Abstract
We report a 3-component reaction between N-benzyl ketimines, [1.1.1]propellane, and pinacol boronates to generate benzylamine bicyclo[1.1.1]pentane (BCP) pinacol boronates. These structures are analogous to highly sought diarylmethanamine cores, which are common motifs in bioactive molecules. We demonstrate the versatility of the boronate ester handle via downstream functionalization through a variety of reactions, including a challenging Pd-catalyzed (hetero)arylation that exhibits a broad substrate scope. Together, these methods enable the synthesis of high-value BCP benzylamines which are inaccessible by existing methods. Furthermore, we demonstrate the successful application of these newly developed (hetero)arylation conditions to a variety of challenging tertiary pinacol boronates, including nitrogen-containing heterocycles, 1,1-disubstituted cyclopropanes, and other BCP cores.
We report a 3-component reaction between N-benzyl ketimines, [1.1.1]propellane, and pinacol boronates to generate benzylamine bicyclo[1.1.1]pentane (BCP) pinacol boronates.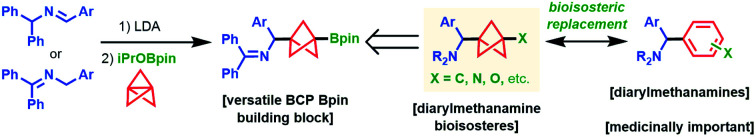
Introduction
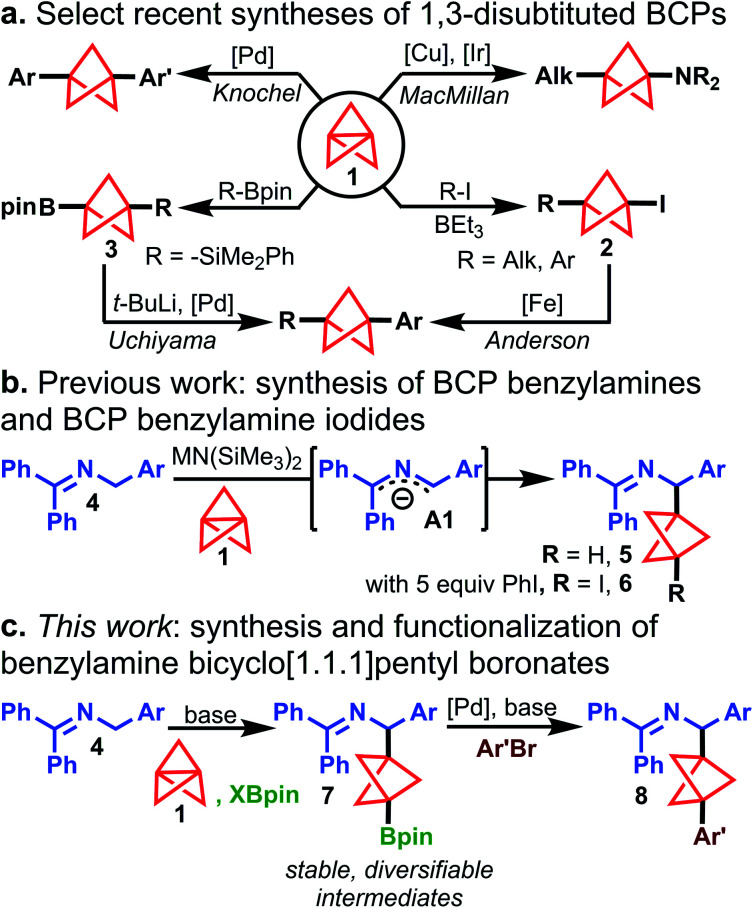
Recent years have witnessed significant developments in the synthesis of 1,3-disubstituted bicyclo[1.1.1]pentanes (BCPs).1,2 Owing largely to an interest from the medicinal chemistry community in the BCP motif as an alkyl bioisostere for aromatic rings in drug-like molecules,3 synthetic chemists have developed new methods for the direct functionalization of [1.1.1]propellane (1). Two common approaches to prepare 1,3-disubstituted BCPs from 1 include: (1) formation of metallated BCP intermediates that can readily form C–C or C–X bonds and (2) synthesis of isolable BCP intermediates that can undergo further structural diversification. For example, Knochel built upon the work of de Meijere and Szeimies4,5 by generating BCP organomagnesium reagents via addition of Grignard reagents to 1. These intermediates were further transmetallated with ZnCl2 and cross-coupled under palladium catalysis (Scheme 1a, top left).6 Zinc enolates were also shown to be effective organometallic reagents for addition to 1 followed by electrophile trapping.7 MacMillan recently demonstrated that BCP radicals (formed from radical addition to 1) could be intercepted by copper-amide species to forge a C–N bond, giving difunctionalized amino BCPs (Scheme 1a, top right).8
Scheme 1. Select recent syntheses of 1,3-disubstituted BCPs. (a) Knochel, top left: (1) ArMgX, 100 °C then ZnCl2 (2) Ar′X, (dppf)PdCl2, 40–65 °C; MacMillan, top right: (Alk-CO2)2IMes, R2NH, Ir(ppy)3, Cu(acac)2, BTMG, blue LEDs; Anderson, bottom right: Fe(acac)3, TMEDA, ArMgX, 20 °C; Uchiyama, bottom left: t-BuLi, –78 °C, then, (dppf)PdCl2, Cu2O, ArBr, 60 °C. (b) Synthesis of BCP benzylamines. (c) This work: synthesis and functionalization of benzylamine bicyclo[1.1.1]pentyl boronates.
