Abstract
Obesity and type 2 diabetes are both chronic, relapsing, progressive diseases that are recognized as risk factors for the development of multiple types of cancer. In a recent symposium “Hitting A Triple—Diabetes, Obesity, and the Emerging Links to Cancer Risk” convened by The Obesity Society (TOS) during ObesityWeek® 2019, experts in the field presented the current science and highlighted existing research gaps. Topics included: 1) the epidemiology of obesity and diabetes and their links to cancer risk; 2) racial and ethnic differences in obesity, diabetes, and cancer risk; 3) biological mechanisms common to obesity and diabetes that may increase cancer risk; and 4) innovative interventions that can be used to prevent the development of cancers related to obesity and diabetes. This report provides an overview of the symposium and describes key research gaps and pressing questions in need of answers to advance the field. The collective burden of obesity, diabetes, and cancer represents one of the largest public health challenges of the century. Although the symposium was titled “hitting a triple” it was recognized that being able to disrupt the linkages among obesity, diabetes, and cancer would be a “grand slam” for public health and medicine.
Keywords: Abdominal Obesity, Metabolism, Molecular Epidemiology, Prediabetes, Randomized Trial
INTRODUCTION
Obesity and type 2 diabetes, hereafter referred to as diabetes, are both chronic, relapsing, progressive diseases (1, 2). By the year 2030, it is projected that 1 in 2 adults in the U.S. will have obesity and 1 in 7 will have diabetes (3, 4). Obesity is a strong risk factor for the development of diabetes, consequently half of people diagnosed with diabetes have obesity (5). Multiple racial and ethnic subgroups experience a disproportionate burden of obesity and diabetes in the U.S. (3, 6).
Obesity and diabetes have long been recognized as risk factors for cardiovascular morbidity and mortality. In 2002, the International Agency for Research on Cancer (IARC) reported that obesity was linked with the development of five different cancers (7). In 2016, IARC and the World Cancer Research Fund (WCRF) reported that obesity was now convincingly linked with the development of 13 different cancers (8). Furthermore, diabetes has recently been recognized as a risk factor for the development of multiple malignancies (9), independent of obesity (10). Similar to obesity and diabetes, underrepresented racial and socioeconomic subgroups experience a disproportionate cancer burden (11).
In a recent symposium titled “Hitting A Triple—Diabetes, Obesity, and the Emerging Links to Cancer Risk” convened by The Obesity Society (TOS) during ObesityWeek® 2019, experts in the field presented the current state of the science and highlighted existing research gaps. Topics that were discussed included: 1) the epidemiology of obesity and diabetes and their links to cancer risk; 2) racial and ethnic differences in obesity, diabetes, and cancer risk; 3) biological mechanisms common to obesity and diabetes that may increase cancer risk; and 4) innovative interventions that can be used to prevent the development of cancers related to obesity and diabetes. The purpose of this report is to provide a concise overview of the symposium; it is not intended to serve as a comprehensive review of all aspects of these individual topics.
THE EPIDEMIOLOGY OF OBESITY
Obesity is a multi-causal chronic disease of excess adipose tissue that can occur throughout the lifespan (12). Obesity is diagnosed using the body mass index (BMI) ≥30 kg/m2 (13). Among all weight-to-height indices, BMI has the strongest correlation with measures of total body adiposity (14). Current estimates are that 34% of adults in the U.S. have obesity (15), and this estimate is projected to increase to 48.9% by 2030 (3). Women are more likely to develop obesity as compared with men, and this risk is highest among black and Hispanic women (prevalence ratio: 1.44 for black women and 1.21 for Hispanic women, as compared with non-Hispanic white women) (16). Body fat distribution, particularly intra-abdominal visceral adipose tissue, is a strong determinant of the adverse metabolic effects of obesity (17). Abdominal obesity can be defined using the waist circumference or the waist-to-hip ratio. Similar to BMI, rates of abdominal obesity are increasing in the U.S. (18). The combined use of BMI and waist circumference or the waist-to-hip ratio can identify population subgroups with a high cancer risk (19, 20).
THE EPIDEMIOLOGY OF DIABETES
Diabetes is the result of a progressive loss of adequate β-cell insulin secretion, frequently occurring on the background of insulin resistance (21). Diabetes is diagnosed by plasma glucose concentrations, either fasting or during an oral glucose tolerance test, or by glycated hemoglobin. Current estimates are that 9.1% of adults in the U.S. have diabetes, and this estimate is projected to increase to 13.9% by 2030 (4). Newly released data indicate that the prevalence of diabetes is highest among people of Hispanic origin (14.7%) and non-Hispanic black (16.4%) as compared with other racial and ethnic subgroups (11.9% non-Hispanic white) (22). Overt diabetes is often preceded by a period of prediabetes, characterized by fasting glucose and glucose intolerance that is above normal but below diabetes thresholds, that affects 34.5% of U.S. adults (23). The annual rate of progression from prediabetes to diabetes is 5−10% (24).
