Abstract
Previous studies have linked brain oscillation and timing, with evidence suggesting that alpha oscillations (10 Hz) may serve as a “sample rate” for the visual system. However, direct manipulation of alpha oscillations and time perception has not yet been demonstrated. To test this, we had 18 human subjects perform a time generalization task with visual stimuli. Additionally, we had previously recorded resting-state EEG from each subject and calculated their individual alpha frequency (IAF), estimated as the peak frequency from the mean spectrum over posterior electrodes between 8 and 13 Hz. Participants first learned a standard interval (600 ms) and were then required to judge if a new set of temporal intervals were equal or different compared with that standard. After learning the standard, participants performed this task while receiving occipital transcranial Alternating Current Stimulation (tACS). Crucially, for each subject, tACS was administered at their IAF or at off-peak alpha frequencies (IAF ± 2 Hz). Results demonstrated a linear shift in the psychometric function indicating a modification of perceived duration, such that progressively “faster” alpha stimulation led to longer perceived intervals. These results provide the first evidence that direct manipulations of alpha oscillations can shift perceived time in a manner consistent with a clock speed effect.
Keywords: EEG, milliseconds, tACS, time generalization, visual stimuli, young adults
Introduction
Despite temporal processing having a fundamental role in daily living, the neural mechanisms underlying time perception are still being uncovered. Recent research in this area has begun to focus on the role of endogenous oscillations in temporal perception and action. Yet, the role of any particular neural oscillation in temporal perception remains to be determined (Wiener and Kanai 2016). In search for an internal timekeeper, some theories (Treisman 1963) have suggested that the rate of an internal pacemaker would be driven by neural oscillations in the alpha range (8–12 Hz). Faster alpha rhythms would result in longer estimates of time than slower alpha rhythms, considering that more pulses would accumulate during the same physical time interval (Treisman et al. 1990, 1994). Given recent findings that individual alpha frequency (IAF) correlates with temporal illusions (Cecere et al. 2015), it remains plausible that fluctuations of alpha peaks could modulate perceived duration. However, the relationship between IAF and modification in subjective timing has not been clearly established.
Associations between cognitive performance and endogenous modulations of oscillatory neural activity in the IAF range have been established in a number of studies (Klimesch 1999). Indeed, previous research has revealed that IAF predicts the performance on a variety of perceptual (e.g., Cecere et al. 2015; Samaha and Postle 2015) and cognitive (e.g., Bornkessel et al. 2004; Klimesch et al. 2006) tasks. If these associations are functionally relevant, then it should be possible to influence cortical oscillations with noninvasive brain stimulation techniques (Helfrich et al. 2014; Ruhnau et al. 2016) and thereby modulate behavioral performance (Klimesch 2012). Here, we applied transcranial alternating current stimulation (tACS), under the hypothesis that it can induce predictable changes in oscillatory cortical activity and alter perception in a temporal generalization task.
Previous studies have shown that the phase of alpha oscillations at the onset of 2 temporally proximal visual stimuli predicted whether they were perceived as occurring simultaneously or asynchronously (Varela, et al., 1981; Samaha and Postle 2015) suggesting that alpha cycles reflect sequential discrete “frames” of visual perception (Busch et al. 2009; Busch and van Rullen 2014). More recently, Cecere et al. (2015) observed that stimulus temporal proximity determines whether or not inputs coming from different modalities are bound or not. Using a flash-sound illusion paradigm, in which a single flash bound by 2 tones produces an illusory second flash, the authors were able to modify the temporal window of integration, indexed by how close in time the tones had to be to induce an illusory percept, by applying tACS during task performance at IAF or IAF ±2 Hz. Compared with performance at tACS IAF, the temporal window was wider at IAF-2 Hz and narrower at IAF + 2 Hz, indicating that a faster alpha rate was associated with less susceptibility to the illusion. Similarly, Zhang et al. (2019) showed that applying tACS at different frequency in the α-band changed subjects’ perceptual switch rate in the binding of bistable color-motion stimuli. Taken together, previous studies provided converging evidence for a causal link of IAF and temporal resolution of visual perception (see also Samaha et al. 2015; Samaha and Postle 2015; Ronconi et al. 2018).
The present study aims to investigate the link between modification in IAF and variation in perceived duration. We first recorded EEG during a 5-min resting state, which was used to extract IAF; then, participants performed a sub-second visual time generalization task while receiving tACS either at their IAF or at individually accelerated or decelerated rates (IAF ± 2 Hz). We hypothesized that driving IAF toward slower versus faster oscillations should result in under- or over-temporal estimation, respectively (Fig. 1). As an alternative hypothesis, we note that altering IAF may not shift perceptual duration per se but instead alter the resolution at which time intervals are perceived. Under this instance, no biases should be observed, but instead a sharpening/blunting of temporal estimates, leading to differences in the precision of generalization gradients across conditions. We further note that both patterns may occur, such that tACS shifts both temporal estimation with a concomitant change in precision. Indeed, a faster internal pacemaker may also be more precise (Gibbon 1992), and so a change in both measures would support a shift in pacemaker speed. We further note that behavioral shifts attributable to pacemaker changes do not necessarily entail changes in variability (Matell et al. 2004).