The synthesis of complex 1,3-disubstituted amino BCPs has been a frequent target of recent work: photochemical,9 metal-mediated radical chain processes,10 and “turbo amide”11 approaches to BCP amine synthesis have been reported. Anderson demonstrated the straightforward synthesis of BCP iodides (2) under Atom Transfer Radical Addition catalysis12,13 and developed an Fe-catalyzed Kumada coupling with aryl Grignards to access all-carbon 1,3-disubstituted BCP products (Scheme 1a, bottom right).14 Uchiyama reported the silaboration of 1 to give isolable intermediate 3, which could be subsequently arylated under Pd catalysis (Scheme 1a, bottom left).15 This work was the first to demonstrate arylation of BCP boronates; however, the arylation protocol requires pre-activation of the boronate with stoichiometric tert-butyllithium, which highlights the synthetic difficulty of functionalizing BCP Bpin compounds. Additionally, t-BuLi is undesirable from the standpoints of safety, functional group compatibility, and ease of operation. Moreover, general methods for the rapid synthesis of BCP boronates are scarce: Anderson12 and Aggarwal16 reported the quenching of BCP organometallics with boronate electrophiles. Decarboxylative borylation of a BCP N-hydroxypthalimide ester furnished the BCP boronate, but this method was not general due to the limited availability of diverse BCP carboxylic acids.17,18 Thus, general methods to prepare high value BCP boronate intermediates with diverse substitution patterns are still limited.19
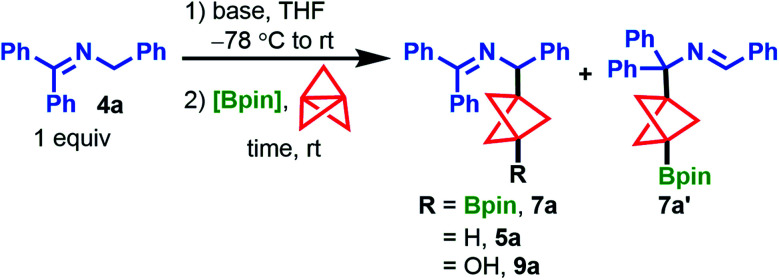
Despite these recent developments, synthetic routes to BCP analogues of many motifs prevalent in drug-like molecules remain elusive. For example, 1,3-difunctionalized BCP analogues of diarylmethanamines, which are privileged structures in medicinal chemistry,20 are unreported in the current literature and cannot be accessed by the methods described above. To address a synthetic gap in preparing complex BCP diarylmethanamine analogues, we sought to expand our earlier work on the synthesis of BCP benzylamines (5) from N-benzyl benzophenone imines (4) and [1.1.1]propellane (1) (Scheme 1b).21 In this reaction, ketimines 4 are deprotonated under mild conditions to form 2-azaallyl anions A1,22,23 which are powerful nucleophiles that add to [1.1.1]propellane to give BCP benzylamines 5. During this study, we found the addition of iodobenzene to the reaction mixture enabled iodination of the intermediate BCP anion to give BCP benzylamine iodides (6). The successful capture of this BCP carbanion suggested that a two-electron mechanism was operative for the cleavage of 1 and that capture of this intermediate with other electrophiles might also be possible. Since the functionalization of tertiary iodides generally requires organometallic reagents (e.g. t-BuLi, Grignard reagents),12,14 we explored BCP carbanion capture with other electrophiles with the hope of accessing bench-stable, functionalized BCP benzylamines that could be readily elaborated to more complex substrates. We were particularly attracted to the idea of accessing pinacol boronates, which are both bench-stable and highly versatile synthetic handles.24,25 Herein, we describe a novel method for the synthesis of 1,3-disubstituted BCP benzylamine pinacol boronates (7) via capture of Bpin electrophiles in the propellylation of 2-azaallyl anions (Scheme 1c). The reaction proceeds with high chemoselectivity to access 1,3-difunctionalized products (7). Additionally, various functional groups on the nucleophilic ketimine are tolerated (e.g. –CN, –Br, and –Bpin substituents), which are generally incompatible with difunctionalization methods employing Grignard or organolithium reagents. Furthermore, we present the discovery and development of the first cross-coupling of BCP pinacol boronates with aryl bromides that does not rely on pre-activation of the boronate ester or the use of organometallic reagents. This operationally simple method enables access to complex arylated BCP benzylamines (8) and can be applied to the arylation of other BCP Bpin derivatives as well as select tertiary boronate esters for which no cross-coupling methods are currently known.
Results and discussion
Discovery of the propellylation/borylation reaction began by including 2 equiv. of bis(pinacolato)diboron (B2pin2) under our standard propellylation conditions with the 2-azaallyl anion derived from 4a (Table 1, entry 1).21 By 1H NMR analysis of the crude mixture, we observed the borylated BCP product, 7a, in 43% assay yield (AY, determined by 1H NMR integration against an internal standard) accompanied by 13% AY of protonated product 5a and traces of isomeric product 7a′. Throughout optimization, we aimed to eliminate the formation of 5a, both to improve the product yield and to circumvent difficulty in separating 7a and 5a by column chromatography. Although reducing the equivalents of LiN(SiMe3)2 from 2.0 to 1.0 improved the yield of 7a, a similar yield of 5a was observed (entry 2). In contrast, switching to NaN(SiMe3)2 promoted a sluggish reaction (24 h to reach completion) that primarily generated the protonated product, resulting in 25% and 70% AY of 7a and 5a, respectively (entry 3). We hypothesized that 5a arose from reaction of the BCP carbanion with in situ generated HN(SiMe3)2. To eliminate this proton source, we employed 1.1 equiv. of n-BuLi as the base which led to the formation of 7a in 49% AY and, as expected, 5a was not detected (entry 4). Switching boron sources from the costly B2pin2 to inexpensive iPrOBpin (1.1 equiv.) led to a mixture of products, including a 33% AY of 7a and a significant amount of oxidation side product 9a (27% AY) (entry 5), which is presumed to form with traces of oxygen via the highly reactive BCP carbanion. Gratifyingly, increasing the equivalents of iPrOBpin to five gave 7a in 62% AY, with small amounts of byproducts 5a, 9a, and 7a’ detected (entry 6). Cooling the reaction mixture to −78 °C prior to n-BuLi addition further improved the AY of 7a to 73% (entry 7). However, while the model reaction using ketimine 4a gave a high AY of product 7a, an initial survey of the reaction scope using these conditions showed poor functional group compatibility with halogenated ketimines due to competing Li–halogen exchange. To mitigate this issue, we replaced n-BuLi with lithium diisopropylamide (LDA), which delivered 7a in good yield (74% AY, 61% IY) and improved functional group tolerance during subsequent scope analysis (entry 8). Notably, heating the reaction mixture to 50 °C for 2 h did not improve the yield of 7a further (60% AY, entry 9).