THE TRIPLE THREAT OF OBESITY AND DIABETES AND CANCER RISK
Obesity defined using BMI is associated with an increased risk of developing at least 13 cancers throughout the body (Figure 1) (8). These 13 obesity-related cancers represent 40% of all malignancies diagnosed in the U.S. (25). Between 2005-2014, with exception of colorectal cancer, the annual incidence of obesity-related cancers increased among persons aged 20-74 years (25). Independent of baseline BMI, weight gain across the lifespan is associated with risk of cancer (26, 27). A higher lifetime BMI and longer duration of obesity are positively related to cancer risk (28, 29, 30, 31). Observational studies of Mendelian randomization that use genetic markers known to be associated with obesity or adiposity have reported associations with various types of cancer (32, 33).
Figure 1.
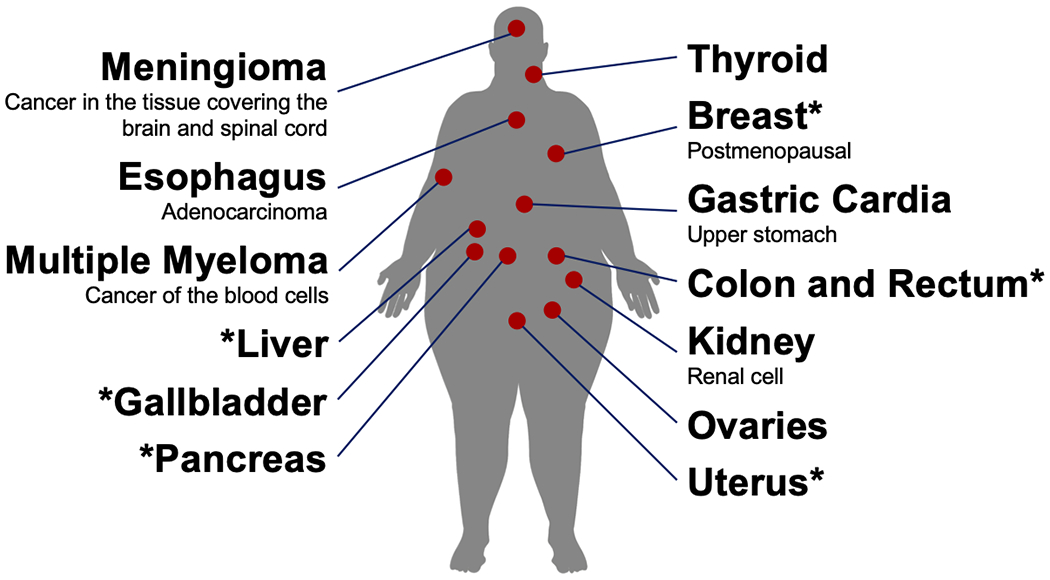
Cancers that have an established relationship with obesity. Cancers that also have an established relationship with diabetes have an asterisk.
Diabetes is associated with an increased risk of developing at least six cancers, including breast, endometrial, and several gastrointestinal malignancies in the colorectum, pancreas, gallbladder, and liver (9). After diagnosis of diabetes, risk of cancer is elevated for ≥20 years, with the highest degree of risk occurring approximately 4–8 years after diabetes diagnosis (34). Importantly, prediabetes is associated with an increased cancer risk (35, 36). Mendelian randomization studies report that genetic predisposition to diabetes and insulin resistance is associated with cancer risk (37).
Disentangling the joint and independent effects of obesity and diabetes on cancer risk has been challenging, as obesity and diabetes are strongly related. Among women in the multi-ethnic cohort study, obesity increased risk of breast cancer [HR: 1.33 (95% CI: 1.24, 1.43)] and was unchanged after adjustment for diabetes [HR: 1.31 (95% CI: 1.22, 1.41)] (38). In a meta-analysis of 39 studies, diabetes increased the risk of breast cancer in studies that did not adjust for BMI [RR: 1.33 (95% CI: 1.18, 1.51)], and this risk was attenuated, but remained statistically significant, in studies that did adjust for BMI [RR: 1.16 (95% CI: 1.08, 1.24)] (39). It is estimated that as independent risk factors, obesity and diabetes account for 5.7% of all incident cancers (10). After cigarette smoking, obesity is the second strongest modifiable risk factor for cancer (40).
