Abstract
Objective:
Behavioral interventions during early memory decline hold promise in delaying the development of dementia. In the present study, participants in a multimodal behavioral intervention study were assessed for post-intervention adherence and predictors of adherence.
Methods:
Participants (N=272, mean age=75.04 ±7.54) diagnosed with amnestic Mild Cognitive Impairment (aMCI) were assigned to intervention groups receiving four out of five behavioral intervention components, including yoga, memory compensation training, computerized cognitive training, support groups, and/or wellness education. Length of the intervention was 10 days, 4 hours per day, with post-intervention follow-up at 6, 12, and 18 months.
Results:
Two-hundred and thirty-seven participants completed the 6-month post-intervention follow-up measures, 228 participants completed the 12-month measures, and 218 participants completed the 18-month measures. Participants fully adhered to a mean of 2 out of the 4 taught intervention components. Eighty-nine percent of participants were at least partially adherent to one or more taught intervention components at 6-, 12-, and 18-month post-intervention follow-up. Physical activity was the most adhered to intervention while group support was the least adhered to intervention across all three follow-up time-points. Higher educational level, higher baseline depressive symptoms, higher baseline global cognitive functioning, and better baseline and concurrent functional abilities were associated post-intervention adherence.
Conclusion:
Changes in functional abilities are associated with disease progression among persons with aMCI. In the present study, individuals with aMCI who have higher education, higher depressive symptoms, and better baseline functioning abilities are more likely to adhere to behavioral intervention components over time. Post-intervention adherence also associates with concurrent daily function.
Keywords: MCI, cognitive intervention, multimodal behavioral intervention, post-intervention adherence, aging
Introduction:
Mild Cognitive Impairment (MCI) is the prodromal phase that, for many, precedes the development of dementia. In this phase individuals show cognitive impairment in psychometric testing, with moderately retained or mild functional impairments in activities of daily living (ADLs) (Albert et al., 2011). A recent meta-analysis suggests that the cognitive issues experienced by people with MCI negatively impact their mood, relationships, compliance to medical treatment, and independence (Chandler, Parks, Marsiske, Rotblatt, & Smith, 2016). In a qualitative interview persons with aMCI and their study partners rated patient-related outcomes (quality of life, self-efficacy, memory-based activities of daily living, daily functioning, and mood) as more important than partner-related outcomes (burden, self-efficacy, anxiety, and mood) (Barrios et al., 2016). The use of behavioral interventions to delay or prevent the transition from MCI to dementia, as well as reduce distress associated with cognitive impairment, is a major focus of inquiry. Interventions such as exercise, brain training, calendar training, and group support/education (either individually or in combination [multi-modal]), have demonstrated a positive effect on cognition, mood, and ADLs (Chandler et al., 2016; Dannhauser et al., 2014; Suzuki et al., 2012). In a pilot study, we found patient memory-related activities of daily living (ADLs) improved among participants who received calendar training over those in the no-treatment group (Chandler et al., 2017).
Due to potential functional impairment impact of MCI on adherence to medical treatments in general it is important to assess adherence and its determinants in behavioral interventions. Interventional studies involving older adults with normal to impaired cognition have measured adherence as the number of enrolled participants completing a certain percentage of the designed program during the active learning phase of the intervention. For example, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was a multi-domain randomized control trial that aimed to prevent cognitive decline in at-risk healthy elderly people. In that trial, reported participation (i.e., adherence during the active phase) in the individual intervention components ranged from 47% to 95% but only 39% participated in all 4 interventions (Coley et al., 2019; Ngandu et al., 2015). They also found baseline smoking status, age, educational attainment, and depressive symptoms consistently impacted active-phase adherence to some of the individual intervention components and combined intervention components (Coley et al., 2019). Randomized controlled trials in older adults with MCI (Dannhauser et al., 2014; Suzuki et al., 2012) have used a similar approach as the FINGER trial when reporting adherence to interventions, but have not discussed factors that potentially affected the level of adherence during the active phase of the study. When considering behavioral interventions in older adults at risk for MCI (amnestic and non-amnestic), few studies of adherence to physical exercise programs have considered factors contributing to active-phase adherence or intervention participation. The results suggest that age, higher socioeconomic status, living alone, better health status, better physical abilities, better cognition, lower Clinical Dementia Rating (CDR) score, fewer depressive symptoms, physical activity level and absence of APOE-4 allele were all predictors of better adherence (Garmendia et al., 2013; Lam, Chan, Leung, Fung, & Leung, 2015; Pandya, Lacritz, Weiner, Deschner, & Woon, 2017; Picorelli, Pereira, Pereira, Felício, & Sherrington, 2014).
To our knowledge, no behavioral intervention study investigating persons with MCI has reported the mean number of intervention components of the multimodal intervention engaged in by participants after study completion (post-intervention). Likewise, no study has assessed the concurrent association between continuous involvement in a multimodal intervention and everyday functioning and cognitive functioning outcome measures in persons with aMCI post-intervention. Thus, the goal of this paper is to report the average number of intervention components participants adhered to (participated in) after completing the interventional classes by experimental research arm, as well as identify predictors and outcome measures associated with higher post-intervention adherence in a multimodal behavioral study in persons with aMCI. We hypothesize that within the restricted range of MCI, severity of illness and caregiver variables would predict adherence. The primary trial was a multicomponent comparative effectiveness trial supported by the Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306–01897, ClinicalTrials.gov Identifier: NCT02265757) (Smith et al., 2017).
Methods:
A complete description of the study protocol was reported by Smith et al. (2017) and the primary outcome analysis is reported in (Chandler et al., 2019). A brief description is summarized below.
Participants:
Two hundred and seventy-two dyads consisting of the study participant (person with aMCI) and study partner (spouse, family member, or friend) participated in the study. Recruitment took place at neuropsychology clinics and Alzheimer’s Disease Research Centers at Mayo Clinic in Minnesota, Arizona, and Florida, and at the University of Washington. Eligible participants had a diagnosis of amnestic MCI (single or multi-domain) according to the diagnostic guidelines from the National Institute on Aging – Alzheimer’s Association (Albert et al., 2011). They also met the inclusion criteria including: a Clinical Dementia Rating (CDR) (Morris, 1993) score of ≤0.5, fluency in English, not taking or stable on nootropics for at least 3 months, and a cognitively normal (per cognitive assessment) study partner who has at least twice-weekly contact with the participant. Exclusion criteria included clinically significant visual or auditory impairments that would render the potential participant or study partner unable to participate, or inclusion in another clinical trial. Participants’ baseline characteristics are described in Table 1.