Figure 1 .
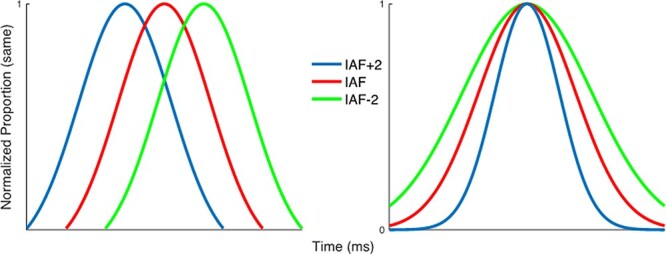
Two possible hypotheses for the manipulation of IAF and time estimation. Left: if IAF indexes the rate of temporal estimation, akin to altering the speed of an internal pacemaker, then increasing/decreasing the speed of this pacemaker would shift temporal estimates, such that a faster/slower IAF leads to intervals perceived as longer/shorter. Right: if IAF indexes the sample rate or resolution of the temporal estimator, then increasing/decreasing the resolution would sharpen temporal estimates, such that a faster/slower IAF leads to more/less precise estimates of duration.
Materials and Methods
Participants
Thirty-two right-handed, healthy volunteers (mean age = 22.95, SD = 4.64; range 19–36 years) who met inclusion criteria for brain stimulation were initially contacted for participation in the experiment. Of those contacted, 19 responded and agreed to participate (mean age = 23.15, SD = 9.80); among these, 1 was excluded due to a misunderstanding of the task resulting in uninterpretable data. The final sample included 18 right-handed healthy volunteers (9 males, 9 females; mean age, 22.89, SD = 4.47 years; range, 19–36 years) who met inclusion criteria for brain stimulation participated in the experiment. All subjects gave their informed consent as approved by the Institutional Review Board of George Mason University.
Procedure
Participants were tested in 2 separate experimental sessions on separate days. One the first day, participants underwent 5-min EEG recording and we calculated IAF. On the second testing day, in 3 separate blocks, participants received tACS over occipital cortex to modulate occipital alpha oscillations at their IAF or at slower (IAF −2 Hz) or faster (IAF +2 Hz) rates while they were performing the time generalization task; stimulation condition (IAF and ± 2 IAF) was counterbalanced between participants. The tACS blocks were 40 min apart from each other (Fig. 2, Top).
Figure 2 .
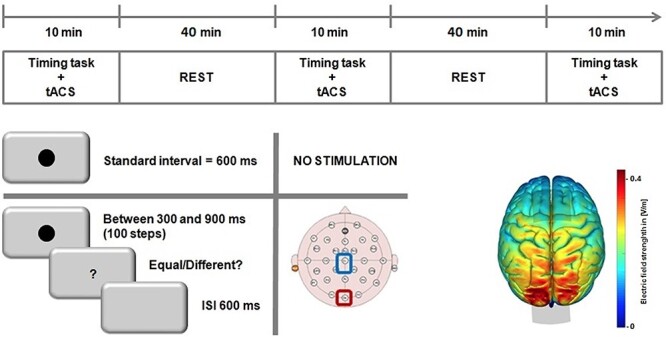
Graphical representation of experimental procedure. Top: timeline of the experiment for the tACS sessions; stimulation levels were counterbalanced across subjects/blocks. Bottom Left: schematic for the temporal generalization task, in which subjects first learned a standard 600-ms interval, prior to stimulation, and were then tested on a range of linearly spaced intervals around the standard. Bottom Right: a simulation of the normalized electric field strength of tACS electrodes, run via SimNIBS displaying maximal field strength over occipital cortex.