DISPARITIES IN OBESITY, DIABETES, AND CANCER RISK
Racial and ethnic minority subgroups experience a disproportionate burden of cancers (11), including the malignancies that are related to obesity and diabetes (25). BMI does not quantify excess adiposity and metabolic abnormalities consistently across racial populations (41, 42, 43). At a specific BMI, black individuals have less visceral adiposity (BMI by race interaction for visceral adipose tissue, P<0.05) and lower insulin sensitivity (BMI by race interaction for skeletal muscle insulin sensitivity, P=0.04) than white individuals (44, 45). Among black women, for example, abdominal obesity (e.g., waist circumference or the waist-to-hip ratio) is a stronger predictor of breast cancer risk than general adiposity measured by BMI (46). Similar findings have been reported for Hispanic women (47). The joint and independent effects of obesity and diabetes on cancer risk may vary by race and ethnicity (38, 48). Molecular pathological epidemiology studies have provided important mechanistic insights about how obesity and diabetes impact the molecular tumor characteristics (49), and how these tumor characteristics may vary by racial and ethnic subgroup (for example, black women are more likely than white women to be diagnosed with estrogen receptor negative and triple negative breast cancer, which are biologically more aggressive and have a poorer prognosis) (50).
MECHANISMS THAT LINK OBESITY AND DIABETES WITH CANCER
The precise biological mechanism through which obesity and diabetes promote tumorigenesis remains incompletely understood but is likely multifactorial. Hypertrophic adipose tissue is associated with altered concentrations of metabolic hormones (e.g., insulin and insulin-like growth factors), adipokines (e.g., leptin, adiponectin), steroid hormones (e.g., estrogen), and inflammatory cytokines (e.g., interleukin-6) (51, 52). Multiple intracellular pathways may be activated including the Janus kinase (JAK)-signal transducers of transcription (STAT), mitogen activated protein kinase (MAPK), and the phosphatidylinositol 3-kinase, protein kinase B, mammalian target of rapamycin (PI3K-Akt-mTOR) pathway, which are often mutated in cancer (53). By activating these signaling pathways, malignant transformation is supported.
PI3K-Akt-mTOR is perhaps the most intriguing signaling pathway underpinning the effects of obesity and diabetes on cancer risk (Figure 2). This pathway is one of the most commonly activated in cancer (54). mTOR is sensitive to the nutrient status surrounding the cell, such high-energy states (e.g., obesity and hyperinsulinemia), which activate mTOR via Akt, and low energy states (e.g., caloric restriction and exercise), which inhibit mTOR via AMPK (55, 56). In preclinical models of mammary cancer, suppressing weight gain and accumulation of lipid in adipose depots via dietary energy restriction and/or physical activity reduce tumor incidence (57), mediated in part by activation of AMPK and downregulation of mTOR (58), that reduce rates of cell proliferation and vascularization and increase apoptosis (59, 60, 61). These studies suggest that the PI3K-Akt-mTOR pathway is a useful bridge between population studies and mechanistically based interventions because of its central role in cell metabolism, and the host systemic and cell autonomous processes that drive selective growth advantage, and response to therapeutic targeting (62).
Figure 2.
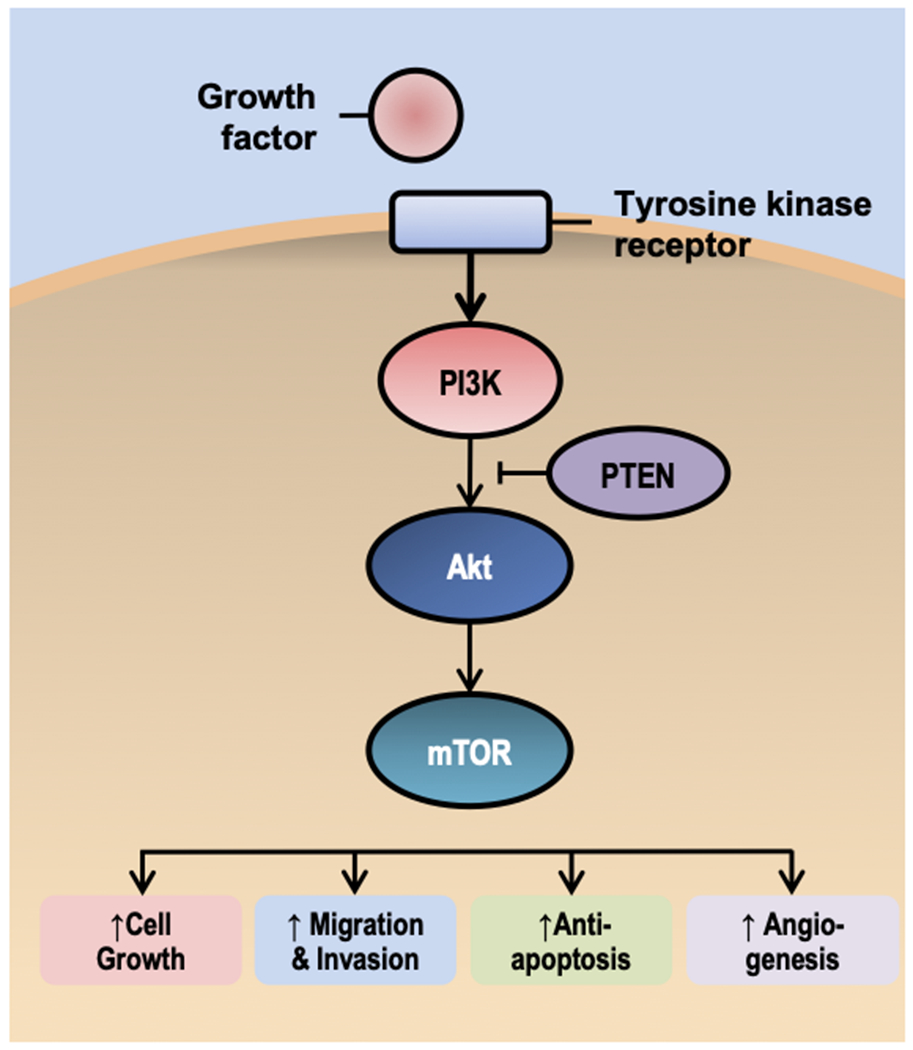
A simplified overview of the phosphatidylinositol 3-kinase, protein kinase B, mammalian target of rapamycin (PI3K-Akt-mTOR) pathway. The PI3K-Akt-mTOR pathway has been implicated in obesity, diabetes, and cancer risk, make this pathway a useful bridge between population studies and mechanistically based interventions.