Table 1:
Participant baseline characteristics by study arm.
| Characteristic | No Computer Exercises (n=54) | No MSS (n=57) | No Support Group (n=53) | No Wellness (n=52) | No Yoga (n=56) |
|---|---|---|---|---|---|
| Age, mean (range) | 75 (71–82) | 75 (69–80) | 75 (71–80) | 76 (72–80) | 75 (70–81) |
| Sex, No. (%) | |||||
| Male | 33 (61.1) | 31 (54.4) | 32 (60.4) | 30 (57.7) | 34 (60.7) |
| Female | 21 (38.9) | 26 (45.6) | 21 (39.6) | 22 (42.3) | 22 (39.3) |
| Years of education, mean (range)b | 16 (14–18) | 16 (14–18) | 16 (14–19) | 16 (14–18) | 17 (16–19) |
| Race, No. (%) | |||||
| Non-white | 1 (1.9) | 5 (8.8) | 2 (3.8) | 1 (1.9) | 3 (5.4) |
| White, non-Hispanic | 53 (98.1) | 52 (91.2) | 51 (96.2) | 51 (98.1) | 53 (94.6) |
| Partner, No. (%)b | |||||
| Spouse | 44 (81.5) | 41 (77.3) | 48 (90.6) | 44 (84.6) | 52 (92.9) |
| Other | 10 (18.5) | 12 (22.7) | 5 (9.4) | 8 (15.4) | 4 (7.1) |
| Current memory medications, No. (%)b | 24 (44.4) | 22 (41.5) | 11 (20.8) | 16 (32.7) | 27 (49.1) |
| DRS-2 total score, mean (range)b | 127.5 (122–135) | 129.9 (124–136) | 130.9 (127–136) | 128 (122–135) | 130.1 (124–138) |
| CDR score, No. (%) | |||||
| 0, None | 1 (1.9) | 7 (12.3) | 4 (7.5) | 5 (9.6) | 4 (7.3) |
| 0.5, Questionable | 53 (98.1) | 50 (87.7) | 49 (92.4) | 47 (90.4) | 51 (92.7) |
| QoL-AD score, mean (SD)b | 40.9 (5.7) | 39.9 (5.5) | 41.5 (5.7) | 39.1 (5.0) | 39.9 (5.9) |
| CES-D total score, mean (SD)b | 12.4 (9.6) | 12.8 (6.8) | 10.5 (7.2) | 12.6 (8.5) | 11.5 (8.2) |
| Self-efficacy score, mean (SD)b | 74.1 (15.7) | 72.6 (15.2) | 75.6 (13.8) | 73.0 (12.1) | 74.1 (13.0) |
| FAQ score, mean (SD)b | 7.13 (5.79) | 7.29 (5.35) | 6.2 (4.71) | 6.32 (5.09) | 5.55 (5.45) |
Abbreviations: DRS-2: Dementia Rating Scale-2, MSS: memory support system, CDR: Clinical Dementia Rating, QoL-AD: Quality of life in Alzheimer’s Disease, CES-D: Center of Epidemiologic Studies Depression Scale, FAQ: Functional Assessment Questionnaire.
P values result from the Kruskal-Wallis test for numeric variables and either the Chi-square test or the Fisher exact test for categorical variables.
Information was not reported for the following variables: years of education (no computer exercises [n=1]); DRS-2 total raw score (no MSS [n=2], no wellness [n=1], and no yoga [n=7]); partner (no MSS [n=4]); QoL-AD score (no computer exercise [n=1], no wellness [n=3]); CES-D total score (no computer training [n=1], no wellness [n=3], no MSS [n=4]); Self-efficacy score (no computer exercise [n=1], no wellness education [n=3], no MSS [n=4]); FAQ total score (no support group [n=1], no wellness education [n=3], no MSS [n=4]); and current memory medications (no MSS [n=4], no wellness [n=3], no yoga [n=1])
Intervention and randomization:
Each study arm consisted of four of five possible behavioral intervention components, producing five possible study arms. Cluster randomization was done by intervention assignment, and each study site ran each study arm at least once within the 16-month enrollment period. Dyads were trained for 45 to 60 minutes per behavioral intervention component. Thus, each study session was run for 4 hours per day for 10 days over a 2-week period. Participants and study partners had the weekend off to practice the first week’s trained components. Program instructors were PhD clinical neuropsychologists, Master’s trained counselors, or cognitive rehabilitation and dementia education specialists who variously conducted the memory support system training, support groups, and wellness programs and oversaw the computerized training. Certified yogini’s conducted the yoga sessions. The same persons would deliver multiple components of the program. For example, the site PIs conducted some of the MSS training, patient support groups, and delivered wellness sessions at their sites. A summarized description of each behavioral intervention component is below:
Yoga:
Participants engaged in 45 to 60 minutes of Hatha yoga daily. Hatha yoga was chosen because it is appropriate for older adults and it is manageable for those with limited mobility. Breathing and meditation exercises were also incorporated. The yoga sessions were led by certified yoga instructors.
After completing the 10-day sessions, study participants and study partners were encouraged to engage in their preferred exercise (yoga, water aerobics, resistance training, walking, swimming, running, etc.) for 150 minutes per week. Participants were provided with a customized yoga DVD if they wished to continue with yoga.
Computer brain fitness training (brain fitness):
Study participants engaged in 45- to 60-minute sessions of brain fitness training daily with the Brain-HQ™ product (Posit Science Corporation, San Francisco CA.). Computerized brain training with the Brain-HQ™ product consisted of six specific modules: target tracker, double decision, visual sweeps, sound sweeps, to-do list training, and syllable stacks. After completion of the 10-day study session, participants were provided with a 1-year subscription to the Brain-HQ product. Study participants were encouraged to engage in 150 minutes of computerized brain training per week for 18 months.
Wellness education:
The wellness education program used in the study included 45 to 60 minutes of discussion on a different topic each day, including: living with MCI, sleep hygiene, healthy brain aging, preventing dementia, MCI and depression, nutrition and exercise, community resources, and other health topics. Dyads were provided with resources to help them incorporate the discussed topics in their daily lives after the study.
Support group:
Separate 45- to 60-minute support groups were held for participants and partners. The participant group focused on emotional processing around the MCI diagnosis, memories, and adaptation, while the partner group focused on building resources for coping with the change in their family member or friend. Sessions were led by one or more professional therapists. At the conclusion of the intervention participants and partners were encouraged to seek out community-based support groups (e.g. Alzheimer’s Association groups).
Memory support system (MSS) compensation training:
Dyads received 45 to 60 minutes of daily training on how to use and incorporate a calendar system in their daily life (Greenaway, Duncan, & Smith, 2013; Sohlberg, & Mateer, 1989). This included written reminders for appointments, family events, daily experiences, and thoughts. A copy of the yearly calendar was provided to dyads to encourage continued use after the study.