Time Generalization Task
We chose a time generalization task in which the response requirements (same/different) are nondirectional in nature and therefore resistant to response bias over tasks relying on a directional response (i.e., longer/shorter) (Yates et al. 2012). Participants were instructed to judge if a probe stimulus was the same or different than a previously learned standard. During an initial learning phase, participants encoded the standard duration (600 ms), presented 10 times (Fig. 2 Bottom left). In the subsequent testing phase, participants were given 4 blocks of 56 trials without feedback. Each block included 8 initial presentations of the standard duration (600 ms) as a “top-up” phase and 48 presentations of different intervals (24 shorter: 300, 400, and 500 ms and 24 longer: 700, 800, and 900 ms). The top-up phase was included to remind subjects of the standard interval; we suggest that the low number of exposures would not override the previously trained memory due to any effect of tACS, as visual stimuli typically require a large number of trials to shift trained intervals in memory (Gamache and Grondin 2010). Participants had to judge whether the comparison duration was similar (equal response) or not similar (different response) to the standard duration by pressing the corresponding key. Visual stimuli (gray circle) were presented centrally on a computer screen in a lit room while participants were sat on a comfortable chair at ~ 60 cm viewing distance. Stimulus presentation and behavioral responses were recorded by a computer using E-Prime software (Psychology Software Tools, Inc. Version 2.0). In the temporal generalization task, participants were instructed to compare the duration of the presented stimulus with a previously learned standard (Droit-Volet 2002). We used a QWERTY keyboard to record responses and response keys were labeled with a yellow sticker for “yes” and green stickers for “no” responses. Participants responded with their index fingers; the yellow sticker was placed over the “S” letter on the keyboard and the green sticker was placed over the “L” letter on the keyboard.
EEG Recording and Analysis
We note that these EEG data were collected from several other experiments concurrently being conducted at our institution; as such, 2 different EEG recording systems were used. Sixteen participants were recorded using a 64-channel active electrode system (BrainProducts, Munich, Germany) with a sample rate of 1000 Hz and impedances kept below 20 kΩ. Two participants were recorded using a 40-channel passive electrode system (NeuroScan) with a sample rate of 1000 Hz and impedances kept below 5 kΩ. In both recordings, the electrodes were placed according to the 10–20 system; the online reference was placed at FCz and the ground was placed at AFz in the 64 channel system, whereas the online reference was the left mastoid in the 40-channel system. Data were recorded using an online band-pass filter of 0.1–100 Hz for the 64-channel system and a high-pass filter of 0.1 Hz for the 40-channel system. For all participants, we recorded 5 min EEG resting state eyes open.
EEG data were preprocessed offline using EEGLAB (version 12.0.2b; Delorme and Makeig 2004). First, the data were down-sampled to 500 Hz and rereferenced to the common average. Then, we performed an automatic rejection of noisy EEG channels on continuous data and confirmed by visual inspection (median = 1; range 0–5 channels rejected). The noisy channels were interpolated using spherical splines. Next, we applied an automatic artifact rejection procedure on the continuous EEG data using ERPLAB (Lopez-Calderon and Luck 2014). A Fourier transform was then conducted on all electrodes using the remaining, artifact-free dataset using the EEGLAB function spectopo with a frequency factor of 10. Finally, we extracted the IAF by first detrending the spectral data for 1/f noise, and then averaging the maximum spectral peak (8–13 Hz) of the following a-priori channels: P1, P2, P3, P4, Pz, P5, P6, P7, P8, PO3, PO4, POz, PO7, PO8, O1, Oz, and O2 (Corcoran et al. 2018). For the 3 subjects using the 40-channel system, the same electrodes were used minus those not available: P3, P4, Pz, PO1, PO2, O1, Oz, and O2. For each subject, the averaged power spectrum was visually inspected for a clear peak around 10 Hz; 3 subjects exhibited a lack of a discernable peak and they were excluded.
tACS
The tACS was conducted within a single session and the 3 blocks of stimulation occurred 40 min apart (cf. Cecere et al. 2015). This separation in time was expected to allow any after-effect of tACS to return to a sufficient baseline before being altered again (Neuling, et al. 2013; Wach, et al. 2013). Positions of Oz and Cz were determined using the 10–20 system. tACS was delivered by NeuroConn DC-Stimulator+ through a pair of rubber electrodes enclosed in saline-soaked sponges and fixed on the head by elastic bands. The reference electrode was placed over Cz and the active electrode was placed over the occipital cortex (Oz). The reference electrode (Cz) had a larger size (35 cm2) than the active electrode (Oz, 9 cm2) to decrease current density delivered over Cz (Cecere et al. 2015). Impedance was maintained within the safety criteria of the tACS device; if they were not met the experimenter added saline solution and tightened the headband to improve electrode contact with the scalp. tACS was delivered at 1.5-mA peak-to-peak; participants were stimulated for a total of 10 min in each block. Subjects completed a poststudy questionnaire after each session to assess side effects (tingling, itching sensation, burning sensation, pain, and fatigue). All participants were actively encouraged to report any perception of tACS-induced phosphenes throughout the experimental sessions. To avoid phosphenes throughout the experimental sessions participants received 30-s stimulation at their IAF and IAF ± 2 Hz before performing the time generalization task; if phosphenes were reported, stimulation would be lowered until none were detected. None of the participants reported phosphenes during any of the 30-s stimulations prior to the testing phase, nor after any of the stimulation sessions, and therefore, the intensity of stimulation was kept at 1.5 mA for all participants.