INTERVENTIONS TO BREAK THE LINK BETWEEN OBESITY AND DIABETES WITH CANCER
The Diabetes Prevention Program (DPP) established that lifestyle modification (7% weight loss and 150 minutes per week of physical activity) or metformin (oral anti-diabetes medication) reduced the progression from prediabetes to diabetes by 58% and 31%, respectively (63). Publication of the cancer risk analyses from the DPP are pending (64). Observational studies report that weight loss, self-reported as intentional, is associated with a 12% reduction in cancer risk (65). The only randomized trial to address the effect of lifestyle modification (7% weight loss and 175 minutes per week of physical activity) on cardiovascular risk was the Look AHEAD (Action for Health in Diabetes) trial (66). After 11 years of follow-up, data from the lifestyle intervention was associated with a 16% relative risk reduction in obesity-related cancer, though this comparison was not statistically significant (HR: 0.84, 95% CI: 0.68, 1.04) (67). The findings from the Look AHEAD trial provide the first evidence that the effects of obesity on cancer risk may be reversable (68).
Metabolic surgery consistently yields ≈25% weight loss at 10 years, induces diabetes remission, with low operative morbidity (5%) and mortality (0.2%) (69, 70). Observational studies report that patients who undergo metabolic surgery are at a lower risk of developing cancer compared with patients who do not undergo surgery (71, 72). A meta-analysis of 13 observational studies reported that surgery was associated with a 44% lower risk of invasive cancer [OR: 0.56 (95% CI: 0.46, 0.68)] (73). Metabolic surgery is also associated with a lower risk of malignancies not traditionally considered to be related to obesity and diabetes, such as melanoma and non-Hodgkin lymphoma (74, 75).
Inconsistency arises with respect to the anti-cancer effects of diabetes and obesity medications (76). Many studies that have examined the anti-cancer effects of diabetes medications use observational designs and have been limited by various types of biases (e.g., immortal time bias) (77). Meta-analyses that combine both observational and randomized designs demonstrate that, dependent on drug class, cancer risk may be increased, decreased, or not changed among observational studies; however randomized studies do not support these findings (78, 79). For example, in a pooled analysis of 21 cohort studies metformin was associated with a lower risk of cancer (Hazard Ratio [HR]: 0.88, 95% CI: 0.83-0.92), whereas no cancer risk reduction was observed in 23 randomized trials (HR: 1.05, 95% CI: 0.94-1.18) (78). An important limitation is that these trials were not designed to assess cancer risk, and definitive conclusions cannot be made (80). There are also several anti-obesity medications that are approved by the FDA (81). Relevant to cancer, lorcaserin was withdrawn from the market in February 2020 due to an increased risk of cancer (82, 83).
PRESSING QUESTIONS TO ADVANCE THE FIELD
Throughout all presentations, existing research gaps and pressing questions in need of answers to advance the field were highlighted. There was agreement by all presenters that in order to move the field forward in a transformative and rapid manner will require the assembly of diverse teams of scientists, such as that made possible by the NCI-sponsored Transdisciplinary Research on Energetics and Cancer (TREC) consortium (84). There was also agreement that because of the diverse causes of obesity, diabetes, and cancer, an array of innovative study designs, such as multilevel or adaptive approaches, would be likely to offer unique and complementary evidence to advance the field (85).