Post-intervention adherence:
Adherence to taught intervention components in each study arm was assessed at 6, 12, and 18 months post-intervention. For MSS and Brain-HQ, post-intervention adherence was assessed by direct evaluation and study partner reported activity log, which is described below. For the other intervention components direct observation of post-intervention adherence was not possible and study partners were asked to report participant activities. This was completed with the partner-report activity logs mailed to study partners in advance of the booster sessions and collected at the booster session, or presented at the 6, 12, and 18 months post-intervention follow-up visits. The partner-report activity log asked the study partner to answer questions about the participant’s level of engagement in the different intervention components. Post-intervention adherence definitions were based on previously published or prevailing treatment standards, and categorized into 3 different levels: adherent, non-adherent, and indeterminate as summarized in Table 2 and described in detail below.
Table 2.
Adherence definitions by intervention.
| Intervention | Adherent (score 1) | Indeterminate (score 0.5) | Non-Adherent (score 0) |
|---|---|---|---|
| Definition | Definition | Definition | |
| Memory Support (MSS)1 | Adherence scale score >7 | 7 times /week use of calendar but score <7 on adherence scale | Calendar use <6 times per week on adherence scale |
| Computerized Brain Fitness2 | >40 hours of training (about 2hrs/week over 6 months) | All else | <60 minutes a week of cognitive activity |
| Physical Exercise3 | > 150 minutes per week of exercise | 60–149 minutes per week of exercise | <60 minutes per week of exercise |
| Support Group | At least 2 engagement in support therapy in the past 2 weeks | All else | No engagement in support therapy |
| Wellness Education | >2 times in the past 2 weeks | All else | An answer of ‘not at all’ |
Rationale for the cut-off adherence standard is referenced as applicable.
Memory support system (MSS):
MSS adherence was evaluated by direct review of the MSS calendar (along with completion of the adherence assessment form) and partner-reported activity log at each post-intervention follow-up time point. The adherence assessment form allocates 1 point for bringing calendar to visit, 1 point for an entry for present date, 2 points on entries happening at a certain time and 2 points for happening on anytime of the day, and 4 points for at least two entries for each of the two days in the journaling section of the calendar (Greenaway, Hanna, Lepore, & Smith, 2008). The participants were asked to present their calendars, and two randomly selected dates in that calendar were evaluated for compliance with calendar training targets. Two random dates were assessed to offset potential deliberate calendar preparation for the visit. The participant was assigned adherent for this intervention if they obtained a raw score of 7 out of 10 on their adherence assessment form (Greenaway, Hanna, Lepore, & Smith, 2008), which was completed by a trained therapist. A raw score of 7 was the minimum score necessary to assure that participants were using all 3 sections of the calendar. Non-adherent was assigned if the participant and study partner reported use of MSS or any calendar system less than once a day. Anything that fell between these adherent and non-adherent standards was considered indeterminate, including participants who used the prescribed MSS calendar but obtained a raw score less than 7 points on the adherence assessment form or used another type of calendar system.
Brain fitness:
A participant was assigned adherent to this intervention if they accumulated at least 40 hours (roughly 2 hours per week) of training/usage time with Brain-HQ post-intervention. Actual training time was tracked through the Brain-HQ group portal (an automated log of time spent training). Study participants whose partners reported they engaged in less than 60 minutes per week of any brain fitness activity were assigned to the non-adherent category for this intervention. Any level of usage that fell between these two categories was assigned to the indeterminate group.
Physical activity:
Participants that engaged in at least 150 minutes of physical activity per week according to partner report were assigned to the post-intervention adherent category. Study participants who engaged in less than 60 minutes per week or engaged in no physical activities at all were assigned to the non-adherent category for this intervention. Participants that fell between the required standards for adherent and non-adherent were assigned to the indeterminate group. The rationale for setting the standards for these categories was based on previous literature (Lautenschlager et al., 2008).
Supportive group therapy:
Post-intervention adherent was assigned if partners reported that the participant consistently engaged in individual or group supportive therapy after the program (at least twice in the past two weeks). Non-adherent was assigned if the participant did not engage in any form of individual or group supportive therapy. A category of indeterminate was assigned if the participant engaged in some form/level of therapy similar to what was provided during the training.
Wellness education:
Post-intervention assessment of adherence was obtained from the activity logs. The wellness education questions in the activity log inquired about the frequency of the participant engagement in wellness education and the participant’s most frequent type of wellness education. The participant was assigned to the adherent category if the participant and/or the study partner answered ‘once’, ‘twice’, or ‘more than twice in the past 2 weeks’ to all of the wellness education questions. Non-adherent category was assigned if the participant and/or study partner answered ‘not at all’ to all of the wellness education questions. Indeterminate was assigned if the answers provided fell between the standards of adherent and non-adherent.
Outcome measures:
Measures completed with/by participant:
Assessments completed by study participants included: Quality of Life-Alzheimer’s Disease (QoL-AD) (Logsdon, Gibbons, McCurry, & Teri, 2002) with scores ranging from 13 to 52 with higher scores representing better QOL; Short Physical Performance Examination (SPPE) (Guralnik et al., 1994) with scores ranging from 0 to 12 with higher scores indicating better physical performance; Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977) with scores ranging from 0 to 60 with higher scores suggestive of more symptoms of depression; modified selected items from the Chronic Disease Self-Efficacy Scale (Lorig, Stewart, Ritter, González, Laurent, 1996) with scores ranging from 0 to 90 with high scores suggesting higher self-efficacy; Clinical Dementia Rating (CDR; patient component, completed over the phone) (Morris, 1993), and Dementia Rating Scale, 2nd edition (DRS-2) (Jurica P.J., Leitten, C.L., and Mattis, 2004).
Measures completed with/by research partner:
The following assessments were completed by the study partner based on their observation of the participant: Clinical Dementia Rating (CDR; informant component, completed over the phone) (Morris, 1993), Everyday Cognition (ECog) (Farias et al., 2008) with scores ranging from 39 to 156 with higher scores suggesting more functional impairment; and Functional Assessment Questionnaire (FAQ) (Teng et al., 2010) with total scores ranging from 0 to 30 with higher scores suggesting more functional impairment. The partner also completed the Care Partner Burden (Bédard et al., 2001).
Research follow-up timeline:
All outcome assessments were completed at baseline, end of treatment, and 6, 12, and 18 months post-intervention. One-day booster sessions which included refresher sessions for each of the 4 intervention components to which a given participant was assigned were completed at 6 and 12 months post-intervention. The activity logs that provided the basis for certain post-intervention adherence assessments were mailed to study partners in advance of the booster sessions and collected at the booster session. Attrition rates were calculated from baseline to 6-month post-intervention, from 6-month to 12-month post-intervention, and 12-month to 18-month post-intervention.