To validate the likely physiological source of stimulation, we simulated the effect of our electrode montage using the SimNIBS 2.0 Toolbox (http://simnibs.de/). As in our main experiment, the placement of 2 electrodes located at Oz and Cz, were simulated with a current of 1.5 mA. The normalized electrical field was simulated via a realistic finite element head model. The results of this analysis revealed a centralized electrical field effect centred over the occipital cortex (Fig. 2, Bottom right).
Statistical Analyses
Performance was analyzed in terms of the Point of Subjective Equality (PSE), calculated as the time value at which subjects were maximally likely to judge the stimulus as equal and the Weber Ratio (WR), calculated as the normalized variability of measurements. Individual PSEs for each of the 3 stimulation conditions were estimated using adapted MATLAB routines for fitting curves to simultaneity judgment tasks (Alcalá-Quintana and García-Pérez 2013). Briefly, this routine is based on an independent-channels model for stimulus timing judgment tasks proposed by García-Pérez and Alcalá-Quintana (2012a). The advantage of this model over fitting a simple Gaussian curve is that it is a psychophysical model that explicitly accounts for response errors arising from nonspecific factors (e.g., lapses of attention, misreports) (Garcia-Perez and Alcalá-Quintana 2012). The results of this analysis yielded the PSE, and the WR calculated as the peak spread of the fitted curve divided by the PSE.
PSE and WR data were included in separate repeated-measures ANOVAs with Condition (IAF, IAF + 2 Hz, and IAF-2 Hz) as within-subjects factors. Significant effects were followed up by post hoc analyses with Bonferroni correction to reduce the Type I error rate, and the effect size was estimated with the partial eta squared index (η2p). An observed shift of the PSE for the different condition (IAF, IAF + 2 Hz, and IAF-2 Hz) can be interpreted as an indicator of differences in these conditions, with smaller bisection point (left shift of the psychometric function) values meaning longer perceived durations. Concerning WR, higher values indicate higher temporal variability.
Results
Each participant underwent a single, 5-min eyes-open resting-state EEG session before the temporal task and we extracted IAF. For the IAF analysis, we measured a mean IAF of 10.4 Hz ± 1.02, in the approximate middle of the alpha band. The IAF data were further determined to be normally distributed via a Shapiro–Wilk test [W(18) = 0.95, P = 0.422] (Fig. 3).
Figure 3 .

IAF values used for the tACS experiment. (A) Topographic plot of the average spectral power across subjects in the alpha band. The distribution exhibited strongest power at posterior electrodes; dark circles indicate electrodes used for calculating IAF. (B) Mean power spectrum (log scale) across the electrodes in (A) exhibiting a characteristic 1/f distribution and typical “peak” in the alpha range. (C) Raincloud plot (Allen et al. 2019) of IAF values calculated for each subject; values were normally distributed across the alpha band.
Subjects then performed a temporal generalization task with visual stimuli, in which they were required to judge if a probe stimulus was the same or different than a previously learned, prior to stimulation, standard (600 ms). When analyzing the mean proportion of trials on which subjects classified durations as “same,” we initially observed a main effect of tested duration [F(6,102) = 15.12, P < 0.001, η2p = 0.471] and a significant interaction between tACS and duration [F(12,204) = 4.613, P < 0.001, η2p = 0.213] (Figs 4 and 5A). Concerning perceived duration (PSE), a main effect of tACS [F(2,34) = 4.232, P = 0.023, η2p = 0.20] was found; post hoc paired t-tests showed significant difference between PSE at IAF-2 Hz and PSE at IAF + 2 Hz [t(17) = 2.869, P = 0.011, Cohen’s d = 0.68] but not between PSE at IAF and PSE at IAF + 2 Hz (P = 0.134) or PSE at IAF and PSE at IAF-2 Hz (P = 0.169) (Fig. 5B). Furthermore, a linear contrast effect was observed across PSE values from slower to faster IAF values [F(1,17) = 8.234, P = 0.011, η2p = 0.326], in which the PSE shifted linearly from left-to-right with faster-to-slower tACS rates. The effect of stimulation acted specifically on perceived duration and did not influence temporal variability (WR) [F(2,34) = 1.206, P = 0.312, η2p = 0.066] (Fig. 5C).
Figure 4 .

Individual psychometric functions for each of the stimulation conditions. Each point represents proportion of responding “same” for that tested interval.
Figure 5 .
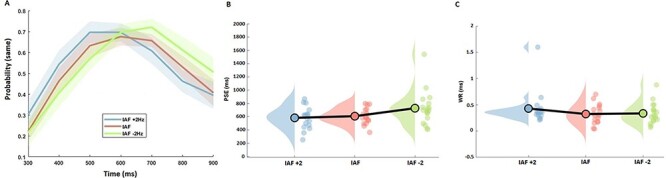
Behavioral results of tACS. (A) Mean temporal generalization gradients for each of the stimulation conditions. Each point represents the average proportion of responding “same” for that tested interval. Subjects exhibited a leftward shift for IAF + 2 Hz and a rightward shift for IAF-2 Hz, relative to IAF tACS. Shaded regions represent standard error. (B) Raincloud plots of PSE values across all 3 conditions exhibited the same pattern as in the average proportion data, with a linear shift in PSE for slower-to-faster tACS. (C) Same plot at in (B), but for WR values, exhibiting no significant shifts across conditions.