There is an important need to determine which measures of obesity and diabetes status are best to prognosticate cancer risk, when, and for whom. The majority of studies to date have used a single assessment of BMI as a measure of adiposity and a single fasting glucose with/without insulin as a measure of insulin resistance. The introduction of optical imaging technology to quantify body composition, continuous glucose monitors, and accelerometry embedded into digital devices, for example, offer the potential to obtain high-dimensional data to glean additional mechanistic insights (86, 87).
The importance of measures across the lifespan have been recently appreciated. Among children, 12.4% have obesity when they enter kindergarten (88), with the most rapid weight gain occurring between 2 and 6 years of age (89). It is predicted that 57.3% of children today will have obesity at the age of 35 years (90). Children with obesity have a similar cardiometabolic risk factors profile as adults, including prediabetes and diabetes (91, 92). Adolescent obesity predicts midlife cancer risk (93), and has been hypothesized as a possible cause of the increase in early-onset cancer (cancers occurring before the age of 50 years) (94).
Many studies to date have been insufficiently racially and ethnically diverse to identify clinically meaningful heterogeneity of effects (95). Diverse subgroups will also enable molecular pathological epidemiology studies to identify differences in tumor subtypes (96). Enrolling study participants from diverse backgrounds in sufficient numbers will enable the identification of distinct obesity and diabetes phenotypes that may offer important clues to social determinants of disparities, mechanisms of action, and identify population subgroups most likely to benefit from intervention (97). Interventions should be tailored to include culturally relevant design approaches to increase their relevance, appeal, and effectiveness (98).
Given the relative infrequency and long latency interval required for cancer to occur, there is a critical need to identify additional biomarkers that can be validated and used as surrogate cancer risk endpoints for intervention trials. The identification and characterization of such surrogate endpoints will reduce study length, sample size, and cost. An example of a unique surrogate measure was the use of recurrent polyps as an endpoint to characterize the potential colorectal cancer benefit of metformin (99). As mechanisms of action continue to be identified, the potential opportunities to identify surrogate endpoints may increase.
It remains unknown how much weight loss or what degree of glycemic control is required to reduce cancer risk. Greater clarity is needed to define the role of anti-obesity or anti-diabetes medications as potential cancer risk reduction strategies. Lastly, the observational data supporting the anti-cancer potential of metabolic surgery should continue to be investigated (100).
CONCLUSIONS
Obesity and diabetes are complex diseases that are independently and jointly risk factors for cancer. The collective burden of obesity, diabetes, and cancer represent one of the largest public health challenges of the century. As the prevalence of obesity and diabetes increase, clinical and public health interventions are urgently needed (Figure 3). Identifying how to disrupt the linkages among obesity, diabetes, and cancer has the potential to transform the health and wellness of society. Although the symposium was titled “hitting a triple” is was recognized that being able to break the linkages among these three chronic diseases would be classified as a “grand slam” for public health and medicine.
Figure 3.
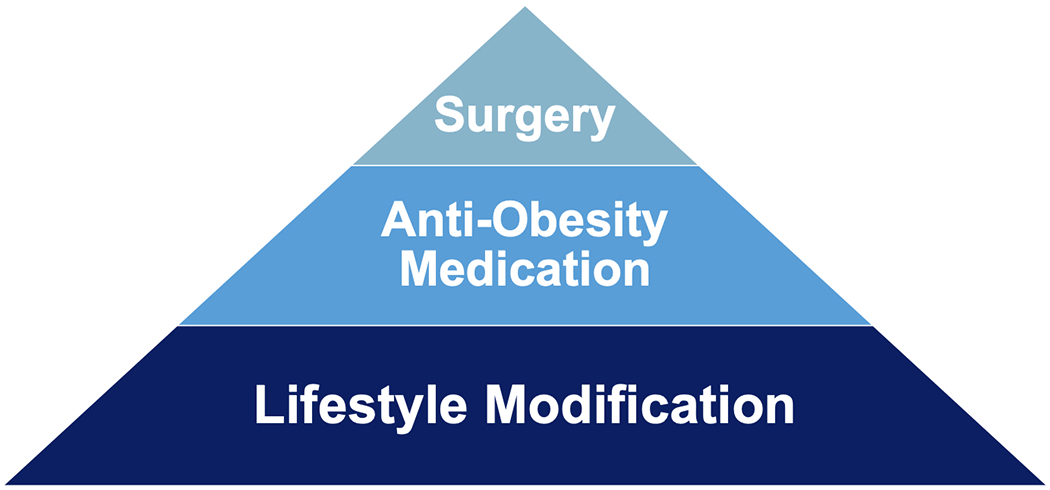
Managing obesity and diabetes as a cancer prevention and control strategy will likely require a multimodal approach that utilizes lifestyle modification as a foundation and offers evidence-based anti-obesity or anti-diabetes medication and metabolic surgery, as appropriate.
Study Importance.
What is already known?
Obesity and type 2 diabetes are both chronic, progressive, and relapsing diseases that increase the risk of developing various types of cancer.