Data analysis:
Derivation of post-intervention adherence scores:
As noted, specific definitions for post-intervention adherence scores for each behavioral intervention component are also summarized in Table 2. These definitions were derived from prior research (Greenaway et al., 2008; Lautenschlager et al., 2008; Smith et al., 2009). The percentage of participants that meet these definitions at each time point is depicted in Figure 1. Note that the ‘stringency’(i.e. time demands, accessibility, compliance with a rigid standard) for meeting post-intervention adherence or non-adherence was highly variable across interventions, e.g., physical exercise showed high post-intervention adherence and low non-adherence, support groups showed low post-intervention adherence and high non-adherence, while MSS showed both low post-intervention adherence and low non-adherence. Thus, for each study component we created a standardized total post-intervention adherence score. This was accomplished by first assigning a score of 1 for fully meeting the adherence standard for the taught intervention, a score of 0.5 for meeting the indeterminate standard or a score of 0 for meeting the non-adherent standard. Per the above cited research, one could reasonably speculate that some engagement (indeterminate) in the intervention components post-intervention could provide benefits when compared with no engagement (non-adherent). An ordinal raw post-intervention adherence total score was calculated as the sum of these individual intervention component scores for each participant. Then a standardized adherence (z-score) was calculated using the mean and standard deviation for each participant assigned arm ([participant raw adherence score – mean for assigned arm] / [standard deviation for assigned arm]). This normalized our dependent variable (adherence scores). These z-scores were calculated at each post-intervention time point: 6, 12, and 18 months. No regression assumptions were violated, and no other adjustments were made.
Figure 1.
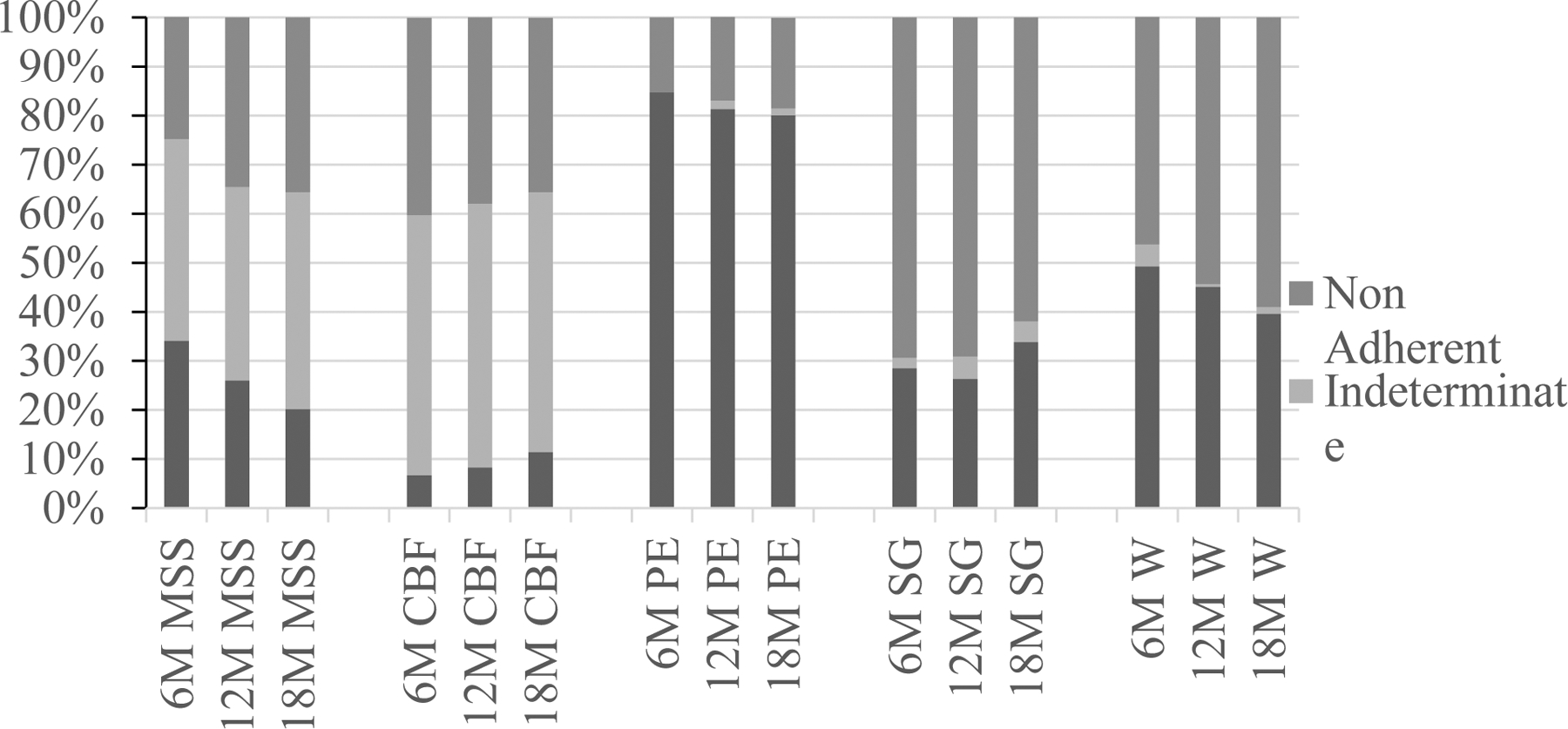
Frequencies of post-intervention adherence, non-adherence or intermediate adherence by intervention component by time. MSS=Memory Support System (6M=6-month follow-up, N= 185; 12M=12-month follow-up, N=177; 18M=18-month follow-up, N= 163); CBF=Computerized Brain Fitness (6M=6-month follow-up, N= 164; 12M=12-month follow-up, N=169; 18M=18-month follow-up, N= 166); PE=Physical Exercise (6M=6-month follow-up, N=176; 12M=12-month follow-up, N=171; 18M=18-month follow-up, N=141); SG=Support Group (6M=6-month follow-up, N=179; 12M=12-month follow-up, N=175; 18M=18-month follow-up, N=139); W=Wellness Education (6M=6-month follow-up, N=179; 12M=12-month follow-up, N=173; 18M=18-month follow-up, N=139).
Statistical analysis:
Post-intervention adherence scores were calculated for each participant using Microsoft Excel 2010 (Microsoft, Redmond, WA). The data was then transferred into IBM SPSS Statistics for Windows, Version 24.0, Armonk, NY. Forward stepwise regression models were used to analyze associates of post-intervention adherence. The independent variables used for the stepwise analysis were participant demographics (age, education, and marital status), depressive symptoms (CES-D total scores), memory-related self-efficacy (Chronic Disease Self-Efficacy Scale scores), functioning abilities (FAQ scores), quality of life (QoL-AD total scores), and global cognitive functioning (DRS-2 raw total score), as well as partner burden scores. These variables were selected based on the literature regarding active-phase adherence to interventions as previously cited (Picorelli et al., 2014). Forward stepping was used to add variables that were significant. The probability required to enter the regression equation (PIN) was set at <0.05. The probability required to be removed from the regression equation (POUT) was set at ≥0.10.