Given the above findings, we further investigated if IAF interacted with the observed effect. To quantify this, we calculated the slope value of a linear regression for each subject between the PSE values and the individually determined levels in stimulation frequency. Accordingly, a negative slope value indicates a shift toward longer/shorter perceived intervals with faster/slower frequencies of stimulation. When correlated with the baseline IAF values, we observed a positive correlation; this effect was quantified by both a Pearson [r(17) = 0.523, P = 0.026] and Spearman [rho(17) = 0.481, P = 0.043] correlations and confirmed using bootstrapped samples for each [Pearson 95% confidence interval: 0.167–0.75; Spearman: 0.019–0.785]. Bootstrapped samples were calculated by sampling with replacement from the original dataset and recalculating the correlation coefficients 10 000 times. Spearman and Pearson coefficients were used to ensure robustness against outliers (Rousselet and Pernet 2012). As a further measure, we removed 2 subjects that could have overly driven the effect (Fig. 6). The Pearson correlation remained significant (0.54, P = 0.025), whereas the Spearman became only marginally significant (0.47, P = 0.057). Thus, while we suggest that the effect may have been influenced by a strong effect in 2 subjects, we note that even without these 2, the same relationship is observed.
Figure 6 .

Correlation between baseline IAF and impact of tACS. (A) Individual IAF values are plotted against the slope of a linear regression of PSE values across stimulation levels; a negative slope indicates that faster/slower tACS increased/decreased estimates of duration. Shaded regions indicate density estimates. A positive correlation was observed, indicating that subjects with a slower baseline IAF exhibited a larger impact of tACS. The effects remained largely significant with the removal of 2 outliers (see text). (B) Bootstrapped distributions of Pearson and Spearman correlation coefficients; dashed lines indicate 95% confidence intervals for each distribution.
Discussion
The present study aimed to investigate the link between modification of IAF and variation in perceived duration. In particular, we hypothesized that applying tACS at IAF or driving IAF toward slower or faster oscillation rates would result in either under- or over-temporal estimation if IAF are involved in perceived duration, or in less- or more-precise estimation if involved in temporal resolution. We note that both possibilities could also have occurred, in which case under- or over-estimations would have become less- or more-precise. Our results confirm the first hypothesis by indicating that tACS applied at IAF ±2 Hz produced a shift in the psychometric function consistent with a variation in perceived duration, with no change in the precision of temporal estimates.
The link between neural oscillations in the alpha range (8–12 Hz) and temporal processing has a long history (Anliker 1963; Wiener and Kanai 2016). Several researchers suggested that α may contribute to defining psychological moments with the hypothesis that 1 α cycle (~100 ms) would form the unit of subjective time, that is, the psychological moment. Here, we extend previous findings by showing the intraindividual modification of IAF results in modified perceived duration. Importantly, we confirmed that the observed modification induced by tACS was specific for perceived duration and not on temporal variability, confirming a causal link between IAF and perceived duration. It is assumed that the speed of internal clock can be modulated by the speed of cortical oscillators, and in particular, the clock speed could be represented by the alpha band regime (Treisman et al. 1990, 1994). Here, we confirm the relationship between IAF and fluctuations in subjective timing. Furthermore, our results demonstrate that baseline IAF rate contributes to the strength of the effect of tACS; subjects with a slower baseline IAF showed a greater influence of tACS than those with a faster IAF. This finding suggests an upper limit for speeding up alpha oscillations, such that, beyond a particular frequency, the visual system is unable to accommodate a faster clock signal.
Our results are consistent with previous studies that observed variation in perceived duration with transcranial Random Noise Stimulation (tRNS; Mioni et al. 2018) compared with variation in temporal variability with transcranial Direct Currect Stimulation (tDCS; Mioni et al. 2016, Vicario et al. 2013). tACS and tRNS are not intended to excite or inhibit cortical activity monotonously as it is for tDCS. Instead, the main goal of tACS is to influence brain oscillations (Woods et al. 2016). tRNS is similar to tACS in that it uses an alternating current; however, instead of stimulating at a fixed frequency throughout the stimulation period, tRNS alternates at a random frequency and amplitude within a specific range. As such, tACS provides the opportunity to test targeted hypotheses regarding specific frequency bands. We note that our results differ from another recent tACS study of time perception (Wiener et al. 2018) in which beta (20 Hz), but not alpha, stimulation induced a change in temporal perception. However, we further note that 1) stimulation in that study was localized to the supplementary motor area; 2) the study employed a temporal bisection task, with very different task demands and response mapping; and 3) the effect of beta oscillations was hypothesized to be a result of altering the memory, rather than perception, of perceived intervals, a finding consistent with beta’s potential role in timing (Mendoza et al. 2018). In particular, we also note that this previous study did not titrate the stimulation frequency to individual values. This last point bears emphasizing: if we had stimulated all subjects at the same frequency within the alpha band (i.e., 10 Hz) in the present study, no effect would have been observed, with some subjects speeding up and others slowing down.