The collective burden of obesity, diabetes, and cancer represents one of the largest public health challenges of the century.
What does this study add?
In a symposium “Hitting A Triple—Diabetes, Obesity, and the Emerging Links to Cancer Risk” experts presented the current science and highlighted research gaps.
Topics included the epidemiology of obesity and diabetes and links to cancer risk; racial and ethnic differences in obesity, diabetes, and cancer risk; biological mechanisms common to obesity and diabetes that increase cancer risk; and interventions to prevent the development of cancers related to obesity and diabetes.
How might these results change the focus of clinical practice?
As the prevalence of obesity and diabetes increases, clinical and public health interventions are urgently needed.
Identifying how to disrupt the linkages among obesity, diabetes, and cancer has the potential to transform the health and wellness of society.
Acknowledgments
Funding: Dr. Brown is supported by the National Institute of General Medicine Sciences of the National Institutes of Health under Award Number U54-GM104940, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30-DK072476, the National Cancer Institute of the National Institutes of Health under Award Numbers R00-CA218603; and R25-CA203650, the Susan G. Komen Foundation, and the American Institute for Cancer Research. Dr. Carson is supported by the National Cancer Institute of the National Institutes of Health under Award Numbers K01-CA190559 and R01-CA253219 and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01-DK125367. Dr. Thompson is Professor and Director of the Cancer Prevention Laboratory at Colorado State University, which provides full salary support for this work.
Disclosure: Dr. Agurs-Collins reports employment by the National Cancer Institute of the National Institutes of Health. All other authors report no relevant relationships.
REFERENCES
- 1.Bray GA, Kim KK, Wilding JPH, World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev 2017;18: 715–723. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32 Suppl 2: S151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med 2019;381: 2440–2450. [DOI] [PubMed] [Google Scholar]
- 4.Lin J, Thompson TJ, Cheng YJ, Zhuo X, Zhang P, Gregg E, et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr 2018;16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg 2011;21: 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, et al. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA 2019;322: 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. The Lancet Oncology 2002;3: 565–574. [DOI] [PubMed] [Google Scholar]
- 8.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375: 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350: g7607. [DOI] [PubMed] [Google Scholar]
- 10.Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. The Lancet Diabetes & Endocrinology 2018;6: e6–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69: 211–233. [DOI] [PubMed] [Google Scholar]
- 12.Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a Disease: The Obesity Society 2018 Position Statement. Obesity (Silver Spring) 2019;27: 7–9. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014;129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis 1972;25: 329–343. [DOI] [PubMed] [Google Scholar]
- 15.Ward ZJ, Long MW, Resch SC, Gortmaker SL, Cradock AL, Giles C, et al. Redrawing the US Obesity Landscape: Bias-Corrected Estimates of State-Specific Adult Obesity Prevalence. PLoS One 2016;11: e0150735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013–2016. JAMA 2018;319: 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93: 359–404. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA 2014;312: 1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra-Soto S, Petermann-Rocha F, Boonpor J, Gray SR, Pell JP, Celis-Morales C, et al. Combined association of general and central obesity with incidence and mortality of cancers in 22 sites. Am J Clin Nutr 2020. [DOI] [PubMed] [Google Scholar]
- 20.Houghton SC, Eliassen H, Tamimi RM, Willett WC, Rosner BA, Hankinson SE. Central adiposity and subsequent risk of breast cancer by menopause status. J Natl Cancer Inst 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes A 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44: S15–S33. [DOI] [PubMed] [Google Scholar]
- 22.Control CfD, Prevention. National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 23.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379: 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78: 305–312. [DOI] [PubMed] [Google Scholar]
- 25.Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2017;66: 1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva M, Weiderpass E, Licaj I, Lissner L, Rylander C. Excess body weight, weight gain and obesity-related cancer risk in women in Norway: the Norwegian Women and Cancer study. Br J Cancer 2018;119: 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chadid S, Singer MR, Kreger BE, Bradlee ML, Moore LL. Midlife weight gain is a risk factor for obesity-related cancer. Br J Cancer 2018;118: 1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold M, Freisling H, Stolzenberg-Solomon R, Kee F, O'Doherty MG, Ordonez-Mena JM, et al. Overweight duration in older adults and cancer risk: a study of cohorts in Europe and the United States. Eur J Epidemiol 2016;31: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women's Health Initiative: A Longitudinal Study from the United States. PLoS Med 2016;13: e1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, et al. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer 2016;138: 2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Lynch BM, Dugue PA, Karahalios A, MacInnis RJ, Bassett JK, et al. Latent Class Trajectory Modeling of Adult Body Mass Index and Risk of Obesity-Related Cancer: Findings from the Melbourne Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev 2021;30: 373–379. [DOI] [PubMed] [Google Scholar]