Results:
Participants:
Participants’ baseline characteristics for each study arm are described in Table 1. Required measures were completed by 237 participants at 6-month follow-up, by 228 participants at 12-month follow-up, and by 218 participants at 18-month follow-up. Attrition rate was 13.2%, 3.4% and 4.4% at 6-, 12-, and 18-month post-intervention respectively.
Post-intervention adherence scores:
The percentage of participants’ adherent to each intervention component at all 3 post-intervention follow-up time points is shown in Figure 1. In general, participants were most adherent to physical activity and least adherent to support group across all three follow-up time points. Incidentally we note little concordance between these adherence rates and the perceived benefit of each intervention as reported from a prior clinical sample wherein memory support was seen as the most helpful intervention followed in order by support group, computerized brain training, physical exercise and wellness education (Smith, Chandler, Fields, Aakre, & Locke, 2018). However, as noted above it may not make conceptual sense to expect concordance when the stringency of adherence rules differs. The frequency of summed raw post-intervention adherence scores is presented in Table 3. At 6-month follow-up, 96.2% (n=228) participants were at least partially adherent to one or more intervention components taught in their study arm. At 12-month follow-up this rate was 91.2% (n=206), and at 18-month follow-up, 88.5% (n =193) of continuing participants were at least partially adherent to one or more intervention components (Table 3). The percentage of the sample that was fully adherent to 2 or more intervention components at 6-, 12-, and 18-month post-intervention follow-up was 48.3%, 46.7%, and 37.8% respectively. Mean and standard deviations for these raw summed adherence scores by treatment arm and time point are presented in Table 4. Standardization of post-intervention adherence scores was verified by univariate analysis between the different study arms at 6-month follow up. No differences were observed.
Table 3:
Frequencies of adherence scores per follow-up.
| Follow-up | Total adherence score | Frequency | Percentage |
|---|---|---|---|
| 6-month N=237 | 0 | 9 | 3.8% |
| 0.5 | 17 | 7.2% | |
| 1 | 40 | 16.9% | |
| 1.5 | 38 | 16.0% | |
| 2 | 48 | 20.3% | |
| 2.5 | 41 | 17.3% | |
| 3 | 21 | 8.9% | |
| 3.5 | 19 | 8% | |
| 4 | 4 | 1.7% | |
| 12-month N=226 | 0 | 20 | 8.8% |
| 0.5 | 19 | 8.4% | |
| 1 | 30 | 13.3% | |
| 1.5 | 37 | 16.4% | |
| 2 | 37 | 16.4% | |
| 2.5 | 37 | 16.4% | |
| 3 | 28 | 12.4% | |
| 3.5 | 16 | 7.1% | |
| 4 | 2 | 0.9% | |
| 18-month N=218 | 0 | 25 | 11.5% |
| 0.5 | 27 | 12.4% | |
| 1 | 36 | 16.5% | |
| 1.5 | 32 | 14.7% | |
| 2 | 35 | 16.1% | |
| 2.5 | 32 | 14.7% | |
| 3 | 14 | 6.4% | |
| 3.5 | 11 | 5.0% | |
| 4 | 6 | 2.8% |
Table 4:
Mean adherence score per study arm.
| Treatment Arm | 6-month Adherence score | 12-month Adherence score | 18-month Adherence score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | S.D. | N | Mean | S.D. | N | Mean | S.D. | |
| No Memory Support | 47 | 1.95 | .96 | 46 | 2.10 | 1.01 | 43 | 1.78 | 1.15 |
| No Computerized Brain Fitness | 47 | 1.90 | 1.01 | 43 | 1.84 | 1.17 | 41 | 1.73 | 1.16 |
| No Yoga | 52 | 1.68 | 0.95 | 50 | 1.41 | 0.96 | 48 | 1.24 | 0.95 |
| No Support Group | 45 | 2.32 | 0.89 | 43 | 2.10 | 0.94 | 43 | 1.94 | 0.99 |
| No Wellness Education | 46 | 1.66 | 0.77 | 44 | 1.65 | 0.83 | 43 | 1.43 | 0.90 |
Do baseline characteristics predict post-intervention adherence?
Forward stepwise regression models examining the association of baseline measures with standardized post-intervention adherence scores are presented in Table 5a–c. The final stepwise analysis model showed that certain baseline variables significantly predicted adherence to intervention components at each post-intervention follow-up point. Specifically, higher educational attainment, and higher depressive symptoms significantly predicted better adherence to intervention components at 6-month post-intervention (R²=0.068, p<.001, ES=.073). Higher global cognitive functioning abilities significantly predicted better adherence to intervention components 12 months post-intervention (R²=0.024, p=.025, ES=.025). Lower baseline functional abilities significantly predicted better adherence to interventions at 18-month post-intervention (R²=0.041, p=.043, ES=.043).
Table 5a:
Table of final forward stepwise regression model for associations of baseline measures with post-intervention adherence scores at 6-months (R²=0.068, p<0.001, ES=.073, N=226).
| Measure | β (95% CI) | p-value |
|---|---|---|
| Constant | 0.000 | |
| Education | .208 (0.08 to 0.33) | 0.002 |
| CES-D | .173 (0.04 to 0.30) | 0.008 |
Abbreviation: CES-D, Center for Epidemiological Studies Depression Scale
Table 5c:
Table of final forward stepwise regression model for associations of baseline measures with post-intervention adherence scores at 18-months (R²=0.041, p=0.003, ES=.043, N=208).
| Measure | β (95% CI) | p-value |
|---|---|---|
| Constant | 0.017 | |
| FAQ | −.203 (−0.34 to −0.07) | 0.003 |
Abbreviation: FAQ, Functional Assessment Questionnaire
Does current adherence predict future adherence?
Linear regression analysis showed that higher adherence scores at 6-month follow-up predicted higher adherence at 12-month post-intervention follow-up (R²=0.29, F (1,221) =89.491, p<0.001). Similarly, adherence to interventions at 12-month predicted adherence at 18-month post-intervention (R²=0.22, F (1,216) = 61.365, p<0.001).