Optimization of the propellylation/borylation reaction. [1.1.1]propellane (1, 1.4 equiv.), reaction concentration = 0.2 M.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Base (equiv.) | Bpin source (equiv.) | Time (h) | % 7aa | % 4aa | % 5a : 9a : 7a′a |
| 1b | LiN(SiMe3)2 (2) | B2pin2 (2) | 4 | 43 | 0 | 13 : 0 : 5 |
| 2b | LiN(SiMe3)2 (1) | B2pin2 (2) | 7 | 55 | 0 | 18 : 0 : 6 |
| 3b | NaN(SiMe3)2 (2) | B2pin2 (2) | 24 | 25 | 0 | 70 : 0 : 4 |
| 4b,c | n-BuLi (1.1) | B2pin2 (2) | 2 | 49 | 6 | 0 : n/d : 4 |
| 5b | n-BuLi (1.1) | iPrOBpin (1.1) | 2 | 33 | 6 | 10 : 27 : 2 |
| 6b | n-BuLi (1.1) | iPrOBpin (5) | 2 | 62 | 19 | 5 : 1 : 6 |
| 7 | n-BuLi (1.1) | iPrOBpin (5) | 2 | 73 | 8 | 0 : 0 : 6 |
| 8 | LDA (1.1) | iPrOBpin (5) | 16 | 74 [61%] | 14 | 0 : 0 : 7 |
| 9d | LDA (1.1) | iPrOBpin (5) | 2 | 60 | 6 | 0 : 0 : 3 |
Determined by 1H NMR analysis of the crude reaction mixture using CH2Br2 as an internal standard.
Base added at room temperature.
1H NMR spectrum of the crude reaction mixture was too complex to accurately quantify 9a and 4a.
Reaction initiated at −78 °C and warmed to 50 °C.
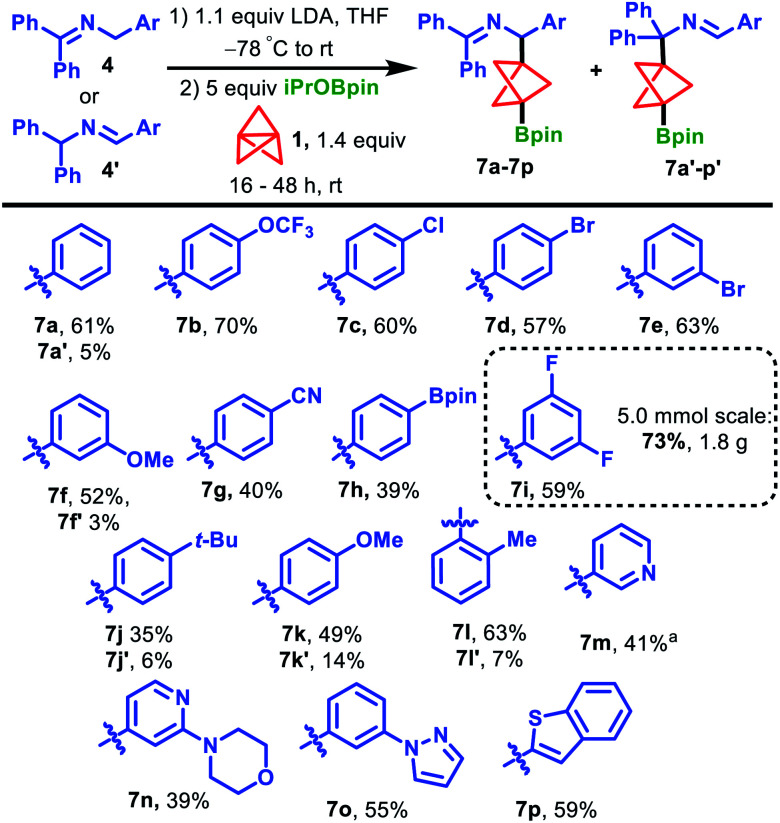
With optimized conditions in hand, we surveyed the scope of the propellylation/borylation reaction with ketimines 4 and aldimines 4′ (Scheme 2). Electron-withdrawing groups were best tolerated in this transformation, producing high yields of products 7. Imine starting materials possessing trifluoromethoxy, chloro and bromo groups were well-tolerated, affording the products 7b–e in 57–70% yield. Substrates bearing 3-methoxy and 4-cyano substituents were also viable, generating products 7f and 7g in 52% and 40% yield, respectively. In these two cases, as well as other lower yielding reactions, no other major BCP-related products were detected even though the reactions reached >90% conversion. Therefore, we attribute these low yields to decomposition of the 2-azaallyl anion intermediates under the reaction conditions. Aldimine 4h′, bearing an aryl boronic ester, underwent the reaction in 39% yield to produce 7h, which possesses two Bpin handles that can be further derivatized via orthogonal functionalization.26,27 Ketimines substituted with electron-donating tert-butyl and methoxy groups were also successful in the reaction but produced higher proportions of isomeric products 7j′ and 7k′ (7j and 7k, 35% and 49%, respectively). Notably, we observed significant amounts of the isomeric products in our study of 2-azaallyl anion propellylation/protonation when ketimines bearing electron-donating groups were used.21 Despite the sterically hindered nature of ortho-methyl substituted imine 4l, the desired product was obtained in good yield, affording a mixture of 7l and 7l’ in 63% and 7% yields, respectively. Importantly, several pharmaceutically relevant heterocycle-derived ketimines also provided products in synthetically useful yields. 3-Pyridyl-substituted 7m was generated in 41% yield after heating at 60 °C for 16 h and morpholine-substituted pyridine product 7n could be formed in 39% yield. Moreover, a pyrrole and benzothiophene group could also be smoothly incorporated into products 7o and 7p, respectively. Finally, while most of these experiments were conducted on 0.2 mmol scale, the reaction could be easily scaled-up without any detrimental impact on the yield. For instance, a 5.0 mmol scale reaction of ketimine 4i produced 1.8 g (73% yield) of the corresponding BCP Bpin product 7i. We note that, despite the indefinite shelf-stability of BCP BPin products 7 to air and moisture, the high sensitivity of these compounds towards silica and alumina necessitates their purification on C18-endcapped reversed-phase silica gel.