Broadly, alpha oscillations have been associated with a variety of perceptual and cognitive processes, including attentional state and focus (Klimesch 2012), perceptual awareness (Mathewson et al. 2009), problem solving (Fink and Neubauer 2006), and working memory (Bonnefond and Jensen 2012). Occipital alpha, which our experiment focuses on, has been hypothesized to underlie phasic transmission from the lateral geniculate nucleus to striate and extrastriate cortex (Bollimunta et al. 2011; Vijayan and Kopell 2012). Beyond the visual cortex, alpha oscillations have been linked with theta (~5–7 Hz) rhythms in coordinated patterns to prefrontal regions (Zhang et al. 2019). The function of this coordination has been associated with perceptual “sampling” of visual information, which may be probed via time-based illusions or behavioral cycles (VanRullen 2016; Brüers and VanRullen 2017; Tomassini et al. 2017). We here suggest, additionally, that this sample rate mediates the individual perception of duration. A limitation of our study is the lack of any EEG measures of alpha oscillation after using tACS. Further work will be necessary to determine how tACS specifically alters IAF and other EEG indices.
Notably, tRNS, which also can shift perceived duration, has been associated with a frequency-specific increase in resting theta oscillations (Van Doren et al. 2014) despite the broad, high range of frequencies employed. As theta oscillations are also involved in time perception (Kononowicz and van Rijn 2015; Zold and Shuler 2015; Wiener and Kanai 2016) and are functionally coupled with alpha oscillations, it is possible that stimulation-specific effects on perceived, rather than remembered duration is the result of interactions between these 2 frequency bands, as tested by each stimulation technique. Consistent with this idea, recent evidence suggests that alpha–theta interactions underlie an intermixing of phasic sampling (alpha) and attentional focus (theta) such that each is necessary for gating of sensory information (Fiebelkorn et al. 2018; Helfrich et al. 2018).
Our results are also informative to neurobiological models of time perception. Recent debate exists in the field regarding the modal-specificity of duration processing, as to whether or not time depends on the modality in which it is perceived (Ivry and Schlerf 2008; van Wassenhove 2009; Merchant et al. 2013). Our results suggest that time is a modal feature, which may be altered by manipulating oscillatory cycling within the visual cortex; however, we note that our findings are limited in that the intervals tested were 1) sub-second and 2) visually demarcated. Yet, to the former point, recent research suggests that visually induced time dilation effects exist at both sub and supra-second durations (Herbst et al. 2013; Shima et al. 2016; Wearden et al. 2017), and both may be linked to occipital alpha (Hashimoto and Yotsumoto 2018); to the latter, if timing is modal-specific, then an auditory version of our task would have resulted in a null effect. Further work will need to dissociate between the role of modality and interval length on the impact of tACS stimulation.
Overall, our results provide the first evidence that we are aware of for a causal link between individual alpha levels and the rate of the internal clock in time estimation. We further suggest that previous studies which could not account for a strong link between alpha and time estimation did not examine individual differences in alpha that may have accounted for heterogeneous results between subjects. Our results suggest a way forward for further studies to dissect an alpha-driven clock signal for the perception of duration.
Notes
The information in this manuscript and the manuscript itself has never been published either electronically or in print. This work was carried out within the scope of the project “use-inspired basic research,” for which the Department of General Psychology of the University of Padova has been recognized as “Dipartimento di Eccellenza” by the Ministry of University and Research. Conflict of Interest: None declared.
Funding
Iniziative di Cooperazione Universitaria 2018 sponsored by University of Padova (to G.M.).
Contributor Information
Giovanna Mioni, Department of General Psychology, University of Padova, 35121 Padova, Italy.
Adam Shelp, Department of Psychology, George Mason University, Fairfax, VA 22030, USA.
Candice T Stanfield-Wiswell, Department of Psychology, George Mason University, Fairfax, VA 22030, USA.
Keri A Gladhill, Department of Psychology, George Mason University, Fairfax, VA 22030, USA.
Farah Bader, Department of Psychology, George Mason University, Fairfax, VA 22030, USA.
Martin Wiener, Department of Psychology, George Mason University, Fairfax, VA 22030, USA.