- 32.Carreras-Torres R, Johansson M, Gaborieau V, Haycock PC, Wade KH, Relton CL, et al. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thrift AP, Shaheen NJ, Gammon MD, Bernstein L, Reid BJ, Onstad L, et al. Obesity and risk of esophageal adenocarcinoma and Barretťs esophagus: a Mendelian randomization study. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Y, Zhang X, Ma Y, Yuan C, Wang M, Wu K, et al. Incident Type 2 Diabetes Duration and Cancer Risk: A Prospective Study in Two US Cohorts. J Natl Cancer Inst 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia 2014;57: 2261–2269. [DOI] [PubMed] [Google Scholar]
- 36.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293: 194–202. [DOI] [PubMed] [Google Scholar]
- 37.Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is Type 2 Diabetes Causally Associated With Cancer Risk? Evidence From a Two-Sample Mendelian Randomization Study. Diabetes 2020;69: 1588–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maskarinec G, Jacobs S, Park SY, Haiman CA, Setiawan VW, Wilkens LR, et al. Type II Diabetes, Obesity, and Breast Cancer Risk: The Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev 2017;26: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer 2012;107: 1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68: 31–54. [DOI] [PubMed] [Google Scholar]
- 41.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr., Ravussin E, et al. Ethnic-specific BMI and waist circumference thresholds. Obesity (Silver Spring) 2011;19: 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ntuk UE, Gill JM, Mackay DF, Sattar N, Pell JP. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care 2014;37: 2500–2507. [DOI] [PubMed] [Google Scholar]
- 43.Heo M, Faith MS, Pietrobelli A, Heymsfield SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am J Clin Nutr 2012;95: 594–602. [DOI] [PubMed] [Google Scholar]
- 44.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tay J, Goss AM, Garvey WT, Lockhart ME, Bush NC, Quon MJ, et al. Race affects the association of obesity measures with insulin sensitivity. Am J Clin Nutr 2020;111: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandera EV, Maskarinec G, Romieu I, John EM. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: a global perspective. Adv Nutr 2015;6: 803–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John EM, Sangaramoorthy M, Hines LM, Stern MC, Baumgartner KB, Giuliano AR, et al. Overall and abdominal adiposity and premenopausal breast cancer risk among hispanic women: the breast cancer health disparities study. Cancer Epidemiol Biomarkers Prev 2015;24: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park SY, Haiman CA, Cheng I, Park SL, Wilkens LR, Kolonel LN, et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the Multiethnic Cohort Study. Cancer Causes Control 2015;26: 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng X, Song M, Preston MA, Ma W, Hu Y, Pernar CH, et al. The association of diabetes with risk of prostate cancer defined by clinical and molecular features. Br J Cancer 2020;123: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat 2015;150: 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011;11: 886–895. [DOI] [PubMed] [Google Scholar]
- 52.Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. J Clin Oncol 2016;34: 4277–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, et al. A census of human cancer genes. Nat Rev Cancer 2004;4: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15: 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018;560: 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Z, Haegele AD, Thompson HJ. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis 1997;18: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 58.Jiang W, Zhu Z, Thompson HJ. Effects of physical activity and restricted energy intake on chemically induced mammary carcinogenesis. Cancer Prev Res (Phila) 2009;2: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang W, Zhu Z, Thompson HJ. Effect of energy restriction on cell cycle machinery in 1-methyl-1-nitrosourea-induced mammary carcinomas in rats. Cancer Res 2003;63: 1228–1234. [PubMed] [Google Scholar]
- 60.Thompson HJ, Zhu Z, Jiang W. Identification of the apoptosis activation cascade induced in mammary carcinomas by energy restriction. Cancer Res 2004;64: 1541–1545. [DOI] [PubMed] [Google Scholar]
- 61.Thompson HJ, McGinley JN, Spoelstra NS, Jiang W, Zhu Z, Wolfe P. Effect of dietary energy restriction on vascular density during mammary carcinogenesis. Cancer Res 2004;64: 5643–5650. [DOI] [PubMed] [Google Scholar]
- 62.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heckman-Stoddard BM, Crandall JP, Edelstein SL, Hamman RF, Prorok PC, Ryan A, et al. Abstract A23: Cancer outcomes in the diabetes prevention program outcomes study. Cancer Prevention Research 2015;8: A23–A23. [Google Scholar]