Scheme 2. Scope of the propellylation/borylation reaction. All products were generated from ketimines 4 except for 7h and 7o, which were generated from aldimines 4h′ and 4o′, respectively, due to ease of synthetic access. a 60 °C, 16 h.
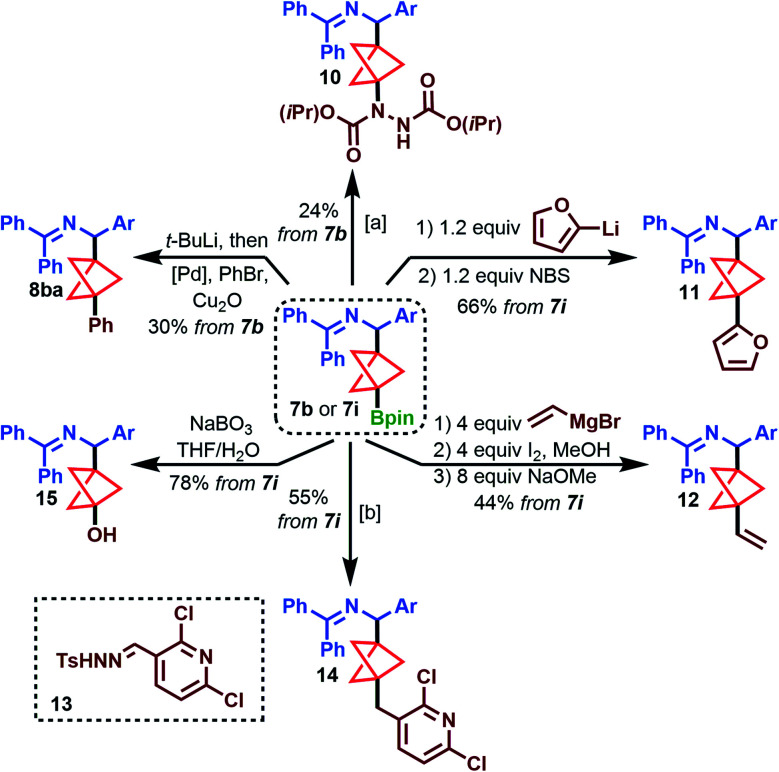
To demonstrate the utility of these borylated products, we turned our attention to functionalization of the tertiary Bpin group, using 7b and 7i as model substrates (Scheme 3). C–N bond formation using DIAD furnished hydrazine dicarboxylate 10 in 24% yield (top).28 Aggarwal's conditions for oxidative arylation with electron-rich arenes were also tolerated, producing furylated product 11 in 66% yield from 7i (top right). To further demonstrate the compatibility of substrates 7 with certain organometallic reagents, 7i was subjected to Zweifel olefination conditions, giving the vinylated product 12 in 44% yield (bottom right).29 A recent modification on the Barluenga–Valdés reaction, which couples alkylboronic acids with sulfonylhydrazones,30 was successful with the boronic acid of 7i and sulfonylhydrazone 13 to produce 14 in 55% yield (bottom). Furthermore, oxidation of 7i to alcohol 15 proceeded smoothly in 78% yield (bottom left).
Scheme 3. Functionalization of the benzylamine BCP Bpin. [a] (i) 1.2 equiv. 4-lithioanisole, THF, −78 °C, 30 m, then rt, 30 m. (ii) 2.5 equiv. diisopropyl azodicarboxylate (DIAD), rt, 16 h. [b] 7i used as 2.2 equiv. relative to limiting reagent, tosylsulfonylhydrazone. (i) 2.2 equiv. NaIO4, THF/H2O, 1 h, then sat'd aq. NH4Cl, 24 h; 1 equiv. 13, 1.5 equiv. Cs2CO3, 1,4-dioxane, 110 °C, 18 h.
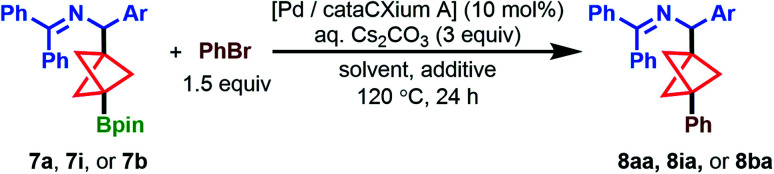
To further explore the utility of these BCP Bpin products, we were interested in examining their cross-coupling with aryl halide partners. Following the state-of-the-art in BCP Suzuki cross-coupling, we attempted Uchiyama's arylation of BCP Bpin derivatives via sequential t-BuLi activation and Pd catalysis (Scheme 3, top left).15 We found that subjecting 7b to these literature conditions gave 30% yield of the bromobenzene-derived arylated product 8ba. This result might reflect some level of incompatibility of benzylamine BCP pinacol boronates with t-BuLi, most likely due to competitive attack of the highly reactive organolithium reagent at the boron center and at the acidic benzylic proton of the ketimine. Moreover, t-BuLi is expected to be incompatible with halogenated substrates such as 7c and 7d due to competitive metal–halogen exchange. Thus, we sought to develop a more general BCP Bpin arylation protocol that did not require pre-activation of the Bpin ester.15
We initially utilized high-throughput experimentation (HTE) to broadly survey potentially viable catalysts and solvents for the arylation of ketimine BCP Bpin 7a with PhBr (Table 2). A screen of six phosphine ligands [cataCXium A (n-BuP(1-Ad)2), P(t-Bu)3, DTBPF, RuPhos, XPhos, and sSPhos] and 4 solvents (PhMe, CPME, t-AmOH, and DMF; 0.1 M) in the presence of aq. Cs2CO3 (3 equiv.) at 100 °C revealed cataCXium A and P(t-Bu)3 to be the most effective ligands for arylation of 7a (2.5 μmol scale; see ESI, S33–S37†). Unfortunately, bench-scale evaluation of the best result (cataCXium A in PhMe, 0.05 mmol scale) showed only 5% AY of the arylation product 8aa with 71% AY of 7a remaining (Table 1, entry 1). Increasing the reaction temperature to 120 °C under otherwise identical conditions improved the AY to 21%, leading us to continue our optimization efforts at this temperature (entry 2). Extensive screening of potential ligands, bases, and solvents at 120 °C confirmed cataCXium A as the top performing ligand and showed CPME/aq. Cs2CO3 to be the most effective solvent/base combination.