References
- Alcalá-Quintana R, García-Pérez MA. 2013. Fitting model-based psychometric functions to simultaneity and temporal-order judgment data: MATLAB and R routines. Behavior Research Methods. 45(4):972–998. [DOI] [PubMed] [Google Scholar]
- Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA. 2019. Raincloud plots: a multi-platform tool for robust data\visualization [version 1; peer review: 2 approved]. Wellcome Open Res. 4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anliker J. 1963. Variations in alpha voltage of the electroencephalogram and time perception. Science. 140(3573):1307–1309. [DOI] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. 2012. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Current Biology. 22(20):1969–1974. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. 2011. Neuronal mechanisms and attentional modulation of corticothalamic alpha oscillations. Journal of Neuroscience. 31(13):4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkessel ID, Fiebach CJ, Friederici AD, Schlesewsky M. 2004. “Capacity” reconsidered: interindividual differences in language comprehension and individual alpha frequency. Experimental Psychology. 51(4):279–289. [DOI] [PubMed] [Google Scholar]
- Brüers S, VanRullen R. 2017. At what latency does the phase of brain oscillations influence perception? eNeuro. 4(3):ENEURO.0078-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. 2009. The phase of ongoing EEG oscillations predicts visual perception. Journal of Neuroscience. 29(24):7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch N, VanRullen R. 2014. Is visual perception like a continuous flow or a series of snapshots. In: Arstila V, Lloyd D, editors. Subjective time: The philosophy, psychology, and neuroscience of temporality. The MIT Press, p. 161–178. [Google Scholar]
- Cecere R, Rees G, Romei V. 2015. Individual differences in alpha frequency drive crossmodal illusory perception. Current Biology. 25(2):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AW, Alday PM, Schlesewsky M, Bornkessel-Schlesewsky I. 2018. Toward a reliable, automated method of individual alpha frequency (IAF) quantification. Psychophysiology. 55(7):e13064. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 134(1):9–21. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S. 2002. Scalar timing in temporal generalization in children with short and long stimulus durations. The Quarterly Journal of Experimental Psychology Section A. 55(4):1193–1209. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, Pinsk MA, Kastner S. 2018. A dynamic interplay within the frontoparietal network underlies rhythmic spatial attention. Neuron. 99(4):842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Neubauer AC. 2006. EEG alpha oscillations during the performance of verbal creativity tasks: differential effects of sex and verbal intelligence. International Journal of Psychophysiology. 62(1):46–53. [DOI] [PubMed] [Google Scholar]
- Gamache PL, Grondin S. 2010. The lifespan of time intervals in reference memory. Perception. 39(11):1431–1451. [DOI] [PubMed] [Google Scholar]
- García-Pérez MA, Alcalá-Quintana R. 2012a. On the discrepant results in synchrony judgment and temporal-order judgment tasks: a quantitative model. Psychonomic Bulletin & Review. 19(5):820–846. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez MA, Alcala-Quintana R. 2012b. Response errors explain the failure of independent-channels models of perception of temporal order. Frontiers in Psychology. 3:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J. 1992. Ubiquity of scalar timing with a Poisson clock. Journal of Mathematical Psychology. 36(2):283–293. [Google Scholar]
- Lopez-Calderon J, Luck SJ. 2014. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Yotsumoto Y. 2018. The amount of time dilation for visual flickers corresponds to the amount of neural entrainments measured by EEG. Frontiers in Computational Neuroscience. 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. 2014. Entrainment of brain oscillations by transcranial alternating current stimulation. Current Biology. 24(3):333–339. [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Fiebelkorn IC, Szczepanski SM, Lin JJ, Parvizi J, Knight RT, Kastner S. 2018. Neural mechanisms of sustained attention are rhythmic. Neuron. 99(4):854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst SK, Javadi AH, van der Meer E, Busch NA. 2013. How long depends on how fast—perceived flicker dilates subjective duration. PLoS One. 8(10):e76074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Schlerf JE. 2008. Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences. 12(7):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. 1999. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 29(2–3):169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W. 2012. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences. 16(12):606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Hanslmayr S. 2006. Upper alpha ERD and absolute power: their meaning for memory performance. Progress in Brain Research. 159:151–165. [DOI] [PubMed] [Google Scholar]
- Kononowicz TW, van Rijn H. 2015. Single trial beta oscillations index time estimation. Neuropsychologia. 75:381–389. [DOI] [PubMed] [Google Scholar]
