissue EMT↔MET is responsive to glycemic status (Talakatta et al., 2018). Further studies unveiling the molecular underpinnings of cutaneous wound epithelial plasticity will reveal regulatory hubs orchestrating wound inflammation, re-epithelialization and vascularization.
Clinical Relevance.
- Management of hyperglycemia will:
- defend skin plasticity necessary for wound closure
- enable cholinergic pathways of the skin to support wound closure
- help achieve functional wound closure
Acknowledgment:
Wound healing research in the authors’ laboratories is supported by DK119099, DK125835, NR015676, NS042617 and DK 114718.
Footnotes
Conflict of interest: “The authors state no conflict of interest.”
REFERENCES
- Chandna AR, Nair M, Chang C, Pennington PR, Yamamoto Y, Mousseau DD, et al. RAGE mediates the inactivation of nAChRs in sympathetic neurons under high glucose conditions. Eur J Neurosci 2015;41(3):341–51. [DOI] [PubMed] [Google Scholar]
- Chernyavsky AI, Kalantari-Dehaghi M, Phillips C, Marchenko S, Grando SA. Novel cholinergic peptides SLURP-1 and −2 regulate epithelialization of cutaneous and oral wounds. Wound Repair Regen 2012;20(1):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BJ, Radek KA. Cholinergic regulation of keratinocyte innate immunity and permeability barrier integrity: new perspectives in epidermal immunity and disease. J Invest Dermatol 2012;132(1):28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Datta S, Roche E, Chaffee S, Jose E, Shi L, et al. Novel mechanisms of Collagenase Santyl Ointment (CSO) in wound macrophage polarization and resolution of wound inflammation. Sci Rep 2018;8(1):1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 2014;192(3):1120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ghatak S, Sinha M, Chaffee S, Ahmed NS, Parinandi NL, et al. Correction of MFG-E8 Resolves Inflammation and Promotes Cutaneous Wound Healing in Diabetes. J Immunol 2016;196(12):5089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol 2015;185(10):2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Meade RD, McNeely BD, Nishiyasu T, Sigal RJ, Kenny GP. Type 2 diabetes specifically attenuates purinergic skin vasodilatation without affecting muscarinic and nicotinic skin vasodilatation and sweating. Exp Physiol 2018;103(2):212–21. [DOI] [PubMed] [Google Scholar]
- Ghatak S, Chan YC, Khanna S, Banerjee J, Weist J, Roy S, et al. Barrier Function of the Repaired Skin Is Disrupted Following Arrest of Dicer in Keratinocytes. Mol Ther 2015;23(7):1201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnyawali SC, Sinha M, El Masry MS, Wulff B, Ghatak S, Soto-Gonzalez F, et al. High Resolution Ultrasound Imaging for Repeated Measure of Wound Tissue Morphometry, Biomechanics and Hemodynamics under Fetal, Adult and Diabetic Conditions. PLoS One 2020;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haensel D, Dai X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev Dyn 2018;247(3):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy JA, Nilsson KR, Hohmeier HE, Berglund J, Davis J, Hoffman J, et al. Cholinergic augmentation of insulin release requires ankyrin-B. Sci Signal 2010;3(113):ra19. [DOI] [PubMed] [Google Scholar]
- Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 2011;269(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi J, Jang JJ, Sundram U, Dayoub H, Fajardo LF, Cooke JP. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Am J Pathol 2002;161(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 2010;5(3):e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Li S, Liu C, Bari AS, Longaker MT, Lorenz HP. Epithelial-mesenchymal transition occurs after epidermal development in mouse skin. Exp Cell Res 2006;312(19):3959–68. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ghatak S, El Masry MS, Das A, Liu Y, Roy S, et al. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound. Mol Ther 2018;26(9):2178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Jiang SK, Wang LL, Zhang MZ, Wang S, Jiang ZF, et al. alpha7-nAChR Activation Has an Opposite Effect on Healing of Covered and Uncovered Wounds. Inflammation 2018;41(2):474–84. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell 2016;166(1):21–45. [DOI] [PubMed] [Google Scholar]
- Paslin D. Grover disease may result from the impairment of keratinocytic cholinergic receptors. J Am Acad Dermatol 2012;66(2):332–3. [DOI] [PubMed] [Google Scholar]
- Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol 2014;233(4):331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Santra S, Das A, Dixith S, Sinha M, Ghatak S, et al. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann Surg 2020;271(6):1174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Roy S. Sociogenomic Approach to Wound Care: A New Patient-Centered Paradigm. Adv Wound Care (New Rochelle) 2019;8(11):523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Sinha M, Pal D, Tabasum S, Gnyawali SC, Khona D, et al. Cutaneous Epithelial to Mesenchymal Transition Activator ZEB1 Regulates Wound Angiogenesis and Closure in a Glycemic Status-Dependent Manner. Diabetes 2019;68(11):2175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talakatta G, Sarikhani M, Muhamed J, Dhanya K, Somashekar BS, Mahesh PA, et al. Diabetes induces fibrotic changes in the lung through the activation of TGF-beta signaling pathways. Sci Rep 2018;8(1):11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MWI, Tan WR, Kong ZQ, Toh JH, Wee WKJ, Teo EML, et al. High glucose restrains acetylcholine-induced keratinocyte EMT is mitigated by p38 inhibition. . J Invest Dermatol 2020;in press. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139(5):871–90. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009;9(6):418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberti F, Bardelli C, Morsanuto V, Ghirlanda S, Cochis A, Molinari C. Stimulation of the Nonneuronal Cholinergic System by Highly Diluted Acetylcholine in Keratinocytes. Cells Tissues Organs 2017;203(4):215–30. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ding XP, Zhao QN, Yang XJ, An SM, Wang H, et al. Role of alpha7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget 2016;7(37):59199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Gu X, Zhang C, Lu Q, Chen H, Xu L. Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC). Cancer Biol Ther 2015;16(4):634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