Optimization of the BCP Bpin cross-coupling reaction.
| |||||
|---|---|---|---|---|---|
| Entry | BCP Bpin | Catalysta | Solv., add. | % 8b | % 7 remainingb |
| 1c | 7a | cataCXium A G2 | PhMe (0.1 M) | 5 | 71 |
| 2 | 7a | cataCXium A G2 | PhMe (0.1 M) | 21 | 55 |
| 3 | 7i | cataCXium A G2 | CPME (0.1 M) | 43 | 46 |
| 4 | 7i | cataCXium A G2 | CPME (0.4 M) | 55 | 25 |
| 5 | 7i | Pd(OAc)2/cataCXium A (1 : 2) | CPME (0.4 M) | 62 | 9 |
| 6 | 7b | Pd(OAc) 2 /cataCXium A (1 : 2) | CPME (0.4 M), Cu 2 O (1.0 equiv.) | 69 (53% IY) | 18 |
| 7 | 7b | Pd(OAc)2/cataCXium A (1 : 2) | CPME (0.4 M), Cu2O (2.0 equiv.) | 56 | 8 |
CataCXium A: (n-Bu)P(1-Ad)2, di(1-adamantyl)-n-butylphosphine.
AY as determined by 1H NMR analysis of the crude reaction mixture against CH2Br2 as an internal standard.
Reaction performed at 100 °C, 0.1 M.
To avoid complications in reaction analysis (with C3 isomer 7a’ being present in our starting material), we opted to utilize substrate 7i during our continued optimization. Under these conditions, arylation of 7i with PhBr gave a promising 43% AY of 8ia, albeit with significant quantities of 7i remaining (entry 3). We found that increasing the reaction concentration 4× (to 0.4 M) improved the yield to 55% (entry 4). During our optimization, we identified an N-arylated carbazole side product, which formed from the Pd precatalyst and co-eluted with 8ia during chromatographic separation. By replacing the precatalyst with Pd(OAc)2 and cataCXium A in a 1 : 2 ratio, we effectively eliminated its presence and improved the AY to 62%. It is worth noting that at this stage we opted to complete our optimization of this arylation reaction on substrate 7b rather than 7i, due to the latter's propensity to undergo C–H arylation between the fluoro groups on the 3,5-F2-C6H3 ring when certain aryl bromides were used.
Copper(i) oxide (Cu2O) has recently been reported to facilitate arylation of sterically encumbered nucleophiles15,31 and we hypothesized that this additive could also prove beneficial for our coupling. Gratifyingly, we found that the addition of 1 equiv. Cu2O further improved the yield to 69% AY (53% IY of 8ba, entry 6). Employing 2 equiv. of Cu2O was found to slightly diminish the yield to 56% AY (entry 7).
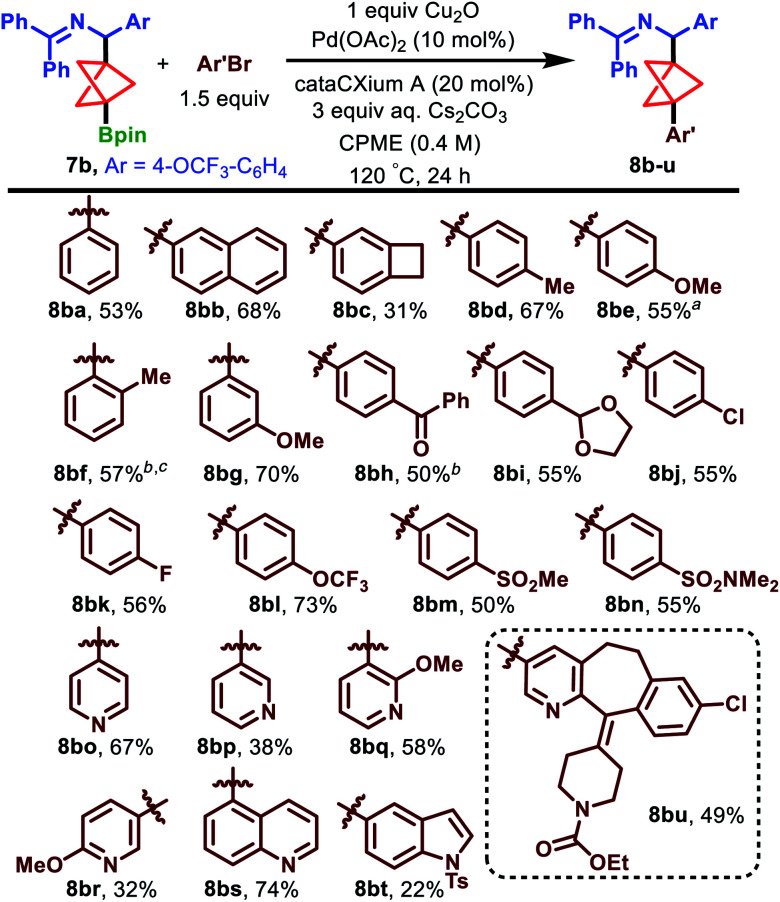
Having optimized the conditions for the challenging arylation of our BCP Bpin building blocks, we probed the scope of this transformation using substrate 7b. We first examined electron-neutral aryl bromides in the coupling reaction (Scheme 4). 2-Naphthyl and 3-benzocyclobutyl groups were tolerated in the reaction, producing 8bb and 8bc in 68% and 31% yield, respectively. The low yield of 8bc is attributed to a high proportion of unreacted starting material (32% AY) as well as significant quantities of homocoupled aryl bromide (Ar′–Ar′), as determined by 1H NMR analysis of the crude mixture.