- Matell MS, King GR, Meck WH. 2004. Differential modulation of clock speed by the administration of intermittent versus continuous cocaine. Behavioral Neuroscience. 118(1):150. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. 2009. To see or not to see: prestimulus α phase predicts visual awareness. Journal of Neuroscience. 29(9):2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza G, Méndez JC, Pérez O, Prado L, Merchant H. 2018. Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nature Communications. 9(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, Meck WH. 2013. Neural basis of the perception and estimation of time. Annual Review of Neuroscience. 36:313–336. [DOI] [PubMed] [Google Scholar]
- Mioni G, Grondin S, Mapelli D, Stablum F. 2018. A tRNS investigation of the sensory representation of time. Scientific Reports. 8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioni G, Grondin S, Forgione M, Fracasso V, Mapelli D, Stablum F. 2016. The role of primary auditory and visual cortices in temporal processing: a tDCS approach. Behavioural Brain Research. 313:151–157. [DOI] [PubMed] [Google Scholar]
- Paulus W. 2011. Transcranial electrical stimulation (tES–tDCS; tRNS, tACS) methods. Neuropsychological Rehabilitation. 21(5):602–617. [DOI] [PubMed] [Google Scholar]
- Ronconi L, Busch NA, Melcher D. 2018. Alpha-band sensory entrainment alters the duration of temporal windows in visual perception. Scientific Reports. 8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet GA, Pernet CR. 2012. Improving standards in brain-behavior correlation analyses. Frontiers in Human Neuroscience. 6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhnau P, Neuling T, Fuscá M, Herrmann CS, Demarchi G, Weisz N. 2016. Eyes wide shut: transcranial alternating current stimulation drives alpha rhythm in a state dependent manner. Scientific Reports. 6(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha J, Postle BR. 2015. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Current Biology. 25(22):2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha J, Bauer P, Cimaroli S, Postle BR. 2015. Top-down control of the phase of alpha-band oscillations as a mechanism for temporal prediction. Proceedings of the National Academy of Sciences. 112(27):8439–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima S, Murai Y, Hashimoto Y, Yotsumoto Y. 2016. Duration adaptation occurs across the sub-and supra-second systems. Frontiers in Psychology. 7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman M. 1963. Temporal discrimination and the indifference interval: implications for a model of the “internal clock”. Psychological Monographs: General and Applied 77:1–31. [DOI] [PubMed] [Google Scholar]
- Treisman M, Cook N, Naish PL, MacCrone JK. 1994. The internal clock: electroencephalographic evidence for oscillatory processes underlying time perception. Quarterly Journal of Experimental Psychology. 47:241–289. [DOI] [PubMed] [Google Scholar]
- Treisman M, Faulkner A, Naish PL, Brogan D. 1990. The internal clock: evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. Perception. 19:705–743. [DOI] [PubMed] [Google Scholar]
- Tomassini A, Ambrogioni L, Medendorp WP, Maris E. 2017. Theta oscillations locked to intended actions rhythmically modulate perception. eLife. 6:e25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario CM, Martino D, Koch G. 2013. Temporal accuracy and variability in the left and right posterior parietal cortex. Neuroscience. 245:121–128. [DOI] [PubMed] [Google Scholar]
- Van Doren J, Langguth B, Schecklmann M. 2014. Electroencephalographic effects of transcranial random noise stimulation in the auditory cortex. Brain Stimulation. 7(6):807–812. [DOI] [PubMed] [Google Scholar]
- Van Wassenhove V. 2009. Minding time in an amodal representational space. Philosophical Transactions of the Royal Society B: Biological Sciences. 364(1525):1815–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R. 2016. Perceptual cycles. Trends in Cognitive Sciences. 20(10):723–735. [DOI] [PubMed] [Google Scholar]
- Vijayan S, Kopell NJ. 2012. Thalamic model of awake alpha oscillations and implications for stimulus processing. Proceedings of the National Academy of Sciences. 109(45):18553–18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearden JH, Williams EA, Jones LA. 2017. What speeds up the internal clock? Effects of clicks and flicker on duration judgements and reaction time. The Quarterly Journal of Experimental Psychology. 70(3):488–503. [DOI] [PubMed] [Google Scholar]
- Wiener M, Kanai R. 2016. Frequency tuning for temporal perception and prediction. Current Opinion in Behavioral Sciences. 8:1–6. [Google Scholar]
- Wiener M, Parikh A, Krakow A, Coslett HB. 2018. An intrinsic role of beta oscillations in memory for time estimation. Scientific Reports. 8(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, et al. 2016. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology. 127(2):1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MJ, Loetscher T, Nicholls ME. 2012. A generalized magnitude system for space, time, and quantity? A cautionary note. Journal of Vision. 12(7):9–9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Cai P, Luo H, Fang F. 2019. The causal role of α-oscillations in feature binding. Proceedings of the National Academy of Sciences. 116(34):17023–17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zold CL, Shuler MGH. 2015. Theta oscillations in visual cortex emerge with experience to convey expected reward time and experienced reward rate. Journal of Neuroscience. 35(26):9603–9614. [DOI] [PMC free article] [PubMed] [Google Scholar]