Association between participants’ concurrent reported outcomes and adherence at post-intervention
Separate forward stepwise regression models examining the association of concurrent outcomes with standardized post-intervention adherence are shown in Tables 6a–b. At 6-month post-intervention, after controlling for education, higher post-intervention adherence to intervention component scores were associated with lower (i.e. better) Functional Assessment Questionnaire (FAQ) score (R²=0.048, p=.005, ES=.050). Additionally, higher functional status at 12 months were associated with higher concurrent adherence (R²=0.083, p<.001, ES=.090). No variables significantly predicted adherence at p<0.05 probability level at 18-month follow-up.
Table 6a:
Table of final forward stepwise regression model for association of concurrent outcomes with standardized post-intervention adherence scores at 6-months (R²=0.048, p=0.005, ES=.050, N=221).
| Measure | β (95% CI) | p-value |
|---|---|---|
| Constant | 0.060 | |
| Education | .172 (0.04 to 0.30) | 0.010 |
| FAQ | −.143(−0.27 to −0.01) | 0.032 |
Abbreviation: FAQ, Functional Assessment Questionnaire
Table 6b:
Table of final forward stepwise regression model for association of concurrent outcomes with standardized post-intervention adherence scores at 12-months (R²=0.083, p<0.001, ES=.090, N=185).
| Measure | β (95% CI) | p-value |
|---|---|---|
| Constant | 0.000 | |
| FAQ | −.288 (−0.43 to −0.15) | 0.000 |
Abbreviation: FAQ, Functional Assessment Questionnaire.
Overall, the results suggest that the ability to perform activities of daily living impacts study participants’ capability to engage in intervention components on a regular basis.
Discussion:
The literature suggests that MCI behavioral interventions can improve cognitive, emotional, and physical functioning, as well as other patient-reported outcomes (Chandler et al., 2016; Chandler et al., 2019; Dannhauser et al., 2014; Greenaway et al., 2013; Lam et al., 2015; Ngandu et al., 2015; Pandya et al., 2017; Suzuki et al., 2012). However, data on the impact of multimodal behavioral intervention adherence (both during the active phase of the intervention and post-intervention) on outcomes are limited. Two multimodal trials reported active-phase of intervention adherence to taught individual intervention components, but did not report the average number of combined intervention components followed by study participants (Dannhauser et al., 2014; Lam et al., 2015). In contrast, the FINGER trial reported the percentage of participants that adhered to the taught interventions (i.e. participated in classes, not engagement outside of classes, (Coley et al., 2019; Ngandu et al., 2015) individually and combined, and these findings are based mainly on active supervision of study staff. Research staff supervision and involvement in behavioral interventions is known to increase intervention participation (adherence) (Ligthart et al., 2015; Van Middelaar et al., 2018). Our reported adherence may differ from that of the FINGER trial because 1) we reported on-going adherence (post-intervention engagement) in intervention components outside of structured in-person sessions and 2), we used a mixture of direct observation (BrainHQ™ portal report and calendar completion) and partner report to determine post-intervention adherence. These methods of assessing adherence appear unique to our study and set a high standard for adherence in that it reflects the practical barriers of incorporating the interventions in the life of persons with aMCI. It is perhaps also more realistic than direct active supervision and involvement of the research team. In the present study, at each post-intervention follow-up time point nearly 45% of participants were fully adherent to at least 2 out of 4 intervention components per study arm. The present study also permitted the possibility of partial post-intervention adherence, reasoning that an all-or-nothing approach to categorizing adherence was overly restrictive. The mean post-intervention adherence score per study arm was approximately 2 as well. Evidently, about 50% of the participants in each study arm fully engaged in 2 out of the 4 intervention components. We are not aware of any comparator in the literature for this result due to the novel reporting method of our study.
It is perhaps expected that various baseline measures of impairment severity and cognitive reserve were associated with post-intervention adherence at follow-up. These included education and depressive symptoms for 6-month adherence, higher global cognitive functioning for 12-month post-intervention, and better functional abilities for 18-month follow-up. These variables share a fair degree of collinearity. The Functional Assessment Questionnaire (FAQ) is known to adequately measure ADL impairments in longitudinal follow-up studies due to its ability to reliably measure functional impairments throughout disease progression (Smith et al., 2017; Teng et al., 2010). Analysis of reported outcomes in the current study found better functional abilities were significantly associated with higher post-intervention adherence at 6-month, 12-month, and 18-month follow up. This supports the importance of early detection and implementation of behavioral interventions as a viable strategy to prevent or delay further decline of impairments associated with aMCI. Delaying interventions until functional impairments accumulate will interfere with adherence to interventions intended to mitigate those functional impairments.
Furthermore, higher education level was found to significantly predict higher post-intervention adherence to intervention components at 6-month follow-up. Higher education level has been shown to be associated with higher cognitive functioning and to delay the onset of cognitive decline in older adults (Alley, Suthers, & Crimmins, 2007; Wilson et al., 2009; Zahodne, Stern, & Manly, 2015). However, its association with adherence in interventional studies has not been well studied. One systematic review paper that analyzed adherence during the active phase in exercise intervention programs among healthy older adults found that higher socioeconomic status was positively associated with adherence (Picorelli et al., 2014). Although this review and the current study are different in many regards, it is generally accepted that higher socioeconomic status is positively associated with educational attainment (Barrera et al., 2017; Zahodne et al., 2015). This could, in turn, provide study participants of higher socioeconomic status more tools (monetary resources, time, understanding of the potential benefits of the intervention, etc.) to fully adhere to interventions. Furthermore, higher education could be associated with a higher cognitive reserve, ultimately delaying the onset of dementia (O’Shea et al., 2018; Stern et al., 2018). Higher cognitive reserve could protect against further cognitive decline, possibly making the participants less impaired and more able to adhere to the interventions. Conversely this finding suggests those with lower education may require substantially more programmatic support to have success with behavioral interventions of the sort used here.
Higher baseline participant depressive symptoms were found to be a significant predictor of post-intervention adherence at 6-month follow-up in the current study. Studies with healthy to cognitively impaired older adults have found lower reported depressive symptoms to be significantly associated with adherence to various interventions (Garmendia et al., 2013; Pandya et al., 2017; Picorelli et al., 2014). It is curious we found the opposite result. We have reported elsewhere (Chandler et al., 2019) that, in general, the program improves mood. This may in turn improve daily function which were described above as associates of adherence. Thus one possibility is that people with high baseline depressive symptoms may be most disposed to benefit from the program not only in terms of outcomes but also adherence. Alternatively, we speculate that deficit awareness maybe a moderator of this finding, i.e. MCI patients with milder deficits may have both greater awareness of impairments, greater willingness to endorse sadness about those deficits and greater capacity to adhere to interventions to address the deficits. Unfortunately we did not formally measure awareness of deficits in this trial. This could be an important future direction.