Scheme 4. Scope of the cross-coupling reaction. aMicrowave irradiation conditions, 100 °C, 24 h, using 7b and 4-bromoanisole, gave 73% AY, compared to 77% AY for standard conditions (as shown, heating in an oil bath). bNo Cu2O additive. cn-Bu2O solvent, 140 °C.
Electron-rich substrates 4-bromotoluene and 4-bromoanisole delivered arylation products 8bd and 8be in 67% and 55% yield, respectively. Notably, 2-bromotoluene was successfully coupled upon heating to 140 °C in n-Bu2O and in the absence of Cu2O, producing the highly encumbered 8bf in 57% yield.
We next turned to electron-poor aryl bromides. 3-Bromoanisole and 4-bromoacetophenone furnished the arylation products 8bg and 8bh in 70% and 50% yield, respectively. Interestingly, the latter substrate performed more effectively in the absence of Cu2O. Moreover, para-1,3-dioxolo-, halo-, trifluoromethoxy-, and sulfonyl-derived aryl bromides each performed well and formed products 8bi–8bn in 50–73% yield.
Heterocyclic aryl bromides are absent in the existing BCP Bpin cross-coupling literature and, given their importance in medicinal chemistry, we were pleased to observe the coupling of 4- and 3-bromopyridine to deliver 8bo and 8bp, respectively, in synthetically useful yields of 67% and 38%.32 Substitution of the pyridine was also tolerated, as demonstrated by the formation of methoxy-substituted pyridines 8bq and 8br in 58% and 32% yield, respectively. Moreover, both quinoline (8bs, 74%) and indole (8bt, 22%) substrates could be coupled under the standard conditions. We observed a 49% yield of 8bu when a loratadine-derived aryl bromide was employed in the reaction, further demonstrating the compatibility of hydrolytically sensitive functional groups, nitrogen heterocycles, and aryl chlorides under these conditions. Lastly, we established that the arylation is also effective at slightly lower temperatures when conducted under microwave irradiation. Specifically, compound 8be was produced in 73% AY after 24 h at 100 °C under microwave irradiation (cf. 77% AY, 55% IY under the standard conditions at 120 °C in an oil bath).
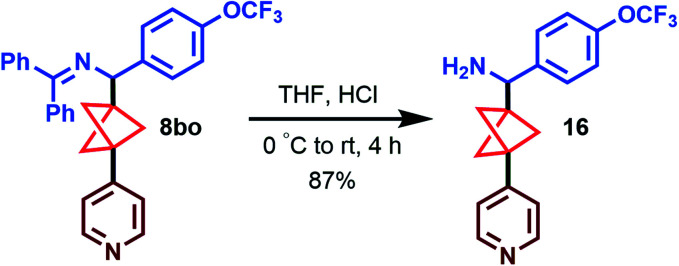
Importantly, the imine products can be readily hydrolyzed to the corresponding free amine, as exemplified by the conversion of 8bo to 16 in 87% yield (Scheme 5). This demonstrates the ease in which complex, high-value arylated BCP benzylamines can be accessed using these new methods.
Scheme 5. Hydrolysis of the arylation product.
Though our arylation conditions had been optimized for benzylamine BCP Bpin substrates (7), they could also be applied to the coupling of other tertiary pinacolboronates. The coupling of tertiary C(sp3) centers and arenes is an important area of research, with several advances being reported recently. Tertiary redox-active esters have been coupled with aryl Grignard and arylzinc reagents.33 Boracene-based alkylborates facilitate coupling of tertiary radicals derived from alkyllithiums under Ni catalysis.34 Zn/Ni-mediated catalysis has also been effective for the coupling of tertiary bromides and tertiary oxalates with aryl and acrylate-based electrophiles.35,36 Boron-based reagents that do not require pre-activation (e.g. t-BuLi activation or coordination of an alkyllithium to an arylboron-based reagent to form a photoexcitable complex), namely tertiary potassium trifluoroborates, undergo arylation in both photoredox and Pd-catalyzed contexts.37–39 We suspected that successful coupling of tertiary Bpin reagents, which are invariably the precursor to tertiary trifluoroborate salts, would enable more rapid assembly of highly desirable C(sp3)-rich scaffolds.40 Facile arylation of tertiary Bpins would also present an attractive synthetic option that precludes the use of added organometallic reagents and/or undesired synthetic manipulations.