- 65.Luo J, Hendryx M, Manson JE, Figueiredo JC, LeBlanc ES, Barrington W, et al. Intentional Weight Loss and Obesity-Related Cancer Risk. JNCI Cancer Spectr 2019;3: pkz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Look ARG, Yeh HC, Bantle JP, Cassidy-Begay M, Blackburn G, Bray GA, et al. Intensive Weight Loss Intervention and Cancer Risk in Adults with Type 2 Diabetes: Analysis of the Look AHEAD Randomized Clinical Trial. Obesity (Silver Spring) 2020;28: 1678–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown JC, McTiernan A. Obesity and Cancer: Iťs Causal and...Reversible? Obesity (Silver Spring) 2020;28: 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Longitudinal Assessment of Bariatric Surgery C, Flum DR, Belle SH, King WC, Wahed AS, Berk P, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg 2018;153: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, et al. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann Surg 2019;269: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10: 653–662. [DOI] [PubMed] [Google Scholar]
- 73.Zhang K, Luo Y, Dai H, Deng Z. Effects of Bariatric Surgery on Cancer Risk: Evidence from Meta-analysis. Obes Surg 2020;30: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 74.Taube M, Peltonen M, Sjoholm K, Anveden A, Andersson-Assarsson JC, Jacobson P, et al. Association of Bariatric Surgery With Skin Cancer Incidence in Adults With Obesity: A Nonrandomized Controlled Trial. JAMA Dermatol 2020;156: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsui ST, Yang J, Zhang X, Docimo S Jr., Spaniolas K, Talamini MA, et al. Development of cancer after bariatric surgery. Surg Obes Relat Dis 2020;16: 1586–1595. [DOI] [PubMed] [Google Scholar]
- 76.Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol 2017;14: 85–99. [DOI] [PubMed] [Google Scholar]
- 77.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012;35: 2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu L, Zhu J, Prokop LJ, Murad MH. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci Rep 2015;5: 10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao M, Chen J, Yuan Y, Zou Z, Lai X, Rahmani DM, et al. Dipeptidyl peptidase-4 inhibitors and cancer risk in patients with type 2 diabetes: a meta-analysis of randomized clinical trials. Sci Rep 2017;7: 8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB, Investigators LPCobotLT. Neoplasms Reported With Liraglutide or Placebo in People With Type 2 Diabetes: Results From the LEADER Randomized Trial. Diabetes Care 2018;41: 1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol 2018;14: 12–24. [DOI] [PubMed] [Google Scholar]
- 82.Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer Risk Associated with Lorcaserin - The FDA's Review of the CAMELLIA-TIMI 61 Trial. N Engl J Med 2020;383: 1000–1002. [DOI] [PubMed] [Google Scholar]
- 83.de Andrade Mesquita L, Fagundes Piccoli G, Richter da Natividade G, Frison Spiazzi B, Colpani V, Gerchman F. Is lorcaserin really associated with increased risk of cancer? A systematic review and meta-analysis. Obes Rev 2021;22: e13170. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz KH, Gehlert S, Patterson RE, Colditz GA, Chavarro JE, Hu FB, et al. TREC to WHERE? Transdisciplinary Research on Energetics and Cancer. Clin Cancer Res 2016;22: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clauser SB, Taplin SH, Foster MK, Fagan P, Kaluzny AD. Multilevel intervention research: lessons learned and pathways forward. J Natl Cancer Inst Monogr 2012;2012: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kennedy S, Hwaung P, Kelly N, Liu YE, Sobhiyeh S, Heo M, et al. Optical imaging technology for body size and shape analysis: evaluation of a system designed for personal use. Eur J Clin Nutr 2020;74: 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res Clin Pract 2017;133: 178–192. [DOI] [PubMed] [Google Scholar]
- 88.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014;370: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med 2018;379: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 90.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N Engl J Med 2017;377: 2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med 2015;373: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 92.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346: 802–810. [DOI] [PubMed] [Google Scholar]
- 93.Furer A, Afek A, Sommer A, Keinan-Boker L, Derazne E, Levi Z, et al. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2.3 million adolescents in Israel. Lancet Diabetes Endocrinol 2020;8: 216–225. [DOI] [PubMed] [Google Scholar]
- 94.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019;4: E137–E147. [DOI] [PubMed] [Google Scholar]
- 95.Martin DN, Lam TK, Brignole K, Ashing KT, Blot WJ, Burhansstipanov L, et al. Recommendations for Cancer Epidemiologic Research in Understudied Populations and Implications for Future Needs. Cancer Epidemiol Biomarkers Prev 2016;25: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishi A, Milner DA Jr., Giovannucci EL, Nishihara R, Tan AS, Kawachi I, et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn 2016;16: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rebbeck TR. Conquering cancer disparities: new opportunities for cancer epidemiology, biomarker, and prevention research. Cancer Epidemiol Biomarkers Prev 2006;15: 1569–1571. [DOI] [PubMed] [Google Scholar]
- 98.Orji R, Mandryk RL. Developing culturally relevant design guidelines for encouraging healthy eating behavior. Int J Hum Comput Stud 2014;72: 207–223. [Google Scholar]
- 99.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol 2016;17: 475–483. [DOI] [PubMed] [Google Scholar]
- 100.Castagneto-Gissey L, Casella-Mariolo J, Casella G, Mingrone G. Obesity Surgery and Cancer: What Are the Unanswered Questions? Front Endocrinol (Lausanne) 2020;11: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]