Higher baseline global cognitive functioning was found to significantly predict post-intervention adherence to intervention components at 12-month follow-up. The DRS-2 was administered both at baseline and 12-month post-intervention to measure overall level of cognitive functioning (Smith et al., 2017). Last year, analysis from the National Alzheimer’s Coordinating Center Uniform Data Set found lower baseline cognitive functioning was significantly associated with reversion from MCI (amnestic and non-amnestic) to normal cognition (Pandya et al., 2017). Although the researchers did not look specifically at adherence to an intervention, the completion of all follow-up tasks were associated with revision from MCI to normal cognition. High cognitive functioning might serve as a proxy for sustained engagement in intervention components post-intervention.
The literature shows that cognitive functioning abilities, depressive symptoms, social support, and physical activity are associated with level of functioning status (Bonnefoy et al., 2012; D. et al., 2012; Dombrowsky, 2017; Hatch & Lusardi, 2010; Song, Meade, Akobundu, & Sahyoun, 2014). Furthermore, frequency and level of engagement is known to be associated with better level of functioning; however, the direction of the impact is unclear (Dombrowsky, 2017). It is unknown whether study participants engaged in the intervention components because they had better level of functioning, or whether they had better functioning abilities because they engaged in the intervention components. With further MCI decline associated with more functional impairments (Lindbergh, Dishman, & Miller, 2016), it is possible that disease severity progression overshadowed the role of our predictive variables when assessing post-intervention adherence over time.
Limitations and future research:
Our method of permitting partial post-intervention adherence and summing post-intervention adherence scores is unique and entails some assumptions that can be challenged. First, this system assigns a value of ‘1” to being fully adherent to any one intervention even though we acknowledge that the stringency of adherence differs across behavioral interventions. Second, this system assigns the same value (i.e., a score of 1) to being partially adherent to 2 interventions as it does to being fully adherent to 1 intervention. However, in the context of a multicomponent comparative effectiveness trial it is indeed adherence with multiple interventions that is at issue. In addition, as noted, standardizing the scores within each treatment arm mitigates the issue of varying stringency. Ultimately, we must qualify statements regarding adherence to include, ‘relative to other participants within the same treatment arm being more adherent to more treatments was associated with X’. This is clearly not identical to saying being adherent to 2.5 hours per week of intervention Y is associated with X.
In addition, our study is only correlational. We cannot say that lower FAQ (better functioning abilities) or high DRS-2 (better global cognitive functioning abilities) ‘caused’, ‘produced’, or ‘resulted’ in better adherence. Perhaps these factors are mediators, moderators or merely share variance with more important factors associated with adherence. In this regard however we note both cognitive impairment severity as measured by the DRS-2 and caregiver factors were included as potential covariates and cognitive impairment severity was found to be associated with adherence. However, our predictors explained a small amount of the variance (less than 7%) in observed post-intervention adherence. There is still a large amount of unexplained variance.
The current study data is from a predominantly non-Hispanic white population. Thus, our predicted variables may not be generalized to minority populations. More research is needed to determine if these results are consistent among other ethno-racial groups. Regardless of this, this paper examining different variables associated with adherence may have relevance to future trials using multimodal behavioral intervention in older adults with MCI.
Behavioral interventions cannot impact important outcomes if people do not adhere to them. Thus, it is crucial to analyze factors influencing behavioral intervention adherence. The present study found that education level, individual functional abilities, and depressive symptoms may influence adherence to a multimodal intervention in older adults with MCI. It is possible that the most influential intervention among each study arm could in turn be influencing overall wellbeing, thus increasing adherence in an iterative fashion. In the present study we focused globally on multi-component adherence, in other word treated adherence as general construct. However it is possible, and perhaps likely that each intervention type has its own associates of adherence. We are actively exploring adherence at the level of the individual interventions (De Wit, L., et al., 2019, manuscript submitted for publication). Clinical trials often mandate a one-size fits all approach to intervention. One implication of our findings for behavioral trials in MCI is that persons with subtly greater impairment may require additional supports in the form of long active interventions and increased aftercare to maintain adherence, i.e. a somewhat adaptive design relative to adherence. Moving forward to clinical applications, the goal to create patient-centered care and personalized behavioral interventions calls for treatment plans attentive to factors associated with adherence in multimodal interventions.
Table 5b:
Table of final forward stepwise regression model for associations of baseline measures with post-intervention adherence scores at 12-months (R²=0.024, p=0.022, ES=.025, N=215).
| Measure | β (95% CI) | p-value |
|---|---|---|
| Constant | 0.024 | |
| DRS-2 | .156 (0.02 to 0.29) | 0.022 |
Abbreviation: DRS-2, Dementia Rating Scale Second Edition
Acknowledgments:
“Research reported in this manuscript was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-1306-01897). Partial support received by National Institute of aging grants T32 AG020499, and P50 AG016574. The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.”
Footnotes
Trial Registration: ClinicalTrials.gov. Identifier: NCT02265757
Disclosure statement:
No conflict of interest was reported by authors.