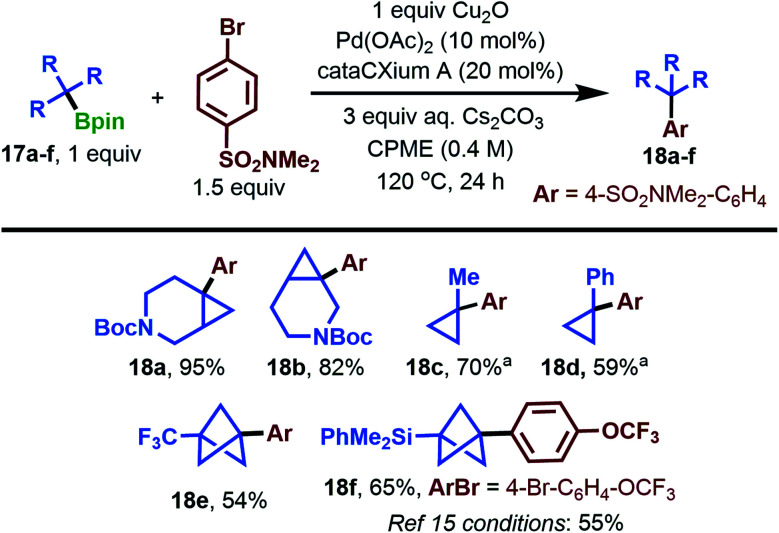
To probe the generality of our cross-coupling conditions on non-BCP substrates, we examined the arylation of several commercially available tertiary pinacol boronates 17a–f with 4-bromo-N,N-dimethylbenzenesulfonamide (Scheme 6). We were pleased to find that 3-azabicyclo[4.1.0]heptanes 17a and 17b coupled in excellent yields of 95% and 82%, respectively, saving a synthetic step from the previously reported coupling of their BF3K derivatives.39 Methylcyclopropane 17c coupled in 70% yield by inverting the ratio of Bpin and aryl bromide, which helped facilitate purification of the product. Notably, coupling of the methylcyclopropane fragment with aryl halides is virtually unreported in the present literature, with a single example producing arylated product in 6% yield.41 We next examined 1-phenylcyclopropane 17d. The BF3K analogue of this substrate was shown previously to couple using cataCXium A G2 precatalyst, Cs2CO3 in PhMe/H2O at 95 °C,39 conditions which returned only starting materials when applied to the coupling of the Bpin substrate 17d. Under our coupling conditions, a 59% yield of 1,1-diarylcyclopropane product 18d from the pinacol boronate was obtained. Efficient coupling of trifluoromethyl-substituted BCP 17e is an unsolved challenge in the current synthetic literature. In a recent report, it could only be converted to the corresponding BF3K in 4.5% yield,17 highlighting the potential utility of a coupling method which obviates the need for BF3K formation. Gratifyingly, under our standard conditions, we observed a 54% yield of the desired arylation product 18e (Scheme 6, bottom left). We also studied the arylation of Uchiyama's silaborated BCP,1517f, under our conditions. Using 1-bromo-4-(trifluoromethoxy)benzene, which was successful in Uchiyama's study to produce 18f, we obtained a 10% improvement in isolated yield (65%). This demonstrates similar efficacy of our reaction conditions versus the current state-of-the-art, but without the need for t-BuLi pre-activation.
Scheme 6. Tertiary pinacol boronate scope in the cross-coupling reaction. a Ratio of Bpin to ArBr is 1.5 : 1.
Conclusions
In summary, we have developed a 2-azaallyl anion addition/borylation reaction of [1.1.1]propellane to prepare new 1,3-difunctionalized benzylamine BCP pinacol boronates of structural relevance to a number of bioactive scaffolds. Functionalization of the boronate ester via oxidation, C–N, and C–C bond formation were shown to be effective. Further, we addressed a gap in the BCP cross-coupling literature by developing an arylation of BCP Bpin derivatives under Pd catalysis that does not require pre-activation of the boronate ester or the use of added organometallic reagents. These conditions enable facile arylation of benzylamine BCP Bpin compounds with a broad range of aryl bromides and were also readily applied to other tertiary Bpin-containing structures, including those possessing nitrogen heterocycles, cyclopropanes, and BCPs for which arylation has not yet been demonstrated. This underscores the broad utility of this method to enable the synthesis of C(sp3)-rich scaffolds in a variety of contexts.
Author contributions
The work was conceptualized by RAS, JMEH, RRM and PJW. Experiments were performed by RAS, AC, YP and JMEH. The first draft of the manuscript was prepared by RAS and the final draft was edited by all the authors.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We are grateful to Drs Cayetana Zarate, Ben Sherry, Jamie McCabe Dunn, Xiaoshen Ma and L. C. Campeau (Merck) for feedback on this manuscript. PJW thanks the NSF (CHE-1902509) and Merck for support.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc01349a
Notes and references
- He F. S. Xie S. Yao Y. Wu J. Chin. Chem. Lett. 2020;31:3065–3072. [Google Scholar]
- Dilmaç A. M. Spuling E. de Meijere A. Bräse S. Angew. Chem., Int. Ed. 2017;56:5684–5718. doi: 10.1002/anie.201603951. [DOI] [PubMed] [Google Scholar]
- Mykhailiuk P. K. Org. Biomol. Chem. 2019;17:2839–2849. doi: 10.1039/c8ob02812e. [DOI] [PubMed] [Google Scholar]
- Messner M. Kozhushkov S. I. de Meijere A. Eur. J. Org. Chem. 2000;2000:1137–1155. [Google Scholar]
- Rehm J. D. D. Ziemer B. Szeimies G. Eur. J. Org. Chem. 2001;2001:1049–1052. [Google Scholar]
- Makarov I. S. Brocklehurst C. E. Karaghiosoff K. Koch G. Knochel P. Angew. Chem., Int. Ed. 2017;56:12774–12777. doi: 10.1002/anie.201706799. [DOI] [PubMed] [Google Scholar]
- Schwärzer K. Zipse H. Karaghiosoff K. Knochel P. Angew. Chem., Int. Ed. 2020;59:20235–20241. doi: 10.1002/anie.202009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Smith R. T. Le C. McCarver S. J. Shireman B. T. Carruthers N. I. MacMillan D. W. C. Nature. 2020;580:220–226. doi: 10.1038/s41586-020-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H. Ruffoni A. Al-Faiyz Y. S. S. Sheikh N. S. Leonori D. Angew. Chem., Int. Ed. 2020;59:8225–8231. doi: 10.1002/anie.202000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa J. Maeda K. Uchiyama M. J. Am. Chem. Soc. 2017;139:17791–17794. doi: 10.1021/jacs.7b11865. [DOI] [PubMed] [Google Scholar]
- Hughes J. M. E. Scarlata D. A. Chen A. C. Y. Burch J. D. Gleason J. L. Org. Lett. 2019;21:6800–6804. doi: 10.1021/acs.orglett.9b02426. [DOI] [PubMed] [Google Scholar]
- Caputo D. F. J. Arroniz C. Dürr A. B. Mousseau J. J. Stepan A. F. Mansfield S. J. Anderson E. A. Chem. Sci. 2018;9:5295–5300. doi: 10.1039/c8sc01355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent J. Arroniz C. Shire B. R. Sterling A. J. Pickford H. D. Wong M. L. J. Mansfield S. J. Caputo D. F. J. Owen B. Mousseau J. J. Duarte F. Anderson E. A. ACS Catal. 2019;9:9568–9574. [Google Scholar]
- Nugent J. Shire B. R. Caputo D. F. J. Pickford H. D. Nightingale F. Houlsby I. T. T. Mousseau J. J. Anderson E. A. Angew. Chem., Int. Ed. 2020;132:11964–11968. doi: 10.1002/anie.202004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M. Kanazawa J. Ichikawa T. Shimokawa T. Nagashima Y. Miyamoto K. Uchiyama M. Angew. Chem., Int. Ed. 2020;59:1970–1974. doi: 10.1002/anie.201909655. [DOI] [PubMed] [Google Scholar]
- Yu S. Jing C. Noble A. Aggarwal V. K. Angew. Chem., Int. Ed. 2020;59:3917–3921. doi: 10.1002/anie.201914875. [DOI] [PubMed] [Google Scholar]
- Vanheyst M. D. Qi J. Roecker A. J. Hughes J. M. E. Cheng L. Zhao Z. Yin J. Org. Lett. 2020;22:1648–1654. doi: 10.1021/acs.orglett.0c00242. [DOI] [PubMed] [Google Scholar]
- Fawcett A. Pradeilles J. Wang Y. Mutsuga T. Myers E. L. Aggarwal V. K. Science. 2017;357:283–286. doi: 10.1126/science.aan3679. [DOI] [PubMed] [Google Scholar]
- Yang Y. Tsien J. Hughes J. M. E. Peters B. K. Rohan R. R. Qin T. ChemRxiv. 2021 [Google Scholar]
- Li M. Yücel B. Adrio J. Bellomo A. Walsh P. J. Chem. Sci. 2014;5:2383–2391. doi: 10.1039/C3SC53526F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelp R. A. Walsh P. J. Angew. Chem., Int. Ed. 2018;57:15857–15861. doi: 10.1002/anie.201810061. [DOI] [PubMed] [Google Scholar]
- Tang S. Zhang X. Sun J. Niu D. Chruma J. J. Chem. Rev. 2018;118:10393–10457. doi: 10.1021/acs.chemrev.8b00349. [DOI] [PubMed] [Google Scholar]
- Panetti G. B. Carroll P. J. Gau M. R. Manor B. C. Schelter E. J. Walsh P. J. Chem. Sci. 2021;12:4405–4410. doi: 10.1039/d0sc04822d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford C. Aggarwal V. K. Chem. Commun. 2017;53:5481–5494. doi: 10.1039/c7cc01254c. [DOI] [PubMed] [Google Scholar]
- Fyfe J. W. B. Watson A. J. B. Chem. 2017;3:31–55. [Google Scholar]
- Crudden C. M. Ziebenhaus C. Rygus J. P. G. Ghozati K. Unsworth P. J. Nambo M. Voth S. Hutchinson M. Laberge V. S. Maekawa Y. Imao D. Nat. Commun. 2016;7:11065. doi: 10.1038/ncomms11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeschger R. Su B. Yu I. Ehinger C. Romero E. He S. Hartwig J. Science. 2020;368:736–741. doi: 10.1126/science.aba6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche-Gauthier R. Elford T. G. Aggarwal V. K. J. Am. Chem. Soc. 2011;133:16794–16797. doi: 10.1021/ja2077813. [DOI] [PubMed] [Google Scholar]
- Bonet A. Odachowski M. Leonori D. Essafi S. Aggarwal V. K. Nat. Chem. 2014;6:584–589. doi: 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
- Merchant R. R. Lopez J. A. Org. Lett. 2020;22:2271–2275. doi: 10.1021/acs.orglett.0c00471. [DOI] [PubMed] [Google Scholar]
- Oi M. Takita R. Kanazawa J. Muranaka A. Wang C. Uchiyama M. Chem. Sci. 2019;10:6107–6112. doi: 10.1039/c9sc00891h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. Lee S. Choi W. Kim N. Hong S. Angew. Chem., Int. Ed. 2021;60:7873–7879. doi: 10.1002/anie.202016156. [DOI] [PubMed] [Google Scholar]
- Toriyama F. Cornella J. Wimmer L. Chen T. G. Dixon D. D. Creech G. Baran P. S. J. Am. Chem. Soc. 2016;138:11132–11135. doi: 10.1021/jacs.6b07172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y. Nakamura K. Sumida Y. Sumida Y. Hashizume D. Hosoya T. Hosoya T. Ohmiya H. Ohmiya H. J. Am. Chem. Soc. 2020;142:9938–9943. doi: 10.1021/jacs.0c04456. [DOI] [PubMed] [Google Scholar]
- Wang X. Wang S. Xue W. Gong H. J. Am. Chem. Soc. 2015;137:11562–11565. doi: 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]
- Ye Y. Chen H. Sessler J. L. Gong H. J. Am. Chem. Soc. 2019;141:820–824. doi: 10.1021/jacs.8b12801. [DOI] [PubMed] [Google Scholar]
- Primer D. N. Molander G. A. J. Am. Chem. Soc. 2017;139:9847–9850. doi: 10.1021/jacs.7b06288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. R. Li Q. Lian Y. Xiao J. Londregan A. T. Org. Lett. 2017;19:2450–2453. doi: 10.1021/acs.orglett.7b01097. [DOI] [PubMed] [Google Scholar]
- Harris M. R. Wisniewska H. M. Jiao W. Wang X. Bradow J. N. Org. Lett. 2018;20:2867–2871. doi: 10.1021/acs.orglett.8b00899. [DOI] [PubMed] [Google Scholar]
- Lovering F. Bikker J. Humblet C. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Dombrowski A. W. Gesmundo N. J. Aguirre A. L. Sarris K. A. Young J. M. Bogdan A. R. Martin M. C. Gedeon S. Wang Y. ACS Med. Chem. Lett. 2020;11:597–604. doi: 10.1021/acsmedchemlett.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.