Data availability statement:
The data that support the findings of this study are available from the corresponding author, G.S, upon reasonable request.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley D, Suthers K, & Crimmins E (2007). Education and cognitive decline in older Americans: Results from the AHEAD sample. Research on Aging. 10.1177/0164027506294245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G, Cases T, Bunout D, de la Maza MP, Leiva L, Rodriguez JM, & Hirsch S (2017). Associations between socioeconomic status, aging and functionality among older women. Geriatric Nursing. 10.1016/j.gerinurse.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Barrios PG, González RP, Hanna SM, Lunde AM, Fields JA, Locke DEC, & Smith GE (2016). Priority of Treatment Outcomes for Caregivers and Patients with Mild Cognitive Impairment: Preliminary Analyses. Neurology and Therapy. 10.1007/s40120-016-0049-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, & O’donnell M (2001). The Zarit Burden Interview: A new short version and screening version. Gerontologist. 10.1093/geront/41.5.652 [DOI] [PubMed] [Google Scholar]
- Bonnefoy M, Boutitie F, Mercier C, Gueyffier F, Carre C, Guetemme G, … Cornu C (2012). Efficacy of a home-based intervention programme on the physical activity level and functional ability of older people using domestic services: A randomised study. Journal of Nutrition, Health and Aging. 10.1007/s12603-011-0352-6 [DOI] [PubMed] [Google Scholar]
- Chandler MJ, Parks AC, Marsiske M, Rotblatt LJ, & Smith GE (2016). Everyday Impact of Cognitive Interventions in Mild Cognitive Impairment: a Systematic Review and Meta-Analysis. Neuropsychology Review. 10.1007/s11065-016-9330-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler Melanie J., Locke DEC, Duncan NL, Hanna SM, Cuc AV, Fields JA, … Smith GE (2017). Computer versus compensatory calendar training in individuals with mild cognitive impairment: Functional impact in a pilot study. Brain Sciences. 10.3390/brainsci7090112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler Melanie J., Locke DE, Crook JE, Fields JA, Ball CT, Phatak VS, … Smith GE (2019). Comparative Effectiveness of Behavioral Interventions on Quality of Life for Older Adults With Mild Cognitive Impairment. JAMA Network Open, 2(5), e193016. 10.1001/jamanetworkopen.2019.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley N, Ngandu T, Lehtisalo J, Soininen H, Vellas B, Richard E, … Olivier-Abbal P (2019). Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimer’s and Dementia. 10.1016/j.jalz.2019.03.005 [DOI] [PubMed] [Google Scholar]
- M. D, L. J, P. N, F. L, H. G, Dobson A, … Dobson A (2012). Social support and subsequent disability: It is not the size of your network that counts. Age and Ageing. 10.1093/ageing/afs036 [doi] [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Cleverley M, Whitfield TJ, Fletcher BC, Stevens T, & Walker Z (2014). A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment-ThinkingFit: Pilot and feasibility study for a randomized controlled trial. BMC Psychiatry. 10.1186/1471-244X-14-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit L, Chandler M, Amofa P, DeFeis B, Mejia A, O’Shea D, Locke DEC, Fields JA, Smith GE (2019). Memory support system training in mild cognitive impairment: Predictors of learning and adherence. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed]
- Dombrowsky TA (2017). Relationship between engagement and level of functional status in older adults. SAGE Open Medicine. 10.1177/2050312117727998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, & DeCarli C (2008). The Measurement of Everyday Cognition (ECog): Scale Development and Psychometric Properties. Neuropsychology. 10.1037/0894-4105.22.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia ML, Dangour AD, Albala C, Eguiguren P, Allen E, & Uauy R (2013). Adherence to a physical activity intervention among older adults in a post-transitional middle income country: A quantitative and qualitative analysis. Journal of Nutrition, Health and Aging. 10.1007/s12603-012-0417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway MC, Duncan NL, & Smith GE (2013). The memory support system for mild cognitive impairment: Randomized trial of a cognitive rehabilitation intervention. International Journal of Geriatric Psychiatry. 10.1002/gps.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway Melanie C., Hanna SM, Lepore SW, & Smith GE (2008). A behavioral rehabilitation intervention for amnestic mild cognitive impairment. American Journal of Alzheimer’s Disease and Other Dementias. 10.1177/1533317508320352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, … Wallace RB (1994). A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journals of Gerontology. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- Hatch J, & Lusardi MM (2010). Impact of participation in a wellness program on functional status and falls among aging adults in an assisted living setting. Journal of Geriatric Physical Therapy. 10.1097/JPT.0b013e3181defe81 [DOI] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, and Mattis S (2004). Dementia Rating Scale-2 (DRS-2). Archives of Clinical Neuropsychology. 10.1016/j.acn.2003.07.003 [DOI] [Google Scholar]
- Lam LCW, Chan WC, Leung T, Fung AWT, & Leung EMF (2015). Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: A cluster randomized controlled trial. PLoS ONE. 10.1371/journal.pone.0118173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, Van Bockxmeer FM, Xiao J, … Almeida OP (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA - Journal of the American Medical Association. 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- Ligthart SA, Van Den Eerenbeemt KDM, Pols J, Van Bussel EF, Richard E, & Moll Van Charante EP (2015). Perspectives of older people engaging in nurse-led cardiovascular prevention programmes: A qualitative study in primary care in the Netherlands. British Journal of General Practice. 10.3399/bjgp15X683149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbergh CA, Dishman RK, & Miller LS (2016). Functional Disability in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Neuropsychology Review. 10.1007/s11065-016-9321-5 [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Gibbons LE, McCurry SM, & Teri L (2002). Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine. 10.1097/00006842-200205000-00016 [DOI] [PubMed] [Google Scholar]
- Lorig K, Stewart A, Ritter P, González V, Laurent D, & L. J (1996). Chronic Disease Self-Efficacy Scales. Outcome Measures for Health Education and Other Health Care Interventions. [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, … Kivipelto M (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- O’Shea DM, Langer K, Woods AJ, Porges EC, Williamson JB, O’Shea A, & Cohen RA (2018). Educational Attainment Moderates the Association Between Hippocampal Volumes and Memory Performances in Healthy Older Adults. Frontiers in Aging Neuroscience, 10(November), 1–9. 10.3389/fnagi.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya SY, Lacritz LH, Weiner MF, Deschner M, & Woon FL (2017). Predictors of Reversion from Mild Cognitive Impairment to Normal Cognition. Dementia and Geriatric Cognitive Disorders. 10.1159/000456070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picorelli AMA, Pereira LSM, Pereira DS, Felício D, & Sherrington C (2014). Adherence to exercise programs for older people is influenced by program characteristics and personal factors: A systematic review. Journal of Physiotherapy. 10.1016/j.jphys.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Smith G, Chandler M, Locke DE, Fields J, Phatak V, Crook J, … Cochran D (2017). Behavioral Interventions to Prevent or Delay Dementia: Protocol for a Randomized Comparative Effectiveness Study. JMIR Research Protocols. 10.2196/resprot.8103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Chandler M, Fields JA, Aakre J, & Locke DEC (2018). A Survey of Patient and Partner Outcome and Treatment Preferences in Mild Cognitive Impairment. Journal of Alzheimer’s Disease. 10.3233/JAD-171161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, & Zelinski EM (2009). A cognitive training program based on principles of brain plasticity: Results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society. 10.1111/j.1532-5415.2008.02167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Meade K, Akobundu U, & Sahyoun NR (2014). Depression as a correlate of functional status of community-dwelling older adults: Utilizing a short-version of 5-item Geriatric Depression Scale as a screening tool. Journal of Nutrition, Health and Aging. 10.1007/s12603-014-0542-0 [DOI] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, … Vuoksimaa E (2018). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s and Dementia. 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, … Park H (2012). Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: A randomized controlled trial. BMC Neurology. 10.1186/1471-2377-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, & Lu PH (2010). Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Disease and Associated Disorders. 10.1097/WAD.0b013e3181e2fc84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Middelaar T, Beishuizen CRL, Guillemont J, Barbera M, Richard E, & Moll Van Charante EP (2018). Engaging older people in an internet platform for cardiovascular risk self-management: A qualitative study among Dutch HATICE participants. BMJ Open. 10.1136/bmjopen-2017-019683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, De Leon CFM, & Evans DA (2009). Educational attainment and cognitive decline in old age. Neurology. 10.1212/01.wnl.0000341782.71418.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, & Manly JJ (2015). Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology. 10.1037/neu0000141 [DOI] [PMC free article] [PubMed] [Google Scholar]


